Red blood cells stored for increasing periods produce progressive impairments in nitric oxide–mediated vasodilation (original) (raw)
. Author manuscript; available in PMC: 2014 Aug 21.
Published in final edited form as: Transfusion. 2013 Mar 11;53(11):2619–2628. doi: 10.1111/trf.12111
Abstract
BACKGROUND
Clinical outcomes in transfused patients may be affected by the duration of blood storage, possibly due to red blood cell (RBC)-mediated disruption of nitric oxide (NO) signaling, a key regulator of vascular tone and blood flow.
STUDY DESIGN AND METHODS
AS-1 RBC units stored up to 42 days were sampled at selected storage times. Samples were added to aortic rings ex vivo, a system where NO-mediated vasodilation could be experimentally controlled.
RESULTS
RBC units showed storage-dependent changes in plasma hemoglobin (Hb), RBC 2,3-diphosphoglycerate acid, and RBC adenosine triphosphate conforming to expected profiles. When freshly collected (Day 0) blood was added to rat aortic rings, methacholine (MCh) stimulated substantial NO-mediated vasodilation. In contrast, MCh produced no vasodilation in the presence of blood stored for 42 days. Surprisingly, the vasoinhibitory effects of stored RBCs were almost totally mediated by RBCs themselves: removal of the supernatant did not attenuate the inhibitory effects, while addition of supernatant alone to the aortic rings only minimally inhibited MCh-stimulated relaxation. Stored RBCs did not inhibit vasodilation by a direct NO donor, demonstrating that the RBC-mediated vasoinhibitory mechanism did not work by NO scavenging.
CONCLUSIONS
These studies have revealed a previously unrecognized vasoinhibitory activity of stored RBCs, which is more potent than the described effects of free Hb and works through a different mechanism that does not involve NO scavenging but may function by reducing endothelial NO production. Through this novel mechanism, transfusion of small volumes of stored blood may be able to disrupt physiologic vasodilatory responses and thereby possibly cause adverse clinical outcomes.
Blood transfusion is the most commonly employed procedure for hospitalized patients in the United States, based on discharge codes.1 Optimal functioning of this system depends on the ability to store blood for up to 42 days before transfusion. Studies used to support 42-day post-donation storage include biochemical measurements (2,3-diphosphoglycerate acid [2,3-DPG] and adenosine triphosphate [ATP]), measures of red blood cell (RBC) integrity (plasma free hemoglobin [Hb]), and quantification of survival of stored RBCs in autologous transfusion recipients at 24 hours after transfusion.2,3 However, there are no specific measurements performed to show that RBCs stored up to 42 days achieve minimal standards of efficacy or produce acceptably low rates of adverse events in transfusion recipients.
Despite the undeniable therapeutic benefits of blood transfusion, numerous studies have demonstrated significant biochemical, structural, and morphologic changes in RBCs during pretransfusion storage.4-6 These changes (the “RBC storage lesion”) may be of negligible impact after short storage periods (“fresh RBCs”), but longer-term storage approaching 42 days (“storage-aged RBCs”; saRBCs) may have deleterious effects on the recipient.
Tinmouth and coworkers7 and Wang and coworkers8 have performed systematic reviews of dozens of studies that investigated the relationship between blood storage and adverse transfusion events. Meta-analyses showed worse recipient outcomes after transfusion of saRBCs. Since the largest clinical studies included in these reviews were retrospective, further elucidation of the possible adverse effects of saRBCs may be provided by prospective randomized trials. The largest to be published to date, ARIPI,9 compared fresh blood (stored up to 7 days; median, 5 days) with standard of care (stored up to 42 days; median, 13 days) in low-birthweight neonates. Although the outcomes showed no difference between study arms, the relatively short storage times in the standard-of-care arm do not allow an assessment of the efficacy of saRBCs stored for long periods (21-42 days). This issue may be better addressed in the ongoing RECESS,10 ABLE,11 and Red Cell Storage Duration and Outcomes in Cardiac Surgery studies.12
As an adjunct to biochemical, molecular, and clinical outcomes studies, investigations of the acute physiologic effects of saRBCs may identify potential mechanisms by which saRBC transfusions could cause adverse outcomes. In addition to oxygen and carbon dioxide transport, another significant physiologic role of RBCs is in the process of hypoxic vasodilation. This activity, which regulates local blood flow to preferentially perfuse and provide oxygen for the most hypoxic tissues, involves combined activities of RBCs and endothelial cells to regulate arteriolar smooth muscle tone.13 Disruption of hypoxic vasodilation by saRBCs represents a viable physiologic hypothesis to explain an association between blood storage and adverse transfusion events.14
Although hypoxic vasodilation is not yet fully understood at a biochemical level, it is likely to involve regulation of nitric oxide (NO) signaling. Theoretically, if saRBCs did not produce or stimulate sufficient NO, then replacement of a patient’s normal RBCs with transfused cells could result in an NO synthesis defect. There is some evidence for this possibility. For example, studies show that _S_-nitrosylation of Hb (SNO-Hb) may produce a storage pool of NO within RBCs that can be released in hypoxic environments,15-18 and _S_-nitrosylation of Hb has been shown to decline with RBC storage.19 Other investigators have shown that nitrite (which is present at high levels in plasma) can be converted to NO by Hb.20-23 In this model, deoxygenated Hb, which is at high concentrations in hypoxic tissues, binds nitrite and a proton to generate NO and methemoglobin. If blood storage altered the nitrite reductase activity of Hb, this NO source would be disrupted. With either of these models, NO must diffuse from the RBC to the smooth muscle to produce vasodilation.24,25 In a third model that could potentially explain reduced NO production with saRBCs, ATP release by RBCs (a robust mechanism for stimulating endothelial NO production26-29) may be impaired by storage.30
As an alternative mechanism that could also underlie reduced hypoxic vasodilation after transfusion, infused saRBCs could produce an inhibitory factor that interrupts normal NO signaling between endogenous RBCs, endothelium, and the underlying smooth muscle. This mechanism, which may function in conjunction with (or instead of) the synthesis defects described above, is attractive because it could allow a small volume of transfused saRBCs to broadly impair hypoxic vasodilation. Disruption of NO signaling by saRBCs could be mediated by free Hb (or other RBC constituents) released into the plasma as a consequence of RBC hemolysis or by Hb or other factors encapsulated in RBC-derived microparticles.4,24,25 The free Hb mechanism is supported by clinical and experimental studies showing that plasma free Hb can scavenge NO, reducing its bioavailability and causing vascular sequelae.31-33 Microparticles may be important because they can flow closer to the endothelium than intact RBCs, bringing Hb close to the sites of NO synthesis, which may further accentuate NO scavenging after transfusion.34 Although intact saRBCs could also potentially interfere with NO-mediated vasodilation, there is currently little evidence for this mechanism.
In these studies, we used an ex vivo system where endothelium-dependent, NO-mediated vasodilation can be experimentally controlled and sensitively monitored to assess the impact of fresh blood versus saRBCs on vasodilation. This system has been previously employed to probe interactions between RBCs and the vasculature and to demonstrate how RBC-derived ATP and deoxyhemoglobin-catalyzed NO production can stimulate vasodilation.14 Using this model, we have instead focused on determining whether saRBCs can interfere with normal NO-mediated vasodilatory activities and to what extent the inhibitory effect is mediated by substances, such as Hb, released from saRBCs, or by an alternate mechanism.
MATERIALS AND METHODS
Blood collection, preparation, and sampling
All protocols were approved by the Emory University Institutional Review Board. Recruited research donors were screened by health history questionnaire and vital signs. 500 mL (±10%) was drawn by peripheral venipuncture into CPD-containing blood collection sets (Fenwal, Inc., Lake Zurich, IL). Bag sets were stored at 2 to 6°C for 1 to 3 hours and then leukoreduced using the integral filter according to manufacturer’s instructions. Filtered blood was then centrifuged at 4530 × g for 10 minutes at 4°C. Platelet-rich plasma was expressed into a satellite bag and discarded. The residual RBC pellet was mixed with AS-1 and resuspended with gentle agitation. The RBCs were then stored at 2 to 6°C for up to 42 days in a monitored refrigerator. Additional blood samples obtained at the time of collection were used for infectious disease testing.
At selected time points during the storage period, RBC bags were gently but thoroughly agitated to assure homogeneity, and then 10-mL samples were aseptically removed by syringe through a site coupler. Five milliliters of each aliquot was used for aortic ring bioassays. The remaining sample volume was used for biochemical measurements, as described below.
Aorta preparation
All animal studies were completed in compliance with protocols approved by the Atlanta VA Institutional Animal Care and Use Committee. Rats were euthanized and aortas were carefully excised, cleaned of loose fat and connective tissue, and maintained in physiologic saline solution (Krebs-Henseleit buffer: 118 mmol/L NaCl, 4.73 mmol/L KCl, 1.2 mmol/L MgSO4, 0.025 mmol/L EDTA, 1.2 mmol/L KH2PO4, 2.5 mmol/L CaCl2, 11 mmol/L glucose, and 25 mmol/L NaH2CO3, pH 7.4, in 95% to 5% CO2 at 37°C). Care was taken to ensure that aortas were free of blood. All chemicals were of highest purity and purchased from Sigma (St Louis, MO).
Blood preparation for ex vivo aortic relaxation
For initial studies, unmanipulated blood samples were added directly to the aortic ring baths at the selected concentration. In subsequent studies, plasma and RBC fractions were tested separately for their effects on aortic ring vasoresponsiveness. For example, to model clinical volume reduction approaches, samples were centrifuged at 5000 × g for 5 minutes, the supernatant was removed, and then the resulting RBC pellet was added to the organ baths. To model cell washing approaches, after centrifugation the supernatant anticoagulant and preservative was removed and replaced with saline (0.9% NaCl). This washing step was repeated three times before adding the washed RBCs to the aortic rings.
Endothelium-dependent relaxation
Studies examining endothelium-dependent relaxation were performed as we have described previously.35 Briefly, 5-mm segments of thoracic aorta were mounted between stainless-steel wires in an organ chamber containing Krebs-Henseleit buffer and connected to a Harvard apparatus differential capacitor force transducer. For each aorta, resting tension was adjusted to 50 mN over a 1-hour period. After precontraction with 300 nmol/L phenylephrine, a concentration that yields 80% maximum contraction, relaxation was examined after addition of the endothelium-dependent vasorelaxant methacholine (MCh; 0.1 nmol/L to 10 μmol/L) and the endothelium-independent NO-donor sodium nitroprusside (SNP; 0.1 to 300 nmol/L). To determine the effects of whole blood or blood fractions, stored for varying durations, aortas were incubated with whole blood at a final concentration of 1% (or equivalent amounts of separated RBC or plasma) for 30 minutes before the addition of each vasorelaxant. In other studies, MCh was added first to induce vasodilation, followed by the addition of RBCs to determine their effects on relaxed vessels. Data were recorded using digital acquisition and analyzed using software (PowerLab and Chart, respectively, AD Instruments, Colorado Springs, CO). Vasodilation was expressed as a mean percentage relaxation relative to the precontracted vessel (defined as 0% relaxation) ± standard error of the mean. Unpaired t tests and Tukey’s post hoc analysis tests were used to compare changes in mean relaxation of vessels in response to graded MCh concentrations in the presence of blood stored between 0 and 42 days.
Hb quantitation
Total Hb was measured in aliquots of the RBC samples using a photometer (HemoCue instrument, HemoCue, Inc., Cypress, CA) according to manufacturer’s instructions. In addition, plasma free Hb was determined by spectrophotometry. Standards utilized were from cyanmethemoglobin standard set (Count-A-Part, Diagnostic Technology, Inc., Hauppage, NY). Drabkin’s reagent was used to make 1:2 dilutions of the Hb standards (24.0 and 77.4 mg/dL) to generate a standard curve. Samples were centrifuged at 1940 × g for 10 minutes at 4°C, and the plasma supernatant was removed to another tube. The plasma was centrifuged again under the same conditions, and the supernatant was removed to a clean tube and then diluted in Drabkin’s reagent and concentrations were calculated in reference to the standard curve. The assay was performed at an absorbance of 540 nm with standards and samples being run simultaneously within the same 96-well plate. Drabkin’s reagent was used as a blank to correct for background and subtracted from the resultant value of each well.
2,3-DPG assay and ATP assays
To stabilize 2,3-DPG and ATP in blood samples for subsequent quantitation, perchloric acid extraction was performed. A 1-mL blood sample was added to a tube containing 2 mL of 5% perchloric acid, which was being continuously mixed. The solution was maintained at 4°C for 20 minutes and then centrifuged at 4°C for 10 minutes at 1940 × g. The clear supernatant was transferred to a clean tube and centrifuged again. Two milliliters of the resulting supernatant was transferred to a clean tube and, while vigorously mixing, 300 μL of 3 mol/L K2CO3 was added. The resulting precipitate was allowed to settle for 20 minutes at 4°C. The extract was then centrifuged at 1940 × g (4°C) for 10 minutes. The resulting clear, neutralized supernatant was transferred to a clean tube; a 0.3-mL aliquot was set aside at 4°C for 2,3-DPG quantitation and the remaining volume was capped tightly and stored frozen at −80°C in aliquots. The perchloric acid extract was tested for 2,3-DPG and ATP content utilizing commercially available kits (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s instructions. 2,3-DPG content of the blood samples was assayed within 24 hours of extraction and the concentration was calculated using correction factors provided by the manufacturer. ATP content was calculated using an internal standard curve.
Analysis of nitrite and nitrate levels in stored blood
Blood samples were collected in distilled water-rinsed centrifuge tubes containing 100 μL of 100 mmol/L _N_-ethylmaleimide and 5 μL of 0.5 mmol/L EDTA. Blood samples were then centrifuged to obtain a plasma sample. Plasma samples were flash frozen and stored at −80°C until further analysis. At the time of measurement, plasma samples were thawed on ice and nitrite and nitrate concentrations were quantified by ion chromatography using an analyzer (ENO20, Eicom USA, San Diego, CA) as previously described.36
RESULTS
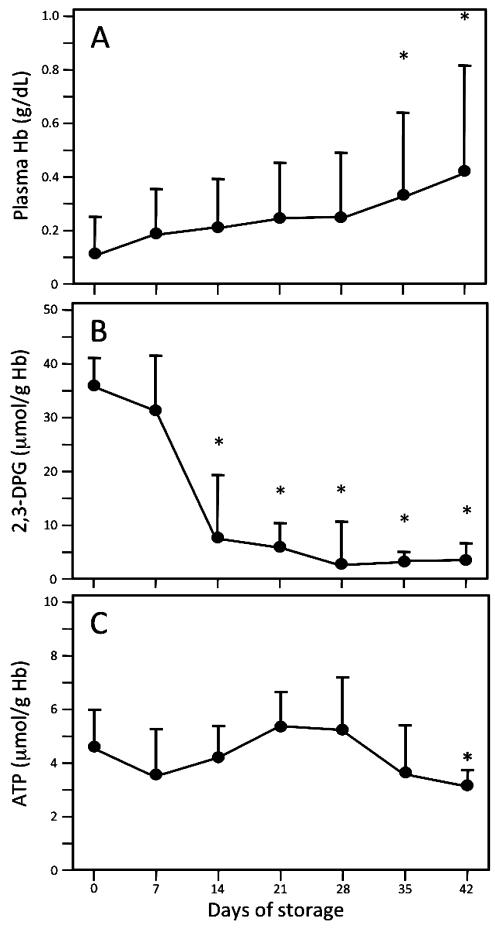
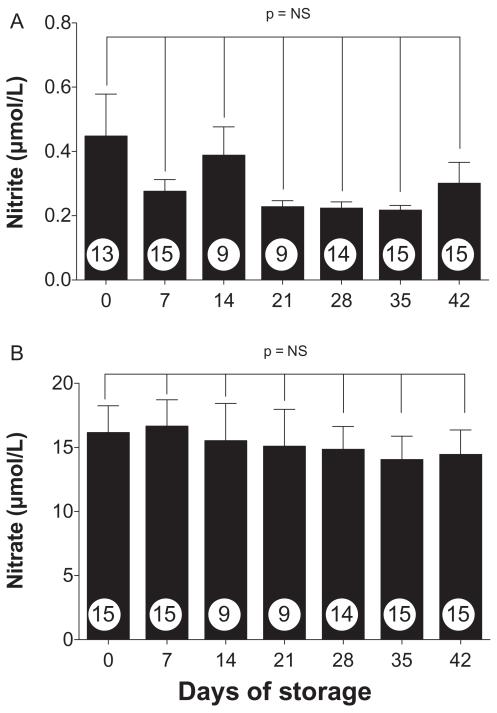
RBC units collected, leukoreduced, and stored according to standard clinical protocols were periodically sampled to confirm that they exhibited the expected biochemical changes with storage. Figure 1 shows alterations in plasma Hb (a marker of hemolysis; Fig. 1A), 2,3-DPG (Fig. 1B), and ATP (Fig. 1C) over the 42-day storage period (n = 8-15 samples per data point). Hemolysis, while increasing progressively during storage (Fig. 1A), remained low during the entire storage time, with mean plasma Hb levels less than 0.45 g/dL (approx. 1% hemolysis) at all time points. Changes in 2,3-DPG and ATP concentrations were comparable to those seen in previous studies.37-40 We also quantified plasma nitrate and nitrite during RBC storage (Fig 2). Since nitrite can be enzymatically converted to NO by deoxyhemoglobin,22 changes in nitrite concentrations during storage could alter the ability of transfusions to influence NO-mediated vasodilation. However, although there was a trend toward reduced nitrite levels with longer storage times (Fig. 2A), it did not reach significance (p > 0.05); similarly, changes in nitrate levels during storage were not significant (Fig. 2B).
Fig. 1.
Alterations in plasma free Hb, 2,3-DPG, and ATP during RBC storage. Serial aliquots, removed from saRBC units every 7 days over 42 days of storage, were assayed as described under Materials and Methods (n = 8-15 samples per data point). (A) Compared to Day 0, hemolysis was significantly greater on Days 35 and 42 of storage (*p < 0.05). However, mean plasma Hb on Day 42 (0.42 g/dL) represents less than 1% hemolysis (approx. 0.45 g/dL). (B) 2,3-DPG levels declined progressively with storage, as previously documented (*p < 0.05 vs. Day 0 sample). (C) ATP levels seen in stored blood were comparable to those previously described in the literature; compared to Day 28, the decrease seen on Day 42 was significant (*p < 0.05).
Fig. 2.
Quantitation of plasma nitrite and nitrate during RBC storage. Plasma samples obtained during the storage period were tested by ion chromatography to quantify nitrite (A) and nitrate (B) concentrations (n = 9-15 samples per data point). No significant storage-related changes were observed at any of the time points (p > 0.05).
To determine whether fresh versus saRBCs exerted differential effects on NO-mediated endothelium-dependent vasodilation, rat aortic rings were tested in the presence of samples collected at different times from RBC units. Before addition of blood, all rings were confirmed to contract and dilate appropriately in response to phenylephrine and MCh, respectively. RBC samples by themselves (in the absence of MCh) did not stimulate vasorelaxation or vasoconstriction (data not shown).
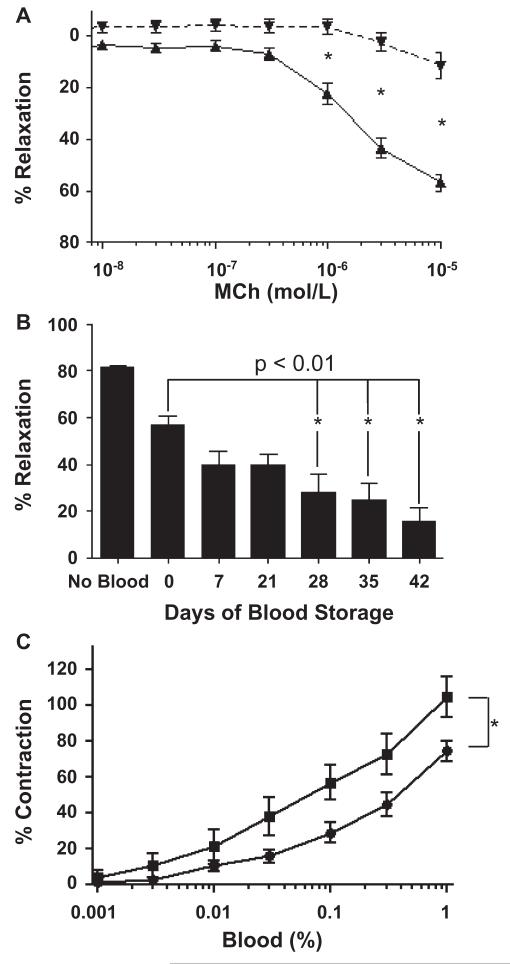
As shown in Fig. 3A, aortic rings in the presence of fresh unstored blood (Day 0) exhibited up to 60% mean relaxation in response to 10−5 mol/L MCh concentrations (n = 14-19 separate experiments per data point). However, in the presence of RBCs stored for 42 days, there was no significant relaxation even at the highest MCh concentrations. The magnitude of MCh-stimulated relaxation was significantly different between fresh versus Day 42 blood (p < 0.001) at MCh concentrations of 1 μmol/L and greater. We next investigated in more detail how the duration of blood storage altered the effects of blood on vasodilation. As seen in Fig. 3B, the mean maximal MCh-stimulated relaxation of vessels in the absence of whole blood was 81.6 ± 6.4%, while the addition of unstored blood (Day 0) modestly attenuated maximal relaxation to 56.9 ± 3.4% (n = 13-19). As blood storage time increased, maximal relaxation markedly decreased. Compared to Day 0 blood, blood storage times of 28 to 42 days produced significantly greater inhibition of vasodilation (p < 0.01). The maximal relaxation in the presence of Day 42 stored blood was only 11.3 ± 4.9%.
Fig. 3.
Blood storage time correlates with decreased endothelium-dependent vasorelaxation. Vasorelaxation of rat aortic segments was quantified in response to increasing concentrations of the endothelium-dependent vasorelaxant MCh. (A) MCh stimulated marked vasorelaxation in the presence of Day 0 saRBCs ( , 1% final concentration), but not Day 42 saRBCs (
, 1% final concentration), but not Day 42 saRBCs ( , *p < 0.001; n = 14-19 experiments per data point). (B) Time course analysis shows that blood stored 28 days or longer significantly inhibits vasodilation compared to Day 0 saRBCs (* p < 0.01; n = 62 [no blood], n = 10-19 [data points with added blood]). (C) Day 42 saRBCs (
, *p < 0.001; n = 14-19 experiments per data point). (B) Time course analysis shows that blood stored 28 days or longer significantly inhibits vasodilation compared to Day 0 saRBCs (* p < 0.01; n = 62 [no blood], n = 10-19 [data points with added blood]). (C) Day 42 saRBCs ( ) were more effective than Day 0 samples (
) were more effective than Day 0 samples ( ) at stimulating vasoconstriction when added to prerelaxed vessels (*p < 0.01 for two curves by two-way analysis of variance; n = 6 samples per data point).
) at stimulating vasoconstriction when added to prerelaxed vessels (*p < 0.01 for two curves by two-way analysis of variance; n = 6 samples per data point).
The studies described above showed that stored blood could inhibit subsequent MCh-stimulated endothelial relaxation. To determine if blood could also reverse existing relaxation (cause contraction), blood was added at increasing concentrations to vessels that were precontracted with phenylephrine and then relaxed with 10 μmol/L MCh (Fig. 3C). Samples from Day 42 blood more effectively reversed vasorelaxation than fresh blood samples (p < 0.01; n = 6).
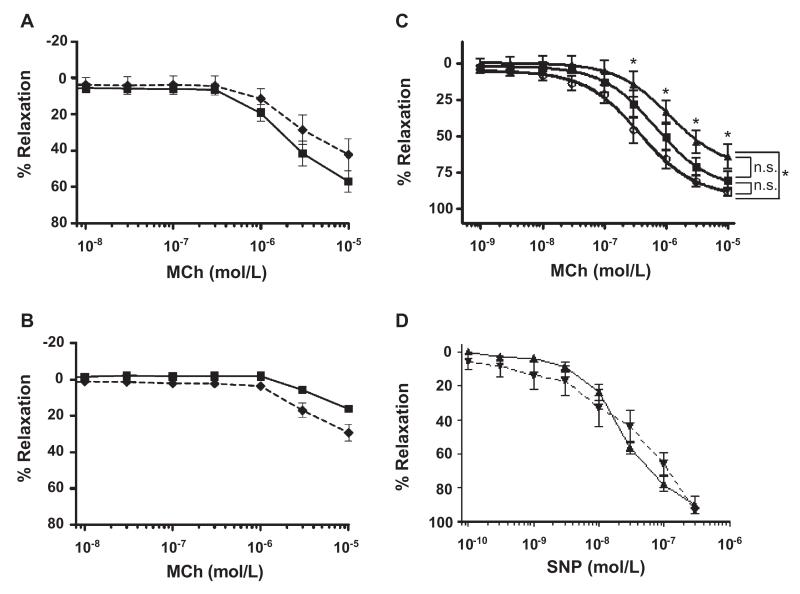
To begin to dissect the vasoinhibitory effects of saRBC samples, we investigated whether the activity was associated with the supernatant or RBC fractions. Published data suggested the former as degenerating stored RBCs release Hb and microparticles, which can reduce NO bio-availability.34 RBC samples were washed to replace the plasma-buffer supernatant with saline and then added to precontracted vessels. Washing has been shown to remove both soluble substances such as Hb and also microparticles (N. Blumberg, S. Spinelli, and R. Phipps, unpublished observations, 2012). MCh dose–response curves for blood at Days 0 and 42 of storage are shown in Figs. 4A and 4B, respectively (n = 5-11). The results for unmanipulated blood (not washed) were similar to those previously shown in Figs. 3A and 3B. Compared to these results, there was no significant effect of washing of RBCs on MCh-stimulated vasodilation. Maximal relaxation was not different between unmanipulated (57.1 ± 5.8%) and washed (42.3 ± 8.7%) Day 0 blood (Fig. 4A; p = 0.178). Values of maximal relaxation were also similar with Day 42 unmanipulated and washed blood, 16.2 ± 2.1 and 29.3 ± 4.6%, respectively (Fig. 4B; p = 0.063). Thus, washing of fresh or Day 42 RBCs does not alter their effects on NO-mediated vasodilation. As an alternative approach to remove the supernatant, which might be less traumatic to RBCs than repetitive washing, we briefly centrifuged additional samples only once and then removed the supernatant before adding the concentrated RBCs to the ring baths (modeling the clinical practice of volume reduction). This approach was likewise ineffective at abrogating the inhibitory effects of Day 42 blood on vasodilation (not shown). To rule out the possibility that washing injured RBCs and the cells then hemolyzed in the organ baths releasing Hb during the assay, we quantified free Hb in the organ baths after the MCh dose–response analysis (n = 10). Only one sample showed detectable free Hb in the bath, and it was an unwashed sample (the amount of free Hb was calculated to represent approx. 1% total RBC hemolysis).
Fig. 4.
The vasoinhibitory effects of saRBCs are primarily mediated by RBCs, not the supernatant, and do not act by scavenging NO. Day 0 (A) and Day 42 (B) saRBC samples were washed to replace the supernatant with 0.9% NaCl ( ), or left unmanipulated (
), or left unmanipulated ( ), and then added to aortic rings before MCh. Removal of supernatant by washing did not significantly affect the vasoinhibition (p > 0.05; n = 5-11 samples per data point). (C) Compared to the no supernatant added control (○), addition of Day 0 saRBC supernatant (■, n = 6) did not inhibit MCh-stimulated vasodilation (p > 0.05; NS). However, Day 42 supernatant (▲) did cause a small but significant inhibition of vasodilation (*p < 0.01) at the highest four MCh concentrations when compared to no supernatant, but not when compared to Day 0 supernatant (p > 0.05; NS). These results suggest that saRBC supernatant accounts for only a small fraction of the vasoinhibitory activity of unmanipulated saRBC samples. (D) In contrast to the inhibitory activity of saRBCs on endothelium-dependent MCh-stimulated vasodilation, saRBCs had no effect on vasodilation induced by the endothelium-independent NO-donor SNP (p > 0.05; n = 4). (
), and then added to aortic rings before MCh. Removal of supernatant by washing did not significantly affect the vasoinhibition (p > 0.05; n = 5-11 samples per data point). (C) Compared to the no supernatant added control (○), addition of Day 0 saRBC supernatant (■, n = 6) did not inhibit MCh-stimulated vasodilation (p > 0.05; NS). However, Day 42 supernatant (▲) did cause a small but significant inhibition of vasodilation (*p < 0.01) at the highest four MCh concentrations when compared to no supernatant, but not when compared to Day 0 supernatant (p > 0.05; NS). These results suggest that saRBC supernatant accounts for only a small fraction of the vasoinhibitory activity of unmanipulated saRBC samples. (D) In contrast to the inhibitory activity of saRBCs on endothelium-dependent MCh-stimulated vasodilation, saRBCs had no effect on vasodilation induced by the endothelium-independent NO-donor SNP (p > 0.05; n = 4). ( ) Day 0; (
) Day 0; ( ) Day 42.
) Day 42.
As an alternative approach to identify vasoinhibitory activity in the supernatant fraction, plasma supernatants were removed from blood units on Day 0 or Day 42 and then added to aortic rings before performing MCh dose-response curves (Fig. 4C; n = 6). The results were compared against aortic rings to which no supernatant was added. Vasodilation in the presence of Day 0 supernatant (80.5%) was not different from the responses seen without supernatant present (88.5%; p > 0.05). Addition of Day 42 supernatant to aortic rings did produce a small decrement in MCh-stimulated vasodilation (63.8% maximal relaxation), which was not different from Day 0 plasma (p > 0.05) but was significantly different from the no-supernatant control (p < 0.01). This inhibitory activity was quite small, however, compared to Day 42 blood (Figs. 3A-3C) and washed RBCs (Fig. 4B), which completely or strongly blocked MCh-stimulated vasodilation (10%-30% maximal relaxation).
As an additional approach to further probe the role of free Hb or other NO scavengers in vasoinhibition, we quantified vasodilation in the presence of blood samples and increasing concentrations of the direct NO donor SNP (Fig. 4D). If saRBC samples scavenged NO, by free Hb or other mechanisms, we would expect them to strongly inhibit SNP-initiated vasodilation. Aortic rings were exposed to increasing concentrations of SNP in the presence of 1% saRBC samples. SNP caused profound vasorelaxation, and there was no effect of blood storage time on vasorelaxation at any SNP dosage: mean maximal relaxations in the presence of Day 0 and Day 42 blood at 5 × 10−6 mol/L SNP were 99.6 ± 4.9 and 93.5 ± 1.6% (p = 0.283), respectively (n = 4). These results indicated that the inhibitory effects of stored blood were mostly accounted for by the RBC fraction, and that the saRBC inhibitory effect did not involve NO scavenging.
DISCUSSION
We and others previously proposed a “double-hit” hypothesis to explain how stored RBC units may cause deleterious outcomes in some transfusion recipients, which we termed the insufficient NO bioavailability (INOBA) hypothesis.41-44 This hypothesis postulates that both changes occurring in the RBC unit during storage and pathologic processes in the transfusion recipient (e.g., endothelial dysfunction) can independently regulate local NO levels. Together, the sum of these factors determines NO bioavailability, which controls vasodilation and blood flow. When NO bioavailability declines below a critical threshold, local blood flow and O2 delivery are insufficient to meet tissue demands, resulting in end-organ complications. Recent studies support this double-hit mechanism to explain adverse effects of old blood.43,45
Regarding the role of RBCs in the INOBA hypothesis, a number of studies have suggested that saRBCs transport and/or synthesize lower amounts of NO than fresh RBCs.14,17,19,43 However, less work has been done to investigate the alternative mechanism, also consistent with the INOBA hypothesis, that saRBCs actively disrupt normal ongoing vascular NO signaling.34 Such a dominant inhibitory effect would be physiologically significant and could be mediated by relatively small volumes of transfused RBCs. For this reason, we focused on investigating whether transfused saRBCs could disrupt vasoregulation through interference with normal NO signaling.
After addition of fresh RBC samples (Day 0 of storage) to the aortic ring baths, MCh produced marked vasodilation. In comparison, the addition of stored samples led to significant inhibition of MCh-stimulated vasodilation. These findings were consistent with the idea that banked blood is altered during storage in such a way that it markedly interferes with normal NO-mediated vasodilatory activity. The storage changes were progressive: longer periods of RBC storage significantly increased interference with physiologic vasodilatory signals to the point that no significant relaxation occurred in the presence of Day 42 saRBCs. These effects could be produced by very small volumes of saRBCs.
From a mechanistic perspective, saRBC samples could interfere with MCh-stimulated vasodilation at a number of distinct signaling points including MCh interactions with endothelial receptors, endothelial production and release of NO, and postendothelial NO signaling in the smooth muscle. While there is ongoing debate about the clinical effects of low levels of plasma free Hb on vasoreactivity,34,46 we initially anticipated that free Hb or other substances released from stored RBCs mediated the inhibitory effects by scavenging postendothelial NO. For example, Donadee and colleagues34 showed that free Hb-containing supernatant from stored human RBCs significantly increased mean arterial blood pressure when infused into anesthetized rats. However, in contrast to those results, our studies indicated that plasma supernatant constituents, including free Hb and microparticles, were not the only cause of the vasoconstrictive effect in our model. Removal of the supernatant from stored RBCs, either through volume reduction or through washing, did not improve NO-mediated vasodilation. While addition of Day 42 supernatant alone to the aortic rings did inhibit NO-mediated vasodilation, the effect was only significant when compared to aortic rings with no supernatant added; there was no difference in ring vasodilation when Day 42 supernatant was compared to Day 0 supernatant. We also considered the possibility that intact RBCs became fragile with storage, subsequently hemolyzing and releasing Hb into the aortic organ baths. However, we excluded this possibility because we could not detect a consistent increase in free Hb in the bath supernatant. Thus, while free Hb exerts some inhibitory effects on NO signaling in this system, it does not appear to account for the majority of the vasoinhibitory activity seen with stored RBC samples. Rather, the saRBCs themselves appear capable of inhibiting NO-mediated vasodilation, as others have recently suggested.47
NO is synthesized in the endothelium by NO synthase and then acts in a paracrine fashion by diffusing into the underlying smooth muscle cells to activate guanylate cyclase resulting in smooth muscle relaxation and vasodilation.48,49 Although other factors released by the endothelium are able to either relax (endothelium-derived hyperpolarizing factor) or contract (thromboxane and endothelin) smooth muscle,48,49 studies have demonstrated that endothelium-dependent relaxation of aortic segments by cholinergic agonists such as MCh is due solely to production of NO.50,51 In conjunction with this accepted mechanism, our results suggest that stored intact RBCs interfere with normal NO signaling between endothelial and smooth muscle cells. The inhibitory effect could theoretically occur at the level of NO production by the endothelium or could interfere with postendothelial signaling in the smooth muscle. To begin to dissect these possibilities, we stimulated endothelium-independent vasodilation in aortic rings using the direct NO donor SNP. In contrast to their effects on MCh-stimulated vasodilation, saRBC samples stored for any duration (Days 0-42) did not inhibit SNP-mediated vasodilation. These results indicate that rather than interfering with postendothelial NO signaling by scavenging NO, saRBCs may instead suppress endothelial NO production, although further work will be necessary to confirm this effect and determine the underlying mechanism.
Our results appear to contradict the study of Donadee and colleagues34 particularly since the levels of free Hb in our stored units (Fig. 1A) are comparable to those seen in that study. However, our results do not specifically argue against RBC-derived free Hb or microparticles causing vasoconstriction in transfusion recipients.34,52 While we have seen small vasoinhibitory effects with the RBC super-natant, the present results argue for the existence of additional distinct vasoconstrictive activity that appears to be due to the RBCs themselves: it depends on the presence of RBCs (not found in plasma), it reduces NO synthesis (does not scavenge NO), and it can completely prevent (or reverse) vasodilation produced by a strong pharmacologic stimulus. This last point is significant in that the RBC-derived inhibitory activity appears much more potent than the vasoconstrictive activity of free Hb. The blood samples were diluted 1:100 when added to the ring bath. Thus, in our studies the vasoinhibitory activities were mediated by RBCs at a final “hematocrit” of approximately 0.65%. By comparison, the Hb-containing plasma was present at a 50-fold higher concentration in the circulation of rats in the study by Donadee and colleagues. Thus the RBC activity is at least manyfold more potent than free Hb-mediated vasoconstriction, the latter of which exerted only a small effect on MCh-stimulated aortic rings (Fig. 4). Consistent with our conclusions, we note that Donadee and colleagues34 focused only on the vasoconstrictive effects of the supernatant fraction and thus did not examine whether stored RBCs could also increase blood pressure. However, Stapley and coworkers47 did specifically examine RBCs and found that they also appear capable of interfering with NO signaling.
While the aortic ring system has a number of advantages, as an experimental model it does have limitations. For example, in circulation RBCs normally only have access to the endothelial surface of blood vessels, while in these studies RBCs could potentially interact with subendothelial cells including smooth muscle. In addition, interactions between RBC and endothelium in circulation are controlled by flow mechanics, while those forces do not occur in this model. Nonetheless, this reductionist model is very useful in that it will allow the mechanisms of saRBC vasoinhibition to be dissected in ways that are not possible with more complex systems. Furthermore, we have now seen comparable inhibition of NO-mediated vasodilation with Day 35 to 42 saRBCs (but not Day 3-7 blood) in hospitalized transfusion recipients (manuscript in preparation), supporting many of the conclusions derived from the aortic ring model.
In summary, studies using in vitro aortic ring models have revealed the unexpected finding that saRBCs interrupt the normal vasodilatory responses mediated by endothelial-derived NO. These effects increase in direct relation to RBC storage time, appear to be mediated by RBCs themselves, and may occur through a novel direct effect on endothelial cells that impairs NO production. Results from the studies may lead to better methods of storing RBCs before transfusion, as well as assays to identify RBCs that could cause adverse effects after transfusion.
ABBREVIATIONS
INOBA
insufficient nitric oxide bioavailability
MCh
methacholine
NO
nitric oxide
saRBC(s)
storage-aged red blood cell(s)
SNP
sodium nitroprusside
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Wier LM, Pfuntner A, Maeda J, Stranges E, Ryan K, Jagadish P, Collins Sharp B, Elixhauser A. Health Care Cost and Utilization Project (HCUP) facts and figures, statistics on hospital-based care in the United States. Exhibit 3.1. Most frequent all-listed procedures. 2009 [cited 2013 Jan 14]. Available from: URL: http://www.hcup-us.ahrq.gov/reports/factsandfigures/2009/exhibit3_1.jsp. [Google Scholar]
- 2.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 3.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 4.Roback JD. Vascular effects of the red cell storage lesion. Hematology Am Soc Hematol Educ Program. 2011;2011:475–9. doi: 10.1182/asheducation-2011.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39:277–81. doi: 10.1046/j.1537-2995.1999.39399219284.x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tinmouth A, Fergusson D, Yee IC, Hébert PC, ABLE Investigators. Canadian Critical Care Trials Group Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, Sankaran K, Tinmouth A, Blajchman MA, Kovacs L, Lachance C, Lee S, Walker CR, Hutton B, Ducharme R, Balchin K, Ramsay T, Ford JC, Kakadekar A, Ramesh K, Shapiro S. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 10.Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, Triulzi D, Stowell CP. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 2010;43:107–16. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacroix J, Hébert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, Cook D, Marshall JC, McIntyre L, Turgeon AF, ABLE study group The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev. 2011;25:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Koch CG. The Red Cell Storage Duration and Outcomes in Cardic Surgery study. 2012 ClinicalTrials.gov. [cited 2013 Jan 14]. Available from: URL: http://clinicaltrials.gov/ct2/show/NCT00458783. [Google Scholar]
- 13.Allen BW, Piantadosi CA. How do red blood cells cause hypoxic vasodilation? The SNO-hemoglobin paradigm. Am J Physiol Heart Circ Physiol. 2006;291:H1507–12. doi: 10.1152/ajpheart.00310.2006. [DOI] [PubMed] [Google Scholar]
- 14.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–74. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–49. [PubMed] [Google Scholar]
- 16.Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992;89:444–8. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pawloski JR, Stamler JS. Nitric oxide in RBCs. Transfusion. 2002;42:1603–9. doi: 10.1046/j.1537-2995.2002.00278.x. [DOI] [PubMed] [Google Scholar]
- 18.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104:17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Popel AS. Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free Radic Biol Med. 2006;41:668–80. doi: 10.1016/j.freeradbiomed.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Chen K, Piknova B, Pittman RN, Schechter AN, Popel AS. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide. 2008;18:47–60. doi: 10.1016/j.niox.2007.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–98. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168:551–9. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36:35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 28.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269(6 Pt 2):H2155–61. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 29.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271(6 Pt 2):H2717–22. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5′-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Crit Care Med. 2011;39:2478–86. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–9. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 32.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 33.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kline ER, Bassit L, Hernandez-Santiago BI, Detorio MA, Liang B, Kleinhenz DJ, Walp ER, Dikalov S, Jones DP, Schinazi RF, Sutliff RL. Long-term exposure to AZT, but not d4T, increases endothelial cell oxidative stress and mitochondrial dysfunction. Cardiovasc Toxicol. 2009;9:1–12. doi: 10.1007/s12012-008-9029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45:468–74. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hess JR, Rugg N, Knapp AD, Gormas JF, Hill HR, Oliver CK, Lippert LE, Greenwalt TJ. The role of electrolytes and pH in RBC ASs. Transfusion. 2001;41:1045–51. doi: 10.1046/j.1537-2995.2001.41081045.x. [DOI] [PubMed] [Google Scholar]
- 38.Hess JR, Rugg N, Joines AD, Gormas JF, Pratt PG, Silberstein EB, Greenwalt TJ. Buffering and dilution in red blood cell storage. Transfusion. 2006;46:50–4. doi: 10.1111/j.1537-2995.2005.00672.x. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida T, AuBuchon JP, Dumont LJ, Gorham JD, Gifford SC, Foster KY, Bitensky MW. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion. 2008;48:2096–105. doi: 10.1111/j.1537-2995.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 40.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over longterm storage. Transfusion. 2011;51:1574–9. doi: 10.1111/j.1537-2995.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 41.Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. 2011;51:859–66. doi: 10.1111/j.1537-2995.2011.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glynn SA. The red blood cell storage lesion: a method to the madness. Transfusion. 2010;50:1164–9. doi: 10.1111/j.1537-2995.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- 43.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion. 2012;52:1388–92. doi: 10.1111/j.1537-2995.2012.03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinella PC, Doctor A, Blumberg N, Holcomb JB. Does the storage duration of blood products affect outcomes in critically ill patients? Transfusion. 2011;51:1644–50. doi: 10.1111/j.1537-2995.2011.03245.x. [DOI] [PubMed] [Google Scholar]
- 45.Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012;52:1410–22. doi: 10.1111/j.1537-2995.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, Ware RE. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116:687–92. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 47.Stapley R, Owusu BY, Brandon A, Cusick M, Rodriguez C, Marques MB, Kerby JD, Barnum SR, Weinberg JA, Lan-caster JR, Jr, Patel RP. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. Biochem J. 2012;446:499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villar IC, Francis S, Webb A, Hobbs AJ, Ahluwalia A. Novel aspects of endothelium-dependent regulation of vascular tone. Kidney Int. 2006;70:840–53. doi: 10.1038/sj.ki.5001680. [DOI] [PubMed] [Google Scholar]
- 49.Walford G, Loscalzo J. Nitric oxide in vascular biology. J Thromb Haemost. 2003;1:2112–8. doi: 10.1046/j.1538-7836.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 50.Chataigneau T, Félétou M, Huang PL, Fishman MC, Duhault J, Vanhoutte PM. Acetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout mice. Br J Pharmacol. 1999;126:219–26. doi: 10.1038/sj.bjp.0702300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu LH, Paul RJ, Sutliff RL, Miller ML, Lorenz JN, Pun RY, Duffy JJ, Doetschman T, Kimura Y, MacLennan DH, Hoying JB, Shull GE. Defective endothelium-dependent relaxation of vascular smooth muscle and endothelial cell Ca2+ signaling in mice lacking sarco(endo)plasmic reticulum Ca2+-ATPase isoform 3. J Biol Chem. 1997;272:30538–45. doi: 10.1074/jbc.272.48.30538. [DOI] [PubMed] [Google Scholar]
- 52.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]