A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010 (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 5.
Summary
Background
Quantification of the disease burden caused by different risks informs prevention by providing an account of health loss different to that provided by a disease-by-disease analysis. No complete revision of global disease burden caused by risk factors has been done since a comparative risk assessment in 2000, and no previous analysis has assessed changes in burden attributable to risk factors over time.
Methods
We estimated deaths and disability-adjusted life years (DALYs; sum of years lived with disability [YLD] and years of life lost [YLL]) attributable to the independent effects of 67 risk factors and clusters of risk factors for 21 regions in 1990 and 2010. We estimated exposure distributions for each year, region, sex, and age group, and relative risks per unit of exposure by systematically reviewing and synthesising published and unpublished data. We used these estimates, together with estimates of cause-specific deaths and DALYs from the Global Burden of Disease Study 2010, to calculate the burden attributable to each risk factor exposure compared with the theoretical-minimum-risk exposure. We incorporated uncertainty in disease burden, relative risks, and exposures into our estimates of attributable burden.
Findings
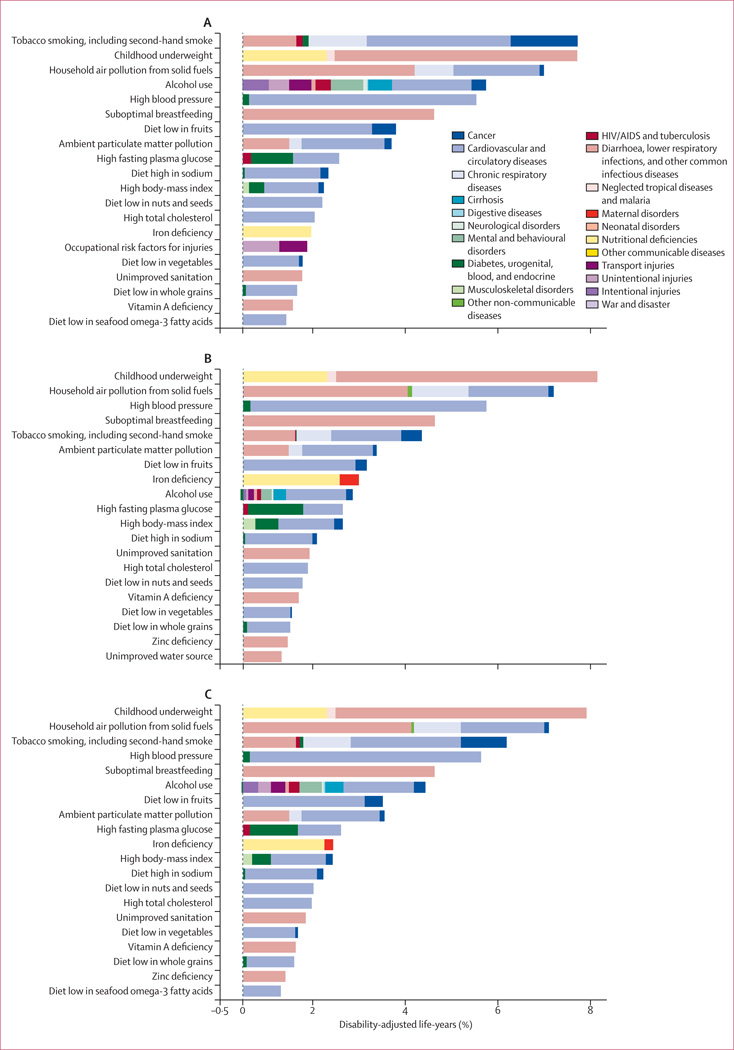
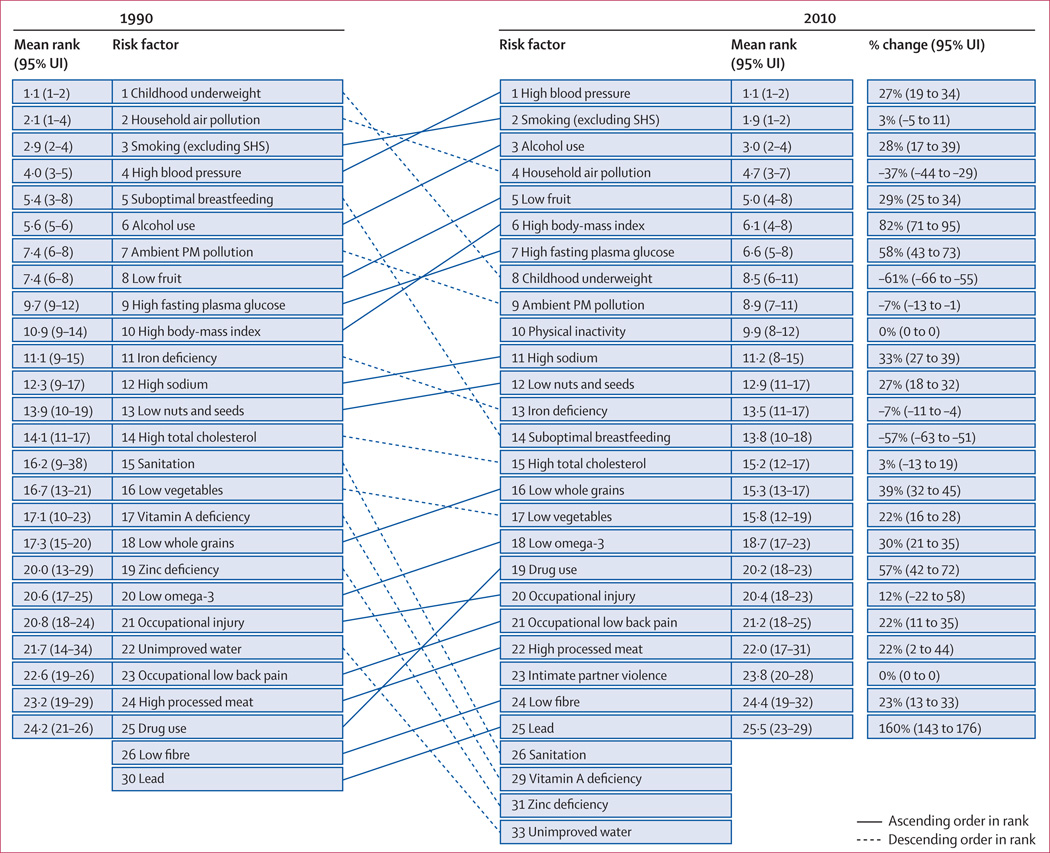
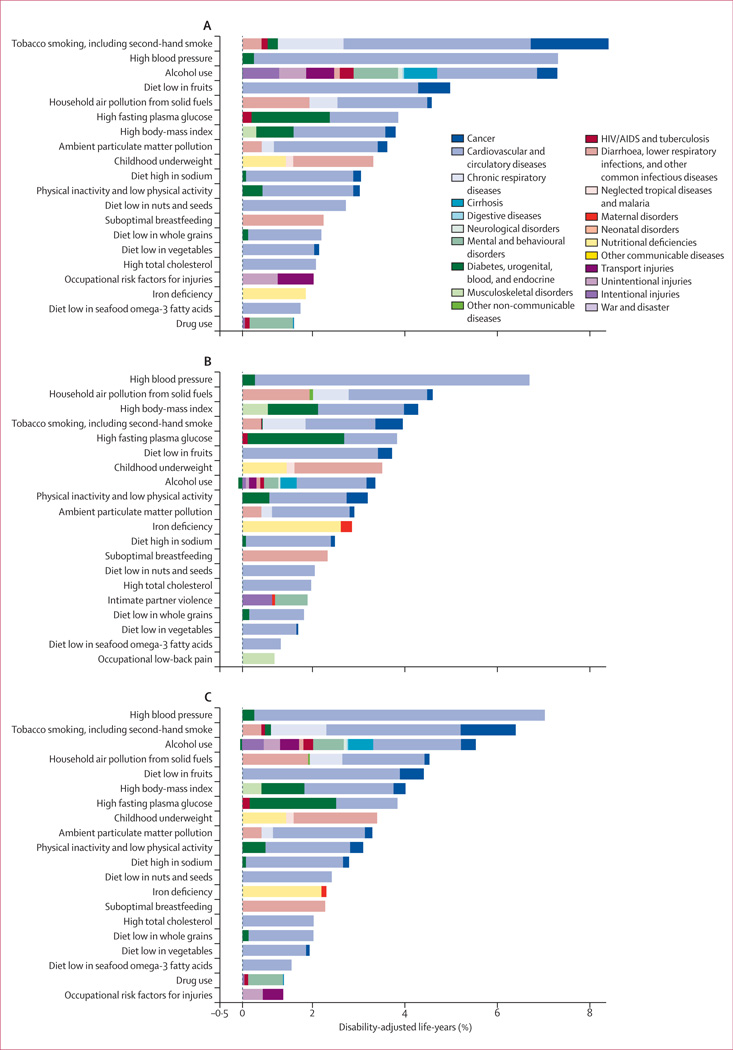
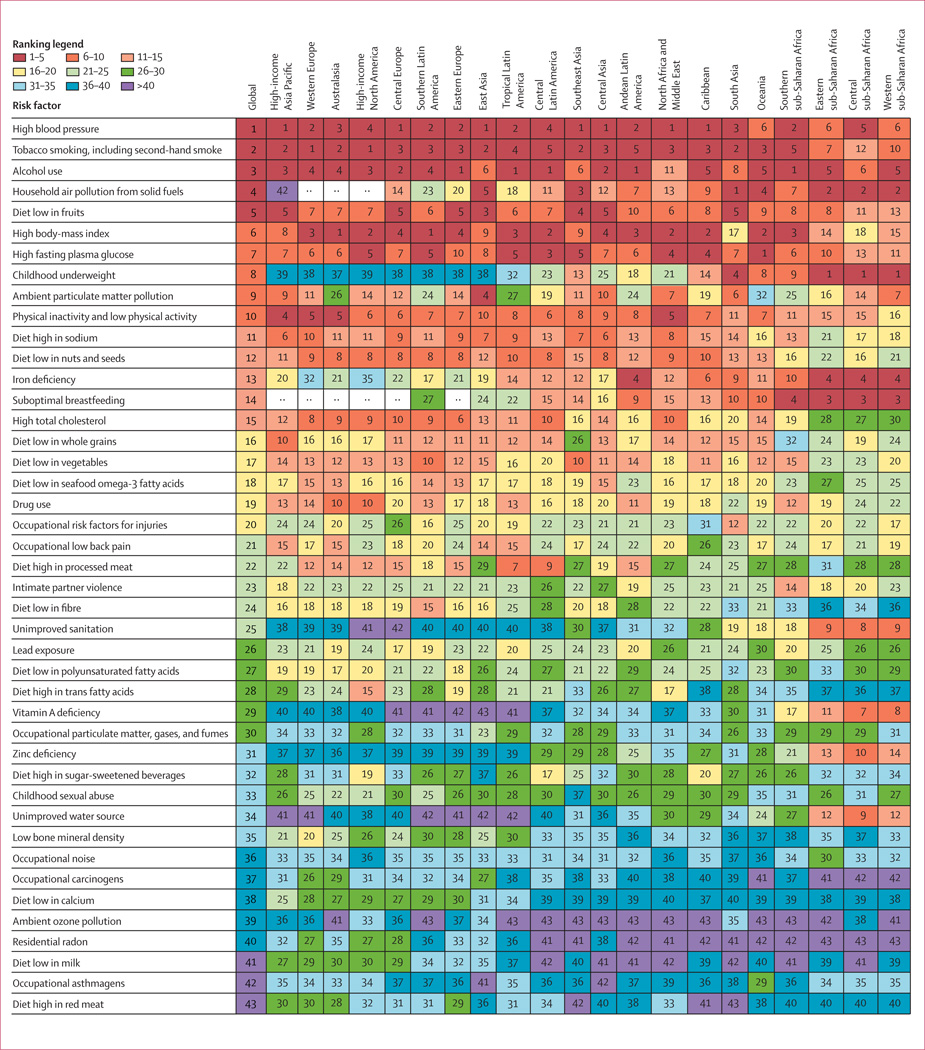
In 2010, the three leading risk factors for global disease burden were high blood pressure (7·0% [95% uncertainty interval 6·2–7·7] of global DALYs), tobacco smoking including second-hand smoke (6·3% [5·5–7·0]), and alcohol use (5·5% [5·0–5·9]). In 1990, the leading risks were childhood underweight (7·9% [6·8–9·4]), household air pollution from solid fuels (HAP; 7·0% [5·6–8·3]), and tobacco smoking including second-hand smoke (6·1% [5·4–6·8]). Dietary risk factors and physical inactivity collectively accounted for 10·0% (95% UI 9·2–10·8) of global DALYs in 2010, with the most prominent dietary risks being diets low in fruits and those high in sodium. Several risks that primarily affect childhood communicable diseases, including unimproved water and sanitation and childhood micronutrient deficiencies, fell in rank between 1990 and 2010, with unimproved water we and sanitation accounting for 0·9% (0·4–1·6) of global DALYs in 2010. However, in most of sub-Saharan Africa childhood underweight, HAP, and non-exclusive and discontinued breastfeeding were the leading risks in 2010, while HAP was the leading risk in south Asia. The leading risk factor in Eastern Europe, most of Latin America, and southern sub-Saharan Africa in 2010 was alcohol use; in most of Asia, North Africa and Middle East, and central Europe it was high blood pressure. Despite declines, tobacco smoking including second-hand smoke remained the leading risk in high-income north America and western Europe. High body-mass index has increased globally and it is the leading risk in Australasia and southern Latin America, and also ranks high in other high-income regions, North Africa and Middle East, and Oceania.
Interpretation
Worldwide, the contribution of different risk factors to disease burden has changed substantially, with a shift away from risks for communicable diseases in children towards those for non-communicable diseases in adults. These changes are related to the ageing population, decreased mortality among children younger than 5 years, changes in cause-of-death composition, and changes in risk factor exposures. New evidence has led to changes in the magnitude of key risks including unimproved water and sanitation, vitamin A and zinc deficiencies, and ambient particulate matter pollution. The extent to which the epidemiological shift has occurred and what the leading risks currently are varies greatly across regions. In much of sub-Saharan Africa, the leading risks are still those associated with poverty and those that affect children.
Funding
Bill & Melinda Gates Foundation.
Introduction
Measurement of the burden of diseases and injuries is a crucial input into health policy. Equally as important, is a comparative assessment of the contribution of potentially modifiable risk factors for these diseases and injuries. The attribution of disease burden to various risk factors provides a different account compared with disease-by-disease analysis of the key drivers of patterns and trends in health. It is essential for informing prevention of disease and injury.
Understanding the contribution of risk factors to disease burden has motivated several comparative studies in the past few decades. The seminal work of Doll and Peto1 provided a comparative assessment of the importance of different exposures, particularly tobacco smoking, in causing cancer. Peto and colleagues2 subsequently estimated the effects of tobacco smoking on mortality in developed countries since 1950. Although these risk factor-specific or cause-specific analyses are useful for policy, a more comprehensive global assessment of burden of disease attributable to risk factors can strengthen the basis for action to reduce disease burden and promote health. The Global Burden of Disease Study (GBD) 1990 provided the first global and regional comparative assessment of mortality and disability adjusted life-years (DALYs) attributable to ten major risk factors.3 However, different epidemiological traditions for different risks limited the comparability of the results. Subsequently, Murray and Lopez4 proposed a framework for global comparative risk assessment, which laid the basis for assessment of 26 risks in 2000.5–7 Since this work, WHO has provided estimates for some risks by the same methods but with updated exposures and some updates of the effect sizes for each risk.8 Analyses have also been done for specific clusters of diseases, like cancers,9 or clusters of risk factors, like maternal and child under-nutrition.10 National comparative risk assessments (including in Australia, Iran, Japan, Mexico, South Africa, Thailand, USA, and Vietnam) have also been undertaken with similar approaches.11–16
GBD 2010 provides an opportunity to re-assess the evidence for exposure and effect sizes of risks for a broad set of risk factors by use of a common framework and methods. Particularly, since this work was done in parallel with a complete re-assessment of the burden of diseases and injuries in 1990 and 2010, for the first time changes in burden of disease attributable to different risk factors can be analysed over time with comparable methods. Since uncertainty has been estimated for each disease or injury outcome,17,18 the comparative risk assessment for GBD 2010 has also enabled us to incorporate uncertainty into the final estimates. We describe the general approach and high-level findings for comparison of the importance of 67 risk factors and clusters of risk factors, globally and for 21 regions of the world, over the past two decades.
Methods
Overview
The basic approach for the GBD 2010 comparative risk assessment is to calculate the proportion of deaths or disease burden caused by specific risk factors—eg, ischaemic heart disease caused by increased blood pressure—holding other independent factors unchanged. These calculations were done for 20 age groups, both sexes, and 187 countries and for 1990, 2005 (results for 2005 not shown, available from authors on request), and 2010. We present aggregated results for 21 regions.
Table 1 shows the included risk factors and their organisation into a hierarchy with three levels. Level 1 risks are clusters of risk factors that are related by mechanism, biology, or potential policy intervention. Most risks are presented at level 2. For occupational carcinogens, a third level is included to provide additional detail about specific carcinogens. For suboptimal breastfeeding we also include a third level to distinguish between nonexclusive breastfeeding during the first 6 months and discontinued breastfeeding from 6 to 23 months.
Table 1.
Risk factors included, exposure variables, theoretical-minimum-risk exposure distributions, and outcomes affected
| Exposure definition | Outcomes | Subgroup | Main data sources forexposure | Exposureestimationmethod | Theoretical-minimum-riskexposuredistribution | Source ofrelative risks | |
|---|---|---|---|---|---|---|---|
| 1. Unimproved water and sanitation | |||||||
| 1.1.Unimprovedwater source | Proportion of households usingan unimproved water source(unprotected wells or springs,vendor-provided water, tankertrucks, surface water, and otherunspecified sources) | Intestinal infectious diseases | All ages | Population surveys andcensuses | SpatiotemporalGaussianprocessregression19–21 | All households usean improved watersource (householdconnection, a publictap or standpipe, atubewell orborehole, aprotected well orspring, or rainwatercollection) | Newmeta-analysis |
| 1.2.Unimprovedsanitation | Proportion of households usingunimproved sanitation(traditional latrines, openlatrines without squatting slabs,bucket latrines, hanginglatrines, open defecation or nofacilities, and other unspecifiedfacilities) | Intestinal infectious diseases | All ages | Population surveys andcensuses | SpatiotemporalGaussianprocessregression19–21 | All households useimproved sanitation(public sewers,septic systems, flushor pour-flushfacilities, ventilatedimproved latrines,simple pit latrineswith squattingslabs, andcomposting toilets) | Newmeta-analysis |
| 2. Air pollution | |||||||
| 2.1. Ambientparticulatematterpollution | Ambient concentration ofparticles with an aerodynamicdiameter smaller than 2·5 µm,measured in µg/m3 | Lower respiratory infections;trachea, bronchus, and lungcancers; IHD; cerebrovasculardisease; COPD | Age <5 yearsfor lowerrespiratorytract infection;≥25 years forall others | Surface monitormeasurements, aerosoloptical depth fromsatellites, and TM5 globalatmospheric chemistrytransport model22–26 | Average ofsatellite andchemistrytransportestimates,calibrated tosurfacemonitormeasurements | 5·8–8·8 µg/m3 | Integratedexposure–response curve |
| 2.2. Householdair pollutionfrom solid fuels | Proportion of households usingsolid fuels for cooking (coal,wood, charcoal, dung, andagricultural residues) | Lower respiratory infections;trachea, bronchus, and lungcancers; IHD; cerebrovasculardisease; COPD; cataracts | Age <5 yearsfor lowerrespiratorytract infection;≥25 years forall others | Population surveys andcensuses | Mixed effectregression | All householdsusing clean fuels forcooking (ventedgas, electricity) | Integratedexposure–response curvefor lowerrespiratorytract infection,IHD, andstroke; newmeta-analysisfor cataracts,COPD, andlung cancer |
| 2.3. Ambientozone pollution | Ambient concentrations ofozone in air, measured in partsper billion | COPD | Age ≥25 years | TM5 global atmosphericchemistry transportmodel22–24 | TM5 globalatmosphericchemistrytransportmodel22–24 | 33·3–41·9 parts perbillion | Jerrett andcolleagues27 |
| 3. Other environmental risks | |||||||
| 3.1. Residentialradon | Residential radon, measured inBq/m3 | Trachea, bronchus, and lungcancers | All ages | Direct householdmeasurements fromsurveys | Mixed effectregression | 10 Bq/m3 | Darby andcolleagues28 |
| 3.2. Leadexposure | Blood lead (measured in µg/dL)and bone lead (measured inµg/g) | Intellectual disability; systolic bloodpressure, which has effects on: RHD;IHD; ischaemic stroke; haemorrhagicand other non-ischaemic stroke;HHD; aortic aneurysm; the aggregateof cardiomyopathy and myocarditisand endocarditis; the aggregate ofatrial fibrillation and flutter, PVD,other CVD; CKD | <15 years forintellectualdisability;≥25 years forall others | Examination surveys andepidemiological studies | DisMod 3 | Bone lead levelexpected from age-specific cumulativeexposure to bloodlead of 0·09652µmol/L29 | Lanphear andcolleagues,30Navas-Acienandcolleagues31 |
| 4. Child and maternal undernutrition | |||||||
| 4.1. Suboptimalbreastfeeding | |||||||
| 4.1.1. Non-exclusivebreastfeeding | Proportion of children youngerthan 6 months withpredominant, partial, or nobreastfeeding | Intestinal infectious diseases; theaggregate of lower respiratoryinfections, upper respiratoryinfections, and otitis media | Age0–5 months | Population surveys | SpatiotemporalGaussianprocessregression19–21 | All childrenexclusivelybreastfed for first6 months | Lamberti andcolleagues,32Black andcolleagues10 |
| 4.1.2.Discontinuedbreastfeeding | Proportion of children aged6–23 months with discontinuedbreastfeeding | Intestinal infectious diseases | Age6–23 months | Population surveys | SpatiotemporalGaussianprocessregression19–21 | Continuedbreastfeeding until2 years | Lamberti andcolleagues,32Black andcolleagues10 |
| 4.2. Childhoodunderweight | Proportion of children less than−3 SDs, −3 to −2 SDs, and −2 to−1 SDs of the WHO 2006standard weight-for-age curve | Intestinal infectious diseases;measles; malaria; the aggregate oflower respiratory infections, upperrespiratory infections, and otitismedia; protein–energymalnutrition | Age <5 years | Examination surveys andepidemiological studies | SpatiotemporalGaussianprocessregression19–21 | Proportion of theWHO 2006referencepopulation in eachSD range | Black andcolleagues10 |
| 4.3. Irondeficiency | Haemoglobin, measure in g/L | The aggregate of maternalhaemorrhage and maternal sepsis;iron-deficiency anaemia | All ages | Examination surveys andepidemiological studies | Mixed effectregression | Country-specific | Stoltzfus andcolleagues33 |
| 4.4. Vitamin Adeficiency | Proportion of children withserum retinol concentration<70 µmol/L | Intestinal infectious diseases;measles; vitamin A deficiency | Age 6 monthsto 5 years | Examination surveys andepidemiological studies | DisMod 3 | No childhoodvitamin Adeficiency | Imdad andcolleagues,34,35adjusted forbackgroundprevalence |
| 4.5. Zincdeficiency | Proportion of the populationwith inadequate zinc intakebased on estimated mean dailyamount of absorbable zinc perhead in the food supplycompared with meanphysiological requirements | Intestinal infectious diseases; lowerrespiratory infections | Age 1–4 years | Food and AgriculturalOrganization food balancesheets | Mixed effectregression | No inadequate zincintake | Yakoob andcolleagues,36adjusted forbackgroundprevalence |
| 5. Tobacco smoking, including second-hand smoke | |||||||
| 5.1. Tobaccosmoking | Smoking impact ratio forcancers and chronic respiratorydisease, 10-year lagged tobaccosmoking prevalence for all othercauses including cardiovasculardiseases | Tuberculosis; oesophageal cancer;nasopharynx cancer; pancreaticcancer; kidney and other urinaryorgan cancers; bladder cancer;stomach cancer; leukaemia; livercancer; trachea, bronchus, and lungcancers; cervical cancer; colon andrectal cancer; mouth cancer;diabetes mellitus; IHD;cerebrovascular disease; theaggregate of HHD, atrial fibrillationand flutter, aortic aneurysm, PVD,and other CVD; COPD; the aggregateof pneumoconiosis, asthma, otherinterstitial lung disease, and otherchronic respiratory diseases | Age ≥25 years | Mortality data includingvital registration, verbalautopsy, and populationsurveys for smokingprevalence | CoDEM37 | No tobaccosmoking | Re-analysis ofthe CancerPreventionStudy 238–40 |
| 5.2. Second-hand smoke | Proportion of children andnon-smoking adults reportingexposure to second-handsmoke | The aggregate of lower respiratoryinfections, upper respiratoryinfections, and otitis media;trachea, bronchus, and lungcancers; IHD; cerebrovasculardisease | Age <5 yearsfor theaggregate oflowerrespiratoryinfections,upperrespiratoryinfections, andotitis media,age ≥25 yearsfor all others | Population surveys | SpatiotemporalGaussianprocessregression19–21 | No second-handsmoke exposure | USDepartment ofHealth andHumanServices,41Oono andcolleagues,42Jones andcolleagues43,44 |
| 6. Alcohol and drug use | |||||||
| 6.1. Alcohol use | Average consumption of purealcohol (measure in g/day) andproportion of the populationreporting binge consumption of0·06 kg or more of pure alcoholon a single occasion | Tuberculosis; lower respiratoryinfections; oesophageal cancer; theaggregate of mouth cancer,nasopharynx cancer, cancer ofother part of pharynx andoropharynx; liver cancer; larynxcancer; breast cancer; colon andrectum cancers; diabetes mellitus;IHD; ischaemic stroke;haemorrhagic and other non-ischaemic stroke; HHD; atrialfibrillation and flutter; cirrhosis ofthe liver; pancreatitis; epilepsy;transport injuries; the aggregate offalls, drowning, fire, heat, and hotsubstances, poisonings, exposureto mechanical forces, intentionalself-harm, and interpersonalviolence; alcohol use disorders | All ages foralcohol usedisorders,transportinjuries, andinterpersonalviolence;≥15 years forall others | Population surveys, alcoholsales, production, and othereconomic statistics | Mixed effectregression45 | No alcoholconsumption | Publishedstudies46–59 |
| 6.2. Drug use | Proportion of the populationreporting use of cannabis,opioids, and amphetamines,proportion of the populationreporting use of injecting drugs | Drug use disorders; schizophrenia;HIV/AIDS; the aggregate of acutehepatitis B, liver cancer secondaryto hepatitis B, and cirrhosis of theliver secondary to hepatitis B; theaggregate of acute hepatitis C,liver cancer secondary to hepatitisC, and cirrhosis of the liversecondary to hepatitis C;intentional self-harm | All ages | Population surveys,registries, and indirectmeasures | DisMod 3 | No use of cannabis,opioid, oramphetamines, nouse of injectingdrugs | New meta-analyses,publishedstudies60,61 |
| 7. Physiological risk factors | |||||||
| 7.1. High fastingplasma glucose | Fasting plasma glucose,measured in mmol/L | Diabetes mellitus; IHD;cerebrovascular disease; CKD;tuberculosis | Age ≥25 years | Examination surveys andepidemiological studies | Bayesianhierarchicalregression62 | Mean4·9–5·3 mmol/L(SD 0·3 mmol/L) | Meta-regression ofpooledprospectivestudies63–66 |
| 7.2. High totalcholesterol | Total cholesterol, measured inmmol/L | IHD; ischaemic stroke | Age ≥25 years | Examination surveys andepidemiological studies | Bayesianhierarchicalregression67 | Mean3·8–4·0 mmol/L(SD 0·9 mmol/L) | Meta-regression ofpooledprospectivestudies68,69 |
| 7.3. High bloodpressure | Systolic blood pressure,measured in mm Hg | RHD; IHD; ischaemic stroke,haemorrhagic and other non-ischaemic stroke; HHD; theaggregate of cardiomyopathy andmyocarditis and endocarditis; theaggregate of atrial fibrillation andflutter, PVD, and other CVD; aorticaneurysm; CKD | Age ≥25 years | Examination surveys andepidemiological studies | Bayesianhierarchicalregression70 | Mean 110–115 mmHg (SD 6 mm Hg) | Meta-regression ofpooledprospectivestudies71–73 |
| 7.4. High body-mass index | Body-mass index, measured inkg/m2 | Oesophageal cancer; gallbladderand biliary tract cancer; pancreaticcancer; kidney and other urinaryorgan cancers; breast cancer;uterine cancer; colon and rectumcancers; diabetes mellitus; IHD;ischaemic stroke; HHD; theaggregate of cardiomyopathy andmyocarditis and endocarditis; theaggregate of atrial fibrillation andflutter, PVD, and other CVD; CKD;osteoarthritis; low back pain | Age ≥25 years | Examination surveys andepidemiological studies | Bayesianhierarchicalregression74 | Mean21·0–23·0 kg/m2(SD 1 kg/m2) | Meta-regression ofpooledprospectivestudies75–78 |
| 7.5. Low bonemineral density | Standardised bone mineraldensity measured at the femoralneck | Hip fracture falls; non-hip fracturefalls | Age ≥50 years | Examination surveys andepidemiological studies | DisMod 3 | 90th percentile ofNHANES-IIIcohort79 by age | Johnell andcolleagues80 |
| 8. Dietery risk factors and physical inactivity | |||||||
| 8.1. Diet low infruits | Dietary intake of fruits (fresh,frozen, cooked, canned, or driedbut excluding fruit juices andsalted or pickled fruits) | The aggregate of oesophagealcancer, mouth cancer, the aggregateof nasopharynx cancer, cancer ofother part of pharynx andoropharynx, and larynx cancer;trachea, bronchus, and lung cancers;IHD; ischaemic stroke; haemorrhagicand other non-ischaemic stroke | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean 300 g/day(SD 30 g/day) | New meta-analysis,publishedstudies81,82 |
| 8.2. Diet low invegetables | Dietary intake of vegetables(fresh, frozen, cooked, canned, ordried vegetables includinglegumes but excluding salted orpickled, juices, nuts and seeds,and starchy vegetables such aspotatoes or corn) | The aggregate of mouth cancer,nasopharynx cancer, cancer ofother part of pharynx andoropharynx, and larynx cancer; IHD;ischaemic stroke; haemorrhagicand other non-ischaemic stroke | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean 400 g/day(SD 30 g/day) | New meta-analysis, Heandcolleagues81 |
| 8.3. Diet low inwhole grains | Dietary intake of whole grains(bran, germ, and endosperm intheir natural proportions) frombreakfast cereals, bread, rice,pasta, biscuits, muffins, tortillas,pancakes, and others | Diabetes mellitus; IHD;cerebrovascular disease | Age ≥25 year | Nutrition and healthsurveys | DisMod 3 | Mean 125 g/day(SD 12·5 g/day) | Mellen andcolleagues,83de Munter andcolleagues84 |
| 8.4. Diet low innuts and seeds | Dietary intake of nut and seedfoods including, for example,peanut butter | IHD | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean 114 g perweek (SD 11·4 g perweek) | Kelly andcolleagues85 |
| 8.5. Diet low inmilk | Dietary intake of milk includingnon-fat, low-fat, and full-fatmilk but excluding soya milkand other plant derivatives | Colon and rectum cancers | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean 450 g/day(SD 45 g/day) | World CancerResearch Fundand AmericanInstitute forCancerResearch82 |
| 8.6. Diet high inred meat | Dietary intake of red meat (beef,pork, lamb, and goat butexcluding poultry, fish, eggs,and all processed meats) | Colon and rectum cancers; diabetesmellitus | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean 100 g perweek (SD 10 g perweek) | World CancerResearch Fundand AmericanInstitute forCancerResearch,82publishedstudies86,87 |
| 8.7. Diet high inprocessed meat | Dietary intake of meatpreserved by smoking, curing,salting, or addition of chemicalpreservatives, including bacon,salami, sausages, or deli orluncheon meats like ham,turkey, and pastrami | Colon and rectum cancers; diabetesmellitus; IHD | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | No dietary intake ofprocessed meat | World CancerResearch Fundand AmericanInstitute forCancerResearch,82Micha andcolleagues87 |
| 8.8. Diet high insugar-sweetenedbeverages | Dietary intake of beverages with≥50 kcal per 226·8 g serving,including carbonated beverages,sodas, energy drinks, fruit drinksbut excluding 100% fruit andvegetable juices | Diabetes mellitus and body-massindex with subsequent effects on:oesophageal cancer; gallbladder andbiliary tract cancer; pancreatic cancer;kidney and other urinary organcancers; breast cancer; uterine cancer;colon and rectum cancers; diabetesmellitus; IHD; ischaemic stroke; HHD;the aggregate of cardiomyopathyand myocarditis and endocarditis;the aggregate of atrial fibrillationand flutter, PVD, and other CVD;CKD; osteoarthritis; low back pain | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | No dietary intake ofsugar-sweetenedbeverages | New meta-analysis |
| 8.9. Diet low infibre | Dietary intake of fibre from allsources including fruits,vegetables, grains, legumes,and pulses | Colon and rectum cancers; IHD | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean of 30 g/day(SD 3 g/day) | World CancerResearch Fundand AmericanInstitute forCancerResearch,82Pereira andcolleagues88 |
| 8.10. Diet lowin calcium | Dietary intake of calcium fromall sources, including milk,yogurt, and cheese | Colon and rectum cancers; prostatecancer | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean of1200 mg/day (SD120 mg/day) | World CancerResearch Fundand AmericanInstitute forCancerResearch,82 Choandcolleagues89 |
| 8.11. Diet lowin seafoodomega-3 fattyacids | Dietary intake ofeicosapentaenoic acid anddocosahexaenoic acid,measured in mg/day | Death caused by IHD | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | 250 mg/day | Updatedpublishedreview ofMozaffarianandcolleagues90 |
| 8.12. Diet low inpolyunsaturatedfatty acids | Dietary intake of omega-6 fattyacids from all sources, mainlyliquid vegetable oils, includingsoybean oil, corn oil, andsafflower oil | IHD | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Substitution ofpresent saturatedfatty acid intake upto a mean intake ofpolyunsaturatedfatty acids of 12%of energy (SD 1·2%) | Jakobsen andcolleagues,91Mozaffarianandcolleagues92 |
| 8.13. Diet highin trans fattyacids | Dietary intake of trans fat fromall sources, mainly partiallyhydrogenated vegetable oilsand ruminant products | IHD | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean of 0·5% ofenergy (SD 0·05%) | Mozaffarianandcolleagues93 |
| 8.14. Diet highin sodium | 24 h urinary sodium, measuredin mg/day | Stomach cancer; systolic bloodpressure which has effects on: RHD;IHD; ischaemic stroke,haemorrhagic and othernon-ischaemic stroke; HHD; theaggregate of cardiomyopathy andmyocarditis and endocarditis; theaggregate of atrial fibrillation andflutter, PVD, and other CVD; aorticaneurysm; CKD | Age ≥25 years | Nutrition and healthsurveys | DisMod 3 | Mean of1000 mg/day (SD100 mg/day) | Re-analysis ofobservationalstudies forstomachcancer andrandomisedstudies forblood pressurelowering82,94 |
| 8.15. Physicalinactivity andlow physicalactivity* | Proportion of the population incategories of physical activity:level 0, <600 MET-minutes perweek (inactive); level 1,600–3999 MET-minutes perweek (low-active); level 2, 4000–7999 MET-minutes per week(moderately active); and level 3,≥8000 MET-minutes per week(highly active) | Breast cancer; colon and rectumcancers; diabetes mellitus; IHD;ischaemic stroke | Age ≥25 years | Population surveys | DisMod 3 | All individuals arehighly active(level 3) | Danaei andcolleagues11 |
| 9. Occupational risk factors | |||||||
| 9.1.Occupationalcarcinogens | |||||||
| 9.1.1.Occupationalexposure toasbestos | Cumulative exposure toasbestos using mesothelioma ina smoking impact ratioanalogue | Ovarian cancer; other neoplasms;larynx cancer; trachea, bronchus,and lung cancers | Age ≥15 years | Vital registration mortalitydata, asbestos production,import, and exportstatistics | SpatiotemporalGaussianprocessregression19–21 | No exposure toasbestos | Publishedstudies95–98 |
| 9.1.2.Occupationalexposure toarsenic | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Lee-Feldstein101 |
| 9.1.3.Occupationalexposure tobenzene | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Leukaemia | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Khalade andcolleagues102 |
| 9.1.4.Occupationalexposure toberyllium | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Schubauer-Berigan andcolleagues103 |
| 9.1.5.Occupationalexposure tocadmium | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Hutchings andcolleagues95 |
| 9.1.6.Occupationalexposure tochromium | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Rosenman andcolleagues104 |
| 9.1.7Occupationalexposure todiesel engineexhaust | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus and lung cancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Lipsett andcolleagues105 |
| 9.1.8.Occupationalexposure tosecond-handsmoke | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Stayner andcolleagues106 |
| 9.1.9.Occupationalexposure toformaldehyde | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Leukaemia; nasopharynx cancer | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Collins andcolleagues,107Hauptmannandcolleagues108 |
| 9.1.10.Occupationalexposure tonickel | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Grimsrud andcolleagues109,110 |
| 9.1.11.Occupationalexposure topolycyclicaromatichydrocarbons | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Armstrongandcolleagues111 |
| 9.1.12.Occupationalexposure tosilica | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Trachea, bronchus, and lungcancers | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Kurihara andcolleagues112 |
| 9.1.13.Occupationalexposure tosulphuric acid | Proportion of population everexposed (by taking into accountworker turnover)99,100 based ondistribution of the population innine industries† | Larynx cancer | Age ≥15 years | Labour force surveys,censuses, and InternationalInformation System onOccupational Exposure toCarcinogens | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure tocarcinogens | Soskolne andcolleagues113 |
| 9.2.Occupationalasthmagens | Proportion of populationexposed based on distribution ofthe population in eightoccupational groups(professional, technical, andrelated workers; administrativeand managerial workers; clericaland related workers; salesworkers; service workers;agriculture, animal husbandry,and forestry workers, fishermenand hunters; production andrelated workers; and transportequipment operators andlabourers) | Asthma | Age ≥15 years | Labour force surveys andcensuses | SpatiotemporalGaussianprocessregression19–21 | Backgroundasthmagenexposures | Publishedstudies114–116 |
| 9.3.Occupationalparticulatematter, gases,and fumes | Proportion of populationexposed based on distributionof the population in nineindustries† | COPD | Age ≥15 years | Labour force surveys andcensuses | SpatiotemporalGaussianprocessregression19–21 | No occupationalexposure toparticulates, gases,or fumes | New meta-analysis |
| 9.4.Occupationalnoise | Proportion of populationexposed based on distributionof the population in nineindustries† | Hearing loss | Age ≥15 years | Labour force surveys andcensuses | SpatiotemporalGaussianprocessregression19–21 | Background noiseexposure | New meta-analysis |
| 9.5.Occupationalrisk factors forinjuries | Fatal occupational injury | ‥ | Age ≥15 years | International LabourOrganization injurydatabase | SpatiotemporalGaussianprocessregression19–21 | Five injury deathsper 1 000 000person-years | ‥ |
| 9.6.Occupationallow back pain | Proportion of populationexposed based on distribution ofthe population in eightoccupational groups(professional, technical, andrelated workers; administrativeand managerial workers; clericaland related workers; salesworkers; service workers;agriculture, animal husbandry,and forestry workers, fishermenand hunters; production andrelated workers; and transportequipment operators andlabourers) | Low back pain | Age ≥15 years | Labour force surveys andcensuses | SpatiotemporalGaussianprocessregression19–21 | All individuals havethe ergonomicfactors of clericaland related workers | New meta-analysis |
| 10. Sexual abuse and violence | |||||||
| 10.1. Childhoodsexual abuse* | Proportion of the populationwho have ever experiencedchildhood sexual abuse,defined as the experience withan older person of unwantednon-contact, contact abuse, orintercourse, when aged 15 yearsor younger | Alcohol use disorders, unipolardepressive disorders, intentionalself-harm | All ages | Population surveys andepidemiological studies | DisMod 3 | No childhoodsexual abuse | New meta-analysis |
| 10.2. Intimatepartnerviolence* | Proportion of the populationwho have ever experienced oneor more acts of physical orsexual violence by a present orformer partner since age15 years | Abortion, unipolar depressivedisorders, intentional self-harm,interpersonal violence | Age15–49 yearsfor abortion,≥15 years forall others | Population surveys andepidemiological studies | DisMod 3 | No intimatepartner violence | New meta-analysis,Beydoun andcolleagues117 |
We calculated burden attributable to all (67) risk factors and clusters of risk factors except for physiological risks and air pollution. These two clusters present analytical challenges for computation of the aggregate burden. For example, the effects of high body-mass index are partly mediated through high blood pressure, high total cholesterol, and high fasting plasma glucose, and household air pollution from solid fuels (wood, crop, residues, animal dung, charcoal, and coal) contributes to ambient particulate matter pollution.
We ranked results for 43 risk factors and clusters of risk factors, grouping together occupational carcinogens, non-exclusive and discontinued breastfeeding, and tobacco smoking with second-hand smoke on the basis of common exposure sources.
Our estimation of disease burden attributable to a risk factor had five steps: 1) selection of risk–outcome pairs to be included in the analysis based on criteria about causal associations; 2) estimation of distributions of exposure to each risk factor in the population; 3) estimation of etiological effect sizes, often relative risk per unit of exposure for each risk–outcome pair; 4) choice of an alternative (counterfactual) exposure distribution to which the current exposure distribution is compared. We selected an optimum exposure distribution, termed the theoretical-minimum-risk exposure distribution for this purpose; and 5) computation of burden attributable to each risk factor, including uncertainty from all sources. Further details about the data and methods used for specific risk factors are available on request.
Selection of risk–outcome pairs
The inclusion criteria for each risk–outcome pair that we applied were: 1) the likely importance of a risk factor to disease burden or policy based on previous work; 2) availability of sufficient data and methods to enable estimation of exposure distributions by country for at least one of the study periods (1990 and 2010); 3) sufficient evidence for causal effects based on high-quality epidemiological studies in which the findings were unlikely to be caused by bias or chance, analogous to the criteria used for assessment of carcinogens with con vincing or probable evidence (panel). Sufficient data to estimate outcome-specific etiological effect sizes per unit of exposure were also needed; and 4) evidence to support generalisability of effect sizes to populations other than those included in the available epidemiological studies or satisfactory models for extrapolating them. Table 1 shows the risk–outcome pairs that were included in the final analysis, on the basis of these criteria.
Distribution of exposure to each risk factor
For most risk factors, a systematic search was done to identify published and, when possible, unpublished data sources to estimate risk factor exposure distributions in 1990 and 2010. Strategies to identify data sources included searching survey databases such as the WHO Global Database on Child Growth and Malnutrition, searching general citation databases such as Google Scholar and PubMed, manual searching of reference lists of articles and conference abstracts, and contacting experts in the relevant fields. Data sources included censuses, health examination and nutrition surveys, and community-based epidemiological studies.
Because data for risk factor exposure are often incomplete or missing for many populations, models were used to generate a complete set of current exposure distributions for risk factors for each country and for both years, including uncertainty. Table 1 shows for each risk factor the main sources of data and the modelling approach used for estimation of present risk factor exposure distributions. Briefly, risk factor models were designed to use available data and information for exposures in countries, for several years, and for different age groups to generate estimates for all countries, for both years, and for all relevant age groups. Estimation of exposure was done with statistical models that used predictors such as time, geography, and other variables that were relevant to the exposure of interest— eg, income per person.
For each risk factor, we tested a wide array of covariates for prediction of exposure distributions, drawing from covariates included in databases created or collated at the Institute for Health Metrics and Evaluation for GBD 2010. If relevant, the model also included age. Finally, each analysis accounted for important study characteristics such as national versus subnational representativeness, and the measures and instruments used for measuring exposure.
In addition to this general approach, specific methods were used for some risk factors. For tobacco including second-hand smoke, much scientific literature exists about alternative methods to estimate cumulative exposure, based on the premise that present prevalence and consumption data do not take into account likely variations in duration and intensity of smoking. In this case, we used the method of Peto and Lopez,2 which uses lung cancer mortality as a marker (ie, smoking impact ratio) of cumulative population exposure to smoking for cancers and chronic respiratory disease. We used epidemiological data to estimate lung cancer mortality in non-smokers separately for China, other countries in the high-income Asia Pacific region, and all remaining countries.119,120 For all other outcomes, we used 10-year lagged tobacco smoking prevalence. We also applied an approach analogous to the smoking impact ratio for occupational exposure to asbestos, for which we used mesothelioma mortality, separately estimated, as a marker of asbestos exposure.
For ambient particulate matter pollution, two complete, high resolution estimates exist of the concentration of particulate matter smaller than 2·5 µm in aerodynamic diameter (PM2·5) in ambient air: TM5 estimates—based on a nested three-dimensional global atmospheric chemistry transport model—which simulates both particulate matter and ozone at a high spatial resolution;22,23,121 and satellite-based estimates, which are based on satellite observations of aerosol optical depth, a measure of light extinction by aerosols in the total atmospheric column.25 TM5 and satellite-based estimates of PM2·5, measured in µg/m3, were averaged at a 0·1° × 0·1° grid cell resolution (equivalent to roughly 11 km × 11 km at the equator) and linked to available measures of PM2·5 from ground-based monitors. We used a regression model with the average of TM5 and satellite-based estimates as the predictor to estimate ground-based PM2·5 for all grid cells.26 For ozone, we relied solely on the TM5 model.
Few population-based surveys have measured zinc deficiency based on serum zinc concentration;122 however, intervention trials show a benefit of zinc supplementation for reduction of diarrhoea and lower respiratory infections in populations that have high zinc deficiency.10 Because of the paucity of data for serum zinc concentrations, we measured zinc deficiency at the population level on the basis of dietary sources of zinc, expanding on previous work of the International Zinc Nutrition Consultative Group.123 This approach uses national food balance sheets produced by the UN Food and Agriculture Organization to estimate a country-specific mean fractional absorption of zinc. The estimated mean daily per person amount of absorbable zinc in the food supply was compared with the mean physiological requirements of the population to calculate the percentage of the population with inadequate zinc intake.
Effects of risk factors on disease outcomes
Table 1 shows the sources of effect sizes per unit of exposure for each risk factor. Some effect sizes were based on meta-analyses of epidemiological studies. For several risk factors without recent systematic reviews or for which evidence had not recently been synthesised, new meta-analyses were done as part of GBD 2010. We used effect sizes that had been adjusted for measured confounders but not for factors along the causal pathway. For example, effect sizes for body-mass index were not adjusted for blood pressure. For some risk–outcome pairs, evidence is only available for the relative risk (RR) of morbidity or mortality. In these cases, we assumed that the reported RR would apply equally to morbidity or mortality, unless evidence suggested a differential effect. For example, studies of ambient particulate matter pollution suggest a smaller effect on incidence of cardio vascular and respiratory disease than on mortality;124–126 the published work on consumption of seafood omega-3 fatty acids suggests an effect on ischaemic heart disease mortality but not on incidence of ischaemic heart disease.90
Evidence for the RR of diarrhoea from unimproved water and sanitation is complicated by the complexity of available epidemiological studies, since the comparison groups varied greatly between studies. The comparison group used varied widely. For example, some studies compare an improved water source (eg, piped water) with an unimproved water source (eg, river water); in other studies the comparison is between two different types of improved water source (eg, piped water vs a protected well). Furthermore, studies often examine a combination of water, sanitation, and hygiene interventions. Previous reviews have yielded conflicting results about the magnitude of the effect sizes.127–131
We re-examined the epidemiological evidence for the effects of water and sanitation by reviewing the relation between water, sanitation and hygiene, and diarrhoea, starting with previous reviews.128–131 We did a meta-regression of 119 studies that was designed to adjust for intervention and baseline group characteristics. First, we compared indicator variables for each of the intervention components (improved sanitation, hygiene, point-of-use water treatment, source water treatment, and piped water) with a reference category (improved water source). Second, we also included indicator variables for the baseline characteristics—ie, whether the baseline was an unimproved or improved water source or sanitation—as covariates to account for the heterogeneous control groups. Our analysis showed a significant effect of both improved water and improved sanitation compared with unimproved water and sanitation; we did not note a significantly greater effect of piped water or point-of-use or source water treatment compared with improved water.
Particulate matter smaller than 2·5 µm is a common useful indicator of the risk associated with exposure to a mixture of pollutants from diverse sources and in different environments, including ambient particulate matter pollution from transportation, wind-blown dust, burning of bio mass, and industrial sources; second-hand smoke; burning of biomass and coal for household energy; and active smoking.132,133 However, existing studies cover only small concentration ranges—for example, ambient particulate matter pollution studies have been restricted to yearly average concentrations of particulate matter smaller than 2·5 µm of roughly 5 µg/m3 to 30 µg/m3,134–137 but much higher concentrations of ambient particulate matter have been recorded in polluted cities in Asia and elsewhere. The relation between concentration of small particulate matter and risk of disease is probably non-linear.132,133
To inform estimates of risk across the full range of concentrations, we used the approach of Pope and colleagues132 and integrated epidemiological evidence for the hazardous effects of particulate matter at different concentrations from different sources and environments. Methods for estimation of the integrated exposure– response curves for each cause are described elsewhere.138 Briefly, we compiled study-level estimates of the RR of mortality associated with any or all of ambient air pollution, second-hand smoke, household air pollution, and active smoking for the following outcomes: ischaemic heart disease, stroke, lung cancer, chronic obstructive pulmonary disease, and acute lower respiratory tract infection in children. We evaluated several non-linear functions with up to three parameters for fitting the integrated exposure– response relation and assessed them by calculation of the root mean squared error. An exponential decay with a power of concentration was the functional form that provided the best fit for all five outcomes. The integrated exposure–response curve was then used to generate effect sizes specific to the amount of ambient particulate matter smaller than 2·5 µm for each population. For ischaemic heart disease and stroke, evidence shows that household air pollution affects intermediate outcomes, such as blood pressure,139 but not clinical events. For acute lower respiratory tract infection, the integrated exposure– response curve enabled us to extrapolate beyond the partial exposure–response measured in the RESPIRE trial.140 For effects of household air pollution on chronic obstructive pulmonary disease and lung cancer we use the effect size based on new systematic reviews and meta-analyses.
Several dietary factors affect ischaemic heart disease and stroke, including consumption of fruits, vegetables, nuts and seeds, whole grains, processed meat, polyunsaturated fats, and seafood omega-3 fatty acids.81,83,85,87,90–92,141,142 We updated earlier systematic reviews and meta-analyses for fruits, vegetables, and seafood omega-3 fatty acids, which included both observational and intervention studies if available. A systematic review143 of randomised clinical trials of supplementation with seafood omega-3 fatty acids reported non-significant effects on several outcomes, and a significant effect for mortality from ischaemic heart disease—the primary outcome in GBD 2010. In view of this finding, we tested whether a significant difference exists between the randomised clinical trials of seafood omega-3 fatty acid supplementation and observational studies of seafood-omega 3 fatty acid intake. The effect of seafood omega-3 fatty acids tended to be lower in randomised controlled trials than in observational studies, however, this difference was not statistically significant (p=0·057). Therefore, we used the effect size based on the combination of randomised clinical trials and observational studies but also did a sensitivity analysis with the effect size based on randomised clinical trials.
Estimates of the RR associated with dietary risk factors are based largely on observational studies that control for age, sex, and other cardiovascular risk factors. However, some early observational studies do not fully control for other dietary components. Protective dietary risk factors such as consumption of fruits, vegetables, and whole grains, tend to be positively correlated with each other and negatively correlated with harmful dietary risk factors such as consumption of processed meat. Therefore, RRs estimated for single risk factors in observational studies could overestimate the protective or harmful effect of that risk factor. In effect, the partially adjusted RR will include some of the effects associated with other correlated diet components, particularly since the exposure measure for dietary risk factors is energy adjusted to a standard calorie intake.
To examine this issue, we did further empirical assessments using studies of dietary patterns and randomised controlled feeding studies. Studies of dietary patterns144–148 have estimated the effects of beneficial diets (prudent or Mediterranean diets) and harmful diets (western diets); these studies capture the overall effects of differences in dietary components. For example, a prudent diet has lots of fruits, vegetables, fish, and whole grains. For each of the dietary pattern studies we computed the estimated RR for dietary pattern groups with the RRs from the meta-analyses of single dietary risk factors, the reported differences in dietary intake, and assuming a multiplicative relation between RRs for individual components. Results of this internal validation study show that overall, estimation of the effect of dietary pattern based on the RRs reported for single risk factors was much the same as the effect reported in the study; across four large cohort studies of seven dietary patterns the average ratio for the estimated RR reduction compared with the measured RR reduction was 0·98.
In addition to the dietary pattern studies, we also investigated the evidence for the effects of dietary risk factors from randomised controlled feeding studies, such as DASH149 and OmniHeart,150 which measured the effect of dietary changes on blood pressure and LDL cholesterol. We used meta-regression to estimate the pooled effect of fruits, vegetables, nuts and seeds, whole grains, fish, and dietary fibre on systolic blood pressure and LDL cholesterol, based on all randomised controlled feeding studies (six treatment groups from three studies for blood pressure and six treatment groups from two studies for cholesterol). When translated into an effect using the RRs of blood pressure and cholesterol for ischaemic heart disease, the average ratio of the estimated to measured RR reduction was 1·07 for all components and 0·85 when excluding fish, which has mechanisms additional to lowering blood pressure and cholesterol.151 These two supplementary analyses suggest that the RRs estimated in the meta-analyses of single dietary risk factors are unlikely to be significantly biased because of residual confounding due to other diet components.
Pooled epidemiological studies of cardiovascular disease risks show that the RR decreases with age, and that the inverse age association is roughly log-linear. Based on a pooled analysis of several risk factors (high blood pressure, high fasting plasma glucose, high total cholesterol, and tobacco smoking), the age at which the RR reaches 1 is often between 100 and 120 years. We therefore estimated age-specific RRs for all cardiovascular risk factors by meta-regression of available data with logRR as the dependent variable and median age at event as the independent variable with an age intercept (RR=1) at age 110 years. Uncertainty in the RR was generated by simulation analyses.152
The causal association between a risk factor and a disease outcome is often informed by a wider body of evidence than epidemiological studies of RRs for specific measures of exposure, especially when disease-specific and age-specific RRs are needed. For example, although smoking is an established cause of cardiovascular diseases, when cohorts are analysed in fine age groups, the 95% CI for the effect of smoking on stroke spans 1·0 in several age groups.38 Similarly, randomised trials of zinc supplementation were designed to detect effects on total mortality.36,153 Re-analysis of the same trials for disease-specific outcomes, which is necessary to extrapo late effects to populations with different causes of death, reduced their statistical power and gave 95% CIs that spanned 1·0. To use the broad evidence while accounting for the uncertainty of the subgroup RRs, we included in the uncertainty analysis all draws of the RR distribution, including those that show a protective effect as long as the overall relation for the risk factor across all ages is significant. In other cases, if there are different degrees of exposure for a risk factor, in some exposure categories the RR might not be significant. We have included draws from these posterior distributions if the mean values show a dose–response relation. To fairly represent the extent of our epidemiological knowledge, we have included in the uncertainty analysis draws from the posterior distribution for those exposure categories that show a protective effect.
Theoretical-minimum-risk exposure distributions for counterfactual comparison
In the comparative risk assessment framework, disease burden attributable to risk factors is calculated with reference to an alternative (counterfactual) distribution of exposure; in GBD 2010, we used an optimal exposure distribution (in terms of effect on population health), termed the theoretical-minimum-risk exposure distribution. For several risk factors, such as tobacco smoking, the choice of theoretical-minimum-risk exposure distribution is clear—ie, 100% of the population being lifelong non-smokers. However, for many of the other risk factors zero exposure is not possible (eg, blood pressure), or the lowest amount of exposure that is still beneficial is not yet established. In these cases the theoretical-minimum-risk exposure distribution was informed by two considerations: the availability of convincing evidence from epidemiological studies that support a continuous reduction in risk of disease to the chosen distribution; and a distribution that is theoretically possible at the population level (table 1).
For some risk factors, new evidence has resulted in a revision of the theoretical-minimum-risk exposure distribution compared to the previous comparative risk assessment. For example, the previous distribution for systolic blood pressure was a mean of 115 mm Hg (SD 6).6 However, subsequent randomised trials154 of blood pressure-lowering medication suggest that the benefits of lowering blood pressure could continue to 110 mm Hg or lower. On this basis, we changed the theoretical-minimum-risk exposure distribution to a mean of 110–115 mm Hg (SD 6). For other exposures, the distribution was increased because of data from new epidemiological studies75— eg, for mean body-mass index we used 21–23 kg/m2, compared with 21 kg/m2 used previously.
For ambient particulate matter pollution, we did a sensitivity analysis with an alternative theoretical-minimum-risk exposure distribution that included the effect of regional dust particulate matter. We did so because although particulate exposure from dust could theoretically be reduced, it would probably be prohibitively expensive and could only be done over a very long period. This factor is particularly relevant in areas with high amounts of dust—eg, deserts. Dusty grid cells were identified as those with an ambient air concentration of PM2·5 of 36 µg/m3 or more and where the dust fraction from the TM5 chemical transport model was 50% or more.
Mortality and disease burden attributable to individual and clusters of risk factors
We calculated the burden attributable to risk factors with continuous exposure by comparing the present distribution of exposure to the theoretical-minimum-risk exposure distribution for each age group, sex, year (1990 and 2010), and cause according to the following formula:
PAF=∫x=0mRR(x)P1(x)dx−∫x=0mRR(x)P2(x)dx∫x=0mRR(x)P1(x)dx
Where PAF is the population attributable fraction (burden attributable to risk factor), RR(×) is the RR at exposure level ×, P1(×) is the (measured or estimated) population distribution of exposure, P2(×) is the counterfactual distribution of exposure (ie, the theoretical-minimum-risk exposure distribution), and m the maxi mum exposure level.4
Burden attributable to categorical exposures was calculated by comparing exposure categories to a reference category for each age, sex, year, and cause according to the following formula:
PAF=∑i=1nPi(RRi−1)∑i=1nPi(RRi−1)+1
Where RRi is the RR for exposure category i, Pi is the fraction of the population in exposure category i, and n is the number of exposure categories.4
We calculated the burden attributable to clusters of risk factors by computing the combined population attributable fraction for risk factors for each age, sex, year, and cause according to the following formula:
Where r is each individual risk factor, and R is the number of risk factors. This approach assumes that risk factors are independent—ie, it does not account for mediation, exposure correlation, or effect size modification that might exist between risk factors in a cluster.155
To represent uncertainty in the estimates we used simulation analysis to take 1000 draws from the posterior distribution of exposure, RR, and each relevant outcome for each age, sex, country, year. We accounted for the correlation structure of uncertainty (ie, whether exposure in a country, age group, and sex is high or low might be related to whether it is high or low in other subgroups) by use of the same draw of exposure across different outcomes and the same draw of RR across country, age, and sex subgroups when the RR does not vary by country, age, or sex. We otherwise assumed that the uncertainties in exposure, RR, and underlying burden attributable to the outcome were independent.
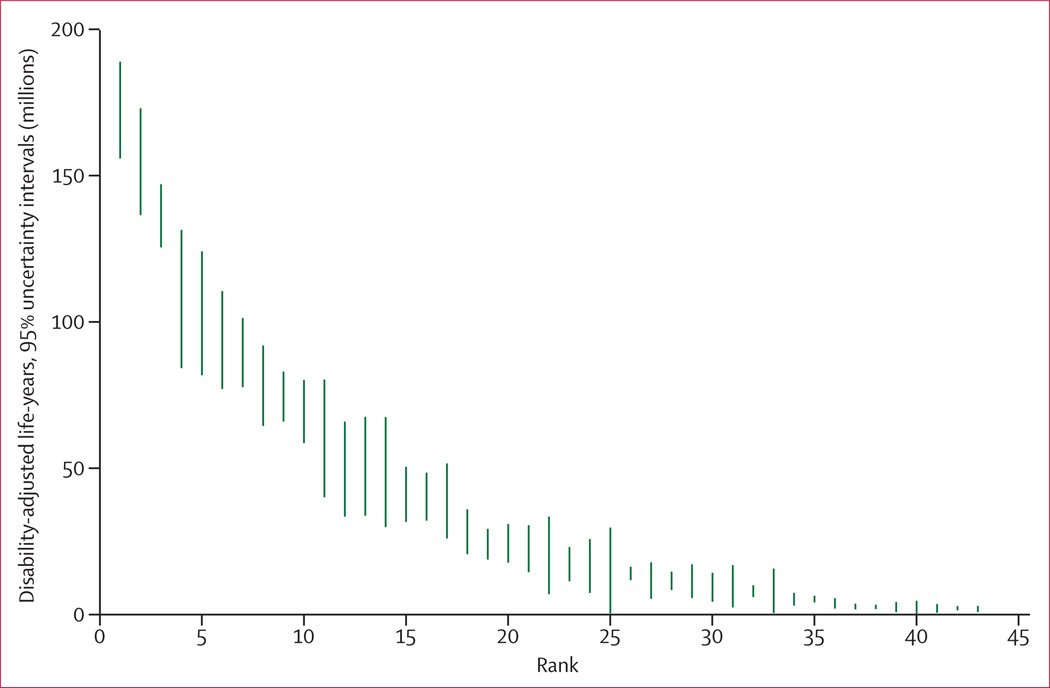
We computed the mean deaths and DALYs attributable to each risk factor and risk factor cluster from the 1000 draws. The 95% uncertainty intervals (95% UI) were calculated as the 2·5th and 97·5th percentiles of the 1000 draws. We also computed the mean rank and 95% UI for the 43 risk factors included in the ranking list. The mean of the ranks for a risk factor was not necessarily equivalent to the rank of the mean deaths or mean DALYs attributable to the risk factor.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
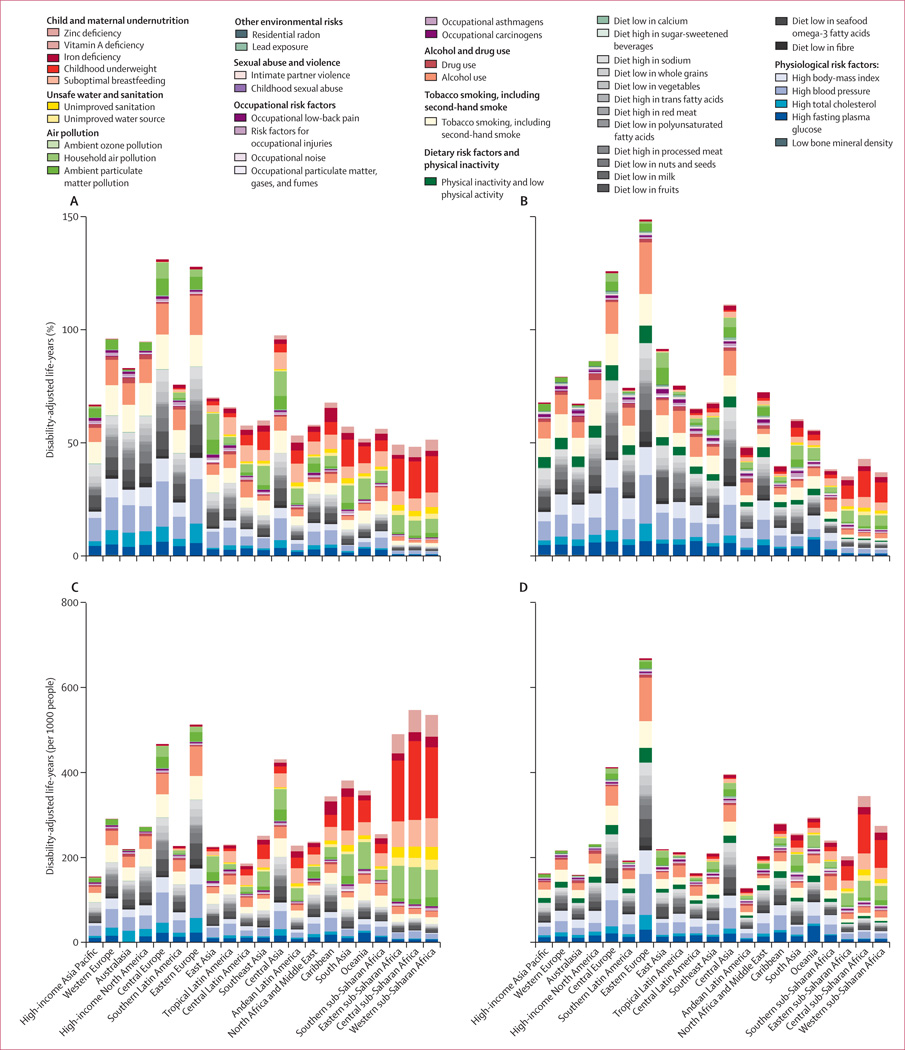
Quantification of risk factors in this analysis represents the effects of each individual risk factor, holding all other independent factors constant. The effects of multiple risk factors are not a simple addition of the individual effects and are often smaller than their sums,156 especially for cardiovascular diseases, which are affected by several risk factors (eg, table 2). The sum of the individual effects of just the metabolic risk factors at the global level is 121% and the summation of all the risks is greater than 400%.
Table 2.
Proportion of ischaemic heart disease disability-adjusted life-years attributable to individual risk factors, worldwide, 2010
| Disability-adjustedlife-years (%) | |
|---|---|
| Physiological risk factors | |
| High blood pressure | 53% |
| High total cholesterol | 29% |
| High body-mass index | 23% |
| High fasting plasma glucose | 16% |
| Alcohol use | 33% |
| Tobacco smoking, including second-hand smoke | 31% |
| Dietary risk factors and physical inactivity | |
| Diet low in nuts and seeds | 40% |
| Physical inactivity and low physical activity | 31% |
| Diet low in fruits | 30% |
| Diet low in seafood omega-3 fatty acids | 22% |
| Diet low in whole grains | 17% |
| Diet high in sodium | 17% |
| Diet high in processed meat | 13% |
| Diet low in vegetables | 12% |
| Diet low in fibre | 11% |
| Diet low in polyunsaturated fatty acids | 9% |
| Diet high in trans fatty acids | 9% |
| Diet high in sugar-sweetened beverages | 2% |
| Air pollution | |
| Ambient particulate matter pollution | 22% |
| Household air pollution from solid fuels | 18% |
| Other environmental risks | |
| Lead exposure | 4% |
We estimated global attributable mortality and DALYs with uncertainty for 1990, and 2010, for each of the 67 risk factors and clusters of risk factors (table 3, 4). The appendix shows full results by region, year, age, and sex for attributable deaths and DALYs. Because of the interest in the combined effects of multiple risk factors, we have approximated the joint effects of clusters of risk factors assuming that risk factors included in each cluster are independent. However, risk factors included in a cluster are not necessarily independent; for example, a substantial part of the burden attributable to high body-mass index is mediated through high blood pressure and high fasting plasma glucose. Others act together and risk factor exposures might be correlated at the individual level,155 especially household air pollution and ambient particulate matter pollution, which might have common sources.
Table 3.
Deaths attributable to risk factors and risk factor clusters, worldwide
| Men | Women | Both sexes | ||||
|---|---|---|---|---|---|---|
| 1990 | 2010 | 1990 | 2010 | 1990 | 2010 | |
| Unimproved water and sanitation | 365 244 (18 940–662 551) | 171 097 (6841–326 262) | 350 629 (17 531–638 433) | 166 379 (6690–326 989) | 715 873 (36 817–1 279 220) | 337 476 (13 150–648 205) |
| Unimproved water source | 147 857 (10 566–282 890) | 59 463 (3880–120 264) | 140 150 (10 042–271 546) | 56 663 (3604–115 704) | 288 007 (20 641–553 293) | 116 126 (7518–233 136) |
| Unimproved sanitation | 252 779 (8032–480 822) | 123 255 (2924–242 588) | 244 207 (7348–460 913) | 120 851 (3104–242 452) | 496 986 (15 380–927 845) | 244 106 (6027–478 186) |
| Air pollution | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| Ambient particulate matter pollution | 1 549 448 (1 345 894–1 752 880) | 1 850 428 (1 614 010–2 082 474) | 1 360 712 (1 166 992–1 559 747) | 1 373 113 (1 187 639–1 563 793) | 2 910 161 (2 546 184–3 286 508) | 3 223 540 (2 828 854–3 619 148) |
| Household air pollution from solid fuels | 2 309 166 (1 720 246–2 824 893) | 1 900 443 (1 378 832–2 518 572) | 2 270 549 (1 889 651–2 655 411) | 1 645 956 (1 265 509–2 089 785) | 4 579 715 (3 717 711–5 365 013) | 3 546 399 (2 679 627–4 516 722) |
| Ambient ozone pollution | 77 087 (25 256–134 021) | 86 335 (30 551–153 776) | 66 274 (22 424–116 663) | 66 100 (21 362–115 225) | 143 362 (47 539–251 885) | 152 434 (52 272–267 431) |
| Other environmental risks | 109 224 (91 805–131 511) | 426 280 (341 744–541 465) | 100 699 (82 720–119 745) | 346 751 (281 555–413 370) | 209 923 (177 673–243 565) | 773 030 (640 893–929 935) |
| Residential radon | ‥ | 70 014 (9140–154 460) | ‥ | 28 978 (4098–64 387) | ‥ | 98 992 (13 133–215 237) |
| Lead exposure | 109 224 (91 805–131 511) | 356 266 (292 587–435 046) | 100 699 (82 720–119 745) | 317 772 (265 722–376 431) | 209 923 (177 673–243 565) | 674 038 (575 858–779 314) |
| Child and maternal undernutrition | 1 805 224 (1 479 043–2 219 888) | 739 863 (570 560–909 248) | 1 668 365 (1 396 689–1 986 532) | 698 442 (569 013–832 012) | 3 473 589 (2 906 896–4 175 138) | 1 438 305 (1 175 257–1 713 103) |
| Suboptimal breastfeeding | 693 103 (427 028–972 440) | 293 449 (175 623–429 772) | 581 921 (370 598–814 551) | 251 368 (155 884–359 651) | 1 275 024 (802 142–1 772 745) | 544 817 (338 453–775 077) |
| Non-exclusive breastfeeding | 612 059 (354 236–875 230) | 257 771 (143 116–382 459) | 505 849 (302 585–720 858) | 218 117 (126 383–319 470) | 1 117 908 (663 274–1 576 633) | 475 888 (272 493–684 422) |
| Discontinued breastfeeding | 81 044 (8643–178 237) | 35 678 (3475–79 940) | 76 073 (7809–165 395) | 33 251 (3091–73 804) | 157 117 (16 188–341 702) | 68 929 (6445–153 290) |
| Childhood underweight | 1 198 178 (997 627–1 484 105) | 458 639 (366 866–561 352) | 1 065 774 (898 859–1 299 715) | 401 478 (325 516–484 452) | 2 263 952 (1 927 356–2 735 821) | 860 117 (715 742–1 033 573) |
| Iron deficiency | 39 409 (30 677–47 108) | 32 287 (21 925–37 449) | 128 675 (92 036–156 884) | 87 321 (62 505–107 021) | 168 084 (130 444–197 085) | 119 608 (93 261–139 985) |
| Vitamin A deficiency | 181 151 (85 775–341 439) | 63 291 (32 070–104 030) | 168 203 (80 696–298 163) | 56 472 (28 192–91 464) | 349 354 (170 504–632 149) | 119 762 (61 723–191 846) |
| Zinc deficiency | 143 518 (27 797–276 850) | 52 390 (9382–105 728) | 132 071 (23 716–253 841) | 44 940 (7696–87 711) | 275 590 (51 274–529 451) | 97 330 (17 575–190 527) |
| Tobacco smoking (including second-hand smoke) | 3 680 571 (3 213 427–4 229 530) | 4 507 059 (3 757 779–5 092 460) | 1 649 238 (1 380 504–2 144 408) | 1 790 228 (1 278 666–2 094 260) | 5 329 808 (4 778 526–6 049 296) | 6 297 287 (5 395 769–7 006 942) |
| Tobacco smoking | 3 332 192 (2 871 957–3 840 033) | 4 251 424 (3 503 674–4 850 554) | 1 244 106 (961 356–1 781 819) | 1 443 924 (920 763–1 743 849) | 4 576 298 (4 068 753–5 312 438) | 5 695 349 (4 755 779–6 421 611) |
| Second-hand smoke | 348 378 (273 555–425 310) | 255 634 (191 587–314 541) | 405 132 (310 224–500 100) | 346 304 (252 702–439 439) | 753 510 (585 131–912 313) | 601 938 (447 705–745 328) |
| Alcohol and drug use | 2 367 579 (2 201 233–2 555 818) | 3 249 978 (3 004 655–3 488 393) | 1 394 778 (1 245 021–1 545 612) | 1 768 073 (1 588 197–1 935 072) | 3 762 356 (3 508 021–4 030 022) | 5 018 051 (4 680 954–5 321 362) |
| Alcohol use | 2 325 747 (2 153 733–2 512 207) | 3 140 109 (2 902 204–3 376 483) | 1 374 578 (1 223 155–1 522 080) | 1 720 059 (1 541 469–1 886 125) | 3 700 324 (3 451 511–3 967 436) | 4 860 168 (4 533 106–5 153 283) |
| Drug use | 46 682 (33 063–78 398) | 109 420 (82 297–152 421) | 21 895 (15 984–31 023) | 48 385 (36 780–64 303) | 68 577 (50 706–102 395) | 157 805 (124 639–209 873) |
| Physiological risk factors | ||||||
| High fasting plasma glucose | 1 051 401 (865 949–1 250 550) | 1 749 058 (1 455 169–2 039 206) | 1 052 773 (881 704–1 230 327) | 1 607 214 (1 367 465–1 839 764) | 2 104 174 (1 797 633–2 401 170) | 3 356 271 (2 917 520–3 782 483) |
| High total cholesterol | 936 749 (767 684–1 128 051) | 961 614 (714 774–1 236 023) | 1 009 172 (829 163–1 218 442) | 1 057 196 (793 595–1 350 633) | 1 945 920 (1 625 929–2 318 054) | 2 018 811 (1 572 853–2 479 097) |
| High blood pressure | 3 412 588 (3 089 548–3 769 223) | 4 750 581 (4 272 529–5 273 576) | 3 880 598 (3 559 634–4 250 099) | 4 645 279 (4 198 029–5 092 003) | 7 293 185 (6 701 203–7 859 894) | 9 395 860 (8 579 630–10 147 805) |
| High body-mass index | 887 047 (698 599–1 079 235) | 1 632 766 (1 328 501–1 941 988) | 1 076 502 (878 065–1 286 482) | 1 738 466 (1 454 008–2 036 059) | 1 963 549 (1 590 282–2 345 133) | 3 371 232 (2 817 774–3 951 127) |
| Low bone mineral density | 52 816 (43 822–69 605) | 103 440 (67 743–124 596) | 50 455 (40 408–62 110) | 84 146 (57 863–102 441) | 103 270 (90 672–124 230) | 187 586 (140 636–219 906) |
| Dietary risk factors and physical inactivity | 4 473 276 (4 110 262–4 852 556) | 6 687 621 (6 172 230–7 206 283) | 4 057 558 (3 704 325–4 431 571) | 5 815 748 (5 380 274–6 261 225) | 8 530 835 (7 907 898–9 150 862) | 12 503 370 (11 710 741–13 324 770) |
| Diet low in fruits | 2 013 415 (1 570 347–2 435 112) | 2 837 481 (2 203 651–3 414 649) | 1 653 787 (1 269 335–2 006 693) | 2 064 761 (1 593 495–2 507 876) | 3 667 202 (2 870 267–4 394 152) | 4 902 242 (3 818 356–5 881 561) |
| Diet low in vegetables | 779 747 (535 472–1 041 517) | 1 017 500 (687 787–1 378 721) | 674 309 (441 649–910 150) | 779 754 (521 285–1 040 304) | 1 454 057 (978 665–1 924 334) | 1 797 254 (1 205 059–2 394 366) |
| Diet low in whole grains | 649 676 (503 984–787 057) | 963 640 (748 116–1 162 721) | 580 600 (447 140–706 303) | 762 171 (592 879–919 709) | 1 230 276 (958 136–1 489 812) | 1 725 812 (1 342 896–2 067 224) |
| Diet low in nuts and seeds | 1 041 726 (667 481–1 349 266) | 1 389 433 (890 869–1 817 734) | 872 483 (541 757–1 147 258) | 1 082 390 (663 158–1 441 054) | 1 914 209 (1 216 363–2 487 874) | 2 471 823 (1 559 603–3 226 994) |
| Diet low in milk | 34 838 (10 464–58 211) | 54 093 (16 106–91 527) | 33 312 (9745–57 799) | 46 858 (13 085–80 413) | 68 150 (20 479–114 435) | 100 951 (29 728–171 340) |
| Diet high in red meat | 13 888 (3859–23 763) | 21 330 (6175–37 340) | 12 551 (3425–22 054) | 16 762 (4306–29 007) | 26 439 (7374–45 232) | 38 092 (10 749–65 727) |
| Diet high in processed meat | 397 198 (85 536–688 905) | 473 562 (103 608–842 923) | 334 476 (71 692–584 050) | 367 296 (83 446–637 120) | 731 675 (158 044–1 257 423) | 840 857 (188 952–1 460 279) |
| Diet high in sugar-sweetened beverages | 100 250 (69 485–134 139) | 161 042 (111 700–219 563) | 83 548 (53 949–117 567) | 138 480 (91 257–203 236) | 183 799 (127 938–240 028) | 299 521 (212 310–403 716) |
| Diet low in fibre | 333 603 (149 007–521 712) | 441 895 (201 062–693 234) | 250 541 (111 867–394 088) | 300 994 (134 201–470 634) | 584 144 (260 065–914 729) | 742 888 (334 379–1 166 933) |
| Diet low in calcium | 48 975 (32 814–66 562) | 76 413 (51 653–103 188) | 33 330 (23 008–43 904) | 49 181 (34 016–63 592) | 82 305 (57 324–108 535) | 125 594 (88 323–164 800) |
| Diet low in seafood omega-3 fatty acids | 576 646 (418 376–735 746) | 793 650 (574 241–1 010 930) | 466 440 (337 205–601 988) | 596 246 (437 287–764 762) | 1 043 085 (757 418–1 327 627) | 1 389 896 (1 010 300–1 781 401) |
| Diet low in polyunsaturated fatty acids | 248 677 (117 929–381 787) | 306 296 (140 873–473 149) | 199 388 (95 418–305 733) | 227 307 (108 675–350 194) | 448 065 (213 262–687 396) | 533 603 (245 096–820 854) |
| Diet high in trans fatty acids | 202 725 (144 395–260 843) | 293 087 (209 155–371 284) | 164 736 (117 395–211 588) | 222 173 (160 511–283 740) | 367 461 (265 936–467 609) | 515 260 (371 081–649 451) |
| Diet high in sodium | 1 197 713 (776 962–1 589 448) | 1 732 870 (1 122 107–2 301 781) | 1 047 642 (666 779–1 397 486) | 1 371 438 (878 780–1 834 541) | 2 245 355 (1 459 900–2 966 107) | 3 104 308 (2 016 734–4 105 019) |
| Physical inactivity and low physical activity | ‥ | 1 547 833 (1 264 464–1 835 192) | ‥ | 1 636 107 (1 369 722–1 899 182) | ‥ | 3 183 940 (2 657 204–3 718 963) |
| Occupational risk factors | 694 403 (541 113–858 435) | 749 857 (580 954–941 322) | 116 743 (74 642–164 679) | 102 250 (68 744–140 097) | 811 146 (623 674–1 010 107) | 852 107 (659 652–1 062 443) |
| Occupational carcinogens | 55 306 (37 867–80 887) | 92 154 (57 261–127 678) | 16 766 (11 866–24 842) | 25 943 (15 498–37 074) | 72 073 (50 753–101 233) | 118 097 (77 249–160 431) |
| Occupational exposure to asbestos | 17 024 (11 044–26 605) | 26 563 (14 454–36 593) | 6033 (4012–9397) | 7047 (3312–9681) | 23 057 (16 939–33 009) | 33 610 (20 317–43 647) |
| Occupational exposure to arsenic | 1155 (446–2210) | 1915 (717–3496) | 463 (176–915) | 747 (275–1402) | 1618 (622–3039) | 2662 (1011–4860) |
| Occupational exposure to benzene | 993 (426–1757) | 1542 (618–2706) | 770 (292–1422) | 1189 (434–2156) | 1764 (741–3085) | 2731 (1111–4811) |
| Occupational exposure to beryllium | 61 (24–110) | 114 (44–192) | 26 (10–47) | 49 (19–86) | 87 (35–152) | 163 (65–276) |
| Occupational exposure to cadmium | 214 (97–370) | 410 (179–670) | 74 (33–130) | 145 (62–245) | 288 (131–494) | 555 (249–901) |
| Occupational exposure to chromium | 729 (431–1133) | 1361 (720–2014) | 293 (171–490) | 570 (295–858) | 1022 (618–1578) | 1931 (1140–2799) |
| Occupational exposure to diesel engine exhaust | 10 979 (6241–17 555) | 18 773 (9641–28 714) | 2060 (1180–3422) | 3413 (1709–5262) | 13 040 (7494–20 486) | 22 187 (12 180–33 213) |
| Occupational exposure to second-hand smoke | 10 171 (6878–15 272) | 17 189 (10 127–23 037) | 3854 (2637–6207) | 7046 (3935–9630) | 14 025 (10 058–19 715) | 24 235 (16 094–31 803) |
| Occupational exposure to formaldehyde | 299 (117–584) | 486 (185–939) | 179 (77–325) | 245 (97–456) | 478 (202–877) | 731 (301–1361) |
| Occupational exposure to nickel | 3578 (935–7585) | 6443 (1616–13 317) | 1425 (369–3031) | 2702 (743–5679) | 5004 (1331–10 489) | 9145 (2449–18 834) |
| Occupational exposure to polycyclic aromatic hydrocarbons | 1638 (772–2817) | 3092 (1394–5028) | 492 (230–864) | 993 (441–1661) | 2130 (1018–3613) | 4086 (1909–6567) |
| Occupational exposure to silica | 7870 (5154–11 902) | 14 205 (8244–19 702) | 1185 (797–1975) | 2072 (1102–2948) | 9056 (6140–13 213) | 16 277 (9875–22 272) |
| Occupational exposure to sulphuric acid | 1964 (531–4383) | 2606 (718–5761) | 193 (55–452) | 239 (74–509) | 2157 (626–4707) | 2845 (833–6109) |
| Occupational asthmagens | 31 666 (15 305–62 856) | 25 364 (15 642–48 748) | 10 485 (5116–19 129) | 8352 (4854–13 425) | 42 151 (24 425–76 872) | 33 716 (22 844–58 659) |
| Occupational particulate matter, gases, and fumes | 207 366 (92 516–320 244) | 171 553 (79 656–270 369) | 68 281 (29 408–112 504) | 47 311 (20 330–77 499) | 275 647 (121 774–429 427) | 218 864 (100 403–344 633) |
| Occupational noise | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Occupational risk factors for injuries | 400 064 (308 482–507 787) | 460 785 (343 904–618 319) | 21 211 (16 479–27 705) | 20 644 (15 628–27 414) | 421 275 (329 209–529 004) | 481 429 (363 778–639 590) |
| Occupational low back pain | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Sexual abuse and violence | ‥ | 37 429 (21 366–56 607) | ‥ | 200 930 (113 070–292 802) | ‥ | 238 359 (143 200–325 690) |
| Childhood sexual abuse | ‥ | 37 429 (21 366–56 607) | ‥ | 27 009 (14 290–43 424) | ‥ | 64 438 (37 339–94 174) |
| Intimate partner violence | ‥ | ‥ | ‥ | 186 365 (92 028–280 059) | ‥ | 186 365 (92 028–280 059) |
Table 4.
Disability-adjusted life-years (1000s) attributable to risk factors and risk factor clusters, worldwide
| Men | Women | Both sexes | ||||
|---|---|---|---|---|---|---|
| 1990 | 2010 | 1990 | 2010 | 1990 | 2010 | |
| Unimproved water and sanitation | 27 045 (1409–49 439) | 11 022 (458–21 162) | 25 123 (1262–45 792) | 10 165 (428–19 650) | 52 169 (2700–93 073) | 21 187 (866–40 957) |
| Unimproved water source | 11 075 (792–21 250) | 4080 (266–8172) | 10 097 (722–19 424) | 3694 (242–7511) | 21 172 (1517–40 491) | 7775 (514–15 705) |
| Unimproved sanitation | 18 610 (593–35 486) | 7735 (190–15 338) | 17 441 (522–32 889) | 7192 (187–14 099) | 36 050 (1115–66 871) | 14 927 (377–29 705) |
| Air pollution | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| Ambient particulate matter pollution | 46 667 (40 185–53 381) | 46 732 (41 393–52 602) | 35 032 (29 974–40 402) | 29 431 (25 722–33 273) | 81 699 (71 012–92 859) | 76 163 (68 086–85 171) |
| Household air pollution from solid fuels | 94 276 (73 721–113 071) | 61 645 (45 944–77 497) | 81 632 (66 415–96 472) | 49 317 (38 818–60 315) | 175 909 (141 870–207 095) | 110 962 (86 848–137 813) |
| Ambient ozone pollution | 1409 (460–2456) | 1440 (506–2563) | 1125 (375–1990) | 1016 (331–1758) | 2534 (851–4426) | 2456 (837–4299) |
| Other environmental risks | 2876 (2406–3459) | 9434 (7476–12 045) | 2489 (1974–3015) | 6617 (5322–7938) | 5365 (4534–6279) | 16 051 (13 212–19 503) |
| Residential radon | ‥ | 1514 (191–3383) | ‥ | 600 (84–1355) | ‥ | 2114 (273–4660) |
| Lead exposure | 2876 (2406–3459) | 7920 (6491–9683) | 2489 (1974–3015) | 6017 (4915–7231) | 5365 (4534–6279) | 13 936 (11 750–16 327) |
| Child and maternal undernutrition | 175 366 (146 049–211 406) | 83 202 (67 963–99 704) | 164 599 (139 926–192 077) | 82 894 (69 171–98 757) | 339 965 (289 845–402 489) | 166 095 (139 685–193 981) |
| Suboptimal breastfeeding | 59 902 (36 953–84 059) | 25 572 (15 540–37 260) | 50 359 (32 186–70 526) | 21 965 (13 717–31 340) | 110 261 (69 615–153 539) | 47 537 (29 868–67 518) |
| Non-exclusive breastfeeding | 52 729 (30 540–75 288) | 22 258 (12 464–32 936) | 43 601 (26 173–62 072) | 18 850 (10 926–27 569) | 96 330 (57 274–135 861) | 41 108 (23 668–58 913) |
| Discontinued breastfeeding | 7173 (767–15 819) | 3314 (324–7377) | 6758 (696–14 710) | 3114 (296–6915) | 13 931 (1443–30 062) | 6429 (605–14 426) |
| Childhood underweight | 104 713 (87 668–128 697) | 41 270 (33 478–50 007) | 93 028 (78 656–112 766) | 36 045 (29 430–43 394) | 197 741 (169 224–238 276) | 77 316 (64 497–91 943) |
| Iron deficiency | 21 451 (14 947–30 321) | 19 974 (13 595–28 289) | 30 390 (22 473–40 703) | 28 251 (20 195–39 063) | 51 841 (37 477–71 202) | 48 225 (33 769–67 592) |
| Vitamin A deficiency | 15 689 (7475–29 165) | 5672 (2904–9348) | 14 598 (7068–25 637) | 5098 (2566–8168) | 30 288 (14 884–54 488) | 10 770 (5625–17 149) |
| Zinc deficiency | 12 666 (2938–23 883) | 4880 (1203–9316) | 11 709 (2640–22 049) | 4256 (1131–7821) | 24 375 (5385–45 685) | 9136 (2458–16 903) |
| Tobacco smoking (including second-hand smoke) | 104 840 (91 849–119 255) | 115 496 (98 595–130 090) | 46 926 (39 634–58 092) | 41 342 (30 473–48 563) | 151 766 (136 367–169 522) | 156 838 (136 543–173 057) |
| Tobacco smoking | 84 956 (73 038–97 937) | 105 635 (88 332–120 347) | 28 784 (21 829–40 090) | 31 272 (19 859–38 467) | 113 740 (100 454–131 675) | 136 907 (117 201–153 778) |
| Second-hand smoke | 19 884 (14 493–25 591) | 9861 (7669–12 312) | 18 142 (13 748–22 355) | 10 070 (7931–12 429) | 38 026 (28 832–47 544) | 19 931 (15 707–24 223) |
| Alcohol and drug use | 88 000 (79 774–96 333) | 119 130 (107 244–130 842) | 33 648 (30 426–37 108) | 42 178 (38 018–46 786) | 121 648 (111 426–132 342) | 161 308 (146 934–175 282) |
| Alcohol use | 77 550 (70 706–84 835) | 101 875 (92 405–111 165) | 28 479 (25 762–31 372) | 34 188 (30 882–37 479) | 106 029 (98 299–114 763) | 136 063 (125 361–146 788) |
| Drug use | 10 178 (7787–13 073) | 16 248 (12 679–20 132) | 4993 (3811–6417) | 7562 (5922–9471) | 15 171 (11 714–19 369) | 23 810 (18 780–29 246) |
| Physiological risk factors | ‥ | ‥ | ‥ | ‥ | ‥ | ‥ |
| High fasting plasma glucose | 30 177 (25 148–34 980) | 49 148 (41 619–57 197) | 26 181 (22 243–30 349) | 39 864 (34 103–45 972) | 56 358 (48 720–65 030) | 89 012 (77 743–101 390) |
| High total cholesterol | 22 519 (18 230–27 029) | 23 179 (17 148–29 650) | 17 006 (13 940–20 640) | 17 721 (13 153–22 508) | 39 526 (32 704–47 202) | 40 900 (31 662–50 484) |
| High blood pressure | 73 120 (65 538–81 302) | 99 566 (88 193–110 943) | 63 897 (57 903–70 789) | 73 991 (66 161–81 931) | 137 017 (124 360–149 366) | 173 556 (155 939–189 025) |
| High body-mass index | 25 391 (19 752–31 108) | 48 310 (39 429–57 750) | 26 174 (20 911–31 642) | 45 300 (37 218–54 219) | 51 565 (40 786–62 557) | 93 609 (77 107–110 600) |
| Low bone mineral density | 1764 (1448–2208) | 3105 (2295–3831) | 1361 (1102–1686) | 2111 (1627–2618) | 3125 (2589–3811) | 5216 (4133–6418) |
| Dietary risk factors and physical inactivity | 102 663 (94 539–111 011) | 149 576 (138 035–160 263) | 74 611 (68 196–81 173) | 104 757 (97 047–112 535) | 177 274 (164 710–190 286) | 254 333 (237 748–270 495) |
| Diet low in fruits | 47 979 (37 530–57 842) | 65 523 (51 056–78 959) | 32 474 (25 061–39 155) | 38 573 (29 923–46 512) | 80 453 (63 298–95 763) | 104 095 (81 833–124 169) |
| Diet low in vegetables | 18 755 (12 859–24 939) | 24 169 (16 503–32 480) | 12 803 (8412–17 503) | 14 389 (9434–19 284) | 31 558 (21 349–41 921) | 38 559 (26 006–51 658) |
| Diet low in whole grains | 17 033 (13 513–20 522) | 24 881 (19 486–29 709) | 12 370 (9625–14 895) | 15 881 (12 615–18 949) | 29 404 (23 097–35 134) | 40 762 (32 112–48 486) |
| Diet low in nuts and seeds | 24 918 (16 268–31 946) | 32 615 (21 258–41 958) | 15 607 (9915–20 208) | 18 674 (11 716–24 404) | 40 525 (26 308–51 741) | 51 289 (33 482–65 959) |
| Diet low in milk | 818 (248–1366) | 1171 (350–1977) | 710 (210–1210) | 931 (264–1605) | 1527 (461–2555) | 2101 (619–3544) |
| Diet high in red meat | 642 (306–1014) | 1026 (484–1629) | 566 (263–903) | 827 (374–1362) | 1208 (571–1909) | 1853 (870–2946) |
| Diet high in processed meat | 10 477 (2801–17 479) | 12 901 (4012–21 421) | 6882 (2340–11 119) | 8038 (2932–12 685) | 17 359 (5137–27 949) | 20 939 (6982–33 468) |
| Diet high in sugar-sweetened beverages | 3085 (2120–4151) | 4858 (3154–6549) | 2358 (1586–3484) | 3695 (2356–5255) | 5443 (3769–7373) | 8553 (5823–11 418) |
| Diet low in fibre | 8485 (3787–13 262) | 10 893 (4903–17 191) | 4862 (2188–7562) | 5559 (2500–8639) | 13 347 (5970–20 751) | 16 452 (7401–25 783) |
| Diet low in calcium | 1083 (752–1406) | 1570 (1113–2058) | 753 (521–975) | 1019 (720–1319) | 1836 (1316–2368) | 2590 (1873–3322) |
| Diet low in seafood omega-3 fatty acids | 13 620 (9915–17 307) | 18 300 (13 267–23 201) | 8120 (5900–10 388) | 9899 (7241–12 596) | 21 740 (15 869–27 537) | 28 199 (20 624–35 974) |
| Diet low in polyunsaturated fatty acids | 6185 (2891–9362) | 7521 (3455–11 583) | 3727 (1788–5709) | 4159 (1973–6396) | 9912 (4655–14 976) | 11 680 (5360–17 798) |
| Diet high in trans fatty acids | 4979 (3571–6413) | 7339 (5240–9300) | 3085 (2226–3944) | 4253 (3106–5416) | 8064 (5893–10 305) | 11 592 (8395–14 623) |
| Diet high in sodium | 26 807 (17 646–35 273) | 37 378 (24 639–49 428) | 19 376 (12 521–25 596) | 23 852 (15 544–31 682) | 46 183 (30 363–60 604) | 61 231 (40 124–80 342) |
| Physical inactivity and low physical activity | ‥ | 37 007 (30 583–43 466) | ‥ | 32 311 (27 698–37 217) | ‥ | 69 318 (58 646–80 182) |
| Occupational risk factors | 42 660 (35 146–50 545) | 48 317 (38 407–58 677) | 12 754 (9357–16 658) | 14 171 (10 344–18 842) | 55 414 (45 312–66 718) | 62 488 (49 471–76 240) |
| Occupational carcinogens | 1346 (917–1958) | 2087 (1315–2928) | 412 (284–611) | 594 (368–855) | 1758 (1220–2477) | 2681 (1773–3689) |
| Occupational exposure to asbestos | 362 (236–555) | 521 (279–709) | 122 (78–189) | 132 (61–184) | 484 (354–695) | 653 (389–840) |
| Occupational exposure to arsenic | 29 (11–56) | 45 (17–84) | 12 (5–24) | 18 (7–33) | 41 (16–77) | 63 (24–114) |
| Occupational exposure to benzene | 36 (15–64) | 52 (21–92) | 28 (11–52) | 40 (15–72) | 65 (27–112) | 92 (39–163) |
| Occupational exposure to beryllium | 2 (1–3) | 3 (1–5) | 1 (0–1) | 1 (0–2) | 2 (1–4) | 4 (2–6) |
| Occupational exposure to cadmium | 5 (2–9) | 10 (4–16) | 2 (1–3) | 3 (1–6) | 7 (3–12) | 13 (6–21) |
| Occupational exposure to chromium | 18 (11–28) | 32 (17–48) | 8 (4–13) | 13 (7–21) | 26 (16–40) | 45 (27–66) |
| Occupational exposure to diesel engine exhaust | 278 (158–436) | 442 (232–682) | 54 (31–88) | 81 (42–126) | 332 (192–517) | 523 (292–789) |
| Occupational exposure to second-hand smoke | 257 (173–383) | 405 (244–544) | 100 (69–162) | 167 (95–228) | 358 (255–500) | 572 (386–762) |
| Occupational exposure to formaldehyde | 11 (4–20) | 17 (6–31) | 7 (3–13) | 9 (4–16) | 18 (8–32) | 25 (11–47) |
| Occupational exposure to nickel | 90 (24–191) | 151 (38–312) | 37 (10–79) | 64 (18–132) | 128 (34–266) | 215 (58–443) |
| Occupational exposure to polycyclic aromatic hydrocarbons | 41 (19–71) | 73 (33–119) | 13 (6–23) | 23 (10–39) | 54 (26–92) | 96 (45–156) |
| Occupational exposure to silica | 199 (129–297) | 333 (199–463) | 31 (21–52) | 49 (26–71) | 230 (154–328) | 382 (239–526) |
| Occupational exposure to sulphuric acid | 52 (14–114) | 66 (19–143) | 5 (1–12) | 6 (2–13) | 57 (16–122) | 71 (21–152) |
| Occupational asthmagens | 1467 (874–2439) | 1359 (917–2153) | 662 (366–1062) | 661 (407–994) | 2129 (1419–3222) | 2020 (1441–2871) |
| Occupational particulate matter, gases, and fumes | 6808 (3162–10 425) | 6682 (3293–10 311) | 2745 (1216–4406) | 2460 (1105–4025) | 9552 (4385–14 636) | 9142 (4377–14 250) |
| Occupational noise | 1936 (1149–3103) | 2284 (1348–3649) | 933 (550–1489) | 1167 (696–1870) | 2869 (1698–4582) | 3451 (2072–5574) |
| Occupational risk factors for injuries | 20 175 (15 588–25 639) | 22 434 (16 711–29 943) | 1090 (836–1437) | 1010 (771–1331) | 21 265 (16 644–26 702) | 23 444 (17 736–30 904) |
| Occupational low back pain | 10 929 (7340–15 116) | 13 471 (8968–18 945) | 6912 (4487–9835) | 8279 (5502–11 602) | 17 841 (11 846–24 945) | 21 750 (14 492–30 533) |
| Sexual abuse and violence | ‥ | 3588 (2669–4679) | ‥ | 19 931 (14 524–26 397) | ‥ | 23 519 (17 961–30 322) |
| Childhood sexual abuse | ‥ | 3588 (2669–4679) | ‥ | 4244 (3082–5533) | ‥ | 7833 (5964–10 005) |
| Intimate partner violence | ‥ | ‥ | ‥ | 16 794 (11 373–23 087) | ‥ | 16 794 (11 373–23 087) |
For these reasons we have not computed the joint effects for physiological risk factors or air pollution. However, the combined effects of physiological risk factors are probably large, with high blood pressure the leading single risk factor globally, accounting for 9·4 million (95% UI 8·6 million to 10·1 million) deaths and 7·0% (6·2–7·7) of global DALYs in 2010, followed by high body-mass index (3·4 million [2·8 million to 4·0 million deaths] and 3·8% [3·1–4·4] of global DALYs in 2010), high fasting plasma glucose (3·4 million [2·9 million to 3·7 million] deaths and 3·6% [3·1–4·0] of DALYs), high total cholesterol (2·0 million [1·6 million to 2·5 million] deaths and 1·6% [1·3–2·0] of DALYs), and low bone mineral density (0·2 million [0·1 million to 0·2 million] deaths and 0·21% [0·17–0·25] of DALYs).
The joint effects of air pollution are also likely to be large. Household air pollution from solid fuels accounted for 3·5 million (2·7 million to 4·4 million) deaths and 4·5% (3·4–5·3) of global DALYs in 2010 and ambient particulate matter pollution accounted for 3·1 million (2·7 million to 3·5 million) deaths and 3·1% (2·7–3·4) of global DALYs. For ambient particulate matter pollution, we also did a post-hoc sensitivity analysis excluding the effects of dust, which had a small effect worldwide—attributable global DALYs decreased by 2%—but large effects in north Africa and Middle East. Household air pollution is an important contributor to ambient particulate matter pollution; we estimate that it accounted for 16% of the worldwide burden from ambient particulate matter pollution in 2010. The effects of ambient ozone pollution, which increases the risk of chronic obstructive pulmonary disease, were smaller than those of household air pollution from solid fuels or ambient particulate matter pollution (0·2 million [0·1 million to 0·3 million] deaths and 0·1% [0·03–0·2] of global DALYs in 2010).
For other clusters of risk factors for which we approximated the joint effects assuming independence, dietary risk factors and physical inactivity were responsible for the largest disease burden: 10·0% (9·2–10·8) of global DALYs in 2010. Of the individual dietary risk factors, the largest attributable burden in 2010 was associated with diets low in fruits (4·9 million [3·8 million to 5·9 million] deaths and 4·2% [3·3–5·0] of global DALYs), followed by diets high in sodium (4·0 million [3·4 million to 4·6 million]; 2·5% [1·7–3·3]), low in nuts and seeds (2·5 million [1·6 million to 3·2 million]; 2·1% [1·3–2·7]), low in whole grains (1·7 million [1·3 million to 2·1 million]; 1·6% [1·3–1·9]), low in vegetables (1·8 million [1·2 million to 2·3 million]; 1·5% [1·0–2·1]), and low in seafood omega-3 fatty acids (1·4 million [1·0 million to 1·8 million]; 1·1% [0·8–1·5]). Our sensitivity analysis of omega-3 fatty acids using relative risks from randomised trials reduced the attributable burden by more than half, to 0·6 million (–0·6 million to 1·7 million) deaths, and 0·5% (−0·5 to 1·4) of global DALYs in 2010. Physical inactivity and low physical activity accounted for 3·2 million (2·7 million to 3·7 million) deaths, and 2·8% (2·4–3·2) of DALYs in 2010.
Child and maternal undernutrition was responsible for the next largest attributable burden of the risk factor clusters (1·4 million [1·2 million to 1·7 million] deaths; 6·7% [5·7–7·7] of global DALYs in 2010), with childhood underweight the largest individual contributor (0·9 million [0·7 million to 1·0 million]; 3·1% [2·6–3·7]), followed by iron deficiency (0·1 million [0·09 million to 0·14 million]; 1·9% [1·4–2·6]), and suboptimal breast feeding (0·5 million [0·3 million to 0·8 million]; 1·9% [1·2–2·7]). Vitamin A and zinc deficiencies amongst children accounted for less than 0·8% of the disease burden.
The burdens of disease attributable to tobacco smoking including second-hand smoke (6·3 million [5·4 million to 7·0 million] deaths and 6·3% [5·5–7·0] of DALYs) as well as alcohol and drug use (5·0 million [4·7 million to 5·3 million] deaths and 6·5% [6·0–7·0] of DALYs) were substantial in 2010. These burdens are mainly driven by active smoking, which accounts for 87% of the combined burden with second-hand smoke, and alcohol use which accounted for 4·9 million (4·5 million to 5·2 million) deaths and 5·5% (5·0–5·9) of global DALYs in 2010. Of the remaining risk factor clusters, occupational risk factors accounted for 0·9 million (0·7 million to 1·1 million) deaths and 2·5% (2·0–3·0) of global DALYs in 2010, followed by sexual abuse and violence (0·2 million [0·1 million to 0·3 million] deaths and 0·9% [0·7–1·2] DALYs), unimproved water and sanitation, (0·3 million [0 to 0·6 million] deaths and 0·9% [0·04–1·6] DALYs), and other environmental risks (0·7 million [0·6 million to 0·9 million] deaths and 0·6% [0·5–0·8] DALYs).
The rest of the results section refers to the 43 risk factors and clusters of risk factors in the rank list. The predominance of non-communicable disease risks in 2010 highlights the global epidemiological transition that has occurred since 1990 (figures 1, 2, 3). In 1990, the leading risks were childhood underweight (7·9% [6·8–9·4] of global DALYs), household air pollution from solid fuels (7·0% [5·6–8·3]), and tobacco smoking including second-hand smoke (6·1% [5·4–6·8]), high blood pressure (5·5% [4·9–6·0]), and suboptimal breast feeding (4·4% [2·8–6·1]). With the exception of house hold air pollution, which is a significant contributor to childhood lower respiratory tract infections, the five leading risk factors in 2010 (high blood pressure, tobacco smoking including secondhand smoke, alcohol use, household air pollution, and diets low in fruits) are mainly causes of adult chronic disease, especially cardio vascular diseases and cancers (figures 1, 2). The burden of disease attributable to other chronic disease risk factors also increased substantially between 1990 and 2010; for example, the global disease burden attributable to high body-mass index increased from 52 million to 94 million DALYs and that of high fasting plasma glucose increased from 56 million to 89 million DALYs over this period.
Figure 1. Burden of disease attributable to 20 leading risk factors in 1990, expressed as a percentage of global disability-adjusted life-years.
For men (A), women (B), and both sexes (C).
Figure 3. Global risk factor ranks with 95% UI for all ages and sexes combined in 1990, and 2010, and percentage change.
PM=particulate matter. UI=uncertainty interval. SHS=second-hand smoke. An interactive version of this figure is available online at http://healthmetricsandevaluation.org/gbd/visualizations/regional.
Figure 2. Burden of disease attributable to 20 leading risk factors in 2010, expressed as a percentage of global disability-adjusted life-years.
For men (A), women (B), and both sexes (C).
The rise in global disease burden attributable to chronic disease risk factors has been accompanied by a decrease in the relative importance of risk factors that largely or exclusively cause communicable diseases in children. The global disease burden attributable to childhood underweight halved between 1990 (7·9% [6·8–9·4] of global DALYs) and 2010 (3·1% [2·6–3·7]; table 3). Although the fraction of disease burden attributable to iron deficiency fell relatively little, suboptimal breastfeeding, unimproved water, unimproved sanitation, vitamin A deficiency, and zinc deficiency all decreased substantially between 1990, and 2010.
The transition from childhood communicable to non-communicable disease burden is also exemplified by the fall in DALYs caused by household air pollution from solid fuels (despite the rise in its effects on cardiovascular diseases). Although the burden attributable to ambient particulate matter pollution has largely remained unchanged (3·2% [2·8–3·7] of global DALYs in 1990 vs 3·0% [2·6–3·4] in 2010), the contribution of lower respiratory tract infections had fallen sharply by 2010, with chronic diseases of adults being the dominant health outcome caused by this exposure.
Figure 4 shows the 95% uncertainty interval in global DALYs attributable to each risk factor and the overall rank for each risk factor. The uncertainty intervals for many risk factors overlap, especially those not in the top five. Unimproved water, unimproved sanitation, vitamin A deficiency, and zinc deficiency have large uncertainty, which reflects the substantial uncertainty in the estimates of etiological effect sizes for these risks.
Figure 4. 95% uncertainty intervals for risk factors ranked by global attributable disability-adjusted life-years, 2010.
An interactive version of this figure is available online at http://healthmetricsandevaluation.org/gbd/visualizations/regional
Some risks were quantified for women only—for example, intimate partner violence, which accounted for 1·5% (1·0—2·1) of DALYs among women in 2010. Important differences between men and women also exist for disease burden attributable to other risk factors, most notably, for tobacco smoking including secondhand smoke and alcohol use (figures 1, 2). These risks cause substantially lower burden in women than in men, because women drink less and in less harmful ways than do men, and fewer smoke or have smoked for a shorter time than have men in most regions.157 In 2010, tobacco smoking including second-hand smoke accounted for 8·4% of worldwide disease burden among men (the leading risk factor) compared with 3·7% among women (fourth highest risk factor). For alcohol use, these sex differences were similarly sub stantial: 7·4% (third) versus 3·0% (eighth). The effect of occupational risk factors on population health also differed between sexes—for example, the fraction of disease burden attributable to occupational risk factors for injuries was 18·5 times higher for men than for women in 2010 (20 175 000 DALYs for men vs 1 090 000 for women). Dietary risk factors had broadly similar effects for men and women with the exception of diet low in fruits, for which the fraction of disease burden attributable was 1·5 times larger for men than for women in 2010 (47 979 000 DALYs for men vs 32 474 000 for women). This effect is caused by lower fruit consumption and a larger disease burden from cardiovascular disease in men.
Further disaggregation of mortality and disease burden attributable to risk factors reveals several patterns by age group (appendix). Among children younger than 5 years, childhood underweight was the leading risk factor worldwide in 2010 (12·4% [10·4–14·7] of global DALYs), followed by non-exclusive or discontinued breast feeding (7·6% [4·8–10·9]) and household air pollu tion from solid fuels (6·3% [4·4–8·1]). Vitamin A and zinc deficiencies, unimproved sanitation, and unimproved water each accounted for less than 2% of disease burden in children younger than 5 years.
For people aged 15–49 years, the leading risk factor worldwide was alcohol use, followed by tobacco smoking including second-hand smoke, high blood pressure, high body-mass index, diet low in fruits, drug use, and occupational risk factors for injuries. Risk factor rankings in this age group stayed broadly similar between 1990, and 2010, with the exception of iron deficiency, which dropped from the fourth leading risk factor in 1990, to ninth in 2010.
High blood pressure, tobacco smoking including second-hand smoke, alcohol use, and diet low in fruits were all in the top five risk factors for adults aged 50–69 years and adults older than 70 years, in both 1990, and 2010, accounting for a large proportion of disease burden in both age groups. Globally, high blood pressure accounted for more than 20% of all health loss in adults aged 70 years and older in 2010, and around 15% in those aged 50–69 years. Tobacco smoking including secondhand smoke accounted for more than 10% of global disease burden in each of these age groups in 2010.
In all 21 regions, and worldwide, a shift has occurred, from risk factors for childhood communicable disease to risk factors for non-communicable disease. The size of this shift and which risk factors account for the largest burden varies highly between regions (figure 5, appendix).
Figure 5. Risk factors ranked by attributable burden of disease, 2010.
Regions are ordered by mean life expectancy. No data=attributable disability-adjusted life-years were not quantified.
In central, eastern, and western sub-Saharan Africa, the share of disease burden attributable to childhood underweight, household air pollution from solid fuels, and suboptimal breastfeeding has fallen sub stantially. However, these risk factors continue to be the leading three causes of disease burden in 2010. The disease burden attributable to risk factors for childhood communicable diseases, such as micronutrient deficiencies and unimproved water and sanitation, has decreased, both as a proportion of total disease burden and in their rank order: risk factors for some non-communicable diseases and injury accounted for a larger disease burden in 2010. The most notable of these factors were alcohol use and high blood pressure (appendix).
Compared with other regions of sub-Saharan Africa, southern sub-Saharan Africa had a more mixed pattern of risk factor burden in 1990 (appendix). In 2010, alcohol use was the leading risk factor in southern sub-Saharan Africa, followed by high blood pressure and high bodymass index (figure 6). In addition to high exposure to harmful alcohol use, the effects of alcohol were particularly large because it increases the risk of road traffic and other unintentional and intentional injuries, as well as of tuberculosis,47 all of which are large causes of disease and injury burden in this region.
Figure 6. Attributable burden for each risk factor.
As percentage of disability-adjusted life-years in 1990 (A), and 2010 (B), and as disability-adjusted life-years per 1000 people in 1990 (C), and 2010 (D). Regions ordered by mean life expectancy. Burden of disease attributable to individual risk factors are shown sequentially for ease of presentation. In reality, the burden attributable to different risks overlaps because of multicausality and because the effects of some risk factors are partly mediated through other, more proximal, risks. An interactive version of this figure is available online at http://healthmetricsandevaluation.org/gbd/visualizations/regional.
In south Asia, the rise of risk factors for non-communicable diseases is shown by the substantial increase in the burden attributable to tobacco smoking including second-hand smoke, high blood pressure and other metabolic risk factors, dietary risk factors, and alcohol use. However, household air pollution from solid fuels was, despite decreases, the leading risk factor in 2010. Childhood underweight was still the fourth leading risk factor in 2010, despite its share of disease burden having more than halved from 11·9% [95% UI 10·1–14·4] of DALYs in 1990, to 4·0% [3·2–4·9] in 2010. Other risk factors for communicable disease, such as suboptimal breastfeeding and micronutrient deficiencies, fell sub stantially in the region as child mortality decreased.
In southeast, east, and central Asia, the epidemiological transition was already well advanced in 1990, and by 2010, high blood pressure (which is commonly associated with diets high in sodium as a prominent underlying cause94,158), tobacco smoking including second-hand smoke, and diets low in fruits were all among the five leading risk factors in these regions. The disease burden attributable to childhood underweight and sub optimal breastfeeding had been largely eliminated in east Asia by 2010, although they remain important in southeast Asia. In these three regions, despite decreases, household air pollution from solid fuels was still a leading risk factor in 2010, ranked third in south-east Asia, sixth in east Asia, and 12th in central Asia. Ambient particulate matter pollution accounted for a larger disease burden than did household air pollution in central and east Asia in 2010, although household solid fuels is an important source of ambient particulate matter pollution in these regions.
The North Africa and Middle East region also had a large shift from risk factors for communicable to non-communicable diseases. In 2010, risk factors for noncommunicable disease almost exclusively dominated the region’s causes of loss of health, with high blood pressure and high body-mass index each accounting for roughly 8% of disease burden, followed by tobacco smoking including second-hand smoke, high fasting plasma glucose, and physical inactivity or low physical activity. Ambient particulate matter pollution (seventh leading risk factor) is a notable cause of disease burden in this region, caused by a combination of polluted cities and dust from the Sahara desert.
Alcohol use was an important cause of disease burden in most of Latin America. It was ranked first in central Latin America, fourth in tropical Latin America, and sixth in Andean Latin America in 1990, and first in all these regions in 2010. Risk factors for childhood communicable disease had been largely replaced by those causing non-communicable diseases in these regions by 2010, although household air pollution from solid fuels was still an important risk factor in Andean Latin America in 2010.
One of the most notable findings was the effect of alcohol use in Eastern Europe, where it accounts for almost a quarter of total disease burden. Other risk factors, such as high blood pressure, tobacco smoking including second-hand smoke, high body-mass index, and dietary risks, also feature prominently, underscoring the large underlying burden of cardiovascular disease in the region.
In North America, Australasia, southern Latin America, and western Europe, the share of disease burden attributable to tobacco smoking including second-hand smoke has fallen slightly; it has stayed almost constant in central Europe and high-income Asia Pacific. Tobacco smoking including second-hand smoke was still the leading risk factor in 2010 in North America and western Europe. Important decreases in disease burden are evident for high blood pressure and total cholesterol in North America, Australasia, and western Europe. High blood pressure is a leading risk for health in high-income Asia Pacific (accounting for 8·5% [95% UI 7·1–10·1] of disease burden) and central Europe (18·9% [16·8–20·8]); evidence from individual-level trials of salt and blood pressure and from cross-population studies indicates that this result is likely to be driven partly by high salt consumption in these regions.94,158 Falls in disease burden attributable to tobacco smoking including second-hand smoke, high blood pressure, and high total cholesterol in high-income regions have been partly offset by the increasing burden caused by high body-mass index. In southern Latin America, high body-mass index accounted for almost 10% of overall disease burden in 2010, and is the leading risk factor in southern Latin America and Australasia.
Figure 6 summarises these regional patterns, in relation to the proportion of regional burden and attributable DALYs per 1000 people. Regions in figure 6 are ordered by mean age of death, a marker of the epidemiological transition. Figure 6 shows the clear transition away from risk factors for childhood communicable disease towards risk factors for non-communicable disease, with increasing mean age at death. This change is apparent from the decrease in burden of disease attributable to undernutrition and unimproved water and sanitation, with increased mean age at death, especially when the effect of risks is assessed by DALYs per 1000 people (figure 6C, D). A clear general shift occurs towards a larger proportion of overall burden arising from risk factors for non-communicable diseases, particularly metabolic risks and dietary risk factors (figure 6A, B). However, the absolute burden of risk factors for non-communicable disease does not increase with increasing mean age at death. Rather, its magnitude is lower in high-income regions than in sub-Saharan Africa and south Asia (figure 6C, D), showing the double burden of communicable and non-communicable disease in regions early in the epidemiological transition.
Some risk factors deviated from the pattern of the proportional burden (percent of region-specifc DALYs attributable to a risk factor) being closely associated with epidemiological and demographic transition (shift from communicable to non-communicable disease with increasing mean age of death). The proportion of DALYs attributable to tobacco smoking including second-hand smoke was largest in North America—where smoking among women has generally been prevalent for a long time—and central and eastern Europe. Central and eastern Europe and central Asia also had the largest proportion of disease burden attributable to risk factors with large effects on cardiovascular diseases, which are disproportionately high in these regions. Exposure to particulate matter from household and ambient sources had the most varied pattern with respect to the epidemiological transition, partly because of the heterogeneous pattern of exposure and the effects on both children and adult causes of ill health. Household air pollution from solid fuels accounted for a large proportion of disease burden in central, eastern, and western sub-Saharan Africa and it is a leading risk factor in some Asian regions and Oceania. In central and east Asia in 2010, ambient particulate matter pollution surpassed household air pollution in terms of its burden.
Discussion
The results of GBD 2010 suggest that the contributions of risk factors to regional and global burden of diseases and injuries has shifted substantially between 1990, and 2010, from risk factors that mainly cause communicable diseases in children to risk factors that mainly cause non-communicable diseases in adults. The proportion of overall disease burden attributable to childhood underweight— the leading risk factor worldwide in 1990—had more than halved by 2010, making childhood underweight the eighth risk worldwide, behind six behavioural and physiological risks, and household air pollution from solid fuels. Other risks for child mortality, such as nonexclusive and discontinued breastfeeding, micronutrient deficiencies, and unimproved water and sanitation, have also fallen. However, child and maternal undernutrition risks collectively still account for almost 7% of disease burden in 2010, with unimproved water and sanitation accounting for almost 1%. Of the non-communicable disease risks, high blood pressure, high body-mass index, high fasting plasma glucose, alcohol use, and dietary risks have increased in relative importance. This overall shift has arisen from a combintion of the ageing population, substantial achievements in lowering mortality of children aged younger than 5 years, and changes in risk factor exposure.
These broad global patterns mask enormous regional variation in risks to health. In sub-Saharan Africa, risks such as childhood underweight, household air pollution from solid fuels, and suboptimal breastfeeding continue to cause a disproportionate amount of health burden, despite decreasing. The shift to risk factors for non-communicable disease was clear in east Asia, North Africa and Middle East, and Latin America. This regional heterogeneity underestimates even greater differences in exposure to, and health effects of, risk factors in national and subnational populations. These differences should be further elucidated in country-specific analyses using the framework and methods reported here.
Our analysis shows the large burden of disease attributable to primary and secondary tobacco smoking and to particulate matter pollution in household and ambient environments. The magnitude of disease burden from particulate matter is substantially higher than estimated in previous comparative risk assessment analyses. For example, ambient particulate matter pollution was estimated in the previous comparative risk assessment7 to account for 0·4% of DALYs in 2000 compared with 3·1% in GBD 2010 based on interpolating our 1990 and 2005 results; for household air pollution from solid fuels the comparison is 2·7% in the previous comparative risk assessment versus 5·3% based on GBD 2010.
Several reasons could account for this difference. First, accumulation of evidence from epidemiological studies about diseases caused by particulate matter, and the use of an integrated exposure–response curve, has led to the inclusion of more outcomes than before. For example, health effects for ischaemic heart disease and stroke were not previously included for household air pollution from solid fuels, and lung cancer was included for coal smoke only. Second, the previous assessment of ambient particulate matter pollution was restricted to medium and large cities. High-resolution satellite data and chemical transport models have enabled us to quantify exposure and burden for all rural and urban populations. Third, the previous assessment of ambient particulate matter pollution did not include additional increments of risk above a concentration of 50 µg/m3 for PM2·5, because of the narrow range of ambient particulate matter pollution levels reported in epidemiological studies. The use of an integrated exposure–response curve enabled us to estimate a continuous risk function across the full range of particulate matter concentrations, which covers the very high concentrations of ambient particulate matter exposure measured in, for example, parts of east Asia.
Our integrated exposure–response curve, however, does not address how different sources of particulate matter interact in terms of effects and overlapping exposures. Studies124,159,160 have reported broadly similar effect sizes for ambient particulate matter by smoking status (never, former, and current smokers). Other evidence161 shows that the effects diminish with increasing exposure for active smoking, a pattern incorporated into our exposure– response curves. We applied the effects of ambient particulate matter to both smokers and non-smokers alike to be consistent with the epidemiological evidence that emphasises independent effects of ambient particulate matter. The reasons for the independent effects of different sources of particulate matter should be further investigated. They might include different compositions of particulate matter by source, or different time patterns of exposure162—eg, exposure to particulate matter from active smoking is characterised by episodic, high doses whereas exposure to ambient particulate matter is more constant over time.
These limitations aside, the large attributable burden documented in our analysis represents a major shift in our understanding of disease burden arising from particulate matter and emphasises the need to design alternative fuels for household cooking and heating,163 implement more stringent regulation of vehicle and industrial emissions,164–166 reduce agricultural burning or land clearing by fire,167 and curb and reverse deforestation and desertification to reduce ambient particulate matter from dust.168–171 A large share of ambient particulate matter in Asia and sub-Saharan Africa originates from solid fuel.172,173 Therefore the two exposures are related, and alternative cooking and heating fuels would have benefits for people who currently use solid fuels as well as those who do not, but live in the same community.173
Unimproved water and unimproved sanitation together accounted for 0·9% of DALYs in 2010, compared with 2·1% in 1990. These proportions are substantially smaller than the 6·8% for 1990, and 3·7% for 2000, estimated in previous GBD studies for water, sanitation, and hygiene combined.3,7 The relatively small burden estimated for 2010 is partly related to decreases in diarrhoeal disease mortality since 1990, and partly to differences in the distributions of deaths by underlying cause of death. We have also done an updated meta-analysis of quasi experimental and experimental studies. Historical demographic analyses suggest that the introduction of piped water into cities in the late 19th and early 20th centuries had a large beneficial effect on mortality.174 However, our re-analysis both when restricted to experimental studies and when also including quasi experimental studies did not detect a significantly improved effect of household water connections over improved water sources. Similarly, we did not find a significantly improved effect of water quality interventions, consistent with the findings reported by Cairncross and colleagues,128 which showed that masked point-of-use water quality interventions did not have a significant effect on self-reported diarrhoea. As a result of this reassessment, we restricted our analysis to improved water and improved sanitation compared with unimproved sources following the MDG definitions. However, the interventions used in previous studies might not have achieved their full efficacy because of the quality of implementation. The real burden from water and sanitation could therefore be underestimated if well-implemented household connect ions and water quality interventions have a larger effect than improved water sources alone, and if the combination of poor water and sanitation has a larger effect than a sample interaction of individual effects. More definitive epidemiological evidence is needed to assess the effects of low quality versus high quality water, household connections versus improved water sources, and exposure based on travel time to water source.175 Also, we could not include an assessment of personal hygiene because of the paucity of national exposure data.
Our findings on the burden of micronutrients are also substantially smaller than those in the previous comparative risk assessment for 2000 and in estimates for 2004 by Black and colleagues10 in The Lancet's Maternal and Child Undernutrition Series. For example, Black and colleagues estimated 668 000 deaths caused by vitamin A deficiency in 2004; we estimated a quarter (168 000 deaths) for 2005; for zinc deficiency, the differences are similarly large (453 000 vs 120 000). These differences stem from many sources. First, the estimates of Black and colleagues were based on 10·3 million child deaths worldwide, itself based on WHO estimates of global child deaths for 2004. This estimate is substantially larger than those reported by UNICEF176 and the Institute for Health Metrics and Evaluation177 at the time of Black and colleagues' publication.
Large differences also exist for cause-specific mortality, especially in relation to diarrhoea and lower respiratory tract infections (which can be affected by both of these risks) versus malaria (which is not).176 The estimates also differ because of differences in the availability and interpretation of epidemiological evidence for disease outcomes and effect sizes. Maternal mortality and malaria as outcomes of vitamin A deficiency were included in the 2000 comparative risk assessment but they were not included in the present report because recent epidemiological evidence did not show a significant effect of supplementation on these outcomes. Furthermore, we excluded neonatal vitamin A deficiency since it is the subject of three ongoing randomised trials. The age at which the effects of zinc deficiency begin was increased from birth in the 2000 comparative risk assessment, to 6 months in 2004,10 and to 12 months in the present analysis based on a reassessment of existing and new supplementation trials. Furthermore, we quantified the proportion of the population who are vitamin A or zinc deficient instead of classing whole countries as exposed or non-exposed. The evolving epidemiology of exposure to micronutrient deficiency and the subsequent health effects suggests a need to systematically reconsider most single nutrient supplementation for children in preventive strategies to lower child mortality, as suggested by the 2000 comparative risk assessment and later analyses. 10 Therapeutic zinc supplementation in health-care settings is feasible, as is iron supplementation during pregnancy.174–179 Our findings support the need for strengthened policy about promotion of optimal breastfeeding practices and nutritional programmes that improve child growth. The estimated number of child deaths caused by underweight has also changed substantially over successive studies: in GBD 1990 it was estimated to be 5·9 million deaths in 1990,180 in the comparative risk assessment study for 2000 as 3·7 million deaths,7 and 1·9 million deaths in 2004.10 In GBD 2010 we estimated 2·3 million deaths for 1990 and 0·9 million deaths for 2010.
The evolution of estimates for deaths caused by childhood under weight is because of improvements in assessment of the population at risk. These improvements come from systematic analysis of the available data on underweight, a major modification of RRs after the change in the WHO standard in 2006, and differences in estimates of total and cause-specific mortality. We have also assessed the burden attributable to childhood wasting and childhood stunting. These analyses produce quite similar findings, for example, worldwide, childhood wasting accounted for 0·7 million deaths in 2010, and childhood stunting for 0·9 million deaths, compared with 0·9 million deaths for childhood under weight (the effects of these risks cannot be added).
The global burden of disease attributable to tobacco smoking including second-hand smoke has changed little, with decreases in high-income regions offset by increases in regions such as south east Asia and, to a lesser extent, east and south Asia. The burden attributable to alcohol use has increased substantially in eastern Europe since 1990, mainly because of a rise in the effects of heavy drinking on cardiovascular diseases.181 The high burden in eastern Europe was also identified in the 2000 comparative risk assessment but the data for patterns of alcohol consumption and their effects were weaker, whereas now they are supported by more surveys and epidemiological studies.182 High blood pressure, high body-mass index, and high fasting plasma glucose are leading risk factors for disease worldwide, with blood pressure having large effects on population health in all regions, including low-income regions in sub-Saharan Africa and south Asia. This finding is consistent with previous comparative risk assessment analyses. The disease burden in south Asia and sub-Saharan Africa, caused by increased blood pressure,70 has increased its absolute and relative importance in risk factor rankings. The large burden of high blood pressure emphasises the importance of implementing both population-wide and high-risk approaches to reduction of blood pressure.183,184 The worldwide increase in body-mass index and blood glucose is of particular concern in view of the absence of effective interventions.62,74 In contrast to these risks, the burden of high total cholesterol is lower than that estimated in the 2000 comparative risk assessment, because the effects on ischaemic stroke were negligible at old ages when data from the Asia-Pacific Cohort Studies Collaboration and Prospective Studies Collaboration were pooled,68,185 and because exposure has fallen in high-income countries.67
A recent study estimated that 5·3 million deaths were attributable to physical inactivity in 2008.186 This number, which has been widely quoted and equated with the number of deaths attributable to tobacco smoking,187 used effect sizes for all-cause mortality obtained from cohorts of adults mainly from North America and Europe and applied these effects to deaths at all ages. This approach not only assumes that the cause distribution is the same in all populations, irrespective of region and age structure, but also extends the effects to people younger than those in the cohort study, including to infants and children. In other words, a proportion of deaths from maternal causes, neonatal causes, and children’s infectious diseases and HIV were attributed to physical inactivity.186 The prevalence of inactivity also included people who had sedentary patterns as well as those in the low (insufficient) activity group. By contrast, our approach—calculating attributable burden by cause and age group, and accounting for exposure in four categories—estimated substantially fewer attributable deaths: 3·2 million (2·7 million to 3·7 million) in 2010, 56% of what we attribute to tobacco smoking when second-hand smoke is excluded. This discrepancy shows the importance of comparable risk factor assessments and the importance of estimation of attributable burden taking into account differences in underlying disease and injury patterns across populations.
We have expanded the set of components of diet included from a combined category of fruits and vegetables in the 2000 comparative risk assessment to 15 components in GBD 2010; together these dietary risk factors account for a tenth of global disease burden. Of the dietary risk factors, the aetiological effect sizes for sodium, polyunsaturated fatty acids replacing saturated fatty acids, and seafood omega-3 fatty acids were informed fully (for sodium) or partly by randomised controlled trials. Disease burden attributable to diet high in sodium was a third of that for high blood pressure. The theoretical-minimum-risk exposure distribution was selected on the basis of values reported in randomised trials; studies of populations with low prevalence of cardiovascular disease suggest that benefits are likely to continue to lower levels.158
The large attributable burden for dietary risk factors such as diets low in fruits, vegetables, whole grains, nuts and seeds, and seafood omega-3 fatty acids might surprise some readers. The large burden is caused by both high exposure—eg, low intake of fruits in many regions—and large effect sizes. We did supplementary analyses using information from studies of dietary patterns and randomised controlled feeding studies to examine the robustness of the effect sizes used in GBD 2010. The findings of these supplementary analyses were consistent with those from the meta-analyses of single risk factors. However, we stress that these results should still be interpreted with caution, particularly because of the debate surrounding the effects of seafood omega-3 fatty acids.143,188 Empirical assessments show that the pooled effect of risks and interventions trends towards a null result over time189,190 and this pattern could apply to seafood omega-3 fatty acids since the earlier, primarily observational effect sizes tended to show a larger effect than did the more recent randomised controlled trials. Because the difference between results of observational studies and randomised controlled trials is not statistically significant we have quantified the attributable burden by use of the combined effect size. However, the validity of this approach could change as new evidence accumulates. Also, evidence from randomised controlled trials does not exist for several of the dietary components with a large attributable burden—fruits, vegetables, and nuts and seeds—although, as previously noted, evidence from randomised controlled trials does exist for inter mediate outcomes. Further work is needed to confirm the effect size of dietary components and to establish to what degree the benefits continue, preferably through intervention studies of fatal and non-fatal events.
The extended analysis of components of diet does not include saturated fat beyond its replacement by polyunsaturated fats. Ecological studies suggest that saturated fat intake is a significant risk factor for mortality from ischaemic heart disease.191 However, observational studies indicate that there might be no benefits if saturated fat reduction is associated with an increase in carbohydrates,91 which is also supported by the absence of benefits from a low fat diet in the Women’s Health Initiative.192 Together with data for seafood omega-3 fatty acids, these findings show the complexity of the relation between dietary fat and health and suggest that the traditional health education message focused on lowering saturated fat alone needs to be expanded greatly to encompass several other key components of diet, including increased consumption of healthy foods that are presently missing from most diets.
The strengths of our study include a more comprehensive set of risk factors than any previous global or national analysis, consistent analyses in 1990, and 2010, which enables assessment of changes in risk factor burden, the incorporation of substantially more data for risk-factor exposure, improved methods to deal with missing and incomparable data, strong emphasis on comparability of methods related to exposure, disease outcomes, and effect sizes, and use of theoretical-minimum-risk exposure distribution as the consistent alternative exposure distribution with which current exposures are compared.
Like all population-based analysis, our study also has some limitations. First, despite the massive improvement in the availability of exposure data and methods, exposure estimates for many risk factors are affected by data limitations, especially for 2010, since fewer data could be included. This limitation will become even more salient in applications of our methods to individual countries and shows the importance of surveillance of national risk factors as a crucial component of national health information systems. More importantly, for some risk factors we have less direct measures of exposure than for others. For example, for household air pollution from solid fuels we measured exposure on the basis of household fuel use rather than personal exposure to particulate matter; for other risks, such as blood pressure, we have direct biological measurements of exposure.
Second, the presence of residual confounding in the estimates of effect sizes cannot be definitively ruled out, particularly for those without evidence from intervention studies, either because they have not yet been done or the risk is not amenable to intervention. For example, no large-scale trials have been done of interventions for high body-mass index that measured cause-specific deaths although effects on disease incidence have been investigated in trials.193 Observational studies of the effect sizes for body-mass index have controlled for some potential confounders.75–77 As noted, the pooled effect of risks and interventions trends towards the null result over time;189,190 the implication being that risks for which only a few studies have been done might have their effect overestimated compared with risks for which a large body of evidence exists.
Third, with the exception of risk factors for which much evidence has been accumulated across diverse populations and age groups, such as the metabolic risks, uncertainty remains as to the extent to which effect sizes are generalisable to different populations. Similarly, the large body of epidemiological evidence for cardiovascular risk factors shows a relation between age and the effect size of risk factors for cardiovascular disease. Such age-related changes might be present for other outcomes. Fourth, we have combined epidemiological evidence for effect sizes using studies across different periods, which could mask underlying temporal changes in risk; no data presently exist to enable an examination of the extent to which effect sizes might change over time.
Fifth, we have excluded risks for which insufficient information exists to enable estimation of exposure, or for which the evidence of effect sizes is scarce. This approach excludes several risk–outcome pairs that have been previously included in global and regional assessments of risk factor attributable burden, such as unsafe sex and global climate change. Unsafe sexual practices were included in the 2000 comparative risk assessment but we excluded it because of the absence of robust estimates of exposure or available approaches to determine the proportion of HIV infection that is attributable to unsafe sexual practices by country over time. If quantifiable, unsafe sexual practices would probably account for a large fraction of global health burden; the direct burden of HIV is 3·3% of DALYs in 2010; other sexually transmitted infections account for 0·4% of DALYs. Similarly, we have been unable to control for confounding in observational studies of late initiation of breastfeeding, which is associated with an increased risk of neonatal mortality. Infants who might too ill or weak to breastfeed are more likely to die. In our analysis, we could not assess low birthweight as an outcome for maternal iron deficiency, despite evidence from random ised trials. Similarly, we could not assess low birthweight as an outcome for maternal alcohol use. Low birthweight was not a disease outcome in GBD 2010 but is associated with an increased risk of neonatal mortality. We excluded several other risk–outcome pairs that had insufficient evidence to estimate effect sizes or that had substantial potential of residual confounding— eg, the effect of addictive drugs (cannabis, amphetamines, and opioids) on unintentional and intentional injuries; or the effects of intimate partner violence, on HIV or other sexually transmitted infections.
Sixth, we included few risks that affected three of the leading communicable diseases—HIV/AIDs, tuberculosis, and malaria (beyond deaths in childhood). Overall, we have not included risks for 126 of the 241 most detailed causes included in the GBD, which account for 26·3% of global disease health burden. This shortcoming emphasises the need for a more deliberate research focus to identify and quantify risk factors for the outcomes for which there are presently no risks or few large risks.
Seventh, we have quantified the attributable burden of risk factors, holding all other independent factors constant. For clusters of risk factors we have approximated the joint effects, assuming that risk factors within each cluster are independent. A more accurate quantification of the joint effects of multiple risk factors is an important area for future research. Finally, it is important to stress that the size of the attributable risk factor burden does not equal priority for action since prioritisation also depends on availability, cost, and effectiveness of inter vention strategies to reduce exposures to these risks.
Public policy to improve the health of populations will be more effective if it addresses the major causes of disease burden. Even small reductions of population exposure to large risks will yield substantial health gains.194 The principal advantage of doing a comprehensive and comparable scientific assessment of disease burden caused by different risk factors is that it provides the evidence base for informing discussion about policy. Coupled with evidence of their present burden, most of the leading risk factors, except high body-mass index and high fasting plasma glucose, have decreased in at least some regions and countries, showing that substantial reduction of their effect through targeted prevention strategies is feasible. If predictions about huge increases in disease burden worldwide are to be proved wrong, then countries, with appropriate global public health leadership, must urgently implement measures to control exposure to leading hazards, particularly risks for non-communicable diseases.
Supplementary Material
Appendix
Panel: The World Cancer Research Fund grading system118.
Convincing evidence
Evidence based on epidemiological studies showing consistent associations between exposure and disease, with little or no evidence to the contrary. The available evidence is based on a substantial number of studies including prospective observational studies and where relevant, randomised controlled trials of sufficient size, duration, and quality showing consistent effects. The association should be biologically plausible.
Probable evidence
Evidence based on epidemiological studies showing fairly consistent associations between exposure and disease, but for which there are perceived shortcomings in the available evidence or some evidence to the contrary, which precludes a more definite judgment. Shortcomings in the evidence may be any of the following: insufficient duration of trials (or studies); insufficient trials (or studies) available; inadequate sample sizes; or incomplete follow-up. Laboratory evidence is usually supportive. The association should be biologically plausible.
Possible evidence
Evidence based mainly on findings from case-control and cross-sectional studies. Insufficient randomised controlled trials, observational studies, or non-randomised controlled trials are available. Evidence based on non-epidemiological studies, such as clinical and laboratory investigations, is supportive. More trials are needed to support the tentative associations, which should be biologically plausible.
Insufficient evidence
Evidence based on findings of a few studies which are suggestive, but insufficient to establish an association between exposure and disease. Little or no evidence is available from randomised controlled trials. More well-designed research is needed to support the tentative associations.
Acknowledgments
We thank the countless individuals who have contributed to the Global Burden of Disease Study 2010 in various capacities. We specifically acknowledge the important contribution to this work from multiple staff members of the World Health Organization. We also thank the following organisations that hosted consultations during the final stages of the analytical process, providing valuable feedback about the results and the data to improve the study’s findings overall: Pan American Health Organization; Eastern Mediterranean Regional Office of WHO; UNAIDS; Ministry of Health, Brazil; China Centers for Disease Control; and the University of Zambia. We thank Regina Guthold, Jordis Ott, Annette Pruss-Ustun, and Gretchen A Stevens for their collaboration and input into the analyses and estimates. Finally, we acknowledge the extensive support from all staff members at the Institute for Health Metrics and Evaluation and specifically thank: James Bullard, Andrew Ernst, and Serkan Yalcin for their tireless support of the computational infrastructure required to produce the results; Linda A Ettinger for her expert administrative support to facilitate communication and coordination amongst the authors; Peter Speyer, Abigail McLain, Katherine Leach-Kemon, and Eden Stork for their persistent and valuable work to gain access to and catalogue as much data as possible to inform the estimates; and Erin C Mullany for her systematic efforts in organising drafts of papers, formatting correspondence with expert groups, and preparing the final manuscript. J Balmes, Z Chafe, and K R Smith acknowledge that their aspects of the research were also supported by USEPA and the Shell Foundation, neither of which had any role in design, data collection, analysis, interpretation, or decisions related to publication. R Bourne acknowledges Institutional Support: Vision & Eye Research Unit, Postgraduate Medical Institute, Anglia Ruskin University, Cambridge, UK. Funding support: Fight for Sight (Dr Hans and Mrs Gertrude Hirsch award). R Buchbinder was partially supported by an Australian National Health and Medical Research Council Practitioner Fellowship, Monash University, and Cabrini Health. A J Cohen received support from the Health Effects Institute and The William and Flora Hewlett Foundation. S Darby was supported by Cancer Research UK. L Degenhardt was supported by an Australian National Health and Medical Research Council Senior Research Fellowship. T Driscoll was supported in part by funding from the National Occupational Health and Safety Commission (now Safe work Australia). K M Hanafiah’s work for the GBD hepatitis C prevalence study was funded partly by Johns Hopkins Vaccine Initiative Scholarship and partly by WHO. H W Hoek acknowledges the support of: the Parnassia Psychiatric Institute, The Hague, Netherlands; the Department of Psychiatry, University Medical Center Groningen, University of Groningen, Netherlands; and the Department of Epidemiology, Columbia University, New York, USA. D Hoy was supported by the Bill & Melinda Gates Foundation and the Australian National Health and Medical Research Council. N Kawakami notes that data used in the study was collected through support from the following grants: The World Mental Health Japan is supported by the Grant for Research on Psychiatric and Neurological Diseases and Mental Health (H13-SHOGAI-023, H14-TOKUBETSU-026, H16-KOKORO-013) from the Japan Ministry of Health, Labour, and Welfare. He thanks staff members, filed coordinators, and interviewers of the WMH Japan 2002–2004 Survey. Q Lan was supported in part by the Intramural Research Program of the NIH (National Cancer Institute). S London is supported by the Division of Intramural Research, National Institute of Environmental Health Sciences, USA. T R Merriman acknowledges the Health Research Council of New Zealand. B Neal was supported in his contribution to this work by an Australian Research Council Future Fellowship and a National Health and Medical Research Council of Australia Senior Research Fellowship. C Olives was supported in his contribution to this work by an Australian Research Council Future Fellowship and a National Health and Medical Research Council of Australia Senior Research Fellowship. E A Rehfuess acknowledges financial support from the Munich Centre of Health Sciences. R Room’s position at the University of Melbourne and Turning Point Alcohol and Drug Centre is funded by the Foundation for Alcohol Research and Education and the Victorian Department of Health. J A Salomon received support from the Burke Global Health Fellowship while working on this study. U Sampson was supported in part by: The Harold Amos Medical Faculty Development Award of the Robert Wood Johnson Foundation; The Vanderbilt Clinical and Translational Scholars Award. L Sanchez-Riera acknowledges the Spanish Society of Rheumatology for their funds. S Seedat is supported by the South African Research Chairs Initiative, hosted by the Department of Science and Technology and the National Research Foundation. G D Thurston was supported in part by grant ES00260 from the National Institute of Environmental Health Sciences. J M Zielinski acknowledges institutional support from: Health Canada, University of Ottawa, and WHO (International Radon Project). M Ezzati’s research is supported by a Strategic Award from the UK Medical Research Council (MRC) and by the National Institute for Health Research Comprehensive Biomedical research Centre at Imperial College Healthcare NHS Trust. Work on micronutrient deficiencies was supported by the Nutrition Impact Model Study (NIMS) funded by the Bill & Melinda Gates Foundation. The GBD Osteoporosis Expert Group was supported by the Spanish Rheumatology Association, Institute of Bone and Joint Research, University of Sydney. The GBD Osteoporosis Expert Group also acknowledges the contributions made by Professor Philip Sambrook who passed away in April, 2012.
Conflicts of interest
A Davis is employed by the NHS on works for the UK Dept of Health as lead adviser on audiology. E R Dorsey has been a consultant for Medtronic and Lundbeck and has received grant support from Lundbeck and Prana Biotechnology. M Ezzati chaired a session and gave a talk at the World Cardiology Congress (WCC), with travel cost reimbursed by the World Heart Federation. At the WCC, he also gave a talk at a session organised by Pepsico with no financial remuneration. G A Mensah is a former employee of PepsiCo. D Mozaffarian has received: ad hoc travel reimbursement and/or honoraria for one-time specific presentations on diet and cardiometabolic diseases from Nutrition Impact (9/10), the International Life Sciences Institute (12/10), Bunge (11/11), Pollock Institute (3/12), and Quaker Oats (4/12; modest); and Unilever’s North America Scientific Advisory Board (modest). B Neal is the Chair of the Australian Division of World Action on Salt and Health. He has consulted to Roche and Takeda. He has received lecture fees, travel fees, or reimbursements from Abbott, Amgen, AstraZeneca, George Clinical, GlaxoSmithKline, Novartis, PepsiCo, Pfizer, Pharmacy Guild of Australia, Roche, Sanofi-Aventis, Seervier, and Tanabe. He holds research support from the Australian Food and Grocery Council, Bupa Australia, Johnson and Johnson, Merck Schering-Plough, Roche, Servier, and United Healthcare Group. He is not employed by a commercial entity and has no equity ownership or stock options, patents or royalties, or any other financial or non-financial support that might be viewed as a conflict of interest. L Rushton received honorarium for board membership of the European Centre for Ecotoxicology and Toxicology of Chemicals and research grants to Imperial College London (as PI) from the European Chemical Industry Council and CONCAWE.
Footnotes
Contributors
CJLM, SSL, and ME wrote the first draft. SSL, TV, AF, GD, KS, ADL, CJLM, and ME revised the report. ME, CJLM, and ADL designed the study and provided overall guidance. SSL, EC, GF, CA, ESa, KA, REE, and LCR did comparative analyses of risk factors. All other authors developed the estimates of risk-specific exposure, theoretical-minimum-risk exposure distribution, and RR inputs, and checked and interpreted results.
References
- 1.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 2.Peto R, Boreham J, Lopez AD, Thun M, Heath C. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- 3.Murray C, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected in 2020. Cambridge: Harvard University Press; 1996. [Google Scholar]
- 4.Murray CJ, Lopez AD. On the comparable quantification of health risks: lessons from the Global Burden of Disease Study. Epidemiology. 1999;10:594–605. [PubMed] [Google Scholar]
- 5.WHO. Geneva: World Health Organization; 2002. The World Health Report 2002—Reducing Risks, Promoting Healthy Life. [DOI] [PubMed] [Google Scholar]
- 6.Ezzati M, Lopez AD, Rodgers A, Murray CJ. Comparative quantification of health risks: global and regional burden of diseases attributable to selected major risk factors. Geneva: World Health Organization; 2004. [Google Scholar]
- 7.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ the Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Geneva: World Health Organization; 2009. Global health risks: morality and burden of disease attributable to selected major risks. [Google Scholar]
- 9.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M the The Comparative Risk Assessment collaborating group. (Cancers). Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 10.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 11.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens G, Dias RH, Thomas KJA, et al. Characterizing the epidemiological transition in Mexico: national and subnational burden of diseases, injuries, and risk factors. PLoS Med. 2008;5:e125. doi: 10.1371/journal.pmed.0050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg SJ, Vos T, Barker B, Stanley L, Lopez AD. Burden of disease and injury in Australia in the new millennium: measuring health loss from diseases, injuries and risk factors. Med J Aust. 2008;188:36–40. doi: 10.5694/j.1326-5377.2008.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 14.Norman R, Bradshaw D, Schneider M, et al. A comparative risk assessment for South Africa in 2000: towards promoting health and preventing disease. S Afr Med J. 2007;97:637–641. [PubMed] [Google Scholar]
- 15.Ikeda N, Inoue M, Iso H, et al. Adult mortality attributable to preventable risk factors for non-communicable diseases and injuries in Japan: a comparative risk assessment. PLoS Med. 2012;9:e1001160. doi: 10.1371/journal.pmed.1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farzadfar F, Danaei G, Namdaritabar H, et al. National and subnational mortality effects of metabolic risk factors and smoking in Iran: a comparative risk assessment. Popul Health Metr. 2011;9:55. doi: 10.1186/1478-7954-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 18.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLD) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiener N. Extrapolation, interpolation, and smoothing of stationary time series. Cambridge, MA: MIT Press; 1949. [Google Scholar]
- 20.Paciorek P. Nonstationary Gaussian process for regression and spatial modeling. Pittsburgh, PA: Carnegie Mellon University; 2003. [Google Scholar]
- 21.Thompson P. Optimum smoothing of two-dimensional fields. Tellus. 1956;8:84–93. [Google Scholar]
- 22.Dentener F, Drevet J, Lamarque JF, et al. Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cycles. 2006 published online Oct 28. [Google Scholar]
- 23.Fiore AM, Dentener FJ, Wild O, et al. Multimodel estimates of intercontinental source-receptor relationships for ozone pollution. J Geophys Res. 2009;114:21. [Google Scholar]
- 24.Stevenson DS, Dentener FJ, Schultz MG, et al. Multimodel ensemble simulations of present-day and near-future tropospheric ozone. J Geophys Res. 2006;111 [Google Scholar]
- 25.van Donkelaar A, Martin RV, Brauer M, et al. Global Estimates of Ambient Fine Particulate Matter Concentrations from Satellite-Based Aerosol Optical Depth: Development and Application. Environ Health Perspect. 2010;118:847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brauer M, Amann M, Burnett RT, et al. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environ Sci Technol. 2012;46:652–660. doi: 10.1021/es2025752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerrett M, Burnett RT, Pope CA, 3rd, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330:223. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services, Public Health Service, Centers for Disease Control. Preventing lead poisoning in young children. Centers for Disease Control. [accessed Nov 19, 2012];1991 http://wonder.cdc.gov/wonder/prevguid/p0000029/p0000029.asp.
- 30.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navas-Acien A, Schwartz BS, Rothenberg SJ, Hu H, Silbergeld EK, Guallar E. Bone lead levels and blood pressure endpoints: a meta-analysis. Epidemiology. 2008;19:496–504. doi: 10.1097/EDE.0b013e31816a2400. [DOI] [PubMed] [Google Scholar]
- 32.Lamberti LM, Fischer Walker CL, Noiman A, Victora C, Black RE. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11(suppl 3):S15. doi: 10.1186/1471-2458-11-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoltzfus RJ, Mullany L, Black RE. Iron Deficiency Anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJ, editors. Comparative quantification of health risks: Global and regional burden of disease attributable to selected major risk factors. Geneva: World Health Organization; 2004. pp. 163–209. [Google Scholar]
- 34.Imdad A, Herzer K, Mayo-Wilson E, Yakoob MY, Bhutta ZA. Vitamin A supplementation for preventing morbidity and mortality in children from 6 months to 5 years of age. Cochrane Database Syst Rev. 2010;12 doi: 10.1002/14651858.CD008524.pub2. CD008524. [DOI] [PubMed] [Google Scholar]
- 35.Imdad A, Yakoob MY, Sudfeld C, Haider BA, Black RE, Bhutta ZA. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health. 2011;11(suppl 3):S20. doi: 10.1186/1471-2458-11-S3-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakoob MY, Theodoratou E, Jabeen A, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 2011;11(suppl 3):S23. doi: 10.1186/1471-2458-11-S3-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foreman K, Lozano R, Lopez A, Murray C. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oza S, Thun MJ, Henley SJ, Lopez AD, Ezzati M. How many deaths are attributable to smoking in the United States? Comparison of methods for estimating smoking-attributable mortality when smoking prevalence changes. Prev Med. 2011;52:428–433. doi: 10.1016/j.ypmed.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Ezzati M, Henley SJ, Lopez AD, Thun MJ. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer. 2005;116:963–971. doi: 10.1002/ijc.21100. [DOI] [PubMed] [Google Scholar]
- 40.Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation. 2005;112:489–497. doi: 10.1161/CIRCULATIONAHA.104.521708. [DOI] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. [Google Scholar]
- 42.Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf) 2011;33:496–502. doi: 10.1093/pubmed/fdr025. [DOI] [PubMed] [Google Scholar]
- 43.Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. 2011;12:5. doi: 10.1186/1465-9921-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones LL, Hassanien A, Cook DG, Britton J, Leonardi-Bee J. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:18–27. doi: 10.1001/archpediatrics.2011.158. [DOI] [PubMed] [Google Scholar]
- 45.Shield KD, Rehm M, Patra J, Sornpaisarn B, Rehm J. Global and Country Specific Adult per capita Consumption of Alcohol, 2008. SUCHT—Zeitschrift für Wissenschaft und Praxis/Journal of Addiction Research and Practice. 2011;57:99–117. [Google Scholar]
- 46.Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis—a systematic review. BMC Public Health. 2008;8:289. doi: 10.1186/1471-2458-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2010;138:1789–1795. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- 49.Patra J, Bakker R, Irving H, Jaddoe VWV, Malini S, Rehm J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011;118:1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–619. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 51.Baan R, Straif K, Grosse Y, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–293. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 52.Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2009;32:2123–2132. doi: 10.2337/dc09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor B, Irving HM, Kanteres F, et al. The more you drink, the harder you fall: a systematic review and meta-analysis of how acute alcohol consumption and injury or collision risk increase together. Drug Alcohol Depend. 2010;110:108–116. doi: 10.1016/j.drugalcdep.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roerecke M, Rehm J. Alcohol consumption and the risk for morbidity and mortality of ischemic heart disease—a systemic review and meta-analysis. Toronto, ON: Centre for Addiction and Mental Health; 2011. [Google Scholar]
- 55.Roerecke M, Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am J Epidemiol. 2010;171:633–644. doi: 10.1093/aje/kwp451. [DOI] [PubMed] [Google Scholar]
- 56.Patra J, Taylor B, Irving H, et al. Alcohol consumption and the risk of morbidity and mortality for different stroke types—a systematic review and meta-analysis. BMC Public Health. 2010;10:258. doi: 10.1186/1471-2458-10-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for atrial fibrillation: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2010;17:706–712. doi: 10.1097/HJR.0b013e32833a1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irving HM, Samokhvalov AV, Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP. 2009;10:387–392. [PMC free article] [PubMed] [Google Scholar]
- 59.Samokhvalov AV, Irving H, Mohapatra S, Rehm J. Alcohol consumption, unprovoked seizures, and epilepsy: a systematic review and meta-analysis. Epilepsia. 2010;51:1177–1184. doi: 10.1111/j.1528-1167.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 60.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–1462. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167:987–993. doi: 10.1176/appi.ajp.2010.09020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 63.Sarwar N, Gao P, Seshasai SRK, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawes CMM, Parag V, Bennett DA, et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27:2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 65.Balkau B. The DECODE study. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Diabetes Metab. 2000;26:282–286. [PubMed] [Google Scholar]
- 66.Jeon CY, Murray MB. Diabetes Mellitus Increases the Risk of Active Tuberculosis: A Systematic Review of 13 Observational Studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farzadfar F, Finucane MM, Danaei G, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Patel A, Horibe H, et al. Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol. 2003;32:563–572. doi: 10.1093/ije/dyg106. [DOI] [PubMed] [Google Scholar]
- 69.Danesh J, Erqou S, Walker M, et al. the The Emerging Risk Factors Collaboration. analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–869. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 70.Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 71.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 72.Lawes CMM, Rodgers A, Bennett DA, et al. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21:707–716. doi: 10.1097/00004872-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 73.The Renal Risk Collaboration. Foote C, Lin J, et al. The effect of blood pressure on kidney failure: a systematic review and meta-analysis in 2·7 million participants (unpublished) [Google Scholar]
- 74.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ni Mhurchu C, Rodgers A, Pan WH, Gu DF, Woodward M. Body mass index and cardiovascular disease in the Asia-Pacific Region: an overview of 33 cohorts involving 310 000 participants. Int J Epidemiol. 2004;33:751–758. doi: 10.1093/ije/dyh163. [DOI] [PubMed] [Google Scholar]
- 77.Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 79.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 80.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 81.He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 82.World Cancer Research Fund, American Institute for Cancer Research. Washington, D.C.: AICR; 2007. Food, Nutrition, and Physical Activity, and the Prevention of Cancer: A Global Perspective. [Google Scholar]
- 83.Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18:283–290. doi: 10.1016/j.numecd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 84.de Munter JSL, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelly JH, Jr, Sabaté J. Nuts and coronary heart disease: an epidemiological perspective. Br J Nutr. 2006;96(suppl 2):S61–S67. doi: 10.1017/bjn20061865. [DOI] [PubMed] [Google Scholar]
- 86.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94:1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pereira MA, O’Reilly E, Augustsson K, et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med. 2004;164:370–376. doi: 10.1001/archinte.164.4.370. [DOI] [PubMed] [Google Scholar]
- 89.Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 90.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 91.Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7:e1000252. doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr. 2009;63(suppl 2):S22–S33. doi: 10.1038/sj.ejcn.1602976. [DOI] [PubMed] [Google Scholar]
- 94.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761–770. doi: 10.1038/sj.jhh.1001459. [DOI] [PubMed] [Google Scholar]
- 95.Hutchings S, Rushton L. Toward risk reduction: predicting the future burden of occupational cancer. Am J Epidemiol. 2011;173:1069–1077. doi: 10.1093/aje/kwq434. [DOI] [PubMed] [Google Scholar]
- 96.Goodman M, Morgan RW, Ray R, Malloy CD, Zhao K. Cancer in asbestos-exposed occupational cohorts: a meta-analysis. Cancer Causes Control. 1999;10:453–465. doi: 10.1023/a:1008980927434. [DOI] [PubMed] [Google Scholar]
- 97.Rake C, Gilham C, Hatch J, Darnton A, Hodgson J, Peto J. Occupational, domestic and environmental mesothelioma risks in the British population: a case-control study. Br J Cancer. 2009;100:1175–1183. doi: 10.1038/sj.bjc.6604879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Camargo MC, Stayner LT, Straif K, et al. Occupational exposure to asbestos and ovarian cancer: a meta-analysis. Environ Health Perspect. 2011;119:1211–1217. doi: 10.1289/ehp.1003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nelson DI, Concha-Barrientos M, Driscoll T, et al. The global burden of selected occupational diseases and injury risks: Methodology and summary. Am J Ind Med. 2005;48:400–418. doi: 10.1002/ajim.20211. [DOI] [PubMed] [Google Scholar]
- 100.Hutchings SJ, Rushton L. Occupational cancer in Britain. Statistical methodology. Br J Cancer. 2012;107(suppl 1):S8–S17. doi: 10.1038/bjc.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee-Feldstein A. Cumulative exposure to arsenic and its relationship to respiratory cancer among copper smelter employees. J Occup Med. 1986;28:296–302. [PubMed] [Google Scholar]
- 102.Khalade A, Jaakkola MS, Pukkala E, Jaakkola JJK. Exposure to benzene at work and the risk of leukemia: a systematic review and meta-analysis. Environ Health. 2010;9:31. doi: 10.1186/1476-069X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schubauer-Berigan MK, Deddens JA, Couch JR, Petersen MR. Risk of lung cancer associated with quantitative beryllium exposure metrics within an occupational cohort. Occup Environ Med. 2011;68:354–360. doi: 10.1136/oem.2010.056515. [DOI] [PubMed] [Google Scholar]
- 104.Rosenman KD, Stanbury M. Risk of lung cancer among former chromium smelter workers. Am J Ind Med. 1996;29:491–500. doi: 10.1002/(SICI)1097-0274(199605)29:5<491::AID-AJIM7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 105.Lipsett M, Campleman S. Occupational exposure to diesel exhaust and lung cancer: a meta-analysis. Am J Public Health. 1999;89:1009–1017. doi: 10.2105/ajph.89.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stayner L, Bena J, Sasco AJ, et al. Lung cancer risk and workplace exposure to environmental tobacco smoke. Am J Public Health. 2007;97:545–551. doi: 10.2105/AJPH.2004.061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Collins JJ, Lineker GA. A review and meta-analysis of formaldehyde exposure and leukemia. Regul Toxicol Pharmacol. 2004;40:81–91. doi: 10.1016/j.yrtph.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 108.Hauptmann M, Lubin JH, Stewart PA, Hayes RB, Blair A. Mortality from solid cancers among workers in formaldehyde industries. Am J Epidemiol. 2004;159:1117–1130. doi: 10.1093/aje/kwh174. [DOI] [PubMed] [Google Scholar]
- 109.Grimsrud TK, Berge SR, Haldorsen T, Andersen A. Can lung cancer risk among nickel refinery workers be explained by occupational exposures other than nickel? Epidemiology. 2005;16:146–154. doi: 10.1097/01.ede.0000152902.48916.d7. [DOI] [PubMed] [Google Scholar]
- 110.Grimsrud TK, Peto J. Persisting risk of nickel related lung cancer and nasal cancer among Clydach refiners. Occup Environ Med. 2006;63:365–366. doi: 10.1136/oem.2005.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Armstrong B, Hutchinson E, Unwin J, Fletcher T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environ Health Perspect. 2004;112:970–978. doi: 10.1289/ehp.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kurihara N, Wada O. Silicosis and smoking strongly increase lung cancer risk in silica-exposed workers. Ind Health. 2004;42:303–314. doi: 10.2486/indhealth.42.303. [DOI] [PubMed] [Google Scholar]
- 113.Soskolne CL, Jhangri GS, Siemiatycki J, et al. Occupational exposure to sulfuric acid in southern Ontario, Canada, in association with laryngeal cancer. Scand J Work Environ Health. 1992;18:225–232. doi: 10.5271/sjweh.1585. [DOI] [PubMed] [Google Scholar]
- 114.Karjalainen A, Kurppa K, Martikainen R, Klaukka T, Karjalainen J. Work is related to a substantial portion of adult-onset asthma incidence in the Finnish population. Am J Respir Crit Care Med. 2001;164:565–568. doi: 10.1164/ajrccm.164.4.2012146. [DOI] [PubMed] [Google Scholar]
- 115.Karjalainen A, Kurppa K, Martikainen R, Karjalainen J, Klaukka T. Exploration of asthma risk by occupation--extended analysis of an incidence study of the Finnish population. Scand J Work Environ Health. 2002;28:49–57. doi: 10.5271/sjweh.646. [DOI] [PubMed] [Google Scholar]
- 116.Kogevinas M, Antó JM, Sunyer J, Tobias A, Kromhout H, Burney P. Occupational asthma in Europe and other industrialised areas: a population-based study. European Community Respiratory Health Survey Study Group. Lancet. 1999;353:1750–1754. doi: 10.1016/s0140-6736(98)07397-8. [DOI] [PubMed] [Google Scholar]
- 117.Beydoun HA, Beydoun MA, Kaufman JS, Lo B, Zonderman AB. Intimate partner violence against adult women and its association with major depressive disorder, depressive symptoms and postpartum depression: a systematic review and meta-analysis. Soc Sci Med. 2012;75:959–975. doi: 10.1016/j.socscimed.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.World Cancer Research Fund. AICR. Washington, DC: American Institute for Cancer Research; 2007. Food, Nutrition, and Physical Activity, and the Prevention of Cancer: A Global Perspective. [Google Scholar]
- 119.Liu BQ, Peto R, Chen ZM, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huijnen V, Williams J, van Weele M, et al. The global chemistry transport model TM5: description and evaluation of the tropospheric chemistry version 3.0. Geoscientific Model Development. 2010;3:445–473. [Google Scholar]
- 122.Caufield LE, Black RE. Zinc Deficiency. In: Ezzati M, Lopez AD, Rodgers A, Murray C, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva: World Health Organization; 2004. pp. 729–882. [Google Scholar]
- 123.Wessells KP, Brown KH. Estimating global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7:e50568. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 125.Puett RC, Hart JE, Yanosky JD, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117:1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lipsett MJ, Ostro BD, Reynolds P, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184:828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fink G, Günther I, Hill K. The effect of water and sanitation on child health: evidence from the demographic and health surveys 1986–2007. Int J Epidemiol. 2011;40:1196–1204. doi: 10.1093/ije/dyr102. [DOI] [PubMed] [Google Scholar]
- 128.Cairncross S, Hunt C, Boisson S, et al. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol. 2010;39(suppl 1):i193–i205. doi: 10.1093/ije/dyq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Waddington H, Snilstveit B, White H, Fewtrell L. Water, sanitation and hygiene interventions to combat childhood diarrhea in developing countries. Washington, DC: International Initiative for Impact Evaluation; 2009. [Google Scholar]
- 130.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 131.Clasen TF, Bostoen K, Schmidt W-P, et al. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD007180.pub2. CD007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pope CA, 3rd, Burnett RT, Turner MC, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119:1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pope CA, 3rd, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 134.Krewski D, Jerrett M, Burnett RT, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 2009;140:5–114. [PubMed] [Google Scholar]
- 135.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 136.Committee on the Medical Effects of Air Pollutants. Long-Term Exposure to Air Pollution: Effect on Mortality. London: Health Protection Agency; 2009. [Google Scholar]
- 137.Cooke RM, Wilson AM, Tuomisto JT, Morales O, Tainio M, Evans JS. A probabilistic characterization of the relationship between fine particulate matter and mortality: elicitation of European experts. Environ Sci Technol. 2007;41:6598–6605. doi: 10.1021/es0714078. [DOI] [PubMed] [Google Scholar]
- 138.Burnett RT, Pope CA, 3rd, Ezzati M, et al. A unified risk function for estimating the global burden of mortality attributable to fine particulate matter exposure. doi: 10.1289/ehp.1307049. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baumgartner J, Schauer JJ, Ezzati M, et al. Indoor air pollution and blood pressure in adult women living in rural China. Environ Health Perspect. 2011;119:1390–1395. doi: 10.1289/ehp.1003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 141.He K, Song Y, Daviglus ML, et al. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke. 2004;35:1538–1542. doi: 10.1161/01.STR.0000130856.31468.47. [DOI] [PubMed] [Google Scholar]
- 142.He K, Song Y, Daviglus ML, et al. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 143.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 144.Trichopoulou A, Bamia C, Trichopoulos D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ. 2009;338:b2337. doi: 10.1136/bmj.b2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 146.Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161:1857–1862. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- 147.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martínez-González MA, Garcia-Lopez M, Bes-Rastrollo M, et al. Mediterranean diet and the incidence of cardiovascular disease: a Spanish cohort. Nutr Metab Cardiovasc Dis. 2011;21:237–244. doi: 10.1016/j.numecd.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 149.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 150.Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 151.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 152.King G, Tomz M, Wittenberg J. Making the most of statistical analyses: improving interpretation and presentation. Am J Pol Sci. 2000;44:347–361. [Google Scholar]
- 153.Sazawal S, Black RE, Ramsan M, et al. Effect of zinc supplementation on mortality in children aged 1–48 months: a community-based randomised placebo-controlled trial. Lancet. 2007;369:927–934. doi: 10.1016/S0140-6736(07)60452-8. [DOI] [PubMed] [Google Scholar]
- 154.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ezzati M, Vander Hoorn S, Rodgers A, Lopez AD, Mathers CD, Murray CJ. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet. 2003;362:271–280. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- 156.Rothman KJ. Causes. Am J Epidemiol. 1976;104:587–592. doi: 10.1093/oxfordjournals.aje.a112335. [DOI] [PubMed] [Google Scholar]
- 157.Thun M, Peto R, Boreham J, Lopez AD. Stages of the cigarette epidemic on entering its second century. Tob Control. 2012;21:96–101. doi: 10.1136/tobaccocontrol-2011-050294. [DOI] [PubMed] [Google Scholar]
- 158.Elliott P, Stamler J, Nichols R, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ. 1996;312:1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pope CA, 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 160.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120:965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 162.Pope C, Brook R, Burnett R, Dockery D. How is cardiovascular disease mortality risk affected by duration and intensity of fine particulate matter exposure? An integration of the epidemiologic evidence. Air Qual Atmos Health. 2011;4:5–14. [Google Scholar]
- 163.Bailis R, Ezzati M, Kammen DM. Mortality and greenhouse gas impacts of biomass and petroleum energy futures in Africa. Science. 2005;308:98–103. doi: 10.1126/science.1106881. [DOI] [PubMed] [Google Scholar]
- 164.Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.HEI Accountability Working Group. Communication 11. Boston: Health Effects Institute; 2003. Assessing Health Impact of Air Quality Regulations: Concepts and Methods for Acountability Research. [Google Scholar]
- 166.Health Effects Institute. Communication 15. Boston: Health Effects Institute; 2010. Proceedings of an HEI Workshop on Further Research to Assess the Health Impacts of Actions Taken to Improve Air Quality. [Google Scholar]
- 167.Johnston FH, Henderson SB, Chen Y, et al. Estimated global mortality attributable to smoke from landscape fires. Environ Health Perspect. 2012;120:695–701. doi: 10.1289/ehp.1104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Normile D. Ecology. Getting at the roots of killer dust storms. Science. 2007;317:314–316. doi: 10.1126/science.317.5836.314. [DOI] [PubMed] [Google Scholar]
- 169.Amiraslani F, Dragovich D. Combating desertification in Iran over the last 50 years: an overview of changing approaches. J Environ Manage. 2011;92:1–13. doi: 10.1016/j.jenvman.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 170.Wang XM, Zhang CX, Dong ZB, Hasi E. Has the Three Norths Forest Shelterbelt Program solved the desertification and dust storm problems in arid and semiarid China? J Arid Environ. 2010;74:13–22. [Google Scholar]
- 171.Wang X, Dong Z, Zhang J, Liu L. Modern dust storms in China: an overview. J Arid Environ. 2004;58:559–574. [Google Scholar]
- 172.Dionisio KL, Rooney MS, Arku RE, et al. Within-neighborhood patterns and sources of particle pollution: mobile monitoring and geographic information system analysis in four communities in Accra, Ghana. Environ Health Perspect. 2010;118:607–613. doi: 10.1289/ehp.0901365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou Z, Dionisio KL, Arku RE, et al. Household and community poverty, biomass use, and air pollution in Accra, Ghana. Proc Natl Acad Sci USA. 2011;108:11028–11033. doi: 10.1073/pnas.1019183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Preston SH, Van de Walle E. Urban French mortality in the nineteenth century. Popul Stud (Camb) 1978;32:275–297. [PubMed] [Google Scholar]
- 175.Wang X, Hunter PR. A systematic review and meta-analysis of the association between self-reported diarrheal disease and distance from home to water source. Am J Trop Med Hyg. 2010;83:582–584. doi: 10.4269/ajtmh.2010.10-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lukacik M, Thomas RL, Aranda JV. A meta-analysis of the effects of oral zinc in the treatment of acute and persistent diarrhea. Pediatrics. 2008;121:326–336. doi: 10.1542/peds.2007-0921. [DOI] [PubMed] [Google Scholar]
- 178.Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003;78:773–781. doi: 10.1093/ajcn/78.4.773. [DOI] [PubMed] [Google Scholar]
- 179.Zeng L, Dibley MJ, Cheng Y, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001. doi: 10.1136/bmj.a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Murray CJ, Lopez AD. Boston: Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1996. The Global Burden of Disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. [Google Scholar]
- 181.Zaridze D, Maximovitch D, Lazarev A, et al. Alcohol poisoning is a main determinant of recent mortality trends in Russia: evidence from a detailed analysis of mortality statistics and autopsies. Int J Epidemiol. 2009;38:143–153. doi: 10.1093/ije/dyn160. [DOI] [PubMed] [Google Scholar]
- 182.Rehm J, Baliunas D, Borges GLG, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053. doi: 10.1016/S0140-6736(07)61698-5. [DOI] [PubMed] [Google Scholar]
- 184.Lim SS, Gaziano TA, Gakidou E, et al. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet. 2007;370:2054–2062. doi: 10.1016/S0140-6736(07)61699-7. [DOI] [PubMed] [Google Scholar]
- 185.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 186.Lee I-M, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Das P, Horton R. Rethinking our approach to physical activity. Lancet. 2012;380:189–190. doi: 10.1016/S0140-6736(12)61024-1. [DOI] [PubMed] [Google Scholar]
- 188.Hu FB, Manson JE. Omega-3 fatty acids and secondary prevention of cardiovascular disease—is it just a fish tale?: Comment on ‘efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease’. Arch Intern Med. 2012;172:694–696. doi: 10.1001/archinternmed.2012.463. [DOI] [PubMed] [Google Scholar]
- 189.Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ioannidis JPA. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218–228. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- 191.Slattery ML, Randall DE. Trends in coronary heart disease mortality and food consumption in the United States between 1909 and 1980. Am J Clin Nutr. 1988;47:1060–1067. doi: 10.1093/ajcn/47.6.1060. [DOI] [PubMed] [Google Scholar]
- 192.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 193.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30:427–432. doi: 10.1093/ije/30.3.427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix