Inflammation-induced effector CD4+ T cell interstitial migration is alpha-v integrin dependent (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 9.
Published in final edited form as: Nat Immunol. 2013 Aug 11;14(9):949–958. doi: 10.1038/ni.2682
Abstract
Leukocytes must traverse inflamed tissues to effectively control local infection. Although motility in dense tissues appears to be integrin-independent actin-myosin based, during inflammation changes to the extracellular matrix (ECM) may necessitate distinct motility requirements. Indeed, we found that T cell interstitial motility was critically dependent on RGD-binding integrins in the inflamed dermis. Inflammation-induced deposition of fibronectin was functionally linked to increased αv integrin expression on effector CD4+ T cells. Using intravital multi-photon imaging, we found that CD4+ T cell motility was dependent on αv expression. Selective αv blockade or knockdown arrested TH1 motility in the inflamed tissue and attenuated local effector function. These data show a context-dependent specificity of lymphocyte movement in inflamed tissues that is essential for protective immunity.
Pathogen clearance requires effector T cells to migrate into inflamed tissues to locate the infected microenvironment. The molecular mechanisms involved in the migration of leukocytes into tissues via extravasation have been well characterized1. However, the interactions controlling movement through peripheral tissues are less well understood.
Integrins play a vital role in leukocyte trafficking from blood into tissues. Chemokine-mediated integrin activation leads to firm adhesion of leukocytes to the endothelium, essential for tranendothelial migration. In contrast, leukocyte interstitial migration is thought to be largely integrin-independent2. Migration studies using artificial collagen matrices or confinement chambers suggest that the dense three-dimensional ECM scaffold supports the use of non-adhesive, actin-based traction mechanisms by leukocytes3, 4. In the lymph node (LN), T cells migrate along networks of fibroblastic reticular cells (FRCs) that express integrin ligands ICAM-1 and VCAM-1 and chemokines CCL19 and CCL21. However, motility is driven by chemokine-mediated chemotaxis/haptotaxis directly and does not require integrins for adhesion5. Similarly, genetic ablation of all known integrins in DCs failed to perturb DC migration in the LN or skin6. Thus, under a variety of conditions, integrins appear to be largely dispensable for extravascular trafficking of leukocytes.
Inflammatory mediators and pathogens themselves modify ECM density and composition in peripheral tissues which may dictate new requirements for leukocyte motility at sites of inflammation7. T cells expressing distinct integrins accumulate in particular inflammatory settings and in discrete tissues where integrin expression is thought to play a role in tissue-specific homing8 and retention within the tissue9. In contrast to the situation in LNs, where collagen fibers are coated by FRCs, T cells in non-lymphoid tissues such as the skin and lung are directly exposed to collagen fibers and associated ECM components that could act as guidance cues for movement through the interstitial space. The use of multiphoton microscopy has enabled the visualization of leukocyte motility within peripheral tissues with studies highlighting a close association between T cells and matrix fibers in skin, brain and tumors10–16. Lymphocyte movement in the skin during delayed type hypersensitivity showed a correlation between collagen-binding integrins on T cells and their migration along fibers requiring calcium signaling by Kv1.3 potassium-channels14. During infection with Toxoplasma in the brain, effector T cells also migrate along reticular fibers, although the brain was largely devoid of infection-induced increases in collagen13. In many studies, blockade of G protein-coupled receptor signaling attenuated leukocyte interstitial motility, but it is not clear if this is due to blockade of chemotaxis/kinesis per se or the absence of chemokine-induced integrin activation. Although neutrophil interstitial migration in the mesentery was shown to be integrin dependent17, there have been no studies that directly test the role of integrins in T cell motility within inflamed peripheral tissues.
Given the critical role that physical confinement plays in three-dimensional cell motility4 and the extent of inflammation-driven ECM remodeling, we addressed mechanisms of interstitial motility of effector CD4+ T lymphocytes in the inflamed dermis. Inflammation was associated with a reduction in the density of collagen fibers and with extensive fibronectin deposition. Using intravital multiphoton microscopy (IV-MPM) we found that effector CD4+ T cell movement within the inflamed dermis is integrin-dependent and the expression of αv-integrins is essential for interstitial motility and pathogen clearance. Thus, movement through inflamed interstitial tissue requires the coordinate expression of specific integrins on effector T cells with ECM changes in the tissue.
Results
Inflammation-induced migration along ECM fibers
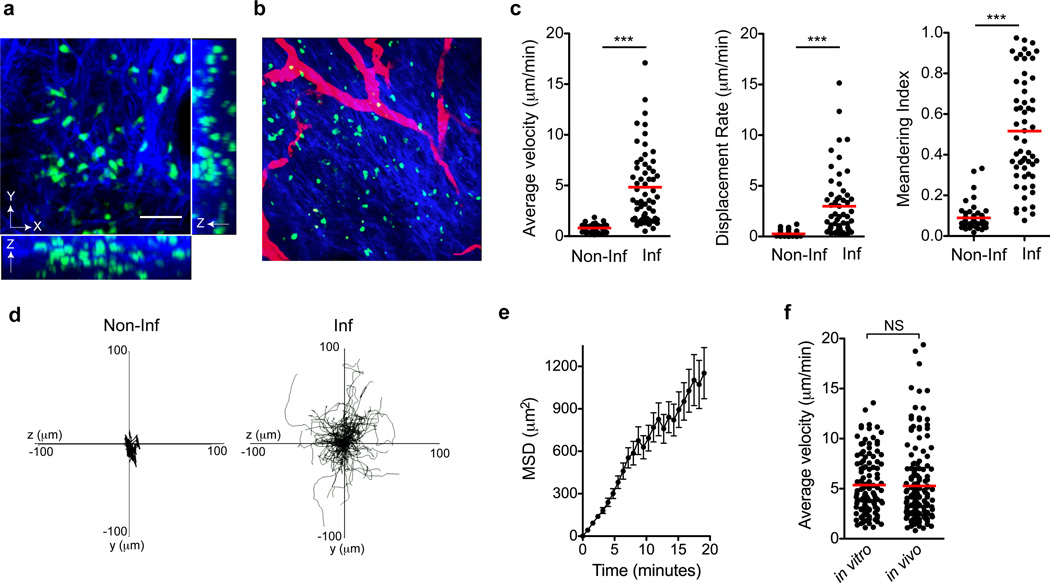
Analysis of T cell interstitial migration was performed in the inflamed and non-inflamed dermis using intravital multiphoton microscopy (IV-MPM). Dermal location was assessed using the second harmonic generation (SHG) to distinguish the collagen-rich dermis from collagen-replete epidermis and from blood vessels using intravenous Texas-red dextran (Fig 1a, b). Cells were tracked over time in three-dimensional space using semi-automated software. T cells in the non-inflamed dermis were identified by using CD4-Cre+ Stop-floxed YFP mice (Gt(ROSA)26Sortm1(EYFP)Cos mice) (86% αβ+ T cells, the majority being CD4+ and Foxp3−, Supplementary Figure 1). In the steady state non-inflamed dermis T cells were observed at low frequencies and were uniformly non-motile (Fig 1c). Average velocities for dermal CD4+ T cells (predominantly αβ T cells) were 0.803 ± 0.05 SEM µm/min; noticeably less than that observe for actively surveying dermal γδ T cells in the steady state (average speeds of 2–5 µm/min)18, 19. For T cell migration in the inflamed dermis, we followed a population of antigen-specific CD4+ T cells. Naïve CD4+ T cells from WT15 T cell receptor (TCR)-transgenic mice20 were primed in vitro under TH1 differentiation conditions. Primed cells were labeled with CFSE and transferred into naïve BALB/c mice that were immunized in the ear dermis with cognate peptide emulsified in CFA. Kinetic analysis in the inflamed dermis defined the peak of effector T cell accumulation in the tissue to be 3–4 days after immunization (data not shown). Inflammation led to striking changes in dermal T cell velocities (Fig 1c, Supplementary movie 1), consistent with previous models14, 21, 22. The T cells exhibited no preferential directionality in their crawling patterns and the mean squared displacement (MSD) of their crawling tracks increased linearly over time, suggesting that these cells were not following a chemotactic gradient within the tissue (Fig 1d, e). T cells crawled preferentially in the lateral plane (XY) of this tissue, with limited vertical (Z-axis) translocation (Supplementary Fig 2). _In vivo_-primed effector cells were visualized using donor T cells from a YFP-IFN-γ reporter mouse23 and revealed crawling velocities and patterns similar to the adoptive transfer of _in vitro_-primed effector T cells (Fig 1f, Supplementary Fig 3). Thus, inflammation drives the recruitment and interstitial motility of effector T cells in the dermis. This enhanced T cell motility in the presence of inflammation and/or microbial stimuli is in contrast to dermal dendritic cells that are motile in non-inflamed dermis but immobile when exposed to microbial stimuli24 suggesting cell type-specific cues for motility and arrest.
Figure 1. Migratory patterns of effector TH1 T cells in the CFA-inflamed ear dermis.
(a) Maximum intensity projections across X, Y, and Z axes of WT15 T cells (green) and collagen (second harmonic generation, blue) in the CFA-inflamed dermis. Scale bar, 40µm. (b) X-Y projection of T cells and SHG with vasculature shown in red using intravenous Texas Red-labeled dextran. (c) Steady-state (non-inflamed, Non-Inf) and CFA-inflamed (Inf) T cell motility by average velocity, displacement and meandering index. (d) X–Y projections of the T cell migration tracks over a 20-minute time span with CFA. (e) Mean squared displacement (MSD) of the T cell tracks show a linear increase over time. (f) Comparison of average velocities of T cells using adoptively transferred effector WT15 T cells (in vitro) or effector T cells primed in vivo using WT15 IFN-γ-YFP reporter cells to identify putative IFN-γ-producing cells (in vivo). Statistics by Mann-Whitney: ***p<0.001; NS, not significant, _p_>0.05. Data are representative of more than ten independent imaging experiments.
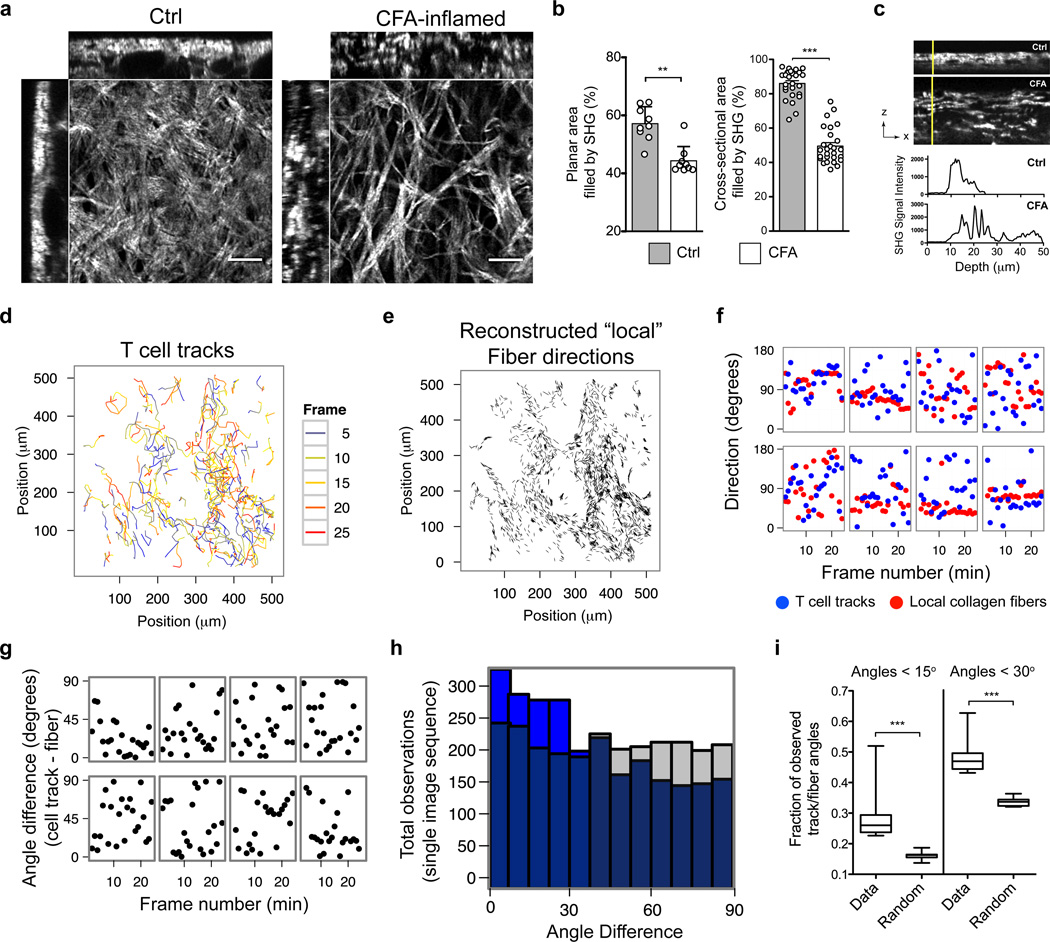
Imaging revealed dramatic changes in the collagen fibers of the inflamed skin, as visualized by second harmonic generation (SHG) (Fig 2a). Compared to collagen structure in untreated skin, characterized by dense layers of interwoven collagen fibers, the collagen in CFA-inflamed skin was a loose network of thick collagen bundles (Fig 2a–c). The collagen fibers were also preferentially oriented in the XY plane (Supplementary Fig 4). Many T cells appeared to be crawling in directional patterns that closely followed the local collagen fibers (Supplementary Fig 5, Supplementary movie 1). To test if there was preferential migration of T cells along the matrix scaffold, we developed an unbiased quantitative technique that utilized computational image analysis to compare frame-by-frame directional vectors of individual T cell migration patterns with the orientation of the matrix fibers (Fig 2d, e). Briefly, SHG images were translated into a map of discrete collagen fibers based on local SHG intensity gradients (Supplementary Fig 5, and On-line methods). We then compared the migratory directions of the T cells with directions of the “local” collagen fibers (those fibers within a 6 µm radius around the T cell centroid) (Fig 2f) and calculated the angle differences between the vectors representing the fibers and those representing the migrating cell (Fig 2g). If T cells preferentially moved parallel to the local ECM fibers, we would expect more observations in close alignment (small angle differences) between ECM fibers and T cell migration than if T cell migration was random. This was indeed the case (Fig 2h). We quantified the close alignment by calculating the fraction of observations with an angle difference smaller than 15° or 30° between fiber and migrating T cell (_n_=10 experiments). The fraction of closely aligned observations was significantly larger (p<0.0001) than would be expected from random T cell movement (Fig 2i). We conclude that T cells preferentially migrated in directions parallel to the local collagen structures, suggesting that T cells were utilizing a form of contact guidance. These data are consistent with a haptokinetic model of motility, in which cells migrate along a three-dimensional surface, interacting with bound ligand through cell-surface receptors.
Figure 2. T cells migration is guided by ECM-fibers.
BALB/c mice immunized with KLH/CFA in the ear dermis and analyzed by IV-MPM on day 3. Collagen structure by SHG. (a) Sample image volumes of non-immunized (Ctrl) and CFA-inflamed ears. (b) Analysis of planar area (XY) (left) and cross-sectional area (XZ) filled by SHG in control and CFA-inflamed ears. Left, each circle represents a unique region (150 µm × 150 µm area, avoiding hair follicles and blood vessels) from three different ears for each group. Right, each circle represents one of three orthogonal views analyzed for every one planar region analysis on the left. Bars represent mean ± SEM. (c) Cross-sectional images of SHG signal intensity in ear (top) and SHG across the vertical yellow lines (bottom). (d) Projection of cell tracks in x-y plane. Color code, frame at which T cell locations were observed. (e) Reconstructed fiber directions. (f) Migration and fiber directions along eight representative T-cell tracks. Directions are measured as angle relative to the x-axis of the image data: blue dots, T cell tracks; red dots, fibers. (g) Angle differences between fiber and migration directions in (f). (h) Histogram of angle differences for all tracks time steps from a single representative experiment (blue) compared to the distribution of angle differences that would result from random migration (grey). (i) Frequency of directional differences smaller than 15° and 30°, _n_=10 experiments. Statistical significances evaluated by Mann-Whitney: **p<0.01; ***p<0.001.
Integrin-dependent motility in the inflamed dermis
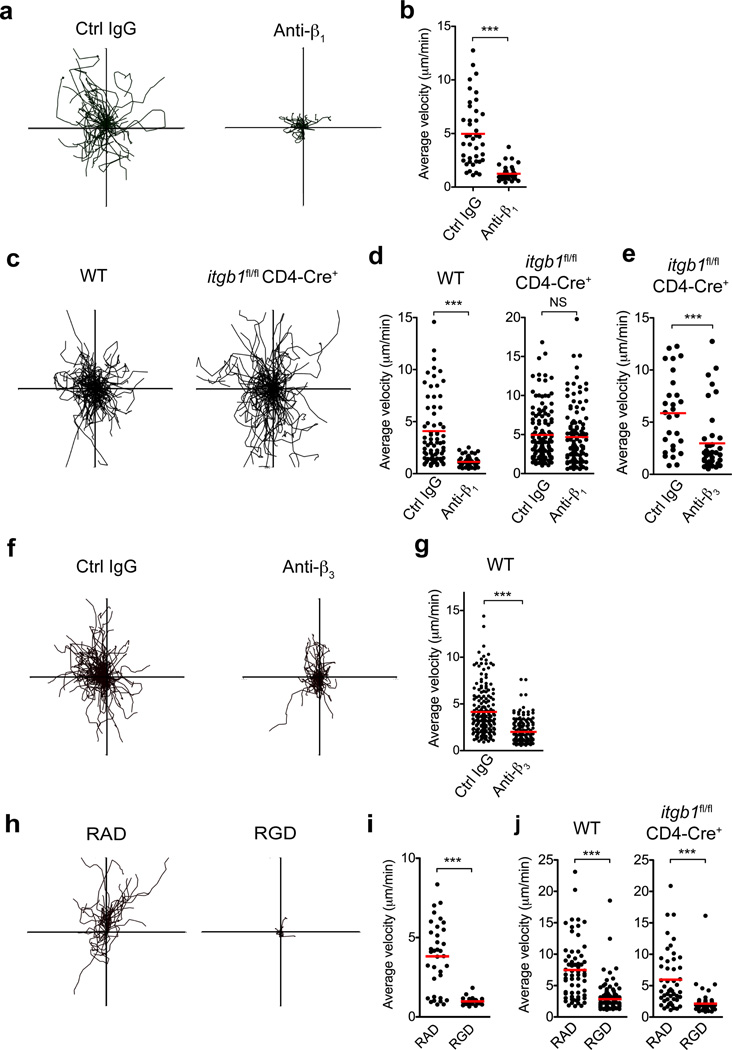
The density of the matrix appears to be a key determinant of the mode of interstitial motility, with integrin-dependency increasing as the density of the matrix decreases4. Therefore, the inflammation-based changes in collagen density and the crawling pattern of T cells along the matrix fibers prompted us to revisit integrin-dependency for T cell interstitial migration. While imaging T cell migration in the dermis we administered a blocking antibody to β1 integrin, a common receptor chain that pairs with various α-chains for binding to extracellular matrix components25, 26. Soon after i.v. injection of anti-β1 all intradermal T cells stopped migrating (Fig 3a,b) and adopted a rounded morphology (Supplementary Fig 6, Supplementary movie 2), consistent with a loss of adhesion to the surrounding matrix. Blockade of LFA-1 (ICAM-binding) had no effect on interstitial motility (data not shown).
Figure 3. RGD-dependent integrin blockade impairs T cell motility in the inflamed dermis.
(a, b) While imaging WT15 T cells in the inflamed ear dermis, control (Ctrl) IgG or a blocking antibody to β1 integrin were administered intravenously. (a) XY projections of T cell migratory paths over 25 minutes immediately following control IgG or anti-β1 integrin treatment (100 µg antibody). (b) Average velocity of T cells. (c) WT or β1-deficient (_itgb1_fl/fl × CD4-Cre+) OT-II T cells were imaged in the dermis and the resultant T cell crawling patterns are shown. (d) Blocking antibodies to β1 integrin were administered intravenously and OT-II T cell migration velocities shown for both WT and β1-deficient cells. (e) β1-deficient T cell migration velocities after blocking antibodies to β3 integrin. (f–g) While imaging WT OT-II T cells in the dermis, blocking antibodies to β3 integrin were administered intravenously and the resultant T cell crawling patterns (f) and migration velocities (g) are shown. (h–j) Immediately prior to imaging of adoptively transferred WT effector WT15 T cells, control RAD or integrin-blocking RGD peptides were injected into the CFA-inflamed ear dermis. (h) XY projections of T cell migratory paths over 25 minutes. (i) Average velocity of T cells over the same time period. (j) Immediately prior to imaging of WT and β1-deficient OT-II T cells in the inflamed dermis, control RAD or blocking RGD peptides were injected into the ear dermis and resultant T cell migration velocities are shown. Statistics by Mann-Whitney: ***p<0.001; NS, not significant, _p_>0.05.
To confirm that β1 was necessary for motility in the inflamed skin, we analyzed T cells genetically deficient in the β1-subunit, using OTII _itgb1_flox/flox _CD4_-Cre mice. β1-deficient T cells displayed no functional defects in clonal expansion, cytokines, or tissue infiltration; as previously noted27 (data not shown). However, in contrast to acute β1 blockade, β1-deficient T cells showed no defects in migration within the dermal interstitium (Fig 3c,d). The disconnect between acute blockade and genetic deficiency raised two possibilities: that antibody treatment leads to migratory arrest through activating T cell signaling or that there is genetic compensation. To test effects of anti-β1 signaling independent of receptor-ligand blockade we treated effector T cells with antibody and monitored migration in vitro on β2-dependent ICAM-1 and CXCL12 coated-plates (Supplementary Fig 7). Anti-β1 treatment did not lead to cell arrest, suggesting cross-linking β1 is not sufficient to halt migration. To address redundancy/compensation, β3 appeared to be the likely alternative matrix-binding integrin for lymphocytes. Indeed, anti-β3 Ab treatment halted migration by β1-deficient T cells in the inflamed dermis, showing β3 can compensate in the genetic absence of β1 (Fig 3e), but not during acute β1-blockade (Fig 3a,b). Surprisingly, anti-β3 treatment also led to acute migratory arrest of wild-type (WT) T cells (Fig 3f,g). Therefore, both β1 and β3 contribute to interstitial motility but neither is sufficient for full migration in WT T cells. Consistent with the ability of both β1 and β3 integrins to bind the RGD sequence found in many ECM components such as fibronectin, local administration of RGD peptides (but not control RAD peptides) led to striking T cell migratory arrest (Fig 3h–j, Supplementary movie 3). Thus, effector T cells require integrin-ECM interactions for T cell migration within the inflamed dermal interstitium.
Selective αv-integrin dependency for dermal motility
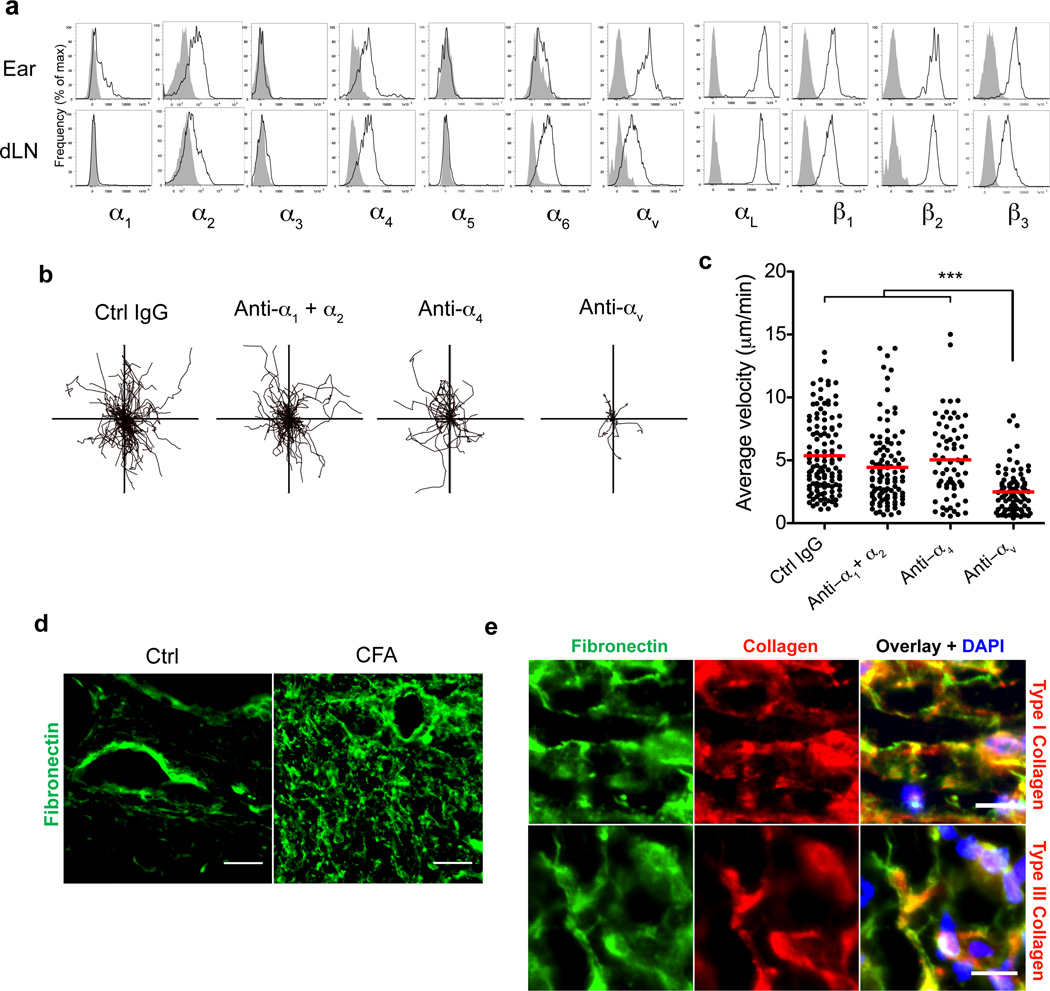
β1 and β3 both pair with the α-subunit, αv, prompting us to analyze effector T cell α-subunit usage in the inflamed dermis. We first monitored receptor expression on a synchronous cohort of effector T cells through the adoptive transfer of naïve WT15 cells followed by immunization with LACK-CFA (Fig 4a). Five days after cell transfer and immunization we observed a restricted pattern of integrin expression on the effector T cells in the inflamed dermis with effector T cells expressing α2, α4 and αv subunits, but little α1 and no α5 (Fig 4a). To rigorously test the role of these individual α-subunits in T cell motility we used acute antibody blockade and IV-MPM imaging. We found a highly restricted requirement for αv in effector T cell motility in the inflamed dermis (Fig 4b, c, Supplementary movie 4); blockade of α1, α2, α1+α2, or α4 failed to arrest T cell motility. Thus, αvβ1 and αvβ3 appear to be uniquely required for T cell interstitial motility. The finding that T cells may use both αvβ1 and αvβ3 to migrate through the CFA-inflamed dermis suggests that αv expression may be limiting: acute perturbations to either β-subunit reduces the surface pool of αv-integrins below a threshold needed for migration.
Figure 4. CD4+ T cells utilize αv integrin for intradermal motility.
(a) Integrin expression by _in vivo_-primed WT15 cells in the immunized ear dermis and draining lymph node five days after immunization. Histograms represent viable donor CD4+Thy-1.1+ (WT15) lymphocytes: integrin staining (black line) and isotype control staining (grey shaded). (b,c) While imaging effector WT15 T cells in the inflamed ear dermis, control (Ctrl) IgG or blocking antibodies (100 µg each) to the indicated integrin subunits were administered intravenously. XY projections of T cell migratory paths over 25 minutes immediately following treatment with indicated blocking antibody. c. Average velocities of T cells over the same time periods and treatments. (d) Immunohistological staining of fibronectin on frozen sections of control (non-inflamed, Ctrl) and KLH/CFA-immunized ears three days after immunization. Scale bar, 25µm. (e) Co-staining of fibronectin and types I or III collagen on KLH/CFA-inflamed ears. Scale bar, 10µm. Data are representative of at least three independent experiments for all antibody-blocking experiments. For (c) statistical significance was evaluated by a Kruskal-Wallis test with Dunn’s post-test; *** p<0.001.
Fibronectin is a primary substrate for αv integrins28. However, dermal fibronectin is sparse in the steady state, non-inflamed tissue (Fig 4d29, 30). Analysis of CFA-inflamed tissue sections revealed widespread increases in dermal fibronectin throughout the tissue (Fig 4d), consistent with previous observations in humans and hamsters29–31. Co-staining for types I and III collagen showed that fibronectin was closely associated with the interstitial collagen fibers (Fig 4e and Supplementary Fig 8) as previously observed32. Thus, in the inflamed dermis, dramatic changes in the collagen density are accompanied by increases in tissue fibronectin, consistent with a role for the RGD-dependent αv-integrin in T cell motility.
Coordinate expression of integrins and ECM
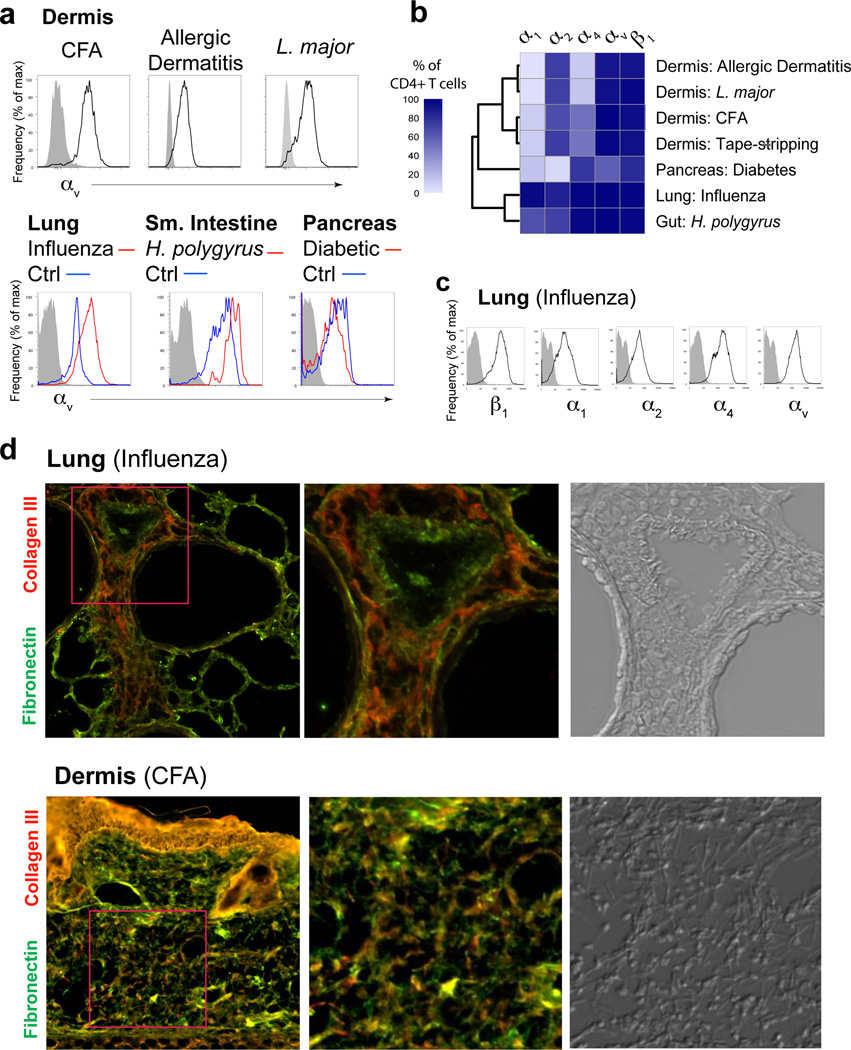
We next asked if expression of αv was restricted to a particular inflammation or tissue type. Robust expression of αv was seen in the dermis in numerous inflammatory settings including a Th2 model of atopic or allergic inflammation and infection with the protozoa Leishmania major (Fig 5a, b) in both C57BL/6 (TH1-dominated) and BALB/c mice (Th2 dominated) (data not shown) and also acute barrier disruption from tape stripping (Fig 5b). Similarly, pathogen-induced inflammation in the lung (Influenza) and gut (H. polygyrus) and autoimmune infiltrates in the diabetic pancreas all showed uniformly high expression of αv on infiltrating CD4+ T cells. Therefore, αv expression on effector CD4+ T cells is observed across tissues and inflammation types. To identify possible context-dependent changes in T cell integrin expression we examined other matrix-binding α-integrin subunits. Using an unbiased hierarchical clustering analysis of the frequency of integrin-expressing T cells, T cells clustered into two principal groups based on tissue type (Fig 5b). Expression of collagen-binding integrins, α1 and α2, were markedly different between tissues: α2 but not α1 was expressed on effector CD4+ T cells in the skin under all inflammatory settings (Fig 5b) while effector T cells in the lung expressed high levels of both α1 and α2 (Fig 5c)9, 33. T cells from the autoimmune inflamed pancreas segregated away from T cells in strong microbial-induced inflammatory settings, expressing little α1 or α2.
Figure 5. Broad αv integrin expression by effector CD4+ T cells across tissues and types of inflammation.
(a) Histogram profiles of αv integrin expression on total CD4+ T cells extracted from the ear dermis (top) and other tissues (bottom). Where indicated, αv expression on CD4+ T cells from non-inflamed (control) tissues are shown (blue line), compared to inflamed/infected tissue (red line) and isotype control staining (grey shaded). (b) Hierarchical clustering of the frequency of integrin-expressing T cell populations from different tissues. (c) Histogram expression profiles of indicated alpha chain integrin subunits on CD4+ T cells extracted from influenza-infected lung tissue (day 8 post-infection) compared to isotype control staining (grey shaded). (d) Comparison of fibronectin and collagen immunohistochemistry on tissues from influenza-infected lung (day 8) (top row) and CFA-inflamed dermis (bottom row). Second panels are larger representations of boxed areas from first panel. Third panel is a DIC image of same boxed area.
To examine the relationship between T cell matrix-binding integrins and the type of ECM components, we compared two examples of differential integrin expression and evaluated collagen and fibronectin in the respective inflamed tissues (Fig 5d). As noted above, in the CFA/OVA inflamed dermis αv was expressed by effector T cells and was critical for motility (Fig 4a–c) consistent with co-localization of fibronectin and collagen fibers (Fig 4e, 5d). Expression of α2, but not α1, correlated with inflammation-induced expression of collagens I and III (α2 ligands) but not collagen IV (α1 ligand) (Supplementary Fig 8). In contrast, in the influenza infected lung (day 8 post-infection) high α1 and α2 expression by effector T cells (Fig 5c) was associated with large areas of the lung parenchyma that were rich in collagen III but low in fibronectin (Fig 5d, left panels) with extensive collagen IV around blood vessels, airways and alveoli as described33. The collagen III rich areas were coincident with the leukocytic infiltrates (Fig 5d, DIC image) and were not seen in uninfected lungs (data not shown). Similarly, widespread T cell-collagen IV co-localization was previously seen around the airways33. Thus, the co-expression of both α1 and α2 and αv integrins may facilitate migration through distinct collagen-rich and fibronectin-rich regions in the influenza-infected lung33.
αv-expression upregulated during T cell priming
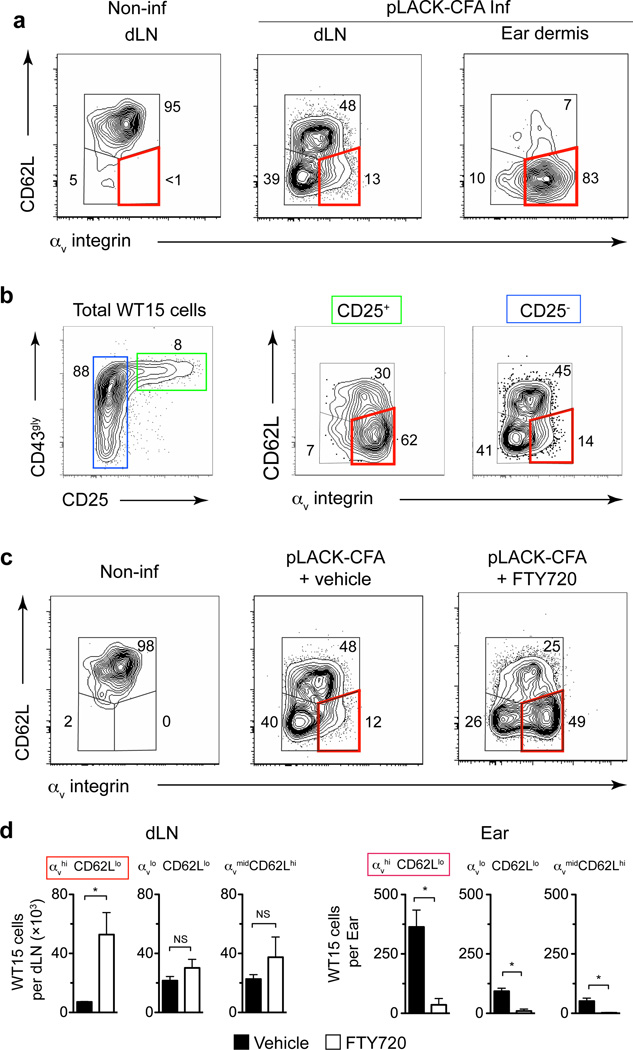
αv expression was consistently higher on T cells in the inflamed dermis compared to the LN (Fig 6a, Supplementary Fig 9). The regulation of matrix-binding integrins could occur directly in the inflamed tissue through local induction or selection of integrin-expressing effector T cells, or could be programmed during T cell activation in the LN, as seen for tissue-specific homing molecules8, 34, 35. To explore these possibilities, we examined the phenotype of activated T cells in the LN and in the inflamed tissue. Following intradermal immunization, LN antigen-specific T cells segregated into three distinct populations based on αv and CD62L expression, with the majority of CD62Llo cells expressing low levels of αv (Fig 6a). In contrast, effector T cells that had migrated to the dermis were predominantly αvhiCD62Llo (Fig 6a). Cells expressing the highest amounts of activation markers CD25 and glycosylated CD43 in the LN were preferentially αvhi CD62Llo (Fig 6b). Blockade of LN egress using the S1P1 receptor agonist, FTY720, led to a selective increase in the number of αvhiCD62Llo cells in the LN and a reciprocal loss of αvhiCD62Llo cells in the inflamed dermis (Fig 6c, d). Similar results were observed in a polyclonal CD4+ T cell response to KLH/CFA and Leishmania major infection (Supplementary Fig 9). Thus effector CD4+ T cells destined to exit the LN and patrol peripheral tissues express high amounts of αv integrin and low CD62L, consistent with “emigrant” effector T cells36.
Figure 6. LN “emigrant” effector T cells express high levels of αv integrin.
Naïve WT15 cells were transferred into BALB/c mice that were then immunized with pLACK/CFA (LACK-CFA Inf) in ear dermis the following day or not immunized (Non-inf). (a) αv integrin and CD62L expression on WT15 cells in the draining lymph node and ear dermis five days after immunization. Numbers represent cell frequency (%). The red box highlights the αvhi CD62Llo population throughout all panels in the figure. (b) Expression of αv on activated cells in the draining lymph node. Transferred cells gated on high or low expression of CD25 (left panel) and analyzed for αv and CD62L. (c, d) FTY720 (or vehicle) was administered days 3 and 4 post-immunization and αv and CD62L expression was evaluated on WT15 cells in the lymph node on day five. (d) Total numbers of WT15 cells in the draining lymph node and immunized ear dermis in mice treated with FTY720 (open bars) or vehicle (filled bars). Bars represent mean ± SEM. For (d) statistical significance was evaluated by a Mann-Whitney test: * p<0.05; NS, not significant, _p_>0.05. Data are representative of two independent experiments with similar results. _n_= 4–5 mice per group per experiment.
Essential role for αv integrin in interstitial migration and effector function
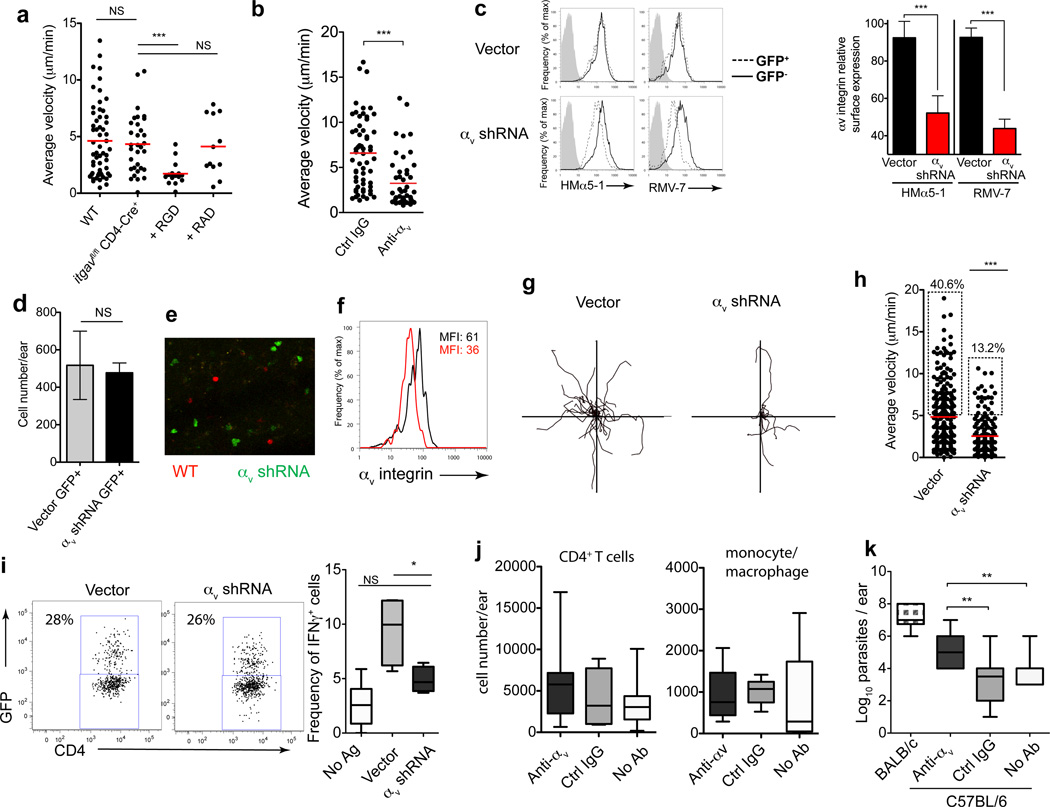
As seen in β1-deleted T cells (Fig 3), T cells genetically deficient in αv (OTII _itgav_flox/flox _CD4_-Cre+) remained motile in the inflamed dermis but were still susceptible to migratory arrest by RGD peptides (Fig 7a). Thus there appears to be a fundamental requirement for integrin-mediated motility in inflamed tissues as highlighted by the high degree of compensation following genetic ablation. To directly test the requirement for αv in interstitial migration we turned to acute gene knock down using αv-specific shRNA. β1-deficient OT-II T cells (whose motility remains αv-dependent, Fig 7b) were retrovirally-transduced with a control vector or an αv-specific shRNA (Fig 7c) and transferred into C57BL/6 congenic mice for imaging. Testing two shRNA constructs, αv-9 and αv-10, (see Supplementary Fig 10 for αv-10 data) we found that knockdown of αv did not hinder effector T cell recruitment to the inflamed dermis (Fig 7d) or change T cell location within the tissue as both αv-sufficient and αv-deficient effector T cells could be found in the same dermal regions (Fig 7e). However, the stable loss of αv (Fig 7c, f) significantly impaired the motility of the interstitial T cells (Fig 7g, h, Supplementary Fig 10, Supplementary movie 5). These data are especially remarkable because surface expression of αv was only reduced by ~60% (Fig 7c, f), indicating that only a fraction of the αv surface receptors need to be compromised to alter migration, thus pointing to an essential role for αv integrins in T cells interstitial migration within the inflamed dermis.
Figure 7. Non-redundant role for αv in effector CD4+ T cell intradermal motility and anti-microbial clearance.
(a) αv-deficient (_itgav_fl/fl × CD4-Cre+) OT-II T cell average velocity in CFA-inflamed dermis and blockade with RGD peptides as in Fig 3. (b) β1-deficient OT-II T cell velocity in the CFA-inflamed ear after anti-αv antibody. (c) TH1-primed β1-deficient OT-II T cells transduced with MSCV-GFP encoding shRNA for αv integrin (αv shRNA) or empty vector (Vector). Left, surface expression of αv integrin day 4 after retroviral transduction on GFP+ and GFP- cells, with two αv antibody clones or isotype control(grey). Right, αv MFI of GFP+ and GFP- cells, normalized to the MFI of αv on untreated T cells. Bars represent mean ± SEM, four independent experiments. (d–i) Transduced T cells transferred to C57BL/6 mice and mice immunized intra-dermally with pOVA-CFA in one ear and KLH-CFA in the other ear (no cognate antigen, No Ag). Transduced T cells (GFP+) in the ear dermis imaged by IV-MPM day 3. (d) Number of vector or αv shRNA GFP+ T cells in the antigen-bearing dermis. (e) Dermal location of effector T cells following co-injection of SNARF-labeled WT (red) and GFP+ expressing αv shRNA (green) cells to pOVA-CFA immunized mice. (f) ex vivo αv expression on dermal GFP+ cells. Black line, control vector; red line, αv shRNA. (g) XY projections of T cell migration patterns and average velocities (h) (30 minutes). (i) Vector control and αv shRNA GFP+ expression in pOVA-CFA inflamed ears (left). Right, frequency of IFNγ producing vector or αv shRNA GFP+ TH1 cells, gated as in dot plots, by ex vivo intracellular cytokine staining for IFN-γ. No Ag, frequency of IFN-γ+-transferred cells in KLH-OVA inflamed ears (j) Mice were infected with L. major in the ear dermis. Four weeks post-infection, C57BL/6 mice were given anti-αv or control antibody i.p. (1mg/mouse) three times/week for 3 weeks. Mice were harvested at week 7 post-infection and CD4+ T cell numbers (j) and parasite load (k) determined in the infected ear dermis. Statistics by Mann-Whitney: *p<0.05; **_p_<0.01; ***_p_<0.001; NS, not significant, _p_>0.05. (k) ANOVA across C57BL/6 groups, p<0.0001 for parasite load.
To determine the functional significance of αv expression we used both gene knockdown and antibody blockade approaches. OVA-specific β1-deficient effector TH1 cells were transduced with control or αv-9 shRNA expressing retrovirus and transferred to C57BL/6 congenic mice immunized with OVA/CFA. Both control vector and αv-9 shRNA GFP+ expressing T cells homed to the inflamed dermis with similar frequency (Fig 7i) and number (Fig 7d). To determine ‘in vivo’ function, cells were harvested from tissues in buffers containing Brefeldin-A to block cytokine secretion and intracellularly stained for IFN-γ, without additional in vitro stimulation37, 38. Knockdown of αv had no effect on the frequency of ex vivo IFN-γ-producers in the dLN (mean: 3,500 GFP+ IFN-γ producers, control vector; 3,540 GFP+ IFNγ producers, αv-9 shRNA) highlighting αv-deleted T cells are competent to make IFN-γ. Nonetheless, a reduction in T cell αv expression led to a marked reduction in the frequency of IFN-γ-producers in the inflamed dermal tissue (Fig 7i). Thus, T cell interstitial motility is dependent on αv and disruption of interstitial motility limits T cell effector function, as represented here by IFN-γ production, presumably by limiting their ability to encounter antigen-bearing APCs in the inflamed tissue. Clearance of the protozoa L. major is critically dependent on IFN-γ-production by CD4+ T cells localized to the infected dermis. Therefore, to test if the reduction in IFN-γ production might impact on pathogen clearance, we infected WT mice with the protozoa L. major and administered blocking anti-αv antibody. Anti-αv or control antibody was administered 4 weeks after L. major infection, for a 3 week period, when the C57BL/6 mice are mounting a protective IFN-γ-dependent anti-parasite immune response. As with shRNA knock-down, antibody blockade of αv did not limit the generation of anti-Leishmania T cells in the LN (similar IL-4/IFNγ ratio in all C57BL/6 groups, data not shown) or limit the number of CD4+ T cells and macrophages that accumulated at the infection site (Fig 7j). However, blocking αv had a significant impact on the ability to clear the pathogen (Fig 7k); with αv blockade leading to significantly higher parasite titers. Attenuated pathogen clearance is consistent with the reduced ability of T cells to mount an efficient IFN-γ–response. However, we cannot exclude alternative mechanisms such as an effect of αv-blockade on macrophage localization or function. Our studies find that integrin-dependent interstitial motility is essential for efficient IFN-γ effector T cell function in inflamed tissues and contributes, in part, to pathogen clearance.
DISCUSSION
Here we identify αv (with β1 or β3) as a key receptor for intradermal motility of effector CD4+ T cells. Integrin-dependency in interstitial motility of leukocytes has been controversial with previous studies supporting a largely integrin-independent mode of migration5,6. However, more recent studies suggest migration modes appear to be highly dynamic, with dendritic cells seamlessly moving between integrin-dependent and integrin-independent motility39, 40. Thus the requirement for integrin-driven motility is likely to be context-dependent and influenced by inflammation-induced changes in the tissue microenvironment. We found that T cells are essentially non-motile in non-inflamed tissue but motility is dramatically enhanced during inflammation in a manner dependent on integrins αvβ1 and/or αvβ3. In the CFA-inflamed dermis, significant structural changes in the density and composition of the tissue extracellular matrix occur, which may create an environment where lymphocytes are dependent on integrins for motility. Integrin specificity appears to be coordinately regulated with the type of ECM changes in the inflamed tissue. Fibronectin has long been known to bind to collagen and serve as a cell adhesion substrate32 and we speculate that the inflammation-driven increase in fibronectin of the dermal interstitium observed across species29–31 provides a substrate for infiltrating lymphocytes. Intriguingly, TH1 effector cells are unique among leukocytes in their ability to synthesize fibronectin41 possibly suggesting an active role for effector T cells in contributing to substrate deposition to facilitate interstitial migration. Our data show that the upregulation of αv can be initiated during T cell activation in the LN, although further selection or regulation of integrin-expressing cells may occur locally as the effector T cells enter the inflamed microenvironment. Using IV-MPM, we found that the expression of αv is critical for the rapid amoeboid movement of effector T cells in the inflamed dermis and that perturbation of αv limits T cell effector function and attenuates efficient L. major clearance.
Chemokines direct tissue-specific homing and localization within tissues and are critical for the activation of leukocyte integrins (LFA-1, Mac-1) for firm adhesion during transendothelial migration (TEM). Although not directly tested here, we speculate that the integrin-dependent interstitial movement in the dermis is also contingent on chemokines for integrin activation. Indeed, many studies show a requirement for pertussis toxin-sensitive G-protein coupled receptors for interstitial motility. Lymphocyte crawling during TEM occurs via the dynamic assembly-disassembly of high affinity LFA-1 in adhesive filopodia and is essential for crawling under shear flow42, 43. Future studies with fluorescently-tagged αv should reveal the dynamics of αv expression in migrating T cells in inflamed tissues. During TEM, shear forces promote migration through increases in invasive filopodia and accelerated dissociation of LFA-1:ICAM-1 interactions42. The edema associated with inflammation in tissues may also elevate ‘interstitial’ shear forces to promote integrin-dependent motility44. It is also possible that integrin activation could be regulated independently of chemokines, perhaps via CD44 as observed for TEM. Alternatively, it is possible that the integrin dependency underlies a need to adhere to ECM fibers to come into contact with immobilized chemokines rather than direct integrin-driven motility: disrupting integrin adhesion to ECM fibers could lead to an inability of cells to follow haptotactic gradients imposed by chemokines coating the fibers.
Integrins play a variety of roles in the immune response including promoting stable T cell-DC interactions for effector function, extravasation and tissue retention or survival. Distinct integrins direct tissue-specific homing (α4β7 integrin and gut accumulation) and may determine location within the tissue as suggested by T cell α1-integrin expression and their accumulation in the epidermis in the lung and skin33,45. Our data highlight an essential role for integrins in T cell movement through the inflamed tissue and suggest specific integrin expression is dictated by the changes in ECM components. As concluded from in vitro models2, the density of the ECM scaffold may also influence the mode of motility with the degree of confinement dictating the utility of non-adhesive actin-myosin-based movement versus adhesive integrin-based migration4. Thus the mode of motility at sites of inflammation may differ depending on the extent of ECM remodeling, density or composition.
Interestingly, αv itself plays a critical role in shaping the inflammatory microenvironment through modulating cytokine and matrix expression. αv-binding to latent TGF-β via its RGD motif leads to TGF-β release from the ECM and biological activation46 important in wound healing and fibrotic events. αv also modulates induction of TH17 and Treg differentiation via changes in dendritic cell function47–49. More recently the IL-4 and IL-13-induced matricellular protein, periostin50, binds αv integrin on keratinocytes to induce proinflammatory cytokines. Blockade of either periostin or αv potently suppressed the allergic skin inflammation. Indeed, blockade or genetic ablation of αv (mainly in myeloid cells) can inhibit inflammation in EAE, allergic asthma and lung scleroderma47, 48, 51 although it precipitates ulcerative colitis52. The requirement for αv for effector T cell interstitial migration through such inflamed sites may compound the therapeutic effects of targeting the αv pathway in inflammation. Interestingly, this αv-based motility is not required for gain of effector function or localization to the dermis but does limit robust execution of effector function, suggesting attenuation of interstitial migration limits the ability of effector T cells to scan the microenvironment of the inflamed tissue in search of specific antigen-bearing APCs.
Acute disruption of αv via antibody blockade or shRNA knockdown revealed functional requirements for αv in T cell interstitial motility. However, these were not recapitulated by genetic ablation experiments, with αv-deficient T cells remaining highly motile through a compensatory mechanism dependent on RGD-binding integrins or other RGD-binding proteins. A similarly high degree of functional compensation for fibronectin-binding integrin subunits in knockout mice has been reported for developmental roles of αv and α553, 54 and also for β1 and β3 in knockout cell lines55. These data therefore suggest a fundamental requirement for integrin-fibronectin (or other RGD-containing matrix proteins) interactions during both development and immune cell migration during inflammation.
Here we have show that inflammation in the dermis necessitates an integrin-based mechanism for effector T cell interstitial migration that is critical for full effector T cell function in the inflamed and infected tissue. As an intriguing perspective, it may be possible to predict the integrin requirements for interstitial migration in a given inflamed tissue by identifying the specific changes to the ECM landscape. Understanding the coordinate expression of leukocyte integrins and ECM substrates within inflamed tissues should provide novel therapeutic strategies for inflammation-specific and/or tissue-specific mitigation of immune-mediated tissue damage in autoimmunity and chronically inflamed pathologies.
On-line METHODS
Mice, Immunizations, and Infections
BALB/c and C57BL/6 mice were purchased from National Cancer Institute. LACK-specific TCR-Tg WT15 mice and IFN-γ reporter (Yeti) mice were provided by Drs. Nigel Killeen and Richard Locksley, respectively. CD11c-YFP mice were provided by Dr. Michel Nussenzweig. _itgb1_fl/fl mice. Albino B6 mice and Gt(ROSA)26Sortm1(EYFP)Cos mice were purchased from Jackson Laboratories and CD4-Cre mice from Taconic Farms. itga5fl/fl mice provided by Dr. Richard Hynes. itgavfl/fl mice were provided by Dr Adam Lacy-Hulbert. Mice were bred and maintained in the pathogen-free animal facility at the University of Rochester. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Rochester. Mice were immunized intradermally in the ear with 1 µg pLACK, 1 µg pOVA, or 5 ug Keyhole Limpit Hemacyanin (KLH) emulsified in Complete Freund’s Adjuvant (CFA). For Leishmania major infection, mice were infected subcutaneously in the ear with 2 × 105 infectious parasites/ear. For Influenza infection, C57BL/6 mice were sedated with avertin and given a 30 uL intranasal inoculation of Influenza A/HK/X31 (9.2×107 PFU/ml). Eight days after infection, lungs were perfused and airway-resident cells were removed by bronchoalveolar lavage. Lymphocytes were then isolated from the lung tissue by collagenase digestion and Ficoll separation of viable cells. For H. polygrus infection, C57BL/6 mice were infected by gavage with 200 L3 Heligmosomoides polygyrus larvae. Ten days later, lamina propria lymphocytes were extracted from the small intestines using collagenase and a Percoll gradient after removal of Peyer's Patches. For tape-stripping, the ventral skin of the ear was tape stripped 10× by applying and gently removing adhesive tape (Scotch brand 610) followed by the application of 50µg OVA protein in 10uL PBS directly onto the stripped ear surface. Ears were harvested 5 days after tape stripping. For chronic allergic dermal sensitization, the ears were tape stripped three times, spaced three weeks apart. Samples were collected 48 hours after the final tape stripping and application of OVA. For the pancreas, diabetic NOD mice were identified when blood glucose levels exceeded 300mg/dl (usually 10–12 weeks of age). Young (6 weeks) non-diabetic mice were used as controls. Cells were isolated from the pancreas following collagenase digestion as for the ear dermis.
Antibodies and Reagents
All conjugated antibodies were purchased from eBioscience or Biolegend unless otherwise noted. For integrin expression on transferred WT15 T cells in the dermis, leukocytes were extracted from the ear and stained with CD45-Pacific Blue, CD4-AlexaFluor700, Thy-1.1-FITC and antibodies against one of the following: β1-PE (Hmβ1-1), α1-Alexa 488 or α2-FITC (Ha31/8 and Ha1/29, respectively), α3-APC (polyclonal, R&D Systems), α4-PE (R1-2), purified α5 (5H10-27), α6-APC (GoH3) and αv-PE (Hmα5-1 or RMV-7). Note that the anti-αv specificity of the commercially available Hmα5-1 antibody clone (Biolegend) is different from that of the originally-described anti-α5 clone (see supplementary Fig 10). Hmα5-1 binds to CD4+ T cells from itga5fl/fl CD4-Cre+ mice but not itgavfl/fl CD4-Cre+ mice (Fig 13a) and precipitates αv but not α5 from activated T cell lysates (Fig 13b). Moreover, the Biolegend Hmα5-1 clone competes for binding with commercially available anti-αv clone, RMV-7 (Supplementary Fig 10). Expression of α5 on in vitro activated CD4+ T cells was used to determine the specificity of Biolegend Hmα5-1 clone as expression of α5 could not be detected on T cells activated in vivo (Supplementary Fig 10) under the variety of stimulatory conditions outlined in Fig 5. Of note, the absence of α5 staining in vivo was not due to an α5-specific sensitivity to enzymatic tissue digestion as positive α5 staining on CD11b+ myeloid cells remained intact with/without enzymatic digestion (data not shown). Supplementary figure 10 directly compares the α5-integrin specificity of the Biolegend Hmα5-1 clone and the original anti-α5 clone from Yagita. For blocking in vivo, the following azide-free low endotoxin antibodies were injected through a tail vein catheter: anti-β1 (Hmβ1-1, eBioscience), anti-α1 (Ha31/8), anti-α2(Ha1/29), anti-α4 (R1-2, Biolegend), anti-αv (HMα5-1, Biolegend), anti-αL (M17/4), and control Armenian Hamster IgG (H4/8). For FACS, cells were analyzed on an LSR II or a FACSCalibur (BD Biosciences, San Jose, CA). Data analyzed using FlowJo software (TreeStar).
Cell Culture and Adoptive Transfers
For in vitro TH1 priming, CD4+ cells were enriched from lymph node and spleen from WT15 Tg mice by complement-mediated lysis of CD8+ (3.155), CD24+ (J11D), and MHC-II+ (M5/114) cells. Naïve T cells were then enriched by positive selection for CD62L+ cells using a MACS column (Miltenyi Biotec): routinely >85% CD4+ and >95% CD62L+. Naïve T cells were stimulated with peptide-pulsed irradiated T cell-depleted splenocytes, IL-2 (10 U/mL), IL-12 (20 ng/mL), and anti-IL-4 (11B11, 40 µg/mL). Cells were split three days later and harvested on day five. The effector TH1 cells were labeled with CFSE (Invitrogen, Carlsbad, CA) and transferred into mice (5×106 cells per mouse) prior to immunization. For WT15-Yeti cells, naïve CD4+ T cells were enriched as described above and transferred directly into BALB/c mice without in vitro stimulation or labeling. The following day, mice were immunized with 5 µg pLACK/CFA in the ear dermis.
Extraction of T cells from the ear dermis
Ear tissue was processed as previously described33. Ears were excised, ventral and dorsal sheets separated and placed in collagenase D (1 mg/ml in PBS with 3mM CaCl2, Roche) and incubated for 30 minutes at 37°C. EDTA was added (5mM) and the digested tissue disrupted using a pestle and metal strainer. Insoluble material was removed by filtration and the pellet was washed twice in HBSS with 5% FCS. For ex vivo intracellular cytokine staining, Brefeldin A (1µg/ml) was added to all extraction and wash buffers to block cytokine secretion.
Intravital Multiphoton Microscopy and Image Analysis
Mice were anesthetized with isoflurane (induction ~5%, maintenance ~1.5%) (isoflurane vaporizer/ventilation machine, Lei Medical M3000R, VAP2100). Once anesthetized, a venous catheter was inserted in the lateral tail vein and the ventral side of the ear pinna was affixed to a coverslip. Mice were placed on a custom-made platform for imaging. Body temperature was maintained using a heated water pad and a heating block, monitored with a rectal thermometer. The microscope objective was heated (Bioptechs) to maintain normal dermal temperature during imaging. Images were acquired using an Olympus FV1000-AOM multiphoton system. Fluorescence was collected using an Olympus XLPlanN 25× NA1.05 objective for deep tissue multiphoton imaging and detected using two proprietary external photomultipliers (Hamamatsu, Japan). Fluorescence excitation was achieved by a Spectra-Physics DeepSee-MaiTai HP Ti:Sa laser tuned to 900 nm for excitation of CFSE and Texas Red. CFSE and Texas Red fluorescence was collected using 519/26nm and 607/36nm bandpass filters (Olympus and Semrock, respectively). YFP fluorescence was collected using 535/30nm bandpass filter (Chroma). Second harmonic signals were collected using a 447/60nm bandpass filter (Semrock). Z-stack images (512×512 pixels) were acquired with a vertical resolution of 3–5 µm and a lateral pixel size of 994nm. For time series, three-dimensional stacks were acquired every 40–45 seconds with a Kalman filter.
Raw imaging data were processed with Volocity software (Perkin Elmer) and ImageJ. A median filter was used to reduce noise and T cells were tracked using automated algorithms with manuals corrections. Raw tracking data was exported and processed in Excel. Cell tracks lasting for less than eight minutes were excluded from analyses. No minimum cell displacement criteria were imposed that would have excluded non-motile cells. Movies were created using both Volocity and ImageJ. For analysis of the effects of blocking antibodies, images were acquired for 20 minutes prior to treatment, followed by 25 minutes following treatment.
For quantitative analysis of collagen density, SHG images were obtained from the dermis of anesthetized mice that had been immunized three days previously in one ear with KLH/CFA. Control images were taken from the contralateral ear. Images stacks were taken at 512×512 resolution (994 nm/pixel) at 1 µm vertical intervals through the ear. Laser intensities and PMT voltages were adjusted with tissue depth to maintain visible SHG signal. Once acquired, 150×150 µm regions of the image stacks were selected to avoid hair follicles and major vasculature.
Analysis tool for migrating T cell—ECM association
ECM-fiber directions were estimated using ImageJ. The Differentials plug-in was used to calculate intensity gradients, which were rotated by 90 degrees and mapped to a range of [0..180] degrees to represent fiber directions. Our approach took advantage of the fact that the direction of strongest variation (intensity gradients) must be perpendicular to the fiber direction: any directions pointing away from the fiber have a lower intensity than the directions that follow the fiber. We only determined lateral directions for this analysis (Fig 3a,b), since both T-cell movement and ECM-fiber direction are preferentially aligned in the X-Y plane (Supplementary Fig. 2,4,5). Cell locations were imported from tracking data and used to export fiber directions and gradient magnitudes from spheres with 6 µm radius around cell centroids. Extracted ECM-direction data and cell tracks were imported in R. Cell velocities were calculated using central differences and tracks with sedentary cells, which were defined as cells with a mean velocity of less than 2.5 µm/min were removed. For each observation of a cell in a track, we disregarded irrelevant direction samples stemming from background noise instead of ECM-fibers by considering only those samples that were in the 90th percentile of the intensity gradient magnitude (Supplementary Fig 5). The local ECM-direction around the cell centroid was calculated as the gradient weighted mean of the selected direction samples.
FTY720 Treatment
Mice received FTY20 (1 mg/kg, Cayman Chemicals) i.p. 72 and 96 hours post-immunization. Control mice received vehicle alone.
RGD Peptide Treatment
BALB/c mice received CFSE-labeled effector TH1 cells and were immunized as described above. Immediately prior to imaging, RGD or control RAD peptide was injected directly into the ear dermis (50 µg peptide, Peptides International).
Immunohistochemistry
Three days after immunization, ears were excised and frozen in OCT. Frozen sections were cut, fixed, and stained for ECM components. Primary antibodies against fibronectin (Abcam) and types I, III, and IV collagen (Southern Biotec) were used, followed by staining with Cy3- and AlexaFluor647-conjugated secondary antibodies (Jackson and Molecular Probes, respectively). For IHC analysis of influenza-infected lung tissue, lungs were excised eight days post-infection.
Retroviral Transduction
Naive β1−/− OT-II cells were purified from spleen and LN and stimulated with peptide/APC under TH1 conditions. One day after the initiation of the culture, cells were transduced with MSCV encoding αv shRNA or MSCV vector alone. Cells were then split on day 3 and harvested on day 5, similar to conditions with WT15 cells. On the day of harvest, transduction efficiency (indicated by % GFP+ cells) and αv expression levels were measured by FACS. Transduction efficiencies were routinely between 15–30% and were similar between control vector and αv shRNA MSCV.
Statistics
All statistical tests were done with GraphPad Prism or R software. Many analyses used the non-parametric Mann Whitney U test. For multiple analyses of variance, the Kruskal-Wallis test was used with Dunn’s post-test. For pairwise comparisons, Wilcoxon signed-rank test was used. ANOVA was used across multiple treatment groups.
Supplementary Material
1
ACKNOWLEDGEMENTS
The authors thank Ron Germain and Jackson Egen for technical assistance; Karl Kasischke, Linda Callahan, Ed Brown and the URMC Multiphoton Core for support; Beatriz Leon and Frances Lund for H.polygyrus infected tissue; Kihong Lim for technical assistance; Jim Miller and Steve Georas for comments on the manuscript; and members of the Fowell and Kim labs for discussion and support. This work was supported by National Institutes of Health grants AI072690 and AI088427 to D.J.F, HL018208 and HL087088 to M.K, AI089079 to M.G.O, and American Heart Association grant 11SDG7520018 to Y-M.H.
Footnotes
AUTHOR CONTRIBUTIONS
M.G.O, A.G. and D.J.F designed the study. M.G.O., A.G, K.L., M.A., A.B-M., A.H. and Y-M.H. performed the experiments. M.G.O., A.G. B.A., A.R., M.M-S and D.J.F analyzed the data. Y-M.H., M.K., D.J.T., A.L-H., H.Y., M.M-S provided reagents and conceptual advice. M.G.O. and D.J.F. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 3.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Jacobelli J, et al. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol. 2010;11:953–961. doi: 10.1038/ni.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf E, et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 6.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 7.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 8.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray SJ, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 10.Okada T. Two-photon microscopy analysis of leukocyte trafficking and motility. Semin Immunopathol. 2010;32:215–225. doi: 10.1007/s00281-010-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumann K, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Bajenoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson EH, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matheu MP, et al. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mrass P, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Werr J, Xie X, Hedqvist P, Ruoslahti E, Lindbom L. beta1 integrins are critically involved in neutrophil locomotion in extravascular tissue In vivo. J Exp Med. 1998;187:2091–2096. doi: 10.1084/jem.187.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumaria N, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, et al. CD4 promotes breadth in the TCR repertoire. J Immunol. 2001;167:4311–4320. doi: 10.4049/jimmunol.167.8.4311. [DOI] [PubMed] [Google Scholar]
- 21.Filipe-Santos O, et al. A dynamic map of antigen recognition by CD4 T cells at the site of Leishmania major infection. Cell Host Microbe. 2009;6:23–33. doi: 10.1016/j.chom.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Egawa G, et al. In vivo imaging of T-cell motility in the elicitation phase of contact hypersensitivity using two-photon microscopy. J Invest Dermatol. 2011;131:977–979. doi: 10.1038/jid.2010.386. [DOI] [PubMed] [Google Scholar]
- 23.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng LG, et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4:e1000222. doi: 10.1371/journal.ppat.1000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 26.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeNucci CC, Shimizu Y. beta1 integrin is critical for the maintenance of antigen-specific CD4 T cells in the bone marrow but not long-term immunological memory. J Immunol. 2011;186:4019–4026. doi: 10.4049/jimmunol.1003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 29.Clark RA, Dvorak HF, Colvin RB. Fibronectin in delayed-type hypersensitivity skin reactions: associations with vessel permeability and endothelial cell activation. J Immunol. 1981;126:787–793. [PubMed] [Google Scholar]
- 30.Clark RA, et al. Fibronectin deposition in delayed-type hypersensitivity. Reactions of normals and a patient with afibrinogenemia. J Clin Invest. 1984;74:1011–1016. doi: 10.1172/JCI111468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusubata M, et al. Spatiotemporal changes of fibronectin, tenascin-C, fibulin-1, and fibulin-2 in the skin during the development of chronic contact dermatitis. J Invest Dermatol. 1999;113:906–912. doi: 10.1046/j.1523-1747.1999.00802.x. [DOI] [PubMed] [Google Scholar]
- 32.Klebe RJ. Isolation of a collagen-dependent cell attachment factor. Nature. 1974;250:248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- 33.Richter M, et al. Collagen distribution and expression of collagen-binding alpha1beta1 (VLA-1) and alpha2beta1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J Immunol. 2007;178:4506–4516. doi: 10.4049/jimmunol.178.7.4506. [DOI] [PubMed] [Google Scholar]
- 34.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigmundsdottir H, et al. DCs metabolize sunlight-induced vitamin D3 to 'program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 36.Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat Immunol. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30:277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sojka DK, Fowell DJ. Regulatory T cells inhibit acute IFN-gamma synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10. Proc Natl Acad Sci U S A. 2011;108:18336–18341. doi: 10.1073/pnas.1110566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renkawitz J, et al. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol. 2009;11:1438–1443. doi: 10.1038/ncb1992. [DOI] [PubMed] [Google Scholar]
- 40.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandig H, et al. Fibronectin is a TH1-specific molecule in human subjects. J Allergy Clin Immunol. 2009;124:528–535. doi: 10.1016/j.jaci.2009.04.036. 535 e521–525. [DOI] [PubMed] [Google Scholar]
- 42.Shulman Z, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park EJ, et al. Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood. 2010;115:1572–1581. doi: 10.1182/blood-2009-08-237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends Cell Biol. 2007;17:44–50. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Conrad C, et al. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 46.Yang Z, et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luzina IG, et al. Regulation of pulmonary inflammation and fibrosis through expression of integrins alphaVbeta3 and alphaVbeta5 on pulmonary T lymphocytes. Arthritis Rheum. 2009;60:1530–1539. doi: 10.1002/art.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acharya M, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120:4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paidassi H, et al. Preferential expression of integrin alphavbeta8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology. 2011;141:1813–1820. doi: 10.1053/j.gastro.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masuoka M, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kudo M, et al. IL-17A produced by alphabeta T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacy-Hulbert A, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Mol Biol Cell. 1996;7:1737–1748. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Flier A, et al. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–2449. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wennerberg K, et al. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1