Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 23.
Published in final edited form as: Cochrane Database Syst Rev. 2012 Apr 18;4:CD002042. doi: 10.1002/14651858.CD002042.pub3
Abstract
Background
Most clinical practice guidelines recommend restrictive red cell transfusion practices, with the goal of minimising exposure to allogeneic blood. The purpose of this review is to compare clinical outcomes in patients randomised to restrictive versus liberal transfusion thresholds (triggers).
Objectives
To examine the evidence for the effect of transfusion thresholds on the use of allogeneic and/or autologous red cell transfusion, and the evidence for any effect on clinical outcomes.
Search methods
We identified trials by searching: the Cochrane Injuries Group Specialised Register (searched 1 February 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1), MEDLINE (Ovid) 1948 to January Week 3 2011, EMBASE (Ovid) 1980 to 2011 (Week 04), ISI Web of Science: Science Citation Index Expanded (1970 to February 2011) and ISI Web of Science: Conference Proceedings Citation Index - Science (1990 to February 2011). We checked reference lists of other published reviews and relevant papers to identify any additional trials.
Selection criteria
Controlled trials in which patients were randomised to an intervention group or to a control group. We included trials where intervention groups were assigned on the basis of a clear transfusion ‘trigger’, described as a haemoglobin (Hb) or haematocrit (Hct) level below which a red blood cell (RBC) transfusion was to be administered.
Data collection and analysis
We pooled risk ratios of requiring allogeneic blood transfusion, transfused blood volumes and other clinical outcomes across trials using a random-effects model. Two people performed data extraction and assessment of the risk of bias.
Main results
We included 19 trials involving a total of 6264 patients and they were similar enough that results could be combined. Restrictive transfusion strategies reduced the risk of receiving a RBC transfusion by 39% (risk ratio (RR) 0.61, 95% confidence interval (CI) 0.52 to 0.72). This equates to an average absolute risk reduction (ARR) of 34% (95% CI 24% to 45%). The volume of RBCs transfused was reduced on average by 1.19 units (95% CI 0.53 to 1.85 units). However, heterogeneity between trials was statistically significant (P < 0.00001; I2 ≥ 93%) for these outcomes. Restrictive transfusion strategies did not appear to impact the rate of adverse events compared to liberal transfusion strategies (i.e. mortality, cardiac events, myocardial infarction, stroke, pneumonia and thromboembolism). Restrictive transfusion strategies were associated with a statistically significant reduction in hospital mortality (RR 0.77, 95% CI 0.62 to 0.95) but not 30-day mortality (RR 0.85, 95% CI 0.70 to 1.03). The use of restrictive transfusion strategies did not reduce functional recovery, hospital or intensive care length of stay. The majority of patients randomised were included in good-quality trials, but some items of methodological quality were unclear. There are no trials in patients with acute coronary syndrome.
Authors’ conclusions
The existing evidence supports the use of restrictive transfusion triggers in most patients, including those with pre-existing cardiovascular disease. As there are no trials, the effects of restrictive transfusion triggers in high-risk groups, such as acute coronary syndrome, need to be tested in further large clinical trials. In countries with inadequate screening of donor blood, the data may constitute a stronger basis for avoiding transfusion with allogeneic red cells.
Medical Subject Headings (MeSH): *Practice Guidelines as Topic; Erythrocyte Transfusion [adverse effects; mortality; *standards]; Hematocrit [standards]; Hemoglobin A [analysis]; Randomized Controlled Trials as Topic; Reference Values; Transplantation, Autologous [standards]; Transplantation, Homologous [mortality; standards]
MeSH check words: Humans
BACKGROUND
Blood is an indispensable product in modern medical practice (Amin 2004). Red blood cells (RBC) are used to improve oxygen delivery to tissues in situations of haemorrhage and anaemia (Napolitano 2009). Red blood cell transfusion constitutes one of the mainstays of therapy in the management of anaemic patients and is one of the few treatments that adequately restores tissue oxygenation when oxygen demand exceeds supply (Klein 2007; Wang 2010).
The risk and availability of red blood cell transfusion varies throughout the world. In most developed countries with well-regulated blood supplies, the safety of allogeneic red cell transfusion has improved significantly over the past 30 years. This has been primarily due to improvements in donor blood screening procedures and the implementation of more stringent quality control measures (Klein 2007). It has been estimated that the residual risk of transmission through transfusion of HIV, HCV and HBV in Canada is 1 per 7.8 million donations, 1 per 2.3 million donations and 1 per 153,000 donations respectively (O’Brien 2007). Globally, the estimated risks of infection per blood unit range from 1 per 100,000 to 1 per 400,000 for HBV, 1 per 1.6 million to 1 per 3.1 million for HCV, 1 per 1.4 million to 1 per 4.7 million for HIV, and 1 per 500,000 to 1 per 3.0 million for human T cell lymphotropic virus (Goodnough 2008). Data from seven developed countries from 2000 to 2005 showed the residual risk of transfusion-transmitted viral infections ranged from 0.22 to 2.48 per 1 million donations for HIV, 0.05 to 3.94 per 1 million donations for HCV and 1.51 to 9.78 per 1 million donations for HBV (Kitchen 2008). In the USA, the estimated risk per unit for HIV is 1:1,467,000 (Zou 2010), for HCV is 1:1,149,000 (Zou 2010) and for HBV is 1:282,000 to 357,000 (Zou 2009). In the USA, life-threatening reactions were also estimated to occur in 1: 139,908 patients transfused (Whitaker 2011).
In developing countries, the supply of blood is inadequate and may not be safe because it often is not tested for viral pathogens. Blood donations are not routinely tested in 39 countries for transfusion-transmissible infections including HIV, hepatitis B, hepatitis C and syphilis (WHO 2011). In 40 countries, less than 25% of the blood supply is collected from voluntary unpaid blood donors, with most coming from family or paid blood donors (WHO 2011). The prevalence of HIV in low-income countries is 2.3% of blood donations compared to 0.001% in high-income countries (WHO 2011).
Blood transfusion is expensive. In 2008, the mean payment for one unit of leukoreduced red blood cells in the United States was USD 223 (Whitaker 2011). However, if the costs of administration as well as the acquisition expenses of red blood cell transfusion are considered, the estimated cost derived from four US and European hospitals rises to USD 761 per unit (standard deviation +/− USD 294) (Shander 2010).
Description of the intervention
Historically, the widely accepted clinical standard was to transfuse patients when the haemoglobin level dropped below 10.0 g/dL or the haematocrit fell below 30%. This ‘10/30 rule’ was first proposed by Adams and Lundy in 1942 and served as a RBC transfusion trigger for decades (Madjdpour 2005; Wang 2010). However, the 1988 National Institutes of Health Consensus Conference in the United States reported that the evidence did not support a single criterion for transfusion (NIH 1988). Since then, several published guidelines have advised against a single threshold for red cell transfusion, recommending that a range of haemoglobin values between 6.0 and 10.0 g/dL can be used, depending on the presence of serious co-morbidity (AAGBI 2008; ASA 2006; ASBT 2001; BCTMAG 2003; Napolitano 2009; NBUGI 2001).
Clinical trials evaluating transfusion thresholds usually compare two transfusion groups: 1) liberal transfusion where patients receive blood at a higher haemoglobin concentration, and 2) restrictive transfusion where patients receive blood at a lower haemoglobin concentration.
Why it is important to do this review
The purpose of the review was to find, appraise and summarise the data from high-quality trials that studied the clinical impact of varying thresholds for transfusion with red cells. We were particularly interested in whether the results of randomised controlled trials support the trend for increasingly restrictive red cell transfusion practices and if red cell transfusions can be withheld in some circumstances without harming patients. We have updated the review because two recent trials (Carson 2011; Hajjar 2010) were published, increasing the number of patients included in this review from 3746 to 6264.
OBJECTIVES
To examine the evidence for the effect of transfusion thresholds on the use of red cell transfusions and the evidence for any change in clinical outcomes.
METHODS
Criteria for considering studies for this review
Types of studies
Randomised controlled trials with a concurrent control group. We included trials if the comparison groups were assigned on the basis of a clear transfusion ‘trigger’ or ‘threshold’, described as a haemoglobin or haematocrit level (with or without a specified level of haemodynamic instability) that had to be reached before a red cell transfusion was administered. Control group patients were required to be either transfused with allogeneic and/or autologous red blood cells at higher Hb or Hct levels (transfusion threshold) than the intervention group or transfused in accordance with current transfusion practices, which may not have included a well-defined transfusion threshold, but involved liberal rather than restrictive transfusion practices.
Types of participants
We included trials of surgical or medical patients, involving adults and/or children. We excluded neonates.
Types of interventions
The intervention considered was the use of transfusion thresholds (‘triggers’) as a means of guiding allogeneic and/or autologous red blood cell transfusion.
Types of outcome measures
Primary outcomes
- The proportion of patients ‘at risk’ who were transfused with allogeneic and/or autologous red blood cells.
Secondary outcomes
- The amounts of allogeneic and autologous blood transfused.
- Morbidity (non-fatal myocardial infarction, cardiac events, pulmonary oedema, cerebral vascular accident, thromboembolism, renal failure, infection, haemorrhage, mental confusion), mortality, haematocrit levels (postoperative/discharge) and length of hospital stay (LOS). We expected the definitions of each of the morbidity events to vary between studies.
Search methods for identification of studies
We did not restrict our search for trials by date, language or publication status.
Electronic searches
The Cochrane Injuries Group Trials Search Co-ordinator conducted the latest search for trials and collated the results. We searched the following databases:
- the Cochrane Injuries Group’s Specialised Register (searched 1 February 2011);
- the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1);
- MEDLINE (Ovid) 1950 to January Week 3 2011;
- EMBASE (Ovid) 1980 to 2011 Week 04;
- ISI Web of Science: Science Citation Index Expanded (SCI-EXPANDED) (1970 to February 2011);
- ISI Web of Science: Conference Proceedings Citation Index - Science (CPCI-S) (1990 to February 2011).
The search strategies are presented in Appendix 1.
Searching other resources
We contacted experts in the field to identify information relevant to the review. Where possible, we contacted authors of published studies for clarification of trial methodology and data. This was only possible where a contact address was reported in the published study. We searched the reference lists of relevant reviews and published papers as well as the reference lists of all included trials for further studies.
Data collection and analysis
Selection of studies
Two authors (JLC and PAC) independently screened the titles, abstracts, or both, of the search results and selected trials that met the previously defined inclusion criteria. We discussed inclusion of studies until consensus was reached; there were no disagreements on the inclusion of studies. We identified trials in which patients were randomised to a restrictive transfusion strategy (transfusion threshold and/or protocol), or to a control group which was randomised to a liberal transfusion strategy. JLC and PAC independently extracted study characteristics and outcomes using a data extraction form. The extraction form recorded information regarding: study type, methodology descriptions, the presence of a transfusion threshold, transfusion protocol, the type of surgery involved, clinical setting, treatment outcomes and general comments.
Data extraction and management
JLC and PAC performed data extraction on articles that met the inclusion criteria. JLC then entered data into Review Manager; data were checked by PAC. We contacted authors of trials to request missing data.
We used a data extraction form to record data on the following outcomes: the number of patients exposed to allogeneic blood, the amount of allogeneic blood transfused, the number of patients receiving any transfusion (allogeneic blood, autologous blood, or both). For trials involving surgical patients, we recorded the following outcomes: postoperative complications (infection, haemorrhage, non-fatal myocardial infarction, cardiac events, renal failure, stroke, thromboembolism, pulmonary oedema, mental confusion), mortality and length of hospital stay (LOS). We recorded data for blood loss and haemoglobin and haematocrit levels (on admission, pre-post transfusion and at discharge). We recorded information regarding demographics (age, sex), type of surgery or medical condition on the data extraction form. We extracted data for allogeneic blood transfusion if it was expressed as packed red blood cells (RBC). We documented information regarding the use of fresh frozen plasma (FFP) and/or platelets.
Assessment of risk of bias in included studies
The Cochrane Collaboration’s tool for assessing risk of bias is described in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
JLC and PAC assessed the following domains for each study:
- sequence generation;
- allocation concealment;
- blinding;
- incomplete outcome data;
- selective outcome reporting;
- other potential sources of bias.
We completed a ‘Risk of bias’ table for each study, incorporating a description of the study’s performance against each of the above domains and our overall judgement of the risk of bias for each entry as follows: ‘Low’, ‘Unclear’ (indicating unclear or unknown risk of bias) and ‘High’ risk of bias.
Measures of treatment effect
We calculated the risk ratio (RR) for allogeneic blood transfusion in the intervention group as compared with the control group and the corresponding 95% confidence intervals for each trial using the random-effects model (Der Simonian 1986). We adopted a similar approach to examine the other outcomes of transfusion. We also entered the mean number of units of red blood cells transfused to each group and the corresponding standard deviations. We used the mean difference (MD) and 95% confidence intervals (CI) to express the average reduction in the number of units of RBC administered to the intervention group, compared with the control.
Unit of analysis issues
The unit of analysis was the patient. We converted data expressed in millilitres (ml) for the volume of blood transfused to units of blood by dividing by 300.
Dealing with missing data
All analyses were on an intention-to-treat basis. We imputed no missing data.
Assessment of heterogeneity
There was significant clinical heterogeneity. The trials included surgical, medical and critical care patients. We pooled the data for all outcomes and presented data stratified by subgroups for the primary outcome only. The subgroups evaluated were allogeneic transfusion, autologous transfusion and clinical settings (cardiac surgery, orthopaedic surgery, vascular surgery, acute blood loss/trauma, cancer and critical care). We also examined the proportion of patients exposed to transfusion stratified by the transfusion threshold (difference ≥ 2 g/dL, < 2 g/dL), risk of bias and units of blood transfused.
We examined statistical heterogeneity using both the I2 statistic and Chi2 test. The I2 statistic describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity and larger values show increasing heterogeneity; substantial heterogeneity is considered to exist when the I2 > 50% (Higgins 2011). For the Chi2 test, we used a P value of < 0.10 to indicate the presence of statistically significant heterogeneity.
Assessment of reporting biases
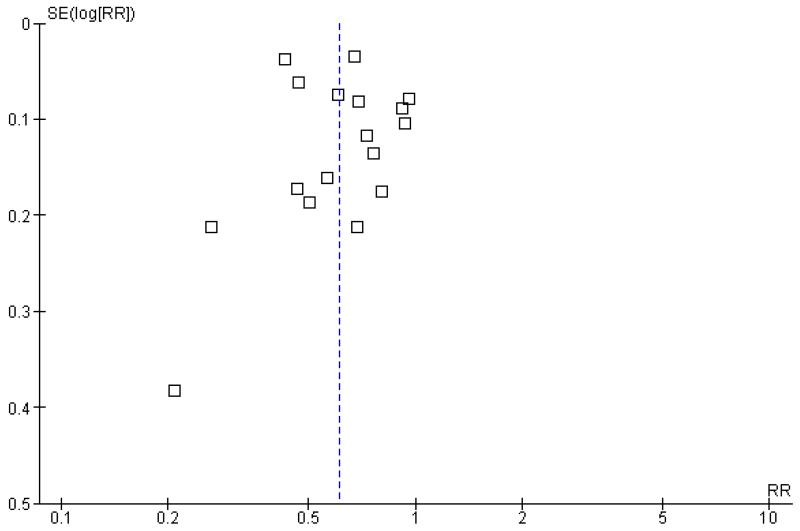
We examined funnel plots for evidence of publication bias. Although the small number of trials hampered funnel plot assessment, the outcome with the largest data set could be assessed for the presence of publication bias. The funnel plot for this outcome is presented in Figure 1.
Figure 1. Funnel plot of comparison 1.1 Patients exposed to blood transfusion (all studies).
Data synthesis
Due to the anticipated significant clinical heterogeneity of the trials, we analysed data using a random-effects model.
We performed all analyses using Review Manager software. We entered data on the numbers of patients exposed to allogeneic blood and the numbers of patients in each treatment group into Review Manager. We converted data in millilitres (ml) to units by dividing by 300. We converted studies reporting haematocrit to haemoglobin concentration by dividing by three.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses to explore treatment effects by blood product (allogeneic versus autologous, units of blood transfused), clinical setting (cardiac surgery, orthopaedic surgery, vascular surgery, acute blood loss/trauma, cancer, critical care), transfusion threshold (difference ≥ 2 grams per decilitre and difference less than 2 grams per decilitre) and risk of bias.
Sensitivity analysis
We performed a sensitivity analysis to assess the effects of study allocation concealment on the results.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The original literature search conducted in 1999 identified 110 full-text articles. Of these 110 eligible studies, 99 were excluded from further assessment (transfusion audits and reviews n = 94; observational studies - cohort or case-control studies n = 5). We considered 11 full-text articles for review. Of these 11 studies one was excluded because the trigger was based on the level of HbS not the haemoglobin or haematocrit level.
We conducted an updated search in November 2004. No new trials were identified by this search.
An updated search conducted in 2009 identified an additional seven trials. A further search conducted in February 2011 identified an additional two trials.
Included studies
We identified and included 19 eligible studies in this review. Among the 19 included trials the clinical settings were variable. Eight studies took place within the context of surgery: cardiac, vascular or orthopaedic (Bracey 1999; Bush 1997; Carson 1998; Carson 2011; Foss 2009; Grover 2005; Hajjar 2010; Johnson 1992; Lotke 1999; So-Osman 2010). Five trials were in the context of acute blood loss and/or trauma (Blair 1986; Colomo 2008; Fortune 1987; Topley 1956; Zygun 2009), three trials involved patients in critical care units (Hebert 1995; Hebert 1999; Lacroix 2007) and one trial involved leukaemia patients undergoing chemotherapy or stem cell transplantation (Webert 2008).
There was considerable variation with regard to the restrictive transfusion strategies used. These varied from 7.0 to 9.0 g/dL, with two further trials specifying haematocrit values of 25% or 30% (equivalent to haemoglobin levels of around 8.0 and 10.0 g/dL respectively). The liberal transfusion triggers varied: 100% of ‘normal red cell volume’ (Topley 1956), two units of blood (immediately in one trial (Blair 1986), postoperatively in another (Lotke 1999)) irrespective of clinical state; transfusion sufficient to maintain haemoglobin levels at or above 12.0 g/dL (Webert 2008), 10.0 g/dL (Bush 1997; Carson 1998; Carson 2011; Foss 2009; Grover 2005; Hebert 1995; Hebert 1999); Hajjar 2010, 9.5 g/dL (Lacroix 2007) and 9.0 g/dL (Bracey 1999; Colomo 2008; Zygun 2009). Two trials specified the liberal triggers as haematocrit levels of 32% (Johnson 1992) and 40% (Fortune 1987). One trial compared a new uniform, restrictive transfusion policy with more liberal standard care (So-Osman 2010).
In these trials random allocation was at the level of the patient, not the clinician or clinical unit. Consequently, participating clinicians may have been responsible for patients in both arms of the trials. Ten trials included more than 100 patients. Only one trial included over 1000 patients (Carson 2011). A total of 6264 trial participants were included in this systematic review.
Excluded studies
One randomised controlled trial was confined to patients with sickle cell disease and was excluded as the trigger was based on the level of HbS, not the haemoglobin or haematocrit level (Vichinsky 1995).
Risk of bias in included studies
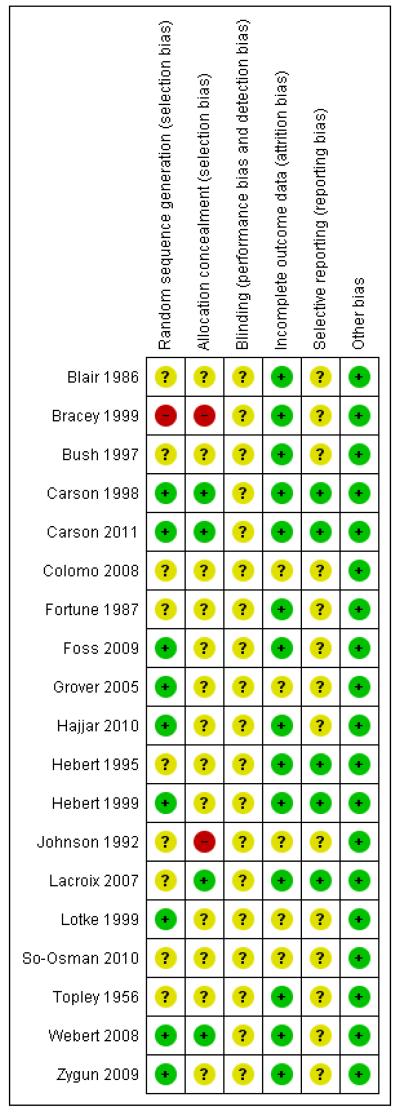
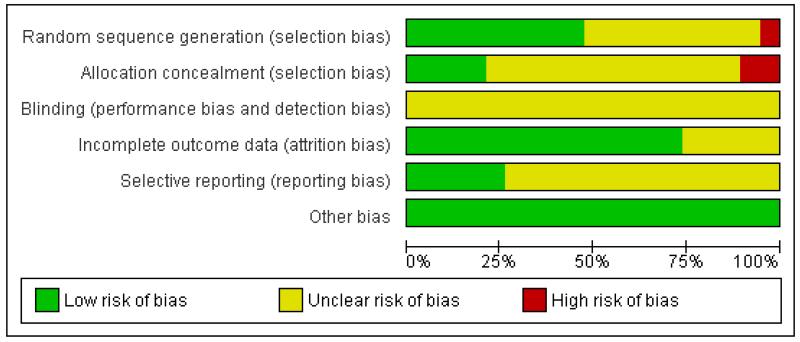
For further details regarding the performance of the studies against each domain, please see the ‘Risk of bias’ tables. A summary of the information in the tables is given below. Additionally, a visual summary of judgements about each methodological quality item for each included trial is shown in Figure 2 and Figure 3.
Figure 2. ‘Risk of bias’ summary: review authors’ judgements about each methodological quality item for each included study.
Figure 3. ‘Risk of bias’ graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies. Nineteen studies are included in this review.
Sequence generation (selection bias)
We judged the risk of bias for this item to be low for nine trials, eight of which used computer randomisation and one which used a table of random numbers to generate the allocation sequence for the patients. One trial based the randomisation sequence on hospital record number and we judged it to be at high risk of bias, while the remaining nine trials presented insufficient information to assess the adequacy of sequence generation and we rated them as unclear.
Allocation
We judged the risk of bias for this item to be low for four trials which used central allocation. We rated 13 trials as unclear; seven used sealed envelopes, however, it was not clear if they were used with appropriate safeguards (e.g. sequentially numbered) to adequately conceal allocation. The other six rated as unclear did not present any information regarding allocation concealment. We rated two trials as being at high risk of bias for this domain.
Blinding
The nature of the intervention means that blinding of clinicians involved in the care and administration of blood transfusions would not have been feasible. The extent to which this could have biased the results is unclear, thus we have rated none of the studies as being at low risk of bias for this domain. However, blind outcome assessment was reported as being used in eight trials.
Incomplete outcome data
We rated 14 trials as being at low risk of bias for this domain as they either had no missing data or performed intention-to-treat analyses. A small number of exclusions were reported in the remaining five trials, although the extent to which this may have introduced bias is uncertain, thus we rated these trials as unclear.
Selective reporting
We could not find any evidence of reporting bias. Although we did not have access to the trial protocols for the majority of trials, the results for the primary and secondary outcomes, as described in the methods sections of each trial, were clearly and concisely reported. Trial protocols were available for the trials with which the authors had some degree of involvement (Carson 1998; Carson 2011; Hebert 1995; Hebert 1999; Lacroix 2007).
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
Eighteen of the 19 trials presented data suitable for inclusion in the meta-analyses. One trial evaluated changes in brain tissue oxygen and other metabolic parameters (Zygun 2009).
Despite the heterogeneity in the methods and transfusion triggers reported in these randomised trials, it was possible to aggregate data from the following outcomes: exposure to red cell transfusion, exposure to red cell transfusion (allogeneic), average volume of red cells transfused in all patients, average volume of red cells transfused in transfused patients, haemoglobin concentration, cardiac events, myocardial infarction, pulmonary oedema, cerebrovascular accident, pneumonia, infection, mortality at 30 days, hospital mortality, overall length of hospital stay and intensive care unit length of hospital stay.
Proportion of patients transfused
Data on the frequency of transfusions were available from 17 trials. On average, the implementation of a restrictive transfusion trigger reduced the risk of receiving a red cell transfusion by a relative 39% (risk ratio (RR) 0.61, 95% confidence interval (CI) 0.52 to 0.72). Heterogeneity between these trials was statistically significant (Chi2 = 238.95, df = 16 (P < 0.00001); I2 = 93%).
The funnel plot displays an absence of smaller studies showing patients at elevated risk of receiving a transfusion with more restrictive transfusion triggers.
Quantity of red cells transfused
The quantities of blood transfused were reported in eight trials. The use of a restrictive transfusion trigger resulted in an average saving of 1.19 units of red cells per transfused patient (mean difference (MD) −1.19, 95% CI −1.85 to −0.53). Heterogeneity between these trials was statistically significant (Chi2 = 84.46, df = 7 (P < 0.00001); I2 = 92%). One trial transfused autologous blood (Lotke 1999).
Haemoglobin or haematocrit concentration
Postoperative haemoglobin or haematocrit levels were reported in 12 trials. In five trials the timing of measurement varied (the average measured over a number of days after hospitalisation (or operation)). In five trials a single value prior to discharge was recorded and in one trial a single value after the first transfusion was recorded. When we pooled data (without regard to timing, which was consistent within studies) patients assigned to a restrictive strategy had haemoglobin concentration on average 1.5% lower than patients assigned to a liberal transfusion strategy (MD −1.48, 95% CI −1.92 to −1.03). Heterogeneity between these trials was statistically significant (Chi2 = 752.16, df = 11 (P < 0.00001); I2 = 99%).
Mortality
Thirty-day mortality data were reported for 11 trials. There was no statistically significant difference in 30-day mortality between restrictive and liberal transfusion strategies (RR 0.85, 95% CI 0.70 to 1.03). Heterogeneity between these trials was not statistically significant (Chi2 = 5.90, df = 9 (P = 0.75); I2 = 0%). It should be noted that one study of patients in intensive care (Hebert 1999) contributed 52.0% and the study in patients with hip fracture (Carson 2011) contributed 23.4% of the weight in the meta-analysis of this outcome. Hospital mortality was 23% lower in patients in the restrictive compared to the liberal transfusion strategy (RR 0.77, 95% CI 0.62 to 0.95). Heterogeneity between the trials was not significant (Chi2 = 1.35, df = 3 (P = 0.72); I2 = 0%).
Hospital length of stay
Eight trials reported data for length of hospital stay. These data indicated that the reduction in red blood cell transfusion was not associated with a prolongation of hospital stay (MD 0.11 days, 95% CI −0.16 to 0.38 days). Heterogeneity between these trials was not statistically significant (Chi2 = 6.28, df = 7 (P = 0.51); I2 = 0%). It is unclear if length of hospital stay included only patients who survived.
Functional recovery
Two trials reported functional outcomes in hip fracture patients (Carson 2011; Foss 2009). The functional measures were different in the trials and could not be combined. Death or inability to walk at 30 days (RR 1.05, 95% CI 0.95 to 1.15) or 60 days (RR 0.99, 95% CI 0.88 to 1.11) was not significant between transfusion strategies. No other measures of function were significantly different between transfusion strategies.
Cardiac events
Seven trials reported data on cardiac events. The rates of cardiac events (myocardial infarction, cardiac arrhythmias, cardiac arrest, pulmonary oedema and angina) were not increased significantly by the use of restrictive transfusion strategies (RR 0.96, 95% CI 0.70 to 1.32). Heterogeneity between these trials was statistically significant (Chi2= 16.87, df = 6 (P = 0.010); I2 = 64%). It is likely that patients were counted in more than one category of this composite outcome.
Myocardial infarction
Eight trials reported data on myocardial infarction (fatal and non-fatal). The use of a restrictive transfusion threshold did not appear to impact adversely on the rates of myocardial infarction (RR 0.88, 95% CI 0.38 to 2.04). There was no statistical heterogeneity between trials (Chi2= 10.42, df = 7 (P = 0.17); I2 = 33%).
Pulmonary oedema
Five trials reported data for pulmonary oedema. There was no significant difference between the restrictive and liberal transfusion strategies (RR 0.72, 95% CI 0.31 to 1.70). Heterogeneity between the trials was significant (Chi2 = 11.40, df = 4 (P = 0.02); I2 = 65%).
Cerebrovascular accident - stroke
Five trials reported on stroke. There was no significant difference between transfusion strategies (RR 0.84, 95% CI 0.47 to 1.49). Heterogeneity between the trials was not significant (Chi2 = 2.61, df = 4 (P = 0.63); I2 = 0%).
Pneumonia
Five trials reported data for pneumonia. In contrast to overall infections; there was no significant difference between transfusion strategies (RR 0.93, 95% CI 0.76 to 1.15). Heterogeneity between these trials was not statistically significant (Chi2= 2.51, df = 4 (P = 0.64); I2 = 0%).
Infections
Six trials reported data for infections. The rate of infections was decreased by 19% with the use of restrictive transfusion strategies although the results are not significant (RR 0.81, 95% CI 0.66 to 1.00). Heterogeneity between these trials was not statistically significant (Chi2 = 5.70, df = 5 (P = 0.34); I2 = 12%).
Other outcomes
A number of other potentially relevant clinical outcomes were reported in individual trials, including thromboembolism, multi-organ failure, mental confusion and delayed wound healing. Although there were no statistically significant differences between restrictive and liberal transfusion strategies for any of these outcomes, the overall event rates were low. Interestingly, one trial (Blair 1986) reported a decreased risk of re-bleeding in patients randomised to a restrictive transfusion strategy compared to patients randomised to a liberal transfusion strategy (RR 0.10, 95% CI 0.01 to 0.75). Where reported, heart rates, cardiac index and systemic vascular resistance also appeared to be unaffected (Bush 1997; Johnson 1992).
Sensitivity analyses
We performed a post hoc sensitivity analysis to explore the effects of the inclusion of data from the Webert 2008 trial in the pooled analyses. Webert 2008 explored whether a higher transfusion threshold would be beneficial for patients with acute leukaemia, unlike the other included studies which investigated the safety of a lower transfusion threshold. When we excluded data from Webert 2008 from the pooled analysis of blood transfusion exposure, the risk ratio was reduced slightly from 0.63 (95% CI 0.54 to 0.74) to 0.61 (95% CI 0.53 to 0.71). Heterogeneity between these trials remained statistically significant (Chi2 = 96.82, df = 13, P < 0.00001; I2 = 87%).
DISCUSSION
We identified 19 randomised controlled trials evaluating different red cell transfusion triggers carried out over a 55-year time period. These trials enrolled 6264 patients from divergent patient populations. The results of the meta-analyses indicated that, on average, restrictive transfusion strategies were associated with a reduction of more than one-third in the number of patients receiving blood, a red cell transfusion requirement that was approximately one unit lower, and a haemoglobin concentration (average postoperative) that was around 1.5 g/dL lower than in the liberal transfusion group. However, such results need tempering against the significant heterogeneity of the trials assessed.
Sources of heterogeneity
For the primary outcome (the number of patients exposed to blood transfusion) we observed substantial heterogeneity. The variation was in terms of the size (but not the direction) of the treatment effect. The individual trials (with five exceptions: Bush 1997; Grover 2005; So-Osman 2010; Topley 1956; Webert 2008) found that a restrictive transfusion trigger statistically significantly reduced the probability of receiving a red cell transfusion, with the risk ratio estimates ranging from 0.21 to 0.96. However, some confidence intervals were non-overlapping. Heterogeneity might have been anticipated, as the clinical settings and the transfusion triggers differed between trials. In addition, the primary outcome in the meta-analysis, the decision to transfuse, is a practice variable and involves a degree of subjectivity. It cannot be argued that the treatment effect varied according to the rate of red cell transfusion in the control groups, as most patients (84%) in the liberal transfusion groups received red cell transfusions.
The level of the transfusion trigger between trials does not seem to account for the variation in treatment effect size; the relative risk appeared unrelated to it. However, the degree of difference within trials, between the transfusion triggers of the intervention and control groups, may account for some of the variation observed in the treatment effect size. The effect estimates for trials comparing well-defined transfusion rates that differed by 2.0 g/dL tended to be larger than the estimates for trials comparing thresholds differing by less than 2.0 g/dL. Although these apparent ‘associations’ may also be due to the play of chance, such observations warrant further discussion.
Two trials (Blair 1986; Lotke 1999) showed greater benefit (in favour of restrictive transfusion strategies) in reducing exposure to red cell transfusion, than any of the other trials. These two trials appeared to add considerably to the observed heterogeneity. In Blair 1986 the control group were routinely transfused (as dictated by the trial protocol) at least two units of blood within 24 hours of hospital admission, regardless of their Hb level and clinical state, whereas the intervention group were only transfused blood when their Hb concentration fell below 8.0 g/dL or they displayed signs of shock. For this trial (Blair 1986) the transfusion exposure rate for the intervention group was 19% compared to 100% for the control group. For the trial conducted by Lotke 1999 the control group received all of their preoperatively donated autologous (PAD) blood (2 units/patient) immediately after surgery (as dictated by the trial protocol) whereas the patients in the intervention group were not transfused their PAD blood unless their Hb concentration fell to less than 9.0 g/dL. For this trial (Lotke 1999) the transfusion exposure rate for the intervention group was 26% compared to 100% in the control group.
Five trials (Bush 1997; Grover 2005; So-Osman 2010; Topley 1956; Webert 2008) failed to show a statistically significant reduction in red cell transfusion rates. For Bush 1997 and Webert 2008 protocol violations may have impacted significantly on the rates of transfusion in the intervention groups. In Bush 1997, patients randomised to the intervention group were to be transfused allogeneic red cells, and in some instances autologous red cells, when their Hb concentration fell below 9.0 g/dL; the control group were transfused when their Hb concentration fell below 10.0 g/dL. The authors of Bush 1997 conceded that not all the patients randomised to the restrictive transfusion strategy reached the transfusion threshold level of Hb < 9.0 g/dL because they either had minimal intra-operative blood loss or were excessively transfused by the anaesthetists or surgeons. The later may account for the relatively small difference in transfusion rates between the intervention and control groups (88% versus 80%, respectively). In Webert 2008, patients were allocated to receive RBC transfusion when their Hb level fell below 8.0 g/dL in the intervention group or 12.0 g/dL in the control. The trial authors note that a number of patients received transfusion before their assigned threshold had been reached; compliance with the assigned threshold was achieved only 64% of the time in the intervention and 70% of the time in the control group. This also may explain the similar transfusion rates observed in the two groups (90% and 94% for the restrictive and liberal groups, respectively). The trial by So-Osman 2010 compared a new age-dependent restrictive transfusion policy with the standard policy used in the three participating hospitals. Deviation from the assigned trigger was not found to be a problem, however differences in the transfusion threshold forming the standard policy of the hospitals may explain the lack of difference observed in transfusion rates (36% and 39% for intervention and control, respectively). The trial by Topley 1956 was designed so that one group of patients (‘under-transfused’ group) would have a red cell volume (RCV) of 70% to 80% of normal at the end of resuscitation, whilst the control group (‘adequately transfused’ group) would have a RCV of 100% of normal or over at the end of resuscitation. However, as reported, in practice these objectives were achieved with an accuracy of only ± 20%.
There is no evidence to suggest that clinical setting or adequacy of allocation concealment explains the variability in the effect estimates.
Adverse events and other outcomes
None of the outcomes evaluated, including mortality, cardiac morbidity, infections and length of hospital stay, appear to be adversely affected by the lower use of red cell transfusions. In contrast, the evidence raises the possibility of harm associated with liberal transfusion. In-hospital mortality and infections were 23% and 19% higher in patients receiving liberal transfusion, respectively. However, these findings should be interpreted cautiously. Mortality was not significantly different for the other time periods examined. The infection results were borderline significant and the risk of pneumonia was not elevated. Although very little heterogeneity was seen for the outcome variable mortality, the meta-analysis was dominated by two trials (Carson 2011; Hebert 1999) that contributed 75% of the statistical information.
These data are quite informative and support the recent move to more restrictive transfusion practices. Trials have now been performed in several settings where transfusion is widely used. Trials in adult and paediatric intensive care unit (ICU) patients confirm the safety of a 7.0 g/dL threshold in patients with severe acute illness. One trial in elderly hip fracture patients undergoing surgery and with extensive co-morbidity including underlying cardiovascular disease suggests that restrictive transfusion to 8.0 g/dL is safe as well. This trial is also the first to demonstrate that liberal transfusion does not improve functional recovery.
Sources of bias
We performed extensive searches in an attempt to identify all eligible trials irrespective of publication status. Despite these efforts, inspection of the funnel plot (Figure 1) raises the possibility of publication bias or other small study biases affecting the exposure to blood transfusion outcome.
Our analyses demonstrate that only two trials in adults (Carson 2011; Hebert 1999) were adequately powered to evaluate the impact of different transfusion strategies on mortality, morbidity and function. Carson 2011 was the largest trial performed and included 2016 patients undergoing surgical repair for hip fracture. Hebert 1999 was the next largest study, involving 838 intensive care patients. Given this, the meta-analysis of mortality is dominated by studies in elderly surgical and intensive care patients and therefore it is uncertain if the results can be applied to other clinical settings. In paediatric intensive care unit patients there was no difference in new or progressive multiple-organ dysfunction syndrome (Lacroix 2007).
Several important clinical outcomes have not been adequately evaluated in the trials published to date. The studies evaluating myocardial infarction are too small to detect moderate differences and infection results are inconsistent. Observational data suggest that higher blood counts may be associated with less postoperative delirium (Weiskopf 2000).
This systematic review found new evidence of the safety of restrictive transfusion triggers in important subsets of patients with underlying cardiovascular disease (Carson 2011). Overall, the rates of cardiac events in this meta-analysis were not increased by the use of restrictive transfusion triggers; the trial was too small to detect small to moderate effects. However, evidence is still lacking in other important subsets of patients, including those with acute cardiovascular disease, renal failure and haematological disorders. Some guidelines recommend transfusion for symptoms or haemodynamic instability, rather than for a specific trigger haemoglobin level (AAGBI 2008; ASA 2006; ASBT 2001; Napolitano 2009; NBUGI 2001). This approach to transfusion was tested in a pilot study involving 84 patients (Carson 1998) and in a trial involving 2016 patients (Carson 2011) in which patients could be transfused with symptoms (cardiac chest pain, congestive heart failure and orthostatic hypotension or tachycardia unresponsive to adequate fluid challenge) or haemoglobin concentration less than 8.0 g/dL. These studies found no difference in functional recovery, mortality or morbidity in patients in the restrictive (symptomatic) transfusion group.
The results of these trials need to be viewed against six large observational studies that compared clinical outcomes at varying haemoglobin levels in transfused and non-transfused patients, and found conflicting results. In a study of 2202 patients undergoing coronary bypass surgery, the liberal transfusion group had a higher incidence of myocardial infarction than the conservative transfusion group (Spiess 1998). In a study of 8787 hip fracture patients, there was no difference in short or long-term mortality between patients transfused and not transfused down to a post-operative haemoglobin of 8.0 g/dL (Carson 1998). In a study of 4470 ICU patients, mortality was reduced in patients receiving transfusion of up to six units of blood (Hebert 1997). A retrospective study of 78,974 Medicare beneficiaries (Wu 2001) found that blood transfusion was associated with a lower short-term mortality rate among elderly patients with acute myocardial infarction if the haematocrit on admission was 30% or lower and that blood transfusion may be effective with a haematocrit as high as 33% on admission. A study of 310,311 patients 65 years or older who underwent major non-cardiac surgery found a 1.6% increase in 30-day postoperative mortality for each 1% decrease in preoperative haematocrit (Wu 2007). A study of 239,286 patients 65 years or older who underwent major non-cardiac surgery found intraoperative blood transfusion was associated with a reduction in mortality in patients with preoperative haematocrit levels of < 24% or in those with blood loss > 500 cc (Wu 2010). The main limitation of these observational studies is that there may be residual confounding by indication, despite the extensive statistical adjustment of the results. It is possible that differences in patient characteristics between transfused and non-transfused patients may not be identified, or adequately adjusted for. This point is emphasised by the fact that a randomised controlled trial (Hebert 1999) and an observational study (Hebert 1997) in intensive care patients, performed by the same group of investigators, came to opposite conclusions. Despite recent assertions to the contrary (Benson 2000; Concato 2000), we believe that adequately powered, rigorously performed, randomised clinical trials are the only way of overcoming these limitations.
A study presented at the Cochrane Colloquium in Lyon, France (9-13 October 2001) (Henry 2001a) highlighted the significant discrepancies in the results reported by randomised controlled trials compared to those reported by observational studies. This and other studies (Ioannidis 2001) have shown that disagreements in the magnitude of treatment effect between randomised controlled trials (RCTs) and observational studies are common. The authors of Henry 2001a analysed the data from studies of various interventions including: preoperative autologous donation (PAD), acute normovolemic haemodilution, cell salvage, laparoscopic cholecystectomy, hormone replacement therapy and antioxidant therapy. For PAD alone, the observational studies’ (n = 41) estimate of treatment effect (risk ratio) for the number of patients exposed to allogeneic blood transfusion was 0.30 (95% confidence interval (CI) 0.26 to 0.35) compared to 0.39 (95% CI 0.27 to 0.57) for the RCTs (n = 7). For this intervention (PAD) there appears to be reasonable agreement between the results of the observational studies and the randomised controlled trials. However, the observational studies have appeared to over-estimate the magnitude of the treatment effect. Observational studies of the other interventions have tended to under-estimate the magnitude of treatment effect. Although the results obtained from well-conducted observational studies are extremely valuable, making inferences from observational data sets is problematic, as the sources of error and bias that afflict observational studies do not afflict randomised trials (Henry 2001a).
Conducting randomised clinical trials, where one intervention is a clinical policy regarding red cell transfusion, is demanding. Masking (blinding) the use of transfusion at the bedside is difficult to achieve unless study personnel are assigned to each patient, an expensive procedure. Outcomes that are determined by observers who are blind to the treatment group is probably the most rigorous approach that is practical. This approach was reported in only eight of the trials reviewed here (Carson 1998; Carson 2011; Foss 2009; Grover 2005; Hajjar 2010; Johnson 1992; Lotke 1999; Webert 2008). Maintaining the integrity of the randomisation process becomes important if the trial is not to over-estimate the benefit of the intervention (Schulz 1995). Some studies in this review did not report the methods used to conceal the allocation sequence from the treating clinicians. Four trials (Carson 1998; Carson 2011; Lacroix 2007; Webert 2008) used a centralised allocation and four others (Bush 1997; Foss 2009; Hebert 1999; So-Osman 2010) used randomisation codes in sealed envelopes. The latter method has the potential to be unmasked, leading to the potential for selection bias in the inclusion of patients in the trials (Schulz 1995).
The transfusion policies reviewed here represent fairly small modifications to routine clinical practice. They are consistent with the recommendations of published clinical practice guidelines (AAGBI 2008; ASA 2006; ASBT 2001; BCTMAG 2003; Napolitano 2009; NBUGI 2001). The transfusion triggers (in terms of haemoglobin levels) were most often in the range of 8.0 to 9.0 g/dL, although values as low as 7.0 g/dL were assessed. In fact, the ‘restrictive’ transfusion triggers in some trials were equivalent to the ‘liberal triggers’ used in other trials. Nevertheless, the trials documented significant reductions in the rates of red cell transfusion and worthwhile blood conservation. These effects are similar to what has been documented in meta-analyses of trials of blood sparing techniques, such as cell salvage and anti-fibrinolytic drugs (Carless 2010; Henry 2011b). Adoption of a conservative transfusion threshold appears to be as effective as these technologies in avoiding the need for transfusion and is likely to cost less. In summary, a restrictive transfusion trigger reduces the risk of exposure to red blood cell transfusion and the total number of units transfused. The currently published evidence suggests that restrictive transfusion triggers do not adversely affect mortality, cardiac morbidity, function or length of hospital stay. For the present we recommend the use of a restrictive transfusion trigger, but suggest using caution in patients from high-risk groups such as acute coronary syndrome as there is currently no evidence from randomised controlled trials to guide treatment. In countries where there are serious doubts about the safety of donated blood, because of inadequate testing for viral pathogens, the existing data may constitute a stronger basis for avoiding red cell transfusion in many clinical settings.
AUTHORS’ CONCLUSIONS
Implications for practice
In patients who do not have acute coronary artery disease, blood transfusion can probably be withheld in the presence of haemoglobin levels as low as 7.0 g/dL to 8.0 g/dL as long as there is no notable bleeding. The benefits of minimising allogeneic red cell transfusion are likely to be greatest where there is doubt about the safety of the blood supply.
Implications for research
Future trials of transfusion ‘triggers’ should include patients with acute coronary syndrome, elderly patients recovering from acute illness, patients with gastrointestinal bleeding, coagulopathy or haemorrhagic shock, and patients with traumatic brain injury. Trials are also needed that evaluate lower haemoglobin concentrations such as 6.0 g/dL. Trials should be large enough to measure the impact that lower thresholds have on clinical outcomes.
PLAIN LANGUAGE SUMMARY.
Restricting the use of blood transfusion
Many people are given a transfusion of blood from an unrelated donor as part of their medical treatment. There are, however, risks involved. In particular, infections (including HIV and certain types of hepatitis) may be passed on to the person receiving the blood. This risk is very small in high-income countries but much larger in poor countries which do not test the blood for infections. Because of the risks, doctors try to avoid giving blood unless it is really necessary. One approach is to give the transfusion only if the amount of haemoglobin in the patient’s blood has dropped below a certain ‘threshold’ level. We looked for controlled studies comparing the effectiveness of giving more versus less blood. We found 19 studies, with a total of 6264 patients. We conclude that, for most patients, giving less blood is safe and blood transfusion is probably not essential until haemoglobin levels drop below 7.0 to 8.0 grams per decilitre. As no trials have been done involving patients with an acute heart problem, it is not currently known how much blood to give these patients.
ACKNOWLEDGEMENTS
We acknowledge the contribution of Suzanne Hill (World Health Organization), the first author of the original version and the 2004 update of the review. We also acknowledge the contribution of Kim Henderson in the original review first published in 2000. We also acknowledge David Henry (Institute of Clinical Evaluative Sciences) and Brian McClelland who co-wrote reviews up to 2010; Katharine Ker (London School of Hygiene & Tropical Medicine) undertook the following tasks for the 2010 update: screened search output, obtained articles, applied inclusion/exclusion criteria to retrieved papers, assessed risk of bias, extracted data, performed data analysis and revised the text of the review. We thank Karen Blackhall (Injuries Group Trials Search Co-ordinator) who ran the electronic database searches in 2009 and 2011.
SOURCES OF SUPPORT
Internal sources
- No sources of support supplied
External sources
- NSW Ministerial Advisory Committee on Quality in Health Care, Australia.
- NSW Health Department, Australia.
Appendix 1. Search strategy
Cochrane Injuries Group’s Specialised Register (searched 1 February 2011)
(Blood or “Red blood cell” or “Red blood cells” or RBC) and (therap* or transfus*) and (polic* or practice or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard* or restrict* or liberal* or management or program*)
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1)
- #1
MeSH descriptor Blood Transfusion, this term only with qualifiers: MT,ST
- #2
transfus* near5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)
- #3
(Red blood cell* or RBC) near5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*) and (therap* or transfus*)
- #4
(H?emoglobin or h?emocrit or HB or HCT) near5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)
- #5
transfus* near5 (restrict* or liberal*)
- #6
(blood transfus*) near3 (management or program*)
- #7
(#1 OR #2 OR #3 OR #4 OR #5 OR #6)
MEDLINE (Ovid) 1948 to January Week 3 2011
- *Blood Transfusion/
- ((Red blood cell* or RBC) adj3 (therap* or transfus*)).mp.
- 1 or 2
- exp Reference Standards/
- standards.fs.
- methods.fs.
- 4 or 5 or 6
- 3 and 7
- (transfus* adj5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)).mp.
- ((Red blood cell* or RBC) adj5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)).mp.
- ((H?emoglobin or h?emocrit or HB or HCT) adj5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)).mp.
- (transfus* adj5 (restrict* or liberal*)).mp.
- ((blood or transfus*) adj3 (management or program*)).mp.
- 8 or 9 or 10 or 11 or 12 or 13
- randomi?ed.ab,ti.
- randomized controlled trial.pt.
- controlled clinical trial.pt.
- placebo.ab.
- clinical trials as topic.sh.
- randomly.ab.
- trial.ti.
- 15 or 16 or 17 or 18 or 19 or 20 or 21
- (animals not (humans and animals)).sh.
- 22 not 23
- 24 and 14
EMBASE (Ovid) 1980 to 2011 Week 04
- *Blood Transfusion/
- ((Red blood cell* or RBC) adj3 (therap* or transfus*)).mp.
- 1 or 2
- exp standard/
- 3 and 4
- (transfus* adj5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)).mp.
- ((Red blood cell* or RBC) adj5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)).mp.
- ((H?emoglobin or h?emocrit or HB or HCT) adj5 (polic*or practic* or protocol* or trigger* or threshold*or indicator* or strateg* or criteri* or standard*)).mp.
- (transfus* adj5 (restrict* or liberal*)).mp.
- ((blood or transfus*) adj3 (management or program*)).mp.
- 5 or 6 or 7 or 8 or 9 or 10
- exp Randomized Controlled Trial/
- exp controlled clinical trial/
- randomi?ed.ab,ti.
- placebo.ab.
- *Clinical Trial/
- randomly.ab.
- trial.ti.
- 12 or 13 or 14 or 15 or 16 or 17 or 18
- exp animal/not (exp human/and exp animal/)
- 19 not 20
- 11 and 21
ISI Web of Science: Science Citation Index Expanded (SCI-EXPANDED) (1970 to February 2011) and ISI Web of Science: Conference Proceedings Citation Index - Science (CPCI-S) (1990 to February 2011)
- #1
TS=((Blood or “Red blood cell” or “Red blood cells” or RBC or Hemoglobin* or haemoglobin* or haemocrit or hemocrit or HB or HCT) SAME transfus*)
- #2
TS=(polic* or practice or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard* or restrict* or liberal* or management or program*)
- #3
#1 and #2
- #4
TS=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial) OR Topic=(controlled clinical trial OR controlled trial OR clinical trial OR placebo)
- #5
TS=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*))
- #6
#2 or #3
- #7
#3 and #6
- #8
Topic=(human*)
- #9
#7 and #8
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 50 consecutive patients with severe upper gastrointestinal haemorrhage were randomised to 1 of 2 groups: Liberal group: n = 24; mean (SD) age = 64 (17.6) years Restrictive group: n = 26; mean (SD) age = 60 (17.8) years | |
| Interventions | Liberal group received at least 2 units of red blood cells immediately at admission and during their first 24 hours in hospital. Restrictive group were not transfused red blood cells unless the Hb was less than 8.0 g/dL or shock persisted after initial resuscitation with Haemaccel | |
| Outcomes | Outcomes reported: blood usage (units), re-bleeding, mortality, clotting times, Hct on admission/discharge, kaolin cephalin clotting time after 24 hours, impedance clotting time after 24 hours | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information reported |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 428 consecutive patients undergoing elective primary coronary artery bypass graft surgery were randomly assigned to 1 of 2 groups: Liberal group: n = 212; M/F = 82/18; mean (SD) age = 61 (11) years Restrictive group: n = 216; M/F = 83/17; mean (SD) age = 62 (11) years | |
| Interventions | Liberal group received transfusions on the instructions of their individual physicians, who considered the clinical assessment of the patient and the institutional guidelines, which propose a Hb level < 9.0 g/dL as the postoperative threshold for RBC transfusion Restrictive group received a RBC transfusion in the postoperative period at a Hb level < 8.0 g/dL | |
| Outcomes | Outcomes reported: mortality, length of hospital stay, blood usage (units), blood loss, complications, infection rates, cardiac events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randomly assigned on the basis of the last digit of their medical record number |
| Allocation concealment (selection bias) | High risk | Inadequately concealed (record number) |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Intention-to-treat analysis used. A small number of exclusions were reported |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 99 patients undergoing elective aortic or infrainguinal arterial reconstruction were randomised to 1 of 2 groups: Liberal group: n = 49; M/F = 41/8; mean (SD) age = 64 (11) years Restrictive group: n = 50; M/F = 32/18; mean (SD) age = 66 (10) years | |
| Interventions | Liberal group had their Hb concentrations maintained at or above 10.0 g/dL Restrictive group were transfused only when their Hb concentration fell below 9. 0 g/dL | |
| Outcomes | Outcomes reported: 30-day mortality, length of ICU stay, length of hospital stay, blood use (units), postoperative blood loss, cardiac events, Hct/Hb on admission | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were chosen at random for patient assignment |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Both surgeons and anaesthesiologists were informed as to the group of randomisation |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Appears to be complete |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 84 hip fracture patients undergoing surgical repair who had postoperative Hb levels < 10.0 g/dL were randomly assigned to 1 of 2 groups: Liberal group: n = 42; M/F = 9/33; mean (SD) age = 81.3 (8.1) years Restrictive group: n = 42; M/F = 11/31; mean (SD) age = 83.3 (10.8) years | |
| Interventions | Liberal group received 1 unit of packed RBC at the time of random assignment and as much blood as necessary to keep the Hb level above 10.0 g/dL Restrictive group received a RBC transfusion for symptoms of anaemia or for a Hb level that dropped below 8.0 g/dL | |
| Outcomes | Outcomes reported: mortality, length of hospital stay, blood usage (units), complications, pneumonia, stroke, thromboembolism | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules were stratified by clinical site and cardiovascular disease state. The randomisation was designed in blocks of 2 to 8 patients to avoid imbalance within a site |
| Allocation concealment (selection bias) | Low risk | Study personnel at the clinical sites randomly assigned patients by contacting the data co-ordinating centre’s 24-hour automated telephone service |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Blinding of observers |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Intention-to-treat analysis used |
| Selective reporting (reporting bias) | Low risk | - |
| Other bias | Low risk | - |
| Methods | Randomised, unblinded, parallel, 2-group multicentre trial | |
|---|---|---|
| Participants | Patients 50 years or older, who are undergoing surgical repair of a hip fracture, with Hb concentrations below 10.0 g/dL within 3 days after surgery and who have clinical evidence for cardiovascular disease or cardiovascular risk factors Sample size = 2016 | |
| Interventions | Liberal group - receive packed RBC when haemoglobin level dropped below 10.0 g/dL Restrictive (’symptomatic strategy’) group - receive transfusion if develop symptoms of anaemia or if Hb falls below 8.0 g/dL | |
| Outcomes | Primary outcome is inability to walk 10 feet (or across a room) without human assistance or death prior to closure of the window for the 60-day, 30 and 60-day mortality. Other outcomes are Hb concentration, acute coronary syndrome (ACS), in-hospital myocardial infarction, unstable angina or death, disposition on discharge, survival, functional measures, fatigue/energy, readmission to hospital, pneumonia, wound infection, thromboembolism, stroke or transient ischaemic attack | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Data Co-ordinating Center staff prepared randomisation schedules for each site using randomly ordered block sizes of 2, 4, 6 or 8 |
| Allocation concealment (selection bias) | Low risk | Used an automated telephone randomisation system |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | After random allocation, clinical site staff, clinicians and patients were not blinded to treatment assignment. Primary and secondary outcomes were assessed blinded to treatment assignment |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Intention-to-treat analysis |
| Selective reporting (reporting bias) | Low risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 214 patients with acute gastrointestinal bleeding and cirrhosis were randomly allocated to 1 of 2 groups: Liberal group: n = 105 Restrictive group: n = 109 NB: no demographic information was presented, although stated that baseline characteristics were similar in the 2 groups | |
| Interventions | Liberal group received packed RBC when Hb level dropped below 9.0 g/dL (to maintain Hb concentration at 9.0 to 10.0 g/dL) Restrictive group received packed RBC when Hb level dropped below 7.0 g/dL (to maintain Hb concentration at 7.0 to 8.0 g/dL) | |
| Outcomes | Outcomes reported: mortality, therapeutic failures, transfusion, Hb concentration, side effects | |
| Notes | Conference abstract | |
| Risk Of Bias | ||
| Bias | Author’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias)All outcomes | Unclear risk | Insufficient information presented to permit judgement of ’Yes’ or ’No’ |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 25 patients were studied prospectively following acute injury and haemorrhage. These patients were randomised to 1 of 2 groups: Liberal group: n = 13; mean age = 46.9 years Restrictive group: n = 12; mean age = 46.5 years | |
| Interventions | Liberal group had their Hct brought up to 40% slowly over a period of several hours by the infusion of packed red cells Restrictive group had their Hct maintained close to 30% by the appropriate administration of packed red cells NB: all patients had sustained a Class III or Class IV haemorrhage and had clinical signs of shock (systolic blood pressure < 90 torr, heart rate >100 bpm or urine output < 20 ml/hr) before entry into the study. Patients were resuscitated according to the clinical protocol of the centre first using crystalloid to re-establish organ perfusion and haemodynamic stability and then giving sufficient packed red cells to achieve a Hct close to 30%. Patients were studied twice a day for 3 days after the period of haemorrhagic shock | |
| Outcomes | Outcomes reported: RBC consumption (units), cardiopulmonary parameters: pulmonary capillary wedge pressure (PCWP), intrapulmonary shunt, tissue oxygenation/perfusion, oxygen consumption/delivery, arterial and venous O2 saturations, arterial and venous O2 contents, cardiac index (CI), heart rate, systemic vascular resistance, left ventricular stroke work index | |
| Notes | ||
| Risk Of Bias | ||
| Bias | Author’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Appears to have been complete |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | ||
|---|---|---|
| Participants | 120 hip fracture patients were randomly allocated to 1 of 2 groups: Liberal group: n = 60; M/F = 14/46; mean (SD) age = 81 (6.8) years Restrictive group: n = 60; M/F = 14/46; mean (SD) age = 81 (7.3) years | |
| Interventions | Liberal group received packed RBC when Hb level dropped below 10.0 g/dL Restrictive group received packed RBC when Hb level dropped below 8.0 g/dL | |
| Outcomes | Outcomes reported: ambulatory capacity, mortality, length of stay, cardiac complications, infectious complications | |
| Notes | ||
| Risk Of Bias | ||
| Bias | Author’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated list |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Reported as being double-blind |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Intention-to-treat analysis used |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 260 patients undergoing elective lower limb joint replacement surgery were randomly allocated to 1 of 2 groups: Liberal group: n = 109; M/F = 55/54; mean (SD) age = 71.5 (7.6) years Restrictive group: n = 109; M/F = 48/61; mean (SD) age = 70.7 (7.1) years | |
| Interventions | Liberal group received packed RBC when Hb level dropped below 10.0 g/dL, and Hb concentration maintained between 10.0 to 12.0 g/dL Restrictive group received packed RBC when Hb level dropped below 8.0 g/dL and Hb concentration maintained between 8.0 to 9.5 g/dL | |
| Outcomes | Outcomes reported: ischaemic load, blood load, Hb concentration, number of units transfused, length of hospital stay, adverse events, new infections requiring antibiotic therapy | |
| Notes | ||
| Risk Of Bias | ||
| Bias | Author’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Anaesthetists and surgical team responsible for treatment were aware of allocation. Outcome assessment was blind |
| Incomplete outcome data (attrition bias)All outcomes | Unclear risk | Of a recruited 260 patients, outcome data presented for 218. Missing 42 did not have analysable tape recordings |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised clinical trial | |
|---|---|---|
| Participants | 502 adult patients who underwent cardiac surgery with cardiopulmonary bypass Liberal group: n = 257; M/F = 161/92; mean (SD) age 60.7 (12.5) years Restrictive group: n = 255; M/F = 149/100; mean (SD) age 58.6 (12.5) | |
| Interventions | Liberal group were transfused RBC if the haematocrit was less than 30% at any time from the start of surgery until discharge from the ICU Restrictive group were transfused if haematocrit values were less than 24% | |
| Outcomes | Outcomes reported: primary outcome composite endpoint that included 30-day all-cause mortality and severe morbidity (cardiogenic shock, ARDS or acute renal injury requiring dialysis or haemofiltration. Respiratory, cardiac, neurologic and infectious complications; inflammatory complications; bleeding; ICU and hospital lengths of stay, RBC transfusions.) | |
| Notes | ||
| Risk Of Bias | ||
| Bias | Author’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random-number table prepared by chief statistician |
| Allocation concealment (selection bias) | Unclear risk | Opaque envelopes |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Patient and outcome assessors were blinded; clinicians were not blinded |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Intention-to-treat. Complete follow-up. |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 69 normovolaemic critically ill patients admitted to 1 of 5 tertiary level intensive care units with Hb values < 9.0 g/dL within 72 hours of admission were randomly assigned to 1 of 2 groups: Liberal group: n = 36; M/F = 19/17; mean (SD) age = 59 (21) years Restrictive group: n = 33; M/F = 14/19; mean (SD) age = 58 (15) years | |
| Interventions | Liberal group were transfused RBC if the Hb level fell to between 10.0 to 10.5 g/dL. Hb level maintained between 10.0 to 12.0 g/dL. Restrictive group were transfused RBC if the Hb level fell to between 7.0 to 7.5 g/dL. Hb level was maintained between 7.0 to 9.0 g/dL. | |
| Outcomes | Outcomes reported: mortality, length of hospital stay, length of ICU stay, blood usage (units), complications, Hb levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned to 1 of 2 groups by consecutive allocation from a random listing stratified by centre and disease severity |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection biasAll outcomes | Unclear risk | “Blinding of treatment allocation was not feasible” |
| Incomplete outcome data (attrition biasAll outcomes | Low risk | Intention-to-treat analysis used |
| Selective reporting (reporting bias) | Low risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 838 critically ill patients with euvolemia after initial treatment who had Hb concentrations <9.0 g/dL within 72 hours after admission to the intensive care unit were randomly assigned to 1 of 2 groups: Liberal group: n = 420; M/F = 255/165; mean (SD) age = 58.1 (18.3) years Restrictive group: n = 418; M/F = 269/149; mean (SD) age = 57.1 (18.1) years | |
| Interventions | Liberal group were transfused RBC when the Hb concentration fell below 10.0 g/dL. The Hb concentration was maintained between 10.0 to 12.0 g/dL. Restrictive group were transfused RBC if the Hb concentration dropped below 7. 0 g/dL. The Hb concentration was maintained between 7.0 to 9.0 g/dL. | |
| Outcomes | Outcomes reported: mortality, length of hospital stay, length of ICU stay, blood usage (units), complications, infection rates, cardiac events, pulmonary oedema, pneumonia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random order |
| Allocation concealment (selection bias) | Unclear risk | Sealed, opaque envelopes prepared by the data-coordinating centre and distributed to each participating institution where they were opened up sequentially to determine the patients treatment assignment. The envelopes were returned periodically to the co-ordinating centre for auditing |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | “It was not feasible to mask the assigned transfusion strategy from health care providers” |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Intention-to-treat analysis used |
| Selective reporting (reporting bias) | Low risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 39 autologous blood donors undergoing elective myocardial revascularisation were randomised to 1 of 2 groups: Liberal group: n = 18; M/F = 16/2; mean (SD) age = 60.5 (6.9) years Restrictive group: n = 20; M = 20; mean (SD) age = 58.2 (7.5) years | |
| Interventions | Liberal group received blood to achieve a Hct value of 32% Restrictive (conservative) group received transfusions for a Hct value less than 25% NB: operative management included sequestration of 1 or more units of fresh autologous blood in patients with a Hct value greater than 35% who were haemodynamically stable after anaesthetic induction. Red cell conservation was practised through salvage of oxygenator contents and reinfusion of postoperatively shed mediastinal blood. On the 5th postoperative day all patients were asked to complete an exercise treadmill test. A second test was performed the following day | |
| Outcomes | Outcomes reported: cardiac events, complications, postoperative blood loss, blood use (total units), allogeneic blood use (units), autologous blood use (units), all product blood use (units), number of patients receiving transfusions, mean cardiac index, mean systemic resistance, exercise capacity, Hct levels, length of ICU stay, length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised with the aid of a table of random numbers and an odd-even designation |
| Allocation concealment (selection bias) | High risk | Inadequately concealed |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Surgeons and anaesthesiologists were blinded as to the group of randomisation until the patient reached the intensive care unit (ICU) |
| Incomplete outcome data (attrition bias)All outcomes | Unclear risk | A small number of exclusions were reported |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 637 stable, critically ill children with Hb concentrations below 9.5 g/dL within 7 days after admission to an ICU were randomly allocated to 1 of 2 groups: Liberal group: n = 317; M/F = 191/126; mean (SD) age = 39.6 (51.9) months Restrictive group: n = 320; M/F = 190/130; mean (SD) age = 35.8 (46.2) months | |
| Interventions | Liberal group were transfused RBC when the Hb concentration fell below 9.5 g/dL, with a target range of 11.0 to 12.0 g/dL Restrictive group were transfused RBC ifthe Hb concentration dropped below 7. 0 g/dL, with a target range of 8.5 to 9.5 g/dL | |
| Outcomes | Outcomes reported: 28-day mortality, sepsis, transfusion reactions, infections, length of stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Internet-based, central allocation |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Clinical staff and parents of the patients were aware of the assignments to study groups, but the statistician and members of the data and safety monitoring committee were unaware of the assignments |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Intention-to-treat analysis used |
| Selective reporting (reporting bias) | Low risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 152 patients undergoing primary total knee arthroplasty (TKA) were randomly assigned to 1 of 2 groups: Liberal group: n = 65; M/F = 19/46; mean age = 69.7 years Restrictive group: n = 62; M/F = 20/42; mean age = 68.7 years | |
| Interventions | Liberal group were transfused autologous blood immediately after TKA, beginning in the recovery room postoperatively Restrictive group were transfused autologous blood when the Hb level had fallen to < 9.0 g/dL | |
| Outcomes | Outcomes reported: complications, cardiac events, Hb levels, blood usage (units), mental confusion, lethargy, orthostatic hypotension, number of patients transfused | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Assessments were made by a person blind to the group to which the patient was assigned |
| Incomplete outcome data (attrition bias)All outcomes | Unclear risk | Appears to have been complete |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 619, patients undergoing elective orthopaedic hip/knee replacement surgery were randomised to 1 of 2wo groups: Liberal (standard care) group: n = 304; M/F = 118/186; mean (SD) age = 70.3 (9. 7) years Restrictive (new transfusion policy) group: n = 299; M/F = 84/215; mean (SD) age = 70.7 (10.2) years | |
| Interventions | Liberal group received standard care Restrictive group were treated using a ’new transfusion policy’ | |
| Outcomes | Outcomes reported: red blood cell usage, length of hospital stay, Hb levels, mobilisation delay, postoperative complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | Clinicians caring for the patients were aware of allocation status, however, the study investigators were not |
| Incomplete outcome data (attrition bias)All outcomes | Unclear risk | Intention-to-treat analysis was not performed, although unclear if this would have biased the results |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 22 trauma patients were randomly allocated to 1 of 2 groups: Liberal group: n = 10 Restrictive group: n = 12 NB: no demographic data were reported | |
| Interventions | Liberal group: the aim was to achieve 100% or more of the red cell volume at the end of resuscitation Restrictive group: an attempt was made to leave the red cell volume at the end of resuscitation at 70% to 80% of normal | |
| Outcomes | Outcomes reported: blood usage (units), blood loss, wound healing, elevated temperature, number of patients transfused, Hb levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | When the patient was considered eligible for the trial, they were placed in a severity grade and an envelope opened to decide which transfusion schedule was to be used |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | Appears to be complete |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 60 adult patients with acute leukaemia were randomly allocated to 1 of 2 groups: Liberal group: n = 31; M/F = 14/17; mean (SD) age = 45.3 (16.8) years Restrictive group: n = 29; M/F = 1811; mean (SD) age = 50.8 (15.3) years | |
| Interventions | Liberal group were transfused 2 units of RBC when the Hb concentration fell below 12.0 g/dL Restrictive group were transfused 2 units of RBC if the Hb concentration dropped below 8.0 g/dL, with a target range of85 to 95 g/dL | |
| Outcomes | Outcomes reported: transfusions, bleeding risk | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated sequence generation |
| Allocation concealment (selection bias) | Low risk | Internet-based, central allocation |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | “Single-blinded” - blind outcome assessment |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | 30 patients with severe traumatic brain injury were randomly allocated to 1 of 3 groups: Liberal group 1: n = 10 Liberal group 2: n = 10 Restrictive group: n = 10 NB: Mean (SD) age = 39 (15) years, 70% of trial participants were male | |
| Interventions | Liberal group 1 were transfused 2 units of RBC when the Hb concentration fell below 9.0 g/dL Liberal group 2 were transfused 2 units of RBC when the Hb concentration fell below 10.0 g/dL Restrictive group were transfused 2 units of RBC if the Hb concentration dropped below 8.0 g/dL | |
| Outcomes | Outcomes reported: change in brain tissue oxygen, brain pH, mortality | |
| Notes | Additional data were obtained from lead trialist for inclusion in the meta-analysis. Data from liberal groups 1 and 2 combined for analysis | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias)All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias)All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | - |
| Other bias | Low risk | - |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Vichinsky 1995 | Intervention not relevant |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised clinical trial |
|---|---|
| Participants | Patients with acute myocardial infarction and haematocrit less than 30%Sample size = 45 patients |
| Interventions | Liberal transfusion: transfuse when haematocrit < 30% to maintain 30% to 33%)Conservative transfusion: transfuse when haematocrit < 24% to maintain 24% to 27% |
| Outcomes | The primary clinical safety measurement of in-hospital death, recurrent myocardial infarction, or new or worsening congestive heart failure |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Myocardial ischemia and transfusion |
|---|---|
| Methods | Randomised, single-blinded, parallel trial |
| Participants | Anaemic patients with acute coronary syndrome, aged 18 years or over Sample size =110 |
| Interventions | Liberal group - receive 1 unit of packed RBC following randomisation and received enough blood to raise Hb concentration above 10 g/dL, during hospitalisation for up to 30 days Restrictive group - receive transfusion if develop symptoms of anaemia or if Hb falls below 8.0 g/dL |
| Outcomes | Trial performance and feasibility, Hb concentration, mortality or myocardial ischaemia, unscheduled hospital admission, stroke, congestive hear failure, stent thrombosis, deep vein thrombosis, pulmonary embolism, pneumonia, blood stream infection |
| Starting date | September 2009 |
| Contact information | Jeffrey Carson (carson@umdnj.edu) |
| Notes |
| Trial name or title | A multi-centre randomised controlled trial of Transfusion Indication Threshold Reduction on transfusion rates, morbidity and healthcare resources use following cardiac surgery |
|---|---|
| Methods | Multicentre randomised controlled trial |
| Participants | Patients aged 16 years and over undergoing cardiac surgery |
| Interventions | Liberal group - receive packed RBC if Hb concentration falls below 9 g/dL, objective is to maintain Hb above 9 g/dL Restrictive group - receive packed RBC if Hb concentration falls below 7.5 g/dL, objective is to maintain Hb above 7.5 g/dL |
| Outcomes | Infectious events, ischaemic events, units of RBC transfused, duration of hospital stay, all-cause mortality, resource use |
| Starting date | December 2008 |
| Contact information | |
| Notes |
DATA AND ANALYSES
Comparison 1. Blood transfusions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients exposed to blood transfusion (all studies) | 17 | 6125 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.52, 0.72] |
| 2 Patients exposed to allogeneic blood transfusion | 10 | 4146 | Risk Ratio (M-H, Random, 95% CI) | 0.58 [0.46, 0.72] |
| 3 Patients exposed to autologous blood transfusion | 2 | 165 | Risk Ratio (M-H, Random, 95% CI) | 0.46 [0.12, 1.82] |
| 4 Patients exposed to blood transfusion (by clinical setting) | 17 | 6127 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.52, 0.72] |
| 4.1 Cardiac surgery | 3 | 968 | Risk Ratio (M-H, Random, 95% CI) | 0.67 [0.58, 0.78] |
| 4.2 Orthopaedic surgery | 6 | 3170 | Risk Ratio (M-H, Random, 95% CI) | 0.53 [0.37, 0.75] |
| 4.3 Vascular | 1 | 99 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.77, 1.08] |
| 4.4 Acute blood loss/trauma | 3 | 286 | Risk Ratio (M-H, Random, 95% CI) | 0.52 [0.30, 0.89] |
| 4.5 Cancer | 1 | 60 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.82, 1.12] |
| 4.6 Critical care | 3 | 1544 | Risk Ratio (M-H, Random, 95% CI) | 0.56 [0.42, 0.75] |
| 5 Patients exposed to blood transfusion (by transfusion threshold) | 12 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 Difference ≥ 2 g/dL | 10 | 4758 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.49, 0.72] |
| 5.2 Difference < 2 g/dL | 2 | 527 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.63, 1.07] |
| 6 Patients exposed to blood transfusion (by allocation concealment) | 17 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 6.1 Low risk of bias | 4 | 2797 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.37, 0.80] |
| 6.2 Unclear risk ofbias | 11 | 2862 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.54, 0.74] |
| 6.3 High risk of bias | 2 | 466 | Risk Ratio (M-H, Random, 95% CI) | 0.74 [0.62, 0.88] |
| 7 Units of blood transfused | 8 | 2715 | Mean Difference (IV, Random, 95% CI) | −1.19 [−1.85, −0.53] |
| 8 Units of blood transfused in those transfused | 8 | 1555 | Mean Difference (IV, Random, 95% CI) | −0.75 [−1.30, −0.20] |
Comparison 2. Haemoglobin concentration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin concentration | 12 | 5302 | Mean Difference (IV, Random, 95% CI) | −1.48 [−1.92, −1.03] |
Comparison 3. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≤14-day mortality | 2 | 821 | Risk Ratio (M-H, Random, 95% CI) | 0.44 [0.06, 2.96] |
| 2 30-day mortality | 11 | 4979 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.70, 1.03] |
| 3 60-day mortality | 3 | 2938 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.72, 1.06] |
| 4 120-day mortality | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5 Hospital mortality | 5 | 3411 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.62, 0.95] |
| 6 ICU mortality | 3 | 736 | Risk Ratio (M-H, Random, 95% CI) | 1.15 [0.59, 2.23] |
| 7 Mortality (unspecified follow-up period) | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only |
Comparison 4. Length of stay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital length of stay | 8 | 4226 | Mean Difference (IV, Random, 95% CI) | 0.11 [−0.16, 0.38] |
| 2 ICU length of stay | 5 | 2114 | Mean Difference (IV, Random, 95% CI) | −0.15 [−0.66, 0.37] |
Comparison 5. Function and fatigue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Inability to walk or death at 30 days | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 2 Inability to walk or death at 60 days | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3 Lower extremity physical activities of daily living at 30 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Lower extremity physical activities of daily living at 60 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Instrumental activities of daily living at 30 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Instrumental activities of daily living at 60 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Energy/fatigue at 30 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Energy/fatigue at 60 days | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 6. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac events | 7 | 4048 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.70, 1.32] |
| 2 Myocardial infarction | 8 | 3884 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.38, 2.04] |
| 3 Pulmonary oedema | 5 | 3649 | Risk Ratio (M-H, Random, 95% CI) | 0.72 [0.31, 1.70] |
| 4 Cerebrovascular accident (CVA) - stroke | 5 | 2760 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.47, 1.49] |
| 5 Pneumonia | 5 | 3695 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.76, 1.15] |
| 6 Thromboembolism | 3 | 2220 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.32, 1.59] |
| 7 Rebleeding | 2 | 552 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.03, 4.99] |
| 8 Infection | 6 | 4306 | Risk Ratio (M-H, Random, 95% CI) | 0.81 [0.66, 1.00] |
| 9 Renal failure | 3 | 1567 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.56, 2.18] |
| 10 Mental confusion | 2 | 247 | Risk Ratio (M-H, Random, 95% CI) | 1.86 [0.63, 5.44] |
Analysis 1.1. Comparison 1 Blood transfusions, Outcome 1 Patients exposed to blood transfusion (all studies).
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 1 Patients exposed to blood transfusion (all studies)
Analysis 1.2. Comparison 1 Blood transfusions, Outcome 2 Patients exposed to allogeneic blood transfusion.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 2 Patients exposed to allogeneic blood transfusion
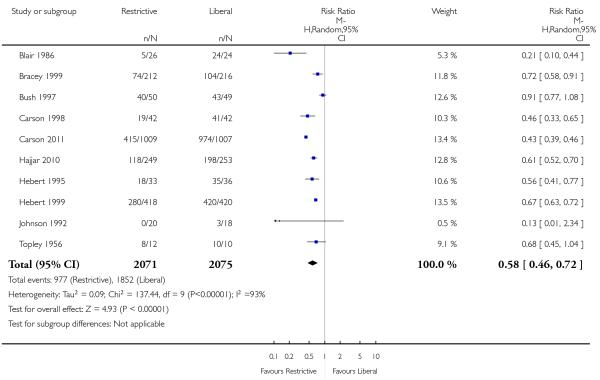
Analysis 1.3. Comparison 1 Blood transfusions, Outcome 3 Patients exposed to autologous blood transfusion.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 3 Patients exposed to autologous blood transfusion
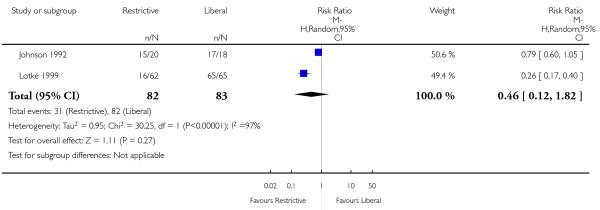
Analysis 1.4. Comparison 1 Blood transfusions, Outcome 4 Patients exposed to blood transfusion (by clinical setting).
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 4 Patients exposed to blood transfusion (by clinical setting)
Analysis 1.5. Comparison 1 Blood transfusions, Outcome 5 Patients exposed to blood transfusion (by transfusion threshold).
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 5 Patients exposed to blood transfusion (by transfusion threshold)
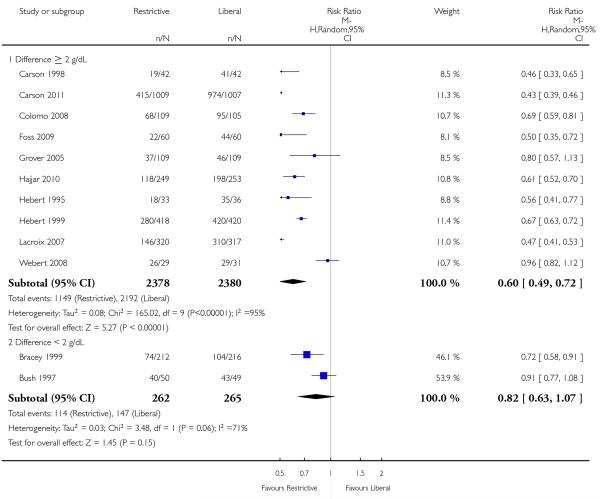
Analysis 1.6. Comparison 1 Blood transfusions, Outcome 6 Patients exposed to blood transfusion (by allocation concealment).
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 6 Patients exposed to blood transfusion (by allocation concealment)
Analysis 1.7. Comparison 1 Blood transfusions, Outcome 7 Units of blood transfused.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 7 Units of blood transfused
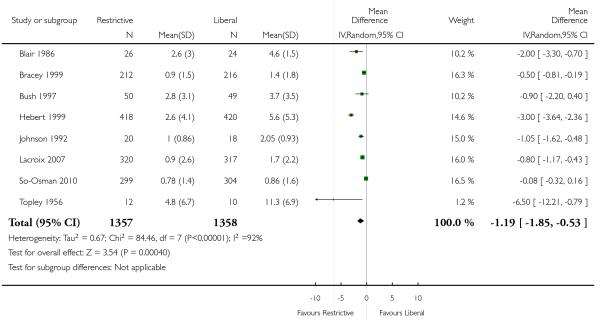
Analysis 1.8. Comparison 1 Blood transfusions, Outcome 8 Units of blood transfused in those transfused.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 1 Blood transfusions
Outcome: 8 Units of blood transfused in those transfused
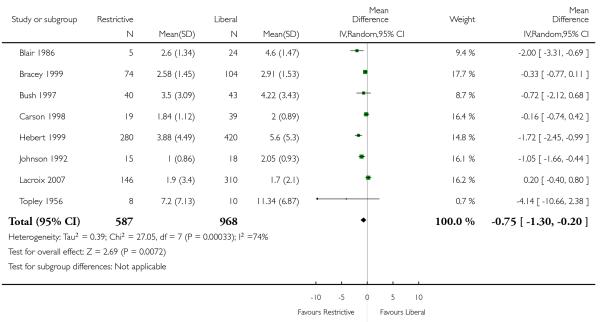
Analysis 2.1. Comparison 2 Haemoglobin concentration, Outcome 1 Haemoglobin concentration.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 2 Haemoglobin concentration
Outcome: 1 Haemoglobin concentration
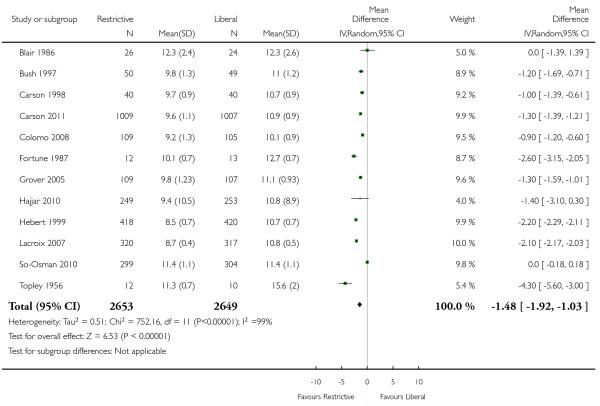
Analysis 3.1. Comparison 3 Mortality, Outcome 1 ≤14-day mortality.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 3 Mortality
Outcome: 1 ≤14-day mortality
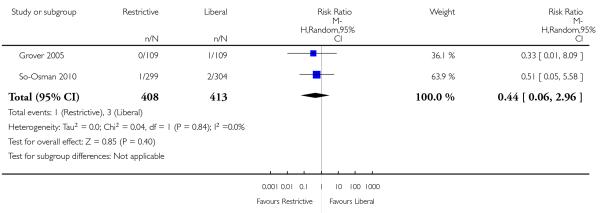
Analysis 3.2. Comparison 3 Mortality, Outcome 2 30-day mortality.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 3 Mortality
Outcome: 2 30-day mortality
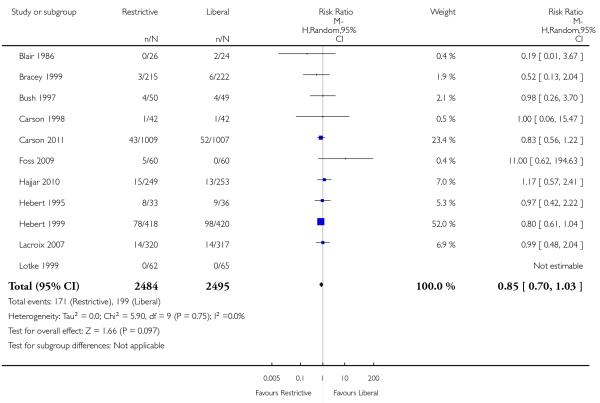
Analysis 3.3. Comparison 3 Mortality, Outcome 3 60-day mortality.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 3 Mortality
Outcome: 3 60-day mortality
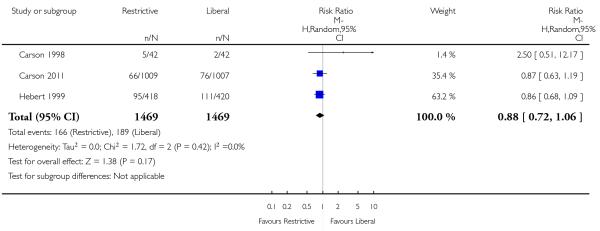
Analysis 3.4. Comparison 3 Mortality, Outcome 4 120-day mortality.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 3 Mortality
Outcome: 4 120-day mortality
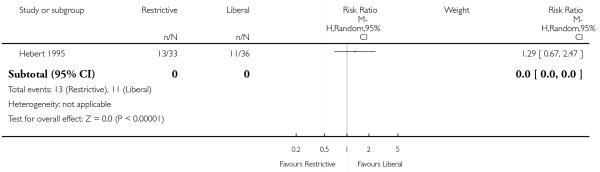
Analysis 3.5. Comparison 3 Mortality, Outcome 5 Hospital mortality.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 3 Mortality
Outcome: 5 Hospital mortality
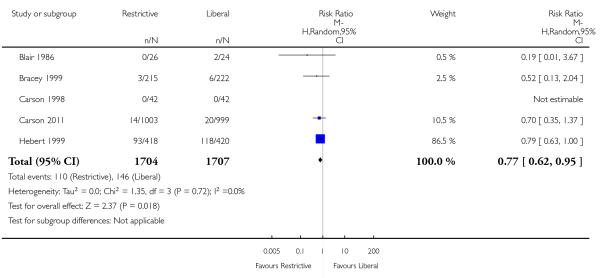
Analysis 3.6. Comparison 3 Mortality, Outcome 6 ICU mortality.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 3 Mortality
Outcome: 6 ICU mortality
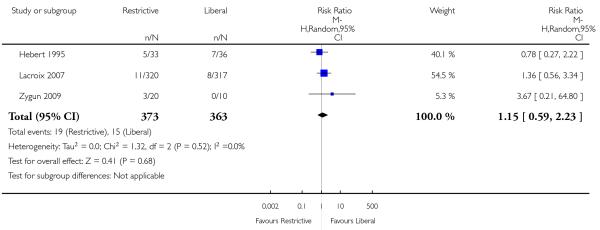
Analysis 3.7. Comparison 3 Mortality, Outcome 7 Mortality (unspecified follow-up period).
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 3 Mortality
Outcome: 7 Mortality (unspecified follow-up period)
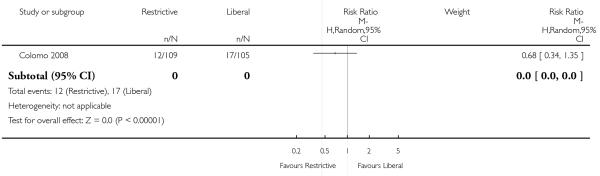
Analysis 4.1. Comparison 4 Length of stay, Outcome 1 Hospital length of stay.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 4 Length of stay
Outcome: 1 Hospital length of stay
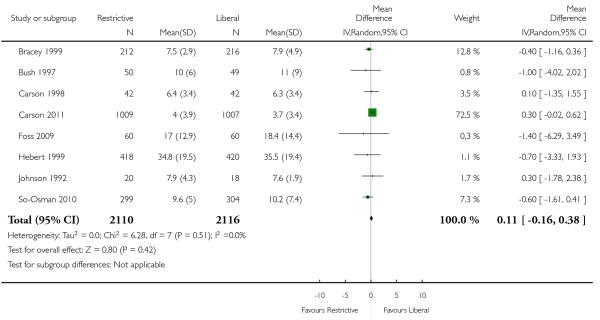
Analysis 4.2. Comparison 4 Length of stay, Outcome 2 ICU length of stay.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 4 Length of stay
Outcome: 2 ICU length of stay
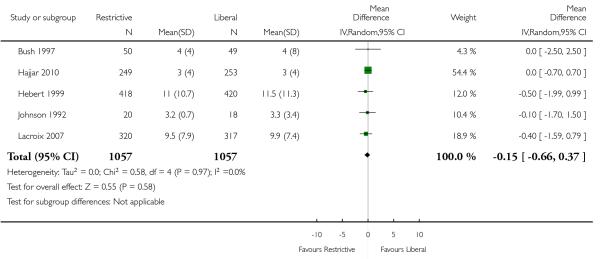
Analysis 5.1. Comparison 5 Function and fatigue, Outcome 1 Inability to walk or death at 30 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 1 Inability to walk or death at 30 days
Analysis 5.2. Comparison 5 Function and fatigue, Outcome 2 Inability to walk or death at 60 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 2 Inability to walk or death at 60 days
Analysis 5.3. Comparison 5 Function and fatigue, Outcome 3 Lower extremity physical activities of daily living at 30 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 3 Lower extremity physical activities of daily living at 30 days
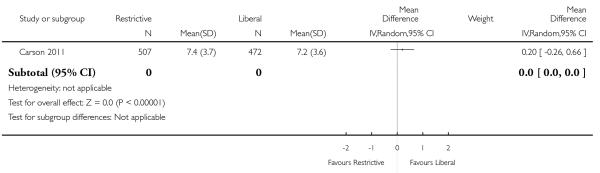
Analysis 5.4. Comparison 5 Function and fatigue, Outcome 4 Lower extremity physical activities of daily living at 60 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 4 Lower extremity physical activities of daily living at 60 days
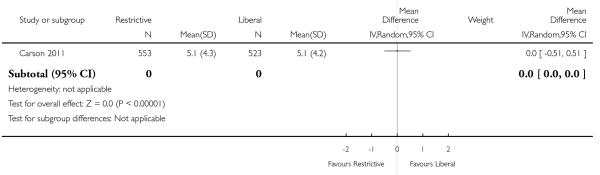
Analysis 5.5. Comparison 5 Function and fatigue, Outcome 5 Instrumental activities of daily living at 30 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 5 Instrumental activities of daily living at 30 days
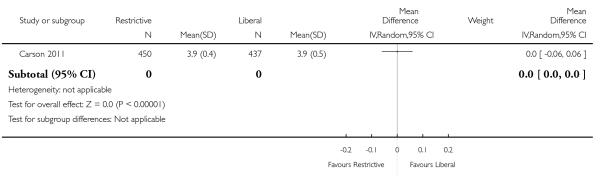
Analysis 5.6. Comparison 5 Function and fatigue, Outcome 6 Instrumental activities of daily living at 60 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 6 Instrumental activities of daily living at 60 days
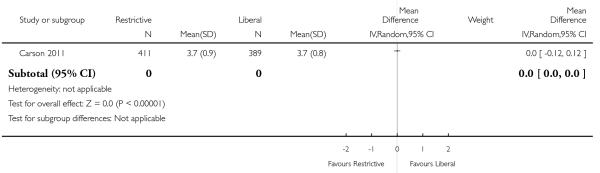
Analysis 5.7. Comparison 5 Function and fatigue, Outcome 7 Energy/fatigue at 30 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 7 Energy/fatigue at 30 days
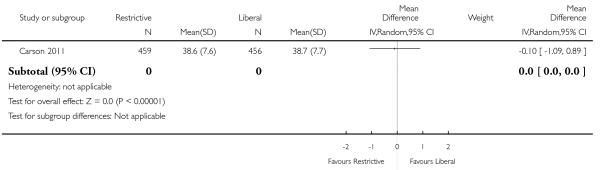
Analysis 5.8. Comparison 5 Function and fatigue, Outcome 8 Energy/fatigue at 60 days.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 5 Function and fatigue
Outcome: 8 Energy/fatigue at 60 days
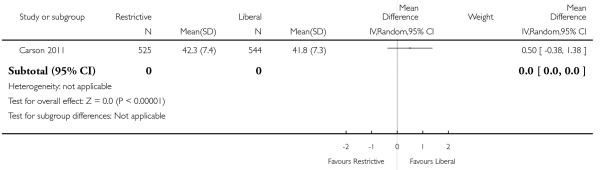
Analysis 6.1. Comparison 6 Adverse events, Outcome 1 Cardiac events.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 1 Cardiac events
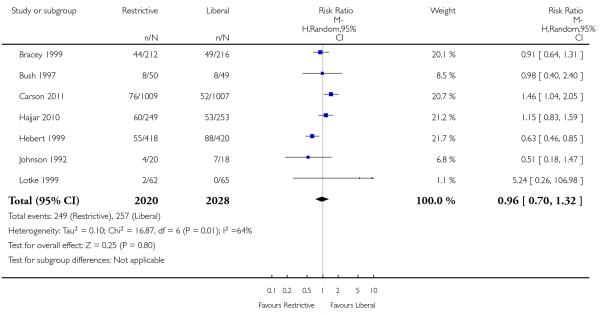
Analysis 6.2. Comparison 6 Adverse events, Outcome 2 Myocardial infarction.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 2 Myocardial infarction
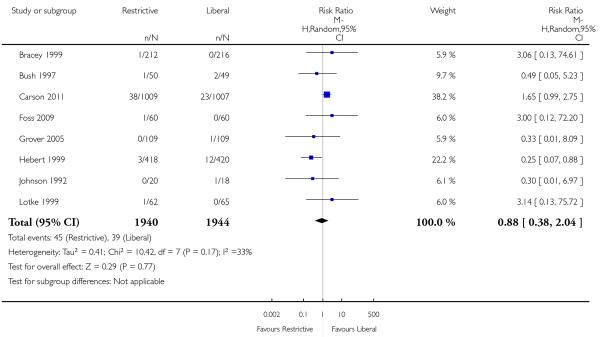
Analysis 6.3. Comparison 6 Adverse events, Outcome 3 Pulmonary oedema.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 3 Pulmonary oedema
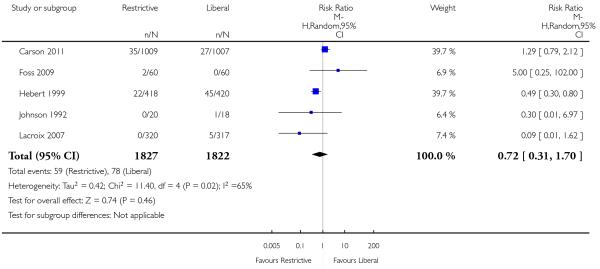
Analysis 6.4. Comparison 6 Adverse events, Outcome 4 Cerebrovascular accident (CVA) - stroke.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 4 Cerebrovascular accident (CVA) - stroke
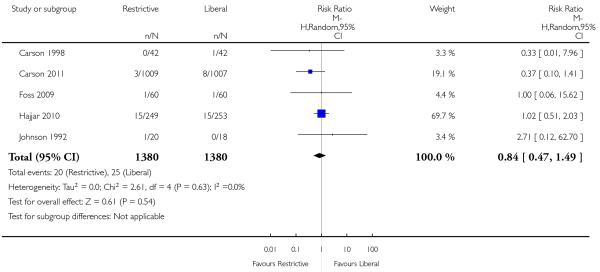
Analysis 6.5. Comparison 6 Adverse events, Outcome 5 Pneumonia.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 5 Pneumonia
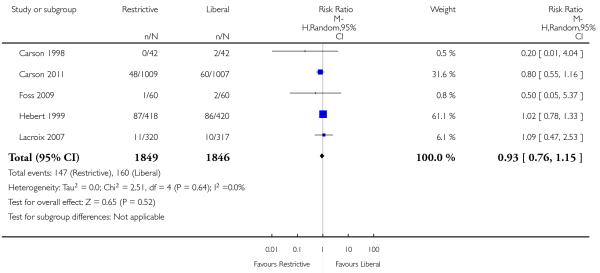
Analysis 6.6. Comparison 6 Adverse events, Outcome 6 Thromboembolism.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 6 Thromboembolism
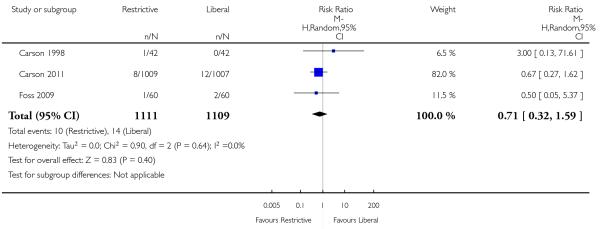
Analysis 6.7. Comparison 6 Adverse events, Outcome 7 Rebleeding.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 7 Rebleeding
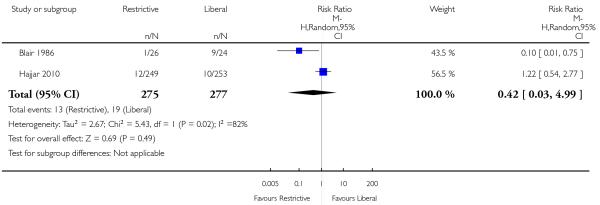
Analysis 6.8. Comparison 6 Adverse events, Outcome 8 Infection.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 8 Infection
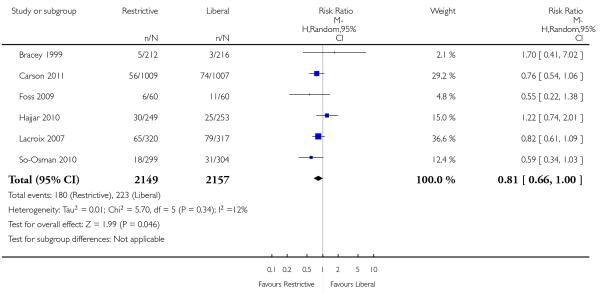
Analysis 6.9. Comparison 6 Adverse events, Outcome 9 Renal failure.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 9 Renal failure
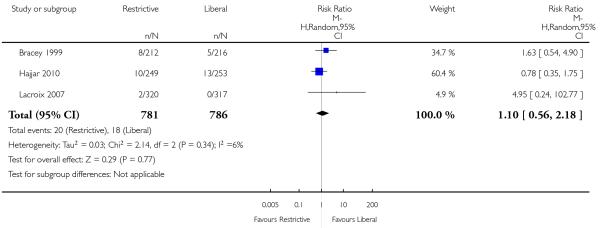
Analysis 6.10. Comparison 6 Adverse events, Outcome 10 Mental confusion.
Review: Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion
Comparison: 6 Adverse events
Outcome: 10 Mental confusion
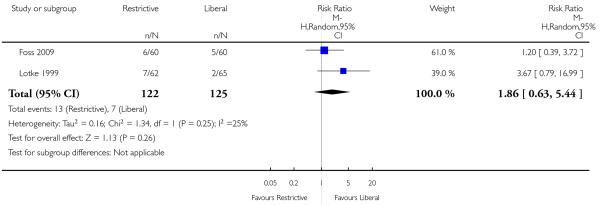
WHAT’S NEW
Last assessed as up-to-date: 1 February 2011.
| Date | Event | Description |
|---|---|---|
| 17 April 2012 | Amended | Minor copy edits were made to the text. |
HISTORY
Protocol first published: Issue 2, 2000
Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 20 December 2011 | New citation required and conclusions have changed | The searches were updated to February 2011. Data from two new trials are included and the results have been amended accordingly. One trial was identified through the updated search; the other had previously been included as an ongoing trial and the results recently became availableThe Background section of the review has been updated. The overall conclusions of the review remain similar but the clinical settings for which the results can be generalised have been extendedAs part of this update the assessment of methodological quality used in earlier versions of this review has been replaced with an assessment of the risk of bias. This amendment is in accordance with a change in the Cochrane Collaboration’s methodological guidance The authors of the review have changed. |
| 1 February 2011 | New search has been performed | The search for studies was updated to February 2011. |
| 9 September 2008 | Amended | Converted to new review format. |
| 17 November 2004 | New search has been performed | An updated search for new trials was conducted in November 2004. No new trials for inclusion were identified |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
There were no differences identified.
Footnotes
DECLARATIONS OF INTEREST
Dr. Carson reports receiving grant support to his institution from Amgen and US National Institutes of Health. He is involved with guideline development. Dr. Hebert reports receiving grant support from Amgen and Johnson and Johnson.
References to studies included in this review
- Blair 1986 {published data only} .Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. British Journal of Surgery. 1986;73(10):783–5. doi: 10.1002/bjs.1800731007. [DOI] [PubMed] [Google Scholar]
- Bracey 1999 {published data only} .Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39(10):1070–7. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- Bush 1997 {published data only} .Bush RL, Pevec WC, Holcroft JW. A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. American Journal of Surgery. 1997;174(2):143–8. doi: 10.1016/s0002-9610(97)00073-1. [DOI] [PubMed] [Google Scholar]
- Carson 1998 {published data only} .Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion. 1998;38(6):522–9. doi: 10.1046/j.1537-2995.1998.38698326331.x. [DOI] [PubMed] [Google Scholar]
- Carson 2011 {published data only} .Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GR, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. New England Journal of Medicine. 2011;365(26):2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomo 2008 {published data only} .Colomo A, Hernandez-Gea V, Muniz-Diaz E, Madoz P, Aracil C, Alvarez-Urturi C, et al. Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology. 2008;48(4 (Suppl)):413A. [Google Scholar]
- Fortune 1987 {published data only} .Fortune JB, Feustel PJ, Saifi J, Stratton HH, Newell JC, Shah DM. Influence of hematocrit on cardiopulmonary function after acute hemorrhage. Journal of Trauma. 1987;27(3):243–9. doi: 10.1097/00005373-198703000-00003. [DOI] [PubMed] [Google Scholar]
- Foss 2009 {published data only} .Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion. 2009;49(2):227–34. doi: 10.1111/j.1537-2995.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- Grover 2005 {published data only} .*; Grover M, Talwalkar S, Casbard A, Boralessa H, Contreras M, Boralessa H, et al. Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sanguinis. 2006;90:105–12. doi: 10.1111/j.1423-0410.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- Grover M, Talwalker SA, Contreras Soni N. Blood transfusion threshold and silent myocardial ischemia after lower limb arthroplasty: a randomized controlled trial of transfusion strategy. Transfusion Alternatives in Transfusion Medicine. 2005;7(1 (Suppl)):62–3. [Google Scholar]
- Hajjar 2010 {published data only} .Hajjar LA, Vincent JL, Galas FRGB, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion requirements after cardiac surgery. The TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- Hebert 1995 {published data only} .Hebert PC, Wells G, Marshall J, Martin C, Tweeddale M, Pagliarello G, et al. Transfusion requirements in critical care. A pilot study. Canadian Critical Care Trials Group. JAMA. 1995;273(18):1439–44. doi: 10.1001/jama.1995.03520420055038. published erratum appears in JAMA 1995 Sep 27;274(12): 944. [DOI] [PubMed] [Google Scholar]
- Hebert 1999 {published data only} .*; Hebert PC. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. New England Journal of Medicine. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- McIntyre L, Hebert PC, Wells G, Fergusson D, Marshall J, Yetisir E, et al. Canadian Critical Care Trials Group. Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? Journal of Trauma. 2004;57(3):563–8. doi: 10.1097/01.ta.0000136158.93864.54. [DOI] [PubMed] [Google Scholar]
- McIntyre LA, Fergusson DA, Hutchison JS, Pagliarello G, Marshall JC, Yetisir E, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion. 1998;38(6):522–9. doi: 10.1046/j.1537-2995.1998.38698326331.x. [DOI] [PubMed] [Google Scholar]
- Johnson 1992 {published data only} .Johnson RG, Thurer RL, Kruskall MS, Sirois C, Gervino EV, Critchlow J, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. Journal of Thoracic & Cardiovascular Surgery. 1992;104(2):307–14. [PubMed] [Google Scholar]
- Lacroix 2007 {published data only} .Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. New England Journal of Medicine. 2007;356(16):1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- Lotke 1999 {published data only} .Lotke PA, Barth P, Garino JP, Cook EF. Predonated autologous blood transfusions after total knee arthroplasty: immediate versus delayed administration. Journal of Arthroplasty. 1999;14(6):647–50. doi: 10.1016/s0883-5403(99)90216-4. [DOI] [PubMed] [Google Scholar]
- So-Osman 2010 {published data only} .So-Osman C. A restrictive transfusion trigger is a method for blood saving in elective orthopaedic surgery. Vox Sanguinis. 2004;87(Suppl 3):52. [Google Scholar]
- *; So-Osman C, Nelissen R, Te Slaa R, Coene L, Brand R, Brand A. A randomized comparison of transfusion triggers in elective orthopaedic surgery using leucocyte-depleted red blood cells. Vox Sanguinis. 2010;98:56–64. doi: 10.1111/j.1423-0410.2009.01225.x. [DOI] [PubMed] [Google Scholar]
- Topley 1956 {published data only} .Topley ET, Fisher MR. The illness of trauma. British Journal of Clinical Practice. 1956;10(11):770–6. [PubMed] [Google Scholar]
- Webert 2008 {published data only} .Webert KE, Cook RJ, Couban S, Carruthers J, Lee KA, Blajchman MA, et al. A multicenter pilot-randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion. 2008;48(1):81–91. doi: 10.1111/j.1537-2995.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Zygun 2009 {published data only} .Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Critical Care Medicine. 2009;37(3):1074–8. doi: 10.1097/CCM.0b013e318194ad22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Vichinsky 1995 {published data only} .Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, et al. The Preoperative Transfusion in Sickle Cell Disease Study Group A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. New England Journal of Medicine. 1995;333(4):206–13. doi: 10.1056/NEJM199507273330402. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Cooper 2011 {published data only} .Cooper HA, Rao SV, Greenberg MD, Rumsey MP, Mckenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT randomized pilot study) American Journal of Cardiology. 2011;108:1108–11. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- MINT {published data only} .Carson JL, Noveck H. Myocardial Ischemia and Transfusion (MINT) http://www.ClinicalTrials.gov. [: NCT01167582]
- TITRe 2 {published data only} .Reeves B. A multi-centre randomised controlled trial of the effects of a reduction in the threshold for blood transfusion following heart surgery. http://www.controlled-trials.com. [: ISRCTN70923932]
Additional references
- AAGBI 2008 .Association of Anaesthetists of Great Britain and Ireland Blood transfusion and the anaesthetist - red cell transfusion. 2008 Jun 1-20; http://www.aagbi.org/publications/guidelines/docs/redcell08.pdf
- Amin 2004 .Amin M, Fergusson D, Wilson K, Tinmouth A, Aziz A, Coyle D, et al. The societal unit cost of allogenic red blood cells and red blood cell transfusion in Canada. Transfusion. 2004;44(10):1479–86. doi: 10.1111/j.1537-2995.2004.04065.x. [DOI] [PubMed] [Google Scholar]
- ASA 2006 .American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies Practice guidelines for perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105(1):198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- ASBT 2001 .National Health and Medical Research Council (NHMRC) Australasian Society of Blood Transfusion (ASBT) Clinical Practice Guidelines: Appropriate Use of Red Blood Cells. 2001 [Google Scholar]
- BCTMAG 2003 .Guidelines for Red Blood Cell Transfusion. British Columbia Transfusion Medicine Advisory Group; Nov 1-3, 2003. [Google Scholar]
- Benson 2000 .Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. New England Journal of Medicine. 2000;342(25):1878–86. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- Carless 2010 .Carless PA, Henry DA, Moxey AJ, O’Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2010;(4) doi: 10.1002/14651858.CD001888.pub4. DOI: 10.1002/14651858.CD001888.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concato 2000 .Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. New England Journal of Medicine. 2000;342(25):1887–92. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Simonian 1986 .Der Simonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Goodnough 2008 .Goodnough LT, Shander A. Risks and complications of blood transfusions: optimizing outcomes for patients with chemotherapy-induced anemia. Advanced Studies in Medicine. 2008;8(10):357–62. [Google Scholar]
- Hebert 1997 .Hebert PC, Wells G, Tweeddale M, Martin C, Marshall J, Pham B, et al. Does transfusion practice affect mortality in critically ill patients? Transfusion Requirements in Critical Care (TRICC) Investigators and the Canadian Critical Care Trials Group. American Journal of Respiratory & Critical Care Medicine. 1997;155(5):1618–23. doi: 10.1164/ajrccm.155.5.9154866. [DOI] [PubMed] [Google Scholar]
- Henry 2001a .Henry DA, Moxey AJ, O’Connell D. Agreement between randomised and non-randomised studies - the effects of bias and confounding; Poster presentation - 9th Cochrane Colloquium; Lyon, France. Oct 9-13, [Google Scholar]
- Henry 2011b .Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Ferugsson DA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews. 2011;(3) doi: 10.1002/14651858.CD001886.pub4. DOI: 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2011 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. updated March 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Ioannidis 2001 .Ioannidis JPA, Haidich AB, Pappa M, Pantazis N, Kokori SI, Tektonidou MG, et al. Comparison of evidence of treatment effects in randomised and nonrandomised studies. JAMA. 2001;286:821–30. doi: 10.1001/jama.286.7.821. [DOI] [PubMed] [Google Scholar]
- Kitchen 2008 .Kitchen AD, Barbara JAJ. Current information on the infectious risks of allogeneic blood transfusion. Transfusion Alternative in Transfusion Medicine. 2008;10:102–11. [Google Scholar]
- Klein 2007 .Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415–26. doi: 10.1016/S0140-6736(07)61197-0. [DOI] [PubMed] [Google Scholar]
- Madjdpour 2005 .Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. British Journal of Anaesthesia. 2005;95(1):33–42. doi: 10.1093/bja/aeh290. [DOI] [PubMed] [Google Scholar]
- Napolitano 2009 .Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Critical Care Medicine. 2009;37(12):3124–57. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- NBUGI 2001 .National Blood Users Group Ireland A Guideline for Transfusion of Red Blood Cells in Surgical Patients. 2001 Jan 1-20; http://www.dohc.ie/publications/transfusionofredbloodcells.html
- NIH 1988 .National Institutes of Health Perioperative red blood cell transfusion. JAMA. 1988;260:2700–3. [PubMed] [Google Scholar]
- O’Brien 2007 .O’Brien SF, Yi QL, Fan W, Scalia V, Kleinman SH, Vamvakas EC. Current incidence and estimated residual risk of transfusion-transmitted infections in donations made to Canadian Blood Services. Transfusion. 2007;47(2):316–25. doi: 10.1111/j.1537-2995.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- Review Manager .The Nordic Cochrane Centre. The Cochrane Collaboration . Review Manager (RevMan). 5.1. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2011. [Google Scholar]
- Schulz 1995 .Schulz KF, Chalmers I, Hayes RJ, Altman DJ. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled clinical trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Shander 2010 .Shander A, Hofmann A, Ozawa S, Theusinger OM, Gomboz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- Spiess 1998 .Spiess BD, Ley C, Body SC, Siegel LC, Stover EP, Maddi R, et al. The Institutions of the Multicenter Study of Perioperative Ischemia (McSPI) Research Group Hematocrit value on intensive care unit entry influences the frequency of Q-wave myocardial infarction after coronary artery bypass grafting. Journal of Thoracic & Cardiovascular Surgery. 1998;116(3):460–7. doi: 10.1016/s0022-5223(98)70012-1. [DOI] [PubMed] [Google Scholar]
- Wang 2010 .Wang JK, Klein HG. Red blood cell transfusion in the treatment and management of anaemia: the search for the elusive transfusion trigger. Vox Sanguinis. 2010;98(1):2–11. doi: 10.1111/j.1423-0410.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- Weiskopf 2000 .Weiskopf RB, Kramer JH, Viele M, Neumann M, Feiner JR, Watson JJ, et al. Acute severe isovolemic anemia impairs cognitive function and memory in humans. Anesthesiology. 2000;92(6):1646–52. doi: 10.1097/00000542-200006000-00023. [DOI] [PubMed] [Google Scholar]
- Whitaker 2011 .U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health . Report of the US Department of Health and Human Services. The 2009 national blood collection and utilization survey report. U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. www.hhs.gov/ophs/bloodsafety/2007nbcussurvey.pdf [Google Scholar]
- WHO 2011 .WHO Blood safety: key global facts and figures in 2011. 2011;279:1–9. http://www.who.int/worldblooddonorday/media/whobloodsafetyfactsheet2011.pdf Fact Sheet. [Google Scholar]
- Wu 2001 .Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. New England Journal of Medicine. 2001;345(17):1230–6. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- Wu 2007 .Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297(22):2481–8. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- Wu 2010 .Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, et al. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Annals of Surgery. 2010;252(1):11–7. doi: 10.1097/SLA.0b013e3181e3e43f. [DOI] [PubMed] [Google Scholar]
- Zou 2009 .Zou S, Stramer SL, Notari EP, Kuhns MC, Krysztof D, Musavi F, et al. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion. 2009;49(8):1609–20. doi: 10.1111/j.1537-2995.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Zou 2010 .Zou S, Dorsey KA, Notari EP, Foster GA, Krysztof DE, Musavi F, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion. 2010;50(7):1495–504. doi: 10.1111/j.1537-2995.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study