Gut Epithelial Barrier Dysfunction and Innate Immune Activation Predict Mortality in Treated HIV Infection (original) (raw)
Abstract
Background. While inflammation predicts mortality in treated human immunodeficiency virus (HIV) infection, the prognostic significance of gut barrier dysfunction and phenotypic T-cell markers remains unclear.
Methods. We assessed immunologic predictors of mortality in a case-control study within the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), using conditional logistic regression. Sixty-four case patients who died within 12 months of treatment-mediated viral suppression were each matched to 2 control individuals (total number of controls, 128) by duration of antiretroviral therapy–mediated viral suppression, nadir CD4+ T-cell count, age, sex, and prior cytomegalovirus (CMV) retinitis. A similar secondary analysis was conducted in the SCOPE cohort, which had participants with less advanced immunodeficiency.
Results. Plasma gut epithelial barrier integrity markers (intestinal fatty acid binding protein and zonulin-1 levels), soluble CD14 level, kynurenine/tryptophan ratio, soluble tumor necrosis factor receptor 1 level, high-sensitivity C-reactive protein level, and D-dimer level all strongly predicted mortality, even after adjustment for proximal CD4+ T-cell count (all P ≤ .001). A higher percentage of CD38+HLA-DR+ cells in the CD8+ T-cell population was a predictor of mortality before (P = .031) but not after (P = .10) adjustment for proximal CD4+ T-cell count. Frequencies of senescent (defined as CD28−CD57+ cells), exhausted (defined as PD1+ cells), naive, and CMV-specific T cells did not predict mortality.
Conclusions. Gut epithelial barrier dysfunction, innate immune activation, inflammation, and coagulation—but not T-cell activation, senescence, and exhaustion—independently predict mortality in individuals with treated HIV infection with a history of AIDS and are viable targets for interventions.
Keywords: HIV, gut epithelial cell barrier, intestinal fatty acid binding protein (I-FABP), zonulin-1, sCD14, IL-6, D-dimer, hsCRP, cytomegalovirus, CD57, CD28, CD38, HLA-DR, T-cell activation, mortality, antiretroviral therapy, immune activation
Despite antiretroviral therapy (ART), human immunodeficiency virus type 1 (HIV-1)–infected individuals have a shorter life expectancy and greater morbidity than the general population, particularly when ART is delayed until disease is advanced [1–4]. Levels of inflammatory and coagulation markers remain abnormally high despite suppressive ART and predict this increased morbidity and mortality [5–17]. It remains unclear, however, whether these biomarkers are the most appropriate direct targets for interventions or whether they simply reflect the presence of persistent inflammatory stimuli that drive morbidity and mortality through parallel pathways. A more complete understanding of the immunologic pathways that predict mortality in this setting might help prioritize interventions to pursue in trials.
The role of gut mucosa in HIV pathogenesis has been intensively studied. Soluble CD14 (sCD14), the receptor for lipopolysaccharide (LPS) and a microbial translocation and monocyte activation marker, is predictive of mortality among patients with treated HIV infection [18, 19]. Nevertheless, sCD14 may be nonspecific for LPS-driven monocyte activation, so it has remained unclear whether persistent gut epithelial barrier dysfunction and microbial translocation truly predict mortality (and should remain targets for interventions). The induction of the kynurenine pathway of tryptophan catabolism by indoleamine 2,3-dioxygenase-1 (IDO) in activated myeloid cells (and other enzymes elaborated by gut-resident microbes [20]) has also been proposed as an important pathway contributing to HIV pathogenesis. Several catabolites in the kynurenine pathway suppress T-cell proliferation and T-helper cell 17 (Th17) development, potentially driving persistent gut barrier dysfunction and microbial translocation and a vicious cycle of further IDO induction and immune activation [21, 22]. While this pathway predicted mortality in a cohort of HIV-infected Ugandans starting ART [23], its prognostic significance in individuals with treated HIV infection in resource-rich settings remains unclear. It is also unclear whether T-cell activation, which predicts disease progression in untreated HIV infection [24, 25] and has served as the most commonly used surrogate outcome for pilot trials of immune-based therapeutics in treated HIV infection, also predicts mortality in treated disease. Finally, while T-cell senescence and cytomegalovirus (CMV)–specific immune responses predict increased mortality in elderly HIV-uninfected populations [26], their prognostic significance in treated HIV infection is unknown.
To address these issues, we performed a nested case-control study of individuals with ART-suppressed HIV infection in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA) to assess the relationship between these immunologic factors and mortality. Since the LSOCA is restricted to individuals with a history of AIDS, we performed a smaller secondary case-control study within the San Francisco SCOPE cohort to begin to address the generalizability of our findings to those with less-advanced disease.
METHODS
Participants
For the primary nested case-control study, we sampled HIV-infected participants in the LSOCA with a plasma HIV RNA level of <400 copies/mL. The LSOCA a multicenter cohort of >2200 HIV-infected participants who initiated ART with an AIDS diagnosis (25% had an ocular opportunistic infection, and 75% had a nonocular opportunistic disease or CD4+ T-cell count of <200 cells/mm3). We used a threshold of <400 copies/mL to define viral suppression, as several deaths occurred before assays with lower detection limits were available. All participants with available peripheral blood mononuclear cell (PBMC) and plasma specimens that had been collected during a visit that occurred (1) while HIV was suppressed by ART and (2) within 12 months of death (not known to be accidental) were included as case patients. Two control participants were matched to each case by age, sex, duration of viral suppression, history of CMV retinitis, and nadir CD4+ T-cell count. Identical inclusion and matching criteria were used for the secondary case-control study performed in the San Francisco SCOPE cohort, except the lag between sampling and death was extended to 48 months to include cases who lacked PBMC specimens collected within 12 months of death (n = 8). All participants provided written informed consent. This research was approved by the Institutional Review Board of the University of California, San Francisco.
Laboratory Methods
Thawed PBMCs were assessed for the following surface markers: CD3, CD4, CD8, CD28, CD45RA, CD31, CCR7, CD57, and CD27 [27]. T-cell expression of HLA-DR, CD38, CCR5, and PD-1 was performed in a separate panel [28], with the addition of CD45RA-PE and CCR7-Alexa Fluor 700 (BD Pharmingen) to assess these markers on memory subsets. The frequency of CMV-specific interferon γ–expressing T cells was assessed by cytokine flow cytometry on rested thawed PBMCs by stimulating the cells for 18–22 hours at 37°C with overlapping CMV pp65/IE peptide pools (cells in control wells were not stimulated) in the presence of 0.5 µg/mL brefeldin A and 0.5 µg/mL monensin (Sigma-Aldrich) [29].
Cryopreserved plasma was assessed by immunoassay for the gut barrier markers intestinal fatty acid binding protein (I-FABP; Human FABP2 DuoSet, R&D Systems) and zonulin-1 (Immundiagnostik), sCD14 (R&D Systems), interleukin 6 (IL-6; R&D Systems), soluble tumor necrosis factor receptor-1 (sTNF-R1; R&D Systems), high-sensitivity C reactive protein (hsCRP; UBI Magiwel), D-dimer (Diagnostica Stago), and anti-CMV immunoglobulin G (IgG) levels (GenWay Biotech). Plasma tryptophan and kynurenine levels were assessed by high-performance liquid chromatography-tandem mass spectrometry [30]. Kynurenine pathway activity was assessed as the plasma kynurenine to tryptophan (KT) ratio. KT ratios, zonulin-1 levels, and CMV IgG levels were unavailable in SCOPE because of resource constraints.
Statistical Methods
Predictors of mortality were assessed using conditional logistic regression; 1:1 matching was allowed if only 1 control per matched set had available data. While potential confounding by age, sex, nadir CD4+ T-cell count, prior history of CMV retinitis, and duration of ART-mediated viral suppression was controlled for by matching in the primary analysis, potential confounding by proximal (ie, date of sampling) CD4+ T-cell count was evaluated in secondary adjusted models. Proximal CD4+ T-cell count was not considered a matching variable because some immunologic mediators could be a cause and not simply a consequence of poor ART-mediated recovery in the CD4+ T-cell count. Relationships between continuous variables were assessed with Spearman correlation coefficients. When modeled continuously (per interquartile range [IQR]), all levels of biomarkers except proximal CD4+ T-cell count were log transformed to satisfy model assumptions. Analyses were performed using Stata, version 11 (StataCorp).
RESULTS
Participant Characteristics
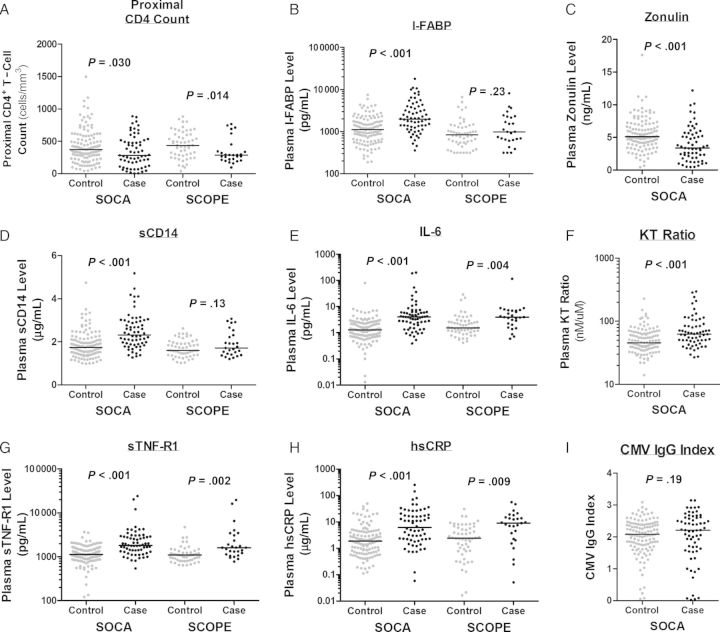
Sixty-four cases with ART-suppressed HIV infection who died within a median of 5 months (IQR, 3–8 months) after PBMC and plasma samples were obtained and 128 controls matched for age, sex, duration of ART-mediated viral suppression, nadir CD4+ T-cell count, and prior CMV retinitis were included in the primary LSOCA analysis. Most participants were 40–50-year-old men; the median nadir CD4+ T-cell count was 30 cells/mm3, and an ART-mediated HIV RNA level of <400 copies/mL was maintained for a median duration of 21 months (Table 1). The majority of LSOCA cases died of non–AIDS-defining causes (Supplementary Table 1). Cases had a lower median proximal CD4+ T-cell count than controls (283 vs 374 cells/mm3; P = .03), confirming that poor CD4+ T-cell recovery predicts increased mortality (Figure 1A). There was no evidence for a difference in history of hypertension, diabetes, hepatitis C virus (HCV) infection, or injection drug use (IDU) between cases and controls, but cases tended to more commonly report chronic renal insufficiency (13% vs 5%; P = .09), and controls more commonly reported hyperlipidemia (31% vs 17%; P = .04). Smoking history was unavailable for the majority of case-control sets.
Table 1.
Characteristics of Case Patients With Antiretroviral Therapy (ART)–Suppressed Human Immunodeficiency Virus (HIV) Infection Who Died and Matched Control Patients
| Characteristic | LSOCA Cohort | SCOPE Cohort | ||
|---|---|---|---|---|
| Deaths (n = 64) | Controls (n = 128) | Deaths (n = 27) | Controls (n = 54) | |
| Age, y | 47 (40–54) | 44 (39–50) | 54 (49–59) | 53 (49–57) |
| Male sex | 54 (84) | 108 (84) | 22 (81) | 44 (81) |
| Pre-ART nadir CD4+ T-cell count, cells/mm3 | 25 (9–104) | 31 (10–83) | 81 (37–131) | 89 (28–153) |
| Pre-ART HIV RNA level, log10 copies/mL | 5.2 (4.6–5.7) | 5.2 (4.4–5.7) | 4.9 (4.5–5.5) | 5.0 (4.6–5.5) |
| History of CMV retinitis | 14 (22) | 26 (20) | … | … |
| Proximal CD4+ T-cell count, cells/mm3 | 283 (123–515) | 374 (247–541) | 286 (214–344) | 437 (286–606) |
| Proximal HIV RNA level, copies/mL | <400 | <400 | <400 | <400 |
| Duration of viral suppression, mo | 23 (7–37) | 19 (6–35) | 12 (4–65) | 17 (6–51) |
| Months between sampling date and death | 5 (3–8) | … | 5 (2–18) | … |
| Hypertension | 15 (23) | 26 (20) | … | … |
| Type 2 diabetes | 6 (9) | 14 (11) | … | … |
| Hyperlipidemia | 11 (17) | 40 (31) | … | … |
| Chronic renal insufficiency | 8 (13) | 7 (5) | … | … |
| History of injection drug usea | 10 (16) | 13 (10) | 11 (41) | 11 (20) |
| HCV status | ||||
| HCV IgG positive, HCV RNA positive | 15 (25) | 19 (15) | 15 (58) | 14 (26) |
| HCV IgG positive, HCV RNA negative | 5 (8) | 7 (6) | 1 (4) | 5 (9) |
| HCV IgG negative | 40 (67) | 97 (79) | 10 (38) | 34 (64) |
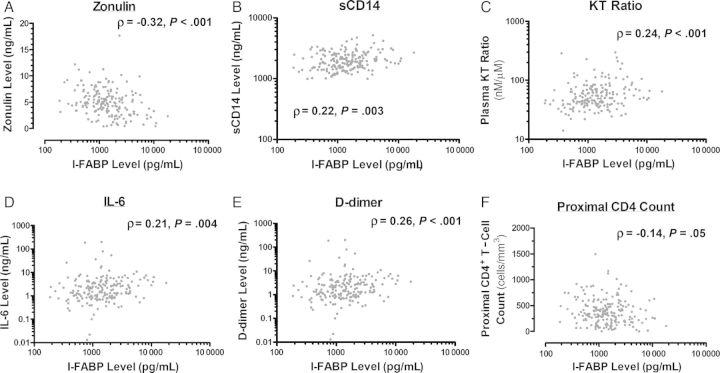
Figure 2.
Relationship between plasma markers of gut epithelial barrier integrity and innate immune activation. The relationships between plasma intestinal fatty acid binding protein (I-FABP) and the plasma zonulin-1 level (A), soluble CD14 (sCD14) level (B), kynurenine/tryptophan (KT) ratio (C), interleukin 6 (IL-6) level (D), and D-dimer level (E) and the proximal CD4+ T-cell count (F) were assessed using Spearman rank order correlations.
The 27 case and 54 control participants in the secondary SCOPE analysis were similarly well matched in age, sex, duration of viral suppression, and nadir CD4+ T-cell count but tended to have started ART at higher median nadir CD4+ T-cell counts than the LSOCA participants (88 vs 30 cells/mm3; P < .001). Compared with LSOCA participants, SCOPE participants tended to be slightly older, maintained ART-mediated viral suppression for a somewhat shorter time, were more likely to report IDU and have chronic active HCV infection. Most SCOPE cases also died of non–AIDS-defining causes (Supplementary Table 1). Compared with SCOPE controls, SCOPE cases had lower median proximal CD4+ T-cell counts (286 vs 437 cells/mm3; P = .014), were more likely to have chronic HCV infection (58% vs 26%; P = .026), and were more likely to report a history of IDU (41% vs 20%; P = .07). Data on traditional cardiovascular risk factors were unavailable.
Gut Epithelial Cell Barrier Markers Predict Mortality During Treated HIV Infection
We first assessed gut epithelial integrity markers in LSOCA participants. Plasma I-FABP is a systemic marker of gut epithelial cell death, while zonulin-1 is expressed by viable gut epithelial cells to disassemble tight junctions between cells, increasing permeability and macromolecule absorption [31]. Plasma I-FABP and zonulin-1 levels were negatively correlated (ρ = −0.32; P < .001; Figure 2A). A higher plasma I-FABP level was also associated with a higher sCD14 level, KT ratio, IL-6 level, and D-dimer level (all P ≤ .004) and a lower proximal CD4+ T-cell count (P = .05; Figure 1B_–_F). I-FABP and zonulin-1 levels also strongly predicted mortality in LSOCA participants (Figure 2B and 2C). Each IQR increase in the I-FABP level was associated with a 3.5-fold increased odds of death, while each IQR increase in the zonulin-1 level was associated with a 57% lower odds of death (both P < .001; Table 2). These relationships were unaffected by adjustment for proximal CD4+ T-cell count (Table 2) and/or by adjustment for self-reported history of chronic renal insufficiency, hyperlipidemia, or HCV status (data not shown).
Figure 1.
Relationships between soluble immunologic markers and mortality in Longitudinal Study of the Ocular Complications of AIDS (LSOCA) and SCOPE participants. The distribution of proximal CD4+ T-cell counts (A) and plasma intestinal fatty acid binding protein (I-FABP) levels (B), zonulin-1 levels (C), soluble CD14 (sCD14) levels (D), interleukin 6 (IL-6) levels (E), kynurenine/tryptophan (KT) ratio (F), soluble tumor necrosis factor receptor-1 (sTNF-R1) levels (G), high-sensitivity C-reactive protein (hsCRP) levels (H), and cytomegalovirus (CMV) immunoglobulin G (IgG) indexes are plotted for case patients who died after confirmed antiretroviral therapy–mediated viral suppression and matched controls in the LSOCA and SCOPE cohorts. P values represent the statistical significance for each marker modeled continuously in conditional regression models (Tables 2 and 3).
Table 2.
Soluble Biomarker Predictors of Mortality Among 192 Participants in the Longitudinal Study of the Ocular Complications of AIDS Who Had Antiretroviral Therapy–Suppressed Human Immunodeficiency Virus Infection
| Characteristic, Analysisa | OR (95% CI) for Death, by Quartileb | OR per IQR Increase (95% CI) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Second | P Value | Third | P Value | Fourth | P Value | |||
| Proximal CD4+ T-cell count, cells/mm3 | ||||||||
| Primary | 0.50 (.22–1.1) | .099 | 0.41 (.17–.98) | .045 | 0.44 (.18–1.1) | .076 | 0.62 (.40–.95) | .030 |
| I-FABP level, pg/mL | ||||||||
| Primary | 1.76 (.61–5.1) | .30 | 4.5 (1.5–13.3) | .007 | 8.3 (2.8–25.1) | <.001 | 3.5 (2.0–6.1) | <.001 |
| Adjusted | 1.69 (.56–5.1) | .35 | 4.2 (1.4–12.8) | .011 | 8.6 (2.7–27.8) | <.001 | 3.5 (1.9–6.1) | <.001 |
| Zonulin-1 level, ng/mL | ||||||||
| Primary | 0.29 (.12–.69) | 0.005 | 0.21 (.08–.53) | .001 | 0.24 (.09–.60) | .002 | 0.43 (.28–.64) | <.001 |
| Adjusted | 0.28 (.12–.69) | .005 | 0.20 (.08–.53) | .001 | 0.25 (.09–.64) | .004 | 0.43 (.28–.66) | <.001 |
| sCD14 level, μg/mL | ||||||||
| Primary | 2.7 (.82–9.1) | .10 | 7.7 (2.3–25.7) | .001 | 17.6 (4.4–55.1) | <.001 | 5.4 (2.8–10.4) | <.001 |
| Adjusted | 4.5 (1.09–18.6) | .038 | 11.4 (2.9–46) | .001 | 30.1 (6.2–145) | <.001 | 7.5 (3.4–16.5) | <.001 |
| KT ratio, nM/μM | ||||||||
| Primary | 1.48 (.51–4.3) | .47 | 2.3 (.82–6.5) | .11 | 4.6 (1.72–12.3) | .002 | 2.3 (1.45–3.5) | <.001 |
| Adjusted | 1.50 (.50–4.4) | .47 | 2.4 (.82–6.9) | .11 | 4.3 (1.51–12.4) | .006 | 2.3 (1.40–3.7) | .001 |
| IL-6 level, pg/mL | ||||||||
| Primary | 6.4 (1.33–30.6) | .020 | 9.8 (1.89–50.5) | .007 | 69.7 (12.4–392) | <.001 | 6.1 (2.9–12.9) | <.001 |
| Adjusted | 12.0 (1.42–102) | .023 | 17.8 (2.1–154) | .009 | 139 (14–1362) | <.001 | 6.6 (2.9–15.0) | <.001 |
| sTNF-RI level, pg/mL | ||||||||
| Primary | 1.42 (.44–4.5) | .55 | 3.8 (1.3–11.0) | .012 | 9.0 (3.1–26) | <.001 | 4.6 (2.5–8.5) | <.001 |
| Adjusted | 1.25 (.38–4.2) | .71 | 3.6 (1.23–10.8) | .02 | 10.2 (3.2–32) | <.001 | 5.2 (2.6–10.4) | <.001 |
| hsCRP level, ng/mL | ||||||||
| Primary | 1.61 (.57–4.6) | .37 | 2.1 (.78–5.6) | .14 | 10.9 (3.7–33) | <.001 | 3.7 (2.1–6.7) | <.001 |
| Adjusted | 1.58 (.53–4.7) | .42 | 2.3 (.81–6.6) | .12 | 10.9 (3.4–35) | <.001 | 3.8 (2.0–7.0) | <.001 |
| D-dimer level, ng/mL | ||||||||
| Primary | 1.33 (.35–5.1) | .68 | 5.9 (1.80–19.1) | .003 | 30.3 (7.2–128) | <.001 | 7.7 (3.6–16.7) | <.001 |
| Adjusted | 1.23 (.31–4.9) | .77 | 6.2 (1.8–21.3) | .003 | 29.4 (6.6–131) | <.001 | 7.7 (3.5–17.3) | <.001 |
| CMV IgG index | ||||||||
| Primary | 0.42 (.17–1.05) | .064 | 0.54 (.22–1.30) | .17 | 1.37 (.58–3.2) | 0.47 | 0.91 (.80–1.05) | .19 |
| Adjusted | 0.38 (.15–.98) | .045 | 0.43 (.17–1.12) | .085 | 1.07 (.42–2.7) | .89 | 0.89 (.77–1.03) | .11 |
The plasma I-FABP level appeared to be less predictive of mortality in SCOPE participants in unadjusted analysis (odds ratio [OR], 1.4 per IQR increase; P = .23), but after adjustment for CD4+ T-cell count, HCV status, and IDU history, the plasma I-FABP level tended to predict increased mortality in SCOPE participants (OR, 2.4 per IQR increase; 95% confidence interval, .99–5.8; P = .052).
Innate Immune Activation, Inflammation, and Coagulation Strongly Predict Mortality During Treated HIV Infection
We next asked whether soluble markers of monocyte activation (sCD14), IDO activity (KT ratio), inflammation (IL-6, sTNF-R1, and hsCRP), and coagulation (D-dimer) predicted mortality in LSOCA participants. Each biomarker strongly predicted mortality both in the primary analysis and after adjustment for proximal CD4+ T-cell count (OR range, 2.3–7.7 per IQR increase; all P < .001; Table 2). Adjustment for proximal CD4+ T-cell count had no effect on the relationship between each biomarker and mortality, nor did adjustment for self-reported chronic renal insufficiency, hyperlipidemia, or HCV (not shown). The associations between several biomarkers (particularly IL-6 and D-dimer) and mortality also appeared to be stronger than observed in several other recently reported cohort studies and trials [5, 9, 19]. For example, participants whose IL-6 levels were in the highest quartile had a 70-fold greater odds of mortality than those in the lowest quartile (P < .001).
We hypothesized that advanced disease stage at ART initiation might have contributed to the strength of these associations in LSOCA participants. We therefore contrasted these relationships to those observed in the SCOPE cohort, where immunodeficiency among participants was comparatively less advanced. The strength of associations between these biomarkers and mortality did appear to be weaker in SCOPE participants (OR range, 1.33–3.8 per IQR increase; Supplementary Table 2), compared with LSOCA participants, although the SCOPE analysis had less power and a longer lag between biomarker measurement and death than the LSOCA analysis. While CIs of most of these associations overlap those for the LSOCA cohort, plasma sCD14 level was not significantly associated with mortality in the SCOPE cohort either in the primary analysis or analyses adjusted for proximal CD4+ T-cell count, HCV status, and/or IDU history (not shown).
T-Cell Activation, Senescence, and CMV-Specific Responses Are Less Predictive of Mortality During Treated HIV Infection
We next assessed the association between phenotypic and functional T-cell markers that predict morbidity and mortality in either untreated HIV-infected patients or elderly HIV-uninfected individuals. As observed in Ugandans with ART-suppressed HIV infection [32], the frequency of activated (CD38+HLA-DR+) CD8+ T cells predicted increased mortality in the primary LSOCA analysis (OR, 4.2 per IQR increase; P = .031), but this association lost significance after adjustment for proximal CD4+ T-cell count (OR, 3.3 per IQR increase; P = .10; Table 3). Similar inferences were observed with the frequencies of activated CD4+ T cells and activated central memory CD8+ and CD4+ T-cell subsets. Greater naive CD4+ T-cell frequency predicted decreased mortality, although this association lost significance after adjustment for CD4+ T-cell count. Similar trends were observed for the frequency of recent thymic emigrant (CD31+) CD4+ T cells. While power was considerably lower, there was no evidence for an association between any of these markers and mortality in SCOPE participants.
Table 3.
Phenotypic and Functional T-Cell Predictors of Mortality Among 166 Participants in the Longitudinal Study of the Ocular Complications of AIDS Who Had Antiretroviral Therapy–Suppressed Human Immunodeficiency Virus Infection
| Characteristic, Analysisa | OR (95% CI) for Death, by Quartileb | OR per IQR Increase (95% CI) | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Second | P Value | Third | P Value | Fourth | P Value | |||
| D38+HLA-DR+ cells among CD8+ T cells, % | ||||||||
| Primary | 2.3 (.67–8.0) | .18 | 3.8 (1.17–12.3) | .027 | 3.1 (1.04–9.1) | .043 | 4.2 (1.14–15.6) | .031 |
| Adjusted | 2.4 (.66–8.6) | .19 | 3.4 (.98–11.6) | .053 | 2.7 (.86–8.6) | .090 | 3.3 (.79–13.6) | .10 |
| CD38+HLA-DR+ cells among CM CD8+ T cells, %c | ||||||||
| Primary | 2.1 (.70–6.4) | .19 | 3.5 (1.09–11.1) | .035 | 3.4 (1.18–10.0) | .024 | 4.1 (1.13–14.8) | .032 |
| Adjusted | 2.1 (.67–6.3) | .21 | 3.1 (.88–10.9) | .078 | 3.0 (.92–10.6) | .069 | 3.2 (.78–13.2) | .11 |
| CD38+HLA-DR+ cells among CD4+ T cells, % | ||||||||
| Primary | 0.57 (.18–1.79) | .34 | 1.23 (.41–3.7) | .71 | 1.97 (.72–5.4) | .18 | 3.4 (1.04–10.9) | .043 |
| Adjusted | 0.51 (.15–1.67) | .27 | 1.04 (.33–3.29) | .95 | 1.51 (.47–4.9) | .49 | 2.8 (.70–11.1) | .15 |
| CD38+HLA-DR+ cells among CM CD4+ T cells, %c | ||||||||
| Primary | 0.70 (.23–2.1) | .52 | 1.61 (.62–4.2) | .33 | 2.0 (.71–5.8) | .18 | 3.8 (1.11–13.3) | .034 |
| Adjusted | 0.66 (.21–2.1) | .48 | 1.41 (.50–4.0) | .52 | 1.68 (.48–5.8) | .42 | 3.4 (.74–12.2) | .12 |
| Naive cells among CD4+ T cells, %d | ||||||||
| Primary | 0.54 (.20–1.46) | .22 | 0.52 (.18–1.49) | .22 | 0.64 (.23–1.82) | .40 | 0.66 (.44–.97) | .033 |
| Adjusted | 0.67 (.24–1.93) | .46 | 0.61 (.21–1.79) | .37 | 0.83 (.27–2.52) | .74 | 0.71 (.47–1.06) | .091 |
| CD31+ naive cells among CD4+ T cells, %d,e | ||||||||
| Primary | 0.33 (.11–.99) | .050 | 0.78 (.29–2.1) | .62 | 0.52 (.16–1.6) | .27 | 0.41 (.16–1.06) | .065 |
| Adjusted | 0.33 (.11–.98) | .047 | 0.75 (.28–2.1) | .58 | 0.59 (.17–2.1) | .41 | 0.41 (.15–1.11) | .079 |
| Naive cells among CD8+ T cells, %d | ||||||||
| Primary | 0.59 (.21–1.61) | .30 | 0.42 (.14–1.25) | .12 | 0.55 (.18–1.67) | .29 | 0.49 (.22–1.13) | .095 |
| Adjusted | 0.66 (.24–1.85) | .43 | 0.50 (.16–1.53) | .22 | 0.65 (.21–2.1) | .47 | 0.55 (.24–1.27) | .16 |
| CD28− cells among CD8+ T cells, % | ||||||||
| Primary | 0.90 (.33–2.5) | .84 | 0.76 (.28–2.0) | .58 | 1.48 (.57–3.9) | .42 | 1.01 (.71–1.45) | .94 |
| Adjusted | 0.75 (.26–2.2) | .60 | 0.61 (.21–1.81) | .38 | 1.15 (.42–3.15) | .78 | 0.93 (.64–1.36) | .71 |
| CD28−CD57+ cells among CD8+ T cells, % | ||||||||
| Primary | 0.75 (.26–2.2) | .61 | 0.86 (.32–2.4) | .78 | 0.44 (.16–1.22) | .11 | 0.74 (.50–1.10) | .14 |
| Adjusted | 0.58 (.18–1.84) | .35 | 0.77 (.27–2.22) | .63 | 0.33 (.11–1.00) | .050 | 0.65 (.42–1.00) | .051 |
| CD28− cells among CD4+ T cells, % | ||||||||
| Primary | 0.43 (.15–1.25) | .12 | 0.90 (.31–2.7) | .86 | 0.57 (.21–1.56) | .27 | 0.89 (.49–1.61) | .69 |
| Adjusted | 0.36 (.12–1.15) | .084 | 0.87 (.28–2.8) | .82 | 0.51 (.18–1.45) | .21 | 0.87 (.47–1.63) | .67 |
| PD1+ cells among CD8+ T cells, % | ||||||||
| Primary | 0.73 (.22–2.4) | .60 | 0.89 (.36–2.2) | .79 | 1.31 (.45–3.8) | .62 | 1.20 (.70–2.1) | .51 |
| Adjusted | 0.70 (.21–2.3) | .56 | 0.93 (.37–2.3) | .87 | 1.19 (.40–3.5) | .76 | 1.16 (.67–2.0) | .59 |
| PD1+ cells among CD4+ T cells, % | ||||||||
| Primary | 0.88 (.34–2.3) | .80 | 0.49 (.15–1.64) | .25 | 1.04 (.34–3.01) | .95 | 1.16 (.69–1.94) | .58 |
| Adjusted | 0.74 (.26–2.09) | .56 | 0.38 (.10–1.38) | .14 | 0.61 (.17–2.2) | .45 | 0.95 (.53–1.69) | .86 |
| CMV-specific cells among CD8+ T cells, %f | ||||||||
| Primary | 2.4 (.53–11.2) | .25 | 1.33 (.29–6.1) | .71 | 0.54 (.08–3.7) | .53 | 1.12 (.64–1.97) | .69 |
| Adjusted | 2.7 (.51–14.0) | .25 | 1.64 (.27–9.9) | .59 | 0.66 (.08–5.1) | .69 | 1.25 (.67–2.3) | .48 |
| CMV-specific cells among CD4+ T cells, %f | ||||||||
| Primary | 3.6 (.57–23) | .17 | 2.0 (.44–9.2) | .37 | 0.92 (.17–4.9) | .92 | 1.61 (.77–3.4) | .21 |
| Adjusted | 4.4 (.63–31.5) | .14 | 1.92 (.40–9.3) | .42 | 0.72 (.12–4.4) | .72 | 1.56 (.73–3.3) | .25 |
While T-cell activation and naive CD4+ T-cell frequencies predicted mortality, albeit inconsistently and not entirely independent of CD4+ T-cell count, classical markers of immunosenescence failed to predict mortality. The percentages of CD8+ and CD4+ T cells that were CD28− cells, the percentage of CD8+ T cells that were CD28−CD57+ cells, and the percentage of CD8+ T cells that were CMV-specific cells all failed to predict mortality in each cohort (Table 3 and Supplementary Table 2). CMV-specific antibody levels also failed to predict mortality in LSOCA participants (Table 2). Notably, the percentage of CD8+ T cells that were CD28−CD57+ cells, a senescence marker that predicts increased mortality in elderly HIV-uninfected individuals, actually appeared to predict decreased mortality in LSOCA participants, after adjustment for proximal CD4+ T-cell count (OR, 0.65 per IQR increase; P = .051). The percentages of CD4+ and CD8+ T cells that were PD1+ cells also failed to predict mortality in both cohorts.
DISCUSSION
While innate immune activation, inflammation, and coagulation markers predict mortality in treated HIV infection, it has remained unclear whether gut epithelial barrier integrity, T-cell activation, exhaustion, and senescence markers also predict mortality in this setting. In our study, gut epithelial barrier function markers (I-FABP and zonulin-1) strongly predicted mortality in individuals with ART-suppressed HIV infection and a history of AIDS, supporting microbial translocation as an interventional target. We also demonstrated that soluble innate immune activation, inflammation, and coagulation markers tended to predict mortality more strongly in cohorts with greater immunodeficiency, consistent with the hypothesis that the inflammatory state may be a more important mediator of mortality in this setting. While T-cell activation also predicted mortality, this effect was inconsistent between cohorts and may have been confounded (or mediated) by proximal CD4+ T-cell count. Last, T-cell senescence phenotypes that predict mortality in elderly individuals failed to predict mortality in treated HIV infection, suggesting distinct immunologic mechanisms mediating disease in these settings.
This is the first study to demonstrate that gut epithelial barrier integrity markers predict mortality in treated HIV infection. Sandler et al previously reported that while plasma sCD14 levels predicted mortality in the SMART trial, I-FABP levels did not [18]. Since sCD14 may be released from monocytes in response to stimuli other than LPS, question remained as to whether microbial translocation predicted mortality. Our study demonstrated that among individuals with ART-suppressed HIV infection and a history of AIDS, the gut epithelial barrier function markers I-FABP and zonulin-1 correlated with soluble markers of monocyte activation, inflammation, and coagulation and strongly predicted increased mortality. Zonulin-1 is made by gut epithelial cells; disassembles tight junctions, thereby increasing intestinal permeability; and is present in increased levels in celiac and other inflammatory diseases [31]. It is unclear why lower zonulin-1 levels predicted mortality in LSOCA participants, but greater gut epithelial cell death or dysfunction during AIDS [33] might decrease its expression. While I-FABP levels also appeared to predict mortality in an adjusted analysis of data from SCOPE participants, the association between I-FABP levels (and sCD14 levels) and mortality appeared weaker in SCOPE participants than in LSOCA participants. While these results could be explained by differential power or demographic characteristics between cohorts, they are also consistent with the hypothesis that gut mucosal damage and microbial translocation is a less important driver of mortality in individuals with less advanced pre-ART immunodeficiency. If confirmed in other studies, this might suggest that interventions to decrease microbial translocation might be best targeted to individuals with lower nadir CD4+ T-cell counts.
Our finding that the plasma KT ratio, a marker of IDO activity (and/or gut microbe–induced tryptophan catabolism [20]) predicted mortality in HIV-infected individuals maintaining ART-mediated viral suppression for nearly 2 years in the LSOCA extends earlier observations from a cohort of HIV-infected Ugandans initiating ART [23] to a resource-rich setting and to individuals with a longer duration of suppressive ART receipt. We also showed that the plasma KT ratio was strongly associated with gut epithelial barrier dysfunction markers, consistent with the hypothesis that IDO induction may be both a cause (via Th17 depletion) and/or a consequence of microbial translocation [22]. These results support kynurenine pathway enzymes as targets for future interventional studies.
Innate immune activation, inflammation, and coagulation markers also appeared to predict mortality more strongly in LSOCA participants than in SCOPE participants, raising the possibility that these immunologic defects may be even more important drivers of disease among those who initiated ART at a more advanced disease stage. While differential power, lag between biomarker measurement and death, and demographic factors may have also contributed to differential inferences between cohorts, several prior studies in populations with less advanced immunodeficiency have observed weaker associations between these biomarkers and mortality than in the LSOCA population [5, 7–11]. This is consistent with a possible immunologic cost to delaying ART even if viral suppression is eventually achieved and CD4+ T-cell counts restored, a hypothesis supported by recent observational studies [34–36] but yet to be proven in a clinical trial.
While our LSOCA analysis confirmed recent observations from our Ugandan cohort that CD8+ T-cell activation predicts mortality during ART-mediated viral suppression [32], this effect was no longer significant after adjustment for proximal CD4+ T-cell count. It is possible that the association between CD8+ T-cell activation and mortality is partially mediated by blunted recovery in the CD4+ T-cell count, as has been suggested in several prior studies [32, 37–40]. Nevertheless, T-cell activation failed to predict mortality in the SCOPE cohort, which had participants with less advanced immunodeficiency. Other recently reported studies have also failed to find an association between T-cell activation and mortality in treated HIV infection [10, 41]. Thus, T-cell activation may predict mortality in untreated HIV infection and to a lesser degree in patients with advanced immunodeficiency who are undergoing treatment, settings in which persistent T-cell immunodeficiency may play an important role in susceptibility to opportunistic infections and malignancies, but not necessarily in treated patients with less advanced immunodeficiency. Conversely, innate immune activation and coagulation markers may be more robust predictors of typical non–AIDS-defining morbidities, such as cardiovascular conditions and other end points, that now explain the majority of deaths in the modern treatment era. Thus, for most treated HIV-infected individuals, innate immune activation and its determinants are probably more appropriate targets for interventions than T-cell activation. Furthermore, T-cell activation may no longer be the most appropriate surrogate marker to use as a primary outcome measure in pilot trials of immune-based interventions in treated HIV infection, despite its smaller within-subject variability and established responsiveness to interventions [42–44].
Last, our study demonstrated that T-cell senescence and CMV-specific immune responses, which predict morbidity and mortality in elderly HIV-uninfected individuals [26, 45–47], fail to predict mortality in treated HIV infection. This may suggest that the immunologic defects that drive disease in treated HIV infection may be distinct from those that drive disease in elderly populations. The lack of an association between CMV-specific antibody titers or T-cell frequencies and mortality in our study also suggests that the adaptive immune responses directed against CMV is unlikely to be a direct mediator of morbidity and mortality in this setting. Nevertheless, asymptomatic CMV replication may contribute in some other way to immune activation and morbidity and mortality in this setting, a hypothesis supported by a recent clinical trial of valganciclovir [44] and by several observational studies [48, 49].
Our study has important limitations. First, participants in both cohorts had relatively advanced AIDS before ART initiation. Thus, it is unclear whether the mortality associations reported here will be generalizable to populations with less advanced immunodeficiency. Few cohorts of individuals with ART-suppressed, less advanced immunodeficiency from whom viable PBMC specimen are regularly obtained and stored have sufficient numbers of deaths to address this question. Second, the relatively short period between biomarker measurement and death in our study raises the possibility that biomarker abnormalities are simply a consequence of a soon-to-be-fatal illness rather than a cause. This seems less likely, however, since the most common cause of death among known causes in our study was cardiovascular disease. Third, since no direct microbial translocation measures were performed, we cannot confirm whether the gut barrier integrity associations with mortality are mediated by microbial translocation. Fourth, since smoking history and other potentially important lifestyle factors were not measured in the cohorts, we cannot definitively exclude confounding by these factors, but this seems unlikely to explain the dramatic biomarker associations we observed here, as these same factors are prevalent in HIV-uninfected populations in which inflammatory markers predict mortality much less robustly. Fifth, since this study was observational, we cannot provide assurance that the immunologic processes represented by the biomarkers reported here are causally associated with mortality. Such evidence can only come from large clinical end point trials of targeted interventions. Nevertheless, our study may help narrow and prioritize the list of interventional targets to pursue. Finally, many of the biomarkers reported here are interrelated and represent not just discrete linear pathways, but complex biological systems with multiple intermediary steps and feedback loops. Thus, the biomarker most strongly associated with mortality may not necessarily be the most appropriate direct target for interventions. More-sophisticated mediation modeling approaches may prove useful in further prioritizing targets.
In summary, our study demonstrated that gut epithelial barrier integrity markers predict mortality in patients with ART-suppressed HIV infection and a history of AIDS, supporting gut barrier dysfunction and microbial translocation as important targets for interventions in this setting. We also demonstrated strong and consistent associations between mortality and innate immune activation, inflammation, and coagulation markers—but not necessarily between mortality and T-cell activation, senescence, and exhaustion—which should narrow the list of potential interventional targets and surrogate outcomes to consider in future clinical trials of immune-based therapies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Data
Notes
Acknowledgments. We thank the study participants who contributed to this work; the clinical research staff of the LSOCA and SCOPE cohorts who made this research possible; and the Cleveland Immunopathogenesis Consortium, for facilitating helpful scientific discussion (in particular Netanya Sandler Utay, Jason Brenchley, and Danny Douek).
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH; grants R56AI100765, R21 AI087035, RO1 AI087145, K24AI069994, 1K99HL108743, and P01AI076174); the UCSF/Gladstone Institute of Virology and Immunology CFAR (grant P30 AI027763); the UCSF Clinical and Translational Research Institute Clinical Research Center (grant UL1 RR024131); the Center for AIDS Prevention Studies (grant P30 MH62246); the CFAR Network of Integrated Systems (grant R24 AI067039); and the National Eye Institute, NIH (grants U10EY008052, U10EY008057, and U10EY008067).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. Hogg R, Lima V, Sterne JAC, et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 3.van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527–35. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 4.Lewden C, Bouteloup V, De Wit S, et al. All-cause mortality in treated HIV-infected adults with CD4 >/=500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol. 2012;41:433–45. doi: 10.1093/ije/dyr164. [DOI] [PubMed] [Google Scholar]
- 5.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duprez DA, Kuller LH, Tracy R, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207:524–9. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–46. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenorio A, Zheng E, Bosch R, et al. Soluble markers of inflammation & coagulation, but not T-cell activation, predict non–AIDS-defining events during suppressive antiretroviral therapy [abstract 790]. Program and abstracts of the 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. 2013. [Google Scholar]
- 11.Ledwaba L, Tavel JA, Khabo P, et al. Pre-ART levels of inflammation and coagulation markers are strong predictors of death in a South African cohort with advanced HIV disease. PLoS One. 2012;7:e24243. doi: 10.1371/journal.pone.0024243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–9. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–17. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–95. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–9. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letendre S, Marcotte T, Deutsch R, et al. A concise panel of biomarkers diagnoses and predicts neurocognitive status in HIV-infected individuals [abstract 82]. Program and abstracts from the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington.2012. [Google Scholar]
- 18.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–94. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006438. 193ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000632. 32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byakwaga H, Boum Y, II, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210:383–91. doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 25.Giorgi JV, Lyles RH, Matud JL, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29:346–55. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 26.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–53. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee SA, Sinclair E, Jain V, et al. Low proportions of CD28- CD8+ T cells expressing CD57 can be reversed by early ART initiation and predict mortality in treated HIV infection. J Infect Dis. 2014;210:374–82. doi: 10.1093/infdis/jiu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208:50–6. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatano H, Scherzer R, Wu Y, et al. A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. J Acquir Immune Defic Syndr. 2012;61:317–25. doi: 10.1097/QAI.0b013e31826e7d0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Louie A, Yang Q, et al. A simple LC-MS/MS method for determination of kynurenine and tryptophan concentrations in human plasma from HIV-infected patients. Bioanalysis. 2013;5:1397–407. doi: 10.4155/bio.13.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–75. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 32.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–31. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 34.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain V, Hartogensis W, Bacchetti P, et al. Antiretroviral therapy initiated within six months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208:1202–11. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinikoor M, Cope A, Gay C, et al. ART started during acute HIV infection failed to prevent persistent immune activation [abstract 554]. Program and abstracts of the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington, 2012. [Google Scholar]
- 37.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 38.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 39.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Hunt PW, Hammer SM, Cespedes MS, Patterson KB, Bosch RJ. Immune activation while on potent antiretroviral therapy can predict subsequent CD4+ T-cell increases through 15 years of treatment. HIV Clin Trials. 2013;14:61–7. doi: 10.1310/hct1402-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lok J, Hunt P, Collier A, et al. The impact of age on the prognostic capacity of CD8+ T-cell activation during suppressive antiretroviral therapy. AIDS. 2013;27:2101–10. doi: 10.1097/QAD.0b013e32836191b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunt PW, Shulman NS, Hayes TL, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood. 2013;121:4635–46. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Read SW, DeGrezia M, Ciccone EJ, et al. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS One. 2010;5:e11937. doi: 10.1371/journal.pone.0011937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–83. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadrup SR, Strindhall J, Kollgaard T, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–53. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 46.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–83. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vescovini R, Biasini C, Telera AR, et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol. 2010;184:3242–9. doi: 10.4049/jimmunol.0902890. [DOI] [PubMed] [Google Scholar]
- 48.Deayton JR, Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. 2004;363:2116–21. doi: 10.1016/S0140-6736(04)16500-8. [DOI] [PubMed] [Google Scholar]
- 49.El Amari EB, Combescure C, Yerly S, et al. Clinical relevance of cytomegalovirus viraemia. HIV Med. 2011;12:394–402. doi: 10.1111/j.1468-1293.2010.00900.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data