Recurrent de novo mutations implicate novel genes underlying simplex autism risk (original) (raw)
. Author manuscript; available in PMC: 2015 May 24.
Published in final edited form as: Nat Commun. 2014 Nov 24;5:5595. doi: 10.1038/ncomms6595
Abstract
Autism spectrum disorder (ASD) has a strong but complex genetic component. Here we report on the resequencing of 64 candidate neurodevelopmental disorder risk genes in 5,979 individuals: 3,486 probands and 2,493 unaffected siblings. We find a strong burden of de novo point mutations for these genes and specifically implicate nine genes. These include CHD2 and SYNGAP1, genes previously reported in related disorders, and novel genes TRIP12 and PAX5. We also show that mutation carriers generally have lower IQs and enrichment for seizures. These data begin to distinguish genetically distinct subtypes of autism important for etiological classification and future therapeutics.
Introduction
Over the past several years, we and others have used whole-exome sequencing of families with a single ASD proband to identify de novo coding mutations1, 2, 3, 4, 5, yielding hundreds of new candidate ASD risk genes. However, recurrent disruptions have only been observed in a handful of genes. Furthermore, cohort sizes are approaching a scale where even recurrence is not necessarily significant when sequencing the entire exome. We previously demonstrated using modified molecular inversion probes (MIPs) that we could economically resequence a modest number of candidate genes across large cohorts6. We concurrently developed a statistical approach to rigorously implicate ASD risk genes based on a recurrent disruption model that takes into account known and predicted mutational biases and multiple hypothesis testing.
Here we further explore this paradigm by successfully resequencing 64 genes in 4,260 new and 1,719 previously exome-sequenced individuals from two large family-based simplex ASD cohorts. We compare de novo mutation burden between probands and unaffected siblings finding a strong enrichment for new mutations overall, especially those that are predicted to be more severe. Probands with mutations have a lower IQ distribution and are enriched for reports of seizures. Based on our recurrent disruption model, we specifically implicate nine individual genes with ASD risk. We believe that firmly implicating new genes that are mutated in a sizable fraction of children with ASD provides the motivation for further functional and translational studies of these specific genes.
Results
Resequencing of 64 genes in large simplex ASD cohorts
We selected candidate genes from previously published reports and our own unpublished data (discovery set). These included de novo mutation calls from exome sequencing of 1,308 probands (1,157 ASD (208 (ref 9)) and 151 intellectual disability (ID)) and 803 unaffected siblings or controls (195 (ref 9)))1, 2, 3, 4, 5, 7, 8, 9. We selected genes that were recurrently disrupted or with at least one putative severe (nonsense, canonical splice site, or small insertion/deletion (indel)) event found exclusively in probands. We excluded genes based on high-predicted mutability or known associations with likely unrelated conditions (OMIM). We ranked genes based on recurrence, presence in protein-protein interaction (PPI) networks, predicted role in chromatin regulation, and our previously unpublished exome data (Methods). The final set consisted of 64 candidate genes (Supplementary Table 1)—56 novel neurodevelopmental candidates and eight genes screened previously in a subset of the samples6.
We designed MIPs using an improved design implementation10 and also incorporated molecular tagging11. Specifically, the MIP backbone contains a degenerate region, such that each independent MIP capture event is associated with a single-molecular (sm) tag that can be used to identify PCR duplicates and to more accurately count mutation allele frequencies—analogous to PCR subcloning. The final design included 2,928 smMIPs that were individually synthesized, pooled, and tested using control DNA. Probe concentrations were modified based on empirical performance to generate a rebalanced working probe set.
We targeted two well-described simplex (sporadic) ASD cohorts that meet ASD criteria on both of the current gold-standard autism diagnostic assessments (ADI-R and ADOS), the Simons Simplex Collection (SSC)12 and The Autism Simplex Collection (TASC)13. Exome sequencing was available for a portion of the SSC individuals, which was used as the discovery set (Supplementary Table 2). While 8 of the 64 targeted genes overlap our previous study6, here we captured and sequenced ~2,600 individuals that had not been previously MIP or exome sequenced, including all of TASC (n = 921 probands, 124 siblings), unaffected siblings from the SSC (n = 1,638) and a small number of additional SSC probands. In addition, to identify de novo mutations that may have been missed by exome sequencing, we resequenced 974 ASD probands and 773 unaffected siblings from this exome discovery set using our smMIP pool.
In total, 3,486 probands (2,527 MIP sequenced; 959 MIP and exome sequenced) and 2,493 siblings (1,733 MIP sequenced; 760 MIP and exome sequenced) passed capture and other QC measures (Supplementary Table 2, Methods). We discovered >1,400 rare variants predicted to alter protein sequence or gene splicing and evaluated these sites in relevant parents by resequencing with smMIP subpools or the full probe set. Potential de novo events were confirmed using Sanger sequencing and paternity was firmly established using common variant calls across the full probe set.
Mutation analysis in the MIP only ASD dataset
For our primary analysis of mutation burden and individual gene recurrence, we restricted our analysis to only the newly sequenced 2,527 probands and 1,733 siblings. In probands, we discovered 56 de novo mutations in 27 of the 64 candidate genes (Table 1 and Supplementary Data 1). This total includes 16 mutations in the eight genes screened previously in a subset of the samples6. The most mutated gene was CHD8 with 11 total mutations (20% of all proband de novo mutations). In addition, we observed three or more mutations in PTEN, TBR1, SYNGAP1, CHD2, ADNP, GRIN2B, and TRIP12. In stark contrast, we observed only 14 de novo mutations in unaffected siblings for 11 of the 64 genes. No genes were found to have more than two de novo events in siblings. Importantly, the increased sensitivity of the smMIPs allowed for identification of five events (three in probands and two in siblings) with skewed allele frequencies likely reflecting mosaic mutations (Supplementary Data 1). Most notable was a heterozygous nonsense mutation in ADNP identified in a proband that shows evidence of low-level mosaicism (~10% allele frequency) in the child’s mother (Supplementary Fig. 1).
Table 1.
Summary of proband de novo mutations for exome and MIP sequencing results for individual genes that were significantly deviated from the simulation-based expectation
| n = Probands | Exome Events 1,157 | MIP Events 2,757 | Total ASD Events 3,681 | ||||
|---|---|---|---|---|---|---|---|
| Significant Genes% | severe | missense | severe | missense | severe | missense | combined |
| CHD8 | 5 (2) | 0 | 7 | 4 | 12 | 4 | 16 |
| CHD2 | 1 (1) | 1 | 3 | 0 | 4 | 1 | 5 |
| PTEN | 0 | 1 | 1 | 4 | 1 | 5 | 6 |
| TBR1 | 2 (1) | 1 | 0 | 3 | 2 | 4 | 6 |
| ADNP | 1 | 0 | 3 | 0 | 4 | 0 | 4 |
| GRIN2B^ | 2 (1) | 0 | 1 | 2 | 3 | 2 | 5 |
| SYNGAP1 | 1 (1) | 0 | 2 | 1 | 3 | 1 | 4 |
| TRIP12 | 1 | 0 | 1 | 2 | 2 | 2 | 4 |
| PAX5 | 1 | 0 | 1 | 1 | 2 | 1 | 3 |
The overall rate of protein-altering mutations for these 64 genes is 2.7-fold greater in the probands compared to the unaffected siblings (Supplementary Table 3; 0.022 vs. 0.0081 mutations/individual; rate ratio test, one-sided p-value = 0.000199; 1.4% differential). The distribution of proband mutations is markedly skewed toward mutations predicted to be severe (nonsense, splice site, and indels) relative to missense changes (observed 46.4% severe; expected 16% severe; binomial P(X ≥ 26) = 9.1×10−8), while the sibling mutations fit the null expectation (observed 14.3% severe). Consistent with this, the rate of severe mutations is more markedly skewed in probands compared to unaffected siblings (0.010 vs. 0.0012 mutations/individual; 8.92-fold; 95% CI 2.61-Inf; rate ratio test, one-sided p-value = 8.8 ×10−5), while the rate of missense mutations is only modestly greater (0.012 vs. 0.0069 mutations/individual; 1.71-fold; 95% CI 0.94-Inf; rate ratio test, one-sided p-value = 0.07285).
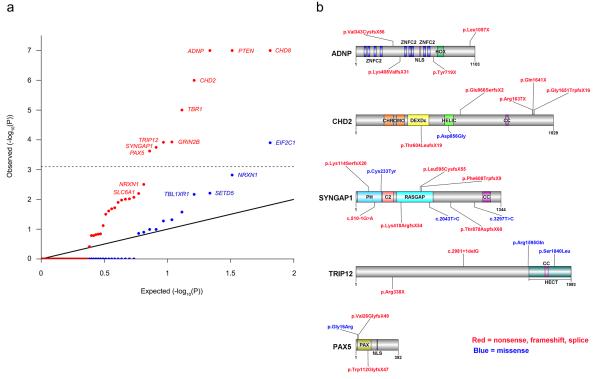
We also evaluated the observed results in the context of a recurrent mutation simulation model that takes into account mutation type (missense vs. severe) and differences in relative mutation rates between genes6. Importantly, this model can provide relative confidence estimates for each gene based on the current evidence. Others have proposed similar models and approaches14, 15, 16. Again, we observed a marked mutation burden in probands, as simulation reported on average only 6.6 missense mutations (vs. 30 observed) and 0.82 severe mutations (vs. 26 observed) (Supplementary Table 4). When evaluating the individual genes, nine were significantly mutated in the probands, implicating these loci in ASD risk to various degrees (Fig. 1 and Supplementary Fig. 2). We previously reported four of these—CHD8, PTEN, TBR1, _GRIN2B_—as significant in a smaller ASD cohort and one additional gene, ADNP, was previously insignificant6. The other significant genes include CHD2 and SYNGAP1, previously reported in related disorders with potential ASD phenotypic overlap14, 17, 18, 19, 20, 21, and novel genes TRIP12 and PAX5 (Fig. 1b). In contrast, in the sibling analysis only one gene, EIF2C1, was marginally significant (Fig. 1a).
Figure 1. smMIP resequencing of 64 genes implicates specific genes in ASD.
(a) Quantile-Quantile plot comparing the individual gene recurrence mutation simulation results from the MIP-only samples (2,757 ASD probands, 1,733 unaffected siblings) to a uniform distribution (Methods). Proband data are plotted in red, sibling data in blue. Proband data implicate nine genes as significantly disrupted in ASD. Dashed line indicates a Holm-Bonferroni corrected significance level. (b) Protein diagrams of five genes with significant recurrent de novo mutations. Annotated protein domains are shown (colored blocks) for the largest protein isoforms. Mutations shown above the protein structure were newly identified in this study using MIPs. Mutations shown below the protein structure have been previously reported from exome sequencing of ASD/ID cohorts or MIP-based resequencing6. Red variants are nonsense, frameshifting, or splice site. Domain abbreviations: ZNFC2, zinc finger; NLS, nuclear localization signal; HOX, homeodomain; CHROMO, chromatin organization modifier; DEXDc, DEAD-like helicases superfamily; HELIC, helicase superfamily C-terminal; CC, coiled coil; PH, pleckstrin homology; RASGAP, GTPase-activator for Ras-like GTPases; HECT, homologous to E6-AP carboxyl terminus; PAX, paired box.
Mutation analysis in combined ASD datasets
As a secondary analysis we evaluated the full data set of ASD exome plus MIP-identified events. Overall smMIPs showed good sensitivity, detecting 54/58 de novo events previously identified and 10 new de novo mutations not previously identified in these individuals by exome sequencing. The smMIP false negatives were the result of low coverage or poor quality data for these specific sites. The severe mutation rate is ~28-fold higher in probands (Supplementary Table 3; pro: 0.022, sib: 0.00079, diff: 0.021), and missense mutations are ~2-fold in excess (pro: 0.0136, sib: 0.0060, diff: 0.0076) (3% differential overall, though likely an overestimate as it is inclusive of nominating events in all genes). In the proband simulation, eight genes with recurrent mutations reach a conservative Bonferroni genome-wide significant threshold, including DYRK1A6 (Supplementary Fig. 3 and Supplementary Table 5). Despite not reaching significance in the resequencing data alone, DSCAM has three severe de novo mutations (Supplementary Fig. 4). If eventually implicated further, DSCAM would join DYRK1A as genes in the Down syndrome critical region22, 23 that are also risk factors for ASD, suggesting a dosage reciprocity effect for these two critical genes—where increases in copy associate with Down syndrome/ID and reductions associate with ASD.
Common mutation comorbidities
The association of lower IQ with more severe de novo mutations en masse has been described repeatedly1, 24, 25. We discover similar skewed (from the Gaussian distribution) mean and median IQ distributions for the non-mutation carriers in both SSC and TASC cohorts [SSC: 85.6/89, TASC: 84.6/86]. We find the IQ distribution of the mutation carriers (n = 52) to be significantly lower compared with the mutation negative samples, with approximately half in the ID range [mean/median: 69.2/72, Wilcox P < 1×10−4] (Supplementary Fig. 5). This distribution deviation is true for both missense and severe mutations, which is notable given the weak signal for missense mutations and lower IQ exome-wide. Among the nine significant genes from the primary analysis, only three have mutation carriers exclusively in the ID IQ range: ADNP [range 19-55], GRIN2B [55-65], and TRIP12 [53-72]. Based on the strict autism inclusion criteria (ADI-R+ADOS), we can be confident that these cases meet the current clinical criteria for ASD. How generalizable these genetic risk factors will be to individuals with ID or developmental delay that do not meet autistic diagnostic criteria is an important area of future research that will require targeted sequencing of large cohorts of patients with developmental delay26.
Seizures are a comorbid condition often associated with ASD27. In the primary data (MIP only) 98/2,282 (4.3%) probands without mutations (with reports) have a history of seizures or have a parent report of possible seizures. Among de novo mutation carriers, 6/51 (12%) are positive for seizures, which is a significant enrichment (Fisher’s exact test, p = 0.024, Odds ratio: 2.971, 95% CI: 1.112-7.481). In the full data set (including the exome data samples, which may include some selection bias) for those with available seizure history data, a stronger association is seen. Seizures are reported at a much higher rate in the mutation carriers [23/123 (19%)] versus those without mutations [151/3611 (4%)] (Fisher’s exact test, p < 0.00001, Odds ratio: 5.2, 95% CI: 3.3-8.5).
Discussion
Moving from candidate gene discovery to gene validation has been a major challenge for neurodevelopmental/psychiatric genetics. With sets of high-confidence risk genes and the ability to recontact and evaluate individual patients, it is now feasible to use this type of genotype-first approach to link significant genotypes to clinical phenotypes in ASD and related disorders28. Moreover, specific genotypes may span our current diagnostic categories in unexpected ways. Initial efforts in this vein with relatively small cohorts (<10) have already proven fruitful. Through a parallel study, we showed that CHD8, a chromatin remodeling factor, is linked to a clinical phenotype, including significant macrocephaly, distinct facial features, and gastrointestinal defects, which can be modeled in zebrafish29. In this study we identified seven novel CHD8 mutations, some presenting with phenotypes similar to those previously reported (Supplementary Data 2). However, we also identified missense mutations (not previously observed), 3/4 of which do not demonstrate reported macrocephaly. Similarly, mutations in ADNP, a transcription factor involved in the SWI/SNF remodeling complex, are linked to a shared clinical phenotype including ID and a characteristic facial dysmorphism30. In our case, the four probands with ADNP mutations all have a confirmed ASD diagnosis and are also severely impaired cognitively (Supplementary Data 2).
Two genes reported here, CHD2 and SYNGAP1, have been implicated now across several neurodevelopmental disorders. SYNGAP1 de novo mutations have been observed in patients with ID, with or without autism and/or seizures, and epileptic encephalopathies, again with or without ASD features17, 18, 19. CHD2, chromodomain helicase DNA binding protein 2, is a chromatin remodeling factor for which de novo deletions have been associated with recurrent clinical symptoms, including developmental delay, ID, epilepsy, behavioral problems and ASD-like features without characteristic facial gestalt or brain malformations14, 20 and de novo point mutations have been associated again in epileptic encephalopathies with ID and occasionally ASD17. In our four CHD2 mutation carriers, all have confirmed autism that is skewed toward higher ASD severity, according to the clinician-derived ADOS-calibrated severity scores as well as parent and teacher reporting on the Social Responsiveness Scale (SRS), three have reports of seizures, and there are a range of cognitive impairments. In contrast, of the four SYNGAP1 mutation carriers none report seizures (one unknown). Interestingly, similar methods have now firmly implicated SYNGAP1 in both ID21 and ASD (presented here) based on independent datasets/analyses.
These efforts have also contributed novel candidates for clinical follow-up, including the genes TRIP12 and PAX5. Very little is known about what role TRIP12, thyroid hormone receptor interactor 12, may play in neurodevelopment having been primarily described as a protein with E3 ubiquitin-protein ligase activity involved in the ubiquitin fusion degradation pathway and the regulation of DNA repair31. PAX5, paired box protein 5, is a transcription factor and has been shown as significantly downregulated in bipolar disorder postmortem hippocampal extracts32. Conditional knockout of Pax5 in GABAergic neurons in mice highlight the necessity for this gene in normal ventricular development33 further promoting a specific role for Pax5 in the etiology of ASD. Preliminary analysis based on existing data shows TRIP12 mutation carriers all have ASD and ID ranging from mild to moderate severity while the individuals with PAX5 demonstrate a higher range of cognitive ability from borderline to average intelligence concomitant with ASD. Individuals with both genes display variation in physical features (e.g., head size), presence of regression, language ability, and other psychiatric features. While this study focused on autism cohorts, we note that combining our results with other comorbid phenotypes, such as ID, reveals additional genes such as PPP2R5D. In addition to autism, the preliminary data on both individuals with PPP2R5D highlight short stature and problems with excessive sleepiness, warranting further follow up. The ongoing genetic classification of distinct subtypes of neurodevelopmental disorders promises to revolutionize not only our understanding of the biologic nature of these conditions and their expressivity but also the precision treatment of patients moving forward.
Methods
Human subjects
All participants completed informed consent/assent before participation in the original data collection study12, 13. Approval for sequencing was obtained from each local institutional review board. All samples and phenotypic data were de-identified prior to release. DNA samples were obtained from the Rutgers University Cell and DNA Repository through the Simons Foundation Autism Research Initiative (SSC) or National Institute on Mental Health (TASC). This project was deemed human subjects exempt by the University of Washington Human Subjects Division. MIP only samples were excluded from families if: the proband they did not meet criteria for ASD on ADI-R and ADOS, possible multiple affected individuals was indicated, or and if both parents were not available. We did not exclude any individuals from the previously published exome datasets.
Gene selection
We combined and harmonized annotations of de novo mutation calls from exome sequencing of 1,308 probands (1,157 ASD (208 (ref 9))) and 151 ID) and 803 unaffected siblings or controls (ref 9 in press) 1, 2, 3, 4, 5, 7, 8, 9. We further annotated the 954 genes with at least one predicted protein-altering mutation in probands with mutability estimates and known disease/disorder associations. Similar to reported previously, we conducted a protein–protein interaction (PPI) analysis using either the set of genes as nodes with either (1) one proband truncating or splice-disrupting event (trunc network) or (2) missense (Grantham score ≥ 50 and GERP score ≥ 3 or Grantham score ≥ 85), other indels, and truncating events (severe network). Human PPI data were collected from GeneMANIA34 on 29 August 2011. Only direct physical interactions from the Homo sapiens database were considered. We then selected 64 genes prioritizing those that were recurrently disrupted or with at least one putative severe (non-missense) event found exclusively in probands, low predicted mutability, membership in the major connected component of either PPI analysis set, predicted role in chromatin regulation, or novelty (i.e., for our own unpublished exome data) (Supplementary Tables 6-7, Supplementary Fig. 6-7). We excluded a small number of strong candidates being screened by other studies. For example, SCN2A, POGZ, and KATNAL2 were all identified with multiple mutations in the discovery set. We elected not to include these in our set because we were aware on an ongoing effort by others to sequence these genes. The final set included eight genes screened previously in a subset of the samples6.
MIP design and testing
All smMIPs were designed with an updated scoring algorithm described recently10. MIP arms + predicted gap fill were set to total a fixed length of 162 base pairs (bp). Individual arms ranged from 15-30 bp. Oligonucleotides (IDT, Coralville, IA) were ordered with five degenerate bases between the end of the common linker and the extension arm allowing for a maximum theoretical non-duplicate coverage of (4^5 = 1,024). Probes were pooled by gene and then combined in equal molar ratios and phosphorylated (1X pool). After initial testing, the bottom 400 performing probes were repooled and phosphorylated (50X pool). The next 242 poorest performing probes were repooled and phosphorylated (10X pool). A final working probe pool was then generated by combining the three pools so that the final concentration of each MIP in the 10X and 50X initial pools was a 10- or 50-fold excess relative to the 1X pool concentration.
Multiplex capture and amplification of targeted sequences
Hybridization of smMIPs to genomic DNA, gap filling and ligation were performed in one 25 μl reaction of 1X Ampligase buffer (Epicentre, Madison, WI) with: 120 ng of genomic DNA, 0.32 μM dNTPs, 0.5X of Hemo KlenTaq (0.32μl) (New England Biolabs, Inc., Ipswich, MA), one unit of Ampligase (Epicentre), and MIPs. MIP concentration was based on a ratio of 667 copies of each MIP to each haploid genome copy, based on the 1X pool concentration. Reactions were incubated at 95°C for 10 min then at 60°C for 18 h. Exonuclease treatment and amplification of the captured DNA was performed as previously described6. We pooled 5 μl of ~96 different barcoded libraries together and purified the pools with 0.8X AMPure XP beads (Beckman Coulter, Brea, CA) according to the manufacturer’s protocol. Libraries were resuspended in 100 μl of 1X EB (Qiagen, Valencia, CA). Pools were quantified in duplicate using the Qubit dsDNA HS Assay (Life Technologies, Grand Island, NY). Multiple libraries were combined to create the final megapools of ~192 individual capture reactions for sequencing. One lane of 101 bp paired-end reads was generated for each megapool on an Illumina HiSeq 2000 according to manufacturer’s instructions.
MIP sequencing analysis
Sequencing reads were analyzed as described previously6 with minor modifications. The 5 bp degenerated sequence was removed from the beginning of read2 and added to the index barcode to create a sample-tag index. For primary single nucleotide variation (SNV), indel, and target coverage analysis, reads were trimmed to 81 bp prior to mapping. Indels were also called by mapping the full-length reads. After mapping, read-pairs with incorrect pairs and insert sizes were removed. For capture events with identical tag sequences only the read-pair with the highest sum of quality scores was used. MIP targeting arm sequences were then removed. On average, >95% of target coding regions had ≥10X coverage. Coverage distributions were generally consistent across plates/samples (Supplementary Fig. 8-11) and between proband and sibling samples when analyzed by gene (Supplementary Fig. 12-15). Captures with less than 75% of the target at greater than 10-fold coverage were removed from analysis. SNV calls required a minimum of 8-fold coverage with a consensus or variant quality score of 30 or higher and an allele balance less than or equal to 0.80 (considering Q20 bases only). Indel calls required at least 25% of reads to support an event. Variants were removed from the potential de novo validation set if present in Exome Variant Server (ESP6500SI-V2), multiple independent families, or siblings from the same family.
Validations
Rare, potentially protein-altering events (nonsense, splice site, indel, and missense) in children were tested for inheritance status by MIP-based resequencing of their parents using subpools of MIPs grouped by gene, containing approximately 6-13 genes per pool. Parent samples/sites that failed using the subpools were captured using the full probe set. Variants that appeared de novo or failed the full pool capture were further validated with PCR and Sanger sequencing. Samples were removed if non-paternity was suspected or parental DNA was absent or failed to amplify.
Statistics
Comparisons of de novo rates in probands versus siblings were compared using the exact rate ratio test (one-sided) implemented in the R package rateratio.test. For recurrence mutation simulation, we used the same probabilistic framework developed previously6 that incorporates the overall rate of mutation in coding sequences, estimates of relative locus-specific rates based on human-chimpanzee fixed differences in each gene’s coding and splice sequences, and other factors that may influence the distribution of mutation classes, e.g., codon structure. We simulated the location of random mutations using this framework based on the number of samples screened and an overall protein-altering mutation rate of 0.9187 events per proband1. We then compared our observed (obs) data to the simulation (sim) using counts of two classes of events (1) missense and (2) severe (nonsense, splice site, or indel). For gene i, we calculated the probability of observing Xiabs or more of any protein-altering events, and among them Yiabs or more severe events, from Z simulations (equation (1)).
| Pi,severe=#(Xisim◻XiobsandYisim◻Yiobs)Z | (1) |
|---|
Supplementary Material
1
2
3
Acknowledgements
This work was supported by grants from the Simons Foundation Autism Research Initiative (SFARI 191889, 191889EE, E.E.E.). We are grateful to all of the families at the participating Simons Simplex Collection (SSC) sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, R. Goin-Kochel, E. Hanson, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman). We appreciate obtaining access to phenotypic data on SFARI Base. Approved researchers can obtain the SSC population dataset described in this study (http://sfari.org/resources/simons-simplex-collection) by applying at https://base.sfari.org. We are grateful for manuscript preparation from T. Brown. E.E.E. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Competing financial interests
E.E.E. is on the scientific advisory board (SAB) of DNAnexus, Inc. and was an SAB member of Pacific Biosciences, Inc. (2009-2013) and SynapDx Corp. (2011-2013). J.S. is a member of the SAB or serves as a consultant for Adaptive Biotechnologies, Ariosa Diagnostics, Stratos Genomics, GenePeeks, Gen9, Good Start Genetics, Ingenuity Systems and Rubicon Genomics. B.J.O. is an inventor on patent PCT/US2009/30620: Mutations in contactin associated protein 2 are associated with increased risk for idiopathic autism. The remaining authors declare no competing financial interests.
Accession codes
Raw sequencing data have been deposited in the National Database for Autism Research under the accession code NDARCOL2130.
References
- 1.O'Roak BJ, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Roak BJ, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nature Genetics. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders SJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iossifov I, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neale BM, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Roak BJ, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch A, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 8.de Ligt J, et al. Diagnostic exome sequencing in persons with severe intellectual disability. The New England journal of medicine. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 9.Iossifov I, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. doi: 10.1038/nature13908. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle EA, O'Roak BJ, Martin BK, Kumar A, Shendure J. MIPgen: optimized modeling and design of molecular inversion probes for targeted resequencing. Bioinformatics. 2014;30:2670–2672. doi: 10.1093/bioinformatics/btu353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiatt JB, Pritchard CC, Salipante SJ, O'Roak BJ, Shendure J. Single molecule molecular inversion probes for targeted, high-accuracy detection of low-frequency variation. Genome research. 2013;23:843–854. doi: 10.1101/gr.147686.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010;68:192–195. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Buxbaum J, et al. The Autism Simplex Collection: an international, expertly phenotyped autism sample for genetic and phenotypic analyses. Mol Autism. 2014;5:34. doi: 10.1186/2040-2392-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen AS, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fromer M, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samocha KE, et al. A framework for the interpretation of de novo mutation in human disease. Nature Genetics. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvill GL, et al. Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nature Genetics. 2013;45:825–830. doi: 10.1038/ng.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamdan FF, et al. Mutations in SYNGAP1 in autosomal nonsyndromic mental retardation. N Engl J Med. 2009;360:599–605. doi: 10.1056/NEJMoa0805392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berryer MH, et al. Mutations in SYNGAP1 cause intellectual disability, autism, and a specific form of epilepsy by inducing haploinsufficiency. Human mutation. 2013;34:385–394. doi: 10.1002/humu.22248. [DOI] [PubMed] [Google Scholar]
- 20.Chenier S, et al. CHD2 haploinsufficiency is associated with developmental delay, intellectual disability, epilepsy and neurobehavioural problems. J Neurodev Disord. 2014;6:9. doi: 10.1186/1866-1955-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samocha KE, et al. A framework for the interpretation of de novo mutation in human disease. Nature genetics. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estivill X, et al. Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome. Human Molecular Genetics. 2001;10:1915–1923. doi: 10.1093/hmg/10.18.1915. [DOI] [PubMed] [Google Scholar]
- 23.Yamakawa K, et al. DSCAM: a novel member of the immunoglobulin superfamily maps in a Down syndrome region and is involved in the development of the nervous system. Human Molecular Genetics. 1998;7:227–237. doi: 10.1093/hmg/7.2.227. [DOI] [PubMed] [Google Scholar]
- 24.Girirajan S, et al. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. American Journal of Human Genetics. 2013;92:221–237. doi: 10.1016/j.ajhg.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronemus M, Iossifov I, Levy D, Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nature reviews Genetics. 2014;15:133–141. doi: 10.1038/nrg3585. [DOI] [PubMed] [Google Scholar]
- 26.Coe BP, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nature genetics. 2014;46:1063–1071. doi: 10.1038/ng.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkmar FR, Nelson DS. Seizure disorders in autism. J Am Acad Child Adolesc Psychiatry. 1990;29:127–129. doi: 10.1097/00004583-199001000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Stessman HA, Bernier R, Eichler EE. A genotype-first approach to defining the subtypes of a complex disease. Cell. 2014;156:872–877. doi: 10.1016/j.cell.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernier R, et al. Disruptive CHD8 Mutations Define a Subtype of Autism Early in Development. Cell. 2014;158:263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helsmoortel C, et al. A SWI/SNF-related autism syndrome caused by de novo mutations in ADNP. Nature genetics. 2014;46:380–384. doi: 10.1038/ng.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen EG, Steinhauer C, Lees M, Lauridsen AM, Ellgaard L, Hartmann-Petersen R. HUWE1 and TRIP12 collaborate in degradation of ubiquitin-fusion proteins and misframed ubiquitin. PloS one. 2012;7:e50548. doi: 10.1371/journal.pone.0050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtsuka N, Badurek S, Busslinger M, Benes FM, Minichiello L, Rudolph U. GABAergic neurons regulate lateral ventricular development via transcription factor Pax5. Genesis. 2013;51:234–245. doi: 10.1002/dvg.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warde-Farley D, et al. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic acids research. 2010;38:W214–220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3