Adult Bone Marrow Cell Therapy Improves Survival and Induces Long-Term Improvement in Cardiac Parameters: A Systematic Review and Meta-Analysis (original) (raw)
. Author manuscript; available in PMC: 2015 Jan 2.
Abstract
Background
Despite rapid clinical translation and widespread enthusiasm, the therapeutic benefits of adult bone marrow cell (BMC) transplantation in patients with ischemic heart disease (IHD) continue to remain controversial. A synthesis of the available data is critical to appreciate and underscore the true impact of this promising approach.
Methods and Results
A total of 50 studies (enrolling 2,625 patients) identified by database searches through January 2012 were included. Weighted Mean Differences for changes in left ventricular (LV) ejection fraction (LVEF), infarct size, LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV) were estimated using random effects meta-analysis. Compared with controls, BMC-treated patients exhibited greater LVEF (3.96%, 95% confidence interval (CI): 2.90, 5.02; P<0.00001), and smaller infarct size (–4.03%, CI: –5.47, –2.59; P<0.00001), LVESV (–8.91 ml, CI: –11.57, –6.25; P<0.00001), and LVEDV (–5.23 ml, CI: – 7.60, –2.86; P<0.0001). These benefits were noted irrespective of the study design (RCT vs. Cohort study) and the type of IHD (acute myocardial infarction vs. chronic IHD), and persisted during long-term follow-up. Importantly, the all-cause mortality, cardiac mortality, and the incidence of recurrent MI and stent thrombosis were significantly lower in BMC-treated patients compared with controls.
Conclusions
Transplantation of adult BMCs improves LV function, infarct size, and remodeling in patients with IHD compared with standard therapy, and these benefits persist during long-term follow-up. BMC transplantation also reduces the incidence of death, recurrent MI, and stent thrombosis in patients with IHD.
Keywords: bone marrow mononuclear cells, ischemic heart disease, myocardial infarction, remodeling, stem cells
Introduction
Coronary artery disease and myocardial infarction (MI) cause significant mortality, morbidity, and economic burden1. Despite current medical and interventional therapies, myocardial tissue lost during MI is replaced by noncontractile scar followed by remodeling of the left ventricle (LV) and gradual progression to heart failure. Based on promising results from preclinical studies and clinical trials, a new therapeutic approach has gained vigorous momentum over the past decade – transplantation of adult bone marrow-derived cells (BMCs) for heart repair. However, while BMC injection resulted in significant improvement in LV function and structure in many studies2,3, these benefits were mixed or absent in several others4-8. Although results from clinical trials and meta-analyses have documented that BMC transplantation is feasible and safe7, the efficacy of this approach for cardiac repair continues to remain unclear and controversial. In addition, the long-term persistence of benefits of BMC transplantation remains uncertain9.
Because of the relatively small number of patients even in pooled datasets7,10, satisfactory analysis of several key aspects of outcomes could not be achieved previously. These include the impact of BMC transplantation on long-term patient-important clinical outcomes, and the persistence of benefits during prolonged follow-up. While surrogate endpoints demonstrate benefit with BMC transplantation7, understanding the clinical impact of this new therapy on hard clinical endpoints is quintessential before mainstream application. With the reporting of several newer clinical trials5,6,11-47 since our prior review, we sought to systematically review the effects of adult BMC transplantation in patients with ischemic heart disease on clinical and surrogate endpoints.
Methods
Search Strategy
We searched MEDLINE, the Web of Science, the Cochrane Central Register of Controlled Trials, and the reference lists of retrieved reports through January 2012 for studies of BMC transplantation in patients with ischemic heart disease (IHD) using the following terms: “stem cells”, “progenitor cells”, “bone marrow cells”, “coronary artery disease”, “myocardial infarction”, “acute myocardial infarction”, “ischemic cardiomyopathy”, “cardiomyopathy”, and “heart failure”. The complete search strategy is provided in Appendix 1.
Study Selection
Studies were included if they were: (i) randomized controlled trials or cohort studies with a control group; (ii) conducted in patients with acute myocardial MI or chronic IHD; (iii) conducted in patients who received percutaneous coronary intervention or thrombolysis or coronary artery bypass surgery; and (iv) designed such that patients in the intervention arm received BMC therapy either via intracoronary injection or intramyocardial injection, and patients in the control arm received standard therapy. Studies that had at least one month of follow-up and ≥10 patients as the total sample size were included. Because we used mean and standard deviation, studies that reported data using median and range, could not be included. Search criteria were set to include only human studies conducted in adults ≥18 years of age.
Studies that used circulating progenitor cells following granulocyte colony-stimulating factor (G-CSF) mobilization were excluded in order to avoid confounding direct effects of GCSF on the myocardium and BMCs. Studies that did not report pre- and post-intervention outcomes of interest were excluded. Studies published in languages other than English were excluded, except those for which abstracts were available in English.
Data extraction
Three investigators (VJ, MB, and AS) independently screened all titles and abstracts to identify studies that met the inclusion criteria and extracted relevant data using a standardized form. The outcome measures included changes in left ventricular (LV) ejection fraction (LVEF), infarct size, LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV). The clinical outcome measures included: all-cause mortality, cardiac mortality, heart failure, stent thrombosis, in-stent restenosis, target vessel revascularization, cerebrovascular event, and ventricular arrhythmia. Data with the longest duration of follow-up were included for primary and secondary outcome measures. LV volumes were estimated from LV volume indices when appropriate. Modes of imaging included echocardiography, cardiac MRI, left ventriculography (LVG), radionuclide ventriculography (RNV), and single-photon emission computed tomography (SPECT) (Table 1). MRI and SPECT data were preferred over echocardiographic data for primary analysis when available. When multiple imaging modalities were used in one study, data by each modality were extracted to be included in subgroup analysis. Clinical trials with multiple publications with sequential follow-up durations or different outcomes were considered as one study. For studies with two intervention arms12,23,24,32,48 which involved two different doses (low dose and high dose of BMCs) or different routes of administration (intracoronary and intramuscular), data were combined using methods described in the Cochrane Handbook49.
Table 1.
Characteristics of studies included in the meta-analysis
| Source | Sample size | Mean follow-up duration (months) | Study design | Cell type | BMC preparation, suspension, injection | No. of cells transplanted | Route of Injection | Type of IHD | Location of MI | DES use | Time from PCI and/or MI to transplantation | Imaging modalities* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akar et al,11 2009 | 50 | 18 | Cohort | BMMNC | COBE spectra,injected same day | 1.29 ± 0.09 × 109 | IM w/CABG | CIHD | Multiple | - | 397 ± 467 d | Echo (EF),SPECT (Vol) |
| Ang et al,12 2008 | 25 | 6 | RCT | BMMNC | Lymphoprep,autologous serum,injected same day | 85 ± 56 × 106 (IM)115 ± 73 ×106 (IC) | IM or ICw/ CABG | CIHD | NR | - | >6 wk | MRI |
| Assmus et al,532006 | 46 | 3 | RCT | BMMNC | Ficoll, X-vivo 10,3-d culture beforeinjection | 205 ± 110 × 106 | IC | CIHD | Multiple | 2470 ± 2196 d | LVG | |
| Bartunek et al,542005 | 35 | 4 | Cohort | CD133+BMMNC | CliniMacs, PBS+1%HSA, injectedwithin 10 h | 12.6 ± 2.2 × 106 | IC | AMI | Multiple | NR | 11.6 ± 1.4 d | LVG (EF,Vol), SPECT(IS) |
| Cao et al,15 2009 | 86 | 48 | RCT | BMMNC | Lymphoprep,heparinized saline | 5 ± 1.2 × 107 | IC | AMI | Anterior | DES78-85% | 7 d | Echo (EF,Vol), SPECT(IS) |
| Chen et al,55 2004 | 69 | 6 | RCT | MSC | Culture expanded,heparinized saline | 48-60 × 109 | IC | AMI | Multiple | NR | 18.4 ± 0.5 d | LVG (EF),PET (IS) |
| Colombo et al,162011 | 10 | 12 | RCT | CD133+BMMNC | CliniMacs,saline+10% HSA | 5.9 (4.9 to 13.5) × 106 | IC | AMI | Anterior | BMS | 10 to 14 d | Echo (EF, Vol) |
| Ge et al,57 2006 | 20 | 6 | RCT | BMMNC | Lymphoprep,heparinized saline | 40 × 106 | IC | AMI | Multiple | NR | 1 d | Echo (EF),SPECT (IS) |
| Grajek et al, 17 2010 | 45 | 12 | RCT | BMMNC | Ficoll, X-vivo15+2% plasma,injected next day | 2.34 ± 1.2 × 109 | IC | AMI | Anterior | BMS | 5-6 d | Echo |
| Hendrikx et al,582006 | 20 | 4 | RCT | BMMNC | Lymphoprep,heparinized salineinjected next day | 60.25 ± 31.35 × 106 | IM | CIHD | Multiple | 217 ± 162 d | MRI | |
| Herbots et al,18 2009 | 67 | 4 | RCT | BMMNC | Ficoll, saline+5%autologous serum,injected within 4-6 h | 17.2 ± 7.2 × 107 | IC | AMI | Multiple | NR | <1 d | Echo |
| Huang et al,19 2006(abstract only) | 40 | 6 | RCT | BMMNC | NA | NA | IC | AMI | Inferior | NA | NA | MRI |
| Huikuri et al,202008 | 80 | 6 | RCT | BMMNC | Ficoll-Hypaque,heparinizedsaline+autologousserum, injectedwithin 3 h | 402 ± 196 × 106 /2.6 ± 1.6 × 106 | IC | AMI | Multiple | DES | 2 to 6 d | Echo (EF),LVG (Vol) |
| Janssens et al,592006 | 67 | 4 | RCT | MSC | Ficoll,saline+autologousserum, injectedwithin 24 h | 172 ± 72 × 106 | IC | AMI | Multiple | NR | 1 to 2 d | MRI |
| Katritsis et al,602005 | 22 | 4 | Cohort | MSC &EPC | Culture expanded,saline | 2-4 × 106 | IC | AMI &CIHD | Anteroseptal | NR | 224 ± 470 d | Echo |
| Lipiec et al,21 2009 | 36 | 6 | RCT | BMMNC | Ficoll-Paque Plus,saline, injectedwithin2-3 h | 0.33 ± 0.17 × 106 (CD133+)3.36 ± 1.87 × 106 (CD34+) | IC | AMI | Anterior | NR | 3 to10 d | SPECT |
| Lunde et al,4,13,14,612006, 2008, 2009 | 100 | 36 | RCT | BMMNC | Ficoll, heparin-plasma, injectednext day | 87 ± 47.7 × 106 | IC | AMI | Anterior | DES4-6% | 6 ± 1.3 d | SPECT (EF,EDV, IS),Echo (ESV) |
| Manginas et at,222007 | 24 | 11 | Cohort | CD133+andCD133-/CD34+ | Ficoll, FC-Macs,injected within 1-2 hof isolation | 16.9 ± 4.9 × 106 (CD133+)8.0 ± 4.0 ×106(CD 34+) | IC | CIHD | Anterior | - | 43.9 ± 38.4 months | Echo |
| Meluzin et al,23,24,262006, 2008 | 66 | 12 | RCT | BMMNC | Histopaque,cultivated overnight | (High Dose) 1 × 108(Low Dose) 1 × 107 | IC | AMI | Multiple | NR | 7 ± 0.3 d | SPECT |
| Meyer et al,9,67,68,702006 | 60 | 18 | RCT | BMC | Gelatinpolysuccinatedensity gradient,injected within 6-8h | 24.6 ± 9.4 × 108 | IC | AMI | Multiple | NR | 4.8 ± 1.3 d | MRI |
| Mocini et al,62 2006 | 36 | 3 | Cohort | BMMNC | Centrifugation, PBS | 292 ± 232 × 106 | IM | CIHD | Multiple | - | NA | Echo |
| Nogueira et al, 252009 | 20 | 6 | RCT | BMMNC | Ficoll-Paque Plus,saline+5% HSA,injected within 8.5 h | 1.0× 108 | IC | AMI | Multiple | NR | 5.5 ± 1.2 d | Echo |
| Penicka et al,5 2007 | 27 | 4 | RCT | BMMNC | BMNC concentrate | 26.4 × 108 / 1.3 × 106 | IC | AMI | Anterior | NR | 4 to 11 d | Echo (EF,Vol), SPECT(IS) |
| Perin et al,63,64 2003, 2004 | 20 | 12 | Cohort | BMMNC | Ficoll-Paque Plus,saline+5% HSA,injected within 4 h | 25.5 ± 6.3 × 106 | IM | CIHD | Multiple | NA | Echo (EF,Vol), SPECT(IS) | |
| Piepoli et al,27 2010 | 38 | 12 | RCT | BMMNC | Ficoll-Hypaque,PBS+5% HSA,injected same day | 24.88 × 107 (mononuclear)41.88 × 107 (CD45+) | IC | AMI | Anterior | NR | 4 to 7 d | SPECT |
| Plewka et al,28 2009 | 56 | 6 | RCT | BMMNC | Ficoll-Paque plus,saline, injectedwithin 2 h | 14.4 ± 4.9 × 107 | IC | AMI | Anterior | NR | 7 ± 2 d | Echo |
| Pokushalov et al,292010 | 109 | 12 | RCT | BMMNC | Ficoll-Paque Plus,heparinized saline,injected same day | 41 ± 16 × 106 | IM | CIHD | Multiple | - | 9 ± 8 years | Echo |
| Quyyumi et al,302011 | 31 | 6 | RCT | CD34+ | Dynabeads (Isolox300i), heparinizedPBS+40%autologous serum,injected within 24-48 h | 5-15 × 106 | IC | AMI | NR | DES50-60% | 8.3 d (median) | MRI |
| Ramshorst et al,41,422009 | 49 | 3 | RCT | BMMNC | Ficoll, PBS+0.5%HSA, injected sameday | 100×106 | IM | CIHD | NA | - | >6 months | MRI |
| Rivas-Plata et al,312010 | 34 | 27 | Cohort | BMMNC | Lymphoprep,Hank's medium,injected same day | 407 ×106 | IM w/CABG | CIHD | NR | - | >4 wk | Echo |
| Ruan et al,65 2005 | 20 | 6 | RCT | BMC | NR | NR | IC | AMI | Anterior | NR | 1 d | Echo |
| Schachinger etal,3,56,66 2006 | 204 | 4 | RCT | BMMNC | Ficoll-Hypaue, X-vivo 10, injected thesame or next day | 236 ± 174 × 106 | IC | AMI | Multiple | DES13-16% | 4.3 ± 1.3 d | LVG |
| Silva et al,32 2009 | 30 | 6 | RCT | BMMNC | Ficoll-Paque Plus,saline, injectedwithin 8.5 h | 1 × 108 | IC | AMI | Multiple | NR | 5.5 ± 1.3 d | RNV |
| Srimahacho-ta etal,33 2011 | 23 | 6 | RCT | BMMNC | Isoprep, saline+2%autologous serum,injected same day | 420 ± 221 × 106 | IC | AMI | Multiple | DES17-18% | 57 ± 122 d | MRI |
| Stamm et al,34 2007 | 43 | 6 | Cohort | CD133+ | CliniMacs, injectednext day | 1.08 × 106 to 8.35 ×107 | IM w/CABG | CIHD | Multiple | - | 7.9 wk (median) | Echo |
| Strauer et al,2 2002 | 20 | 3 | Cohort | BMMNC | Ficoll, X-vivo15,heparinized saline,overnight cultivation | 28 ± 22 × 106 | IC | AMI | Multiple | NR | 8 ± 2 d | LVG |
| Strauer et al,69 2005 | 36 | 3 | Cohort | BMMNC | Ficoll, X-vivo15,heparinized saline,overnight cultivation | 90 × 106 | IC | CIHD | Multiple | - | 823.5 ± 945.5 d | LVG |
| Strauer et al, 352010 | 391 | 60 | Cohort | BMMNC | Ficoll, X-vivo15,heparinized saline | 6.6 ± 3.3 × 107 | IC | CIHD | Multiple | - | 8.5 ± 3.2 y | LVG |
| Suarez de Lezo etal,36 2007 | 20 | 3 | RCT | BMMNC | Ficoll-Hypaque,heparinized saline,injected same day | 9 ± 3 × 108 /17 ± 13 × 106 | IC | AMI | Anterior | NR | 7 ± 2 d | LVG |
| Traverse et al,6 2010 | 40 | 6 | RCT | BMMNC | Ficoll, saline+5%HSA, deliveredwithin 8 h | 1 × 108 | IC | AMI | Anterior | DES95% | 3 to 10 d | MRI |
| Traverse et al, 372011 | 87 | 6 | RCT | BMC | Automated cellprocessor (Sepax)saline+5% HSA,injected within 12 h | 1.47 ± 17 × 108 | IC | AMI | Multiple | DES69-78% | 14-21 d (17.4) | MRI |
| Tse et al,38 2007 | 28 | 6 | RCT | BMMNC | Ficoll, PBS+10%autologous serum,injected same day | 1.67 ± 0.34 ×107 (low)4.20 ± 2.80 ×107 (high) | IM | CIHD | NA | - | NA | MRI |
| Turan et al,39 2011 | 32 | 6 | Cohort | BMC | BMAC, freshlyisolated BMCs | 101 ± 20 × 106 | IC | AMI | Multiple | NR | 7 d | LVG |
| Turan et al, 402011 | 62 | 12 | RCT | BMC | BMAC, freshlyisolated BMCs | 9.6 ± 3.2 × 107 | IC | AMI | Multiple | NR | 7 days | LVG |
| Wohrle et al,43 2010 | 42 | 6 | RCT | BMMNC | Ficoll, saline+2%albumin, injected at6 h (median) | 381 ± 130 × 106 | IC | AMI | Multiple | DES28-31% | 6.3 ± 0.8 d | MRI |
| Yao et al,44 2008 | 47 | 6 | RCT | BMMNC | Ficoll-Hypaque,heparin-treatedplasma, injectedsame day | 180 ×106 | IC | CIHD | Multiple | DES57-58% | 13 ± 8 months | MRI |
| Yao et al,48 2009 | 39 | 12 | RCT | BMMNC | Ficoll-Hypaque,heparin-treatedplasma, injectedsame day | 1.9 ± 1.2 × 108(single transfusion)2.0 ± 1.4 × 108(repeat transfusion) | IC | AMI | Anterior | DES33-47% | 3 to 7 drepeat at 3 months | MRI |
| Yerebakan et al,45 2011 | 55 | 18 | Cohort | CD133+ | CliniMacs, injectednext day | 6 × 106 | IM w/CABG | CIHC | Multiple | - | >14 days (2-1,215weeks) | Echo |
| Yousef et al,46 2009 | 124 | 60 | Cohort | BMMNC | Ficoll, heparinizedsaline | 6.1 ± 3.9 × 107 | IC | AMI | Multiple | NR | 7 ± 2 d | LVG |
| Zhao et al,47 2008 | 36 | 6 | RCT | BMMNC | Ficoll, heparinizedsaline, injected sameday | 6.59 ± 5.12 × 108 | IM w/CABG | CIHD | Multiple | - | NA | Echo |
Quality Assessment
The quality of included RCTs was assessed by using criteria established by Juni et al.50, and the quality of cohort studies was assessed by using the modified Newcastle-Ottawa scale51.
Data Analysis
Statistical analyses were performed using the Cochrane RevMan version 5, and the results expressed as weighted mean differences (WMDs) for continuous outcomes, with 95% confidence intervals (CIs). Data were pooled using the DerSimonian-Laird random-effects model, but a fixed-effects model was also employed to ensure the robustness of the model chosen and the susceptibility to outliers. Heterogeneity was analyzed using I2 statistic, with a significance level alpha = 0.05. For I2 statistic, heterogeneity was defined as low (25-50%), moderate (50-75%), and high (>75%). We planned to conduct sensitivity analysis if significant heterogeneity was found (I2 > 50%) for any one of the outcomes. For studies that reported mean±standard deviation (SD) at baseline and follow-up, but did not report the actual change (from baseline to follow-up) as (mean±SD), the change in SD was calculated using a standardized formula used previously to calculate changes in mean and standard deviation52. Peto odds ratio was calculated for clinical outcomes (all-cause mortality, cardiac mortality, recurrent myocardial infarction, stent thrombosis, heart failure, in-stent restenosis, target vessel revascularization, cerebrovascular event, and ventricular arrhythmias).
Subgroup analysis and Sensitivity Analysis
Planned subgroup analyses were conducted based on: (i) type of study design (RCT vs. Cohort study); (ii) type of IHD (acute MI vs. chronic IHD); (iii) duration of follow-up; (iv) baseline LVEF of <43% vs. >43% (43% was the median LVEF at baseline in included studies); and <50% vs. ≥50% (LVEF <50% represents LV dysfunction); (v) timing of BMC transplantation after acute MI and/or PCI (<7 days vs. 7 to 30 days [7 days after acute MI/PCI was the median in included studies]); (vi) number of cells injected (<100×106 vs. >100×106 BMCs injected [100×106 was the median number of BMCs injected]; and <40×106 vs. >40×106 BMCs injected); (vii) type of BMC (bone marrow mononuclear cells [BMMNCs] vs. other select cell populations [CD133+ and CD34+ BMCs]); and (viii) method of cell preparation (Lymphoprep vs. other Ficoll-based methods), and the use of heparin in the final cellular suspension; (ix) location of MI (anterior vs. multiple areas); and (x) route of injection. Sensitivity analyses were conducted to explore heterogeneity (investigating the effects of route of injection, sample size in studies, median LVEF, and median number of BMCs injected).
Results
Search Results
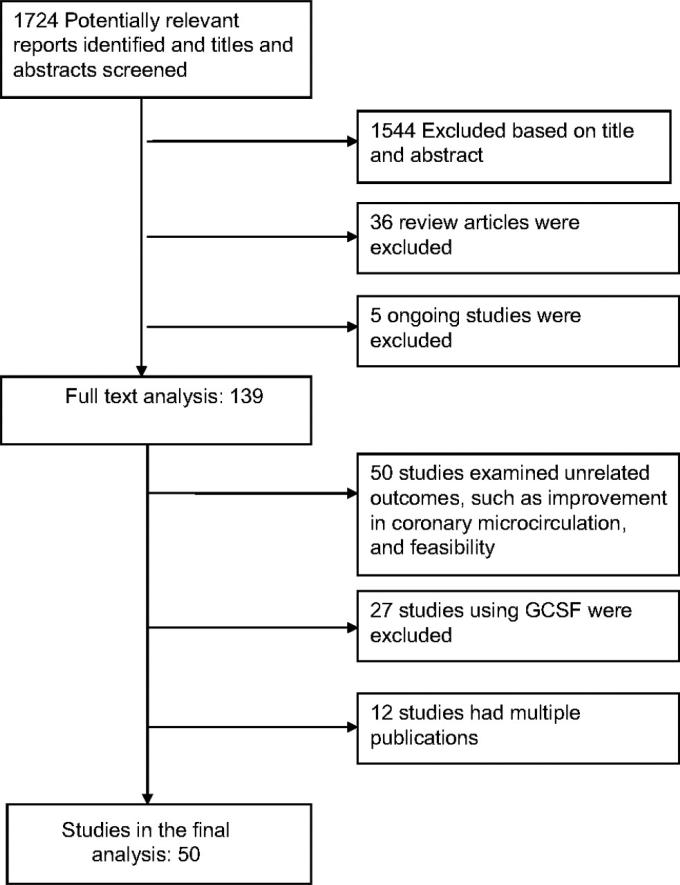
The initial search retrieved 1,724 reports, of which 1,544 were excluded based on the title and abstract. Following the exclusion of 36 review articles and 5 reports of ongoing trials, full-text analysis was performed on 139 reports, of which 89 were excluded because of unrelated outcomes and the use of G-CSF and circulating progenitor cells. The remaining 50 studies (36 RCTs and 14 cohort studies enrolling a total of 2,625 patients)2-6,9,11-47,53-70 that reported changes in LVEF, infarct size, LVESV, or LVEDV in patients who underwent BMC transplantation compared with standard therapy were included in the final analysis (Figure 1).
Figure 1.
Flow diagram of eligible studies of bone marrow–derived cell (BMC) transplantation in patients with acute myocardial infarction and chronic ischemic heart disease. GCSF indicates granulocyte colony-stimulating factor; and RCT, randomized controlled trial. IV, inverse variance.
Study Characteristics
Table 1 summarizes the characteristics of included studies. The median follow-up duration was 6 months (range: 3 months to 60 months) and the median sample size was 39 patients (range: 10 to 391 patients). The timing of BMC transplantation in patients with acute MI varied among the included studies (median 6.7 days; range: 1 day to 18.4 days), and the median number of BMCs injected was 100×106 (range: 2×106 to 60×109). The median EF of patients at baseline was 43% (range: 21% to 62%).
Study Quality
The quality metrics of included RCTs are shown in Table 2, while Table 3 summarizes the quality of cohort studies. All cohort studies and at least 15 RCTs failed to blind participants and/or caregivers; 7 RCTs did not provide adequate information on blinding of participants and caregivers; and blinding of outcome assessors was unclear in at least 3 RCTs. The loss and adequacy of follow-up in the eligible studies are provided in Tables 2 and 3. The follow-up was complete in most studies with shorter follow-up duration. In studies with longer follow-up, the percent of patients lost to follow-up was acceptable. The inter-reviewer agreement on these quality domains was greater than 90%.
Table 2.
Quality assessment scale for the randomized controlled trials included in the meta-analysis
| Selection | Performance | Detection | Attrition | ||||
| Source of bias | Was allocation adequate?* | Was an adequate method of randomization described? | Were groups similar at the start of the study? | Were the patients/caregivers blinded to the intervention? | Was the outcome ascertained blindly? | What percent was lost to follow-up? | Were all patients analyzed in the group to which they were assigned (intention-to-treat analysis)? |
| Ang et al,12 2008 | Y | N | Y | Y | Y | 8% | N |
| Assmus et al,53 2006 | Y | N | Y | N | Y | 8.6% | Y |
| Cao et al,15 2009 | Y | Y | Y | NR | Y | 0 | Y |
| Chen et al,55 2004 | Y | N | Y | Y | Y | 0 | Y |
| Colombo et al,16 2011 | Y | Y | Y | Y | Y | 0 | Y |
| Ge et al,57 2006 | Y | Y | Y | N | Y | 0 | Y |
| Grajek et al.172010 | Y | Y | Y | N | Y | 0 | N |
| Hendrikx et al,58 2006 | Y | Y | Y | N | Y | 0 | Y |
| Herbots et al,18 2009 | Y | Y | Y | Y | Y | 1% | Y |
| Huang et al,19 2006 (abstract only) | NA | NA | NA | NA | NA | NA | NA |
| Huikuri et al,20 2008 | Y | Y | Y | Y | Y | 3.7% | Y |
| Lunde et al,4,13,14,61 2006, 2008, 2009 | Y | Y | Y | N | Y | 0 | Y |
| Janssens et al,59 Lancet 2006 | Y | Y | Y | Y | Y | 10% | Y |
| Lipiec et al,21 2009 | Y | Y | Y | N | Y | 5% | N |
| Meluzin et al,23,24 2006, 2008 | Y | N | Y | NR | Y | 9% | N |
| Meyer et al,9,67,68,70 2006 | Y | Y | Y | Y | Y | 0 | Y |
| Nogueira et al 25 2009 | Y | Y | Y | N | Y | 0 | Y |
| Penicka et al,5 2007 | Y | Y | NR | NR | NR | 11% | N |
| Piepoli et al,27 2010 | Y | Y | Y | NR | NR | 0 | Y |
| Plewka et al,28 2009 | Y | N | Y | NR | Y | 0 | Y |
| Pokushalov et al,29 2010 | Y | Y | Y | N | Y | 24% | Y |
| Quyyumi et al,30 2011 | Y | N | Y | N | Y | 8% | N |
| Ramshorst et al,41,42 2009 | Y | Y | Y | Y | Y | 18% | Y |
| Ruan et al,65 2005 | Y | N | Y | Y | Y | 0 | Y |
| Schachinger et al,3,56,66 2006 | Y | Y | Y | Y | Y | 0 | Y |
| Silva et al,32 2009 | Y | Y | Y | N | Y | 0 | Y |
| Srimahachota et al,33 2011 | N | Y | Y | N | Y | 0 | Y |
| Suarez de Lezo et al,36 2007 | Y | Y | Y | NR | Y | 0 | Y |
| Traverse et al,6 2010 | Y | Y | Y | Y | Y | 0 | Y |
| Traverse et al, 372011 | Y | N | Y | N | Y | 1.1% | Y |
| Turan et al, 40 2011 | Y | N | Y | N | Y | 0 | Y |
| Tse et al,38 2007 | Y | Y | Y | Y | Y | 7% | Y |
| Wohrle et al,43 2010 | Y | Y | Y | Y | Y | 4.7% | Y |
| Yao et al,44 2008 | Y | N | Y | N | Y | 0 | Y |
| Yao et al,48 2009 | Y | Y | Y | NR | Y | 13% | N |
| Zhao et al,47 2008 | Y | Y | Y | N | Y | 5.5% | Y |
Table 3.
Modified Newcastle-Ottawa Quality Assessment Scale for the Cohort Studies included in the meta-analysis
| Selection | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|
| Source | Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | Incident disease | Comparability | Assessment of outcome | Length of follow-up | Adequacy of follow-up |
| Akar et al,11 2009 | B | A | A | A | B | B | A | A |
| Bartunek et al,54 2005 | A | A | A | A | A | B | A | A |
| Katritsis et al,60 2005 | A | A | A | A | A | A | A | A |
| Manginas et al,22 | B | A | A | A | B | NR | A | A |
| Mocini et al,62 2006 | A | A | A | A | A | A | A | A |
| Perm et al,63,64 2003, 2004 | A | A | A | NR | A | A | A | A |
| Rivas-Plata et al,31 2010 | B | A | A | A | A | NR | A | A |
| Stamm et al,34 2007 | B | A | A | A | C | A | A | B |
| Strauer et al,2 2002 | A | A | A | A | A | B | A | A |
| Strauer et al,69 2005 | A | A | A | A | A | B | A | A |
| Strauer et al,35 2010 | A | A | A | A | A | B | A | A |
| Turan et al,39 2011 | A | A | A | A | A | A | A | A |
| Yerebakan et al, 45 2011 | A | A | A | A | A | A | A | B |
| Yousef et al,46 2009 | A | A | A | A | A | A | A | A |
Cardiac parameters.
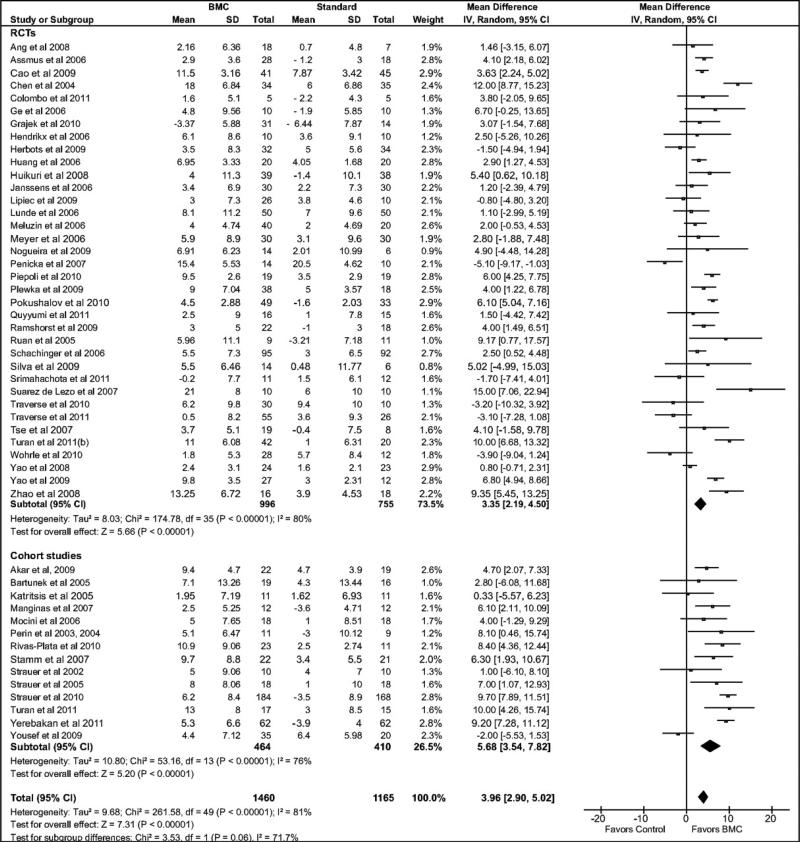
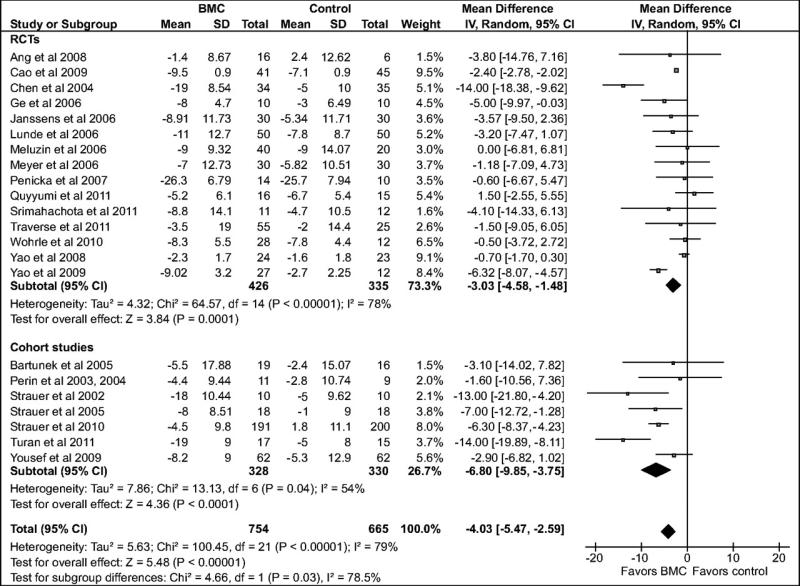
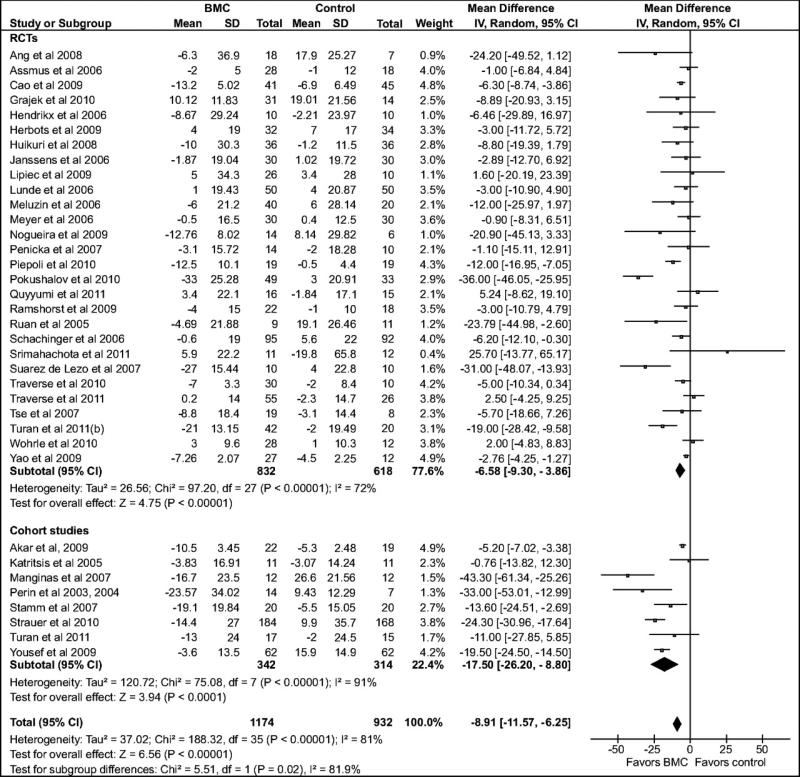
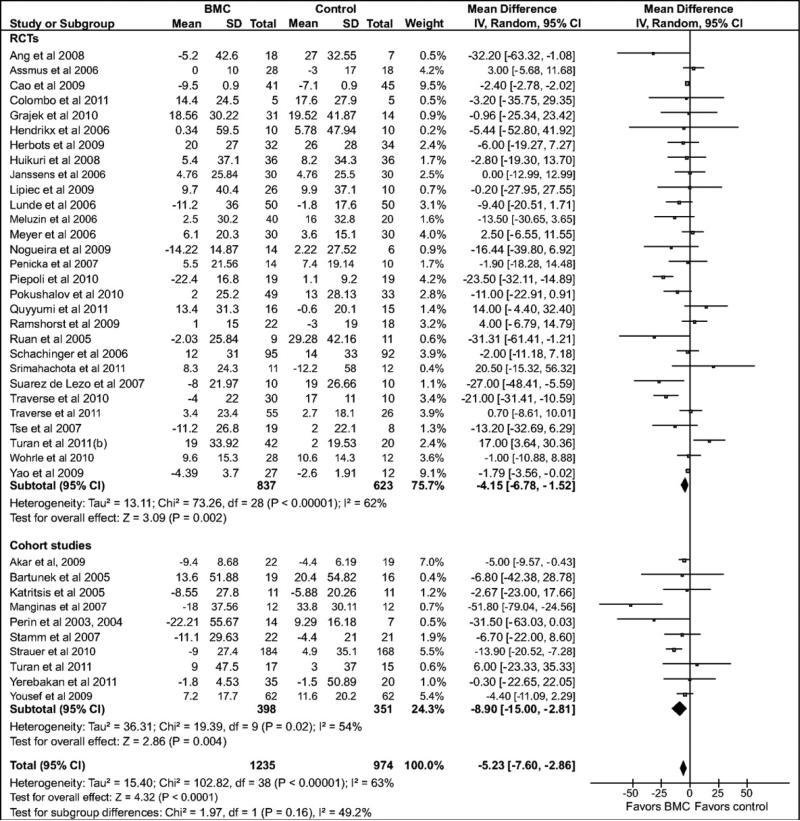
Compared with the standard treatment group, BMC transplantation improved LVEF by 3.96% (95% confidence interval [CI]: 2.90, 5.02; P<0.00001; Figure 2), reduced infarct size by 4.03% (CI: –5.47, –2.59; P<0.00001, Figure 3), reduced LVESV by 8.9 ml, (CI: –11.57, –6.25; P<0.00001, Figure 4), and reduced LVEDV by 5.23 ml (CI: –7.6, –2.86; P<0.0001, Figure 5).
Figure 2.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in left ventricular ejection fraction (LVEF) in patients treated with bone marrow-derived cells (BMCs) compared with controls. The figure shows the summary of randomized controlled trials (RCTs) and cohort studies. Transplantation of BMCs resulted in a 3.96% (CI: 2.90, 5.02; P<0.00001) increase in mean LVEF. The overall effect was statistically significant in favor of BMC transplantation. WMD indicates weighted mean difference. IV, inverse variance.
Figure 3.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in infarct scar size in patients treated with bone marrow-derived cells (BMCs) compared with controls. The figure shows the summary of randomized controlled trials (RCTs) and cohort studies. Transplantation of BMCs resulted in a 4.03% (CI: –5.47, –2.59; P<0.00001) decrease in mean infarct scar size. The overall effect was statistically significant in favor of BMC transplantation. WMD indicates weighted mean difference. IV, inverse variance.
Figure 4.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in left ventricular end-systolic volume (LVESV) in patients treated with bone marrow-derived cells (BMCs) compared with controls. The figure shows the summary of randomized controlled trials (RCTs) and cohort studies. Transplantation of BMCs resulted in a 8.91 ml (CI: – 11.57, –6.25; P<0.00001) decrease in LVESV. The overall effect was statistically significant in favor of BMC transplantation. WMD indicates weighted mean difference. IV, inverse variance.
Figure 5.
Forest plot of unadjusted difference in mean (with 95% confidence intervals [CIs]) change in left ventricular end-diastolic volume (LVEDV) in patients treated with bone marrow-derived cells (BMCs) compared with controls. The figure shows the summary of randomized controlled trials (RCTs) and cohort studies. BMC transplantation resulted in a 5.23 ml (CI: – 7.60, –2.86; P<0.001) decrease in mean LVEDV. The overall effect was statistically significant in favor of BMC transplantation. WMD indicates weighted mean difference. IV, inverse variance.
Persistence of benefits during long-term follow-up
With analyses based on the duration of follow-up, the improvement in LVEF persisted for at least more than 24 months, and improvement in infarct size, LVESV, and LVEDV persisted for at least more than 12 months (Table 4).
Table 4.
Unadjusted difference in mean change in parameters in BMC-treated patients compared with controls based on the duration of follow-up
| Follow-up duration | Difference in mean | 95% Confidence Interval | P value |
|---|---|---|---|
| LVEF | |||
| 0 – 3 months | 4.78 | 3.22 to 6.34 | <0.00001 |
| 4 – 6 months | 3.47 | 2.35 to 4.59 | <0.00001 |
| 7 – 12 months | 5.93 | 4.56 to 7.30 | <0.00001 |
| 13 – 24 months | 2.14 | 0.25 to 4.02 | <0.03 |
| > 24 months | 6.91 | 3.37 to 10.45 | 0.0001 |
| Infarct size | |||
| 0 – 3 months | –6.19 | –9.73 to –2.64 | 0.0006 |
| 4 – 6 months | –2.94 | –4.60 to –1.29 | 0.0005 |
| 7 – 12 months | –5.60 | –9.67 to –1.53 | 0.007 |
| > 12 months | –2.39 | –2.78 to –2.01 | <0.00001 |
| LVESV | |||
| 0 – 3 months | –9.33 | –13.66 to –5.00 | <0.00001 |
| 4 – 6 months | –5.68 | –7.83 to –3.54 | <0.00001 |
| 7 – 12 months | –14.52 | –19.35 to –9.68 | <0.00001 |
| > 12 months | –9.47 | –14.51 to –4.44 | 0.0002 |
| LVEDV | |||
| 0 – 3 months | –2.92 | –7.09 to 1.26 | 0.17 |
| 4 – 6 months | –2.90 | –4.92 to –0.89 | 0.005 |
| 7 – 12 months | –7.65 | –12.48 to –2.83 | 0.002 |
| > 12 months | –4.37 | –7.84 to –0.90 | 0.01 |
Subgroup Analysis
Subgroup analysis showed that improvements in LV function, scar size, and LV volumes were significant irrespective of the type of IHD (acute MI vs. chronic IHD), except that BMC transplantation produced greater reduction in LVESV in patients with chronic IHD (Table 5). The benefits of BMC therapy were similar in patients with MI in any territory compared with those with anterior MI, although improvement in LVEDV was greater in the latter (Table 5). The impact of baseline LVEF was analyzed separately based on the median LVEF (43%) and the presence of LV systolic dysfunction (LVEF <50%). Results from both analyses showed that recipients of BMC transplantation with lower LVEF at baseline experienced significantly greater improvement in LVESV and LVEDV, with no significant reduction in LVEDV in recipients with baseline LVEF >43% and > 50% (Table 5). In patients with acute MI, BMC injection <7 days after acute MI and/or PCI produced similar improvements in EF, scar size, and LVESV compared with BMC injection between 7 and 30 days. The improvement in LVEDV was also significant when cells were injected at <7 days, while BMC injection between 7 to 30 days failed to reduce LVEDV (Table 5).
Table 5.
Subgroup analysis examining the impact of study design, type of ischemic heart disease, timing of transplantation, number of BMCs transplanted, and route of BMC transplantation, and left ventricular ejection fraction at baseline on outcome variables.
| Outcome | Difference in mean (95% confidence interval) | P value for subgroup differences | |
|---|---|---|---|
| Acute MI | Chronic IHD | ||
| LVEF | 3.48 (2.05 to 4.91) | 4.94 (3.27 to 6.61) | 0.19 |
| Infarct scar size | –3.73 (–5.29 to –2.18) | –6.09 (–7.96 to –4.21) | 0.06 |
| LVESV | –5.91 (–8.31 to –3.50) | –16.34 (–23.98 to –8.70) | 0.01 |
| LVEDV | –3.76 (–6.38 to –1.15) | –7.81 (–13.8 to –1.83) | 0.22 |
| Anterior wall MI | MI in any territory | ||
| LVEF | 3.37 (1.48 to 5.26) | 4.37 (2.91 to 5.82) | 0.41 |
| Infarct scar size | –3.56 (–6.28 to –0.84) | –4.85 (–7.27 to –2.42) | 0.49 |
| LVESV | –8.15 (–12.03 to –4.27) | –10.08 (–14.56 to –5.60) | 0.52 |
| LVEDV | –13.73 (–22.2 to –5.27) | –3.14 (–5.87 to –0.41) | 0.02 |
| Baseline LVEF <43 % | Baseline LVEF ≥43% | ||
| LVEF | 4.83 (3.37 to 6.29) | 3.61 (2.05 to 5.18) | 0.26 |
| Infarct scar size | –3.84 (–6.14 to –1.55) | –4.52 (–7.07 to –1.97) | 0.70 |
| LVESV | –13.93 (–18.27 to –9.59) | –4.70 (–7.34 to –2.07) | 0.0004 |
| LVEDV | –10.01 (–14.59 to –5.43) | –2.19 (–6.08 to 1.69) | 0.01 |
| Baseline LVEF <50 % | Baseline LVEF ≥50 % | ||
| LVEF | 4.06 (2.87 to 5.24) | 3.75 (0.81 to 6.69) | 0.85 |
| Infarct scar size | –4.55 (–6.32 to –2.77) | –3.03 (–5.84 to –0.23) | 0.37 |
| LVESV | –9.88 (–12.91 to –6.86) | –4.49 (–8.73 to –0.26) | 0.04 |
| LVEDV | –7.18 (–10.69 to –3.68) | –1.05 (–5.42 to 3.31) | 0.03 |
| BMCs injected <7 d after acute MI and/or PCI | BMCs injected 7 to 30 d after acute MI and/or PCI | ||
| LVEF | 3.91 (1.40 to 6.42) | 0.43 | |
| Infarct scar size | 2.68 (0.87 to 4.48) | –4.78 (–7.91 to –1.64) | 0.55 |
| LVESV | –3.56 (–6.0 to –1.12) | –7.48 (–12.24 to –2.72) | 0.35 |
| LVEDV | –4.89 (–7.48 to –2.3)–7.14 (–12.29 to –1.99) | –0.12 (–4.48 to 4.24) | 0.04 |
| No. of BMCs <100 × 106 | No. of BMCs ≥100 × 106 | ||
| LVEF | 4.69 (3.22 to 6.16) | 3.54 (2.04 to 5.04) | 0.28 |
| Infarct scar size | –4.35 (–6.45 to –2.25) | –3.71 (–6.65 to –0.78) | 0.73 |
| LVESV | –13.46 (–18.78 to –8.15) | –4.52 (–6.67 to –2.37) | 0.002 |
| LVEDV | –5.1 (–9.45 to –0.76) | –4.52 (–8.30 to –0.75) | 0.84 |
| No. of BMCs <40 × 106 | No. of BMCs ≥40 × 106 | ||
| LVEF | 1.88 (–0.49 to 4.25) | 4.19 (3.06 to 5.32) | 0.09 |
| Infarct scar size | –3.48 (–10.13 to 3.17) | –4.22 (–5.73 to –2.71) | 0.83 |
| LVESV | –13.59 (–32.68 to 5.49) | –7.78 (–10.21 to –5.35) | 0.55 |
| LVEDV | –7.30 (–20.31 to 5.72) | –4.31 (–6.60 to –2.03) | 0.66 |
| BMMNC | CD133+/CD34+ | ||
| LVEF | 3.84 (2.68 to 5.00) | 3.05 (–0.19 to 6.29) | 0.65 |
| Infarct scar size | –3.47 (–4.86 to –2.07) | 0.94 (–2.85 to 4.74) | 0.03 |
| LVESV | –9.13 (–12.08 to –6.17) | –16.53 (–40.47 to 7.41) | 0.55 |
| LVEDV | –6.47 (–9.00 to –3.94) | –8.01 (–25.02 to 9.00) | 0.86 |
| Other Ficoll-based methods | Lymphoprep | ||
| LVEF | 3.94 (2.57 to 5.31) | 4.44 (2.06 to 6.82) | 0.72 |
| Infarct scar size | –3.81 (–5.98 to –1.65) | –2.42 (–2.80 to –2.04) | 0.21 |
| LVESV | –9.75 (–13.83 to –5.68) | –6.46 (–8.88 to –4.05) | 0.17 |
| LVEDV | –7.5 (–11.46 to –3.54) | –9.54 (–27.93 to 8.85) | 0.83 |
| No heparin | Heparinized Saline | ||
| LVEF | 2.58 (1.22 to 3.95) | 6.15 (4.30 to 8.01) | 0.002 |
| Infarct scar size | –4.29 (–6.66 to –1.92) | –4.58 (–6.37 to –2.79) | 0.85 |
| LVESV | –4.84 (–8.4 to –1.27) | –13.07 (–19.17 to –6.96) | 0.02 |
| LVEDV | –6.41 (–12.29 to –0.52) | –4.43 (–7.05 to –1.80) | 0.55 |
| IC - Chronic IHD | IM - Chronic IHD | ||
| LVEF | 3.43 (0.33 to 6.53) | 4.94 (3.27 to 6.12) | 0.40 |
| Infarct scar size | –3.99 (–8.3 to 0.32) | –3.42 (–10.23 to 3.39) | 0.89 |
| LVESV | –19.24 (–37.92 to –0.56) | –15.64 (–24.95 to –6.33) | 0.74 |
| LVEDV | –12.91 (–27.96 to 2.14) | –6.39 (–12.78 to 0.00) | 0.43 |
| RCTs | Cohort studies | ||
| LVEF | 3.35 (2.19 to 4.50) | 5.68 (3.54 to 7.82) | 0.06 |
| Infarct scar size | –3.03 (–4.58 to –1.48) | –6.80 (–9.85 to –3.75) | 0.03 |
| LVESV | –6.58 (–9.30 to –3.86) | –17.50 (–26.20 to –8.80) | 0.02 |
| LVEDV | –4.15 (–6.78 to –1.52) | –8.9 (–15 to –2.81) | 0.16 |
Analysis based on the median BMC number (100×106) showed that injection of >100×106 BMCs produced similar improvements in EF, scar size, and EDV compared with <100×106 BMCs; while reduction in ESV was significantly greater with <100×106 BMCs. Additional analyses utilizing progressively lower BMC numbers showed that injection of >40×106 BMCs resulted in significant improvement in all 4 primary outcome measures (LVEF, scar size, LVESV, and LVEDV), while injection of ≤40×106 BMCs did not show improvement in any outcome (Table 5), indicating that 40×106 BMCs may represent the cut-off, below which BMCs fail to exert a majority of the desired benefits.
Regarding cell types, 36 studies used BMMNCs, 5 studies used BMCs, 6 studies used CD133+ and/or CD34+ cells, and 3 studies used MSCs and/or EPCs. Subgroup analysis showed that while BMMNC therapy improved LVEF, scar size, and LV volumes, the pooled effects of CD133+ and/or CD34+ cell therapy were not significantly different compared with controls (Table 5). The reduction in scar size with BMMNC therapy was significantly greater compared with CD133+/CD34+ cells. Analysis based on the methods of cell preparation showed similar benefits in LVEF, scar size, and LVESV when cells were isolated using Lymphoprep compared with other Ficoll-based methods (Table 5). Further subgroup analysis comparing studies that used heparinized saline vs. saline-based solutions without heparin in the final cell suspension showed greater improvement in EF and LVESV with heparinized saline, while improvements in scar size and LVEDV were comparable with both methods (Table 5). In 26 studies, cells were injected on the same day as BM harvest, and in 9 studies, cells were injected by the next day (Table 1). BMCs were cultured or cell injection was delayed for up to 48 h in 4 studies, and the time-frame was unclear in 11. Since information regarding storage condition, especially temperature during storage was not available in the vast majority, subgroup analysis was not performed.
Regarding the route of injection, all patients with acute myocardial infarction received intracoronary injection of BMCs. Therefore the impact of intracoronary vs. intramyocardial route of injection was analyzed in patients with chronic IHD. In these patients the outcomes were not significantly different between the two routes of BMC administration (Table 5). With regard to the design of included studies, the benefits remained significant when RCTs and cohort studies were analyzed separately (Figures 2-5), albeit with greater magnitudes in cohort studies compared with RCTs (Table 5).
Impact of BMC therapy on survival and clinical outcomes
Compared with patients who received standard therapy, BMC-treated patients experienced significant decrease in all-cause mortality (OR 0.39, CI: 0.27 to 0.55, _I2_=14%, P<0.00001), cardiac mortality (OR 0.41, CI: 0.22 to 0.79, _I2_=2%, _P_=0.005), recurrent MI (OR 0.25, CI: 0.11 to 0.57, _I2_=22%, _P_=0.001), and stent thrombosis (OR 0.34, CI: 0.12 to 0.94, _I2_=6%, _P_=0.04) (Table 6). There were trends toward reduction in the incidence of heart failure (OR 0.52, CI: 0.27 to 1.00, _I2_=4%, _P_=0.05) and cerebrovascular event (OR 0.28, CI: 0.08 to 1.07, _I2_=0%, _P_=0.06) in BMC-treated patients. The incidence of in-stent restenosis (OR 0.87, CI: 0.47 to 1.62, _I2_=0%, _P_=0.66), target vessel revascularization (OR 0.83, CI: 0.55 to 1.23, _I2_=0%, _P_=0.35), and ventricular arrhythmias (OR 1.14, CI: 0.52 to 2.53, _I2_=18%, _P_=0.74) were similar in BMC-treated patients compared with controls (Table 6).
Table 6.
Clinical outcomes in BMC-treated patients compared with patients receiving standard therapy
| Outcome | Peto OR | 95% Confidence Interval | P value |
|---|---|---|---|
| All-cause mortality | 0.39 | 0.27 to 0.55 | <0.00001 |
| Cardiac deaths | 0.41 | 0.22 to 0.79 | 0.005 |
| Recurrent MI | 0.25 | 0.11 to 0.57 | 0.001 |
| Heart failure | 0.52 | 0.27 to 1.00 | 0.05 |
| Stent thrombosis | 0.34 | 0.12 to 0.94 | 0.04 |
| In-stent restenosis | 0.87 | 0.47 to 1.62 | 0.66 |
| TVR | 0.83 | 0.55 to 1.23 | 0.35 |
| CVA | 0.28 | 0.08 to 1.07 | 0.06 |
| VT / VF | 1.14 | 0.52 to 2.53 | 0.74 |
Imaging modalities and outcomes
Significant differences were noted when the mean changes in LVEF, infarct size, and LVESV were compared among studies that used echocardiography, SPECT, MRI, or LVG for outcomes assessment. Specifically, improvement in LVEF in BMC-treated patients was significant when echocardiography or LVG were used and showed a trend toward improvement with SPECT, whereas the increase was insignificant with MRI (Table 7). Infarct scar size reduction was significant with both SPECT and LVG, but not with MRI (Table 7). Importantly, reduction in LVESV was significant with all imaging modalities, albeit the magnitude varied; while reduction in LVEDV was significant by echocardiography and SPECT, but not by MRI or LVG (Table 7).
Table 7.
Unadjusted differences in mean change in parameters in BMC-treated patients compared with controls based on the mode of imaging
| Difference in mean | 95% Confidence Interval | P value for Z | P value for subgroup differences | |
|---|---|---|---|---|
| LVEF | ||||
| Echo | 3.61 | 2.18 to 5.04 | <0.00001 | 0.001 |
| SPECT | 2.60 | −0.35 to 5.55 | 0.08 | |
| MRI | 1.17 | −0.60 to 2.95 | 0.20 | |
| LVG | 7.08 | 4.77 to 9.38 | 0.0001 | |
| Infarct size | ||||
| SPECT | –2.41 | –2.78 to –2.03 | <0.00001 | 0.04 |
| MRI | –1.48 | –1.48 to 0.91 | 0.22 | |
| LVG | –7.01 | –10.66 to–3.36 | 0.0002 | |
| LVESV | ||||
| Echo | –15.81 | –23.75 to –7.87 | <0.0001 | <0.0001 |
| SPECT | –7.02 | –11.19 to –2.85 | 0.001 | |
| MRI | –2.38 | –3.89 to –0.87 | 0.002 | |
| LVG | –14.44 | –21.61 to –7.27 | <0.0001 | |
| LVEDV | ||||
| Echo | –7.66 | –13.08 to –2.25 | 0.006 | 0.08 |
| SPECT | –14.79 | –24.22 to –5.35 | 0.002 | |
| MRI | –2.39 | –6.84 to 2.06 | 0.29 | |
| LVG | –3.08 | –10.25 to 4.10 | 0.4 |
Sensitivity analysis
Heterogeneity was explored by conducting sensitivity analysis based on the route of injection, sample size, median LVEF and median number of BMCs injected. All clinical trials in patients with acute MI used the intracoronary route for BMC injection. Analysis based on the route of injection, median EF and the median number of BMCs did not explain the heterogeneity (Table 5). Analysis of studies based on sample size (<50 patients vs. ≥50 patients) did not change the results and did not explain the heterogeneity.
Publication Bias
We drew funnel plots to seek evidence of publication bias: where inconsistency was high, the funnel plots were not interpretable; where inconsistency was low, the funnel plots were inconclusive.
Discussion
Salient findings
Our meta-analysis of pooled data from 2,625 patients, the largest to date, demonstrate that adult BMC transplantation results in modest yet significant improvements in LVEF, infarct scar size, LVESV, and LVEDV. These results indicate that BMC transplantation can improve LV function and remodeling beyond those achievable with standard therapy. The persistence of LVEF improvement at least beyond 24 months and other enhancements at least beyond 12 months underscores the long-standing nature of cardiac repair induced by BMC transplantation. Importantly, and although assessed as secondary outcomes, our results also indicate that BMC-treated patients experienced significant reduction in all-cause mortality, cardiac mortality, recurrent MI, and stent thrombosis compared with patients who received standard therapy. While the clinical trials included in this meta-analysis were not designed to assess the impact of BMC transplantation on long-term clinical outcomes as their primary outcome, these findings are highly significant from a therapeutic standpoint, and provide a strong basis for large scale clinical trials.
BMC therapy improves LV function and remodeling
The primary objectives of cell therapy are to improve LV structure and function and ameliorate patient symptoms. In this regard, results from individual clinical trials have been discordant with some trials showing improvement in diverse functional and clinical parameters with BMC transplantation, while others failing to document significant benefits. Based on data from 2,625 patients, the current results indicate that injection of BMCs in patients with IHD results in modest improvements in LVEF, infarct size, LVESV, and LVEDV. The improvement in LV systolic function is noteworthy as LVEF is an important prognostic factor in patients with acute myocardial ischemic injury71. It is also important to note that although the 3.96% increase in LVEF is not large, the other therapeutic options in these patients are able to offer only similar benefits72. In addition, BMC transplantation also improved postinfarct remodeling as evidenced by reduction in infarct scar size and LVEDV. These benefits may translate into superior long-term prognosis in these patients. The mechanisms underlying these benefits remain poorly understood at this time, although enhanced angiogenesis and reduction in apoptosis through paracrine effects of growth factors secreted by BMCs, differentiation of BMCs into cardiac cells, and activation of cardiac stem cells have all been suggested73,74.
The sustained nature of benefits
We performed additional analysis based on duration of follow-up to examine whether the benefits would persist during long-term follow-up. As shown in Table 4, the improvement in LVEF was robust even beyond 24 months, while the reduction in infarct size and LV volumes persisted for at least more than 12 months. These data indicate that the benefits of BMC transplantation on LV structure and function are not transient.
Patient characteristics
Notwithstanding this uncertainty regarding mechanisms, we analyzed data based on pre-defined subgroups attempting to identify the potential factors that may influence the observed benefits. When analyzed based on the type of ischemic heart disease, BMC transplantation in patients with chronic IHD produced greater reduction in LVESV compared with acute MI patients who received BMC therapy (Table 5). These findings indicate that beyond the acute setting, BMC transplantation can also effectively ameliorate LV remodeling, which is a chronic process. Further analysis revealed similar benefits irrespective of the location of MI, although the reduction in LVEDV was more pronounced in patients with anterior MI.
Analysis based on the median LVEF (43%) in recipients showed significantly greater reduction in LV volumes in patients with LVEF <43% at baseline (Table 5**). These differences in outcomes persisted when subgroup data were analyzed using a baseline LVEF of 50%, below which LV dysfunction is considered present. Importantly, BMC therapy failed to reduce LVEDV in patients with a baseline LVEF >43% (Table 5**). Together, these results indicate that LV remodeling outcomes are superior with lower baseline LVEF in recipients. Although no rigid cut-off value below which BMC transplantation would be ineffective could be determined, these data indicate that the benefits of BMC transplantation are greater in recipients with LV dysfunction at baseline.
Timing of cell injection
Following an acute MI, the initial inflammatory myocardial milieu progressively changes to that of a remodeled heart, and understandably the fate of injected BMCs and the outcomes of therapy may depend on the timing of cell injection. Interestingly, when BMCs were injected <7 days (the median interval) after acute MI and/or PCI, the improvements in LVEF, infarct scar size, and LVESV were similar compared with BMC injection within the 7 to 30 day period; however, improvement in LVEDV was absent with delayed BMC injection (Table 5). These results underscore the critical need for direct comparison of different timings of cell therapy after acute MI in prospective trials.
The impact of cell number
Since only a small fraction of injected cells is retained in the myocardium, the total number of BMCs injected may determine the degree of cardiac recovery. While the mean changes in LVEF, infarct size, and LVEDV were similar in patients who received >100×106 BMCs (the median number in included studies) and <100×106 BMCs, there was a greater reduction in LVESV in patients who received <100×106 BMCs. Upon further analysis with progressively lower BMC numbers, none of the benefits (improvement in LVEF, and reduction in infarct size, LVESV, and LVEDV) were observed in patients who received <40×106 BMCs, while improvements in all four outcome parameters were evident in those who received >40×106 cells (Table 5). However, a limitation in this type of subgroup analysis is the fact that these trials did not directly compare the effects of low vs. high dose of BMC transplantation. Moreover, clinical factors such as the timing after MI and the route of injection may also be responsible for the lack of benefits observed with lower number of BMCs.
Comparison of cell types
Since the initial demonstration of cardiac repair with Lin-/c-kit+ BMCs, a number of other BMC subfractions have been used for similar purposes. In subgroup analysis, BMMNC transplantation resulted in improvement in all four primary outcomes, whereas therapy with CD133+ and/or CD34+ cells did not improve LVEF, scar size, or volumes (Table 5). While this could be related to the small number of studies (reduced sample size) with these subsets, the benefits of specific subgroups of BMMNCs need further evaluation.
It is important to note that recent studies have documented the efficacy of myocardial repair with various adult cells from other tissues, including the heart. Indeed, the c-kit+ cardiac stem cells (CSCs)75 are considered optimally suited for myocardial repair because of their cardiac origin and inherent ability to differentiate into cardiac lineages. Consistent with the efficacy of CSCs to repair infarcted myocardial tissue following intravascular delivery76 and in the setting of an old MI77, intracoronary delivery of autologous CSCs improved LVEF by 12.3% and reduced infarct size by 30% after 1 year in patients with ischemic cardiomyopathy in a recent trial78. In a subsequent study79, intracoronary injection of cardiosphere-derived cells reduced infarct mass and improved regional myocardial contractility in patients with acute MI and LV dysfunction. Thus, the efficacy of CSCs for cardiac repair needs to be compared with BMMNCs in future trials.
The importance of cell processing methods
It has been appropriately suggested that cell processing methods impact outcomes80,81. Therefore we performed subgroup analysis based on the specific method of density-gradient centrifugation, and the benefits were comparable with Lymphoprep vs. other Ficoll-based protocols (Table 5). Additional subgroup analysis showed greater improvement in LVEF and LVESV with the use of heparin in the final BMC suspension (Table 5). Importantly, BMCs were stored for various lengths of time, and further studies will be necessary to directly assess the importance of additional factors in this process.
Route of injection
In patients with acute MI, all of the included studies employed the intracoronary route. Therefore, we analyzed the impact of cell delivery approaches in patients with chronic IHD only. There was no significant difference between outcomes with intracoronary compared with intramyocardial administration in patients with CIHD (Table 5). Nonetheless, in clinical scenarios, the applicability and selection of intracoronary and intramyocardial routes will often depend on patient characteristics and logistics.
Improvement in survival and adverse outcomes during follow-up
With the growing number of cell therapy trials, it has become critically important to consider the overall clinical picture, which includes broader endpoints. In this light, and although analyzed as secondary outcomes, the ability of BMC transplantation to reduce all-cause as well as cardiac mortalities, incidence of recurrent MI, and stent thrombosis is noteworthy. The incidence of heart failure and CVA also showed a trend toward reduction. These data suggest that BMC transplantation may modulate other as yet unknown variables that may influence the overall outcomes positively.
The impact of imaging modality
The potential influence of imaging modality was analyzed for all primary outcomes. Interestingly, the improvements in LV functional parameters were more pronounced in studies that used echocardiography or LVG compared with those using MRI. It is important to note that the differences in mean change by MRI were uniformly directionally concordant with other modalities, albeit not statistically significant. Thus, these results need to be interpreted in light of the relative paucity of studies that have employed MRI for assessment of primary outcomes (Table 1). The increasing use of MRI in newer studies may provide additional data for effective comparison among various imaging modalities.
Safety
Our review demonstrates that BMC transplantation is safe in patients with IHD. The incidence of in-stent restenosis, a potential concern in patients treated with intracoronary BMC injection, was similar in BMC-treated and control patients. The incidence of other important clinical adverse outcomes, including target vessel revascularization and ventricular arrhythmia also did not differ between groups.
The selection of outcome variables
In this systematic review, we were able to analyze the primary variables that were reported in a majority of studies. However, it is important to note that these variables have inherent limitations in serving as accurate end-points of BMC therapy. For example, LVEF is known to be load-dependent and may be influenced by hypercontractile segments in the viable myocardium. Further, its prognostic significance diminishes with values >45%. Therefore, in future studies, it will be important to identify a combinatorial set of parameters that will reliably reflect the true impact of BMC therapy in patients with IHD.
Limitations
The degree of heterogeneity observed among trials in this review is a limitation. This heterogeneity may have resulted from the differences in imaging modalities used to determine LV volumes and EF, BMC number and processing, timing and route of injection, and differences in baseline characteristics among the study populations. We conducted predetermined subgroup analyses for the mode of imaging, timing of BMC injection, and the number of BMCs injected. However, a limitation in subgroup analysis, although pre-defined, is that the number of studies included in one subgroup may be less than the other(s). This could lead to smaller sample size which may result in nonsignificant association. Nonetheless, the improvements observed across most of these subgroups (Tables 4, 5, and 7) suggest that the associations are likely valid. Sensitivity analyses based on sample size, baseline LVEF and route of injection also did not explain the heterogeneity. Most of these studies were conducted in small patient populations with a few exceptions, and did not focus on broad clinical outcomes.
In conclusion, the results of our systematic review suggest that BMC transplantation in addition to standard therapy in patients with IHD improves LV function and remodeling as well as patient-important clinical outcomes. Further large scale randomized studies are needed to critically evaluate the multi-faceted benefits of this promising therapeutic approach.
CLINICAL PERSPECTIVE.
Although adult bone marrow cell (BMC) therapy for cardiac repair appears promising, divergent data from smaller clinical trials have generated lingering controversy over the nature and extent of benefits. We performed a systematic review and meta-analysis of pooled data from 50 trials to assess the impact of BMC therapy on clinically important end-points. Our results show that BMC therapy modestly improves left ventricular function and remodeling in patients with IHD, and these benefits persist during long-term follow-up. These data also suggest that BMC therapy is associated with reduced all-cause as well as cardiac mortality, and reduced incidence of recurrent myocardial infarction (MI) and stent thrombosis without any significant increase in adverse events. BMC therapy seems effective for both acute MI and chronic ischemic cardiomyopathy, largely independent of the location of MI. Patients with lower LV ejection fraction at baseline appear to benefit more. To be effective, injection of at least 40 million BMCs seems necessary, and the remodeling benefits seem more pronounced with earlier BMC injection. Although BM mononuclear cells are generally more effective compared with subpopulations, cell processing techniques deserve particular attention, because they influence the outcomes significantly. Finally, the magnitude of changes in various outcome parameters depends on the imaging modality, although the findings remain directionally concordant. Thus, larger clinical trials utilizing stringent methodology and broader array of outcomes are warranted to definitively determine the true utility of this novel therapeutic strategy for cardiac repair.
Acknowledgments
The authors gratefully acknowledge Renee Falsken for expert secretarial assistance.
Funding Sources: This meta-analysis and publication was supported in part by NIH grant R01 HL-89939
Footnotes
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 3.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 4.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 5.Penicka M, Horak J, Kobylka P, Pytlik R, Kozak T, Belohlavek O, Lang O, Skalicka H, Simek S, Palecek T, Linhart A, Aschermann M, Widimsky P. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction: A prematurely terminated randomized study. J Am Coll Cardiol. 2007;49:2373–2374. doi: 10.1016/j.jacc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Traverse JH, McKenna DH, Harvey K, Jorgenso BC, Olson RE, Bostrom N, Kadidlo D, Lesser JR, Jagadeesan V, Garberich R, Henry TD. Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following st-elevation myocardial infarction. Am Heart J. 2010;160:428–434. doi: 10.1016/j.ahj.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 8.Dawn B, Abdel-Latif A, Sanganalmath SK, Flaherty MP, Zuba-Surma EK. Cardiac repair with adult bone marrow-derived cells: The clinical evidence. Antioxid Redox Signal. 2009;11:1865–1882. doi: 10.1089/ars.2009.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months' follow-up data from the randomized, controlled boost (bone marrow transfer to enhance st-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 10.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 11.Akar AR, Durdu S, Arat M, Kilickap M, Kucuk NO, Arslan O, Kuzu I, Ozyurda U. Five-year follow-up after transepicardial implantation of autologous bone marrow mononuclear cells to ungraftable coronary territories for patients with ischaemic cardiomyopathy. Eur J Cardiothorac Surg. 2009;36:633–643. doi: 10.1016/j.ejcts.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 12.Ang KL, Chin D, Leyva F, Foley P, Kubal C, Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT, Galinanes M. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during cabg versus cabg alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–670. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 13.Beitnes JO, Hopp E, Lunde K, Smith HJ, Solheim S, Arnesen H, Forfang K, Brinchmann JE, Aakhus S. Long-term follow-up of left ventricular function after acute myocardial infarction treated with intracoronary injection of autologous bone marrow cells. The astami study. Circulation. 2008;118:S_863. [Google Scholar]
- 14.Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, Forfang K, Aakhus S. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: The astami randomised, controlled study. Heart. 2009;95:1983–1989. doi: 10.1136/hrt.2009.178913. [DOI] [PubMed] [Google Scholar]
- 15.Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, Feng X, Zhang J, Duan Y, Wang J, Liu D, Wang H. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with st-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J. 2009;30:1986–1994. doi: 10.1093/eurheartj/ehp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombo A, Castellani M, Piccaluga E, Pusineri E, Palatresi S, Longari V, Canzi C, Sacchi E, Rossi E, Rech R, Gerundini P, Viecca M, Deliliers GL, Rebulla P, Soligo D, Giordano R. Myocardial blood flow and infarct size after cd133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: Results from a randomized controlled trial. J Cardiovasc Med (Hagerstown) 2011;12:239–248. doi: 10.2459/JCM.0b013e328343d708. [DOI] [PubMed] [Google Scholar]
- 17.Grajek S, Popiel M, Gil L, Breborowicz P, Lesiak M, Czepczynski R, Sawinski K, Straburzynska-Migaj E, Araszkiewicz A, Czyz A, Kozlowska-Skrzypczak M, Komarnicki M. Influence of bone marrow stem cells on left ventricle perfusion and ejection fraction in patients with acute myocardial infarction of anterior wall: Randomized clinical trial: Impact of bone marrow stem cell intracoronary infusion on improvement of microcirculation. Eur Heart J. 2010;31:691–702. doi: 10.1093/eurheartj/ehp536. [DOI] [PubMed] [Google Scholar]
- 18.Herbots L, D'Hooge J, Eroglu E, Thijs D, Ganame J, Claus P, Dubois C, Theunissen K, Bogaert J, Dens J, Kalantzi M, Dymarkowski S, Bijnens B, Belmans A, Boogaerts M, Sutherland G, Van de Werf F, Rademakers F, Janssens S. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: A randomized, double-blind strain rate imaging study. Eur Heart J. 2009;30:662–670. doi: 10.1093/eurheartj/ehn532. [DOI] [PubMed] [Google Scholar]
- 19.Huang RC, Yao K, Zou YZ, Ge L, Qian JY, Yang J, Yang S, Niu YH, Li YL, Zhang YQ, Zhang F, Xu SK, Zhang SH, Sun AJ, Ge JB. [long term follow-up on emergent intracoronary autologous bone marrow mononuclear cell transplantation for acute inferior-wall myocardial infarction]. Zhonghua Yi Xue Za Zhi. 2006;86:1107–1110. [PubMed] [Google Scholar]
- 20.Huikuri HV, Kervinen K, Niemela M, Ylitalo K, Saily M, Koistinen P, Savolainen ER, Ukkonen H, Pietila M, Airaksinen JK, Knuuti J, Makikallio TH. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29:2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 21.Lipiec P, Krzeminska-Pakula M, Plewka M, Kusmierek J, Plachcinska A, Szuminski R, Robak T, Korycka A, Kasprzak JD. Impact of intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction on left ventricular perfusion and function: A 6-month follow-up gated 99mtc-mibi single-photon emission computed tomography study. Eur J Nucl Med Mol Imaging. 2009;36:587–593. doi: 10.1007/s00259-008-0988-6. [DOI] [PubMed] [Google Scholar]
- 22.Manginas A, Goussetis E, Koutelou M, Karatasakis G, Peristeri I, Theodorakos A, Leontiadis E, Plessas N, Theodosaki M, Graphakos S, Cokkinos DV. Pilot study to evaluate the safety and feasibility of intracoronary cd133(+) and cd133(−) cd34(+) cell therapy in patients with nonviable anterior myocardial infarction. Catheter Cardiovasc Interv. 2007;69:773–781. doi: 10.1002/ccd.21023. [DOI] [PubMed] [Google Scholar]
- 23.Meluzin J, Mayer J, Groch L, Janousek S, Hornacek I, Hlinomaz O, Kala P, Panovsky R, Prasek J, Kaminek M, Stanicek J, Klabusay M, Koristek Z, Navratil M, Dusek L, Vinklarkova J. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: The effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152:975, e979–915. doi: 10.1016/j.ahj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Meluzin J, Janousek S, Mayer J, Groch L, Hornacek I, Hlinomaz O, Kala P, Panovsky R, Prasek J, Kaminek M, Stanicek J, Klabusay M, Koristek Z, Navratil M, Dusek L, Vinklarkova J. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128:185–192. doi: 10.1016/j.ijcard.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 25.Nogueira FB, Silva SA, Haddad AF, Peixoto CM, Carvalho RM, Tuche FA, Soares VE, Sousa AL, Rabischoffsky A, Mesquita CT, Borojevic R, Dohmann HF. Systolic function of patients with myocardial infarction undergoing autologous bone marrow transplantation. Arquivos brasileiros de cardiologia. 2009;93:374–379, 367-372. doi: 10.1590/s0066-782x2009001000010. [DOI] [PubMed] [Google Scholar]
- 26.Panovsky R, Meluzin J, Janousek S, Mayer J, Kaminek M, Groch L, Prasek J, Stanicek J, Dusek L, Hlinomaz O, Kala P, Klabusay M, Koristek Z, Navratil M. Cell therapy in patients with left ventricular dysfunction due to myocardial infarction. Echocardiography. 2008;25:888–897. doi: 10.1111/j.1540-8175.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- 27.Piepoli MF, Vallisa D, Arbasi M, Cavanna L, Cerri L, Mori M, Passerini F, Tommasi L, Rossi A, Capucci A. Bone marrow cell transplantation improves cardiac, autonomic, and functional indexes in acute anterior myocardial infarction patients (cardiac study). Eur J Heart Fail. 2010;12:172–180. doi: 10.1093/eurjhf/hfp183. [DOI] [PubMed] [Google Scholar]
- 28.Plewka M, Krzeminska-Pakula M, Lipiec P, Peruga JZ, Jezewski T, Kidawa M, Wierzbowska-Drabik K, Korycka A, Robak T, Kasprzak JD. Effect of intracoronary injection of mononuclear bone marrow stem cells on left ventricular function in patients with acute myocardial infarction. Am J Cardiol. 2009;104:1336–1342. doi: 10.1016/j.amjcard.2009.06.057. [DOI] [PubMed] [Google Scholar]
- 29.Pokushalov E, Romanov A, Chernyavsky A, Larionov P, Terekhov I, Artyomenko S, Poveshenko O, Kliver E, Shirokova N, Karaskov A, Dib N. Efficiency of intramyocardial injections of autologous bone marrow mononuclear cells in patients with ischemic heart failure: A randomized study. J Cardiovasc Transl Res. 2010;3:160–168. doi: 10.1007/s12265-009-9123-8. [DOI] [PubMed] [Google Scholar]
- 30.Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J, Kereiakes D, Gersh BJ, Gregory D, Werner A, Moss T, Chan WS, Preti R, Pecora AL. Cd34(+) cell infusion after st elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Rivas-Plata A, Castillo J, Pariona M, Chunga A. Bypass grafts and cell transplant in heart failure with low ejection fraction. Asian Cardiovasc Thorac Ann. 2010;18:425–429. doi: 10.1177/0218492310379939. [DOI] [PubMed] [Google Scholar]
- 32.Silva SA, Sousa AL, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, Soares AJ, Issa AF, Felipe LR, Branco RV, Addad JA, Moreira RC, Tuche FA, Mesquita CT, Drumond CC, Junior AO, Rochitte CE, Luz JH, Rabischoffisky A, Nogueira FB, Vieira RB, Junior HS, Borojevic R, Dohmann HF. Autologous bone-marrow mononuclear cell transplantation after acute myocardial infarction: Comparison of two delivery techniques. Cell Transplant. 2009;18:343–352. doi: 10.3727/096368909788534951. [DOI] [PubMed] [Google Scholar]
- 33.Srimahachota S, Boonyaratavej S, Rerkpattanapipat P, Wangsupachart S, Tumkosit M, Bunworasate U, Nakorn TN, Intragumtornchai T, Kupatawintu P, Pongam S, Saengsiri AO, Pothisri M, Sukseri Y, Bunprasert T, Suithichaiyakul T. Intra-coronary bone marrow mononuclear cell transplantation in patients with st-elevation myocardial infarction: A randomized controlled study. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2011;94:657–663. [PubMed] [Google Scholar]
- 34.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G. Intramyocardial delivery of cd133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: Safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 35.Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary stem cell transplantation in 191 patients with chronic heart failure: The star-heart study. Eur J Heart Fail. 2010;12:721–729. doi: 10.1093/eurjhf/hfq095. [DOI] [PubMed] [Google Scholar]
- 36.Suarez de Lezo J, Herrera C, Pan M, Romero M, Pavlovic D, Segura J, Sanchez J, Ojeda S, Torres A. [regenerative therapy in patients with a revascularized acute anterior myocardial infarction and depressed ventricular function]. Rev Esp Cardiol. 2007;60:357–365. [PubMed] [Google Scholar]
- 37.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The latetime randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tse HF, Thambar S, Kwong YL, Rowlings P, Bellamy G, McCrohon J, Thomas P, Bastian B, Chan JK, Lo G, Ho CL, Chan WS, Kwong RY, Parker A, Hauser TH, Chan J, Fong DY, Lau CP. Prospective randomized trial of direct endomyocardial implantation of bone marrow cells for treatment of severe coronary artery diseases (protect-cad trial). Eur Heart J. 2007;28:2998–3005. doi: 10.1093/eurheartj/ehm485. [DOI] [PubMed] [Google Scholar]
- 39.Turan RG, Bozdag-Turan I, Ortak J, Akin I, Kische S, Schneider H, Rehders TC, Turan CH, Rauchhaus M, Kleinfeldt T, Chatterjee T, Sahin K, Nienaber CA, Ince H. Improvement of cardiac function by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction. Circulation journal : official journal of the Japanese Circulation Society. 2011;75:683–691. doi: 10.1253/circj.cj-10-0817. [DOI] [PubMed] [Google Scholar]
- 40.Turan RG, Bozdag TI, Turan CH, Ortak J, Akin I, Kische S, Schneider H, Rauchhaus M, Rehders TC, Kleinfeldt T, Belu C, Amen S, Hermann T, Yokus S, Brehm M, Steiner S, Chatterjee T, Sahin K, Nienaber CA, Ince H. Enhanced mobilisation of the bone marrow derived circulating progenitor cells by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, de Roos A, Fibbe WE, Zwaginga JJ, Boersma E, Schalij MJ, Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: A randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 42.van Ramshorst J, Antoni ML, Beeres SL, Roes SD, Delgado V, Rodrigo SF, de Roos A, Holman ER, Fibbe WE, Lamb HJ, Zwaginga JJ, Boersma E, van der Wall EE, Schalij MJ, Atsma DE, Bax JJ. Intramyocardial bone marrow-derived mononuclear cell injection for chronic myocardial ischemia: The effect on diastolic function. Circ Cardiovasc Imaging. 2011;4:122–129. doi: 10.1161/CIRCIMAGING.110.957548. [DOI] [PubMed] [Google Scholar]
- 43.Wohrle J, Merkle N, Mailander V, Nusser T, Schauwecker P, von Scheidt F, Schwarz K, Bommer M, Wiesneth M, Schrezenmeier H, Hombach V. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol. 2010;105:804–812. doi: 10.1016/j.amjcard.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 44.Yao K, Huang R, Qian J, Cui J, Ge L, Li Y, Zhang F, Shi H, Huang D, Zhang S, Sun A, Zou Y, Ge J. Administration of intracoronary bone marrow mononuclear cells on chronic myocardial infarction improves diastolic function. Heart. 2008;94:1147–1153. doi: 10.1136/hrt.2007.137919. [DOI] [PubMed] [Google Scholar]
- 45.Yerebakan C, Kaminski A, Westphal B, Donndorf P, Glass A, Liebold A, Stamm C, Steinhoff G. Impact of preoperative left ventricular function and time from infarction on the long-term benefits after intramyocardial cd133(+) bone marrow stem cell transplant. J Thorac Cardiovasc Surg. 2011;142:1530–1539. e1533. doi: 10.1016/j.jtcvs.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Yousef M, Schannwell CM, Kostering M, Zeus T, Brehm M, Strauer BE. The balance study: Clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262–2269. doi: 10.1016/j.jacc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Q, Sun Y, Xia L, Chen A, Wang Z. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann Thorac Surg. 2008;86:1833–1840. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 48.Yao K, Huang R, Sun A, Qian J, Liu X, Ge L, Zhang Y, Zhang S, Niu Y, Wang Q, Zou Y, Ge J. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur J Heart Fail. 2009;11:691–698. doi: 10.1093/eurjhf/hfp062. [DOI] [PubMed] [Google Scholar]
- 49.Studies with more than two intervention groups. ( http://www.cochrane-handbook.org) CHv. Chapter 16.5. updated March 2011.
- 50.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomized studies in meta-analysis. The ottawa health research institute; Ottawa, ontario: [October 2011]. Http://www.Ohri.Ca/programs/clinical_epidemiology/oxford.Asp. [Google Scholar]
- 52.Hristov M, Heussen N, Schober A, Weber C. Intracoronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: A meta-analysis. J Cell Mol Med. 2006;10:727–733. doi: 10.1111/j.1582-4934.2006.tb00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 54.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, Van Haute I, Lootens N, Heyndrickx G, Wijns W. Intracoronary injection of cd133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: Feasibility and safety. Circulation. 2005;112:I178–183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 55.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 56.Erbs S, Linke A, Schachinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, Dimmeler S, Tonn T, Hambrecht R, Zeiher AM, Schuler G. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: The doppler substudy of the reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction (repair-ami) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 57.Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y, Fan B, Liu X, Zhang S, Sun A, Zou Y. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (tct-stami). Heart. 2006;92:1764–1767. doi: 10.1136/hrt.2005.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P, Vijgen J, Dilling D, Steels P, Mees U, Rummens JL. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: Results from a randomized controlled clinical trial. Circulation. 2006;114:I101–107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 59.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with st-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 60.Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, Voridis EM, Papamichail M. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65:321–329. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 61.Lunde K, Solheim S, Forfang K, Arnesen H, Brinch L, Bjornerheim R, Ragnarsson A, Egeland T, Endresen K, Ilebekk A, Mangschau A, Aakhus S. Anterior myocardial infarction with acute percutaneous coronary intervention and intracoronary injection of autologous mononuclear bone marrow cells: Safety, clinical outcome, and serial changes in left ventricular function during 12-months' follow-up. J Am Coll Cardiol. 2008;51:674–676. doi: 10.1016/j.jacc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 62.Mocini D, Staibano M, Mele L, Giannantoni P, Menichella G, Colivicchi F, Sordini P, Salera P, Tubaro M, Santini M. Autologous bone marrow mononuclear cell transplantation in patients undergoing coronary artery bypass grafting. Am Heart J. 2006;151:192–197. doi: 10.1016/j.ahj.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 64.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Mesquita CT, Belem L, Vaughn WK, Rangel FO, Assad JA, Carvalho AC, Branco RV, Rossi MI, Dohmann HJ, Willerson JT. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:II213–218. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- 65.Ruan W, Pan CZ, Huang GQ, Li YL, Ge JB, Shu XH. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chin Med J (Engl) 2005;118:1175–1181. [PubMed] [Google Scholar]
- 66.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the repair-ami trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 67.Schaefer A, Meyer GP, Fuchs M, Klein G, Kaplan M, Wollert KC, Drexler H. Impact of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: Results from the boost trial. Eur Heart J. 2006;27:929–935. doi: 10.1093/eurheartj/ehi817. [DOI] [PubMed] [Google Scholar]
- 68.Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled boost trial. Eur Heart J. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 69.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kogler G, Wernet P, Muller HW, Kostering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The iact study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 70.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The boost randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 71.Cohn JN, Johnson GR, Shabetai R, Loeb H, Tristani F, Rector T, Smith R, Fletcher R. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The v-heft va cooperative studies group. Circulation. 1993;87:VI5–16. [PubMed] [Google Scholar]
- 72.Reffelmann T, Konemann S, Kloner RA. Promise of blood-and bone marrow-derived stem cell transplantation for functional cardiac repair: Putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–308. doi: 10.1016/j.jacc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Ramos GA, Hare JM. Cardiac cell-based therapy: Cell types and mechanisms of actions. Cell Transplant. 2007;16:951–961. doi: 10.3727/096368907783338208. [DOI] [PubMed] [Google Scholar]
- 74.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 75.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 76.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: A comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 81.Dawn B, Bolli R. Bone marrow for cardiac repair: The importance of characterizing the phenotype and function of injected cells. Eur Heart J. 2007;28:651–652. doi: 10.1093/eurheartj/ehm009. [DOI] [PubMed] [Google Scholar]