A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease (original) (raw)
. Author manuscript; available in PMC: 2015 Nov 1.
Published in final edited form as: Alzheimers Dement. 2014 May 3;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001
Abstract
There is increasing evidence that subjective cognitive decline (SCD) in individuals with unimpaired performance on cognitive tests may represent the first symptomatic manifestation of Alzheimer’s disease (AD). The research on SCD in early AD, however, is limited by the absence of common standards. The working group of the Subjective Cognitive Decline Initiative (SCD-I) addressed this deficiency by reaching consensus on terminology and on a conceptual framework for research on SCD in AD. In this publication, research criteria for SCD in pre-mild cognitive impairment (MCI) are presented. In addition, a list of core features proposed for reporting in SCD studies is provided, which will enable comparability of research across different settings. Finally, a set of features is presented, which in accordance with current knowledge, increases the likelihood of the presence of preclinical AD in individuals with SCD. This list is referred to as SCD plus.
Keywords: Alzheimer’s disease, Subjective cognitive decline, Preclinical Alzheimer’s disease, Mild cognitive impairment, Prodromal Alzheimer ’s disease, Research criteria
1. Introduction
Characterization of at-risk states and detection of early disease are crucial for targeted dementia prevention [1]. The development of Alzheimer’s disease (AD), the most common cause of dementia, is slow and progressive with a presymptomatic course over several years to decades [2,3]. Biomarkers are available for some of the core features of AD pathology. These biomarkers include cerebrospinal fluid Aβ42, total tau, and phosphorylated tau (ptau) concentrations, positron emission tomography of brain amyloid deposition and glucose metabolism, and brain atrophy on magnetic resonance imaging [4].
Recently, two similar concepts have been proposed that subdivide the course of AD into three subsequent stages. The International Working Group (IWG) proposed (1) the asymptomatic at-risk stage of AD (AD pathology evidenced by biomarkers and no symptoms), (2) prodromal AD (episodic memory deficit with impaired cued recall that can be isolated or in association with other cognitive changes and biomarker evidence for AD), and (3) AD dementia (dementia and biomarker evidence for AD) [5,6]. The US National Institute on Aging-Alzheimer’s Association (NIA-AA) group proposed (1) the preclinical stage of AD (no impairment in cognition on standard assessments and biomarker evidence for AD), (2) mild cognitive impairment (MCI) due to AD (impairment on memory or other domains of cognition on a standard assessment and biomarker evidence for AD), and (3) dementia due to AD (dementia and biomarker evidence for AD) [7–10]. Those stages before dementia may serve to define the population for targeted dementia prevention trials. Currently, studies with potentially disease-modifying drugs are being performed in prodromal AD and MCI due to AD (www.clinicaltrials.gov). At these stages, however, progressive neuronal loss and irreversible cognitive impairment may have already occurred. Thus, conceptualization and investigation of the preprodromal or pre-MCI stage of AD is needed to define target populations for interventions at a stage of only mild neuronal damage and with still sufficient functional compensation [11].
In accordance with the NIA-AA criteria, stage 3 of pre-clinical AD is defined by biomarker evidence for AD plus subtle cognitive decline, which does not reach the level of objective impairment required for the MCI diagnosis. This subtle cognitive decline is difficult to detect on standardized cognitive testing because of the requirement of high test sensitivity, robustness of tests against within-subject performance variability, specificity of test results in the differentiation from normal performance, and test stability against rater-related confounds in application. The subtle decline is also associated with at least partly successful compensation, yielding unimpaired performance levels on some individual tests.
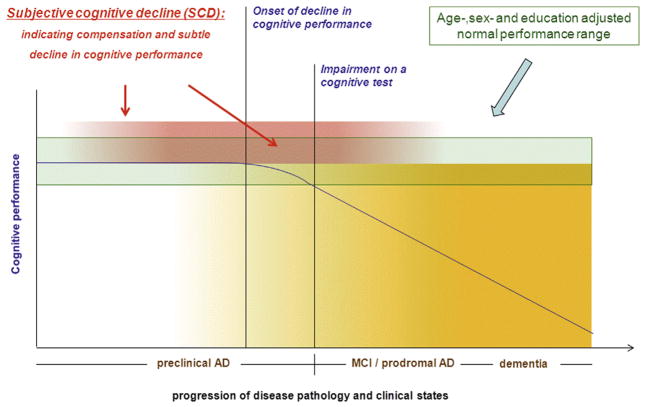
Limitations in detecting this subtle decline on cognitive tests, however, do not preclude self-experience of this decline in individuals with preclinical AD. In fact, subjectively reported change in cognitive performance is a core criterion of the MCI and prodromal AD definition [5,9,12]. There is rapidly increasing evidence that this subjectively experienced decline even at the stage of normal performance on cognitive tests (pre-MCI and preprodromal) is associated with increased likelihood of biomarker abnormalities consistent with AD pathology and with an increased risk for future cognitive decline and AD dementia (e.g., [13–35]). Valid usage of subjective reports on cognitive decline as an indicator of first effects of AD pathology on cognition would therefore be of significant benefit. As the report of subjective decline reflects a longitudinal course, it may even prove to be particularly informative at a very early disease stage, in which detection of decline with cross-sectional cognitive tests is challenging (preclinical AD). At this stage, it may reflect the first effects of AD pathology on cognitive functioning between full compensation and very first decline (Fig. 1). Future effective treatment at this stage would preserve function at a high level.
Fig. 1.
Depicted is the course of cognitive decline in relation to progressive disease pathology in Alzheimer’s disease (AD). After a phase of stable cognitive performance in the presence of increasing pathology, cognitive decline occurs. After crossing the threshold of below normal age-, sex-, and education-adjusted performance, the stage of mild cognitive impairment (MCI) or prodromal AD is reached. Subsequently, cognitive decline progresses onward to the stage of dementia. It is proposed that SCD occurs at the late stage of preclinical AD, which is characterized by increasing compensatory cognitive efforts and subtle cognitive decline. Thus, SCD may indicate the late-stage preclinical AD before the threshold of MCI/prodromal AD is reached. There is evidence that the subjective experience of decline levels off as disease progresses into dementia. That stage, however, is not part of the conceptualization of SCD in preclinical AD and is not addressed in the present publication.
However, subjective decline in cognition is unspecific. It is related to numerous conditions such as normal aging, personality traits, psychiatric conditions, neurologic and medical disorders, substance use, and medication. It may also be affected by the individual cultural background. Refinement of knowledge about the characteristics of subjective decline at the very early (preclinical) stage of AD is therefore needed.
Results of current studies on subjective cognitive decline (SCD) in preclinical AD are highly variable and a conclusive picture with regard to rate of decline, risk for conversion to AD dementia, role of biomarkers in disease course prediction, and other crucial questions is missing [36–38]. This is partly due to a dearth of common terminology and viable research concepts on this topic. For example, after the initial description in 1982 [39], the reported experiences of cognitive decline have been denoted and conceptualized as subjective cognitive impairment, subjective memory decline, subjective memory impairment, and memory complaints, among other terminologies [40]. Different strategies are applied for assessment ranging from a single question or a small number of items with varied content and unknown measurement properties to more comprehensive, psychometrically derived, and validated questionnaires. Moreover, participants are studied in different research environments, including clinical settings, volunteer samples, and population-based cohorts.
A common concept for terminology and research procedures is critically needed to achieve a collective and improved understanding of the subjective experience of cognitive decline, to elaborate its role in identifying individuals with preclinical AD, and to assess its potential usefulness for clinical trials recruitment. To address this need, the Subjective Cognitive Decline Initiative (SCD-I) was started with the aim of facilitating the development of a common SCD research concept.
2. Procedures
The SCD-I was launched in October 2012 and a working group was formed, including researchers from clinical and population-based science on AD who have investigated the topic of SCD. Researchers were identified by systematic literature search. In addition, the initiative sought to include the leaders of the IWG and NIA-AA preclinical AD working groups and key representatives of the large ongoing studies on early AD detection (Alzheimer’s Disease Neuroimaging Initiative (ADNI); Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL); Dementia Competence Network (DCN); Development of screening guidelines and criteria for predementia Alzheimer’s disease (DESCRIPA); Sydney Memory and Ageing Study, Determinants and Evolution of Alzheimer’s Disease and Related Disorders (MEMENTO); DZNE Longitudinal Study og Cognition and Dementia (DELCODE); and Mayo Clinic Study of Aging). All contacted investigators agreed to participate. After confirmation of participation, a set of key points and definitions was distributed. This was followed by three rounds of comments and discussion with subsequent modifications of the key points and definitions. After consenting to the content, the present manuscript was drafted and approved by all participants of the SCD-I working group.
3. Key points on SCD in preclinical AD
The SCD-I working group agreed on the following key points:
- There is evidence that SCD occurs at the preclinical stage of AD and may serve as a symptomatic indicator of preclinical AD because (a) longitudinal data support SCD as a risk factor for future cognitive decline as well as for MCI and AD dementia (e.g., [13,14,33–35]), (b) there is cross-sectional biomarker evidence for an increased prevalence of preclinical AD in those with SCD (e.g. [15–31]), and (c) individuals with SCD and biomarker evidence for AD are at increased risk of future cognitive decline and progression to MCI and AD dementia [41–43].
- Current knowledge is insufficient to comprehensively define the specific features of SCD in preclinical AD. The characteristics of SCD in preclinical AD are probably variable and are expressed heterogeneously.
- Preclinical AD is, by definition, a biomarker diagnosis, and SCD is neither required for the diagnosis of preclinical AD nor is it necessarily present in all cases of preclinical AD. SCD by itself may never be sufficient to diagnose preclinical AD.
- Numerous causes of SCD other than preclinical AD exist. These include, but are not limited to, SCD in MCI due to AD/prodromal AD, dementia, normal aging, psychiatric and neurologic disorders other than AD, or related to effects of medication and substance use.
4. Aims of the SCD framework
The first aim of this framework was to create a common concept and terminology to facilitate research on various aspects of SCD in different research settings and at the same time generate comparability and synergies across studies. This is achieved by defining terms and proposing a broad symptomatic definition of pre-MCI SCD (Table 1).
Table 1.
Research criteria for pre-MCI subjective cognitive decline (SCD)
| Self-experienced persistent decline in cognitive capacity in comparison with a previously normal status and unrelated to an acute event. Normal age-, gender-, and education-adjusted performance on standardized cognitive tests, which are used to classify mild cognitive impairment (MCI) or prodromal AD. |
|---|
| 1 and 2 must be present |
| Exclusion criteria Mild cognitive impairment, prodromal AD, or dementia Can be explained by a psychiatric* or neurologic disease (apart from AD), medical disorder, medication, or substance use |
It is acknowledged that any definition of SCD is a trade-off between being overinclusive (high sensitivity and high false positive rates) and being too restrictive (high specificity and high false negative and high screening failure rates). At the present state, a sensitive and potentially overinclusive definition was considered appropriate as the specific features of SCD in preclinical AD are not yet well known.
In addition, we propose a system for coding essential features of SCD, which investigators may adopt as a common core of future studies of SCD. This coding system will support the standardization of assessments of SCD across studies. Eventually, this will enable the identification of SCD subtypes, which may serve different research purposes (Table 2).
Table 2.
Features suggested for coding in studies on SCD
- Setting in which SCD is expressed
- Medical environment.
* Memory clinic, memory specialist
* General practitioner - Population sample
- Volunteer sample (recruitment by advertisement)
- Other, specify
- Medical environment.
- Association of SCD with medical help seeking (yes/no)
- Report of SCD (spontaneously/on request)
- Onset of SCD (number of years)
- Age at onset of SCD
- Subjective decline in memory (yes/no)
- Subjective decline in nonmemory domains (yes/no), if yes, specify
- Concerns (worries) associated with SCD (yes/no)
- Feeling of worse performance than others of the same age group (yes/no)
- Association of SCD with experience of impairment (yes/no)
- Confirmation of cognitive decline by an informant (yes/no)
- Score on a depression scale, score on an anxiety scale
- APOE genotype, if available
A further aim of the framework is to list specific features associated with SCD, which increase the likelihood of the presence of preclinical AD. This list of features may be useful for studies that wish to use SCD as a symptomatic marker for enrichment of preclinical AD but do not specifically aim at elaborating SCD itself. This list of features is referred to as SCD plus (preclinical AD) (Table 3). It is an open set of criteria reflecting current knowledge, which allows for addition and subtraction of items as research progresses.
Table 3.
Features that increase the likelihood of preclinical AD in individuals with SCD according to current data: SCD plus (preclinical AD)
| Subjective decline in memory, rather than other domains of cognition Onset of SCD within the last 5 y Age at onset of SCD ≥60 y Concerns (worries) associated with SCD Feeling of worse performance than others of the same age group |
|---|
| If available or possible to obtain in the respective study: Confirmation of cognitive decline by an informant Presence of the APOE ε4 genotype Biomarker evidence for AD (defines preclinical AD) |
5. Terminology of SCD
The rationale and meaning of the term of subjective cognitive decline are the following: Subjective refers to the self-perception of cognitive performance. It is conceptually independent of performance on a cognitive test. No “validation” of the subjective experience of cognitive capability by means of cognitive testing is required. The performance on a cognitive test is the objective level of cognitive functioning at a particular point in time. The concurrent and longitudinal relationship between subjective and objective cognitive performance is a research topic of major interest (e.g., [44]).
In the context of SCD in preclinical AD, cognitive testing is required to establish a normal objective performance level, which defines preclinical AD. If SCD is studied in other conditions than preclinical AD, the respective criteria set for these conditions (e.g. MCI) need to be applied.
Cognitive refers to any cognitive domain. It is not restricted to memory. Cognitive as opposed to memory was chosen for the following reasons: (1) the first symptoms of AD are not limited to memory decline and (2) lay people may report memory decline when they actually experience decline in other cognitive domains such as executive function and vice versa (e.g., reporting a “speech problem” when the difficulty is really memory retrieval). Currently, many studies have used questions specifically related to episodic memory, and the available evidence for an association of preclinical AD with questions about memory functioning may be strongest at present. Therefore, a positive response to the question on subjective memory is proposed as an item of the SCD plus category. Broader application of instruments that extend beyond the assessment of subjective memory decline [29,39,45,46], the development and elaboration of questions on subjective change in other cognitive domains (e.g., executive function, attention, language, and visuospatial function), and the association of these with preclinical AD or other diseases are core topics for current and future SCD research.
Decline refers to a subjectively experienced worsening of cognitive capacities. It was chosen because it reflects the progressive nature of cognitive deterioration in AD. It is acknowledged that this term also incorporates conditions such as normal aging. There is evidence that additional characteristics of this decline increase the likelihood of an association with preclinical AD. These include (1) the association of decline with a particular concern (worries) [34] and (2) the appraisal that one’s own cognitive capacity is inferior compared with others of the same age group [19,20]. These two features are proposed for the SCD plus category.
Studies on SCD have often used the term impairment (subjective cognitive impairment) instead of decline. The term impairment does not immediately reflect the temporal course of subjective cognitive change because impairment may also be of a chronic and stable nature. Thus, it requires an additional definition of onset. In contrast, the term decline already includes the fact that an onset has occurred. However, the condition of subjective impairment in cognition may indicate a certain level of severity of SCD, which is characterized by a subjectively experienced impairment (i.e., feeling handicapped or functionally defective) as opposed to just a worse level of cognitive functioning from some prior time [39,47,48]. Therefore, it is proposed that SCD studies code whether an individual experiences impairment because of decline in cognitive capacity. This coding system permits the assessment of the specific prediction of AD based on subjective cognitive impairment within the SCD framework.
6. Onset of SCD
One feature of decline is the time frame of onset. There is evidence that the onset of SCD within a few years may be more predictive of cognitive decline and AD than the presence of SCD for several years [14,49,50]. It is also acknowledged that an individual’s recall of the time of onset of SCD may be vague. It is suggested to code the reported SCD onset in studies on SCD but not to define an onset limit in the core criteria of SCD. An onset within the last 5 years [14,49,50] is suggested as a component of SCD plus.
7. Age at the onset of SCD
Age at the onset of SCD should also be coded in SCD studies. This is defined as the age at which the individual first experienced a significant and persistent decline that does not just occur as transient or short term. No age at onset cutoff is defined as an SCD core criterion because different research settings may require different age ranges. As an example, highly specialized memory clinic settings may sample individuals with AD at a younger age than broad population-based studies. For the SCD plus category, in studies of preclinical AD that are unrelated to autosomal dominant genetic mutations, a reported age of onset at 60 years or older is proposed. The rationale for this cutoff is the increasing prevalence of AD-related neuropathological alterations starting at midlife, which may trigger SCD after neuronal dysfunction affects cognitive abilities. At younger age cutoffs, the likelihood of SCD due to causes other than AD increases. It is acknowledged that this age cutoff is arbitrary and may not be appropriate for all studies. We caution that subjects with early-onset AD may experience SCD earlier and may be missed by this proposed criterion. Thus, studies may apply other age cutoffs, depending on the population of interest, but should code the age at onset to allow comparison with other investigations.
8. Confirmation of cognitive decline by others
SCD conceptually refers to the self-perception of cognitive decline and does not require confirmation by external observation per se. For this reason, confirmation by an informant is not a core criterion of SCD.
However, cognitive decline may be observed by others in contact with the individual. In fact, there is evidence suggesting that informant report may be a better predictor of objective performance than self-report and may facilitate identification of very early decline related to AD (e.g., [44,47,48,51,52]). This may be particularly the case in advanced stages of preclinical AD in proximity to MCI [53]. In contrast, recent data suggest that earliest changes in cognition are best perceived by the individual rather than by an observer [54]. This finding may be related to still successful functional compensation at the stage of early SCD.
Finally, there are individuals at the early AD stage with observed cognitive decline but without self-reported SCD (e.g., [55]). These do not fall into the SCD category. They may represent a group with very early anosognosia or denial in the course of the disease and may be of particular research interest.
It is proposed to code whether SCD is confirmed by an informant in studies on SCD. It has to be acknowledged, however, that the observation and report by others may be affected by several issues such as frequency of contact, quality of the relationship, expectations, affective state of the individual or informant, etc. Thus, refinement and standardization of the assessment of informant report on SCD is an important research topic.
It is suggested to include confirmation by an informant as an SCD plus feature because it may serve as an enrichment strategy for preclinical AD, particularly at the progressed stage of SCD (e.g., [16,44,48,51,52]).
9. Psychiatric comorbidities, subthreshold symptoms, and personality traits
It is acknowledged that various psychiatric disorders can be associated with SCD. If SCD criteria are applied in the context of research on preclinical AD, the presence of major psychiatric disorders should be an exclusion criterion because AD-related SCD should not be confounded by other conditions that clearly affect the subjective experience of cognitive capacity. The delineation of the very first symptomatic manifestation of AD in subjects with major depression or generalized anxiety disorder is, however, a particular research topic, which may yield specific characteristics of AD-related SCD in these conditions. Furthermore, SCD in psychiatric disorders, independent of preclinical AD, is considered an important topic and relevant subject of future conceptualization and research.
In many studies on SCD in preclinical AD, individuals report subthreshold symptoms of depression and anxiety. Slightly higher scores on scales that measure these conditions frequently document this finding. These subjects, however, do not necessarily fulfill the criteria for a psychiatric disorder. It is proposed that subthreshold symptoms of depression and anxiety are assessed with respective scales and to account for them in statistical models but not to exclude subjects with subthreshold symptoms from studies on SCD in preclinical AD. These subthreshold symptoms may also be manifestations of preclinical AD.
There is literature suggesting that SCD is associated with certain personality traits such as neuroticism and anxiety sensitivity and related inversely with measures of openness and conscientiousness [44,56,57]. Personality traits can be captured with respective instruments in studies on SCD, but there is no specific pattern of personality characteristics that at present should be defined as an exclusion criterion.
10. Neurologic and medical comorbidities as well as medications
Several neurologic and medical conditions as well as medications can affect cognition and may be associated with SCD. An explicit list of conditions and drugs will always be incomplete. Therefore, investigators in SCD studies should document comorbidities and medications and report whether these were treated as exclusion criteria or considered otherwise in the respective studies.
11. Apolipoprotein E genotype
There is evidence from some memory clinic and population-based studies of an overrepresentation of the APOE ε4 allele in SCD, a particular association of APOE ε4 and biomarker evidence for AD in SCD, and an effect of APOE ε4 on the prospective risk of cognitive decline in SCD [58–61]. To further elucidate the potential use of the APOE genotype in predicting preclinical AD and cognitive decline in SCD, it should be reported in SCD studies, if available. As APOE ε4 is a valid genetic risk factor for AD, it is proposed as an SCD plus feature.
12. Research setting
The setting in which SCD subjects are recruited is considered a crucial factor in determining the characteristics of the respective samples. All SCD studies should explicitly describe their recruitment strategy and describe the setting. Typical research settings include (1) population-based studies, (2) volunteer samples, and (3) medical help–seeking samples. The latter include, but are not limited to, patients consulting general practitioners, neurologists, psychiatrists, and geriatricians or attending memory clinics. In research in the medical environment, the terms complaint or complainers are frequently used. In many countries, these terms and their equivalents in other languages have negative connotations. It is therefore suggested to preferentially use the term medical help seeking, which can be associated with particular concerns.
13. Perspectives
The proposed framework on SCD in preclinical AD aims at standardizing research on self-perceptions of change and symptom development at very early disease stages before the detection threshold of current neuropsychological instruments is reached. This particular nature of SCD represents a challenge regarding research designs and modes of assessment. However, it can potentially provide highly valuable information for early disease detection and disease course prediction. The common framework will serve as the basis for joint research efforts, synergies across studies, and elaboration of knowledge about SCD.
The application of a refined SCD approach as an enrichment strategy for preclinical AD studies is distinct from other current approaches, which select on the basis of biological criteria (causal mutation carriers, APOE ε4 carriers, and amyloid-positive cognitively normal individuals). Although highly plausible, there may be selection bias associated with these approaches because they depend on an a priori biological hypothesis, and findings from these enrichment approaches may not be generalizable to all AD cases. In comparison, SCD is a much more liberal and broad enrichment strategy that potentially captures additional AD cases. This may, however, be at the expense of specificity. A particular advantage of enrichment by SCD is its practicality and low cost of application. It is applicable in different settings and most likely preselects subjects who are willing to participate in trials and who will receive future treatments.
This proposal focuses on SCD in preclinical AD. As with previous research criteria on prodromal AD and MCI due to AD, the SCD and SCD plus criteria require continuous refinement and validation to eventually serve as a standardized indicator for biomarker-based preclinical AD detection. This research framework is a central step in this process.
RESEARCH IN CONTEXT.
- Systematic review: We reviewed the literature on subjective cognitive decline (SCD) in relation to Alzheimer’s Disease. We included population-based studies and studies in clinical samples.
- Interpretation: We consented a framework for research on SCD in the context of preclinical AD. This framework includes a broad definition of pre-mild cognitive impairment SCD, features to be reported in SCD studies and a set of criteria, which increase the likelihood of the presence of preclinical AD in individuals with SCD (SCDplus). The consented research framework is the basis for increased comparability of research on SCD across settings and studies
- Future directions: Refined understanding of several aspects of SCD is required to employ it as an indicator of the earliest symptomatic manifestation of AD. Further conceptual work and harminisation processes will guide this process.
Acknowledgments
Role of the funding source: There was no specific funding source in this consensus article.
Glossary
Subjective cognitive decline (SCD)
Self-perceived decline in any cognitive domain over time. The category SCD does not require cognitive testing or confirmation of cognitive decline by an informant. SCD is not associated with a particular disease or disease state per se. When reporting on SCD, the specific disease condition to which it refers in the particular context needs to be added (e.g., SCD in pre-clinical AD). If these conditions have specific definitions, these need to be established. As an example, in the case of SCD in preclinical AD, cognitive testing is needed to exclude cognitive impairment (e.g., MCI)
Objective cognitive performance level
Performance on a cognitive test
Preclinical Alzheimer’s disease (AD)
Preclinical AD describes the stage at which AD pathology is present, but the objective cognitive performance still reaches a level within the normal age-, gender-, and education-adjusted range on standardized cognitive tests. It includes the presence of subtle cognitive decline, which has not yet reached the level of a clinical symptom [8]. SCD can be a reflection of subtle cognitive decline in preclinical AD. SCD, however, is not a requirement in the preclinical AD definition [8]
Footnotes
Authors’ contribution: All authors contributed to the definitions and the contents of the article, the provision of views, data, and literature, and drafting of the manuscript, including several steps of revision and to the final consensus process.
References
- 1.Vellas B, Aisen PS, Sampaio C, Carrillo M, Scheltens P, Scherrer B, et al. Prevention trials in Alzheimer’s disease: an EU-US task force report. Prog Neurobiol. 2011;95:594–600. doi: 10.1016/j.pneurobio.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 4.Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–74. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 13.Glodzik-Sobanska L, Reisberg B, De Santi S, Babb JS, Pirraglia E, Rich KE, et al. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord. 2007;24:177–84. doi: 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- 14.Dufouil C, Fuhrer R, Alperovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The Epidemiology of Vascular Aging Study. J Am Geriatr Soc. 2005;53:616–21. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Risacher SL, West JD, McDonald BC, Magee TR, Farlow MR, et al. Altered default mode network connectivity in older adults with cognitive complaints and amnestic mild cognitive impairment. J Alzheimers Dis. 2013;35:751–60. doi: 10.3233/JAD-130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risacher SL, Wudunn D, Pepin SM, MaGee TR, McDonald BC, Flashman LA, et al. Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol Aging. 2013;34:1133–44. doi: 10.1016/j.neurobiolaging.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, West JD, Flashman LA, Wishart HA, Santulli RB, Rabin LA, et al. Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta. 2012;1822:423–30. doi: 10.1016/j.bbadis.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–9. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 19.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–9. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–6. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rami L, Fortea J, Bosch B, Solé-Padullés C, Lladó A, Iranzo A, et al. Cerebrospinal fluid biomarkers and memory present distinct associations along the continuum from healthy subjects to AD patients. J Alzheimers Dis. 2011;23:319–26. doi: 10.3233/JAD-2010-101422. [DOI] [PubMed] [Google Scholar]
- 22.Fortea J, Sala-Llonch R, Bartres-Faz D, Lladó A, Solé-Padullés C, Bosch B, et al. Cognitively preserved subjects with transitional cerebrospinal fluid ss-amyloid 1-42 values have thicker cortex in Alzheimer’s disease vulnerable areas. Biol Psychiatry. 2011;70:183–90. doi: 10.1016/j.biopsych.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Chetelat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67:317–24. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- 24.Chetelat G, Villemagne VL, Pike KE, Baron JC, Bourgeat P, Jones G, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133:3349–58. doi: 10.1093/brain/awq187. [DOI] [PubMed] [Google Scholar]
- 25.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–27. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 26.Tepest R, Wang L, Csernansky JG, Neubert P, Heun R, Scheef L, et al. Hippocampal surface analysis in subjective memory impairment, mild cognitive impairment and Alzheimer’s dementia. Dement Geriatr Cogn Disord. 2008;26:323–9. doi: 10.1159/000161057. [DOI] [PubMed] [Google Scholar]
- 27.Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–18. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang PJ, Saykin AJ, Flashman LA, Wishart HA, Rabin LA, Santulli RB, et al. Regionally specific atrophy of the corpus callosum in AD, MCI and cognitive complaints. Neurobiol Aging. 2006;27:1613–7. doi: 10.1016/j.neurobiolaging.2005.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–42. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–6. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Prichep LS, John ER, Ferris SH, Reisberg B, Almas M, Alper K, et al. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging. 1994;15:85–90. doi: 10.1016/0197-4580(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 32.Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry. 2011;68:845–52. doi: 10.1001/archgenpsychiatry.2011.80. [DOI] [PubMed] [Google Scholar]
- 33.van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MM. Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimers Dement. 2007;3:92–7. doi: 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–22. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 35.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;2006:471–85. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 37.Mol M, Carpay M, Ramakers I, Rozendaal N, Verhey F, Jolles J. The effect of perceived forgetfulness on quality of life in older adults; a qualitative review. Int J Geriatr Psychiatry. 2007;22:393–400. doi: 10.1002/gps.1686. [DOI] [PubMed] [Google Scholar]
- 38.Stewart R. Subjective cognitive impairment. Curr Opin Psychiatry. 2012;25:445–50. doi: 10.1097/YCO.0b013e3283586fd8. [DOI] [PubMed] [Google Scholar]
- 39.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–9. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 40.Abdulrab K, Heun R. Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23:321–30. doi: 10.1016/j.eurpsy.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Prichep LS, John ER, Ferris SH, Rausch L, Fang Z, Cancro R, et al. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiol Aging. 2006;27:471–81. doi: 10.1016/j.neurobiolaging.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 42.van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, et al. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–7. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Peter J, Scheef L, Abdulkadi A, Boecker H, Heneka M, Wagner M, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement. 2014;10:99–109. doi: 10.1016/j.jalz.2013.05.1764. [DOI] [PubMed] [Google Scholar]
- 44.Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, et al. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–10. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 45.Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS) Psychopharmacol Bull. 1988;24:629–36. [PubMed] [Google Scholar]
- 46.Eckerstrom M, Skoogh J, Rolstad S, Göthlin M, Steineck G, Johansson B, et al. Sahlgrenska Academy Self-reported Cognitive Impairment Questionnaire (SASCI-Q)—a research tool discriminating between subjectively cognitively impaired patients and healthy controls. Int Psychogeriatr. 2013;25:420–30. doi: 10.1017/S1041610212001846. [DOI] [PubMed] [Google Scholar]
- 47.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr. 2008;20:1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 48.Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4:98–108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Treves TA, Verchovsky R, Klimovitzky S, Korczyn AD. Incidence of dementia in patients with subjective memory complaints. Int Psychogeriatr. 2005;17:265–73. doi: 10.1017/s1041610205001596. [DOI] [PubMed] [Google Scholar]
- 50.Chary E, Amieva H, Pérès K, Orgogozo JM, Dartigues JF, Jacqmin-Gadda H. Short- versus long-term prediction of dementia among subjects with low and high educational levels. Alzheimers Dement. 2013;9:562–71. doi: 10.1016/j.jalz.2012.05.2188. [DOI] [PubMed] [Google Scholar]
- 51.Tierney MC, Szalai JP, Snow WG, Fisher RH. The prediction of Alzheimer disease. The role of patient and informant perceptions of cognitive deficits. Arch Neurol. 1996;53:423–7. doi: 10.1001/archneur.1996.00550050053023. [DOI] [PubMed] [Google Scholar]
- 52.Rabin LA, Wang C, Katz MJ, Derby CA, Buschke H, Lipton RB. Predicting Alzheimer’s disease: neuropsychological tests, self-reports, and informant reports of cognitive difficulties. J Am Geriatr Soc. 2012;60:1128–34. doi: 10.1111/j.1532-5415.2012.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Risacher SL, Petersen RC, Aisen P, Jack CR, Koeppe R, Jagust W, et al. Self vs. informant-based cognitive complaints: relation of E-Cog scores to imaging, biomarkers and clinical status in ADNI-2. Alzheimers Dement. 2013;9(Suppl):429. [Google Scholar]
- 54.Caselli RJ, Chen K, Locke DE, Lee W, Roontiva A, Bandy D, et al. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement. 2014;10:93–8. doi: 10.1016/j.jalz.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol. 2011;10:213–20. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 56.Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. J Affect Disord. 2002;72:157–65. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 57.Dux MC, Woodard JL, Calamari JE, Messina M, Arora S, Chik H, et al. The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. J Int Neuropsychol Soc. 2008;14:327–36. doi: 10.1017/S1355617708080363. [DOI] [PubMed] [Google Scholar]
- 58.Dik MG, Jonker C, Comijs HC, Bouter LM, Twisk JW, van Kamp GJ, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57:2217–22. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- 59.Laws SM, Clarnette RM, Taddei K, Martins G, Paton A, Hallmayer J, et al. APOE-epsilon4 and APOE-491A polymorphisms in individuals with subjective memory loss. Mol Psychiatry. 2002;7:768–75. doi: 10.1038/sj.mp.4001083. [DOI] [PubMed] [Google Scholar]
- 60.van der Flier WM, Pijnenburg YA, Schoonenboom SN, Dik MG, Blankenstein MA, Scheltens P. Distribution of APOE genotypes in a memory clinic cohort. Dement Geriatr Cogn Disord. 2008;25:433–8. doi: 10.1159/000124750. [DOI] [PubMed] [Google Scholar]
- 61.Striepens N, Scheef L, Wind A, Meiberth D, Popp J, Spottke A, et al. Interaction effects of subjective memory impairment and ApoE4 genotype on episodic memory and hippocampal volume. Psychol Med. 2011;41:1997–2006. doi: 10.1017/S0033291711000067. [DOI] [PubMed] [Google Scholar]