ADVANCES IN UNDERSTANDING THE LEUKEMIA MICROENVIRONMENT (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 1.
Published in final edited form as: Br J Haematol. 2014 Jan 9;164(6):767–778. doi: 10.1111/bjh.12725
SUMMARY
Dynamic interactions between leukemic cells and cells of the bone marrow are a feature of hematological malignancies. Two distinct microenvironmental niches in the bone marrow, the “osteoblastic (endosteal)” and “vascular” niches, provide a sanctuary for subpopulations of leukemic cells to evade chemotherapy-induced death and allow acquisition of a drug-resistance. Key components of the bone marrow microenvironment as a home for normal hematopoietic stem cells and the leukemia stem cell niches, and the molecular pathways critical for microenvironment/leukemia interactions via cytokines, chemokines, and adhesion molecules as well as hypoxic conditions, are described in this review. Finally, the genetic abnormalities of leukemia-associated stroma are discussed. Further understanding of the contribution of the bone marrow niche to the process of leukemogenesis may provide new targets that allow destruction of leukemia stem cells without adversely affecting normal stem cell self-renewal.
Keywords: Bone marrow microenvironment, leukemia, stem cell niche
INTRODUCTION
Despite significant progress achieved over the past decade in the chemotherapy-based and targeted treatments of several leukemia subsets, the relapse remains common after an initial response, indicating resistance of leukemia stem/progenitor cells to current therapies. The proof for the concept that a subpopulation of leukemic stem cells (LSCs) is solely responsible for maintenance of the leukemia was derived from the study of human acute myeloid leukemia (AML). In 1997, Bonnet and Dick established the concept of the hierarchical organization of AML. They showed that only CD34++CD38− cells within the leukemic clone, regardless of the heterogeneity in maturation characteristics of the leukemic blasts, had the capacity to initiate AML growth after transplant into NOD/SCID mice (Bonnet & Dick, 1997). LSCs exhibit the unique characteristics as stem cells, including quiescence, pluripotency, and self-renewal within the bone marrow (BM) microenvironment (Warner et al, 2004). On the other hand, a specialized regulatory microenvironmental “niche” had been proposed more than 30 years ago (Schofield, 1978). Schofield suggested that stem cells reside in this sanctuary niche, where they receive appropriate support for maintaining self-renewal and multi-lineage differentiation capacity and are protected from environmental stress.
Subsequent studies have demonstrated that hematopoietic stem cells (HSC) reside in two distinct BM niches: the “osteoblastic (endosteal)” and “vascular” niches (Calvi et al, 2003; Kiel et al, 2005; Adams et al, 2006; Ding et al, 2012). These niches are part of a complex of BM cells that includes bone-lining cells (osteoblasts and osteoclasts), mesenchymal stem cells (MSCs), sinusoidal endothelium and perivascular stromal cells, and immune cells that play different roles in hematopoietic regulation (Nwajei & Konopleva, 2013). Cytokines and chemokines produced by BM stromal cells concentrate in particular niches secondary to varying local production and through the effects of cytokine-binding glycosaminoglycans. Chemokine (C X C motif) ligand 12 (CXCL12) positively regulates HSC homing, while transforming growth factor-β (TGF-β) and angiopoietin-1 (ANG-1) function as quiescence factors. The BM stromal cell–produced complex extracellular matrix (ECM) is involved in BM engraftment of HSCs and LSCs via vascular cell–adhesion molecule-1 (VCAM-1) or fibronectin (Miyake et al, 1991).
Like normal HSCs, LSCs remain dependent on signals from the hematopoiesis-regulating stromal environment for survival and proliferation (Dührsen & Hossfeld, 1996). Although the biology of LSCs shares many similarities with that of HSCs, LSCs are able to outcompete HSCs, hijacking the BM microenvironment. In the context of the “seed and soil” hypothesis, BM niches fuel the growth of leukemia cells and contribute to the therapy resistance and metastatic potential of leukemia cells by shielding LSCs (Hanahan & Coussens, 2012). There is increasing evidence that this microenvironment plays a critical role in the disease process. Not only a “microenvironment-induced oncogenesis”, but also a “malignancy-induced microenvironment” has been proposed (Raaijmakers et al, 2010).
The key components and regulatory mechanisms (via cytokines, chemokines, adhesion molecules, and hypoxic conditions) of the two kinds of BM niche, osteoblastic and vascular, are described in this review. The genetic abnormalities of leukemia-associated stroma also are discussed. Further understanding of the contribution of the BM niches to the process of leukemogenesis may provide new targets that allow destruction of LSCs without adversely affecting normal stem cell self-renewal.
Microenvironmental niches of normal HSCs
HSCs reside and self-renew in the specialized area of the BM microenvironment called the niche. The BM niche modulates HSC quiescence, proliferation, differentiation, and migration. HSCs interact with the niche by exchanging various molecular signals, including adhesion mechanisms. The two distinct microenvironmental niches within the BM, the osteoblastic (endosteal) and vascular niches, have been demonstrated to work in concert (Perry & Li, 2007).
The osteoblastic niche localized at the inner surface of the bone cavity with abundant bone-forming osteoblasts. In the osteoblastic niche, HSCs and osteoblasts bind to each other via adhesion molecules, and this binding contributes to the maintenance of stem cell quiescence (Iwasaki & Suda, 2009).
Kiel et al demonstrated that HSCs, in this case a highly purified population of CD150+CD244−CD48− cells isolated via a combination of SLAM (signaling lymphocyte activation molecule) family markers, mainly reside adjacent to sinusoidal endothelium in spleen and BM (Kiel et al, 2005). Although a few HSCs show a preference for the BM endosteum, Kiel et al estimated that two thirds of HSC in the BM are adjacent to sinusoids. The vascular niche consists of sinusoidal endothelial cells lining blood vessels; it promotes proliferation and differentiation of actively cycling, short-term HSCs (Passegue et al, 2005).
Thus, HSCs occupy multiple niches, including the endosteum and sinusoidal endothelium, in the BM microenvironment. Coordination between the osteoblastic and vascular niches regulates HSC self-renewal, proliferation, differentiation, and mobilization in and out of the BM. Several reports demonstrated that the vascular niche may localize within close proximity to the osteoblastic niche; in such cases the endosteum forms a well-vascularized special zone which is localized near N-cadherin–positive preosteoblastic cells, and this special niche promotes expansion of HSCs in response to BM damage (Jin et al, 2006). A recent comprehensive study has demonstrated that quiescent HSCs associate with NG2+ periarteriolar niches found within the endosteal bone marrow, while cycling HSC re-localize to LEPR+ perisinusoidal niches (Kunisaki et al, 2013). Elucidating the anatomical and functional diversity of the bone marrow microenvironment is crucial to understanding the behavior of HSCs and to exploiting this knowledge for clinical applications.
Components of the osteoblastic (endosteal) niche
Osteoblasts
The surface of the endosteum is lined by osteoblasts and osteoclasts. Osteoblasts are progenitor bone-forming cells that work in tandem with osteoclasts in the process of osteogenesis (Schroder et al, 2012).
In the osteoblastic niche, signaling through Jagged-1 (Jag-1) on osteoblasts and its receptor NOTCH on HSCs is involved in the expansion of the HSC pool (Calvi et al, 2003). On the other hand, angiopoietin-1 (Ang-1) in osteoblasts interacts with its receptor Tie-2, a type of receptor tyrosine kinase (RTK) expressed in HSCs, which results in activation of β1-integrin and N-cadherin. This enhanced adhesion between the niche cell and the stem cell also contributes to the maintenance of stem cell quiescence (Arai et al, 2004).
Osteoblasts express osteopontin, a negative regulator of HSC pool size that inhibits HSC proliferation, promotes HSC apoptosis, and affects the expression of Jag-1 and Ang-1 by stromal cells (Nilsson et al, 2005).
CXCL12 produced by osteoblasts is the major chemoattractant for hematopoietic stem and progenitor cells (HSPCs) (Christopher et al, 2009). Several studies demonstrated that mice deficient in CXCL12 or its receptor CXCR4 displayed impaired BM engraftment by hematopoietic cells (Sugiyama et al, 2006). All of these data underline the essential role of MSCs and osteoblasts in the BM HSC niche.
Osteoclasts
Bone-resorbing osteoclasts play a major role in endochondral ossification and coordinate with osteoblasts in bone formation (Schroder et al, 2012).
Osteoclasts regulate osteoblastic development in establishing HSC niches and, more importantly, form the cavities that constitute the endosteal niche (Schroder et al, 2012). Mice lacking osteoclast activity developed severe osteopetrosis, whch is associated with extramedullary hematopoiesis (Mansour et al, 2012), and osteoclast inhibition by bisphosphonates in mice caused severe depression of HSC formation and delay of hematopoietic recovery (Lymperi et al, 2010). These observations indicate that osteoclasts participate in the initial formation as well as the maintenance of the HSC niche (Mansour et al, 2012). On the other hand, granulocyte colony-stimulating factor (G-CSF) increased osteoclastic activity, driving HSPCs from the BM to the periphery (Kollet et al, 2006). The activity of osteoclasts further elevates the local and systemic calcium ion concentration, and published studies have demonstrated that HSC engraftment at the endosteal niche is specified by the calcium-sensing receptor (CaR) expressed on HSCs (Adams et al, 2006). Reduced cellularity and HSC content and increased mobilization of progenitor cells have been observed in the BM of CaR-deficient mice (Adams et al, 2006), suggesting the relevance of osteoclasts in retaining high calcium concentration, which is critical to keep HSCs localized in close physical proximity to the endosteal surface and the regulatory niche components.
Potential role of the nervous system
Glial nonmyelinating Schwann cells, a component of the BM niche, were shown to be responsible for activation of latent TGF-β produced by a variety of BM cells (Yamazaki et al, 2011). Nonmyelinating Schwann cells are ensheathed autonomic nerves in contact with a substantial proportion of HSCs. The critical role of TGF-β/Smad signaling in HSC maintenance was demonstrated by the impaired long-term repopulating activity of HSCs deficient in the TGF-β type II receptor. Autonomic denervation reduced the number of active TGF-β–producing cells and led to rapid loss of HSCs from the BM, which suggests that glial cells maintain HSC hibernation by regulating activation of latent TGF-β.
Sympathetic nervous system regulation of HSCs residing in BM niches via norepinephrine signaling has been reported (Katayama et al, 2006). Katayama et al showed that G-CSF–induced adrenergic activity resulted in suppression of osteoblasts, decreasing CXCL12 synthesis by osteoblasts and thus increasing HSPC mobilization in the BM microenvironment. Lucas et al further demonstrated that chemotherapy-induced nerve injury impaired hematopoietic regeneration and that neuroprotection induced by deletion of TP53 in sympathetic neurons or neuroregeneration induced by administration of 4-methylcatechol or glial-derived neurotrophic factor promoted hematopoietic recovery in a murine model (Lucas et al, 2013). These reports indicate that sympathetic nerves in the BM promote the survival of constituents of the BM niche. In humans, however, Bonig and Papayannopoulou (2013) reported observing no difference in mobilization of stem cells by G-CSF in stem cell donors taking a noradrenalin-reuptake inhibitor or beta-receptor blocker.
Regulatory T cells
In immune-suppressive stem cell niches called immune-privileged sites, multiple mechanisms cooperate to prevent immune attack and enable prolonged survival of foreign allografts without immunosuppression. Fujisaki et al (2011) demonstrated co-localized accumulation of HSPCs with regulatory T (T(reg)) cells on the endosteal surface in the calvarial and trabecular BM, which was lost after the depletion of T(reg) cells in their non-immunosuppressed mouse model. These results suggest that T(reg) cells participate in creating the BM niche, which provides a relative sanctuary from immune attack and supports stem-cell function.
Components of the vascular niche
CXCL12-abundant reticular cells
CXCL12 (SDF-1α), a chemokine elaborated by stromal cells, functions through its receptor CXCR4, a seven-transmembrane G-coupled receptor protein. CXCL12 attracts CXCR4-expressing HSCs to stromal surfaces. CXCL12–CXCR4 signaling is involved in homing of HSC into BM, activates several integrins, and supports survival of colony-forming progenitor cells (Sugiyama et al, 2006). CXCL12-secreting cells include osteoblasts (Christopher et al, 2009), CXCL12-abundant reticular (CAR) cells (Sugiyama et al, 2006), and Nestin-positive stromal cells (Mendez-Ferrer et al, 2010), all components of the BM niches.
Most CAR cell populations express PPARγ, Runx2, and Osterix in the BM, and short-term ablation of CAR cells in vivo severely impaired the adipogenic and osteogenic differentiation potential of BM cells, indicating that CAR cells are adipo-osteogenic bipotential progenitors (Omatsu et al, 2010). In the sinusoidal areas of the BM in which HSCs predominantly reside, the HSCs have direct contact with CAR cells, which secrete higher levels of CXCL12 than osteoblasts. Moreover, depletion of CXCR4 leads to reduction of the HSC population, suggesting that CXCL12–CXCR4 chemokine signaling plays an essential role in maintaining the HSC pool (Sugiyama et al, 2006).
Similarly, the mobilization of HSCs into the peripheral blood induced by CXCL12 downregulation or CXCR4-selective antagonism further indicates a role for CXCL12 in retaining HSC in BM niches (Sugiyama et al, 2006).
Nestin-positive mesenchymal stem cell and leptin receptor–positive cells
Whereas the nature of the true “mesenchymal stem cells” remains enigmatic, specific MSCs named Nestin-positive MSCs have been reported to participate in the regulation of BM niches (Raaijmakers et al, 2010). Nestin-positive MSCs constitute an essential HSC niche component, co-localizing with HSCs and adrenergic nerve fibers (Mendez-Ferrer et al, 2010). Frenette and colleagues reported that depletion of Nestin-positive MSCs in an in vivo model significantly reduced BM homing of hematopoietic progenitors and HSC content in the BM (Mendez-Ferrer et al, 2010). They also reported that the selective downregulation of HSC retention genes in Nestin-positive MSCs was induced by the reduction of CD169-positive BM macrophages, which led to reduced BM CXCL12 levels and egress of HSPCs to the bloodstream (Chow et al, 2011). It has been further demonstrated that the conditional deletion of stem cell factor (SCF) from endothelial cells or leptin receptor–expressing perivascular stromal cells, including Nestin-positive stromal cells and CAR cells, significantly reduced HSC number, whereas SCF deletion from hematopoietic cells, osteoblasts, and Nestin-cre– or Nestin-creER–expressing cells did not affect HSC number (Ding et al, 2012).
Thus, heterogeneous stromal cells contribute to HSC maintenance through various mechanisms.
LEUKEMIC MICROENVIRONMENT
Bone marrow niche as a “foster home” for LSCs
The leukemic clone is organized as a hierarchy, and LSC behavior is modulated by interactions and signals received within their BM microenvironment. For LSC survival, proliferation, and differentiation, both the osteoblastic and vascular niches are critical (Calvi et al, 2003; Arai et al, 2004; Nilsson et al, 2005; Kiel et al, 2005). LSCs share certain features of self-renewal and differentiation with HSCs, and the molecules that mediate the interaction between LSCs and the BM niche, including adhesion molecules (Messinger et al, 1996), and CXCL12-mediated CXCR4 signaling for homing and mobilization within the BM (Messinger et al, 1996) are similar to those of HSCs.
However, LSCs differ from HSCs in their dysregulated activation of key pathways regulating proliferation, survival, and abilities to invade and spread (Lane, 2012). Recent studies indicate that BM niche components contribute to LSC engraftment into the niches; to leukemia development, survival, and drug resistance; and to determination of leukemia phenotype by providing the necessary cytokines and cell contact–mediated signals to LSCs (Raaijmakers et al, 2010). These findings suggest the roles of the normal HSC niche in leukemia pathogenesis (Lane, 2012). On the other hand, LSCs themselves create their “foster home,” inducing reversible changes in BM stromal cell function or composition that result in survival of the leukemic cells (Dührsen et al, 1996). Suppression of normal hematopoiesis in leukemia patients with relatively low tumor burden may reflect disruption of normal hematopoietic progenitor cell (HPC) BM niches and creation of leukemia niches by leukemic cells (Colmone et al, 2008).
In a murine model, transplanted leukemic cells initially migrated toward the CXCL12-positive vascular niches, which overlap with normal HPC niches; after leukemia growth in vivo, CXCL12 production in the leukemia vascular niche was markedly downregulated, and newly transplanted normal CD34+ cells migrated to tumor niches by virtue of SCF abundantly secreted by leukemic cells (Colmone et al, 2008). These findings indicate that the signaling mechanisms of BM niches are altered such that the niches are “hijacked” by LSCs (Li & Neaves, 2006) and remodeled as their “foster home.”
CXCR4–CXCL12 interactions and leukemic cell migration to bone marrow niches
The poor prognosis of acute leukemia afforded by current treatments is mainly due to the relapse of the disease following chemotherapy. Interaction of LSCs and BM niches is recognized as the major cause of this acute leukemia relapse. Chemokine CXCL12 elaborated by osteoblasts, CAR cells, or Nestin-positive MSCs is one of the key factors mediating the crosstalk between leukemic cells and the BM niches and regulates the homing and engraftment of LSCs into the BM niche.
Levels of the CXCR4 receptor for CXCL12 are significantly elevated and highly responsive to CXCL12 in primary leukemic cells, including B-cell chronic lymphocytic leukemia (B-CLL) (Mohle et al, 2000), B-cell acute lymphocytic leukemia (ALL) (Shen et al, 2001), and to a lesser degree AML (Raaijmakers et al, 2010). Associations between CXCR4 expression and poor outcome in patients with B-CLL (Ishibe et al, 2002), pre-B-ALL (Crazzolara et al, 2001), or AML (Rombouts et al, 2004; Konoplev et al, 2007) have been reported.
Inhibition of CXCL12–CXCR4 interactions resulted in abolishment of CXCL12-induced chemotaxis; inactivation of prosurvival signaling pathways, including phosphorylation of p44/42 mitogen-activated protein kinase (MAPK) and signal tranducer and activator of transcription 3 (STAT3); and decreases in stromal protective effects on chemotherapy-induced apoptosis in CLL and AML cells (Zeng et al, 2006). The small-molecule reversible CXCL12–CXCR4 inhibitor plerixafor completely blocked CXCL12-induced chemotaxis, attenuated the migration of pre-B-ALL cells into BM stromal cell layers, and enhanced the cytotoxic and antiproliferative effects of vincristine and dexamethasone (Juarez et al, 2003). In a murine model of acute promyelocytic leukemia (APL), administration of plerixafor in combination with chemotherapy triggered an increase of circulating APL cells with decreased tumor burden and improved overall survival compared to chemotherapy alone (Nervi et al, 2009). Novel fully human antibody to CXCR4 BMS-936564/MDX-1338 induced apoptosis in AML cell lines and exhibited single agent antitumor activity in the in vivo AML models (Kuhne et al, 2013).
CXCR4 expression has been reported to be higher in Flt3/internal tandem duplication AML than in FLT3/wild-type AML (Rombouts et al, 2004). Additional preclinical data indicate that the Flt3 axis participates in the CXCR4-mediated trafficking of transformed hematopoietic cells and that CXCR4 inhibition increased sensitivity of FLT3-mutated leukemic cells to the FLT3 inhibitor sorafenib under stromal co-culture conditions. Furthermore, CXCR4 inhibitor AMD3465, alone or in combination with G-CSF, induced mobilization of AML progenitor cells into circulation and reduced AML burden in mice, which resulted in prolonged survival in response to sequenced sorafenib treatment, presumably through recruitment of leukemic cells out of their protective microenvironmental niches (Zeng et al, 2009). Similarly, treatment with plerixafor combined with TGFβ-neutralizing antibody 1D11 and cytarabine decreased leukemia burden and prolonged survival in a leukemia mouse model, proving that TGFβ and CXCL12, produced abundantly in the BM niche, play a role in AML chemoresistance (Tabe et al, 2013).
Primary chronic myelogenous leukemia (CML) blasts show attenuated migration to CXCL12 and decreased CXCR4 expression (Peled et al, 2002). BCR-ABL tyrosine kinase inhibitor imatinib restored CXCR4 expression under MSC co-culture conditions, which in turn induced migration of CML cells to the BM microenvironment niches, where quiescent CML progenitor cells acquired stroma-mediated chemoresistance (Jin et al, 2008). Pathological crosstalk between BCR/ABL and the CXCR4 pathway is modulated by Src family tyrosine kinase Lyn in CML cells, which disrupts chemokine signaling and chemotaxis and increases the ability of immature cells to escape from the BM (Tabe et al, 2012).
These results suggest that CXCL12–CXCR4 interactions in the BM microenvironment contribute to the chemoresistance of leukemic cells and that disruption of these interactions by CXCR4 inhibitors represents a rational strategy for blocking LSC homing to a BM niche and/or sensitizing leukemic cells to chemotherapy or kinase inhibitors.
LSC niche and adhesion molecules
Adhesion to the stromal niche is crucial for LSCs because it directly supports self-renewal, proliferation, and arrest of differentiation and protects from damage by chemotherapy or kinase inhibitors. The transmembrane glycoprotein CD44, existing as a standard isoform (CD44s) and a range of variant isoforms (CD44v), is a key regulator of LSC homing to BM niches and maintenance of their primitive state (Jin et al, 2006). CD44 modulates interactions of LSCs with hyaluronan; ECM components, including heparin sulfate; and a range of growth factor ligands to promote CD44/ligand/RTK complex formation and signal transduction (Nervi et al, 2009). The glycosaminoglycan hyaluronan is highly concentrated in the endosteal region (Avigdor et al, 2004). CD44–hyaluronan interactions contribute to self-renewal, proliferation, differentiation, homing to BM, and preservation of the integrity of the stem cell genome by decreasing DNA damage and enahancing DNA repair (Williams et al, 2013).
CD44/ligand/RTK signaling has been shown to modulate microRNA expression to regulate promoter methylation status and gene expression (Williams et al, 2013). Through this mechanism, CD44 participates in reprogramming of leukemia cells to exhibit a more stem cell-like LSC phenotype, which could be an elemental mechanism in promoting leukemic progression and chemoresistance (Williams et al, 2013). On the other hand, cancer stem cells (CSCs) have been shown to further synthesize hyaluronan to recruit tumor-associated macrophages into the CSC niche (Jinushi et al, 2011), which then recruits adjacent stromal cells into the CSC niche.
Niche stromal cells secrete numerous growth factors, many of which are known to moderate stem cell functions such as self-renewal and stem cell fate. CD44s and CD44v bind to growth factors, activating an impressive range of RTKs. Although activities of CD44s or CD44v have been reported to be similar in hyaluronan-mediated regulation of HSC differentiation (Herrlich et al, 2000) and MSC homing to BM (Avigdor et al, 2004), the mechanisms of preferential CD44v expression have been shown to be potentially useful in enforced maturation of self-renewing LSCs. For example, variant isoforms of CD44v4–10 have been demonstrated as the primary CD44 isoform expressed during maturation of CML progenitor cells into myeloid cells (Herrlich et al, 2000).
Cells in the BM niche also express integrins, which are cell adhesion receptors that link extracellular adhesion molecules with the intracellular actin cytoskeleton (Redondo-Munoz et al, 2008). Integrins are known to be required for lodging of LSCs in the BM niche (Redondo-Munoz et al, 2008). Integrin heterodimers, made up of one of 18α subunits and one of 8β subunits, regulate cell–cell adhesion, growth factor receptor signaling, cell lineage specification, differentiation, survival, proliferation, and migration (Prowse et al, 2011). Many of these functions parallel with CD44 expression, suggesting integrin–CD44 interactions (Williams et al, 2013). Indeed, integrins are known to link to CD44 through their interactions with selectins. Homing of HSPC to BM requires a coordinated sequence of four steps, including E-selectin receptor/ligand interaction; engagement of CXCL12–CXCR4 signaling, resulting in activation of very late antigen–4 (VLA-4; integrin α4β1); VLA-4 adherence to VCAM-1; and transmigration on endothelium (Sackstein et al, 2011). VLA-4 binds to CD44v to form a docking complex for pro–matrix metalloproteinase-9, which is associated with transendothelial migration and invasion through Matrigel of B-CLL cells (Redondo-Munoz et al, 2008). Mudry et al (2000) showed that the maximum viability of ALL cells during exposure to cytarabine and etoposide required interaction with the MSC adhesion molecule VCAM-1. Conditional deletion of alpha4 sensitized BCR-ABL(+) leukemias to nilotinib, and pharmacological VLA4 blockade with antibody Natalizumab prolonged survival of NOD/SCID recipients of primary ALL when combined with chemotherapy, indicating the role of this integrin in chemoresistance of lymphoid malignancies (Hsieh et al, 2013). Very recently, Miller et al demonstrated that ITGB3 knockdown impaired homing, downregulated LSC transcriptional programs, and induced differentiation via the intracellular kinase Syk without affecting normal HSPCs (Miller et al, 2013).
Modulation of the LSC stem cell niche via hypoxia/HIF-1α signaling
The endosteum of the bone–BM interface has been shown to be hypoxic: the average pO2 in BM is approximately 55 mmHg, and the mean O2 saturation 87.5% (Harrison et al, 2002). Leukemic cells are able to proliferate even under hypoxic conditions, indicating that the cells are able to adapt to these conditions. Abnormalities including elevated CXCL12, vascular endothelial growth factor (VEGF), and SCF levels, as well as increased acidity and hypoxia, were reported in leukemia BM (Mohle et al, 2000; Benito et al, 2011). It has been reported that the oxygen-regulated component hypoxia-inducible transcription factor alpha (HIF-1α) was overexpressed in clusters of leukemic cells in BM specimens from ALL patients (Wellmann et al, 2004).
Notably, HIF-1α was demonstrated to regulate CXCL12 gene expression in endothelial cells, resulting in selective in vivo expression of CXCL12 in ischemic tissue, which increased migration and homing of circulating CXCR4-positive progenitor cells into the ischemic tissue (Ceradini et al, 2004). In AML, total and surface CXCR4 expression were upregulated under hypoxic conditions in leukemic cell lines and patient samples (Fiegl et al, 2009). Consistent with the findings that HIF-1α regulates CXCR4 (Staller et al, 2003), these data suggest that a hypoxic BM microenvironment represents a conditional stem and progenitor cell niche in which HIF-1α–induced stabilization and activation of CXCL12–CXCR4 signaling facilitates recruitment and retention of leukemic progenitor cells.
One of the most advertised functions of hypoxia and HIF-1α is upregulation of growth factor VEGF and stimulation of angiogenesis. The microvasculature is an active component of the BM microenvironment and is responsible for supplying appropriate oxygen and nutrients. VEGF secreted by leukemic cells activates receptors on both leukemic and endothelial cells and plays a vital role in the growth of leukemia cells (Ferrara et al, 2003). Increased angiogenesis is observed in myelodysplastic syndrome (Korkolopoulou et al, 2001), AML (Hussong et al, 2000), ALL (Koomagi et al, 2001) and multiple myeloma (Rajkumar et al, 2000). VEGF was found to inhibit apoptosis in leukemic cells after exposure to etoposide and doxorubicin by inducing Mcl-1, a member of the prosurvival Bcl-2 family, and to promote the survival of multiple myeloma cells by inducing Bcl-2 via VEGF receptor 2 (Dias et al, 2002). The direct HIF-1α inhibitor PX-478 decreased expression of hypoxia-mediated, but not normoxic, VEGF expression with antitumor activity against tumor xenografts (Koh et al, 2008).
CML-associated oncogene BCR-AB1L1 has been shown to induce VEGFA and HIF1A gene expression via a phophoinositide-3 kinase (PI3K)/mTOR–dependent pathway (Mayerhofer et al, 2002). Tyrosine kinase inhibitor imatinib inhibited c-KIT–induced HIF-1α activity and VEGF expression in small cell lung cancer cells (Litz et al, 2006). These findings indicate that the activation of c-KIT by SCF could be followed by HIF-1α –mediated VEGF expression.
In a very recent review, Bonig and Papayannopoulou (2013) discussed the significance of the observation that, although the lower oxygen tension in the BM than in arterial blood is an inevitable consequence of physics, it is difficult to define the BM stem cell niche as severely hypoxic. They argued that the high abundance of capillaries in cancellous bone is incompatible with the hypoxia in BM niches, and that histological images of HSCs indicate that HSCs reside in proximity to the proliferative progenitor cells throughout the BM (Kiel & Morrison, 2008) but not in anatomically separate compartments. This notion is supported by a recent quantitative imaging study of HSCs which demonstrated that despite their preferential endosteal localization, HSCs closely interact with bone marrow microvessels, and yet exhibit a hypoxic profile, indicating regulation of the hypoxic HSC state through cell-intrinsic mechanisms rather than lack of blood supply (Nombela-Arrieta et al, 2013). It remains to be determined whether abundant marrow hypoxia reported in leukemic bone marrows (Benito et al, 2011) regulated through similar or distinct mechanisms. Whereas both leukemia progenitor cells and LSCs reside in the “hypoxic” BM microenvironment, they have distinct metabolic states; progenitor cells are engaged in active cycling and contain many mitochondria, whereas LSCs are quiescent and exhibit few mitochondria (Suda et al, 2011). The transition from stem to progenitor cell corresponds to a critical metabolic change, namely from glycolysis to oxidative phosphorylation (Suda et al, 2011). For the stress resistance of LSCs, long-term quiescence and self-renewal may be crucial, and leukemic cells’ survival and proliferation is critically regulated by the transition mechanisms from stem to progenitor cell, corresponding to metabolic alteration, oxygen concentration, cytokine stimulation, or cell contact regulation (Suda et al, 2011).
Leukemogenesis: role of the bone marrow microenvironment
Dysfunction of a BM niche may contribute to leukemogenesis via interaction through adhesion, supplying abundant growth factors and immunosuppression that promote proliferation and/or inhibit apoptosis (Jones & Wagers, 2008). Overproduction of apoptosis-inducing cytokines by T cells triggers BM failure and then leads to transformation through clonal selection and adaption of modified BM microenvironment-resistant HSCs, which develop into clonal neoplasms such as AML, myelodysplastic syndromes, and paroxysmal nocturnal hemoglobinuria (Tavor & Petit, 2010). In a recent report, Schepers et al. dscribe how malignant myeloid cells profoundly reprogram the endosteal BM osteoblasts into the pro-inflammatory bone marrow niche that supports LSC while creating an inhospitable environment for normal HSCs (Schepers et al, 2013). Leukemogenic transformation of normal cells triggered by alterations of the BM microenvironment has been observed in patients with BM failure syndromes such as aplastic anemia (Sands et al, 2013). Conditional knockout of DICER1, a gene that regulates microRNA processing, in osteoblastic precursors has been shown to result in BM failure and leukemia predisposition. DICER1 deletion caused reduced expression of SBDS, the gene mutated in Schwachman-Bodian-Diamond syndrome. Deletion of SBDS in mouse osteoprogenitors induced myelodysplasia and the development of AML (Raaijmakers et al, 2010). These findings highlight the suggestion that primary stromal dysfunction can result in secondary neoplastic disease, supporting the concept of niche-induced oncogenesis.
LSCs that receive the support of a BM niche for their survival may in turn associate with deregulation of the BM niche by their dominant proliferation-promoting signals. It has been shown that beta-catenin signaling has a central role in the self-renewal of CML and AML stem cells. Zhao et al demonstrated that beta-catenin deletion caused a profound reduction in the ability of mice to develop BCR-ABL–induced CML (Zhao et al, 2007). Wang et al showed that, in murine LSCs derived from MLL-AF9-induced leukemias, the Wnt/beta-catenin signaling pathway was required for self-renewal (Wang et al, 2010). Thus, activation of the self-renewal pathways through Wnt/beta-catenin signaling can be caused by microenvironmental stimuli (Konopleva & Jordan, 2011). N-cadherin and Wnt-β-catenin axis was recently shown to play an important role in microenvironment- mediated protection of CML LSCs from tyrosine kinase inhibitor treatment, suggesting this axis a potential; new target for eradication of residual leukemia in CML patients (Zhang et al, 2013). On the other hand, myeloma cells inhibited Wnt activation in the microenvironment through release of soluble dickkopf homolog 1 (DKK1), which caused an increase in the concentration of RANKL and a decrease in osteoprotegerin production, resulting in increased activation of osteoclasts and bone destruction (Qiang et al, 2008). It has been reported that activation of NF-κB or the absence of its inhibitor IκBα in myelopoietic cells changed the nonhematopoietic compartment, resulting in increased numbers of dysplastic hematopoietic cells with progression into secondary AML via upregulated perinatal expression of Jag-1 in IκBαΔ/Δ hepatocytes and activation of NOTCH1 in neutrophils (Rupec _et a_l, 2005). These findings indicate that a premalignant hematopoietic disorder can be initiated by non-hematopoietic cells with inactive IκBα, conceivably via activation of the Notch pathway. Jagged/Notch activation has been shown to result in increased numbers of HSCs and niche expansion (Calvi et al, 2003). Additional studies demonstrated that the tumor suppressor FBXW7, which negatively regulates cyclin E, NOTCH, and c-MYC protein levels, plays a role in maintaining HSC quiescence and repressing potential oncogenic activity of HSCs (Matsuoka et al, 2008).
Several lines of experimental evidence have suggested that genetic changes in the BM microenvironment contribute to or are required for leukemogenesis. Walkley et al reported that dysfunction of the retinoblastoma protein (RB), a central regulator of the cell cycle and a tumor suppressor, or of retinoic acid receptor γ (RARγ) in the BM microenvironment contributes to development of preleukemic myeloproliferative disease. They demonstrated that the widespread inactivation of RB but not myeloid-specific loss of RB resulted in extramedullary hematopoiesis and myeloproliferative disease in the murine hematopoietic system (Walkley et al, 2007a). The microenvironment-induced myeloproliferative-like disorder was also observed in _RARγ_−/− mice because of the RARγ-deficient microenvironment (Walkley et al, 2007b )
Similarly, deficiency of phosphatase and tensin homolog (PTEN), a tumor suppressor and an antagonist of the PI3K pathway, in both hematopoietic cells and the microenvironment resulted in myeloproliferation that progressed to overt leukemia/lymphoma (Yilmaz et al, 2006). However, inducible PTEN deletion in hematopoietic cells in the presence of a wild-type BM microenvironment promoted HSC depletion without evidence of myeloproliferation or leukemic development (Yilmaz et al, 2006). These findings indicate the importance of interactions between hematopoietic cells and the BM niche/microenvironment and suggest that additional genetic mutations within the BM microenvironment may be necessary for leukemic transformation.
Intriguingly, several studies have implicated a previously unrecognized link between microenvironment and cancer metabolism. A high level of asparagine secretion by MSCs has been shown to cause asparaginase resistance of ALL cells that reside in MSC niches, and this protective effect correlated with levels of asparagine synthetase expression in MSCs (Iwamoto et al, 2007). Further, recent study by Zhang et al. has shown that bone marrow stroma conversion of cystine to cysteine is crucial for the survival of CLL cells ex vivo and their protection against oxidative damage (Zhang et al, 2012). In turn, AML blasts alter the immune microenvironment via release of high concentrations of arginase II, which suppresses T cell proliferation, polarizes surrounding monocytes into a suppressive M2-like phenotype, and finally inhibits proliferation and differentiation of murine granulocyte-monocyte progenitors and human CD34+ progenitors (Mussai et al, 2013). These findings directly implicate metabolic features of the perturbed bone marrow microenvironment as a prerequisite for leukemia-stroma interplay. Further characterization of the key mechanisms governing this metabolic exploitation of the supporting bone marrow niche may yield novel therapeutic targets to render the microenvironment less promiscuous for the genetically altered leukemia cell.
CONCLUSION
By elucidating the role of the BM microenvironment in the pathogenesis of hematologic tumors, recent studies have provided insight into the molecular mechanisms involved in stem cell activation and homing to the BM niche (Table I, Figure 1). This better understanding of the nature of HSCs and their niches is expected to provide an alternative approach to the treatment of various serious diseases, including leukemia, in clinical practice. Further understanding of the contribution of the BM niche to the process of leukemogenesis may provide new targets that allow destruction of LSCs without adversely affecting normal stem cell self-renewal.
Table I.
Cellular components of hematopoietic bone marrow niches.
| Components of Niche | Molecule *(receptor molecule in HSC and LSC) | Reference |
|---|---|---|
| Osteoblastic (endosteal) niche | ||
| Osteoblasts | Jagged1 *(Notch) | Calvi et a l, 2003 |
| Angiopoietin-1 (Tie-2) | Arai et al , 2004 | |
| Osteopontin (β1-integrin) | Nilsson et al , 2005 | |
| Osteoclasts | Kong et al , 1999; Schroder et al , 2012 | |
| Regulatory T cells | Fujisaki et al , 2011 | |
| Vascular niche | ||
| CXCL12-abundant reticular (CAR) cells | CXCL12 (CXCR4) | Nagasawa et al , 1996; Sugiyama et al , 2006; Nagasawa et al , 2011 |
| Nestin-positive mesenchymal stem cells | Mendez-Ferrer et al , 2010 | |
| Leptin receptor–expressing stromal cells** | Ding et al , 2012 | |
| CD169-positive macrophages | Chow et al , 2011 | |
| Glial cells | Yamazaki et al , 2011 | |
| Extracellular matrix | Hyaluronan (CD44) | Jin et a l, 2006; Krause et al , 2006 |
| Fibronectin, VCAM-1 (VLA-4) | Miyake et al , 1991; Garcia-Gila et al , 2002 | |
| Hypoxic environment | (HIF-1α) | Mortensen et al , 1998; Jensen et al , 2000 |
| High-calcium environment | (Calcium-sensing receptor) | Adams et al , 2006 |
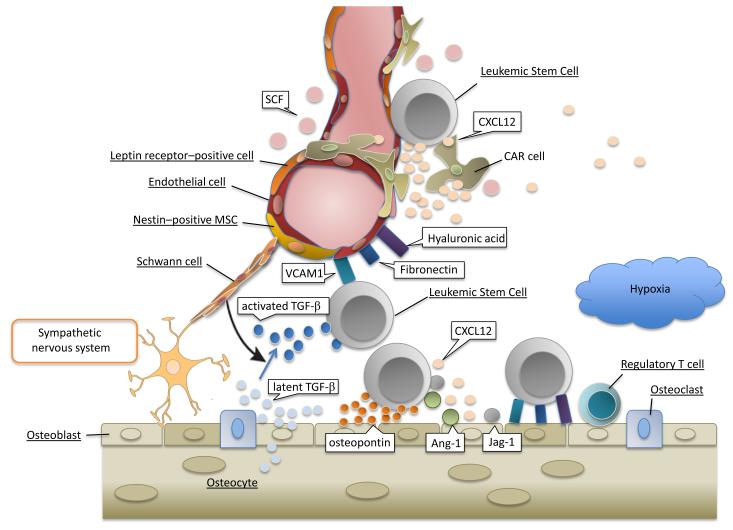
Figure 1. Key components of the leukemic bone marrow microenvironment.
Components of normal HSC niches consist of multiple cell types including osteoblasts, Cxcl12-abundant reticular (CAR) cells, nestin-positive mesenchymal stem cells (MSCs), _Lepr_-expressing perivascular cells, endothelial cells and Schwann cells wrapping sympathetic nerve fibers. LSCs hijack HSC marrow spaces including perivascular and endosteal niches. The BM stromal cells and osteoblasts produce complex extracellular matrix (ECM) such as vascular cell–adhesion molecule-1 (VCAM-1), fibronectin and hyaluronic acid, which facilitate engraftment and adhesion of LSCs. Osteoblasts within endosteal niches generate transforming growth factor-β (TGF-β), angiopoietin-1 (Ang-1) and Jagged-1 (Jag-1) that in turn promote leukemia cells dormancy and decrease their chemosensitivity. CAR cells, nestin-positive MSCs, Leptin receptor-positive perivascular cells, and endothelial cells may play role for leukemia cells migration to perivascular microenvironment via cytokines, chemokines, and adhesion molecules. Inhibition of leukemia / stroma interactions causes increased leukemia cells cycling and homing to perivascular niches which can potentially be used for chemosensitization to target domant LSCs.
References
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Benito J, Shi Y, Szymanska B, Carol H, Boehm I, Lu H, Konoplev S, Fang W, Zweidler-McKay PA, Campana D, Borthakur G, Bueso-Ramos C, Shpall E, Thomas DA, Jordan CT, Kantarjian H, Wilson WR, Lock R, Andreeff M, Konopleva M. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One. 2011;6:e23108. doi: 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonig H, Papayannopoulou T. Hematopoietic stem cell mobilization: updated conceptual renditions. Leukemia. 2013;27:24–31. doi: 10.1038/leu.2012.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. Journal of Experimental Medicine. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Crazzolara R, Kreczy A, Mann G, Heitger A, Eibl G, Fink FM, Mohle R, Meister B. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. British Journal of Haematoogy. 2001;115:545–553. doi: 10.1046/j.1365-2141.2001.03164.x. [DOI] [PubMed] [Google Scholar]
- Dias S, Shmelkov SV, Lam G, Rafii S. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood. 2002;99:2532–2540. doi: 10.1182/blood.v99.7.2532. [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dührsen U, Hossfeld DK. Stromal abnormalities in neoplastic bone marrow diseases. Annual Hematology. 1996;73:53–70. doi: 10.1007/s002770050203. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–1512. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H, Sato T, Côté D, Sykes M, Strom TB, Scadden DT, Lin CP. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gila M, Lopez-Martin EM, Garcia-Pardo A. Adhesion to fibronectin via alpha4 integrin (CD49d) protects B cells from apoptosis induced by serum deprivation but not via IgM or Fas/Apo-1 receptors. Clinical & Experimental Immunology. 2002;127:455–462. doi: 10.1046/j.1365-2249.2002.01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- Herrlich P, Morrison H, Sleeman J, Orian-Rousseau V, Konig H, Weg-Remers S, Ponta H. CD44 acts both as a growth- and invasiveness-promoting molecule and as a tumor-suppressing cofactor. Annals of the New York Academy of Sciences. 2000;910:106–118. doi: 10.1111/j.1749-6632.2000.tb06704.x. [DOI] [PubMed] [Google Scholar]
- Hsieh YT, Gang EJ, Geng H, Park E, Huantes S, Chudziak D, Dauber K, Schaefer P, Scharman C, Shimada H, Shojaee S, Klemm L, Parameswaran R, Loh M, Kang ES, Koo HH, Hofmann WK, Andrade J, Crooks GM, Willman CL, Müschen M, Papayannopoulou T, Heisterkamp N, Bönig H, Kim YM. Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy. Blood. 2013;121:1814–1818. doi: 10.1182/blood-2012-01-406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–313. [PubMed] [Google Scholar]
- Ishibe N, Albitar M, Jilani IB, Goldin LR, Marti GE, Caporaso NE. CXCR4 expression is associated with survival in familial chronic lymphocytic leukemia, but CD38 expression is not. Blood. 2002;100:1100–1101. doi: 10.1182/blood-2002-03-0938. [DOI] [PubMed] [Google Scholar]
- Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. Journal of Clinical Investigation. 2007;117:1049–1057. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Science. 2009;100:1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PO, Mortensen BT, Hodgkiss RJ, Iversen PO, Christensen IJ, Helledie N, Larsen JK. Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Proliferation. 2000;33:381–395. doi: 10.1046/j.1365-2184.2000.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, Kimura S, Ohsaka A, Rios MB, Calvert L, Kantarjian H, Andreeff M, Konopleva M. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Molecular Cancer Therapeutics. 2008;7:48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nature Medicine. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A, Tahara H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proceedings of the National Academy of Sciences of U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nature Reviews Molecular Cell Biology. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Juarez J, Bradstock KF, Gottlieb DJ, Bendall LJ. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nature Reviews Immunology. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL, Powis G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Molecular Cancer Therapeuitics. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nature Medicine. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Konoplev S, Rassidakis GZ, Estey E, Kantarjian H, Liakou CI, Huang X, Xiao L, Andreeff M, Konopleva M, Medeiros LJ. Overexpression of CXCR4 predicts adverse overall and event-free survival in patients with unmutated FLT3 acute myeloid leukemia with normal karyotype. Cancer. 2007;109:1152–1156. doi: 10.1002/cncr.22510. [DOI] [PubMed] [Google Scholar]
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. Journal of Clinical Oncology. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomagi R, Zintl F, Sauerbrey A, Volm M. Vascular endothelial growth factor in newly diagnosed and recurrent childhood acute lymphoblastic leukemia as measured by real-time quantitative polymerase chain reaction. Clinical Cancer Research. 2001;7:3381–3384. [PubMed] [Google Scholar]
- Korkolopoulou P, Apostolidou E, Pavlopoulos PM, Kavantzas N, Vyniou N, Thymara I, Terpos E, Patsouris E, Yataganas X, Davaris P. Prognostic evaluation of the microvascular network in myelodysplastic syndromes. Leukemia. 2001;15:1369–1376. doi: 10.1038/sj.leu.2402220. [DOI] [PubMed] [Google Scholar]
- Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013 Oct 9; doi: 10.1038/nature12612. (in press) doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, Cohen LJ, Korman AJ, Cardarelli PM. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clinical Cancer Research. 2013;19:357–366. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- Lane SW. Bad to the bone. Blood. 2012;119:323–325. doi: 10.1182/blood-2011-10-383901. [DOI] [PubMed] [Google Scholar]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- Litz J, Krystal GW. Imatinib inhibits c-Kit-induced hypoxia-inducible factor-1alpha activity and vascular endothelial growth factor expression in small cell lung cancer cells. Molecular Cancer Therapeutics. 2006;5:1415–1422. doi: 10.1158/1535-7163.MCT-05-0503. [DOI] [PubMed] [Google Scholar]
- Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, Tessarollo L, Frenette PS. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nature Medicine. 2013;19:695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2010;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. Journal of Experimental Medicine. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Oike Y, Onoyama I, Iwama A, Arai F, Takubo K, Mashimo Y, Oguro H, Nitta E, Ito K, Miyamoto K, Yoshiwara H, Hosokawa K, Nakamura Y, Gomei Y, Iwasaki H, Hayashi Y, Matsuzaki Y, Nakayama K, Ikeda Y, Hata A, Chiba S, Mortensen BT, Jensen PO, Helledie N, Iversen PO, Ralfkiaer E, Larsen JK, Madsen MT. Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. British Journal of Haematology. 1998;102:458–464. doi: 10.1046/j.1365-2141.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Suda T. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes and Development. 2008;22:986–991. doi: 10.1101/gad.1621808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer M, Valent P, Sperr WR, Griffin JD, Sillaber C. BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood. 2002;100:3767–3775. doi: 10.1182/blood-2002-01-0109. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger Y, Chelstrom L, Gunther R, Ukmi FM. Selective homing of human leukemic B-cell precursors to specific lymphohematopoietic microenvironments in SCID mice: a role for the beta 1 integrin family surface adhesion molecules VLA-4 and VLA-5. Leukemia Lymphoma. 1996;23:61–69. doi: 10.3109/10428199609054803. [DOI] [PubMed] [Google Scholar]
- Miller PG, Al-Shahrour F, Hartwell KA, Chu LP, Järås M, Puram RV, Puissant A, Callahan KP, Ashton J, McConkey ME, Poveromo LP, Cowley GS, Kharas MG, Labelle M, Shterental S, Fujisaki J, Silberstein L, Alexe G, Al-Hajj MA, Shelton CA, Armstrong SA, Root DE, Scadden DT, Hynes RO, Mukherjee S, Stegmaier K, Jordan CT, Ebert BL. In vivo RNAi screening identifies a leukemia-specific dependence on integrin beta 3 signaling. Cancer Cell. 2013;24:45–58. doi: 10.1016/j.ccr.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, Medina K, Ishihara K, Kimoto M, Auerbach R, Kincade PW. A VCAM-like adhesion molecule on murine bone marrow stromal cells mediates binding of lymphocyte precursors in culture. Journal of Cell Biology. 1991;114:557–565. doi: 10.1083/jcb.114.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohle R, Shittenhelm M, Faienschmid C, Bautz F, Kratz-Albers K, Serve H, Brugger W, Kanz L. Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. British Journal of Haematology. 2000;110:563–572. doi: 10.1046/j.1365-2141.2000.02157.x. [DOI] [PubMed] [Google Scholar]
- Mudry RE, Fortney JE, York T, Hall BM, Gibson LF. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96:1926–1932. [PubMed] [Google Scholar]
- Mussai F, De Santo C, Abu-Dayyeh I, Booth S, Quek L, McEwen-Smith RM, Qureshi A, Dazzi F, Vyas P, Cerundolo V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122:749–758. doi: 10.1182/blood-2013-01-480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: the role of reticular cells. Trends Immunol. 2011;32:315–320. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, DiPersio JF. Chemosensitization of AML following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature Cell Biology. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwajei F, Konopleva M. The bone marrow microenvironment ironment as niche retreats for hematopoietic and leukemic stem cells. Advances in Hematology. 2013;2013:953982. doi: 10.1155/2013/953982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. Journal of Experimental Medicine. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A, Hardan I, Trakhtenbrot L, Gur E, Magid M, Darash-Yahana M, Cohen N, Grabovsky V, Franitza S, Kollet O, Lider O, Alon R, Rechavi G, Lapidot T. Immature leukemic CD34+CXCR4+ cells from CML patients have lower integrin-dependent migration and adhesion in response to the chemokine SDF-1. Stem Cells. 2002;20:259–266. doi: 10.1634/stemcells.20-3-259. [DOI] [PubMed] [Google Scholar]
- Perry JM, Li L. Disrupting the stem cell niche: good seeds in bad soil. Cell. 2007;129:1045–1047. doi: 10.1016/j.cell.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Prowse AB, Chong F, Gray PP, Munro TP. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Research. 2011;6:1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J, Barlogie B, Shaughnessy JD., Jr. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Leong T, Roche PC, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Kyle RA, Gertz MA, Greipp PR. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clinical Cancer Research. 2000;6:3111–3116. [PubMed] [Google Scholar]
- Redondo-Munoz J, Ugarte-Berzal E, Garcia-Marco JA, del Cerro MH, Van den Steen PE, Opdenakker G, Terol MJ, Garcia-Pardo A. Alpha4beta1 integrin and 190-kDa CD44v constitute a cell surface docking complex for gelatinase B/MMP-9 in chronic leukemic but not in normal B cells. Blood. 2008;112:169–178. doi: 10.1182/blood-2007-08-109249. [DOI] [PubMed] [Google Scholar]
- Rombouts EJ, Pavic B, Löwenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550–557. doi: 10.1182/blood-2004-02-0566. [DOI] [PubMed] [Google Scholar]
- Rupec RA, Jundt F, Rebholz B, Eckelt B, Weindl G, Herzinger T, Flaig MJ, Moosmann S, Plewig G, Dörken B, Förster I, Huss R, Pfeffer K. Stroma-mediated dysregulation of myelopoiesis in mice lacking I kappa B alpha. Immunity. 2005;22:479–491. doi: 10.1016/j.immuni.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Sackstein R. The biology of CD44 and HCELL in hematopoiesis: the “step 2-bypass pathway” and other emerging perspectives. Current Opinion in Hematology. 2011;18:239–248. doi: 10.1097/MOH.0b013e3283476140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands WA, Copland M, Wheadon H. Targeting self-renewal pathways in myeloid malignancies. Cell Communication and Signaling. 2013;11:33. doi: 10.1186/1478-811X-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T, Wagers AJ, Hsiao EC, Passegué E. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell. 2013;13:285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schroder HC, Wang XH, Wiens M, Diehl-Seifert B, Kropf K, Schloßmacher U, Müller WE. Silicate modulates the cross-talk between osteoblasts (SaOS-2) and osteoclasts (RAW 264.7 cells): inhibition of osteoclast growth and differentiation. Journal of Cellular Biochemistry. 2012;113:3197–3206. doi: 10.1002/jcb.24196. [DOI] [PubMed] [Google Scholar]
- Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrow. Experimental Hematology. 2001;29:1439–1447. doi: 10.1016/s0301-472x(01)00741-x. [DOI] [PubMed] [Google Scholar]
- Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Tabe Y, Jin L, Iwabuchi K, Wang RY, Ichikawa N, Miida T, Cortes J, Andreeff M, Konopleva M. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia. 2012;26:883–892. doi: 10.1038/leu.2011.291. [DOI] [PubMed] [Google Scholar]
- Tabe Y, Shi YX, Zeng Z, Jin L, Shikami M, Hatanaka Y, Miida T, Hsu FJ, Andreeff M, Konopleva M. TGF-β-neutralizing antibody 1D11 enhances cytarabine-induced apoptosis in AML cells in the bone marrow microenvironment. PLoS One. 2013;8:e62785. doi: 10.1371/journal.pone.0062785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor S, Petit I. Can inhibition of the SDF-1/CXCR4 axis eradicate acute leukemia? Seminars in Cancer Biology. 2010;20:178–185. doi: 10.1016/j.semcancer.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007a;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007b;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JK, Wang JC, Hope KJ, Jin L, Dick JE. Concepts of human leukemic development. Oncogene. 2004;23:7164–7177. doi: 10.1038/sj.onc.1207933. [DOI] [PubMed] [Google Scholar]
- Wellmann S, Guschmann M, Griethe W, Eckert C, von Stackelberg A, Lottaz C, Moderegger, Seeger K. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18:926–933. doi: 10.1038/sj.leu.2403332. [DOI] [PubMed] [Google Scholar]
- Williams K, Motiani K, Giridhar PV, Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Experimental Biology and Medicine (Maywood) 2013;238:324–338. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Samudio IJ, Munsell M, An J, Huang Z, Estey E, Andreeff M, Konopleva M. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Molecular Cancer Therapeutics. 2006;5:3113–3121. doi: 10.1158/1535-7163.MCT-06-0228. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, Konoplev S, Andreeff M, Konopleva M. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, Shultz L, Bhatia R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-β-catenin signaling. Blood. 2013;121:1824–1938. doi: 10.1182/blood-2012-02-412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W, Keating MJ, Huang P. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nature Cell Biology. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]