A randomized trial of a computer-tailored decision aid to improve prostate cancer screening decisions: results from the Take the Wheel Trial (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 6.
Published in final edited form as: Cancer Epidemiol Biomarkers Prev. 2010 Aug 17;19(9):2172–2186. doi: 10.1158/1055-9965.EPI-09-0410
Abstract
Objective
To evaluate a decision aid (DA) designed to promote informed decision-making for prostate cancer screening.
Methods
Twelve worksites were randomly assigned to an intervention or non-intervention comparison condition. Intervention sites received access to a computer-tailored DA at the workplace. Male employees age 45+ (n=625) completed surveys at baseline and at three-month follow-up, documenting aspects of informed decision-making.
Results
Using an intention-to-treat analysis, men in the intervention group were significantly more likely to have made a screening decision and to have improved knowledge without increased decisional conflict, relative to men in the comparison group. These changes were observed despite the fact that only 30% of men in intervention sites used the DA. Among DA users, similar improvements were observed, although the magnitudes of changes were substantially greater, and significant improvements in decision self-efficacy were observed.
Conclusions
A DA offered in the workplace promoted decision-making, improved knowledge and increased decision self-efficacy among users, without increasing decisional conflict. However, participation was suboptimal, suggesting that better methods for engaging men in workplace interventions are needed.
INTRODUCTION
Prostate cancer (CaP) is the most commonly diagnosed cancer among men in the United States. Established risk factors (age, family history, Black race) are not modifiable (1). Therefore, cancer control efforts have focused on early detection with the prostate specific antigen (PSA) test for men ages 50 and over. However, PSA screening remains controversial, and data from two long-awaited trials have only intensified debate about the role of routine screening (2, 3).
Until recently, routine screening for prostate cancer was recommended only by a few organizations, including the American Cancer Society (4) and the American Urological Association (5). Other medical organizations advised men to learn about the potential benefits, limitations and harms of the test, through a process termed “informed decision-making” (IDM) (6–9). In 2010, the American Cancer Society revised their recommendations, emphasizing the importance of informed decision-making rather than mass screening (4). According to the U.S. Preventive Services Task Force (USPSTF), an informed decision is one in which an individual: (1) understands the nature of the disease being addressed as well as the risks, limitations and benefits of clinical services (i.e., adequate knowledge); (2) is confident in his ability to participate in decision-making at a personally-desired level (decision self-efficacy); and (3) has considered his personal preferences and makes a decision consistent with his values (decisional consistency) (10, 11).
Recently, there has been a proliferation of interventions to promote IDM, particularly in the form of decision aids. Decision aids (DAs) are tools designed to help individuals make choices by providing pertinent information, presenting data about the likelihood of potential outcomes, and elucidating personal values associated with each option, with a goal of promoting decisional consistency (12, 13). There are few published trials of computer-based DAs for CaP screening (14–17), and with one exception (17), have all been conducted in clinical settings. The present study was conducted in worksites, a setting that affords access to a large segment of men who are age-appropriate for IDM regarding CaP screening, has existing communication channels that can facilitate promotion efforts, and offers an infrastructure through which intervention activities may be institutionalized and sustained. By targeting particular industries, it is also possible to gain access to workers in particular occupations. We recruited manufacturing industries with a goal of including high proportions of workers, who, by nature of their income and educational level, may have diminished access to information regarding CaP screening.
The purpose of this randomized trial was to evaluate the efficacy of a computer-tailored DA designed to promote IDM for CaP screening among employed men. Primary outcomes were decisional status (decided/undecided) and individual components of IDM as defined by the USPSTF: knowledge, decision self-efficacy and decisional consistency (6). Secondary outcomes included desire for involvement in decision-making (control preferences) and decisional conflict. We hypothesized that at the conclusion of the study, relative to men in comparison worksites, men in intervention worksites would: (a) be more likely to have made a screening decision; and (b) demonstrate significantly higher levels of knowledge, decision self-efficacy and decisional consistency. Further, we anticipated that men in the intervention group would desire greater involvement in the decision-making process and would report lower decisional conflict.
METHODS
In this trial, worksites were the unit of randomization and intervention, while individual employees constitute the unit of measurement. Twelve worksites were randomly assigned to a three-month intervention or to a non-intervention comparison group. Using an intention-to-treat analysis, the efficacy of the DA was evaluated by comparing mean changes in decisional status (decided/undecided) and IDM variables between baseline and follow-up in intervention and comparison sites. A process tracking system documented intervention delivery and characteristics of DA users.
Setting
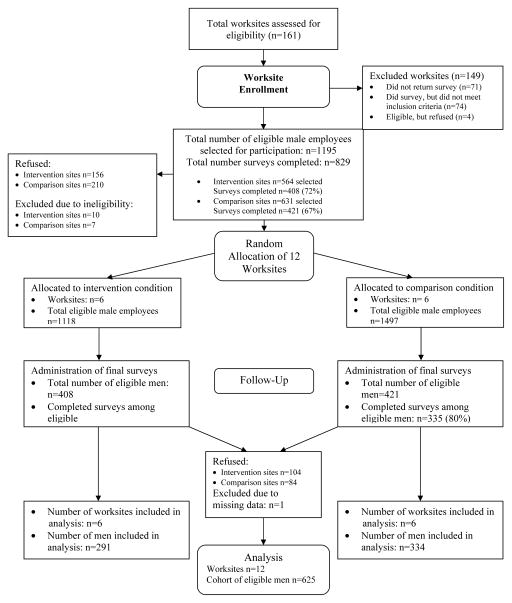
The Dun & Bradstreet database was used to identify worksites with Standard Industrial Classification (SIC) codes 20–39 that represent manufacturing industries. Additional criteria for site selection included: (1) ≥ 100 men in the targeted age range (45–70); (2) ≤ 20% employee turn-over in the year prior to study initiation; and (3) location within 90 minutes of the study center (Boston, MA). A total of 161 companies were contacted to determine eligibility, which was assessed through a five-page mailed survey documenting organizational and workforce characteristics. Seventy-one companies (44%) did not return the survey; 74 companies (46%) completed the survey but were ineligible (n=45 due to size). Four sites (n=3%) declined participation, citing lay-offs, acquisitions, or re-structuring as reasons for non-participation. See Figure 1. The 12 participating worksites ranged in size from 100 to 1000 employees (mean=650). Sites were blocked on size (total employees <500; ≥500) and percent of male employees (<50%; ≥50%), and randomly assigned by computer-generated random numbers to condition within blocks.
Figure 1.
Sampling schema- worksites & employees
Intervention Development
The DA development process followed a strategy described by Bartholomew et al.(18) for the development of theory-based interventions. The first step was articulation of a conceptual framework. We employed the Ottawa Decision Support Framework (DSF) which identifies factors that influence decision-making and are amenable to modification through decision support (13). The DSF also suggests a step-by-step approach for decision-making: identification of available options, acquisition of information necessary for decision-making, clarification of values relevant to the decision, and development of a plan for action.
Content of the DA was based on expert opinion regarding information necessary for informed decision making (10, 19), as well as guidelines from the International Patient Decision Aid Standards (IPDAS). IPDAS is an international group of experts in the field of decision-making that has developed guidelines for the creation of high-quality DA tools (20). Criteria include the provision of: (1) balanced information about various options based on evidence; (2) information about the probabilities of potential outcomes associated with each option; (3) exercises to elucidate values relevant to available options; and (4) guidance regarding development of a plan that will facilitate progress toward the chosen option. Factual content considered “required” for IDM included: prevalence of CaP; CaP risk factors; methods used for early detection and their operating characteristics (i.e., sensitivity, specificity); potential advantages and disadvantages of screening, and men’s evaluation of the relative importance of these factors; the recommendation of major medical organizations that men make individualized decisions; and meaning of an elevated PSA test and methods for diagnosis. After viewing the aforementioned “required” IDM content, men were able to select from a menu of additional topics (e.g., treatment options) according to their interests.
During the development process, focus groups (k=4; n=28) were conducted to gauge men’s responses to educational messages and to assess their reactions to various communication strategies. All participants were recruited from worksites not participating in the trial. During these discussions, several themes became evident, including the importance of: taking charge of one’s health; making independent decisions; and masculinity. In addition, some men used a “road map” analogy to communicate about the various decision points in screening. Therefore, we conceived of the study name “Take the Wheel,” to reflect a masculine theme in which men were taking an active role in decisions about their own health care and “steering their own course” (i.e., making decisions). Based on focus group feedback, a functional version of the computer DA was developed and tested among men (n=10) recruited from non-participating worksites to assess acceptability of the graphics, actors, narrator and script. Following revisions based on audience feedback, the prototype was further refined. Usability testing was conducted with an additional 15 men recruited from non-participating sites to assess navigability, efficiency of use, error frequency and severity, and satisfaction among end-users. The final DA consisted of interactive video and audio components, with a computer touch screen and minimal on-screen text.
Based on our conceptual model and prior work, the DA was tailored on three characteristics: personal risk for CaP; individual ratings of the pros and cons of screening; and decisional consistency. Personalized risk was calculated using the YourDiseaseRisk® algorithm (21). Men input data in response to questions about risk factors and were provided with an on-screen graphic of their risk relative to other men their age (i.e., greater than average, average, less than average). In addition, men were asked to weigh the pros and cons of screening. This information was then pictorially presented as a balance scale, with pros on one side and cons on the other. Men were then asked about their screening preference (“decided to be screened,” “undecided” or “decided not to be screened”). If their rating of the pros/cons was not compatible with their screening decision, the user was informed that these positions were “inconsistent.” In this case, the individual was encouraged to return to the menu of topics to learn more, to take more time to think things over, or to discuss their decision with their health care provider or significant other. To promote self-efficacy with respect to decision-making, the DA presented several scenarios with different men going through the decision-making process to role model a variety of decision-making approaches. Men were subsequently coached through the steps of decision-making, as outlined by the DSF. Additional information about the DA is available elsewhere (22).
Intervention Delivery
Between November 2006 and June 2007, the DA was delivered in intervention sites via tablet computers made available in common gathering areas (e.g., break rooms, cafeterias). We elected to make computers available in public spaces, with the assumption that visibility would generate interest, and thus promote participation. Each location afforded sufficient privacy so that DA users could sit individually and view the computer screen without their responses being seen by others. Headphones were provided. The DA was designed to be independently administered—even for those with minimal or no computer skills. A health educator was available to provide assistance with computers if needed, although no individual required assistance, other than initial start-up of the program.
We employed multiple strategies to publicize and promote the intervention, including posters placed in high-visibility areas, distribution of fliers, announcements made at regularly-scheduled meetings, and provision of small incentives (e.g., key ring flashlights). Computers were made available in worksites at pre-specified days, based on agreements between management and study staff. The computers were available during the day, generally in six-hour periods, based on managements’ request. Each site had at least three computers available on site for a minimum of 15 days over the three-month intervention period (roughly once per week). Men were allowed release-time from work to use the DA. Information was saved at each time of use; men could either complete the DA session at one time or return at multiple time points to complete it (mean time spent=28 minutes). At the conclusion of the session, men were provided with a printed tailored report summarizing their estimated risk for CaP, assessment of pros/cons, decisional status, and pages visited during DA use. This report was designed to facilitate communication about screening with primary care providers.
Data Collection
Baseline data were collected through self-administered pencil-and-paper surveys between September 2006 and March 2007; follow-up assessments were conducted between March 2007 and July 2007. Those eligible were men age ≥45 who were permanent employees working ≥20 hours per week. Forty-five years was selected as a minimum because some medical organizations advise that men be offered screening at <50 years if they are at higher-than-average risk for CaP (23). Men employed temporarily, or those who worked less than half-time were excluded from data collection because these factors would diminish access to the intervention. Employee rosters were used to identify eligible men. In sites that employed more than 100 eligible men, a random sample was selected. In sites with 100 men, a census was surveyed.
Eligible men were sent a letter inviting them to participate and that provided informed consent information. Two weeks later, surveys were distributed and collected via company mail or by hand by study staff. The survey cover sheet reiterated informed consent information; completion of surveys was taken as consent to participate. All written communication was at the sixth-grade level. Men were allowed to complete the survey on site during work hours, and were provided a financial incentive ($25) after each time point. Non-respondents were contacted up to three times by electronic mail, telephone or in person. Across the 12 sites, the mean baseline response rate was 72% (n=812; range = 59%–86%). At follow-up, the mean response rate among those who completed the baseline survey was 79% (n=639; range = 61–85%), resulting in a final cohort of 625 men. All procedures were approved by Institutional Review Board at the Dana-Farber Cancer Institute.
Measures
Primary Outcomes
Decisional Status
The validated Stage of Decision-making scale (24) asked respondents to rate their readiness to make a decision, with five response options ranging from “I haven’t thought about it before” to “I have made a decision and I am not likely to change my mind.” Men were classified as having “decided” if they stated either that they had made a decision, but were willing to reconsider, or if they responded that they had made a decision, but were unlikely to change their mind. Those “undecided” reported that they hadn’t thought about the decision, or were uncertain.
Prostate Cancer Knowledge
Men’s recognition of the PSA test was assessed using a standard single item: “The prostate specific antigen test (PSA) is a blood test that is used to find prostate cancer. Before now, had you ever heard of the PSA test?” (yes/no). Fourteen validated questions assessed men’s knowledge of CaP prevalence, risk factors, screening modalities, diagnostic procedures and treatment-related complications (25). The proportion of accurate responses was transformed to a percentage scale ranging from 0% (no correct responses) to 100% (all correct responses). The internal reliability in our sample was adequate (Cronbach’s α=0.69).
Decision Self-Efficacy
The validated 11-item Decision Self-Efficacy Scale with three response categories was used to assess confidence in one’s ability to participate in decision-making to the extent desired (26). Respondents were asked to reflect on their confidence level regarding various aspects of the decision-making process, with response options of “very confident” (score=4), to “not at all confident” (score=0). Scores were summed, divided by 11 and multiplied by 25, to arrive at a range of scores from 0 (no self-efficacy) to 100 (higher self-efficacy). In this sample, the internal consistency coefficient was high (α=0.91).
Consistency between Values and Screening Decision
We assessed the congruence between screening preference and personal values relevant to the screening decision, an approach similar to that used by Sepucha and colleagues in a recent study of breast cancer treatment decisions (27). First, to assess screening preference, men were asked “If you had to decide now, what would you choose?” Options included: “to get a PSA test,” “not to get a PSA test,” and “I could not decide.” In the literature, assessment of values has primarily been measured with probability-based risk-benefit trade-offs (28). We pre-tested these items in focus groups (k=1; n=15) and found them unacceptable to a majority of men. Therefore, we developed items to assess the personal importance or relative worth of the advantages and limitations of screening, based on focus groups themes and published literature (e.g., importance of information, accuracy of test, potential side-effects of treatment). Further information about scale development is available elsewhere (29). Individual items are presented in Appendix 1. High positive scores reflect values strongly in favor of screening and high negative scores reflect low importance of screening (range +16 to −16). Principle components analysis identified a single factor, providing support for combining all items in one scale. The internal reliability of the values questions was good (α= 0.81). In test-retest assessments six months apart, men (n=812) rated their values consistently (concordance coefficient=0.682) (29).
Based on an ROC curve of the sensitivity and specificity of responses to questions related to values, we set the cut-off for favorable versus unfavorable value ratings of screening at “zero.” An individual’s screening decision was considered “consistent” with their values if they reported an intention to be screened and their values score was greater than zero. Having a negative value score and lack of intention to be screened was considered “consistent.” Those considered “inconsistent” had value ratings that did not align with screening preference.
Secondary Outcomes
Preference for Control in Decision-making was assessed via the Control Preference Scale (30) with a single item. Individuals were asked “Who should make medical decisions?” Response options included: (a) “I make the follow-up make decision on my own”; (b) “I make the decision after seriously considering my doctor’s opinion”; (c) “My doctor and I share responsibility for the decision”; (d) “I prefer that the doctor make the decision after seriously considering my opinion”; and (e) “I prefer that the doctor make the decision.” In analyses, responses were collapsed to reflect active decision-making styles (options a and b), collaborative styles (option c), and passive styles (options d and e) (30).
Decisional Conflict was measured via the validated Decisional Conflict Scale (13). Respondents were asked to rate statements such as: “The decision I made was the best decision possible for me personally.” Reponses were gauged on a five-point scale ranging from “strongly agree” to “strongly disagree.” Scales were standardized from 0 (no conflict) to 100 (extreme conflict). In this sample, internal consistency was good (α=0.84).
Socio-demographic characteristics, health behaviors, screening history, and access to health care were also assessed, using standard items from the CDC’s Behavioral Risk Factor Surveillance Surveys.
Statistical Analysis
The worksite was the unit of recruitment and intervention. All analyses incorporated the clustering of respondents within worksites sites (ICC = 0.0068) using generalized estimating equations to fit generalized linear models. An intention-to-treat approach was used in all analyses, using all available data. P-values were two-sided and a p-value < 0.05 was termed significant. With this sample size, assuming a type one error of 0.05, the design had 79% power when the coefficient of variation (ratio of standard deviation to mean) was 1.2 (31). All analyses were carried out using SAS statistical software, version 9.1 (SAS Institute, Cary, NC).
Discrete variables, such as age group, race/ethnicity and marital status, were compared using Chi-square tests. The Wilks-Shapiro test was used to assess normality of continuous variables. Linear regression was used to examine mean change between baseline and follow-up for the outcomes of knowledge, decision self-efficacy and decisional conflict across intervention and comparison groups. Logistic regression was used to examine mean change between baseline and follow-up or the outcomes of decisional status, decisional consistency, and control preferences, controlling for baseline values across intervention and comparison groups.
Control variables included age, race/ethnicity, income, education, marital status, family history of prostate cancer, and previous PSA test. We included some of these variables even though they were not significantly associated with outcomes at the p>0.05 level in bivariate analyses, with a goal of controlling for potential confounding. However, some outcomes had very limited variability (i.e., decision self-efficacy, decisional consistency and control preferences). In these cases, we were unable to fit adjusted models with all of the aforementioned control variables. Adjusted analyses for decisional status include only age and education. Adjusted models were not calculable for control preferences or decisional consistency, due to limited variability in responses.
RESULTS
Characteristics of Cohort
Table 1 summarizes characteristics of the samples at baseline and follow-up, by intervention group. Overall, the cohort was characterized by a slightly larger percentage of non-Hispanic white men, compared with the total baseline sample (92% versus 89%, p<0.01). Other socio-demographic and health characteristics were not significantly different between baseline and follow-up.
Table 1.
Demographic characteristics of samples at baseline (N=812) and follow-up (N=625) by intervention arm, Take the Wheel Trial
| Baseline (N=812) | Follow-up (N=625) | ||||
|---|---|---|---|---|---|
| Intervention | Comparison | P-value* | Intervention | Comparison | P-value** |
| Characteristic | N=398N (%) | N=414N (%) | N=291N (%) | N=334N (%) | |
| Age | 0.98 | <0.01 | |||
| 45–49 | 151 (38) | 120 (29) | 116 (40) | 96 (29) | |
| 50–54 | 106 (27) | 126 (30) | 78 (27) | 104 (31) | |
| ≥55 | 93 (23) | 153 (37) | 64 (22) | 126 (38) | |
| Missing | 48 (12) | 15 (4) | 33 (11) | 8 (2) | |
| Race/Ethnicity | <0.01 | 0.41 | |||
| White, non-Hispanic | 354 (89) | 372 (90) | 259 (89) | 309 (93) | |
| Other | 37 (9) | 39 (9) | 26 (9) | 22 (7) | |
| Missing | 7 (2) | 3 (1) | 6 (2) | 3 (1) | |
| Household Income | 0.37 | 0.01 | |||
| < $50,000 | 65 (16) | 59 (14) | 43 (15) | 46 (14) | |
| $50,000–74,999 | 117 (29) | 80 (19) | 86 (30) | 63 (19) | |
| ≥ $75,000 | 195 (49) | 247 (60) | 146 (50) | 201 (60) | |
| Missing | 21 (5) | 28 (7) | 16 (5) | 24 (7) | |
| Education | 0.08 | 0.05 | |||
| High school or less | 131 (33) | 74 (18) | 93 (32) | 55 (16) | |
| Some college | 129 (32) | 141 (34) | 94 (32) | 123 (37) | |
| 4-yr degree or more | 134 (34) | 196 (47) | 102 (35) | 154 (46) | |
| Missing | 4 (1) | 3 (1) | 2 (1) | 2 (1) | |
| Marital Status | 0.79 | ||||
| Married/living as married | 320 (80) | 341 (82) | 236 (81) | 272 (81) | 0.91 |
| Other | 75 (19) | 70 (17) | 53 (18) | 60 (18) | |
| Missing | 3 (1) | 3 (1) | 2 (1) | 2 (1) | |
| Family History | 0.95 | 0.85 | |||
| Yes | 45 (11) | 51 (12) | 34 (12) | 40 (12) | |
| No/Don’t know | 348 (87) | 353 (85) | 253 (87) | 285 (85) | |
| Missing | 5 (1) | 10 (2) | 4 (1) | 9 (3) | |
| Previous PSA | 0.60 | 0.69 | |||
| Yes | 177 (44) | 196 (47) | 127 (44) | 161 (48) | |
| No | 88 (22) | 89 (21) | 62 (21) | 72 (22) | |
| Missing | 133 (33) | 129 (31) | 102 (35) | 101 (30) | |
| Screening Preference | 0.79 | 0.88 | |||
| Want to be screened | 315 (79) | 325 (79) | 225 (77) | 264 (79) | |
| Does not want to be screened | 33 (8) | 40 (10) | 29 (10) | 32 (10) | |
| Undecided | 46 (12) | 46 (11) | 34 (12) | 36 (11) | |
| Missing | 4 (1) | 3 (1) | 3 (1) | 2 (1) |
Changes in Primary Outcomes by Treatment Group
Table 2 presents bivariate relationships among socio-demographic and health characteristics across the range of primary and secondary outcomes. Older mean were more likely to demonstrate decisional consistency and had higher decisional conflict. White race was associated with decreased decisional self-efficacy. Those who had a prior PSA were more likely to be decided, to demonstrate decisional consistency, and in addition, had higher levels of decisional conflict.
Table 2.
Bivariate regression results for association between socio-demographic variables and outcomes
| Characteristic | Decisional Status | CaP Knowledge | Decision Self-Efficacy | Decisional Consistency | Control Preferences | Decisional Conflict |
|---|---|---|---|---|---|---|
| OR (95% CI) | Regression Coefficient (95% CI) | Regression Coefficient (95% CI) | OR (95% CI) | OR (95% CI) | Regression Coefficient (95% CI) | |
| Age | ||||||
| 45–49 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| 50–54 | 1.23 (0.80, 1.89) | −0.98 (−3.59, 1.64) | 2.88 (−0.15, 5.92) | 1.63 (0.82, 3.25) | 0.63 (0.23, 1.70) | 3.32 (−0.58, 7.21) |
| ≥55 | 1.42 (0.84, 2.14) | −1.52 (−4.20, 1.16) | 1.32 (−2.43, 5.08) | 2.37 (0.98, 5.20) | 0.65 (0.28, 1.48) | 9.04 (5.18, 12.89)** |
| Race/Ethnicity | ||||||
| White, non-Hispanic | 0.86 (0.52, 1.42) | −0.19 (−4.07, 3.70) | −7.35 (−13.44,−1.26)* | 0.63 (0.63, 2.94) | 1.38 (0.41, 4.58) | 4.80 (−0.92, 10.57) |
| Other | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Household Income | ||||||
| < $50,000 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| $50,000–74,999 | 1.18 (0.76,1.83) | 0.49 (−2.90, 3.88) | −2.33 (−7.81, 3,15) | 1.07 (0.44, 2.63) | 2.10 (0.80, 5.55) | −3.13 (−9.61, 3.35) |
| ≥ $75,000 | 1.60 (0.96, 2.69) | 0.88 (−1.56, 3.31) | −5.41 (−11.00, 0.16) | 0.99 (0.52, 1.87) | 1.72 (0.58, 5.09) | −0.07 (−6.60, 6.45) |
| Education | ||||||
| High school or less | 0.52 (0.36, 0.76) | 1.20 (−3.05, 5.45) | 1.89 (−2.62, 6.40) | 1.23 (0.94, 1.61) | 0.48 (0.21, 1.10) | 1.68 (−3.33, 6.69) |
| Some college | 0.68 (0.47, 0.98)* | 1.41 (−0.73, 3.56) | −0.76 (−3.73, 2.22) | 0.98 (0.58, 1.66) | 0.56 (0.31, 1.02) | 2.94 (−1.34, 7.21) |
| 4-yr degree or more | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Marital Status | ||||||
| Married/living as married | 0.86 (0.47, 1.59) | 0.83 (−2.96. 4.62) | −0.01 (−4.16, 4.14) | 1.15 (0.72, 1.83) | 0.60 (0.33, 1.09) | 3.11 (−7.90, 4.30) |
| Other | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Family History | ||||||
| Yes | 1.55 (0.81, 2.99) | 2.19 (−1.84, 6.22) | −0.68 (−4.71, 3.34) | 2.48 (0.87, 6.80) | 1.82 (0.88, 3.76) | 0.40 (−5.11, 5.90) |
| No/Don’t know | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| Previous PSA | ||||||
| Yes | 2.57 (1.72, 3.85)** | 0.39 (−2.73, 3.51) | −2.52 (−5.65, 0.61) | 2.04 (1.18, 3.51)* | 1.76 (0.64, 4.89) | 7.89 (4.25, 11.54)** |
| No | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
Relative to the comparison group, there was a greater percentage of men in the intervention group who were undecided about screening at baseline and had made a screening decision at follow-up (21% vs 13%, respectively; p=0.01) (see Table 3). Although not an outcome of the study, 77–79% of men in both groups preferred CaP screening at both time points. Only 23% of men changed in their screening preferences between baseline and follow-up and this did not vary by intervention arm. Approximately equal percentages of men in both group changed from preferring to be screened, to preferring not to be screened (56 men changed from undecided/against screening to want screening and 66 men changed the preferences in the reverse
Table 3.
Changes in primary and secondary outcomes between baseline and follow-up surveys, by intervention arm, Take the Wheel Trial (N=625)
| Intervention (N=291) | Comparison (N=334) | Bivariate Regression | Adjusted Regression | |||
|---|---|---|---|---|---|---|
| Primary Outcomes | Baseline | Follow-up | Baseline | Follow-up | Coefficient or OR (95% CI)P-value | Coefficient or OR (95% CI)P-value |
| Decisional Status (%) | ||||||
| Decided | 31% | 43% | 40% | 43% | ||
| Undecided | 69 | 57 | 60 | 57 | ||
| Missing | 0 | 0 | 0 | 0 | ||
| Changes between baseline & follow-up1 | ||||||
| Remained decided | 22% | 30% | 1.26 | 1.53 | ||
| Remained undecided | 49 | 48 | (0.99, 1.61) | (1.15 2.05) | ||
| Became decided | 21 | 13 | 0.07 | < 0.01 | ||
| Became undecided | 8 | 9 | ||||
| CaP Knowledge2 | ||||||
| Mean (%) | 56% | 66% | 56% | 60% | 3.25 | 4.24 |
| SE | 2.68 | 2.08 | 1.23 | 1.6 | (−0.52, 7.02) | (0.40, 8.07) |
| % of men w/improved score | 54% | 39% | 0.09 | 0.03 | ||
| Decision Self-efficacy3 | ||||||
| Mean (%) | 83% | 83% | 79% | 79% | 0.17 | 1.35 |
| SE | 2.46 | 2.36 | 1.59 | 1.81 | (−2.70, 3.03) | (−1.24, 3.93) |
| % of men w/improved score | 39% | 40% | 0.91 | 0.31 | ||
| Decisional Consistency (%) | ||||||
| Consistent | 71% | 69% | 73% | 74% | ||
| Inconsistent | 28 | 31 | 26 | 24 | ||
| Missing | 1 | 0.3 | 1 | 2 | ||
| Changes between baseline & follow-up4 | ||||||
| Remained consistent | 53% | 58% | 0.73 | |||
| Remained inconsistent | 13 | 10 | (0.48, 1.12) | |||
| Became consistent | 15 | 16 | 0.15 | |||
| Became inconsistent | 17 | 13 | ||||
| Secondary Outcomes | ||||||
| Control Preferences (%) | ||||||
| Active/collaborative | 94% | 95% | 91% | 92% | ||
| Passive | 5 | 4 | 8 | 7 | ||
| Missing | 1 | 0.3 | 0.3 | 1 | ||
| Changes between baseline & follow-up5 | ||||||
| Did not change | 92% | 87% | 1.36 | |||
| Change to passive | 3 | 5 | (0.66, 2.82) | |||
| Change to active/collaborative | 3 | 7 | 0.41 | |||
| Decisional Conflict6 | ||||||
| Mean (%) | 25% | 14% | 28% | 20% | −3.33 | −2.06 |
| SE | 2.08 | 2.01 | 2.12 | 2.07 | (−6.69, 0.02) | (−4.42, 0.29) |
| % of men w/improved score | 53% | 49% | 0.05 | 0.09 |
Men in the intervention group experienced greater improvements in knowledge scores than men in the comparison group; more than half (54%) of men in intervention sites had improved knowledge scores versus 39% of men in comparison sites. The average increase was ten percentage points in the intervention group versus four percentage points in the comparison group (p=0.03). There were no discernable changes in mean decision self-efficacy or decisional consistency scores in either group across time points.
Changes in Secondary Outcomes by Treatment Group
Overall, at both time points across intervention and comparison sites, the majority of men (91–95%) wanted an active or collaborative role in decision-making. Preferred role in decision-making did not change significantly between baseline and follow-up in either intervention or comparison sites. Mean scores in decisional conflict were low in both intervention and comparison groups at baseline (mean scores = 25 vs. 18, respectively). In multivariate analyses, men in the intervention group had marginally reduced decisional conflict scores (p=0.09).
Subgroup Analyses among DA Users
Across the six intervention sites, 30% of the cohort reported using the DA (86/291). However, since we used an “intention to treat” model, use of the DA was not required for cohort membership. According to our process tracking system, overall use of the tool among all age-eligible men in intervention worksites ranged from 23% to 59% (total n=335 out of a possible 1,118 eligible men across sites). DA users did not differ significantly by socio-demographic or health characteristics compared with non-users (data not shown).
Men who used the DA significantly were more likely to have made a decision about screening at the follow-up survey, compared to non-users (35% vs. 14%, p<0.01) (Table 4). In addition, 79% of users improved their knowledge scores, whereas only 43% of non-users had improved knowledge at the time of the follow-up survey (+12% points vs. +1% points; p <0.01). DA users also had a greater sense of self-efficacy in decision-making; 41% of men in the intervention group vs. 38% in the comparison group improved in their decision self-efficacy score (p<0.01 in bivariate analyses). Nonetheless, decisional consistency was not different between the two groups. While there were no significant differences between users and non-users in preferences for control in decision-making, DA users experienced a significant decrease in decisional conflict, compared with non-DA users (p=0.03).
Table 4.
Changes in primary and secondary outcomes between baseline and follow-up surveys in intervention worksites, by DECISION AID USE, Take the Wheel Trial (N=289)
| Intervention (N=86) | Comparison (N=203) | Bivariate Regression | Adjusted Regression | |||
|---|---|---|---|---|---|---|
| Primary Outcomes | Baseline | Follow-up | Baseline | Follow-up | Coefficient or OR (95% CI)P-value | Coefficient or OR (95% CI)P-value |
| Decisional Status (%) | ||||||
| Decided | 28% | 57% | 32% | 37% | ||
| Undecided | 72 | 43 | 68 | 63 | ||
| Missing | 0 | 0 | 0 | 0 | ||
| Changes between baseline & follow-up1 | ||||||
| Remained decided | 22 | 23 | 2.93 | |||
| Remained undecided | 37 | 54 | (1.49, 5.79) | |||
| Became decided | 35 | 14 | < 0.01 | |||
| Became undecided | 6 | 9 | ||||
| CaP Knowledge2 | ||||||
| Mean (%) | 59% | 73% | 62% | 64% | 13.34 | 10.98 |
| SE | 3.04 | 2.44 | 3.60 | 3.43 | (10.09, 16.58) | (6.56, 15.39) |
| % of men w/improved score | 79% | 43% | < 0.01 | < 0.01 | ||
| Decision Self-efficacy3 | ||||||
| Mean (%) | 80% | 77% | 79% | 76% | 3.30 | −2.53 |
| SE | 4.36 | 3.65 | 4.04 | 2.95 | (0.76, 5.84) | (−1.24, 6.30) |
| % of men w/improved score | 41% | 38% | 0.01 | 0.19 | ||
| Decisional Consistency (%) | ||||||
| Consistent | 77% | 70% | 69% | 68% | ||
| Inconsistent | 22 | 30 | 30 | 31 | ||
| Missing | 1 | 0 | 1 | 0.5 | ||
| Changes between baseline & follow-up4 | ||||||
| Remained consistent | 56% | 53% | 1.01 | |||
| Remained inconsistent | 8 | 15 | (0.50, 2.04) | |||
| Became consistent | 14 | 15 | 0.98 | |||
| Became inconsistent | 21 | 16 | ||||
| Secondary Outcomes | ||||||
| Control Preferences (%) | ||||||
| Active | 58% | 67% | 70% | 67% | ||
| Collaborative | 34 | 28 | 25 | 29 | ||
| Passive | 6 | 5 | 4 | 4 | ||
| Missing | 2 | 0 | 1 | 0.5 | ||
| Changes between baseline & follow-up5 | ||||||
| Did not change | 62% | 70% | 0.98 | |||
| Change to passive | 16 | 16 | (0.26, 3.62) | |||
| Change to active/collaborative | 20 | 12 | 0.97 | |||
| Decisional Conflict6 | ||||||
| Mean (%) | 29% | 13% | 30% | 22% | −14.77 | −8.29 |
| SE | 2.73 | 3.54 | 2.26 | 2.32 | (−24.55, −4.99) | (−15.54, −1.04) |
| % of men w/improved score | 59% | 45% | < 0.01 | 0.03 |
When asked about their satisfaction with the DA, 67% reported that the tool was “very helpful” in making screening decisions, and 70% of users were “very” or “mostly” satisfied with the DA. Importantly, 71% reported that the main message was that “men need to make an individual decision about prostate cancer screening with their medical providers.” However, 26% thought the main message was that “men should get an annual PSA test.”
DISCUSSION
Access to a computer-tailored DA over a three-month period at the workplace resulted in a significant increase in the proportion of men who made a decision about CaP screening. In addition, the intervention produced a significant improvement in CaP knowledge, yet mean knowledge scores remained fairly low in both intervention and control groups. Neither decision self-efficacy nor decisional consistency was measurably improved. Yet men in the intervention group reported marginally reduced decisional conflict. Unlike some prior studies, we used an “intention-to-treat” model in the primary analyses. In subgroup analyses among DA users, men were much more likely to have made a screening decision, have striking improvements in their levels of knowledge, and have increased decision self-efficacy.
Our findings align with some previous IDM trials. The previously cited review found that of 18 trials, 14 reported improvements in knowledge (12). The impact of DAs on decision self-efficacy has received far less attention; only two prior studies assessed this construct, each using one item for assessment. Gattellari et al. (32) found that patients who received DAs had greater confidence in their decision compared to patients who received a leaflet describing CaP screening risks and benefits. In contrast, Frosch et al. (33) reported reduced confidence in screening decisions among DA users compared with individuals in the control condition. Reductions in decisional conflict following DA use have been demonstrated in several studies (32, 34–36). Six (14, 36–40) of nine CaP DA studies (32, 35, 38–44) have also produced a decreased preference for screening, although this was not the case among men in our study. We have been unable to locate any DA trials that evaluated consistency of decision-making with one’s values; our study found no measurable change in decisional consistency. Only four prior studies have evaluated computer-based DAs (14–17). Of these, two demonstrated improved knowledge (15, 17) and one reported increased desire for decisional control (15). In terms of screening preferences, results have been mixed, with some finding a decreased desire to be screened and others finding an increased desire to be screened (14–16). Of note, only one of these interventions was tailored to individual user characteristics (although only on the basis of family history) (17).
Prior to a discussion of implications, limitations of our study must be acknowledged. First, overall use of the DA among men in the intervention group was lower than we had hoped. Prior research has documented low rates of health program participation among males and “blue collar workers” (45). National data show that only 9% of eligible workers participate in available worksite wellness programs (45). Thus, the participation rate among eligible men in this study should not have been entirely unexpected, particularly given that CaP is a highly personal topic. Moreover, given production demands in manufacturing worksites, it may have been difficult for men to take time away from their jobs, despite management agreement to let them do so. Still, improving rates of participation is an important goal for future efforts.
Second, findings from this sample of predominantly white, employed men may not be generalizable to men of varied racial/ethnic backgrounds or men who are not employed. In particular, African American men, who are at high risk for CaP, may have unique information needs and concerns (46) that were not addressed here. Given the disproportionate burden of CaP borne by African American men, development and testing of CaP screening interventions specifically for this audience should be a priority. Third, we used a previously unvalidated measure of decisional consistency. Existing strategies to assess values are based on probability assessments and require a high level of numeracy (28). Although we implemented several strategies (i.e., cognitive testing) to enhance the face and content validity of the measure we developed, additional work to fully evaluate its psychometric properties is needed. Also, given the high scores on this scale across the sample, a more sensitive measure of values may be needed. Fourth, we used self-reported PSA, which can underreport rates of screening (47). We did find a substantial amount of missing data on PSA history. However, our primary aims were to examine IDM outcomes, not screening participation. Finally, we examined only short-term changes in IDM after a three-month period. Since PSA screening may be offered annually, it is important to examine how various aspects of decision-making change over time.
Nevertheless, this study makes important contributions to the existing literature on interventions to promote IDM for CaP. First, we examined multiple aspects of IDM, as defined by the USPSTF. Prior studies have tended to focus on knowledge, and there has been insufficient study of other features of IDM (48). It is important that interventions assess all aspects of IDM, since knowledge is not the sole criterion for high quality decision-making. Second, we report results from a computer-tailored DA. To date, the vast majority of DAs have been non-tailored, and have taken the form of videos or written materials. As noted, there are numerous advantages to utilizing computer technology to deliver tailored IDM messages. Finally, we present data from an intervention offered in the work setting. To our knowledge, this is the first trial of a workplace intervention directed at IDM for CaP. Most interventions have been conducted in clinical settings despite the call for providing interventions in community settings (10).
The results of this study point to several important issues for consideration in future interventions. First, use of the DA resulted in an increase in decision-making, improved knowledge and enhanced self-efficacy. However, decisional consistency was consistently high and unaffected. This may have been due to “ceiling effects” in the measurement of this variable. Alternatively, it may be that many men overestimate the efficacy of screening and their providers’ endorsement of PSA, leading them to disregard the potential disadvantages and harms (34). We found that men are confident in their decision-making capabilities and are making decisions consistent with their values, even in the absence of what most would consider adequate knowledge. Are these truly “informed” decisions? Future research should consider whether it would be reasonable to identify a required level of knowledge regarding the benefits, risks and limitations of CaP screening as a pre-requisite for IDM, such is the case for informed consent (19).
Second, more research is needed on how to create ‘balanced’ messages in the context of IDM interventions. Communicating the uncertain balance between potential benefits (e.g., early detection) and harms (e.g., risk of over-treatment) of CaP screening presents a major challenge. In our study, nearly a quarter of DA users thought the main message was that “men should be screened annually.” Other IDM studies have similarly reported that men interpreted educational messages as promoting screening (50). There is ample evidence that message framing and varied formats for presenting risk information can impact decisions (51). Experimental manipulation of these factors may be informative for development of future DAs.
Based on these findings, we conclude that a computer-tailored DA offered at the workplace can be effective in promoting IDM, but participation among male, blue collar workers in this study was suboptimal. A recent review of health promotion programs offered in the worksite found that less than ten percent of employees accessed program components required for successful interventions (45). It is clear that better strategies for engaging men, particularly those of diverse racial/ethnic backgrounds, are needed. Worksites are a logical venue for reaching men, and many already offer health promotion programs (45). Integrating health topics, such as IDM for CaP screening, into existing programs or health issues that are more highly utilized (e.g., blood pressure screening) could potentially enhance programmatic dose and reach. Framing interventions in terms of “men’s health” rather than “cancer screening” may also draw a larger audience. Efforts might include engaging men in more socially relevant or trusted environments (e.g., barbershops, sporting events) where they may be more likely to discuss health issues with one another. Given the impact of the DA among those who used it, we are optimistic that employing interactive state-of-the-art technology will help to better engage men, and will facilitate the ultimate goal of preparing them to participate in complex decisions about their medical care.
IMPACT STATEMENT.
This trial demonstrates the efficacy of a computer-tailored decision aid in promoting informed decisions regarding prostate cancer screening. The decision aid was delivered through worksites, thereby providing access to resources required to participate in informed decision-making without requiring a medical appointment. However, participation rates were suboptimal and additional strategies for engaging men are needed.
Acknowledgments
This study was funded by the Centers for Disease Control and Prevention (Grant 3U48DP000064-01S1, SIP 21-04 Community Intervention to Increase IDM for Prostate Cancer). We gratefully acknowledge the contributions of the following individuals: Christian Brown, Emily Chasson, Stephen Flaherty, Josh Gagne, Elizabeth Harden, Kerry Kokkinogenis, Ruth Lederman, Susan McCabe, Jodi Saia-Witte, Rachel Shelton, Larry Shiman, Laura Tom, Jamielle Walker, and David Wilson. We are indebted to the men who took part in this study and to the participating worksites.
Appendix 1
| Values Questions | Would choose to be screened* (N=489) | Would choose NOT to be screened* (N=131) |
|---|---|---|
| It is important to me to have a PSA test, even if my doctors are not sure that screening can save lives. | 92% | 61% |
| It is important to me to have a PSA test, even if there is a chance the results could be wrong. | 91% | 54% |
| Finding prostate cancer early and getting treatment is worth any possible side effect, including difficult having sex or leaking urine. | 91% | 76% |
| If I had prostate cancer, I would want to know - even if it wasn’t going to kill me. | 97% | 93% |
| I would not want to have a PSA test unless doctors are reasonably sure that it can save lives. | 18% | 53% |
| I prefer not to be screened for prostate cancer if there is a chance the results could be wrong. | 11% | 39% |
| If getting treated for prostate cancer mean that I wouldn’t be able to have sex or that I might not be able to control my urine, I might choose not to get screened. | 24% | 93% |
| If I had prostate cancer, I would rather not know - especially if it wasn’t going to kill me. | 10% | 19% |
References
- 1.American Cancer Society. American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2006–2008. Atlanta: 2006. [Google Scholar]
- 2.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 4.Brawley OW, Gansler T. Introducing the 2010 American Cancer Society prostate cancer screening guideline. CA Cancer J Clin. 60:68–9. doi: 10.3322/caac.20067. [DOI] [PubMed] [Google Scholar]
- 5.Greene KL, Albertsen PC, Babaian RJ, Carter HB, Gann PH, Han M, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182:2232–41. doi: 10.1016/j.juro.2009.07.093. [DOI] [PubMed] [Google Scholar]
- 6.Recommendations for client- and provider-directed interventions to increase breast, cervical, and colorectal cancer screening. Am J Prev Med. 2008;35:S21–5. doi: 10.1016/j.amepre.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Screening for prostate cancer. American College of Physicians. Ann Intern Med. 1997;126:480–4. [PubMed] [Google Scholar]
- 8.Lim LS, Sherin K. Screening for prostate cancer in U.S. men ACPM position statement on preventive practice. Am J Prev Med. 2008;34:164–70. doi: 10.1016/j.amepre.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Family Physicians (AAFP) Summary of recommendations for clinical preventive services. Leawood, KS: 2007. [Google Scholar]
- 10.Briss P, Rimer B, Reilley B, Coates RC, Lee NC, Mullen P, et al. Promoting informed decisions about cancer screening in communities and healthcare systems. Am J Prev Med. 2004;26:67–80. doi: 10.1016/j.amepre.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101:1214–28. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 12.Volk RJ, Hawley ST, Kneuper S, Holden EW, Stroud LA, Cooper CP, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33:428–434. doi: 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2003:CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 14.Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing internet and video to facilitate patient education for men considering the prostate specific antigen test. J Gen Intern Med. 2003;18:781–7. doi: 10.1046/j.1525-1497.2003.20911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007;5:112–9. doi: 10.1370/afm.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lic D, Egberts K, BiomedSc H, McKenzie J, Risbridger G, Green S. Informing men about prostate cancer screening: A randomized controlled trial of patient education materials. J Gen Intern Med. 2007;23:466–71. doi: 10.1007/s11606-007-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison GL, Weinrich SP, Lou M, Xu H, Powell IJ, Baquet CR. A randomized trial comparing web-based decision aids on prostate cancer knowledge for African-American men. J Natl Med Assoc. 2008;100:1139–45. doi: 10.1016/s0027-9684(15)31481-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory- and evidence-based health education programs. Health Educ Behav. 1998;25:545–63. doi: 10.1177/109019819802500502. [DOI] [PubMed] [Google Scholar]
- 19.Talcott JA. What patients should be told before agreeing to a blood test that could change their lives. Urology. 2003;61:7–9. doi: 10.1016/s0090-4295(02)02148-9. [DOI] [PubMed] [Google Scholar]
- 20.Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Bmj. 2006;333:417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colditz GA, Atwood KA, Emmons K, Monson RR, Willett WC, Trichopoulos D, et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Vol. 11. Risk Index Working Group, Harvard Center for Cancer Prevention Cancer Causes Control; 2000. pp. 477–88. [DOI] [PubMed] [Google Scholar]
- 22.Bowen D, Hart A, Ludwig A, Allen JD. Development of a computer-tailored decision aid to promote informed decision-making for prostate cancer screening. Under review. [Google Scholar]
- 23.Prostate-specific antigen (PSA) best practice policy. American Urological Association (AUA) Oncology (Williston Park) 2000;14:267–72. 277–8. 280 passim. [PubMed] [Google Scholar]
- 24.O’Connor A, Jacobsen M, Stacey D. Stage of Decision Making Scale. Ottawa: Ottawa Health Research Institute; 2008. [Google Scholar]
- 25.Radosevich DM, Partin MR, Nugent S, Nelson D, Flood AB, Holtzman J, et al. Measuring patient knowledge of the risks and benefits of prostate cancer screening. Patient Educ Couns. 2004;54:143–52. doi: 10.1016/S0738-3991(03)00207-6. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor AM, Jacobsen MJ, Stacey D. An evidence-based approach to managing women’s decisional conflict. J Obstet Gynecol Neonatal Nurs. 2002;31:570–81. doi: 10.1111/j.1552-6909.2002.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 27.Sepucha K, Ozanne E, Silvia K, Partridge A, Mulley AG., Jr An approach to measuring the quality of breast cancer decisions. Patient Educ Couns. 2007;65:261–9. doi: 10.1016/j.pec.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Mullen PD, Allen JD, Glanz K, Fernandez ME, Bowen DJ, Pruitt SL, et al. Measures used in studies of informed decision making about cancer screening: a systematic review. Ann Behav Med. 2006;32:188–201. doi: 10.1207/s15324796abm3203_4. [DOI] [PubMed] [Google Scholar]
- 29.Allen JD, Othus MK, Hart A, Jr, Mohllajee AP, Li Y, Bowen D. Do Men Make Informed Decisions about Prostate Cancer Screening? Baseline Results from the “Take the Wheel” Trial. Med Decis Making. doi: 10.1177/0272989X10369002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29:21–43. [PubMed] [Google Scholar]
- 31.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–26. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 32.Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Educ Couns. 2005;57:168–82. doi: 10.1016/j.pec.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Frosch DL, Kaplan RM, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med. 2001;16:391–8. doi: 10.1046/j.1525-1497.2001.016006391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor KL, Davis JL, 3rd, Turner RO, Johnson L, Schwartz MD, Kerner JF, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiol Biomarkers Prev. 2006;15:2179–88. doi: 10.1158/1055-9965.EPI-05-0417. [DOI] [PubMed] [Google Scholar]
- 35.Gattellari M, Ward JE. Does evidence-based information about screening for prostate cancer enhance consumer decision-making? A randomised controlled trial. J Med Screen. 2003;10:27–39. doi: 10.1258/096914103321610789. [DOI] [PubMed] [Google Scholar]
- 36.Davison BJ, Kirk P, Degner LF, Hassard TH. Information and patient participation in screening for prostate cancer. Patient Educ Couns. 1999;37:255–63. doi: 10.1016/s0738-3991(98)00123-2. [DOI] [PubMed] [Google Scholar]
- 37.Wilt TJ, Paul J, Murdoch M, Nelson D, Nugent S, Rubins HB. Educating men about prostate cancer screening. A randomized trial of a mailed pamphlet. Eff Clin Pract. 2001;4:112–20. [PubMed] [Google Scholar]
- 38.Flood AB, Wennberg JE, Nease RF, Jr, Fowler FJ, Jr, Ding J, Hynes LM. The importance of patient preference in the decision to screen for prostate cancer. Prostate Patient Outcomes Research Team. J Gen Intern Med. 1996;11:342–9. doi: 10.1007/BF02600045. [DOI] [PubMed] [Google Scholar]
- 39.Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1:22–8. doi: 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partin MR, Nelson D, Radosevich D, Nugent S, Flood AB, Dillon N, et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. J Gen Intern Med. 2004;19:835–42. doi: 10.1111/j.1525-1497.2004.30047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Arch Fam Med. 1999;8:333–40. doi: 10.1001/archfami.8.4.333. [DOI] [PubMed] [Google Scholar]
- 42.Watson E, Hewitson P, Brett J, Bukach C, Evans R, Edwards A, et al. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: a randomised controlled trial exploring the impact of a brief patient decision aid on men’s knowledge, attitudes and intention to be tested. Patient Educ Couns. 2006;63:367–79. doi: 10.1016/j.pec.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Ruthman JL, Ferrans CE. Efficacy of a video for teaching patients about prostate cancer screening and treatment. Am J Health Promot. 2004;18:292–5. doi: 10.4278/0890-1171-18.4.292. [DOI] [PubMed] [Google Scholar]
- 44.Wilkins E, Lowery J, Hamill J. The impact of shared decision-making in prostate specific antigen (PSA) screening. Med Decis Making. 1999;19:A525. [Google Scholar]
- 45.Goetzel RZ, Ozminkowski RJ. The health and cost benefits of work site health-promotion programs. Annu Rev Public Health. 2008;29:303–23. doi: 10.1146/annurev.publhealth.29.020907.090930. [DOI] [PubMed] [Google Scholar]
- 46.Allen JD, Kennedy M, Wilson-Glover A, Gilligan TD. African-American men’s perceptions about prostate cancer: implications for designing educational interventions. Soc Sci Med. 2007;64:2189–200. doi: 10.1016/j.socscimed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:748–57. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 48.Bowen DJ, Allen JD, Vu T, Johnson RE, Fryer-Edwards K, Hart A., Jr Theoretical foundations for interventions designed to promote informed decision making for cancer screening. Ann Behav Med. 2006;32:202–10. doi: 10.1207/s15324796abm3203_5. [DOI] [PubMed] [Google Scholar]
- 49.Allen JD, Mohllajee AP, Shelton RC, Drake BF, Mars DR. A computer-tailored intervention to promote informed decision making for prostate cancer screening among African American men. Am J Mens Health. 2009;3:340–51. doi: 10.1177/1557988308325460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheridan SL, Felix K, Pignone MP, Lewis CL. Information needs of men regarding prostate cancer screening and the effect of a brief decision aid. Patient Educ Couns. 2004;54:345–51. doi: 10.1016/j.pec.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Albada A, Ausems MG, Bensing JM, van Dulmen S. Tailored information about cancer risk and screening: a systematic review. Patient Educ Couns. 2009;77:155–71. doi: 10.1016/j.pec.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Health Educ Res. 2008;23:454–66. doi: 10.1093/her/cyn004. [DOI] [PMC free article] [PubMed] [Google Scholar]