Hyperbaric oxygenation promotes neural stem cell proliferation and protects the learning and memory ability in neonatal hypoxic-ischemic brain damage (original) (raw)
. 2015 Feb 1;8(2):1752–1759.
Abstract
The aim of our study was to evaluate whether hyperbaric oxygenation (HBO) was an effective therapy for neonatal hypoxic ischemic brain damage (HIBD). Seven-day-old rat pups were divided into 3 groups: sham, hypoxia-ischemia (HI) control and HI-HBO group. HBO was administered for HI rats daily. The pathologic changes in brain tissues were observed by hematoxylin-eosin (H-E) staining. The immunohistochemical staining was applied to detect the Nestin and 5-bromo-2-deoxyuridine (BrdU) positive cells in hippocampal dentate gyrus region. The learning and memory function of rats was examined by Morris water maze. The HI rats showed obvious pathologic changes accompanied by levels decreasing and disorder arrangement of pyramidal cells, glial cells proliferation in postoperative, and nerve nuclei broken, while pathologic changes of rats in sham group was approximate to that in the HI + HBO group that was opposite to the HI group. Compared with the sham group, the Nestin and BrdU positive cells in HBO + HI group at different time points increased significantly (P < 0.01). Learning and memory function of rats in HI group was poor compared with the sham/HI + HBO group (_P_ < 0.01), while that in HI + HBO group was approximate to that in sham group (_P_ > 0.05). HBO treatment improved the learning and memory ability of the HI rats. HBO therapy may be effective for neonatal HIBD treatment.
Keywords: Hypoxic ischemic brain damage, hyperbaric oxygenation, hematoxylin-eosin staining, Nestin, 5-bromo-2-deoxyuridine, Morris water maze
Introduction
Hypoxic ischemic brain damage (HIBD) is a common and severe complication resulting from reduced supply of oxygen in brain [1]. The neonatal HIBD may cause metal impairment, seizures, motor deficits, cognize and learning disability [2,3]. Besides, HI brain damage is always associated with high rate of morbidity and mortality in newborns [4]. The HIBD especially in newborns is a heath concern highlighted all over the world.
Hyperbaric oxygenation (HBO) has been applied in neonatal disease treatment for many years [5]. Recently, there is an issue on debate whether hyperbaric oxygenation (HBO) is an effective therapy for HIBD treatment. Some studies reported that HBO stimulated the proliferation and migration of neural stem cells (NSCs) in brain tissues, which contributed to central nervous system recovery and repaired the brain damage [6]. While others are anxious about the safety and validity of HBO treatment due to the oxygen toxicity, which may increase the infract area [7]. However, there is no confirming verdict about this issue.
The HIBD model was successfully constructed with 7-day-postnatal rats and widely used to investigate the mechanism underlying HIBD development in neonates [8]. In the present study, we constructed the HIBD model with Sprague-Dawley rats. Combined with BrdU (5-bromo-2’-deoxyuridine) labeling technology, we assessed the Nestin and BrdU positive cells to trace the proliferation of NSCs. The learning and memory disability of HBO treatment rats were evaluated by Morris water maze (MWM) test. The purpose of this work was to explore the therapeutic effect of HBO on HIBD neonates.
Materials and methods
Animals and group
Unsexed 7-day-postnatal Sprague-Dawley (SD) rats (purchased from the Animal Department of the Third Military Medical University, Chongqing, China) were randomly divided into 3 groups: sham group (underwent surgery but no carotid ligation and no hypoxia) (n = 40), HI control group (carotid ligation and hypoxia, no intervention) (n = 40) and HI-HBO group (carotid ligation and hypoxia, HBO treatment) (n = 40). In each group, the pups were confirmed to be from each litter for parity. After surgery for 15-30 min, the pups in HI-HBO group were placed in the baby HBO chamber (YLC0.5/1A, Wuhan, China) and treated with HBO. The HBO treatment was performed daily as previously described [9]. All the pups were maintained with food and water ad libitum normally throughout the study. At postnatal day 30, 8 pups from each group underwent Morris water maze behavior measurement and same numbers of rats were used at different time points for hematoxylin-eosin (H-E) and immunohistochemical staining.
Hypoxia-ischemia (HI) animal model construction
The protocol was approved by the local Animal and Ethics Review Committee. The 7-day-postnatal (SD) rats were subjected to the construction of HIBO animal model based on the modified Rice-Vannucci procedure [8]. Briefly, neonatal rats were administrated with ether inhalationally. At a temperature of 37°C, the left common carotid artery of the pups were exposed and double ligatured with 0 surgical sutures. The pups were placed in self-controlled hypoxic device perfused with a mixed gas of 8% O2 + 92% N2 for 2 h. After hypoxia exposure, the pups were returned to their dams with food and water available ad libitum.
BrdU (5-bromo-2’-deoxyuridine) labeling
All the rats were administrated with BrdU (Sigma, USA) (50 μg/g) intradermally at one day before being killed per 4 h for 3 times. At 12 h after the last injection, the rats were put to death for further study.
Tissue preparation for microscopy and H-E staining
Animals (n = 8) were death by lethal injection with 4% paraformaldehyde at 4 sequential time intervals after surgery (4 d, 7 d, 14 d and 21 d). The brain was removed and post-fixed in paraformaldehyde for 24 h, followed by embedding in paraffin wax. The dentate gyrus (DG) areas were cut into 4 μm sections coronally for HE and immumohistochemical staining. The paraffin sections were stained with H-E for cell and observed under microscope (Olympus, Japan).
Immunohistochemical staining
The immunohistochemical staining was performed using the modified streptavidin-biotin complex (SABC) method previously reported [10]. Briefly, sections were de-waxed, steeped in 3% H2O2-methyl alcohol solution for 15 min and blocked in 10% goat serum albumin for 20 min. Then the sections were incubated with primary antibodies: Mouse anti-Nestin monoclonal antibody (1:100, Chemicon, USA) and Mouse anti-BrdU monoclonal antibody (1:300, Chemicon, USA) at 37°C for 90 min. For BrdU processing, antigens were retrieved by high pressure repairing (0.01 M Tris-EDTA, PH: 9.0) for 2 min. Sections were incubated with the second antibodies: Biotin-conjugated goat anti-mouse (1:100, Shenzhen Jingmei, China ) for Nestin and biotin-conjugated goat anti-rabbit (1:100, Shenzhen Jingmei, China) for BrdU, at 37°C for 30 min. Sections were treated with 3, 3’-duaminobenzidine (DAB) for 2-5 min, rinsed and analyzed by DM2500 microscope (Leica, Germany) equipped with Leica Qwin cell image analysis software.
BrdU and Nestin cells that colored into brown colored-nucleus or brown colored-cytoplasm cells were considered as positive cells, respectively. The 4-5 discontinued sections from different kinds brain tissues at each time point (dentate gyrus granular cell layer and hyperplasia of the grain belt) of each rat were selected for positive cells counted with magnifications up to 400-fold.
Morris water maze (MWM) test
In order to assess the spatial learning and memory of the experimental animals, we performed the MWM test. The maze was composed of a circular tank (130 cm diameter, 45 m depth) and a computerized tracking system (MT-200, Chengdou Taimeng, China). The tank with white interior was filled with milk powder solution with a temperature of 23-25°C. The rats were labeled with picric acid at the head. The tank and surrounding environment remained the same throughout the MWM test.
In the spatial navigation test, the animals were released in the water from 4 different initial points: N (north), E (east), S (south), W (west) to locate the hidden platform (11.5 cm diameter, 30 cm depth, 2 cm below the water surface) in the NE (Northeast) of the tank. Before the test, the rats were allowed to swim freely in the circular tank for 2 min to be familiar with the maze environment. Every rat was subjected to 4 swimming trials in morning and afternoon time period for 5 consecutive days. The rats started the test randomly from four positions and the escape latency time (from starting point to platform) was recorded for each trial. If the rats failed to reach the hidden platform within 120 s, they were guided to the platform and the escape latency time was recorded as 120 s for each rat in this trail. The time interval was set as 60 s between two trails.
On day 6 of the MWM test, the spatial probe trial was carried out to evaluate the spatial memory. The platform was removed before the rats were released to the water from random starting points at the same time. The number of times that one rat crossed the former platform area within 2 min was recorded as the platform crossing frequencies. In the test process, the computerized tracking system was applied for automatic video and data acquisition.
Data analysis
All the data were displayed as mean ± standard deviation (SD) and analyzed by SPSS 16.0 software. The immunohistochemical data was examined by one-way ANOVA combined with Fisher’s Least Significant Difference (LSD) test. Differences among groups at different time points in the escape latency time and platform crossing frequencies were evaluated by the two-way ANOVA with repeated measures and one-way ANOVA, respectively. P < 0.05 was considered as significant.
Results
HI animal model construction
After the model construction, 5 rats became weak for infection and other factors. Although these animals did not die immediately, all the 5 rats were dead within 4 days after surgery, among which, 2 ones died from bites of the mother rat and 3 ones for disability to suck. The dead animals were replenished to maintain the number of rats as 40. But during the experimental period, no animal died unexpectedly.
HE staining
The pathologic changes in brain tissues were observed by HE staining. As shown in Figure 1, the pyramidal cells of DG region in sham group grew in multiple layers and arranged in neat rows. The cell contour was normal and nucleoli in the center of cells were observed clearly. In the HI group, the lesions were mainly occurred in cortex, CA1 and CA3 region of DG. The cell layers in brain tissues reduced and cells arranged in disorder. At day 4 and day 7 after surgery, the gliocyte proliferation, karyopyknosis and cell fragmentation were found in brain tissues. In addition, the neurons degenerated and glial scar formed. Compared with the brain tissues in HI group, the lesion was alleviated after HBO treatment. The pyramidal cells arranged loosely and reduced inconspicuously. The phenomena of karyopyknosis and fragmentation were relatively less.
Figure 1.
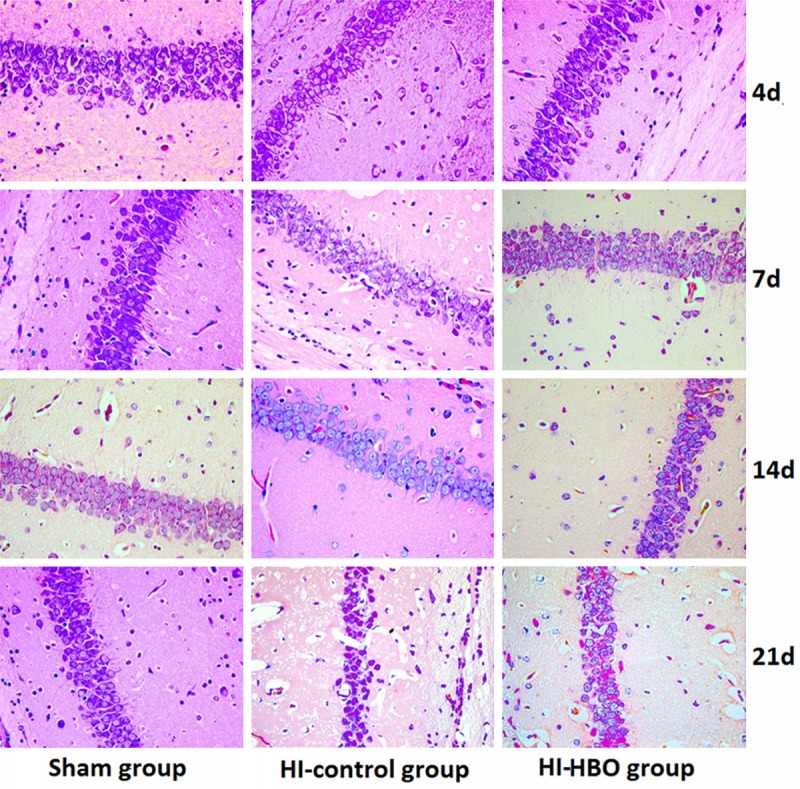
Pathologic damage in hippocampal DG region after HI in neonatal rats (HE × 400).
Expression of Nestin in hippocampal DG region
Nestin protein is expressed by the differentiating neural stem cell (NSC) [11] and commonly considered as the marker for NSC [12]. Nestin positive cells in sham group displayed thinner and shorter protuberances compared with those in HI and HI-HBO group (Figure 2). The difference in the number of Nestin positive cells was significant (P < 0.01) between sham and HI group at different time points. And the Nestin positive cells in HI-HBO group increased significantly among groups (P < 0.01). The number of Nestin positive cells peaked 4 days after surgery in the three groups, then decreased significantly at 7 d and 21 d after surgery (P < 0.01) (Table 1).
Figure 2.
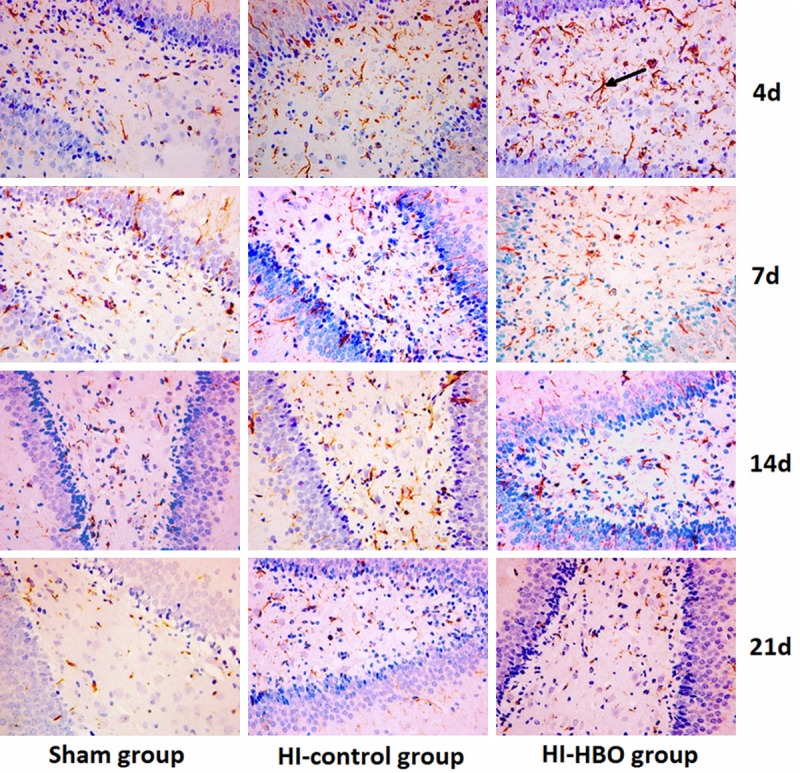
Immunohistochemical staining demonstrated Nestin expression in the hippocampus of each group (× 400).
Table 1.
Numbers of Nestin positive cells in hippocampal DG region at different time points
| Groups | 4 d | 7 d | 14 d | 21 d |
|---|---|---|---|---|
| Sham | 30.50 ± 2.45 | 20.38 ± 1.69 | 8.63 ± 1.60 | 2.88 ± 1.25 |
| HI control | 40.00 ± 4.81* | 29.50 ± 2.45* | 16.63 ± 1.69* | 4.75 ± 1.28* |
| HI-HBO | 51.25 ± 6.04*,# | 40.00 ± 4.90*,# | 24.75 ± 3.11*,# | 8.00 ± 1.31*,# |
Expression of BrdU in hippocampal DG region
The positive-BrdU cells mainly distributed in the polymorphous cell layer and adopt round shape, fusiform shape and pole shape (Figure 3). The BrdU positive cells peaked 4 days after surgery and began to decrease at 7 d after surgery in all of the three groups and there was significant difference in the number of BrdU positive cells between different time points (P < 0.01). A significant difference of the number of BrdU positive cells was observed at different time points compared the sham group with HI group. And there were significant increases of BrdU positive cells at different time points between HI group and HI-HBO group (P < 0.01) (Table 2).
Figure 3.
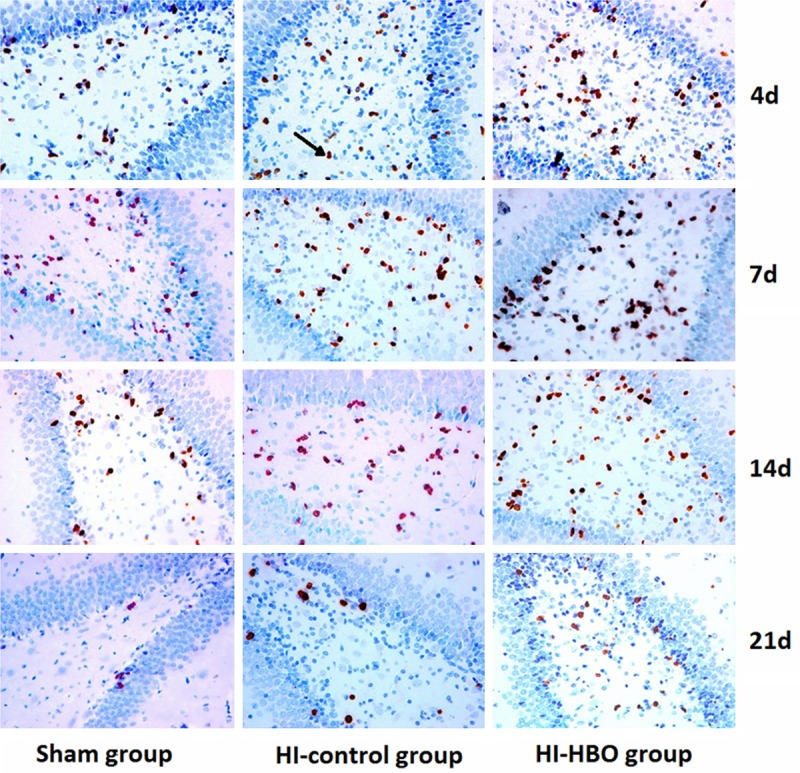
Immunohistochemical staining demonstrated BrdU labeled cells in the hippocampus of each group (× 400).
Table 2.
Numbers of BrdU labeled cells in hippocampal DG region at different time points
| Group | 4 d | 7 d | 14 d | 21 d |
|---|---|---|---|---|
| Sham | 50.00 ± 4.54 | 34.50 ± 2.45 | 15.13 ± 2.03 | 2.13 ± 0.64 |
| HI control | 64.88 ± 4.67* | 46.00 ± 4.07* | 24.50 ± 3.34* | 6.75 ± 1.28* |
| HI-HBO | 80.00 ± 4.34*,# | 56.88 ± 4.29*,# | 35.00 ± 3.12*,# | 12.75 ± 1.83*,# |
MWM test analysis
The learning and memory abilities of the rats were manifested by delayed escape latency time and platform crossing frequencies. In the spatial navigation test, all the animals were likely to find the platform more quickly after the first trial constructed. Among the three groups, rats in HI group spent the statistically longest time to search for the platform hidden in water (P < 0.05). Compared with HI-HBO group and sham group, difference was not observed in the escape latency time of the rats (_P_ > 0.05) (Table 3). In the spatial probe trial, rats exposed to the HBO showed significant higher platform crossing frequencies compared with HI rats (P < 0.05). And sham-surgery rats showed similar memory ability with HBO-treated rats (_P_ > 0.05) (Table 4).
Table 3.
Changes in escape latency time
| Groups | 1 d | 2 d | 3 d | 4 d | 5 d |
|---|---|---|---|---|---|
| Sham | 44.75 ± 2.82 | 32.25 ± 2.12 | 19.00 ± 2.00 | 15.50 ± 2.45 | 12.75 ± 2.31 |
| HI-HBO | 48.63 ± 3.78 | 34.13 ± 3.60 | 21.30 ± 2.12 | 18.13 ± 3.14 | 15.63 ± 2.33 |
| HI control | 72.00 ± 2.00*,# | 64.88 ± 2.23*,# | 59.00 ± 3.12*,# | 50.00 ± 2.83*,# | 42.25 ± 2.38*,# |
Table 4.
Changes in platform crossing frequencies among three groups
| Groups | Sham | HI-HBO | HI control |
|---|---|---|---|
| Frequencies | 8.13 ± 0.64 | 7.38 ± 1.06 | 5.00 ± 0.76*,# |
Discussion
Currently, an important understanding of HIBD pathogenesis is the strong activation of neuronal apoptosis in brain [13]. NSCs have potential of self-renewal, migration and multiple differentiations into neurons, astrocyte and oligodendrocyte for injury repair. There is a hypothesis that the increasing renewal capability of the endogenous NSCs contributes to the repair in brain damage [6]. Since, the NSCs in mammalian brain persist mainly in hippocampal DG region [14] and the hippocampus is susceptible to HI injury [15], we chose the hippocampal DG region for endogenous NSCs analysis. Nestin is an intermediate filament protein which is known as the biological marker for NSCs in central nervous system. BrdU served as a synthetic nucleoside, can be incorporated into the newly synthesized DNA during the S phase of cell cycle. BrdU is widely used to detect the proliferation cells in living tissues [16]. In the present study, we used the Nestin and BrdU staining to evaluate the NSC proliferation.
Our results showed that the number of Nestin positive cells was significantly larger in HI and HBO group than that in sham group. It is reported that the HIBD can induce the proliferation of NSCs in situ [17,18]. We speculated that the proliferation of NSCs in situ was involved with the Nestin expression after HIBD. Figure 2 indicated that the shape of Nestin positive cells was similar with astrocyte. A previous report revealed that a majority of Nestin expression was observed in astrocytes following brain injury [19]. The Nestin expression in astrocytes consisted with the embryonal protein played key role in brain remodeling and recovery [20,21]. In this study the Nestin positive cells observed may be reactive hyperplasia astrocytes differentiated by SNC after HIBD. Our results also showed that HBO treatment predominately increased the BrdU labeled cells in DG region of pups, which suggested that HBO promoted the proliferation and differentiation in HI rats. Besides, reports documented that the optimum time of HBO treatment was at 6h after injury [22] and in contrast, HBO treatment aggravated the injury after 12 h [23,24]. We found that the both Nestin and BrdU positive cells peaked 4 days after surgery and then decreased significantly. The most effect of HBO on HIBD treatment may be for 4 days. Thus, there is a problem which is the best treatment of HBO for HIBD that needs to be further investigated.
Furthermore, hippocampus has close association with learning and memory ability [25]. Then HIBD can affect the hippocampal function and declined the learning and memory ability. Our paper designed MWM test for pups at 30 days after surgery to test HBO effect on the learning and memory abilities by delayed escape latency time and platform crossing frequencies. The MWM test showed that the HIBD significantly induced the cognitive dysfunction evidenced by long escape latency time and less platform crossing frequencies. The rats exposed to HBO retained similar learning and memory function compared with those in sham group. Therefore, HBO therapy can improve the learning and memory ability following HIBD.
In summary, HBO treatment promoted the repair and regeneration of the nervous system and contributed to the self-recovery and protection of the damaged brain. The improvements in learning and memory function were observed by HBO treatment. HBO may be a promising therapy for HIBD in newborn based on the appropriate treatment time and pressure. However, the optimum treatment condition needs to be further studies.
Acknowledgements
This work is supported by Special foundation for Taishan Scholars No. ts20110814.
Disclosure of conflict of interest
None.
References
- 1.Mattiesen WR, Tauber SC, Gerber J, Bunkowski S, Brück W, Nau R. Increased neurogenesis after hypoxic-ischemic encephalopathy in humans is age related. Acta Neuropathol. 2009;117:525–534. doi: 10.1007/s00401-009-0509-0. [DOI] [PubMed] [Google Scholar]
- 2.Gulczyńska E, Gadzinowski J. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy] . Ginekol Pol. 2012;83:214. [PubMed] [Google Scholar]
- 3.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 4.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:F152–F155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudge LCFW. Carbon monoxide poisoning in infants: treatment with hyperbaric oxygen. South Med J. 1993;86:334–337. doi: 10.1097/00007611-199303000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Wang X, Yu X, Wang X, Xie M, Liu C. Hyperbaric oxygen induces endogenous neural stem cells to proliferate and differentiate in hypoxic-ischemic brain damage in neonatal rats. Undersea Hyperb Med. 2008;35:113–29. [PubMed] [Google Scholar]
- 7.Badr A, Yin W, Mychaskiw G, Zhang J. Dual effect of HBO on cerebral infarction in MCAO rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R766–R770. doi: 10.1152/ajpregu.2001.280.3.R766. [DOI] [PubMed] [Google Scholar]
- 8.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 9.Yujia LL. Relationship of Dose-Effect and Time-Effect to Hyperbaric Oxygen Therapy on Hypoxic-Ischemic Brain Damage in the Neonatal Rat. Zhongguo Dang Dai Er Ke Za Zhi. 2001;3:355–358. [Google Scholar]
- 10.Santini D, Ceccarelli C, Mazzoleni G, Pasquinelli G, Jasonni VM, Martinelli GN. Demonstration of cytokeratin intermediate filaments in oocytes of the developing and adult human ovary. Histochemistry. 1993;99:311–319. doi: 10.1007/BF00269104. [DOI] [PubMed] [Google Scholar]
- 11.Strojnik T, Røsland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki S, Gerhold LM, Böttner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors α and β. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 13.Kilicdag H, Daglioglu YK, Erdogan S, Zorludemir S. Effects of caffeine on neuronal apoptosis in neonatal hypoxic-ischemic brain injury. J Matern Fetal Neonatal Med. 2014:1–21. doi: 10.3109/14767058.2013.878694. [DOI] [PubMed] [Google Scholar]
- 14.Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- 15.Calvert JW, Zhou C, Nanda A, Zhang JH. Effect of hyperbaric oxygen on apoptosis in neonatal hypoxia-ischemia rat model. J Appl Physiol. 2003;95:2072–2080. doi: 10.1152/japplphysiol.00630.2003. [DOI] [PubMed] [Google Scholar]
- 16.Lehner B, Sandner B, Marschallinger J, Lehner C, Furtner T, Couillard-Despres S, Rivera FJ, Brockhoff G, Bauer HC, Weidner N. The dark side of BrdU in neural stem cell biology: detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011;345:313–328. doi: 10.1007/s00441-011-1213-7. [DOI] [PubMed] [Google Scholar]
- 17.Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13:127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 18.He Z, Cui L, Meschia JF, Dickson DW, Brott TG, Simpkins JW, Day AL, Mckinney M. Hippocampal progenitor cells express nestin following cerebral ischemia in rats. Neuroreport. 2005;16:1541–1544. doi: 10.1097/01.wnr.0000179074.32035.46. [DOI] [PubMed] [Google Scholar]
- 19.Douen A, Dong L, Vanance S, Munger R, Hogan M, Thompson C, Hakim A. Regulation of nestin expression after cortical ablation in adult rat brain. Brain Res. 2004;1008:139–146. doi: 10.1016/j.brainres.2003.08.070. [DOI] [PubMed] [Google Scholar]
- 20.Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768:1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- 21.Sahin Kaya S, Mahmood A, Li Y, Yavuz E, Chopp M. Expression of nestin after traumatic brain injury in rat brain. Brain Res. 1999;840:153–157. doi: 10.1016/s0006-8993(99)01757-6. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Zhao Y, Ma Q, Zhou X, Wang Y. Optimal therapeutic window of hyperbaric oxygenation in neonatal rat with hypoxic-ischemic brain damage] . Zhonghua Er Ke Za Zhi. 2006;44:177. [PubMed] [Google Scholar]
- 23.Lou M, Eschenfelder CC, Herdegen T, Brecht S, Deuschl G. Therapeutic window for use of hyperbaric oxygenation in focal transient ischemia in rats. Stroke. 2004;35:578–583. doi: 10.1161/01.STR.0000111599.77426.A0. [DOI] [PubMed] [Google Scholar]
- 24.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 25.Sakimura K, Kutsuwada T, Itot I, Manabel T, Takayama C, Suglyamat H, Mishina M. Reduced hippocampal LTP and spatial learning in mice lacking NM DA receptor si subunit. Nature. 1995;373:151. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]