Liberal versus restrictive blood transfusion strategy: 3-year survival and cause of death results from the FOCUS randomised controlled trial (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 10.
Summary
Background
Blood transfusion might affect long-term mortality by changing immune function and thus potentially increasing the risk of subsequent infections and cancer recurrence. Compared with a restrictive transfusion strategy, a more liberal strategy could reduce cardiac complications by lowering myocardial damage, thereby reducing future deaths from cardiovascular disease. We aimed to establish the effect of a liberal transfusion strategy on long-term survival compared with a restrictive transfusion strategy.
Methods
In the randomised controlled FOCUS trial, adult patients aged 50 years and older, with a history of or risk factors for cardiovascular disease, and with postoperative haemoglobin concentrations lower than 100 g/L within 3 days of surgery to repair a hip fracture, were eligible for enrolment. Patients were recruited from 47 participating hospitals in the USA and Canada, and eligible participants were randomly allocated in a 1:1 ratio by a central telephone system to either liberal transfusion in which they received blood transfusion to maintain haemoglobin level at 100 g/L or higher, or restrictive transfusion in which they received blood transfusion when haemoglobin level was lower than 80 g/L or if they had symptoms of anaemia. In this study, we analysed the long-term mortality of patients assigned to the two transfusion strategies, which was a secondary outcome of the FOCUS trial. Long-term mortality was established by linking the study participants to national death registries in the USA and Canada. Treatment assignment was not masked, but investigators who ascertained mortality and cause of death were masked to group assignment. Analyses were by intention to treat. The FOCUS trial is registered with ClinicalTrials.gov, number NCT00071032.
Findings
Between July 19, 2004, and Feb 28, 2009, 2016 patients were enrolled and randomly assigned to the two treatment groups: 1007 to the liberal transfusion strategy and 1009 to the restrictive transfusion strategy. The median duration of follow-up was 3·1 years (IQR 2·4–4·1 years), during which 841 (42%) patients died. Long-term mortality did not differ significantly between the liberal transfusion strategy (432 deaths) and the restrictive transfusion strategy (409 deaths) (hazard ratio 1·09 [95% CI 0·95–1·25]; p=0·21).
Interpretation
Liberal blood transfusion did not affect mortality compared with a restrictive transfusion strategy in a high-risk group of elderly patients with underlying cardiovascular disease or risk factors. The underlying causes of death did not differ between the trial groups. These findings do not support hypotheses that blood transfusion leads to long-term immunosuppression that is severe enough to affect long-term mortality rate by more than 20–25% or cause of death.
Funding
National Heart, Lung, and Blood Institute.
Introduction
In recent years, substantial progress has been made in our understanding of the effect of blood transfusion on clinical outcomes. Several published clinical trials mostly show that a restrictive transfusion strategy with a haemoglobin concentration threshold of 70–80 g/L is safe,1,2 and in one case superior,3 to a liberal transfusion strategy with a threshold of 90–100 g/L. These trials have focused on short-term outcomes such as 30-day mortality and infection complications. However, transfusion is thought to have long-term consequences related to changes in immune function. These effects have been postulated to increase the risk of subsequent infections and cancer.4,5 Thus, transfusion could possibly increase the rate of long-term mortality by increasing the frequency of two of the most common causes of death: infections and cancer. Alternatively, a more liberal transfusion strategy might reduce cardiac complications2,6 by reducing short-term clinical or subclinical myocardial damage by increasing oxygen delivery to the heart, which could have long-term health implications.
We did secondary analyses of our previously published randomised clinical trial Transfusion Trigger Trial for Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS).7 A full list of the investigators who worked on the FOCUS trial is available in the appendix. The aim of our analyses was to establish the effect of a liberal red blood cell transfusion strategy on long-term survival (with median follow-up of 3 years) compared with a restrictive transfusion strategy. When gathering the mortality data, we recognised that information about cause of death could be obtained, so we planned and undertook an additional analysis of cause of death before we examined the results by transfusion group. Our hypotheses were that compared with a restrictive transfusion strategy, liberal transfusion might reduce long-term mortality and cardiovascular deaths but could increase the risk of death from infections and cancer. To our knowledge, this clinical trial is the first to assess the long-term effects of blood transfusion.
Methods
Study design and participants
For this randomised controlled trial, participants were recruited from 47 hospitals across the USA and Canada. Patients aged 50 years and older with a haemoglobin concentration lower than 100 g/L within 3 days after undergoing surgery to repair a hip fracture, and a history of cardiovascular disease or risk factors for cardiovascular disease, were eligible for enrolment into the study. We defined cardiovascular disease as history of coronary artery disease, congestive heart failure, stroke, or peripheral vascular disease. We defined cardiovascular risk factors as history of diabetes mellitus, hypertension, hypercholesterolaemia, smoking, or creatinine concentration of 2 mg/dL or higher. In the original protocol, only patients with cardiovascular disease were eligible for inclusion. On Nov 17, 2005, the Director of the National Heart, Lung, and Blood Institute approved the data safety monitoring board’s recommendation to expand the eligibility criteria to improve recruitment by including patients with cardiovascular risk factors. We excluded patients from the study if they were unable to walk independently before the hip fracture (although use of an assistive device was allowed), refused red blood cell transfusion, had a myocardial infarction within the preceding 30 days, were actively bleeding, or if the hip fracture was the result of multiple trauma (ie, patients also required surgery at a site other than the hip). We used a central telephone system to randomly assign consenting patients in a 1:1 ratio to either the liberal transfusion strategy (transfusion to maintain a haemoglobin level ≥ 100 g/L) or restrictive transfusion strategy (transfusion for symptoms of anaemia or at the doctor’s discretion if the patient had a haemoglobin concentration <80 g/L). The assigned transfusion strategy was followed during the hospital stay (for up to 30 days). Symptoms of anaemia for which transfusion was allowed were chest pain thought to be cardiac in origin, symptoms and signs of congestive heart failure, or hypotension or tachycardia unresponsive to fluid challenge. We collected detailed information including baseline clinical status, red blood cell transfusion, morbidity, and mortality during the hospital stay (for up to 30 days). We ascertained functional status and vital status at 30 and 60 days by telephone query, which was done by nurses at the clinical coordinating centre who were masked to treatment assignment. The Data Coordinating Center at the University of Maryland (MD, USA) prepared randomisation schedules for each site with use of randomly ordered permuted block sizes of two, four, six, or eight. After random allocation, treatment assignment was not masked, but ascertainment of mortality and cause of death was assessed by investigators who were masked to group assignment. The full details of the trial, including short-term mortality up to 60 days, have previously been published.7,8
The trial was approved by institutional review boards or ethics committees at all the hospitals and monitored by the data and safety monitoring boards. All participants, or a member of their family, provided written informed consent.
Procedures
To ascertain long-term survival, we identified deaths that occurred by linking the study participants to national mortality registries that are maintained by both the USA and by Canada. All deaths that occur in the USA are reported to the National Center for Health Statistics National Death Index by the vital statistics offices of each individual state.9 The National Death Index database is updated annually once the successive calendar year data become available. Similarly, all deaths that occur in Canada are reported to the Statistics Canada Mortality Database by the local provinces. Both registries compare key identifying variables (ie, name, date of birth, sex, and social security number [only in the USA]) in the research cohort to those of the deaths in electronic database and, based on the degree of correspondence, identify likely matches.
At the conclusion of the active follow-up of the FOCUS study (on May 4, 2009) and before long-term survival information was available from registry linkage, vital status at 60 days was verified by available methods. At that time (2009), an online database of deaths that had been reported to the Social Security Administration was publicly available. The identifiers for each participant recruited within the USA were compared against those in the database and deaths that matched to the study participants were identified. In Canada, where no such centralised databases were available, each site searched hospital records and locally available databases and identified deaths for their patients. Deaths detected through this process, including those that occurred after the 60-day follow-up, were recorded.
To ascertain the final long-term mortality, deaths identified from the registry matches were compared with those detected by the vital status verification during the overlapping search periods. A death that was identified through the earlier verification procedure but not matched to a death file in the registry linkage was retained as a true death. Thus, the final measure of long-term survival included the deaths identified by the systematic search of the national registries and the additional deaths that were identified by the verification process. We calculated survival as the number of days from randomisation to death or, in the absence of a death, to the last day of the calendar year for which the registry data were complete.
The registries provided underlying cause of death for each identified death in accordance with WHO criteria.10 We grouped the specific underlying causes into seven categories: cardiovascular disease, cancer, infection, stroke, dementia, pulmonary, and others.
Statistical analysis
In this study, we analysed long-term mortality, which was a prespecified secondary endpoint of the original FOCUS trial. When gathering the mortality data, we recognised that cause of death information could also be obtained. This additional analysis was then planned and conducted before we examined the results by transfusion group.
We compared survival time between the two transfusion treatment strategies using the unadjusted log-rank test, and we used Cox proportional hazard models to obtain hazard ratios. We first did an unadjusted Cox model with treatment group as the only independent variable. We confirmed that the hazards are proportional in a model in which a time-dependent product term between transfusion strategy (liberal vs restrictive) and time was included in the models. Proportionality is also visually apparent by examination of the survival curves. This finding was replicated when we used log-transformed time. We then did a second adjusted proportional hazards model that included an a-priori list of potentially confounding variables (sex, age, coronary artery disease, congestive heart failure, stroke, peripheral vascular disease, diabetes mellitus, hypertension, creatinine concentration ≥ 2·0 mg/dL, American Society of Anesthesiologists risk score, chronic lung disease, cancer, dementia, nursing home residence, clinical site, and country) to ensure that randomisation had accurately isolated the treatment effect. We assessed the homogeneity of transfusion group effect across prespecified subgroups with Cox models that included transfusion group, subgroup, and their interaction. Participants with missing variables were excluded from the Cox proportional hazards models. We used the χ2 test statistic to compare causes of death between the two transfusion treatment strategies. The long-term mortality outcome was pre-specified in the protocol. Before we did the cause of death analysis, we created the categories of cause of death and the a-priori hypotheses. Sample size was established based on the primary outcome in the trial.7 SAS version 9.2 was used for all analyses.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
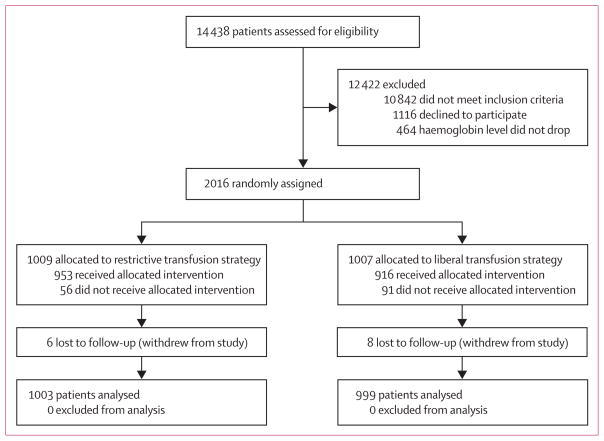
Between July 19, 2004, and Feb 28, 2009, we assessed 14 438 patients for eligibility and enrolled 2016 patients from 47 sites in the USA and Canada into the trial (figure 1). 1222 of these patients were enrolled at sites in the USA, nine of whom withdrew from the study and were excluded from the analysis population. Another three patients were visitors to USA from other countries at the time of the hip fracture. Long-term survival for these individuals was censored at 60 days since the National Death Index would not capture deaths that occur in their home countries. For one patient, the site provided a date of death but did not provide the necessary personal identifiers to do a mortality search. The National Death Index database was complete through Dec 31, 2010, at the time of the registry search for the remaining 1209 patients from the USA. Of the 551 deaths in these 1209 American patients, 545 (99%) were identified by the National Death Index search. Six deaths occurred that were identified only in the social security database.
Figure 1.
Trial profile
Of the 794 patients enrolled in Canada, five withdrew from the study and were excluded from the study population. Two patients who were not Canadian had long-term survival censored at 60 days. The site provided a date of death for two patients but did not submit the necessary personal identifiers to enable us to do a registry search. The Statistics Canada Mortality Database, which has a longer lag time than the National Death Index, was complete through to Dec 31, 2009, for the remaining 785 patients from Canada. Of the 287 deaths in these 785 Canadian patients, 284 (99%) were identified by a Statistics Canada Mortality Database search. Three deaths were identified only by site report.
The total population of 2016 enrolled patients had a mean age of 81·6 years (range 51–103 years), 1527 (76%) were women, and 1268 (63%) had cardiovascular disease. Baseline characteristics and clinical status were similar between the two groups (table 1). The mean pre-transfusion haemoglobin concentration was 92 g/L (SD 5) in the liberal transfusion strategy group and 79 g/L (SD 6) in the restrictive transfusion strategy group. The number of units of red blood cells transfused post-randomisation in the liberal transfusion strategy was 2·9-times larger than that in the restrictive transfusion strategy (table 1; p<0·0001). 970 (97%) patients in the liberal transfusion strategy group received post-randomisation blood transfusions, whereas 413 (41%) patients in the restrictive transfusion strategy group received post-randomisation transfusions and 593 (59%) received transfusions either pre-randomisation or post-randomisation. Transfusion status was missing for four patients in the liberal strategy group and two in the restrictive strategy group. 2234 (90%) of the 2489 units of blood transfused post-randomisation were leukoreduced.
Table 1.
Baseline characteristics
| Liberal transfusion strategy (n=1007) | Restrictive transfusion strategy (n=1009) | |
|---|---|---|
| Female sex | 757 (75%) | 770 (76%) |
| White ethnic origin | 944 (94%) | 947 (94%) |
| Age group (years) | ||
| 50–74 | 177 (18%) | 188 (19%) |
| 75–79 | 160 (16%) | 177 (18%) |
| 80–84 | 231 (23%) | 228 (23%) |
| ≥85 | 439 (44%) | 416 (41%) |
| US patients | 609 (60%) | 613 (61%) |
| Nursing home residents before hospital admission | 104/1005 (10%) | 110/1008 (11%) |
| Cardiovascular disease | 637 (63%) | 631 (63%) |
| Coronary artery disease | 402 (40%) | 403 (40%) |
| Congestive heart failure | 184 (18%) | 167 (17%) |
| Cerebrovascular disease | 249 (25%) | 224 (22%) |
| Peripheral vascular disease | 117 (12%) | 102 (10%) |
| Cardiovascular risk factors | ||
| Treated hypertension | 824/1003 (82%) | 821/1005 (82%) |
| Treated diabetes | 252/1003 (25%) | 256/1005 (25%) |
| Treated hypercholesterolaemia | 347/1002 (35%) | 360/1001 (36%) |
| Creatinine concentration ≥ 2·0 mg/dL | 83/1001 (8%) | 86/1003 (9%) |
| Chronic lung disease | 189/1003 (19%) | 188/1007 (19%) |
| History of dementia | 309/1004 (31%) | 325/1008 (32%) |
| History of cancer | 181/1003 (18%) | 189/1008 (19%) |
| ASA score | ||
| 1 and 2 combined | 168/968 (17%) | 194/971 (20%) |
| 3 | 660/968 (68%) | 658/971 (68%) |
| 4 | 140/968 (14%) | 119/971 (12%) |
| Malnourished or cachectic | 36/1004 (3·6%) | 50/1008 (5%) |
| Body-mass index* | 24·3 (5·0) | 24·3 (5·9) |
| In-hospital aspirin use | 258/1005 (26%) | 254/1008 (25%) |
| In-hospital β-blocker use | 597/1005 (59%) | 6003/1008 (60%) |
| Transfusion units of red blood cells | ||
| Pre-randomisation | 452 | 531 |
| Post-randomisation† | 1866 | 652 |
We established long-term survival for 2002 (99%) of the overall study population, of whom 999 were randomly allocated to the liberal transfusion strategy and 1003 to the restrictive transfusion strategy. 841 (42%) of this study population died during the long-term follow-up period. Median follow-up was 3·1 years (IQR 2·4–4·1) overall, 3·6 years (2·7–4·4) for the USA, and 2·6 years (1·9–3·3) for Canada. In the American patients, 281 (46·4%) deaths occurred in the liberal treatment group and 271 (44·6%) deaths occurred in the restrictive group. In the Canadian patients, 151 (38·3%) deaths occurred in the liberal group and 138 (34·9%) in the restrictive group.
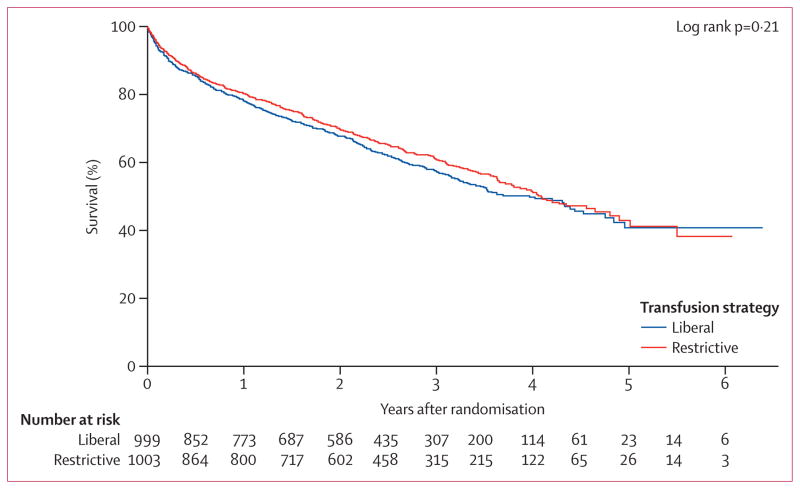
Long-term mortality did not differ significantly between the two treatment groups. 432 (43·2%) of 999 patients died in the liberal transfusion strategy group and 409 (40·8%) of 1003 died in the restrictive strategy group (hazard ratio 1·09 [95% CI 0·95–1·25], p=0·21 by the log-rank test; figure 2). The results were unchanged in the adjusted model that compared the liberal and restrictive transfusion strategies (hazard ratio 1·04 [95% CI 0·91–1·20], p=0·56). The hazard ratio of death between the two transfusion groups was consistent across all subgroups assessed (table 2).
Figure 2.
Long-term survival with liberal versus restrictive transfusion strategies
Table 2.
Subgroup analysis and interaction between transfusion groups and mortality
| Mortality for liberal vs restrictive strategy within the subgroup, hazard ratio (95% CI) | p value | |
|---|---|---|
| Overall | 1·09 (0·95–1·25) | 0·21 |
| Sex | 0·07 | |
| Male | 1·34 (1·04–1·73) | |
| Female | 1·01 (0·86–1·19) | |
| White race | 0·95 | |
| White | 1·09 (0·95–1·26) | |
| Not white | 1·06 (0·60–1·89) | |
| Age group | 0·14 | |
| Age 50–84 years | 1·19 (0·98–1·45) | |
| Age ≥85 years | 0·97 (0·81–1·17) | |
| Nursing home residence before hospital admission | 0·84 | |
| Nursing home residence | 1·05 (0·75–1·46) | |
| Non-nursing home residence | 1·11 (0·96–1·29) | |
| Cardiovascular disease | 0·59 | |
| Cardiovascular disease | 1·06 (0·91–1·24) | |
| No cardiovascular disease | 1·16 (0·89–1·50) | |
| Coronary artery disease | 0·06 | |
| Coronary artery disease | 1·26 (1·04–1·54) | |
| No coronary artery disease | 0·97 (0·80–1·17) | |
| Congestive heart failure | 0·27 | |
| Congestive heart failure | 0·93 (0·71–1·22) | |
| No congestive heart failure | 1·11 (0·95–1·30) | |
| Cerebrovascular disease | 0·40 | |
| Cerebrovascular disease | 1·19 (0·92–1·55) | |
| No cerebrovascular disease | 1·05 (0·89–1·23) | |
| Peripheral vascular disease | 0·74 | |
| Peripheral vascular disease | 1·01 (0·70–1·45) | |
| No peripheral vascular disease | 1·09 (0·94–1·26) | |
| Treated hypertension | 0·29 | |
| Treated hypertension | 1·06 (0·91–1·23) | |
| No hypertension | 1·28 (0·93–1·77) | |
| Treated diabetes | 0·48 | |
| Treated diabetes | 1·19 (0·91–1·56) | |
| No diabetes | 1·07 (0·91–1·25) | |
| Creatinine concentration ≥2·0 mg/dL | 0·33 | |
| Creatinine ≥2·0 mg/dL | 1·31 (0·90–1·92) | |
| No creatinine ≥2·0 mg/dL | 1·07 (0·93–1·24) | |
| Chronic lung disease | 0·20 | |
| Chronic lung disease | 0·92 (0·70–1·22) | |
| No chronic lung disease | 1·14 (0·98–1·33) | |
| History of dementia | 0·78 | |
| History of dementia | 1·13 (0·93–1·39) | |
| No history of dementia | 1·09 (0·91–1·31) | |
| History of cancer | 0·47 | |
| History of cancer | 0·98 (0·74–1·31) | |
| No history of cancer | 1·12 (0·96–1·31) | |
| ASA score | 0·98 | |
| 1 and 2 combined | 1·28 (0·83–1·97) | |
| 3 | 0·98 (0·83–1·16) | |
| 4 | 1·14 (0·85–1·54) | |
| General anaesthesia | 0·30 | |
| General anaesthesia | 1·16 (0·96–1·39) | |
| Other anaesthesia | 1·00 (0·82–1·23) | |
| Country | 0·68 | |
| USA | 1·07 (0·90–1·26) | |
| Canada | 1·13 (0·90–1·42) |
The cause of death was obtained for the 829 deaths identified from the registry searches (98·6% of the total 841 deaths). The most common cause of death was cardiovascular disease, which was the cause in about a third of patients who died (table 3). The cause of death did not differ between the transfusion strategies (p=0·99); the proportion of deaths from cardiovascular disease, cancer, and infections were nearly identical in the two groups of the trial.
Table 3.
Underlying primary cause of death overall and by transfusion strategy
| Total deaths (n=841) | Deaths in the liberal transfusion group (n=432) | Deaths in the restrictive transfusion group (n=409) | |
|---|---|---|---|
| Cardiovascular disease | 278 (33%) | 141 (33%) | 137 (34%) |
| Cancer | 103 (12%) | 54 (13%) | 49 (12%) |
| Infection | 78 (9%) | 41 (9%) | 37 (9%) |
| Stroke | 57 (7%) | 27 (6%) | 30 (7%) |
| Dementia | 108 (13%) | 56 (13%) | 52 (13%) |
| Pulmonary | 58 (7%) | 29 (7%) | 29 (7%) |
| Other | 147 (17%) | 79 (18%) | 68 (17%) |
| Unknown | 12 (1%) | 5 (1%) | 7 (2%) |
Discussion
We did a clinical trial comparing transfusion strategies in more than 2000 elderly patients with substantial comorbidity undergoing hip fracture surgery. Long-term mortality did not differ significantly between patients receiving transfusion to maintain haemoglobin concentration higher than 100 g/L (liberal transfusion strategy) versus those who received transfusion to maintain haemoglobin level higher than 80 g/L or because of anaemia symptoms (restrictive transfusion strategy). Furthermore, no evidence suggested that the transfusion strategy used affected the cause of death. We did not find that patients receiving liberal (more) transfusion had more deaths from infections or cancer, or fewer deaths from cardiac disease, than those who received restrictive transfusion. These results do not support hypotheses that transfusion is harmful and leads to long-term immunosuppression that is severe enough to affect mortality from infection or cancer, or that transfusion reduces cardiac complications by increasing oxygen supply to vulnerable myocardium and thus reduces deaths from cardiovascular disease.
Panel: Research in context.
Systematic review
We updated the systematic review done in 2011 for a Cochrane review to compare clinical outcomes in patients randomly assigned to restrictive versus liberal blood transfusion thresholds (triggers for transfusion).1 We further assessed any subsequently published trials by searching for clinical trials comparing transfusion thresholds in the Cochrane Central Register of Controlled Trials, Ovid Medline, and ISI Web of Science: Science Citation Index Expanded from 2010 to 2014. The original search strategy is detailed on page 68 of the Cochrane review.1 The primary outcome of interest in our searches was long-term mortality and cause of death.
Interpretation
In our searches, we identified a total of nine additional trials comparing transfusion thresholds but no other trials assessed mortality beyond 180 days. Most trials analysed mortality up to 30 days, and none assessed cause of death. In the original meta-analysis,1 restrictive transfusion strategies were associated with a statistically significant reduction in hospital mortality (risk ratio 0·77 [95% CI 0·62–0·95]) but not 30-day mortality (0·85 [0·70–1·03]). The FOCUS trial is the largest study done so far and showed no statistically significant difference in long-term mortality between the liberal transfusion strategy and the restrictive transfusion strategy, even with consideration of the underlying cause of death (cardiovascular disease, cancer, or infection). This study differs from the trials identified in our systematic review because it is the first to assess long-term mortality and cause of death. Our results suggest that liberal transfusion does not increase the risk of death over a median follow-up of 3·1 years and does not affect the cause of death.
Previous studies linking blood transfusion to increased infection and cancer recurrence have reported inconsistent findings. Many experimental studies show changes in immune function, including reduced production of cytokines, and reduced numbers of CD4 helper cells, natural killer cells, transforming growth factor beta, iron, and other factors.5,11,12 However, clinical studies have been inconsistent in linking transfusion with cancer recurrence.13–17 A meta-analysis of observational studies in colon cancer showed an association between transfusion and cancer recurrence and mortality.15 By contrast, two recent studies in prostate cancer showed no association between transfusion and outcome.16,17 The strongest evidence linking transfusion to infection comes from a meta-analysis of clinical trials comparing liberal versus restrictive transfusion that showed an increased risk of serious infections in the liberal transfusion group.18 We did not record an association with infection in the FOCUS trial. Why the meta-analysis showed an association between liberal transfusion and infection and this analysis did not is unclear. One possible explanation is that transfusion might increase the risk of infection but the infections are not sufficiently severe to cause death.
To our knowledge, no previous clinical trials of red blood cell transfusion have assessed long-term mortality or cause of death (panel). However, some observational studies have analysed long-term mortality in transfused patients compared with those not transfused.19–23 Similar to most observational studies analysing short-term outcomes, these studies consistently reported a raised risk of long-term mortality in the patients who had transfusions compared with those who did not. In many of these studies, investigators attempted to control for differences between patients transfused and those not transfused by using statistical methods such as propensity scores. However, such techniques might not be able to control for all the risk factors for mortality and morbidity. Thus, randomised clinical trials are the best way to provide an unbiased assessment of the effect of blood transfusion on clinical outcomes such as death.
Our randomised clinical trial has several strengths and some potential weaknesses. We enrolled more than 2000 vulnerable elderly patients with a high prevalence of comorbidities. In view of the high death rate in this population, if clinically meaningful adverse effects of blood transfusion on mortality are present, they should be demonstrable. Our trial methods minimised bias by using a central randomisation to protect concealment, masking for assessment of deaths and causes of death, and had almost complete follow-up. Compliance with the protocol was excellent.7 The mortality databases from both the USA and Canada have low error rates24,25 and we verified deaths using the social security database in the USA and hospital records in Canada. These results are likely to be generalisable to elderly patients with cardiovascular disease and risk factors undergoing surgery.
The liberal transfusion strategy received nearly three-times as many red blood cell transfusions post-randomisation as did the restrictive transfusion strategy. This large difference in blood use between the two groups of the trial is clinically significant and provides an excellent test of the hypothesis, although more than half of the patients in the restrictive transfusion strategy group received transfusions. We did not compare the outcomes of patients receiving transfusion versus those in patients not receiving transfusion or by number of units of blood (dose response) because this would break randomisation and might lead to a biased assessment of outcomes because of reverse causality from the most ill patients selectively receiving more blood.7
Although this trial included more than 2000 patients, we might have missed a small increase in long-term mortality from transfusion because the sample size was too small. The upper 95% CI of the unadjusted and adjusted Cox proportional hazards models suggests that we are 95% certain to have excluded a 20–25% or higher rate of death but we might have missed smaller effects. The classification of cause of death might have been incorrect in some cases even though we used a widely applied WHO algorithm based on death certificate data to establish the cause of death. However, modest misclassification would be unlikely to differ by treatment group or change the results in view of how similar the causes of death were in the two groups of the trial. It is also possible that median follow-up of 3 years is not sufficiently long to detect a latent effect of transfusion on risk of cancer and this adverse effect of transfusion might be detected in a younger or healthier population of patients who do not have as many competing causes of death as our elderly patients.
In summary, we recorded no evidence to suggest that a liberal transfusion strategy has a moderate adverse effect on long-term mortality or affects cause of death. Alternative pathophysiological mechanisms should be sought for the apparent increased risk of death in some populations of patients.
Supplementary Material
Acknowledgments
This trial was supported in part by grants from the National Heart, Lung, and Blood Institute (grants U01 HL073958 and U01 HL 074815).
Footnotes
See Online for appendix
Declaration of interests
JM has received grants from National Heart, Lung, and Blood Institute; personal fees from Ammonett, Novartis, Sanofi, Regeneron, GlaxoSmithKline, OrgaNext, and the American Orthopaedic Association; and grants and personal fees from Eli Lilly. The other authors declare no competing interests.
Contributors
JLC was the primary author and obtained funding for the project, did the literature search, designed the trial, and supervised data collection, data analysis, interpretation, and writing of the report. HN did the data analysis and writing of the report. DRH supervised the data analysis, interpretation, and writing of the report. JM and BRC helped with obtaining funding, study design, data analysis, interpretation, and writing. The remaining authors enrolled patients in the trial, and helped to interpret the results and write the report.
References
- 1.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–71. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 4.Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Refaai MA, Blumberg N. Transfusion immunomodulation from a clinical perspective: an update. Expert Rev Hematol. 2013;6:653–63. doi: 10.1586/17474086.2013.850026. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HA, Rao SV, Greenberg MD, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT Randomized Pilot Study) Am J Cardiol. 2011;108:1108–11. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson JL, Terrin ML, Magaziner J, et al. Transfusion trigger trial for functional outcomes in cardiovascular patients undergoing surgical hip fracture repair (FOCUS) Transfusion. 2006;46:2192–206. doi: 10.1111/j.1537-2995.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [accessed July 20, 2014];National Death Index. 2014 http://www.cdc.gov/nchs/ndi.htm.
- 10.WHO. Manual of the international statistical classification of diseases, injuries, and causes of death: based on the recommendations of the ninth Revision Conference, 1975, and adopted by the twenty-ninth World Health Assembly. Geneva: World Health Organization; 1977. 1975 revision. [Google Scholar]
- 11.Clark DA, Gorczynski RM, Blajchman MA. Transfusion-related immunomodulation due to peripheral blood dendritic cells expressing the CD200 tolerance signaling molecule and alloantigen. Transfusion. 2008;48:814–21. doi: 10.1111/j.1537-2995.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- 12.Hod EA, Spitalnik SL. Stored red blood cell transfusions: Iron, inflammation, immunity, and infection. Transfus Clin Biol. 2012;19:84–89. doi: 10.1016/j.tracli.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiss MM, Mempel W, Jauch KW, et al. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet. 1993;342:1328–33. doi: 10.1016/0140-6736(93)92247-q. [DOI] [PubMed] [Google Scholar]
- 14.Busch OR, Hop WC, Hoynck van Papendrecht MA, Marquet RL, Jeekel J. Blood transfusions and prognosis in colorectal cancer. N Engl J Med. 1993;328:1372–76. doi: 10.1056/NEJM199305133281902. [DOI] [PubMed] [Google Scholar]
- 15.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 16.Chalfin HJ, Frank SM, Feng Z, et al. Allogeneic versus autologous blood transfusion and survival after radical prostatectomy. Transfusion. 2014;54:2168–74. doi: 10.1111/trf.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeoh TY, Scavonetto F, Weingarten TN, et al. Perioperative allogeneic nonleukoreduced blood transfusion and prostate cancer outcomes after radical prostatectomy. Transfusion. 2014;54:2175–81. doi: 10.1111/trf.12595. [DOI] [PubMed] [Google Scholar]
- 18.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–26. doi: 10.1001/jama.2014.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shishehbor MH, Madhwal S, Rajagopal V, et al. Impact of blood transfusion on short- and long-term mortality in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2009;2:46–53. doi: 10.1016/j.jcin.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 20.van Straten AH, Bekker MW, Soliman Hamad MA, et al. Transfusion of red blood cells: the impact on short-term and long-term survival after coronary artery bypass grafting, a ten-year follow-up. Interact Cardiovasc Thorac Surg. 2010;10:37–42. doi: 10.1510/icvts.2009.214551. [DOI] [PubMed] [Google Scholar]
- 21.Bhaskar B, Dulhunty J, Mullany DV, Fraser JF. Impact of blood product transfusion on short and long-term survival after cardiac surgery: more evidence. Ann Thorac Surg. 2012;94:460–67. doi: 10.1016/j.athoracsur.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Engoren M. The effect of red blood cell transfusion on 90-day mortality in patients with acute lung injury. J Intensive Care Med. 2012;27:112–18. doi: 10.1177/0885066610394326. [DOI] [PubMed] [Google Scholar]
- 23.Shaw RE, Johnson CK, Ferrari G, et al. Blood transfusion in cardiac surgery does increase the risk of 5-year mortality: results from a contemporary series of 1714 propensity-matched patients. Transfusion. 2014;54:1106–13. doi: 10.1111/trf.12364. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–39. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 25.Statistics Canada. [accessed Nov 16, 2014];Vital statistics—death database. 2013 Sep 24; http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3233.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.