A MODEL TO PREDICT CARDIOVASCULAR EVENTS IN PATIENTS WITH NEWLY DIAGNOSED WEGENER’S GRANULOMATOSIS AND MICROSCOPIC POLYANGIITIS (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 22.
Published in final edited form as: Arthritis Care Res (Hoboken). 2011 Apr;63(4):588–596. doi: 10.1002/acr.20433
Abstract
Objectives
To create a prognostic tool to quantify the 5 year cardiovascular (CV) risk in patients with newly diagnosed Wegener’s granulomatosis (WG) and microscopic polyangiitis (MPA) without pre-morbid CV disease.
Methods
We reviewed CV outcomes during the long term follow up of patients in the first 4 European Vasculitis Study Group (EUVAS) trials of WG and MPA. CV events were defined as: CV-death, stroke, myocardial infarction, coronary artery bypass graft, or percutaneous coronary intervention. Logistic regression was performed to create a model to predict the absolute risk of a CV event. The model was tested using the Wegener’s Granulomatosis Etanercept Trial (WGET) cohort.
Results
74/535 (13.8%) of the patients with 5 years of follow up from the EUVAS trials had at least one CV event: 33/281 (11.7%) WG vs. 41/254 (16%) MPA. The independent determinants of CV outcomes were; older age [OR 1.45 (95%CI 1.11 – 1.90)]; diastolic hypertension [OR 1.97 (95%CI 0.98 – 3.95)], and positive PR3 ANCA status [OR 0.39 (95%CI 0.20 – 0.74)]. The model was validated using the WGET cohort (Area under ROC curve = 0.80).
Conclusion
Within 5 years of diagnosis of WG or MPA, 14% of patients will have a cardiovascular event. We have constructed and validated a tool to quantify the risk of a cardiovascular event based on age, diastolic hypertension and PR3 ANCA status in patients without prior CV disease. In patients with vasculitis, PR3 ANCA is associated with reduced cardiovascular risk compared to MPO ANCA or negative ANCA status.
Keywords: Vasculitis, Cardiovascular, Wegener’s granulomatosis, microscopic polyangiitis, ANCA, myocardial infarction, stroke
Introduction
Wegener’s granulomatosis (WG) and microscopic polyangiitis (MPA) are the two most common types of small and medium vessel vasculitis with an estimated combined prevalence of 49–254 per million in Europe and North America.[1] Untreated these diseases are fatal, but modern therapy has dramatically improved survival.[2–8] However, patients continue to experience long-term morbidity and mortality from persistent low grade activity and permanent damage caused by the acute phase of vasculitis or its treatment.[9] An important component of this increased long-term morbidity and mortality is from cardiovascular (CV) disease which was highlighted in a retrospective review of the Danish National Hospital Register, where patients with WG showed an increased rate of a myocardial infarction within the 1st 5 years after a diagnosis of WG compared to the general population (hazard ratio 3.6).[10] In addition, a retrospective study showed that patients with WG and MPA when matched for renal function and other traditional risk factors, had double the rate of CV events.[11] Theories for the increased CV events in vasculitis include systemic inflammation and endothelial dysfunction,[12, 13] factors associated with increased cardiovascular risk in other inflammatory diseases.[14–16]
In 1995 the European Vasculitis Study Group (EUVAS) launched randomised controlled trials for the treatment of WG and MPA with unified trial protocols and data collection procedures; 4 of these trials have been published.[4, 6–8, 17] Patient enrolment in the 4 trials was stratified by the degree of renal involvement. Subsequent to the completion of these trials, EUVAS has performed a long-term follow-up study on patients enrolled in these original trials. Together, this cohort represents the largest ever prospectively studied group of patients with WG and MPA.
The aims of this study were to 1) review the cardiovascular events in the first 5 years from the long-term follow-up of the 4 EUVAS trials; 2) use this data to create a prognostic tool aimed at predicting the 5 year risk of cardiovascular events in patients with newly diagnosed WG and MPA who have no cardiovascular disease at diagnosis; 3) validate the tool in a second separate cohort of vasculitis patients.
Patients and Methods
The patients and methods of the first 4 EUVAS randomised controlled trials included in the long-term follow-up (LTFU) study have previously been described.[4, 6–8, 17] In summary, all patients had a new diagnosis of WG, MPA or renal limited vasculitis (RLV). Patients were stratified into the 4 studies by the severity of renal involvement, listed here in order: 1) MEPEX: Comparison of Plasma Exchange to Methyl prednisone in patients with severe renal disease (Cr >500mmol/L or requiring dialysis).[7] 2) CYCAZAREM: Maintenance therapy with azathioprine vs. cyclophosphamide in patients with a renal manifestation (Cr<500mmol/L) or with generalised life threatening disease.[4] 3) CYCLOPS: Induction therapy comparing daily oral cyclophosphamide with pulsed intravenous cyclophosphamide in patients with generalised disease (Creatinine <500mmol/L).[8] 4) NORAM: A comparison of methotrexate to cyclophosphamide for induction treatment in patients with a Cr<150mmol/L and without critical organ threatening disease.[6] The study methods, data collection procedures and disease scoring were consistent between the 4 trials.
For the validation set we used anonymised data from patients enrolled in the Wegener’s granulomatosis etanercept trial (WGET): 180 patients with active Wegener’s granulomatosis who were randomized to receive etanercept or placebo in addition to standard maintenance therapy.[18] Long-term follow-up of this cohort is now available including cardiovascular events. Data from 136 patients without pre-existing CV disease was available for testing our regression model. 16/136 patients in the WGET cohort had a CV event: 14 with a non fatal cardiac event (myocardial infarction, coronary artery bypass graft, or percutaneous coronary intervention), 1 non fatal stroke and 1 CV death. Baseline demographics for the EUVAS and WGET cohorts are shown in table 1.
Table 1.
Baseline demographics in the EUVAS long-term follow-up and WGET cohorts
| EUVAS LTFU (n=535) | WGET (n=180) | P* | |
|---|---|---|---|
| Age in years, mean (SD) | 58 (14) | 47 (16) | <0.001 |
| Male gender, n (%) | 288 (54) | 108 (60) | 0.17 |
| Wegener’s granulomatosis, n (%) | 281 (53) | 180 (100) | <0.001 |
| Microscopic polyangiitis, n (%) | 254 (47) | 0 (0) | <0.001 |
| New diagnosis of vasculitis, n (%) | 535 (100) | 80 (44) | <0.001 |
| Existing diagnoses of vasculitis, n (%) | 0 (0) | 100 (56) | <0.001 |
| Serum creatinine μmol/L, mean(SD) | 341 (321) | 153 (177) | <0.001 |
| BVAS v2 (new/worse) score, mean (SD) | 16.9 (9.2) | Not available | - |
| BVAS/WG score, mean (SD) | Not available | 6.9 (3.4) | - |
| MPO ANCA positive, n(%) | 190 (35.5) | 21 (12) | <0.001 |
| PR3 ANCA positive, n (%) | 302 (56.5) | 131 (73) | <0.001 |
| ESR, mean (SD) | 76 (36) | Not available | - |
| CRP, mean (SD) | 88 (139) | Not available | - |
Ethics approval was obtained for each participating site as per local requirements. A questionnaire on vital status and CV events was completed on all eligible patients by clinical investigators at each participating site. Information was collected at 5 years from entry into the original study, and also at the last available follow-up. The databases containing baseline information for the 4 trials were merged with the long-term follow-up results. All patients with an entry diagnosis of RLV were considered to have MPA for this analysis.
The primary outcome measure of interest in this sub-analysis was a CV event within 5 years after enrolment into the original EUVAS trial. We defined a CV event as death from any CV cause, non-fatal stroke, non-fatal myocardial infarction (MI), and coronary artery bypasses graft (CABG) or percutaneous coronary intervention (PCI). The identification of cardiovascular death was based on the local investigator reporting death and cause of death. The cause of death was adjudicated by an independent panel. The observed CV death rate in the EUVAS trials was compared to the predicted CV death rate (adjusted for age and country of origin). The relevant European and Mexican CV death rates for the year 2002 based on the World Health Organisation Statistical Information System (WHOSIS) database (http://www.who.int/whosis/en/) are provided in Appendix A.
Determination of non fatal MI required evidence of ECG changes or cardiac enzyme elevation. Non fatal stroke was defined as focal neurological deficit present for at least 3 months (i.e. transient ischemic events were not included). All items recorded at baseline from entry into each trial such as patient demographics, clinical features, blood results, disease activity scores (Birmingham Vasculitis Activity Score (BVAS 1&2))[19] and disease damage scores (Vasculitis Damage Index (VDI))[20] including individual components of these instruments were available as predictor variables. Hypertension in the development cohort (EUVAS trials) was defined as a diastolic blood pressure > 95 mmHg at the time of entry into the trial which was attributable to active vasculitis (irrespective of whether the patient had a previous history of hypertension or were on antihypertensive treatment). This definition of hypertension was chosen because of how the data was captured in the original trials (based on a BVAS item). For the validation sample (WGET cohort), hypertension was defined as a diastolic blood pressure >95mmHg at trial entry irrespective of previous history of hypertension or antihypertensive therapy. Attribution of the diastolic hypertension to vasculitis in the WGET cohort was not included in the definition because it was not captured in the original trial.
A points based Cox Framingham cardiovascular risk prediction tool,[21] which uses body mass index instead of total and HDL cholesterol was used to test how well a general population risk model performed in patients with vasculitis. We simplified Systolic Blood Pressure to < 140 mmHg (0 points on the scoring system) versus ≥140 mmHg (3 points women, 2 points men) because of the way in which hypertension was recorded in the dataset.
We used two methods to validate our new regression model: 1) Bootstrapping - an internal validation technique used to obtain a bias-corrected estimate in the development sample.[22]; and 2) Testing the model in the validation cohort using receiver operator characteristics.
Data analysis
Data was analysed using the statistical software package Stata, release 10.1 (College Station, Texas, USA). Univariate logistic regression modelling was used to examine the association between baseline variables and cardiovascular events. Fractional polynomial regression modelling was used to model non-linear relationships for continuous variables. A multivariate logistic regression model was then fitted including all predictor variables, regardless whether or not they were statistically associated with a CV event in univariate analysis. Having fitted the full multivariate model, a backwards selection process was used to exclude variables that did not improve model fit. Likelihood ratio tests were used to compare model fit. The results of complete case analyses can be biased because the cumulative effect of missing data in several variables often leads to exclusion of a substantial proportion of the original sample, causing a loss of precision and power. [23] This bias can be overcome by using multiple imputation methods which we did using the ICE procedure in Stata.[23–26] For logistic regression, 10 times as many outcome events as predictor variables are required to avoid model over fit. Using clinical judgment, we initially created a model using traditional risk factors (age, gender, body mass index, smoking status, hypertension and diabetes), and then a model with only disease specific items (Birmingham Vasculitis Activity Score (BVAS), proteinase 3 (PR3) and myeloperoxidase (MPO) anti neutrophil cytoplasm antibody (ANCA) status, baseline estimated glomerular filtration rate (eGFR), age and gender). Regression diagnostics were performed to identify outlying data that may overly influence the model. Cholesterol measurements were not recorded at baseline therefore could not be included in the traditional risk factor model.
Performance of the predictive model was assessed in terms of calibration and discrimination. Calibration measures how closely predicted risk agrees with observed risk. This was assessed for each tenth of predicted risk ensuring 10 equally sized groups, and a Hosmer-Lemeshow goodness of fit test performed.[27] Discrimination is the ability of the model to differentiate between patients who experienced a CV event during the 5 years of follow-up in this study and those who did not. We tested discrimination by calculating the area under the receiver operating characteristic (AUROC) curve in a) the original dataset, b) by bootstrapping and c) testing in the WGET cohort.[18] To assess the goodness of fit of a logistic model we used McKelvey-Zavoina pseudo R2.[28, 29]
In addition, CART (Classification and Regression Tree) analysis, a binary recursive partitioning method was performed in the R statistical software package (R Foundation, Vienna, Austria), using the ‘party’ package. In each node of the tree a significance test was made between any of the covariates and the response, and a split established when the p-value was < 0.05.
Results
A total of 535 patients (281 WG, 254 MPA) including patients who died, had 5 years of follow-up; 74/535 (13.8%) of the patients had at least one CV event; 33/281 (11.7%) WG vs. 41/254 (16.1%) MPA. There were 32 (6.0%) CV deaths, 25 (4.7%) non fatal strokes and 42 (7.9%) had a non fatal myocardial infarction, coronary artery bypass graft or a percutaneous coronary intervention. The observed age standardised CV death rate for the EUVAS cohort was 699 compared to the predicted rate of 190 per 100,000 population per annum.
When developing the predictive model, 31 patients were excluded due to missing baseline values; 32 due to missing outcome records, and 45 due to CV disease prior to trial entry. The remaining 427 patients, 50 with a CV event (19/237 WG vs. 31/190 MPA, P<0.01) were used to develop the model. A summary of the baseline variables analysed for the 427 patients is provided in table 2. Smoking status was available in 292/427 patients in the study: 7.3% were current smokers, 23.7% ex-smokers and 37.5% had never smoked. For analysis we combined the current and ex-smokers as an ever smoked group. Comparing those that had never smoked to ever smoked gave an OR 0.61 (95%CI 0.32 – 1.18), P = 0.14, for any CV event within 5 years. Re-analysing the data comparing never smoked to current smokers gave OR 0.90 (95%CI 0.24 – 3.36), P = 0.87.
Table 2.
Baseline variables for patients included in the development of the cardiovascular risk prediction model. (N=427)
| Total number of patients | Number of patients with at least one cardiovascular event (%) | Multiple Imputation [n=427]Crude OR (95%CI) | P-value | |
|---|---|---|---|---|
| Trial name: | ||||
| CYCAZAREM | 131 | 14 (10.7%) | 1.00 | |
| CYCLOPS | 107 | 14 (13.1%) | 1.26 (0.57 – 2.77) | 0.57 |
| MEPEX | 99 | 18 (18.2%) | 1.86 (0.87 – 3.95) | 0.11 |
| NORAM | 90 | 4 (4.4%) | 0.39 (0.12 – 1.22) | 0.11 |
| Diagnosis: | ||||
| MPA | 190 | 31 (16.3%) | 1.00 | |
| WG | 237 | 19 (8.0%) | 0.45 (0.24 – 0.82) | 0.009 |
| ANCA PR3: | ||||
| No | 168 | 33 (19.6%) | 1.00 | |
| Yes | 257 | 17 (6.6%) | 0.29 (0.16 – 0.54) | <0.001 |
| ANCA MPO: | ||||
| No | 279 | 22 (7.9%) | 1.00 | |
| Yes | 146 | 28 (19.2%) | 2.78 (1.52 – 5.06) | 0.001 |
| Age*: | 1.58 (1.22 – 2.06) | 0.001 | ||
| Sex: | ||||
| Female | 207 | 23 (11.1%) | 1.00 | |
| Male | 220 | 27 (12.3%) | 1.12 (0.62 – 2.02) | 0.71 |
| BMI: | 1.03 (0.93 – 1.14) | 0.57 | ||
| Ever smoked: | ||||
| No | 160 | 15 (9.4%) | 0.61 (0.32 – 1.18) | 0.14 |
| Yes | 132 | 20 (15.2%) | 1.00 | |
| Diabetes: | ||||
| No | 339 | 40 (11.8%) | 1.00 | |
| Yes | 14 | 3 (21.4%) | 2.06 (0.57 – 7.40) | 0.27 |
| New hypertension: | ||||
| No | 350 | 33 (9.4%) | 1.00 | |
| Yes | 72 | 16 (22.2%) | 2.74 (1.41 – 5.30) | 0.003 |
| Haemoglobin: | 426 | 0.86 (0.75 – 1.00) | 0.051 | |
| log WBC*: | 427 | 1.20 (0.56 – 2.59) | 0.63 | |
| Platelets**: | 427 | 0.97 (0.95 – 0.99) | 0.011 | |
| eGFR* (MDRD): | 426 | 0.83 (0.74 – 0.93) | 0.001 | |
| BVAS score: | 422 | 1.02 (0.99 – 1.05) | 0.23 | |
| VDI 6-month score: | 238 | 1.38 (1.09 – 1.74) | 0.007 |
All of the traditional and disease-specific risk factors were considered for inclusion in the final model. To avoid over-fitting we considered the effect of each predictor independently, and assessed the effect of confounding for that predictor with each risk factor. Variables that were significantly associated with cardiovascular events in univariate analysis, or were important confounders were included in the full multivariable regression model. Older age was associated with an increased risk of a CV event, however the effect was slightly attenuated due to confounding by eGFR. Crude analyses suggested that increasing eGFR was associated with reduced CV risk, but this was attenuated after adjustment for age and PR3 ANCA. As MPO ANCA and PR3 ANCA were strongly inversely associated with each other, only PR3 ANCA was considered for inclusion in the full model because it was a statistically stronger predictor. PR3 ANCA was associated with reduced CV risk and MPO with an increased CV risk, in both cases this was attenuated slightly after adjusting for age, eGFR and diastolic hypertension. Patients with diastolic hypertension were at increased risk of CV events. This effect was attenuated after adjusting for age, eGFR and PR3 ANCA, but strengthened if adjusting for gender and smoking status. Gender, BMI, smoking status, baseline BVAS score and diabetes were not associated with cardiovascular events.
Age, gender, eGFR, PR3 ANCA status, smoking status, and diastolic hypertension at baseline, were included in creating the full multivariable regression model. After backward selection: age, PR3 ANCA status and diastolic hypertension remained in the final model. The final model is shown in Figure 1. A cut off of <10% as low risk, 10–20% as moderate risk and >20% as high risk were chosen to provide memorable cut-offs. In the EUVAS cohort: 245 (57.4%) of patients fell into the low risk, 106 (24.8%) into the moderate risk, and 76 (17.8%) into the high risk group using our new 5 year risk prediction model. Observed CV events were: 15/245 (6.1%) for the low risk group, 14/106 (13.2%) for the medium risk group and 21/76 (27.6%) for the high risk group.
Figure 1.
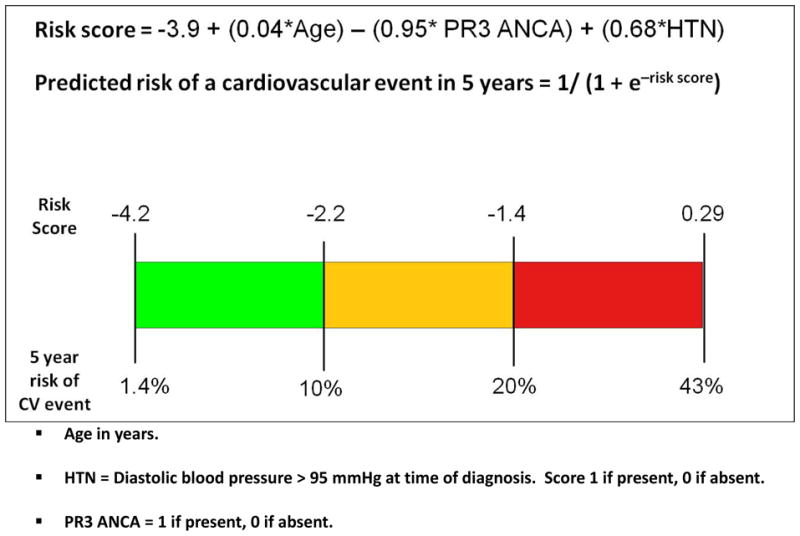
Final model to predict the risk of a cardiovascular event in the first 5 years from diagnosis with microscopic polyangiitis or Wegener’s granulomatosis
Five year CV risk is shown as: green = low risk (<10%), orange = moderate risk (10–20%), Red = high risk (>20%).
The Hosmer-Lemeshow goodness of fit test suggested that the model was well calibrated (P = 0.55). The model demonstrated good discrimination with an AUROC of 0.73 for the original dataset; bootstrapped analysis gave a bias-corrected AUROC of 0.72; and validation with the WGET cohort demonstrated very good discrimination with an area under the ROC of 0.80; Figure 2. The pseudo R2 (McKelvey-Zavonia) for the final model was 18.5%. The model was better at predicting coronary events than strokes; AUROC 0.85 (95%CI 0.77 – 0.92) with a pseudo R2 of 49.7% vs. AUROC 0.70 (95%CI 0.60 – 0.80) and a pseudo R2 of 19.6% respectively for the EUVAS cohort. In comparison, the AUROC for any cardiac event using the points based Cox Framingham model was 0.65 (95%CI 0.57 – 0.72), Figure 2.
Figure 2.
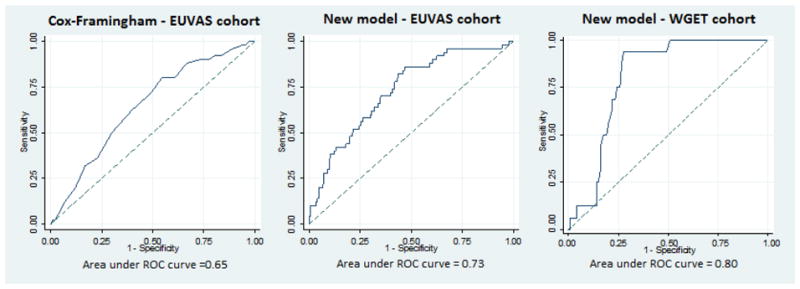
Receiver operating characteristic curves comparing our new disease specific model with the traditional points based Cox-Framingham model
Figure 3 shows the conditional inference trees we created using CART analysis to predict CV events. In this tree, PR3 ANCA status was the most discriminative starting point. Thereafter, age, eGFR, and BVAS items were important predictors of the risk of a CV event. Using this inference tree, a patient that was PR3 ANCA positive, had eGFR > 32.3, and no items present on the Cardiovascular section of the BVAS, had the lowest risk (1.4%) of a CV event in the following 5 years. In the EUVAS cohort, 146 individuals fitted these criteria and 2 of them (1.4%) had a CV event. Conversely, 12 of 47 (44.4%) of patients that were PR3 ANCA negative and older than 72.4 years had a CV event.
Figure 3.
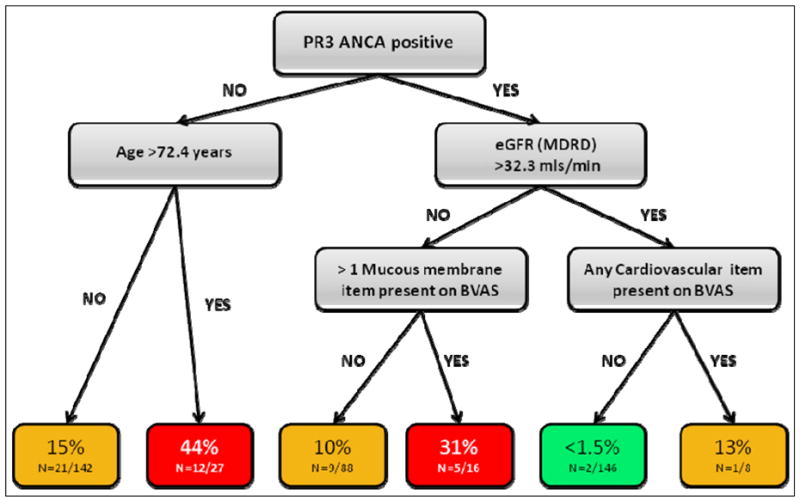
Conditional inference tree showing approximate risk of a cardiovascular event within 5 years based on baseline clinical features
% shown is the risk of a cardiovascular event within 5 years of diagnosis of Wegener’s granulomatosis or microscopic polyangiitis.
N=Cardiovascular events/number of individuals in Group
Discussion
Within 5 years of diagnosis of WG or MPA, approximately 14% of patients will have a major cardiovascular event. The age standardised annual CV mortality rate of 699 per 100,000 in this cohort is 3.7 (95% CI 3.2–4.3) times higher than we would expect in the background population. The result of our study adds further weight to the growing body of evidence that individuals with a diagnosis of WG or MPA are at significantly increased risk of cardiovascular morbidity and mortality.[10, 11]
There are many potential reasons for the increased CV risk. Endothelial dysfunction which is a recognised risk factor for CV disease[16, 30–32] has been shown to be present in ANCA associated vasculitis and is independent of disease activity or renal involvement.[33] Renal dysfunction, which occurs frequently in patients with MPA and WG, is an established contributor to cardiovascular disease by affecting metabolic, inflammatory and hemodynamic pathways.[34] In addition, vasculitis represents a chronic inflammatory state, and other inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosis have strong associations with CV disease.[35, 36] Animal models suggest that vessels following arteritis are more prone to atherosclerotic change.[37] Further, corticosteroids, which are a routine part of treatment for vasculitis, present an interesting conflict: they increase cardiovascular risk by accelerating the development of diabetes,[38] dyslipidemia[39] and hypertension,[40] but may also have a protective role in vasculitis by reducing systemic inflammation and improving endothelial dysfunction.[13, 15, 41]
We have developed 2 complementary models (logistic regression and CART) to predict the 5-year cardiovascular risk at the time of first presentation with WG and MPA. The regression model was validated in the WGET cohort and performed better than existing generic risk tools available for the general population. Both models indentified a positive PR3 ANCA status as an important determinant of lower risk among those with vasculitis. However, almost all patients in the EUVAS trials were either MPO or PR3 ANCA positive implying that those with a positive MPO ANCA are associated with an increased risk of CV disease. Interestingly, neither model identified gender as a predictor of risk, suggesting that vasculitis may remove the CV risk benefit usually observed in women. A potential explanation for this is that a large proportion of the women enrolled in the EUVAS trials were in the peri- or post-menopausal age group (mean age was 58, SD 14, i.e. 84% were over the age of 46 at baseline) and others may have been pushed into premature menopause by the use of cyclophosphamide. In the regression model, a positive PR3 ANCA status decreased the risk of a CV event, older age and the presence of diastolic hypertension at time of enrolment into the study increased the risk. The discrimination of this model compares very favourably with the QRISK score (area under ROC curve of 0.76 and 0.79 for males and females respectively) and the 1991 Framingham equation (area under ROC curve 0.74 for male and 0.76 for females).[42–44] Our second model used CART analysis to create an inference tree to determine the cardiovascular risk which provides insight into possible interactions between variables. For example, the inference tree shows that patients who are PR3 ANCA positive and have good renal function are generally at low risk for cardiovascular events, however, if these patients have any cardiovascular system involvement at baseline then their risk of a major cardiovascular event is 9-fold higher.
The main reason for developing risk algorithms is not just prognostication, but prognostication that allows clinicians to develop and test preventative strategies. For vasculitis we do not yet know whether treating traditional risk factors such as smoking and dyslipidemia would change the cardiovascular outcome or whether it is more important to treat the inflammation and renal disease. Our model could be used to do the power calculation to determine how many patients would be needed in an interventional study to detect a 20% reduction in CV events. For example, you would need X high risk patients vs. Y low risk patients or Z all patients.
There are limitations to our study. Lipid results, glycosylated haemoglobin levels and specific cardiovascular medications associated with prevention of CV disease (e.g. aspirin, statins, ACE inhibitors) were absent from the baseline dataset which may have omitted important predictor variables. The original EUVAS trials were not designed to evaluate cardiovascular risk and therefore a study designed to look specifically at this issue may have resulted in an even stronger model. This is something to aspire to for future studies. However, ours is the only disease specific model available for vasculitis currently. When interpreting our statistical model it is important to remember that we are trying to predict a specific outcome and the key objective is to predict the risk accurately. There are potentially a large number of variables that have overlapping contributions to the cardiovascular risk but if a few variables are able to predict the risk with the same accuracy, then whether or not we include the other variables in the equation should not matter. For example ‘hypertension’ in our model would very likely have an overlapping contribution to cardiovascular risk with renal impairment. The patients included in our development dataset comprised a larger proportion of patients with mild WG (NORAM trial), and more patients with severe MPA (MEPEX trial), therefore patient selection bias may have unduly influenced the CV outcomes despite our best efforts to correct for renal function and disease severity in our statistical modelling. In addition, there are some major differences between the development and validation groups; all patients had newly diagnosed WG or MPA in the EUVAS trials whereas the WGET cohort only included WG but comprised patients with new and existing disease. We acknowledge that a large mixed cohort with both WG and MPA patients would have been the best cohort for testing and validation. However, there are currently no other suitable cohorts available (i.e. with sufficient number of patients, long enough duration of follow-up and with accurate recording of cardiovascular events). Therefore we have used the best alternative possible. There was only 1 stroke in the WGET cohort so validation of this outcome needs to be interpreted with caution. Despite these potential biases and cohort differences our model still works very well in both groups, confirming the strength of our model and reinforcing that age, diastolic hypertension and PR3 ANCA status are strong predictors of cardiovascular outcomes in patients with ANCA associated vasculitis. A further consideration is that renal function my change dramatically from baseline to later in the disease, therefore its use as a predictive variable may depend on when it is measured. The effect of disease flares, cumulative dose of steroids and changes in renal function will need to be taken into account when evaluating cardiovascular risk at different time points in the disease course.
In conclusion, we have shown that the risk of a cardiovascular event in the first 5 years after the diagnosis of WG or MPA is raised. To quantify this risk for an individual patient we have created and validated a statistical model using baseline clinical features. Identifying those at highest risk may help target those who require closer monitoring and further intervention.
Table 3.
Predictor variables included in developing the final regression model.
| Complete case | Multiple Imputation [n=427] | Multiple Imputation [n=427] | Multiple Imputation [n=427] | ||
|---|---|---|---|---|---|
| UnivariableOR (95%CI) | UnivariableOR (95%CI) | Pseudo R2 | MultivariableOR (95%CI)R2 = 22.1% | Reduced modelOR (95%CI)R2 = 18.5% | |
| Age [n=427]* | 1.58 (1.22, 2.06) | 1.58 (1.22, 2.06) | 11.7% | 1.35 (1.02, 1.79) | 1.45 (1.11, 1.90) |
| Gender [n=427]: | |||||
| Female | 1.00 | 1.00 | 1.00 | - | |
| Male | 1.12 (0.62, 2.02) | 1.12 (0.62, 2.02) | 0.1% | 1.10 (0.56, 2.15) | - |
| BMI [n=196] | 1.03 (0.93, 1.14) | 1.03 (0.93, 1.14) | 1.3% | - | - |
| BVAS1 [n=422] | 1.02 (0.99, 1.05) | 1.02 (0.99, 1.05) | 1.0% | - | - |
| eGFR [n=426]* | 0.83 (0.74, 0.93) | 0.83 (0.74, 0.93) | 11.3% | 0.93 (0.82, 1.06) | - |
| ANCA MPO [n=425] | |||||
| No | 1.00 | 1.00 | - | - | |
| Yes | 2.77 (1.52, 5.05) | 2.78 (1.52, 5.06) | 6.7% | - | - |
| ANCA PR3 [n=425] | |||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | |
| Yes | 0.29 (0.16, 0.54) | 0.29 (0.16, 0.54) | 10.0% | 0.41 (0.21, 0.80) | 0.39 (0.20, 0.74) |
| Ever smoked [n=427]: | |||||
| Yes | 1.00 | 1.00 | 1.00 | - | |
| No | 0.58 (0.28, 1.18) | 0.61 (0.32, 1.18) | 2.3% | 0.62 (0.29, 1.31) | - |
| New Hypertension [n=422]: | |||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | |
| Yes | 2.74 (1.42, 5.32) | 2.74 (1.41, 5.30) | 4.0% | 1.92 (0.93, 3.95) | 1.97 (0.98, 3.95) |
| Previous diabetes [n=353]: | |||||
| No | 1.00 | 1.00 | - | - | |
| Yes | 2.04 (0.55, 7.62) | 2.06 (0.57, 7.40) | 0.9% | - | - |
Acknowledgments
Funding
The long term follow up study of the EUVAS trials was funded by a project grant from the European League Against Rheumatism (EULAR). RS was supported by the Rose Hellaby Medical Scholarship, New Zealand. AJ, RB, MKJ and RL were supported by Oxford NIHR BRU Musculoskeletal Research Group, University of Oxford. DJ was supported by the Cambridge Biomedical Research Centre. The WGET was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Research Resources, and the Office of Rare Diseases Research 1 U54-RR01949, U54-AR057319, and U01 AR1874, NIH N01-AR92240 and the Office of Orphan Products, FDA (grant FD-R-001652), General Clinical Research Center Grants M01-RRO-00533 (Boston University), M01-RRO-0042 (The University of Michigan), MO1-RR-30 (Duke University), and M01-RRO-2719 (Johns Hopkins University School of Medicine), from the National Center for Research Resources/NIH. Drs. Merkel, Stone, and St. Clair were supported by NIAMS grants K24 AR049185-01, K24 AR2224-01A1, and K24 AR02126-04.
APPENDIX A. A comparison of this EUVAS cohort’s age standardised cardiovascular mortality rate with selected European countries and Mexico
| Country | Age-standardized mortality rate for cardiovascular diseases (per 100 000 population)* | Country of origin of patients in EUVAS cohort as percentage of whole group (n=535) |
|---|---|---|
| Belgium | 162 | 3.9 |
| Czech Republic | 315 | 7.5 |
| Denmark | 182 | 1.7 |
| Finland | 201 | 2.6 |
| France | 118 | 4.7 |
| Germany | 211 | 10.7 |
| Ireland | 214 | 3.4 |
| Italy | 174 | 7.7 |
| Lithuania | 391 | 1.1 |
| Mexico | 163 | 0.9 |
| Netherlands | 171 | 8.2 |
| Spain | 137 | 6.2 |
| Sweden | 176 | 9.7 |
| United Kingdom | 182 | 31.6 |
| Predicted CV mortality rate for EUVAS cohort adjusted for country of origin | 190 | |
| Observed CV mortality rate for EUVAS cohort | 699 |
APPENDIX B. The physicians involved in the EUVAS trials
Abramowicz D, Abuzakouk M, Adu D, Andrassy K, Bacon, P, Ballarin J, Bataille P, Blockmans D, Bruchfeld A, Burns A, Calero F, Chabova V, Chizzolini C, Cohen Tervaert JW, Confalonieri R, Dadoniene J, de Groot K, de Lind van Wijngaarden R, de Souza R, Dhaene M, Ekstrand A, Eriksson P, Esnault VL, Feehally J, Feighery C, Ferrario F, Flores Suarez L, Garibotto G, Gaskin G, Germanis G, Gibson J, Gregorini G, Grönhagen C, Gross WL, Guillevin L, Hagen EC, Harper L, Haubitz M, Heigl Z, Heimburger M, Hergesell O, Jayne DR, Kallenberg CGM, Kieley P, Leblau J, Lesavre P, Lundberg I, Luqmani R, Madhoun P, Mason P, Mathieson P, Mirapeix E, Natusch A, Nowack R, Olveira D, Oudejans I, Pettersson E, Poisetti G, Poveda R, Puechal X, Pusey C, Rasmussen N, Reinhold-Keller E, Riska, Rupprecht H, Rychlik I, Santostefano M, Savage C, Schmitt W, Schneider M, Segelmark M, Selga D, Sennesael J, Serra A, Siegert C, Sinico A, Specker C, Stahl-Hallengren C, Stegeman CM, Sterner G, Stevens J, Stolear J, Svenungsson E, Tesar V, Theander E, Tidman M, Tomson C, Turner N, Valles M, Valles M, van der Woude F, van Gurp E, Vanhille P, Verburgh C, Vischedyck M, Watts R, Weber P, Weidner S, Westman K, Wiik A, Williams A, Wissing M.
Appendix C. The WGET research group
WGET Chairman: Stone J (The Johns Hopkins Vasculitis Center), WGET Co-Chairman: Hoffman G (The Cleveland Clinic Foundation Center for Vasculitis Research and Care). The Johns Hopkins University Center for Clinical Trials (co-ordinating center): Holbrook JT, Meiner CL, Doge J, Donithan J, Min N, Murrow L, Smith J, Tibbs A, Van Natta M. The Beth Israel Medical Center, New York: Spiera R, Berman R, Enuha S. Boston University: Merkel PA, Gelbard R, Nuite M, Schiller A. The Cleveland Clinic Foundation: Hoffman G, Blumenthal D, Bork D, Clark T, Crook S, Calabrese L, Farkas S, Sridharan S, Strom K, Wilke. Duke University: St. Clair EW, Allen N, Rodin K, Scarlett E. Johns Hopkins University: Stone J, Hellman D, Moore A, Pinachos L, Regan M, Uhlfelder M. The Mayo Clinic: Specks U, Bradt K, Carlson K, Fisher S, Hammel B, Mieras K, Ytterberg S. University of California, San Francisco: Davis JC, Fitzpatrick M, Fye K, Lund S. University of Michigan: McCune J, Coomer B, Gilson B, Haftel H, Morrel-Samuels A, Neckel S. The Johns Hopkins University Immune Diseases Laboratory: Rose N, Burek CL, Barin J, Talor M. Data and Safety Monitoring Board: Canner P (Maryland Medical Research Institute), Conn DL (Emory University), Klippel JH (Arthritis Foundation), Landis JR (University of Pennsylvania).
Footnotes
Competing interests: none
References
- 1.Mahr AD. Epidemiological features of Wegener’s granulomatosis and microscopic polyangiitis: two diseases or one ‘anti-neutrophil cytoplasm antibodies-associated vasculitis’ entity? APMIS Suppl. 2009:41–7. doi: 10.1111/j.1600-0463.2009.02476.x. [DOI] [PubMed] [Google Scholar]
- 2.Godman GC, Churg J. Wegener’s granulomatosis: pathology and review of the literature. AMA Arch Pathol. 1954;58:533–53. [PubMed] [Google Scholar]
- 3.Hollander D, Manning RT. The use of alkylating agents in the treatment of Wegener’s granulomatosis. Ann Intern Med. 1967;67:393–8. doi: 10.7326/0003-4819-67-2-393. [DOI] [PubMed] [Google Scholar]
- 4.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 5.Langford CA, Talar-Williams C, Barron KS, Sneller MC. Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener’s granulomatosis: extended follow-up and rate of relapse. Am J Med. 2003;114:463–9. doi: 10.1016/s0002-9343(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 6.De Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 7.Jayne DR, Gaskin G, Rasmussen N, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–8. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 8.de Groot K, Harper L, Jayne DR, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 9.Seo P, Min YI, Holbrook JT, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET) Arthritis Rheum. 2005;52:2168–78. doi: 10.1002/art.21117. [DOI] [PubMed] [Google Scholar]
- 10.Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum. 2009;60:1187–1192. doi: 10.1002/art.24386. [DOI] [PubMed] [Google Scholar]
- 11.Morgan MD, Turnbull J, Selamet U, et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: A matched-pair cohort study. Arthritis Rheum. 2009;60:3493–500. doi: 10.1002/art.24957. [DOI] [PubMed] [Google Scholar]
- 12.Booth AD, Jayne DR, Kharbanda RK, et al. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation. 2004;109:1718–23. doi: 10.1161/01.CIR.0000124720.18538.DD. [DOI] [PubMed] [Google Scholar]
- 13.Raza K, Thambyrajah J, Townend JN, et al. Suppression of inflammation in primary systemic vasculitis restores vascular endothelial function: lessons for atherosclerotic disease? Circulation. 2000;102:1470–2. doi: 10.1161/01.cir.102.13.1470. [DOI] [PubMed] [Google Scholar]
- 14.Schachinger V, Zeiher AM. Atherosclerosis-associated endothelial dysfunction. Z Kardiol. 2000;89(Suppl 9):IX/70–4. doi: 10.1007/s003920070033. [DOI] [PubMed] [Google Scholar]
- 15.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 16.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–92. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 17.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68:310–7. doi: 10.1136/ard.2008.088096. [DOI] [PubMed] [Google Scholar]
- 18.Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 19.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Qjm. 1994;87:671–8. [PubMed] [Google Scholar]
- 20.Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 21.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 22.Harrell F. Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. 1. New York: Springer; 2001. [Google Scholar]
- 23.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roysten P. Multiple imputation of missing values: update. The Stata Journal. 2005:5. [Google Scholar]
- 25.Roysten P. Multiple imputation of missing values: Update of ice. The Stata Journal. 2005:5. [Google Scholar]
- 26.Royston P. Multiple imputation of missing values. The Stata Journal. 2004:4. [Google Scholar]
- 27.Archer K. Goodness-of-fit test for a logistic regression model fitted using survey sample data. The Stata Journal. 2006;6:97–105. [Google Scholar]
- 28.DeMaris A. Explained Variance in Logistic Regression: A Monte Carlo Study of Proposed Measures. Sociological Methods Research. 2002;31:27. [Google Scholar]
- 29.What are pseudo R-squareds? UCLA: Academic Technology Services, Statistical Consulting Group; [cited 14 December 2009]; Available from: http://www.ats.ucla.edu/stat/mult_pkg/faq/general/Psuedo_RSquareds.htm. [Google Scholar]
- 30.Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation. 17:192–205. doi: 10.1111/j.1549-8719.2009.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanasescu C, Jurcut C, Caraiola S, Nitescu D, Copaci I, Jurcut R. Endothelial dysfunction in inflammatory rheumatic diseases. Rom J Intern Med. 2009;47:103–8. [PubMed] [Google Scholar]
- 32.Zeiher AM. Endothelial vasodilator dysfunction: pathogenetic link to myocardial ischaemia or epiphenomenon? Lancet. 1996;348 (Suppl 1):s10–2. doi: 10.1016/s0140-6736(96)98004-6. [DOI] [PubMed] [Google Scholar]
- 33.Filer AD, Gardner-Medwin JM, Thambyrajah J, et al. Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann Rheum Dis. 2003;62:162–7. doi: 10.1136/ard.62.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hage FG, Venkataraman R, Zoghbi GJ, Perry GJ, DeMattos AM, Iskandrian AE. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;53:2129–40. doi: 10.1016/j.jacc.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 35.Von Feldt JM. Premature atherosclerotic cardiovascular disease and systemic lupus erythematosus from bedside to bench. Bull NYU Hosp Jt Dis. 2008;66:184–7. [PubMed] [Google Scholar]
- 36.Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309–13. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Onouchi Z, Sakata K, Ikuta K. An Experimental Study on the Role of Smooth Muscle Cells in the Pathogenesis of Atherosclerosis of the Coronary Arteries. Nippon Shonika Gakkai Zasshi. 1996:1453–1458. [Google Scholar]
- 38.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469–74. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 39.Nashel DJ. Is atherosclerosis a complication of long-term corticosteroid treatment? Am J Med. 1986;80:925–9. doi: 10.1016/0002-9343(86)90639-x. [DOI] [PubMed] [Google Scholar]
- 40.Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6:493–9. doi: 10.1007/s11906-004-0046-0. [DOI] [PubMed] [Google Scholar]
- 41.Souverein PC, Berard A, Van Staa TP, et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart. 2004;90:859–65. doi: 10.1136/hrt.2003.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 43.Collins GS, Altman DG. An independent external validation and evaluation of QRISK cardiovascular risk prediction: a prospective open cohort study. Bmj. 2009;339:b2584. doi: 10.1136/bmj.b2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. Bmj. 2007;335:136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]