The developing human preterm neonatal immune system: A case for more research in this area (original) (raw)
. Author manuscript; available in PMC: 2015 Sep 1.
Published in final edited form as: Clin Immunol. 2012 Aug 17;145(1):61–68. doi: 10.1016/j.clim.2012.08.006
Abstract
Neonates, particularly those born prematurely, are among the most vulnerable age group for morbidity and mortality due to infections. Immaturity of the innate immune system and a high need for invasive medical procedures in the context of a preterm birth make these infants highly susceptible to common neonatal pathogens. Preterm infants who survive may also suffer permanent disabilities due to organ damage resulting from either the infection itself or from the inflammatory response generated under an oxidative stress. Infections in preterm infants continue to pose important healthcare challenges. Yet, developmental maturation events in the innate immune system that underlie their excessively high vulnerability to infection remain largely understudied. In this review article, we identify pertinent knowledge gaps that must be filled in order to orient future translational research.
Keywords: Innate immunity, Infant, Premature, Toll-like receptors, Infection, Oxidative stress
1. Introduction
Neonates are one of the highest risk age groups for mortality and morbidity from infection. According to the World Health Organization, 3.7 million neonates less than 28 days of age died in 2010 – 37% of these deaths were due to infectious causes [1]. Among those, preterm infants are undoubtedly at highest risk and the health consequences that ensue are broad and serious [2]. Eleven percent of all infants are born premature (i.e. before 37 weeks of gestation), representing about 12.9 million infants born prematurely each year worldwide [3]. Unlike respiratory distress-related mortalities, which have shown a constant decline, the mortality from neonatal infection in preterm infants has increased over the last 20 years [4]. In North America, nearly one in six premature infant develops an invasive infection (i.e. bacteremia, pneumonia or meningitis) in their first weeks of life - many of which are fatal - and this risk is even higher in smallest preterm infants [5; 6; 7]. Unfortunately, neonatal infection can also cause irreversible damage to preterm infants’ organs (like their lungs, brain and intestines) also resulting in a greater risk of long-term neurodevelopmental impairment in survivors [8]. Finally, illnesses due to infection in this age group impose considerable pressure on health care resources, adding approximately twenty-five thousand of dollars per episode of infection per infant, out of the $25 billion annual economic impact of prematurity in the US alone [9; 10].
Preterm infants’ vulnerability to infection can be attributed to two main reasons: a frequent need for life-saving medical interventions that interfere with the body’s protective mucosal and epithelial barriers (e.g. mechanical ventilation, intravenous lines, etc.), as well as developmental immaturity leading to deficits of the immune system (e.g. lack of maternally transferred antibodies, which normally occur late in pregnancy, see below). A better understanding of human innate immune system at the very earliest stage in life is necessary in order to orient the development of safe, targeted drug therapies to improve health of these children. Yet, despite serious outcomes translational immunology research on the human newborn immune system remains limited. For example, at the time of writing of this manuscript, a search through the US National Institutes of Health public database (Pubmed) identified 905,094 publications related to the field of human immunology, of which 63,110 (7%) were concentrated on newborns and infants, whereas only a relatively minuscule proportion (2,413 publications, or <0.3%), concerned the premature infant immune system1. Detailed literature reviews of the neonatal [11] and particularly, the preterm immune system have been recently published [12]. Here, we present our perspective on the latest research in this area. We identify knowledge gaps as well as some of the emerging research areas on the characteristics of the innate immune system in infants born very early in gestation.
2. The developmentally immature preterm innate immune system
Protection against pathogens is achieved through the coordinated actions of the innate and the adaptive arms of the immune system. Newborns rely heavily on their innate immune defenses as their “educated” adaptive immunity fully develops only later, in the early years of life [13]. Infants born at term benefit from supplemental protection afforded by maternal antibodies transferred to them through the placenta (figure 1) [14]. Extreme preterm infants, however, substantially lack trans-placental transfer of maternal antibodies, which largely occurs during the third trimester of gestation [15; 16].
Figure 1.
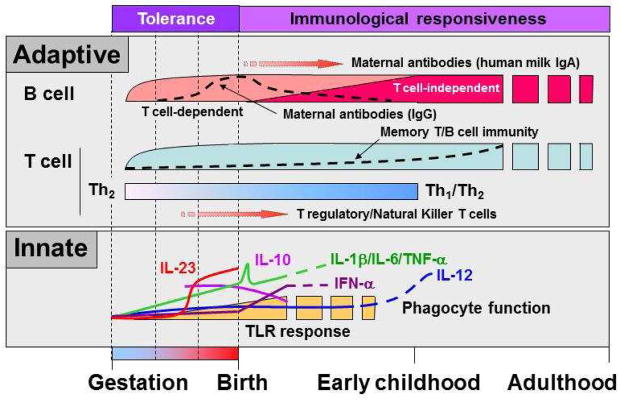
Developmental changes occurring in the human immune system early in life. This figure illustrates maturational events occurring in major adaptive and innate immune functions as the human transitions from a fetal tolerance state and becomes exposed to microorganisms as well as other environmental antigens de novo after birth. Adaptive immune functions [top panel]: Maternal transplacental antibody transfer (IgG) mainly occurs during late gestation, followed by maternal antibody protection (IgA) acquired through breast-milk after birth. Infants’ own antibody response become fully mature later during early childhood. Neonatal T cells are largely biased towards helper type II responses and humans display high proportions of T regulatory and Natural Killer T cells at birth [91]. Innate immune functions [bottom panel]: Pro-inflammatory (IL-1β, IL-6, TNF-α, IL-12, IL-23) and anti-viral (IFN-α) cytokine responses are largely attenuated in preterm infants, whereas production of the anti-inflammatory IL-10 cytokine is relatively high during late gestation and at birth.
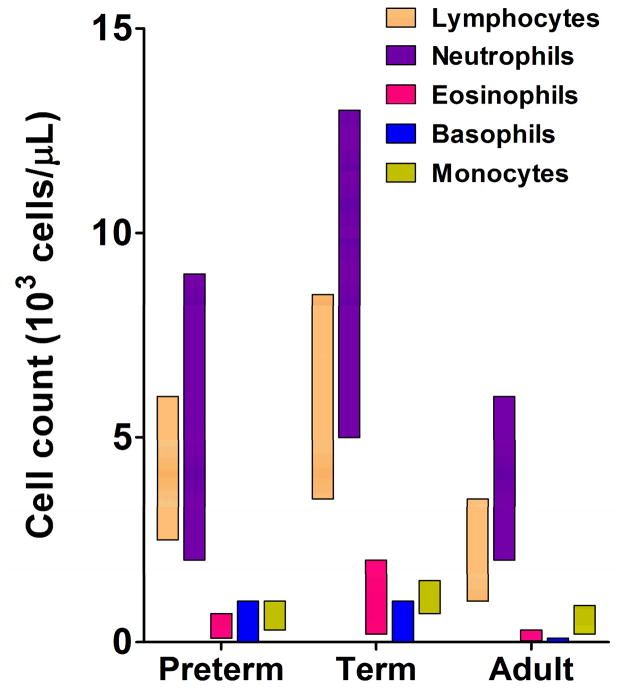
Even though the high vulnerability of preterm infants to infection cannot be ascribed to individual immune components, major functional differences exist when comparing to adults, term and preterm neonates (figure 1). Generally speaking, the composition of circulating white blood cells numerically differs among neonates of different gestations, likely reflecting dynamic developmental phases (figure 2). The proportions of both lymphoid and myeloid cells are generally higher in the peripheral blood of a healthy term neonate [17; 18; 19; 20]. Other major functional deficiencies in preterm innate immune functions have been recently reported include reduced Pattern-Recognition Receptor (PRR) function (discussed below), extracellular bacterial elimination [21] and leukocyte endothelial adhesive/rolling [22].
Figure 2.
Blood leukocyte count in preterm and term newborns and adults. Adapted from reference [18].
2.1 Attenuated Toll-like receptor function in preterm neonates
Innate immune surveillance is achieved through activation of cell surface receptors called Pattern Recognition Receptors (PRRs), which include Toll-like receptors (TLR), the Nucleotide Oligomerization Domain (NOD)-like receptors (NLR) and the retinoic acid-inducible gene I (RIG-I)-like receptors (RLR). TLRs have been a major focus of neonatal immunological research due to the extent of basic science knowledge in this area. Humans have ten known TLRs whose biological responses are dictated by the structural characteristics, cell-type specific expression and cellular localization profiles [23]. TLR cytokine responses are markedly attenuated in preterm cord blood compared to their term counterparts or to adults, when examined either in populations of cells or on a per-cell basis ([12] and below). Specifically, preterm neonates have significantly reduced pro-inflammatory cytokine (i.e. IL-1β, IL-6, TNF-α, figure 1) responses when stimulated with endotoxin (lipopolysaccharide, a potent TLR4 agonist) [24; 25; 26; 27; 28], IL-1 [29] or whole micro-organisms in vitro [30; 31; 32; 33]. They also produce limited amounts of anti-viral interferon-α even though their proportion of blood plasmacytoid dendritic cells, main source of this cytokine, is comparable to term neonates or adults [34]. In contrast, neonatal anti-inflammatory responses (e.g. IL-10 or TGF-β) are high, especially in preterm neonates at the lower end of gestation, although discrepancies exist among studies most likely due to methodological differences (e.g. sample size, stimulating conditions, etc) [27; 30; 31; 34; 35; 36] (figure 1). The attenuated cytokine responses observed in preterm neonates are also consistent with reduced protective antibody responses following administration of routine immunizations [37; 38].
2.2 Mechanisms of innate immune response attenuation
Some investigators have attributed part of the attenuation in pro-inflammatory cytokine responses to a gestational age-dependent reduction in surface expression of TLR4 and its co-receptor CD14 [39; 40; 41], as well as to reduced expression of MyD88 and IRF5 (two key components of the TLR signaling cascade [41; 42]). Lack of these proteins has also been associated with a functional reduction in the activity and nuclear translocation of the major pro-inflammatory NF-κB and p38/JNK transcription factors [41; 42]. However, most recent data suggest that the mechanisms responsible for the diminished cytokine response in preterm infants lie downstream of gestational changes in PRR expression ([32; 43] and below).
The fundamental mechanisms underlying the developmental attenuation of preterm innate immune cytokine responses have not been investigated. Epigenetic mechanisms have been largely implicated in the differentiation of hematopoietic cells [44]; therefore, it is plausible that similar mechanisms may play a role sustaining the developmental program regulating cytokine gene expression in early life. In newborns, lack of expression at the IL12A locus (encoding the p35 IL-12 subunit) in dendritic cells primarily occurs through nucleosome remodeling [45]. This lack of IL-12 expression is compensated for by a high production of IL-23 (through pairing of p19 with the p40 molecular subunit) in term neonates [46; 47; 48]. Recently, we have shown that preterm infants lack expression of p40 and therefore have a markedly reduced capacity to produce both IL-12 and IL-23 [34] (illustrated also in figure 1). In another recent study, reduced TLR3 expression in cord blood monocyte-derived dendritic cells was associated with allelic skewing of the TLR3 gene expression and modifications in chromatin structure, again suggesting a common role for epigenetic regulation of TLR function in early life [49]. However, whether and how epigenetic mechanisms regulate the maturation of innate immune functions during fetal life and in preterm neonates deserve more direct investigations.
3. Clinical impact of attenuated preterm innate immune functions
Fragile preterm infants are particularly susceptible to organ damage which may result from sepsis (reviewed in [50]). Infection can result in meningitis, which carries a major risk of permanent neurological impairment. Hemodynamic instability also carries a high risk of causing additional hypoxic-ischemic organ injury as it may occurs at critical developmental stages in preterm infants [51]. Given the unique vulnerability of preterm infants’ developing organs, it is likely that a reduced inflammatory response in fetal life serves to protect against the potential damaging effects of an excessive immune activation. However, the evolutionary advantage of attenuated innate immune defenses in utero clearly becomes a major clinical disadvantage following a preterm birth. Nonetheless, direct evidence for the implication of specific innate immune deficits in increasing preterm infants’ risk of infection in humans is lacking and inference from data in mice is complicated by significant functional differences across species [52]. Moreover, the presence of compensatory mechanisms (i.e. adaptive immune functions, etc.) at a later age may mask our ability to infer knowledge obtained from adults or from older children with primary immune deficiencies [53]. Despite these limitations some recent examples shed light on the clinical impact of specific immune deficits in human preterm infants. For example, the PRR Mannose-Binding Lectin (MBL) recognizes carbohydrate structures on the surface of a wide variety of pathogenic micro-organisms. The main function of MBL is to facilitate phagocytosis through antibody-mediated and C1-independent complement pathways [54]. In addition, MBL directly enhances TLR activity in the phagosome [55] and thereby can modulate inflammatory responses [56]. Reduced MBL serum levels have been linked to an increased risk of infection [57; 58; 59; 60; 61]. Preterm infants generally display lower MBL serum levels [58]. Moreover, common functional polymorphisms in the MBL2 gene resulting in variations in MBL serum levels may further increase susceptibility to infection in some infants [62; 63].
As mentioned above, preterm neonates who developed early-onset neonatal sepsis also display significantly lower serum levels of the IL-12/23 p40 protein subunit at birth compared to control neonates who did not go on to develop sepsis [34]. P40 mainly functions in conjunction with either p35 (to form the IL-12 cytokine) or p19 (to form IL-23), to support differentiation and maintenance of the major T helper 1 (Th1) or T helper 17 (Th17) cell regulatory subsets, respectively [64]. Data obtained from p40-deficient mice demonstrate a key role of these cytokines in protecting against mucosal pathogens [64]. Altogether, these data indicate an important role of specific innate immune defense pathways in protecting preterm infants against infection. However, data have largely been obtained from cord blood and a more detailed characterization of immunological deficits during the neonatal period, in humans is required in order to fully understand the factors responsible for their unique vulnerability to infection.
3.1 Pathogen-specific preterm innate immune responses
Although specific preterm innate immune deficits have been reported, little is known also about how these deficits interact while combating common neonatal pathogens such as Escherichia coli, Candida, Staphylococcal species or group B Streptococci (GBS). The lack of data stems, in part, from great ethical challenges with the conduct of studies requiring peripheral blood in vulnerable newborns. However, recent improved methods for polyfunctional assessment of immune functions from amounts of blood obtainable in small preterm neonates greatly facilitate investigations of pathogen-specific responses to common neonatal infections [32; 33; 43; 65; 66].
Coagulase-negative staphylococci (CoNS) species is one of the most common source of blood stream infection in the neonatal intensive care unit [33]. In mice, TLR2 appears to be particularly important in the early detection and clearance of Staphylococcus epidermis especially in conditions of reduced bacterial load [66]. TLR2 appears to play an important role in the innate immune recognition of this pathogen by human neonatal blood cells [66; 67]. On the other hand, adult- or term-like levels of TLR2 expression is preserved in preterm neonates, suggesting that their higher vulnerability to CoNS arise from more downstream functional differences [30; 41; 43].
The responses to GBS, another major neonatal pathogen, have also been recently examined more specifically in vitro [32]. Interestingly, the ability of preterm infants’ cord blood monocytes to phagocytose GBS is comparable to term neonates or adults [32]. However, the cytokine response produced upon exposure of preterm cord blood mononuclear cells to live or heat-killed GBS was significantly impaired, again suggesting downstream attenuation of the innate immune response to this pathogen [32]. Altogether, these data highlight the importance of a more thorough characterization of innate immune deficits in the context of neonatal pathogens to facilitate a targeted development of pharmacological strategies either to promote immune defenses or to limit invasive medical interventions at times when infants are most vulnerable immunologically.
4. Potential adverse effects of an excessive innate immune activation in preterm infants
Dysregulated innate immune responses play a major role in the etiology of common and serious preterm neonatal complications, such as bronchopulmonary dysplasia (a form of neonatal chronic lung disease) and necrotizing enterocolitis (a surgical intestinal complication) ([68; 69] for some recent studies). Apart from the direct effect of infection, most recent evidence indicate that non-infectious stimulation of the innate immune system may also present additional risks to preterm infants [70]. Supplemental oxygen, mechanical ventilation and intravenous nutrition are often used as life-supporting therapies in their initial weeks of life. Because of a close interplay between cellular oxidative stress and innate immune sensors these intensive care interventions also lend infants to experience significant inflammation resulting from an oxidative stress [70; 71; 72]. According to recent data in human infants, brief periods of oxygen administered at birth may generate sufficient inflammation and oxidative injury to cause sustained organ damage [71]. This oxidative stress is likely exacerbated by a profound immaturity of anti-oxidant defenses [73], which continues to drive a controversy in neonatal care about the amount of supplemental oxygen that is safe to administer to preterm newborns [74]. These data clearly mandate a proper understanding of the impact of therapeutically altering the normal preterm homeostatic innate immune balance in infants exposed to intensive care, before responses can be safely manipulated for the purpose of preventing infection.
5. Other emerging research areas
Other recent findings in neonatal immunology have expanded the scope of future translational investigations, while highlighting a number of important research questions. For example, in small series the intestinal microbial flora appeared distinct in preterm infants who develop sepsis, suggesting a potentially acquired predisposition [75]. Feeding human breast milk to premature infants reduces the incidence of necrotizing enterocolitis and favorably impacts their health in a number of other ways [76], whereas broad-spectrum antibiotic exposure was associated with a higher incidence of necrotizing enterocolitis [77; 78; 79]. Also, exogenous administration of certain types of bacteria through the administration of probiotics (e.g. Bifidobacteria and Lactobacillus) reduces the incidence of necrotizing enterocolitis in high-risk preterm infants [75; 80; 81; 82; 83]. In mice, exposure to breast milk immediately postpartum modulates TLR reactivity in the intestinal epithelium [84]. Altogether, these data highlight the potential therapeutic benefit of manipulating preterm infants’ own microbial flora in order to reduce serious complications such as infection and/or necrotizing enterocolitis. These data also raise more fundamental questions about the importance of the human microbiome in shaping the developmentally immature immune system.
Finally, some recent data indicate that early life exposure to infection may have life-long effects on the immune system [85; 86]. Based on animal studies, neonatal infections have significant long-term “programming” effects resulting in long-term inflammatory diseases [87; 88; 89; 90]. However, whether similar long-term effects occur in humans is completely unknown. Given their high rate of infection and their unique immunological vulnerability, the long-term immunological consequences of an early life exposure to infection in preterm infants certainly deserve more investigations.
6. Conclusions
In summary, a focused drive to improve knowledge of the human innate immune system at the very early stage in life is important in order to reduce neonatal mortality and achieve better health outcomes in preterm neonates. Major knowledge gaps remain to be filled in order to orient future clinical research, mainly in our understanding of the fundamental mechanisms regulating specific developmental innate immune pathways and how deficits in these pathways contribute to the overall risk of infection during the neonatal period and in the context of common neonatal infections. Finally, it is unclear how the preterm immune system can be safely modulated in order to avoid potential harmful consequence of an excessive immune activation on fragile immature organs. From a basic science perspective, a functional characterization of the immune system before the emergence of protective adaptive immunity also offers unique opportunities to understand the hierarchical importance of innate immune functions in humans.
Acknowledgments
The corresponding author’s (PML) research is funded by Hospital for Sick Children Foundation (XG09-015R) and Canadian Institutes of Health Research grants (MOP-110938). We thank Linda Dix-Cooper for an editorial revision of this manuscript. AAS is supported by a Child & Family Research Institute Graduate Studentship. PML is support by a Clinician-Scientist Award from the Child & Family Research Institute and a Career Investigator Award from the Michael Smith Foundation for Health Research.
Abbreviations
CoNS
Coagulase-negative staphylococcus
GBS
Group B streptococcus
MBL
Mannose-binding lectin
miRNA
regulatory micro-ribonucleic acids
NLR
Nucleotide Oligomerization Domain (NOD)-like receptors (NLR)
PRR
Pattern-Recognition Receptor
RLR
retinoic acid-inducible gene I (RIG-I)-like receptors
TLR
Toll-like receptor
Footnotes
1
Using keywords: [Human AND (immunity OR immunology)], [newborn OR neonate OR infant] and [premature OR preterm], respectively.
References
- 1.WHO. World Health Statistics: 2010. World Health Organization; 2010. [Google Scholar]
- 2.UNICEF. The state of the world’s health 2009: maternal and newborn health. 2009. [Google Scholar]
- 3.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PFA. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization. 2010;88:31–8. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND. Deaths in Preterm Infants: Changing Pathology Over 2 Decades. J Pediatr. 2011 doi: 10.1016/j.jpeds.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 7.Lavoie PM. Earlier initiation of enteral nutrition is associated with lower risk of late-onset bacteremia only in most mature very low birth weight infants. J Perinatol. 2009;29:448–54. doi: 10.1038/jp.2009.8. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292:2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 9.Gray JE, Richardson DK, McCormick MC, Goldmann DA. Coagulase-negative staphylococcal bacteremia among very low birth weight infants: relation to admission illness severity, resource use, and outcome. Pediatrics. 1995;95:225–30. [PubMed] [Google Scholar]
- 10.Thomas RE, Baker P. A cost-outcome description of the septic work-up for bacterial infection in neonates in a tertiary care hospital. Int J Technol Assess Health Care. 1995;11:11–25. doi: 10.1017/s0266462300005225. [DOI] [PubMed] [Google Scholar]
- 11.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 12.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011 doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 13.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 14.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–9. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 15.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Berg JP, Westerbeek EA, Berbers GA, van Gageldonk PG, van der Klis FR, van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J. 2010;29:801–5. doi: 10.1097/inf.0b013e3181dc4f77. [DOI] [PubMed] [Google Scholar]
- 17.Walker JC, Smolders MA, Gemen EF, Antonius TA, Leuvenink J, de Vries E. Development of lymphocyte subpopulations in preterm infants. Scand J Immunol. 2011;73:53–8. doi: 10.1111/j.1365-3083.2010.02473.x. [DOI] [PubMed] [Google Scholar]
- 18.Milcic TL. The complete blood count. Neonatal Netw. 2010;29:109–15. doi: 10.1891/0730-0832.29.2.109. [DOI] [PubMed] [Google Scholar]
- 19.Christensen RD, Henry E, Jopling J, Wiedmeier SE. The CBC: reference ranges for neonates. Semin Perinatol. 2009;33:3–11. doi: 10.1053/j.semperi.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Berrington JE, Barge D, Fenton AC, Cant AJ, Spickett GP. Lymphocyte subsets in term and significantly preterm UK infants in the first year of life analysed by single platform flow cytometry. Clin Exp Immunol. 2005;140:289–92. doi: 10.1111/j.1365-2249.2005.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, Chandler NB, Rodesch CK, Albertine KH, Petti CA, Weyrich AS, Zimmerman GA. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neonates. Blood. 2009;113:6419–27. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nussbaum C, Sperandio M. Innate immune cell recruitment in the fetus and neonate. J Reprod Immunol. 2011;90:74–81. doi: 10.1016/j.jri.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weatherstone KB, Rich EA. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res. 1989;25:342–6. doi: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Yachie A, Takano N, Ohta K, Uehara T, Fujita S, Miyawaki T, Taniguchi N. Defective production of interleukin-6 in very small premature infants in response to bacterial pathogens. Infect Immun. 1992;60:749–53. doi: 10.1128/iai.60.3.749-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schultz C, Rott C, Temming P, Schlenke P, Moller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. 2002;51:317–22. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P. Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine. 2003;21:200–6. doi: 10.1016/s1043-4666(02)00498-2. [DOI] [PubMed] [Google Scholar]
- 28.Strunk T, Temming P, Gembruch U, Reiss I, Bucsky P, Schultz C. Differential maturation of the innate immune response in human fetuses. Pediatr Res. 2004;56:219–26. doi: 10.1203/01.PDR.0000132664.66975.79. [DOI] [PubMed] [Google Scholar]
- 29.Liechty KW, Koenig JM, Mitchell MD, Romero R, Christensen RD. Production of interleukin-6 by fetal and maternal cells in vivo during intraamniotic infection and in vitro after stimulation with interleukin-1. Pediatr Res. 1991;29:1–4. doi: 10.1203/00006450-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Hartel C, Osthues I, Rupp J, Haase B, Roder K, Gopel W, Herting E, Schultz C. Characterisation of the host inflammatory response to Staphylococcus epidermidis in neonatal whole blood. Arch Dis Child Fetal Neonatal Ed. 2008;93:F140–5. doi: 10.1136/adc.2007.124685. [DOI] [PubMed] [Google Scholar]
- 31.Tatad AM, Nesin M, Peoples J, Cheung S, Lin H, Sison C, Perlman J, Cunningham-Rundles S. Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes. Neonatology. 2008;94:8–15. doi: 10.1159/000112541. [DOI] [PubMed] [Google Scholar]
- 32.Currie AJ, Curtis S, Strunk T, Riley K, Liyanage K, Prescott S, Doherty D, Simmer K, Richmond P, Burgner D. Preterm infants have deficient monocyte and lymphocyte cytokine responses to group B streptococcus. Infect Immun. 2011;79:1588–96. doi: 10.1128/IAI.00535-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strunk T, Richmond P, Simmer K, Currie A, Levy O, Burgner D. Neonatal immune responses to coagulase-negative staphylococci. Curr Opin Infect Dis. 2007;20:370–5. doi: 10.1097/QCO.0b013e3281a7ec98. [DOI] [PubMed] [Google Scholar]
- 34.Lavoie PM, Huang Q, Jolette E, Ladd M, Nuyt AM, Audibert F, Speert DP, Lacaze-Masmonteil T, Soudeyns H, Kollmann TR. Profound lack of IL-12/23p40 in neonates born early in gestation influencing the risk of early-onset sepsis. J Infect Dis. 2010;202:1754–63. doi: 10.1086/657143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultz C, Temming P, Bucsky P, Gopel W, Strunk T, Hartel C. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135:130–6. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belderbos ME, van Bleek GM, Levy O, Blanken MO, Houben ML, Schuijff L, Kimpen JL, Bont L. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: Low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–37. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Angio CT. Active immunization of premature and low birth-weight infants: a review of immunogenicity, efficacy, and tolerability. Paediatr Drugs. 2007;9:17–32. doi: 10.2165/00148581-200709010-00003. [DOI] [PubMed] [Google Scholar]
- 38.Baxter D. Vaccine responsiveness in premature infants. Hum Vaccin. 2010;6:506–11. doi: 10.4161/hv.6.6.12083. [DOI] [PubMed] [Google Scholar]
- 39.Forster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, Hayde M, Prusa AR, Herkner K, Boltz-Nitulescu G, Pollak A, Spittler A. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–4. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 40.Henneke P, Osmers I, Bauer K, Lamping N, Versmold HT, Schumann RR. Impaired CD14-dependent and independent response of polymorphonuclear leukocytes in preterm infants. J Perinat Med. 2003;31:176–83. doi: 10.1515/JPM.2003.024. [DOI] [PubMed] [Google Scholar]
- 41.Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 42.Al-Hertani W, Yan SR, Byers DM, Bortolussi R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin Invest Med. 2007;30:E44–53. doi: 10.25011/cim.v30i2.979. [DOI] [PubMed] [Google Scholar]
- 43.Strunk T, Prosser A, Levy O, Philbin V, Simmer K, Doherty D, Charles A, Richmond P, Burgner D, Currie A. Human monocyte responsiveness to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr Res. 2012 doi: 10.1038/pr.2012.48. [DOI] [PubMed] [Google Scholar]
- 44.Cedar H, Bergman Y. Epigenetics of haematopoietic cell development. Nat Rev Immunol. 2011;11:478–88. doi: 10.1038/nri2991. [DOI] [PubMed] [Google Scholar]
- 45.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonte D, Willems F, Goldman M. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199:1011–6. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36:21–6. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 47.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corbett NP, Blimkie D, Ho KC, Cai B, Sutherland DP, Kallos A, Crabtree J, Rein-Weston A, Lavoie PM, Turvey SE, Hawkins NR, Self SG, Wilson CB, Hajjar AM, Fortuno ES, 3rd, Kollmann TR. Ontogeny of Toll-like receptor mediated cytokine responses of human blood mononuclear cells. PLoS One. 2010;5:e15041. doi: 10.1371/journal.pone.0015041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porras A, Kozar S, Russanova V, Salpea P, Hirai T, Sammons N, Mittal P, Kim JY, Ozato K, Romero R, Howard BH. Developmental and epigenetic regulation of the human TLR3 gene. Mol Immunol. 2008;46:27–36. doi: 10.1016/j.molimm.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19:290–7. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- 51.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, Moldawer LL. Defective innate immunity predisposes murine neonates to poor sepsis outcome, but is reversed by TLR agonists. Blood. 2008;112:1750–8. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bustamante J, Boisson-Dupuis S, Jouanguy E, Picard C, Puel A, Abel L, Casanova JL. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 55.Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008;205:169–81. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fidler KJ, Wilson P, Davies JC, Turner MW, Peters MJ, Klein NJ. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intensive Care Med. 2004;30:1438–45. doi: 10.1007/s00134-004-2303-8. [DOI] [PubMed] [Google Scholar]
- 57.de Benedetti F, Auriti C, D’Urbano LE, Ronchetti MP, Rava L, Tozzi A, Ugazio AG, Orzalesi MM. Low serum levels of mannose binding lectin are a risk factor for neonatal sepsis. Pediatr Res. 2007;61:325–8. doi: 10.1203/pdr.0b013e318030d12f. [DOI] [PubMed] [Google Scholar]
- 58.Dzwonek AB, Neth OW, Thiebaut R, Gulczynska E, Chilton M, Hellwig T, Bajaj-Elliott M, Hawdon J, Klein NJ. The role of mannose-binding lectin in susceptibility to infection in preterm neonates. Pediatr Res. 2008;63:680–5. doi: 10.1203/PDR.0b013e31816fdbff. [DOI] [PubMed] [Google Scholar]
- 59.Frakking FN, Brouwer N, van Eijkelenburg NK, Merkus MP, Kuijpers TW, Offringa M, Dolman KM. Low mannose-binding lectin (MBL) levels in neonates with pneumonia and sepsis. Clin Exp Immunol. 2007;150:255–62. doi: 10.1111/j.1365-2249.2007.03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Israels J, Frakking FN, Kremer LC, Offringa M, Kuijpers TW, van de Wetering MD. Mannose-binding lectin and infection risk in newborns: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2010;95:F452–61. doi: 10.1136/adc.2009.172122. [DOI] [PubMed] [Google Scholar]
- 61.Frakking FN, Brouwer N, Zweers D, Merkus MP, Kuijpers TW, Offringa M, Dolman KM. High prevalence of mannose-binding lectin (MBL) deficiency in premature neonates. Clin Exp Immunol. 2006;145:5–12. doi: 10.1111/j.1365-2249.2006.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oudshoorn AM, van den Dungen FA, Bach KP, Koomen I, Fetter WP, Catsburg A, Savelkoul PH, van Elburg RM. Mannose-binding lectin in term newborns and their mothers: genotypic and phenotypic relationship. Hum Immunol. 2008;69:344–8. doi: 10.1016/j.humimm.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Hilgendorff A, Heidinger K, Pfeiffer A, Bohnert A, Konig IR, Ziegler A, Merz C, Frey G, Chakraborty T, Gortner L, Bein G. Association of polymorphisms in the mannose-binding lectin gene and pulmonary morbidity in preterm infants. Genes Immun. 2007;8:671–7. doi: 10.1038/sj.gene.6364432. [DOI] [PubMed] [Google Scholar]
- 64.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 65.Strunk T, Curtis S, Richmond P, Currie AJ, Simmer K, Levy O, Otto M, Burgner D. Innate immune responses of preterm infants to neonatal bacterial pathogens and Toll-like receptor agonists. European Society of Pediatrics Research Annual Meeting; Prague, Czech Republic. 2007. [Google Scholar]
- 66.Strunk T, Power Coombs MR, Currie AJ, Richmond P, Golenbock DT, Stoler-Barak L, Gallington LC, Otto M, Burgner D, Levy O. TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One. 2010;5:e10111. doi: 10.1371/journal.pone.0010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang JP, Yang Y, Levy O, Chen C. Human neonatal peripheral blood leukocytes demonstrate pathogen-specific coordinate expression of TLR2, TLR4/MD2, and MyD88 during bacterial infection in vivo. Pediatr Res. 2010;68:479–83. doi: 10.1203/PDR.0b013e3181f90810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nathe KE, Parad R, Van Marter LJ, Lund CA, Suter EE, Hernandez-diaz S, Boush EB, Ikonomu E, Gallington L, Morey JA, Zeman AM, McNamara M, Levy O. Endotoxin-directed innate immunity in tracheal aspirates of mechanically ventilated human neonates. Pediatr Res. 2009;66:191–6. doi: 10.1203/PDR.0b013e3181aa33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. 2011;6:e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang BA, Huang Q, Quan J, Chau V, Ladd M, Kwan E, McFadden DE, Lacaze-Masmonteil T, Miller SP, Lavoie PM. Early inflammation in the absence of overt infection in preterm neonates exposed to intensive care. Cytokine. 2011;56:621–6. doi: 10.1016/j.cyto.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107:642–7. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- 72.Lavoie PM, Lavoie JC, Watson C, Rouleau T, Chang BA, Chessex P. Inflammatory response in preterm infants is induced early in life by oxygen and modulated by total parenteral nutrition. Pediatr Res. 2010;68:248–51. doi: 10.1203/PDR.0b013e3181eb2f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, Roberts LJ, 2nd, Arduini A, Escobar JJ, Sastre J, Asensi MA. Preterm Resuscitation With Low Oxygen Causes Less Oxidative Stress, Inflammation, and Chronic Lung Disease. Pediatrics. 2009;124:e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 74.Saugstad OD. Why are we still using oxygen to resuscitate term infants? J Perinatol. 2011;30(Suppl):S46–50. doi: 10.1038/jp.2010.94. [DOI] [PubMed] [Google Scholar]
- 75.Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH, Sogin ML, Foster JA, Edwards WH, Palumbo P, Hibberd PL. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012 doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011:CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 77.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–7. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–5. doi: 10.1016/j.jpeds.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, Ambalavanan N, Benjamin DK., Jr Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. :CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 81.Fernandez-Carrocera LA, Solis-Herrera A, Cabanillas-Ayon M, Gallardo-Sarmiento RB, Garcia-Perez CS, Montano-Rodriguez R, Echaniz-Aviles MO. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. doi: 10.1136/archdischild-2011-300435. [DOI] [PubMed] [Google Scholar]
- 82.Manzoni P, Stolfi I, Messner H, Cattani S, Laforgia N, Romeo MG, Bollani L, Rinaldi M, Gallo E, Quercia M, Maule M, Mostert M, Decembrino L, Magaldi R, Mosca F, Vagnarelli F, Memo L, Betta PM, Stronati M, Farina D. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. 2012;129:116–23. doi: 10.1542/peds.2011-0279. [DOI] [PubMed] [Google Scholar]
- 83.Wang Q, Dong J, Zhu Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg. 47:241–8. doi: 10.1016/j.jpedsurg.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 84.LeBouder E, Rey-Nores JE, Raby AC, Affolter M, Vidal K, Thornton CA, Labeta MO. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. J Immunol. 2006;176:3742–52. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- 85.Conroy ME, Shi HN, Walker WA. The long-term health effects of neonatal microbial flora. Curr Opin Allergy Clin Immunol. 2009;9:197–201. doi: 10.1097/ACI.0b013e32832b3f1d. [DOI] [PubMed] [Google Scholar]
- 86.Taylor A, Hale J, Wiltschut J, Lehmann H, Dunstan JA, Prescott SL. Evaluation of the effects of probiotic supplementation from the neonatal period on innate immune development in infancy. Clin Exp Allergy. 2006;36:1218–26. doi: 10.1111/j.1365-2222.2006.02552.x. [DOI] [PubMed] [Google Scholar]
- 87.Spencer SJ, Galic MA, Pittman QJ. Neonatal programming of innate immune function. Am J Physiol Endocrinol Metab. 2011;300:E11–8. doi: 10.1152/ajpendo.00516.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beloosesky R, Maravi N, Weiner Z, Khatib N, Awad N, Boles J, Ross MG, Itskovitz-Eldor J. Maternal lipopolysaccharide-induced inflammation during pregnancy programs impaired offspring innate immune responses. Am J Obstet Gynecol. 2010;203:185 e1–4. doi: 10.1016/j.ajog.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 89.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci U S A. 2008;105:7528–33. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–50. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ladd M, Sharma A, Huang Q, Wang AY, Genowati I, Levings MK, Lavoie PM. Natural Killer T cells constitutively expressing the IL-2 receptor alpha chain early in life are primed to respond to lower antigenic stimulation. Immunology. 2010;131:289–99. doi: 10.1111/j.1365-2567.2010.03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]