S-Nitrosylation of Calcium-Handling Proteins in Cardiac Adrenergic Signaling and Hypertrophy (original) (raw)
. Author manuscript; available in PMC: 2016 Oct 9.
Abstract
Rationale
The regulation of calcium (Ca2+) homeostasis by beta-adrenergic receptor (βAR) activation provides the essential underpinnings of sympathetic regulation of myocardial function as well as a basis for understanding molecular events that result in hypertrophic signaling and heart failure. Sympathetic stimulation of the βAR not only induces protein phosphorylation but also activates nitric oxide (NO)-dependent signaling, which modulates cardiac contractility. Nonetheless, the role of NO in βAR-dependent regulation of Ca2+ handling has not yet been explicated fully.
Objective
To elucidate the role of protein S-nitrosylation, a major transducer of NO bioactivity, on βAR-dependent alterations in cardiomyocyte Ca2+ handling and hypertrophy.
Methods and Results
Using transgenic mice to titrate the levels of protein SNO, we uncovered major roles for protein S-nitrosylation generally, and for phospholamban (PLN) and cardiac troponin C (cTnC) S-nitrosylation in particular, in βAR-dependent regulation of Ca2+ homeostasis. Notably, S-nitrosylation of PLN consequent upon βAR stimulation is necessary for its inhibitory pentamerization of PLN, which activates sarcoplasmic reticulum Ca2+-ATPase (SERCA2a) and increases cytosolic Ca2+ transients. Coincident S-nitrosylation of cTnC decreases myocardial sensitivity to Ca2+. During chronic adrenergic stimulation, global reductions in cellular S-nitrosylation mitigate hypertrophic signaling resulting from Ca2+ overload.
Conclusions
S-nitrosylation operates in concert with phosphorylation to regulate many cardiac Ca2+-handling proteins, including PLN and cTnC, thereby playing an essential and previously unrecognized role in cardiac Ca2+ homeostasis. Manipulation of the S-nitrosylation level may prove therapeutic in heart failure.
Keywords: Calcium, heart failure, myocardial contraction, nitric oxide, receptors, beta adrenergic
INTRODUCTION
Cardiomyocyte contraction is triggered when an action potential activates voltage-gated Ca2+ channels (e.g., L-type Ca2+ channel; LTCC). The Ca2+ current induces the sarcoplasmic reticulum (SR) to release more Ca2+ into the cytosol. The rise of cytosolic Ca2+ initiates contraction by the binding of Ca2+ to cTnC, which in turn binds and displaces troponin I (cTnI) from its actin-binding site, thereby allowing myosin heads to interact with actin to form cross-bridges. Stimulated βARs activate cAMP-dependent protein kinase A (PKA) which regulates Ca2+ homeostasis by phosphorylating Ca2+ handling proteins1. In particular, phosphorylation of PLN activates SERCA2a, augmenting SR Ca2+ load ([Ca2+]SRT) and transient rises in intracellular Ca2+ (Δ[Ca2+]). Increased Ca2+ subsequently activates Ca2+/calmodulin-dependent protein kinase II (CaMKII) that also phosphorylates additional Ca2+ handling proteins.2 Phosphorylation of cTnI and PLN promotes relaxation of cardiomyocytes by accelerating Ca2+ dissociation from cTnC and Ca2+ re-uptake into the SR by SERCA2a, respectively3. Combined, activated βAR induces positive inotropic and lusitropic effects. On the other hand, chronic βAR activation and sustained increase of intracellular Ca2+ induces left ventricular (LV) hypertrophy and dysfunction,4 which ultimately leads to the development of heart failure.
βAR stimulation activates NO synthases 1 (NOS1) and NOS3 to produce NO, which regulates cardiac function.5, 6 For example, NOS1-derived NO S-nitrosylates and activates the ryanodine receptor (RyR2), resulting in cytosolic Ca2+ release and enhanced catecholamine-stimulated contractility.7 On the other hand, NOS3-derived NO increases LV compliance and attenuates βAR-induced inotropy,5 presumably by activation of soluble guanylate cyclase (sGC) and subsequent production of cGMP. The existence of multiple downstream signaling mechanisms and different sources of NO has lead to conflicting data regarding the specific contribution of NO and its effectors to the regulation of myocardial function.8
Accumulating evidence suggests that protein S-nitrosylation (SNO) is a major effector of NO signaling. By analogy to the role of phosphatases, which regulate the levels of protein phosphorylation, amounts of SNO are set by enzymes that metabolize S-nitrosothiols (denitrosylases). Reduction of S-nitrosoglutathione (GSNO) by S-nitrosoglutathione reductase (GSNOR) controls the intracellular levels of protein SNO, as GSNO and protein S-nitrosothiols are in equilibrium.9 Recent studies suggest that signaling through the βAR is regulated by GSNOR-dependent denitrosylation.10 Nonetheless, the impact of protein SNO on cardiomyocyte function and its regulation by adrenergic signaling is incompletely understood. Given the central role of Ca2+ in cardiomyocyte function, we hypothesized that GSNOR regulates cardiomyocyte function via controlling SNO levels of Ca2+ handling proteins after βAR stimulation. Here, we report that βAR stimulation induces SNO of Ca2+ handling proteins in cardiomyocytes. Our results suggest that, in addition to phosphorylation, SNO of key Ca2+ handling proteins is required for the proper transduction of βAR signaling in cardiomyocytes. Detrimental effects of Ca2+ mishandling induced by chronic hemodynamic stress may be mitigated by enhancing protein denitrosylation.
METHODS
Animal studies
The generation of mice deficient in GSNOR (GSNOR−/−) and PLN (PLN−/−), as well as mice with cardiomyocyte-specific GSNOR overexpression (GSNOR-Tg) has been described elsewhere.11–13 All protocols and experimental procedures were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and performed in accordance with the guidelines of the National Institutes of Health for the use of animals in research.
Isolated cardiomyocytes and langendorff perfused hearts
Isolation of ventricular cardiomyocytes of adult mice and high-speed imaging of Δ[Ca2+] and sarcomere length (SL) shortening (i.e., twitch contraction) was carried out as previously described with minor modifications.14, 15 Myocardial Ca2+ responsiveness was examined in intact cardiomyocytes, as estimated by the ratio of percent SL shortening (%ΔL/L0)/Δ[Ca2+], as well as in permeabilized cardiomyocytes.15 The LTCC current (ICa,L) was measured in isolated cardiomyocytes using the whole-cell voltage-clamp technique, as previously described.16 SR calcium load ([Ca2+]SRT), SR calcium leak (SR[Ca2+]leak), and sodium-calcium exchanger (NCX) activity were measured as previously described.15 All experiments in isolated cardiomyocytes were conducted at 37°C, unless otherwise specified. LV contractility was measured in isolated perfused mouse hearts as described previously.17
In vivo chronic βAR stimulation
DL-isoproterenol (ISO) was administered to mice for 14 days using surgically implanted mini-osmotic pumps (Alzet-20; Durect Corporation, CA). Based on pilot studies, we used a high dose of ISO (60 mg/kg/day) to examine the impact of GSNOR overexpression and a low dose of ISO (30 mg/kg/day) to examine the effects of GSNOR deficiency. After removal of the pumps, mice were observed for an additional 14 days. In vivo cardiac function was evaluated by transthoracic echocardiography, as described previously.15 At the end of the study, hearts were harvested for histological examination and analysis of protein expression.
Analysis of protein S-nitrosylation
A modified biotin switch assay was performed as previously described18 with minor modifications. Detailed experimental protocols of organic mercury resin-assisted capture of S-nitrosylated proteins and peptides have been published elsewhere.19 After purification, specific nitrosylated proteins were detected using standard immunoblot techniques.
Adenovirus specifying mutant PLN and cTnC
Adenoviral (Ad) constructs were generated encoding wild-type (WT) PLN (C36/C41/C46), single cysteine-to-alanine PLN mutants (C36A, C41A, C46A, respectively), triple PLN mutant (C36A/C41A/C46A), WT cTnC (C35/C84), single cysteine-to-serine cTnC mutants (C35S, C84S), and double cTnC mutant (C35S/C84S) protein. Ad-PLN solution was injected in vivo into the myocardium at the left ventricular apex, and Ad-cTnC was used to infect cultured adult mouse cardiomyocytes.
Statistics
All data are presented as mean ± standard error of the mean (s.e.m.). For isolated cardiomyocyte experiments, measurements of all cells from one mouse were averaged and the average value for each mouse was used for statistical comparison between groups unless otherwise specified. Statistical differences among groups were evaluated using the appropriate statistical tests as indicated in the text.
RESULTS
GSNOR modulates Ca2+ handling in cardiomyocytes
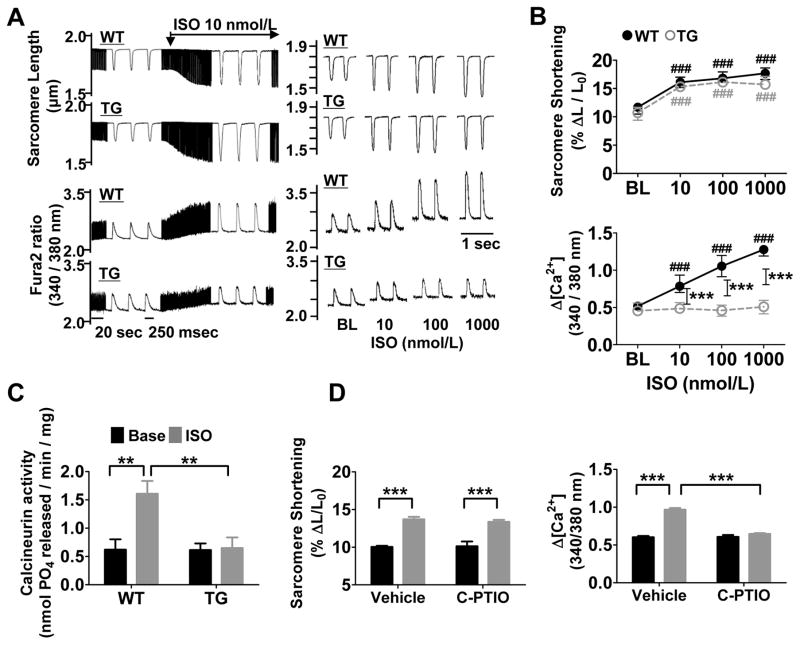
After stimulation with the βAR agonist ISO, twitch contraction was increased to the same extent in WT and GSNOR-Tg cardiomyocytes (Figure 1A and 1B), but attenuated in GSNOR−/− cardiomyocytes at higher doses of ISO (Online Figure IA and IB). In WT cardiomyocytes, the ISO-induced increases in contraction correlated with increases in Δ[Ca2+] (Figure 1B), whereas the attenuated contraction in GSNOR−/− cardiomyocytes was associated with an impaired increase of Δ[Ca2+] to ISO stimulation. In contrast, GSNOR-Tg cardiomyocytes showed no change in Δ[Ca2+] despite a sustained contractile response to ISO stimulation (Figure 1B). In isolated perfused hearts, ISO enhanced LV inotropy in both GSNOR-Tg and WT hearts (Online Figure IC). However, calcineurin, a Ca2+-dependent enzyme, was activated only in WT hearts, suggesting suppressed Δ[Ca2+] in GSNOR-Tg hearts during βAR stimulation (Figure 1C). The NO scavenger carboxy-PTIO also prevented the ISO-induced increase in Δ[Ca2+] in WT cardiomyocytes without affecting inotropy (Figure 1D). These observations suggest that SNO mediates both an augmentation of Δ[Ca2+] and a decrease in myocardial sensitivity to Ca2+ during βAR stimulation.
Figure 1. Enhanced denitrosylation alters the intracellular Ca2+ response to βAR stimulation.
(A) Left, representative continuous recordings of sarcomere length and Ca2+ transients (Δ[Ca2+]) in isolated cardiomyocytes before and during infusion of ISO at 10 nmol/L. Right, representative traces of sarcomere length and Δ[Ca2+] after different doses of ISO in WT and GSNOR-Tg (TG) cardiomyocytes paced at 2 Hz. (B) Dose-dependent changes of sarcomere shortening and Δ[Ca2+] in response to ISO in isolated WT and TG cardiomyocytes. n=4 mice (13–18 cells per mouse) per group. (C) Calcineurin activity in isolated WT and TG hearts treated with or without ISO (10 μmol/l). n=5 mice per group. (D) Sarcomere shortening and Δ[Ca2+] in response to ISO at 10 nmol/L with or without Carboxy-PTIO (C-PTIO, 300 μmol/L) in isolated WT cardiomyocytes. n=4 mice (11–15 cells per mouse) per group. ##P<0.01 and ###P<0.001 (vs baseline), *P<0.05, **P<0.01, ***P<0.001.
Enhanced denitrosylation prevents LV hypertrophy and dysfunction during chronic βAR stimulation
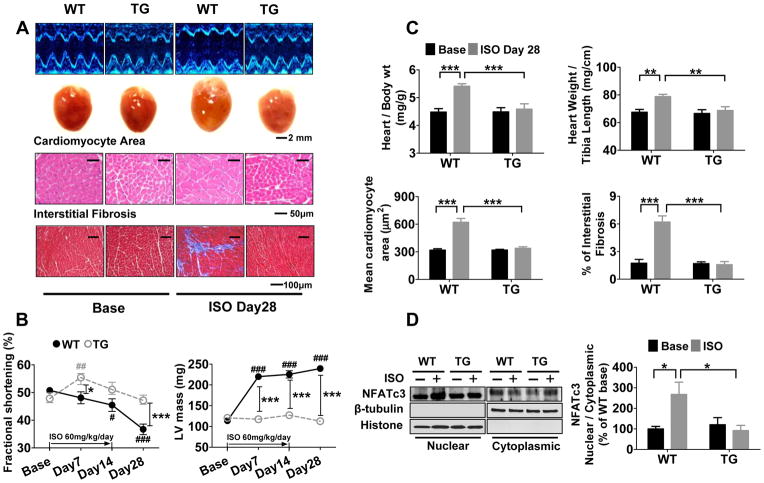
Increased Δ[Ca2+] activates calcineurin, leading to the translocation of nuclear factor of activated T-cells (NFAT) and the activation of downstream hypertrophic signaling. Because GSNOR-Tg inhibited ISO-induced increases in both Δ[Ca2+] and calcineurin activity (Figure 1B and 1C), we surmised that denitrosylation may mitigate LV remodeling in response to adrenergic stimulation. Infusion of ISO (60 mg/kg/day) in WT mice for 2 weeks resulted in marked LV and cardiomyocyte hypertrophy and myocardial fibrosis, associated with the nuclear translocation of NFATc3 (Figure 2). GSNOR-Tg prevented ISO-induced translocation of NFATc3 and the associated LV remodeling and dysfunction. In contrast, when we challenged WT and GSNOR−/− mice with a lower dose of ISO (30 mg/kg/day), GSNOR deficiency worsened LV remodeling and dysfunction (Online Figure II). These observations suggest that S-nitrosothiol homeostasis plays an important role in limiting pathological hypertrophy by titrating Δ[Ca2+].
Figure 2. Enhanced denitrosylation mitigates LV remodeling after chronic βAR stimulation.
(A) Representative echocardiography images (Top), heart images (middle) and histological images of hematoxylin and eosin staining for cardiomyocyte area (upper histological image) and Masson’s Trichrome staining of interstitial fibrosis (lower histological image) at baseline and 28 days after starting ISO infusion for 14 days (ISO Day28, i.e., 14 days after the end of ISO infusion) in WT and TG mice. (B) Fractional shortening and left ventricular mass calculated by echocardiography at baseline, and 7, 14 and 28 days after the start of ISO infusion for 14 days in WT and TG mice. n=5–7 mice per group. (C) Heart weight/body weight ratio, heart weight/tibia length ratio, mean cardiomyocyte area in LV sections (average of 207±12 cells were measured from 20 fields per mouse), and interstitial fibrosis in LV section (11 fields per mouse) in WT and TG mice at baseline and 28 days after starting ISO infusion for 14 days. N=4–7 mice per group. (D) Representative western blots and summary data showing the amount of NFATc3, β-tubulin, and histone of purified nuclear and cytoplasmic fraction in WT and TG hearts. β-tubulin and histone were used as a loading control for purified nuclear and cytoplasmic fractions, respectively. n=4–6 mice per group. ##P<0.01 and ###P<0.001 (vs baseline), *P<0.05, **P<0.01, ***P<0.001.
GSNOR overexpression or NOS inhibition prevents S-nitrosylation of Ca2+ handling proteins after βAR stimulation
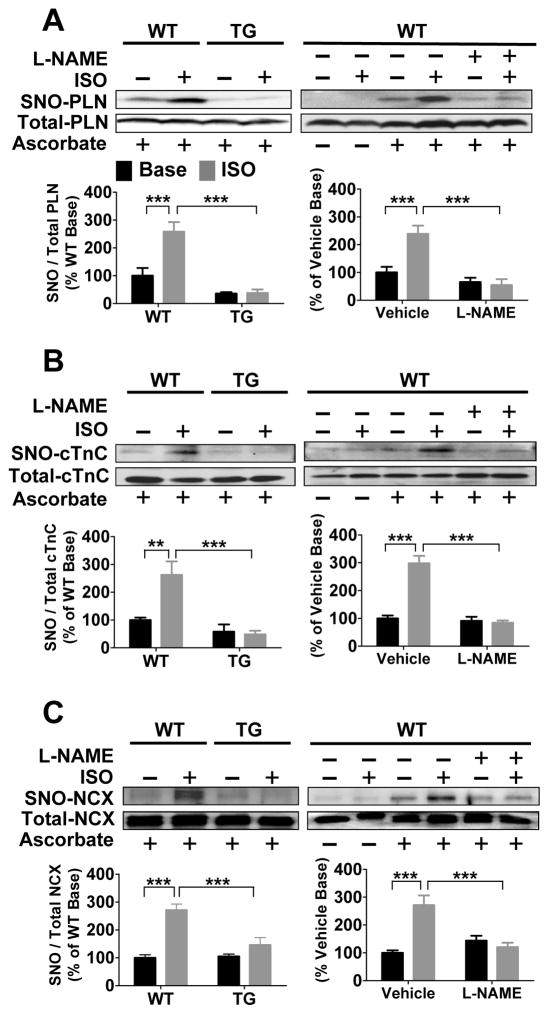
We used both the modified biotin switch (mBS) assay and phenyl mercury resin-assisted capture (MRC) method to identify Ca2+ handling proteins that undergo SNO after βAR stimulation in lysates of isolated WT and GSNOR-Tg cardiomyocytes incubated with ISO (10 nmol/l). Stimulation by ISO increased the SNO of PLN, NCX, and cTnC, but not RyR2, SERCA2a, LTCC, cTnI, tropomyosin, or actin, in WT cardiomyocytes, as detected by mBS (Figure 3, Online Figure III). GSNOR-Tg or inhibition of NO synthesis with L-NG-nitroarginine-methylester (L-NAME) blocked ISO-induced SNO of PLN, NCX, and cTnC (Figure 3). On the other hand, we found that all Ca2+ handling proteins probed were S-nitrosylated in ISO-treated WT, but not in GSNOR-Tg, cardiomyocytes, as determined by MRC (Online Figure IV). Thus βAR stimulation induces GSNOR coupled, reversible SNO of multiple Ca2+ handling proteins including PLN, NCX, and cTnC.
Figure 3. GSNOR overexpression or inhibition of NOS prevents isoproterenol-induced S-nitrosylation of Ca2+ handling proteins.
Representative modified biotin switch assay blots of S-nitrosylated PLN (A), cTnC (B), and NCX (C) in GSNOR-Tg cardiomyocytes, and WT cardiomyocytes with or without L-NAME at baseline and after ISO treatment. n=5–6 mice per group. **P<0.01, ***P<0.001.
Phosphorylation of PLN is insufficient for βAR-induced PLN pentamerization
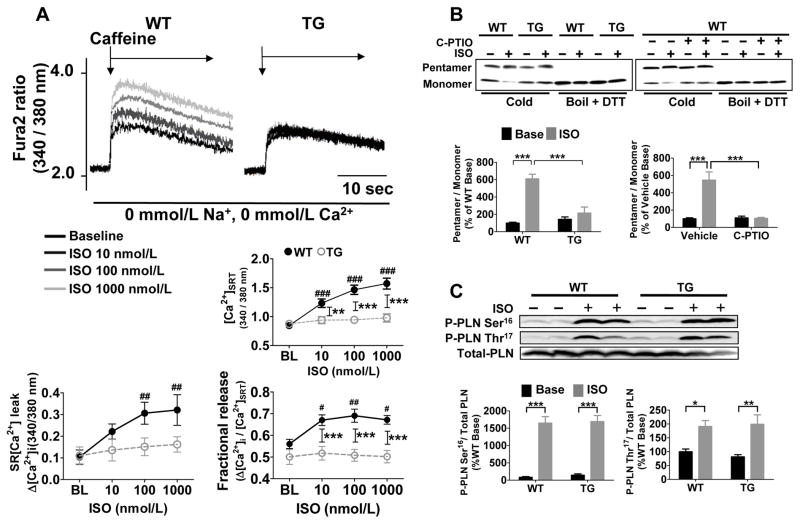
The amount of Ca2+ released into the cytosol is predominantly determined by the function of LTCC and SR.2 Because GSNOR-Tg did not affect LTCC current with or without ISO (Online Figure V), we tested the effect of GSNOR on SR function. βAR stimulation increased [Ca2+]SRT, SR[Ca2+]leak, and fractional Ca2+ release in a dose-dependent manner in WT, but not in GSNOR-Tg, cardiomyocytes (Figure 4A). These results suggest that ISO failed to activate SERCA2a in GSNOR-Tg myocytes. The monomeric form of PLN inhibits SERCA2a, while the pentameric form is functionally inactive.20 Under basal conditions, the PLN pentamer/monomer ratio was similar in WT and GSNOR-Tg hearts (Figure 4B). In WT cardiomyocytes, ISO markedly increased the PLN pentamer/monomer ratio, whereas in GSNOR-Tg myocytes or in WT cardiomyocytes treated with carboxy-PTIO, ISO failed to alter the PLN pentamer/monomer ratio (Figure 4B). Of note, the levels of ISO-induced PLN phosphorylation at Ser16 and Thr16 did not differ between WT and GSNOR-Tg cardiomyocytes (Figure 4C), indicating that PKA and CaMKII-mediated phosphorylation of PLN is not altered by GSNOR-Tg, and that it is not sufficient to cause pentamerization without concomitant SNO.
Figure 4. PLN S-nitrosylation is required for isoproterenol to induce PLN pentamerization and activate SERCA2a.
(A) Representative tracings of SR Ca2+ load measurements in WT and GSNOR-Tg (TG) cardiomyocytes at baseline and after ISO stimulation (10, 100 and 1000 nmol/L). Dose-dependent changes of SR Ca2+ load, SR Ca2+ leak, and fractional Ca2+ release in WT and TG cardiomyocytes in response to ISO stimulation. n=18–25 cells per group. ##P<0.01, ###P<0.001 (vs baseline), **P<0.01, ***P<0.001. (B) Representative immunoblots showing levels of pentamer and monomer PLN in TG hearts, and WT hearts with or without carboxy-PTIO (C-PTIO) at baseline and after ISO treatment. Samples that were boiled and treated with dithiothreitol (DTT) are shown as all monomer control. Effects of ISO on PLN pentamer/monomer ratio calculated from immunoblots in TG and WT with and without carboxy-PTIO. Data were normalized to the levels in WT hearts at baseline. (C) Representative immunoblots and relative quantification of PLN phosphorylation at Ser16 and Thr16 versus total PLN in WT and TG cardiomyocytes without (Base) or with ISO treatment. n=5 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs. baseline.
S-nitrosylation of PLN at Cys36 and/or Cys41 is required for SERCA2a activation
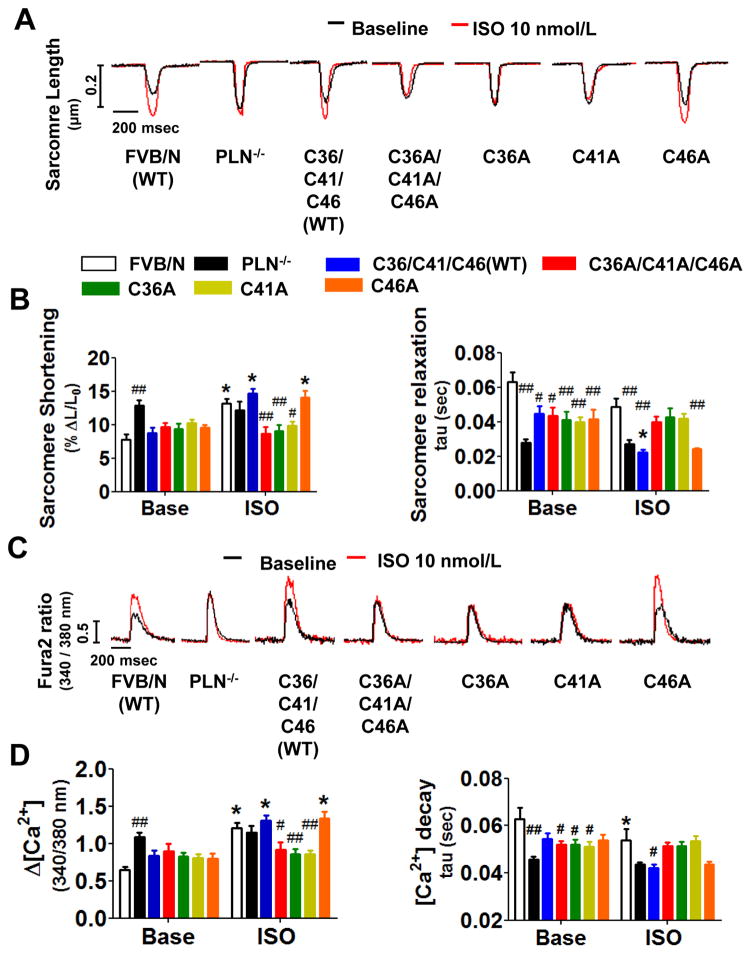
PLN has three cysteine residues (Cys36, Cys41, and Cys46), which are located in the hydrophobic C-terminal transmembrane region (Online Figure VIA-VIC), and likely modulate oligomerization of PLN.21 To elucidate the role of βAR-induced SNO of PLN on Ca2+ handling, we constructed recombinant adenovirus vectors encoding mutant PLN in which cysteines are replaced by alanines (Online Figure VID and VIE). In HEK293 cells expressing Ad-PLN, mutation of all cysteines to alanines (C36A/C41A/C46A) abolished SNO of PLN by exogenous CysNO, a cell permeable S-nitrosothiol (Online Figure VIF). Moreover, in HUVEC (with intact βAR) expressing Ad-PLN, ISO nitosylated WT PLN, but not mutant PLNC36A/C41A/C46A (Online Figure VIG). We examined the effects of expressing SNO resistant PLN mutants on Ca2+ handling in PLN−/− cardiomyocytes. As reported previously, ISO failed to augment Δ[Ca2+] and SL shortening or to accelerate sarcomere relaxation and Δ[Ca2+] decay in PLN−/− cardiomyocytes transfected with empty adenovirus encoding the RFP marker alone (Figure 5).22 Ad-mediated expression of WT PLN or PLNC46A, but not PLNC36A, PLNC41A, or PLNC36A/C41A/C46A, restored the ability of ISO to enhance Δ[Ca2+] and contraction in PLN−/− cardiomyocytes (Figure 5). These observations suggest that ISO-induced SNO of Cys36 and/or Cys41 (in addition to phosphorylation of Ser16 and Thr16) is required for PLN pentamerization that leads to SERCA2a activation.
Figure 5. S-nitrosylation of PLN at Cys36 and/or Cys41 is required for βAR-induced SERCA2a activation.
(A) Representative tracings of sarcomere length of wild-type FVB/N transfected with empty virus (FVB/N), PLN−/− cardiomyocytes transfected with empty virus (PLN−/−), and PLN−/− cardiomyocytes expressing wild-type (C36/C41/C46 WT) or mutant PLN with or without ISO treatment. (B) Summary of percent sarcomere shortening and relaxation rate. N=11–19. (C) Representative Ca2+ transient tracing and (D) summary of Δ[Ca2+] and Ca2+ decay rate. N=11–19 *P<0.01 (vs baseline), #P<0.01 and ##P<0.001 (vs FVB/N).
Denitrosylation activates NCX
Although ISO-induced SERCA2a activation was impaired in GSNOR-Tg cardiomyocytes, GSNOR-Tg did not affect the rates of Δ[Ca2+] decay and SL relengthening at baseline or after βAR stimulation (Online Figure VIIA and VIIB). This suggests that an alternative mechanism of Ca2+ removal compensates for the lack of SERCA2a activity in GSNOR-Tg cardiomyocytes treated with ISO. We found that ISO dose-dependently increased NCX activity in GSNOR-Tg, but not in WT cardiomyocytes (Online Figure VIIC and VIID). Inhibition of NCX with SEA-0400 slowed Δ[Ca2+] decay more markedly in GSNOR-Tg than in WT cardiomyocytes (Online Figure VIIE and VIIF). Together with the lack of ISO-induced SNO of NCX in GSNOR-Tg cardiomyocytes (Figure 3C), these results suggest that βAR-induced upregulation of NCX activity (presumably due to PKA-induced phosphorylation) is counteracted by SNO resulting in minimal changes in NCX activity in WT hearts. The increased NCX activity in GSNOR-Tg hearts may therefore be an underlying mechanism contributing to the accelerated Δ[Ca2+] decay during βAR stimulation, at least partially taking over the Ca2+ removal function normally performed by SERCA2a.
Protein S-nitrosylation is required for the ability of βAR stimulation to reduce myocardial Ca2+ responsiveness
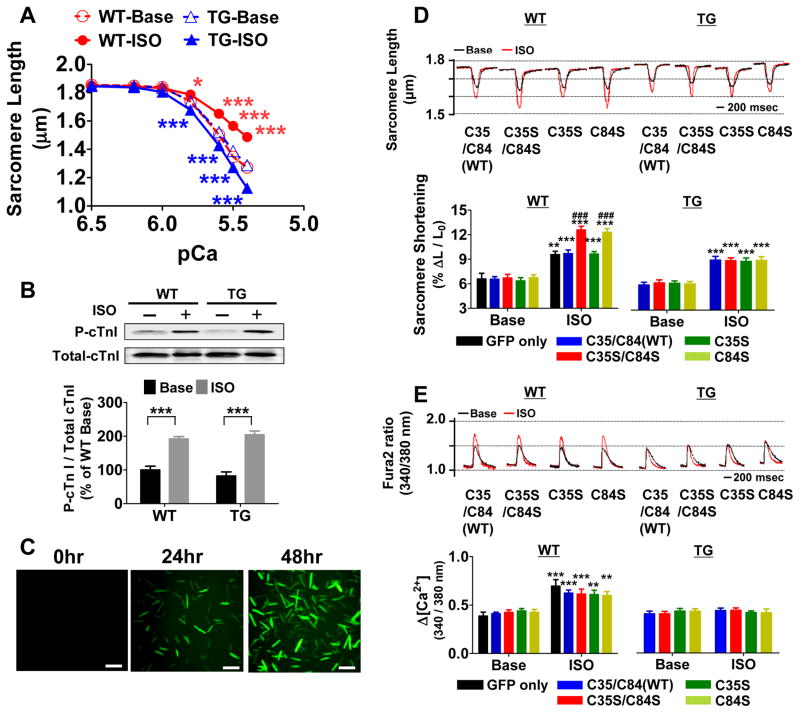
Increments in extracellular [Ca2+] concentration-dependently increased contraction in GSNOR-Tg and WT cardiomyocytes, whereas GSNOR-Tg markedly attenuated the ability of extracellular [Ca2+] to increase Δ[Ca2+] suggesting an increased myocardial Ca2+ responsiveness in GSNOR-Tg (Online Figure VIIIA and VIIIB). To assess the myofilament Ca2+ sensitivity, we measured Ca2+-induced changes in SL in unloaded permeabilized cardiomyocytes. At baseline, Ca2+ sensitivity was similar between WT and GSNOR-Tg cardiomyocytes (Figure 6A). Incubation with ISO decreased myofilament Ca2+ sensitivity in WT cardiomyocytes, while ISO increased Ca2+ sensitivity in GSNOR-Tg cardiomyocytes (Figure 6A). Of note, phosphorylation of cTnI at Ser22/23, an important regulator of Ca2+ sensitivity, was increased to a similar extent by ISO in WT and GSNOR-Tg hearts (Figure 6B). These results suggest that protein SNO is required for the ability of ISO to reduce myfilament Ca2+ sensitivity.
Figure 6. De-nitrosylation of cTnC increases myocardial Ca2+ sensitivity during βAR activation.
(A) Myofilament Ca2+ sensitivity in Triton-permeabilized WT and GSNOR-Tg (TG) cardiomyocytes at baseline and after ISO challenge (10 nmol/L). (B) Ser22,23 phosphorylation of cTnI in WT and TG cardiomyocytes without (base) or with ISO. n=5 mice per group. *P<0.05, **P<0.01, ***P<0.001 vs. baseline. (C) Representative photomicrographs of cardiomyocytes culture before and 24 and 48 hours after infection with Ad.cTnC.EGFP. (D) Representative sarcomere length tracings and summary of percent sarcomere shortening in WT (n=20–28 cells per group) and TG (n=24–36 cells per group) cardiomyocytes expressing WT or mutant cTnC with or without ISO treatment. (E) Representative Δ[Ca2+] tracing and summary of Δ[Ca2+] of WT and TG cardiomyocytes expressing WT or mutant cTnC with or without ISO treatment. **P<0.01, ***P<0.001 (* vs each baseline), and ###P<0.001 (# vs other data points).
Enhanced de-nitrosylation augments the temperature-dependent increase of myocardial Ca2+ responsiveness
To define the mechanisms responsible for the increased myocardial Ca2+ sensitivity in ISO-treated GSNOR-Tg cardiomyocytes, we examined the impact of temperature on myocardial Ca2+ responsiveness with or without ISO, as assessed by the ratio of %ΔL/L0/Δ[Ca2+], during twitch contraction in intact cardiomyocytes. Myocardial Ca2+ responsiveness was similar at 37°C and 25°C in WT cardiomyocytes with or without ISO, although there was a trend towards lower Ca2+ responsiveness with ISO at both temperatures (Table 1). Increase of temperature from 25°C to 37°C markedly increased Ca2+ responsiveness in GSNOR-Tg cardiomyocytes (Table 1). ISO increased Ca2+ responsiveness at 37°C, but not at 25°C, in GSNOR-Tg cardiomyocytes. Since increases in Ca2+ responsiveness with temperature is largely determined by the temperature dependence of cross-bridge cycling rates,23 these results suggest that enhanced de-nitrosylation increases myocardial Ca2+ responsiveness during βAR stimulation by accelerating cross-bridge cycling at physiological temperature.
Table 1.
Percent SL shortening (%ΔL/L0), Δ[Ca2+], and ratio of %ΔL/L0/Δ[Ca2+] (Ratio) in WT and GSNOR-Tg (TG) cardiomyocytes at baseline (Base) and after ISO stimulation at 25 or 37°C. n=14–18 cells.
| 25°C | 37°C | ||||
|---|---|---|---|---|---|
| Base | ISO | Base | ISO | ||
| WT | %ΔL/L0 | 13.9±0.5 | 17.2±0.3## | 10.9±0.3** | 15.6±0.3##* |
| Δ[Ca2+] | 0.7±0.1 | 1.0±0.1# | 0.5±0.1 | 0.8±0.1## | |
| %ΔL/L0/Δ[Ca2+] | 22.8±2.6 | 20.3±2.3 | 24.8±3.1 | 19.4±1.2 | |
| TG | %ΔL/L0 | 11.8±0.7 | 18.1±0.5## | 10.5±0.9 | 15.7±0.6## |
| Δ[Ca2+] | 0.7±0.1 | 0.9±0.1# | 0.5±0.1 | 0.6±0.1** | |
| %ΔL/L0/Δ[Ca2+] | 18.2±1.7 | 20.4±1.3 | 21.3±2.1 | 29.7±2.2#* |
S-nitrosylation of cTnC at Cys84 is required for the βAR-induced reduction of myocardial Ca2+ sensitivity
To examine the impact of SNO of cTnC on myocardial Ca2+ responsiveness, we constructed recombinant adenoviral vectors encoding mutant cTnC in which cysteines (Cys35 and or Cys84) are replaced with serines (Online Figure VIIIC and VIIID). In HEK293 cells expressing cTnC, mutation of both cysteines to serines (C35S/C84S) abolished SNO of cTnC by exogenous CysNO (Online Figure VIIIE). In HUVEC, ISO-dependent SNO of cTnC was eliminated by the C35S/C84S mutation (Online Figure VIIIF). Wild-type cardiomyocytes expressing cTnCC84S or cTnCC35S/C84S exhibited a marked increase in twitch contraction in response to ISO compared to myocytes expressing wild-type cTnC or cTnCC35S (Figure 6D). Expression of any of the mutant cTnCs did not affect ISO-induced increases in Δ[Ca2+], implying an increased myocardial Ca2+ responsiveness as the basis of the effects of C84S mutation (cTnCC84S) (Figure 6E). Notably, mutation of cTnC did not alter the responses to ISO in GSNOR-Tg cardiomyocytes, which show impaired SNO (Figure 6D). Expression of mutant cTnC did not affect sarcomere relaxation or Δ[Ca2+] decay in either genotype (Online Figure IXA and IXB). These results suggest that βAR-induced SNO of cTnC-Cys84 is required for the reduction of myocardial Ca2+ sensitivity in normal hearts stimulated with ISO.
DISCUSSION
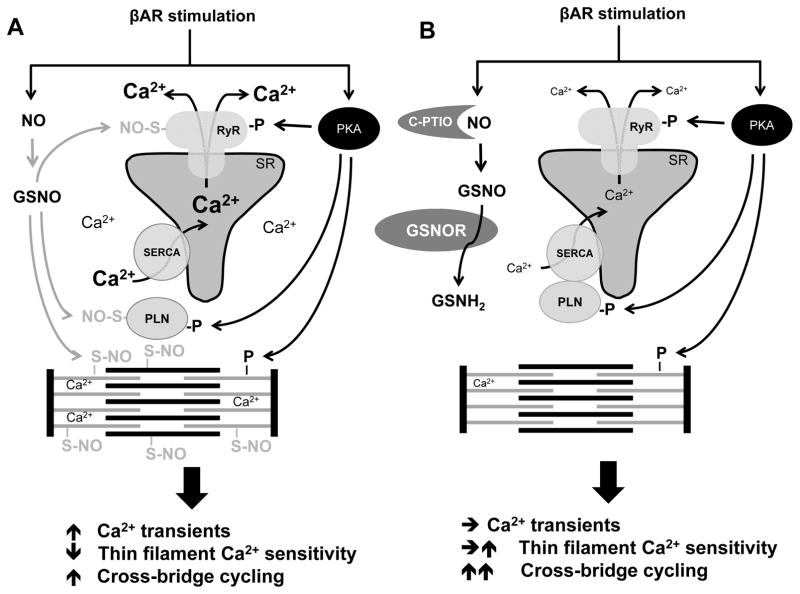
The current observations uncover the existence of previously unrecognized signaling events dependent on S-nitrosylation of key Ca2+ handling proteins during βAR stimulation. While multiple Ca2+ handling SNO-proteins including PLN have been previously reported,24 our findings reveal a far more important role for S-nitrosylation in transduction of adrenergic activation and hypertrophic signaling than previously thought. Phosphorylation and S-nitrosylation in fact work in concert to control calcium homeostasis (Figure 7).
Figure 7. Hypothetical impact of protein SNO on Ca2+ handling in βAR stimulation.
(A) Normal cardiomyocytes with intact PKA-dependent phosphorylation and NO-dependent SNO. (B) Cardiomyocytes with GSNOR-Tg or treated with carboxy-PTIO (C-PTIO) with impaired SNO.
As is the case for phosphorylation, S-nitrosylation is pervasive. Therefore, many proteins are potential targets for both S-nitrosylation and phosphorylation.25 Using two distinct enrichment techniques for S-nitrosylated proteins, we found that many, if not all, cardiac Ca2+ handling proteins can be S-nitrosylated by βAR stimulation. Although different sets of S-nitrosylated proteins were detected by mBS and MRC (reflecting the differences in reactivities of assay reactants i.e. ascorbate vs. organic mercury, respectively), ISO-induced S-nitrosylation of Ca2+ handling proteins was completely inhibited by GSNOR-Tg, establishing the specificity of both techniques. Furthermore, three Ca2+ handling proteins (i.e., PLN, NCX, and cTnC) were found to be S-nitrosylated in WT cardiomyocytes after βAR stimulation by both methods, suggesting the robustness of these SNO-proteins. Consistent with this, SNO-resistant Cys mutants in PLN and cTnC recapitulate the phenotype of GSNOR-Tg cardiomyocytes (i.e., impairment of ISO-induced increase of Δ[Ca2+] and ISO-induced enhancement of Ca2+ sensitivity, respectively). Our data suggest that S-nitrosylation of PLN and cTnC is critically important for the transduction of βAR signaling in cardiomyocytes.
The central role of PLN in myocardial Ca2+ handling has been established by studies using PLN−/− mice.26 Previous studies using a non-phosphorylatable mutant of PLN showed that phosphorylation of PLN at Ser16 is necessary for mediating the maximal myocardial response to βAR stimulation.3, 22 However, the SNO status of PLN SNO was not addressed in that study. The current results show that βAR stimulation induces both phosphorylation and SNO of PLN. Moreover, when S-nitrosylation of PLN was prevented by GSNOR-Tg or NO scavenging, βAR stimulation failed to induce PLN pentamerization and SERCA2a activation despite intact phosphorylation of PLN. These results indicate that phosphorylation is necessary but not sufficient for PLN pentamerization and activation of SERCA2a during βAR stimulation. It has been postulated that cysteine residues in the transmembrane domain of PLN play a role in pentamer formation and stability.27 Whether or not SNO-PLN leads to disulfide bond formation between PLN monomers remains to be determined.28
βAR stimulation exerts inotropic effects as a net consequence of increased Δ[Ca2+], reduced thin filament Ca2+ sensitivity, and enhanced cross-bridge cycling (Figure 7). While the relative contribution of these three components to βAR-induced inotropy is still debated, mathematical modeling suggests that enhanced cross-bridge cycling plays a central role in the βAR-induced twitch responses.29 Without it, the Δ[Ca2+] is only able to offset the intrinsic negative inotropic effect of ISO-induced reduction in thin filament Ca2+ sensitivity. In the current study, we observed that GSNOR-Tg abolished the ability of ISO to increase Δ[Ca2+] without impairing inotropic twitch responses. Similarly, cTnCC84S mutation further augmented ISO-induced inotropy without augmenting Δ[Ca2+]. These observations suggest that GSNOR-Tg and cTnCC84S either prevented the reduction of thin filament Ca2+ sensitivity and/or augmented the cross-bridge cycling in response to ISO.
βAR signaling is transduced through the cTnI N-terminus (residues 1–30), which is thought to be bound to the N-domain of cTnC when unphosphorylated and to dissociate upon phosphorylation at Ser23/24.30 The dissociation of cTnI N-terminus decreases the affinity of the cTnI switch region (residues 147–163) to the N-domain of cTnC thus decreasing the Ca2+ sensitivity of thin filaments.31 The crystal structure of cTnC shows that Cys84 is located in the hydrophobic core at the D-helix of the N-domain that interacts with the switch region of cTnI (Online Figure IX C).31, 32 It has been reported that Cys84 contributes to formation of intramolecular (within cTnC) or intermolecular (between cTnC and cTnI) disulfide bonds, which may affect the cTnI-cTnC interaction.33 These data support the idea that βAR-induced nitrosylation of cTnC at Cys84 may regulate cTnI-cTnC interactions thereby influencing myofilament Ca2+ sensitivity. Indeed, a missense mutation of cTnC (cTnCC84Y) has been found to be associated with hypertrophic cardiomyopathy and to increase the Ca2+ sensitivity of reconstituted skinned fibers, indicating an important role of Cys84 in modulating Ca2+ sensitivity.34
Alternatively, SNO may alter myocardial Ca2+ sensitivity by modulating cross-bridge cycling kinetics. It is known that physiological temperature (e.g., 37°C) increases myocardial Ca2+ responsiveness primarily due to increased cross-bridge cycling rates compared to lower temperatures (e.g., 15–25°C).23, 35 We observed that the temperature-dependent increase of Ca2+ responsiveness was markedly exaggerated in ISO-treated GSNOR-Tg cardiomyocytes. These results suggest that ISO-induced twitch inotropy in GSNOR-Tg cardiomyocytes is at least partly mediated by enhanced cross-bridge cycling. This hypothesis is consistent with a previous report that NO reduces myocardial Ca2+ sensitivity by slowing cross-bridge cycling rates.36 The temperature dependency of the effects of SNO-myofilament proteins also explains why the effects of SNO to reduce myofilament Ca2+ sensitivity were not observed in most previous studies, where myofilament Ca2+ sensitivity was typically measured only at or below room temperature (e.g., 15°C).37
In addition to its well-documented role in myocardial contractile function, Ca2+ plays an important role in programming myocardial hypertrophy including the calcineurin-NFAT signaling.2, 38 Because GSNOR-Tg attenuated ISO-induced increases in Δ[Ca2+], hypertrophic signaling, and LV dysfunction, whereas GSNOR−/− worsened LV remodeling after chronic ISO infusion, we suggest that protein de-nitrosylation protects against pathologic LV remodeling and heart failure in response to chronic βAR activation. Collectively, these results suggest that cellular levels of S-nitrosothiols may determine the fate of cardiomyocytes under chronic adrenergic stimulation by modulating Ca2+ handling.
Protein SNO may exert harmful or beneficial effects depending on the cellular context. GSNOR−/− mice were shown to have increased tissue damage and mortality after sepsis,11 whereas GSNOR-Tg prevented sepsis-induced LV dysfunction in mice.13 Consistent with our current results, βAR-induced hyper-S-nitrosylation of RyR2 was shown to increase SR[Ca2+]leak and depress the myocardial response to βAR stimulation in GSNOR−/− mice.9 On the other hand, GSNOR deficiency prevented oxidative stress-induced increase in SR[Ca2+]leak,39 and reduced myocardial injury after permanent left coronary artery ligation.40 Although the current study showed that enhanced de-nitrosylation has protective effects against LV remodeling in response to chronic βAR stimulation, the effects of GSNOR on the development of LV dysfunction remain to be determined in more clinically relevant models of heart failure.
It has been reported that NOS1 deficiency depresses SERCA2a function at baseline and blunts positive inotropic response to βAR stimulation.41, 42 Because NOS1 is localized to the SR, PLN S-nitrosylation is likely impaired in NOS1−/− cardiomyocytes, in line with our results in GSNOR-Tg mice in which ISO failed to activate SERCA2a and nitrosylate PLN. Nonetheless, the phenotype of GSNOR-Tg cardiomyocytes is unlikely to be the same as NOS1−/− or NOS1/NOS3 double knockout mice.8 GSNOR only de-nitrosylates a subset of the nitrosoproteome,43 whereas SNO depletion may be more complete in NOS1/NOS3 double knockout cardiomyocytes. In fact, we observed no difference in the SNO levels of Ca2+ handling proteins at baseline between WT and GSNOR-Tg cardiomyocytes. In addition, deficiency of NOS abolishes both cGMP-dependent and –independent effects (i.e., SNO) of NO, whereas GSNOR-Tg attenuates protein SNO and leaves cGMP-dependent effects intact.13 These differences may explain the discrepancies in Ca2+ handling parameters obtained in our current studies in GSNOR-Tg mice and previous studies using NOS deficient mice.
Calcium is the primary determinant of cardiac force production. Alterations in phosphorylation levels of Ca2+-handling proteins downstream of the βAR not only control myocardial contraction and relaxation but also represent molecular signatures of failing hearts. Our observations reveal previously unrecognized roles for S-nitrosylation of Ca2+-handling proteins, particularly PLN and cTnC, in transducing adrenergic and hypertrophic responses of the heart. S-nitrosylation may thus operate in parallel with protein phosphorylation to exert widespread control over cardiac function and both may go awry in disease.44 Denitrosylation-based approaches may represent a new therapeutic strategy to improve cardiac function in settings of increased nitrosative stress, including heart failure,45 myocardial ischemia,46 and sepsis.13
Supplementary Material
307157DR1 Online Data Supplement
Novelty and Significance.
What Is Known?
- Regulation of calcium (Ca2+) homeostasis by beta-adrenergic receptor (βAR) activation is a fundamental mechanism of sympathetic regulation of myocardial function, as well as a basis for understanding molecular events that result in hypertrophic signaling and heart failure.
- Sympathetic activation of βAR not only induces protein phosphorylation but also stimulates nitric oxide (NO) production.
- NO-dependent protein S-nitrosylation modulates myocardial function.
What New Information Does This Article Contribute?
- S-nitrosylation provides an essential parallel pathway that acts in concert with phosphorylation to regulate cardiac Ca2+ homeostasis.
- βAR stimulation-induced S-nitrosylation of phospholamban (PLN) is necessary for its pentamerization, which increases Ca2+ transients, whereas S-nitrosylation of troponin C (cTnC) is required to decrease myocardial Ca2+ sensitivity.
- Enhanced de-nitrosylation prevents pathological left ventricular hypertrophy induced by chronic βAR activation by limiting Ca2+ overload.
Sympathetic activation critically regulates myocardial function via βAR-dependent signaling. Activation of βAR modulates calcium handling of cardiac myocytes typically via protein phosphorylation. Although NO modulates cardiac function by S-nitrosylating proteins, how S-nitrosylation affects βAR signaling was incompletely understood. Using transgenic mice to titrate the levels of protein S-nitrosylation, we uncovered unanticipated roles for protein S-nitrosylation in general, and of PLN and cTnC in particular, in the βAR-dependent regulation of Ca2+ homeostasis. Notably, S-nitrosylation of PLN consequent upon βAR activation is necessary to augment Ca2+ release from sarcoplasmic reticulum during contraction. In parallel, S-nitrosylation of cTnC is required to decrease myocardial Ca2+ sensitivity in response to βAR stimulation. Global inhibition of cellular S-nitrosylation prevents hypertrophic signaling in response to chronic adrenergic stimulation, whereas selective inhibition of cTnC S-nitrosylation recapitulates the impact of naturally occurring mutation (cTnCC84Y). Thus S-nitrosylation provides a previously unrecognized parallel pathway, at least in part through PLN and cTnC, which acts in concert with phosphorylation to regulate cardiac Ca2+ homeostasis. Inhibition of S-nitrosylation of specific protein targets may prove to be a therapeutic option for the treatment of heart failure.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the American Heart Association – Philips Resuscitation Fellowship Award (PYS), Research Training Fellowship for Medical Students from the Howard Hughes Medical Institute (PJ), and National Institute of Health grants HL26057 (EGK), HL54926 (HI), HL075443 (JSS), and HL110378 (FI).
Nonstandard Abbreviations and Acronyms
βAR
beta-adrenergic receptor
cAMP
cyclic adenosine monophosphate
cGMP
cyclic guanosine monophosphate
CaMKII
calcium/calmodulin-dependent protein kinase II
cTnC
cardiac Troponin C
cTnI
cardiac Troponin I
CysNO
S-nitrosocysteine
Δ[Ca2+]
calcium transients
GSNO
S-nitrosoglutathione
GSNOR
S-nitrosoglutathione reductase
GSNOR-Tg
cardiomyocyte-specific GSNOR overexpression
ISO
isoproterenol
L-NAME
L-NG-nitroarginine-methylester
LTCC
L-type calcium channel
LV
left ventricle
mBS
modified biotin switch
MRC
mercury resin-assisted capture
NCX
sodium-calcium exchanger
NO
nitric oxide
NOS
NO synthase
PKA
protein kinase A
PLN
phospholamban
RFP
red fluorescent protein
RyR2
ryanodine receptor
SERCA2
sarcoplasmic reticulum Ca2+ ATPase
sGC
soluble guanylate cyclase
SL
sarcomere length
SNO
S-nitrosylation
SR
sarcoplasmic reticulum
[Ca2+]SRT
SR calcium load
SR[Ca2+]leak
SR calcium leak
TPM
tropomyosin
WT
wild-type
%ΔL/L0
percent sarcomere length shortening
Footnotes
References
- 1.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin i in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000;278:H769–779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- 4.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godecke A, Heinicke T, Kamkin A, Kiseleva I, Strasser RH, Decking UK, Stumpe T, Isenberg G, Schrader J. Inotropic response to beta-adrenergic receptor stimulation and anti-adrenergic effect of ach in endothelial no synthase-deficient mouse hearts. J Physiol. 2001;532:195–204. doi: 10.1111/j.1469-7793.2001.0195g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley EA, Sears CE, Bryant SM, Watkins HC, Casadei B. Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation. 2002;105:3011–3016. doi: 10.1161/01.cir.0000019516.31040.2d. [DOI] [PubMed] [Google Scholar]
- 7.Hare JM, Stamler JS. No/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 9.Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM. Dynamic denitrosylation via s-nitrosoglutathione reductase regulates cardiovascular function. Proc Natl Acad Sci U S A. 2012;109:4314–4319. doi: 10.1073/pnas.1113319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, Stamler JS. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Essential roles of s-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 12.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 13.Sips PY, Irie T, Zou L, Shinozaki S, Sakai M, Shimizu N, Nguyen R, Stamler JS, Chao W, Kaneki M, Ichinose F. Reduction of cardiomyocyte s-nitrosylation by s-nitrosoglutathione reductase protects against sepsis-induced myocardial depression. American Journal of Physiology - Heart and Circulatory Physiology. 2013;304:H1134–H1146. doi: 10.1152/ajpheart.00887.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shioya T. A simple technique for isolating healthy heart cells from mouse models. J Physiol Sci. 2007;57:327–335. doi: 10.2170/physiolsci.RP010107. [DOI] [PubMed] [Google Scholar]
- 15.Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res. 2007;100:130–139. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- 16.Iyer V, Heller V, Armoundas AA. Altered spatial calcium regulation enhances electrical heterogeneity in the failing canine left ventricle: Implications for electrical instability. J Appl Physiol (1985) 2012;112:944–955. doi: 10.1152/japplphysiol.00609.2011. [DOI] [PubMed] [Google Scholar]
- 17.Sips PY, Brouckaert P, Ichinose F. The alpha1 isoform of soluble guanylate cyclase regulates cardiac contractility but is not required for ischemic preconditioning. Basic Res Cardiol. 2011;106:635–643. doi: 10.1007/s00395-011-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrester MT, Foster MW, Benhar M, Stamler JS. Detection of protein s-nitrosylation with the biotin-switch technique. Free Radic Biol Med. 2009;46:119–126. doi: 10.1016/j.freeradbiomed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doulias PT, Greene JL, Greco TM, Tenopoulou M, Seeholzer SH, Dunbrack RL, Ischiropoulos H. Structural profiling of endogenous s-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein s-nitrosylation. Proc Natl Acad Sci U S A. 2010;107:16958–16963. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/serca2a regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLennan DH, Kimura Y, Toyofuku T. Sites of regulatory interaction between calcium atpases and phospholamban. Ann N Y Acad Sci. 1998;853:31–42. doi: 10.1111/j.1749-6632.1998.tb08254.x. [DOI] [PubMed] [Google Scholar]
- 22.Chu G, Lester JW, Young KB, Luo W, Zhai J, Kranias EG. A single site (ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to beta -agonists. J Biol Chem. 2000;275:38938–38943. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- 23.de Tombe PP, Stienen GJ. Impact of temperature on cross-bridge cycling kinetics in rat myocardium. J Physiol. 2007;584:591–600. doi: 10.1113/jphysiol.2007.138693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garofalo F, Parisella ML, Amelio D, Tota B, Imbrogno S. Phospholamban s-nitrosylation modulates starling response in fish heart. Proc Biol Sci. 2009;276:4043–4052. doi: 10.1098/rspb.2009.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohr MJ, Aponte AM, Sun J, Wang G, Murphy E, Gucek M, Steenbergen C. Characterization of potential s-nitrosylation sites in the myocardium. Am J Physiol Heart Circ Physiol. 2011;300:H1327–1335. doi: 10.1152/ajpheart.00997.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiss E, Edes I, Sato Y, Luo W, Liggett SB, Kranias EG. Beta-adrenergic regulation of camp and protein phosphorylation in phospholamban-knockout mouse hearts. Am J Physiol. 1997;272:H785–790. doi: 10.1152/ajpheart.1997.272.2.H785. [DOI] [PubMed] [Google Scholar]
- 27.Fujii J, Maruyama K, Tada M, MacLennan DH. Expression and site-specific mutagenesis of phospholamban. Studies of residues involved in phosphorylation and pentamer formation. J Biol Chem. 1989;264:12950–12955. [PubMed] [Google Scholar]
- 28.Burgoyne JR, Eaton P. Transnitrosylating nitric oxide species directly activate type i protein kinase a, providing a novel adenylate cyclase-independent cross-talk to beta-adrenergic-like signaling. J Biol Chem. 2009;284:29260–29268. doi: 10.1074/jbc.M109.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negroni JA, Morotti S, Lascano EC, Gomes AV, Grandi E, Puglisi JL, Bers DM. Beta-adrenergic effects on cardiac myofilaments and contraction in an integrated rabbit ventricular myocyte model. J Mol Cell Cardiol. 2015;81:162–175. doi: 10.1016/j.yjmcc.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finley N, Abbott MB, Abusamhadneh E, Gaponenko V, Dong W, Gasmi-Seabrook G, Howarth JW, Rance M, Solaro RJ, Cheung HC, Rosevear PR. Nmr analysis of cardiac troponin c-troponin i complexes: Effects of phosphorylation. FEBS Lett. 1999;453:107–112. doi: 10.1016/s0014-5793(99)00693-6. [DOI] [PubMed] [Google Scholar]
- 31.Baryshnikova OK, Li MX, Sykes BD. Modulation of cardiac troponin c function by the cardiac-specific n-terminus of troponin i: Influence of pka phosphorylation and involvement in cardiomyopathies. J Mol Biol. 2008;375:735–751. doi: 10.1016/j.jmb.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 32.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the ca(2+)-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 33.Putkey JA, Dotson DG, Mouawad P. Formation of inter- and intramolecular disulfide bonds can activate cardiac troponin c. J Biol Chem. 1993;268:6827–6830. [PubMed] [Google Scholar]
- 34.Pinto JR, Parvatiyar MS, Jones MA, Liang J, Ackerman MJ, Potter JD. A functional and structural study of troponin c mutations related to hypertrophic cardiomyopathy. J Biol Chem. 2009;284:19090–19100. doi: 10.1074/jbc.M109.007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puglisi JL, Bassani RA, Bassani JW, Amin JN, Bers DM. Temperature and relative contributions of ca transport systems in cardiac myocyte relaxation. Am J Physiol. 1996;270:H1772–1778. doi: 10.1152/ajpheart.1996.270.5.H1772. [DOI] [PubMed] [Google Scholar]
- 36.Heunks LM, Cody MJ, Geiger PC, Dekhuijzen PN, Sieck GC. Nitric oxide impairs ca2+ activation and slows cross-bridge cycling kinetics in skeletal muscle. J Appl Physiol (1985) 2001;91:2233–2239. doi: 10.1152/jappl.2001.91.5.2233. [DOI] [PubMed] [Google Scholar]
- 37.Albury AN, Swindle N, Swartz DR, Tikunova SB. Effect of hypertrophic cardiomyopathy-linked troponin c mutations on the response of reconstituted thin filaments to calcium upon troponin i phosphorylation. Biochemistry. 2012;51:3614–3621. doi: 10.1021/bi300187k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goonasekera SA, Molkentin JD. Unraveling the secrets of a double life: Contractile versus signaling ca2+ in a cardiac myocyte. J Mol Cell Cardiol. 2012;52:317–322. doi: 10.1016/j.yjmcc.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Dulce RA, Mayo V, Rangel EB, Balkan W, Hare JM. Interaction between neuronal nitric oxide synthase signaling and temperature influences sarcoplasmic reticulum calcium leak: Role of nitroso-redox balance. Circ Res. 2015;116:46–55. doi: 10.1161/CIRCRESAHA.116.305172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima B, Lam GKW, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous s-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Kohr MJ, Traynham CJ, Wheeler DG, Janssen PM, Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294:C1566–1575. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan SA, Skaf MW, Harrison RW, Lee K, Minhas KM, Kumar A, Fradley M, Shoukas AA, Berkowitz DE, Hare JM. Nitric oxide regulation of myocardial contractility and calcium cycling: Independent impact of neuronal and endothelial nitric oxide synthases. Circulation Research. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 43.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 44.Haldar SM, Stamler JS. S-nitrosylation: Integrator of cardiovascular performance and oxygen delivery. J Clin Invest. 2013;123:101–110. doi: 10.1172/JCI62854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canton M, Menazza S, Sheeran FL, Polverino de Laureto P, Di Lisa F, Pepe S. Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol. 2011;57:300–309. doi: 10.1016/j.jacc.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 46.Heinzel FR, Gres P, Boengler K, Duschin A, Konietzka I, Rassaf T, Snedovskaya J, Meyer S, Skyschally A, Kelm M, Heusch G, Schulz R. Inducible nitric oxide synthase expression and cardiomyocyte dysfunction during sustained moderate ischemia in pigs. Circ Res. 2008;103:1120–1127. doi: 10.1161/CIRCRESAHA.108.186015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
307157DR1 Online Data Supplement