Recurrent antibiotic exposure may promote cancer formation – another step in understanding the role of the human microbiota? (original) (raw)
. Author manuscript; available in PMC: 2016 Nov 1.
Published in final edited form as: Eur J Cancer. 2015 Aug 31;51(17):2655–2664. doi: 10.1016/j.ejca.2015.08.015
Abstract
Background
Bacterial dysbiosis was previously described in human malignancies. In a recent animal model, tumor susceptibility was transmitted using fecal transplantation. Our aim was to further evaluate possible association between antibiotic exposure and cancer risk.
Methods
We conducted nested case-control studies for 15 common malignancies using a large population-based electronic medical records database. Cases were defined as those with any medical code for the specific malignancy. Individuals with familial cancer syndromes were excluded. For every case, four eligible controls matched on age, sex, practice site, and duration of follow-up before index-date were selected using incidence-density sampling. Exposure of interest was antibiotic therapy >1 year before index-date. Adjusted odds-ratios (AORs) and 95%CIs were estimated for each antibiotic type using conditional logistic regression.
Results
125,441 cases and 490,510 matched controls were analyzed. For gastro-intestinal malignancies, the use of penicillin was associated with an elevated risk of esophageal, gastric, and pancreatic cancers. The association increased with the number of antibiotic courses and reached 1.4 for gastric cancers associated with >5 courses of penicillin (95%CI 1.2–1.8). Lung cancer risk increased with the use of penicillin, cephalosporines, or macrolides (AOR for >5 courses of penicillin: 1.4 95%CI 1.3–1.6). The risk of prostate cancer increased modestly with the use of penicillin, quinolones, sulphonamides and tetracyclines. The risk for breast cancer was modestly associated with exposure to sulphonamides. There was no association between use of anti-virals and anti-fungals and cancer risk.
Conclusion
Recurrent exposure to certain antibiotics may be associated with cancer risk in specific organ sites.
Keywords: Antibiotic, penicillin, colorectal, breast, lung, melanoma, prostate, pancreas, cancer, risk factor
Introduction
Antibiotic therapy is commonly used with up to 15% of the western population receiving at least one antibiotic course per-year1. In addition to targeting pathogenic bacteria, antibiotics alter the composition and decrease the diversity of human microbiota.2,3
Bacterial dysbiosis has been described in gastrointestinal4–6, genito-urinary7 and breast cancers8 as well as pre-malignant lesions in the colon.5 Numerous mechanisms have been proposed to explain the association between bacterial dysbiosis and cancer risk, including induction of chronic inflammation; changes in the normal tissue metabolism; direct genotoxic effects; and weakening of the immune response.5,9–13
Previous epidemiologic studies in humans evaluated the possible impact of antibiotic exposure on cancer risk in the lung14, breast15, prostate16, colon17, and skin17 with conflicting results. Important limitations of these studies included lack of adjustment for common cancer risk factors, reverse causality (cancer patients are at higher risk for infections), confounding by indication (infection may be a risk factor for cancer), protopathic bias (medication was prescribed due to symptom of undiagnosed cancer) and failure to account for changes in trends of antibiotic prescription over time as well as changes in antibiotic types used.
In a recent study in mice that were genetically susceptible to CRC, a distinct microbiota composition following high fat diet had a causative role in tumor progression. This phenotype could be transmitted to healthy mice using fecal samples while antibiotics were able to block tumor progression18. In addition, we recently reported a higher risk for colorectal cancer (CRC) associated with penicillin use >1 year before diagnosis date. The risk increased with increasing number of antibiotic courses prescribed (> 10 courses, adjusted OR 1.2, 95%CI 1.1–1.3).19 These data suggest the possibility that repeated antibiotic exposure and a subsequent change in microbiota diversity, both in the gut as well as in other body sites may promote cancer formation.
The current study aims to further evaluate the association between antibiotic exposure and cancer risk in multiple organ sites including the lung, breast, skin, gastrointestinal and genitourinary tract. As possible negative controls for this association, we selected melanoma (associated with exposure to ultraviolet radiation) and cervical cancer (associated with human papilloma virus). By analyzing different time intervals of prescriptions, detailed information regarding previous infectious events, and thorough cancer risk factors, we tried to avoid the bias that impaired the conclusions of previous studies.
Methods
Study Design
We conducted nested case-control studies for 15 different epithelial, mesenchymal and hematologic malignancies using The Health Improvement Network (THIN) database. This design is computationally more efficient than a cohort-study, and produces odds-ratios (ORs) that are unbiased estimates of incidence-rate-ratios.20 The study was approved by the Institutional Review Board at the University of Pennsylvania and by the Scientific Review Committee of THIN.
Data source
THIN is a large population-based electronic medical records database from the UK that contains data on approximately ten million patients treated by general practitioners (http://www.thin-uk.com/). THIN includes information on patient demographics, socioeconomic status, medical diagnoses, lab results, and drug prescriptions. Registration date is defined as the date when patients were first registered with a practice in THIN and Vision date is the date that a practice began using in-practice Vision software that collects information for the THIN database.21 Data quality is monitored through routine analysis of the entered data.22 The database has been previously used for pharmaco-epidemiology studies, showing excellent quality of information.23
Study cohort
All people receiving medical care from 1995–2013 from a THIN practitioner were potentially eligible for inclusion. Patients without acceptable medical records (i.e., incomplete documentation or out of sequence date of birth, registration date, date of death, or date of exit from the database) were excluded. Follow-up started at the later of either the Vision date or 183 days after the date on which the patient registered with the general practitioner24, and ended on the earliest of cancer diagnosis date, date of death, transferring out of the database, or the end date of the database.
In order to focus our analysis on sporadic cancer cases we excluded subjects that were diagnosed with any cancer before the age of 20 as well as individuals with familial cancer syndromes.
Case selection
Cases were defined as all individuals in the cohort with at least one Read code for the specific cancer during follow-up. Subjects who were diagnosed with cancer within the first 183 days after registration were excluded in order to avoid prevalent cases.24 The date of cancer diagnosis was regarded as the index-date for each case.
Selection of controls
Controls were selected based on incidence-density sampling.20 The eligible control pool for each case consisted of all individuals who remained at risk for the cancer at the time when the case was diagnosed. Up to four eligible controls were matched with each case on age, sex, practice site and both duration and calendar period of follow-up. Controls were assigned the same index-date as their matched cases.
Exposures and Covariates
The primary exposure of interest was number of antibiotic courses (0,1,2–5 and >5 courses), >1 year prior to index-date, with one of seven antibiotic classes commonly used in the outpatient setting: penicillins, cephalosporins, macrolides, tetracyclines, sulphonamides, quinolones, and nitroimidazole. The analysis was performed for each antibiotic class separately. We also assessed anti-viral and antifungal medications as potential negative exposure controls.
As potential confounders, we examined a comprehensive list of variables including obesity (BMI>30), smoking history (ever/never), and alcohol consumption (non-users, any use and alcoholism/alcohol dependence); medical co-morbidities including diabetes mellitus, chronic obstructive pulmonary disease (COPD), gastro-esophageal reflux disease, Barrett’s esophagus, and H.Pylori infection, number of past respiratory and urinary infections, as well as medications which may influence cancer risk such as aspirin/NSAIDs, hormone replacement therapy and anti-diabetic drugs. Each medical diagnosis was identified using Read codes and each medication was identified using multilex codes. All covariates were measured prior to index-date. The adjusted analysis included all covariates. Table 1 specify the list of variables used for adjustment in each malignancy.
Table 1.
Risk factors introduced to the multivariable models evaluating the association between antibiotic exposure and specific cancer risk
| Cancer type | Risk factor introduced to the multivariable model1 |
|---|---|
| Breast | BMI, smoking status, alcohol consumption, diabetes, use of hormone replacement therapy |
| Lung | Smoking status, diabetes, COPD, number of previous respiratory infections |
| Esophagus | BMI, smoking status, alcohol consumption, diabetes, gastro-esophageal reflux disease, barrett’s esophagus |
| Gastric | BMI, diabetes, H.pylori infection |
| Hepatocellular | BMI, smoking status, cirrhosis, alcohol consumption, diabetes |
| Biliary | BMI, smoking status, alcohol consumption, diabetes |
| Gallbladder | BMI, diabetes |
| Pancreas | BMI, smoking status, diabetes |
| Prostate | BMI, smoking status, diabetes, number of previous urinary tract infections |
| Renal | BMI, smoking status, diabetes |
| Bladder | BMI, smoking status, diabetes, chronic NSAIDs/aspirin use, anti-diabetic medications (thiazolidinediones, metformin and insulin), number of previous urinary tract infections |
| Melanoma | - |
| Cervix | Smoking status |
| Osteosarcoma | BMI, smoking status, alcohol consumption, height |
| Multiple myeloma | BMI, diabetes |
Statistical Analysis
The primary analysis was a conditional logistic regression to estimate OR and 95% confidence intervals (CIs) for the association between number of antibiotic courses and cancer risk. The reference group consisted of individuals without documented therapy with that specific antibiotic. The analysis was performed separately for each cancer type and was adjusted to all potential confounders for the specific cancer. We performed Bonferroni correction to account for multiple comparisons due to assessment of multiple antimicrobial classes. We also analyzed the association between timing of last antibiotic prescription (1–5, >5–10 and >10 years) and cancer risk among penicillin users. This analysis was done only among penicillin users since this was the most commonly used antibiotic group, allowing large statistical power.
Since bladder cancer is known to be associated with recurrent urinary-tract infections (UTI), we performed a secondary analysis for this malignancy only among individuals with recurrent infections (three or more UTI) and an additional analysis only among individuals without documented infection prior to index-date, in order to exclude confounding by indication. All analyses were performed using STATA 13 (Stata Corp., College Station, Tx, USA).
Results
The study population consisted of 125,441 cases with 15 different cancer types and 490,510 matched controls (Table 2). The most common cancer types were breast among females (31,131 cases) and prostate among males (27,212 cases). Penicillin was the most common antibiotic group used during the follow-up period with more than 45% of individuals (279,777) having at least one prescription.
Table 2.
Characteristics of cases and controls according to specific cancer type
| Cancer Type | AgeYears (SE) | Male genderN (%) | Durationof follow-upYears (SE) | DiabetesN (%) | BMI >30N (%) | Ever smokersN (%) |
|---|---|---|---|---|---|---|
| Breast | ||||||
| Cases(31,252) | 62.8 (<0.1) | 221 (0.7%) | 5.9 (<0.1) | 2,238 (7.1%) | 6,455 (20.6%) | 11,264 (35.9%) |
| Controls(123,285) | 62.5 (<0.1) | 864 (0.7%) | 5.9 (<0.1) | 7,676 (6.2%) | 24,282 (19.7%) | 41,298 (33.5%) |
| Lung | ||||||
| Cases(19,143) | 71.2 (0.1) | 10,947 (57.2%) | 6.2 (<0.1) | 2,380 (12.4%) | 3,299 (17.2%) | 14,750 (77.1%) |
| Controls(74,473) | 71.0 (<0.1) | 42,535 (57.1%) | 6.3 (<0.1) | 7,930 (10.7%) | 14,640 (19.7%) | 32,958 (44.3%) |
| Esophagus | ||||||
| Cases(6,108) | 70.7 (0.1) | 4,081 (66.8%) | 6.2 (0.1) | 792 (13.0%) | 1,234 (20.2%) | 3,502 (57.3%) |
| Controls(23,850) | 70.5 (0.1) | 15,931 (66.8%) | 6.2 (<0.1) | 2,512 (10.5%) | 4,266 (17.9%) | 10,283 (43.1%) |
| Gastric | ||||||
| Cases(3,859) | 73.0 (0.2) | 2,439 (63.2%) | 6.1 (<0.1) | 530 (13.7%) | 729 (18.9%) | 2,047 (53.0%) |
| Controls(15,022) | 72.8 (0.1) | 9,494 (63.2%) | 6.1 (<0.1) | 1,594 (10.6%) | 2,657 (17.7%) | 6,504 (43.3%) |
| Hepatocellular | ||||||
| Cases(1,299) | 68.3 (0.3) | 917 (70.6%) | 6.2 (0.1) | 424 (32.6%) | 401 (30.9%) | 793 (61.1%) |
| Controls(5,055) | 68.0 (0.2) | 3,563 (70.5%) | 6.3 (0.1) | 542 (10.7%) | 1,011 (20.0%) | 2,293 (45.4%) |
| Biliary | ||||||
| Cases(910) | 71.0 (0.4) | 456 (50.1%) | 6.9 (0.1) | 141 (15.5%) | 215 (23.6%) | 486 (53.4%) |
| Controls(3,543) | 70.6 (0.2) | 1,786 (50.4%) | 7.0 (<0.1) | 375 (10.6%) | 684 (19.3%) | 1,498 (42.3%) |
| Gallbladder | ||||||
| Cases(365) | 71.2 (0.6) | 118 (32.3%) | 6.9 (0.2) | 51 (14.0%) | 99 (27.1%) | 179 (49.0%) |
| Controls(1,426) | 71.0 (0.3) | 464 (32.5%) | 7.0 (0.1) | 156 (10.9%) | 310 (21.7%) | 593 (41.6%) |
| Pancreas | ||||||
| Cases(4,113) | 71.1 (0.2) | 1,999 (48.6%) | 6.3 (<0.1) | 931 (22.6%) | 860 (20.9%) | 2,053 (49.9%) |
| Controls(16,072) | 70.8 (0.1) | 7,794 (48.5%) | 6.3 (<0.1) | 1,598 (9.9%) | 3,013 (18.8%) | 6,625 (41.2%) |
| Prostate | ||||||
| Cases(27,212) | 72.0 (0.1) | NA | 6.3 (<0.1) | 3,035 (11.2%) | 4,733 (17.4%) | 13,718 (50.4%) |
| Controls(105,940) | 71.8 (<0.1) | NA | 6.4 (<0.1) | 12,735 (12.0%) | 18,831 (17.8%) | 51,381 (48.5%) |
| Renal | ||||||
| Cases(1,547) | 67.2 (0.3) | 959 (62.0%) | 6.3 (0.1) | 204 (13.2%) | 402 (26.0%) | 803 (51.9%) |
| Controls(6,066) | 67.0 (0.2) | 3,752 (61.9%) | 6.4 (0.1) | 535 (8.8%) | 1,187 (19.6%) | 2,521 (41.6%) |
| Bladder | ||||||
| Cases(13,440) | 71.9 (0.1) | 9,792 (72.9%) | 6.1 (<0.1) | 1,836 (13.7%) | 2,611 (19.4%) | 7,672 (57.1%) |
| Controls(52,421) | 71.6 (0.1) | 38,170 (72.8%) | 6.1 (<0.1) | 5,626 (10.7%) | 9,160 (17.5%) | 22,756 (43.4%) |
| Melanoma | ||||||
| Cases(9,226) | 60.4 (0.2) | 4,193 (45.5%) | 6.0 (<0.1) | 632 (6.9%) | 1,607 (17.4%) | 3,374 (36.6%) |
| Controls(36,346) | 60.2 (0.1) | 16,466 (45.3%) | 6.0 (<0.1) | 2,567 (7.1%) | 6,254 (17.2%) | 14,009 (38.5%) |
| Cervix | ||||||
| Cases(3,394) | 43.5 (0.3) | NA | 4.7 (0.1) | 133 (3.9%) | 530 (15.6%) | 1,550 (45.7%) |
| Controls(13,416) | 43.3 (0.1) | NA | 4.7 (<0.1) | 397 (3.0%) | 2,060 (15.4%) | 4,426 (33.0%) |
| Osteosarcoma | ||||||
| Cases(353) | 60.0 (1.0) | 179 (50.7%) | 5.8 (0.2) | 33 (9.4%) | 71 (20.1%) | 160 (45.3%) |
| Controls(1,380) | 60.0 (0.5) | 691 (50.1%) | 5.8 (0.1) | 108 (7.8%) | 243 (17.6%) | 541 (39.2%) |
| Multiple Myeloma | ||||||
| Cases(3,120) | 71.1 (0.2) | 1,673 (53.6%) | 6.1 (0.1) | 311 (10.0%) | 613 (19.7%) | 1,283 (41.1%) |
| Controls(12,215) | 71.0 (0.1) | 6,553 (53.6%) | 6.1 (<0.1) | 1,149 (9.4%) | 2,184 (17.9%) | 4,941 (40.5%) |
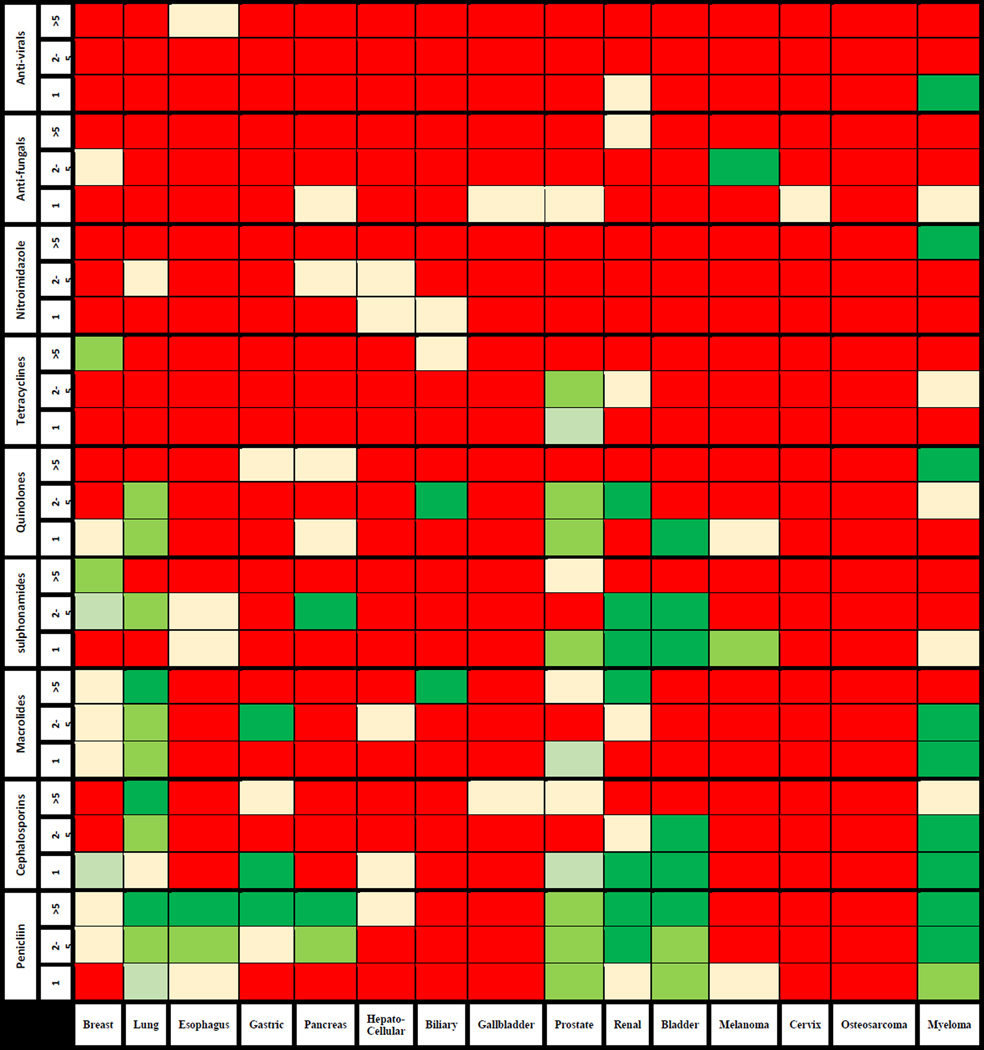
The association between cancer risk, according to organ site, and the number of specific antibiotic courses is presented in Figure 1 and Supplementary index 1.
Figure 1.
Adjusted cancer risk according to organ site as a function of number of specific antibiotic courses
In the gastrointestinal tract, exposure to penicillin was associated with an elevated risk of esophageal, gastric, and pancreatic cancers. The association increased in strength with the number of antibiotic courses. For example, the adjusted OR for esophageal cancer was 1.1 (95%CI 1.0–1.2) among individuals with a single course of penicillin compared to 1.2 for >5 courses (95%CI 1.0–1.4). The risk for biliary tumors was elevated only among individuals with >5 courses of macrolides (AOR 2.5 95%CI 1.3–4.9). There was no increase in risk of Hepatocellular carcinoma.
The risk for lung cancer increased with the use of penicillins, cephalosporines, or macrolides and was the highest among individuals who were exposed to >5 courses (AOR of 1.4, 95%CI 1.3–1.6; 1.3, 95%CI 1.0–1.6 and 1.3, 95%CI 1.1–1.6, respectively). Multiple myeloma was also associated with exposure to penicillins, cephalosporines, and macrolides with an AOR of 1.8 (95%CI 1.4–2.3) for individuals with >5 antibiotic courses of penicillin.
The risk for breast cancer increased only among users of sulphonamides with an AOR of 1.2 for >5 courses (95%CI 1.0–1.4). For tumors of the urinary-tract, the risk of prostate cancer increased modestly with the use of penicillins, quinolones, sulphonamides, and tetracyclines with an OR of 1.2 (95%CI 1.1–1.3) for >5 courses of penicillin. The risk for bladder and renal cancers also increased with the use of penicillins, sulphonamides, quinolones, and macrolides with AOR for renal cancer of up to 1.6 (95%CI 1.1–2.2) for >5 courses of penicillin.
In a sensitivity analysis, we further analyzed bladder cancer risk in individuals with and without bladder infections prior to the index date. Among those without prior bladder infection, cancer risk increased with the number of penicillin and sulphonamides prescriptions up to an AOR of 1.3 (95% CI 1.1–1.5) and 2.0 (95% CI 1.1–3.4) for >5 antibiotic courses respectively. Among those with prior bladder infection, the risk remained elevated among individuals with exposure to >5 courses of penicillin and macrolides with an AOR of 1.4 (95% CI 0.7–2.6) and 2.1 (95% CI 0.5–8.1) respectively, however the statistical power for this analysis was low.
Of note, there was no association between antibiotic use and carcinoma of the cervix, gallbladder, osteosarcoma and melanoma. Among the antibiotic classes, tetracyclines were not associated with increased cancer risk, except for prostate cancer. The use of anti-virals and anti-fungals was not associated with increased cancer risk. There was no change in risk when only individuals with >5 years of follow-up were included in the analysis (Supplementary index 2).
Additionally, among penicillin users we evaluated the association between timing of last penicillin prescription and specific cancer risk. In most malignancies, there was no difference in the direction of association between individuals with last prescription 1–5, >5–10 and >10 years before index-date (Table 3). For gastric, hepatocellular and pancreatic cancers there was no increase in cancer risk for individuals with last prescription >10 years before index date.
Table 3.
Association between time from last penicillin prescription and specific cancer risk
| Cancer Type | Time from last penicillin prescription to index date1 | ||
|---|---|---|---|
| 1–5 yr | >5–10 yr | >10 yr | |
| Breast | |||
| Univariate | 1.1 (1.0–1.1) | 1.0 (1.0–1.1) | 1.0 (0.9–1.1) |
| Multivariate | 1.0 (1.0–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| Lung | |||
| Univariate | 1.6 (1.5–1.7) | 1.4 (1.3–1.5) | 1.5 (1.3–1.8) |
| Multivariate | 1.2 (1.1–1.3) | 1.1 (1.0–1.2) | 1.3 (1.1–1.5) |
| Esophagus | |||
| Univariate | 1.3 (1.2–1.4) | 1.2 (1.1–1.4) | 1.3 (1.0–1.7) |
| Multivariate | 1.1 (1.0–1.3) | 1.1 (0.9–1.3) | 1.1 (0.8–1.4) |
| Gastric | |||
| Univariate | 1.2 (1.0–1.3) | 1.1 (0.9–1.3) | 1.0 (0.7–1.5) |
| Multivariate | 1.1 (1.0–1.3) | 1.1 (0.9–1.3) | 1.0 (0.7–1.4) |
| Hepatocellular | |||
| Univariate | 1.4 (1.1–1.7) | 1.4 (1.0–1.9) | 1.0 (0.6–1.9) |
| Multivariate | 1.1 (0.9–1.5) | 1.2 (0.9–1.8) | 0.8 (0.4–1.7) |
| Biliary | |||
| Univariate | 1.2 (1.0–1.6) | 1.1 (0.8–1.6) | 1.8 (1.0–3.3) |
| Multivariate | 1.1 (0.9–1.4) | 0.9 (0.7–1.4) | 1.6 (0.9–3.0) |
| Gallbladder | |||
| Univariate | 1.1 (0.7–1.6) | 1.1 (0.7–1.9) | 0.6 (0.2–2.0) |
| Multivariate | 1.1 (0.7–1.6) | 1.1 (0.6–1.8) | 0.7 (0.2–2.0) |
| Pancreas | |||
| Univariate | 1.2 (1.1–1.4) | 1.2 (1.0–1.4) | 1.0 (0.7–1.4) |
| Multivariate | 1.1 (1.0–1.3) | 1.1 (0.9–1.3) | 1.0 (0.7–1.3) |
| Prostate | |||
| Univariate | 1.2 (1.2–1.3) | 1.2 (1.1–1.3) | 1.1 (1.0–1.3) |
| Multivariate | 1.2 (1.1–1.2) | 1.1 (1.1–1.2) | 1.1 (1.0–1.3) |
| Renal | |||
| Univariate | 1.4 (1.1–1.7) | 1.4 (1.1–1.9) | 1.4 (0.8–2.3) |
| Multivariate | 1.3 (1.0–1.5) | 1.3 (1.0–1.7) | 1.3 (0.8–2.1) |
| Bladder | |||
| Univariate | 1.4 (1.3–1.4) | 1.3 (1.1–1.4) | 1.4 (1.2–1.7) |
| Multivariate | 1.2 (1.1–1.3) | 1.1 (1.0–1.2) | 1.3 (1.1–1.6) |
| Melanoma | |||
| Univariate | 1.1 (1.0–1.2) | 1.0 (0.9–1.2) | 1.1 (0.9–1.4) |
| Multivariate | - | - | - |
| Cervix | |||
| Univariate | 1.1 (0.9–1.2) | 1.1 (0.9–1.3) | 1.2 (0.8–1.8) |
| Multivariate | 1.0 (0.9–1.1) | 1.0 (0.8–1.3) | 1.1 (0.7–1.8) |
| Osteosarcoma | |||
| Univariate | 1.1 (0.7–1.7) | 1.3 (0.7–2.3) | 1.0 (0.3–3.8) |
| Multivariate | 0.9 (0.5–1.8) | 1.6 (0.7–3.8) | 0.5 (0.1–3.9) |
| Multiple Myeloma | |||
| Univariate | 1.4 (1.2–1.6) | 1.3 (1.1–1.6) | 1.4 (1.0–2.1) |
| Multivariate | 1.4 (1.2–1.6) | 1.3 (1.1–1.6) | 1.4 (1.0–2.1) |
Discussion
In the current population based nested case-control study we evaluated the association between exposure to antibiotic classes commonly used in the community and 15 different cancers. We observed a differential association according to tumor type and antibiotic group used. In the gastrointestinal system, the use of penicillin was associated with an elevated risk of esophageal, gastric and pancreatic cancers in addition to the higher risk for CRC we previously reported19. The risk for biliary tumors was elevated only among individuals with >5 courses of macrolides. Breast cancer risk was mildly increased with exposure to sulphonamides. Lung cancer risk increased with the use of penicillins, cephalosporines, and macrolides and similar increase in risk was seen in myeloma patients as well. For tumors of the urinary-tract, the risk of prostate cancer increased slightly with the use of penicillins, quinolones, sulphonamides, and tetracyclines. Similar risk was also observed in bladder and renal cancers. There was a correlation between number of antibiotic courses and cancer risk, specifically for gastro-intestinal and lung tumors. In most malignancies there was no association between any single antibiotic course and cancer risk. We observed no association between antibiotic use and melanoma, carcinoma of the cervix, osteosarcoma, hepatocellular and gallbladder carcinoma. Importantly, there was also no association between use of anti-virals and anti-fungals and increased cancer risk.
The current study had several unique strengths that support the possibility that the results are not secondary to confounding. Recurrent infections were suggested to correlate with elevated cancer risk, as in recurrent UTI and bladder cancer.25 Thus, for prostate and bladder cancers we adjusted for the number of UTI as part of the analysis. For bladder cancer we also performed two additional sensitivity analyses in order to exclude confounding by indication, the first only among individuals with recurrent UTI and the second only among individuals without UTI prior to index-date. In both cases there were no changes in results. For multiple myeloma, since there is no specific type of infection that is associated with the disease, we performed an additional analysis of the association between recurrent antibiotic exposure and the risk of Myeloma precursor Monoclonal Gammopathy of Undetermined Significance (MGUS) observing the same increase in risk (data not shown).
Cancer might also develop years before the actual diagnosis date causing immune suppression or infection through disturbance to normal body barriers. For this reason we included in our analysis only antibiotic prescriptions >1 year before diagnosis. The fact that the direction of association remained even with last prescriptions >5–10 years before index-date points against such reverse causality.
Additionally, people with more intense use of medical services might have higher likelihood for cancer diagnosis and antibiotic prescription. Infection can also be the reason for a medical encounter and secondarily tumor diagnosis. However, in such a case we would expect increased cancer risk in association with anti-viral and anti-fungal treatments as well. Moreover, we would expect similar association for all cancer types. The lack of association between exposure to any antibiotic group and cancer risk in melanoma, known to be caused by exposure to ultraviolet radiation, and cervical cancer, caused by HPV, as well as the lack of breast cancer risk among penicillin users would seem to argue against this possibility as well as against residual confounding.
The nested case-control design with incidence-density sampling provided us with ORs that are unbiased estimates of incidence-rate-ratios20 and allowed us to evaluate and adjust for multiple risk factors for each cancer type. The matching of cases and controls on duration and calendar period of follow-up prevented time-window bias and bias due to changes in frequency and antibiotic classes used. Matching on practice site minimized inter-clinic differences related to antibiotic prescription rate and cancer screening policy. Furthermore, we evaluated only prescriptions that were given >1 year before cancer diagnosis date in order to avoid protopathic bias due to drug prescriptions for early, non-specific disease manifestations.26 We also excluded cancer cases occurring in the first six months after registration with a clinic in order to avoid prevalent cases.
Since antibiotics has no known direct carcinogenic effect, our main hypothesis focus on antibiotic’s influence on the composition of the human microbiota. Such a mechanism may also explain the need for repeated antibiotic exposures, known to cause a lasting change in bacterial diversity2,3,19, as well as the differential effect of different antibiotic groups. The microbiome can induce chronic inflammation11; influence human metabolism by activating genes that are related to both insulin resistance and cell proliferation20; influence the immune-system response against cancer27 and certain bacteria can even have a direct carcinogenic effect on the epithelium.28 It is not clear whether such effects are unique only to the gut microbiota or can occur by microbiota of other organs as well, such as the skin and lung. These mechanisms explain the differential effects of specific antibiotic groups on different tumor types. Since the microbiota varies among populations, between individuals and within individuals, and because we had no information regarding the microbial composition of study subjects, we were unable to assess the suggested biological mechanism. It is also possible that the microbiota is involved in carcinogenesis only in individuals with a specific genetic or immunologic predisposition.
There are several potential limitation associated with the current study. Our diagnosis relies on Read codes alone and not on pathology reports. The validity of at least one Read code as a method to detect cancer patients was previously reported using the General Practice Research Database (GPRD), a related database that contains some practices which overlap with THIN.29 Garcia et al. report a confirmation rate greater than 95% for CRC using pathology report as the gold standard. Recent evidence indicated that the validity of data in THIN is comparable to GPRD.30 Lack of pathology reports with detailed histopathology prevented the differentiation between cancer types in specific organs, such as adenocarcinomas and squamous cell carcinomas in the lung and esophagus. We also decided to avoid analysis of head and neck cancers due to lack of information regarding HPV status and inability to properly differentiate between oropharyngeal and oral cancers.
Information about BMI was not available for up to 30% of the study population. We categorized those individuals as a separate category in our analysis. Moreover, an analysis restricted among subjects with complete BMI information did not change the results (data not shown). Of note, the percent of obese individuals with BMI above 30 in our study is similar to the one reported by the Health Survey for England (HSE) for the general UK population.
Antibiotics are commonly used in the community and many of the individuals in our study received more than one antibiotic type during follow-up. The differential association seen among antibiotic classes with different spectrum of bacterial coverage, such as high cancer risk among penicillin users compared to almost no cancer risk among tetracyclines users, shows that despite the described overlap we can still distinguish the unique association with each antibiotic group in contrast to previous works that assessed any antibiotic treatment.17
We also assumed 100% compliant with prescribed antibiotic treatment. Even if such assumption is incorrect, any misclassification would likely be non-differential and thus would tend to bias the results towards the null hypothesis. Another potential bias might result from lack of information regarding the amount of unintentional environmental exposure to antibiotics in the food. The amount of such an exposure could not be assessed in the current epidemiological work. Although antibiotic prescriptions during hospital admissions were not available, most of those courses were continued by the general practitioners after discharge, thus they were included in the analysis.
Finally, the current work required multiple comparisons between different antibiotic classes in each cancer type, for this reaso6n we applied the conservative Bonferroni correction for the analysis.
In summary, this large population based study demonstrated a differential increase in cancer risk (mainly gastro-intestinal, genito-urinary, and lung cancers) secondary to repeated exposures to certain antibiotic types. There was almost no increase in risk with a single course of antibiotic. There was almost no change in association when last antibiotic exposure more than five years before index-date was evaluated. Penicillins were associated with the most significant cancer risk while tetracyclines as well as anti-virals and anti-fungals were not correlated with cancer risk. There was no association between antibiotic exposure and melanoma or carcinoma of cervix. Since the incidence of cancers in THIN reflects the true incidence of cancer in the UK population we were able to estimate the absolute risk increase in cancer incidence and found it to be around 20:100,000. One could argue whether this is a small difference, however it demonstrate the mechanistic relevance of gut microbiota in certain cancers. Further studies should focus on evaluating the specific biological pathways behind the association. Since the microbiome is more prone to change during the first years of life, additional investigation evaluating the impact of antibiotic exposure in childhood would be informative. Given their high prevalence of use, it is of utmost significance to understand the influence of antibiotics on our commensal bacteria as well as the possible secondary impact on other disease states including cancer.
Supplementary Material
Highlights.
- Antibiotic exposure can alter the diversity of the human microbiota.
- Bacterial dysbiosis was described in cancer patients.
- In our study recurrent exposure to certain antibiotics increased cancer risk.
- No association was found with anti-virals and anti-fungals.
- No association was found with melanoma or carcinoma of the cervix.
Acknowledgement
Dr. Yang and Dr. Boursi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Yang YX, Boursi B, Haynes K and Mamtani R contributed to conception and design of the study; Yang YX and Boursi B acquired the data; Yang YX, Boursi B, Haynes K and Mamtani R contributed to analysis and interpretation of data, drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Funding: The work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Dr. Boursi would like to thank the Djerassi family for supporting his post-doctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
*
This work was performed in partial fulfilment of the requirements for a Ph.D. degree of Ben Boursi, Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Conflict of interest statement: None of the authors has any relevant conflict of interest to declare.
References
- 1.Haeseker MB, Dukers-Muijrers N, Hoebe C, Bruggeman CA, Cals JW, Verbon A. Trends in antibiotic prescribing in adults in Dutch general practice. PLoS One. 2012;7(12):e51860. doi: 10.1371/journal.pone.0051860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. Plos. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobhani I, Tap J, Roudot-Thoraval F, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. Plos. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulal S, Keku TO. Gut microbiome and colorectal adenomas. The Cancer Jourrnal. 2014;20(3):225–231. doi: 10.1097/PPO.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61(4):582–588. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutcliffe S, Giovannucci E, Isaacs WB, Willett WC, Platz EA. Acne and risk of prostate cancer. Int J Cancer. 2007;121(12):2688–2692. doi: 10.1002/ijc.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xuan C, Shamonki JM, Chung A, et al. Microbial dysbiosis is associated with human breast cancer. PLoS One. 2014;9(1):e83744. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abreu MT, Peek RM. Gastrointestinal malignancy and the microbiome. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cario E. Microbiota and innate immunity in intestinal inflammation and neoplasia. Gastroenterology. 2013;29:85–91. doi: 10.1097/MOG.0b013e32835a670e. [DOI] [PubMed] [Google Scholar]
- 11.Kamada N, Seo S, Chen G, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 12.Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8(8):e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajishengallis G, Darveau R, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Garcia-Rodriguez L, Hernandez-Diaz S. Antibiotic use and the risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1308–1315. doi: 10.1158/1055-9965.EPI-07-2817. [DOI] [PubMed] [Google Scholar]
- 15.Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291(7):827–835. doi: 10.1001/jama.291.7.827. [DOI] [PubMed] [Google Scholar]
- 16.Tamim HM, Hajeer AH, Boivin JF, Collet JP. Association between antibiotic use and risk of prostate cancer. Int J Cancer. 2010;127(4):952–960. doi: 10.1002/ijc.25139. [DOI] [PubMed] [Google Scholar]
- 17.Kilkkinen A, Rissanen H, Klaukka T, et al. Antibiotic use predicts an increased risk of cancer. Int J Cancer. 2008;123:2152–2155. doi: 10.1002/ijc.23622. [DOI] [PubMed] [Google Scholar]
- 18.Schulz MD, Atay C, Heringer J, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514(7523):508–512. doi: 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boursi B, Haynes K, Mamtani R, Yang YX. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2015 doi: 10.1002/pds.3765. in press. [DOI] [PubMed] [Google Scholar]
- 20.Lubin JH, Gail MH. Biased selection of controls for case-control analysis of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 21.Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Safe. 2009;18:76–83. doi: 10.1002/pds.1688. [DOI] [PubMed] [Google Scholar]
- 22.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Safe. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 23.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the general practice research database. Pharmacoepidemiol Drug Saf. 2005;14:443–451. doi: 10.1002/pds.1115. [DOI] [PubMed] [Google Scholar]
- 25.Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, Fraumeni JF., Jr Urinary tract infection and risk of bladder cancer. Am J Epidemiol. 1984;119(4):510–515. doi: 10.1093/oxfordjournals.aje.a113768. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case control studies. Am J Med. 1980;68(2):255–258. doi: 10.1016/0002-9343(80)90363-0. [DOI] [PubMed] [Google Scholar]
- 27.Cheng M, Qian L, Shen G, et al. Microbiota modulate tumoral immune surveillance in lung through a gamma delta T17 immune cell dependent mechanism. Cancer Res. 2014 doi: 10.1158/0008-5472.CAN-13-2462. ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Bue E, Dubois D, Sauvanet P, et al. High prevalence of mucosa associated E.Coli producing cyclomodulin and genotoxin in colon cancer. PLoS One. 2013;8(2):e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of CRC among long-term users of aspirin and nonaspirin NSAIDS. Epidemiology. 2001;1(12):88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Haynes K, Forde KA, Schinnar R, Wong P, Strom BL, Lewis JD. Cancer incidence in The Health Improvement Network. PDS. 2009;18:730–736. doi: 10.1002/pds.1774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.