Changes in Incidence and Antifungal Drug Resistance in Candidemia: Results From Population-Based Laboratory Surveillance in Atlanta and Baltimore, 2008–2011 (original) (raw)
. Author manuscript; available in PMC: 2016 Jan 4.
Published in final edited form as: Clin Infect Dis. 2012 Aug 14;55(10):1352–1361. doi: 10.1093/cid/cis697
Abstract
Background
Candidemia is common and associated with high morbidity and mortality; changes in population-based incidence rates have not been reported.
Methods
We conducted active, population-based surveillance in metropolitan Atlanta, Georgia, and Baltimore City/County, Maryland (combined population 5.2 million), during 2008–2011. We calculated candidemia incidence and antifungal drug resistance compared with prior surveillance (Atlanta, 1992–1993; Baltimore, 1998–2000).
Results
We identified 2675 cases of candidemia with 2329 isolates during 3 years of surveillance. Mean annual crude incidence per 100 000 person-years was 13.3 in Atlanta and 26.2 in Baltimore. Rates were highest among adults aged ≥65 years (Atlanta, 59.1; Baltimore, 72.4) and infants (aged <1 year; Atlanta, 34.3; Baltimore, 46.2). In both locations compared with prior surveillance, adjusted incidence significantly declined for infants of both black and white race (Atlanta: black risk ratio [RR], 0.26 [95% confidence interval {CI}, .17–.38]; white RR: 0.19 [95% CI, .12–.29]; Baltimore: black RR, 0.38 [95% CI, .22–.64]; white RR: 0.51 [95% CI: .29–.90]). Prevalence of fluconazole resistance (7%) was unchanged compared with prior surveillance; 32 (1%) isolates were echinocandin-resistant, and 9 (8 Candida glabrata) were multidrug resistant to both fluconazole and an echinocandin.
Conclusions
We describe marked shifts in candidemia epidemiology over the past 2 decades. Adults aged ≥65 years replaced infants as the highest incidence group; adjusted incidence has declined significantly in infants. Use of antifungal prophylaxis, improvements in infection control, or changes in catheter insertion practices may be contributing to these declines. Further surveillance for antifungal resistance and efforts to determine effective prevention strategies are needed.
Bloodstream infections (BSIs) caused by Candida species, also known as candidemia, are an important public health problem in the United States. Candida species are among the most common causes of BSIs and are associated with high morbidity and mortality, as well as increases in hospital cost and length of stay [1–5].
Although most reports that describe the epidemiology of candidemia are from individual institutions or among specific patient groups, few reports in the United States have described the epidemiology of candidemia at a population level. Population-based data are important for describing infections in whole populations and across the entire spectrum of healthcare settings, and can be used to determine group-specific incidence rates to monitor and compare rates of infection over time. Previous population-based surveillance performed by the Centers for Disease Control and Prevention (CDC) and partners in Atlanta, Georgia (1992–1993), and Baltimore, Maryland (1998–2000), described incidence rates of 8.7 per 100 000 population in Atlanta and 24 per 100 000 population in Baltimore [6, 7]. During both surveillance periods, drug resistance to fluconazole was low: 3% of isolates were resistant in 1992, and 3.7% were resistant in 1998 [6–8].
Over the past decade, Candida species with reduced fluconazole susceptibility, such as Candida glabrata, have become more prevalent in some patient populations [8–12]. The newest class of antifungal medications, the echinocandins, are considered first-line empiric therapy for candidemia, and there are reports of echinocandin-resistant invasive infection [13, 14].
To evaluate changes in the epidemiology and antifungal drug resistance of candidemia, we conducted population-based prospective surveillance in metropolitan Atlanta, Georgia, and Baltimore City and County, Maryland, 2 areas where prior surveillance had been conducted.
MATERIALS AND METHODS
Surveillance Population
Surveillance for candidemia was conducted among residents of Atlanta, Georgia (Fulton, DeKalb, Cobb, Gwinnett, Clayton, Douglas, Newton, and Rockdale counties; 25 hospitals, population: 3.8 million) and Baltimore City and County, Maryland (14 hospitals, population: 1.4 million). Each catchment area is the same as prior surveillance (Atlanta, 1992–1993 [6]; Baltimore, 1998–2000 [7]).
Case Definitions
We defined an incident case of candidemia as the first blood culture positive for a Candida species collected from a resident of the surveillance area. An episode of candidemia was defined by the 30-day period following the day of collection of the incident blood culture. Blood cultures with Candida collected >30 days after the incident blood culture were defined as new incident cases.
Candidemia episodes were categorized as hospital-onset (HO), healthcare-associated community-onset (HACO), or community-onset (CO). Hospital-onset candidemia was defined as candidemia occurring in patients whose incident blood cultures were collected on or after the third day of hospital admission, where admission date is the first day. Candidemia episodes obtained before the third day of hospital admission were categorized as either HACO or CO: candidemia episodes in patients with recent healthcare exposure were categorized as HACO if the patient (1) was a resident of a nursing home at the time of culture collection; (2) had a central venous catheter in place 2 days before, the day before, or on the day of culture collection; (3) had documentation of at least 1 of the following in the 90 days prior to candidemia: hospitalization, surgery, or hemodialysis; (4) was transferred from another acute care hospital; or (5) was a neonate (aged ≤30 days) at the time of candidemia, Patients without any of these criteria were classified as CO.
Data Collection
Surveillance data were collected for 3 years at each location (1 March 2008–28 February 2011 in Atlanta; 1 June 2008–31 May 2011 in Baltimore). Basic demographic and clinical information was collected on all cases, and additional clinical information (referred to as “enhanced surveillance”) on antifungal treatment and severity of illness was collected for the first 2 years of surveillance in each location (1 March 2008–28 February 2010 in Atlanta; 1 June 2008–31 May 2010 in Baltimore). Surveillance personnel used standardized case report forms to abstract data from medical records. Laboratory records from all participating laboratories were audited monthly, ensuring complete capture of all cases.
Isolate Collection, Identification, and Antifungal Susceptibility Testing
All available isolates were sent to the CDC for species confirmation and antifungal drug susceptibility testing. Isolates were identified using a Luminex assay or DNA sequencing of the D1-D2 subunit of the 28S recombinant DNA [15]. CDC-confirmed species are reported; if no isolate was received at the CDC, local laboratory species identifications are reported.
Antifungal susceptibility testing was performed by broth microdilution with fluconazole, itraconazole, voriconazole, posaconazole, flucytosine, anidulafungin, caspofungin, and micafungin as described by Clinical and Laboratory Standards Institute (CLSI) M27-A3 guidelines [16] using frozen RPMI microbroth trays (TREK Diagnostics, Cleveland, Ohio). We used the recently approved, species-specific CLSI 24-hour resistance breakpoints for fluconazole, voriconazole, and echinocandins [16]. Isolates of Candida krusei were considered intrinsically resistant to fluconazole. Amphotericin B susceptibility was performed using Etest as per the manufacturer’s instructions (bioMérieux, Durham, North Carolina); minimum inhibitory concentration values were read at 24 hours. Where comparisons were made to previous surveillance data [6, 7] the new breakpoints were applied to the old data [16], and thus resistance reported here differs from resistance previously reported. The testing protocols do not differ.
Statistical Methods and Denominators
All incidence rates were calculated using 2009 population estimates for Baltimore [17] and Atlanta [18] and are presented per 100 000 person-years. We used original case and isolate data to calculate incidence rates in Atlanta (1992–1993) [6] and Baltimore (1998–2000) [7]. Owing to inconsistency between Atlanta denominators obtained from the 1990 US census and prior published data, our reported 1992–1993 Atlanta rates are slightly higher than previously reported [6].
We report crude rates to describe the burden in the current population, as well as adjusted rates to describe changes over time. For adjusted rates, the data were first aggregated by several factors: time period, age group, race, sex, and species. Negative binomial regression models were then fit to the data to examine the relationship between those factors and the candidemia incidence rates, and to evaluate the change in average annual incidence rates between the 2 time periods. To assess the goodness of fit of a proposed model, we compared observed and expected rates under the assumed model. We found evidence of effect modification by age and race, and thus report group-specific relative risks to summarize changes in incidence over time. Patients with missing race data (<4% of all patients) were removed from multivariate modeling analyses.
Categorical variables were analyzed using χ2 tests or Fisher exact tests. We used the Kaplan-Meier method to describe survival 30 days after incident candidemia. In all analyses, the level of significance was set at α = .05.All analyses were done using SAS software (version 9.3; SAS Institute, Cary, North Carolina).
Human Subjects
The CDC conducted ethical review of this surveillance project and deemed it a nonresearch activity; therefore, it was not subject to review by a CDC institutional review board. This activity was also evaluated individually at each location, and was either deemed a public health assessment or human subjects research and was approved by local review boards.
RESULTS
Demographic and Clinical Characteristics
We identified 2675 cases of candidemia in Atlanta and Baltimore during the 3-year surveillance period (Table 1). The median age of patients was 58 years (interquartile range [IQR], 45–71 years), and 51% were male; 217 patients (8%) had >1 episode of candidemia. The most common underlying conditions documented within the 3 months prior to candidemia were surgery (36%), diabetes (34%), and malignancy (21%). Most patients (n = 2265 [85%]) had a central venous catheter (CVC) in place within 2 days prior to incident candidemia, and of those, 1612 (71%) had all CVCs removed within 7 days of incident candidemia.
Table 1.
Selected Demographic and Clinical Characteristics of Persons With Candidemia in Atlanta and Baltimore (Comparison of Prior Surveillance With Current Surveillance Data)
| No. (%) of Patients | ||||
|---|---|---|---|---|
| Atlanta | Baltimore | |||
| Characteristic | 1992–1993 (n = 428) | 2008–2011 (n = 1554) | 1998–2000 (n = 680) | 2008–2011 (n = 1121) |
| Crude incidence rate (per 100 000 person-years) | 9.1 | 13.3 | 24.2 | 26.2 |
| Sexa | ||||
| Female | 173 (40) | 748 (48) | 328 (48) | 540 (48) |
| Male | 253 (59) | 806 (52) | 352 (52) | 579 (52) |
| Racea | ||||
| White | 175 (41) | 525 (34) | 260 (38) | 447 (40) |
| Black | 193 (45) | 929 (60) | 406 (60) | 623 (56) |
| Median age, years (IQR) | 46 (6–69) | 58 (44–70) | 57 (40–73) | 58 (45–72) |
| Age (years)a | ||||
| <1 | 85 (20) | 61 (4) | 39 (6) | 27 (2) |
| 1–19 | 35 (8) | 62 (4) | 34 (5) | 18 (2) |
| 20–44 | 85 (20) | 271 (17) | 143 (21) | 230 (21) |
| 45–64 | 92 (21) | 598 (38) | 198 (29) | 428 (38) |
| ≥65 | 129 (30) | 562 (36) | 265 (39) | 411 (37) |
| Underlying or prior conditions | ||||
| Surgery in the 3 months priorb | 80 (19) | 588 (38) | 319 (47) | 366 (33) |
| Abdominal surgery | 54 (13) | 367 (24) | 178 (26) | 222(20) |
| Nonabdominal surgery | 26 (6) | 312 (20) | NA | 201 (18) |
| Diabetes | 55 (13) | 508 (33) | 193 (28) | 392 (35) |
| Malignancies | 113 (26) | 339 (22) | 145 (51) | 212 (19) |
| Renal conditions | NA | 250 (16) | 202 (30) | 203 (18) |
| Liver diseases | 9 (2) | 126 (8) | 108 (16) | 199 (18) |
| HIV or AIDS | 29 (7) | 55 (4) | 72 (11) | 95 (8) |
| Central venous catheter usage | ||||
| CVC at time of candidemia | NA | 1342 (86) | 520 (76) | 923 (82) |
| CVC was removed within 7 days of candidemia | NA | 907 (68) | 435 (64)c | 705 (76) |
| Onset of candidemia | ||||
| Candidemia ≤2 days after admission or no admission | 110 (28)d | 443 (29) | 229 (34) | 461 (41) |
| Acquisition of candidemia | ||||
| Hospital onset | NA | 1114 (72) | NA | 661 (59) |
| Health care-associated community-onset | NA | 386 (25) | NA | 401 (36) |
| Community-onset | NA | 54 (3) | NA | 59 (5) |
| Severity of illness | ||||
| Crude 30-day all-cause mortalitye | 35% | 29% | 50% | 28% |
| Had >1 episode of candidemia | 18 (4) | 99 (6) | 33 (3) | 118 (11) |
| SIRS at time of candidemia (age ≥18 years only)f | NA | 798 (81) | NA | 535 (70) |
| In ICU within 14 days before or after candidemiaf | NA | 756 (49) | NA | 494 (44) |
| In ICU at time of candidemiaf | NA | 508 (33) | 218 (32) | 333 (30) |
| Treatmentf | ||||
| Treatment information available | NA | 1052 (98) | NA | 755 (95) |
| Received antifungals in the 14 days prior to candidemia | NA | 195 (18) | NA | 83 (10) |
| Received antifungals after candidemia | NA | 956 (90) | NA | 660 (83) |
| Speciesg | ||||
| C. albicans | 223 (52) | 629 (40) | 289 (43) | 369 (33) |
| Non-C. albicans | 205 (48) | 925 (60) | 391 (58) | 752 (67) |
| C. glabrata | 51 (12) | 403 (26) | 188 (28) | 325 (29) |
| C. tropicalis | 43 (10) | 129 (8) | 14 (98) | 131 (12) |
| C. parapsilosis | 90 (21) | 262 (17) | 72 (11) | 172 (15) |
Most patients (n = 1775 [66%]) had infection occur during hospitalization (HO), or upon admission with documented exposures to healthcare (HACO; n = 787 [29%]); only 113 patients (4%) lacked recent documented healthcare exposure (CO, Table 1). Among the 787 HACO patients, 642 (82%) had documentation of a CVC within 2 days prior to or on the day of initial culture. CO patients were older than other patients (median age, 68 years vs 58 years in non-CO; P < .001), and more likely to have diabetes (39% vs 33% in non-CO; P < .001); C. glabrata was most frequently isolated in this group (n = 45 [40% of all species found in CO patients]), followed by Candida albicans (n = 36 [32%]) and Candida parapsilosis (n = 15 [13%]).
Enhanced Surveillance Results
During the 2 years of enhanced surveillance, we identified 1863 patients with candidemia. A total of 1143 patients (61%) were in an intensive care unit (ICU) within the 14 days before or after candidemia (Table 1). Among adult (aged ≥18 years) patients (n = 1749), 1333 (76%) met criteria for sepsis [19].
Antifungal medication data were available for 1807 patients (97% of 1863 patients) during the 2-year enhanced surveillance period, of whom 1616 (89%) received an antifungal following candidemia (Table 1); fluconazole (n = 1245 [77%]) or an echinocandin (n = 988 [61%]) was most commonly administered, and median treatment duration was 12 days (IQR, 6–17 days). For initial therapy, patients first received fluconazole (n = 938 patients [52%]) or micafungin (n = 371 [20%]). Most patients (n = 857 [53%]) received >1 antifungal. Among those who did not receive antifungal treatment (n = 191 [10%]), most (n = 109 [57%]) died or were discharged before the culture result was available to the clinician. A total of 278 patients (15%) had received an antifungal medication in the 14 days prior to candidemia; fluconazole was the most common choice (n = 177 [64%]), followed by an echinocandin (n = 41 [15%]).
Changes in Incidence Rates and Mortality
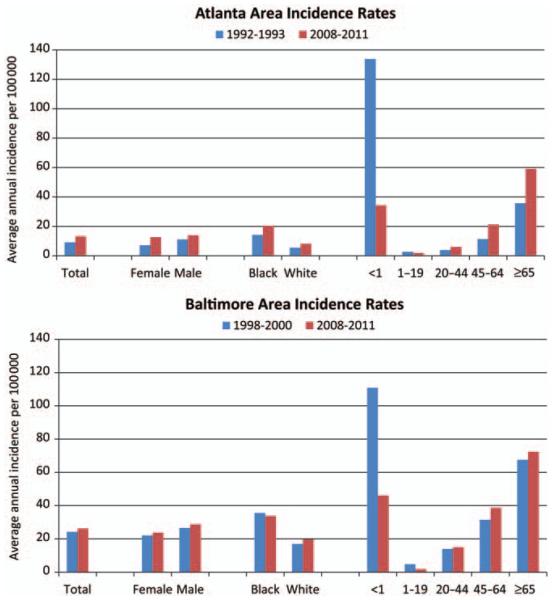
The average annual crude incidence rate per 100 000 person-years during 2008–2011 was 13.3 in Atlanta and 26.2 in Baltimore (Table 1). Rates were highest among persons aged ≥65 years (59.1 in Atlanta, 72.4 in Baltimore), persons <1 year of age (34.3 in Atlanta, 46.2 in Baltimore), and persons of black race (20.5 in Atlanta, 33.8 in Baltimore; Figure 1).
Figure 1.
A comparison of crude average annual incidence rates per 100 000 persons in Atlanta (1992–1993 vs 2008–2011) and Baltimore (1998–2000 vs 2008–2011), overall, and by sex, race, and age group.
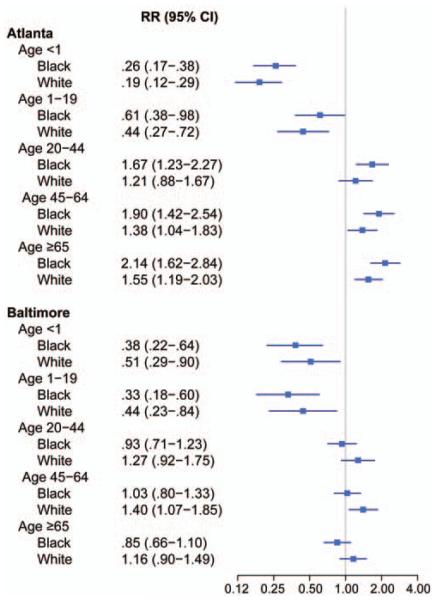
Although compared to prior surveillance periods overall crude incidence was 46% higher in Atlanta and 8% higher in Baltimore (Table 1), changes in rates varied by age group and race. Age- and race-specific strata illustrate variability in these differences between the 2 reporting sites (Figure 1). In both locations, rates significantly declined for all persons <20 years of age (Figure 2). Among persons aged ≥20 years, changes varied by location: In Atlanta, changes in incidence among persons aged ≥20 years were significant for all black persons aged ≥20 years and in all white persons aged ≥45, whereas in Baltimore, rates in persons aged ≥20 years changed little compared to prior surveillance, except for a slight increase in risk among white persons aged 45–64 years (Figure 2).
Figure 2.
A comparison of adjusted incidence rate ratios, by age group and race, comparing rates in the previous time period (1992–1993 in Atlanta, 1998–2000 in Baltimore) to rates in the current time period (2008–2011). 95% confidence interval. The line represents a relative risk of 1. Rates to the right of the line are an increase in risk compared to prior surveillance; rates to the left of the line are a decrease in risk compared to prior surveillance. Abbreviations: CI, confidence interval; RR, relative risk.
Thirty-day case fatality decreased significantly since 1998–2000 in Baltimore (28% in 2008–2011 vs 50% in 1998–2000, P < 0.001), but was similar between the 2 time periods for Atlanta (29% in 2008–2011 vs 35% in 1992–1993 P = .09).
Candida Species and Antifungal Susceptibility
Among 2675 total cases, the CDC received 2329 isolates from 2227 patients (83% of total cases); 99 patients (4%) had >1 isolate of different species available for testing, and 448 patients (17%) had no isolate. Incident isolates were available for 2136 (80%) patients. When species identifications from submitting laboratories were compared to CDC results, 107 isolates (5%) were discrepant (results not shown).
Species distributions and antifungal resistance testing results of the isolates received at the CDC are shown in Table 2. Among all isolates received at the CDC, C. albicans was the most common species (n = 877 [38%]), followed by C. glabrata (n = 670 [29%]), C. parapsilosis (n = 389 [17%]), and Candida tropicalis (n = 241 [10%]; Table 2).
Table 2.
Candida Species Distribution and Antifungal Resistance to Fluconazole and Echinocandins of Total Isolates Received at the Centers for Disease Control and Prevention, 2008–2011, Compared With Prior Surveillance in Atlanta and Baltimore
| Atlanta Area | Baltimore Area | |||||||
|---|---|---|---|---|---|---|---|---|
| 1992–1993 Fluconazole Resistant, No. (%) | 2008–2011 | 1998–2000 Fluconazole Resistant, No. (%) | 2008–2011 | |||||
| Species | Fluconazole Resistant, No. (%) | Echinocandin Resistant, No. (%) | Total Isolates, No. (%) | Fluconazole Resistant, No. (%) | Echinocandin Resistant, No. (%) | Total Isolates, No. (%) | ||
| C. albicans | 1 (1) | 11 (2) | 5 (1) | 489 (41) | 8 (3) | 9 (2) | 1 (0) | 388 (34) |
| Non–C. albicans | 15 (12) | 83 (12) | 10 (1) | 709 (59) | 32 (10) | 62 (9) | 15 (2) | 743 (66) |
| C. glabrata | 7 (20) | 42 (13) | 10 (3) | 318 (27) | 12 (8) | 38 (11) | 10 (3) | 352 (31) |
| C. tropicalis | 1 (3) | 9 (9) | 0 (0) | 103 (9) | 7 (9) | 6 (4) | 3 (2) | 138 (12) |
| C. parapsilosis | 1 (2) | 12 (6) | 0 (0) | 212 (18) | 0 (0) | 4 (2) | 1 (1) | 177 (16) |
| C. krusei | 0 (0) | 19 (100) | 0 (0) | 19 (2) | 0 (0) | 13 (100) | 0 (0) | 13 (1) |
| C. dubliniensis | 0 (0) | 1 (20) | 0 (0) | 5 (<1) | 0 (0) | 1 (3) | 1 (3) | 31 (3) |
| C. lusitaniae | 0 (0) | 0 (0) | 0 (0) | 22 (2) | 0 (0) | 0 (0) | 0 (0) | 12 (1) |
| Other species | 0 (0) | 0 (0) | 0 (0) | 30 (3) | 0 (0) | 0 (0) | 0 (0) | 20 (2) |
| All species | 16 (7) | 94 (8) | 15 (1) | 1198 (100) | 40 (7) | 71 (6) | 16 (1) | 1131 (100) |
Compared with prior surveillance, changes in species-specific incidence rates varied by location and age group (Table 3). In Atlanta, in persons aged ≥20 years, the risk for almost all Candida species increased compared with prior surveillance (Table 3); the increase was greatest in non–C. albicans species, particularly for C. glabrata and C. parapsilosis (increase in risk varied by age group; Table 3). In Baltimore, changes were less robust and limited to increases in C. parapsilosis in 2 of the 5 age groups (Table 3). In both locations, C. albicans decreased among all persons aged <20 years.
Table 3.
Changes in Candida Species, by Age Group: Comparison of Rates in the Previous Time Period (1992–1993 in Atlanta, 1998–2000 in Baltimore) With Rates in the Current Time Period (2008–2011)
| Atlanta | Baltimore | |||||
|---|---|---|---|---|---|---|
| Incidence Densitya | Relative Risk (95% CI) | Incidence Densitya | Relative Risk (95% CI) | |||
| Species by Age Group | 1992–1993 | 2008–2011 | 1998–2000 | 2008–2011 | ||
| <1 year | ||||||
| C. albicans | 66.82 | 17.44 | 0.26 (.16–.42)* | 70.70 | 19.86 | 0.28 (.14–.58)* |
| C. glabrata | 0.00 | 3.61 | … | 12.29 | 7.22 | 0.59 (.15–2.35) |
| C. parapsilosis | 39.11 | 7.22 | 0.18 (.09–.37)* | 27.66 | 12.64 | 0.46 (.17–1.23) |
| Other Candida spp | 6.52 | 3.01 | 0.46 (.12–1.72) | 6.15 | 5.42 | 0.88 (.15–5.27) |
| 1–19 years | ||||||
| C. albicans | 1.06 | 0.59 | 0.23 (.11–.47)* | 2.19 | 0.51 | 0.23 (.08–.64)* |
| C. glabrata | 0.25 | 0.61 | 2.49 (.62–9.96) | 0.44 | 0.20 | 0.46 (.08–2.78) |
| C. parapsilosis | 1.14 | 0.43 | 0.37 (.18–.80)* | 0.29 | 0.41 | 1.39 (.26–7.62) |
| Other Candida spp | 0.25 | 0.40 | 1.61 (.46–5.72) | 2.04 | 0.61 | 0.30 (.11–.78)* |
| 20–44 years | ||||||
| C. albicans | 1.85 | 2.56 | 1.38 (.96–1.99) | 6.31 | 4.98 | 0.79 (.56–1.11) |
| C. glabrata | 0.38 | 3.80 | 10.01 (4.77–21.01)* | 4.24 | 3.25 | 0.77 (.50–1.16) |
| C. parapsilosis | 0.47 | 1.13 | 2.37 (1.20–4.69)* | 1.03 | 2.63 | 2.54 (1.27–5.10)* |
| Other Candida spp | 0.85 | 1.10 | 1.29 (.75–2.22) | 3.00 | 4.22 | 1.41 (.90–2.19) |
| 45–64 years | ||||||
| C. albicans | 6.28 | 8.49 | 1.35 (.99–1.83) | 12.89 | 11.80 | 0.92 (.69–1.21) |
| C. glabrata | 1.38 | 15.06 | 10.89 (5.91–20.06)* | 9.09 | 12.27 | 1.35 (.99–1.85) |
| C. parapsilosis | 1.26 | 3.44 | 2.74 (1.42–5.26)* | 5.19 | 5.11 | 1.35 (.83–2.19) |
| Other Candida spp | 1.88 | 3.25 | 1.73 (1.00–2.98)* | 6.28 | 9.57 | 1.52 (1.05–2.21)* |
| ≥65 years | ||||||
| C. albicans | 14.81 | 22.58 | 1.52 (1.13–2.06)* | 30.13 | 24.27 | 0.81 (.63–1.03) |
| C. glabrata | 4.47 | 28.61 | 6.40 (3.83–10.70)* | 20.52 | 23.18 | 1.13 (.85–1.50) |
| C. parapsilosis | 3.63 | 8.81 | 2.42 (1.35–4.36)* | 5.19 | 9.96 | 1.92 (1.15–3.20)* |
| Other Candida spp | 6.43 | 10.02 | 1.56 (.99–2.46) | 11.43 | 12.68 | 1.11 (.76–1.62) |
Overall, 165 isolates (7%) were resistant to fluconazole (Table 2). Thirty-one isolates (1%) were resistant to an echinocandin antifungal; the majority (n = 20 [65%]) were C. glabrata. Eight of the 9 isolates resistant to both an echinocandin and fluconazole were C. glabrata.
DISCUSSION
This report describes the largest prospective, population-based surveillance for candidemia in the United States and is the first to compare changes in the incidence of candidemia over time in large metropolitan areas. Importantly, we report substantial shifts in the epidemiology of candidemia among specific age groups. Infants, who previously had the highest incidence rates of all high risk groups, are now second to adult patients aged ≥65 years, and non–C. albicans species now comprise two-thirds of all Candida species isolated in blood.
Neonates and infants have historically been populations with some of the highest rates of candidemia [3, 6, 7, 20–23]. Although infants aged <1 year still have high rates, we observed a significant decline in incidence in this group compared to prior surveillance. This decline is consistent with other reports [21, 24]. We also observed a decline in incidence among infants aged <1 year in both locations during the 3 years of surveillance (results not shown). Prophylaxis with fluconazole in high-risk patients, such as neonates, has been shown to decrease risk of disease [21, 25–29]. However, it is unknown if the declines in neonatal candidemia have occurred only because of the increased use of azoles as prophylaxis in neonatal ICUs. Declines were also seen in other pediatric populations, and the possible contributions of improved infection control practices, such as hand hygiene and catheter care, deserve further study.
Persons aged ≥65 are now the highest-risk group for candidemia in both areas under surveillance. The shift in burden from neonates to adults is a major finding of this report. The reasons for the changing incidence are likely multifactorial; some contribution may be due to changes in the prevalence of risk factors in the adult population. Increases in common risk factors such as diabetes [30], ICU admissions [31, 32], or numbers of patients receiving immunosuppressive therapies [33] may have resulted in increases in the overall pool of patients at high risk for candidemia. Additionally, we report that CO patients were older and more likely to have diabetes than other patients. Thus, persons without recent healthcare exposures but with other chronic health conditions that are risk factors for candidemia may also be contributing to the increase in incidence in this age group.
Recent reports have documented significant and dramatic declines in some central line–associated BSIs (CLABSIs), such as those associated with Staphylococcus aureus, following adherence to established central line insertion practices; however, similar dramatic declines have been less evident for CLABSIs due to Candida species [20, 24, 34–36]. Candida species are commensals of the human gastrointestinal tract and can cause BSIs that are not the direct result of poor central line insertion practices, and instead may be more dependent on catheter maintenance after insertion [20, 22, 34] or may be unrelated to the central line. Therefore, interventions to reduce CLABSIs that focus solely on line insertion practices may be less effective in preventing candidemia. Further research is needed to define the proportion of candidemias that are central line related, and to identify catheter care strategies effective in preventing candidemia.
Over the past 20 years, the emergence of non–C. albicans species has been well documented [20, 37–42]. Our surveillance confirms these reports; non–C. albicans species now comprise two-thirds of all candidemias in Atlanta and in Baltimore City/County. We report little change among the overall proportion of isolates that were resistant to fluconazole; however, we also report an increase among some groups in the incidence of candidemia due to species with reduced fluconazole susceptibility, such as C. glabrata. As C. glabrata comprised more than half of the isolates resistant to 1 of the 2 drug classes (azoles and echinocandins), and 8 of the 9 isolates resistant to both classes, it is important to monitor this particular species for drug resistance. Additionally, echinocandin antifungals are a new class of drugs introduced in the past decade. Although the low overall level of resistance to echinocandins reported here is reassuring, especially among species like C. parapsilosis, it will also be important to monitor for the emergence of echinocandin resistance as this drug class is now first-line empiric therapy for candidemia.
This surveillance was subject to several limitations. Large medical centers in each metropolitan area contributed the most cases to the surveillance population, and the overall epidemiology in each area was influenced by the epidemiology in these centers. Thus, these data may not be nationally representative. However, they do represent one of the largest populations evaluated for changes in incidence of candidemia in the United States. The differences described in the epidemiology between the 2 locations surveyed emphasize the geographic and demographic variability of candidemia; surveillance in additional geographic areas will be important to further describe the epidemiology of this infection. We were unable to identify patients who received care outside the catchment area or from healthcare facilities that sent blood cultures to labs outside of the catchment area, and were unable to collect isolates from one major federal institution. For these reasons, we may have underestimated the burden of disease in these areas.
This report demonstrates that the overall incidence of candidemia and the burden in adult populations has not declined in the past 20 years, and emphasizes the significant morbidity and mortality that candidemia contributes to the healthcare system. The changing face of healthcare is likely leading to growing populations of persons at risk for the development of candidemia. Our ability to prevent significant morbidity and mortality from candidemia remains a challenge. Although antifungal prophylaxis has been shown to be effective in selected patient populations, there is still debate on the application of risk prediction tools [43] and other preventive strategies. There is a continued need for surveillance of candidemia to develop and evaluate prevention strategies and to monitor for changes in incidence and resistance.
Acknowledgments
We gratefully acknowledge the many individuals in the hospitals and laboratories in Baltimore and Atlanta for their help in identifying cases and isolates, and also thank the following individuals: Wendy Baughman, MSPH, Janine Ladson, MPH, Lewis Perry, RN, MPH, Georgia Emerging Infections Program; Sandra Muhanuka, MPH, Helen Yoon, MS, Carolyn Kreiner, MS, RN, Debbie Lundy, BSN, Kim Holmes, RN, MS, Kathleen Shutt, MS, Maryland Emerging Infections Program; Scott Fridkin, MD, Yi Mu, PhD, Jonathan Edwards, MStat, Division of Healthcare Quality Promotion; Kaitlin Benedict, MPH, Randy Kuykendall, MPH, Shirley McClinton, Joyce Peterson, Carol Bolden, Naureen Iqbal, Lalitha Gade, Kizee Etienne, MPH, and Mary Brandt, PhD, Mycotic Diseases Branch.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24 179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–9. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 3.Fridkin SK. Candidemia is costly—plain and simple. Clin Infect Dis. 2005;41:1240–1. doi: 10.1086/496935. [DOI] [PubMed] [Google Scholar]
- 4.Morgan JMD, Meltzer MIP, Plikaytis BDM, et al. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol. 2005;26:540–7. doi: 10.1086/502581. [DOI] [PubMed] [Google Scholar]
- 5.Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–7. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 6.Kao AS, Brandt ME, Pruitt WR, et al. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin Infect Dis. 1999;29:1164–70. doi: 10.1086/313450. [DOI] [PubMed] [Google Scholar]
- 7.Hajjeh RA, Sofair AN, Harrison LH, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–27. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller MA, Messer SA, Hollis RJ, et al. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn Microbiol Infect Dis. 1999;33:217–22. doi: 10.1016/s0732-8893(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 9.Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, et al. Risk factors for fluconazole-resistant candidemia. Antimicrob Agents Chemother. 2010;54:3149–54. doi: 10.1128/AAC.00479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klevay MJ, Ernst EJ, Hollanbaugh JL, Miller JG, Pfaller MA, Diekema DJ. Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis. 2008;60:273–7. doi: 10.1016/j.diagmicrobio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Ortega M, Marco F, Soriano A, et al. Candida spp. bloodstream infection: influence of antifungal treatment on outcome. J Antimicrob Chemother. 2010;65:562–8. doi: 10.1093/jac/dkp495. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–31. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis RE. Trends in invasive Candida infections and their treatment: focus on echinocandins. Curr Med Res Opin. 2009;25:1729–62. [Google Scholar]
- 14.Garcia-Effron G, Chua DJ, Tomada JR, et al. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob Agents Chemother. 2010;54:2225–7. doi: 10.1128/AAC.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deak E, Etienne KA, Lockhart SR, Gade L, Chiller T, Balajee SA. Utility of a Luminex-based assay for multiplexed, rapid species identification of Candida isolates from an ongoing candidemia surveillance. Can J Microbiol. 2010;56:348–51. doi: 10.1139/w10-003. [DOI] [PubMed] [Google Scholar]
- 16.Institute CaLS . M27-A3 Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard—third edition. Clinical Laboratory Standards Institute; Wayne, PA: 2008. [Google Scholar]
- 17.Maryland Vital Statistics Annual Report 2009 Division of Health Statistics, Maryland Department of Health and Mental Hygiene. 2009 [Google Scholar]
- 18.National Center for Health Statistics Estimates of the 1 July 2000–1 July 2009, United States resident population from the Vintage 2009 postcensal series by year, county, age, sex, race, and Hispanic origin. Prepared under a collaborative arrangement with the U.S. Census Bureau. 2009 Available at: http://www.cdc.gov/nchs/nvss/bridged_race.htm. Accessed 1 December 2012.
- 19.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;10:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics. 2006;117:1680–7. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 22.Chang A, Neofytos D, Horn D. Candidemia in the 21st century. Future Microbiol. 2008;3:463–72. doi: 10.2217/17460913.3.4.463. [DOI] [PubMed] [Google Scholar]
- 23.Shetty SS, Harrison LH, Hajjeh RA, et al. Determining risk factors for candidemia among newborn infants from population-based surveillance: Baltimore, Maryland, 1998–2000. Pediatr Infect Dis J. 2005;24:601–4. doi: 10.1097/01.inf.0000168751.11375.d6. [DOI] [PubMed] [Google Scholar]
- 24.Schulman J, Stricof R, Stevens TP, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127:436–44. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 25.Burwell LA, Kaufman D, Blakely J, Stoll BJ, Fridkin SK. Antifungal prophylaxis to prevent neonatal candidiasis: a survey of perinatal physician practices. Pediatrics. 2006;118:e1019–26. doi: 10.1542/peds.2006-0446. [DOI] [PubMed] [Google Scholar]
- 26.Pappas P, Kauffman C. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manzoni P, Stolfi I, Pugni L, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356:2483–95. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman DA. Fluconazole prophylaxis: can we eliminate invasive Candida infections in the neonatal ICU? Curr Opin Pediatr. 2008;20:332–40. doi: 10.1097/MOP.0b013e3282f79c48. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman D. Fluconazole prophylaxis decreases the combined outcome of invasive Candida infections or mortality in preterm infants. Pediatrics. 2008;122:1158–9. doi: 10.1542/peds.2008-1837. author reply 1159. [DOI] [PubMed] [Google Scholar]
- 30.Boyle J, Thompson T, Gregg E, Barker L, Williamson D. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010:8, 29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerolle N, Trinquart L, Bornstain C, et al. Increased intensity of treatment and decreased mortality in elderly patients in an intensive care unit over a decade. Crit Care Med. 2010;38:59–64. doi: 10.1097/CCM.0b013e3181b088ec. [DOI] [PubMed] [Google Scholar]
- 32.Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 33.OPTN/SRTR 2010 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR); Rockville, MD: 2011. [Google Scholar]
- 34.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. New Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 35.Marcos M, Soriano A, Iñurrieta A, et al. Changing epidemiology of central venous catheter-related bloodstream infections: increasing prevalence of gram-negative pathogens. J Antimicrob Chemother. 2011;66:2119–25. doi: 10.1093/jac/dkr231. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention Vital signs: central line-associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60:243–8. [PubMed] [Google Scholar]
- 37.Nguyen MH, Peacock JE, Morris AJ, et al. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–23. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 38.Hachem R, Hanna H, Kontoyiannis D, Jiang Y, Raad I. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer. 2008;112:2493–9. doi: 10.1002/cncr.23466. [DOI] [PubMed] [Google Scholar]
- 39.Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP, National Nosocomial Infections Surveillance System Hospitals Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis. 2002;35:627–30. doi: 10.1086/342300. [DOI] [PubMed] [Google Scholar]
- 40.Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14:e954–66. doi: 10.1016/j.ijid.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009) Diagn Microbiol Infect Dis. 2010;68:278–83. doi: 10.1016/j.diagmicrobio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 42.Pfaller MA, Diekema DJ, Gibbs DL, et al. Geographic variation in the frequency of isolation and fluconazole and voriconazole susceptibilities of Candida glabrata: an assessment from the ARTEMIS DISK Global Antifungal Surveillance Program. Diagn Microbiol Infect Dis. 2010;67:162–71. doi: 10.1016/j.diagmicrobio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Ostrosky-Zeichner L, Kullberg BJ, Bow EJ, et al. Early treatment of candidemia in adults: a review. Med Mycol. 2011;49:113–20. doi: 10.3109/13693786.2010.512300. [DOI] [PubMed] [Google Scholar]