First Detection of TR34 L98H and TR46 Y121F T289A Cyp51 Mutations in Aspergillus fumigatus Isolates in the United States (original) (raw)
Abstract
Azole resistance in Aspergillus fumigatus is an increasing problem. The TR34 L98H and TR46 Y121F T289A mutations that can occur in patients without previous azole exposure have been reported in Europe, Asia, the Middle East, Africa, and Australia. Here, we report the detection of both the TR34 L98H and TR46 Y121F T289A mutations in confirmed A. fumigatus isolates collected in institutions in the United States. These mutations, other mutations known to cause azole resistance, and azole MICs are reported here.
TEXT
Invasive aspergillosis remains a major problem in immunocompromised individuals, including solid organ transplant recipients, those undergoing hematopoietic stem cell transplant, and patients receiving highly immunosuppressive chemotherapies (1–3). In patients with structural damage to the lungs, such as those who have had tuberculosis or sarcoidosis, chronic pulmonary aspergillosis is also a significant problem, the prevalence of which has been estimated to be approximately 3 million patients worldwide (4–7). The azoles are a mainstay in the treatment of invasive aspergillosis, as the members of this class that have activity against Aspergillus species, itraconazole, posaconazole, voriconazole, and isavuconazole, may be given orally, and the treatment of this invasive fungal infection is often prolonged. However, prolonged therapy may predispose patients to adverse effects and drug interactions associated with these agents and increase the potential for the development of drug-resistant organisms (8). Over the last several years, concern has been growing regarding azole resistance in Aspergillus fumigatus (9, 10). Azole-resistant A. fumigatus isolates recovered from patients failing therapy have been reported in several countries around the world. Mutations in the CYP51A gene, which encodes the Cyp51 enzyme responsible for the last step in ergosterol biosynthesis, have been the resistance mechanism documented in clinical isolates, and these mutations have been found in isolates recovered from patients who have had long exposures to azoles (11, 12). However, resistant isolates harboring tandem repeats of various sizes in the promoter region of the CYP51A gene, along with nonsynonymous point mutations leading to amino acid changes in the Cyp51 enzyme, have been recovered from azole-naive patients with invasive aspergillosis (8, 12, 13). These resistance mechanisms, which include TR34 L98H and TR46 Y121F T289A, have been linked to the environmental use of azoles in agriculture and the preservation of various materials (14, 15), and they have been found in various countries, including many in Europe, India, China, Iran, Tanzania, and Australia (13, 16–21). However, these mutations have not yet been reported in isolates collected in the United States (22). Our objective was to evaluate the Cyp51-associated mechanisms of azole resistance in a collection of A. fumigatus isolates from institutions across the United States.
The antifungal susceptibility database in the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio was queried for itraconazole, voriconazole, and posaconazole MIC data against isolates claimed to be A. fumigatus from 2001 through 2014. This database is populated with antifungal MIC data against fungal isolates sent to our laboratory from institutions across the United States. Susceptibility testing was performed according to methods in the CLSI M38-A2 standard (23). The MIC was defined as the lowest concentration of each agent that resulted in 100% inhibition of growth after 48 h of incubation at 35°C. Since the CLSI has not established clinical breakpoints against molds, isolates were arbitrarily classified as resistant using the EUCAST breakpoints (voriconazole and itraconazole, ≥4 μg/ml; posaconazole, ≥0.5 μg/ml) (24). Viable isolates with an elevated azole MIC and with a morphology consistent with A. fumigatus were subjected to temperature studies at 50°C, and those that grew at this temperature were confirmed to be A. fumigatus sensu stricto by sequence analysis of the β-tubulin gene (25, 26). The isavuconazole MICs were also measured against the confirmed A. fumigatus isolates. The CYP51A gene and its promoter region were also sequenced to evaluate mutations associated with azole resistance. This was done using previously published primers and methods (27–29), and the sequence results were compared to those for the CYP51A gene (GenBank accession no. AF338659) (27). If more than one isolate was received from the same patient, only one isolate was included in the analysis.
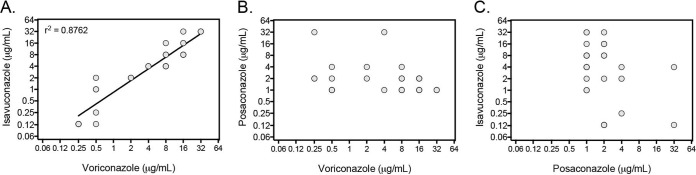
A total of 220 clinical isolates sent to our laboratory between 2001 and 2014 with an elevated MIC for voriconazole, itraconazole (MIC, ≥4 μg/ml), or posaconazole (≥0.5 μg/ml,) and a preliminary identification of A. fumigatus were screened, and 26 nonduplicate isolates with elevated MICs were confirmed to be this species by morphology, growth at 50°C, and β-tubulin sequence analysis. Nonsynonymous point mutations in the CYP51A gene that result in amino acid changes within the Cyp51 enzyme that are associated with azole resistance in A. fumigatus were found in 20 of these isolates, and no point mutations were found in 6 isolates (Table 1). The itraconazole MIC was ≥16 μg/ml against 22 of the 26 isolates, which is consistent with the use of this azole to screen for azole-resistant A. fumigatus (14, 21). Two isolates with the TR34 L98H mutations and two with the TR46 Y121F T289A mutations were also found. The mutations were first observed in an isolate from 2008, with two coming from different cities in Pennsylvania, one from Arizona, and one from another reference laboratory. Similar to previous reports, the MICs for voriconazole and isavuconazole were very high (>16 μg/ml) against the two isolates harboring TR46 Y121F T289A mutations, while those of itraconazole and posaconazole were moderately elevated (16, 18). In addition, several point mutations were found that have been associated with the development of azole resistance in patients following long exposures to these agents. The first of these isolates was received by our laboratory in 2001 and came from various states across the United States. The antifungal susceptibility profiles of these isolates varied, which is consistent with previous observations that the location of the point mutation and the corresponding amino acid change can result in different resistance patterns (8). As reported by others, the MIC profile of isavuconazole was similar to that of voriconazole against these isolates (Pearson correlation coefficient, 0.8824; P < 0.0001), suggesting that CYP51A mutations affect these two antifungals in a similar manner (16, 18). Similar correlations were not observed between posaconazole and voriconazole or isavuconazole (Fig. 1).
TABLE 1.
UTHSCSA isolate information and MICsa
| UTHSCSA isolate no. | Stateb | Yr | Cyp51 mutation(s) | MICs forc: | |||
|---|---|---|---|---|---|---|---|
| Itraconazole | Posaconazole | Voriconazole | Isavuconazole | ||||
| DI15-93 | California | 2001 | M220V | 16 | 1 | 0.5 | 1 |
| DI15-95 | Connecticut | 2007 | None detected | >16 | 2 | 16 | 8 |
| DI15-96 | Arizona | 2008 | TR46 Y121F T289A | 4 | 1 | >16 | >16 |
| DI15-97 | North Carolina | 2008 | None detected | 4 | 1 | 4 | 4 |
| DI15-98 | Reference laboratory | 2008 | None detected | 16 | 2 | 16 | 16 |
| DI15-99 | Maryland | 2008 | G54R | >16 | 2 | 0.5 | 0.125 |
| DI15-100 | Reference laboratory | 2008 | None detected | >16 | 1 | 8 | 4 |
| DI15-101 | Wisconsin | 2009 | G138S | >16 | 2 | 16 | >16 |
| DI15-102 | Pennsylvania | 2010 | TR34 L98H | >16 | 2 | 8 | 16 |
| DI15-103 | Ohio | 2010 | M220I | >16 | 1 | 0.5 | 2 |
| DI15-104 | Ohio | 2011 | G448S | 4 | 1 | 16 | 8 |
| DI15-105 | Reference laboratory | 2012 | None detected | >16 | 2 | 8 | 8 |
| DI15-106 | Reference laboratory | 2012 | TR46 Y121F T289A | 4 | 1 | >16 | >16 |
| DI15-107 | California | 2012 | G448S | 16 | 4 | 0.5 | 0.25 |
| DI15-108 | California | 2012 | G138C | >16 | 1 | 16 | 8 |
| DI15-109 | Washington | 2012 | G54E | >16 | 2 | 0.25 | 0.125 |
| DI15-110 | Connecticut | 2013 | None detected | >16 | 2 | 8 | 4 |
| DI15-111 | Reference laboratory | 2013 | G54W | >16 | >16 | 0.25 | 0.125 |
| DI15-112 | Ohio | 2014 | G54R | >16 | 2 | 0.25 | 0.125 |
| DI15-113 | Massachusetts | 2014 | G448S | >16 | 4 | 8 | 4 |
| DI15-114 | California | 2014 | G54R | >16 | >16 | 4 | 4 |
| DI15-115 | Reference laboratory | 2014 | M220I | >16 | 1 | 0.5 | 1 |
| DI15-116 | Pennsylvania | 2014 | TR34 L98H | >16 | 1 | 8 | 8 |
| DI15-117 | Maryland | 2014 | F219S | >16 | 2 | 2 | 2 |
| DI15-118 | Reference laboratory | 2014 | M220K | >16 | 4 | 2 | 2 |
| DI15-120 | Ohio | 2015 | G448S | >16 | 1 | >16 | >16 |
FIG 1.
Shown are the MICs for voriconazole versus isavuconazole (A), voriconazole versus posaconazole (B), and posaconazole versus isavuconazole (C). All MICs were read as 100% inhibition of growth after 48 h of incubation at 35°C. MICs of >16 μg/ml were plotted at 32 μg/ml.
To our knowledge, this is the first report of the TR34 L98H and TR46 Y121F T289A mutations in A. fumigatus isolates in the United States. A previous study surveillance study of 1,026 clinical A. fumigatus isolates collected from various states across the United States did not find these mutations (22). Although the complete patient histories are not known, additional information is available for 3 of these 4 isolates. Each was a clinical isolate (one from tissue, one from bronchoalveolar lavage fluid, and one from sputum) and was collected from patients living in the United States before and around the time the isolates were collected. Two were known to not have travel histories to countries known to harbor these specific mechanisms of resistance, including the one from which the 2008 isolate was collected. Each was also at risk for invasive aspergillosis, and two had confirmed disease, including one with disseminated infection. It is not known if these isolates may have also been recovered from environmental sources, which has been reported in several studies, mainly in Europe, of A. fumigatus isolates with these mechanisms of azole resistance (9, 14); however, this has not been a consistent finding (30). Of the 26 azole-resistant isolates included in this study, 6 did not contain mutations within the CYP51A gene. Other potential mechanisms of azole resistance that have been reported include higher expression of CYP51B, higher expression or modifications in the efflux transporter genes CDR1B, AfuMDR1, AfuMDR2, AfuMDR3, and AfuMDR4, and a mutation in the CCAAT-binding transcript factor complex subunit HapE (31–35). However, these mechanisms of resistance were not evaluated in this study, and their clinical relevance is unknown.
Our findings demonstrate that the TR34 L98H and TR46 Y121F T289A mutations can be found in the United States and that there is a need for continued surveillance of azole resistance in A. fumigatus.
ACKNOWLEDGMENTS
N.P.W. has received research support from Astellas, Dow, F2G, Merck, Merz, Revolution Medicines, and Viamet and has served on advisory boards for Merck, Astellas, Toyama, and Viamet.
Funding Statement
This project was supported in part by a San Antonio Life Sciences Institute (SALSI) Clusters in Research Excellence Award to Nathan P. Wiederhold.
REFERENCES
- 1.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 2.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 3.Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, Oster RA, Anaissie EJ, Walsh TJ, Schuster MG, Wingard JR, Patterson TF, Ito JI, Williams OD, Chiller T, Pappas PG. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin Infect Dis 50:1559–1567. doi: 10.1086/652768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith NL, Denning DW. 2011. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J 37:865–872. doi: 10.1183/09031936.00054810. [DOI] [PubMed] [Google Scholar]
- 5.Denning DW, Pleuvry A, Cole DC. 2011. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ 89:864–872. doi: 10.2471/BLT.11.089441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning DW, Pleuvry A, Cole DC. 2013. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J 41:621–626. doi: 10.1183/09031936.00226911. [DOI] [PubMed] [Google Scholar]
- 7.Denning DW, Pleuvry A, Cole DC. 2013. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 51:361–370. doi: 10.3109/13693786.2012.738312. [DOI] [PubMed] [Google Scholar]
- 8.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeulen E, Lagrou K, Verweij PE. 2013. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 26:493–500. doi: 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 10.Seyedmousavi S, Mouton JW, Melchers WJ, Bruggemann RJ, Verweij PE. 2014. The role of azoles in the management of azole-resistant aspergillosis: from the bench to the bedside. Drug Resist Updat 17:37–50. doi: 10.1016/j.drup.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Howard SJ, Webster I, Moore CB, Gardiner RE, Park S, Perlin DS, Denning DW. 2006. Multi-azole resistance in Aspergillus fumigatus. Int J Antimicrob Agents 28:450–453. doi: 10.1016/j.ijantimicag.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. 2010. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother 65:2116–2118. doi: 10.1093/jac/dkq279. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snelders E, Huis In 't Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hof H. 2008. Is there a serious risk of resistance development to azoles among fungi due to the widespread use and long-term application of azole antifungals in medicine? Drug Resist Updat 11:25–31. doi: 10.1016/j.drup.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2014. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J Antimicrob Chemother 69:555–557. doi: 10.1093/jac/dkt397. [DOI] [PubMed] [Google Scholar]
- 17.Verweij PE, Mellado E, Melchers WJ. 2007. Multiple-triazole-resistant aspergillosis. N Engl J Med 356:1481–1483. doi: 10.1056/NEJMc061720. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 69:2979–2983. doi: 10.1093/jac/dku259. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, Gaur SN, Hagen F, Klaassen CH, Meis JF. 2012. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PLoS One 7:e52871. doi: 10.1371/journal.pone.0052871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. 2015. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58:350–355. doi: 10.1111/myc.12324. [DOI] [PubMed] [Google Scholar]
- 21.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham CD, Reiss E, Hagen F, Meis JF, Lockhart SR. 2014. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011–2013. Emerg Infect Dis 20:1498–1503. doi: 10.3201/eid2009.140142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Verweij PE, Howard SJ, Melchers WJ, Denning DW. 2009. Azole-resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist Updat 12:141–147. doi: 10.1016/j.drup.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Glass NL, Donaldson GC. 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balajee SA, Houbraken J, Verweij PE, Hong SB, Yaghuchi T, Varga J, Samson RA. 2007. Aspergillus species identification in the clinical setting. Stud Mycol 59:39–46. doi: 10.3114/sim.2007.59.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellado E, Diaz-Guerra TM, Cuenca-Estrella M, Rodriguez-Tudela JL. 2001. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J Clin Microbiol 39:2431–2438. doi: 10.1128/JCM.39.7.2431-2438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiess B, Seifarth W, Merker N, Howard SJ, Reinwald M, Dietz A, Hofmann WK, Buchheidt D. 2012. Development of novel PCR assays to detect azole resistance-mediating mutations of the Aspergillus fumigatus_cyp51A_ gene in primary clinical samples from neutropenic patients. Antimicrob Agents Chemother 56:3905–3910. doi: 10.1128/AAC.05902-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Li H, Li R, Bu D, Wan Z. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother 55:31–37. [DOI] [PubMed] [Google Scholar]
- 30.Astvad KM, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 58:5096–5101. doi: 10.1128/AAC.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arendrup MC, Mavridou E, Mortensen KL, Snelders E, Frimodt-Møller N, Khan H, Melchers WJ, Verweij PE. 2010. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS One 5:e10080. doi: 10.1371/journal.pone.0010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraczek MG, Bromley M, Buied A, Moore CB, Rajendran R, Rautemaa R, Ramage G, Denning DW, Bowyer P. 2013. The cdr1B efflux transporter is associated with non-_cyp51a_-mediated itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother 68:1486–1496. doi: 10.1093/jac/dkt075. [DOI] [PubMed] [Google Scholar]
- 33.Buied A, Moore CB, Denning DW, Bowyer P. 2013. High-level expression of cyp51B in azole-resistant clinical Aspergillus fumigatus isolates. J Antimicrob Chemother 68:512–514. doi: 10.1093/jac/dks451. [DOI] [PubMed] [Google Scholar]
- 34.da Silva Ferreira ME, Capellaro JL, dos Reis Marques E, Malavazi I, Perlin D, Park S, Anderson JB, Colombo AL, Arthington-Skaggs BA, Goldman MHS, Goldman GH. 2004. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob Agents Chemother 48:4405–4413. doi: 10.1128/AAC.48.11.4405-4413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nascimento AM, Goldman GH, Park S, Marras SA, Delmas G, Oza U, Lolans K, Dudley MN, Mann PA, Perlin DS. 2003. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother 47:1719–1726. doi: 10.1128/AAC.47.5.1719-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]