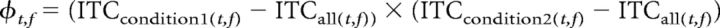Phase of Spontaneous Slow Oscillations during Sleep Influences Memory-Related Processing of Auditory Cues (original) (raw)
Abstract
Slow oscillations during slow-wave sleep (SWS) may facilitate memory consolidation by regulating interactions between hippocampal and cortical networks. Slow oscillations appear as high-amplitude, synchronized EEG activity, corresponding to upstates of neuronal depolarization and downstates of hyperpolarization. Memory reactivations occur spontaneously during SWS, and can also be induced by presenting learning-related cues associated with a prior learning episode during sleep. This technique, targeted memory reactivation (TMR), selectively enhances memory consolidation. Given that memory reactivation is thought to occur preferentially during the slow-oscillation upstate, we hypothesized that TMR stimulation effects would depend on the phase of the slow oscillation. Participants learned arbitrary spatial locations for objects that were each paired with a characteristic sound (eg, cat–meow). Then, during SWS periods of an afternoon nap, one-half of the sounds were presented at low intensity. When object location memory was subsequently tested, recall accuracy was significantly better for those objects cued during sleep. We report here for the first time that this memory benefit was predicted by slow-wave phase at the time of stimulation. For cued objects, location memories were categorized according to amount of forgetting from pre- to post-nap. Conditions of high versus low forgetting corresponded to stimulation timing at different slow-oscillation phases, suggesting that learning-related stimuli were more likely to be processed and trigger memory reactivation when they occurred at the optimal phase of a slow oscillation. These findings provide insight into mechanisms of memory reactivation during sleep, supporting the idea that reactivation is most likely during cortical upstates.
SIGNIFICANCE STATEMENT Slow-wave sleep (SWS) is characterized by synchronized neural activity alternating between active upstates and quiet downstates. The slow-oscillation upstates are thought to provide a window of opportunity for memory consolidation, particularly conducive to cortical plasticity. Recent evidence shows that sensory cues associated with previous learning can be delivered subtly during SWS to selectively enhance memory consolidation. Our results demonstrate that this behavioral benefit is predicted by slow-oscillation phase at stimulus presentation time. Cues associated with high versus low forgetting based on analysis of subsequent recall performance were delivered at opposite slow-oscillation phases. These results provide evidence of an optimal slow-oscillation phase for memory consolidation during sleep, supporting the idea that memory processing occurs preferentially during cortical upstates.
Keywords: memory consolidation, memory reactivation, phase, slow oscillation, slow-wave sleep
Introduction
Sleep, particularly slow-wave sleep (SWS), is known to play an important role in memory consolidation. According to two-stage models of memory consolidation (eg, Marr, 1971; Alvarez and Squire, 1994; McClelland et al., 1995; Frankland and Bontempi, 2005), during wake new information is initially encoded in parallel into both a fast-learning temporary store, namely the hippocampus, as well as a slower-learning, long-term store, namely the neocortex. In subsequent offline periods, newly encoded memory traces are spontaneously and repeatedly reactivated (Sutherland and McNaughton, 2000; cf. O'Neill et al., 2010). These reactivations drive the gradual transformation of newly encoded information stored in cortical networks, such that memory representations become relatively more dependent upon the cortex and less dependent upon the hippocampus. Memory reactivation underlying reorganization and consolidation can occur during wake, but is especially prominent during SWS (Wilson and McNaughton, 1994; Lee and Wilson, 2002; Ji and Wilson, 2007).
SWS is characterized by slow oscillations, which appear as high amplitude, synchronized EEG activity, occurring in humans at a typical frequency of ∼0.8 Hz (Achermann and Borbély, 1997; Mölle et al., 2002; Born and Wilhelm, 2012). Slow oscillations originate in the neocortex and regulate the dialogue between the neocortex and hippocampus thought to underlie memory consolidation. Slow oscillations synchronize neuronal activity into upstates of depolarization, associated with widespread excitability and increased neuronal firing, and downstates of hyperpolarization, corresponding to periods of neuronal quiescence. The up-phase coincides with two additional physiological signals: (1) sharp wave-ripples generated by the hippocampus, associated with reactivations of newly encoded hippocampal memory representations, and (2) 10–15 Hz spindles generated by thalamocortical networks (Diekelmann and Born, 2010; Mölle and Born, 2011). By synchronizing these mechanisms, the slow-oscillation up-phase drives the formation of spindle-ripple events, in which sharp wave-ripples are nested in spindle troughs. These spindle-ripple events are thought to mediate the gradual integration of reactivated information from newly acquired memories into neocortex. With this mechanism, memory-related information from the hippocampus arrives at the cortex, while cortical networks are in the depolarized upstate and in conjunction with spindles, promoting synaptic plasticity (Siapas and Wilson, 1998; Sirota et al., 2003; cf. Mölle and Born, 2011).
Reactivation during sleep can also be induced by covertly presenting memory cues associated with a prior learning episode. This technique, targeted memory reactivation (TMR), can enhance consolidation of declarative and nondeclarative memories (Oudiette and Paller, 2013). In one study, odor re-exposure during SWS, when that odor was present during object-location learning, improved subsequent memory for object locations and elicited hippocampal activation (Rasch et al., 2007). In another study, participants were trained on an object–location association task in which individual objects were paired with characteristic sounds (eg, cat–meow). Presenting these auditory cues during SWS selectively strengthened individual memories (Rudoy et al., 2009). However, it is unknown whether the phase of slow oscillations modulates these effects. Such a finding would be consistent with the idea that slow-oscillation upstates present windows of opportunity for memory consolidation.
In the present study, we used TMR methods to test the hypothesis that memory reactivation occurs differentially as a function of slow-oscillation phase. Participants learned the locations of 50 unique object images, each paired with a characteristic sound, before taking an afternoon nap. During the nap, some of the sounds were covertly presented, presumable triggering reactivation of the associated item memories. Participants were then retested on the item-location associations upon awakening. We hypothesized that learning-related cues would preferentially strengthen associated memories when their processing coincided with the beginning of the slow-oscillation upstate.
Materials and Methods
Participants.
A total of 22 participants (13 female) contributed data to this study. Data were originally collected as part of two previous TMR studies (Rudoy et al., 2009; Creery et al., 2015). Data from these two studies used very similar stimuli, tasks, and learning procedures. Data were combined and reanalyzed in the present study to examine specific hypotheses related to phase angle. Individual item cues were coded during the experiment for only a subset of participants described by Creery et al. (2015), such that only 10 of the 20 original participants could be included in the present study. The combined group of 12 participants from the first experiment and 10 participants from the second experiment were between 19 and 24 years old, were neurologically normal, and did not have any known sleep disorders. They were asked to abstain from caffeine on the day of the experiment.
Task.
The learning task required participants to memorize the spatial locations of 50 unique object images on a 1000 × 800 pixel grid (35.7 × 28.6 cm). At the start of the learning phase, participants were presented with each object once in its correct location. The center of each object was indicated by a red dot. Each object was presented at a screen location randomly determined for each object and each participant. A grid background was provided as reference, but objects could appear anywhere on the screen. Each object was paired with a characteristic sound, presented at stimulus onset (eg, cat with meow and kettle with whistle). Objects (5.3 × 5.3 cm) appeared for 3000 ms with a 1000 ms interstimulus interval. The duration of each sound was 500 ms.
In the second phase of learning, each object appeared at the center of the screen, accompanied by its corresponding sound. Participants were asked to move the object to the correct location using a computer mouse. The object was then displayed in its correct location, providing feedback. Participants completed several rounds of testing with objects presented in random order. After two rounds, objects were included in subsequent rounds of testing only if the learning criterion had not been successfully met. The learning criterion required participants to place the object < 5.3 cm (150 pixels) from the correct location on two consecutive rounds. The second phase concluded when all objects met the learning criterion (mean number of repetitions per item: 3.25, range = 2.14–4.56).
After learning was complete, participants were tested on each object using the same procedure as described above but without feedback, providing a baseline, pre-nap measurement of memory. For each item, error was computed, in pixels, as the distance between the item's actual location and the location selected by the participant. The same test was also administered at the end of the experimental session, providing a post-nap measurement of memory. Our main dependent measure was computed by subtracting pre-nap error from post-nap error for each object. This subtraction yields a pre- to post-nap memory change measure, with higher positive values indicating more forgetting, and negative values indicating improvement in accuracy from pre- to post-nap.
Procedure.
Participants arrived at the laboratory between 11:00 A.M. and 3:00 P.M. After informed consent was obtained, the session consisted of six main phases, as follows: the learning task, the pre-nap test, a ∼10 min break, EEG setup, the nap period, a second short break, and the post-nap test. The protocol was altered for participants run by Creery et al. (2015) in that the pre-nap test was administered after EEG setup rather than immediately after the learning task.
When it was time for the nap, participants reclined in a quiet, darkened room. Background white noise was presented at ∼37 dB(A) sound pressure level, measured from where participants' heads were located. After EEG indications of SWS were observed, sound cues were presented for 25 of the 50 objects at the same intensity as the background noise. The 25 selected sound cues were intermixed with 25 instances of a baseline sound not heard before (a guitar strum). One sound was played every 4.9 s, yielding a stimulation period slightly >4 min. Each sound cue was presented only once. The 25 sound cues presented during the nap were selected such that pre-nap recall accuracy was matched for cued and uncued objects for each participant. Items were ranked as a function of pre-nap recall accuracy, and divided into two lists (odd and even items). The list to be cued was then randomly selected by a computer algorithm. We verified that there was no significant difference in pre-nap recall accuracy between the cued and uncued items (uncued pre-nap error = 75.98 pixels, 4.83 SEM; cued pre-nap error = 74.16 pixels, 3.73 SEM, t(20) = 0.68, p = 0.50).
The nap period ended when participants woke naturally after 60–90 min had elapsed; participants still asleep after 90 min were awakened. After a further 10 min delay, spatial recall was tested as in the pre-nap test. Finally, participants were debriefed about the sound cues presented during their naps, after first being asked whether they thought any sounds had been played while they slept. As part of this debriefing, participants completed a forced-choice task in which all 50 object images were displayed with their corresponding sounds. Participants were required to guess whether each sound had been presented during their nap.
EEG acquisition and analysis.
EEG was recorded from 21 tin electrodes mounted in an elastic cap, along with two electrooculogram channels and one chin electromyogram channel. EEG was acquired at a sampling rate of 250 Hz, amplified with a bandpass of 0.1–100 Hz. EEG analyses were performed using EEGLAB (Delorme and Makeig, 2004). EEG channels were re-referenced off-line to averaged mastoids. Data from noisy electrodes were interpolated when necessary using the spherical interpolation method in EEGLAB. Off-line sleep scoring was conducted using standard criteria by a rater who was blind to when sounds were presented.
For phase analyses, data were initially bandpass filtered from 0.5 to 30 Hz. EEG data epochs were then extracted from −1000 ms before to 1500 ms after sound cue onset. All EEG epochs were visually inspected for possible artifacts; however, no artifacts were detected in any epoch, yielding a total of 25 epochs per participant.
Phase angle and power for individual trials was computed using a continuous Morlet wavelet transformation of single-trial data from 0.5 to 30 Hz, using the newtimef function of EEGLAB. Wavelet transformations were computed in 0.5 Hz steps with 0.5 cycles at the lowest frequency (0.5 Hz) and increasing by a scaling factor of 0.5, reaching 15 cycles at the highest frequency (30 Hz). This approach was selected to optimize the tradeoff between temporal resolution at lower frequencies and frequency resolution at high frequencies (Delorme and Makeig, 2004). For each trial, this computation yields a complex number at each time and frequency point that represents both the amplitude and phase angle of the signal.
First, we empirically evaluated our hypothesis that sound cues linked with high versus low forgetting were associated with opposite phases of spontaneous EEG oscillations in the delta band (0.5–4 Hz) at the end of the prestimulus interval. This hypothesis was tested by computing a phase bifurcation index (φ), which is a measure of the difference in phase angle between two conditions (Busch et al., 2009). As described by Busch et al. (2009), φ is a powerful and sensitive method for testing whether two conditions show significantly different phase distributions from one another. The value for φ provides an unbiased estimate of phase differences between two conditions (i.e., high forgetting vs low forgetting) for an unrestricted number of time and frequency samples. This analysis allowed us to investigate whether memory-related phase effects were maximal within our predicted time (end of prestimulus interval) and frequency range (0.5–4 Hz), as well as the specificity of any such effects.
The phase bifurcation index φ requires comparing between two discrete conditions. Thus, for each participant, the 25 cued trials were divided by median split according to their pre- to post-nap spatial-memory change scores, yielding 12 trials associated with relatively high forgetting and 12 trials associated with relatively low forgetting. Trials with high forgetting and low forgetting from all participants were pooled separately, resulting in a total of 264 trials per condition. The median (i.e., 13th) trial was excluded from median-split analyses. Note that median splits involve converting a continuous variable into a categorical one, resulting in some loss of sensitivity and power. Nonetheless, this median split approach ensured an equal number of trials in each condition, which is important given the overall low number of trials available for analysis (25 per participant), and provided a broad test of whether there are systematic phase differences between presented cues as a function of later memory.
As described by Busch et al. (2009), φ is computed by comparing the intertrial coherence (ITC) within each condition against the ITC across conditions. ITC provides an index of phase synchronization or phase locking across trials relative to time-locking events. The φ computation assumes that the distribution of phases across the whole set of trials should be random, whereas—if there are differences in phases between conditions—the phase distribution within conditions should exhibit stronger phase concentrations. When two conditions are phase-locked, but at opposite phase angles, φ is positive. When only one condition exhibits phase locking, φ is negative. If the phase distribution for both conditions is random (neither one exhibiting phase locking), or if the two conditions are phase-locked at the same phase angle, φ is close to zero. The upper bound of φ is 1, indicating perfect phase locking in both conditions (ITC = 1) but at exactly opposite phases (Busch et al., 2009).
Based on the phase information for each individual trial, we computed ITC within each condition (high forgetting, low forgetting) and across all trials. Using the formula provided by Busch et al. (2009), we then computed φ for each time and frequency sample, averaged across all 21 scalp electrodes as follows:
Because our hypotheses focus specifically on frequencies in the delta band (<4 Hz), for increased visibility we plot results for the restricted frequency range from 0.5 to 10 Hz, including the time interval from −440 to 800 ms after stimulus onset.
We then statistically evaluated the significance of φ at each time and frequency point using a resampling test with 1000 iterations. For each iteration, two sets of trials (i.e., “pseudo-high-forgetting” and “pseudo-low-forgetting”) were drawn randomly from the overall pool of 528 trials (excluding the median trial for each participant), and φ was computed. Next, for each time-frequency sample, a p value was computed as the proportion of these 1000 pseudo-φ values that exceeded the observed φ. Because we were exclusively interested in whether φ was significantly >0 (and not in negative φ values), we used a one-tailed test where p < 0.05 was considered significant. To correct for multiple comparisons within our time and frequency range of interest (prestimulus interval from −400 to 0 ms; 0.5–4 Hz), we used the false discovery rate (FDR) procedure (pFDR ≤ 0.05; Benjamini and Hochberg, 1995), with the number of comparisons equal to the number of time and frequency samples within this range of interest. We focused on the prestimulus interval to isolate the effects of phase on subsequent memory processing, as poststimulus phase differences between conditions may be at least partially driven by physical differences among auditory stimuli that may also play a role in memory processing.
As described above, the purpose of the φ analysis was to examine whether trials with high versus low forgetting were associated with opposite phase angles, and to quantify the frequency and time period over which any such phase effects occurred. After establishing that trials with high versus low forgetting were indeed associated with different phase angles, we completed a second series of analyses, designed to quantify the numerical phase angle values for optimal versus suboptimal memory consolidation. If memory reactivations occur most frequently during the slow-oscillation upstate, we hypothesized that—for optimal memory consolidation—sound cue onset should occur just before the onset of the upstate (i.e., 270°–360°). This timing could conceivably allow the sound cue to be fully presented and to undergo sufficient sensory processing, such that subsequent memory-related processing would coincide with the slow-oscillation upstate. Conversely, the suboptimal phase for sound presentation should occur at the opposite phase angle (i.e., 90°–180°). We tested this hypothesis by computing the phase angle for each trial within the prestimulus time and frequency region in which φ was empirically shown to be maximal (0.5–1 Hz, −100 to 0 ms).
We quantified the relationship between phase and memory change in two main ways. Our first analysis, requiring a median split approach, examined the phase distribution of trials associated with high versus low forgetting. Within each condition (high forgetting, low forgetting), the complex numbers representing the amplitude and phase for each trial at each time and frequency point were averaged across channels and across the time and frequency dimensions within the range of interest. Phase angle was then extracted for each trial, yielding 264 values for each condition. The resulting phase distribution for items with high versus low forgetting was plotted on a circular histogram, and pooled into six equally spaced bins, allowing visualization of phase angles associated with better and worse memory performance. A Kuiper test was used to evaluate whether phases associated with high- and low-forgetting items were significantly different within this restricted time and frequency range.
In a second analysis, which can be considered the mirror image of the previous analysis, we examined forgetting (change in spatial error from pre- to post-nap) as a function of phase. This analysis allowed us to represent change in spatial error as a continuous variable, rather than a categorical one. Phase range was divided into four equal bins, each corresponding to a quadrant of the circle map. We addressed whether objects whose corresponding sound cues were presented during the optimal median phase range (as revealed by the prior analysis) were associated with significantly less pre- to post-nap forgetting compared with objects whose sound cues were presented during the suboptimal median phase range, and to objects that were uncued. The two contiguous angle bins out of the original four that showed the highest ratio of trials with low forgetting versus high forgetting were defined as “optimal.” The other two bins were considered “suboptimal.” Planned t tests evaluated whether the error change scores from pre- to post-nap differed for items whose corresponding sound cues were presented during the optimal phase versus suboptimal phase, and between suboptimal phase items and uncued items. A one-way ANOVA linear contrast with phase bin (1–4) as a between-groups factor was used to examine whether there was a linear effect of phase on change in spatial error.
As an additional characterization of potential pre-stimulus phase differences between conditions, we plotted event-related potentials (ERPs) to sound cues associated with high versus low forgetting. ERPs were baseline corrected to the prestimulus time interval (−1000 to 0 ms). A low-pass filter of 4 Hz was applied, allowing clearer visualization of potential baseline phase differences in the slow delta band specifically. To statistically quantify time-locked EEG differences between conditions during the time interval before and immediately after stimulus onset, mean amplitudes were computed from the original data (i.e., before application of the 4 Hz filter) across a 500 ms window centered on stimulus onset (−250 to 250 ms). We then conducted a repeated-measures ANOVA on mean ERP amplitudes from selected channels (F7, F3, T7, C3, T5, P3, F8, F4, T4, C4, T6, P4), with condition (high forgetting, low forgetting), hemisphere (left, right), anterior/posterior (anterior, middle, posterior), and laterality (lateral, medial) as within-subjects factors. Greenhouse–Geisser corrected values are reported for factors with >2 levels.
Finally, to more completely characterize the relationship between slow oscillations and memory consolidation, we examined the relationship between delta power and change in memory strength for each item from pre- to post-nap. Power analyses paralleled those conducted for phase, using individual items pooled across all participants. First, we plotted the event-related spectrum of the difference between low forgetting and high forgetting trials from 0.5 to 10 Hz and from −440 to 800 ms, averaged across scalp channels. Effects were initially quantified using uncorrected p values. The goal of this analysis was to examine whether there were any significant or marginal power differences between low forgetting and high forgetting trials, specifically in the prestimulus interval and in the delta frequency band. Second, we computed the correlation between slow-wave power (0.5–1 Hz, −100 to 0 ms) and pre- to post-nap forgetting for each item, to examine whether there was a systematic relationship between power and change in memory strength.
Results
Behavioral results
Pre- to post-nap change in spatial error
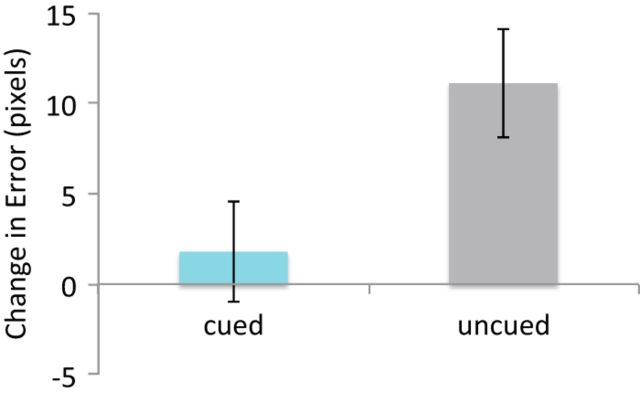
Our main dependent behavioral measure was the change in spatial error from pre-nap to post-nap. To parallel the approach used in our electrophysiological analyses, cued and uncued trials were pooled across participants, yielding a total of 550 trials per condition (cued, uncued). As shown in Figure 1, a significant cueing effect was found, indicating that pooled cued items showed less forgetting compared with pooled uncued items (t(1098) = 2.29, p = 0.022; significant cueing effects were likewise found in our prior studies using participant-level analyses (Rudoy et al., 2009; Creery et al., 2015).
Figure 1.
Behavioral results showed significant cueing effect at the item level, with cued items showing less forgetting from pre- to post-nap compared with uncued items. Positive error scores indicate more forgetting from pre- to post-nap. Error bars indicate SEM (based on pooled means where the number of observations is equal to the total number of trials across all participants).
Within the cued items, high- and low-forgetting categories were determined based on a median split for each participant's change in recall error. High-forgetting items showed a mean decline of 34.7 pixels (SEM = 3.20), and low-forgetting items showed a mean improvement of 31.1 pixels (SEM = 3.85; SEM based on pooled means, where the number of observations is equal to the total number of trials across all participants).
Awareness of sleep cues
At debriefing, no participant reported hearing sounds during the nap when questioned. In the forced-choice task, endorsement rates were 40.4% (SEM = 3.7%) for cued items and 38.9% (SEM = 3.9%) for uncued items. Discrimination between the two conditions did not exceed chance (t(21) = 0.62, p = 0.54). In addition, sleep staging demonstrated that participants were asleep throughout the stimulation period, with 20 participants in slow-wave sleep and two in Stage 2.
Phase bifurcation index (φ)
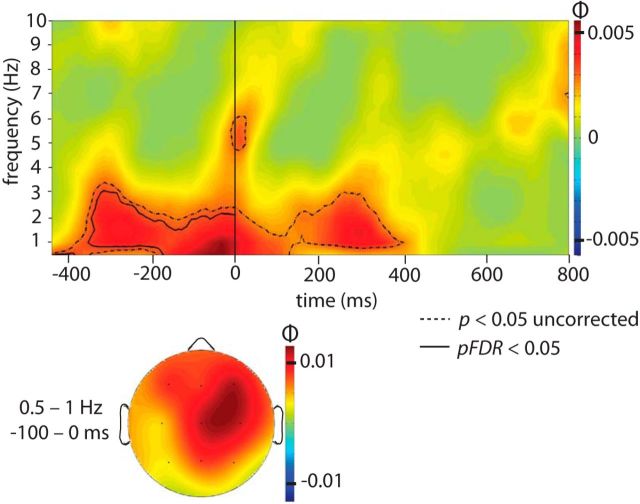
Consistent with our hypothesis, sound cues linked with high versus low forgetting were associated with significantly different phase angles during the prestimulus interval within the delta frequency band. Figure 2 shows φ at each time and frequency sampled from −440 to 800 ms and from 0.5 to 10 Hz, averaged across the 21 scalp electrodes. Overall, φ showed the highest values within lower frequencies and centered around stimulus onset, from ∼−400 to 400 ms. Regions of significance, both uncorrected and FDR-corrected, are indicated via contour lines on this φ plot. A statistically robust prestimulus effect surviving FDR correction was found from ∼−340 to 0 ms, and from 0.5 to 2 Hz. This result suggests that the phase of the slow oscillation at which a sound cue is presented influences memory consolidation for the associated object.
Figure 2.
Phase bifurcation index (ϕ) averaged across all channels, calculated over all pooled trials. A positive ϕ value indicates that cued items with high versus low pre- to post-nap forgetting show phase locking at opposite phase angles. Positive ϕ values were observed in the delta range (0.5–4 Hz) during the prestimulus interval (−400 to 0 ms) and continuing into the poststimulus interval. The dashed outermost contour line indicates that the ϕ at each time and frequency bin within the enclosed region is significant using an uncorrected one-tailed test (p < 0.05). To correct for multiple comparisons within our hypothesized time and frequency range (prestimulus interval from −400 to 0 ms; 0.5–4 Hz), the solid innermost contour line represents a region with significant ϕ that satisfies an FDR of 5%. The topographical plot on a schematic head as viewed from above shows the distribution of ϕ computed from 0.5 to 1 Hz and from −100 to 0 ms relative to stimulus onset (the time and frequency range where ϕ was maximal).
Following Busch et al. (2009), we confirmed that phases showed a uniform distribution across all trials by recombining high-forgetting and low-forgetting trials into a single pool and computing a Rayleigh test on the combined trials. Phase was computed over the time and frequency range where φ was maximal, from 0.5 to 1 Hz and from −100 to 0 ms. The combined phases did not show a significant deviation from uniformity (z = 1.90, p = 0.15). As an additional control, we also tested whether phases in the baseline trials (i.e., the 25 guitar strums, presented intermixed with the 25 critical sound cues) showed a uniform distribution within the φ-maximal time and frequency window. Again, we found no significant deviation from uniformity (z = 0.21, p = 0.82). We thus conclude that it is safe to assume that phases were indeed uniformly distributed in our data.
Relationship between phase angle and change in memory performance
A circular histogram of phase angles in each condition (high forgetting, low forgetting) averaged from 0.5 to 1 Hz and from −100 to 0 ms, where φ is maximal, is shown in Figure 3A. The plot shows that prestimulus slow-oscillation phases for items with high forgetting versus low forgetting were not equally distributed, and that there was a tendency for the two conditions to show opposite phase angles. The phase angle range with the highest proportion of items with low forgetting is centered ∼180° (150°–210°), suggesting that this region corresponds to the optimal phase angle for presenting stimuli during SWS. In contrast, the phase angle range with the highest proportion of items with high forgetting is centered ∼0° (330°–30°), indicating that this region corresponds to a suboptimal phase angle. These results provide evidence in support of our hypothesis that items with high versus low forgetting are associated with opposite phases.
Figure 3.
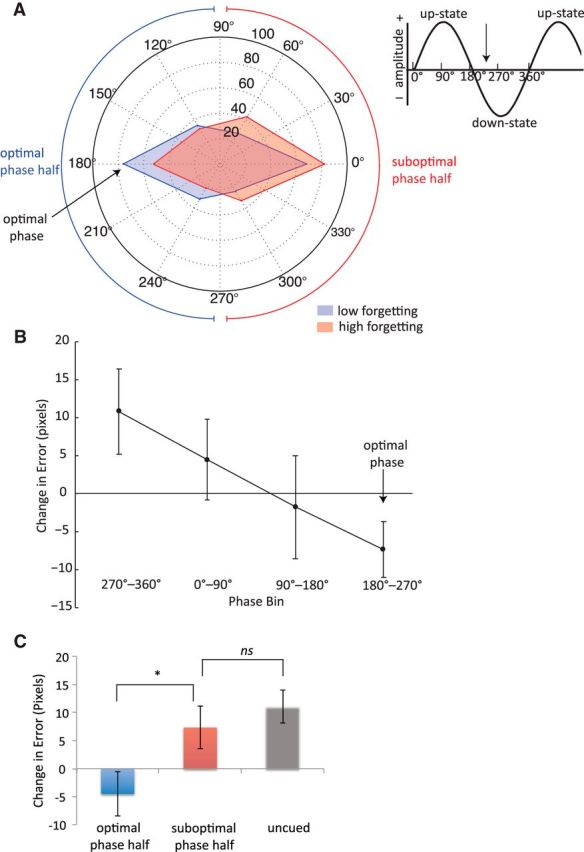
Relationship between slow oscillation phase and associated memory performance for individual sound cues presented during sleep. A, Circular histogram of phases for each condition (more forgetting, less forgetting) averaged from 0.5 to 1 Hz and from −100 to 0 ms, across all scalp channels. Phases were pooled into six bins. Each corner represents the center of each phase angle bin (0°, 60°, 120°, 180°, 240°, and 300°). The distance of each corner from the origin represents the number of items within each phase bin that showed low (blue) and high (red) pre- to post-nap forgetting, divided by median split on a per-subject basis and pooled across all subjects. Optimal phase is defined as the phase range showing the highest proportion of items with low forgetting compared with items with high forgetting. Arrows indicate the approximate semicircle phase range associated with optimal (blue) and suboptimal (red) memory performance. The inset illustrates the correspondence between phase angle and slow oscillation upstates and downstates. The vertical arrow indicates the approximate phase range that may be optimal for auditory cues presentation. B, Change in spatial error from pre- to post-nap as a function of phase. Phases were pooled into four bins. This analysis suggests that the optimal phase for memory consolidation occurs between 180° and 270°. Error bars represent SEM. C, Bar graph showing change in spatial error between cued items presented during the optimal half of the phase range (90°–270°), cued items presented during the suboptimal half of the phase range (270°–90°), and uncued items. Items presented during the optimal phase half of the circle showed significantly improved performance from pre- to post-nap relative to items presented during the suboptimal phase half. There was no significant difference in performance between cued suboptimal phase items and uncued items. Error bars represent SEM.
We evaluated this effect statistically using a Kuiper test. This test indicated that phases associated with high-forgetting versus low-forgetting items were significantly different within the time and frequency range, where φ was maximal (k = 11,616, p = 0.02). To establish that this result would not be expected by chance, we further evaluated the significance of this effect using a resampling test with 1000 iterations. For each iteration, two sets of trials (“pseudo-high-forgetting” and “pseudo-low-forgetting”) were drawn randomly from the overall pool of combined high-forgetting and low-forgetting trials, and the difference in phase distribution between these two categories was evaluated. This resampling analysis indicated that a p value <0.05 occurred <5% of the time, at a rate of 4.2%. Thus, our observed p value of 0.02 is statistically unexpected, providing further confirmation that items with high versus low forgetting are associated with significantly different phases.
Figure 3B displays the results of a related analysis, in which change in spatial error from pre- to post-nap is plotted as a function of phase, divided into four equal bins. Note that in this analysis, change in error was treated as a continuous variable, rather than a categorical one as in the previous analysis. We found that memory strength showed the greatest improvement (i.e., a negative error change value) when the phase angle fell between 180°–270° and showed the largest decline from 270° to 360°, similar to the general ranges implicated in our previous analysis. Interestingly, there was a graded, linear effect of change in spatial error as a function of phase between these two phase bins (linear contrast: t(546) = 2.39, p = 0.017; Fig. 3B). One possible interpretation of this linear effect is that the representation of an auditory stimulus in relevant neural circuitry may gradually fade over time. Auditory stimuli presented immediately before the optimal phase range may still be maintained well enough to often elicit a reactivation event. In contrast, for auditory stimuli presented immediately after the optimal range, the representation of the stimulus may have largely degraded by the time the next up-phase arrives, preventing reactivation from occurring. Only sound cues presented during the optimal phase angle quadrant (between 180° and 270°) were associated with a significant gain in memory performance, as indicated by a negative change in error (t(122) = −2.00, p = 0.048). For sound cues presented during the other three phase angle quadrants, memory performance either did not significantly change from pre- to post-nap (p > 0.39), or, for cues presented between 270° and 360°, showed a marginal decline (t(132) = 1.93, p = 0.056).
Finally, we compared change in error for items presented during the optimal half of the phase range (90°–270°), items presented during the suboptimal half of the phase range (270°–90°), and uncued items (Fig. 3C). There was no significant difference in performance between items presented during the suboptimal phase half and uncued items (t(840) = 0.76, p = 0.45), though uncued items showed numerically worse performance. Sound cues presented during the optimal phase half were associated with significantly lower forgetting compared with sounds presented during the suboptimal phase half (t(548) = 2.14, p = 0.033), and compared with uncued sounds (t(806) = 3.04, p = 0.0024). These results indicate that cues presented at an optimal phase led to significantly greater improvements in memory consolidation relative to cues presented at a suboptimal phase.
Event-related potentials
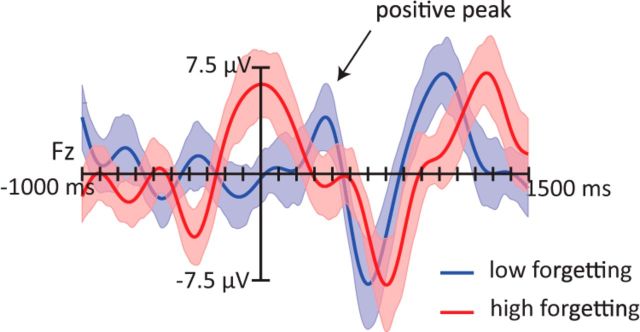
ERPs provide another way to investigate phase-locked EEG signals, in that two conditions systematically associated with different phases of slow oscillations will produce different ERPs. We thus computed ERPs for trials associated with high and low forgetting. Consistent with our previous analyses, these ERPs revealed consistent differences (Fig. 4). On average, sound cues associated with high forgetting were presented during a time interval coinciding with a positive ERP peak, whereas sound cues associated with low forgetting were presented shortly after a negative trough. These observations were confirmed statistically. ERP amplitudes were significantly more positive from −250 to 250 ms for items with high forgetting compared with low forgetting (condition: F(1,21) = 5.88, p = 0.024). This effect was fairly widespread, and marginally larger over anterior electrode regions (condition × anterior/posterior: F(2,42) = 3.87, p = 0.059). All other distributional interactions did not reach statistical significance (p values >0.29).
Figure 4.
Grand average ERPs from the Fz location time locked to sound cues presented during SWS, computed separately for objects that showed less forgetting (blue) versus more forgetting (red) pre- to post-nap. Trials were divided on the basis of median split by subject. A low-pass filter of 4 Hz was applied. On average, sound cues associated with more forgetting were presented at the peak of the positive half-wave, whereas the items associated with less forgetting were presented during the positive going slope of the slow oscillation, shortly before the next positive peak. Shaded regions around waveforms represent error bars (SEM).
Relationship between power and change in memory performance
In contrast to phase results, no significant relationship was found between slow oscillation power and pre- to post-nap forgetting. The event-related spectrum of the difference effect between high-forgetting and low-forgetting trials revealed no significant clusters in the delta band during the prestimulus interval, even when the uncorrected significance cluster was lowered to a more lenient threshold (p < 0.1). Similarly, there was no significant correlation between power from 0.5 to 1 Hz in the time interval immediately preceding stimulus onset (−100 to 0 ms) and pre- to post-nap change in error (r = −0.061, p = 0.15). Slow oscillation power was quite robust across all trials (mean = 31.1 dB, range = 23.8–38.7 dB; computed from 0.5 to 1 Hz across each epoch).
Discussion
The present results demonstrated that the phase of spontaneous slow oscillations influenced memory-related processing of auditory cues presented during sleep. Participants learned the spatial locations of 50 different objects, each paired with a characteristic sound, before taking an afternoon nap. A subset of the sounds was covertly presented during sleep to produce TMR. Upon awakening, participants showed significantly improved memory for objects whose associated sounds had been cued during sleep. This improvement varied across trials, and so we contrasted results for trials with improvement greater than the median versus less than the median for each participant. Critically, the behavioral benefit of TMR was predicted by slow-oscillation phase. The highest proportion of items associated with high forgetting versus low forgetting over the retention interval showed opposite phases in the slow-frequency band around the time of stimulation (Fig. 3A). These results suggest that TMR stimuli are more likely to be processed and trigger memory reactivation when they occur at the optimal phase of a slow oscillation.
These results support a model of “active system consolidation” during slow-wave sleep (Diekelmann and Born, 2010; Mölle and Born, 2011; Born and Wilhelm, 2012). According to this model, slow oscillations at ∼0.75 Hz play a key role in synchronizing memory consolidation. Slow oscillations group neuronal activity in the neocortex into depolarizing upstates and hyperpolarizing downstates. Depolarizing upstates in turn drive thalamocortical spindles and sharp wave-ripples in the hippocampus, underlying the repeated reactivation of hippocampal memory representations. Information from multiple brain regions thus reaches neocortical networks when neurons are generally depolarized, facilitating the plasticity needed for forming enduring memory traces in neocortical networks, ultimately shifting the reliance of memory representations from hippocampus to neocortex. Thus, this model predicts that memory consolidation takes place preferentially during phasic time intervals corresponding to the neocortical upstate, consistent with the present findings showing a phase effect on spatial memory associated with learning-related cues.
The present observations support the idea that cues presented during slow-wave sleep trigger reactivation of associated memories, which then increase in strength (Oudiette and Paller, 2013). Evidence for this notion has also been obtained in animal studies. For example, Bendor and Wilson (2012) trained rats to associate an auditory signal with a spatial location and then presented the auditory cue covertly during sleep while recording from neuronal ensembles in the hippocampus. They found that hippocampal place cells representing the trained spatial location fired preferentially in response to the cue, indicating that external stimulation can control the content of memory reactivation. Similar results have been found in zebra finches, in which presenting a bird's own song during sleep triggers firing patterns in sensorimotor regions similar to those observed during daytime singing (Dave and Margoliash, 2000). However, direct evidence that sensory cues drive specific neural networks responsible for memory reactivation has not yet been shown in humans.
Our results support the idea that similar mechanisms are operative in humans by demonstrating that the timing of the cue is critical for memory consolidation effects of TMR. Based on studies of memory reactivation in rats, Bendor and Wilson (2012) proposed that the content of hippocampal memory reactivations can be biased by cortical activity occurring before a replay event. Supporting this account, synchronized periods of increased multiunit activity, or “frames,” occur in both the cortex and hippocampus, with cortical frames starting ∼50 ms earlier than corresponding hippocampal frames (Ji and Wilson, 2007). This observation implies an initial feedforward interaction from the cortex to the hippocampus, whereby cortical networks actively control hippocampal activity. Similarly, learning-related cues may work by evoking a cortical response (Portas et al., 2000; Issa and Wang, 2008), which then biases hippocampal replay activity and selectively strengthens associated memories. The finding that cortical frames precede hippocampal frames by ∼50 ms suggests that spontaneous memory reactivations require fairly precise timing between cortical and hippocampal activity. The present study supports this idea, demonstrating that timing is also an important factor for externally induced memory reactivations, as elicited by auditory cues.
Our results provide evidence that task-related cues influence which memories are replayed by the hippocampus preferentially when they occur within a restricted time interval. Presumably, the timing of the auditory cue must allow sensory information to be processed by the cortex during the cortical upstate and also reach the hippocampus during the hippocampal upstate, when sharp wave-ripple events are likely to occur. If the timing of these events is misaligned, cortical signals will arrive at the hippocampus while neurons are in the hyperpolarized downstate, and ultimately fail to influence hippocampal replay.
It is also important to note that slow oscillation phase, in addition to modulating memory processing, may also affect processing at an earlier, sensory level. For example, a recent fMRI study demonstrated that phase of the slow oscillation during auditory tone presentation influences the sensory response in higher-order auditory association cortex (Schabus et al., 2012). Specifically, tones presented during the positive-going slow oscillation slope elicited greater activation in the superior temporal gyrus relative to tones presented during the negative-going slope, though no phase-related activation differences were observed in primary auditory cortex. In this same study, tones presented toward the upstate also elicited a larger ERP positivity from 200 to 600 ms poststimulus. Another study showed that auditory stimuli presented during the slow-oscillation upstate elicited an enhanced late ERP positivity as well as a larger spindle and beta power response relative to stimuli presented during the downstate (Cox et al., 2014). The spindle and beta effects occurred quite late (700–1200 ms) and were proposed to reflect greater reprocessing of the stimulus, which may have been unable to proceed until the next upstate. Thus, the present results may be driven by effects of phase on sensory processing as well as on later memory processing. If the brain is less responsive to external stimuli during certain phase intervals, auditory stimuli presented during these times may fail to be adequately processed at a basic sensory level and consequently fail to reach subsequent processing stages.
Our results provide insight into the precise slow-oscillation phase at which cue presentation is maximally effective for influencing subsequent memory. We hypothesized that the optimal phase for sound onset would occur shortly before the onset of the slow-oscillation upstate (eg, 270°–360°). Somewhat at odds with this hypothesis, our data suggest that the optimal phase angle falls ∼180°–270° (i.e., during the first half of the downstate, well before the beginning of the upstate; Fig. 3A). Assuming a slow-oscillation frequency of 0.75 Hz, this interval occurs ∼500 ms before the onset of the slow oscillation upstate. Although this result may initially appear somewhat surprising, upon further consideration this lag may be accounted for by the time required both for stimulus presentation as well as for early auditory processing. Sounds in our study had a duration of 500 ms, and likely did not become recognizable until at least several hundred milliseconds after stimulus onset. In addition, auditory stimuli require ∼80 ms to reach auditory cortex (Kraus and Nicol, 2009), and phase effects occur in higher-order auditory association cortex following initial processing in primary auditory cortex (Schabus et al., 2012). Together, these factors may account for why the optimal phase for sound delivery (stimulus onset) was found to occur so far in advance of the next slow-oscillation up-phase. However, given that this is the first study to link a specific phase of a slow oscillation at stimulus presentation time to later behavioral outcomes, our estimate of optimal phase will require verification by future studies. In addition to the approach we used here, it could be possible to determine optimal phase for TMR by delivering stimuli at a specific slow-oscillation phase rather than the usual method of stimulating at a random phase (Ngo et al., 2013; Cox et al., 2014; Santostasi et al., 2015). In addition, it is important to note that the optimal phase for stimulus presentation is likely to vary depending upon features of the auditory stimuli, such as complexity and duration.
As a final note, these results have implications for future TMR studies, indicating that learning-related cues can be targeted to influence cortical processing during optimal time windows for memory consolidation. This advance in understanding could be combined with other recent experimental advances to synergistically increase potential memory benefits during sleep. For example, it has already been demonstrated that deeper slow oscillations during sleep can be induced via direct current stimulation (Marshall et al., 2006) or through acoustic pulses (Ngo et al., 2013), which in turn enhances declarative memory. In addition, computer algorithms have been developed that can effectively predict slow oscillations in real time and present auditory stimuli targeted at the slow-oscillation upstate (Cox et al., 2014; Santostasi et al., 2015). Combining these technological innovations may yield increased sleep-dependent benefits across a wide range of domains, including spatial memory (Rasch et al., 2007; Rudoy et al., 2009), foreign word learning (Schreiner and Rasch, 2015), visuomotor skills (Antony et al., 2012; Schönauer et al., 2014), and reduction of implicit social biases (Hu et al., 2015). The current era of research on sleep and memory consolidation thus has the potential not merely to understand, but to actively manipulate and harness the capacities of the sleeping brain.
Footnotes
This work was supported by NIH Grants F32 HD 078223 and T32 NS 047987, and NSF Grant BCS-1461088.
The authors declare no competing financial interests.
References
- Achermann P, Borbély AA. Low-frequency (<1 hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. doi: 10.1016/S0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–1116. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The phase of ongoing EEG oscillations predicts visual perception. J Neurosci. 2009;29:7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R, Korjoukov I, de Boer M, Talamini LM. Sound asleep: processing and retention of slow oscillation phase-targeted stimuli. PLoS One. 2014;9:e101567. doi: 10.1371/journal.pone.0101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creery JD, Oudiette D, Antony JW, Paller KA. Targeted memory reactivation during sleep depends on prior learning. Sleep. 2015;38:755–763. doi: 10.5665/sleep.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Hu X, Antony JW, Creery JD, Vargas IM, Bodenhausen GV, Paller KA. Unlearning implicit social biases during sleep. Science. 2015;348:1013–1015. doi: 10.1126/science.aaa3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa EB, Wang X. Sensory responses during sleep in primate primary and secondary auditory cortex. J Neurosci. 2008;28:14467–14480. doi: 10.1523/JNEUROSCI.3086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Kraus N, Nicol T. Auditory evoked potentials. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of neuroscience. Berlin: Springer; 2009. pp. 214–218. [Google Scholar]
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/S0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Mölle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci. 2002;22:10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo HV, Claussen JC, Born J, Mölle M. Induction of slow oscillations by rhythmic acoustic stimulation. J Sleep Res. 2013;22:22–31. doi: 10.1111/j.1365-2869.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn Sci. 2013;17:142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000;28:991–999. doi: 10.1016/S0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santostasi G, Malkani R, Riedner B, Bellesi M, Tononi G, Paller KA, Zee PC. Phase-locked loop for precisely timed acoustic stimulation during sleep. J Neurosci Methods. 2015;259:101–114. doi: 10.1016/j.jneumeth.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Heib DP, Boly M, Desseilles M, Vandewalle G, Schmidt C, Albouy G, Darsaud A, Gais S, Degueldre C, Balteau E, Phillips C, Luxen A, Maquet P. The fate of incoming stimuli during NREM sleep is determined by spindles and the phase of the slow oscillation. Front Neurol. 2012;3:40. doi: 10.3389/fneur.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönauer M, Geisler T, Gais S. Strengthening procedural memories by reactivation during sleep. J Cogn Neurosci. 2014;26:143–153. doi: 10.1162/jocn_a_00471. [DOI] [PubMed] [Google Scholar]
- Schreiner T, Rasch B. Boosting vocabulary learning by verbal cueing during sleep. Cereb Cortex. 2015;25:4169–4179. doi: 10.1093/cercor/bhu139. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/S0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/S0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]