Marked Regional Variation in Acute Stroke Treatment among Medicare Beneficiaries (original) (raw)
. Author manuscript; available in PMC: 2016 Jul 1.
Abstract
Background
Little is known about how regions vary in their use of thrombolysis (Intravenous (IV) tissue plasminogen activator (tPA) and intra-arterial treatment) for acute stroke. We sought to determine regional variation in thrombolysis treatment and investigate the extent to which regional variation is accounted for by patient demographics, regional factors and elements of stroke systems of care.
Methods
Retrospective cross-sectional study of all fee-for-service Medicare ischemic stroke patients admitted via the Emergency Department from 2007–2010 who were assigned to one of 3,436 hospital service areas. Multi-level logistic regression was used to estimate regional thrombolysis rates, determine the variation in thrombolysis treatment attributable to the region and estimate thrombolysis treatment rates and disability prevented under varied improvement scenarios.
Results
There were 844,241 ischemic stroke admissions of which 3.7% received IV tPA and 0.5% received intra-arterial stroke treatment without or without IV tPA over the four year period. The unadjusted proportion of ischemic stroke patients who received thrombolysis varied from 9.3% in the highest treatment quintile compared with 0% in the lowest treatment quintile. Measured demographic and stroke system factors were weakly associated with treatment rates. Region accounted for 7–8% of the variation in receipt of thrombolysis treatment. If all regions performed at the level of 75th percentile region, almost 7,000 additional ischemic stroke patients would be treated with thrombolysis.
Conclusion
There is substantial regional variation in thrombolysis treatment. Future studies to determine features of high performing thrombolysis treatment regions may identify opportunities to improve thrombolysis rates.
Thrombolysis (Intravenous (IV) tissue plasminogen activator (tPA) and acute intra-arterial treatment (IAT)) treatment reduces post-stroke disability but is underutilized.1, 2 While it is known that US hospitals vary widely in their use of thrombolysis, the extent to which this reflects differences in eligibility or differences in thrombolytic use among eligible patients is unknown.3 It is likely that hospital thrombolysis rates are, at least in part, dependent on regional factors that influence thrombolysis eligibility.
In this context, we sought to explore regional variation in thrombolysis treatment and determine the extent that patient demographics, regional factors and elements of stroke systems of care influence treatment rates. Determining thrombolysis rates in high performing regions and understanding the role of immutable regional factors will establish real world regional benchmarks. Ultimately, a better understanding of regional influences on thrombolysis may inform future interventions to increase thrombolysis treatment rates and inform the magnitude of the opportunity for nationwide improvement in thrombolysis treatment.
Methods
This is a retrospective cross-sectional study of regional differences in thrombolysis rates among Medicare fee-for-service beneficiaries. We used Medicare MedPAR files from 2007–2010 to identify all patients with a primary diagnosis of ischemic stroke using ICD-9 codes ICD-9 433.x1, 434.x1, 436 admitted from the emergency department (ED). Hospital-to-hospital transfers were excluded. The majority of the hospital care in the US is provided at the hospital closest to the patient’s home.4 Thus, the primary exposure was the hospital service area (HSA) determined from the home zip code of the ischemic stroke patient. There are 3,436 HSAs in the US and over 60% contain more than 1 hospital.5 HSAs were chosen as the unit of regional analysis because they represent local markets for healthcare whereas hospital referral regions (HRRs) represent tertiary referral regions. Given the time constraints in thrombolysis treatment, we hypothesized that regional factors would be better measured at the more granular HSAs level rather than in HRRs.
The primary outcome was any thrombolysis which included both IV tPA (DRG 559, MS-DRG 61–63 or ICD-9 procedure code 99.10), IAT (CPT codes 37184-6, 37201, 75896)) and the combination (IV+IAT) identified using DRG and procedure codes from MedPAR and CPT codes from the Medicare Carrier file. Drip and ship cases were identified with ICD-9 procedure code V4588 and counted as IV cases if no IAT code was identified or combination therapy if there was also an IAT code. We included IAT as part of our primary outcome for two reasons. First during the study period, some regions may have preferentially treated some patients with IAT, particularly as acute stroke trials comparing the benefits of IV and IAT were ongoing during the study period. Second, guidelines suggested IAT was an option for major middle cerebral artery stroke under 6 hours and in patients who had contraindications to IV tPA.6 To exclude IAT from the primary outcome would have potentially penalized regions with greater IAT rates.
Regional and Patient factors
Regional factors included elements of stroke-systems of care such as primary stroke centers (PSCs) and emergency medical service (EMS) bypass systems. The number of PSCs per region was determined by counting the number of PSCs in a given zip code (using the PSC website),7 mapping zip codes to HSAs using the Dartmouth atlas and summing the number of PSCs within each HSA. EMS bypass was determined by mapping patient zip codes to a prior systematic review of regions with EMS bypass.8 Both PSCs and bypass variables reflect the time at which they were established. For example, a region that established bypass in 2008, would have been categorized as without bypass prior to that time and with bypass subsequent to that time. To explore regional factors that may be associated with pre-hospital delay and that are intrinsic to the region, US census data was linked to Medicare data using patient zip code to capture regional education, median household income, proportion of population unemployed and population density. For this analysis, we focused on regional effects to be able to obtain the broadest possible perspective on regional and hospital effects given their co-dependence and the intrinsic challenges in separating regional and hospital level effects in the absence of thrombolysis eligibility. Patient demographics, risk factors (vascular risk factors and all Charlson comorbidities) were obtained from Medicare files.
Statistical analysis
We first determined the proportion of ischemic stroke patients who received thrombolysis treatment over the four year period. Individual and regional characteristics were then compared using descriptive statistics across quintiles of thrombolysis treatment rates. To estimate the effect of region on the proportion of ischemic stroke patients treated with thrombolysis, multi-level logistic regression was used with and without adjustment for patient, regional and elements of stroke systems of care with a random regional-level intercept. The proportion of variance at the regional level was estimated using the inter-class correlation coefficient (ICC). Regional thrombolysis rates were estimated for each region using shrunken means from the empty model and regions were divided into quintiles on the basis of mean treatment rates. The impact of each regional factor was estimated using average marginal effects and compared using Wald tests from the fully adjusted model.
Finally, we developed a simple model to estimate the societal impact of increasing regional thrombolysis rates using one of two strategies: 1. targeted increases in thrombolysis rates in low-performing regions and 2. nationwide increases in thrombolysis rates. For each model, to represent the entire stroke population as opposed to only the Medicare fee-for-service population, we linearly scaled the number of patients in each region upwards so that there were 750,000 strokes per year.9 Each region’s baseline rate was estimated using the shrunken mean rate from our logistic regression model for 2010. We used only the most recent year of data given the known increase in thrombolysis rates over time. The reduction in disability after thrombolysis was based on a summary estimate of number needed to treat of 8.7 from a recent meta-analysis.10 We then explored the number of patients treated, regions impacted and patients who would be disability free if thrombolysis treatment increased under simple modifications of the regional treatment rates. We explored this by evaluating two different approaches. First, focusing on regions with the lowest thrombolysis rates and increasing them up to a given percentile rank (i.e. improving the lowest performing regions until they were performing at the 25th percentile level). The second approach focused on national thrombolysis rates which meant increasing regional rates by a fixed percentage (i.e. 25% overall increase in treatment rates). All analysis were completed in Stata. (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.)
Results
Between 2007–2010, there were 844,241 ischemic stroke ED admissions among Medicare fee-for-service beneficiaries of which 3.9% received thrombolysis treatment — 3.7% with IV tPA only and 0.5% received IAT with or without IV tPA. 20.1% of regions did not administer thrombolysis treatment over the 4 year time period. Regional variation in the proportion of strokes receiving thrombolysis ranged from 9.3% to 0% in the highest to lowest utilization quintiles and from 5.9% to 2.2% after accounting for variation in the number of strokes per region. There were no marked differences in demographics or comorbidities across thrombolysis treatment quintiles (Table 1). Regions with higher treatment rates generally had higher population density, more bachelor’s degrees, and EMS bypass.
Table 1.
Demographics, Vascular Risk Factors, Co-Morbidities and Procedures among Ischemic Stroke Patients by Quintiles of Thrombolysis Treatment.
| Quintiles | ||||||
|---|---|---|---|---|---|---|
| 1LowestN=25,256(%) | 2N=175,535(%) | 3N=215,248(%) | 4N=245,277(%) | 5HighestN=182,925(%) | TotalN=844,141 | |
| Demographics | ||||||
| Age Mean (SD) | 77.5(10.6) | 77.1(10.8) | 77.7(10.6) | 78.2(10.5) | 78.9(10.3) | 78.0(10.6) |
| Female | 14,358(56.8%) | 99,914(56.9%) | 123,141(57.2%) | 140,605(57.3%) | 104,885(57.3%) | 482,903(57.2%) |
| Non-Hispanic White | 21,278(84.2%) | 135,844(77.4%) | 170,305(79.1%) | 199,086(81.2%) | 153,108(83.7%) | 679,621(80.5%) |
| African American | 2,916(11.5%) | 30,717(17.5%) | 32,881(15.3%) | 34,422(14.0%) | 19,112(10.4%) | 120,048(14.2%) |
| Hispanic | 326(1.3%) | 4,416(2.5%) | 4,415(2.1%) | 4,829(2%) | 3,016(1.6%) | 17,002(2%) |
| Asian | 90(0.4%) | 1,392(0.8%) | 3,081(1.4%) | 2,455(1%) | 3,510(1.9%) | 10,528(1.2%) |
| Unknown/ Other | 646(2.6%) | 3,166(1.8%) | 4,566(2.1%) | 4,485(1.8%) | 4,179(2.3%) | 17,042(2%) |
| Comorbidities | ||||||
| Hypertension | 17,656(69.9%) | 126,492(72.1%) | 154,194(71.6%) | 175,984(71.7%) | 128,390(70.2%) | 602,716(71.4%) |
| Hyperlipidemia | 7,596(30.1%) | 56,253(32%) | 74,552(34.6%) | 87,880(35.8%) | 64,751(35.4%) | 291,032(34.5%) |
| Diabetes | 7,649(30.3%) | 55,038(31.4%) | 64,427(29.9%) | 70,723(28.8%) | 49,322(27%) | 247,159(29.3%) |
| Atrial Fibrillation | 5,964(23.6%) | 39,501(22.5%) | 53,229(24.7%) | 63,058(25.7%) | 50,807(27.8%) | 212,559(255.2%) |
| Smoking | 1,689(6.7%) | 11,943(6.8%) | 14,049(6.5%) | 15,432(6.3%) | 10,921(6%) | 54,034(6.4%) |
| Myocardial Infarction | 1,558(6.2%) | 10,716(6.1%) | 13,942(6.5%) | 15,664(6.4%) | 12,339(6.7%) | 54,219(6.4%) |
| Peripheral VascularDisease | 1,605(6.4%) | 11,744(6.7%) | 14,888(6.9%) | 16,667(6.8%) | 11,904(6.5%) | 56,808(6.7%) |
| Congestive Heart Failure | 4,282(17%) | 30,193(17.2%) | 36,393(16.9%) | 40,911(16.7%) | 29,798(16.3%) | 141,577(16.8%) |
| Dementia | 1,418(5.6%) | 10,235(5.8%) | 12,647(5.9%) | 14,499(5.9%) | 10,711(5.9%) | 49,510(5.9%) |
| Chronic ObstructivePulmonary Disease | 3,826(15.1%) | 26,785(15.3%) | 31.795(14.8%) | 35,673(14.5%) | 25,287(13.8%) | 123,366(14.6%) |
| Regional Factors | ||||||
| Population Density1,000 people/square mileMean (sd) | 0.3 (1.4) | 1.8 (3.9) | 2.6 (4.1) | 3.2 (6.5) | 6.5 (15.8) | 3.4 (8.8) |
| Bachelor's degree | 16.8(8.9%) | 20.6(11,7%) | 24.4(14.5%) | 28.2(15.5%) | 32.2(17.3%) | 26.2(15.5%) |
| Unemployed | 8.8(5%) | 10.2(5.1%) | 9.5(4.7%) | 9.1(4.1%) | 8.4(3.7%) | 9.2(4.5%) |
| Median Income per $10,000Mean (sd) | 4.2 (1.4) | 4.6 (1.7) | 5.2 (2.1) | 5.6 (2.2) | 6.0 (2.5) | 5.4 (2.2) |
| Stroke systems of Care | ||||||
| Emergency Medical ServiceBypass | 8,670(34.3%) | 68,990(39.3%) | 112,793(52.4%) | 131,659(53.7%) | 111,372(60.9%) | 433,484(51.3%) |
| Primary StrokeCenter(s)/regionMean (sd) | 2.7 (3.8) | 3.3 (4.2) | 4.9 (5.0) | 5.2 (4.8) | 4.6 (4.2) | 4.5 (4.7) |
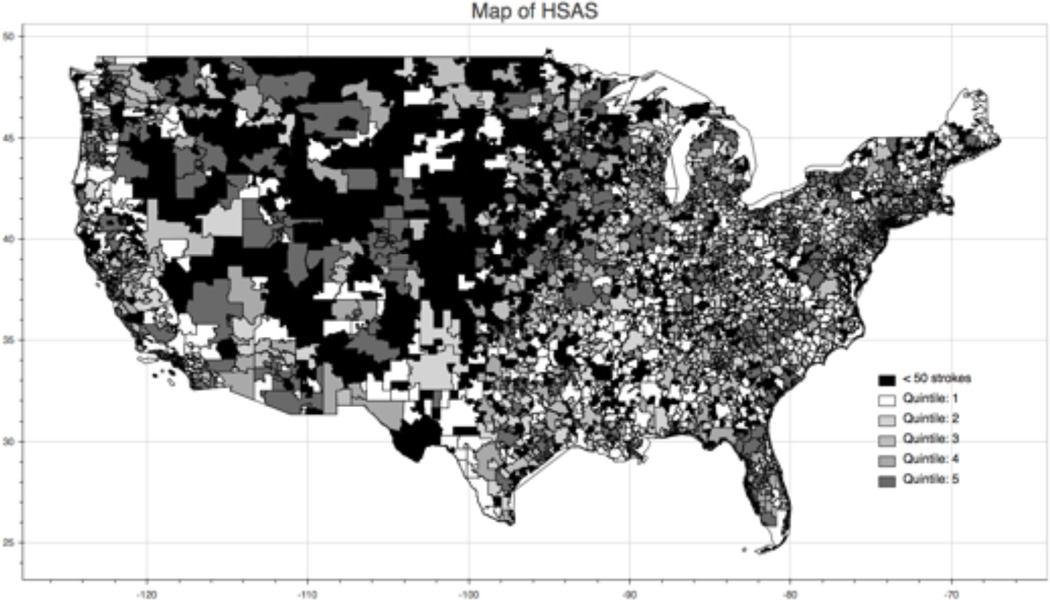
The proportion of ischemic stroke patients who received thrombolysis varied from 14% of patients in the highest region compared with some regions where thrombolysis treatment was not administered during the study period. The top 10 regions spanned from North Carolina to California, and include large and small stroke volume regions (table 2). Figure 1 shows the regional variability thrombolysis treatment in the US.
Table 2.
The 20 regions with the highest proportion of ischemic stroke patients treated with thrombolysis from 2007–2010.
| Rank | AnnualMeanTreatmentRate% | LowerCI% | UpperCI% | City | State | TotalStrokes | Untreated | IV | IA | IV+IA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 14.3 | 11.0 | 18.3 | Stanford | CA | 317 | 266 | 43 | 2 | 6 |
| 2 | 13.8 | 11.7 | 16.1 | Asheville | NC | 910 | 779 | 113 | 1 | 17 |
| 3 | 12.9 | 9.1 | 18.1 | Waconia | MN | 183 | 154 | 24 | 1 | 4 |
| 4 | 11.9 | 10.0 | 14.2 | Langhorne | PA | 862 | 754 | 106 | 1 | 1 |
| 5 | 11.2 | 7.2 | 17.0 | Brevard | NC | 127 | 108 | 17 | 0 | 2 |
| 6 | 11.2 | 8.6 | 14.4 | Iowa City | IA | 422 | 370 | 47 | 4 | 1 |
| 7 | 11.1 | 7.0 | 17.0 | Hastings | NE | 120 | 102 | 18 | 0 | 0 |
| 8 | 10.8 | 8.0 | 14.5 | Wheat Ridge | CO | 317 | 278 | 37 | 0 | 2 |
| 9 | 10.7 | 7.8 | 14.7 | Provo | UT | 274 | 240 | 19 | 3 | 12 |
| 10 | 10.7 | 7.5 | 15.0 | Holyoke | MA | 228 | 199 | 27 | 1 | 1 |
| 11 | 10.7 | 6.4 | 17.4 | Hutchinson | MN | 88 | 74 | 12 | 1 | 1 |
| 12 | 10.7 | 7.0 | 16.0 | Marion | NC | 145 | 125 | 19 | 0 | 1 |
| 13 | 10.6 | 8.1 | 13.7 | St Louis Park | MN | 430 | 380 | 46 | 0 | 4 |
| 14 | 10.5 | 8.5 | 12.9 | Cedar Rapids | IA | 716 | 636 | 68 | 4 | 8 |
| 15 | 10.3 | 7.2 | 14.6 | Encinitas | CA | 219 | 192 | 23 | 1 | 3 |
| 16 | 10.3 | 8.9 | 11.7 | San Francisco | CA | 1790 | 1602 | 169 | 3 | 16 |
| 17 | 10.1 | 5.7 | 17.4 | Southbridge | MA | 66 | 55 | 10 | 1 | 0 |
| 18 | 10.0 | 7.0 | 14.2 | Pekin | IL | 236 | 208 | 25 | 0 | 3 |
| 19 | 10.0 | 8.9 | 11.3 | Denver | CO | 2396 | 2152 | 222 | 5 | 17 |
| 20 | 9.8 | 6.3 | 15.0 | American Fork | UT | 141 | 123 | 10 | 2 | 6 |
Figure 1.
Regional Thrombolysis utilization
Region was an important predictor of receipt of thrombolysis, intra-class correlation coefficient (ICC) in an empty model was 0.075, suggesting that region explains about 8% of all variance in thrombolysis treatment rates. The ICC decreased to 0.070 in the fully adjusted model, suggesting that all factors included in our model explained a modest amount of the variance in the empty model, and that most of the regional variance was not explained by factors measured in this study. Older Americans, women and racial/ethnic minorities were less likely to receive thrombolysis (table 3). EMS bypass and the number of PSCs in a region were associated with increased thrombolysis. Among regions with the lowest number of PSCs, 4.0% (95% CI 3.9%–4.1%) of stroke patients received thrombolysis compared with 4.8% (95% CI 4.4%–5.1%) in regions with the highest number of PSCs (table 4). In areas without EMS bypass systems 4.0% (95% CI 3.9%–4.1%) of stroke patients received thrombolysis compared with 4.4% (95% CI 4.2%–4.5%) of stroke patients in regions with EMS bypass systems. Of regional demographic factors, education was the most important. Regions with the lowest proportion of college graduates had a smaller proportion of patients treated with thrombolysis (3.7%, 95% CI, 3.6–3.9%) compared to regions with the highest proportion of college graduates (5.0%, 95% CI 4.8–5.2%).
Table 3.
The association of patient demographics, regional factors and elements of stroke systems of care with thrombolysis treatment.*
| OddsRatio | p-value | LowerConfidenceInterval | UpperConfidenceInterval | |
|---|---|---|---|---|
| Age (Continuous) | 0.986 | < 0.001 | 0.984 | 0.987 |
| Female | 0.918 | < 0.001 | 0.898 | 0.939 |
| Race/ethnicity (ref:White) | ||||
| Black | 0.709 | < 0.001 | 0.681 | 0.738 |
| Other | 0.823 | < 0.001 | 0.752 | 0.900 |
| Asian | 0.779 | < 0.001 | 0.703 | 0.862 |
| Hispanic | 0.882 | 0.006 | 0.807 | 0.964 |
| Native American | 0.738 | 0.005 | 0.596 | 0.915 |
| Population Density (per1000 population/squaremile) | 1.004 | < 0.001 | 1.002 | 1.007 |
| Bachelor's Degree orHigher (%) | 1.006 | < 0.001 | 1.005 | 1.007 |
| Unemployment (%) | 1.001 | 0.667 | 0.997 | 1.005 |
| Median householdincome (per $10,000) | 1.010 | 0.045 | 1.000 | 1.019 |
| Number of primary strokecertified center(s) | 1.011 | < 0.001 | 1.005 | 1.017 |
| Emergency MedicalService Bypass | 1.097 | < 0.001 | 1.043 | 1.152 |
Table 4.
Adjusted* proportion of ischemic stroke patients treated with thrombolysis across regional factors
| Percentile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regional Factors | |||||||||
| 1st | 5th | 10th | 25th | 50th | 75th | 90th | 95th | 99th | |
| Population Density (pop/square mile) | |||||||||
| PopulationDensity | 0.002 | 0.006 | 0.013 | 0.041 | 0.121 | 0.647 | 2.692 | 4.437 | 10.890 |
| MeanTreatmentRate | 4.1% | 4.1% | 4.1% | 4.1% | 4.1% | 4.1% | 4.2% | 4.2% | 4.3% |
| LCI | 4.0% | 4.0% | 4.0% | 4.0% | 4.0% | 4.0% | 4.1% | 4.1% | 4.2% |
| UCI | 4.2% | 4.2% | 4.2% | 4.2% | 4.2% | 4.2% | 4.3% | 4.3% | 4.5% |
| Median Income | |||||||||
| Income $ | 25685 | 31169 | 34074 | 38947 | 45166 | 53689 | 68519 | 81146 | 105689 |
| MeanTreatmentRate | 4.1% | 4.1% | 4.1% | 4.1% | 4.1% | 4.2% | 4.2% | 4.3% | 4.4% |
| LCI | 3.9% | 4.0% | 4.0% | 4.0% | 4.0% | 4.1% | 4.1% | 4.1% | 4.2% |
| UCI | 4.2% | 4.2% | 4.2% | 4.2% | 4.3% | 4.3% | 4.4% | 4.4% | 4.6% |
| Unemployment | |||||||||
| Unemployme nt (%) | 1.5 | 3.1 | 4.4 | 6.2 | 8.1 | 10.3 | 12.7 | 14.4 | 18.6 |
| MeanTreatmentRate | 4.2% | 4.2% | 4.2% | 4.2% | 4.2% | 4.2% | 4.2% | 4.2% | 4.2% |
| LCI | 4.0% | 4.0% | 4.1% | 4.1% | 4.1% | 4.1% | 4.1% | 4.1% | 4.0% |
| UCI | 4.3% | 4.3% | 4.3% | 4.3% | 4.3% | 4.3% | 4.3% | 4.4% | 4.4% |
| Education | |||||||||
| Bachelor's Degree (%) | 7.6 | 10.0 | 11.4 | 14.2 | 18.3 | 25.2 | 35.6 | 43.7 | 60.6 |
| MeanTreatmentRate | 3.7% | 3.8% | 3.8% | 3.9% | 4.0% | 4.1% | 4.4% | 4.6% | 5.0% |
| LCI | 3.6% | 3.7% | 3.7% | 3.8% | 3.9% | 4.0% | 4.2% | 4.4% | 4.8% |
| UCI | 3.9% | 3.9% | 3.9% | 4.0% | 4.1% | 4.2% | 4.5% | 4.7% | 5.2% |
| Stroke systems of Care | |||||||||
| Number of Primary Stroke Center(s) | 0 | 0 | 0 | 1 | 2 | 4 | 9 | 13 | 17 |
| MeanTreatmentRate | 4.0% | 4.0% | 4.0% | 4.0% | 4.1% | 4.2% | 4.4% | 4.6% | 4.8% |
| LCI | 3.9% | 3.9% | 3.9% | 3.9% | 4.0% | 4.1% | 4.2% | 4.3% | 4.4% |
| UCI | 4.1% | 4.1% | 4.1% | 4.2% | 4.2% | 4.3% | 4.5% | 4.8% | 5.1% |
| Emergency Medical Service Bypass | |||||||||
| No | Yes | ||||||||
| Rate | 4.0% | 4.4% | |||||||
| LCI | 3.9% | 4.2% | |||||||
| UCI | 4.1% | 4.5% |
If lower performing regions administered thrombolysis treatment at the rates of higher performing regions, considerable disability could be prevented (Table 5). For example, if the 1,670 regions that currently perform below the median were increased to the median rate, we estimate that 2,717 additional patients would be treated annually resulting in approximately an additional 236 stroke patients without disability. An optimistic ceiling for acute thrombolysis treatment in the United States can be estimated by increasing all regional treatment rates to that of the highest performing region which would yield an additional 92,847 patients treated with thrombolysis and 8,078 stroke patients without disability.
Table 5.
Projected Thrombolysis Treatment Rates and Disability Prevented Under Various Regional and National Improvement Scenarios. (Based on 2010 regional thrombolysis rates and rounded to integers)
| Number ofRegionsChangingRates | NationalMeanRegionalTreatmentRate | Number ofPatientsTreated | Increase innumber ofpatientstreated | Numberalive,favorableoutcome(MRS 0–1)assumingall treatedwithin 3hours | NumberofPatientsdisability-free duetoTreatmentIncrease | |
|---|---|---|---|---|---|---|
| Baseline | 5.56% | 41,416 | 3,603 | |||
| Increase thrombolysis treatment rates in under-performing regions | ||||||
| Increase below 10th percentile to 10 percentile (3.1%) | 334 | 5.62% | 41,838 | 422 | 3,640 | 37 |
| Increase below 25th percentile to 25th percentile (3.8%) | 835 | 5.72% | 42,621 | 1,205 | 3,708 | 105 |
| Increase below median quintiles to median (4.4%) | 1670 | 5.92% | 44,133 | 2,717 | 3,840 | 236 |
| Increase below 75th percentile quintiles to 75 percentile (5.6%) | 2505 | 6.48% | 48,279 | 6,863 | 4,200 | 597 |
| Increase below 90th percentile quintiles to 90th percentile (7.3%) | 3006 | 7.67% | 57,139 | 15,724 | 4,971 | 1,368 |
| Increase all regions to 99th percentile (11.3%) | 3306 | 11.37% | 84,729 | 43,313 | 7,371 | 3,768 |
| Increase all regions to highest region, Stanford (18.0%) | 3340 | 18.02% | 134,262 | 92,847 | 11,681 | 8,078 |
| Across the board increases in treatment rates in all regions | ||||||
| Increase all regional rates by 10% | 3340 | 6.12% | 45,557 | 4,142 | 3,963 | 360 |
| Increase all regional rates by 25% | 3340 | 6.95% | 51,769 | 10,354 | 4,504 | 901 |
| Increase all regional rates by 50% | 3340 | 8.34% | 62,123 | 20,708 | 5,405 | 1,802 |
| Increase all regional rates by 100% | 3340 | 11.12% | 82,831 | 41,416 | 7,206 | 3,603 |
| Increase all regional rates by 200% | 3340 | 16.68% | 124,247 | 82,831 | 10,809 | 7,206 |
Discussion
This study is the first to assess regional variation in thrombolysis treatment in the United States. There are regions that have more double the national thrombolysis treatment average. Conversely, 20% of regions did not treat any patients over the 4 year period. These results suggest considerable opportunity to improve outcomes in acute ischemic stroke patients—if all regions performed like those in the top 10%, about 16,000 additional patients would receive thrombolysis treatment annually.
Given the exclusions to thrombolysis treatment, every stroke patient should not receive thrombolysis. A previous study estimated that about 6% of stroke patients in the greater Cincinnati region were eligible for thrombolysis, with the major exclusion being delay in hospital presentation.11 Yet, we found that almost 20% of regions, had actual thrombolysis rates higher than this estimate and that the highest performing regions performed at more than double this rate. This, and other limited data on regional variation in time of stroke onset to hospital presentation,12 suggest that eligibility rates likely vary considerably by region and that improving eligibility, presumably by reducing delays to presentation, could have considerable benefits.
By identifying high performing regions within the context of the current US healthcare environment, our findings are the first step toward improving US regional thrombolysis treatment rates.13 Immutable regional factors and stroke systems of care accounted for little of the regional variation suggesting further work is needed to understand how high-performing regions achieved their success and whether that success can be replicated in other regions. This work should include determination of the relative importance of hospital and regional factors. Such work is challenging given the inability to differentiate hospital effects from regional effects in the absence of national patient-level data on time to arrival and other important eligibility criteria. For example, regional characteristics such as EMS bypass may increase the proportion of eligible stroke patients at a given hospital which could artificially attribute the effect of a regional intervention (EMS bypass) to the hospital.
Consistent with prior work,14–17 we found that an increased numbers of PSCs and EMS bypass systems were associated with increased thrombolysis rates. Whether PSCs or EMS bypass are the key causal element in increased treatment rates18 and, if so, the specific mechanisms by which they increase thrombolysis rates are unknown. Regional measures of socioeconomic status including education, median income and unemployment were modestly associated with thrombolysis. Explanations for the association of increased thrombolysis in higher socioeconomic regions are unknown. A previous study found small, clinically insignificant differences in time from 911 call to hospital arrival between diverse economic areas but did not explore differences in time of stroke onset to hospital arrival.19 We also found that women, older Americans, and racial/ethnic minorities were less likely to receive thrombolysis; a finding that is consistent with other studies.20, 21 While population density was associated with thrombolysis treatment, the association was very small. Given the intrinsic differences in rural and urban stroke care, and lack of detailed measures of both urban (e.g. hospital pre-notification, hospital-based quality initiatives) and rural (e.g. telestroke or emergency air evacuation programs) stroke systems, our analysis may understate the role of rural/urban differences.
Our study has several limitations. IV tPA treatment was based on ICD-9, and DRG codes which while broader than previous definitions may still underestimate thrombolytic treatment when compared to pharmacy data.22 This underestimate is unlikely to explain the measured regional variation for two reasons. First, the introduction of DRG 559 in 2005 and MS-DRG 061–063 created a strong incentive for hospitals to improve thrombolysis coding.23 Second, the magnitude of regional variation is considerably greater than the overall amount of miscoding previously documented. Although previous studies of IV tPA have included TIA and hemorrhagic stroke patients in estimates of thrombolysis,22, 23 these patients were excluded in our study given the concern for miscoding. In addition, because procedure codes have a low sensitivity for identifying IAT,24 we linked hospitalization data, to physician payment data, Medicare Part B files. Similar approaches have been used to identify other procedures that are unreliably recorded in hospital-based claims records.25 While the sensitivity and specificity of this approach for IAT is uncertain, there is strong financial incentive for institutions and providers to accurately code these claims. The study population was limited to Medicare fee-for-service enrollees and thus our results do not apply to working age stroke patients or Medicare managed care beneficiaries. While the majority of Americans receive care at their local hospital, there are those who do not which may result in some misclassification.4 Comprehensive stroke center certification was not in existence during the study period and thus was not included in the analysis. Thrombolysis treatment eligibility and stroke severity were not available in our dataset. However, because our primary analysis was at the regional level, it is less likely that differences in severity per se, would lead to major differences in regional treatment rates.26
In conclusion, there is substantial regional variation in thrombolysis treatment. Further study of regions with high thrombolysis treatment rates may identify opportunities to increase nationwide thrombolysis treatment.
Acknowledgments
Funding: Dr. Skolarus is supported by NINDS K23NS073685. Dr. Burke is supported by NINDS K08 NS082597.
Footnotes
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. New England Journal of Medicine. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 3.Kleindorfer D, Xu Y, Moomaw CJ, Khatri P, Adeoye O, Hornung R. Us geographic distribution of rt-pa utilization by hospital for acute ischemic stroke. Stroke. 2009;40:3580–3584. doi: 10.1161/STROKEAHA.109.554626. [DOI] [PubMed] [Google Scholar]
- 4.Appendix on the geography of health care in the united states. [Accessed January 22, 2015]; http://www.dartmouthatlas.org/downloads/methods/geogappdx.pdf. [Google Scholar]
- 5.The dartmouth atlas of health care: Data by region. [Accessed June 12, 2014]; http://www.dartmouthatlas.org/data/region/ [Google Scholar]
- 6.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 7.The joint commision. [Accessed October 16, 2014]; http://www.qualitycheck.org/consumer/searchQCR.aspx. [Google Scholar]
- 8.Song S, Saver J. Growth of regional acute stroke systems of care in the united states in the first decade of the 21st century. Stroke. 2012;43:1975–1978. doi: 10.1161/STROKEAHA.112.657809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics-2014 update: A report from the american heart association. Circulation. 2014;129:e28. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. The Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleindorfer D, Kissela B, Schneider A, Woo D, Khoury J, Miller R, et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: A population-based study. Stroke. 2004;35:e27–e29. doi: 10.1161/01.STR.0000109767.11426.17. [DOI] [PubMed] [Google Scholar]
- 12.Tong D, Reeves MJ, Hernandez AF, Zhao X, Olson DM, Fonarow GC, et al. Times from symptom onset to hospital arrival in the get with the guidelines–stroke program 2002 to 2009. Stroke. 2012 doi: 10.1161/STROKEAHA.111.644963. [DOI] [PubMed] [Google Scholar]
- 13.Marsh DR, Schroeder DG, Dearden KA, Sternin J, Sternin M. The power of positive deviance. BMJ: British Medical Journal. 2004;329:1177. doi: 10.1136/bmj.329.7475.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahr MMH, Luijckx G-J, Vroomen PCAJ, van der Zee D-J, Buskens E. Proportion of patients treated with thrombolysis in a centralized versus a decentralized acute stroke care setting. Stroke. 2012;43:1336–1340. doi: 10.1161/STROKEAHA.111.641795. [DOI] [PubMed] [Google Scholar]
- 15.Hunter RM, Davie C, Rudd A, Thompson A, Walker H, Thomson N, et al. Impact on clinical and cost outcomes of a centralized approach to acute stroke care in london: A comparative effectiveness before and after model. PloS one. 2013;8:e70420. doi: 10.1371/journal.pone.0070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prabhakaran S, O’Neill K, Stein-Spencer L, Walter J, Alberts MJ. Prehospital triage to primary stroke centers and rate of stroke thrombolysis. JAMA Neurology. 2013;70:1126–1132. doi: 10.1001/jamaneurol.2013.293. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakaran S, McNulty M, O'Neill K, Ouyang B. Intravenous thrombolysis for stroke increases over time at primary stroke centers. Stroke. 2012;43:875–877. doi: 10.1161/STROKEAHA.111.640060. [DOI] [PubMed] [Google Scholar]
- 18.Lichtman JH, Allen NB, Wang Y, Watanabe E, Jones SB, Goldstein LB. Stroke patient outcomes in us hospitals before the start of the joint commission primary stroke center certification program. Stroke. 2009;40:3574–3579. doi: 10.1161/STROKEAHA.109.561472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleindorfer DO, Lindsell CJ, Broderick JP, Flaherty ML, Woo D, Ewing I, et al. Community socioeconomic status and prehospital times in acute stroke and transient ischemic attack. Stroke. 2006;37:1508–1513. doi: 10.1161/01.STR.0000222933.94460.dd. [DOI] [PubMed] [Google Scholar]
- 20.Reeves M, Bhatt A, Jajou P, Brown M, Lisabeth L. Sex differences in the use of intravenous rt-pa thrombolysis treatment for acute ischemic stroke: A meta-analysis. Stroke. 2009;40:1743–1749. doi: 10.1161/STROKEAHA.108.543181. [DOI] [PubMed] [Google Scholar]
- 21.Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- 22.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National us estimates of recombinant tissue plasminogen activator use: Icd-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 23.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the united states. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi AI, Harris-Lane P, Siddiqi F, Kirmani JF. International classification of diseases and current procedural terminology codes underestimated thrombolytic use for ischemic stroke. Journal of clinical epidemiology. 2006;59:856–858. doi: 10.1016/j.jclinepi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Lin GA, Dudley RA, Lucas F, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. Jama. 2008;300:1765–1773. doi: 10.1001/jama.300.15.1765. [DOI] [PubMed] [Google Scholar]
- 26.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder ÉL. The implications of regional variations in medicare spending. Part 1: The content, quality, and accessibility of care. Annals of Internal Medicine. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]