Adherence to oral glucose lowering therapies and associations with one year HbA1c: a retrospective cohort analysis in a large primary care database (original) (raw)
. Author manuscript; available in PMC: 2016 Jun 6.
Published in final edited form as: Diabetes Care. 2015 Dec 17;39(2):258–263. doi: 10.2337/dc15-1194
Abstract
Objectives
The impact of taking oral glucose-lowering medicines intermittently, rather than as recommended, is unclear. We conducted a retrospective cohort study using community-acquired United Kingdom clinical data (CPRD and GoDARTS databases) to examine the prevalence of non-adherence to treatment for type 2 diabetes, and investigate its potential impact on HbA1c reduction stratified by type of glucose-lowering medication.
Research design and methods
Data for patients treated between 2004 and 2014 were extracted for those newly-prescribed metformin, sulfonylurea, thiazolidinedione or dipeptidyl peptidase-4 inhibitors who continued to obtain prescriptions over one year, were extracted. Cohorts were defined by prescribed medication type, and good adherence as a medication possession ratio ≥0.8. Linear regression was used to determine potential associations between adherence and one-year baseline-adjusted HbA1c reduction.
Results
In CPRD and GoDARTS, 13% and 15% of patients respectively were non-adherent. Proportions of non-adherent patients varied by the oral glucose-lowering treatment prescribed (range 8.6% (thiazolidinedione) to 18.8% (metformin)). Non-adherent, compared with adherent, patients had a smaller HbA1c reduction (0.4%[4.4mmmol/mol] and 0.46%[5.0mmol/mol] for CPRD and GoDARTs respectively). Difference in HbA1c response for adherent compared with non-adherent patients varied by drug (range: 0.38%[4.1mmol/mol] to 0.75%[8.2mmol/mol] lower in adherent group). Decreasing levels of adherence were consistently associated with a smaller reduction in HbA1c.
Conclusions
Reduced medication adherence for commonly used glucose lowering therapies among patients persisting with treatment is associated with smaller HbA1c reductions, compared with those taking treatment as recommended. Differences observed in HbA1c responses to glucose lowering-treatments may be explained in part by their intermittent use.
Keywords: type 2 diabetes, adherence, HbAlc, oral glucose lowering treatment, retrospective cohort
Between a third and a half of medicines prescribed for type 2 diabetes (T2DM), a condition in which multiple medications are used to control cardiovascular risk factors and blood glucose [1,2], are not taken as prescribed [3–6]. However, estimates vary widely depending on the population being studied and the way in which adherence to recommended treatment is defined.
The impact of continuing to take glucose lowering medicines intermittently, but not as recommended is unknown. Medication possession (expressed as a ratio of actual compared to expected possession), derived from prescribing records has been identified as a valid adherence measure for people with diabetes [7]. Previous studies have been limited to small populations in managed care systems in the United States and focused on metformin and sulfonylurea oral glucose-lowering treatments [8,9]. Further studies need to be carried out in larger groups of people that are more representative of the general population.
The Clinical Practice Research Database (CPRD) is a long established repository of routine clinical data from over 13 million patients registered with primary care services in the England. These data now include demographic data, diagnostic codes, biochemistry results and prescribing records from clinicians that have achieved high standards of data completeness. The GoDARTS database is derived from integrated health records in Scotland with primary care, pharmacy and hospital data on 9400 patients with diabetes.
We sought to establish the prevalence of medication non-adherence, measured from routinely collected data in patients with T2DM, and its relationship to reductions in glycated hemoglobin (HbA1c) with prescribed glucose-lowering medications. Specifically, we wished to determine whether poor response to oral glucose-lowering drugs could be due in part to reduced adherence. We hypothesized that there would be an association between the number of tablets potentially available for a newly prescribed glucose-lowering medication (if all prescriptions were filled) over one year (expressed as a percentage) and the observed reduction in HbA1c.
Research Design and Methods
Design
We performed a retrospective cohort study using routine clinical data obtained from community-acquired United Kingdom clinical data. The primary endpoint of the study was HbA1c reduction over one year.
Data sources
We selected patients from the Clinical Practice Research Datalink (CPRD) and the GoDARTS cohort. CPRD contains anonymized longitudinal health records from 680 general practices across the UK amounting to 13.2 million patients [10]. We selected patients prescribed metformin, sulfonylurea, dipeptidyl peptidase-4 inhibitors (DPP4i), or thiazolidinediones. Prescriptions for glucagon-like peptide 1 receptor agonists (GLP-1 RA) were not included in the analysis given the limited information available and difficulty in determining daily doses for injectable therapies.
GoDARTS contains electronic health record data from primary care, hospitals and pharmacies [11]. It includes over 9,400 patients with T2DM who have given explicit consent for use of the information in research. In addition to the types of data available through CPRD, it includes information on the dispensing of prescribed medication.
Population
In both data-sets the date of diagnosis was based on the earliest record of either a first prescription of a glucose lowering drug prescribed, a diabetes diagnostic code or the first HbA1c>6.5% [48mmol/mol]. Patients were included in a cohort for analysis if there was a record of treatment with a glucose-lowering medication for a period of at least one year
Patients were excluded from the analysis if they: were younger than 35 years old when diagnosed; had forms of diabetes other than T2DM, including gestational diabetes; had a diabetes duration <1 year; had previously been prescribed or were currently taking insulin; or were prescribed a thiazolidinedione or a DPP4i as monotherapy (where the characteristics of patients prescribed these treatments as monotherapy differed from other treatments and treatment combinations). We restricted consideration to the first period of treatment on each drug for each person. An individual patient could contribute 12-month periods of observation for different drugs, but could not contribute data for two or more drugs concurrently.
Derived variables
The medication possession ratio (MPR) was calculated by using prescription data (or for GoDARTS the dispensing data) from the date of the first prescription for that patient to the next prescription after 365 days from the first prescription date. The one year period for calculating adherence was considered valid if: i) there were no gaps between prescriptions longer than 6 months (a gap of more than 6 months was considered “stopping” the drug); ii) there was no change in treatment (either a drug being started or stopped) in the period of three months prior to the drug start date up until the date of the first prescription after one year, and iii) data were available from three or more prescriptions (with non-zero daily dose information). MPR was defined as the number of days of available medication divided by the number of days between the first and last prescription dates, multiplied by 100. The number of days of available medication was calculated by dividing the quantity prescribed by the daily dose. In some instances (25% of 15,336,948 prescription records in CPRD) the daily dose was recorded as zero. Where dose information was not available for any of the prescriptions for a patient over the 12-month prescription period, MPR was not calculated. For those where there were at least three valid prescriptions, but dose was missing from others, we removed the prescription with missing dose and the time between that prescription and the next from the denominator (seven per cent of cases). Patients with MPR ≥120% were excluded from the analysis.
HbA1c response at one year
Baseline HbA1c was the closest HbA1c value to the drug start date in a time-window between three months before to seven days after. The twelve-month HbA1c values used were those nearest to the date one year after the drug was started, with a time window from three months before to three months after. Response was calculated as 12 month HbA1c minus baseline HbA1c. For the response to be valid, there had to be no change in treatment (either a drug being started or stopped) in the period from the baseline HbA1c date to the twelve-month HbA1c date. Invalid responses were excluded from analysis. Response could not be calculated on 6% patients due to missing HbA1c data.
Additional clinical variables
We extracted age and body mass index (BMI), from the data as baseline variables at the time a glucose-lowering drug was started. The values used were those that were the closest to the drug start date in a time-window between six months before to seven days after. Medication doses over one year were calculated as a mean percentage maximum dose for that medication, weighted by the number of days the patient was on that dose.
Statistical Analyses
We categorized adherence into MPR groups; <70%, 70 to <80%, 80 to <90%, 90 to <100%, >100%. For the analyses presented here we defined non-adherence as a MPR <80% and adherence as a MPR ≥80% [12]. .
To assess the impact of MPR category on response we used regression models, adjusted for baseline HbA1c, for each drug, and all data combined. Models used MPR category (coded as a factor) and baseline HbA1c as independent variables and change in HbA1c over 12 months as the outcome. Only baseline HbA1c was adjusted for, as this explains the most variation in response (R2=0.32). We did not adjust for other features in these models. For each drug and overall we plotted 95% confidence intervals for change in HbA1c adjusted by baseline HbA1c and p values for comparison of the extreme categories were derived from the regression analysis. All analyses were carried out in R version 3.0.2 [13].
Ethics
Approval for the study was granted by the CPRD Independent Scientific Advisory Committee (ISAC 13_177R) and for GoDARTS by the East of Scotland Regional Ethics Committee (09/21402/44).
Role of the funding source
The funder of the trial had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Results
Analysis of CPRD dataset
32,634 patients were included for analysis with 38,100 instances of starting a new treatment (periods of treatment) and continuing for at least one year. The characteristics of included patients are shown by treatment cohort in Table 1. The mean duration of diabetes was shorter for those on the metformin and sulfonylurea cohorts, consistent with use of metformin earlier in the course of the disease. The mean proportion of the maximum dose of each drug also varied by treatment. Overall, 28.7% of patients were taking no other non-insulin glucose lowering treatments, 51.8% were taking one other treatment and 19.1% were taking two other treatments (Supplementary Table 1). Additional glucose lowering drug treatment for patients in each cohort is shown in Supplementary Table 2, with the majority taking metformin alongside other treatments.
Table 1.
Clinical characteristics of included patients (CPRD) within each cohort at baseline
| CPRD | Metformin N=13,823 | Sulfonylurea N=10,070 | Thiazolidinedione N=9088 | DPP4i‡ N=5119 |
|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age (years), mean (SD) | 65.1 (10.8) | 64.0 (10.8) | 63.4 (10.4) | 64.1 (10.3) |
| Female, n (%) | 5351 (39) | 3924 (39) | 3398 (37) | 1894 (37) |
| Duration of diabetes (years), mean (SD) | 4.9 (4.0) | 5.2 (3.8) | 6.7 (4.8) | 7.8 (4.9) |
| BMI (Kg/m2), mean (SD) | a30.5 (5.7) | b 31.1 (6.0) | c 31.4 (6.0) | d 32.5 (6.1) |
| HbA1c (mmol/mol), mean (SD) | 70.5 (15.0) | 72.0 (15.5) | 72.0 (13.9) | 71.1 (13.6) |
| HbA1c (%), mean (SD) | 8.6 (1.4) | 8.7 (1.4) | 8.7 (1.3) | 8.7 (1.2) |
| Dose (% maximum dose), mean (SD) | e59.7 (19) | †32.1 (16.7) | g56.2 (18.4) | h97.8 (10.3) |
| Weight change over 12m (kg), mean (SD) | i-1.4 (4.0) | j2.0 (4.5) | k2.8 (4.5) | l-0.9 (4.0) |
| GoDARTS | Metformin N=927 | Sulfonylurea N=729 | Thiazolidinedione N=677 | DPP4i‡ N=244 |
|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age (years), mean (SD) | 66.3 (10.2) | 65.3 (10.5) | 63.7 (9.8) | 65.3 (9.8) |
| Female, n (%) | 41 | 40 | 39 | 43 |
| Duration of diabetes (years), mean (SD) | 5.3 (4.0) | 6.0 (3.7) | 8.4 (5.2) | 6.7 (4.6) |
| BMI (Kg/m2), mean (SD) | m 31.4 (5.7) | n 31.6 (5.8) | o 32.1 (5.7) | p 32.9 (5.9) |
| HbA1c (mmol/mol) mean (SD) | 67.9 (13.4) | 70.2 (14.7) | 72.9 (13.0) | 70.5 (11.3) |
| HbA1c (%) mean (SD) | 8.3 1.23 | 8.6 1.34 | 8.8 1.18 | 8.6 1.10 |
| Dose (% maximum dose), mean (SD) | q 55 (18) | r 28 (16) | s 73 (41) | t 99.6 (0.1) |
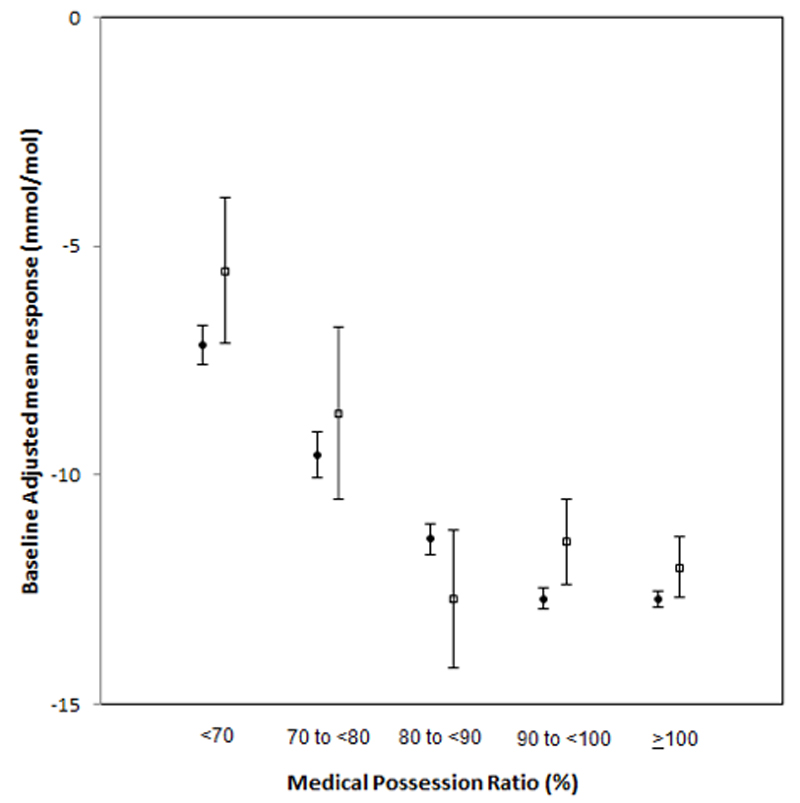
The proportion of non-adherent patients was 13.3% (n=38,100 periods of treatment; 32,634 patients), ranging from 9.1% and 8.6% for DPP4i inhibitors and thiazolidinediones respectively up to 18.8% with metformin (Table 2). For all therapies, participants with MPRs ≥90% experienced the greatest reductions in baseline-adjusted HbA1c (Figure 1). With lower MPRs, there was a consistent observed smaller reduction in HbA1c changes with all therapies (Figure 1).
Table 2.
Adherence by treatment and HbA1c response from baseline to one year showing overall decrement in HbA1c by group
| Drug | N | Percentage of patients with MPR<80% (%) | Change in HbA1c % [mmol/mol] from baseline to 12 months for patients with MPR <80% (95% CI) | Change in HbA1c % [mmol/mol] from baseline to 12 months for patients with MPR ≥80% (95% CI) | Difference between change in HbA1c % [mmol/mol] from baseline to 12 months for patients with MPR<80% and MPR≥80% (95% CI) | P |
|---|---|---|---|---|---|---|
| CPRD | ||||||
| All treatments | 38,100 | 13.3 | -0.75 (-0.78, -0.72) [-8.2 (-8.5, -7.9)] | -1.14 (-1.16, -1.13) [-12.5 (-12.7, -12.4)] | -0.40 (-0.43, -0.37) [-4.4 (-4.7, -4.0)] | <0.001 |
| Metformin | 13,823 | 18.8 | -0.78 (-0.82, -0.74) [-8.5 (-9.0, -8.1)] | -1.16 (-1.18, -1.14) [-12.7 (-12.9, -12.5)] | -0.38 (-0.42, -0.34) [-4.2 (-4.6, -3.7)] | <0.001 |
| Sulfonylurea | 10,070 | 11.9 | -0.85 (-0.91, -0.79) [-9.3 (-10.0, -8.6)] | -1.23 (-1.24, -1.20) [-13.4 (-13.6, -13.1) | -0.38 (-0.44, -0.31) [-4.1 (-4.8, -3.4)] | <0.001 |
| Thiazolidinedione | 9088 | 8.6 | -0.66 (-0.73, -0.59) [-7.2 (-8.0, -6.4)] | -1.24 (-1.25, -1.21) [-13.4 (-13.7, -13.2)] | -0.57 (-0.64, -0.49) [-6.2 (-7.0, -5.4)] | <0.001 |
| DPP4i ‡ | 5119 | 9.1 | -0.40 (-0.50, -0.30) [-4.4 (-5.5, -3.3)] | -0.83 (-0.87, -0.81) [-9.1 (-9.5, -8.8)] | -0.44 (-0.54, -0.33 [-4.8 (-5.9, -3.6)] | <0.001 |
| GoDARTS | ||||||
| All | 2622 | 15.1 | -0.63 (-0.74, -0.52) [-6.9 (-8.1, -5.7)] | -1.09 (-1.14, -1.04) [-11.9 (-12.5, -11.4)] | -0.46 (-0.58, -0.34) [-5.0 (-6.3, -3.7)] | <0.001 |
| MFN | 972 | 18.1 | -0.59 (-0.73, -0.43) [-6.4 (-8.0, -4.7)] | -1.08 (-1.14, -1.01) [-11.8 (-12.5, -11.0)] | -0.49 (-0.66, -0.32) [-5.4 (-7.2, -3.5)] | <0.001 |
| SU | 729 | 16.2 | -0.77 (-0.99, -0,56) [-8.4 (-10.8, -6.1)] | -1.11 (-1.20, -1.02) [-12.1 (-13.1, -11.1)] | -0.34 )-0.58, -0.1) [-3.7 (-6.3, -1.1)] | <0.005 |
| TZD | 677 | 11.4 | -0.70 (-0.95, -0.44) [-7.6 (-10.4, -4.8)] | -1.28 (-1.37, -1.18) [-14.0 (-15.0, -12.9)] | -0.59 (-0.86, -0.32) [-6.4 (-9.4, -3.5)] | <0.001 |
| DPP4i ‡ | 244 | 10.7 | 0.12 (-0.32, 0.56) [1.3 (-3.5, 6.1)] | -0.63 (-0.79, -0.48) [-6.9 (-8.6, -5.3)] | -0.75 (-1.22, -0.28) [-8.2 (-13.3, -3.1)] | <0.005 |
Figure 1.
Plot of HbA1c (mmol/mol) change over all drugs in 12 months for each data set by Medication Possession Ration (MPR) category (baseline adjusted absolute change in HbA1c)
“Difference in HbA1c change for both CPRD and DARTS p<0.00001)
Black circles = CPRD, white squares = GoDARTS
Adherent, compared with non-adherent, patients consistently had greater HbA1c reductions in all drug classes (Table 2). Overall, HbA1c decrements (95% CI) were -0.75 (-0.78 to -0.72)% [-8.2 (-8.5 to -7.9)] mmol/mol) and -1.15 (-1.16 to -1.14)% [-12.5 (-12.7 to -12.4) mmol/mol] for non-adherent and adherent patients respectively (Table 2). The mean (95% CI) difference overall between adherent and non-adherent groups in baseline-adjusted one-year HbA1c was -0.40 (-0.43 to -0.37) % [-4.4 (-4.7 to -4.0) mmol/mol]. This between-group difference varied across drugs from -0.37 (-0.44 to -0.31)% [-4.1 (-4.8, -3.4) mmol/mol] with sulfonylureas to -0.57 (-0.64 to -0.49)% [-6.2 (-7.0, -5.4) mmol/mol] with thiazolidinediones (Table 2).
Analysis of GoDARTS cohort
2284 patients were included for analysis with 2622 instances of starting a new treatment (periods of treatment) and continuing for at least one year. The characteristics of included patients are shown by treatment cohort in Table 1 and, similarly to CPRD, the different characteristics of the cohort reflect the stage of treatment at which the treatment was started. Overall, 34% of patients were taking no other non-insulin glucose lowering treatments, 44% were taking one other treatment and 22% were taking two other treatments (Supplementary Table 3 and 4).
The proportion of non-adherent patients was 15.1% (n=2622 periods of treatment; 2284 patients) ranging from 10.7% with DPP4i up to 18.1% with metformin (Table 2). For all therapies, participants with an MPR≥90% experienced the greatest reductions in baseline-adjusted HbA1c (Figure 1). With lower MPRs, there was a consistent observed smaller reduction in HbA1c across all therapies (Figure 1). There was no clear evidence of a differential response to any one therapy for groups of patients with differing MPR.
Overall HbA1c decrements were -0.67 (-0.79 to -0.55)% [6.9 (-8.1 to -5.7) mmol/mol] and -1.16 (-1.21 to -1.11)% [-11.9 (-12.5 to -11.4) mmol/mol] for non-adherent and adherent patients respectively (Table 2). The mean (95% CI) difference overall between adherent and non-adherent groups in baseline-adjusted one-year HbA1c was -0.49 (-0.62 to -0.36)% [-5.0 (-6.3 to -3.7) mmol/mol]. This between-group difference varied across drugs from -0.34 (-0.58 to -0.10)% [-3.7 (-6.3 to -1.5) mmol/mol] with sulfonylurea to -0.75 (-1.22 to -0.28)% [-8.2 (-13.3, -3.1) mmol/mol] with DPP4i (Table 2).
Conclusions
These findings show an association between adherence to oral glucose lowering treatment, measured by the proportion of medication obtained on prescription over one year, and the corresponding decrement in HbA1c, in a population of patients newly starting treatment and continuing to collect prescriptions. The association is consistent across all commonly used oral glucose lowering therapies and the findings are consistent between the two data sets examined, CPRD and GoDARTS. Non-adherent patients, taking on average less than 80% of the intended medication, had about half the expected reduction in HbA1c.
This is the largest study that we are aware of to examine the association between adherence to oral glucose lowering treatment in type 2 diabetes over one year and change in HbA1c. The study uses two independent datasets: a representative primary care data-set from across England and an electronic medical record data-set from a region in Scotland. Both data sets have detailed information about dosage adjustment over the period of the study[14] and include systematic records of clinical and laboratory measurements at three to six monthly intervals. Results were consistent across both studies. Although the Scottish data utilize prescription encashment, the similar results in CPRD support the use of issued prescription data for studying medication adherence.
There are a number of issues that could be further explored in greater depth to provide information about factors associated with non-adherence. These include whether adherence differs when a medication is used as first, second or third line, and the extent to which there is an interaction with the type of medication. In addition, there is potential for stating a drug to change adherence to other concurrent medication. However, because our study is limited by use of different populations in examining the different drugs, we are unable to directly address these questions, and our study does not provide evidence of comparative effectiveness between the drugs.
Although direct comparisons of adherence rates are not possible, high adherence levels reported for patients taking drugs commonly found in combination therapy (e.g. DPP4i) may reflect a greater personal investment in disease self-management. Differences with baseline characteristics of patients using DPP4i between England and Scotland may reflect differences in local usage of the class of drugs, as there is no difference in national guidance. Similarly, the higher levels of change in HbA1c found for patients taking metformin and sulfonylurea may also reflect an earlier stage in the course of their diabetes.
There is the possibility for time-dependent confounding and this is something we have not explored in this paper. We have limited our data to only simple models, and those cases where there were no changes in diabetes drugs during the one-year period of interest. However, this does not exclude other potential changes that may impact on adherence, such as changes in dose, incidence of side effects, or changes in other medications or comorbidities. It will be of interest to examine predictors of adherence, both at baseline when starting medication, and over the time-course of taking the drug. Development of in depth models to explore this would be an important next step for further research focusing on the relationship between adherence and achieved HbA1c.
There are a number of different ways to measure adherence to medication. Overall adherence rates are consistently overestimated by self-report [15]. Direct measurement (for example with electronic dispensing measurement) has potential to modify behavior because it is intrusive. Data on medication prescribing and dispensing is widely used, has evidence to support its validity [7], and medicine availability is a necessary prerequisite for being able to take the medicine. Medication hoarding (obtaining medicine but not then using it) has been identified as an issue in the psychiatric literature, but not more generally for long-term conditions [16]. In another study, perceptions of higher levels of hoarding in older people relating to medicines’ non-adherence were not supported [17].
The focus of this study is on the relationship of quality of taking medication and impact on HbA1c. The Medication Possession Ratio is used to report adherence as an increasingly accepted measure of medicines use. There are some patterns of medication use that may result in misleading metrics for MPR, but the most common, failure to persist with medication is accounted for in this analysis by exclusion of patients with a six month or longer break in prescribing [18]. It also excludes individuals starting treatment in the first year after diagnosis, again a group where overall adherence and persistence is likely to differ from those at a later stage of their illness.
In addition, this retrospective study was not able to identify sufficient patients within the data set to provide information about GLP-1 RA treatment. We have not addressed adherence to insulin therapy in this analysis.
A number of previous studies have used retrospective databases of electronic health records to examine factors that might predict adherence. A recent large cohort database examined overall adherence to oral therapy for type 2 diabetes, taking into account changes of therapy. It concluded that overall adherence was 69%, with individuals newly started on treatment being significantly less likely to adhere [19]. There are few studies providing estimates of adherence to treatment and its relationship to glycaemic control: we have identified only two, both focussed on sulfonylurea and metformin treatment, and of relatively small size. In one, based in south-west Michigan, 677 patients with diabetes were reviewed and a 10% increase in non-adherence was associated with a 0.14% (1.5 mmol/mol) rise in HbA1c [9]. A larger study in South Carolina of 1668 patients found that the mean medication possession ratio for those reaching an HbA1c target of 7.0% [53mmol/mol] was 81% [8].
Low medication adherence is related to increased mortality [20]. The mean difference in HbA1c between patients with MPR<80% and ≥80% is between 0.37 and 0.55% (4 and 6mmol/mol), equivalent to up to a 10% reduction in death or an 18% reduction in diabetes complications [21]. The small numbers with adherence <70% mean that exploring the impact of this on HbA1c and its relationship to other lifestyle factors requires a larger study population. Further work is now needed to demonstrate the extent to which improved compliance leads to improvements in HbA1c.
Data obtained from real-time monitoring of medication collection may provide feedback to patients and their clinicians to indicate whether medication use might be sub-optimal. Further work is needed to establish the intervals over which data becomes meaningful and whether analysis with other routinely collected data improves identification of low adherence to treatment.
Supplementary Material
Supplementary table 1: Proportion of patients using other glucose lowering drugs (CPRD) in each cohort
Supplementary table 2: Additional treatment being used by patients (CPRD) included in the analysis
Supplementary table 3 Proportion of patients using other glucose lowering drugs (GoDARTS) in each cohort
Supplementary table 4: Additional treatment being used by patients (GoDARTS) included in the analysis.
Acknowledgements
BS, LRR and ML researched data. AF wrote the manuscript. BS ML and LRR reviewed or edited the manuscript. AF, LRR, ML, BS, MW, LD, RH, EP, and AH all contributed to the discussion and reviewed or edited the manuscript. Dr. Andrew Farmer is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Medical Research Council UK (MR-K005707-1)
AF and RRH are NIHR Senior Investigators and receive additional support from the Oxford NIHR Biomedical Research Centre. MNW was supported by a Wellcome Trust Institutional Strategic Support Award (WT097835MF). ERP holds a Wellcome Trust New Investigator award. The MASTERMIND consortium is funded by the MRC MR-K005707-1.
Footnotes
The MASTERMIND Consortium
University of Exeter Andrew T Hattersley, Beverley Shields Michael Weedon, Lauren R Rodgers; University of Dundee Ewan R. Pearson, Mike Lonergan, Louise Donnelly; University of Oxford Rury R. Holman, Andrew J. Farmer.
Declaration of interests
We declare no competing interests.
Diabetes Care
This is an author-created, un-copy-edited electronic version of an article accepted for publication in Diabetes Care. The American Diabetes Care Association (ADA), publisher of Diabetes Care, is not responsible for any errors or omissions in this version of the manuscript or any version derived from it by third parties. The definitive publisher-authenticated version will be available in a future issue of Diabetes Care in print and online at http://care.diabetesjournals.org,
References
- 1.The National Collaborating Centre for Chronic Conditions. Type 2 diabetes: National clinical guideline for management in primary and secondary care (update) National Institute for Health and Clincial Excellence; London: 2008. [Google Scholar]
- 2.American Diabetes Association. Standards of Medical Care in Diabetes Mellitus 2015. Diabetes (2015 (Supplement.
- 3.World Health Organization. Adherence to long term therapies: evidence for action. WHO; Geneva: 2003. [Google Scholar]
- 4.Evans J, Donnan P, Morris A. Adherence to oral hypoglycaemic agents prior to insulin therapy in Type 2 diabetes. Diabet Med. 2002;19:685–8. doi: 10.1046/j.1464-5491.2002.00749.x. [DOI] [PubMed] [Google Scholar]
- 5.Bryson CL, Au DH, Maciejewski ML, et al. Wide Clinic-Level Variation in Adherence to Oral Diabetes Medications in the VA. J Gen Int Med. 2013;28:698–705. doi: 10.1007/s11606-012-2331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin EHB, Korff Von M, Ciechanowski P, et al. Treatment Adjustment and Medication Adherence for Complex Patients With Diabetes, Heart Disease, and Depression: A Randomized Controlled Trial. Ann Fam Med. 2012;10:6–14. doi: 10.1370/afm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen HW, Shmukler C, Ullman R, et al. Measurements of medication adherence in diabetic patients with poorly controlled HbA1c. Diabet Med. 2010;27:210–6. doi: 10.1111/j.1464-5491.2009.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence DB, Ragucci KR, Long LB, et al. Relationship of oral antihyperglycemic (sulfonylurea or metformin) medication adherence and hemoglobin A1c goal attainment for HMO patients enrolled in a diabetes disease management program. J Manag Care Pharm. 2006;12:466–71. doi: 10.18553/jmcp.2006.12.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pladevall M, Williams LK, Potts LA, et al. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27:2800–5. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams T, van Staa T, Puri S, et al. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Therapeutic Advances in Drug Safety. 2012;3:89–99. doi: 10.1177/2042098611435911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans JM, MacDonald TM. Record-linkage for pharmacovigilance in Scotland. BrJClinPharmac. 1999;47:105–10. doi: 10.1046/j.1365-2125.1999.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25:2303–10. doi: 10.1185/03007990903126833. [DOI] [PubMed] [Google Scholar]
- 13.Team RC. R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2012. 2014 Open access available at: http://cran.r-project.org.
- 14.Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why Don’t Diabetes Patients Achieve Recommended Risk Factor Targets? Poor Adherence versus Lack of Treatment Intensification. J Gen Intern Med. 2008;23:588–94. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Fonseca V, Walker P, et al. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life …. 2010 doi: 10.1186/1477-7525-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsalman AJ, Smith WR. Expanding the Framework of Assessing Adherence and Medication-Taking Behavior. J Pain Palliat Care Pharmacother. 2013;27:114–24. doi: 10.3109/15360288.2013.765532. [DOI] [PubMed] [Google Scholar]
- 17.Ellis JC, Mullan J, Worsley T. Prescription medication hoarding and borrowing or sharing behaviours in older residents in the Illawarra, New South Wales, Australia. Australasian Journal on Ageing. 2011;30:119–23. doi: 10.1111/j.1741-6612.2010.00457.x. [DOI] [PubMed] [Google Scholar]
- 18.Fairman K, Matheral B. Evaluating medication adherence: which measure is right for your program? Journal of Managed Care Pharmacy. 2000;6:499–504. [Google Scholar]
- 19.Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of Adherence to Diabetes Medications: Findings From a Large Pharmacy Claims Database. Diabetes Care. 2015 doi: 10.2337/dc14-2098. dc142098–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. 2006;333:15–0. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stratton I, Adler A, Neil H, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]