The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway (original) (raw)
Abstract
Our previous study showed that circulating microvesicles (cMVs) of diabetic mice have negative effects on the function of endothelial progenitor cells (EPCs). Whether this is true in diabetic patients deserves further study. In this study, the effects of cMVs and EPC-derived MVs (EPC-MVs) on EPC migration, apoptosis, and reactive oxygen species (ROS) production in healthy controls, well-controlled, and uncontrolled diabetic patients were investigated. The levels of miR-126 and vascular endothelial growth factor receptor 2 (VEGFR2) in cMVs, EPC-MVs, and/or EPCs were analyzed. Moreover, miR-126 inhibitor or mimic was applied to EPCs to modulate the miR-126 level in EPC-MVs. We found the following: 1) the circulating EPC level was reduced but the circulating EPC-MV level increased in uncontrolled diabetic patients; 2) the cMVs and EPC-MVs of healthy controls had beneficial effects on EPCs (migration, apoptosis, ROS), whereas the effects were reversely changed in the cMVs and EPC-MVs of uncontrolled diabetic patients; and 3) the cMVs and EPC-MVs of uncontrolled diabetic patients carried less miR-126 and had downregulated VEGFR2 expression in EPCs. Manipulating the miR-126 level in EPC-MVs with inhibitor or mimic changed their function. The effects of cMVs and EPC-MVs are compromised in diabetes due to the reduction of their carried miR-126, which might provide a therapy target for diabetic vascular complications.
Keywords: vascular endothelial growth factor receptor 2, type 2 diabetes, microvesicles, endothelial progenitor cells, miR-126, oxidative stress
diabetes mellitus (DM) is considered one of the major risk factors for various cardiovascular complications. Endothelium dysfunction is a key initiator for vascular disease, which results from increased oxidative stress in the vascular cells (39). Endothelial progenitor cells (EPCs) are known to play important roles in maintaining vascular function and structure by repairing or replacing dysfunctional or injured endothelial cells (ECs) (33). Impaired EPC proliferation, differentiation, adhesion, mobilization, and survival have been reported in DM (1, 26). Our previous study has demonstrated that the circulating EPC level is decreased and the function of EPCs impaired in db/db diabetic mice (8). Moreover, we found that the circulating microvesicles (cMVs) of db/db diabetic mice compromised the functions of EPCs. Investigation on the regulatory effects of cMVs on EPCs in diabetic patients could provide novel therapeutic avenues for vascular complications of diabetes.
Extracellular MVs are submicrometric fragments released from the cells in response to activation and apoptosis (22, 37). cMVs are the MVs released from the cells in the blood and from the vascular wall. An elevation of cMV levels has been reported in vascular diseases such as thrombotic diseases, diabetes, and cardiovascular diseases (2, 8, 25). Furthermore, one study has shown that the level of cMVs could predict the severity of vascular diseases (30). MVs released from EPCs (EPC-MVs) have been reported to serve as an index for EPC loss and functional incompetence (27). Moreover, the level of circulating EPC-MVs can predict aortic stiffness in atherosclerotic patients (27). Therefore, circulating MV and EPC-MV levels could serve as biomarkers and predictors for vascular diseases. On the other hand, accumulating evidence suggests that MVs mediate cell-cell communication via transferring proteins, mRNAs, and miroRNAs (miRs) from their parent cells to the target cells (5, 17, 29). The functions of MVs are complex and multifactorial, depending on the stimulator and origin. Previously, we demonstrated that cMVs of diabetic mice impair the function of EPCs, whereas cMVs of healthy controls do not have detrimental effects on EPCs (8). However, whether this is true in diabetic patients remains unclear.
Previous studies have shown that miR-126 governs vascular integrity (35) and is a biomarker or mediator of vascular diseases (11, 32). Downregulation of miR-126 impairs EPC function (23). EPC-MVs have been shown to improve ischemia-reperfusion injury of hindlimb and kidney through the transfer of miR-126 to target cells (6, 28). In the meantime, miR-126 has been reported to regulate angiogenic process and EC/EPC function by modulating vascular endothelial growth factor receptor 2 (VEGFR2) (13, 15). Our previous study found that EPC-MVs affect EC functions and apoptosis via their carried miR-126 (34). However, it is unknown whether EPC-MVs, as one type of MVs, would affect EPC functions through the miR-126 and its downstream VEGFR2 pathway.
In the present study, we determined the effects of cMVs and EPC-MVs of diabetic patients on EPC survival and functions and explored whether miR-126/VEGFR2 is involved in the mechanism.
METHODS
Human subjects.
A total of 45 subjects (42–51 yr old) were included in the study with 24 type 2 diabetes patients (14 were well controlled and 10 were uncontrolled) and 21 healthy controls. Patients were recruited from the Endocrinology Department at the Boonshoft School of Medicine, Wright State University, Dayton, OH, and the Affiliated Hospital of Guangdong Medical College, Zhanjiang, China. The protocols were approved by Institutional Review Boards at both Wright State University and Guangdong Medical College. Each patient signed an informed consent form after a thorough explanation. Exclusion criteria included pregnancy, patients with history of myocardial infarction, stroke, unstable angina, renal or heart failure, cancer, chronic alcohol abuse, hypertension requiring more than three antihypertensive medication, and use of any multivitamin supplement, and patients with proliferative diabetic retinopathy or nephropathy or painful diabetic neuropathy requiring chronic narcotic analgesic therapy.
Experimental design.
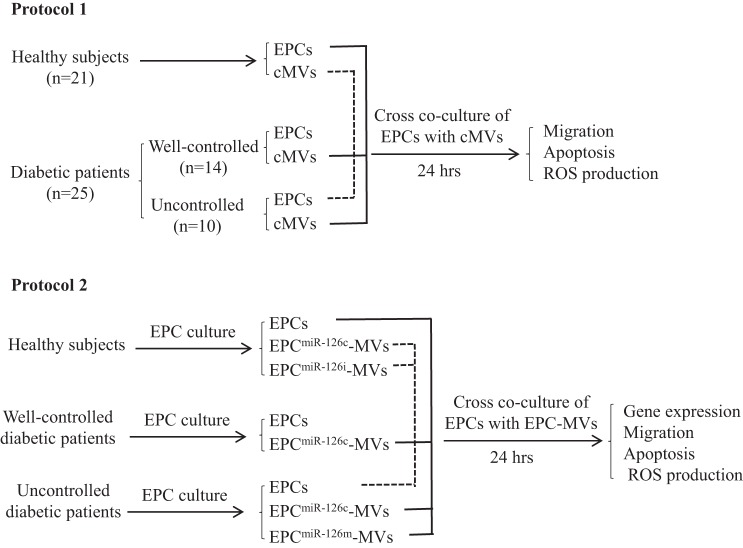
After an 8-h fast, blood samples were taken from patients and healthy controls. Approximately 60 ml of blood was collected from each subject in a tube with a 3.13% citrate buffer. The blood samples were transferred directly to the laboratory and processed within 1 h of collection. The levels of glucose, Hb A1c, and lipid of blood samples were measured. Periphery blood mononuclear cells (PBMCs) isolated from blood samples were used for determining the circulating EPC levels and EPC culture. The plasma was used for isolating circulating MVs. Cross-coculture studies were performed as described in Fig. 3. After 24 h of coculture, EPC migration, apoptosis, and reactive oxygen species (ROS) production were measured. The miR-126 and VEGFR2 expressions were also examined in cMVs and EPCs.
Fig. 3.
Diagram depicts the design of cross-coculture experiments for determining the effects of circulating MVs (cMVs) and EPC-MVs on EPCs. In protocol 1, EPCs from HC were cocultured with cMVs from well-controlled or uncontrolled diabetic patients, whereas EPCs from uncontrolled diabetic patients were cocultured with cMVs from HC. In protocol 2, EPCs from healthy controls were cocultured with EPC-MVs released from EPCs of diabetic patients transfected with miR-126c or miR-126m, whereas EPCs from uncontrolled diabetic patients were cocultured with EPC-MVs released from EPCs of healthy controls transfected with miR-126c or miR-126i. After coculture, the expressions of miR-126 and VEGFR2 and migration, apoptosis, and ROS production of EPCs were measured. EPCmiR-126c-MVs, EPCmiR-126i-MVs, and EPCmiR-126m-MVs, microvesicles released from EPCs transfected with miR-126 control, inhibitor, and mimic, respectively.
cMV isolation.
cMVs were isolated as described in our previous paper (8). Platelet-rich plasma was centrifuged (1,500 g, 15 min) at 4°C to get platelet-free plasma (PFP). PFP was divided to numerous tubes (1 ml/tube) and then centrifuged at 30,000 g for 30 min (4°C). The MV pellet was resuspended in 100 μl of PBS for flow cytometric analysis.
Flow cytometric analyses of the levels of circulating EPCs and EPC-MVs.
The levels of circulating EPCs and EPC-MVs were determined per our previous study (8). For analysis of circulating EPC level, isolated PBMCs were incubated with 1 μl of FITC-conjugated anti-human CD34 (eBioscience, San Diego, CA) and 5 μl of PE-conjugated anti-human VEGFR2 (BD Bioscience, San Jose, CA) antibodies for 30 min at 4°C in the dark. Isotype-matched (IgG) nonspecific antibodies were used as negative controls. Labeled EPCs were analyzed in a flow cytometer (Accuri Cytometers, Ann Arbor, MI). EPCs were defined as CD34+VEGFR+ cells. The numbers of circulating EPCs were described as the CD34+VEGFR2+ cells per microliter of whole blood.
For analysis the of EPC-MV level, isolated cMVs were incubated with antibodies (CD34 and VEGFR2) under the same conditions as those for EPCs. The size of particles was calibrated using 1- and 2-μm flow cytometry beads (Molecular Probes, Invitrogen, Eugene, OR). MVs were defined as particles with a diameter of <1.5 μm. The numbers of circulating EPC-MVs were determined as CD34+VEGFR2+ events in the gate of MVs. The data were described as the number of EPC-MVs per microliter of whole blood.
EPC isolation, culture, and characterization.
EPCs were cultured from peripheral blood, as described in previous reports (4, 21). Peripheral blood taken from patients was diluted in PBS (2 times) and then gently layered over 4 ml of lymphocyte separation liquid (Sigma) for centrifugation (800 g, 30 min at 4°C). The PBMCs in the interface layer were transferred to a new tube and washed with PBS buffer by centrifugation at 400 g for 5 min at 4°C. EPCs were cultured from PBMCs in endothelial cell basal medium-2 (Lonza, Walkersville, MD) on a 24-well plate (5 × 106 cells/well). Cells were cultured continuously for 10 days for coculture studies. The EPCs in the cultures were characterized by assays of double-staining with Di-LDL and BS-lectin according to our previous reports (7–9). In brief, the adherent cells were incubated with PE-labeled Di-LDL (Biomedical Technologies, Stoughton, MA) for 2 h at 37°C. After that, cells were fixed with 2% paraformaldehyde and then counterstained with FITC-labeled BS-lectin (Sigma, Fairfax, VA). Cells were viewed under an inverted fluorescent microscope (EVOS).
EPC apoptosis and migration assays.
To determine the effective dose of cMVs for coculture experiments, EPCs were treated with different doses of cMVs (0.25, 0.5, 0.75, and 1 dilution to the total original cMV concentration from plasma of each individual). After 24 h of coculture, apoptotic rate of EPCs was assessed using an apoptosis assay kit (BD Bioscience) according to our previous studies (7–9). The EPC apoptotic rate was analyzed by a flow cytometer. Based on dose-response studies, a dilution of 1:1 was used for coculture experiments, as described in Experimental design section. EPC migration was evaluated using the Boyden chamber system (Millipore, Temecula, CA), as we have reported previously (7–9).
Intracellular ROS production assay.
Intracellular ROS production was determined by dihydroethidium (DHE; Sigma-Aldrich, St. Louis, MO) staining (10, 14). Cells were incubated with the DHE working solution (1 μM) at 37° for 2 h in dark. Then, the cells were observed under an inverted fluorescence microscope (EVOS), and the percentage of DHE-positive cells was analyzed using a flow cytometer.
Gene expression analysis.
miRNA from cMVs was extracted using a mirVana miRNA isolation kit (Ambion) by following the manufacturer's instructions. cDNA was synthesized using a miScript reverse transcription kit (Qiagen). Quantitative real-time PCR was conducted with miR-126-specific primers (forward: GGCTCGTACCGTGAGTAAT; reverse: GTGCAGGGTCCGAGGT) and a miScript SYBR Green PCR Kit (Qiagen) on a real-time PCR system (Bio-Rad). Small nuclear RNA U6 was used as an internal control. Relative expression of miR126 was calculated using the 2−ΔΔCT method (34).
Transfection of EPCs.
To generate EPCmiR-126c-MVs, EPCmiR-126i-MVs, and EPCmiR-126m-MVs, EPCs were transfected with miR-126 control (1 nM; Applied Biosystems), inhibitor (cat. no. 4464084), or mimic (cat. no. 4464066) using lipofectamine 2000 for 16 h and exposed to media without serum and growth factors for 24 h to generate modified EPC-MVs (18).
Western blotting.
Proteins from EPCs were isolated with lysis buffer (Roche Diagnostic) containing protease inhibitor. The proteins were subjected to SDS-PAGE electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked by incubating with 5% dry milk for 1 h and then incubated with antibodies against VEGFR2 (1:1,000; Cell Signaling Technology). β-Actin (1:4,000; Sigma) was used to normalize protein loading. After being washed thoroughly, membranes were incubated with horseradish peroxidase-conjugated IgG (1:40,000; The Jackson Laboratory) for 1 h at RT. Blots were then developed with enhanced chemiluminescence-developing solutions and quantified.
Statistical analysis.
All data are expressed as means ± SE. Comparisons for two groups were performed by Student's _t_-test. Multiple comparisons of the data, including the ethic factor, were analyzed by two-way ANOVA, followed by a Tukey post hoc test (SPSS version 19.0; SPSS, Chicago, IL). For all tests, a P value of <0.05 was considered significant.
RESULTS
Study subject characteristics.
The study subject characteristics are summarized in Table 1. There were no significant differences in average age between healthy controls, well-controlled patients, and uncontrolled diabetic patients (P > 0.05). The Hb A1c and glucose levels were significantly higher in uncontrolled diabetic patients compared with healthy controls and well-controlled diabetic patients (P < 0.05). Also, the levels of triglycerides and very low-density lipoprotein were significantly higher in uncontrolled diabetic patients (_P_ < 0.05). However, there were no significant differences between diabetes and healthy controls on the levels of total cholesterol, HDL, or LDL (_P_ > 0.05).
Table 1.
Clinical characteristics of the study population
| Patients with T2DM | |||
|---|---|---|---|
| Characteristics | Healthy Controls | Well controlled | Uncontrolled |
| Total nos. (n = 45) | 21 | 14 | 10 |
| Average age, yr | 42 ± 5 | 44 ± 6 | 43 ± 8 |
| Sex ratio (male/female) | 12:9 | 9:5 | 7:3 |
| Fasting blood glucose, mg/dl | 84.1 ± 11.4 | 90.4 ± 9.4 | 165.5 ± 24.5*+ |
| Hb A1c, %total HGB | 4.2 ± 0.3 | 5.6 ± 0.2 | 7.8 ± 0.8*+ |
| Lipid profile, mg/dl | |||
| Total cholesterol | 168.8 ± 26.5 | 175.2 ± 34.2 | 214.4 ± 12.8 |
| Triglycerides | 98.2 ± 10.7 | 100.9 ± 22.5 | 185.2 ± 24.2*+ |
| HDL | 57 ± 7.9 | 53 ± 8.7 | 38.8 ± 8.3 |
| LDL | 92 ± 14.1 | 102 ± 28.1 | 138.5 ± 12.8* |
| VLDL | 18.9 ± 3.3 | 21.2 ± 5.8 | 32.3 ± 8.4 |
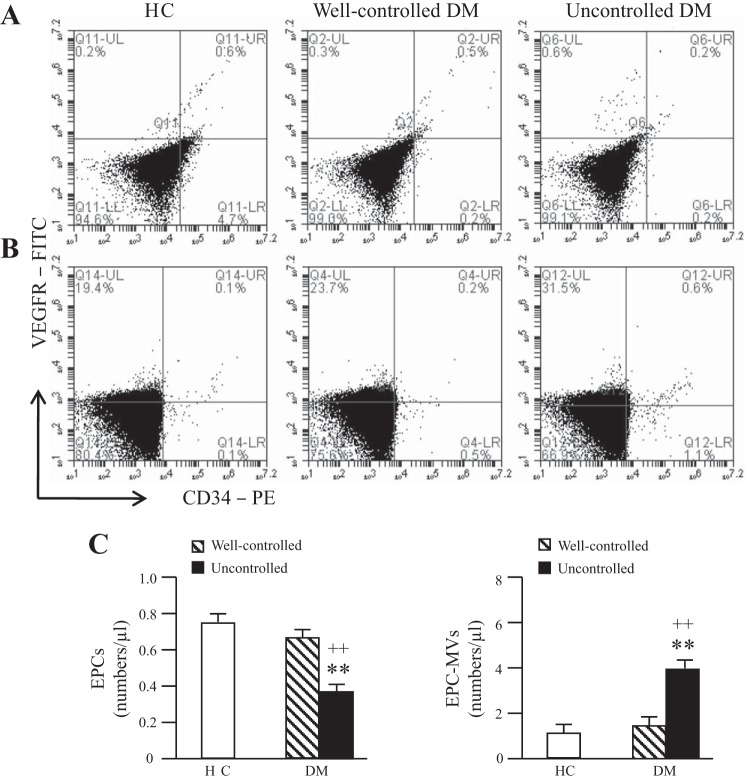
The level of circulating EPCs was decreased and the level of EPC-MVs increased in uncontrolled diabetic patients.
By using flow cytometric analysis, we found that the numbers of circulating EPCs were significantly decreased in uncontrolled diabetic patients compared with heathy controls and well-controlled diabetic patients (Fig. 1, A and C). However, the numbers of circulating EPC-MVs were significantly increased in uncontrolled diabetic patients (Fig. 1, B and C). There were no significant differences in circulating EPC or EPC-MV levels between heathy controls and well-controlled diabetic patients (Fig. 1).
Fig. 1.
The levels of circulating endothelial progenitor cells (EPCs) and EPC-derived microvesicles (EPC-MVs). A and B: representative flow cytometry plots of circulating EPCs (A) and EPC-MVs (B) in healthy controls (HC) and diabetes mellitus (DM) patients. C: summarized data on circulating EPC and EPC-MV levels; n = 21 for HC, 14 for well-controlled DM, and 10 for uncontrolled DM. **P < 0.01 vs. HC; ++P < 0.01 vs. well-controlled DM by 2-way ANOVA. VEGFR, VEGF receptor; PE, phycoerythrin.
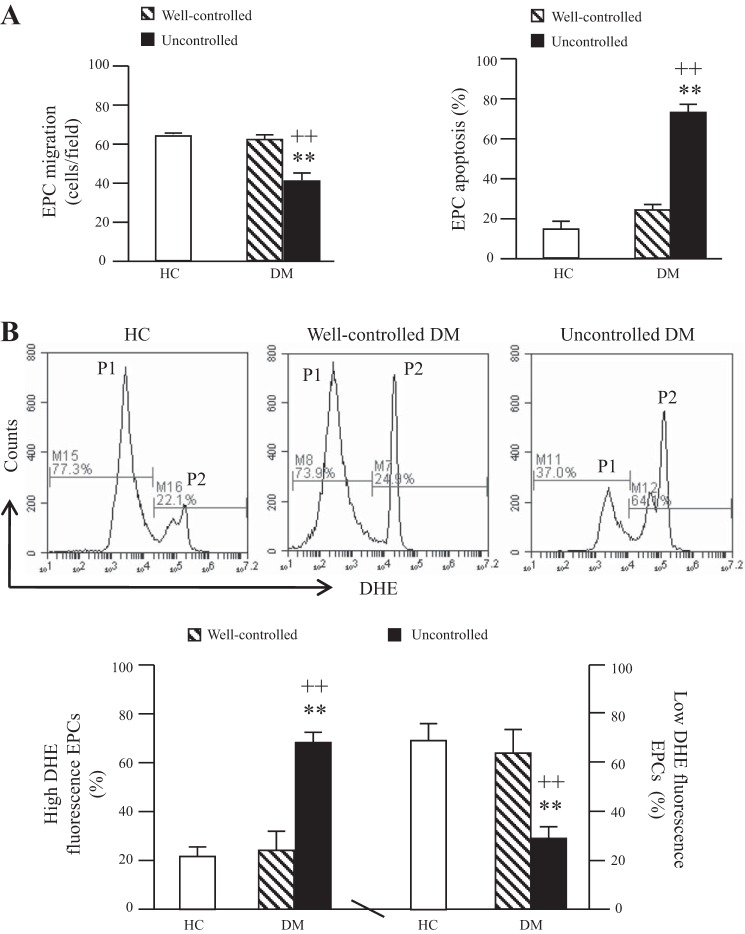
The migration was decreased and the apoptotic rate increased in cultured EPCs from uncontrolled diabetic patients.
Cultured EPCs were characterized by double-stained assay for Di-LDL uptake and BS-lectin binding (data not shown). The migration ability of cultured EPCs from uncontrolled diabetic patients was significantly lower than that of EPCs from healthy controls and well-controlled diabetic patients (Fig. 2_A_). On the contrary, the apoptotic rate of EPCs from uncontrolled diabetic patients was significantly higher than that of EPCs from healthy controls and well-controlled diabetic patients (Fig. 2_A_). There were no significant differences in migration or apoptosis between EPCs from healthy controls and well-controlled diabetic patients (Fig. 2_A_).
Fig. 2.
Migration ability, apoptotic rate, and reactive oxygen species (ROS) production of EPCs from diabetic patients and HC. A: summarized data on migration ability and apoptotic rate. B: representative flow cytometry plots and summarized data of ROS production. P1, EPCs with low ROS; P2, EPCs with high ROS; n = 21 for HC, 14 for well-controlled DM, and 10 for uncontrolled DM. **P < 0.01 vs. HC; ++P < 0.01 vs. well-controlled DM by 2-way ANOVA. DHE, dihydroethidium.
The ROS production was elevated in cultured EPCs from uncontrolled diabetic patients.
DHE staining was used to determine the level of ROS in EPCs. As shown in Fig. 2_B_, there were two ROS production populations, high ROS production population and low ROS production population. The percentage of high ROS production of EPCs from uncontrolled diabetic patients was significantly higher than that of EPCs from healthy controls and well-controlled diabetic patients. However, the percentage of the low ROS production population of EPCs from uncontrolled diabetic patients was significantly decreased. There were no significant differences in ROS production between EPCs from healthy controls and well-controlled diabetic patients.
The detrimental effects of cMVs from uncontrolled diabetes on the migration, apoptosis, and ROS production of EPCs from healthy controls.
To determine the effective dose of cMVs for coculture, EPCs were treated with different doses of cMVs for 24 h. As seen in Fig. 4_A_, cMVs from uncontrolled diabetes dose-dependently increased the apoptotic rate of EPCs from healthy controls. Based on these data, we chose dilution 1:1 for the coculture experiments (Fig. 3). Moreover, we found that co-incubation with cMVs from uncontrolled diabetes decreased the migration ability but increased apoptotic rate of EPCs from healthy controls (P < 0.01 vs. vehicle, P < 0.05 or 0.01 vs. cMVs from well-controlled diabetes; Fig. 4_B_). These changes were accompanied with the increase of high ROS production population (P < 0.01) and decrease of low ROS production population (P < 0.01; Fig. 4_B_). However, the cMVs from well-controlled diabetes did not have effects on healthy EPC migration, apoptosis, or ROS production. These data indicate that cMVs from uncontrolled diabetes could impair the function and survival of heathy EPCs by increasing oxidative stress.
Fig. 4.
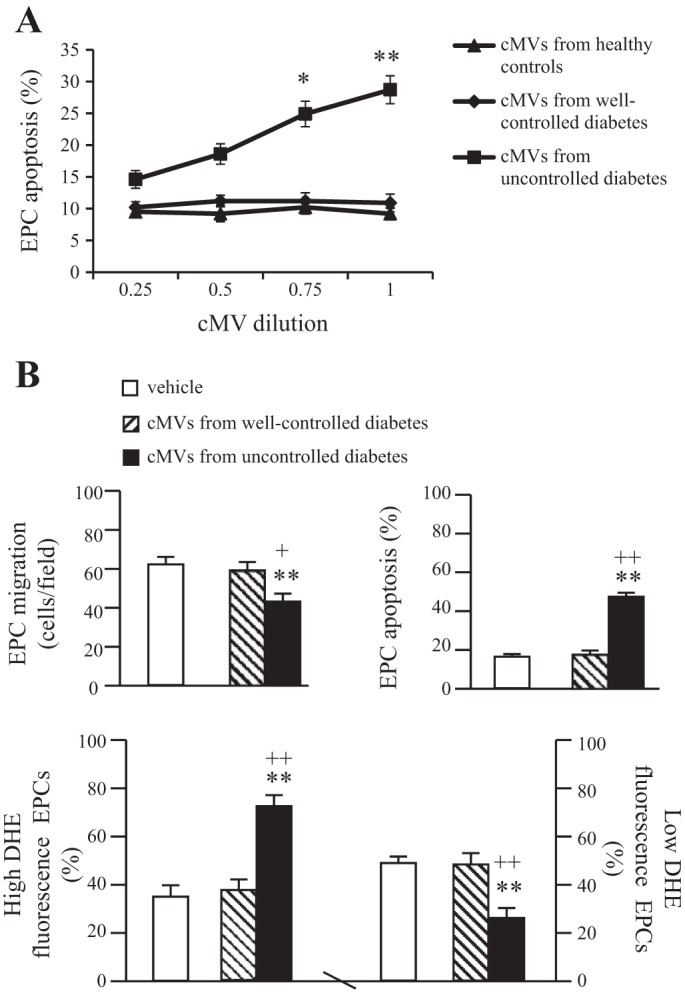
Coculture study of cMVs from uncontrolled diabetic patients with EPCs from healthy controls. A: dose-dependent effect of cMVs from HC and diabetic patients on heathy EPC apoptotic rate; n = 3. *P < 0.05 and **P < 0.01 vs. 0.25. B: effects of cMVs from diabetic patients on healthy EPC migration, apoptotic rate, and ROS production; n = 6. **P < 0.01 vs. vehicle, +P < 0.05 and ++P < 0.01 vs. cMVs from well-controlled diabetic patients.
The protective effects of cMVs from healthy controls on the migration, apoptosis, and ROS production of EPCs from uncontrolled diabetes.
As shown in Fig. 5, coculture with cMVs from healthy controls significantly improved the migration ability and decreased the apoptotic rate of EPCs from uncontrolled diabetes (P < 0.01 vs. vehicle; Fig. 5, A and B). These changes were accompanied with the decrease of the high ROS production (P < 0.05) and the increase of the low ROS production (P < 0.05 vs. vehicle; Fig. 5_C_). These data indicate that cMVs from healthy controls could improve the function and survival of EPCs from uncontrolled diabetes by decreasing oxidative stress.
Fig. 5.
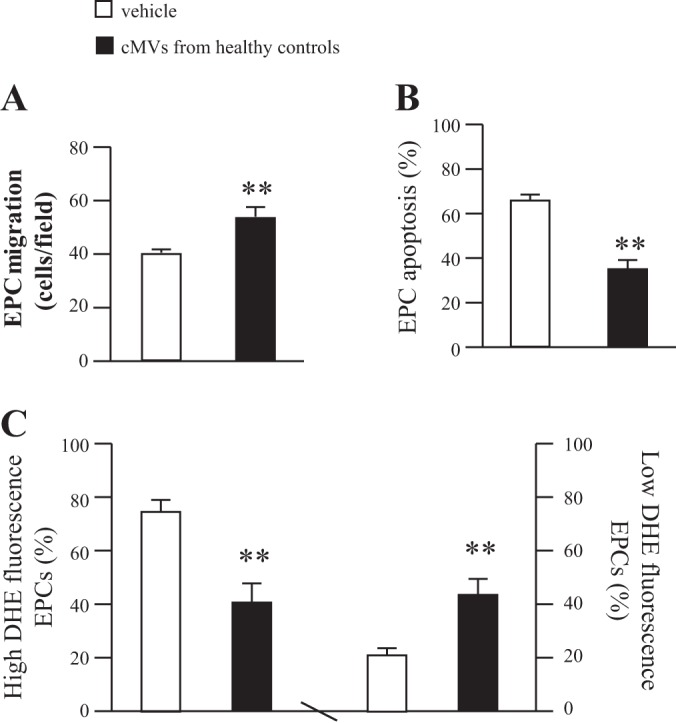
Coculture study of cMVs from HC with EPCs from uncontrolled diabetic patients. A: effects of cMVs from HC on the migration of EPCs from uncontrolled diabetic patients. B: effects of cMVs from HC on the apoptotic rate of EPCs from uncontrolled diabetic patients. C: the effects of cMVs from HC on the ROS production of EPCs from uncontrolled diabetic patients; n = 6. **P < 0.01 vs. vehicle.
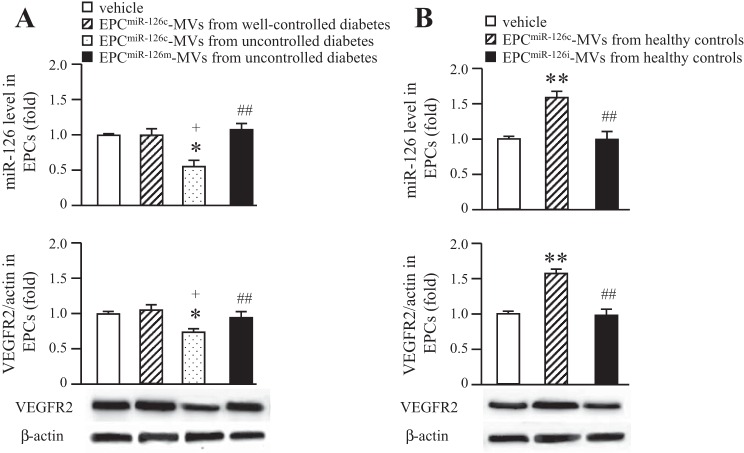
The expressions of miR-126 in cMVs and EPC-MVs.
The miR-126 expression was decreased in the cMVs from uncontrolled diabetes (Fig. 6_A_). However, there was no significant difference in miR-126 expression between cMVs from healthy controls and well-controlled diabetes (Fig. 6_A_). Moreover, we isolated EPC-MVs from EPCs cultured from healthy controls or diabetic patients and found that the miR-126 level was decreased in EPC-MVs released from EPCs of uncontrolled diabetes (Fig. 6_B_). miR-126 inhibitor transfection on healthy EPCs downregulated miR-126 levels in their released EPC-MVs, whereas the miR-126 levels mimic transfection on EPCs from uncontrolled diabetes upregulated the miR-126 level in their released EPC-MVs.
Fig. 6.
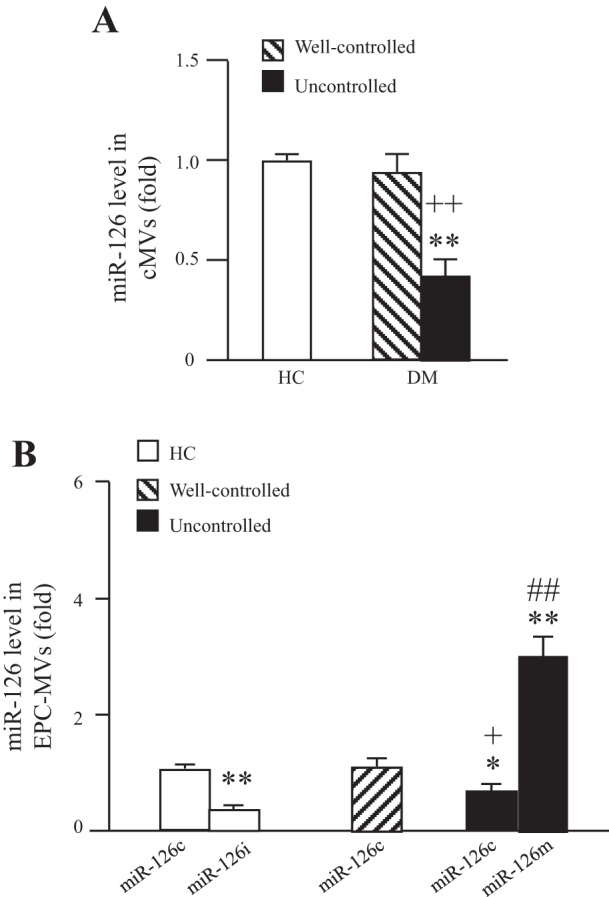
The expression of miR-126 in cMVs and EPC-MVs. A: expression of miR-126 in cMVs isolated HC and well-controlled or uncontrolled DM. B: miR-126 expression in EPC-MVs released from EPCs of HC and well-controlled or uncontrolled DM. EPCs were transfected with miR-126 control, mimic, or inhibitor; n = 6. *P < 0.05 and **P < 0.01 vs. cMVs or EPC-MVs from healthy controls; +P < 0.05 and ++P <0.01 vs. cMVs or EPC-MVs from well-controlled diabetes; #P < 0.05 and ##P < 0.01 vs. cMVs or EPC-MVs from uncontrolled diabetes.
The expressions of miR-126 and VEGFR2 in EPCs.
EPCs were cultured from healthy controls, well-controlled patients, and uncontrolled diabetic patients to generate EPC-MVs and used for coculture. Results showed that both miR-126 and VEGFR2 levels were decreased in heathy EPCs after coculture with EPC-MVs released from EPCs of uncontrolled diabetes (Fig. 7_A_). On the contrary, the miR-126 and VEGFR2 levels were increased in uncontrolled diabetic EPCs after coculture with EPC-MVs released from EPCs of healthy controls (Fig. 7_B_). Coincubation with EPC-MVs released from EPCs of well-controlled diabetes did not change the levels of miR-126 or VEGFR2 in healthy EPCs. Moreover, EPCmiR-126m-MVs from uncontrolled diabetes were able to increase the miR-126 and VEGFR2 levels in heathy EPCs, whereas EPCmiR-126i-MVs from healthy controls were able to decrease the miR-126 and VEGFR2 levels in EPCs from uncontrolled diabetes.
Fig. 7.
Expressions of miR-126 and VEGFR2 in EPCs after coincubation with EPC-MVs. A: expressions of miR-126 and VEGFR2 in EPCs from HC after coculture with EPC-MVs released from EPCs of well-controlled or uncontrolled diabetes. Data are expressed as a fold change of vehicle; n = 6. *P < 0.05 vs. vehicle; +P < 0.05 vs. EPCmiR-126c-MVs from well-controlled diabetes; ##P < 0.01 vs. EPCmiR-126c-MVs from uncontrolled diabetes. B: EPC-MVs released from EPCs of HC increase the expression of miR-126 and VEGFR2 in EPCs from uncontrolled diabetes, which can be reversed by miR-126i transfection. Data are expressed as a fold change of vehicle; n = 6. **P < 0.01 vs. vehicle; ##P < 0.01 vs. EPCmiR-126c-MVs from HC.
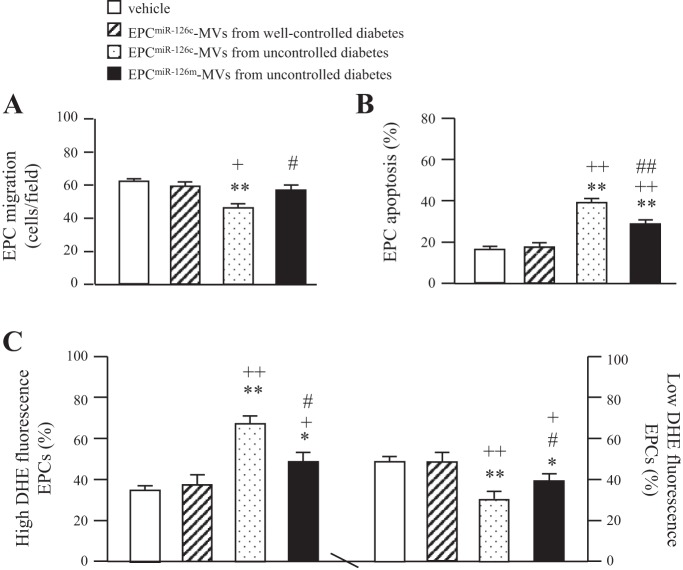
The detrimental effects of EPC-MVs released from EPCs of uncontrolled diabetes on the migration, apoptosis, and ROS production of EPCs from healthy controls.
Coculture with EPC-MVs from uncontrolled diabetes decreased the migration ability but increased apoptotic rate of heathy EPCs (P < 0.01 vs. vehicle, P < 0.05 or 0.01 vs. EPC-MVs from well-controlled diabetes; Fig. 8, A and B). These changes were accompanied with the increase in high ROS production population and decrease in low ROS production population (Fig. 8_C_). However, the EPC-MVs released from EPCs of well-controlled diabetes did not have effects on heathy EPC migration, apoptosis, or ROS production (Fig. 8). These data indicate that EPC-MVs released from EPCs of uncontrolled diabetes could impair the function and survival of heathy EPCs by increasing ROS production. Interestingly, EPCmiR-126m-MVs from uncontrolled diabetes with miR-126 upregulation in EPC-MVs significantly decreased their detrimental effects on healthy EPCs (Fig. 8).
Fig. 8.
Effects of EPC-MVs released from EPCs of uncontrolled diabetes on the EPCs from HC. A: the effects of EPC-MVs released from EPCs of uncontrolled diabetes on the migration of EPCs from HC. B: effects of EPC-MVs released from EPCs of uncontrolled diabetes on the apoptotic rate of EPCs from HC. C: effects of EPC-MVs released from EPCs of uncontrolled diabetes on the ROS production of EPCs from HC; n = 6. *P < 0.05 and **P < 0.01 vs. vehicle; +P < 0.05 and ++P < 0.01 vs. EPCmiR-126c-MVs from well-controlled diabetes; #P < 0.05 and ##P < 0.01 vs. EPCmiR-126c-MVs from uncontrolled diabetes.
The protective effects of EPC-MVs released from EPCs of healthy controls on the migration, apoptosis, and ROS production of EPCs from uncontrolled diabetes.
As shown in Fig. 9, coculture with EPC-MVs released from EPCs of healthy controls significantly improved the migration ability and decreased the apoptotic rate of EPCs from uncontrolled diabetes compared with vehicle (Fig. 9, A and B). These changes were accompanied with the decrease in high ROS production population and the increase in low ROS production population (Fig. 9_C_). These data indicate that EPC-MVs released from EPCs of healthy controls could improve the function and survival of EPCs from uncontrolled diabetes by increasing ROS production. More importantly, EPCmiR-126-MVs from healthy controls with miR-126 downregulation in EPC-MVs could diminish their protective effects on EPCs from uncontrolled diabetes. These data suggest that EPC-MVs could be the modulator for EPC function, apoptosis, and oxidative stress via their carried miR-126.
Fig. 9.
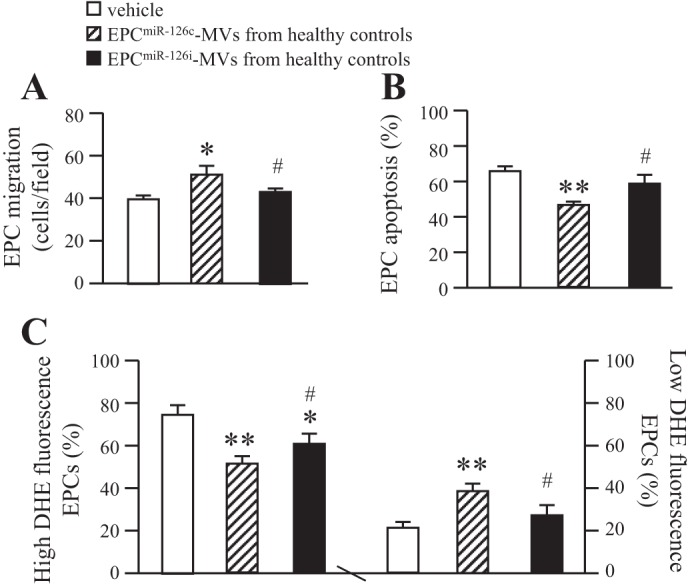
Effects of EPC-MVs released from EPCs of HC on the EPCs from uncontrolled diabetes. A: effects of EPC-MVs released from EPCs of HC on the migration of EPCs from uncontrolled diabetes. B: effects of EPC-MVs released from EPCs of HC on the apoptotic rate of EPCs from uncontrolled diabetes. C: effects of EPC-MVs released from EPCs of HC on the ROS production of EPCs from uncontrolled diabetes; n = 6. *P <0.05 and **P <0.01 vs. vehicle; #P <0.05 vs. EPCmiR-126c-MVs from HC.
DISCUSSION
EPCs play an important role in maintaining endothelium hemostasis and integrity. Our previous studies found that circulating EPCs are decreased and bone marrow-derived EPCs dysfunctional in db/db type 2 diabetic mice (7, 8). In the present study, we confirmed the decrease and dysfunction in EPCs in uncontrolled diabetic patients (Figs. 1 and 2). However, the underlying mechanism responsible for EPC dysfunction in diabetes still needs clarification. Previous studies have shown that high glucose could cause EPC apoptosis and dysfunction (7, 19). Interestingly, we demonstrated previously that cMVs from the db/db diabetic mice induce dysfunction of EPCs from normal control mice (8). cMVs may be a biomarker or predictor for vascular diseases (2, 8, 25, 30) as well as functional by transferring the content from parent cells or tissue to distant cells (5, 29). Moreover, cMVs have distinguished function based on the stimulator and the origin of MV release (3). This suggests that cMVs could be a factor modulating EPC function in diabetes according to their origins. Our present study extends the investigation to human subjects.
In the current study, we conducted a cross study to investigate the role of cMVs in modulating the EPC function in diabetic patients. We cocultured EPCs from uncontrolled diabetes with cMVs from healthy controls and healthy EPCs with cMVs from well-controlled or uncontrolled diabetes. Interestingly, we found that cMVs from uncontrolled diabetes have negative effects on healthy EPCs by decreasing migration ability and increasing apoptosis and ROS production, (Figs. 4 and 5). This is in agreement with our previous study in a db/db type 2 diabetic animal model (8). Indeed, migration process plays an important role in diabetic vascular complication by enhancing EPC's ability to restore endothelium. On the other hand, apoptosis and enhanced oxidative stress are two key alterations responsible for EPC number and function reduction in diabetes and cardiovascular diseases (16, 20, 31). Our data indicate that MVs may link these processes and adversely affect EPC numbers and functions in uncontrolled diabetic patients.
In addition to identifying the function of cMVs on EPCs, we investigated the role of EPC-MVs in diabetes. We found that the level of circulating EPC-MVs is increased in uncontrolled diabetic patients. Circulating EPC-MVs have been reported to be the indicator of impairment or incompetence in EPCs (12). A possible interpretation for the decrease in EPC numbers and increase in EPC-MV level is a higher rate of EPC breakdown into EPC-MVs in diabetes. Although we know that the functions of EPCs are impaired in diabetes, it is unknown whether the functions of EPC-MVs are also impaired. We have demonstrated previously that EPC-MVs have beneficial functions on ECs and cardiomyocytes (14, 34). Recent studies also suggest that EPC-MVs promote EC survival and proliferation and enhance EC function (12, 38). These studies provide a rational basis for our hypothesis that EPC-MVs might play an important role in mediating the EPC functions in diabetes. To explore the role of EPC-MVs in modulating EPC function in diabetes, we generated EPC-MVs from cultured EPCs of diabetic patients and healthy controls and then performed cross studies cocultured with EPCs. Interestingly, we found that the EPC-MVs had similar effects as cMVs on EPCs. Hence, we believe that EPC-MVs might be another mediator of modulating EPC survival and function in diabetes.
Furthermore, we showed the ability of cMVs and EPC-MVs to alter EPC survival and functions depending on their origins. cMVs and EPC-MVs from uncontrolled diabetes decreased EPC survival and functions; in contrast, cMVs and EPC-MVs from healthy controls improved the survival and functions of EPCs from uncontrolled diabetic patients. These data suggest that the origin of MVs and the environment involved in their release are considered the most critical factors that determine the function of MVs. We believe that the different functions might be due to the different contents they carried. Our recent work shows the expression of miR-126 in EPC-MVs, and its high expression is accompanied by the protective effects of EPC-MVs on ECs (34). In the present study, we found that the expression of miR-126 is decreased in cMVs from uncontrolled diabetes and EPC-MVs released from EPCs of uncontrolled diabetes (Fig. 6). EPC-MVs have been reported to promote angiogenesis through the transfer of miR-126 to target cells (6, 28). miR-126 has been reported to regulate angiogenic process and EC/EPC function by targeting VEGFR2-related signal transduction (13, 15). In the present study, we found that the miR-126 and VEGFR2 expressions are increased in EPCs from uncontrolled diabetes after coincubation with EPC-MVs released from EPCs of healthy controls, whereas they are decreased in healthy EPCs after coincubation with EPC-MVs released from EPCs of uncontrolled diabetes (Fig. 7), suggesting the role of EPC-MVs in the transfer of miR-126 and activation of the downstream VEGFR2 pathway in EPCs. A recent study reported that modulation of miR-126 by anti-miR-126 or miR-mimic-126 treatment resulted in significant loss of or an increase in proangiogenic effects of CD34(+) PBMCs in diabetes (24). To further confirm the role of miR-126 in mediating the function of EPC-MVs, we modified the EPC-MVs by transfecting EPCs with miR-126 mimic or inhibitor to generate miR-126-upregulated or -downregulated EPC-MVs. A functional study found that the miR-126 mimic diminished the detrimental effects of EPC-MVs released from EPCs of uncontrolled diabetes on healthy EPCs, whereas the miR-126 inhibitor decreased the protective effects of EPC-MVs released from EPCs of healthy controls on EPCs from uncontrolled diabetes (Figs. 8 and 9). For exploring the mechanisms responsible for these effects, we observed the ROS production of EPCs after different MV treatment. It has become widely accepted that enhanced oxidative stress is responsible for EPC number and function reduction in diabetes and cardiovascular diseases (16, 31). MVs could be the link in this process; they might be involved in the regulation of ROS production. Burger and Touyz (3) reported that MVs are able to modulate ROS production; both the stimulator and the origin of MV release play crucial roles in this process. Moreover, miR-126 has been reported to have implications in oxidative stress. Overexpression of miR-126 decreased ROS production and apoptosis in ECs (36). These data suggest that the function of EPC-MVs on EPCs is mediated by their carried miR-126 and that oxidative stress might be one of the mechanisms responsible for EPC function changes.
Our studies indicate that the cMVs and EPC-MVs of uncontrolled diabetic patients may be the factors to induce the reduction of EPC numbers and functions, which is due to the reduction of their carried miR-126. On the contrary, cMVs and EPC-MVs from healthy controls may improve survival and function of EPC from uncontrolled diabetic patients. This may lead to a novel therapeutic agent capable of reducing diabetic vascular complications.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (HL-098637; Y. Chen) and the National Natural Science Foundation of China (nos. 81300079, 81270195, and 81570259).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.W., Y.Y., Y.Z., H.M.A., P.Z., R.G., H.L., C.C., and J.C.B. performed experiments; K.W., Y.Y., Y.Z., H.M.A., P.Z., R.G., H.L., C.C., and J.C.B. analyzed data; K.W., Y.Z., H.M.A., P.Z., R.G., H.L., C.C., and J.C.B. interpreted results of experiments; K.W., Y.Y., Y.Z., H.M.A., and J.C.B. prepared figures; Y.Y., Y.Z., and J.C.B. drafted manuscript; T.M.K., Y.C., S.L., and J.C.B. conception and design of research; T.M.K., Y.C., S.L., and J.C.B. edited and revised manuscript; T.M.K., Y.C., S.L., and J.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Gengxin Li (Department of Mathematics and Statistics, Wright State University) for helping with data analysis.
REFERENCES
- 1.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34, Suppl 2: S285–S290, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 31: 1898–1907, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens 6: 85–99, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Cai HY, Li L, Guo T, Wang YU, Ma TK, Xiao JM, Zhao L, Fang Y, Yang P, Zhao HU. Cardiac shockwave therapy improves myocardial function in patients with refractory coronary artery disease by promoting VEGF and IL-8 secretion to mediate the proliferation of endothelial progenitor cells. Exp Ther Med 10: 2410–2416, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C, Camussi G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82: 412–427, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Chen J, Chen S, Zhang C, Zhang L, Xiao X, Das A, Zhao Y, Yuan B, Morris M, Zhao B, Chen Y. Transfusion of CXCR4-primed endothelial progenitor cells reduces cerebral ischemic damage and promotes repair in db/db diabetic mice. PLoS One 7: e50105, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Chen S, Chen Y, Zhang C, Wang J, Zhang W, Liu G, Zhao B, Chen Y. Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: possible implications in cerebral ischemic damage. Am J Physiol Endocrinol Metab 301: E62–E71, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Xiao X, Chen S, Zhang C, Chen J, Yi D, Shenoy V, Raizada MK, Zhao B, Chen Y. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension 61: 681–689, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Zhao Y, Chen S, Wang J, Xiao X, Ma X, Penchikala M, Xia H, Lazartigues E, Zhao B, Chen Y. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology 79: 550–558, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JJ, Zhou SH. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol J 18: 675–681, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Deregibus MC, Cantaluppi V, Calogero R, Lo IM, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110: 2440–2448, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15: 272–284, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu S, Zhang W, Chen J, Ma R, Xiao X, Ma X, Yao Z, Chen Y. EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS One 9: e85396, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen TF, Andersen CL, Nielsen BS, Spindler KL, Sorensen FB, Lindebjerg J, Brandslund I, Jakobsen A. Elevated microRNA-126 is associated with high vascular endothelial growth factor receptor 2 expression levels and high microvessel density in colorectal cancer. Oncol Lett 2: 1101–1106, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol 24: 2021–2027, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14: 249–256, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK, Nickenig G, Werner N. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 128: 2026–2038, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Krankel N, Adams V, Linke A, Gielen S, Erbs S, Lenk K, Schuler G, Hambrecht R. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol 25: 698–703, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lin CP, Lin FY, Huang PH, Chen YL, Chen WC, Chen HY, Huang YC, Liao WL, Huang HC, Liu PL, Chen YH. Endothelial progenitor cell dysfunction in cardiovascular diseases: role of reactive oxygen species and inflammation. Biomed Res Int 2013: 845037, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda H, Tanaka R, Fujimura S, Ishikawa M, Akimaru H, Shizuno T, Sato A, Okada Y, Iida Y, Itoh J, Itoh Y, Kamiguchi H, Kawamoto A, Asahara T. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J Am Heart Assoc 3: e000743, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 107: 1047–1057, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol 53: 64–72, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Mocharla P, Briand S, Giannotti G, Dorries C, Jakob P, Paneni F, Luscher T, Landmesser U. AngiomiR-126 expression and secretion from circulating CD34(+) and CD14(+) PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood 121: 226–236, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Nomura S. Dynamic role of microparticles in type 2 diabetes mellitus. Curr Diabetes Rev 5: 245–251, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Petrelli A, Maestroni A, Fadini GP, Belloni D, Venturini M, Albiero M, Kleffel S, Mfarrej BG, Maschio AD, Maffi P, Avogaro A, Ferrero E, Zerbini G, Secchi A, Fiorina P. Improved function of circulating angiogenic cells is evident in type 1 diabetic islet-transplanted patients. Am J Transplant 10: 2690–2700, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Pirro M, Schillaci G, Bagaglia F, Menecali C, Paltriccia R, Mannarino MR, Capanni M, Velardi A, Mannarino E. Microparticles derived from endothelial progenitor cells in patients at different cardiovascular risk. Atherosclerosis 197: 757–767, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, Deregibus MC, Tetta C, Segoloni GP, Camussi G. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol 25: 75–85, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20: 1487–1495, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, Boulanger CM. Microparticles, vascular function, and atherothrombosis. Circ Res 109: 593–606, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Rossig L, Urbich C, Dimmeler S. Endothelial progenitor cells at work: not mature yet, but already stress-resistant. Arterioscler Thromb Vasc Biol 24: 1977–1979, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Zhang M, Sanagawa A, Mori C, Ito S, Iwaki S, Satoh H, Fujii S. Circulating microRNA-126 in patients with coronary artery disease: correlation with LDL cholesterol. Thromb J 10: 16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes 56: 666–674, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Chen S, Ma X, Cheng C, Xiao X, Chen J, Liu S, Zhao B, Chen Y. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid Med Cell Longev 2013: 572729, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15: 261–271, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Wang F, Wu Y, Zuo L, Zhang S, Zhou Q, Wei W, Wang Y, Zhu H. MicroRNA-126 attenuates palmitate-induced apoptosis by targeting TRAF7 in HUVECs. Mol Cell Biochem 399: 123–130, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Xiao X, Ma X, Liu L, Wang J, Bi K, Liu Y, Fan R, Zhao B, Chen Y, Bihl JC. Cellular Membrane Microparticles: Potential Targets of Combinational Therapy for Vascular Disease. Curr Vasc Pharmacol 13: 449–458, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, Kalka-Moll W, Baumgartner I, Di SS, Kalka C. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis 211: 103–109, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de CE, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol 28: 1627–1633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]