Phase I and Pharmacologic Study of SNS-032, a Potent and Selective Cdk2, 7, and 9 Inhibitor, in Patients With Advanced Chronic Lymphocytic Leukemia and Multiple Myeloma (original) (raw)
Abstract
Purpose
SNS-032 is a highly selective and potent inhibitor of cyclin-dependent kinases (Cdks) 2, 7, and 9, with in vitro growth inhibitory effects and ability to induce apoptosis in malignant B cells. A phase I dose-escalation study of SNS-032 was conducted to evaluate safety, pharmacokinetics, biomarkers of mechanism-based pharmacodynamic (PD) activity, and clinical efficacy.
Patients and Methods
Parallel cohorts of previously treated patients with chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) received SNS-032 as a loading dose followed by 6-hour infusion weekly for 3 weeks of each 4-week course.
Results
There were 19 patients with CLL and 18 with MM treated. Tumor lysis syndrome was the dose-limiting toxicity (DLT) for CLL, the maximum-tolerated dose (MTD) was 75 mg/m2, and the most frequent grade 3 to 4 toxicity was myelosuppression. One patient with CLL had more than 50% reduction in measurable disease without improvement in hematologic parameters. Another patient with low tumor burden had stable disease for four courses. For patients with MM, no DLT was observed and MTD was not identified at up to 75 mg/m2, owing to early study closure. Two patients with MM had stable disease and one had normalization of spleen size with treatment. Biomarker analyses demonstrated mechanism-based PD activity with inhibition of Cdk7 and Cdk9, decreases in Mcl-1 and XIAP expression level, and associated CLL cell apoptosis.
Conclusion
SNS-032 demonstrated mechanism-based target modulation and limited clinical activity in heavily pretreated patients with CLL and MM. Further single-agent, PD-based, dose and schedule modification is warranted to maximize clinical efficacy.
INTRODUCTION
B-cell malignancies such as chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) are characterized by accumulation of malignant cells resistant to apoptosis. Resistance to apoptosis is hypothesized to be due to overexpression of the Bcl-2 family of antiapoptotic proteins. Indeed, reduction in these proteins in vitro in primary CLL or MM cells through use of oligonucleotides1,2 or interference with their function using small molecule inhibitors3–6 resulted in rapid onset of apoptosis. The recognition that antiapoptotic Bcl-2 family members, such as Mcl-1, have short-lived transcripts and proteins, together with the observation that some cyclin-dependent kinase (Cdk) inhibitors in clinical development potently inhibited Cdk9 and Cdk7, led to the strategy of transient inhibition of transcription as a means of initiating apoptosis in malignant B cells. Cdk9 and its cyclin partner, cyclinT1, are highly expressed and play important survival roles in CLL and MM.7,8 Indeed, inhibition of these Cdks by flavopiridol,9 SNS-032,10 or R-roscovitine11 quickly reduced transcription, resulting in the rapid reduction of Mcl-1 and induction of apoptosis in primary CLL cells.
In addition to transcriptional control, the Cdk family of protein serine/threonine kinases includes key regulators of cell cycle progression.12,13 Cdk2 controls entry and progression through the DNA synthesis phase of the cell cycle and is altered in many cancer types.14 Aberrant expression of key regulators of Cdk2, such as cyclin E and p27, is associated with a poor prognosis and shorter survival in patients with cancer.15
Flavopiridol was the first Cdk inhibitor to enter clinical trial. Despite promising in vitro activity,16,17 initially, flavopiridol demonstrated no significant clinical activity in phase I/II studies in patients with relapsed CLL.18,19 Subsequent investigations revealed significant binding to human plasma proteins that altered free drug level, target cell exposure, and therefore therapeutic activity.20,21 Subsequently, a pharmacokinetic (PK) -derived schedule of a 30-minute intravenous bolus followed by 4-hour continuous infusion that sustained half maximal inhibitory concentration levels achieved a 50% response rate in patients with refractory CLL.22,23
SNS-032 (BMS-387032) is a synthetic small-molecule inhibitor of Cdks 2, 7, and 9 that exhibited potent in vitro cytotoxicity against cancer cell lines and primary cancer cells that was associated with down-modulation of Mcl-1.10,24,25 SNS-032 effectively killed primary CLL cells in vitro, independent of prognostic factor status or patient treatment history.10 This was associated with rapidly reversible inhibition of phosphorylation of RNA polymerase II (Pol II) and rapid downregulation of Mcl-1 and XIAP. SNS-032 was more potent at inhibiting RNA synthesis and inducing apoptosis than flavopiridol. Activity of SNS-032 was confirmed in vivo with human leukemia (HL-60 and MV 4-11) and MM (RPMI-8226) xenograft tumor mouse models.25 Previously, three phase I trials of SNS-032 given as either 1- or 24-hour infusions to patients with metastatic refractory solid tumors or refractory lymphoma demonstrated tolerability, but did not show significant antitumor activity.24,26
We conducted a phase I clinical study to assess the safety and tolerability of escalating doses of SNS-032, administered as a 5-minute loading dose (LD) followed by a 6-hour maintenance infusion weekly for 3 weeks of each 4-week course in patients with advanced CLL and MM. The dose schedule was based on PK data derived from previous clinical studies with SNS-032 aiming to maintain or exceed a target threshold plasma concentration of 115 ng/mL (0.3 μmol/L) for 6 hours, which equals the concentration that inhibits 90% determined in in vitro assays.10,24,26
PATIENTS AND METHODS
The trial was approved by the institutional review boards of all five participating institutions; all patients provided written informed consent. The study was conducted according to the Declaration of Helsinki.
Patients
Eligible patients had histologically confirmed CLL or MM; were ≥ 18 years of age; had an Eastern Cooperative Oncology Group performance status of 0 to 2; and had adequate hepatic and renal function. Patients with CLL had measurable, relapsed disease after one or more prior treatment regimens. Patients with secretory MM had serum paraprotein more than 0.5 g/dL or 24-hour urine paraprotein more than 0.2 gm; patients with nonsecretory MM had detectable free serum light chain or plasmacytoma measurable by computed tomography or magnetic resonance imaging scan. Patients with MM also had two or more prior therapies, including (1) thalidomide, bortezomib, or lenalidomide and (2) autologous stem-cell transplantation. Patients with the following were excluded: prior SNS-032; thromboembolic event within 28 days; QT interval corrected for heart rate using Frederica's method more than 500 milliseconds; prior pelvic radiation or radiation to more than 25% of bone marrow reserve; or WBC count more than 200,000/μL, absolute neutrophil count less than 500/μL, hemoglobin less than 8.5 g/dL, or platelet less than 30,000/μL.
Trial Design and Treatment Plan
The trial was planned as a two-stage, open-label, multicenter study of escalating doses of SNS-032 in parallel dose cohorts of patients with CLL and MM. SNS-032 was given as a 5-minute LD followed by a 6-hour infusion weekly for 3 consecutive weeks of each 4-week course. The primary end point was to determine dose-limiting toxicities (DLT) and identify a maximum-tolerated dose (MTD) for both cohorts. DLTs and MTD were identified during course 1; patients could continue courses if they did not experience DLT. Secondary end points were to estimate PK parameters and evaluate pharmacodynamic (PD) biomarkers.
Stage I was a standard 3 + 3 dose escalation according to Table 1. No dose modification was allowed during course 1. Dose delays were allowed for subsequent courses; patients must have received all three doses for course 1 within 28 days. Dose escalation occurred in the absence of protocol-defined DLT, until identification of the MTD. Adverse events (AEs) were identified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. A DLT must have been an SNS-032 treatment–related event that occurred during course 1, including absolute neutrophil count less than 500/μL or platelet less than 30,000/μL lasting more than 14 days or not recovering to within 20% of baseline beyond day 28 of course 1; liver function test or electrolyte abnormality ≥ grade 3 persisting more than 2 days; acute tumor lysis syndrome (TLS) requiring hemodialysis; any nonhematologic AE ≥ grade 3; any abnormal nonhematologic laboratory value or AE requiring a dose delay of more than 14 days for recovery; inability to receive all three doses for course 1 within 28 days due to clinically significant abnormal laboratory values and/or nonhematologic AEs; or death. The MTD was defined as the dose at which fewer than two of six patients experienced DLT.
Table 1.
SNS-032 Dose Levels and Number of Patients Treated
| Cohort | Dose (mg/m2) | CLL (n = 19) | MM (n = 18) | ||
|---|---|---|---|---|---|
| LD | 6-Hour Infusion | Total | |||
| 1 | 5 | 10 | 15 | 0 | 3 |
| 2 | 10 | 12 | 22 | 2 | 1 |
| 3 | 10 | 23 | 33 | 5 | 4 |
| 4 | 10 | 40 | 50 | 3 | 5 |
| 5 | 10 | 65 | 75 | 6 | 5 |
| 6 | 10 | 90 | 100 | 3 | 0 |
Prophylaxis for TLS was required for all patients with CLL for course 1, dose 1, and included admission to the inpatient service with cardiac monitoring, oral allopurinol 300 mg daily beginning 48 hours, and intravenous hydration beginning 24 hours before SNS-032 drug administration. For the first dose, vital signs were obtained before the 5-minute LD; at 30 and 60 minutes; at 2, 3, 4, 5, and 6 hours after starting the 6-hour maintenance infusion; at 1 hour after completing the 6-hour infusion; and thereafter every 2 hours until discharge. In the absence of TLS during the first dose, vital signs were obtained for all subsequent infusions before starting the 5-minute LD and 15 minutes after completing the 6-hour infusion. If TLS occurred anytime after the first dose, rasburicase could be given and TLS prophylaxis was required, regardless of TLS severity. If TLS did not occur with dose 1, prophylaxis could subsequently be omitted. Because TLS was not expected in MM, inpatient precautions were not mandated, but all patients with MM received intravenous hydration, oral allopurinol, and close outpatient monitoring with each dose.
Response to treatment was assessed before the start of each course and at the end of treatment. Response was assessed using 1996 National Cancer Institute–Sponsored Working Group criteria27 for CLL and European/International Bone Marrow Transplantation Registry guidelines28 for MM. Patients with evidence of stable disease or better at the end of course 3 could receive three additional courses of treatment. Formal response assessment occurred every three courses, with continued treatment for stable disease or better.
PK
Blood samples were obtained on day 1 of course 1 at predose, end of LD, and 0.25, 0.5, 2, 4, 6, 8, 12, 24 and 30 hours after infusion. Plasma SNS-032 concentrations were determined by a validated liquid chromatography tandem mass spectrometry assay with a lower limit of quantification of 0.5 ng/mL. PK parameters were calculated by noncompartmental analysis using WINNonlin (Scientific Consultant, Apex, NC) software, version 5.2 (Pharsight, Mountain View, CA).
PD
Mechanism-based biomarkers of SNS-032 PD activity were measured in blood mononuclear cells from 31 treated patients (19 with CLL) collected at the initial screen up to 1 hour before the 5-minute LD and at 2, 6, and 24 hours after starting the 6-hour maintenance infusion on course 1, day 1. The biomarkers evaluated were phosphorylation state of the C-terminal domain of the RNA Pol II; levels of the antiapoptotic proteins Mcl-1, Bcl-2, and XIAP; and cleavage of poly (adenosine diphosphate–ribose) polymerase (PARP) by immunoblotting as previously described in detail.10
RESULTS
Patient Characteristics
Characteristics of the 37 treated patients are summarized in Table 2. Patients in both cohorts were heavily pretreated, and virtually all patients with CLL (95%) had prior fludarabine therapy; 26% had fludarabine-refractory disease.
Table 2.
Patient Characteristics
| Characteristic | CLL (n = 19) | MM (n = 18) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 63 | 61 | ||
| Range | 45-82 | 45-82 | ||
| Male sex | 90 | 40 | ||
| Performance status | ||||
| 0 | 2 | 3 | ||
| 1 | 16 | 13 | ||
| 2 | 1 | 2 | ||
| Prior treatments | ||||
| Median | 4 | 9 | ||
| Range | 1-8 | 2-16 | ||
| WBC count, ×1,000/μL | ||||
| Median | 6.9 | 3.5 | ||
| Range | 2.4-112.5 | 1.8-7.2 | ||
| ANC, ×1,000/μL | ||||
| Median | 1.7 | 2.4 | ||
| Range | 0.7-12.6 | 0.8-5.4 | ||
| ALC, ×1,000/μL | ||||
| Median | 2.3 | 0.8 | ||
| Range | 0.7-105 | 0.3-2.7 | ||
| Platelets, ×1,000/μL | ||||
| Median | 87.5 | 135 | ||
| Range | 22-566 | 34-243 | ||
| Hemoglobin, g/dL | ||||
| Median | 11.9 | 10 | ||
| Range | 8.4-15 | 8.3-12 | ||
| β-2 microglobulin, mg/L | ||||
| Median | 5.1 | 4.2 | ||
| Range | 2.4-15.3 | 1.6-2812 | ||
| Prior stem-cell transplantation | ||||
| Allogeneic | 2 | 11 | 1 | 6 |
| Autologous | 17 | 94 | ||
| For patients with CLL | ||||
| Rai stage III to IV | 13 | 68 | ||
| Fludarabine refractory | 18 | 95 | ||
| Prior therapy | ||||
| FCR | 10 | 53 | ||
| FR | 5 | 26 | ||
| Alemtuzumab | 6 | 32 | ||
| CD38 positive by flow | 12 | 63 | ||
| FISH analysis, hierarchical, n = 13 | ||||
| 17p deletion | 3 | 23 | ||
| 11q deletion | 2 | 15 | ||
| Trisomy 12 | 0 | |||
| None | 4 | 31 | ||
| 13q deletion | 4 | 31 | ||
| For patients with MM | ||||
| Serum total protein, g/dL | ||||
| Median | 8.9 | |||
| Range | 5.8-14.2 | |||
| Serum M-spike, g/dL | ||||
| Median | 3.8 | |||
| Range | 0.14-8.8 | |||
| Prior therapy | ||||
| VAD | 11 | 61 | ||
| Dex/Thal | 16 | 89 | ||
| Bortezomib | 16 | 89 | ||
| Melphalan | 9 | 50 |
SNS-032 Dose Level and Associated Toxicities
The dose levels evaluated and number of patients treated at each level are summarized in Table 1. Patients with CLL received SNS-032 at 22 to 100 mg/m2 total per dose. Biochemical evidence of TLS was observed in four patients with CLL treated at 75 mg/m2, including one patient with grade 3 TLS. Grade 3 TLS was observed in two of three patients with CLL treated at 100 mg/m2 and was the DLT; no patients required dialysis, and there were no treatment-related deaths. Therefore, the MTD identified for patients with CLL was 75 mg/m2. SNS-032-related fever and fatigue were minimized with 100 mg of hydrocortisone pretreatment. Patients with MM received total SNS-032 from 15 to 75 mg/m2 per dose. For patients with MM, the MTD was not established, and no DLTs were observed. Further enrollment and dose escalation was not continued as a result of early closure of the study. Biochemical TLS was not observed in any patient with MM as a result of low risk for TLS in this group.
SNS-032 infusions were well-tolerated; Table 3 lists all patients with grade 3 to 4 adverse events, related and unrelated, for all courses by diagnosis. Myelosuppression, including neutropenia, thrombocytopenia, and anemia, was the most frequently observed toxicity during treatment and seemed to be more common in patients with MM (Table 3). Other grade 3 to 4 adverse events were uncommon and sporadic across organ-system categories. Generally, similar proportions of patients experienced grade 3 to 4 adverse events between CLL (74%) and MM (78%). Appendix Table A1 (online only) reports all related and unrelated grade 1 to 2 adverse events that occurred in more than 10% of patients across all treatment courses. The most common grade 1 to 2 adverse events were GI-related, including nausea, vomiting, constipation, and diarrhea.
Table 3.
All Grade 3 and 4 Toxicities Reported With SNS-032 Treatment
| Adverse Events | CLL (n = 19) | MM (n = 18) | ||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| No. of patients reporting toxicity | 14 | 74 | 14 | 78 |
| Hematologic | ||||
| Neutropenia | 3 | 16 | 7 | 39 |
| Anemia | 1 | 5 | 6 | 33 |
| Thrombocytopenia | 0 | 0 | 1 | 6 |
| Febrile neutropenia | 0 | 0 | 4 | 22 |
| Gastrointestinal/hepatic | ||||
| Abdominal pain | 2 | 11 | 0 | 0 |
| Diarrhea | 1 | 5 | 0 | 0 |
| Vomiting | 1 | 5 | 0 | 0 |
| Cholecystitis | 0 | 0 | 1 | 6 |
| Hyperbilirubinemia | 1 | 5 | 0 | 0 |
| Transaminitis (ALT/AST) | 2 | 11 | 0 | 0 |
| Renal/electrolyte abnormal | ||||
| Acute renal failure | 1 | 5 | 1 | 6 |
| Hypokalemia | 1 | 5 | 1 | 5 |
| Hypocalcemia | 1 | 5 | 0 | 0 |
| Hypercalcemia | 0 | 1 | 0 | |
| Hyperuricemia | 1 | 5 | 0 | 0 |
| Respiratory | ||||
| Hypoxia | 2 | 11 | 0 | 0 |
| Dyspnea | 1 | 5 | 0 | 0 |
| Lung infiltration | 1 | 5 | 0 | 0 |
| Cardiovascular | ||||
| Hypotension | 2 | 11 | 0 | 0 |
| Capillary leak syndrome | 1 | 5 | 0 | 0 |
| Nervous system | ||||
| Dizziness | 1 | 5 | 0 | 0 |
| Infection | ||||
| Pneumonia | 2 | 11 | 2 | 11 |
| Cellulitis | 0 | 1 | 6 | |
| Sepsis | 0 | 1 | 6 | |
| Streptococcal bacteremia | 0 | 1 | 6 | |
| Otitis media | 1 | 5 | 0 | 0 |
| Other | ||||
| Asthenia | 2 | 11 | 0 | 0 |
| Fatigue/weakness | 2 | 11 | 0 | 0 |
| Noncardiac chest pain | 1 | 5 | 0 | 0 |
| Dehydration | 1 | 5 | 0 | 0 |
| Tumor lysis syndrome | 4 | 21 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 1 | 6 |
Treatment-associated prolongation of QTc interval occurred in nine and eight of the patients with CLL and MM, respectively; all were asymptomatic and not clinically significant. Prolongation of QTc was not dose-dependent. The maximal QTc after infusion was less than 450 milliseconds for all nine patients with CLL and included seven patients with prolongation less than 30 milliseconds, one patient with 30 to 60 milliseconds, and one patient with more than 60 milliseconds of prolongation. In the eight patients with MM with QTc prolongation, seven patients had prolongation of less than 30 milliseconds, and one patient had more than 60 milliseconds of prolongation. The maximal QTc after infusion was less than 450 milliseconds in five patients, between 450 and 480 milliseconds in two patients, and between 480 and 500 milliseconds in one patient with MM.
PK Analysis
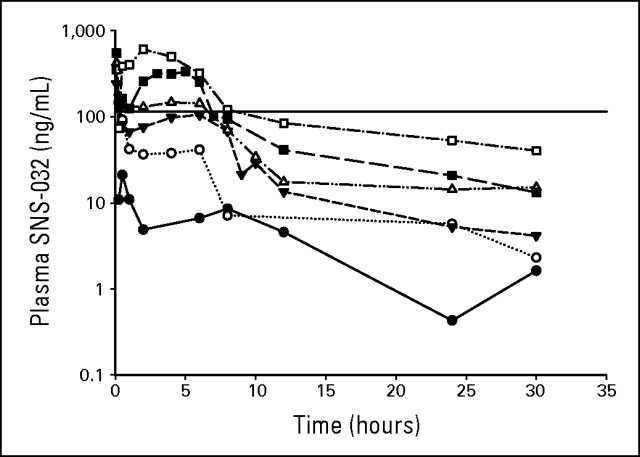
Plasma concentrations of SNS-032 at different dose levels indicated that target SNS-032 concentrations (≥ in vitro concentration that inhibits 90%, 115 ng/mL, approximately 300 nmol/L, for at least 6 hours) were achieved and exceeded with the 50 mg/m2 dose level (cohort 4) with maximum plasma concentration at 6 hours of 142.7 ng/mL (Fig 1). The maximum plasma concentration values at 6 hours were 251.5 and 319 ng/mL for the 75 mg/m2 (cohort 5) and 100 mg/m2 (cohort 6) dose levels, respectively (Table 4). These concentrations decreased rapidly to less than 115 ng/mL on completion of the 6-hour infusion. Greater than dose-proportional increase in exposure (area under the concentration versus time curve from time zero to infinity) was observed in both patients with CLL and MM, in contrast to prior studies of SNS-032 administered to patients with advanced solid tumors, in which PK values were dose proportional.26
Fig 1.
SNS-032 plasma concentration-time profiles. Mean pharmacokinetic profiles for all dose cohorts from patients with multiple myeloma and chronic lymphocytic leukemia are shown. Filled circles, 15 mg/m2, n = 3; open circles, 22 mg/m2, n = 2; filled inverted triangles, 33 mg/m2, n = 8; open triangles, 50 mg/m2, n = 7; filled squares, 75 mg/m2, n = 10; open squares, 100 mg/m2, n = 2; solid line, concentration that inhibits 90%.
Table 4.
Pharmacokinetic Parameters
| Dose (mg/m2) | No. of Patients | AUCINF (h × ng/mL) | CL (h × ng/mL) | Cmax 6 h (ng/mL) | T½ (hours) | MRTINF (hours) | Vss (L/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | Mean | CV% | ||
| 15 | 3 | 376 | 22 | 41.22 | 23 | 38.13 | 17 | 8.68 | 23 | 8.42 | 23 | 348 | 34 |
| 22 | 2 | 630 | 36.74 | 41.5 | 7.39 | 5.73 | 229 | ||||||
| 33 | 8 | 1,099 | 16 | 30.74 | 17 | 105.1 | 22 | 8.39 | 41 | 5.3 | 39 | 165 | 45 |
| 50 | 7 | 1,668 | 37 | 35.12 | 51 | 142.7* | 19 | 9.77 | 75 | 7.89 | 78 | 228 | 43 |
| 75 | 10 | 2,767 | 25 | 28.71 | 26 | 251.5 | 47 | 9.02 | 30 | 6.55 | 31 | 185 | 34 |
| 100 | 2 | 6,049 | 18.12 | 319 | 15.95 | 14.23 | 194 |
PD Analysis
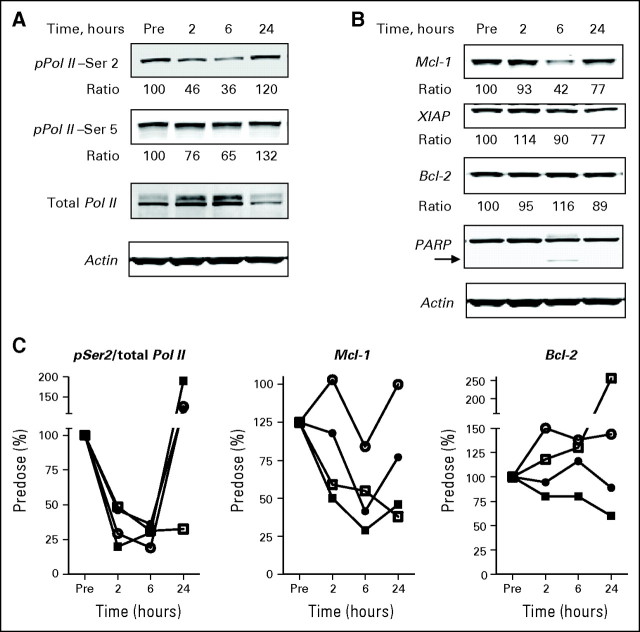
PD studies were possible in patients with CLL owing to the availability of circulating leukemia cells. The decrease in phosphorylation of Ser5 and Ser2 in the C-terminal domain of RNA Pol II during infusion of 75 mg/m2 total dose (Fig 2A) was indicative of inhibition of Cdk9 and Cdk7 by SNS-032. Inhibition was seen as early as 2 hours after starting the SNS-032 infusion and was pronounced after 6 hours, with a return to baseline after 24 hours. The inhibition of pSer2 (64%) was more significant than for pSer5 (35%) at 6 hours after SNS-032 infusion, consistent with a more potent half maximal inhibitory concentration for Cdk9 inhibition as compared with that for Cdk7 (4 v 62 nmol/L).
Fig 2.
Target modulation after SNS-032 infusion in patients with chronic lymphocytic leukemia. (A) Representative immunoblots from a patient treated with 75 mg/m2 of SNS-032. The ratio indicates the densitometry analysis between pPol II-Ser2 or pPol II-Ser5 and total Pol II, normalized to predose controls. (B) Representative immunoblots showing the protein levels of Mcl-1, XIAP, and Bcl-2 after SNS-032 infusion. The ratio indicates the densitometry analysis between Mcl-1, XIAP or Bcl-2, and actin, normalized to predose controls. Cleavage of poly (adenosine diphosphate–ribose) polymerase (PARP; arrow) was used as an indicator of apoptosis. (C) The summarized quantitations of phosphorylation of Ser2 at the C-terminal domain of RNA Pol II and protein levels of Mcl-1 and Bcl-2 for two patients treated with 75 mg/m2 (closed symbols) and two patients treated with 100 mg/m2 (open symbols) of SNS-032.
Transcriptional inhibition by SNS-032 was shown by marked downregulation of Mcl-1 protein level (56% at 6 hours) and modest downregulation of XIAP protein level (10% at 6 hours and 23% at 24 hours; Fig 2B). There was no significant decrease in Bcl-2 protein level after SNS-032 infusion. Apoptosis was detected by PARP cleavage seen 6 hours after the start of infusion. Similar target modulation was observed for patients 1504 (75 mg/m2), 1601, and 1602 (both at 100 mg/m2; Fig 2C), with more pronounced increase in PARP cleavage at 100 mg/m2 (not shown). A complete set of results were available for four of the nine patients with CLL at the 75 mg/m2 and 100 mg/m2 dose levels, as shown in Figure 2C. Results for three additional samples showed decreases in Mcl-1 expression, and seven additional samples showed cleavage of PARP after SNS-032 infusion. The consistency of these observations in evaluable samples indicates that these patterns are representative of the response of CLL to SNS-032.
Antitumor Activity of SNS-032
Overall, there was limited evidence of antitumor activity for SNS-032. For patients with CLL, one patient in cohort 4 (75 mg/m2) had a short-lived decrease in measurable disease, but anemia and thrombocytopenia did not improve to qualify as a partial remission by 1996 National Cancer Institute–Sponsored Working Group response criteria. The patient was taken off study after course 4 with progressive disease. Another patient in the same cohort had stable disease for four courses, with a small decrease in tumor volume. The patient was taken off study after course 5 on patient request. One patient with MM in cohort 3 (33 mg/m2) had stable disease after three courses. Another patient with MM in cohort 4 (75 mg/m2) had normalization of spleen size and stable disease, and the patient was taken off study after course 5 on the basis of investigator decision.
DISCUSSION
This phase I and pharmacology study was conducted to assess the safety and tolerability of SNS-032 in patients with advanced CLL and MM and to relate these findings to the PK and PD of this agent. For patients with CLL, the DLT was TLS, and the MTD was 75 mg/m2. The DLT and MTD were not identified for patients with MM because the study was closed early. PK results from this study indicated that target plasma levels were exceeded and sustained for 6 hours in patients who received SNS-032 doses of 50 mg/m2 and greater. In CLL, pharmacodynamic studies demonstrated mechanism-based activity against the target Cdks that was associated with decreased levels of antiapoptotic proteins and CLL cell death.
In vitro studies with SNS-032 and primary CLL cells demonstrated reversible inhibition of Cdks with exposures for less than 10 hours and removal of SNS-032 from the culture medium after treatment for ≤ 6 hours permitted rephosphorylation of RNA Pol II, which led to re-expression of Mcl-1 protein and cell survival.10 Indeed, rephosphorylation of RNA Pol II was observed in patient samples at a time when plasma SNS-032 had decreased below target levels (Fig 2). Therefore, longer exposure to the target plasma concentration of 115 ng/mL may be required to maximize antitumor activity, which could be achieved by lengthening the infusion duration.
The limited clinical efficacy of SNS-032, despite observing TLS and potent in vitro activity with target modulation, could be explained by several reasons. First, the biochemical TLS involves not only the death of CLL cells, but other cell types. However, there were no significant electrolyte abnormalities, symptoms, or indications of TLS in patients with MM at doses studied, making this an unlikely explanation. Second, in vitro studies showing reversible inhibition of Cdks by SNS-032 suggested that target levels may need to be maintained for ≥ 8 hours and that the 6-hour infusion may have been insufficient for antitumor effects. Third, patients in this study were heavily pretreated; clinical responses with an improved therapeutic index may be observed in patients with earlier-stage disease.
Overall, SNS-032 was well tolerated by most of the treated patients, with grade 3 to 4 neutropenia and thrombocytopenia as the most common toxicities. Serious infections were infrequent with antibiotic prophylaxis. Patients who developed TLS did not require hemodialysis, and no mortality was associated with TLS, although aggressive prehydration and prophylactic monitoring for TLS were used in this trial.
SNS-032 works by a p53-independent mechanism of action, making it a potentially relevant drug in treating patients with CLL and chromosome 17p deletion, a high-risk feature for which there are limited treatment options. It also blocks vascular endothelial growth factor, thereby inhibiting angiogenesis and potentially contributing to cytotoxicity.29 Rational combinations with SNS-032 for patients with CLL may include an inhibitor of protein synthesis, such as homoharringtonine, to affect a sequential blockade of the antiapoptotic protein expression.30 A previous study with roscovitine, a small-molecule Cdk inhibitor, confirmed its synergistic effect with alemtuzumab and restored alemtuzumab sensitivity in alemtuzumab-resistant CLL cells.31
In conclusion, SNS-032 is a highly selective and potent inhibitor of Cdks 2, 7, and 9. In this phase I trial, single-agent SNS-032 demonstrated mechanism-based target modulation as well as modest clinical activity in heavily pretreated patients with CLL and MM. The dose schedule of a loading dose followed by a 6-hour infusion achieved the predicted plasma concentrations and was well-tolerated. The most commonly observed toxicity was myelosuppression, although TLS was observed in patients with CLL treated at ≥ 75 mg/m2, which was managed conservatively without dialysis or fatalities. The schedule of single-agent SNS-032 could be further optimized by longer duration of exposure at doses that achieve the threshold plasma level and warrants further clinical study.
Appendix
Table A1.
All Grade 1 to 2 Toxicities for All Courses Occurring in > 10% of Patients by Diagnosis
| Adverse Events | CLL (n = 19) | MM (n = 18) | Total (n = 37) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Gastrointestinal | ||||||
| Nausea | 8 | 42 | 9 | 50 | 17 | 46 |
| Diarrhea | 9 | 47 | 7 | 39 | 16 | 43 |
| Vomiting | 5 | 26 | 4 | 22 | 9 | 24 |
| Constipation | 2 | 11 | 2 | 11 | 4 | 11 |
| Anorexia | 2 | 11 | 2 | 11 | 4 | 11 |
| General | ||||||
| Pyrexia | 8 | 42 | 5 | 28 | 13 | 35 |
| Fatigue | 4 | 21 | 4 | 22 | 8 | 22 |
| Mucosal inflammation | 2 | 11 | 3 | 17 | 5 | 14 |
| Edema | 1 | 5 | 3 | 17 | 4 | 11 |
| Electrolyte abnormalities | ||||||
| Hypokalemia | 3 | 16 | 9 | 50 | 12 | 32 |
| Hypomagnesemia | 3 | 16 | 2 | 11 | 5 | 14 |
| CNS disorders, headache | 1 | 5 | 3 | 17 | 4 | 11 |
| Respiratory, cough | 5 | 26 | 1 | 6 | 6 | 16 |
| Skin disorders, rash | 3 | 16 | 1 | 6 | 4 | 11 |
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00446342.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Judith A. Fox, Sunesis Pharmaceuticals (C); Kristi Mahadocon, Sunesis Pharmaceuticals (C); Tianling Chen, Sunesis Pharmaceuticals (C); Peggy Kegley, Sunesis Pharmaceuticals (C) Consultant or Advisory Role: David Siegel, Celgene (C), Millenium (C); William G. Wierda, GlaxoSmithKline (C), Trubion (C), Ligand (C), Genentech (C), Medimmune/Micromet (C), Abbott (C) Stock Ownership: Kristi Mahadocon, Sunesis Pharmaceuticals; Tianling Chen, Sunesis Pharmaceuticals Honoraria: David Siegel, Celgene, Millenium; William G. Wierda, Genentech Research Funding: William Plunkett, Sunesis Pharmaceuticals; Leslie Popplewell, Sunesis Pharmaceuticals; Steven Coutre, Sunesis Pharmaceuticals; William G. Wierda, Bayer, Sanofi-Aventis, Abbott, GlaxoSmithKline, Sunesis Pharmaceuticals Expert Testimony: None Other Remuneration: William G. Wierda, Celgene
AUTHOR CONTRIBUTIONS
Conception and design: Rong Chen, William Plunkett, Steven Coutre, Ute Hoch, William G. Wierda
Provision of study materials or patients: David Siegel, R. Donald Harvey, Ashraf Z. Badros, Leslie Popplewell, Steven Coutre, William G. Wierda
Collection and assembly of data: Wei-Gang Tong, Rong Chen, William Plunkett, David Siegel, R. Donald Harvey, Peggy Kegley, William G. Wierda
Data analysis and interpretation: Wei-Gang Tong, Rong Chen, William Plunkett, Judith A. Fox, Kristi Mahadocon, Tianling Chen, Ute Hoch, William G. Wierda
Manuscript writing: Wei-Gang Tong, Rong Chen, William Plunkett, Rajni Sinha, Judith A. Fox, William G. Wierda
Final approval of manuscript: Wei-Gang Tong, Rong Chen, William Plunkett, David Siegel, Rajni Sinha, R. Donald Harvey, Ashraf Z. Badros, Leslie Popplewell, Steven Coutre, Judith A. Fox, Kristi Mahadocon, Tianling Chen, Peggy Kegley, Ute Hoch, William G. Wierda
REFERENCES
- 1.Cheson BD. Oblimersen for the treatment of patients with chronic lymphocytic leukemia. Ther Clin Risk Manag. 2007;3:855–870. [PMC free article] [PubMed] [Google Scholar]
- 2.Soundararajan S, Chen W, Spicer EK, et al. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008;68:2358–2365. doi: 10.1158/0008-5472.CAN-07-5723. [DOI] [PubMed] [Google Scholar]
- 3.Del Gaizo Moore V, Brown JR, Certo M, et al. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule Obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakrishnan K, Wierda WG, Keating MJ, et al. Gossypol, a BH3 mimetic, induces apoptosis in chronic lymphocytic leukemia cells. Blood. 2008;112:1971–1980. doi: 10.1182/blood-2007-12-126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammad RM, Goustin AS, Aboukameel A, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 7.Blagden S, de Bono J. Drugging cell cycle kinases in cancer therapy. Curr Drug Targets. 2005;6:325–335. doi: 10.2174/1389450053765824. [DOI] [PubMed] [Google Scholar]
- 8.Senderowicz AM. Development of cyclin-dependent kinase modulators as novel therapeutic approaches for hematological malignancies. Leukemia. 2001;15:1–9. doi: 10.1038/sj.leu.2401994. [DOI] [PubMed] [Google Scholar]
- 9.Chen R, Keating MJ, Gandhi V, et al. Transcription inhibition by flavopiridol: Mechanism of chronic lymphocytic leukemia cell death. Blood. 2005;106:2513–2519. doi: 10.1182/blood-2005-04-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R, Wierda WG, Chubb S, et al. Mechanism of action of SNS-032, a novel cyclin-dependent kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2009;113:4637–4645. doi: 10.1182/blood-2008-12-190256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahntow IN, Schneller F, Oelsner M, et al. Cyclin-dependent kinase inhibitor Roscovitine induces apoptosis in chronic lymphocytic leukemia cells. Leukemia. 2004;18:747–755. doi: 10.1038/sj.leu.2403295. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GK, Shah MA. Targeting the cell cycle: A new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 14.Senderowicz AM. Small-molecule cyclin-dependent kinase modulators. Oncogene. 2003;22:6609–6620. doi: 10.1038/sj.onc.1206954. [DOI] [PubMed] [Google Scholar]
- 15.Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776–2786. doi: 10.1038/sj.onc.1208613. [DOI] [PubMed] [Google Scholar]
- 16.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin Cancer Res. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 17.Byrd JC, Shinn C, Waselenko JK, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 18.Byrd JC, Peterson BL, Gabrilove J, et al. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: Results from Cancer and Leukemia Group B study 19805. Clin Cancer Res. 2005;11:4176–4181. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 19.Flinn IW, Byrd JC, Bartlett N, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res. 2005;29:1253–1257. doi: 10.1016/j.leukres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Tan AR, Headlee D, Messmann R, et al. Phase I clinical and pharmacokinetic study of flavopiridol administered as a daily 1-hour infusion in patients with advanced neoplasms. J Clin Oncol. 2002;20:4074–4082. doi: 10.1200/JCO.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 21.Rudek MA, Bauer KS, Jr, Lush RM, III, et al. Clinical pharmacology of flavopiridol following a 72-hour continuous infusion. Ann Pharmacother. 2003;37:1369–1374. doi: 10.1345/aph.1C404. [DOI] [PubMed] [Google Scholar]
- 22.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conroy A, Stockett DE, Walker D, et al. SNS-032 is a potent and selective CDK 2, 7 and 9 inhibitor that drives target modulation in patient samples. Cancer Chemother Pharmacol. 2009;64:723–732. doi: 10.1007/s00280-008-0921-5. [DOI] [PubMed] [Google Scholar]
- 25.Reddy M, Arbitrario JP, Jones J, et al. SNS-032 has potent antitumor activity in vivo against human leukemia and multiple myeloma xenografts. AACR Meeting Abstracts. 2007 abstr A258. [Google Scholar]
- 26.Heath EI, Bible K, Martell RE, et al. A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Invest New Drugs. 2008;26:59–65. doi: 10.1007/s10637-007-9090-3. [DOI] [PubMed] [Google Scholar]
- 27.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 29.Ali MA, Choy H, Habib AA, et al. SNS-032 prevents tumor cell-induced angiogenesis by inhibiting vascular endothelial growth factor. Neoplasia. 2007;9:370–381. doi: 10.1593/neo.07136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R, Gandhi V, Plunkett W. A sequential blockade strategy for the design of combination therapies to overcome oncogene addiction in chronic myelogenous leukemia. Cancer Res. 2006;66:10959–10966. doi: 10.1158/0008-5472.CAN-06-1216. [DOI] [PubMed] [Google Scholar]
- 31.Weingrill E, Wolfler A, Strunk D, et al. Roscovitine in B-chronic lymphocytic leukemia cells: High apoptosis-inducing efficacy and synergism with alemtuzumab independent of the patients' pretreatment status. Haematologica. 2007;92:1286–1288. doi: 10.3324/haematol.10680. [DOI] [PubMed] [Google Scholar]