Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (original) (raw)
. Author manuscript; available in PMC: 2017 Jan 28.
Published in final edited form as: N Engl J Med. 2016 Jun 13;375(4):311–322. doi: 10.1056/NEJMoa1603827
Abstract
BACKGROUND
The cardiovascular effect of liraglutide, a glucagon-like peptide 1 analogue, when added to standard care in patients with type 2 diabetes, remains unknown.
METHODS
In this double-blind trial, we randomly assigned patients with type 2 diabetes and high cardiovascular risk to receive liraglutide or placebo. The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. The primary hypothesis was that liraglutide would be noninferior to placebo with regard to the primary outcome, with a margin of 1.30 for the upper boundary of the 95% confidence interval of the hazard ratio. No adjustments for multiplicity were performed for the prespecified exploratory outcomes.
RESULTS
A total of 9340 patients underwent randomization. The median follow-up was 3.8 years. The primary outcome occurred in significantly fewer patients in the liraglutide group (608 of 4668 patients [13.0%]) than in the placebo group (694 of 4672 [14.9%]) (hazard ratio, 0.87; 95% confidence interval [CI], 0.78 to 0.97; P<0.001 for noninferiority; P=0.01 for superiority). Fewer patients died from cardiovascular causes in the liraglutide group (219 patients [4.7%]) than in the placebo group (278 [6.0%]) (hazard ratio, 0.78; 95% CI, 0.66 to 0.93; P=0.007). The rate of death from any cause was lower in the liraglutide group (381 patients [8.2%]) than in the placebo group (447 [9.6%]) (hazard ratio, 0.85; 95% CI, 0.74 to 0.97; P =0.02). The rates of nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure were nonsignificantly lower in the liraglutide group than in the placebo group. The most common adverse events leading to the discontinuation of liraglutide were gastrointestinal events. The incidence of pancreatitis was nonsignificantly lower in the liraglutide group than in the placebo group.
CONCLUSIONS
In the time-to-event analysis, the rate of the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke among patients with type 2 diabetes mellitus was lower with liraglutide than with placebo. (Funded by Novo Nordisk and the National Institutes of Health; LEADER ClinicalTrials.gov number, NCT01179048.)
Type 2 diabetes is a complex metabolic disorder that is characterized by hyperglycemia and associated with a high risk of cardiovascular, microvascular, and other complications.1,2 Although glycemic control is associated with reductions in the risk of microvascular complications, the macrovascular benefits of glycemic control are less certain. Furthermore, concern has been raised about the cardiovascular safety of antihyperglycemic therapies.3 Consequently, regulatory authorities have mandated cardiovascular safety assessments of new diabetes treatments.4,5
Liraglutide, an analogue of human glucagon-like peptide 1 (GLP-1),6 has been approved for the treatment of type 2 diabetes. Its efficacy in lowering glucose levels has been established, and it has been associated with slight reductions in weight and blood pressure.6–8 It has been associated with an increase in pulse rate.7,8 To assess the long-term effects of liraglutide on cardiovascular outcomes and other clinically important events, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial was initiated in 2010.9
METHODS
TRIAL DESIGN AND OVERSIGHT
We performed this multicenter, double-blind, placebo-controlled trial at 410 sites in 32 countries. Detailed methods of the trial have been published previously,9 and the trial protocol is available with the full text of this article at NEJM.org. The trial protocol was reviewed and approved by the institutional review board or ethics committee at each participating center. All the patients provided written informed consent before participation. Patients with type 2 diabetes who were at high risk for cardiovascular disease were randomly assigned, in a 1:1 ratio, to receive liraglutide or placebo. The minimum planned follow-up was 42 months, with a maximum of 60 months of receiving the assigned regimen and an additional 30 days of follow-up afterward.
The trial was overseen by a steering committee consisting of 11 academic investigators and 4 employees of the sponsor. The steering committee, in collaboration with the sponsor and regulatory authorities, was responsible for designing the trial protocol. An independent data and safety monitoring committee performed ongoing safety surveillance and had access to all the data in an unblinded fashion. The protocol for the treatment of risk factors and the concomitant use of medications was developed by a global expert panel (Table S1 in the Supplementary Appendix, available at NEJM.org). The data were gathered by the site investigators, and the sponsor performed site monitoring and data collection. The data were analyzed by Statogen Consulting and the sponsor.
All the authors had access to the final results and vouch for the fidelity of the trial to the protocol. The first and last authors wrote the first draft of the manuscript, which was revised and approved by all the authors, who also assume responsibility for the accuracy and completeness of its content and for the decision to submit the manuscript for publication. Editorial support, funded by the sponsor, was provided by an independent medical writer under the guidance of the authors.
PATIENTS
Patients with type 2 diabetes who had a glycated hemoglobin level of 7.0% or more were eligible if they either had not received drugs for this condition previously or had been treated with one or more oral antihyperglycemic agents or insulin (human neutral protamine Hagedorn, long-acting analogue, or premixed) or a combination of these agents. The major inclusion criteria were the following: an age of 50 years or more with at least one cardiovascular coexisting condition (coronary heart disease, cerebrovascular disease, peripheral vascular disease, chronic kidney disease of stage 3 or greater, or chronic heart failure of New York Heart Association class II or III) or an age of 60 years or more with at least one cardiovascular risk factor, as determined by the investigator (microalbuminuria or proteinuria, hypertension and left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or an ankle–brachial index [the ratio of the systolic blood pressure at the ankle to the systolic blood pressure in the arm] of less than 0.9).9 Major exclusion criteria were type 1 diabetes; the use of GLP-1–receptor agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, pramlintide, or rapid-acting insulin; a familial or personal history of multiple endocrine neoplasia type 2 or medullary thyroid cancer; and the occurrence of an acute coronary or cerebrovascular event within 14 days before screening and randomization. The complete inclusion and exclusion criteria are listed in the Supplementary Appendix.
PROCEDURES
After a 2-week placebo run-in phase to establish whether patients were able to adhere to the injection regimen, patients were randomly assigned, in a 1:1 ratio, to receive either 1.8 mg (or the maximum tolerated dose) of liraglutide or matching placebo once daily as a subcutaneous injection in addition to standard care (Fig. S1 in the Supplementary Appendix). Randomization was stratified according to the estimated glomerular filtration rate (eGFR) at screening (<30 or ≥30 ml per minute per 1.73 m2 of body-surface area), as calculated with the use of the Modification of Diet in Renal Disease equation. For patients who did not meet the recommended target for glycemic control (glycated hemoglobin level ≤7% or individualized target at the investigator’s discretion) after randomization, the addition of any antihyperglycemic agents except for GLP-1–receptor agonists, DPP-4 inhibitors, or pramlintide was permitted. Patients were scheduled for follow-up visits at months 1, 3, and 6 and every 6 months thereafter.
OUTCOMES
The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, nonfatal (including silent) myocardial infarction, or nonfatal stroke. Prespecified exploratory outcomes included an expanded composite cardiovascular outcome (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina pectoris or heart failure), death from any cause, a composite renal and retinal microvascular outcome (nephropathy [defined as the new onset of macroalbuminuria or a doubling of the serum creatinine level and an eGFR of ≤45 ml per minute per 1.73 m2, the need for continuous renal-replacement therapy, or death from renal disease] and retinopathy [defined as the need for retinal photocoagulation or treatment with intravitreal agents, vitreous hemorrhage, or the onset of diabetes-related blindness]), neoplasms, and pancreatitis — all of which were adjudicated in a blinded fashion by an external, independent event-adjudication committee. The definitions that were used for the clinical events and the members of the committee are listed in the Supplementary Appendix.
The glycated hemoglobin level was measured at randomization, at month 3, and then every 6 months thereafter. Other laboratory tests were performed at randomization, at months 6 and 12, and annually thereafter. Prespecified comparisons between groups were performed at 36 months, which was the last annual visit with laboratory testing that was prespecified for the entire trial population, given the minimum follow-up of 42 months.
STATISTICAL ANALYSIS
The statistical analysis plan is available with the protocol at NEJM.org. We based the required sample size for the trial on an assumed annual primary-event rate of 1.8% in each group. Uniform enrollment was projected over the period of 1.5 years. Assuming a withdrawal rate of less than 10%, a minimum exposure to the trial regimen of 42 months, a null hypothesis hazard ratio of 1.30 or more, 90% power, and a one-sided alpha level of 0.025, we calculated that 8754 patients would need to undergo randomization if we were to observe at least 611 primary outcomes.
The primary and exploratory analyses for the outcomes in the time-to-event analyses were based on a Cox proportional-hazards model with treatment as a covariate. The primary hypothesis was that liraglutide would be noninferior to placebo with regard to the primary outcome, with a margin of 1.30 for the upper boundary of the 95% confidence interval of the hazard ratio. We used a hierarchical testing strategy for the liraglutide group versus the placebo group, first testing for noninferiority and subsequently for superiority. Noninferiority was established for the primary outcome if the upper limit of the two-sided 95% confidence interval of the hazard ratio was less than 1.30, and superiority was established if the upper limit was less than 1.00. In addition, prespecified sensitivity analyses were conducted (see the protocol). For exploratory outcomes, no adjustments of P values for multiplicity were performed. All the patients who underwent randomization were included in the primary and exploratory analyses, and data from the patients who completed or discontinued the trial without having an outcome were censored from the day of their last visit; events occurring after that visit were not included. Two-sided P values are presented throughout. We estimated the mean differences between the trial groups in the glycated hemoglobin level, weight, systolic and diastolic blood pressure, and pulse using a mixed model for repeated measurements, with adjustment for baseline covariates.
RESULTS
OVERVIEW OF TRIAL CONDUCT
A total of 9340 patients underwent randomization from September 2010 through April 2012; 4668 patients were randomly assigned to receive liraglutide and 4672 to receive placebo. The planned closeout of follow-up of the patients was from August 2014 through December 2015. The vital status was known in 99.7% of the patients. A total of 96.8% of the patients completed a final visit, died, or had a primary outcome. The median time of exposure to liraglutide or placebo was 3.5 years. The mean percentage of time that patients received the trial regimen was 84% for liraglutide and 83% for placebo. The median follow-up was 3.8 years in each group. The median daily dose of liraglutide was 1.78 mg (interquartile range, 1.54 to 1.79), including periods during which the patients did not receive liraglutide. The screening, randomization, and follow-up of the patients are shown in Figure S2 in the Supplementary Appendix.
The demographic and clinical characteristics of the patients were similar in the two groups (Table S2 in the Supplementary Appendix). Of the 9340 patients, the majority (7598 [81.3%]) had established cardiovascular disease (6764 patients [72.4%]), chronic kidney disease of stage 3 or higher (2307 [24.7%]), or both (1473 [15.8%]). At baseline, the mean duration of diabetes was 12.8 years, and the mean glycated hemoglobin level was 8.7%.
CARDIOVASCULAR OUTCOMES
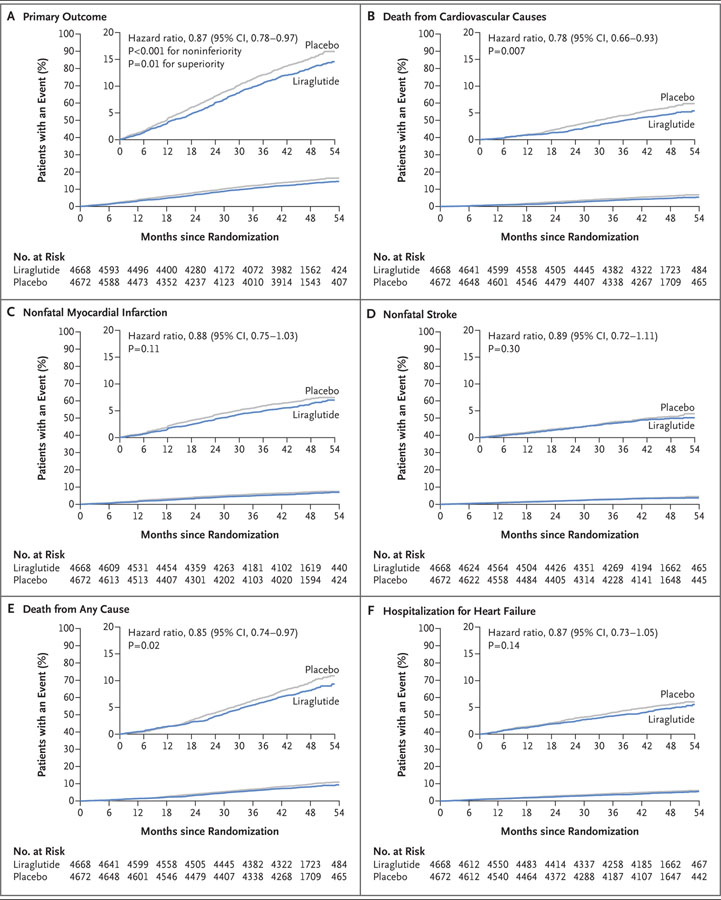
The primary composite outcome occurred in fewer patients in the liraglutide group (608 of 4668 patients [13.0%]) than in the placebo group (694 of 4672 [14.9%]) (hazard ratio, 0.87; 95% confidence interval [CI], 0.78 to 0.97; P<0.001 for noninferiority; P = 0.01 for superiority) (Table 1 and Fig. 1A). Death from cardiovascular causes occurred in fewer patients in the liraglutide group (219 patients [4.7%]) than in the placebo group (278 [6.0%]) (hazard ratio, 0.78; 95% CI, 0.66 to 0.93; P = 0.007) (Fig. 1B). The rate of death from any cause was also lower in the liraglutide group (381 patients [8.2%]) than in the placebo group (447 [9.6%]) (hazard ratio, 0.85; 95% CI, 0.74 to 0.97; P = 0.02). The frequencies of nonfatal myocardial infarction and nonfatal stroke were lower in the liraglutide group than in the placebo group, although the differences were not significant (Fig. 1C and 1D and Table 1). The magnitude of the differences was similar in sensitivity analyses with alternative censoring, including the per-protocol analysis (Fig. S3 in the Supplementary Appendix). Findings for the remaining adjudicated cardiovascular outcomes and the expanded composite outcome are provided in Table 1, and Figure S4 in the Supplementary Appendix.
Table 1.
Primary and Secondary Outcomes.*
| Outcome | Liraglutide (N = 4668) | Incidence Rate | Placebo (N = 4672) | Incidence Rate | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| no. of patients (%) | no. of events/100 patient-yr | no. of patients (%) | no. of events/100 patient-yr | |||
| Primary composite outcome† | 608 (13.0) | 3.4 | 694 (14.9) | 3.9 | 0.87 (0.78–0.97) | 0.01 |
| Expanded composite outcome‡ | 948 (20.3) | 5.3 | 1062 (22.7) | 6.0 | 0.88 (0.81–0.96) | 0.005 |
| Death from any cause | 381 (8.2) | 2.1 | 447 (9.6) | 2.5 | 0.85 (0.74–0.97) | 0.02 |
| Death from cardiovascular causes | 219 (4.7) | 1.2 | 278 (6.0) | 1.6 | 0.78 (0.66–0.93) | 0.007 |
| Death from noncardiovascular causes | 162 (3.5) | 0.9 | 169 (3.6) | 1.0 | 0.95 (0.77–1.18) | 0.66 |
| Myocardial infarction§ | 292 (6.3) | 1.6 | 339 (7.3) | 1.9 | 0.86 (0.73–1.00) | 0.046 |
| Fatal§ | 17 (0.4) | 0.1 | 28 (0.6) | 0.2 | 0.60 (0.33–1.10) | 0.10 |
| Nonfatal | 281 (6.0) | 1.6 | 317 (6.8) | 1.8 | 0.88 (0.75–1.03) | 0.11 |
| Silent§ | 62 (1.3) | 0.3 | 76 (1.6) | 0.4 | 0.86 (0.61–1.20) | 0.37 |
| Stroke§ | 173 (3.7) | 1.0 | 199 (4.3) | 1.1 | 0.86 (0.71–1.06) | 0.16 |
| Fatal§ | 16 (0.3) | 0.1 | 25 (0.5) | 0.1 | 0.64 (0.34–1.19) | 0.16 |
| Nonfatal | 159 (3.4) | 0.9 | 177 (3.8) | 1.0 | 0.89 (0.72–1.11) | 0.30 |
| Transient ischemic attack§ | 48 (1.0) | 0.3 | 60 (1.3) | 0.3 | 0.79 (0.54–1.16) | 0.23 |
| Coronary revascularization | 405 (8.7) | 2.3 | 441 (9.4) | 2.5 | 0.91 (0.80–1.04) | 0.18 |
| Hospitalization for unstable angina pectoris | 122 (2.6) | 0.7 | 124 (2.7) | 0.7 | 0.98 (0.76–1.26) | 0.87 |
| Hospitalization for heart failure | 218 (4.7) | 1.2 | 248 (5.3) | 1.4 | 0.87 (0.73–1.05) | 0.14 |
| Microvascular event | 355 (7.6) | 2.0 | 416 (8.9) | 2.3 | 0.84 (0.73–0.97) | 0.02 |
| Retinopathy | 106 (2.3) | 0.6 | 92 (2.0) | 0.5 | 1.15 (0.87–1.52) | 0.33 |
| Nephropathy | 268 (5.7) | 1.5 | 337 (7.2) | 1.9 | 0.78 (0.67–0.92) | 0.003 |
Figure 1. Primary and Exploratory Outcomes.
The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or non-fatal stroke. The cumulative incidences were estimated with the use of the Kaplan–Meier method, and the hazard ratios with the use of the Cox proportional-hazard regression model. The data analyses are truncated at 54 months, because less than 10% of the patients had an observation time beyond 54 months. The insets show the same data on an enlarged y axis.
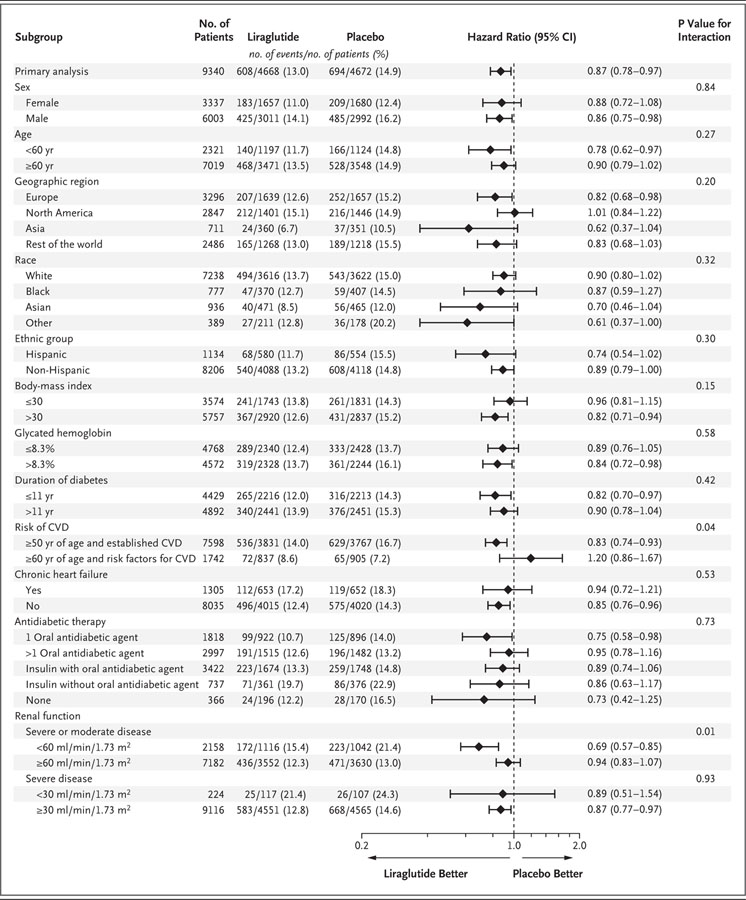
Subgroup analyses are shown in Figure 2. Significant interactions were observed for an eGFR of 60 ml or more per minute per 1.73 m2 versus an eGFR of less than 60 ml per minute per 1.73 m2, with a benefit favoring the lower eGFR, and for the presence versus absence of established cardiovascular disease at baseline, with benefit for those with cardiovascular disease at baseline. Additional subgroup analyses regarding the eGFR are provided in Table S3 in the Supplementary Appendix.
Figure 2. Primary Composite Outcomes in Various Demographic and Clinical Subgroups.
Prespecified Cox proportional-hazard regression analyses were performed for subgroups of patients with respect to the primary outcome (first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke). P values signify tests of homogeneity for between-group differences with no adjustment for multiple testing. The percentages of patients with a first primary outcome between the randomization date and the date of last follow-up are shown. Race and ethnic group were self-reported. There were missing data for the body-mass index (the weight in kilograms divided by the square of the height in meters) in 5 patients in the liraglutide group and 4 in the placebo group and for the duration of diabetes in 11 patients in the liraglutide group and 8 in the placebo group. Renal function was assessed by means of the estimated glomerular filtration rate, as calculated by the Modification of Diet in Renal Disease equation. CVD denotes cardiovascular disease.
GLYCEMIC CONTROL
Changes in the glycated hemoglobin values over time are shown in Figure S5A in the Supplementary Appendix. The prespecified analysis at 36 months showed a mean difference between the liraglutide group and the placebo group of −0.40 percentage points (95% CI, −0.45 to −0.34). Changes in the use of diabetes medication during the trial are shown in Table S4 in the Supplementary Appendix.
CARDIOVASCULAR RISK FACTORS
There were significant mean differences between the liraglutide group and the placebo group in the change from baseline to 36 months in the following variables: weight loss was 2.3 kg (95% CI, 2.5 to 2.0) higher in the liraglutide group, the systolic blood pressure was 1.2 mm Hg (95% CI, 1.9 to 0.5) lower in the liraglutide group, the diastolic blood pressure was 0.6 mm Hg (95% CI, 0.2 to 1.0) higher in the liraglutide group, and the heart rate was 3.0 beats per minute (95% CI, 2.5 to 3.4) higher in the liraglutide group (Fig. S5B, S5C, and S5D in the Supplementary Appendix). The use of cardiovascular medications at baseline and during the trial is shown in Table S4 in the Supplementary Appendix.
MICROVASCULAR OUTCOMES
The incidence of a composite outcome of renal or retinal microvascular events was lower in the liraglutide group than in the placebo group (hazard ratio, 0.84; 95% CI, 0.73 to 0.97; P = 0.02), a difference that was driven by a lower rate of nephropathy events in the liraglutide group (1.5 vs. 1.9 events per 100 patient-years of observation; hazard ratio, 0.78; 95% CI, 0.67 to 0.92; P = 0.003) (Table 1). The incidence of retinopathy events was nonsignificantly higher in the liraglutide group than in the placebo group (0.6 vs. 0.5 events per 100 patient-years; hazard ratio, 1.15; 95% CI, 0.87 to 1.52; P = 0.33).
SAFETY AND ADVERSE EVENTS
Adverse events are listed in Table 2. The overall rates of benign or malignant neoplasms were higher in the liraglutide group than in the placebo group, but the difference was not significant (Fig. S6 in the Supplementary Appendix). There were 13 patients with pancreatic cancer in the liraglutide group and 5 in the placebo group. Additional data regarding pancreatic cancer are provided in Table S5 in the Supplementary Appendix. There were fewer patients with prostate cancer in the liraglutide group than in the placebo group (26 vs. 47) and also fewer patients with leukemia (5 vs. 14) (Fig. S6 in the Supplementary Appendix). Medullary thyroid carcinoma occurred in no patient in the liraglutide group and in 1 in the placebo group. Calcitonin levels over time were similar in the two groups (data not shown).
Table 2.
Selected Adverse Events Reported during the Trial.*
| Event | Liraglutide (N = 4668) | Placebo (N = 4672) | P Value |
|---|---|---|---|
| no. of patients (%) | |||
| Adverse event | |||
| Any adverse event | 2909 (62.3) | 2839 (60.8) | 0.12 |
| Serious adverse event | 2320 (49.7) | 2354 (50.4) | 0.51 |
| Confirmed hypoglycemia† | 2039 (43.7) | 2130 (45.6) | 0.06 |
| Severe adverse event | 1502 (32.2) | 1533 (32.8) | 0.51 |
| Severe hypoglycemia† | 114 (2.4) | 153 (3.3) | 0.02 |
| Acute gallstone disease | 145 (3.1) | 90 (1.9) | <0.001 |
| Cholelithiasis | 68 (1.5) | 50 (1.1) | 0.09 |
| Acute cholecystitis | 36 (0.8) | 21 (0.4) | 0.046 |
| Hypothyroidism | 44 (0.9) | 33 (0.7) | 0.21 |
| Hyperthyroidism | 13 (0.3) | 8 (0.2) | 0.27 |
| Diabetic foot ulcer | 181 (3.9) | 198 (4.2) | 0.38 |
| Allergic reaction | 59 (1.3) | 44 (0.9) | 0.14 |
| Injection-site reaction | 32 (0.7) | 12 (0.3) | 0.002 |
| Adverse event leading to permanent discontinuation of trial regimen | |||
| Any adverse event | 444 (9.5) | 339 (7.3) | <0.001 |
| Serious adverse event | 192 (4.1) | 245 (5.2) | 0.01 |
| Severe adverse event | 164 (3.5) | 188 (4.0) | 0.20 |
| Nausea | 77 (1.6) | 18 (0.4) | <0.001 |
| Vomiting | 31 (0.7) | 2 (<0.1) | <0.001 |
| Diarrhea | 27 (0.6) | 5 (0.1) | <0.001 |
| Increased lipase level‡ | 15 (0.3) | 11 (0.2) | 0.43 |
| Abdominal pain | 11 (0.2) | 3 (0.1) | 0.03 |
| Decreased appetite | 11 (0.2) | 2 (<0.1) | 0.01 |
| Abdominal discomfort | 10 (0.2) | 0 | 0.002 |
| Pancreatitis or neoplasm§ | |||
| Acute pancreatitis | 18 (0.4) | 23 (0.5) | 0.44 |
| Chronic pancreatitis | 0 | 2 (<0.1) | 0.16 |
| Any benign neoplasm | 168 (3.6) | 145 (3.1) | 0.18 |
| Any malignant neoplasm | 296 (6.3) | 279 (6.0) | 0.46 |
| Pancreatic carcinoma | 13 (0.3) | 5 (0.1) | 0.06 |
| Medullary thyroid carcinoma | 0 | 1 (<0.1) | 0.32 |
Acute pancreatitis occurred in 18 patients in the liraglutide group and in 23 in the placebo group. The mean levels of serum amylase and lipase were higher in the liraglutide group than in the placebo group (Fig. S7 in the Supplementary Appendix). Acute gallstone disease was more common with liraglutide than with placebo (in 145 vs. 90 patients), including severe events (in 40 vs. 31). During the trial, fewer patients in the liraglutide group were treated with hypoglycemic medications (insulin, sulfonylurea, and glinides) than in the placebo group (Table S4 in the Supplementary Appendix). Severe hypoglycemia occurred in 114 patients in the liraglutide group and in 153 in the placebo group (rate ratio, 0.69; 95% CI, 0.51 to 0.93). Similarly, the rate ratio for confirmed hypoglycemia (plasma glucose level, <56 mg per deciliter [3.1 mmol per liter]) was 0.80 (95% CI, 0.74 to 0.88). Additional details regarding severe hypoglycemia are provided in Table S6 in the Supplementary Appendix.
Adverse events leading to the permanent discontinuation of the trial regimen were more common with liraglutide than with placebo (Table 2). This result appears to have been driven by gastrointestinal disorders in the liraglutide group.
DISCUSSION
In the present trial, patients in the liraglutide group had a lower risk of the primary composite outcome — first occurrence of cardiovascular death, nonfatal myocardial infarction, or non-fatal stroke in the time-to-event analysis — and lower risks of death from cardiovascular causes, death from any cause, and microvascular events than did those in the placebo group. The number of patients who would need to be treated to prevent one event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause.10 There has been concern about the risk of hospitalization for heart failure with various agents that have been used to treat diabetes mellitus, including DPP-4 inhibitors.11 In the present trial, there were fewer hospitalizations for heart failure among patients in the liraglutide group than among those in the placebo group, although the difference was not significant.
Sensitivity analyses suggested that our findings were robust to baseline adjustment and alternative censoring. Cardiovascular benefits were observed in the context of generally acceptable levels of cardiovascular risk-factor management at baseline and during the trial. There were fewer add-on therapies for diabetes medications, lipid-lowering medications, and diuretics in patients in the liraglutide group than in those in the placebo group. Subgroup analyses suggest a greater benefit of liraglutide with respect to the primary outcome in patients with an eGFR of less than 60 ml per minute per 1.73 m2 and possibly in patients with a history of cardiovascular disease. A sensitivity analysis of data for patients with an eGFR of less than 60 ml per minute per 1.73 m2 did not support a clinically meaningful interaction (Table S3 in the Supplementary Appendix).
The pattern of cardiovascular benefits that were associated with liraglutide in our trial appears to differ from that with the sodium–glucose cotransporter 2 inhibitor empagliflozin in the previously reported EMPA-REG OUTCOME trial.12 The time to benefit emerged earlier in that trial than in the present trial, and the heterogeneity of the direction and magnitude of the effects on the components of the primary composite outcome in that trial contrasts with the consistency of the effect in the present trial. Although these differences may reflect patient populations or chance, the observed benefits in that trial may be more closely linked to hemodynamic changes, whereas in the present trial, the observed benefits are perhaps related to the modified progression of atherosclerotic vascular disease.13
It should be noted that in the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial,14 the GLP-1–receptor agonist lixisenatide, which is shorter-acting than and structurally dissimilar to liraglutide, did not show any cardiovascular benefit in patients with diabetes and a recent acute coronary syndrome. There are a number of other trials regarding cardiovascular outcomes in high-risk cohorts of patients with type 2 diabetes in which similar magnitude effects on glycemic control have been shown but without significant benefits with respect to rates of cardiovascular events or death.15–20 These include trials with insulin,16 thiazolidinediones,15,18 and DPP-4 inhibitors.17,19,20 Our trial had greater statistical power and included patients with a higher baseline glycated hemoglobin level than did most previous studies. However, no obvious single explanation in terms of either the study designs or the included populations is apparent to explain the divergent findings across this body of medical literature.
The prespecified primary microvascular outcome in our trial was a composite of nephropathy and retinopathy outcomes. The benefit with liraglutide was driven by lower rates of renal outcomes, such as new-onset persistent macro-albuminuria in particular. There was a higher rate of retinopathy events with liraglutide than with placebo, although the difference was not significant. With moderate differences in glycemic control between the trial groups over a median 3.8 years of follow-up, the achievement of renal microvascular benefits is surprising. It is uncertain whether this finding relates to the direct effects of liraglutide on kidney function.21,22
More patients in the liraglutide group than in the placebo group permanently discontinued the trial regimen owing to adverse events (difference, 2.2 percentage points). There has been considerable interest in a potential association between the use of GLP-1–receptor agonists and pancreatitis and pancreatic cancer, although there is no consistent preclinical, pharmacovigilance, or epidemiologic evidence to date.23–25 Higher levels of lipase and amylase were observed in the liraglutide group, a finding that is similar to results in other studies.24 Blinded medications were to be stopped only in relation to confirmed pancreatitis as evaluated by the investigator. There were 1.5 episodes of pancreatitis per 1000 patient-years of observation in both regimens combined, and there were numerically fewer acute or chronic pancreatitis events with liraglutide than with placebo. There were more episodes of gallstone disease with liraglutide, a finding that has been reported previously.26
An excess in adjudicated cancers of pancreatic origin was observed in the liraglutide group, although the finding was not significant; there were small overall numbers and no between-group difference in the number of overall cancers. Among rodents receiving liraglutide, higher rates of thyroid C-cell tumors and hyperplasia have been observed than were observed among control animals.27 In the present trial, no episodes of C-cell hyperplasia or medullary thyroid carcinoma were observed in patients in the liraglutide group. Randomized trials of this type, despite their size, are not powered to determine the effect of drugs on cancer risk and can therefore neither confirm nor exclude such a possibility.
Many patients in each group were treated with sulfonylureas or insulin at baseline, but fewer patients in the liraglutide group than in the placebo group added insulin during the trial. There was a 31% lower rate of severe hypoglycemia and a 20% lower rate of the combination of severe and confirmed hypoglycemia (plasma glucose level, <56 mg per deciliter) in the liraglutide group than in the placebo group.
A limitation of our trial is that patients were followed for only 3.5 to 5.0 years, so the safety and efficacy data are restricted to that time period. Also, because our trial recruited a population of patients who were at high risk for cardiovascular events and who had a baseline glycated hemoglobin level of 7% or more, the observed benefits and risks may not apply to patients at lower risk. Furthermore, no adjustments were made for multiplicity of exploratory outcomes.
In conclusion, among patients with type 2 diabetes who were at high risk for cardiovascular events while they were taking standard therapy, those in the liraglutide group had lower rates of cardiovascular events and death from any cause than did those in the placebo group.
Supplementary Material
Supplement1
Acknowledgments
Supported by Novo Nordisk and by grants from the National Institutes of Health.
We thank Dr. Søren Rasmussen (Novo Nordisk) for statistical support; Dr. Florian Baeres (Novo Nordisk) for scientific input; Dr. Alan Moses (Novo Nordisk) for assistance in protocol development and trial conduct; Mr. Joseph Murphy for editorial support (funded by the sponsor) under the direct supervision of Drs. Marso and Buse, as specified contractually; and the participants, investigators, trial-site staff, and the leadership, employees, and contractors of the sponsor who were involved in the conduct of the trial.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Dr. Marso reports receiving consulting fees from Novo Nordisk and AstraZeneca, honoraria for physician education from Abbott, and grant support to his institution from Novo Nordisk; Drs. Brown-Frandsen, Kristensen, Ravn, and Stockner, being employees of and holding stock in Novo Nordisk and holding pending patents (EP 16158737.3, EP 16158735.7, EP 16158738.1, and EP 16158739.9) related to liraglutide methods; Dr. Mann, receiving fees for serving on committees from AstraZeneca, Braun, ACI Clinical, Fresenius, Celgene, AbbVie, Novo Nordisk, Roche, Sandoz, Lanthio Pharma, Sanifit, Relypsa, and ZS Pharma, lecture fees from AstraZeneca, Amgen, Braun, Fresenius, Celgene, Gambro, AbbVie, Medice, Novo Nordisk, Roche, Sandoz, Relypsa, and ZS Pharma, and grant support from Celgene, AbbVie, Novo Nordisk, Roche, and Sandoz; Dr. Nauck, receiving fees for serving on advisory boards from Berlin-Chemie, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Versartis, Intarcia Therapeutics, AstraZeneca, Roche, Novartis, and Janssen, lecture fees from Berlin-Chemie, Boehringer Ingelheim, Eli Lilly, Glaxo-SmithKline, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, AstraZeneca, Novartis, Janssen, and Medscape, travel support from Berlin-Chemie, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Versartis, Intarcia Therapeutics, AstraZeneca, Roche, Novartis, Janssen, and Medscape, and grant support from Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, Novo Nordisk, AstraZeneca, and Novartis; Dr. Nissen, receiving reimbursement from Boehringer Ingelheim and grant support from Pfizer, Amgen, Esperion Therapeutics, Cerenis Therapeutics, the Medicines Company, AstraZeneca, Takeda Pharmaceuticals, Orexigen Therapeutics, and Eli Lilly; Dr. Poulter, receiving fees for serving on steering committees from AstraZeneca and Novo Nordisk, and lecture fees from Novo Nordisk and Takeda Pharmaceuticals; Dr. Steinberg, receiving fees for consulting from and owning stock in Novo Nordisk; Dr. Zinman, receiving consulting fees from Merck, Novo Nordisk, Sanofi-Aventis, Eli Lilly, AstraZeneca, Janssen, and Boehringer Ingelheim and grant support to his institution from Merck, Novo Nordisk, and Boehringer Ingelheim; Dr. Bergenstal, receiving contracted consulting fees paid to his institution and travel support from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb–AstraZeneca, Calibra Medical, Eli Lilly, Hygieia, Johnson & Johnson, Medtronic, Novo Nordisk, Roche, Sanofi, and Takeda Pharmaceuticals, and grant support from Abbott, Becton Dickinson, Boehringer Ingelheim, Bristol-Myers Squibb–AstraZeneca, Calibra, Eli Lilly, Hygieia, Johnson & Johnson, Medtronic, Merck, Novo Nordisk, Roche, Sanofi, Takeda Pharmaceuticals, and ResMed, and holding stock in Merck; and Dr. Buse, receiving contracted consulting fees paid to his institution and travel support from Novo Nordisk, Eli Lilly, Bristol-Myers Squibb, GI Dynamics, Elcelyx, Merck, Metavention, vTv Therapeutics, PhaseBio, AstraZeneca, Dance Biopharm, Quest Diagnostics, Sanofi-Aventis, Lexicon Pharmaceuticals, Orexigen Therapeutics, Takeda Pharmaceuticals, Adocia, and Roche, grant support from Eli Lilly, Bristol-Myers Squibb, GI Dynamics, Merck, PhaseBio, AstraZeneca, Medtronic, Sanofi, Tolerex, Osiris Therapeutics, Halozyme Therapeutics, Johnson & Johnson, Andromeda, Boehringer Ingelheim, GlaxoSmithKline, Astellas Pharma, MacroGenics, Intarcia Therapeutics, Lexicon, Scion NeuroStim, Orexigen Therapeutics, Takeda Pharmaceuticals, Theracos, and Roche, and receiving fees and holding stock options in PhaseBio and serving on the boards of the AstraZeneca Healthcare Foundation and the Bristol-Myers Squibb Together on Diabetes Foundation. No other potential conflict of interest relevant to this article was reported.
References
- 1.Introduction. Diabetes Care. 2016;39(Suppl 1):S1–2. doi: 10.2337/dc16-S001. [DOI] [PubMed] [Google Scholar]
- 2.IDF diabetes atlas. 7. Brussels: International Diabetes Federation; 2015. [Google Scholar]
- 3.Holman RR, Sourij H, Califf RM. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet. 2014;383:2008–17. doi: 10.1016/S0140-6736(14)60794-7. [DOI] [PubMed] [Google Scholar]
- 4.Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. London: European Medicines Agency; 2012. [Google Scholar]
- 5.Guidance for industry: diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Silver Spring, MD: Department of Health and Human Services; 2008. [Google Scholar]
- 6.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–16. doi: 10.1111/dom.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Q, Wang YJ, Yang S, Zhao YY, Han P. Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Adv Ther. 2014;31:1182–95. doi: 10.1007/s12325-014-0164-2. [DOI] [PubMed] [Google Scholar]
- 8.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marso SP, Poulter NR, Nissen SE, et al. Design of the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial. Am Heart J. 2013;166(5):823–30. e5. doi: 10.1016/j.ahj.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–5. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire DK, Van de Werf F, Armstrong PW, et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus. JAMA Cardiol. 2016;2:126–35. doi: 10.1001/jamacardio.2016.0103. [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114:1788–803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 15.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 16.The ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 17.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 18.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 19.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 20.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 21.Muskiet MH, Smits MM, Morsink LM, Diamant M. The gut-renal axis: do incretin-based agents confer renoprotection in diabetes? Nat Rev Nephrol. 2014;10:88–103. doi: 10.1038/nrneph.2013.272. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T, Higashijima Y, Wada T, Nangaku M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014;86:701–11. doi: 10.1038/ki.2014.236. [DOI] [PubMed] [Google Scholar]
- 23.Chalmer T, Almdal TP, Vilsbøll T, Knop FK. Adverse drug reactions associated with the use of liraglutide in patients with type 2 diabetes — focus on pancreatitis and pancreas cancer. Expert Opin Drug Saf. 2015;14:171–80. doi: 10.1517/14740338.2015.975205. [DOI] [PubMed] [Google Scholar]
- 24.Jensen TM, Saha K, Steinberg WM. Is there a link between liraglutide and pancreatitis? A post hoc review of pooled and patient-level data from completed liraglutide type 2 diabetes clinical trials. Diabetes Care. 2015;38:1058–66. doi: 10.2337/dc13-1210. [DOI] [PubMed] [Google Scholar]
- 25.Tseng CH, Lee KY, Tseng FH. An updated review on cancer risk associated with incretin mimetics and enhancers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33:67–124. doi: 10.1080/10590501.2015.1003496. [DOI] [PubMed] [Google Scholar]
- 26.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314:687–99. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 27.Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–86. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1