Immunogenicity of a Meningococcal B Vaccine during a University Outbreak (original) (raw)
. Author manuscript; available in PMC: 2017 Jan 21.
Published in final edited form as: N Engl J Med. 2016 Jul 21;375(3):220–228. doi: 10.1056/NEJMoa1514866
Abstract
BACKGROUND
In December 2013, a multicomponent meningococcal serogroup B (4CMenB) vaccine was used before licensure on the basis of special consideration by the Food and Drug Administration to respond to an outbreak of Neisseria meningitidis B at a U.S. university. Data suggested that vaccination would control the outbreak because isolates expressed antigens that were closely related to the vaccine antigens (factor H–binding protein [fHbp] and neisserial heparin-binding antigen). We quantified the immune responses induced by 4CMenB during the outbreak.
METHODS
We conducted a seroprevalence survey among students to assess vaccination status and collect serum specimens to quantify titers of serum bactericidal antibodies (SBA) with an assay that included human complement (hSBA). We compared the proportion of vaccinated and unvaccinated participants who were seropositive for the outbreak strain and for one closely related reference strain (44/76-SL, which included fHbp) and one mismatched reference strain (5/99, which included neis-serial adhesin A), both of which were used in vaccine development. Seropositivity was defined as an hSBA titer of 4 or higher.
RESULTS
Among the 499 participants who received two doses of the 4CMenB vaccine 10 weeks apart, 66.1% (95% confidence interval [CI], 61.8 to 70.3) were seropositive for the outbreak strain, although the geometric mean titer was low at 7.6 (95% CI, 6.7 to 8.5). Among a random subgroup of 61 vaccinees who also received two doses but did not have a detectable protective response to the outbreak strain, 86.9% (95% CI, 75.8 to 94.2) were seropositive for the 44/76-SL strain, for which there was a geometric mean titer of 17.4 (95% CI, 13.0 to 23.2), whereas 100% of these vaccinees (95% CI, 94.1 to 100) were seropositive for the 5/99 strain and had a higher geometric mean titer (256.3; 95% CI, 187.3 to 350.7). The response to the outbreak strain was moderately correlated with the response to the 44/76-SL strain (Pearson's correlation, 0.64; P<0.001) but not with the response to the 5/99 strain (Pearson's correlation, −0.06; P = 0.43).
CONCLUSIONS
Eight weeks after the second dose of the 4CMenB vaccine was administered, there was no evidence of an hSBA response against the outbreak strain in 33.9% of vaccinees, although no cases of meningococcal disease caused by N. meningitidis B were reported among vaccinated students. (Funded by Princeton University and others.)
In the United States, meningococcal disease, caused primarily by Neisseria meningitidis serogroups B, C, and Y, presents a substantial threat to public health, especially among infants and young adults.1-5 Although the incidence has been declining,6,7 in part because of the routine administration of meningococcal A, C, W, and Y vaccines in adolescents,8 the prevention of serogroup B disease has presented particular challenges; it is not possible to use the meningococcal B polysaccharide as a vaccine antigen owing to its similarity to human glyco-proteins, the presence of which could lead to an autoimmune response.9 Meningococcal B vaccines that are derived from the outer-membrane vesicles of specific outbreak strains have been developed, but these vaccines have not provided broad protection beyond the outbreak strain.8
Between 2009 and 2015, seven meningococcal B outbreaks occurred at U.S. universities.7 From March 2013 through March 2014, a meningococcal B outbreak at a university in New Jersey led to nine cases of disease, including one death.10 No meningococcal B vaccine was licensed in the United States at that time, although the multicomponent meningococcal serogroup B (4CMenB) vaccine, Bexsero (GlaxoSmithKline), was licensed elsewhere.
4CMenB is a recombinant meningococcal B vaccine containing factor H–binding protein (fHbp), an fHbp-GNA2091 fusion protein (fHbp subvariant 1.1); neisserial adhesin A (NadA), subvariant 3.1; neisserial heparin-binding antigen (NHBA), an NHBA-GNA1030 fusion protein (NHBA subvariant 1.2); and outer-membrane vesicles from outbreak strain NZ 98/254 (B:4:P1.7-2,4; ST-42 [cc41/44]). Because sustained transmission occurred during 2 academic years, the Food and Drug Administration approved the use of 4CMenB before licensure.11
The vaccine was offered to nearly 6000 students, beginning in December 2013. Within 6 months, 95% of eligible students had received at least one dose and 89% had completed the two-dose series.10 According to test results from the Meningococcal Antigen Typing System,12-15 outbreak isolates expressed two of the antigens used in vaccine development (fHbp and NHBA).10 Titers of serum bactericidal antibodies (SBA) obtained with assays that included human complement (hSBA) from a small number of pooled serum specimens from participants in a Chilean trial indicated that vaccination induced immunity that was specific to the outbreak strain.10,11,16
There is little indication of how broadly 4CMenB protects people against the diverse strains of meningococcal B.17 The Meningococcal Antigen Typing System predicts that 4CMenB will protect against 91% of U.S. meningococcal B strains.18 Although the system is designed to quantify the expression of antigen-using polyclonal antibodies against the fHbp, NHBA, and NadA components of 4CMenB and to determine whether bacterial expression is sufficient to elicit a vaccine response, the system cannot determine the degree to which heterogeneity in vaccine-induced immunity can be expected within populations. In addition, the results of the typing system cannot be generalized to vaccinees of other ages or to different schedules of administration because the results are based on pooled serum specimens from infants who received four doses of 4CMenB. SBA testing of individual serum specimens is the reference standard for quantifying immune responses and is thought to be more informative with regard to protection against disease. No large-scale assessment of the breadth of individual vaccine-induced immunity has been conducted. It is not yet known whether 4CMenB will induce sufficient immunity against diverse strains, especially during outbreaks. Here we evaluate the effect of 4CMenB on individual immunity during an outbreak among U.S. college students.
Methods
Vaccination Campaign
The 4CMenB vaccine was offered to students in clinics beginning in December 2013; a second dose was recommended 10 weeks later, in February 2014 (Fig. 1). Vaccination was voluntary and was provided at the university independently from this study, as reported previously.10 Students were eligible for vaccination if they were currently enrolled as an undergraduate or a graduate student and lived on campus.
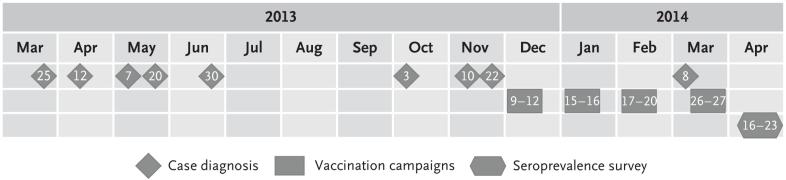
Figure 1. Relationships among the Timing of Diagnosis, 4CMenB Vaccine Campaigns, and the Seroprevalence Survey.
The figure shows the timing of the diagnosis of cases of meningococcal disease associated with the outbreak (with numbers indicating the date of diagnosis), the timing of the 4CMenB vaccination campaigns held at the university (with numbers indicating the days of the month of vaccination),10 and the timing of the seroprevalence survey (with numbers indicating the dates during which the survey was conducted).
Study Design
We conducted a seroprevalence survey among students 4 months after the introduction of 4CMenB. Students were eligible to participate in the survey if they confirmed that they were currently enrolled as an undergraduate or a graduate student who had been eligible to receive 4CMenB and were 18 years of age or older. Students were ineligible if they were ill or had a condition that prevented blood collection. Initially, a random sample of students was invited to participate, but ultimately all students eligible for the vaccination campaigns were invited in order to reach the target sample size of 607. Participants provided a small blood sample and answered a questionnaire.
The institutional review boards at Princeton University and the University of Minnesota approved the research. All participants provided written informed consent.
Evaluation of Immunity
Serum extracted from blood samples was stored at −80°C within 4 hours after the samples were collected. Serum specimens were analyzed at the Vaccine Evaluation Unit, Public Health England, Manchester, United Kingdom. Members of the unit were not aware of participants’ vaccination status. As described previously,19 hSBA assays were used to quantify the immune response against the outbreak strain, which was characterized as ST 409 [cc41/44/lineage3], PFGE 429, PorA P1.5-1,2.2, fHbp 1.276, NHBA p0002, and NadA-negative.10 The hSBA titers were expressed as the reciprocal of the final interpolated dilution that killed at least 50% of bacteria after 60 minutes. SBAs are the standard correlate of protection for the evaluation and licensing of meningococcal vaccines. An hSBA titer of 4 or higher is an accepted threshold for putative protection against serogroup B.19-22
A subset of serum samples were analyzed to assess immune responses to an fHbp vaccine reference strain (44/76-SL) that was previously found to have a genetic similarity of 96% with the outbreak isolate23 and an NadA vaccine reference strain (5/99) that did not match the outbreak strain, which was NadA-negative.10 The subset included samples from all unvaccinated participants and a random sample from those vaccinated with the first dose in December and the second dose in February, according to the recommended schedule. This subgroup of participants who received two doses of the vaccine was stratified into three groups: no detectable protective response to the outbreak strain (hSBA titer, <4), low response to the outbreak strain (hSBA titer, 4 to 8), and high response to the outbreak strain (hSBA titer, >8). Different complement lots were used to conduct the assays for each of the three strains tested. Assessment of the immunity elicited by the NHBA component of the 4CMenB vaccine could not be undertaken because there is no known meningococcal strain that can be used in the hSBA assay for adolescent serum specimens that is matched to the NHBA component of 4CMenB but mismatched to the other antigens. Therefore, it is not possible to quantify the immune response elicited by the NHBA component in isolation.
Data Collection and Analysis
Participants completed an 18-question survey and were asked to provide access to their vaccination records. Data were recorded on paper forms and double-entered into electronic data-bases (Adobe FormsCentral). For all analyses, documented vaccination status was used unless only self-reported status was available (self-reported status applied to 3.0% of the participants). For the primary analysis, we compared all participants who received two doses of the vaccine (the first in December and the second in February, according to the recommended schedule), those who received the first dose in December but did not complete the series, and those who remained unvaccinated. In secondary analyses, we compared vaccinees who chose to be vaccinated according to other schedules. The lower limit of quantitation (LLQ) for the hSBA titer was 2. When complement-independent killing was observed, the hSBA titer was repeated with beta-lactamase added to the serum specimens to counteract the effect of any penicillin that may have been present, and the LLQ was 4. Results below the LLQ were assigned a value of one half of the LLQ.
Statistical Analysis
Data were analyzed to determine the proportion of participants who were seropositive, which was defined as the proportion with an hSBA titer of 4 or more, along with Clopper–Pearson 95% confidence intervals. Chi-square and Fisher's exact tests were used to compare proportions. We calculated hSBA geometric mean titers along with 95% confidence intervals. Welch's two-sample t-test was used to compare geometric mean titers. The Pearson correlation coefficient between the log-transformed responses to each pair of strains was calculated. All data were analyzed with the use of Stata software, version 13.1 (StataCorp) and the statistical software system R, version 3.2.1.
Results
Participants
A total of 607 students were enrolled in the study; 99.8% completed the questionnaire, 99.5% provided a blood sample, and 99.2% of the samples were analyzed. Vaccination status was verified through vaccination records for 97.0% of participants. The 18 (3.0%) participants who did not give researchers permission to access their vaccination records all self-reported receiving two doses of 4CMenB. The groups of participants who received two doses of vaccine, those who received one dose, and those who were not vaccinated were similar in age, sex, ethnic group, and race. Unvaccinated participants included a larger proportion of graduate students than did vaccinated participants (Table 1). In the week preceding the survey, 5.3% reported having taken antibiotics. Only 2.5% reported having received antibiotic prophylaxis after being identified as a contact of a person who had received a diagnosis of meningococcal disease during the outbreak. Overall, 143 (23.6%) reported that they had met at least one person who had received a diagnosis of meningococcal disease during the outbreak, although 87 (14.3%) were not sure whether they had met anyone who had received the diagnosis during the outbreak.
Table 1.
Characteristics of the Participants, According to Vaccination Status.*
| Characteristic | Two Doses (N = 566) | One Dose (N = 21) | No Vaccination (N = 20) |
|---|---|---|---|
| Age — yr | 20.5±1.3 | 20.7±1.8 | 22.6±2.3 |
| Sex — no. (%) | |||
| Female | 321 (56.7) | 8 (38.1) | 12 (60.0) |
| Male | 244 (43.1) | 13 (61.9) | 8 (40.0) |
| Race — no. (%) | |||
| Asian or Asian American | 175 (30.9) | 8 (38.1) | 9 (45.0) |
| Black | 50 (8.8) | 1 (4.8) | 3 (15.0) |
| White | 299 (52.8) | 10 (47.6) | 8 (40.0) |
| Mixed race | 34 (6.0) | 2 (9.5) | 0 |
| Ethnic group — no. (%) | |||
| Hispanic | 39 (6.9) | 2 (9.5) | 2 (10.0) |
| Non-Hispanic | 512 (90.5) | 18 (85.7) | 18 (90.0) |
| Class year — no. (%) | |||
| First | 181 (32.0) | 6 (28.6) | 1 (5.0) |
| Second | 139 (24.6) | 5 (23.8) | 5 (25.0) |
| Third | 120 (21.2) | 6 (28.6) | 1 (5.0) |
| Fourth | 126 (22.3) | 2 (9.5) | 5 (25.0) |
| Graduate student | 0 | 2 (9.5) | 8 (40.0) |
The majority of participants (93.2%) completed the two-dose vaccination series, whereas 3.5% received only one dose and 3.3% remained unvaccinated. The majority of the vaccinees who received two doses (89.4%) were vaccinated in December and February, according to the recommended schedule. The majority of those receiving one dose only (81.0%) were vaccinated in December. Secondary analyses of immunity against the outbreak strain for the small number who were vaccinated according to other schedules are included in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Immune Response According to N. Meningitidis B Strain
Outbreak Strain
Among the vaccinees who received two doses, 330 (66.1%; 95% confidence interval [CI], 61.8 to 70.3) were seropositive (hSBA titer, ≥4), along with 10 (58.8%; 95% CI, 32.9 to 81.6) of the vaccinees who received one dose and 4 (21.1%; 95% CI, 6.1 to 45.6) participants who had not been vaccinated (Table 2). Participants who received one dose or two doses were significantly more likely than unvaccinated participants to be seropositive (P = 0.03 and P<0.001, respectively). There was no significant difference in seropositivity between those receiving one or two doses (P = 0.53), although the confidence interval was wide. Students who received two doses of vaccine had significantly higher geometric mean titers than those who were unvaccinated (P<0.001), but there was no significant difference in geometric mean titers between those receiving two doses and those receiving one dose (P = 0.38) or between those receiving one dose and those who remained unvaccinated (P = 0.10) (Table 2).
Table 2.
Seropositivity and Geometric Mean Titers for the Meningococcal B Outbreak Strain According to Vaccination Status.*
| Characteristic | Two Doses (N = 499) | One Dose (N = 17) | No Vaccination (N = 19) |
|---|---|---|---|
| hSBA ≥4 | |||
| No. of participants | 330 | 10 | 4 |
| % (95% CI) | 66.1 (61.8–70.3) | 58.8 (32.9–81.6) | 21.1 (6.1–45.6) |
| GMT (95% CI) | 7.6 (6.7–8.5) | 5.4 (2.5–11.7) | 2.8 (2.3–3.5) |
5/99 Reference Strain
The seropositivity for 5/99 was 96.7% (95% CI, 88.7 to 99.6) among two-dose vaccinees with high hSBA titers against the outbreak strain and 100% (95% CI, 94.1 to 100) among those with a low outbreak response and those with no detectable protective response. Among unvaccinated participants, 5.6% (95% CI, 0.1 to 27.3) were seropositive for 5/99 (Table 3). Vaccinees who received two doses were significantly more likely than unvaccinated students to be seropositive for 5/99, regardless of their level of response to the outbreak strain (P<0.001 for the comparisons between those with high, low, or no response to the outbreak strain and unvaccinated students). There was no significant difference in seropositivity for 5/99 among participants with high, low, or no detectable outbreak response (P = 0.13). Two-dose vaccinees with high, low, or no outbreak response all had high 5/99 geometric mean titers (Table 3); there was no significant difference between the geometric mean titers for those who had high outbreak titers and those who had low titers (P = 0.42) or titers in which no response could be detected (P = 0.44). The geometric mean titers for students who received two doses of vaccine, regardless of outbreak response, were significantly higher than those among students who were not vaccinated (P<0.001 for all comparisons).
Table 3.
Seropositivity and Geometric Mean Titers for the Meningococcal B Vaccine Reference Strains 5/99 and 44/76-SL, According to Vaccination Status.*
| Reference-Strain Characteristic | Two Doses | No Vaccination (N = 18) | ||
|---|---|---|---|---|
| Outbreak-Strain hSBA Response | ||||
| hSBA >8 (N = 61) | hSBA 4-8 (N = 61) | hSBA <4 (N = 61) | ||
| hSBA response ≥4 against 5/99 including NadA | ||||
| No. of participants | 59 | 61 | 61 | 1 |
| % (95% CI) | 96.7 (88.7–99.6) | 100 (94.1–100) | 100 (94.1–100) | 5.6 (0.1–27.3) |
| GMT (95% CI) | 214.2 (152.7–300.5) | 261.6 (181.3–377.3) | 256.3 (187.3–350.7) | 1.2 (1.0–1.5) |
| hSBA response ≥4 against 44/76-SL including fHbp | ||||
| No. of participants | 61 | 59 | 53 | 6 |
| % (95% CI) | 100 (94.1–100) | 96.7 (88.7–99.6) | 86.9 (75.8–94.2) | 33.3 (13.3–59.0) |
| GMT (95% CI) | 178.8 (129.1–247.8) | 36.4 (26.6–49.9) | 17.4 (13.0–23.2) | 3.2 (1.7–5.8) |
44/76-SL Reference Strain
Among participants who received two doses of vaccine and had a high response to the outbreak strain, 100% (95% CI, 94.1 to 100) were seropositive for 44/76-SL, as were 96.7% (95% CI, 88.7 to 99.6) of those with a lower response to the outbreak strain and 86.9% (95% CI, 75.8 to 94.2) of those with no detectable protective titer for the outbreak strain. Among unvaccinated participants, 33.3% (95% CI, 13.3 to 59.0) were seropositive for the 44/76-SL strain (Table 3). Participants with high antibody titers against the outbreak strain were more likely than those with no detectable titers to be seropositive for the 44/76-SL strain (P = 0.006). There was no significant difference in seropositivity for the 44/76-SL strain between those with high titers and those with low titers for antibodies to the outbreak strain (P = 0.50) or between those for whom the titers for the outbreak strain were low or undetectable (P = 0.10). Vaccinated participants who received two doses regardless of outbreak response were significantly more likely than unvaccinated participants to have seropositivity for 44/76-SL (P<0.001 for all comparisons). The hSBA geometric mean titer against 44/76-SL was 178.8 (95% CI, 129.1 to 247.8) among vaccinees who received two doses and had a high response to the outbreak strain, 36.4 (95% CI, 26.6 to 49.9) among those with a low response, and 17.4 (95% CI, 13.0 to 23.2) among those with no detectable response. The hSBA geometric mean titer for unvaccinated participants was 3.2 (95% CI, 1.7 to 5.8) (Table 3). The geometric mean titers for antibodies to the 44/76-SL strain differed significantly between each group of participants who received two doses of vaccine (P<0.001 for all comparisons) and were significantly higher than the geometric mean titers for those who were not vaccinated (P<0.001 for all).
Predictive Value of Response to 44/76-SL
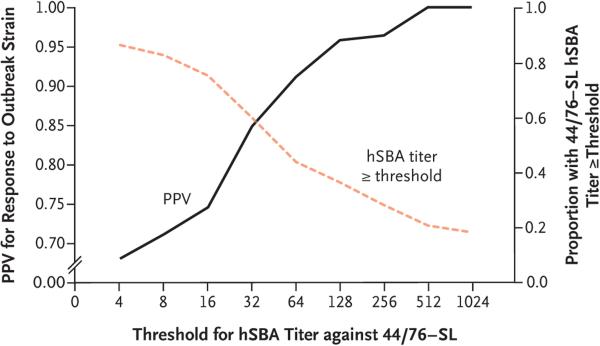
For participants vaccinated with two doses of 4CMenB in accordance with the recommended schedule, there was minimal correlation between the hSBA titers for either of the reference strains (Pearson's correlation coefficient, 0.15; P = 0.04), moderate correlation between the outbreak strain and 44/76-SL (Pearson's correlation coefficient, 0.64; P<0.001), and no correlation between the outbreak strain and 5/99 (Pearson's correlation coefficient, −0.06; P = 0.43). Among all participants, regardless of vaccination status, the response to 44/76-SL was not a reliable indicator of seropositivity for the outbreak strain. Figure 2 summarizes the positive predictive value to illustrate the probability that a person with an hSBA titer against 44/76-SL at or above a range of thresholds will be seropositive (have a titer of ≥4) for the outbreak strain. The assumption that participants with antibody titers of 4 or higher against the 44/76-SL strain will be seropositive for antibodies against the outbreak strain produced a positive predictive value of less than 70%. A positive predictive value of 90% was achieved when there was an hSBA threshold of 64 or higher against the 44/76-SL strain. However, only about half the participants had a hSBA titer of 64 or higher against this strain, yielding a negative predictive value of 50% (with the negative predictive value representing the probability that participants with titers below this range were truly seronegative against the outbreak strain).
Figure 2. Positive Predictive Value for hSBA Immunity against the Outbreak Strain, According to the 44/76-SL Antibody Titer.
The black curve shows the positive predictive value (PPV) of the human serum bactericidal assay (hSBA) for the 44/76-SL reference strain (i.e., the probability that those with a certain hSBA titer against 44/76-SL will be seropositive for the outbreak strain). The orange dashed line shows the proportion of participants with 44/76-SL responses that were greater than or equal to each titer value.
Discussion
Our data indicate that only 66.1% of U.S. university students who were fully vaccinated with 4CMenB had putatively protective immunity against a meningococcal B outbreak strain. This level of seropositivity was lower than expected, given the antigenic similarity between the outbreak strain and the components of the vaccine and given that the Meningococcal Antigen Typing System predicted that 4CMenB would induce responses against the outbreak strain.10,18 We also analyzed immune responses to two of the 4CMenB vaccine reference strains and found that for the 44/76-SL and 5/99 strains, 86.9 to 100% and 96.7 to 100% of students, respectively, who were vaccinated with two doses had putatively protective hSBA titers.
No immunogenicity data on 4CMenB from U.S.-based studies have been published to date, but results from studies conducted elsewhere suggest that the vaccine is immunogenic against the vaccine reference strains after a two-dose series is administered in adolescents and young adults.16,24-27 Our results are similar to those from a clinical trial conducted in Chile among adolescents who had received two doses of 4CMenB, 1 month apart.16 We also found higher seropositivity for the vaccine reference strains (86.9 to 100%) in a subgroup of participants who had no detectable hSBA titer against the outbreak strain, yet there was only moderate correlation between the hSBA response to the outbreak strain and the response to the 44/76-SL strain and no correlation between the outbreak strain and the 5/99 strain. Our results indicate that knowledge of hSBA immunity against the vaccine reference strains is not sufficient to predict individual-level immunity against an outbreak strain, even when the strain expresses one or more antigens that are closely related to the vaccine antigens.
Our comparison of vaccine-induced immunity between those who received two doses of vaccine and those who remained unvaccinated clearly indicates that immunogenicity was higher among those who were vaccinated for all three strains tested. However, 33.9% of the participants who were vaccinated with two doses of 4CMenB did not show evidence of a putatively protective response against the outbreak strain and the geometric mean titer was very low, which shows that not all persons who complete the two-dose vaccination series may have a response. Further evaluation of 4CMenB efficacy is needed because of the limitations in drawing inferences about protection from immunogenicity data alone. It is possible that vaccinees who had no detectable SBA titer against the outbreak strain but who had an immune response to the meningococcal antigens that were used to develop the vaccine may benefit from some degree of protection. Our findings also raise questions about whether a third dose of 4CMenB might increase the proportion of seropositive responses against strains that were not perfectly matched to the vaccine. The prelicensure study that reported the noninferiority of two doses versus three doses of 4CMenB and led to the two-dose recommendation involved vaccine reference strains only.16 The benefit of a third dose against mismatched strains has since been documented.28
These findings have implications for vaccination policies aimed at preventing and controlling meningococcal B disease. The Advisory Committee on Immunization Practices voted for a category A recommendation for meningococcal B vaccine for those 10 years of age or older who are at an increased risk for disease26 and a category B recommendation for those 16 to 23 years of age for short-term protection against most strains causing disease.7 The need for additional data regarding the breadth and duration of protection provided by meningococcal B vaccines was key to the decision-making process. We conducted this study because of the unique opportunity to investigate 4CMenB immunity against a meningococcal strain in which the antigens did not completely match the antigens used in vaccine development. However, our study is limited in that it was an observational study conducted after nearly all students chose to be vaccinated, resulting in a small unvaccinated comparison group. Our findings highlight the need for further postlicensure serologic studies to assess immunity against diverse meningococcal B strains and to better understand the breadth of 4CMenB-induced immunity.
Supplementary Material
Supplement1
Acknowledgments
Supported by grants from the Program on U.S. Health Policy at Princeton University and the Health Grand Challenge at Princeton University (to Dr. Basta), a National Institutes of Health (NIH) Early Independence Award from the Office of the Director (1DP5OD009162, to Dr. Basta), and a grant from the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate of the Department of Homeland Security and the NIH Fogarty International Center (to Drs. Basta and Grenfell).
Dr. Johnsen reports receiving travel support from Pfizer; and Drs. Findlow, Bai, and Borrow, performing contract research for GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur on behalf of their institution.
We thank the research participants for making this study possible and all members of Dr. Ploss's laboratory at Princeton University for processing the blood samples before the analysis was conducted.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9:285–98. doi: 10.1586/erv.10.3. [DOI] [PubMed] [Google Scholar]
- 3.Poland GA. Prevention of meningococcal disease: current use of polysaccha-ride and conjugate vaccines. Clin Infect Dis. 2010;50(Suppl 2):S45–53. doi: 10.1086/648964. [DOI] [PubMed] [Google Scholar]
- 4.Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367:397–403. doi: 10.1016/S0140-6736(06)67932-4. [DOI] [PubMed] [Google Scholar]
- 5.Chang Q, Tzeng YL, Stephens DS. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 2012;4:237–45. doi: 10.2147/CLEP.S28410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR-2):1–28. [PubMed] [Google Scholar]
- 7.MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171–6. doi: 10.15585/mmwr.mm6441a3. [DOI] [PubMed] [Google Scholar]
- 8.Strikas RA. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 0 through 18 years — United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:93–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis: implications for vaccine development and pathogenesis. Lancet. 1983;2:355–7. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 10.McNamara LA, Shumate AM, Johnsen P, et al. First use of a serogroup B meningococcal vaccine in the US in response to a university outbreak. Pediatrics. 2015;135:798–804. doi: 10.1542/peds.2014-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oviedo-Orta E, Ahmed S, Rappuoli R, Black S. Prevention and control of meningococcal outbreaks: the emerging role of serogroup B meningococcal vaccines. Vaccine. 2015;33:3628–35. doi: 10.1016/j.vaccine.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly J, Medini D, Boccadifuoco G, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A. 2010;107:19490–5. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claus H, Jördens MS, Kriz P, et al. Capsule null locus meningococci: typing of antigens used in an investigational multicomponent meningococcus sero-group B vaccine. Vaccine. 2012;30:155–60. doi: 10.1016/j.vaccine.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 14.Plikaytis BD, Stella M, Boccadifuoco G, et al. Interlaboratory standardization of the sandwich enzyme-linked immunosorbent assay designed for MATS, a rapid, reproducible method for estimating the strain coverage of investigational vaccines. Clin Vaccine Immunol. 2012;19:1609–17. doi: 10.1128/CVI.00202-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogel U, Taha MK, Vazquez JA, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis. 2013;13:416–25. doi: 10.1016/S1473-3099(13)70006-9. [DOI] [PubMed] [Google Scholar]
- 16.Santolaya ME, O'Ryan ML, Valenzuela MT, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 ran domised, observer-blind, placebo-controlled study. Lancet. 2012;379:617–24. doi: 10.1016/S0140-6736(11)61713-3. [DOI] [PubMed] [Google Scholar]
- 17.Poolman JT, Richmond P. Multivalent meningococcal serogroup B vaccines: challenges in predicting protection and measuring effectiveness. Expert Rev Vaccines. 2015;14:1277–87. doi: 10.1586/14760584.2015.1071670. [DOI] [PubMed] [Google Scholar]
- 18.Medini D, Stella M, Wassil J. MATS: global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine. Vaccine. 2015;33:2629–36. doi: 10.1016/j.vaccine.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Maslanka SE, Gheesling LL, Libutti DE, et al. Standardization and a multi-laboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–67. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection — serum bactericidal antibody activity. Vaccine. 2005;23:2222–7. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Borrow R, Aaberge IS, Santos GF, et al. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol. 2005;12:970–6. doi: 10.1128/CDLI.12.8.970-976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borrow R, Miller E. Surrogates of protection. In: Frosch M, Maiden MCJ, editors. Handbook of meningococcal disease. Wiley-VCH; Weinheim, Germany: 2006. pp. 323–51. [Google Scholar]
- 23.Rossi R, Beernink PT, Giuntini S, Granoff DM. Susceptibility of meningococcal strains responsible for two sero-group B outbreaks on U.S. university campuses to serum bactericidal activity elicited by the MenB-4C vaccine. Clin Vaccine Immunol. 2015;22:1227–34. doi: 10.1128/CVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Ryan M, Stoddard J, Toneatto D, Wassil J, Dull PM. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74:15–30. doi: 10.1007/s40265-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384:2123–31. doi: 10.1016/S0140-6736(14)60842-4. [DOI] [PubMed] [Google Scholar]
- 26.Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at increased risk for serogroup B meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608–12. [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan T, O'Ryan M, Wassil J, Abitbol V, Dull P. Vaccination with a multicomponent meningococcal B vaccine in preven tion of disease in adolescents and young adults. Vaccine. 2015;33:4437–45. doi: 10.1016/j.vaccine.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Findlow J, Bai X, Findlow H, et al. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine. 2015;33:3322–30. doi: 10.1016/j.vaccine.2015.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1