Comparative Analysis of Metallic Nanoparticles as Exogenous Soft Tissue Contrast for Live In Vivo Micro-Computed Tomography Imaging of Avian Embryonic Morphogenesis (original) (raw)
. Author manuscript; available in PMC: 2017 Oct 1.
Published in final edited form as: Dev Dyn. 2016 Aug 18;245(10):1001–1010. doi: 10.1002/dvdy.24433
Abstract
Background
Gestationally survivable congenital malformations arise during mid-late stages of development that are inaccessible in vivo with traditional optical imaging for assessing long term abnormal patterning. MicroCT is an attractive technology to rapidly and inexpensively generate quantitative 3D datasets but requires exogenous contrast media. Here we establish dose dependent toxicity, persistence, and biodistribution of three different metallic nanoparticles in day 4 chick embryos.
Results
We determined that 110nm alkaline earth metal particles were non-toxic and persisted in the chick embryo for up to 24 hours post injection with contrast enhancement levels at high as 1600HU. 15nm gold nanoparticles persisted with x-ray attenuation higher than that of the surrounding yolk and albumen for up to 8 hours post injection, while 1.9nm particles resulted in lethality by 8 hours. We identified spatial and temporally heterogeneous contrast enhancement ranging from 250-1600HU. With the most optimal 110nm alkaline earth metal particles, we quantified an exponential increase in the tissue perfusion versus distance from the dorsal aorta into the flank over 8 hours with a peak perfusion rate of 0.7um2/s measured at a distance of 0.3mm.
Conclusion
These results demonstrate the safety, efficacy, and opportunity of nanoparticle based contrast media in live embryos for quantitative analysis of embryogenesis.
Keywords: embryo, development, nanoparticles, toxicity, biodistribution, dorsal aorta, perfusion
Introduction
Embryonic development is a rapid and dynamic three-dimensional process characterized by the growth and differentiation of progenitor cell populations into a complex network of organs and supporting tissues. Though much has been learned about the gross anatomical changes that occur during morphogenesis, elucidating the mechanistic interplays between underlying biomechanical and biochemical cues is critical for integrating the genetic and environmental influences on tissue behavior. Outside of the earliest stages of development, our understanding of this highly dynamic process has been gleaned primarily from static reconstructions of animal models. This gap obscures our knowledge of relative growth rates, prioritization, and distinction between association and causality. Genetic mutations that alter early stage patterning are nearly always embryonic lethal, but gestationally survivable malformations arise during the mid-late stages of development after initial fate specification and tissue patterning have begun (Roger et al., 2010). Propagation of even subtle abnormalities at these stages can lead to congenital defects with a range of severities, origins that are largely unknown, and potentially bleak prognoses (Gregg & Butcher, 2013).
High resolution, quantitative dynamic imaging technologies are therefore essential for capturing these emerging knowledge needs in embryonic development. Although high resolution 3D reconstructions from histological sections are commonplace, these are static representations of post-vital organisms. Optical resolution is unparalleled within the sectioning plane but out of plane 3D interpolation between multiple sections is time consuming and suffers from inherent error in embedding and sectioning. The vast majority of live embryonic inquiry has focused on the early events of development spanning gastrulation and heart tube formation with the onset of beating (Cui et al., 2013; Czirók, Rongish, & Little, 2004; Zamir, Czirók, Cui, Little, & Rongish, 2006). Confocal and multiphoton microscopy with excitable and infrared fluorophores is capable of imaging up to 2mm in live samples (Diaspro, Chirico, & Collini, 2005; Dickinson, Simbuerger, Zimmermann, Waters, & Fraser, 2003; Squirrell, Wokosin, White, & Bavister, 1999; Supatto et al., 2005; Supatto, Mcmahon, Fraser, & Stathopoulos, 2009) with limited capacity once the tissue becomes dense and light scattering beyond the earliest stages of development. Live embryonic studies conducted during mid-late stages of development are more limited, requiring a reliable animal model and high resolution, wide field, deep tissue imaging.
Live in vivo imaging with ultrasound and magnetic resonance imaging (MRI) often suffer from restrictions in field of view, signal-to-noise ratio, and differential tissue contrast (Metscher, 2009). Ultrasound is widely used for screening hemodynamic profiles in developing embryos and couples nicely with three dimensional reconstructions for further computation analysis of fluid-solid tissue interactions (Shen et al., 2005; Wessels & Sedmera, 2003; B. Yu et al., 2015; Q. Yu et al., 2004). Whole specimen MRI studies are emerging as radio frequency (rf) coil design and post-processing techniques improve, but MRI remains an expensive and a lengthy scanning process (especially for very high resolutions) requiring additional considerations for environmental control to maintain healthy, viable samples (Gregg & Butcher, 2012). Micro-computed tomography (microCT) is an attractive three dimensional whole specimen imaging technology for morphogenetic inquiry having the resolution and depth of field required to capture mid-late stages of embryonic development with high fidelity at a fraction of the cost of MRI (Gregg & Butcher, 2012).
MicroCT has been a valuable imaging tool for the past 15 years in the field of embryological research characterizing tortuous anatomy with high spatial and temporal resolution (Butcher, Sedmera, Guldberg, & Markwald, 2007). MicroCT imaging produces high resolution three dimensional datasets within minutes at a fraction of the cost of similar technologies (Kim, Min, Recknagel, Riccio, & Butcher, 2011). Advancements in CT dense contrast media and sophisticated protocols enable high tissue boundary identification with general and molecularly targeted exogenous soft tissue contrast (Butcher et al., 2007; Gregg, Recknagel, & Butcher, 2015; Metscher & Müller, 2011; Metscher, 2009; Yalcin, Shekhar, McQuinn, & Butcher, 2011). MicroCT imaging can readily identify multiple and broad three dimensional morphological consequences of transgenic modifications on fixed developing embryos (Degenhardt, Wright, Horng, Padmanabhan, & Epstein, 2010; Johnson et al., 2006). Furthermore, whole mount staining with antibody probes and metal immunodetection has yielded tissue patterning data in the developing chick embryo (Gregg et al., 2015; Metscher & Müller, 2011). Coupled three dimensional microCT anatomical data with Doppler ultrasound has shown to be a power tool for computational analysis of hemodynamic patterning in the developing chick heart (Yalcin et al., 2011). While microCT has served a much needed purpose for high resolution, deep tissue imaging of embryos, the inability to gather longitudinal (time course) data from the same embryo necessitates the preparation of multiple samples for single time points, increasing experimental variability particularly if genetic perturbations or microsurgical manipulations are present. It is essential to extend microCT imaging to live samples to address these limitations. We previously established the possibility for non-toxic live microCT imaging using Visipaque™ (VP) in an avian embryo model (Henning, Jiang, Yalcin, & Butcher, 2011). We showed that radiation from scans up to 7X higher than typical scan dosage did not cause morphological defects for embryos up to day 10 of development. Iodine based molecules, such as VP, have been the standard contrast media for x-ray and CT images proving themselves safe and effective (Scheller et al., 1999). Such free molecule contrast media are < 1 nm, and therefore extravasate quickly with dramatic reduction in contrast after a few hours, too short for meaningful longitudinal analysis from a single injection. Injectable nanoparticle technology has achieved long residence times and high levels of x-ray attenuation, motivating their potential as embryonic imaging contrast (Ashton et al., 2014; Boll et al., 2011; Lee et al., 2014; Nebuloni, Kuhn, & Müller, 2013). Nanoparticles, which can be fabricated out of multiple materials (e.g. gold, earth metals, tungsten, liposomes encapsulating VP), have been used extensively in cancer research as an imaging and delivery tool (Melancon et al., 2014; Zarschler et al., 2014). Additionally, biomolecule conjugation to the nanoparticle surface enables targeting of different tissue types (Melancon et al., 2014; Wen et al., 2001). Nanoparticle based CT contrast media have not been extended to live embryonic imaging to date. In this study we address longitudinal use of nanoparticle based exogenous soft tissue contrast for live microCT imaging of embryonic development in the avian embryo. Intravenous delivery and toxicity of three different metallic nanoparticles were assessed. Furthermore, we analyzed particle material and size contributions on spatial and temporal contrast enhancement of day 4-5 embryos. Lastly, we quantified relative perfusion characteristics via bulk particle movements in embryonic tissue.
We demonstrate that alkaline earth 110nm metallic nanoparticles are more radiopaque within the embryo than their 15nm and 1.9nm gold nanoparticle counterparts. Additionally, we found that the 110nm particles persist longest in the embryo and yields high tissue boundary delineation with time dependent and organ dependent contrast enhancement. The 15nm gold nanoparticles persisted for at least 8 hours post injection and the 1.9nm gold nanoparticles extravasated quickly out of the vasculature into the embryonic tissue and were toxic to the embryo by 8 hours post injection. Lastly, we quantified relative perfusion of the 110nm alkaline earth metal particles moving out of the dorsal aorta into the surrounding tissue based on transient contrast enhancement changes.
Results
Temporal nanoparticle toxicity and x-ray attenuation during in vivo micro-CT imaging
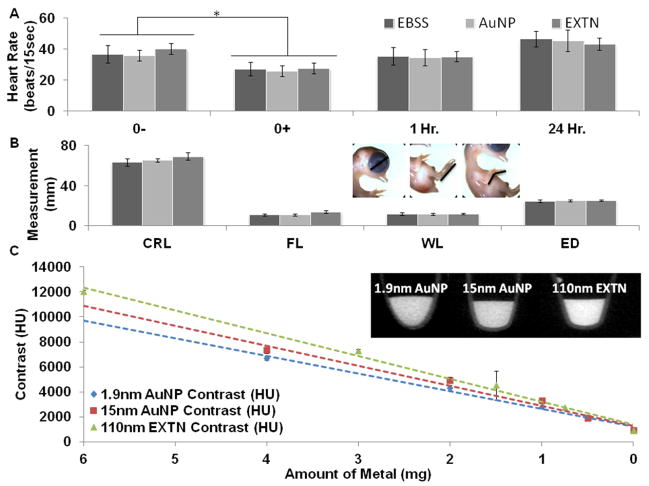
Acute and long-term toxicity from metallic nanoparticle exposure were assessed using heart rate and anatomical analysis, respectively. Embryonic heart rate was recorded immediately before (0-), immediately after (0+), 1 hour, and 24 hours post injection (Figure 1A). Exposure to the microinjection procedure caused a significant but transient drop in heart rate from baseline pre-injections levels in 15nm AuNPs from 143bpm to 104bpm, in 110nmEXTNs from 161bpm to 111bpm, and in EBSS from 147bpm to 109bpm in injected samples (Figure 1A). All heart rates recovered within 1 hour post injection with no significant difference between the 0- pre-injection levels for each respective injected media and between different types of media. This small transient dip is consistent with previous observations from nearly identical experimental procedures resulting in no long term, deleterious consequences to the samples (Henning et al 2011).
Figure 1.
(A) Acute heart rate analysis of day 3 chicks following treatment with metallic nanoparticle contrast media injected at 10% blood volume with EBSS serving as a negative control. A significant heart rate decline was observed immediately post injection (0+) across all contrast medias and EBSS controls (P<0.05) but heart rates returned to their initial values within 1 hour post injection (n = 10, 10, 4). (B) Anatomical analysis one week post exposure to metallic nanoparticle contrast media (day 10 embryos) reveals no morphological defects for either the 15nm AuNP or the 110nm EXTN particles when compared to EBSS controls (P<0.05, n = 9, 9, 4). (C) Modeled particle contrast degradation versus to the amount of metal (mg) present in the solution determined through a dilution series ranging from 100% particle solution to 0% (1X PBS) with inset representative media diluted at 12.5% concentration from the stock solution (n=3).
All samples injected with the 1.9nm AuNP particles died within 8 hours post injection. The concentration of metallic gold material in the 1.9nm AuNP is identical to the 15nm AuNP particles with the only difference being the size of particles themselves, indicating that toxicity is not only dose dependent but also size dependent of the particle (Pan et al., 2009). With the exception of embryos injected with the 1.9nm AuNP particles, viability of embryos injected with either the 15nm AuNP or the 110nm EXTN nanoparticles was no different than that of controls. We next analyzed resulting embryonic morphology through anatomical measurements of day 10 embryos to see if particles induced malformations during development. No gross anatomical malformations were observed between samples injected with particles as compared to EBSS control (Figure 1B). The WL, FL, ED, and total CRL were consistent with no statistically significant differences between the samples injected with 15nm AuNPs or 110nm EXTNs as compared to measurements obtained from the EBSS controls suggesting that development proceeded normally in all nanoparticle exposed embryos. Subsequent scans on Day 10 embryos that were injected at Day 4 were inspected for gross internal organ malformations (e.g. heart, eye, brain, liver size/shape/positions), of which none were found. This was consistent with our previous contrast injection study (Henning et al., 2011).
As expected, we identified a linear reduction of x-ray attenuation with contrast dilution for all particle types considered (Figure 1C). The amount of metal was extrapolated from known metal concentrations in all particle stock solutions. The upper limit of contrast enhancement achieved for each particle type at a 10% injection volume is 1800HU, 1940HU, and 2500HU for the 1.9nm AuNP, 15nm AuNP, and 110nm EXTN respectively.
Comparison of local contrast enhancement between particle types
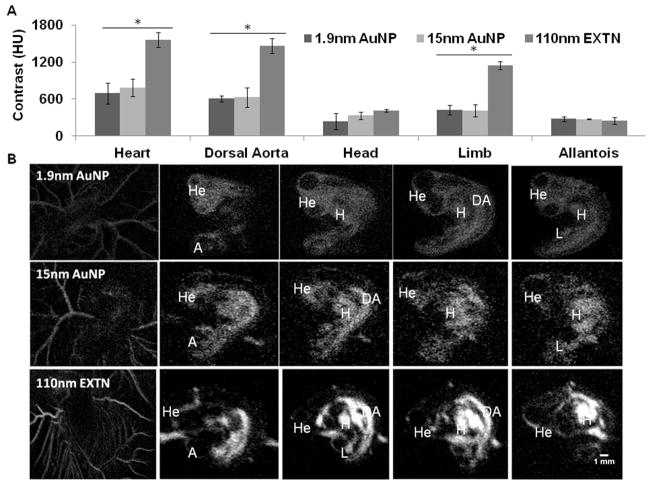
Comparison of spatial particle distribution demonstrates greater heterogeneity with the 110nm EXTN particles as compared to the smaller 1.9nm and 15nm AuNP particles. Structures associated with the vascular system, particularly the heart, dorsal aorta, and extra embryonic vasculature were visualized immediately post injection with representative images being taken within the first 30 minutes post injection (Figure 2B). Contrast enhancement was measured in the heart, dorsal aorta, head measured at the mesencephalon, limb, and allantoic sac immediately post injection (Figure 2A). The contrast enhancement produced by the 110nm EXTN particles was significantly higher in the heart, dorsal aorta, and limb with levels as high as 1560HU immediately post injection (p<0.05). Contrast enhancement was the same in the head and allantois between all particle types considered with no significant differences measured immediately post injection (Figure 2A). Visualization of sagittal image sections in representative embryos (Figure 2B) demonstrates qualitative spatial contrast enhancement differences within the same embryo and across particle types. The 1.9nm particles quickly extravasate from the vasculature and luminal spaces into the surrounding tissue, an observation not comparatively seen with the 110nm EXTN particles suggesting a size specific component to particle perfusion into the tissue.
Figure 2.
(A) Comparison of peak contrast enhancement between three different metallic nanoparticle contrast media microinjected into day 4 chick embryos at 10% blood volume. Contrast enhancement was measured within the heart, dorsal aorta, head, limb, and allantoic sac (n = 3). Contrast was significantly higher in embryos treated with 110nm EXTN particles (P<0.05) for the heart, dorsal aorta, and limb. (B) Representative 2D grayscale cross section images through the sagittal plane labeled with developing organ systems of day 4 chick embryos. 110nm EXTN particles demonstrate higher x-ray attenuation and soft tissue contrast enhancement as compared to the gold nanoparticles. Furthermore, a size dependent extravasation is suggested due to larger particles retained in the vasculature whereas the smallest 1.9nm AuNP particles have extravasated into the surrounding tissue.
H = heart, He = head, DA = dorsal aorta, L = limb, and A = allantois
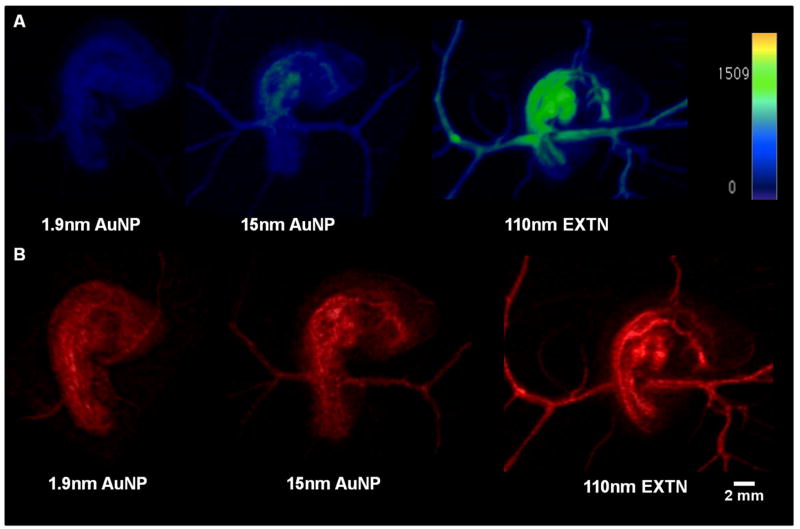
Three dimensional renderings show maximum intensity and volumetric information of the embryo and surrounding extra embryonic vasculature (Figure 3). Maximum intensity projections (MIP) were generated and mapped to the same color intensity legend (Figure 3A) between all particle types allowing direct comparison of spatial contrast intensity revealing particle locations and their relative concentrations. The 1.9nm AuNP MIP rendering has homogenous particle distribution within the embryo with no clear concentrations throughout the volume suggesting fast extravasation and distribution of particles through the tissue (Figure 3A). 3D analysis of embryos injected with the 15nm AuNPs reveals minimal concentration of the particles within the heart and dorsal aorta that is most proximal to the heart indicated through the color map (Figure 3A). These results are also consistent with the measured contrast values (Figure 2A) with highest enhancement found in the cardiovascular system. The largest heterogeneity of spatial particle contrast enhancement is visualized with the 110nm EXTN particles (Figure 3A). High enhancement is observed in the heart, dorsal aorta, and vitelline vascular network denoted by the shift in the color map whereas the head and allantois have minimal particle concentration (Figure 3A, supplement video). Volumetric renderings reveal all density information of the embryo (Figure 3B). Subtleties of soft tissues around highly vascularized tissues are revealed in more detail, particularly soft tissues surrounding the dorsal aorta and somites on the dorsal side of the embryo (Figure 3B) best seen with the 110nm EXTN particles.
Figure 3.
Representative 3D renderings of day 4 chick embryos microinjected with different metallic nanoparticle contrast media and imaged at 50um resolution with 800 projection scans. (A) Maximum intensity projections (MIP) of day 4 chicks calibrated to a single contrast enhancement color map given in the image denoting localized higher and lower contrast values measured in HU and (B) volumetric renderings of day 4 chicks injected with different contrast media.
Supplement: 3D MIP movie
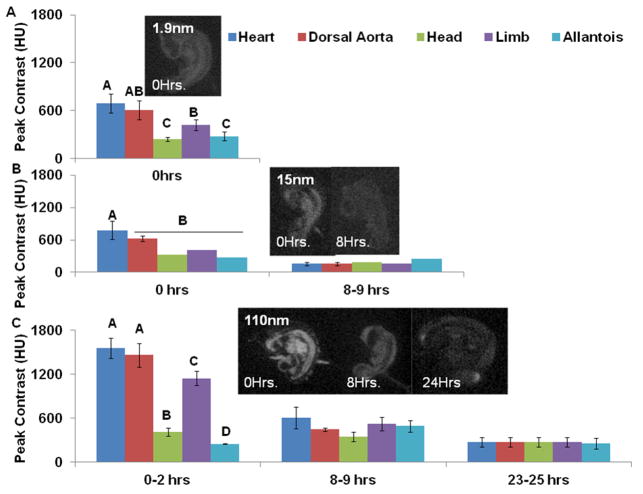
Temporally changing organ specific contrast enhancement
Contrast persistence and distribution was monitored for up to 24 hours post injection. The 1.9nm AuNP embryos did not survive to the 8-9 hour time point but the 15nm AuNP and 110nm EXTN embryos survived for 24 hours post injection for a total of three imaging time points. Heterogeneous spatial contrast enhancement was observed in all three particle types immediately post injection with the highest contrast enhancement associated with the cardiovascular system (Figure 4 A-C). Over a 24 hour period of time, contrast enhancement degraded in both 15nm AuNP and 110nm EXTN particles in the heart, dorsal aorta, limb, and head. At 8 hours post injection with the 15nm AuNPs, all organs considered were statistically the same with contrast levels ranging from 160HU – 250HU (Figure 4B). However, the 110nm EXTN particles at 8 hours post injection had statistically similar contrast enhancement in all organs but the overall contrast intensity was higher than that of the 15nm AuNPs with levels ranging from 350HU – 600HU (Figure 4C). At 24 hours post injection, contrast enhancement in the 15nm AuNP embryos was not distinguishable from background noise suggesting that the particles had extravasated out of the embryonic body into the extra embryonic space with homogenous distribution between the embryo and the albumen. At 24 hours post injection, contrast enhancement of 110nm EXTN embryos was nearly identical in all organs considered with an average value of 272 +/- 7 HU Figure 4C). Specific tissue delineation in the 24 hour 110nm EXTN embryos was not readily visualized given the equal distribution of the particles within all tissues but the overall embryonic body was seen as compared to the background.
Figure 4.
Temporal contrast enhancement comparison (A) Contrast enhancement of the 1.9nm AuNP particles across multiple organ systems. Contrast extravasated quickly from the vasculature into the surround tissue and all samples died within 8 hours post injection. (B) Contrast enhancement of the 15nm AuNP particles over 8 hours post injection. Cardiovascular structures were visualized readily immediately post injection and by 8 hours contrast enhancement was the same across the embryo. Embryos survived for 24 hours post injection but contrast enhancement at 24 hours was not different from the background resulting in zero visualization of the embryo. (C) Contrast enhancement in day 4 embryos treated with 110nm EXTN particles produced the highest levels of contrast with strong attenuation in structures with high vascularity but enhancement across the embryo equalized by 8 hours post injection but tissue boundaries were still readily visualized. At 24 hours post injection, tissue boundaries were not readily seen but the embryonic body was visualized as compared to background.
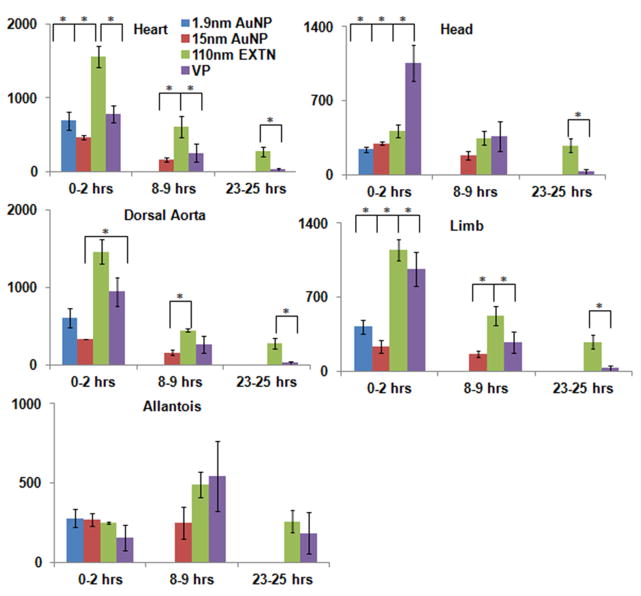
Comparison to previously published VP data (Henning et al., 2011) reveals that the 110nm EXTN particles outperform all other nanoparticle types and VP. The heart and dorsal aorta have significantly higher contrast enhancement in the 110nm EXTN embryos versus the VP embryos with values of 1557HU and 1462HU for 110nm EXTN and 786HU and 945HU for VP in the heart and dorsal aorta respectively immediately post injection (Figure 5). Furthermore, analysis 24 hours later reveals persistence of the 110nm EXTN levels significantly higher than all organs considered except the allantois than the VP (Figure 5).
Figure 5.
Direct metallic nanoparticle comparison as compared to Visipaque. Contrast enhancement in each organ system considered for this study was compared across all three particles types presented against the previously published VP. The 110nm EXTN particles outperformed VP particularly in the cardiovascular structures, maintaining significantly high attenuation at 8 hours post injection in the hearts, dorsal aorta, and limb. At 24 hours post injection, the 110nm EXTN particles outperformed the VP demonstrating high levels of contrast enhancement in the embryonic body.
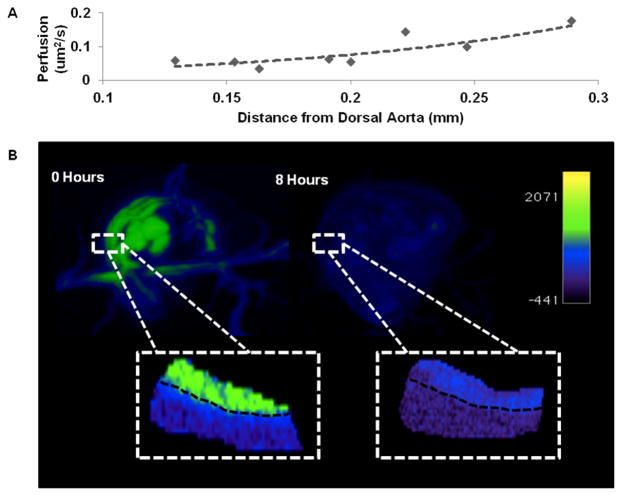
Modeled particle perfusion from the dorsal aorta into the surrounding tissue
Nanoparticles perfuse across the endothelium of the vasculature into the surrounding tissue of the embryonic body prior to perfusing into the remaining extra-embryonic space. Unique to the 110nm EXTN particles, perfusion of the particles from the vascular system into the surrounding tissue can be quantified within the first 8 hours post injection where tissue boundaries are still visualized, enabling quantification of particle movement rates out of major vascular structures. Contrast enhancement per amount of metal is known from the dilution data previously given (Figure 1C) and the stock solution of the EXTN particles is 300mg/mL of metal. We chose to quantify perfusion from the dorsal aorta into the flank mesenchyme. The perfusion coefficient versus distance (Figure 6A) increases exponentially with increasing distance from the dorsal aorta. A faster rate of perfusion farther from the dorsal aorta is correlative to lower contrast enhancement away from the dorsal aorta (Figure 6B). Measured perfusion rates ranged from 0.06um2/s to 0.7um2/s for distances ranging from 0.1mm – 0.3mm respectively. MIP 3D reconstructions reveal overall contrast enhancement based on a controlled color map (Figure 6B) at the two time points considered. Inset images, magnified to the same portion of the dorsal aorta, reveal changes in contrast enhancement over an 8 hour period. These images reveal a significant decrease in contrast enhancement within the dorsal aorta and decreasing contrast enhancement as distance increases away from the dorsal aorta indicating the increasing rate of perfusion with distance (Figure 6).
Figure 6.
(A) Modeled particle diffusion over an 8 hour period of time from the dorsal aorta, sampled above the vitelline vein. Contrast enhancement was measured at known distances away from the dorsal aorta at 8 hours post injection. Concentration and amount of metal values were derived from standard curves previously established (Figure 1). Fick’s first law of diffusion was used to interpolate the diffusion coefficient as a function of distance from the dorsal aorta 8 hours post injection. (B) representative maximum intensity projections of immediately post injection (0 hours) and 8 hour post injection chick embryos with magnified insets of the dorsal aorta (black dashed line) and surrounding tissue. Color map based on contrast enhancement (HU) given in the legend.
Discussion
We demonstrate that nanoparticle based contrast enhancement is safe and effective for live in vivo microCT imaging of embryonic development. We show that the 110nm EXTN particles surpass the gold nanoparticles in contrast enhancement, residence time, and identifiable tissue boundaries. Furthermore, we show that contrast enhancement of the embryo remains at high levels 24 hours post injection than the previously published VP. The best images of the cardiovascular structures was seen immediately post injection whereas the best analysis of the allantois is 8 hours post injection when contrast has reached its peak. Furthermore, our data suggests alternate clearance mechanisms than that observed with the VP studies, indicating extravasation of particles into the tissue where it further moves down a concentration gradient out of the embryonic body. Additionally, particles may also be transported into the cell via endocytosis, a mechanism that is out of the scope of this study but indicates future experimental opportunities. Given the increased time in reliable tissue boundary recognition, quantitative analysis into transport processes is possible. Here we present a method for characterizing relative perfusion coefficients from the dorsal aorta into the surround tissue demonstrating the ability for powerful insights into passive and active transport phenomena within live embryos.
MicroCT is a promising tool in 3D embryonic imaging for mid-late stage in vivo studies fulfilling the needed resolution and depth of field required for capturing these stages of development (Gregg & Butcher, 2012). Previous studies established the dose dependent toxicity of x-ray radiation on avian embryos (Henning et al., 2011) determining that a radiation dose of 798mGy would be required to induce morphological defects within the time period being considered, a level far above what is required for microCT imaging and what was presented in this study. The most pressing challenge associated with live imaging of embryos using microCT is the lack of available non-toxic, non-teratogenic contrast media. A number of exogenous soft tissue contrast agents have been used in embryonic imaging (Butcher et al 2007) but nearly all contrast media has proven to be lethal. A detailed explanation of all contrast media, excluding the media used in this study, has been given in previous literature (Gregg et al., 2015). Prior to this study, the only non-toxic contrast media for live embryonic microCT imaging to our knowledge was VP (Henning et al., 2011) becoming the standard of comparison for live embryonic microCT imaging. In this study, the 110nm EXTN particles outperformed all other contrast media including VP. EXTN particles remained in the vasculature the longest with enhancement remaining up to 8 hours post injection. After 24 hours post injection, while vascular contrast was not distinguishable from the embryonic tissue, contrast enhancement remained within the embryonic body. Differences between nanoparticles in rates of extravasation and clearance from the embryo proper suggest a size dependent mechanism. For the metallic particles to extravaste into the surrounding embryonic tissue, they must cross the endothelium. The primitive vasculature is active and present throughout the time period of the presented study (days 4-5) but the blood vessels are immature in structure and function. Basic vascular architecture arises in the earliest stages of development prior to blood flow initiation (Sabin, 1917). Fusion and transformation of endothelial and angioblast progenitor cells into lamina produce the primitive vascular networks (vasculogenesis), from which sprouting and capillary formation occurs (angiogenesis). Primary blood vessels undergo much remodeling to eventually form the mature vascular system at the end of development (Risau & Flamme, 1995). A vast majority of vessels that arise throughout development regress, serving functions only for the development of a variety of different organ systems such as capillaries found in the prechongrogenic areas of the embryo (Risau & Flamme, 1995). Continuous sprouting and regression of blood vessels results in an immature endothelium during much of development. Endothelial lamina and other structural component (organelles, junction components) are found in the chick embryo vessels by day 4 of development (Roy, Hirano, Kochen, & Zimmerman, 1974) but basement membrane structure is ill formed until day 18 of chick development and the tight junction complexes are “leaky” until the latest stages of development (Roy et al., 1974) indicating that extravasation of molecules, and in the case of this study, particles will readily occur across the endothelium.
Without a developed renal system, primitive filtering in embryos at the stages considered is in part attributed to the allantois. In early mid stage embryos (days 4-5), the allantois serves to store and excrete nitrogenous metabolites from the embryo (Bellairs & Osmond, 2014) with increasing ability of active transport across the allantoic epithelium (Graves, Dunn, & Brown, 1986). Henning et al found that VP was readily filtered into the allantoic sac. These observations are contrary to what was found in this study, suggesting a different mechanism for nanoparticle clearance from the embryonic body through either passive or active means. While contrast in the allantois was significantly less than other organ systems considered immediately post injection, the transient increase in enhancement can be due to many reasons namely slower transport and less permeable blood vessels into the allantois resulting in a time lag for contrast enhancement of the allantois.
The use of nanoparticle based soft tissue contrast presents the questions of particle toxicity and stipulations for the use in live embryonic systems. Previous studies and literature reviews outline the known toxicity of gold nanoparticles with several metrics including MTT assays for metabolic disruption, apoptosis, and oxidative stress (Aillon, Xie, El-Gendy, Berkland, & Forrest, 2009; Jia et al., 2009; Pan et al., 2009). Some of the most influential attributes of nanoparticles that result in toxic effects to cell populations include particle size, dose, and surface charge. Pan et al found that smaller particles (<2nm) exhibit mitochondrial damage, necrosis, and oxidative stress whereas 15nm gold particles did not (Pan et al., 2009). Additionally, particles less than a couple of nanometers in diameter have shown to have chemical reactivity that is not observed with larger sized particles (Turner et al., 2008). Goodman and colleagues indicated that cationic particles display toxic effects suspected of malignant interactions with the negatively charged cellular membrane whereas the anionic counterpart to these particles were non-toxic (Goodman, McCusker, Yilmaz, & Rotello, 2004). We have found that approximately 10% blood volume bolus injections with material concentrations as high as 300mg/mL to be non-toxic. Preliminarily, we have observed that volume injections approaching or exceeding 20% blood volume result in a non-recoverable decrease in heart rate
The data presented here captures static live imaging at a single stage of development, future studies and the development of new technology could address such questions as the biodistribution and residence time of particles in older stage embryos (days 4-10) with changing tissue architecture and material properties affecting the behavior of the particles. Extension of the 110nm EXTN particles into later stage embryos will elucidate changes in material properties and extracellular environments. Differential particle movements within the tissue and changes in clearance patterns – indicative of renal system development – will give critical insights into key morphogenetic events occurring simultaneously through the entire embryo. Furthermore, avian embryos develop in a similar manner as mammals capable of developing relevant congenital malformations through surgical manipulation or genetic perturbation (Hogers, DeRuiter, Gittenberger-de Groot, & Poelmann, 1999). Parsing out tissue contributions and changes in morphogenesis within live, diseased embryos with clinically relevant defects is paramount for quantitative 3D analysis of dysregulation during abnormal development. With the development of novel and adaptation of current technologies for mid-late stage live in vivo microCT imaging of embryonic development, our understandings and capabilities of studying this elusive stage of morphogenesis will be realized. Coupling high resolution 3D imaging technologies with long term tissue contrast will profoundly increase our current understanding of fundamental morphogenetic analysis.
Experimental Procedures
Preparation of embryonic samples and in vivo microinjections
White leghorn eggs (Cornell Poultry) were incubated for 3 days at 99.5°F and 55% humidity. After three days, embryos were cultured in ovo from a method previously described in (Nakamura & Funahashi, 2001). Eggs were cleaned with 70% ethanol and a small opening was created at the narrow end of the egg with the point of sterile dissection scissors. Approximately 6-7mL of albumen was removed using a 21 gauge needle and the opening was secured with a small piece of masking tape. Two large strips of masking tape were placed down the egg and a small window was made in the egg shell using curved dissection scissors allowing visualization of the embryo. The window was covered with sterilized plastic wrap. Following in ovo culture, the embryos were housed in sterilized Styrofoam incubators maintained at appropriate temperature and humidity for proper development for the duration of the experiment.
A gravity driven pressure gradient and micromanipulator were used to microinject chick embryos as previously described by Butcher and colleagues (Butcher et al., 2007). Borosilicate capillary tubes (OD 1.00mm, ID 0.75mm) were drawn into microneedles and beveled at a 45° angle using a microforge forming needles 20μm in diameter. Soft tissues were visualized through a dissecting light microscope and contrast media was injected through extraembryonic vessels in the vitelline network.
Contrast media toxicity based on heart rate and morphology
Day 3 chick embryos were injected with approximately 10% blood volume, in line with previously described literature (Henning et al., 2011). Three different nanoparticles were tested: a 110nm alkaline earth metal particles (110nm EXTN, Miltenyi Biotec. Inc., n=4) and two gold nanoparticles (1.9nm AuNP, 15nm AuNP, Nanoprobes Inc., n=9). The heart rate was monitored immediately before and after injection, 1 hour, and 24 hour post-injection. Earl’s balanced salt solution (EBSS, n=9) served as an injection sham control. At day 10 of development, the embryos were sacrificed and fixed in 4% paraformaldehyde (PFA). Images of the embryos were taken on a stereo Zeiss microscope and anatomical measurements were analyzed in ImageJ. The wing length (WL), foot length (FL), eye diameter (ED), and crown-rump length (CRL) were measured in triplicate for each sample. The WL and FL were measured from the bend the limb to the furthest tip following the curvature of the limb. The eye diameter was measured across the middle of the eye with the head aligned flat with the microscope and the CRL was measured from the tip of the beak to the tail following the curvature from the ventral side of the face, following cranial to the top of the head and then caudally down the backside to the tail. All sample measurements were compared against EBSS injected controls.
In vivo microCT scanning
Day 4 (n=3) nanoparticle injected embryos were taken from the portable Styrofoam incubator and placed in a custom built polycarbonate imaging chamber. All imaging was completed on a GE Healthcare eXplore CT 120 scanner. Embryos were scanned at 50μm for 5 minutes with a total of 800 projections with a voltage of 80kV. Post-processing was completed in MicroView (GE Healthcare) for quantification of contrast levels and OsiriX (Apple) for three dimensional reconstructions. Contrast intensity gray scale values were converted to Hounsfield units (HU), correlating to bone material density (Badea, M, Holdsworth, Johnson, & Angiography, 2008) from calibration to a bone standard phantom with known gross densities reflective of cortical bone (SB3, GE). Contrast enhancement measurements reported reflect the attenuation within the embryo above that of the surrounding yolk and albumen attenuation.
Image analysis and quantification
Virtual 2D cross sections were analyzed in MicroView using the Line Tool, measuring the contrast intensity of the heart, dorsal aorta, limb, head, and allantois. Contrast enhancement and degradation were modeled over a 24 hour period of time. DICOM image stacks were imported into OsiriX and used to produce maximum intensity projections (MIP) and volumetric renderings (VR) of representative day 4 samples.
Particle perfusion quantification from the dorsal aorta immediately post injection and 8 hour post injection images were used for contrast enhancement measurements at known distance from the dorsal aorta. Measurements were taken parallel to the dorsal aorta at known variable distances in multiple (n= 3) embryos. Contrast enhancement inside the dorsal aorta at 0 hours was used as the initial concentration value based on the contrast enhancement. All measurements were normalized to the background attenuation (albumen and yolk). We approximated the flank perfusion as a radially symmetric process (emanating from the vessel wall) resulting in a one-dimensional axi-symmetric system. Fick’s First Law (Equation 1) was used to calculate the relative perfusion with respect to a perpendicular distance away from the dorsal aorta.
The flux (J) was determined through a 1mm2 area where individual contrast measurements were taken. The change in concentration (dC) with respect to the change in distance (dX) was determined based on the differences between the 8 hour post injection image versus the 0 hour image with the initial concentration being the amount of contrast inside the dorsal aorta and the initial distance being the wall of the aorta. The perfusion (P, reported in μm2/s) was determined from Equation 1 with the flux (J), change in concentration (dC), and change in distance (dX) determined from the image data.
Statistics
One way and two way ANOVA and t-test was used to compare contrast enhancement as described in the results. Heart rate and anatomical measurements were compared to EBSS controls via t-test. Data are presented as a mean and standard deviation. P<0.05 denoted statistical significance.
Supplementary Material
Supp Movie S1
Acknowledgments
The authors acknowledge Heming Zhao for her laboratory assistance and dedication to the project. Furthermore, the authors acknowledge Mark Riccio and Dr. Fred VonStein for their technical assistance in the operation of the microCT machine and image reconstructions along with the Cornell Institute for Biotechnology. J.T.B is funded by the National Heart, Lung, and Blood Institute, National Science Foundation, and the Hartwell Foundation. C.L.G. is funded by the National Heart, Lung, and Blood Institute (HL110328, HL128745) and the National Science Foundation (CBET-0955172).
References
- Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Advanced Drug Delivery Reviews. 2009;61(6):457–466. doi: 10.1016/j.addr.2009.03.010. http://doi.org/10.1016/j.addr.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JR, Clark DP, Moding EJ, Ghaghada K, Kirsch DG, West JL, Badea CT. Dual-energy micro-CT functional imaging of primary lung cancer in mice using gold and iodine nanoparticle contrast agents: a validation study. PloS One. 2014;9(2):e88129. doi: 10.1371/journal.pone.0088129. http://doi.org/10.1371/journal.pone.0088129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea C, D M, Holdsworth D, Johnson G, Angiography S. In vivo small animal imaging using micro-CT and digital subtraction angiography. Physics in Medicine and Biology. 2008;53(19):1–36. doi: 10.1088/0031-9155/53/19/R01. http://doi.org/10.1088/0031-9155/53/19/R01.In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs R, Osmond M. Atlas of Chick Development. 2014 http://doi.org/10.1016/B978-0-12-384951-9.00013-7.
- Boll H, Nittka S, Doyon F, Neumaier M, Marx A, Kramer M, Brockmann Ma, et al. Micro-CT based experimental liver imaging using a nanoparticulate contrast agent: a longitudinal study in mice. PloS One. 2011;6(9):e25692. doi: 10.1371/journal.pone.0025692. http://doi.org/10.1371/journal.pone.0025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JT, Sedmera D, Guldberg RE, Markwald RR. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Developmental Dynamics : An Official Publication of the American Association of Anatomists. 2007;236(3):802–9. doi: 10.1002/dvdy.20962. http://doi.org/10.1002/dvdy.20962. [DOI] [PubMed] [Google Scholar]
- Cui C, Filla MB, Jones EaV, Lansford R, Cheuvront T, Al-Roubaie S, Little CD, et al. Embryogenesis of the first circulating endothelial cells. PloS One. 2013;8(5):e60841. doi: 10.1371/journal.pone.0060841. http://doi.org/10.1371/journal.pone.0060841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czirók A, Rongish BJ, Little CD. Extracellular matrix dynamics during vertebrate axis formation. Developmental Biology. 2004;268(1):111–22. doi: 10.1016/j.ydbio.2003.09.040. http://doi.org/10.1016/j.ydbio.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Wright AC, Horng D, Padmanabhan A, Epstein Ja. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circulation Cardiovascular Imaging. 2010;3(3):314–22. doi: 10.1161/CIRCIMAGING.109.918482. http://doi.org/10.1161/CIRCIMAGING.109.918482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaspro A, Chirico G, Collini M. Two-photon fluorescence excitation and related techniques in biological microscopy. Quarterly Reviews of Biophysics. 2005;38(2):97–166. doi: 10.1017/S0033583505004129. http://doi.org/10.1017/S0033583505004129. [DOI] [PubMed] [Google Scholar]
- Dickinson ME, Simbuerger E, Zimmermann B, Waters CW, Fraser SE. Multiphoton excitation spectra in biological samples. Journal of Biomedical Optics. 2003;8(3):329–38. doi: 10.1117/1.1583734. http://doi.org/10.1117/1.1583734. [DOI] [PubMed] [Google Scholar]
- Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chemistry. 2004;15(4):897–900. doi: 10.1021/bc049951i. http://doi.org/10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- Graves JS, Dunn BE, Brown SC. Embryonic chick allantois: functional isolation and development of sodium transport. The American Journal of Physiology. 1986;251(5 Pt 1):C787–94. doi: 10.1152/ajpcell.1986.251.5.C787. Retrieved from http://ajpcell.physiology.org/content/251/5/C787.abstract. [DOI] [PubMed] [Google Scholar]
- Gregg CL, Butcher JT. Quantitative In Vivo Imaging of Embryonic Development: Opportunities and Challenges. Differentiation. 2012;84(1):149–162. doi: 10.1016/j.diff.2012.05.003. http://doi.org/10.1016/j.diff.2012.05.003.Quantitative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg CL, Butcher JT. Translational paradigms in scientific and clinical imaging of cardiac development. Birth Defects Research Part C, Embryo Today : Reviews. 2013;99(2):106–20. doi: 10.1002/bdrc.21034. http://doi.org/10.1002/bdrc.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg CL, Recknagel AK, Butcher JT. Micro/Nano-Computed Tomography Technology for Quantitative Dynamic, Multi-scale Imaging of Morphogenesis. Methods in Molecular Biology (Clifton, NJ) 2015;1189:47–61. doi: 10.1007/978-1-4939-1164-6_4. http://doi.org/10.1007/978-1-4939-1164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning AL, Jiang MX, Yalcin HC, Butcher JT. Quantitative three-dimensional imaging of live avian embryonic morphogenesis via micro-computed tomography. Developmental Dynamics : An Official Publication of the American Association of Anatomists. 2011;240(8):1949–57. doi: 10.1002/dvdy.22694. http://doi.org/10.1002/dvdy.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovascular Research. 1999;41(1):87–99. doi: 10.1016/s0008-6363(98)00218-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10325956. [DOI] [PubMed] [Google Scholar]
- Jia HY, Liu Y, Zhang XJ, Han L, Du LB, Tian Q, Xu YC. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. Journal of the American Chemical Society. 2009;131(1):40–41. doi: 10.1021/ja808033w. http://doi.org/10.1021/ja808033w. [DOI] [PubMed] [Google Scholar]
- Johnson JT, Hansen MS, Wu I, Healy LJ, Johnson CR, Jones GM, Keller C, et al. Virtual histology of transgenic mouse embryos for high-throughput phenotyping. PLoS Genetics. 2006;2(4):e61. doi: 10.1371/journal.pgen.0020061. http://doi.org/10.1371/journal.pgen.0020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Min J, Recknagel AK, Riccio M, Butcher JT. Quantitative Three- Dimensional Analysis of Embryonic Chick Morphogenesis Via Microcomputed Tomography. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2011;294(1):1–10. doi: 10.1002/ar.21336. http://doi.org/10.1002/ar.21276. [DOI] [PubMed] [Google Scholar]
- Lee C-L, Min H, Befera N, Clark D, Qi Y, Das S, Kirsch DG, et al. Assessing Cardiac Injury in Mice wit hDual Energy-microCT, 4D-microCT and microSPECT Imaging Following Partial-Heart Irradiation. Int J Radiat Oncol Biol Phys. 2014;88(3):686–693. doi: 10.1016/j.ijrobp.2013.11.238. http://doi.org/10.1016/j.ijrobp.2013.11.238.Assessing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon MP, Zhou M, Zhang R, Xiong C, Allen P, Wen X, Li C, et al. Selective uptake and imaging of aptamer- and antibody-conjugated hollow nanospheres targeted to epidermal growth factor receptors overexpressed in head and neck cancer. ACS Nano. 2014;8(5):4530–8. doi: 10.1021/nn406632u. http://doi.org/10.1021/nn406632u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metscher BD. MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Developmental Dynamics : An Official Publication of the American Association of Anatomists. 2009;238(3):632–40. doi: 10.1002/dvdy.21857. http://doi.org/10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- Metscher BD, Müller GB. MicroCT for molecular imaging: quantitative visualization of complete three-dimensional distributions of gene products in embryonic limbs. Developmental Dynamics : An Official Publication of the American Association of Anatomists. 2011;240(10):2301–8. doi: 10.1002/dvdy.22733. http://doi.org/10.1002/dvdy.22733. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Funahashi J. Introduction of DNA into chick embryos by in ovo electroporation. Methods (San Diego, Calif) 2001;24(1):43–8. doi: 10.1006/meth.2001.1155. http://doi.org/10.1006/meth.2001.1155. [DOI] [PubMed] [Google Scholar]
- Nebuloni L, Kuhn Ga, Müller R. A comparative analysis of water-soluble and blood-pool contrast agents for in vivo vascular imaging with micro-CT. Academic Radiology. 2013;20(10):1247–55. doi: 10.1016/j.acra.2013.06.003. http://doi.org/10.1016/j.acra.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Jahnen-Dechent W, et al. Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small. 2009;5(18):2067–2076. doi: 10.1002/smll.200900466. http://doi.org/10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. V asculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Wylie-Rosett J, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 2010:1–193. doi: 10.1161/CIR.0b013e3182009701. http://doi.org/10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed]
- Roy S, Hirano A, Kochen JA, Zimmerman HM. The fine structure of cerebral blood vessels in chick embryo. Acta Neuropathologica. 1974;30(4):277–285. doi: 10.1007/BF00697010. [DOI] [PubMed] [Google Scholar]
- Sabin F. Origin and Development of the Primitive Vessels of the Chick and of the Pig. Carnegie Institution of Washington. 1917;6(226):64. [Google Scholar]
- Scheller B, Hennen B, Thünenkötter T, Mrowietz C, Markwirth T, Schieffer H, Jung F. Effect of X-ray contrast media on blood flow properties after coronary angiography. Thrombosis Research. 1999;96(4):253–60. doi: 10.1016/s0049-3848(99)00108-5. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10593427. [DOI] [PubMed] [Google Scholar]
- Shen Y, Leatherbury L, Rosenthal J, Yu Q, Pappas Ma, Wessels A, Lo CW, et al. Cardiovascular phenotyping of fetal mice by noninvasive high-frequency ultrasound facilitates recovery of ENU-induced mutations causing congenital cardiac and extracardiac defects. Physiological Genomics. 2005;24(1):23–36. doi: 10.1152/physiolgenomics.00129.2005. http://doi.org/10.1152/physiolgenomics.00129.2005. [DOI] [PubMed] [Google Scholar]
- Squirrell JM, Wokosin DL, White JG, Bavister BD. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nature Biotechnology. 1999;17(8):763–7. doi: 10.1038/11698. http://doi.org/10.1038/11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supatto W, Débarre D, Moulia B, Brouzés E, Martin JL, Farge E, Beaurepaire E. In vivo modulation of morphogenetic movements in Drosophila embryos with femtosecond laser pulses. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(4):1047–52. doi: 10.1073/pnas.0405316102. http://doi.org/10.1073/pnas.0405316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supatto W, Mcmahon A, Fraser SE, Stathopoulos A. Quantitative imaging of collective cell migration during Drosophila gastrulation: multiphoton microscopy and computational analysis. 2009;4(10):1397–1412. doi: 10.1038/nprot.2009.130. http://doi.org/10.1038/nprot.2009.130.Quantitative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Golovko VB, Vaughan OPH, Abdulkin P, Berenguer-Murcia A, Tikhov MS, Lambert RM, et al. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature. 2008;454(7207):981–983. doi: 10.1038/nature07194. http://doi.org/10.1038/nature07194. [DOI] [PubMed] [Google Scholar]
- Wen X, Wu QP, Lu Y, Fan Z, Charnsangavej C, Wallace S, Li C, et al. Poly(ethylene glycol)-conjugated anti-EGF receptor antibody C225 with radiometal chelator attached to the termini of polymer chains. Bioconjugate Chemistry. 2001;12(4):545–53. doi: 10.1021/bc0001443. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11459459. [DOI] [PubMed] [Google Scholar]
- Wessels A, Sedmera D. Developmental anatomy of the heart: a tale of mice and man. Physiological Genomics. 2003;15(3):165–76. doi: 10.1152/physiolgenomics.00033.2003. http://doi.org/10.1152/physiolgenomics.00033.2003. [DOI] [PubMed] [Google Scholar]
- Yalcin HC, Shekhar A, McQuinn TC, Butcher JT. Hemodynamic patterning of the avian atrioventricular valve. Developmental Dynamics : An Official Publication of the American Association of Anatomists. 2011;240(1):23–35. doi: 10.1002/dvdy.22512. http://doi.org/10.1002/dvdy.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Mao Y, Bai L, Herman SEM, Wang X, Ramanunni A, Muthusamy N, et al. Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. 2015;121(1):136–148. doi: 10.1182/blood-2012-01-407742. http://doi.org/10.1182/blood-2012-01-407742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Shen Y, Chatterjee B, Siegfried BH, Leatherbury L, Rosenthal J, Lo CW, et al. ENU induced mutations causing congenital cardiovascular anomalies. Development (Cambridge, England) 2004;131(24):6211–23. doi: 10.1242/dev.01543. http://doi.org/10.1242/dev.01543. [DOI] [PubMed] [Google Scholar]
- Zamir Ea, Czirók A, Cui C, Little CD, Rongish BJ. Mesodermal cell displacements during avian gastrulation are due to both individual cell-autonomous and convective tissue movements. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(52):19806–11. doi: 10.1073/pnas.0606100103. http://doi.org/10.1073/pnas.0606100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarschler K, Prapainop K, Mahon E, Rocks L, Bramini M, Kelly PM, Dawson Ka, et al. Diagnostic nanoparticle targeting of the EGF-receptor in complex biological conditions using single-domain antibodies. Nanoscale. 2014;6(11):6046–56. doi: 10.1039/c4nr00595c. http://doi.org/10.1039/c4nr00595c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Movie S1