Increasing exercise capacity and quality of life of patients with heart failure through Wii gaming: the rationale, design and methodology of the HF‐Wii study; a multicentre randomized controlled trial (original) (raw)
Abstract
Aims
Exercise is known to be beneficial for patients with heart failure (HF), and these patients should therefore be routinely advised to exercise and to be or to become physically active. Despite the beneficial effects of exercise such as improved functional capacity and favourable clinical outcomes, the level of daily physical activity in most patients with HF is low. Exergaming may be a promising new approach to increase the physical activity of patients with HF at home. The aim of this study is to determine the effectiveness of the structured introduction and access to a Wii game computer in patients with HF to improve exercise capacity and level of daily physical activity, to decrease healthcare resource use, and to improve self‐care and health‐related quality of life.
Methods and results
A multicentre randomized controlled study with two treatment groups will include 600 patients with HF. In each centre, patients will be randomized to either motivational support only (control) or structured access to a Wii game computer (Wii). Patients in the control group will receive advice on physical activity and will be contacted by four telephone calls. Patients in the Wii group also will receive advice on physical activity along with a Wii game computer, with instructions and training. The primary endpoint will be exercise capacity at 3 months as measured by the 6 min walk test. Secondary endpoints include exercise capacity at 6 and 12 months, level of daily physical activity, muscle function, health‐related quality of life, and hospitalization or death during the 12 months follow‐up.
Conclusion
The HF‐Wii study is a randomized study that will evaluate the effect of exergaming in patients with HF. The findings can be useful to healthcare professionals and improve our understanding of the potential role of exergaming in the treatment and management of patients with HF.
Trial registration
Keywords: Exergaming, Heart Failure, Serious gaming, Exercise capacity, Physical activity, Self‐care
Introduction
The number of patients with heart failure (HF) is increasing due to ageing of the population and the therapeutic advancements which improve survival of patients with heart disease.1, 2 Recent HF guidelines advise regular exercise for patients with HF to improve their functional capacity and decrease their symptoms.1, 2, 3 A meta‐analysis on exercise training including 801 patients with chronic HF (the ExTraMATCH trial) found that those patients randomized to exercise were less often admitted to hospital and had a better prognosis than those in the control group.4 The most recent large study on exercise in patients with HF was the HF‐ACTION study (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) including 2331 patients with HF. In that study, participants who were engaged in a combined hospital and home‐based exercise programme had a non‐significant reduction in the primary endpoint of all‐cause mortality or hospitalization,5 but had a better self‐reported health status.6 The two most up to date systematic reviews on the outcomes of exercise in HF are a Cochrane systematic review7 and a meta‐analysis of randomized controlled trials between 1999 and 2013.8 Although these reviews did not find an effect of cardiac rehabilitation on mortality they did find an effect for quality of life and hospitalization.7, 8 Despite positive outcomes on exercise capacity and quality of life, adherence to exercise in patients with HF is as low as 50%,9 a problem that warrants high priority in healthcare.
Becoming physically active might be the first step to reach the optimal exercise dose to improve health outcomes. Barriers to physical activity are often related to motivation and practical issues, such as time, the possibility to travel to a fitness or rehabilitation centre, or costs.10, 11 Another barrier to physical activity could be climate, for example the cold and rainy weather in countries such as Sweden and The Netherlands or the heat and humidity in countries such as Italy and Israel. To increase adherence to exercise, activity at home has been studied in patients with HF, showing both the feasibility and positive effects on physical capacity in elderly patients with HF.12 However, even in home‐based physical activity programmes, adherence might not be optimal due to behavioural and motivational issues.13
A new approach to increase daily physical activity at home is the use of exergaming. The word ‘exergaming’ is a portmanteau of ‘exercise’ and ‘gaming’, a term for video games that are also a form of exercise. In these kinds of games patients can be encouraged to become active at their own pace, reducing climate impact and social barriers. In a recent scoping review on exergames in older adults, exergaming was described as safe and feasible, resulting in more energy expenditure compared with rest.14 Although the research field of exergaming is small and under development, initial results indicate that exergaming might be promising in order to enhance physical activity in patients with HF. In the available studies, the Nintendo Wii exergame platform was the most tested exergame in older adults, showing higher energy expenditure and motor function, better balance and cognitive function, a decrease in depressive symptoms, a high level of enjoyment, and a feeling of being connected with family members (especially grandchildren) without an increase in HF symptoms.14 Exergaming seems to have much to offer in the fields of prevention and rehabilitation; however, only limited well‐powered randomized controlled trials are available.14, 15, 16 To avoid a new ‘hype’ overestimating the potentials of these games, these new options should be tested in specific groups of patients. Studies of good quality including older chronic patients are scarce, and some evidence shows that older people may have specific playing preferences (e.g. less interest in extreme sports) and particular difficulties handling complex digital games.14 In a pilot study, we studied the feasibility of using a game computer in older patients with HF to increase their exercise capacity.17, 18 In a non‐university hospital setting in Sweden we included 31 patients with a mean age of 63 (±14), 10 of whom were women (32%). No injuries occurred in the pilot study and all patients were able to play the Wii successfully; only one patient needed two additional visits by the instructor. The mean time exergaming a day was 28 min (±13). At baseline, the mean score on the 6‐min walk test (6‐MWT) was 477 m compared with 505 m after 12 weeks access to the Wii. In total, 47% of the patients increased their 6‐MWT distance by >30 m (which is a clinically relevant difference) after 3 months of access to the Wii.18 After this pilot, we set out to determine the effects of structured access to a Wii game computer in patients with HF on exercise capacity and their level of physical activity in an randomized controlled trial, the HF‐Wii study. We also aim to test the effects on outcomes beyond exercise and physical activity, such as self‐care, readmission, mortality, and health‐related quality of life. Finally we aim to describe the costs and experiences of patients with HF playing exergames.
Methods
Study design
This will be a multicentre randomized controlled design with two groups (‘HF‐Wii study’). Patients will be randomized to motivational support only (control) or structured access to a Wii game computer (Wii) (Figure 1). The study will be conducted according to the principles of the Declaration of Helsinki (version 2008) and in accordance with the Medical Research Involving Human Subjects Act of the country where the intervention takes place. In Sweden, ethical approval was obtained (DNR 2012/247‐31) and additional approval is/will be obtained from the local medical ethical committees. The trial is registered in ClinicalTrial.gov, identifier: NCT01785121.
Figure 1.
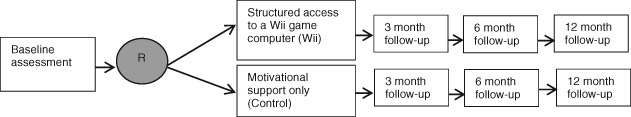
Design of the HF‐Wii study.
Study population
Study population
Patients will be recruited from outpatient HF clinics, cardiology departments, rehabilitation units, or primary care in Sweden, Italy, Israel, and The Netherlands.
Inclusion criteria
- Diagnosed with HF (NYHA I–IV) by a cardiologist according to ESC guidelines1 [independent of EF: both patients with a preserved ejection fraction (HFpEF) and patients with a reduced ejection fraction (HFrEF) will be included].
- Older than 18 years, no upper age limit.
- Speak/understand the language of the country where the intervention takes place.
Exclusion criteria
- Unable to use the Nintendo Wii due to visual impairment (see a TV screen at a distance of 3 m), hearing impairment (the patient is not able to communicate by telephone), cognitive impairment (assessed by a HF nurse or cardiologist), or motor impairment (the patient should be able to swing his arm at least 10 times in a row).
- Unable to fill in data collection material.
- Life expectancy shorter than 6 months
Treatment and control
Motivational support only (control)
Patients will receive regular treatment and information about rehabilitation and daily physical activity. After enrolment in the study, patients will get exercise advice from the HF team (nurse, cardiologist, or physiotherapist). All patients will be advised to be active for 30 min a day. This might not be applicable to all patients, since some patients may only manage to be active for 10 or 20 min and others may manage >30 min. Therefore, the advice is adapted to the capabilities of the individual patient. During the first 3 months, at 2, 4, 8, and 12 weeks after inclusion in the study, patients will be followed up by telephone (in a structured scripted way) to discuss their current activity.
Structured access to a Wii game computer (Wii)
Patients will receive regular treatment and information about rehabilitation and daily physical activity. The patients will also receive activity advice, as described for the control group. In addition, patients in the Wii group will be introduced to the Nintendo Wii game computer in an introductory lesson of ∼1 h in the participating centre led by a dedicated instructor (knowledgeable regarding the Wii but with no further special training). Thereafter, the Wii will be installed at the patient's home. The patients will be taught to move the remote control in a similar way to how the sport is played in real life; for example, holding and swinging the remote control as a bowling ball or tennis racket. At home, the patients will receive additional instruction on the Wii, if needed, and they will be advised how much they should play on the Wii. In general, the advice will be to play for 30 min per day. If needed, they are advised to adapt to their capacity, for example to play more often for shorter periods during a day or even play for longer if they want. The patients will receive written safety guidelines and information on how to use the Wii computer after installation. In the first 3 months after installation, at 2, 4, 8, and 12 weeks after inclusion in the study, patients will be followed up by telephone to discuss their experiences with the Wii, to obtain motivational support, or to resolve unexpected problems. Patients will be given the opportunity to call the research staff during the first 3 months of the study in case they experience any technical problems with the Wii game computer.
Outcomes
Since we have a rather broad aim to test the concept of exergaming, this study can be defined as a complex intervention study.19 The broad evaluation of outcomes is outlined in Figure2 and the research questions and outcome measures are listed below and in Table1.
Figure 2.
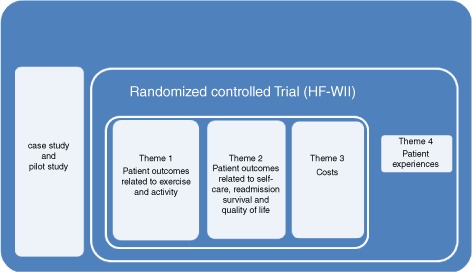
Outcomes in the HF‐Wii study.
Table 1.
Variables and instruments in the HF‐Wii study
| Variable | Instrument | Baseline | 3 months | 6 months | 12 months | Daily 3 months |
|---|---|---|---|---|---|---|
| Theme 1: patient outcomes related to exercise and activity | ||||||
| Exercise capacity | 6‐min walk test20 | X | X | X | X | |
| Muscle function | Shoulder abducton/flexion22 | X | X | X | X | |
| Exercise motivation | Exercise Motivation Index24 | X | X | X | X | |
| Exercise self‐efficacy | Exercise Self‐efficacy Scale25 | X | X | X | X | |
| Perceived physical effort | Borg's scale26 | X | ||||
| Theme 2: patient outcomes related to self‐care, readmission, survival, and quality of life | ||||||
| Heart failure symptoms | Visual analogue scale | X | ||||
| Health‐related quality of life | MLwHFQ27 | X | X | X | X | |
| Global well‐being | Cantril Ladder28 | X | ||||
| Mortality, readmission | Registration | X | X | X | X | |
| Anxiety/depression | HADS29 | X | X | X | X | |
| Insomnia | MISS30 | X | X | X | X | |
| Self‐care | EHFScBS31 | X | X | X | X | |
| Theme 3: Costs and patient experiences | ||||||
| Costs | X |
Theme 1: patient outcomes related to exercise and activity
Primary endpoint
The primary endpoint of the HF‐Wii study is improvement in exercise capacity assessed by the 6‐MWT between baseline and 3 months. The 6‐MWT is a simple, low‐cost method for estimating exercise capacity; only a pre‐measured level surface and a timing device are needed.20 The mode of exercise is familiar to most patients, although it may represent a maximal test for some. The test has appeared to be useful for the assessment of many interventions such as CRT and has strong predictive power for both mortality and morbidity in patients with HF.20, 21
Secondary outcomes
We will also measure the exercise capacity using the 6‐MWT at 6 and 12 months from baseline. Other secondary outcomes include muscle function, exercise motivation, self‐efficacy beliefs, and perceived physical effort in all patient (see Table 1). Muscle function will be assessed with unilateral isotonic heel‐lift, bilateral isometric shoulder abduction, and unilateral isotonic shoulder flexion using pre‐defined protocols.22 The other outcomes such as exercise motivation, self‐efficacy beliefs, and perceived physical effort will be assessed by patient self‐report with validated questionnaires (see Table 1). In a subgroup of 100 patients, the level of physical activity will be monitored with an accelerometer (ActiGraph, Pensacola, FL, USA). An accelerometer will provide information regarding intensity, frequency, and duration of physical activity as well as sedentary time. The ActiGraph GT9X accelerometer (ActiGraph) for hip‐worn placement will be used for this purpose. The ActiGraph has been found to be reliable and valid.23 We will assess the number of times the subjects played on the Nintendo Wii, the mean duration of playing, and other daily activities by a diary during the first 3 months in the study. We will collect information on attendance at exercise‐based rehabilitation
Theme 2: patient outcomes related to self‐care, readmission, survival, and quality of life
To measure the effects of the intervention on outcomes beyond exercise and physical activity, data will be collected on HF symptoms, health‐related quality of life, global well‐being, anxiety and depression, sleep, readmission, mortality, and HF self‐care. Data will be collected from the medical chart and with validated questionnaires (see Table 1).
Theme 3: costs
In both groups we will calculate the costs of visits to healthcare professionals. We also will calculate the cost of the intervention.
Theme 4: patient experiences
Additionally we will collect data on patients' experiences through open‐ended questionnaires. In a subgroup of patients qualitative interviews will be conducted, describing the experience of using an exergame from a broader perspective.
Assessment, randomization, and study protocol
Following the confirmation of suitability and informed consent, patients' baseline characteristics will be assessed from the medical chart, and baseline data will be collected from the 6‐MWT, muscle function test, and questionnaires. After informed consent, patients will be randomized (in a 1:1 ratio in each centre) to one of these conditions: motivational support only (control) or structured access to a Wii game computer (Wii). To achieve balance between study arms and to have similar numbers of patients during the introductory lesson, the randomization will be made with blocks of 12 comprising six intervention and six control conditions. The Linköping Academic Research Centre provides a list of random numbers in blocks of 12 and will generate randomized block allocations for each of the study sites. Due to the nature of the intervention, the study is not blinded, but the assessment of the primary endpoint is blinded.
Statistical issues
Analysis
The primary analysis will consist of comparing the results of the change in 6‐MWT from baseline to 3 months between the group of patients in the Wii group compared with the control group. The statistical analysis will be performed using a two‐sample _t_‐test, and two‐sided 95% confidence intervals will be constructed to describe the treatment differences. An analysis of covariance will be used to test for the treatment effect controlling for different baseline characteristics. The results will be analysed using an intention‐to‐treat analysis including the full set of all randomized patients (primary efficacy population). In addition, a ‘per protocol’ analysis will be performed, including only patients who actually played the Wii (as assessed by the diary and questionnaire) during the study period. Qualitative data will be analysed with content analysis. A multivariate analysis will be performed, with stratification on research centre and NYHA classification.
Power calculation
To achieve a 30 m difference between the control group and the Wii group (which is described to be a clinically significant difference in HF patients based on 80% power, 5% significance), 250 patients in the intervention group and 250 patients in the control group are needed. To ensure appropriate patient numbers at the end of the study, 2× 300 patients will be included.
Organization and progress
In order to recruit the 600 patients, international HF clinics, cardiology departments, and primary care centres have agreed to participate in this research. So far four hospitals in Sweden participate, two hospitals in Italy, one hospital in Israel, and one hospital in The Netherlands.
The first patient was included in September 2013 and, in February 2015, 28% of the required sample was included, mainly from the three Swedish centres that started first. Recruitment is expected to be completed by December 2016 and the study should close at the beginning of 2018.
To guide this investigator‐initiated and driven study, a scientific advisory board was established with the main goal to advise the HF‐Wii study team with regard to issues on data collection, substudies, analysis, future implementation, and publications. Members included in the scientific advisory board are the principle investigators for each Wii‐site and additional experts from primary care, cardiology, physiotherapy, and nursing (M. Bäck RPT PhD, T. Ben Gal MD, J. Boyne RN PhD, Professor K. Dickstein MD PhD, Professor B. Fridlund RN, PhD, Professor A.W. Hoes MD PhD, Professor J. Mårtensson RN PhD, Professor M.F. Piepoli MD PhD, E. Vellone RN, PhD).
Discussion
There is a need for evidence on the long‐term effects of affordable and available interventions that are both ‘patient friendly’ and easy to implement. The proposed study will allow insight into the effects of access and structured introduction of an easy applicable and available virtual reality application. If using a commercially affordable and available game computer is effective in increasing exercise capacity in patients with HF, this might be a recommendation that should be given to patients, in addition to other current recommendations.
Heart failure is a common, costly, and disabling disease with a lot of suffering in patients and their families.32 Improving patients' exercise capacity and increasing their ability to perform more daily physical activity is expected to improve their quality of life. Currently there is no universal agreement on exercise prescription for patients with HF, and thus guidelines recommend an individualized approach with careful clinical evaluation, including behavioural characteristics, personal goals, and preferences.2 The rationale behind the HF‐Wii study is that by introducing an innovative and user‐centred way of performing physical activity we can motivate people to be more physically active in their daily life and feel more confident with exercise. In this way patients will improve their exercise capacity, which in its turn can increase physical activity and participation in everyday life. Increased functional capacity is known to improve relevant outcome measures such as symptoms and quality of life.7, 8
Funding
This work is supported through the Swedish National Science Council (K2013‐69X‐22302‐01‐3), The Swedish Heart and Lung Association E085/12, The Swedish Heart‐Lung Foundation (20130340), the Vårdal Foundation (2014–0018), and FORSS (474681). Swedish National Science Council/Swedish research council for health, working life and welfare (VR‐FORTE) 2014‐4100.
Conflict of interest: none declared.
The copyright line for this article was changed on 30 March 2016 after original online publication.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 2.Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, McMurray J, Pieske B, Piotrowicz E, Schmid JP, Anker SD, Solal AC, Filippatos GS, Hoes AW, Gielen S, Giannuzzi P, Ponikowski PP. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011;13:347–357. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. The 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–e234. [DOI] [PubMed] [Google Scholar]
- 4.Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004;328:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL; HF‐ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP; HF‐ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA 2009;301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson L1, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014;12:CD011273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewinter C, Doherty P, Gale CP, Crouch S, Stirk L, Lewin RJ, LeWinter MM, Ades PA, Køber L, Bland JM Exercise‐based cardiac rehabilitation in patients with heart failure: a meta‐analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol 2015; in press. [DOI] [PubMed] [Google Scholar]
- 9.van der Wal MH, van Veldhuisen DJ, Veeger NJ, Rutten FH, Jaarsma T. Compliance with non‐pharmacological recommendations. Eur Heart J 2010;31:1486–1493. [DOI] [PubMed] [Google Scholar]
- 10.Conraads VM, Deaton C, Piotrowicz E, Santaularia N, Tierney S, Piepoli MF, Pieske B, Schmid JP, Dickstein K, Ponikowski PP, Jaarsma T. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2012;14:451–458. [DOI] [PubMed] [Google Scholar]
- 11.Santaularia N, Jaarsma T. Motivational factors for exercise in cardiac patients? A literature review. Eur J Prev Med 2013;1: 1–19. [Google Scholar]
- 12.Pihl E, Fridlund B, Strömberg A, Cider Å, Mårtensson J. Exercise in elderly patients with chronic heart failure in primary care: effects on physical capacity and health‐related quality of life. Eur J Cardiovasc Nurs 2011;10:150–158. [DOI] [PubMed] [Google Scholar]
- 13.Cowie A, Thow MK, Granat MH. A comparison of home and hospital‐based exercise training in heart failure: immediate and long‐term effects upon physical activity level. Eur J Cardiovasc Prev Rehab 2011;18:158–166. [DOI] [PubMed] [Google Scholar]
- 14.Verheijden Klompstra L, Jaarsma T, Strömberg. Exergaming in older adults: a scoping review and implementation potential for patients with heart failure. Eur J Cardiovasc Nurs 2014;13:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiemeyer J, Kliem A. Serious games in prevention and rehabilitation—a new panacea for elderly people? Eur Rev Aging Phys Act 2012;9:41–50. [Google Scholar]
- 16.Peng W, Lin JH, Crouse J. Is playing exergames really exercising? A meta‐analysis of energy expenditure in active video games. Cyberpsychol Behav Soc Netw 2011;14:681–688. [DOI] [PubMed] [Google Scholar]
- 17.Klompstra L, Jaarsma T, Strömberg A. An in‐depth, longitudinal examination of the daily physical activity of a patient with heart failure using a Nintendo Wii at home: a case report. J Rehabil Med 2013;45:599–602. [DOI] [PubMed] [Google Scholar]
- 18.Klompstra L, Jaarsma T, Strömberg A. Exergaming to increase the exercise capacity and daily physical activity in heart failure patients: a pilot study. BMC Geriatr 2014;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig P, Paul P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 21.Ingle L, Shelton RJ, Rigby AS, Nabb S, Clark AL, Cleland JG. The reproducibility and sensitivity of the 6‐min walk test in elderly patients with chronic heart failure. Eur Heart J 2005;26:1742–1751. [DOI] [PubMed] [Google Scholar]
- 22.Cider A, Carlsson S, Arvidsson C, Andersson B, Sunnerhagen KS. Reliability of clinical muscular endurance tests in patients with chronic heart failure. Eur J Cardiovasc Nurs 2006;5:122–126. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport 2011;14:411–416. [DOI] [PubMed] [Google Scholar]
- 24.Stenström CH, Boestad C, Carlsson M, Edström M, Reuterhäll A. Why exercise? A preliminary investigation of an exercise motivation index among individuals with rheumatic conditions and healthy individuals. Physiother Res Int 1997;2:7–16. [DOI] [PubMed] [Google Scholar]
- 25.Dzewaltowski D. Toward a model of exercise motivation. J Sport Exercise Psychol 1989;11:215–269. [Google Scholar]
- 26.Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 27.Rector TS, Kubo SH, Cohn JN. Patient's self assessment of their congestive heart failure: content, reliability and validity of a new measure: the Minnesota Living with Heart Failure Questionnaire. Heart Fail 1987;3:198–219. [Google Scholar]
- 28.Cantril H. The Pattern of Human Concerns. New Brunswick, NJ: Rutgers University Press; 1965. [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 30.Hellström A, Hagell P, Fagerström C, Willman A. Measurement properties of the Minimal Insomnia Symptom Scale (MISS) in an elderly population in Sweden. BMC Geriatr 2010;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaarsma T, Arestedt KF, Mårtensson J, Dracup K, Strömberg A. The European Heart Failure Self‐care Behaviour scale revised into a nine‐item scale (EHFScB‐9): a reliable and valid international instrument. Eur J Heart Fail 2009;11:99–105. [DOI] [PubMed] [Google Scholar]
- 32.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Siswanto BB, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014;1:1–74. [DOI] [PubMed] [Google Scholar]