Phylogeny and diversification of the largest avian radiation (original) (raw)
Abstract
The order Passeriformes (“perching birds”) comprises extant species diversity comparable to that of living mammals. For over a decade, a single phylogenetic hypothesis based on DNA–DNA hybridization has provided the primary framework for numerous comparative analyses of passerine ecological and behavioral evolution and for tests of the causal factors accounting for rapid radiations within the group. We report here a strongly supported phylogenetic tree based on two single-copy nuclear gene sequences for the most complete sampling of passerine families to date. This tree is incongruent with that derived from DNA–DNA hybridization, with half of the nodes from the latter in conflict and over a third of the conflicts significant as assessed under maximum likelihood. Our historical framework suggests multiple waves of passerine dispersal from Australasia into Eurasia, Africa, and the New World, commencing as early as the Eocene, essentially reversing the classical scenario of oscine biogeography. The revised history implied by these data will require reassessment of comparative analyses of passerine diversification and adaptation.
Major lineages of the ≈5,739 species of passerine birds (1) have diversified on all continents and now occupy nearly all terrestrial ecosystems. The songbirds (oscines, suborder Passeri) alone comprise nearly half of all extant avian species and represent the largest identifiable radiation of birds (2), encompassing a staggering ecological and behavioral diversity. The foundation for understanding passerine diversification and for integrating the spatial, ecological, and temporal history of the group is a comprehensive and robust phylogenetic hypothesis. A decade ago, in a pioneering work, Sibley and Ahlquist (3) used DNA–DNA hybridization to produce the first large-scale phylogenetic hypothesis for passerine birds and suggested a specific temporal history for passerine diversification. Despite often-voiced concerns about the robustness of this phylogeny (colloquially termed the “Tapestry” after its appearance on the wall at an ornithological meeting), it has provided a framework for numerous historical analyses of passerine ecology (4), behavior (5), and diversification (6, 7), and continues to fuel the search for explanations of passerine diversity (8).
Recently, studies have begun to contradict aspects of the DNA hybridization hypothesis for passerines (ref. 9; citations in ref. 10). These investigations, however, used relatively short gene sequences and, more importantly, limited taxon sampling across passerines; thus the phylogenetic, biogeographic, and temporal interpretations of the radiation were incomplete, and broad tests of the DNA hybridization tree were not possible. Here we analyze 4,126 aligned positions of the nuclear genes RAG-1 and -2 from 144 passerine species in 45 families, including representatives of all but one family recognized by current taxonomy (1). We find substantial support for many portions of the passerine tree, as well as quantitative evidence for the relationships of many enigmatic taxa. Moreover, phylogenetic analysis of these data reveals significant conflicts with the DNA–DNA hybridization tree. The phylogenetic structure and temporal scaling we have extracted from these data provide a foundation for interpretations of passerine diversification and critical tests of hypotheses purporting to explain it.
Methods
Data Collection. We sampled exemplars of every passerine family recognized by Sibley and Monroe (1), save one, the monotypic oscine passerine family Hypocoliidae (supporting information, which is published on the PNAS web site). We included samples from many subfamilies and tribes recognized by Sibley and Monroe (1), because these correspond to traditionally recognized families (11). The passerine tree was rooted with two outgroups, Gallus gallus and Coracias caudata, although use of other avian and even mammalian outgroups did not significantly affect the topologies obtained (not shown; discussed in supporting information). We amplified and sequenced a large portion of the single exons of the nuclear genes RAG-1 and -2. Methods of genomic DNA extraction, enzymatic amplification, and sequencing were as described (ref. 9; supporting information). The tandem alignment of RAG-1 and -2, generated by eye, included a total of 4,126 positions.
Phylogenetic Analyses. The tandem gene alignment was analyzed by using parsimony, maximum likelihood (ML), and Bayesian methods, as implemented in paup* Ver. 4.0b10 (12), phyml Ver. 2.1b1 (13), and mrbayes Ver. 3.0 β3 (14, 15). Combined analysis of the data was preceded by a test of congruence between the two genes (the incongruence length difference test; ref. 16), which did not approach significance (P = 0.81; see refs. 17–20 for discussion of the utility of this test). Parsimony analysis of the combined data set was accomplished using the parsimony ratchet (21), as implemented in pauprat (22) and paup* (200 iterations of the ratchet, followed by tree bisection and reconnection branch swapping), yielding 432 minimum-length trees (length = 9,431 steps, ensemble consistency index = 0.36, retention index = 0.56). Nodal robustness under the parsimony criterion was evaluated by using the nonparametric bootstrap (100 replicates; ref. 23). ML and Bayesian analyses of the data were performed using a GTR+I+Γ model of sequence evolution (24), as described (9). ML analyses were accomplished using initial searches with phyml (13), and subsequent tree bisection and reconnection branch swapping with paup*, with nodal robustness estimated by the nonparametric bootstrap (100 replicates), performed with phyml. The probability density of the Bayesian posterior was estimated by Metropolis-coupled Markov chain Monte Carlo, with multiple incrementally heated chains. In total, six independent runs of 106 generations (sampling every 100) were performed, two with three heated chains and four with four, and the results from all chains combined. The goodness of fit of the molecular data to the phylogenetic hypotheses implied by Sibley and Ahlquist's (3) DNA–DNA hybridization phenogram was evaluated under the ML criterion, using the test of Shimodaira and Hasegawa (ref. 25; as implemented in paup*, by using 10,000 reestimated log-likelihood bootstrap replicates; supporting information). Taxon sampling of the current study and that of Sibley and Ahlquist (3) was made equivalent by pruning of genera not held in common between the two (with 112 included in the comparison).
Biogeography. All species of passerines were assigned to higher taxa as defined by our ML tree (Fig. 1; supporting information) and by the taxonomy of Sibley and Monroe (1), as described (ref. 9; supporting information). The ancestral areas for passerine higher taxa were inferred from distributional data, using dispersal–vicariance analysis (26, 27), without constraints on the number of areas allowed for ancestral state reconstructions.
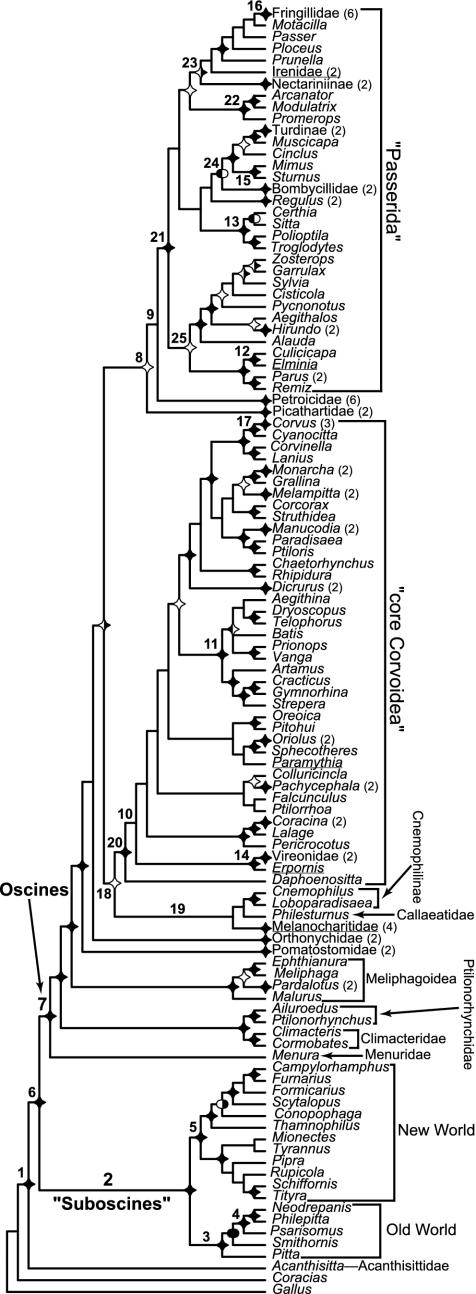
Fig. 1.
Relationships among passerine birds based on analysis of combined RAG-1 and -2 sequences (146 taxa, 4,126 aligned nucleotide positions). The topology presented is the best ML estimate, rooted using Gallus and Coracias. Multiple exemplars of genera and certain higher taxa have been collapsed for clarity (number of species indicated in parentheses after genus or group). Nodal support is indicated by symbols and symbol fills. Stars indicate estimated Bayesian posterior probability ≥0.95 (nodes with oblongs or no symbol have estimated probabilities <0.95), black fill indicates parsimony (left half), and ML (right half) bootstrap percentages ≥75. Numbers refer to selected nodes that are dated in Table 2 or discussed in the text. The quotes around the taxon “Passerida” indicate that Sibley and Ahlquist's (1, 3) definition of the group excludes the underlined taxa within this clade and includes the underlined taxa shown here as falling outside the clade. Their Corvida includes all oscines (node 7) not in the Passerida. Likewise, the quotes around the taxon “Suboscines” indicate that this group traditionally contains the Acanthisittidae, here shown as basal within passerines.
Molecular Clock. A likelihood ratio test (28) rejected the molecular clock for this data set (–2 ln Λ = 492, df = 142, P < 0.001), although the observed rate variation was relatively small (terminals appear at 80–119% of the mean root-to-tip path length), and largely attributable to a rate increase within the oscines (46% of root-to-tip path length variance). To account for this variation, we estimated relative nodal ages both by nonparametric rate smoothing (NPRS) (29) and by penalized likelihood (PL; ref. 30). Bootstrap estimates of the standard error (31) of these relative divergences were derived by application of these analyses to 100 pseudoreplicate data sets. The relative timescale derived from nonparametric analysis was calibrated using the basal divergence of Acanthisitta versus all other passerines, assuming it was coincident with rifting of New Zealand from Antarctica (32, 33). This calibration yielded divergence dates for oscines and suboscines [77–76 million years ago (Ma), Table 2] nearly congruent with an independently derived date (77.1 ± 11.6 Ma; ref. 34). A Cretaceous origin for passerines has been questioned (35), however, and we have provided a second independent calibration reference. We calibrated the passerine tree using pairwise divergences at the mitochondrial locus cytochrome b for closely related sister taxa sampled in our study and a previously established rate for evolution of this gene in passerines (36). This analysis (supporting information) yielded an estimated date for divergence of the Acanthisittidae only 5 million years older than the biogeographic calibration, demonstrating remarkable congruence between estimates derived from very ancient geological events (rifting of New Zealand from Antarctica) and much more recent events (speciation in the Hawaiian archipelago; ref. 36). For comparable phylogenetically independent nodes (n = 6, Table 2), our date estimates are also quite similar to those estimated from DNA–DNA hybridization distances using Sibley and Ahlquist's standard calibration (ref. 3; _r_2 = 0.97 and 0.98 for NPRS and PL, respectively, type II regression), with slope estimates near one (1.3 ± 0.1 SE and 1.2 ± 0.1 SE for NPRS and PL).
Table 2. Inferred dates of major passerine divergences and dispersal events.
| Node | Divergence | NPRS | PL | Sibley and Ahlquist* |
|---|---|---|---|---|
| 1 | Acanthisittidae versus other passerines | 82† | 82† | — |
| 2 | Old World/New World Suboscines | 71 (3.3) | 67 (3.1) | 74 |
| 3 | Pittas/Broadbills | 57 (4.9) | 53 (3.3) | 57 |
| 4 | Origin of Malagasy Philepittidae | 43 (4.7) | 40 (3.0) | — |
| 5 | Tyrannoidea/Furnarioidea | 65 (5.1) | 61 (2.8) | 63 |
| 6 | “Suboscines”/oscines | 77 (1.7) | 76 (2.2) | — |
| 7 | Basal oscine divergence | 65 (2.6) | 62 (2.7) | 60 |
| 8 | Dispersal from Australasia (Picathartidae) | 47 (2.8) | 45 (2.6) | — |
| 9 | Dispersal from Australasia (Passerida) | 45 (2.9) | 44 (2.6) | 55 |
| 10 | Dispersal from Australasia (Vireonidae/Erpornis) | 40 (2.6) | 37 (2.5) | 41 |
| 11 | Dispersal into Africa (Vangini/Malaconotinae) | 29 (3.1) | 26 (2.5) | — |
| 12 | Dispersal into Africa (Culicicapa/Elminia) | 26 (3.2) | 27 (2.2) | — |
| 13 | Dispersal into New World (Troglodytinae) | 34 (3.8) | 34 (2.2) | 42 |
| 14 | Dispersal into New World (Vireonidae) | 28 (2.9) | 25 (2.4) | — |
| 15 | Dispersal into New World (Mimini) | 20 (2.8) | 22 (2.0) | 27 |
| 16 | Dispersal into New World (Emberizinae) | 20 (3.9) | 22 (1.8) | 32 |
| 17 | Dispersal into New World (NW jays‡) | 17 (2.9) | 14 (2.0) | — |
Results and Discussion
Phylogenetic Relationships Among Passerines. Our hypothesis of passerine relationships (Fig. 1) provides important insight into the history of passerine diversification. At the base of the tree, our results provide evidence that the New Zealand endemic family Acanthisittidae is the sister group of all other passerines (Fig. 1, node 1), and that the latter clade can be divided into suboscines (node 2) and the oscine songbirds (node 7; refs. 9 and 33). Within the suboscines, we find additional support for a division into New World (node 5, Sibley and Ahlquist's Tyrannides) and Old World (node 3, Sibley and Ahlquist's Eurylaimides) assemblages (37, 38). DNA–DNA hybridization distances suggested that the highly diverse songbird clade (Fig. 1, node 7) was subdivided into two large clades, the Passerida and the Corvida (3); however, our data corroborate recent studies that demonstrate paraphyly of the Corvida (9, 33, 39). Moreover, the expanded taxon and character sample of the present study demonstrate that the phylogenetic structure among basal oscine lineages is more complex than previously realized.
Analyses of RAG-1 and c-mos sequences (9) previously identified three Australian groups (Menuridae, Ptilonorhynchidae plus Climacteridae, and the superfamily Meliphagoidea) as sequential sister taxa to the other oscines. Our results indicate that at least two additional Australian taxa (the Pomatostomidae and Orthonychidae) are also outside the crown oscine radiation. Although relationships of these ancient lineages violate monophyly of Sibley and Ahlquist's Corvida, we do recover a major clade of “crow-like” songbirds that roughly corresponds to this group (the “core Corvoidea”; Fig. 1, node 20), as well as a large clade roughly corresponding to their Passerida (node 21). In addition to clarifying the contents of these “core” radiations, our data elucidate their early history. First, the Australasian dawn robins and the African endemic rockfowl and rockjumpers (Petroicidae and Picathartidae) are found to be the sister groups to passeridan radiation (Fig. 1, nodes 8 and 9). Second, a newly discovered clade (Fig. 1, node 19) consisting of the cnemophiline “birds of paradise” (Cnemophilus, Loboparadisaea), berrypeckers (Melanocharitidae), and the wattlebirds of New Zealand (Callaeatidae) appears as the sister group to the “core Corvoid” radiation.
Within these two groups, our data corroborate many hypotheses posited on DNA–DNA hybridization evidence, including: (i) a close relationship between New World mockingbirds and Old World starlings (node 15, Fig. 1); (ii) wide separation of New World Vireonidae and wood warblers (Fringillidae); and (iii) separation of “Passerida” into three major groups, the Passeroidea, Muscicapoidea, and Sylvioidea (roughly corresponding to nodes 23–25, Fig. 1). In addition to these, our sampling has allowed us to identify a number of unexpectedly divergent lineages, including (i) the African sugarbirds and allies (Promerops, Arcanator, and Modulatrix; Fig. 1, node 22), (ii) an apparently ancient group of “flycatchers” (Culicicapa and Elminia; Fig. 1, node 12), and (iii) an unsuspected clade of shrike-like corvoid songbirds (node 11, Fig. 1 and Table 2), encompassing species endemic to Australasia, Asia, and Africa.
Comparison to the DNA–DNA Hybridization Tapestry. Although our results bear some rough similarity to those of Sibley and Ahlquist's (3) phenogram, the two differ significantly both in detail and in their implications for passerine evolution. As a first step toward revealing these implications, we have quantified the magnitude and statistical significance of conflict between our hypothesis and theirs. Our best hypothesis of passerine relationships conflicts with 52% (n = 55 paraphyletic groups) of the nodes defined by the Tapestry; 36% of these conflicts (n = 20) are statistically significant (Table 1). Of the nodes we tested, 54 represent higher taxa recognized by Sibley and Ahlquist (3) or Sibley and Monroe (1), of which 39% (n = 21) are not monophyletic on our preferred hypothesis: approximately half of the conflicts appear statistically significant (Table 1). Some of the conflicts observed here are correlated, because shifting individual genera between two higher taxa necessarily violates monophyly of both. However, some cases of nonmonophyly supported by our data involve nesting of large clades within others (e.g., the parvorder Passerida within the Corvida), and a number involve distant relationships among groups of genera such that paraphyly cannot be attributed to the placement of individual taxa (e.g., within Sibley and Ahlquist's subfamily Corvinae). Therefore, our results imply significant pervasive conflicts with the DNA–DNA hybridization estimate of relationships. In addition, a number of species included in the current study represent distinct lineages not included in Sibley and Ahlquist's (3) sampling, the placement of which violates monophyly of recognized taxa from the ranks of tribe to parvorder (see Phylogenetic Relationships; ref. 1). Thus, our results suggest not only that the DNA hybridization hypothesis is problematic, but also that use of the revised taxonomy based partially on it (1) as a proxy for evolutionary relationship is also questionable (6, 8).
Table 1.
Monophyly of named taxa and nodes of the Tapestry (3)
| Node | Number tested | Monophyletic (% of rank) | Paraphyletic, NS* (% of rank) | Paraphyletic, S* (% of rank) |
|---|---|---|---|---|
| Suborder | 2 | 1 (50) | 1 (50) | 0 |
| Infraorder | 2 | 2 (100) | 0 | 0 |
| Parvorder | 3 | 1 (33) | 0 | 2 (67) |
| Superfamily | 7 | 2 (29) | 3 (43) | 2 (29) |
| Family | 22 | 17 (77) | 4 (18) | 1 (5) |
| Subfamily | 9 | 5 (56) | 3 (33) | 1 (11) |
| Tribe | 9 | 5 (56) | 3 (33) | 1 (11) |
| All nodes | 105 | 50 (48) | 35 (33) | 20 (19) |
Diversification of Passerine Lineages. Based on our data and the phylogenetic conclusions drawn from them, we have used quantitative biogeographic methods to infer dispersal patterns of passerine lineages and molecular clock methods to infer the absolute timing of their subsequent radiation across the globe. These analyses provide a previously unavailable set of spatial and temporal constraints on the rates and patterns of passerine diversification.
Basal divergences among Acanthisitta and the suboscine and oscine passerines are trans–Antarctic (32), with deeply diverging lineages distributed in South America, New Zealand, and Australasia (Fig. 2), suggesting a Gondwanan influence on the distribution of these taxa. The earliest fossil passerine is from the early Eocene of Australia (40), and the next known fossil is from the late Oligocene of Europe (41). In contrast, molecular results from this and other studies (3, 33, 34, 42) indicate that passerines began diversifying in the Late Cretaceous (Fig. 2, Table 2), ≈30 million years before their first appearance in the Southern Hemisphere fossil record, and ≈60 million years before their first appearance in the north. The lack of early southern fossils is unsurprising given the historical research bias toward northern paleofaunas (43). We propose that the large gap in the northern record is likely a function of the southern origins of passerines and the temporal history of their radiation out of Gondwana.
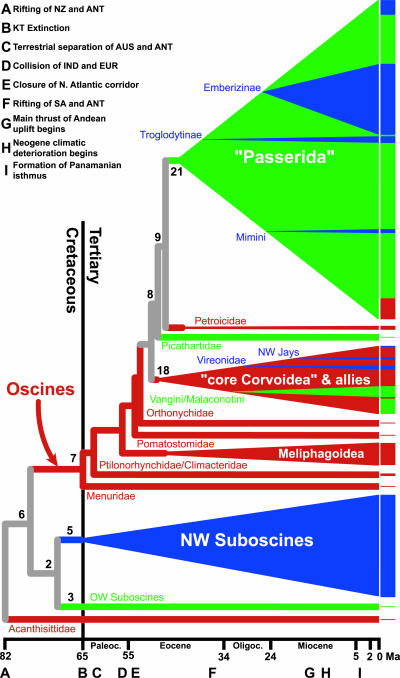
Fig. 2.
Schematic of spatial and temporal patterns in passerine diversification. The height of the bars to the right of the tree is proportional to the number of species in the corresponding clades, and the proportion of colors in each bar represent the current geographic distribution of species (red, Australasia; green, Africa and Eurasia; blue, North and South America). Continuity of the terminal bars does not imply monophyly of species distributed in each region, except for specific clades within the “Passerida” and “core Corvoidea,” which are highlighted by triangles and labeled. Ancestral areas for groups (as inferred by dispersal–vicariance analysis, see Methods) are depicted by coloring of corresponding triangles and subtending branches using the same scheme (gray indicates ambiguous reconstructions). Nodal depths are proportional to inferred dates (Table 2), and the left vertices of individual clades are located at their estimated basal divergence times (major clades; e.g., NW Suboscines, node 5) or at the divergence from their sister taxa (subclades; e.g., Vireonidae). Numbered nodes correspond to Fig. 1. A geological and temporal scale is provided below, and letters associated with this scale indicate the approximate timing of events listed in the upper left-hand corner (references in supporting information; ANT, Antarctica; AUS, Australia; EUR, Eurasia; IND, India; NZ, New Zealand; SA, South America).
Our current sampling of suboscine lineages, although sufficient for testing higher-level hypotheses of relationship, precludes extensive discussion of their biogeography. However, our data suggest that the highly speciose New World suboscines began diversification in South America near the Cretaceous–Tertiary boundary (Table 2), indicating provincialism and vicariance before continental breakup of South America, Antarctica, and Australia. The biogeographic pattern of the remaining suboscines, the relatively species-poor broadbills (including the Malagasy asities; genera Smithornis, Psarisomus, Philepitta, and Neodrepanis in Fig. 1) and pittas (genus Pitta in Fig. 1), currently found on Madagascar, continental Africa, and Asia, seems to implicate tectonic vicariance involving India, Madagascar, and Antarctica followed by diversification in Asia (32). However, the separation of Old and New World suboscines, dated at ≈71 Ma, and the divergence of pittas and broadbills at 57 Ma (nodes 2 and 3 in Fig. 1, Table 2) appear too young for a strictly vicariant explanation. Reconstructing the history of suboscine diversification will require more detailed analysis, with additional taxon and character sampling (37, 38).
Basal oscine (node 7, Fig. 1) relationships and biogeographic history are greatly clarified by our data. In contrast to the classical view of oscine dispersal from the north into Australasia (44), Sibley and Ahlquist (3) hypothesized parallel diversification of northern and southern oscines (their Passerida and Corvida, respectively). The phylogenetic arrangement and inferred biogeography of basal oscine lineages as reconstructed here suggest a more complex history, in which two major sequential diversification events occurred: an ancestral Australasian diversification and a subsequent diversification of the Passerida after dispersal out of Australasia (Fig. 2), essentially reversing the classical scenario. Our phylogenetic results recognize a total of five basal oscine passerine lineages distributed in New Guinea and Australia (Fig. 1; supporting information), firmly rooting the origin of oscines in Australasia (Fig. 2). Additionally, our analyses suggest that three ancient lineages distributed in New Guinea and New Zealand form the sister group (node 19, Fig. 1) to a group we term the “core Corvoidea” (node 20, Fig. 1). Although basal relationships within the latter group are not well supported, >95% of the trees sampled in Bayesian analysis of the data placed an Australasian lineage as sister to the remaining corvoid taxa. Taken together, these relationships support an Australasian ancestry for the entire assemblage. It is from this basal Australasian radiation (from the Menuridae through node 18, Fig. 2) that the extremely diverse secondary radiation of passeridan oscines was derived.
Diversification of oscines appears to have involved dispersal of multiple lineages from Australasia, a single one of which, the Passerida, represents the bulk of passerine diversity (Figs. 1 and 2). The origins of this asymmetry in diversification are thus of particular interest. Analysis of our data identifies the sister taxa of the “Passerida” (Fig. 1) narrowly constraining the age and timing of dispersal of the group. The relatively species-poor families Petroicidae (44 species) and Picathartidae (4 species) are supported as successive sister taxa to the passeridan radiation, although the specific arrangement of these three lineages is only weakly supported (Fig. 1); the alternative resolutions require either one or two dispersals from Australasia. We estimate the divergence of the Picathartidae at 47–45 Ma, with the divergence of the dawn robins from the Passerida nearly coincident (45–44 Ma; Table 2). In contrast, the earliest inferred dispersal of any corvoid from Australia involves the lineage leading to Vireonidae + Erpornis at 40–37 Ma, at least 5 million years after the inferred passeridan dispersal, indicating a substantial head start for the latter group in its occupation of Eurasia. Other corvoids (e.g., the genera Oriolus, Dicrurus, Lanius, and Corvus or their ancestors) appear to have followed even later. Thus, the fundamental asymmetry between the diversity of the “core Corvoidea” and Australasian endemic groups on the one hand (8) and the Passerida on the other may in part be due to differences in the timing of lineage dispersal out of Australasia.
The timing and mechanisms of vertebrate dispersal between Africa and Eurasia remain an area of active research, primarily driven by interpretation of the mammal fossil record (45). We have dated the basal divergence of the newly discovered clade of shrike-like taxa found in Africa, Asia, and Australasia (node 11, Fig. 1) at 29–26 Ma. The divergence of another African/Asian disjunct pair, the sylvioid “flycatchers” Elminia and Culicicapa, appears nearly coincident (node 12; 27–26 Ma). These dates are contemporaneous with, or slightly earlier than, current fossil constraints on Oligocene mammal interchange between Eurasia and Africa (45). The establishment of currently disjunct oscine lineages in Asia and Africa was likely mediated by the presence of mesic forest throughout northern Africa and Eurasia before Miocene desertification (46), which generated disjunct patterns of distribution in many bird groups and other vertebrate lineages (47, 48). More extensive sampling of passerine lineages will undoubtedly reveal additional unrecognized faunal connections between these regions, allowing more detailed evaluation of avian dispersal into Africa.
Another important question for understanding the diversification of passerines is the impact of dispersal between the Old and New Worlds. For instance, one primarily New World group, the subfamily Emberizinae, represents ≈15% of passerines and nearly 8% of all extant bird species (1). Within the oscines, five sister-group comparisons establish maximum ages on invasions into the New World (Table 2). Three of these ages, the mockingbirds and thrashers (Mimini); sparrows, tanagers, and allies (Emberizinae); and vireos (Vireonidae), occur between 28 and 20 Ma; the fourth (the New World jays) is inferred to be somewhat younger (17–14 Ma) and the fifth (the wrens and gnatcatchers, Troglodytinae) somewhat older (34 Ma). Due to the advent of oceanic barriers, in combination with significant climatic deterioration, these times make it unlikely that dispersal of most oscines into the New World occurred via the North Atlantic (49) or Antarctica (ref. 50, contra ref. 3). These findings are in agreement with broad-scale spatial and temporal analyses of the extant Holarctic fauna (51) and with the timing and pattern of mammalian dispersal indicated by the fossil record (52), both of which suggest that dispersal into the New World in the late Oligocene/early Miocene was predominantly through Beringia. We infer that divergence of lineages within the extremely diverse subfamily Emberizinae commenced shortly after invasion of the New World (NPRS: 16 Ma, bootstrap SE = 3.3; PL: 18 Ma, SE = 1.6), a timing in close agreement with dates derived from mitochondrial DNA of this group (53). This is consistent with the hypothesis of relatively recent emberizine dispersal into South America and diversification in concert with the main thrust of Andean uplift (ref. 53; Table 5). Additional sampling of New World songbird lineages will refine the distribution of dispersal times from the Old World and allow more explicit biogeographic and temporal analysis of intrahemispheric dispersal and speciation patterns.
Conclusion
Most previous studies of spatial and temporal patterns in passerine diversification have either been formulated in the absence of a quantitative phylogenetic framework (54, 55) or have used a phylogenetic hypothesis (3), that shows significant and pervasive conflict with available DNA sequence data (refs. 6 and 8, but see ref. 56). The results reported here provide a robust phylogenetic framework for future studies of passerine evolution. In addition to their phylogenetic content, DNA sequence data have proven extremely informative in providing temporal constraints on the origin and diversification of the major passerine clades. These constraints must be corroborated by additional calibrations from fossils and paleogeographic vicariance events, and it is crucial that future phylogenetic analyses incorporate morphological data so that the phylogenetic position of key fossils carrying important temporal data can be established. In the future, increased taxon and character sampling should allow more accurate estimation of diversification rates (57, 58), clarify the role of Earth history in bioegographic patterns and diversification (8, 59), and permit explicit tests of adaptive explanations of passerine diversity (60, 61).
Supplementary Material
Supporting Information
Acknowledgments
We received useful comments on various portions of the manuscript from George Barrowclough, David Fox, Sharon Jansa, Scott Lanyon, Irby Lovette, Georgiana May, Nancy Simmons, Peter Tiffin, and several anonymous reviewers. We gratefully acknowledge loans of genetic material from many institutions, including the Australian Museum; Australian National Wildlife Collection; Field Museum; Museum Victoria; Percy Fitzpatrick Institute of Ornithology; Smithsonian National Zoological Park; University of Queensland; Queensland Museum; Burke Museum; and Zoological Museum, University of Copenhagen. This work was supported in part by National Science Foundation “Assembling the Tree of Life” Grant EAR-0228693. This paper is a contribution from the Monell Molecular Laboratory and the Cullman Research Facility at the American Museum of Natural History and has received support from the Lewis B. and Dorothy Cullman Program for Molecular Systematics Studies, a joint initiative of the New York Botanical Garden and the American Museum of Natural History. Analyses were performed by using resources of the Computational Genetics Laboratory at the University of Minnesota Supercomputing Institute.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ML, maximum likelihood; Ma, million years ago; NPRS, nonparametric rate smoothing; PL, penalized likelihood.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY443260–AY443339, AY443102–AY443247) and the EMBL database (accession nos. ALIGN_000669 and ALIGN_000671).
References
- 1.Sibley, C. G. & Monroe, B. L., Jr. (1990) Distribution and Taxonomy of the Birds of the World (Yale Univ. Press, New Haven, CT).
- 2.Nee, S., Mooers, A. Ø. & Harvey, P. H. (1992) Proc. Natl. Acad. Sci. USA 89**,** 8322–8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sibley, C. G. & Ahlquist, J. E. (1990) Phylogeny and Classification of Birds: A Study in Molecular Evolution (Yale Univ. Press, New Haven, CT).
- 4.Martin, T. E., Martin, P. R., Olson, C. R., Heidinger, B. J. & Fontaine, J. J. (2000) Science 287**,** 1482–1485. [DOI] [PubMed] [Google Scholar]
- 5.Poiani, A. & Pagel, M. (1997) Evolution (Lawrence, Kans.) 51**,** 226–240. [DOI] [PubMed] [Google Scholar]
- 6.Barraclough, T. G., Harvey, P. H. & Nee, S. (1995) Proc. R. Soc. London Ser. B 259**,** 211–215. [Google Scholar]
- 7.Cardillo, M. (1999) Proc. R. Soc. London Ser. B 266**,** 1221–1225. [Google Scholar]
- 8.Ricklefs, R. E. (2003) Proc. R. Soc. London Ser. B 270**,** 2285–2291. [Google Scholar]
- 9.Barker, F. K., Barrowclough, G. F. & Groth, J. G. (2002) Proc. R. Soc. London Ser. B 269**,** 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ericson, P. G. P., Irestedt, M. & Johansson, U. S. (2003) J. Avian Biol. 34**,** 3–15. [Google Scholar]
- 11.Morony, J., Bock, W. & Farrand, J. (1975) Reference List of the Birds of the World (American Museum of Natural History, New York).
- 12.Swofford, D. L. (2002) paup*: Phylogenetic Analysis Using Parsimony (* and Other Methods) (Sinauer, Sunderland, MA).
- 13.Guindon, S. & Gascuel, O. (2003) Syst. Biol. 52**,** 696–704. [DOI] [PubMed] [Google Scholar]
- 14.Huelsenbeck, J. P. & Ronquist, F. (2001) Bioinformatics 17**,** 754–755. [DOI] [PubMed] [Google Scholar]
- 15.Altekar, G., Dwarkadas, S., Huelsenbeck, J. P. & Ronquist, F. (2004) Bioinformatics 20**,** 407–415. [DOI] [PubMed] [Google Scholar]
- 16.Farris, J. S., Källersjö, M., Kluge, A. G. & Bult, C. (1995) Syst. Biol. 44**,** 570–572. [Google Scholar]
- 17.Dolphin, K., Belshaw, R., Orme, C. D. & Quicke, D. L. J. (2000) Mol. Phylogenet. Evol. 17**,** 401–406. [DOI] [PubMed] [Google Scholar]
- 18.Darlu, P. & Lecointre, G. (2002) Mol. Biol. Evol. 19**,** 432–437. [DOI] [PubMed] [Google Scholar]
- 19.Barker, F. K. & Lutzoni, F. M. (2002) Syst. Biol. 51**,** 625–637. [DOI] [PubMed] [Google Scholar]
- 20.Hipp, A. L., Hall, J. C. & Sytsma, K. J. (2004) Syst. Biol. 53**,** 81–89. [DOI] [PubMed] [Google Scholar]
- 21.Nixon, K. C. (1999) Cladistics 15**,** 407–414. [DOI] [PubMed] [Google Scholar]
- 22.Sikes, D. S. & Lewis, P. O. (2001) pauprat (Univ. of Connecticut, Storrs).
- 23.Felsenstein, J. (1985) Evolution (Lawrence, Kans.) 39**,** 783–791. [DOI] [PubMed] [Google Scholar]
- 24.Yang, Z. (1994) J. Mol. Evol. 39**,** 306–314. [DOI] [PubMed] [Google Scholar]
- 25.Shimodaira, H. & Hasegawa, M. (1999) Mol. Biol. Evol. 16**,** 1114–1116. [Google Scholar]
- 26.Ronquist, F. (1996) diva (Uppsala University, Uppsala, Sweden).
- 27.Ronquist, F. (1997) Syst. Biol. 46**,** 195–203. [Google Scholar]
- 28.Felsenstein, J. (1981) J. Mol. Evol. 17**,** 368–376. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson, M. J. (1997) Mol. Biol. Evol. 14**,** 1218–1231. [Google Scholar]
- 30.Sanderson, M. J. (2002) Mol. Biol. Evol. 19**,** 101–109. [DOI] [PubMed] [Google Scholar]
- 31.Efron, B. & Tibshirani, R. J. (1993) An Introduction to the Bootstrap (Chapman and Hall, New York).
- 32.Cracraft, J. (2001) Proc. R. Soc. London Ser. B 268**,** 459–469. [Google Scholar]
- 33.Ericson, P. G. P., Christidis, L., Cooper, A., Irestedt, M., Jackson, J., Johansson, U. S. & Norman, J. A. (2002) Proc. R. Soc. London Ser. B 269**,** 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Tuinen, M. & Hedges, S. B. (2001) Mol. Biol. Evol. 18**,** 206–213. [DOI] [PubMed] [Google Scholar]
- 35.Feduccia, A. (2003) Trends Ecol. Evol. 18**,** 172–176. [Google Scholar]
- 36.Fleischer, R. C., McIntosh, C. E. & Tarr, C. L. (1998) Mol. Ecol. 7**,** 533–545. [DOI] [PubMed] [Google Scholar]
- 37.Irestedt, M., Johansson, U. S., Parsons, T. J. & Ericson, P. G. P. (2001) J. Avian Biol. 32**,** 15–25. [Google Scholar]
- 38.Chesser, R. T. (2004) Mol. Phylogenet. Evol. 32**,** 11–24. [DOI] [PubMed] [Google Scholar]
- 39.Ericson, P. G. P., Christidis, L., Irestedt, M. & Norman, J. A. (2002) Mol. Phylogenet. Evol. 25**,** 53–62. [DOI] [PubMed] [Google Scholar]
- 40.Boles, W. E. (1995) Nature 374**,** 21–22. [Google Scholar]
- 41.Mourer-Chauviré, C., Hugueney, M. & Jonet, P. (1989) CR Acad. Sci. Sér. II 309**,** 843–849. [Google Scholar]
- 42.Cooper, A. & Penny, D. (1997) Science 275**,** 1109–1113. [DOI] [PubMed] [Google Scholar]
- 43.Flynn, J. J. & Wyss, A. R. (1998) Trends Ecol. Evol. 13**,** 449–454. [DOI] [PubMed] [Google Scholar]
- 44.Mayr, E. (1944) Bull. Am. Museum Nat. Hist. 83**,** 123–194. [Google Scholar]
- 45.Kappelman, J., Rasmussen, D. T., Sanders, W. J., Feseha, M., Bown, T., Copeland, P., Crabaugh, J., Fleagle, J., Glantz, M., Gordon, A., et al. (2003) Nature 426**,** 549–552. [DOI] [PubMed] [Google Scholar]
- 46.Axelrod, D. I. & Raven, P. H. (1978) in Biogeography and Ecology of Southern Africa, ed. Werger, M. J. A. (Junk, The Hague), pp. 77–130.
- 47.Darlington, P. J. (1957) Zoogeography: The Geographical Distribution of Animals (Wiley, New York).
- 48.Cracraft, J. (1973) in Implications of Continental Drift to the Earth Sciences, eds. Tarling, D. H. & Runcorn, S. (Academic, London), Vol. 1, pp. 349–389. [Google Scholar]
- 49.McKenna, M. C. (1975) Ann. Mo. Bot. Gard. 62**,** 335–353. [Google Scholar]
- 50.Askin, R. A. & Spicer, R. A. (1995) in Effects of Past Global Change on Life (Natl. Acad. Press, Washington, DC), pp. 156–173.
- 51.Sanmartín, I., Enghoff, H. & Ronquist, F. (2001) Biol. J. Linn. Soc. 73**,** 345–390. [Google Scholar]
- 52.Webb, S. D. & Opdyke, N. D. (1995) in Effects of Past Global Change on Life (Natl. Acad. Press, Washington, DC), pp. 184–208.
- 53.Burns, K. J. (1997) Mol. Phylogenet. Evol. 8**,** 334–348. [DOI] [PubMed] [Google Scholar]
- 54.Beecher, W. J. (1953) Auk 70**,** 270–333. [Google Scholar]
- 55.Feduccia, A. (1976) Syst. Zool. 26**,** 19–31. [Google Scholar]
- 56.Cockburn, A. (2003) Proc. R. Soc. London Ser. B 270**,** 2207–2214. [Google Scholar]
- 57.Purvis, A., Nee, S. & Harvey, P. H. (1995) Proc. R. Soc. London Ser. B 260**,** 329–333. [DOI] [PubMed] [Google Scholar]
- 58.Paradis, E. (1998) Am. Nat. 152**,** 176–187. [DOI] [PubMed] [Google Scholar]
- 59.Cracraft, J. (1994) Am. Zool. 34**,** 33–47. [Google Scholar]
- 60.Barraclough, T. G., Vogler, A. P. & Harvey, P. H. (1998) Proc. R. Soc. London Ser. B 353**,** 241–249. [Google Scholar]
- 61.Barraclough, T. G. & Nee, S. (2001) Trends Ecol. Evol. 16**,** 391–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information