The Ratio of Macronutrients, Not Caloric Intake, Dictates Cardiometabolic Health, Aging, and Longevity in Ad Libitum-Fed Mice (original) (raw)
. Author manuscript; available in PMC: 2016 Oct 31.
Summary
The fundamental questions of what represents a macronutritionally balanced diet and how this maintains health and longevity remain unanswered. Here, the Geometric Framework, a state-space nutritional modeling method, was used to measure interactive effects of dietary energy, protein, fat, and carbohydrate on food intake, cardiometabolic phenotype, and longevity in mice fed one of 25 diets ad libitum. Food intake was regulated primarily by protein and carbohydrate content. Longevity and health were optimized when protein was replaced with carbohydrate to limit compensatory feeding for protein and suppress protein intake. These consequences are associated with hepatic mammalian target of rapamycin (mTOR) activation and mitochondrial function and, in turn, related to circulating branched-chain amino acids and glucose. Calorie restriction achieved by high-protein diets or dietary dilution had no beneficial effects on lifespan. The results suggest that longevity can be extended in ad libitum-fed animals by manipulating the ratio of macronutrients to inhibit mTOR activation.
Introduction
Resolving the effects of dietary macronutrients on aging and health remains a fundamental challenge, with profound implications for human health. As in much of nutrition research, the focus has mainly concerned the effects of individual macronutrients, with proponents of fat, sugar, and protein each claiming primacy in the search for explanations for the global increase in rates of obesity and metabolic disease and dietary solutions for their prevention and treatment. There is, however, growing evidence from studies on a wide range of species that, rather than macronutrients acting singly, it is their interactive effects (i.e., their balance) that are more important for health and aging. In particular, it has emerged that the balance of protein to nonprotein energy in the diet is especially significant, influencing total energy intake, growth and development, body composition, reproduction, aging, gut microbial ecology, the susceptibility to obesity and metabolic disease, immune function, and resistance to infectious diseases (Blumfield et al., 2012; Gosby et al., 2011; Huang et al., 2013; Lee et al., 2008; Mayntz et al., 2009; Piper et al., 2011; Ponton et al., 2011; Simpson and Raubenheimer, 2009, 2012).
Defining what represents a balanced diet—and the consequences of not attaining such balance—is a high priority in nutrition research. Progress has been impeded, however, by the logistical challenges involved in designing dietary experiments able to disentangle the individual and interactive influences of multiple nutrients on the phenotype. The development of the Geometric Framework for nutrition (GF) now provides a platform for taming this complexity (Lee et al., 2008; Mayntz et al., 2005; Piper et al., 2011; Simpson and Raubenheimer, 2009, 2012). GF, a state-space modeling approach that explores how an animal responds to the problem of balancing multiple and changing nutrient needs in a multidimensional and variable nutritional environment, has been successful in solving and reconciling diverse and often conflicting conclusions about how nutrition influences biology across taxa. The GF considers nutrition as an n-dimensional space in which the n components of any diet are represented by separate axes (in the case of macronutrients, there are three axes: protein, carbohydrate, and fat). Responses of individuals, such as lifespan, are phenotypic features that are superimposed on this n-dimensional nutritional space by plotting response surfaces (Lee et al., 2008; Piper et al., 2011; Simpson and Raubenheimer, 2009, 2012).
Here, we have used the GF to investigate how the balance and concentrations of macronutrients affect feeding, aging, and age-related cardiometabolic health in ad libitum-fed mice. We first consider how dietary macronutrients interact to determine chronic food and energy intakes in animals monitored over their lifetime and into old age. A central dogma of aging research is that caloric restriction (reduction of access to dietary energy to about 30%–50% of that consumed by ad libitum-fed controls, supplemented with micronutrients; Everitt et al., 2010) increases lifespan and delays aging, yet recent research has shown that the relationship between diet and aging is complex, particularly in ad libitum-fed animals, in which compensatory feeding regulates the relationship between dietary constituents and the actual dietary intake (Le Couteur et al., 2014; Simpson and Raubenheimer, 2007). Thus, we relate macronutrient intakes to longevity, in particular testing the extent to which lifespan in ad libitum-fed mice is determined by calorie intake per se or by the balance of protein to carbohydrate, as recently proposed from GF studies of insects (Fanson et al., 2009; Lee et al., 2008). These results are then related to known physiological mechanisms that link nutrition with aging biology. We focus on the mammalian target of rapamycin (mTOR) because it is activated by amino acids, thereby plausibly linking diet to a broad suite of cellular responses associated with aging (Kapahi et al., 2010). Moreover, inhibition of mTOR with rapamycin increases lifespan in mice fed a standard diet and represents evidence of pharmacological life extension in normal mammals (Harrison et al., 2009; Miller et al., 2011). We propose that the beneficial effects on aging generated by rapamycin might be replicated by titrating the intake of macronutrients, particularly protein. Finally, we establish the relationship between macronutrient and energy intake with key aspects of late life cardiometabolic phenotype, which is an important determinant of lifespan and likely to contribute to many aspects of the aging process itself (Le Couteur and Lakatta, 2010).
The results provide an overarching schema for the relationship between diet balance, health, and aging. By simultaneously measuring key physiological correlates, we also link the nutritional phenotype to underlying mechanisms and place these mechanisms in a broader nutritional context than hitherto possible.
Results
The data we present derive from 858 mice fed one of 25 diets differing systematically in protein, carbohydrate, and fat content and energy density. By their nature, these data are complex, and their graphical representation in 3D nutrient space is challenging, but can only be appreciated using this methodology. To assist understanding, the narrative of the main text is illustrated by 2D response surfaces, which are provided in figures and supplemental figures. These surfaces are interpreted statistically using General Additive Models (GAM). The complete statistical analyses are provided for each surface in the tables in the Supplemental Information.
Compensatory Feeding Occurred for Protein and Carbohydrate, but Less so for Fat
Mice were fed ad libitum over a lifetime on 1 of 25 diets differing in content of protein (5%–60%), fat (16%–75%), carbohydrate (16%–75%), and energy (8, 13, or 17 kJ/g of food) (Table S1). These dietary compositions were chosen according to the GF to sample a representative number of nutritional vectors (Figure S1 available online) and nutrient concentrations within protein-carbohydrate-fat diet space. One-third of the mice were culled at 15 months of age, and the remainder continued until they died naturally. This age was chosen for the cull because it represents late middle age, but before significant numbers of mice have died, which would introduce survivor bias into the results.
Food (Figures 1A and S2A) and energy intakes (Figures 1C and S2B) were plotted as response surfaces mapped onto diet composition space and interpreted statistically using GAM. Food intake was assessed meticulously, including measurements of discarded food with fecal matter removed, and values are similar to a previous study using this model (Huang et al., 2013). Values reported are per animal per day, averaged over the period of 6–15 months of age, during which time intakes were stable. As the concentrations of either protein or carbohydrate decreased in the diet, chronic food intake increased. This pattern of increasing food intake as nutrient concentrations fall in the food is consistent with compensatory feeding for these macronutrients controlled by nutrient-specific regulatory feedbacks (Sørensen et al., 2008). Regulatory feeding effects were most evident for dietary protein and less marked for dietary carbohydrate. In contrast, fat content in the diet was largely unregulated and thus had negligible influence on food intake (Figures 1A and 1B). Note that both protein and carbohydrate intakes decelerated as their proportions rose in the diet, whereas fat intake continued to increase as the proportion of dietary fat increased (Figure 1B, red lines show the values where half the maximal nutrient intake is reached). Food intake was reduced with high-protein-content diets once protein intake exceeded about 10 kJ/day, whereas carbohydrate intake decelerated once carbohydrate intake exceeded about 15 kJ/day. High-fat diets had minimal impact on food intake.
Figure 1. The Effects of Dietary Macronutrients on Food Intake.
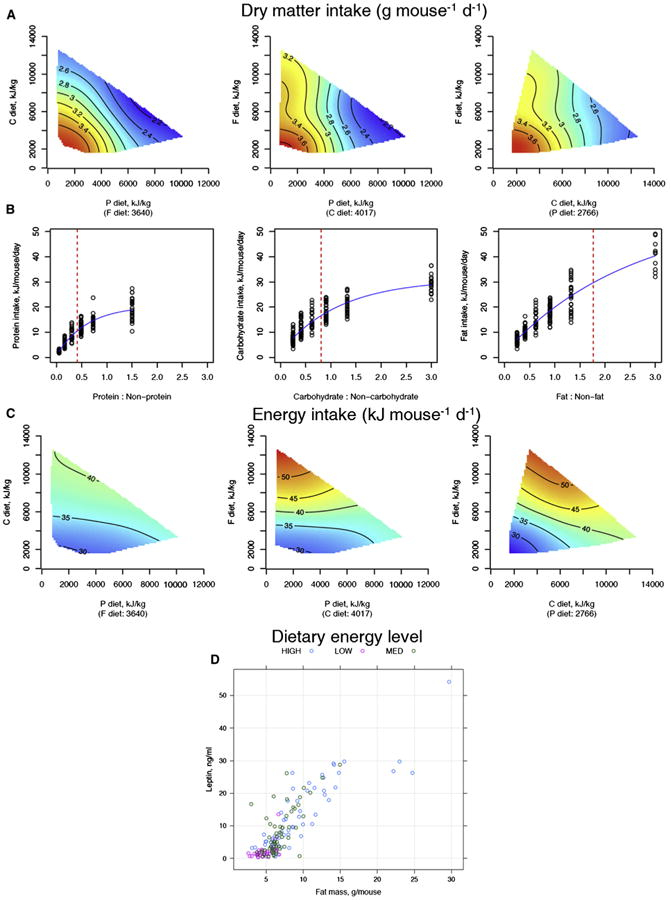
(A) Response surfaces showing the relationship between dry matter (DM) intake versus macronutrient content of the diet. Surfaces were fitted using generalized additive models (GAMs) with thin-plate splines. Three 2D slices are given to show all three nutrient dimensions (protein, P; carbohydrate, C; fat, F). For each 2D slice, the third factor is at its median (shown below the × axis in parentheses). In all surfaces, red indicates the highest value, while blue indicates the lowest value, with the colors standardized across the three slices.
(B) Relationship between protein, carbohydrate, and fat intake versus the proportion of protein, carbohydrate, and fat in the diet relative to the other macronutrients, respectively. A decelerating curve indicates regulation for a given nutrient, with the asymptote reflecting the target intake for that nutrient.
(C) Response surfaces showing the relationship between total energy intake versus macronutrient content of the diet. Energy intake was highest on low P:high F and low C:high F diets, presumably because mice overate in an attempt to achieve their protein or carbohydrate targets, with less compensatory suppression of food intake by fat.
(D) Relationship between body fat and leptin levels, according to the three energy density diets. See also Figure S2 and Table S3.
If nutrient-specific feedback mechanisms exist for fat (as they do, for example, in carnivores; Hewson-Hughes et al., 2011; Mayntz et al., 2009), they are clearly dominated by competing inputs from protein and carbohydrate. Consequently, total energy intake was highest on those diets with low protein and/or low carbohydrate content because compensatory overeating to achieve the protein or carbohydrate intake targets (sensu the GF; Simpson and Raubenheimer, 2012) was not counterbalanced by marked inhibitory effects of fat on total food intake. Leptin titers were positively associated with body fat (Figure 1D) and total macronutrient intake (Figure S2C), implying that leptin was not suppressing appetite.
At 6 and 15 months, mice ate similar amounts of protein regardless of the energy density of the diet, consistent with regulation to a protein intake target. However, at 24 months, which represents old age, mice ate similar amounts of food regardless of the energy density of their diets. This led to a reduction in the intake of energy and macronutrients in the old mice on the low-energy density diets and, conversely, increased intakes in old mice on high-energy density diets (Figure S2D).
The Balance of Dietary Macronutrients Influenced Longevity
The GF was used to establish the effects of macronutrient intakes on longevity. Median lifespan was greatest for animals whose intakes were low in protein and high in carbohydrate, but was not influenced by total calorie intake (Figure 2A). The results are consistent with recent reports in invertebrates showing that the ratio of protein to carbohydrate in the diet influences life-span (Lee et al., 2008; Piper et al., 2011). The survival curves for the different ratios of protein to carbohydrate (Figure 2B) show that the longest median survival occurred in cohorts of mice on the lowest ratio diets, and there was a clear correlation between the ratio and lifespan. Median lifespan increased from about 95 to 125 weeks (approximately 30%; Table S2) as the protein-to-carbohydrate ratio decreased.
Figure 2. The Effects of Dietary Macronutrients and Energy Intake on Longevity.
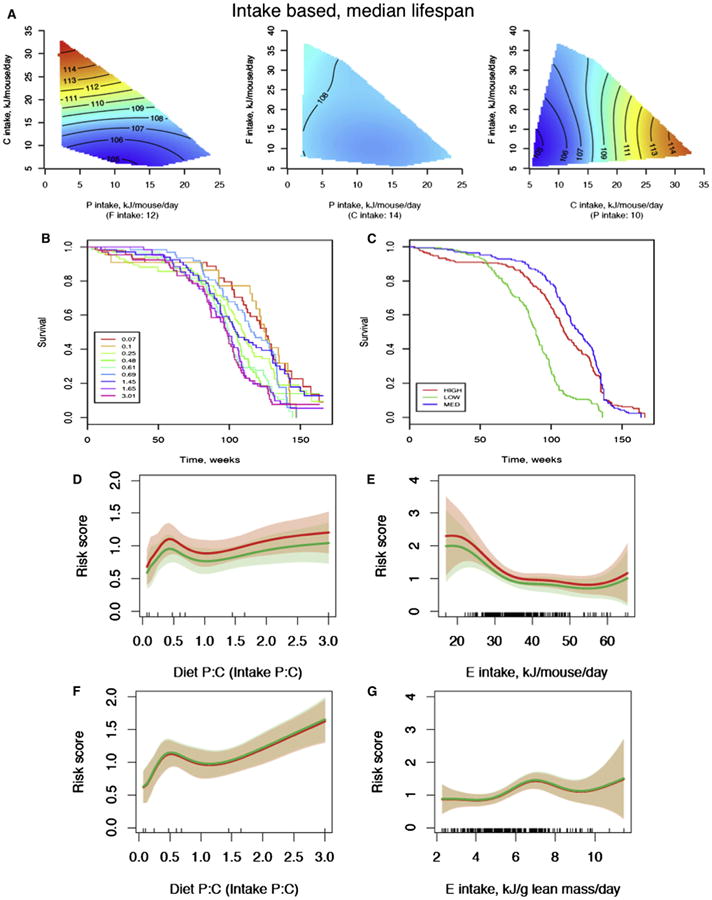
(A) Response surfaces showing the relationship between protein (P), carbohydrate (C), and fat (F) intake versus median lifespan (weeks). For each 2D slice, the third factor is at its median (shown below the × axis in parentheses). In all surfaces, red indicates the highest value, while blue indicates the lowest value, with the colors standardized across the three slices.
(B and C) Kaplan-Meier survival curves according to differing dietary protein:carbohydrate ratios (B) and dietary energy density (C).
(D) The relationship between the ratio of protein to carbohydrate in the diet and the risk of death. A Cox regression analysis was performed with gender and energy intake as covariates for males (red) and females (green). The bands show the 95% confidence intervals.
(E) The relationship between energy intake and the risk of death. A Cox regression analysis was performed with gender and the ratio of dietary protein to carbohydrate as covariates.
(F) As for (D), but based on protein:carbohydrate intakes corrected for lean body mass.
(G) As for (E), with energy intake corrected for lean body mass. See also Table S4.
The presence of compensatory feeding for protein and carbohydrate, but less so for fat, shows that there is a complex interplay between dietary protein, carbohydrates, and total energy intake in ad libitum-fed animals, which may have competing consequences for lifespan and cellular regulatory pathways. Therefore, Cox regression analysis was performed to assess the independent effects of energy intake and protein-to-carbohydrate ratio on lifespan and to take into account individual animal survival data. Analyses were performed both for total macronutrient and energy intakes (Figures 2D and 2E) and for intakes corrected for lean body mass (Figures 2F and 2G). The proportional hazards assumption was tested and found to be valid for each analysis (data not shown). Overall, there was an increase in the hazard ratio for death as the protein to carbohydrate ratio increased, consistent with the univariate analysis (Figures 2D and 2F).
Reduction in calorie intake was achieved by diluting the food with nondigestible cellulose, which allows ad libitum feeding but restricts total energy intake when compensation for dilution by increasing food intake is incomplete. Mice fed experimental diets containing 50% nondigestible cellulose ate a greater bulk of food (3.6 ± 0.4 versus 2.5 ± 0.4 g/day) but ingested about 30% less total energy than mice provided with food containing higher energy content (29.9 ± 3.2 versus 42.1 ± 7.3 kJ/day). Therefore, these mice had a reduction in energy intake similar to those reported in nearly all other studies of calorie restriction in which access to food was restricted (Everitt et al., 2010). Except at the oldest ages, mice on the low-energy diets were able to achieve their protein target through increasing chronic food intake (protein intake: 9.6 ± 4.3 kJ/day with low-energy diets versus 9.6 ± 6.3 kJ/day with high-energy diets). When corrected for lean body mass, the hazard ratio for death was not influenced by calorie intake, except at the highest energy intakes, which were achieved only by low-protein, high-fat diets (Figure 2G). The results suggest that energy restriction achieved by dietary dilution in ad libitum-fed animals does not extend life-span, probably because animals can reach their target protein intake. It should be noted that previous caloric restriction experiments that reduce total food availability and observe longevity extension are unable to conclusively determine whether this effect was due to restriction of total calories or one or more specific nutrients in the diet (such as protein); previous aging studies in which protein content of the diet was reduced in ad libitum-fed animals may not have increased lifespan because of compensatory feeding.
The Relationship between Diet and Longevity Was Associated with Hepatic mTOR Activation
To link these relationships to signaling pathways and mechanisms implicated in appetite, aging, and cardiometabolic health, we measured insulin, activation state of mTOR in the liver, circulating amino acids, and hepatic mitochondrial activity. Insulin and mTOR are strongly implicated in the relationship between diet and aging (Burnett et al., 2011; Fontana et al., 2010; Kapahi et al., 2010). Amino acids, particularly branched-chain amino acids (BCAA), are key signals for insulin release and mTOR activation (Chotechuang et al., 2009; Yang et al., 2010).
Among plasma free amino acids, BCAA levels correlated positively with chronic daily protein intake, with maximum levels occurring at the protein intake of approximately 10–20 kJ/mouse/day (Figures 3A and S3A). In strong contrast to BCAA, all other free amino acids correlated negatively with protein intake (Table 1 and Table S5). For BCAA, there was a sigmoidal relationship with the dietary protein:carbohydrate ratio, reaching a plateau at just below about 45 μg/ml when the protein:carbohydrate ratio was approximately 0.5–0.6 (Figure 3B). Insulin was influenced by both dietary protein and carbohydrate and was minimal when protein intake was lowest and highest when carbohydrate intake was between 15 and 25 kJ/day (Figure 3C).
Figure 3. Mechanisms for the Relationship between Diet and Longevity.
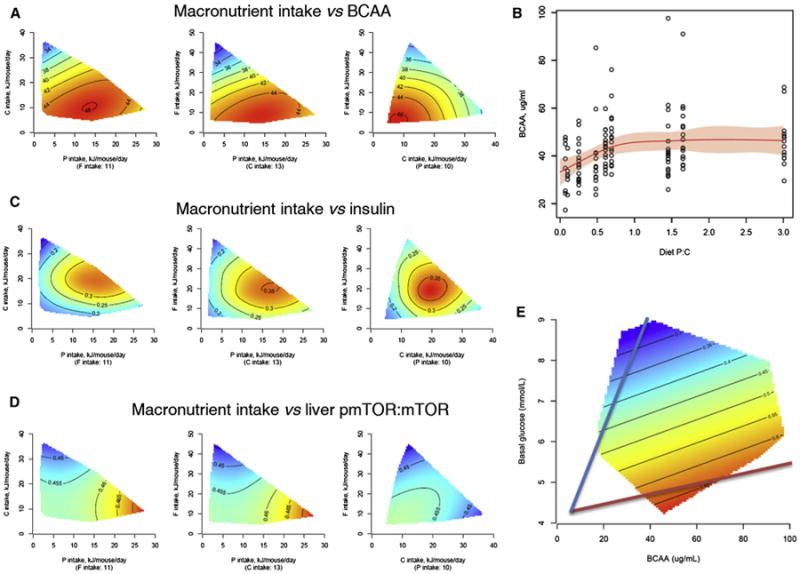
(A) Response surfaces showing the effect of macronutrient intake on the concentration of branched-chain amino acids (BCAAs)at 15 months (μg/ml). For each 2D slice, the third factor is at its median (shown below × axis in parenthesis).
(B) BCAAs in relation to dietary P:C ratio. Red curve is a fitted response by a GAM. Shadowed area shows 95% confidence interval of the fit.
(C) Response surfaces showing the effect of macronutrient intake on the concentration of insulin (ng/ml) at 15 months.
(D) Response surfaces showing the effect of macronutrient intake on mTOR activation (measured as the ratio of phosphorylated mTOR to total mTOR) in the liver at 15 months.
(E) Response surface showing the relationship between blood glucose, BCAA, and mTOR activation. The lowest activation state of mTOR is associated with a low ratio (blue line), and the highest activation state is associated with a high ratio (red line). See also Figure S3 and Table S5.
Table 1. Correlation Coefficients for Relationships between Protein Intake and Circulating Amino Acid Levels.
| aa | Coefficient | aa | Coefficient | aa | Coefficient |
|---|---|---|---|---|---|
| alanine | −0.617 | glutathione | −0.152 | phenylalanine | −0.016 |
| arginine | −0.553 | glycine | −0.159 | proline | −0.358 |
| asparagine | −0.498 | histidine | −0.217 | serine | −0.627 |
| aspartic acid | 0.015 | isoleucine* | 0.176* | taurine | −0.189 |
| citrulline | −0.584 | leucine* | 0.213* | threonine | −0.467 |
| cysteine | −0.170 | lysine | −0.461 | tryptophan | −0.172 |
| glutamic acid | −0.014 | methionine | −0.193 | tyrosine | −0.073 |
| glutamine | −0.346 | ornithine | −0.170 | valine* | 0.328* |
The activation of mTOR (the ratio of phosphorylated mTOR to total mTOR) was influenced by protein intake, albeit the effect, although significant, was small (Figure 3D). However, mTOR activation was strongly influenced by circulating glucose and BCAAs, reflecting protein and carbohydrate intakes, and on this analysis mTOR activation increased 3-fold as the plasma ratio of BCAAs to glucose increased (Figure 3E).
Changes in mitochondrial function and number are implicated in aging (Martin and Loeb, 2004). We used the Seahorse XF Extracellular Flux Analyzer (Horan et al., 2012) to study mitochondrial function in the liver because this is the main metabolic organ. Overall, the results indicate that a low protein intake in ad libitum-fed mice was associated with increased mitochondrial activity and elevated free radical production in the liver, which is similar to effects reported in caloric restriction studies (López-Lluch et al., 2006). There was a general pattern for dietary protein and protein intake to be positively associated with citrate synthase activity (which is a gold-standard marker of mitochondrial number; Larsen et al., 2012) (Figure S4) while negatively associated with state III and IVo mitochondrial respiration and hydrogen peroxide production, except when palmitoyl carnitine was used as a substrate (Figures 4A and 4B and Table S7). State III and IVo respiration rates were increased when fat intake was very high only when palmitoyl carnitine was used as a substrate (Figure 4B). This indicates that liver mitochondria only increased capacity for fat as a substrate when dietary fat was excessive.
Figure 4. The Effects of Macronutrient Balance on Mitochondrial Function in the Liver.
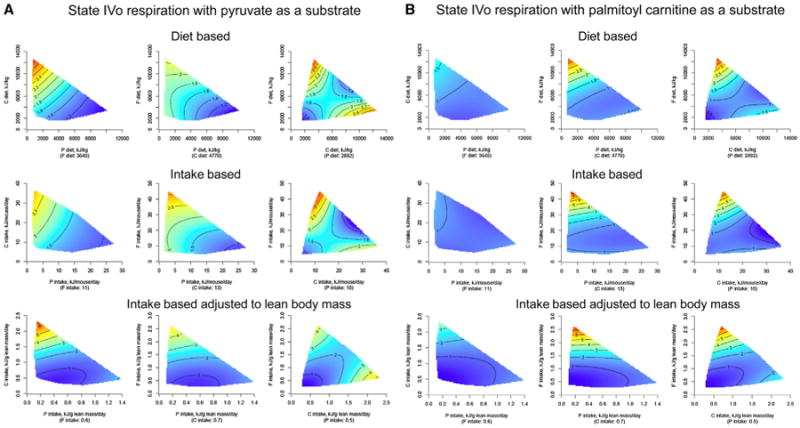
(A) Response surfaces showing the effect of dietary macronutrients on mitochondrial state IVo respiration with pyruvate as substrate (pmol O2 consumed/min/U of citrate synthase) in the liver at 15 months. Surfaces are shown for diet-based data (top), intake-based data (middle), and intake-based data adjusted to lean body mass (bottom). The highest activity is indicated in red (low-protein diets), and the lowest is indicated in blue.
(B) Response surfaces showing the effect of dietary macronutrients on mitochondrial state IVo respiration with palmitoyl carnitine as substrate (pmol O2 consumed/min/U of citrate synthase) in the liver at 15 months. Surfaces are shown for diet-based data (top), intake-based data (middle), and intake-based data adjusted to lean body mass (bottom). The highest activity is indicated in red (high-fat diets), and the lowest is indicated in blue. See also Figure S4 and Tables S6 and S7.
The Balance of Macronutrients Influenced Latelife Cardiometabolic Phenotype
The aging phenotype was assessed at 15 months, when approximately one-third of the mice were culled (Figures 5, 6, and S5). Nutritional geometry showed that the balance of macronutrients influenced several important phenotypic characteristics, including body weight and composition, blood pressure, glucose tolerance, lipids, fatty liver, and bone mineral density.
Figure 5. The Effects of Macronutrients on Latelife Physical Characteristics and Cardiovascular Physiology.
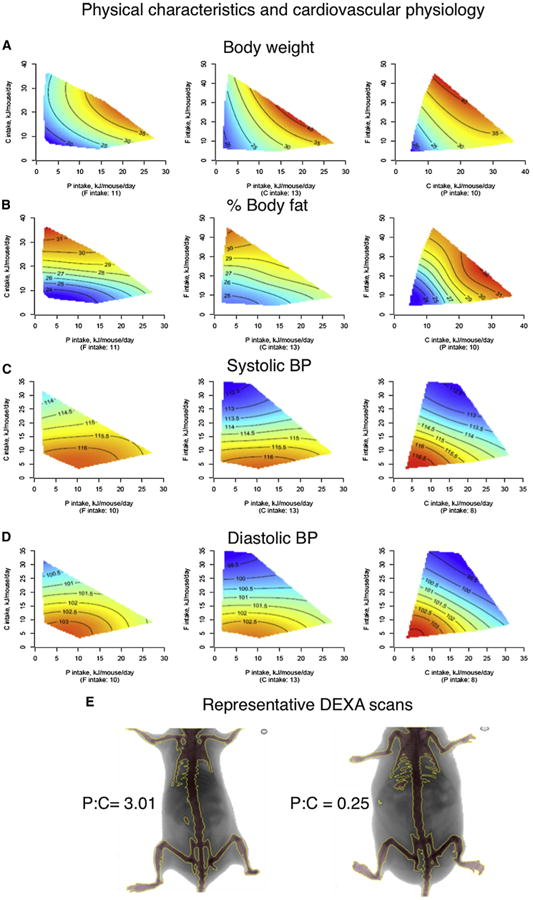
(A–E) Response surfaces show body weight (g) (A), body fat (%) (B), systolic (C) and diastolic blood pressures (mmHg) (D), and representative DEXA scans (E) showing the effect of chronic high P:C and low P:C intakes at 15 months of age. See also Figure S5 and Table S8.
Figure 6. The Effects of Macronutrients on Latelife Metabolic and Bone Parameters.
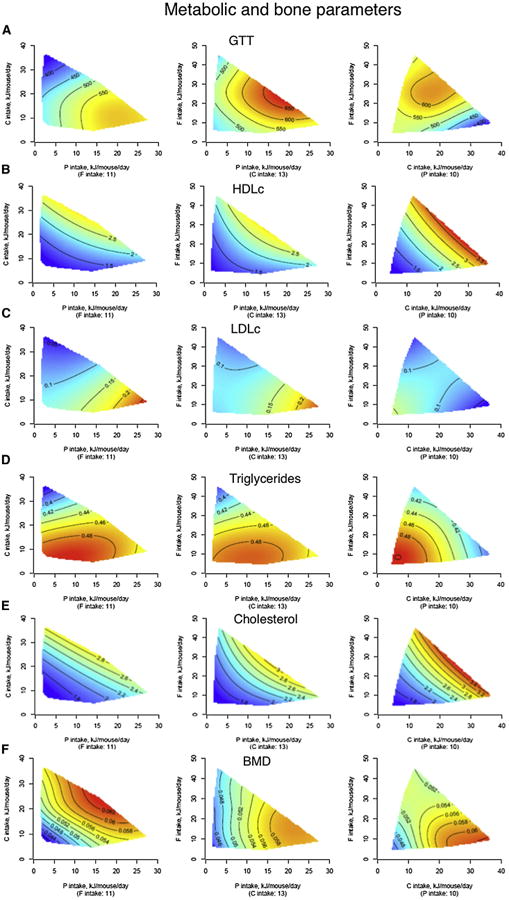
(A–F) Response surfaces show glucose tolerance (incremental area under curve, AUC) (A), HDLc (mmol/l) (B), LDLc (mmol/l) (C), triglycerides (mmol/l) (D), cholesterol (mmol/l) (E), and bone mineral density (g/cm2) (F) at 15 months of age. Low-protein diets were associated with improved glucose tolerance, increased HDLc, and reduced LDLc and cholesterol, all beneficial to the cardiometabolic profile. As expected, triglyceride levels were largely driven by carbohydrate intake. Bone mineral density was adversely affected by both low-protein and low-carbohydrate intakes, being maximal when the combination of protein and carbohydrate intake was high. See also Table S9.
Diets that were low in protein and high in carbohydrate (i.e., those that promoted longest life) were associated with lower blood pressure (Figures 5C and 5D), improved glucose tolerance (Figure 6A), higher levels of high-density lipoprotein (HDLc; Figure 6B), reduced levels of low-density lipoprotein (LDLc; Figure 6C), and lower triglycerides (Figure 6D). This is consistent with human data suggesting that long-term adherence to high-protein, low-carbohydrate diets is linked with increased cardiovascular disease (Floegel and Pischon, 2012; Lagiou et al., 2012) and indicates that the balance of protein to carbohydrate, rather than energy intake, may be the driver of a healthy cardiometabolic profile. However, diets with low protein:carbohydrate ratios were also associated with some characteristics usually considered to be associated with poorer health outcomes, such as increased body fat with reduced body lean (Figure 5B) and fatty liver (Figure S5). These paradoxical results are consistent with some human epidemiological studies that indicate that in old age there is a diminution, or even reversal, of the association between these risk factors and outcomes such as remaining life expectancy (Le Couteur and Simpson, 2011).
Discussion
We have used the Geometric Framework to disentangle the interactive effects of dietary macronutrients on appetite, total energy intake, metabolic health, and longevity outcomes in ad libitum-fed mice. There were regulatory compensatory feeding responses to dietary protein and carbohydrate, but dietary fat had little influence on food intake. This does not necessarily indicate that fat exerted no negative feedback onto intake, but rather that any such feedback is dominated by competing feedbacks from protein and carbohydrate. In this regard, it is interesting to note that a primary feedback emanating from body fat depots, leptin, was not associated with lower energy intake, perhaps indicating development of resistance to leptin in high-fat-, low-protein-fed animals (Pelleymounter et al., 1995). The net result of the asymmetrical interactions between regulatory feedbacks generated by the macronutrients was passive intake of excess fat in response to the drive to ingest target levels of protein and carbohydrate.
When the protein:carbohydrate ratio was very low, mice did not achieve their protein intake target, and subsequently, there were lower circulating BCAAs. As the protein content of the diet increased, energy intake was suppressed, and BCAA levels were stabilized at a higher constant level. In marked contrast, plasma concentrations of all other amino acids fell as protein intake increased. The results support the thesis that BCAAs play a key role in protein appetite and regulation of food intake (Fromentin et al., 2012).
The balance of macronutrients, in particular the protein: carbohydrate ratio, had a marked effect on longevity and latelife health. As the protein:carbohydrate ratio increased, there were concomitant increases in hepatic mTOR activation, apparently associated with the combination of elevated circulating BCAAs and low glucose. Given the evidence that activation of mTOR is proaging (Burnett et al., 2011; Fontana et al., 2010; Kapahi et al., 2010), our results support this as a mechanism to explain the life-extending effects of a low-protein, high-carbohydrate diet. Indeed, the pattern of interactive effects of BCAAs and glucose—as a reflection of dietary protein and carbohydrate—on activation of mTOR (Figure 3E) are as we predicted previously from an understanding of nutritional geometry and the established role of mTOR in regulating the aging process (Simpson and Raubenheimer, 2009).
Both BCAAs and insulin activate mTOR, and both are required for activation of pathways downstream of mTOR (Chotechuang et al., 2009). Chronic exposure to high-protein, low-carbohydrate diets resulted in the lowest food intakes, but elevated both mTOR and insulin, with reduced lifespan. The data indicate that a healthy diet is one that is titrated to generate low mTOR activation and low insulin levels, but must also take into account compensatory feeding, where animals over- or undereat in order to achieve their protein and carbohydrate targets. High-protein diets are recommended for older people to manage sarcopenia, and we noted a positive relationship between protein intake, body lean, and bone mineral density. On the other hand, epidemiological studies have shown that low-protein, high-carbohydrate diets are associated with improved health in humans (Floegel and Pischon, 2012; Lagiou et al., 2012), which is consistent with our overall conclusions.
Our results show that healthy aging is not achieved in mice fed high-protein diets and/or diluted diets to reduce calorie intake, but rather by low-protein diets (especially, we might predict, those low in BCAAs), where additional energy requirements are met by dietary carbohydrates rather than fats. A priority is to establish whether the same applies for humans, especially considering that high-protein diets are widely promoted for weight loss and health. An additional priority is to consider the makeup of lipids in the diet and the quality of carbohydrates. Given the profound effects of the balance of macronutrients on energy intake, health, and longevity, it is clear that dietary interventions aimed at influencing health or aging outcomes must be considered in the context of the underlying dietary landscape.
Experimental Procedures
Animals and Husbandry
C57BL/6 male and female mice (3 weeksold;n = 858) (Animal Resources Centre, WA, Australia) were housed three per cage in standard approved cages (Tecniplast, Varese, Italy) in the Molecular Physiology Unit of the ANZAC Research Institute, which is a specific pathogen-free facility designed for housing transgenic mice. A custom-designed two-chamber Perspex insert, designed to collect food spillage (Sørensen et al. 2008), was placed beneath the food hopper of each cage to collect food waste for quantification. Mice were maintained at 24°C-26°C and 44%–46% humidity under a 12 hr light:12 hr dark photoperiod, with lights on at 0600. All protocols were approved by the Sydney Local Health District Animal Welfare Committee (Protocol No. 2009/003).
Experimental diet treatments were custom designed and manufactured in dry, pelleted form by Specialty Feeds (Table S1). The diet treatments addressed both nutritional quantity and quality. To manipulate diet quantity, indigestible cellulose was added to diet treatments, yielding 3 total energy (caloric) density regimes fixed at 8, 13, and 17 kJ/g (referred to as low, medium, and high energy, respectively).
Mice were provided ad libitum access throughout their lifetime to 1 of 25 diets varying in content of protein, carbohydrate, and fat. Food intake was measured weekly for 6 months, followed by monthly measurements thereafter, and corrected for spillage and water content. Mice were checked daily, and body weight measurements were recorded to correspond with food intake measurements. Animals losing more than 20% body weight were culled.
Glucose Tolerance
At 15 months of age, glucose tolerance tests (GTTs) were performed on 668 mice. Animals were fasted for 4 hr prior to testing. Basal glucose levels were determined (Accu-Chek Performa). Glucose (1.5 g/kg) was then administered via intraperitoneal (i.p.) injection. Blood was sampled at 15, 30, 45, 60, and 90 min. The incremental area under the curve (AUC) was calculated using mean values per cage.
Blood Pressure
Blood pressure was measured indirectly in 240 conscious animals across all dietary groups with an automated multichannel tail-cuff system (MC4000; Hatteras Instruments) at 12 months. Animals were acclimatized to the system for 3 days prior to data collection.
At 15 months, one-third of the animals were culled, and tissues were harvested for further analyses. The mice were assigned a number (1–3) on arrival and housed 3 per cage. At 15 months of age, the number 2 animal from each cage was selected for culling, provided that animals 1 and 3 were still alive. If there were only two animals alive at this time point, no individual was culled (to prevent long-term single housing as much as possible).
Body Composition
Body composition was assessed in 180 mice across all diets by dual-energy X-ray absorptiometry (DEXA) using the GE PIXImus2 Series Densitometer (GE Medical Systems Ultrasound and BMD) under general anesthesia (i.p. ketamine:xylazine) immediately prior to culling.
Plasma Insulin and Leptin
Plasma insulin levels were measured using the Mouse Ultrasensitive Insulin ELISA Kit (ALPCO Diagnostics). Plasma leptin levels were quantified using the Mouse Leptin ELISA Kit (Millipore).
Plasma Amino Acids
Amino acids were analyzed at the Australian Proteome Analysis Facility, Macquarie University, using the Waters AccQ-Tag Ultra Chemistry Kit (Waters Corporation).
Liver Histology
Embedded liver tissue was sectioned at 4 μm and stained with hematoxylin and eosin and Sirius Red. Extent of steatosis was assessed and scored (0, +, ++, +++) by four independent observers blinded to tissue category.
Mitochondrial Function
Mitochondrial functions were assessed in liver tissue using the Seahorse XF Extracellular Flux Analyzer, which generates the key parameters of mitochondrial function using fresh isolated mitochondria from homogenized liver tissue: basal respiration, ATP production, proton leak, maximal respiration, glycolysis, and spare respiratory capacity (Horan et al., 2012). Different conditions were used to measure the mitochondrial oxygen consumption, e.g., by providing different combinations of substrates (pyruvate-malate, glutamate-malate, succinate-rotenone, palmitoyl carnitine-malate) to the electron transport system. State III was monitored after injection of ADP, and State IVo was monitored after injection of oligomycin. Respiratory control ratios (RCRs) were calculated as State III / State IVo. Mitochondrial amount as well as substrate, ADP, and inhibitor concentrations were optimized prior to experiments. Hydrogen peroxide production was measured with an Amplex Red Kit using the same substrates as for mitochondrial respiration. Hydrogen peroxide production and enzymatic activities (3-hydroxyacyl coenzyme A dehydrogenase, aspartate aminotransferase, and citrate synthase) were measured spectrophotometrically on the same mitochondrial isolations. Citrate synthase activity was used to normalize the results, which are therefore expressed as mitochondrial function per mitochondrion.
Blood Lipids and Biochemistry
Blood cholesterol, triglycerides, high-density lipoprotein cholesterol (HDLc), low-density lipoprotein cholesterol (LDLc), liver function tests (alanine transaminase ALT, aspartate aminotransferase AST, gamma-glutamyl transpeptidase GGT), and creatinine were performed at the Concord Hospital Pathology Department.
Liver mTOR and Phospho-mTOR
Frozen liver tissue samples were sectioned into 20 mg blocks and homogenized (QIAGEN TissueLyser LT) in 400 μl RIPA buffer containing Tris-HCl, NaCl, Triton X-100, Na-deoxycholate, SDS, and Roche protease inhibitor tablets (cOmplete, EDTA-free Protease Inhibitor Cocktail). Protein concentration was checked using a bicinchoninic acid (BCA) assay (Thermo Scientific Pierce BCA Protein Assay Kit), and a standard curve was developed with BSA controls. Samples were then diluted into a 2 mg/ml concentration in a 100 μl volume with Laemmli buffer and 50 mM TCEP BondBreaker, and 20 μg of protein were separated on 4%–15% gradient Mini-PROTEAN TGX gels. Gels were transferred onto nitrocellulose membranes at 25 V and 2.5 A for 10 min on a Trans-Blot Turbo Transfer System. Membranes were blocked for 1 hr in 5% skim milk in Tris-buffered saline (TBS). Membranes were washed 3 times for 10 min in wash buffer (TBS 0.1% Tween 20) and incubated overnight in primary antibody solution (5% BSA, TBS 0.1% Tween 20). Membranes were washed 3 times as before and incubated for 1 hr in secondary antibody solution (5% skim milk in TBS 0.1% Tween, 0.01% SDS). Membranes were washed three times as before, rinsed in water, and analyzed on a LI-COR Odyssey System. Densitometry was performed using LI-COR software, and results were analyzed using Prism (GraphPad). Antibodies used were total mTOR (4517 L27D4; Cell Signaling Technology), phospho-Ser2448 mTOR (5536 D9C2; Cell Signaling Technology), and α-tubulin (T6199, clone DM1A; Sigma).
Statistical Modeling and Analysis
Intake Transformations
Macronutrient, energy, and dry matter intake data were analyzed both per mouse with no body size adjustment and by adjusting the intakes to lean body mass. Body fat was determined from 1 mouse of each of 174 cages out of 250 cages at 15 months. These body fat values were used to calculate the lean mass from the cage average body weight. For any missing data, lean mass was estimated from the total body weight by using the average body fat percentage of their specific diet based on the data from the 174 cages. This imputation was used for the phenotypic data, average feed intake data, individual survival data, and blood pressure data. For the median lifespan data, the diet averages of the body fat were used to calculate the lean mass from the average body weight.
Response Surfaces
GAMs with thin-plate splines were used to model the responses either over the diet macronutrient composition or over mice macronutrient intake spaces. GAMs were fitted with the help of the mgcv package of the R language (R Core Team, 2012, v.2.15.13) (Wood, 2006). The mgcv package is based on Wood (2003). An extra penalty was used in the fits to allow a specific term to end up with zero estimated degrees of freedom and thus be excluded from the final model. The macronutrient effects were broken down to main effects and interactions. The responses included dry matter, energy and macronutrient intake, median lifespan, several phenotypic measurements, and mitochondrial function.
Survival Analysis
Survival curves for different dietary energy levels and protein:carbohydrate (P:C) ratios were visualized by Kaplan-Meier estimator using the survival package of the R language. This package is based on Therneau and Grambsch (2000). The same package was used to fit the Cox proportional hazard model to actual lifespans in relation to diet protein:carbohydrate ratio and energy intake. The effects of diet protein:carbohydrate ratio and energy intake were modeled with the help of penalized splines (psplines) as described in the survival package documentation. The proportional hazard assumption was diagnosed by plotting (Therneau and Grambsch, 2000).
Liver Steatosis
Steatosis was assessed as a score ranging from 0 to 3. The scores were modeled with an ordinal regression (proportional odds). The effects of diet composition and macronutrient intakes were modeled with the help of additive splines using the VGAM package of the R language that is based on Yee (1996).
Various Other Methods
Multilevel (mixed-effects) models were used to investigate whether mouse age affected intakes on different dietary energy densities by adding cage as a random effect. A multilevel model was required since the data are repeated measures of the same cages over time. The models were fitted with the help of the lme4 package of R (Bates et al., 2012). The macronutrient intake in relation to diet composition (appetite regulation) was analyzed by fitting an asymptotic regression model with nonlinear least squares. The relationship between some phenotypic responses and diet protein:carbohydrate ratio and energy intake were modeled with single predictor GAMs with the mgcv package of R. Similar GAMs were used to model the protein intake in relation to diet protein:carbohydrate ratio at different ages of the mice. For the GAMs the 95% confidence intervals were approximated as the mean ± 2 standard errors of the predictions. For the mixed-effects and general linear models, the 95% confidence intervals were solved by a posterior simulation with 1,500 random draws from the model as described by Gelman and Hill (2007).
Supplementary Material
1
2
Acknowledgments
Funding was obtained from the Australian National Health and Medical Research Council (NHMRC project grant 571328), the Ageing and Alzheimers Research Fund of Concord RG Hospital, and the Sydney Medical School Foundation. Additionally, S.J.S. was supported by an Australian Research Council Laureate Fellowship; D.R. was part funded by Gravida, the National Research Centre for Growth and Development, New Zealand; and L.E.W. was supported by an early career fellowship from the Cancer Institute NSW (CINSW), Australia. We acknowledge the assistance of the Departments of Biochemistry and Anatomical Pathology, Concord RG Hospital. We thank Leon McQuade for amino acid analyses and Andrew Holmes, Arthur Conigrave, and Patrick Bertolino for their contribution to other aspects of the study protocol. We are extremely grateful to John Speakman and Linda Partridge for their detailed review of earlier versions of the manuscript. There are no conflicts of interest to declare.
Footnotes
References
- Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-0. 2012 http://cran.r-project.org/web/packages/lme4/index.html.
- Blumfield ML, Hure AJ, MacDonald-Wicks LK, Smith R, Simpson SJ, Giles WB, Raubenheimer D, Collins CE. Dietary balance during pregnancy is associated with fetal adiposity and fat distribution. Am J Clin Nutr. 2012;96:1032–1041. doi: 10.3945/ajcn.111.033241. [DOI] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvári M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausserès N, Steiler T, Gaudichon C, Tomé D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297:E1313–E1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Rattan SI, Le Couteur DG, de Cabo R, editors. Calorie Restriction, Aging and Longevity. New York: Springer Press; 2010. [Google Scholar]
- Fanson BG, Weldon CW, Pérez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8:514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Floegel A, Pischon T. Low carbohydrate-high protein diets. BMJ. 2012;344:e3801. doi: 10.1136/bmj.e3801. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromentin G, Darcel N, Chaumontet C, Marsset-Baglieri A, Nadkarni N, Tomé D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr Res Rev. 2012;25:29–39. doi: 10.1017/S0954422411000175. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Gosby AK, Conigrave AD, Lau NS, Iglesias MA, Hall RM, Jebb SA, Brand-Miller J, Caterson ID, Raubenheimer D, Simpson SJ. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS ONE. 2011;6:e25929. doi: 10.1371/journal.pone.0025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson-Hughes AK, Hewson-Hughes VL, Miller AT, Hall SR, Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the adult domestic cat, Felis catus. J Exp Biol. 2011;214:1039–1051. doi: 10.1242/jeb.049429. [DOI] [PubMed] [Google Scholar]
- Horan MP, Pichaud N, Ballard JWO. Review: quantifying mitochondrial dysfunction in complex diseases of aging. J Gerontol A Biol Sci Med Sci. 2012;67:1022–1035. doi: 10.1093/gerona/glr263. [DOI] [PubMed] [Google Scholar]
- Huang X, Hancock DP, Gosby AK, McMahon AC, Solon SM, Le Couteur DG, Conigrave AD, Raubenheimer D, Simpson SJ. Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Obesity (Silver Spring) 2013;21:85–92. doi: 10.1002/oby.20007. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel R, Helge JW, Dela F, Hey-Mogensen M. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–3360. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Lakatta EG. A vascular theory of aging. J Gerontol A Biol Sci Med Sci. 2010;65:1025–1027. doi: 10.1093/gerona/glq135. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Simpson SJ. Adaptive senectitude: the prolongevity effects of aging. J Gerontol A Biol Sci Med Sci. 2011;66:179–182. doi: 10.1093/gerona/glq171. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Wilder SM, de Cabo R, Simpson SJ. The evolution of research on ageing and nutrition. J Gerontol A Biol Sci Med Sci. 2014;69:1–2. doi: 10.1093/gerona/glt130. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Loeb LA. Ageing: mice and mitochondria. Nature. 2004;429:357–359. doi: 10.1038/429357a. [DOI] [PubMed] [Google Scholar]
- Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ. Nutrient-specific foraging in invertebrate predators. Science. 2005;307:111–113. doi: 10.1126/science.1105493. [DOI] [PubMed] [Google Scholar]
- Mayntz D, Nielsen VH, Sørensen A, Toft S, Raubenheimer D, Hejlesen C, Simpson SJ. Balancing of protein and lipid intake by a mammalian carnivore, the mink (Mustela vison) Animal Behaviour. 2009;77:349–355. [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton F, Wilson K, Cotter SC, Raubenheimer D, Simpson SJ. Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 2011;7:e1002223. doi: 10.1371/journal.ppat.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Caloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of aging. J Gerontol A Biol Sci Med Sci. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Macronutrient balance and life-span. Aging (Albany, N Y Online) 2009;1:875–880. doi: 10.18632/aging.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. The nature of nutrition A unifying framework form animal adaption to human obesity. Princeton: Princeton University Press; 2012. [Google Scholar]
- Sørensen A, Mayntz D, Raubenheimer D, Simpson SJ. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 2008;16:566–571. doi: 10.1038/oby.2007.58. [DOI] [PubMed] [Google Scholar]
- Therneau T, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- Wood SN. Thin-plate regression splines. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2003;65:95–114. [Google Scholar]
- Wood S. Generalized additive models: an introduction with R. Boca Raton: Chapman and Hall/CRC; 2006. [Google Scholar]
- Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68:270–279. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee T. Vector generalized additive models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 1996;58:481–493. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2