Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood (original) (raw)
. Author manuscript; available in PMC: 2018 Sep 1.
Published in final edited form as: Addict Biol. 2016 May 15;22(5):1191–1204. doi: 10.1111/adb.12404
Abstract
Alcohol exposure in adolescence is an important risk factor for the development of alcoholism in adulthood. Epigenetic processes are implicated in the persistence of adolescent alcohol exposure-related changes, specifically in the amygdala. We investigated the role of histone methylation mechanisms in the persistent effects of adolescent intermittent ethanol (AIE) exposure in adulthood. Adolescent rats were exposed to 2g/kg ethanol (2 days on/off) or intermittent n-saline (AIS) during post-natal days (PND) 28-41 and used for behavioral and epigenetic studies. We found that AIE exposure caused a long-lasting decrease in mRNA and protein levels of lysine demethylase 1(Lsd1) and mRNA levels of Lsd1+8a (a neuron-specific splice variant) in specific amygdaloid structures compared to AIS-exposed rats when measured at adulthood. Interestingly, AIE increased histone H3 lysine 9 dimethylation (H3K9me2) levels in the central nucleus of the amygdala (CeA) and medial nucleus of the amygdala (MeA) in adulthood without producing any change in H3K4me2 protein levels. Acute ethanol challenge (2g/kg) in adulthood attenuated anxiety-like behaviors and the decrease in Lsd1+8a mRNA levels in the amygdala induced by AIE. AIE caused an increase in H3K9me2 occupancy at the Bdnf exon IV promoter in the amygdala that returned to baseline after acute ethanol challenge in adulthood. These results indicate that AIE specifically modulates epizymes involved in H3K9 dimethylation in the amygdala in adulthood, which are possibly responsible for AIE-induced chromatin remodeling and adult psychopathology such as anxiety.
Keywords: alcohol, amygdala, epigenetics, histone demethylase, histone methylation, lysine demethylase 1, anxiety
INTRODUCTION
Alcohol abuse is a public health concern that significantly impacts individuals and has far-reaching effects on modern society (Grant et al., 2006; Koob, 2003; Room, 1997; Whiteford et al., 2013). Binge drinking during adolescence is of particular concern, as this is considered a risk factor for the development of alcohol use disorder and other psychiatric disorders in adulthood (Comeau et al., 2001; Donovan, 2004; Grant et al., 2006; Witt, 2010). During the period of adolescence, the brain undergoes developmental maturation characterized by both emotional and physiological change including alterations in gene expression, neurotransmitter synthesis and release, axonal outgrowth, dendritic pruning, and synaptic plasticity (Clark et al., 2008; Keshavan et al., 2014; Tau and Peterson, 2010). The amygdala, known as a major regulator of emotion (Davis and Whalen, 2001), is affected by both acute and chronic alcohol exposure in adult and adolescent rats, and specific amygdaloid structures are hypothesized to control the negative affective states experienced during alcohol withdrawal, also known as the “dark side” of alcoholism (Koob, 2003; Koob et al., 2014; Kyzar and Pandey, 2015; Pandey et al., 2015). Despite advancements in this field, the precise neural molecular mechanisms that lead alcohol-consuming adolescents to the development of psychiatric problems in adulthood are not well understood.
Recent evidence has implicated epigenetic processes in the development and preservation of alcoholism and alcohol-related behaviors (Krishnan et al., 2014; Moonat et al., 2013; Starkman et al., 2012). Epigenetic events including histone acetylation, histone methylation, and DNA methylation are involved in widespread neuroadaptive changes occurring during brain development (Fagiolini et al., 2009). Epigenetic modifications can act to either enhance or repress gene transcription, depending on the modification. For example, histone acetylation at a specific genetic locus acts to increase transcription of the underlying gene product, while DNA methylation most often decreases gene transcription (Gräff et al., 2011; Kouzarides, 2007; Krishnan et al., 2014). Recent work in our lab has demonstrated that adolescent intermittent ethanol (AIE) exposure leads to increased histone deacetylase isoform 2 (HDAC2) expression, decreased histone H3 lysine 9 (H3K9) acetylation, and decreased expression of crucial synaptic plasticity genes such as brain-derived neurotrophic factor (Bdnf) and activity-regulated cytoskeletal protein (Arc), leading to decreased dendritic spine density in the central nucleus of the amygdala (CeA) and medial nucleus of the amygdala (MeA) in adulthood (Pandey et al., 2015). Notably, AIE-exposed rats display anxiety-like behaviors and increased alcohol consumption in adulthood compared to adolescent intermittent saline (AIS)-exposed control animals (Pandey et al., 2015; Sakharkar et al., 2016).
The contribution of histone methylation mechanisms to alcohol-induced brain neuroadaptations following adolescent alcohol exposure remains unknown and relatively understudied. In contrast to permissive histone acetylation, histone methylation can be either permissive or repressive depending on the amino acid residue bearing the methyl group. Methylation of histone 3 lysine 4 (H3K4) is a marker of increased gene expression while methylation of H3K9 is a marker of condensed chromatin and decreased gene expression (Kouzarides, 2007; Krishnan et al., 2014). Similar to regulation of histone acetylation by HDACs, a number of enzymes regulate histone methylation at unique residues. Two classes of histone lysine demethylases have been identified - the amine oxidase-related enzymes that include lysine demethylase 1 (LSD1; also known as KDM1A), and the Jumonji C-terminal domain (JmjC)-containing histone demethylases (Allis et al., 2007; Shi et al., 2004). LSD1 specifically removes methyl marks from di- and mono-methylated H3K4 (H3K4me2 and H3K4me1), respectively (Cloos et al., 2008; Shi et al., 2004) and has been shown to be involved in memory formation and cognition (Neelamegam et al., 2012). LSD1 can also demethylate H3K9me2 and H3K9me1, repressive chromatin marks, when in complex with the androgen receptor (AR) (Cloos et al., 2008; Metzger et al., 2005). A neuron-specific isoform of LSD1, termed LSD1+8a for its inclusion of a 4 amino acid mini-exon, participates in neurite growth and axon extension (Wu et al., 2007; Zibetti et al., 2010). Recent work has shown that LSD1+8a specifically demethylates H3K9me2 and does not act on H3K4me2 in vitro, and also regulates neuronal differentiation (Laurent et al., 2015). The JmjC-domain-containing histone demethylases include KDM2A, KDM4A, KDM4C, KDM4D, and PHF8, and have the ability to remove methyl groups from histone residues. Additionally, the Jumonji AT-rich interactive domain 1C (JARID1C) enzyme specifically removes methyl groups from H3K4me2/3. In addition to the demethylases, histone methyltransferases such as MLL1 and MLL2, members of the SET1 family of enzymes, add methyl groups to H3K4 (Allis et al., 2007; Hou and Yu, 2010).
Given the involvement of histone methylation mechanisms in the regulation of synaptic plasticity (Laurent et al., 2015; Wu et al., 2007), we investigated the modulation of epizymes involved in histone H3K4 and H3K9 methylation mechanisms by adolescent binge alcohol exposure in the present study. We measured Lsd1, Lsd1+8a, Mll1, Mll2, and Jarid1c mRNA in the amygdala and bed nucleus of the stria terminalis (BNST) of rats exposed to adolescent intermittent ethanol (AIE). We examined the lasting effect of AIE exposure by measuring histone methylation and associated epizymes in adulthood. We also examined mRNA expression of LSD1-interacting proteins (Jade2, AR, and TLX) and H3K9 demethylases (Kdm4a, Kdm4c, Kdm4d, and Phf8) in the amygdala of AIE adults. It has been shown that Bdnf exon IV expression is regulated by epigenetic mechanisms in the brain (Moonat et al., 2013; Pandey et al., 2015; Roth et al., 2009; Sakharkar et al., 2016), and its expression is decreased in the amygdala after AIE in adulthood (Pandey et al., 2015). To gain insight into the pathogenesis of AIE-induced anxiety-like behaviors (Pandey et al., 2015), we exposed AIS and AIE animals to an acute ethanol challenge in adulthood prior to testing for anxiety-like behavior. We measured Lsd1+8a mRNA and H3K9me2 occupancy at the Bdnf exon IV promoter in the amgydala in these same animals. Our novel results shed light on the complex histone methylation mechanisms responsible for the development of AIE-induced adult psychopathology.
MATERIALS AND METHODS
Experimental animals and ethanol exposure
Pregnant Sprague Dawley (SD) rats were obtained from Harlan Laboratories (Indianapolis, IN, USA). Rats were given ad libitum access to food and water and were maintained on a 12 hr:12 hr light/dark cycle. Procedures utilizing animals followed Institutional Animal Care and Use Committee guidelines and NIH directives for the Care and Use of Laboratory Animals. Male rat pups were weaned from dams at postnatal day (PND) 21 and group-housed with no more than 3 animals per cage. Ad libitum access to food and water was maintained. Adolescent male rats were randomly assigned to receive either AIE or AIS treatment. On PND 28-41, adolescent rats received 8 intraperitoneal (i.p.) injections of ethanol (2 g/kg, 20% w/v) or an equivalent dose of normal saline using a 2 days on-2 days off dosing schedule, similar to previous studies in other labs (Alaux-Cantin et al., 2013; Pascual et al., 2009) and our lab (Pandey et al., 2015; Sakharkar et al., 2016). Notably, the dose of ethanol used in this study was not sufficient to induce sedation, but instead produced anxiolytic–like behaviors in adolescent rats (Sakharkar et al., 2014).
Animals were decapitated under anesthesia (50 mg/kg pentobarbital) for tissue collection exactly 1 hr (AIE group; PND 41) or 24 hr after last AIE during withdrawal (AIW group; PND 42). Brain tissue, specifically the amygdala and bed nucleus of the stria terminalis (BNST), was immediately dissected, frozen and kept at −80°C for further epizyme expression analysis. Some AIS- and AIE-exposed rats were allowed to mature to adulthood (PND 92) to examine the lasting effect of AIE. Rats were decapitated under anesthesia at PND 92 and their brains (amygdala and BSNT) dissected for biochemical studies as described below. A subset of animals from each experimental group was perfused (Pandey et al., 2008; Sakharkar et al., 2012) first with normal saline and then 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB; pH = 7.4). Brains were collected and first fixed in PFA followed by a graded sucrose solution (10% - 20% - 30%) prepared in 0.1M PB. These brains were frozen and stored at −80°C for immunohistochemical analysis.
Acute ethanol challenge exposure in AIE animals in adulthood
Some AIS- and AIE-exposed rats were allowed to mature to PND 101-102 without further exposure to ethanol. AIE and AIS rats were randomly assigned to receive an acute dose of ethanol (2 g/kg; i.p.) or an equivalent dose of normal saline on PND 101 or 102, creating 4 experimental groups (AIS+ Saline, AIS+ EtOH, AIE+ Saline, and AIE+ EtOH). Animals from each of the 4 groups were subjected to anxiety-like measurements in the elevated plus maze (EPM) 1 hr post-injection. EPM testing was performed according to the procedure described by our laboratory and others (File, 1993; Pandey et al., 2008, 2015; Sakharkar et al., 2012). Results are shown as mean ± SEM percent open arm entries and percent time spent in open arms. General activity of rats is represented by mean ± SEM of closed arm entries (File, 1993; Pandey et al., 2015). Immediately after behavioral analysis at PND 101 or 102, rats were decapitated under anesthesia and amygdala tissue was dissected for analysis of Lsd1+8a mRNA and H3K9me2 occupancy at the Bdnf exon IV promoter as described below. Blood was also collected at the time of brain collection to measure blood ethanol levels using an Analox Alcohol Analyzer (Lunenburg, MA, USA).
mRNA quantification by real-time PCR
Total RNA was isolated from rat brain tissue with TRIZOL (Life Technologies, Grand Island, NY, USA) followed by purification using RNeasy mini kit (Qiagen, Valencia, CA, USA) and quantification. RNA was then reverse transcribed in duplicate using random primers and MuLV reverse transcriptase (Life Technologies, Grand Island, NY, USA) to yield a volume of 20 μL. Quantitative real-time PCR was performed using a Mx3000P qPCR system (Agilent Technologies, Santa Clara, CA, USA) and SYBR Green master mix (Fermentas, Glen Burnie, MD, USA). The MxPro software suite was used to analyze qPCR data. Primers corresponding to mRNAs for the genes of interest (Table 1) were added to the master mix, and Hypoxanthine-guanine phosphoribosyltransferase (Hprt1) was utilized as a reference gene. qPCR conditions were a 10 min hold at 95°C, followed by 40 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 1 min. Target cDNA (Lsd1, Lsd1+8a, Mll1, Mll2, Jarid1c, Jade2, AR, TLX, Kdm4a, Kdm4c, Kdm4d, and Phf8) was measured alongside Hprt1. Relative mRNA levels was determined by normalization to Hprt1 using the ΔΔCt method (Livak and Schmittgen, 2001). Results are expressed as fold change in mRNA levels compared to AIS controls.
Table 1.
Primer sets for various gene products studied
| Primer Name | Sequence |
|---|---|
| Lsd1 Forward | CGCCACGGTCTTATCAACTT |
| Lsd1 Reverse | GCCAGAAACACCTGAGCCTA |
| Lsd1+8a Forward | GAGGAAATCCCATGGCTGT |
| Lsd1+8a Reverse | GGAACCTTGACAGTGTCAGCTT |
| Mll1 Forward | TCTTTCCTTGACTCCCGCTT |
| Mll1 Reverse | CAAAGATCGCGATGCTGACA |
| Mll2 Forward | CCAGAGGTCCAAGTCAAGGT |
| Mll2 Reverse | GGCAGAGTTTATGGAGCAGC |
| Jarid1c Forward | GCGCCAAGATGACCATGAGA |
| Jarid1c Reverse | ACACCTCCAGACACCTTTCG |
| Jade2 Forward | CTGGGAGTCCAGCCTAAGTG |
| Jade2 Reverse | TTGGTAATGGGCTCCATCTT |
| AR Forward | TCTGGTTGTCACTACGGAGC |
| AR Reverse | CATTTCCGGAGACGACACGA |
| TLX Forward | GAAGTGAACGGGACCCCAAT |
| TLX Reverse | CAGTTCTCTCCACGCGTCTT |
| Kdm4a Forward | AGCTTTGTCTTTCGCCCTCA |
| Kdm4a Reverse | CAGGGATGACCAGATCGTCG |
| Kdm4c Forward | AAGCCCATGACTGTGAAGGAG |
| Kdm4c Reverse | CCGCTCCATAAATAGGTGCC |
| Kdm4d Forward | TCTCCTGGCCACATGTCAAC |
| Kdm4d Reverse | GTCACGGACTGGGACTGAAG |
| Phf8 Forward | AGCCTCGGGCATGAAGAGA |
| Phf8 Reverse | TACAGGGTCAGAAAGGCAGC |
| Hprt1 Forward | TCCTCAGACCGCTTTTCCCGC |
| Hprt1 Reverse | TCATCATCACTAATCACGACGCTGG |
Immunohistochemistry for LSD1, H3K4me2, and H3K9me2
Gold immunolabeling was utilized for the measurement of LSD1, H3K4me2, and H3K9me2 protein levels in the amygdala of AIS and AIE adult rats as described previously by our group (Moonat et al., 2013; Pandey et al., 2008, 2015). In brief, brains were sliced into 20 μm-thick bregma-matched coronal sections and immunolabeled with antibodies against LSD1 (Abcam, Cambridge, UK; ab17721), H3K4me2 (Abcam; ab32356), and H3K9me2 (Cell Signaling, Beverly, MA, USA; 97953S) at 1:500 dilution. Immunogold particles were visualized and quantified using the Image Analyzer program connected to a light microscope. The threshold for non-immunostained areas in the amygdalar region was set to zero. After threshold determination, the software quantified the amount of immunogold-labeled particles per 100 μm2 brain area at higher magnification (100x). Three object areas of defined brain regions [CeA, MeA, and basolateral amygdala (BLA)] from each of the three brain sections for each animal were counted and the values averaged. Results are represented as number of immunogold particles per 100 μm2 brain area.
Confocal immunofluorescent staining for the localization of H3K9me2 in the amygdala
Immunofluorescence staining was used to determine the presence of H3K9me2 in neurons and glial cells according to the procedure described previously (Sakharkar et al., 2012). 20 μm coronal sections were taken and incubated with an antibody directed against anti-rabbit H3K9me2 (Cell Signaling; 97953S) then co-incubated with an anti-mouse direct-AlexaFlour® 488-conjugated antibody for either neuron-specific nuclear protein (NeuN; Millipore, Billerica, MA, USA; MAB377X) or glial fibrillary acidic protein (GFAP; Cell Signaling; 3655S). Sections were then exposed to an AlexaFlour® 594-conjugated anti-rabbit secondary IgG antibody (Jackson ImmunoResearch, West Grove, PA, USA; 711-585-152) for visualization of H3K9me2 staining. Sections were mounted on slides and visualized with a confocal microscope (LSM 710; Zeiss, Thornwood, NY, USA).
In Situ PCR for Lsd1+8a mRNA
No commercially available antibody specific to the splice variant protein product LSD1+8a was available. Therefore, we performed in situ PCR as previously described (Moonat et al., 2011; Pandey et al., 2008) to visualize any nuclei-specific changes in Lsd1+8a expression in the amygdala of AIS- and AIE-exposed adult animals. Briefly, brains were fixed and frozen before cutting 40μm-thick coronal sections and treatment with 1 μg/mL proteinase K in PBST for 15 min at 37°C. The sections were treated with DNase (Promega, Madison, WI, USA) for 18 hrs and placed in PCR tubes with 100 μL of a reverse transcription mixture (Applied Biosystems, Foster City, CA, USA) for 1 hr at 42°C. Sections were transferred to a clean tube with a PCR mix containing 100 pmol of Lsd1+8a mRNA primers (same as those used for real-time PCR above; Table 1) and digoxigenin (DIG)-11-dUTP (Roche Diagnostics, Indianapolis, IN, USA) rather than dTTP. After PCR cycling, sections were mounted and incubated with anti-DIG antibody conjugated to alkaline phosphotase (Roche). Sections were then stained with nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolylphophate (Roche) for visualization. Lsd1+8a mRNA was quantified using optical density calculations at 20x magnification in the three amygdaloid structures (CeA, MeA, and BLA) measured. The optical density of _Lsd1+8a_-positive cells from three fields in three sections was counted and averaged for each animal for a total of nine object fields per amygdaloid structure. Results are expressed as the mean of optical density per 100 pixel area.
H3K9me2 occupancy at Bdnf exon IV promoter
H3K9me2 occupancy at the Bdnf exon IV promoter was measured in the amgydala of AIE and AIS animals exposed to an acute ethanol challenge in adulthood using the chromatin immunoprecipitation (ChIP) assay as described previously (Moonat et al., 2013; Pandey et al., 2015). Amygdaloid brain tissue was fixed in methanol-free formaldehyde and subjected to DNA shearing by sonication. The resulting DNA-chromatin complex was immunoprecipitated with an antibody directed to H3K9me2 (Abcam, ab1220), and the precipitated DNA was quantified using qPCR with a SYBR Green master mix (BioRad, Berkeley, CA, USA) and primers designed for the Bdnf exon IV promoter (Forward, 5′-GTTCGCTAGGACTGGAAGTGG-3′, Reverse, 5′-CCTCTGCCTCGAAATAGACAC-3′) (Pandey et al., 2015). After subtraction of input DNA Ct value from the Ct value of each respective sample, the ΔΔCt method (Livak and Schmittgen, 2001) was used to determine the fold change in the levels of H3K9me2 at the Bdnf exon IV promoter.
Statistical analysis
Two group experiments, such as all involving AIS- and AIE-exposed adult rat groups, were compared using the Student’s t-test. For adolescent experiments involving 3 groups (adolescent AIS, AIE, and AIW), one-way analysis of variance (ANOVA) was employed to test for differences between groups followed by a post-hoc comparison using Tukey’s test. Experiments involving animals exposed to AIS or AIE in adolescence followed by an acute ethanol or normal saline challenge in adulthood were analyzed with two-way ANOVA followed by post-hoc comparison using Tukey’s test. Significance for all experiments was set at p<0.05.
RESULTS
Effects of AIE on the expression of histone methylation-modifying enzymes in the amygdala and BNST during adolescence and adulthood
We examined the effects of AIE on the expression of histone methyltransferases and demethylases in the amygdala 1 and 24 hrs of last AIE exposure. Rats were divided into three groups: AIS controls, AIE animals sacrificed 1 hr after last ethanol exposure, and AIW animals sacrificed 24 hrs after last ethanol exposure in the withdrawal period (Pandey et al., 2015). We found that AIE treatment significantly altered Lsd1 mRNA expression in the amygdala of adolescent rats (_F_2, 17 = 6.1, p<0.01; Figure 1A), with post hoc testing revealing an increase in Lsd1 mRNA levels in the AIE group (1 hr after last ethanol injection) compared to AIS controls (p<0.01). ANOVA did not find any significant differences between treatment groups in Lsd1+8a mRNA, Mll1 mRNA, Mll2 mRNA, or Jarid1c mRNA in the amygdala (Figure 1A).
Figure 1. mRNA profiling of histone methyltransferases and demethylases in the amygdala (A) and BNST (B) of adolescent rats exposed to AIE.
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) and their brains were collected and amygdaloid as well as BNST tissues were dissected out for mRNA analysis at postnatal day (PND) 41-42 either 1 hr after last ethanol exposure (AIE group) or 24 hrs after last ethanol exposure during withdrawal (AIW group) in the amygdala (A) and BNST (B). Values are represented as mean ± SEM of n=6-7 rats and are significantly different from AIS-exposed controls (*p < 0.05, **p<0.01, one-way ANOVA followed by post hoc Tukey's test).
We measured expression of the same mRNA transcripts in the BNST in adolescence following AIE exposure (Figure 1B). There was a significant effect of AIE exposure on Mll1 mRNA expression in the BNST (_F_2, 15 = 4.0, p<0.05), and post hoc comparison showed an increase in expression of Mll1 specifically in the AIW group (24 hr after last ethanol exposure) compared to AIS controls (p<0.05). Similarly, AIE significantly altered Mll2 mRNA (_F_2, 15 = 5.1, p<0.05), and post hoc analysis revealed an increase (p<0.05) in mRNA levels in the AIE group (1 hr after last ethanol exposure) compared to AIS controls. However, we did not see any significant differences in Lsd1, Lsd1+8a, or Jarid1c mRNA expression in the BNST at adolescence following AIE exposure.
Next, we allowed animals previously exposed to AIE and AIS to grow to adulthood without any further ethanol exposure before tissue collection at PND 92. It was observed that AIE-exposed animals displayed a long-lasting decrease in Lsd1 mRNA expression in the amygdala in adulthood compared to AIS controls (t (13) = 2.4, p<0.05; Figure 2A). Similarly, AIE caused a decrease in the neuron-specific splice variant Lsd1+8a mRNA in the same animals (t (13) = 2.3, p<0.05). However, no changes were found in the adult amygdala for Mll1, Mll2, or Jarid1c mRNA levels after AIE exposure (Figure 2A). We then examined the BNST for alterations in the same mRNA transcripts in adulthood. AIE exposure did not alter the expression of any of the measured transcripts when examined at PND 92 (Figure 2B).
Figure 2. mRNA profiling of histone methyltransferases and demethylases in the amygdala (A) and BNST (B) of adult rats previously exposed to AIE.
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) and allowed to grow to adulthood without further ethanol or saline exposure. Their brains were collected and amygdaloid as well as BNST tissues were dissected out for mRNA analysis at postnatal day (PND) 92. Values are represented as mean ± SEM of n=5-8 rats and are significantly different from AIS-exposed group (*p < 0.05, Student's t test).
Decreased LSD1 and Lsd1+8a in specific amygdaloid structures of adult rats exposed to AIE corresponds to increased H3K9me2 but not H3K4me2
To confirm the results obtained using mRNA expression analysis in Figure 2, we used immunohistochemistry to measure LSD1 protein levels in the amygdala of adult rats after AIE (Figure 3A, B). A decrease in LSD1 protein levels was observed in the CeA of AIE rats when compared to AIS controls (t (8) = 4.7, p<0.001). No change in LSD1 levels was seen in the MeA or the BLA.
Figure 3. Decreased LSD1 protein and Lsd1+8a mRNA in specific amygdaloid structures of adult rats exposed to AIE.
A) Photomicrographs (Scale bar = 50 μm) showing the gold immunolabeling of LSD1 in the central (CeA) and medial nucleus of amygdala (MeA) of adolescent intermittent ethanol (AIE) - or saline (AIS)-exposed adult rats. B) Quantification of LSD1 protein levels by gold immunolabeling in the CeA, MeA and basolateral amygdala (BLA) of AIS- and AIE-exposed adult rats. C) Photomicrographs (Scale bar = 50 μm) showing the Lsd1+8a mRNA positive cells (in-situ PCR) in the CeA and MeA of AIE- and AIS-exposed adult rats. D) Quantification of Lsd1+8a mRNA levels by in situ PCR in the CeA, MeA and BLA of AIS- and AIE-exposed adult rats. Values are represented as mean ± SEM of n=5 rats and are significantly different from AIS-exposed group (**p < 0.01, ***p < 0.001, Student's t test).
The anatomical specificity of the decrease observed in global Lsd1+8a mRNA levels in adult animals exposed to AIE was further probed using in situ PCR (Figure 3C, D). Lsd1+8a mRNA was significantly decreased in the CeA (t (8) = 3.5, p<0.01) and the MeA (t (8) = 4.0, p<0.01), but not the BLA, in AIE-exposed adult rats compared to AIS-exposed adult rats. Therefore, LSD1 protein levels and Lsd1+8a mRNA levels were shown by independent methods to be decreased in the amygdala of AIE-exposed adult rats.
Next, we wanted to determine the significance of decreased LSD1 and Lsd1+8a levels in the CeA and MeA in our model. LSD1 was originally posited to demethylate H3K4me2/1 (Shi et al., 2004). Other work proved that LSD1 can also remove methyl marks from H3K9me2/1 (Metzger et al., 2005), and recent evidence suggests that its effect as an H3K9 demethylase may predominate in postmitotic neurons (Laurent et al., 2015). First, we measured H3K4me2 protein levels by immunohistochemistry in the amygdala of AIE-exposed adult rats (Figure 4A, B). No change was observed in H3K4me2 levels in the CeA, MeA, or BLA. We also measured H3K9me2 protein levels by immunohistochemistry in the same animals (Figure 4C, D). AIE adult rats displayed a significant increase in H3K9me2 protein levels in the CeA (t (8) = 11.1, p<0.001) and the MeA (t (8) = 9.8, p<0.001), but no change was seen in the BLA. Our results indicate that AIE exposure leads to decreased LSD1 and Lsd1+8a levels and increased H3K9me2 levels in the amygdala in adulthood.
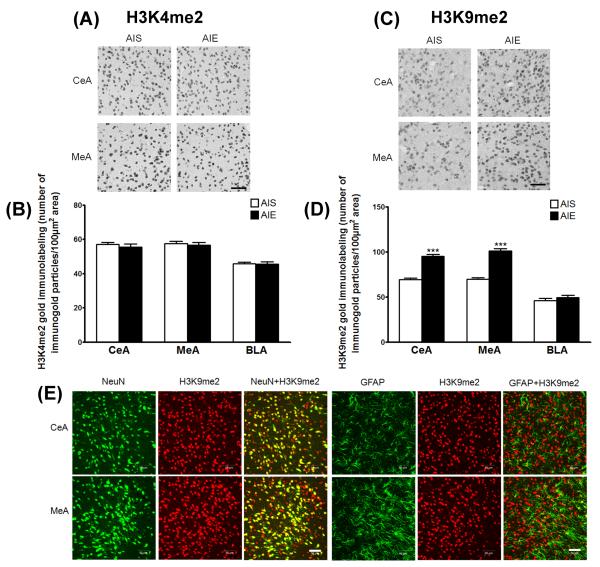
Figure 4. Increased histone H3 lysine 9 dimethlyation (H3K9me2) but no change in H3K4me2 protein levels in the amygdala of AIE adult rats.
A) Photomicrographs (Scale bar = 50 μm) showing the gold immunolabeling of H3K4me2 proteins in the central (CeA) and medial nucleus of amygdala (MeA) of adolescent intermittent ethanol (AIE)- or saline (AIS)-exposed adult rats. B) Quantification of H3K4me2 protein levels by gold immunolabeling in the CeA, MeA and basolateral amygdala (BLA) of AIS- and AIE-exposed adult rats. C) Photomicrographs (Scale bar = 50 μm) showing the gold immunolabeling of H3K9me2 proteins in the CeA and MeA of AIE- and AIS-exposed adult rats. D) Quantification of H3K9me2 protein levels by gold immunolabeling in the CeA, MeA and BLA of AIS- and AIE-exposed adult rats. Values are represented as mean ± SEM of n=5 rats and are significantly different from AIS-exposed group (***p < 0.001, Student's t test). E) Representative confocal photomicrographs of immunefluorescent staining (Scale bar = 50 μm) of H3K9me2 (red) co-localized with either neuron-specific nuclear protein (NeuN; green) or glial fibrillary acidic protein (GFAP; green) in the CeA and MeA of naive adult rats.
We next performed confocal immunofluorescence microscopy to determine if H3K9me2 levels were present in neurons, glial cells, or both in the CeA, MeA, and BLA. Qualitative analysis shows H3K9me2 co-labeling with both NeuN (neurons) and GFAP (glial cells), but to a greater extent in neurons in the CeA, MeA (Figure 4E) and BLA (data not shown).
Expression of specific LSD1-associated transcripts is altered by AIE in adulthood
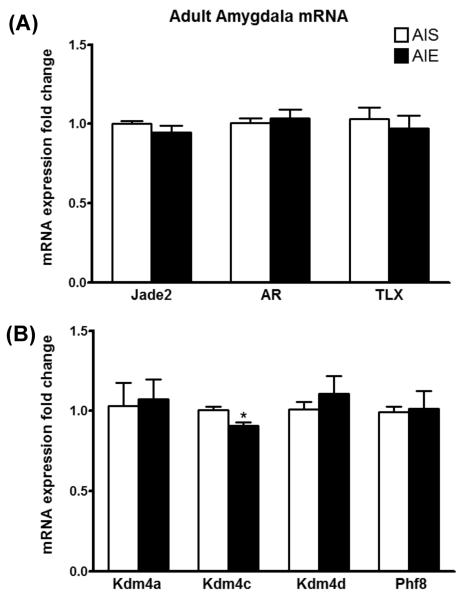
LSD1 is a complex enzyme, and several other proteins (Lan et al., 2008) can modulate the biological responses of LSD1. In order to gain insight into the regulation of LSD1 function after AIE exposure, we measured mRNA transcripts of selected proteins known to interact with LSD1 in the amygdala of adult rats after AIE (Figure 5A). Jade-2 was recently reported to degrade the LSD1 protein and target it for proteasomal degradation (Han et al., 2014). However, our results show that Jade2 mRNA is unaltered in the amygdala after AIE in adulthood. Similarly, although AR mediates H3K9me2/1 demethylation by LSD1 (Metzger et al., 2005), AR mRNA does not change between AIE and AIS groups in the adult amygdala. The nuclear receptor TLX (also known as NR2E1) interacts with LSD1 during neural stem cell proliferation (Sun et al., 2011, 2010). Our results show that TLX mRNA expression in the amygdala is unaltered by AIE exposure when measured in adulthood.
Figure 5. mRNA profiling of (A) _Lsd1_-interacting partners and (B) histone H3 lysine 9 (H3K9) methylation modifying enzymes reveals a specific decrease in Kdm4c in the amygdala of AIE adult rats.
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) and allowed to grow to adulthood without further ethanol exposure. Their brains were collected and amygdaloid tissues dissected out for mRNA analysis at postnatal day (PND) 92. Values are represented as mean ± SEM of n=5-9 rats and are significantly different from AIS-exposed group (*p < 0.05, Student's t test). AR, androgen receptor; TLX, nuclear receptor.
To determine the specificity of Lsd1 regulation in the AIE adult amygdala, we measured mRNA of other demethylases acting on H3K9 (Figure 5B). We found that Kdm4c mRNA was significantly reduced in the amygdala of adult rats exposed to AIE when compared to AIS controls (t (15) = 2.8, p<0.05). Kdm4c (also known as Jmjd2c) is a lysine demethylase that regulates methylated H3K9 modifications (H3K9me3/2) and interacts with LSD1 (Pedersen et al., 2014; Wissmann et al., 2007). mRNA transcripts of Kdm4a, Kdm4d, and Phf8, three demethylases known to act on H3K9me2, were unchanged in the amygdala between AIS and AIE adult rats. These results support our emerging notion that AIE exposure alters chromatin remodeling via H3K9me2 but not H3K4me2 modifications that may produce persistent effects on behavioral and molecular phenotypes in adulthood.
Acute ethanol exposure in adulthood normalizes behavioral and molecular phenotypes of AIE
Acute alcohol exposure can alleviate the increased anxiety seen in models of alcoholism and alcohol abuse, such as alcohol-preferring P rats (Moonat et al., 2011, 2013). Therefore, we exposed AIE and AIS adults to an acute ethanol challenge (2 g/kg) on PND 101 or 102 and tested animals in the EPM (Figure 6A). The mean ± SEM blood ethanol levels (mg %) were 182 ± 10 in the AIS+ EtOH group (n=7) and 191 ± 14 in the AIE+ EtOH group (n=6). There was a significant effect of AIE treatment (_F_1, 26 = 67.1, p<0.001), adult acute ethanol challenge (_F_1, 26 = 89.7, p<0.001), and the interaction between AIE and adult acute ethanol treatment (_F_1, 26 = 8.2, p<0.01) on the percentage of open arm entries. There was also a significant effect of AIE treatment (_F_1, 26 = 41.0, p<0.001), adult acute ethanol challenge (_F_1, 26 = 51.6, p<0.001), and the interaction between AIE and adult acute ethanol treatment (_F_1, 26 = 6.8, p<0.05) on the percent time spent in the open arms. While acute ethanol exposure increased percent of open arm entries and percent time spent in the open arms in AIS adult rats (AIS+ EtOH) compared to normal saline-exposed (AIS+ Saline) rats (p<0.01-0.001), AIE+ EtOH animals returned to baseline levels of anxiety-like behavior after acute ethanol challenge (Figure 6A). As reported earlier (Pandey et al., 2015; Sakharkar et al., 2016), here we also observed that AIE induced anxiety-like behaviors in adulthood. Closed arm entries were not significantly altered between groups, indicating no significant differences in general activity. There were no significant differences in mean ± SEM body weight (g) of rats (n=7-8) among the groups (AIS+ Saline, 395± 6.4; AIE+ Saline, 376±5.4; AIS+ EtOH, 399± 12.0; AIE+ EtOH, 409± 11.9)
Figure 6. Acute ethanol challenge normalizes (A) anxiety-like behavior, (B) amygdala Lsd1+8a expression, and (C) amygdala histone H3 lysine 9 dimethylation (H3K9me2) occupancy at Bdnf exon IV promoter.
Rats were exposed to adolescent intermittent ethanol (AIE) or saline (AIS) in adolescence (postnatal day [PND] 28-41) and no further exposure to ethanol until animals were exposed to an acute ethanol challenge (2 g/kg) at PND 101 or 102. Animals were tested in the elevated plus maze (EPM) for anxiety-like behaviors (activities in open and closed arms), and immediately brains were collected and amygdaloid tissues dissected out for mRNA and chromatin immunoprecipitation (ChIP) analysis. Values are represented as mean ± SEM of n=6-8 rats and are significantly different between groups (*p < 0.05, **p < 0.01, ***p < 0.001, two-way ANOVA followed by post hoc Tukey's test).
We further investigated whether acute ethanol exposure in adulthood could normalize the decreased Lsd1+8a mRNA levels seen in the AIE adult amygdala (Figure 2A). Lsd1+8a mRNA levels in the amygdala were significantly altered (Figure 6B) by both AIE treatment (_F_1, 24 = 6.0, p<0.05) and adult acute ethanol treatment (_F_1, 24 = 8.4, p<0.01), with a significant interaction between AIE and adult acute ethanol treatment (_F_1, 24 = 8.6, p<0.01). AIE+ Saline animals showed a significant reduction in Lsd1+8a mRNA levels compared to AIS+ Saline animals (p<0.001 by post hoc Tukey test), while AIE exposed adult rats challenged with acute ethanol (2 g/kg) displayed levels of Lsd1+8a mRNA in the amygdala that did not differ from that of control (AIS+ Saline) rats. Interestingly, Lsd1+8a mRNA levels in the amygdala of AIE+ EtOH rats were significantly increased (p<0.001) as compared with AIE+ Saline rats. Accordingly, we measured the occupancy of H3K9me2 by ChIP assay at the promoter of Bdnf exon IV (Figure 6C), the expression of which is significantly decreased in the AIE adult amygdala (Pandey et al., 2015). We found that H3K9me2 occupancy at Bdnf IV is significantly altered in the amygdala by the interaction between AIE and adult acute ethanol treatment (_F_1, 22 = 8.8, p<0.01; Figure 6C), but not by either AIE or adult acute ethanol treatment alone. Post hoc analysis reveals a significant (p<0.01) increase in H3K9me2 occupancy in AIE+ Saline animals but no difference in AIE+ EtOH animals as compared to AIS+ Saline controls, suggesting that acute alcohol exposure in adulthood after AIE also normalizes H3K9me2 levels at specific synaptic-plasticity related genes such as Bdnf.
DISCUSSION
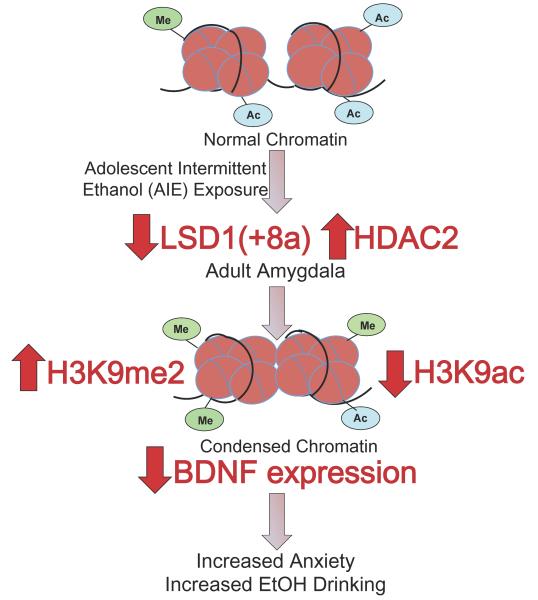
The major findings of the study are that AIE exposure causes a decrease in Lsd1 and Lsd1+8a mRNA and LSD1 protein levels in the amygdala in adulthood. AIE-exposed adult rats show an increase in H3K9me2 protein levels in the CeA and MeA, but no change in H3K4me2 protein levels. The mRNA levels of the H3K9me2/3 demethylase Kdm4c are also decreased in the amygdala of AIE-exposed adult rats. Additionally, acute alcohol challenge in adulthood after AIE normalized the anxiety-like behaviors seen in AIE animals, as well as the decreased Lsd1+8a mRNA levels and increased H3K9me2 occupancy at the Bdnf exon IV promoter in the amygdala. These converging lines of evidence suggest that LSD1, and in particular the neuron-specific isoform Lsd1+8a, may be acting primarily on H3K9me2 rather than H3K4me2 after AIE in adulthood. We recently reported that AIE produces an increase in HDAC2 expression and deficits in global and gene promoter-specific H3K9 acetylation, which is associated with decreases in Bdnf and Arc gene expression and decreased dendritic spines in the amygdala in adulthood. These were associated with increased anxiety-like and alcohol drinking behaviors seen in AIE rats in adulthood (Pandey et al., 2015). Taken together, these data suggest that increased HDAC2 and decreased LSD1+8a levels may be responsible for the AIE-induced decrease in H3K9 acetylation (H3K9ac) and increase in H3K9me2 levels, leading to condensed chromatin architecture and decreased Bdnf expression in the amygdala and adult psychopathology (Figure 7).
Figure 7.
Hypothetical model showing adolescent intermittent ethanol (AIE) exposure causes a lasting decrease in lysine demethylase 1(LSD1) in the central nucleus of amygdala (CeA) and a decrease in Lsd1+8a in the CeA and medial nucleus of amygdala (MeA) in adulthood. Adolescent intermittent ethanol (AIE)-exposed adults show increased histone H3 lysine 9 dimethylation (H3K9me2) in the CeA and MeA with no change in histone H3 lysine 4 dimethylation (H3K4me2) levels. In addition, AIE increased the levels of H3K9me2 at the promoter of Bdnf exon IV in the amygdala. This, along with previous data from our lab showing an increase in histone deacetylase isoform 2 (HDAC2) and the resulting decrease in global and Bdnf exon IV-specific H3K9 acetylation and associated reduced levels of BDNF in the amygdala (Pandey et al., 2015) likely contributes to condensed chromatin architecture and increased anxiety-like and alcohol-drinking behaviors.
mRNA profiling of histone methyltransferases and demethylases in the amygdala and BNST in adolescent animals following AIE showed regional and temporal effects of ethanol exposure. It should be noted that our panel of epizymes was chosen due to their ability to modify H3K4 or H3K9 methylation (Allis et al., 2007) and their proven expression in brain tissue. The expression of the H3K4 methyltransferase Mll2 was increased in the BNST of animals in the AIE group, who were measured 1 hr after the last AIE when ethanol is still present in the body. These levels returned to normal after 24 hrs in the AIW group. In contrast, Mll1 mRNA was significantly elevated in the AIW group but not in the AIE group in the BNST, demonstrating time-point specific effects on gene expression in the BNST after AIE. These findings resemble studies from our group showing dose- and time-specific effects of ethanol exposure to the expression of epigenetic enzymes in the BNST of adolescent rats (Sakharkar et al., 2014). The induction of methyltransferase gene expression in the BNST following AIE may represent an adaptive epigenetic mechanism affecting H3K4 methylation (Shen et al., 2014). Lsd1 mRNA was the only transcript significantly affected by AIE in the amygdala in adolescence. LSD1 was the first enzyme discovered to actively demethylate modified histones, as histone methylation was previously thought to be a permanent mark (Shi et al., 2004). Interestingly, Lsd1 expression levels, but not Lsd1+8a mRNA levels, were increased in the amygdala by AIE but returned to control levels 24 hrs after the last ethanol exposure in adolescent rats, yet both were significantly decreased in animals in adulthood after AIE. The mechanism for differential regulation of Lsd1 and Lsd1+8a expression is not presently clear, but may be related to non-neuronal versus neuronal developmental expression (Zibetti et al., 2010; Laurent et al., 2015), and a longer time period may be needed to gradually decrease expression of these transcripts following AIE. Furthermore, the BNST brain region responded differentially compared to the amygdala in adolescence after AIE. Differences in stress-related peptide levels and expression of epigenetic enzymes between the amygdala and BNST in response to adolescent alcohol exposure have been reported previously (Karanikas et al., 2013; Sakharkar et al., 2014) and may be indicative of differences in the underlying circuitry and neuronal composition.
Lsd1 and Lsd1+8a mRNA levels both were decreased in the amygdala of AIE-exposed adult rats, while no lasting changes were observed in the BNST. AIE animals also show an increase in anxiety-like behaviors in adulthood (Pandey et al., 2015), as the amygdala is a known regulator of anxiety and addiction (Davis and Whalen, 2001; Koob and Volkow, 2010). The decrease in LSD1 protein levels by AIE in adulthood was localized to the CeA, with no change in LSD1 levels in the MeA or BLA, while the decrease in Lsd1+8a mRNA levels was localized to both the CeA and MeA, but not the BLA, by in situ PCR. When we examined the amygdala of AIE-exposed adult rats for levels of H3K4me2 and H3K9me2 (due to the ability of LSD1 to catalyze demethylation of both markers (Metzger et al., 2005; Shi et al., 2004)), we found no change in H3K4me2 immunoreactivity but a marked increase in H3K9me2 immunostaining in the CeA and MeA. We performed immunofluorescent staining to determine the cell type-specificity of H3K9me2 expression and found that this epigenetic mark was present in both neurons (NeuN) and glial cells (GFAP). However, H3K9me2 co-localized to a greater degree with NeuN, suggesting that H3K9me2 is more abundant in neuronal cell populations in the amygdala. As numerous enzymes have been shown to modify H3K9 methylation, we examined gene expression of demethylases known to act on this residue. We found that only the H3K9me3/2 demethylase Kdm4c was decreased in the amygdala of AIE adult rats. Kdm4c complexes with LSD1 to promote gene activation by demethylating H3K9 (Wissmann et al., 2007). Based on these findings we concluded that the decreased LSD1, and the neuron-specific splice variant Lsd1+8a in particular, may be acting primarily to increase H3K9me2 after AIE in the adult amygdala. However, our data is not sufficient to exclude the possibility that other epigenetic enzymes are involved in AIE-induced neuroadaptation in the amygdala. For example, an increase in H3K9me2 due to increased G9a after ethanol exposure has been noted previously in models of neonatal alcohol exposure, leading to hippocampal neurodegeneration and deficits in long-term potentiation (Subbanna and Basavarajappa, 2014; Subbanna et al., 2014, 2013).
The hypothesis that Lsd1+8a may be acting primarily on H3K9 is in line with recent data showing that inclusion of exon 8a switches the substrate specificity of LSD1 away from H3K4me2/1 and towards H3K9me2/1 (Laurent et al., 2015). Other studies have concluded that LSD1 may be primarily affecting H3K9 methylation, and not H3K4 methylation, in a developmentally regulated fashion in the prefrontal cortex (Maćkowiak et al., 2014). Our results seem to support this hypothesis in the amygdala, as H3K4me2 was not altered in AIE adult rats although Lsd1 mRNA and LSD1 protein were decreased. The first reports of neuron-specific Lsd1+8a noted its stimulatory effects on neurite morphogenesis (Zibetti et al., 2010), which may contribute to the well-studied ability of LSD1 to facilitate neural stem cell proliferation (Han et al., 2014; Sun et al., 2010, 2011). Further work showed that the mini-exon 8a, composed of only 4 amino acids, contains a threonine residue that facilitates axonal outgrowth and dendritic branching when phosphorylated (Toffolo et al., 2014). This may explain why AIE adult rats show a stark decrease in dendritic spine density specifically in the CeA and MeA (Pandey et al., 2015), which corresponds anatomically with the decrease in Lsd1+8a presented here. It has been shown that knockdown of Lsd1+8a results in the inhibition of, and over-expression of Lsd1+8a enhances, neurite maturation (Zibetti et al., 2010). Additionally, the balance between Lsd1+8a and non-8a isoforms is involved in neuronal excitation and the response to epileptogenic stimuli, with lower levels of Lsd1+8a leading to decreased excitability (Rusconi et al., 2015). Lsd1+8a may prove a novel target to reverse the decreased dendritic spine density seen in the amygdala of AIE adult rats (Pandey et al., 2015) and in the amygdala and nucleus accumbens of other animal models of alcoholism (Kyzar and Pandey, 2015; You et al., 2014; Zhou et al., 2007), as well as in cortical structures of human patients suffering from alcohol use disorders (Ferrer et al., 1986).
An acute ethanol challenge in adulthood was anxiolytic in AIS rats and attenuated AIE-induced anxiety-like behaviors. This indicates that the increased alcohol drinking behavior exhibited by AIE animals (Pandey et al., 2015) may act to alleviate heightened anxiety, as has been shown in other models of alcohol abuse such as alcohol-preferring P rats (Moonat et al., 2011, 2013). Additionally, acute alcohol exposure normalized the deficits in Lsd1+8a mRNA expression and the increase in H3K9me2 occupancy at the Bdnf exon IV promoter in the amygdala seen in AIE (AIE+ Saline) animals. As Bdnf exon IV expression is decreased in the CeA and MeA of AIE adult rats (Pandey et al., 2015), the increase in repressive H3K9me2 may be acting to decrease Bdnf transcription and thereby affecting dendritic spines in the amygdala. It is interesting to note that, at the same region of the Bdnf exon IV promoter, there is a decrease in acetylated H3K9/14 in the amygdala after AIE in adulthood (Pandey et al., 2015). Previous studies have shown that acute ethanol exposure increased histone H3 acetylation of Bdnf exon IV, BDNF expression and dendritic spines in the amygdala, and produced anxiolytic-like effects in alcohol preferring rats (Moonat et al., 2011, 2013). Thus, the increase in H3K9me2 and HDAC2-induced decrease in H3K9ac in the amygdala after AIE in adulthood may represent important epigenetic regulators of chromatin architecture and AIE-induced adult psychopathology (Figure 7; Pandey et al., 2015). Again, our results do not rule out the influence of other epigenetic enzymes such as Kdm4c that may play a role in the response to adult ethanol challenge after AIE. The neuroadaptative role of LSD1 (+8a) seen here may represent a developmental switch that performs different molecular tasks at various points during maturation. Indeed, LSD1 plays a crucial role in neural stem cell proliferation in early life (Sun et al., 2010), but is also involved in neuronal excitability in adult animals (Rusconi et al., 2015). Therefore, the role of LSD1 (+8a) likely changes and evolves over the lifespan, possibly in a brain-region specific manner. Emerging evidence suggests that adolescent and adult animals respond in fundamentally different ways to the behavioral and molecular effects of alcohol exposure (Spear and Swartzwelder, 2014; Vetreno et al., 2014; Sakharkar et al., 2012, 2014). As acute alcohol exposure did not significantly alter Lsd1+8a mRNA levels or H3K9me2 occupancy in the amygdala of AIS animals, the involvement of Lsd1+8a and H3K9me2 may be specific to the persistent effects of early-life alcohol exposure. It is possible that other epigenetic mechanisms such as increased H3K9 acetylation (Sakharkar et al., 2012) or decreased DNA methylation due to acute ethanol exposure might be responsible for the anxiolysis observed here in AIS adult rats.
Our data suggests that developmental alcohol exposure affects Lsd1+8a in a region-specific manner and causes hypermethylation of H3K9 in particular. Future studies will examine the direct contribution of Lsd1 and Lsd1+8a to anxiety-like behaviors and increased alcohol drinking of rats previously exposed to AIE, as well the regulation of Lsd1 expression by epigenetic and cellular processes. To our knowledge, this is the first study to show that Lsd1 and Lsd1+8a levels are affected by ethanol exposure. The decrease in Lsd1+8a expression is correlated with an increase in the repressive epigenetic marker H3K9me2 specifically in the CeA and MeA, contributing to the condensed chromatin conformation seen in the adult amygdala following AIE (Figure 7; Pandey et al., 2015). Acute ethanol challenge in adulthood was able to attenuate anxiety-like behaviors seen in AIE adults, as well as normalize the decreased Lsd1+8a levels and increased H3K9me2 occupancy at the Bdnf exon IV promoter in the amygdala. We hope that this and other studies of the epigenetic mechanisms (Pandey et al., 2015; Sakharkar et al., 2016) of adolescent alcohol exposure-induced adult psychopathology will shed further light on alcohol addiction and lead to novel and successful treatments of alcohol use disorders.
Acknowledgements
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants UO1AA-019971, U24AA024605 [Neurobiology of Adolescent Drinking in Adulthood (NADIA) project], RO1AA-010005, RO1 AA-013341, P50AA022538 and by the Department of Veterans Affairs (Merit Review Grant, I01BX000143; Senior Research Career Scientist award) to SCP.
Footnotes
Author contributions
Study was conceived, and grant was written and received by SCP. SCP designed the experiments. EJK was also responsible for study design and conducted the majority of experiments. SCP and EJK wrote the manuscript. EJK, HZ, and AS were responsible for the alcohol treatment and generating experimental animals. HZ and EJK conducted all immunohistochemistry and in situ PCR experiments, and EJK performed the mRNA and ChIP analyses. HZ and EJK contributed to the acquisition of behavioral data. All authors have read and approved the final version of this article.
Conflicts of interest: SCP reports that a US patent application entitled “Histone acetyltransferase activators and histone deacetylase inhibitors in the treatment of alcoholism” (serial number 60/848237 filed on September 29th, 2006) is currently pending. Other authors reported no biomedical financial interests or potential conflicts of interest.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Cloos PA, Christensen J, Agger K, Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22:1115–1140. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents’ motivations for alcohol, cigarette, and marijuana use. Addict Behav. 2001;26:803–825. doi: 10.1016/s0306-4603(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Donovan JE. Adolescent alcohol initiation: a review of psychosocial risk factors. J Adolesc Health. 2004;35:529. doi: 10.1016/j.jadohealth.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr Opin Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Fãbregues I, Rairiz J, Galofré E. Decreased numbers of dendritic spines on cortical pyramidal neurons in human chronic alcoholism. Neurosci Lett. 1986;69:115–119. doi: 10.1016/0304-3940(86)90425-8. [DOI] [PubMed] [Google Scholar]
- File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res. 1993;58:199–202. doi: 10.1016/0166-4328(93)90103-w. [DOI] [PubMed] [Google Scholar]
- Gräff J, Kim D, Dobbin MM, Tsai LH. Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol Rev. 2011;91:603–649. doi: 10.1152/physrev.00012.2010. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, True WR, Bucholz KK. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med. 2006;36:109–118. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- Han X, Gui B, Xiong C, Zhao L, Liang J, Sun L, Yang X, Yu W, Si W, Yan R, Yi X, Zhang D, Li W, Li L, Yang J, Wang Y, Sun YE, Zhang D, Meng A, Shang Y. Destabilizing LSD1 by Jade-2 promotes neurogenesis: an antibraking system in neural development. Mol Cell. 2014;55:482–494. doi: 10.1016/j.molcel.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Hou H, Yu H. Structural insights into histone lysine demethylation. Curr Opin Struct Biol. 2010;20:739–748. doi: 10.1016/j.sbi.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas CA, Lu YL, Richardson HN. Adolescent drinking targets corticotropin-releasing factor peptide-labeled cells in the central amygdala of male and female rats. Neuroscience. 2013;249:98–105. doi: 10.1016/j.neuroscience.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck Cl, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:639–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC. The epigenetic landscape of alcoholism. Int Rev Neurobiol. 2014;115:75–116. doi: 10.1016/B978-0-12-801311-3.00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Pandey SC. Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett. 2015;601:11–19. doi: 10.1016/j.neulet.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Nottke AC, Shi Y. Mechanisms involved in the regulation of histone lysine demethylases. Curr Opin Cell Biol. 2008;20:316–325. doi: 10.1016/j.ceb.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent B, Ruitu L, Murn J, Hempel K, Ferrao R, Xiang Y, Liu S, Garcia BA, Wu H, Wu F, Steen H, Shi Y. A specific LSD1/KDM1A isoform regulates neuronal differentiation through H3K9 demethylation. Mol Cell. 2015;57:957–970. doi: 10.1016/j.molcel.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maćkowiak M, Bator E, Latusz J, Mordalska P, Wȩdzony K. Prenatal MAM administration affects histone H3 methylation in postnatal life in the rat medial prefrontal cortex. Eur Neuropsychopharmacol. 2014;24:271–289. doi: 10.1016/j.euroneuro.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Metzger E, Wissmann M, Yin N, Müller JM, Schneider R, Peters AH, Günther T, Buettner R, Schüle R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelamegam R, Ricq EL, Malvaez M, Patnaik D, Norton S, Carlin SM, Hill IT, Wood MA, Haggarty SJ, Hooker JM. Brain-penetrant LSD1 inhibitors can block memory consolidation. ACS Chem Neurosci. 2012;3:120–128. doi: 10.1021/cn200104y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607–619. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pedersen MT, Agger K, Laugesen A, Johansen JV, Cloos PA, Christensen J, Helin K. The demethylase JMJD2C localizes to H3K4me3-positive transcription start sites and is dispensable for embryonic development. Mol Cell Biol. 2014;34:1031–1045. doi: 10.1128/MCB.00864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room R. Alcohol, the individual and society: what history teaches us. Addiction. 1997;92(Suppl 1):S7–S11. [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt D. Lasting epigenetic influence of early-life adversity on BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi F, Paganini L, Braida D, Ponzoni L, Toffolo E, Maroli A, Landsberger N, Bedogni F, Turco E, Pattini L, Altruda F, De Biasi S, Sala M, Battaglioli E. LSD1 neurospecific alternative splicing controls neuronal excitability in mouse models of epilepsy. Cereb Cortex. 2015;25:2729–2740. doi: 10.1093/cercor/bhu070. [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC. A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct. 2016 Mar 3; doi: 10.1007/s00429-016-1196-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson D, Pandey SC. Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol. 2014;17:2057–2067. doi: 10.1017/S1461145714001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E, Shulha H, Weng Z, Akbarian S. Regulation of histone H3K4 methylation in brain development and disease. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman BG, Sakharkar AJ, Pandey SC. Epigenetics-beyond the genome in alcoholism. Alcohol Res. 2012;34:293–305. [PMC free article] [PubMed] [Google Scholar]
- Subbanna S, Basavarajappa BS. Pre-administration of G9a/GLP inhibitor during synaptogenesis prevents postnatal ethanol-induced LTP deficits and neurobehavioral abnormalities in adult mice. Exp Neurol. 2014;261:34–43. doi: 10.1016/j.expneurol.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanna S, Nagre NN, Shivakumar M, Umapathy NS, Psychoyos D, Basavarajappa BS. Ethanol induced acetylation of histone at G9a exon1 and G9a-mediated histone H3 dimethylation leads to neurodegeneration in neonatal mice. Neuroscience. 2014;258:422–432. doi: 10.1016/j.neuroscience.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Umapathy NS, Saito M, Mohan PS, Kumar A, Nixon RA, Verin AD, Psychoyos D, Basavarajappa BS. G9a-mediated histone methylation regulates ethanol-induced neurodegeneration in the neonatal mouse brain. Neurobiol Dis. 2013;54:475–485. doi: 10.1016/j.nbd.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Alzayady K, Stewart R, Ye P, Yang S, Li W, Shi Y. Histone demethylase LSD1 regulates neural stem cell proliferation. Mol Cell Biol. 2010;30:1997–2005. doi: 10.1128/MCB.01116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Ye P, Murai K, Lang MF, Li S, Zhang H, Li W, Fu C, Yin J, Wang A, Ma X, Shi Y. miR-137 forms a regulatory loop with nuclear receptor TLX and LSD1 in neural stem cells. Nat Commun. 2011;2:529. doi: 10.1038/ncomms1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffolo E, Rusconi F, Paganini L, Tortorici M, Pilotto S, Heise C, Verpelli C, Tedeschi G, Maffioli E, Sala C, Mattevi A, Battaglioli E. Phosphorylation of neuronal lysine-specific demethylase 1LSD1/KDM1A impairs transcriptional repression by regulating interaction with CoREST and histone deacetylases HDAC1/2. J Neurochem. 2014;128:603–616. doi: 10.1111/jnc.12457. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT. Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One. 2014;9:e113421. doi: 10.1371/journal.pone.0113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Wissmann M, Yin N, Müller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Günther T, Buettner R, Metzger E, Schüle R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44:119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, Crabtree GR. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC. Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol. 2014;17:313–322. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res. 2007;1134:148–161. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibetti C, Adamo A, Binda C, Forneris F, Toffolo E, Verpelli C, Ginelli E, Mattevi A, Sala C, Battaglioli E. Alternative splicing of the histone demethylase LSD1/KDM1 contributes to the modulation of neurite morphogenesis in the mammalian nervous system. J Neurosci. 2010;30:2521–2532. doi: 10.1523/JNEUROSCI.5500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]