Drought coping strategies in cotton: increased crop per drop (original) (raw)
Summary
The growth and yield of many crops, including cotton, are affected by water deficit. Cotton has evolved drought specific as well as general morpho‐physiological, biochemical and molecular responses to drought stress, which are discussed in this review. The key physiological responses against drought stress in cotton, including stomata closing, root development, cellular adaptations, photosynthesis, abscisic acid (ABA) and jasmonic acid (JA) production and reactive oxygen species (ROS) scavenging, have been identified by researchers. Drought stress induces the expression of stress‐related transcription factors and genes, such as ROS scavenging, ABA or mitogen‐activated protein kinases (MAPK) signalling genes, which activate various drought‐related pathways to induce tolerance in the plant. It is crucial to elucidate and induce drought‐tolerant traits via quantitative trait loci (QTL) analysis, transgenic approaches and exogenous application of substances. The current review article highlights the natural as well as engineered drought tolerance strategies in cotton.
Keywords: ABA, cotton, drought stress, MAPK, ROS
Introduction
Cotton is grown as a leading commercial crop in more than 30 countries of world with major shares from China, India, the United States and Pakistan, and is predominantly cultivated in warmer regions (Riaz et al., 2013). According to statistics, China, India, the United State, Pakistan and Brazil were the top 5 cotton‐producing countries in 2014–2015, generating 6.5 M, 5.4 M, 3.5 M, 2.3 M and 1.5 M tones, respectively (Statista, 2015). As a glycophyte, cotton shows higher tolerance to abiotic stresses than other major crops. However, extreme environmental conditions, such as drought affect growth, productivity, and fibre quality of cotton (Parida et al., 2007). According to a press release from the United States Department of Agriculture (USDA), cotton production is expected to decline due to drought stress (USDA, 2015). Similarly in Pakistan, cotton production declined by 34% to just 9.68 M bales against the production of 14.4 M bales from previous year because of drought and high temperature (Dawn news, 2016). In addition to cotton, other crops were also affected by drought, as approximately 67% of crop losses were due to drought stress over the last 50 years in the United States (Comas et al., 2013). The impacts of drought on cotton are widespread and varied, which makes it difficult to determine accurate financial estimates (Table 1). As shown in Figure 1, world cotton production was very low in 2008 and 2009, which led to a significant decrease in stocks in 2009. Therefore, cotton prices were increased in 2010 and 2011, resulting in the cotton consumption decline of 10% in 2011. Cotton production was higher than demand from 2010 to 2013; however, the production decreased from 2011, with a significant decrease of 6.5% in 2015 from 2014, while consumption is increasing by approximately 6.5 million bales annually (Figure 1). Thus, we need to establish policies for the production and consumption of the cotton. Moreover, it is also necessary to produce stress‐tolerant varieties of cotton due to the uncertain conditions in the future. On the other hand, the emphasis should not be only on stress‐tolerant variety of cotton, although plant survival is very critical in the early stages of growth, stress‐tolerant variety should therefore be based on stability of yield. It is known that improving of yield and maintaining yield stability of cotton crop, under normal as well drought stress conditions, is essential for the growing global population.
Table 1.
Direct and indirect impacts of drought on cotton and its management
| Direct impacts | Indirect impacts | Management |
|---|---|---|
| Damage plants systems | Food scarcity | Drought‐tolerant varieties should develop |
| Reduce crop productivity | Reduce income of farmers and agribusiness | Effective impact assessment procedures should develop |
| Reduce water level | Increase prices of foods and goods | Pro‐active risk management measures |
| Increase insect infestation | Increase unemployment (companies dealing with agriculture will stop working) | Make plans aimed at increasing the coping capacity |
| Increase plant diseases | Increase crime and insecurity | Efficient emergency response programs should be planned which can be used for reducing the impacts of drought |
| Cause pollution in the concern area | Meetings should conduct on national and international level about drought stress | |
| Migration | Early warning system should develop to make decision earlier |
Figure 1.
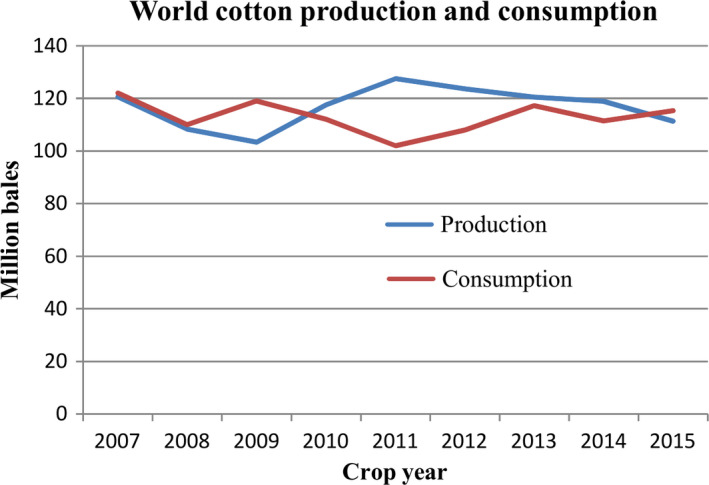
Unstable world cotton production and their consumption since 2007.
Despite the complexity of drought tolerance mechanism in cotton, tremendous progress has been made in understanding the drought tolerance mechanism. Morpho‐physiological, biochemical and molecular adaptations by nature or by genetic engineering can lead to the drought‐tolerant variety of cotton. The current review discusses effective techniques to alleviate the negative effects of drought stress in cotton and maintain the productivity as well as fibre quality. Moreover, the mechanisms of drought tolerance in cotton and strategies to induce tolerance to drought are also discussed.
Morpho‐physiological mechanism of cotton in responses to drought stress
Drought stress causes a wide range of morpho‐physiological and biochemical changes that adversely affect the development as well as the productivity of the cotton (Figure 2). Generally, drought stress severely restricts cotton growth and development, such as affecting plant height, leaf dry weight, stem dry weight, leaf area index, node number, fibre quality, canopy and root development (Loka et al., 2011). Specifically, net photosynthetic rate, transpiration rate, stomata conductance, carboxylation efficiency and water potential of cotton leaves decrease significantly during drought conditions (Kumar et al., 2001). Recently, Hejnák et al. (2015) studied the detrimental effects of drought stress on cotton. According to their results, 50% dry matter accumulation of Gossypium barbadense (G. barbadense) was limited under drought stress. Moreover, the stomata conductance, photosynthetic rate and transpiration rate were also decreased under water deficit. Like other plants, cotton has acquired a wide range of morpho‐physiological, biochemical and molecular mechanisms in response to multiple stresses that enable them to avoid and/or tolerate these stress factors and survive in harsh environments.
Figure 2.
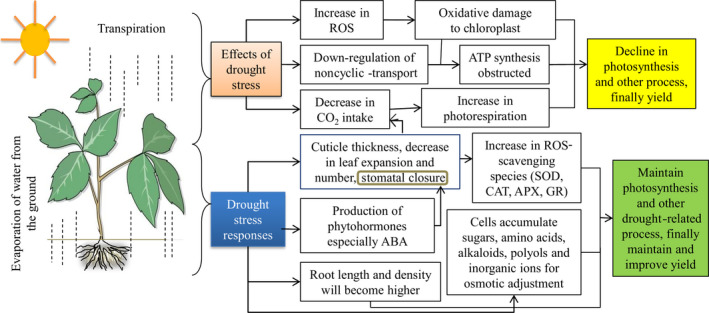
Numerous effects of drought stress on cotton and their responses.
The plant drought tolerance mechanisms can be divided into four strategies: drought avoidance, drought escape, drought tolerance and drought recovery (Fang and Xiong, 2015). Drought avoidance and drought tolerance are the two major strategies of plants against drought stress. Drought avoidance is the maintenance of key physiological processes, such as stomata regulation, root system development and others, during moderate drought conditions. Drought tolerance is the capability of plants to withstand severe dehydration through specific physiological activities, such as osmotic adjustment via osmoprotectants (Luo, 2010). Drought escape is the ability of plants to adjust their growth period or lifecycle, such as the cotton variety with a short life cycle, to avoid the seasonal drought stress (Manavalan et al., 2009). Drought recovery of plants is the capability to resume growth and yield after exposure to severe drought stress. Cotton has evolved several common morpho‐physiological strategies against drought stress, which have been discussed in this section, such as stomata regulation, root development, photosynthetic response and osmotic adjustment.
Stomata regulation
Reduction of water loss through leaves is a crucial phenomenon in cotton plants under drought stress. Wilting and rolling of leaves result in less radiation and thus reduced water loss (Fang and Xiong, 2015). Plants often show various xeromorphic characters and have structures that promote drought tolerance, such as thicker and smaller leaves, a thicker cuticle epidermis, more epidermal trichomes, thicker palisade tissues, smaller and denser stomata, a high ratio of palisades to spongy parenchyma thickness, and a developed vascular bundle sheath (Hetherington and Woodward, 2003; Iqbal et al., 2013). For example, the cotton variety, Gossypium hirsutum (G. hirsutum) YZ1, has smaller leaves as compared to G. hirsutum Y668. Stomata regulation plays a pivotal role in gas exchange between tissues and the atmosphere. It is one of the key mechanisms that allow plants to produce energy and maintain cellular function. Ninety per cent of water losses (transpiration) from plants occur though stomata openings (Wang et al., 2009). In cotton, closure of the stomata is the first step to reduce water loss during drought conditions, when the rate of transpiration is very high. Stomata conductance could be a potential indicator of drought tolerance in cotton as there is a negative correlation between drought tolerance and stomata conductance.
Root development
All root traits are potentially important in the drought stress; however, hydraulic conductance and plant allometry have been of particular interest to researchers. Various scientists have reviewed the potential function of roots under drought stress (Comas et al., 2013). More profuse (higher root length density) and deeper root systems in the soil are often proposed as desirable characteristics for drought adaptation. In a case, Luo et al. (2016) reported that mild and initial‐stage drought stress enhanced root length in cotton, but long‐time water deficit reduced the root activity as compared to control plants. In another study, transgenic cotton plants were more tolerant to drought stress, with a better root system than in wild type (Liu et al., 2014). Similarly, the transgenic cotton plants harboured Arabidopsis that enhanced drought tolerance 1/homodomain glabrous 11 (AtEDT1/HDG11) gene had well‐developed roots in addition to other drought‐tolerant features (Yu et al., 2015).
Photosynthesis
Drought stress causes stomata closure, which leads to the decreased CO2 intake, affecting the rate of photosynthesis and consequently reduces growth and yield (Chaves et al., 2009). However, in some cases, stomata conductance is not always associated with the rate of photosynthesis, but this still needs to be elucidated (Von Caemmerer et al., 2004; Xu et al., 2010). Photosynthesis is severely affected along with growth as the water deficit increases gradually in the field of cotton. For example, it was found that photosynthesis as well as transpiration was affected under drought conditions in cotton (Deeba et al., 2012; Li et al., 2012). Interestingly, it has been reported that young leaves of cotton are photosynthetically more tolerant to drought and heat as compared to mature leaves. When young leaves were subjected to high temperature (37 °C), no decline was observed in net photosynthesis. In contrast, mature leaf net photosynthesis declined 66% under the same conditions (Chastain et al., 2016). In another field study of cotton for two consecutive growing seasons, a decreased lint yield was observed as net photosynthesis declined under water‐deficit conditions in the first growing season. However, no change was observed in the yield of drought‐treated field due to high rainfall in the next growing season (Chastain et al., 2014). These studies revealed that drought stress reduces photosynthesis in cotton which in turn affects growth and yield.
Osmotic adjustment
At the cellular level, water deficit affects turgidity and osmotic balance in the cell. Osmotic adjustment is a critical adaptation to reduce the effects of drought‐induced damage in crop plants. Plant defence mechanisms also include osmoprotectants or osmolytes that regulate homoeostasis following drought and salinity stress on a cellular level. Drought stress has negative effects on osmotic balance, and therefore, plants accumulate different organic and inorganic substances to reduce the osmotic potential in response to drought stress (Fang and Xiong, 2015). Numerous organic compounds, including amino acids (proline, glycine), sugars (trehalose, fructan), sugar alcohols (mannitol, sorbitol, D‐ononitol), amines and polyamines (polyamine, betaines), polyols, ectoine, alkaloids and inorganic ions, known as osmoprotectants/osmolytes, are involved in osmotic adjustment (Fang et al., 2015; Singh et al., 2015). These solutes assist in protecting proteins and membranes from the damage due to high concentrations of inorganic ions and oxidative damage under drought stress (Chen and Murata, 2011) and multiple stresses, such as drought and salinity (Khan et al., 2015). The exogenous application of osmoprotectants (proline and glycinebetaine) has been shown to be effective in reducing the harmful effects of drought stress in cotton (Noreen et al., 2013). The transgenic cotton plants with enhanced glycinebetaine accumulation were more tolerant to drought stress than control plants and had increased photosynthesis, higher relative water content, increased osmotic adjustment, lower lipid membrane peroxidation and a lower percentage of ion leakage (Lv et al., 2007). Ectopic expression of a mustard annexin gene, AnnBj1, enhanced proline content and sucrose, which increased drought tolerance in cotton (Divya et al., 2010). Moreover, overexpression of GhAnn1, a cotton annexin gene, enhanced the tolerance to drought and salt by increasing the activity of superoxide dismutase (SOD) and elevated levels of proline and soluble sugars (Zhang et al., 2015).
Biochemical and molecular mechanism of drought tolerance in cotton
Similar to animals, plants also have a defence mechanism through which they respond to various biotic and abiotic stresses. The drought tolerance mechanism is very complex because it is a multigenic system that is related to various morpho‐physiological, biochemical and molecular processes (Figure 3). Other than morpho‐physiological mechanism, cotton has evolved several signal transduction pathways in response to drought stress.
Figure 3.
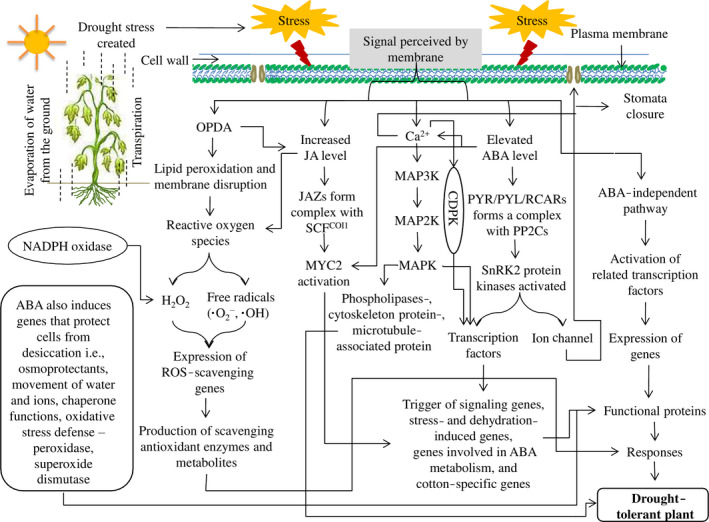
Various signalling pathways connectively enhance drought tolerance in cotton. These pathways work together to maintain their normal activities under drought stress.
Abscisic acid (ABA)
ABA is one of the most important stress hormones and participates in various crucial physiological processes during the plant life cycle, including stress responses, development and reproduction. Studies indicate that osmotic stress occurs due to high drought conditions or salt stress or when water availability is reduced through water loss and turgor pressure (Boudsocq and Lauriere, 2005). Osmotic stress promotes the synthesis of ABA, which activates gene expression and adaptive physiological changes (Yamaguchi‐Shinozaki and Shinozaki, 2006). After stress signal perception by the plasma membrane, ABA synthesis is initiated, which occurs mostly in the plastids, with the exception of xanthoxin conversion to ABA, which takes place in the cytoplasm (Seo and Koshiba, 2002). Generally, ABA synthesis occurs in the roots. It is then transported via vascular tissues, and it shows stomatal closure responses in a variety of cells, such as guard cells (Kuromori et al., 2010). As in other plants, perception and signal transduction of ABA in cotton are mediated by two pathways, which are ABA‐dependent and ABA‐independent. ABA‐dependent signalling pathways play a critical role in stress‐responsive gene expression during various stresses, especially osmotic stress. ABA receptors are important elements for ABA signal transduction. Various receptors have been identified in different subcellular compartments, including the plasma membrane, nucleus, cytosol and chloroplast envelope. Under normal conditions, ABA content is low, and sucrose nonfermenting 1‐related protein kinase 2 (SnRK2) protein activity is inhibited by protein phosphatase 2C (PP2C), which leads to dephosphorylation. When plants suffer drought stress, the cellular ABA level increases, and ABA then binds to PYR/PYL/RCARs, which in turn bind and inactivate PP2Cs. The SnRK2s are autoactivated when they dissociate from PP2Cs. Activated SnRK2s phosphorylate downstream targets and trigger ABA‐induced physiological and molecular responses (Danquah et al., 2014; Dong et al., 2015; Mehrotra et al., 2014; Yoshida et al., 2014)). ABA regulates many stress‐related genes to enhance drought tolerance in cotton plants (Figure 4). Overexpressing an ABA‐induced cotton gene GhCBF3 in Arabidopsis enhanced drought and salinity tolerance in transgenic lines, with higher proline content, relative water content and chlorophyll content in transgenic lines than those in wild type. In the presence of ABA, stomatal aperture was smaller in transgenic lines, and expression level of AREB1 and AREB2 was remarkably higher than wild type. They suggested that GhCBF3 enhance drought and salt tolerance via ABA signalling pathway (Ma et al., 2016).
Figure 4.
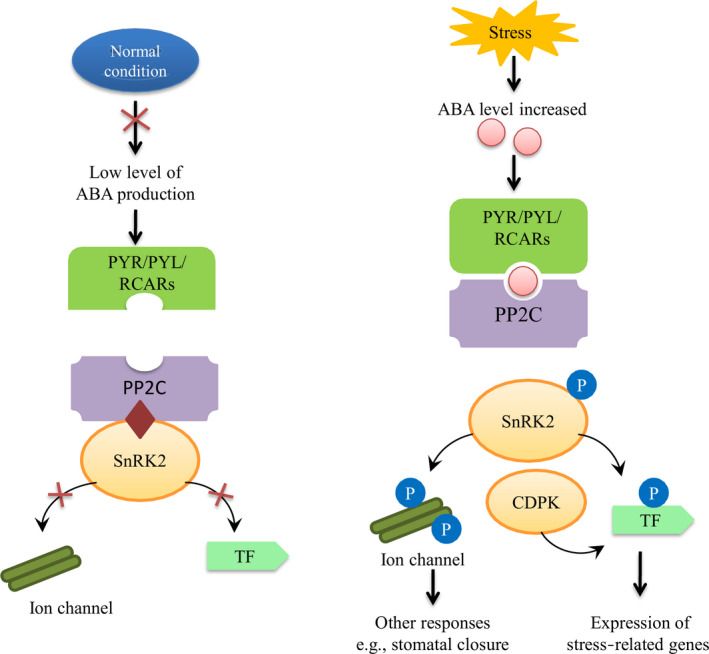
ABA mediated signalling pathway during normal and stress conditions. Under normal conditions, ABA content is low, and SnRK2 protein kinase activity is inhibited by PP2C phosphatases. Under drought stress, the cellular ABA level increases, and ABA then binds to PYR/PYL/RCARs, which in turn bind and inactivate PP2Cs. The SnRK2s autoactivate when they dissociate from PP2Cs. Activated SnRK2s phosphorylate downstream targets and trigger ABA‐induced physiological and molecular responses.
Jasmonic acid (JA)
Jasmonic acid (JA) is another phytohormone derived from α‐linolenic acid. JA and its active derivatives, which are known as jasmonates, have a significant role in regulating stress responses of plants to various biotic as well as abiotic stresses. In addition to plant growth and development, JA is also involved in root growth, fruit ripening, tendril coiling and viable pollen production (Wasternack, 2007). JA has been shown to participate in the response to drought conditions. Genomewide functional analyses of cotton were performed to analyse the molecular mechanism of drought resistance, and they identified various genes related to JA signalling pathways (Chen et al., 2013). Tan et al. (2012) reported that JA application inhibited fibre elongation in cotton. Similarly, several studies have also shown that exogenous application of jasmonates enhances plant resistance to water‐deficit conditions (Bandurska et al., 2003). Similar to ABA, various studies have shown that jasmonates also participate in the regulation of stomatal closure (Riemann et al., 2015).
Although JA signalling pathway has not been fully elucidated, its biosynthesis and signalling pathway have been reviewed extensively in the last few years (Ahmad et al., 2016; Kombrink, 2012; Wasternack and Hause, 2013). The jasmonate‐zim domain (JAZ) repressor proteins have a key role in the JA signalling pathway—they function as a switch for JA signalling. In normal conditions, when JA is absent, jasmonate‐insensitive/jasmonate‐zim (JAI3/JAZ) proteins bind to various transcription factors, including MYC2 (Myelocytomatosis), and limit their activity. However, during stress conditions, when JA and its derivatives are present, degradation of JAZ proteins occurs as described above, resulting in active transcription factors (MYC2) that up‐regulate genes involved in stress responses (Chini et al., 2007). The signalling pathway and JAZ protein interactions in this pathway have been comprehensively reviewed (Wager and Browse, 2012). Generally, plant hormones do not function in discrete pathways but rather depend on each other at different stages to control environmental as well as developmental pathways. This results in signal transduction that can assimilate various processes and respond to the stress in a complex way (Riemann et al., 2015). The jasmonates, similar to ABA signalling, act as a hub where different processes are initiated to appropriately respond to drought stress.
Reactive oxygen species (ROS)
Partial reduction of atmospheric O2 leads to the production of ROS, also known as active oxygen species (AOS) or reactive oxygen intermediates (ROI). Cellular ROS basically consist of four forms, hydrogen peroxide (H2O2), the hydroxyl radical (HO•), superoxide anion radical (O2−) and singlet oxygen (1O2). Two of these forms are especially very reactive, that is HO• and 1O2. They can harm and oxidize various components of the cell, such as lipids, proteins, DNA and RNA. Eventually, they can result in cell death if the oxidation of cellular components is not controlled (Fang et al., 2015). Subcellular locations, such as the mitochondria, plasma membrane, cell wall, chloroplast and nucleus, are responsible for the production of ROS (Gill and Tuteja, 2010). Under drought stress, the production of these ROS increases in various ways. For example, a reduction in CO2 fixation leads to decreased NADP+ regeneration during the Calvin cycle, which will reduce the activity of the photosynthetic electron transport chain. Moreover, during drought conditions, there is excessive leakage of electrons to O2 by the Mehler reaction during photosynthesis (Carvalho, 2008). The Mehler reaction reduces O2 to O2− by donation of an electron in photosystem I. O2− can be converted to H2O2 by SOD which can be further converted to water by ascorbate peroxidase (Heber, 2002). However, it is difficult to evaluate the levels of ROS produced during the Mehler reaction compared to those generated through photorespiration. Drought conditions also enhance the photorespiratory pathway, particularly when RuBP oxygenation is high due to limited CO2 fixation. Noctor et al. (2002) found that approximately 70% of total H2O2 production occurs through photorespiration under drought stress.
Plants have developed complicated scavenging mechanisms and regulatory pathways to monitor the ROS redox homoeostasis to prevent excess ROS in cells. Alterations in antioxidant enzyme metabolism could influence drought tolerance in cotton. The defence mechanism against ROS has been reviewed in detail by Das and Roychoudhury (2014). The antioxidant machinery has been developed by the plants to ensure survival (Figure 5). It has two arms, (i) enzymatic components, such as catalase (CAT), SOD, ascorbate peroxidase (APX), glutathione reductase (GR), guaiacol peroxidase (GPX), dehydroascorbate reductase (NADH) and monodehydroascorbate reductase (MDAR), and (ii) nonenzymatic antioxidants, such as reduced glutathione (GSH), ascorbic acid (AA), α‐tocopherol, flavonoids, carotenoids and the osmolyteproline (Figure 5). To scavenge ROS, these two arms/components work together (Das and Roychoudhury, 2014; Heiber et al., 2007; Wu et al., 2015). APX, along with MDAR, NADH and GR, removes H2O2 via the Halliwell–Asada pathway (Uzilday et al., 2012). APOX reduces H2O2 to water by oxidizing ascorbate to MDHA and thus plays a key role in the ascorbate–glutathione cycle (de Azvedo Neto et al., 2006). MDHA is then reduced to ascorbate by MDHAR. However, two molecules of MDHA can be nonenzymatically converted to MDHA and dehydroascorbate, which is further reduced to ascorbate via the NADH and GR cycle (Szalai et al., 2009). In this cycle, glutathione (GSH) is reduced by GR oxidation to oxidized glutathione at the expense of NADPH (nicotinamide adenine dinucleotide phosphate). Glutathione reductase activity increased during drought stress to keep oxidized and reduced glutathione ratios at adequate level (Chan et al., 2013). The balance between ROS production and antioxidative enzyme activities determines whether oxidative signalling and/or damage will occur (Zhang et al., 2014c). The antioxidative capability of different cotton cultivars determines the resistance capability to drought stress. The drought‐tolerant cultivar M‐503 has constitutively active antioxidative enzymes, including SOD, APX, CAT and POX, which decrease the oxidative stress induced by lipid peroxidation (Sekmen et al., 2014). In cotton, drought induced the production of ROS, but on the other hand, the APX and GR activities also increased and maintained the ROS scavenging process until the plant recovered from stress conditions (Ratnayaka et al., 2003). Supplemental Zn in cotton contributed to alleviating oxidative injuries under polyethylene glycol‐simulated (PEG) drought stress because it enhanced SOD, CAT, APX activities and the content of nonenzymatic antioxidants (Wu et al., 2015). In another example, Zhang et al. (2014c) conducted an experiment on the cotton cultivars: drought‐resistant (CCRI‐60) and drought‐sensitive (CCRI‐27). They found that the CCRI‐60 cultivar was drought tolerant due to increased root length and vigour, antioxidant enzyme activities and significantly increased GR activity and proline content. CCRI‐60 has the ability to scavenge free radicals and provides better protection compared to CCRI‐27; thus, it is more resistant to drought and has increased growth. Down‐regulation of GbMYB5 in G. barbadense resulted in decreased antioxidant enzyme activities such as, SOD, peroxidase (POD), CAT and glutathione S‐transferase (GST), and increased oxidative stress under drought conditions (Chen et al., 2015a). These results show that cotton has numerous genes involved in the antioxidant enzyme‐related pathways that need to be explored in drought‐tolerant cultivars. Moreover, other factors are also involved in improving the antioxidant machinery of cotton plants, such as Zn, (Wu et al., 2015).
Figure 5.
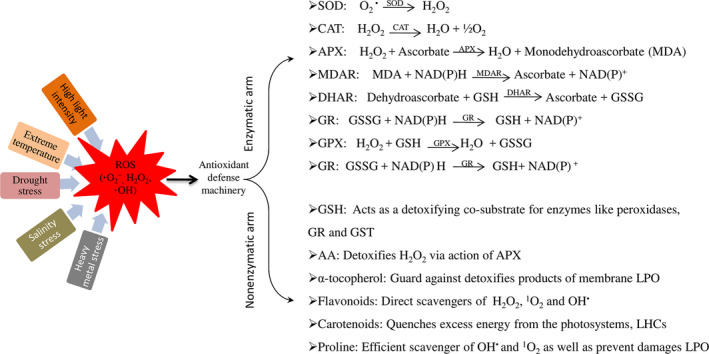
ROS scavenging machinery having two arms: enzymatic arm and nonenzymatic arm. Enzymatic arm contents on various enzymes which converting ROS into other substances. Likewise, Nonenzymatic arm content on other substances which scavenge ROS.
MAPK signalling pathway
Plants have developed various adaptations to environmental stresses that function through a series of molecular networks consisting of stress perception, signal transduction and expression of specific stress‐related genes. The mitogen‐activated protein kinase (MAPK) cascade is one of the key strategies developed by plants against multiple biotic and abiotic stresses that participates in signal transduction of extracellular stimuli and regulates responses. MAPK pathway is a highly conserved central regulator of various processes, including developmental programs, hormonal responses, cell division and apoptosis, proliferation and stress responses. A MAPK cascade is minimally composed of at least three distinct protein kinases, that is MAPKKK, MAPKK and MAPK, which activate each other in a sequential manner via phosphorylation (Ichimura et al., 2002). An activated MAPKKK first phosphorylates two serine and/or threonine residues in a conserved S/T‐X3‐5‐S/T motif located within the activation loop of the MAPKK. The activated MAPKK in turn phosphorylates MAPK on threonine and tyrosine residues in the invariant T‐X‐Y motif in the activation loop, and then MAPK phosphorylate specific targets and modulate the activity of other kinases, transcription factors, phospholipases, cytoskeletal proteins and microtubule‐associated proteins, whose altered activities mediate an extensive range of response (Danquah et al., 2014; Nakagami et al., 2005; Popescu et al., 2009).
To date, many reports have confirmed that MAPKs are involved in plant signal transduction in response to abiotic stresses, such as drought, salinity, cold and oxidative stress, in Arabidopsis and rice (Ning et al., 2010; Shen et al., 2012; Teige et al., 2004; Xing et al., 2008, 2015). In recent years, several genes involved in the MAPK pathway response to abiotic stresses have been identified in cotton (Table 2). Transcriptome analysis revealed that MAPK components are activated by diverse abiotic stresses, such as ABA, cold, drought and pH treatments (Zhu et al., 2013). Twenty‐eight putative MAPK genes distributed on 11 chromosomes were identified in the G. raimondii genome by performing a bioinformatics homology search. These MAPK genes are classified into the four known A, B, C and D groups and have diverse functions (Zhang et al., 2014b). From the above analyses, we conclude that MAPK signalling pathways are involved in the response to multiple environmental stresses in cotton. However, there are no reports on improving plant stress tolerance by engineering MAPK cascades in cotton thus far. We recommend improving cotton stress tolerance by engineering MAPK cascades, and we believed that MKK1, MKK3, MPK6 etc. (Table 2) are effective candidates to improve the tolerance against abiotic stresses because it have been evaluated in Arabidopsis and tobacco.
Table 2.
List of cotton MAPK genes engineered in other plants
| Name | Induced by stress | Transgenic plant | Phenotype/Result | Interaction | References |
|---|---|---|---|---|---|
| GhMKK3 | Drought | N. benthamiana | Enhanced drought tolerance | GhMPK7 and GhPIP1 | Wang et al. (2016) |
| GhMAP3K40 | Low temperature, NaCl, PEG, H2O2 | N. benthamiana | Enhanced drought and salt tolerance at the germination stage but reduced drought and oxidative stresses tolerance at the seedling stage | GhMKK4 | Chen et al. (2015b) |
| GhMPK4 | High salinity, osmotic stress | A. thaliana | Enhanced the sensitivity to salt, osmotic stresses and exogenous ABA | – | Wang et al. (2015) |
| GhMKK4 | NaCl, mannitol, ABA | N. benthamiana | Had no significant effects on salt or drought tolerance | – | Li et al. (2014) |
| GhMPK17 | NaCl, mannitol, ABA | A. thaliana | Enhanced plant tolerance to salt and osmotic stresses | – | Zhang et al. (2014a) |
| GbMPK3 | NaCl, cold, heat, dehydration, oxidative stress | N. benthamiana | Enhanced drought and oxidative stress tolerance | – | Long et al. (2013) |
| GhMPK6a | Cold, NaCl, PEG | N. benthamiana | Reduced drought and salt tolerance | GhMKK4 | Li et al. (2013) |
| GhMKK1 | NaCl, drought, H2O2 | N. benthamiana | Enhanced salt and drought tolerance | – | Lu et al. (2013) |
| GhMKK5 | Low temperature, NaCl, Wounding | N. benthamiana | Reduced the tolerance to salt and drought stresses | – | Zhang et al. (2012) |
| GhMPK2 | ABA, NaCl, PEG, dehydration | N. tobacum | Reduced sensitivity to ABA, enhanced drought and salt tolerance | – | Zhang et al. (2011) |
| GhMPK6 | ABA, NaCl, drought stresses | Arabidopsis mutant atmkk1 | Recovers the wild‐type phenotype of atmkk1 mutant | – | Luo et al. (2011) |
| GhMPK16 | Low and high temperatures, mannitol, NaCl | Arabidopsis | Reduced drought tolerance | – | Shi et al. (2011) |
Calcium signalling pathway
Calcium is a key regulator of various cellular and physiological processes in higher plants. In signal transduction pathways, Calcium (Ca2+) is a universal second messenger that regulates a variety of physiological processes in cotton plants. In addition to other stresses and hormones, drought and ABA are involved in changes of cytoplasmic Ca2+ concentration (Li et al., 2015). Plant cellular calcium signals are detected and transmitted by three major classes of Ca2+ sensor molecules: calmodulin (CaM) and CaM‐related proteins, calcium‐dependent protein kinase (CDPK) and calcineurin B‐like proteins (CBLs). Calmodulin is acidic calcium‐binding protein that contains four EF hand motifs (helix‐loop‐helix structural domains that coordinate with Ca2+ ions). When Ca2+ bind to EF motif, conformational transformation undergoes in CaM that either promotes its own catalytic activity or its interactions with downstream target proteins. As long as calcium sensor genes related to CaM and CaM‐related proteins have been studied for cotton fibre elongation (Cheng et al., 2016; Tang et al., 2014), there are no such reports found on drought stress in cotton. It has been noted that only a few CDPKs in cotton have been characterized extensively. GhCPK1 was identified for the first time that has role in calcium signalling events associated with fibre elongation (Huang et al., 2008). Wang et al. (2012) opened a new door after sequencing the draft genome of G. raimondii. Last year, Li et al. (2015) identified 41 CDPKs gene from the G. raimondii genome. Their study revealed that GhCDPK3, GhCDPK2, GhCDPK11, GhCDPK16, GhCDPK28, GhCDPK35 and GhCDPK14 genes are involved in drought and salt stress. Further, they noted that these genes also respond to ABA. CBLs proteins are another group of calcium sensor which are specific to higher plants and play a significant role in decoding calcium transients by specifically interacting with and regulating a unique family of CBL‐interacting protein kinases (CIPKs). GhCIPK6 was induced by drought, salt and ABA treatments. In addition, overexpression of GhCIPK6 in Arabidopsis significantly enhanced tolerance to drought, salt and ABA stresses (He et al., 2013). These reports indicate that change in the Ca2+ concentration transduce Ca2+ signals via CaMs, CDPKs and CBLs, which phosphorylate downstream targets and subsequently respond to drought and other abiotic stresses.
Stress‐related transcription factors
Transcription factors are master regulators of normal cellular processes as well as respond to biotic and abiotic stresses. Plants including cotton respond and/or adapt to various stresses, and transcriptional modulation is one of the most important ways that induce or repressed a number of genes in plants under biotic and abiotic stresses. Transcription factors play a significant role in the stress signalling, from the perception of drought to the stress‐responsive gene expression by interacting with _cis_‐acting elements present in the promoter region of numerous drought stress‐responsive genes in the signal transduction processes. In this way, transcription factors activate signalling cascade of entire network of drought stress‐responsive genes that operate together in inducing plant tolerance to drought and other abiotic stresses (Guo et al., 2016). As a model plant, more than 1500 transcription factors of the Arabidopsis genome are thought to be involved in stress‐responsive gene expression (Lata and Prasad, 2011).
To increase the tolerance in cotton against drought stress, transcription factors are excellent candidates for the plant scientists. Various transcription factors (such as MYB, WRKY, ERF, NAC, bZIP) are involved in normal development as well as in stress (drought) response (Table 3). These transcription factors have been cloned and proven useful tool for stress tolerance in cotton and/or in other plants. The genetic engineering of transcription factor genes could activate drought tolerance pathways and enhance drought tolerance in cotton. Recently, a bZIP transcription factor gene, GhABF2, has been reported to be involved in the drought and salt tolerance in Arabidopsis and cotton. The transcriptomic analysis revealed that _GhABF2_‐regulating genes related to ABA. Overexpressing GhABF2 cotton increased SOD and CAT activities as compared to wild‐type plants. Moreover, overexpressed plants showed better results in the field and meanwhile its yield were recorded higher than wild‐type plants (Liang et al., 2016). In another case, an R2R3‐type MYB transcription factor gene, GbMYB5, positively involved in response to drought stress in cotton. Overexpressing GbMYB5 tobacco reduced water loss by decreasing the stomatal size and showed hypersensitivity to ABA, and survival rate was higher after drought treatment. In addition, proline content and antioxidant enzymes were enhanced, while malondialdehyde (MDA) production was lower in transgenic lines than in wild‐type plants. Furthermore, the transcript level of drought‐responsive genes (NCED3, BG, RD26), antioxidant genes (SOD, CAT, GST) and polyamine biosynthesis genes (ADC1, SAMDC) were generally higher in _GbMYB_‐overexpressing tobacco (Chen et al., 2015a). Similarly, tobacco plants with ectopic‐expressing GhWRKY41 gene showed higher antioxidant enzyme activity, enhanced stomatal closure and reduced MDA content. In addition, the expression of antioxidant genes was also up‐regulated in transgenic plants exposed to osmotic stress. These characteristics of transgenic plants enhanced plant tolerance to drought stress (Chu et al., 2015).
Table 3.
Transcription factors in cotton playing important role in drought and other abiotic stresses
| Genes encoding transcription factors | Expressing plant | Mode of expression | Environmental condition | Beneficial features under drought and other abiotic stress | Abiotic stress type | References |
|---|---|---|---|---|---|---|
| GhABF2 (bZIP) | A. thaliana and G. hirsutum | Overexpressed and silenced | Greenhouse and field | Regulated genes related to ABA, Increased the activities of SOD and CAT | Drought and salt | Liang et al. (2016) |
| GhNAC2 | A. thaliana and G. hirsutum | Overexpressed | Greenhouse | Higher root length, | Drought | Gunapati et al. (2016) |
| GbMYB5 | G. barbedensis and N. tobacum | Overexpressed and silenced | Greenhouse | Reduced stomatal size, rate of its opening and water loss, while proline content and antioxidant enzymes increased | Drought and salt | Chen et al. (2015a) |
| GhWRKY41 | N. benthamiana | Overexpressed | Greenhouse | Induced stomatal closure, higher antioxidant activity and lower malondialdehyde content | Drought and salt | Chu et al. (2015) |
| GhWRKY17 | N. benthamiana | Overexpressed | Greenhouse | Impaired ABA‐induced stomatal closure, Reduced ABA level, decreased the expression of ROS scavenging genes, reduced proline content, elevated electrolyte leakage, and malondialdehyde | Drought and salt | Yan et al. (2014) |
| GhNAC8‐GhNAC17 | G. hirsutum | Up‐regulation | Greenhouse | NA | Drought, salt, heat and Cold | Shah et al. (2013) |
| GhERF1 | G. hirsutum | Up‐regulation | Greenhouse | Signal regulation during stress and ABA production | Drought, salt and Cold | Qiao et al. (2008) |
| GhERF4 | G. hirsutum | Up‐regulation | Greenhouse | Signal regulation during stress and ABA production | Drought, salt and Cold | Jin and Liu (2008) |
Strategies to induce drought tolerance in cotton
To enhance plant tolerance as well as vigour against drought, alternative solutions must be developed. In this way, we can maintain crop yields under extreme environmental conditions to overcome economic losses. Improvements in cotton productivity are urgently needed, especially in the areas where water availability is scarce. In this regard, cotton crops that require less water but produce higher yields and better fibre quality will be highly desirable. Cotton characteristics should be site‐specific according to the environmental conditions of that area for instance, an area having less rainfall need drought‐tolerant variety of cotton but on other side, saline area need a salt‐tolerant variety of cotton. Along with traditional breeding, development in the field of biotechnology can produce transgenic cotton that performs better in current and future environmental conditions. However, exogenous application of particular substances, including growth regulators, specific osmoprotectants and required minerals, can enhance drought tolerance in otherwise susceptible plants. The aim was to produce more cotton yield per drop, and in this regard, crucial approaches are needed to identify and enhance drought‐tolerant traits, such as quantitative trait loci (QTL) analysis, transgenic approaches and exogenous application of substances.
Marker‐assisted selection (MAS) based on drought‐related QTLs/genes
Various minor genes, that is polygenes, have stronger additive effects against drought tolerance than other biotic and abiotic stresses. Thus, the sections of DNA (locus) located on chromosomes carrying these genes are known as quantitative trait loci (QTL). Natural genetic variability of a crop can be utilized either via direct selection under stress environments whether simulated or natural or through QTL mapping (polygenes) and subsequent marker‐assisted selection (MAS) (Ashraf et al., 2008; Ashraf, 2010). QTL mapping allows us to determine the location, numbers, degree of phenotypic effects and gene action pattern (Vinh and Paterson, 2005). The role of polygenes has been extensively evaluated using traditional methods, but DNA markers as well as QTL mapping have made it possible and convenient to analyse complex traits (Ashraf, 2010). Biological and proteomics analyses have identified drought tolerance‐related QTLs and proteins in crop plants. Furthermore, these drought‐related QTLs and proteins can be used as markers in breeding programmes to develop drought‐tolerant genotypes.
In cotton, using F3 families derived from the cross of G. barbadense cv. F‐177 and G. hirsutum cv. Siv'on, a subset of 33 QTLs identified under water‐deficit conditions, that is five QTLs for different physiological traits, 11 for plant productivity and 17 for fiber quality. Most of these QTLs were located on chromosome c2, 6 and 14 (Saranga et al., 2001). Based on marker‐assisted selection, near‐isogenic lines were produced by exchanging QTL for drought‐ and some yield‐related traits between G. barbadense cv. F‐177 and G. hirsutum cv. Siv'on (Levi et al., 2009a,b). Moreover, metabolite and mineral analyses were conducted for these two species with QTLs for drought‐ and productivity‐related traits. The G. hirsutum cv. Siv'on showed higher levels of metabolites under drought and well‐water conditions compared to G. barbadense cv. F‐177. Under drought stress, Siv'on (Gh) had higher mineral and metabolite content and greater water use efficiency. Moreover, Siv'on also showed stable photosynthesis and a greater assimilation rate than F‐177 under drought conditions. For most of the studied traits, Siv'on showed a marked adaptation to drought (Levi et al., 2011). In another QTL study, five QTLs for osmotic potential (two QTLs were on chromosome c1, while rest of three were on c2, 6 and 25 each contained one), three for chlorophyll (two were on c2 and one on c14), 25 for leaf morphology and various others for yield and biotic stress were identified in cotton. QTLs for leaf morphology were distributed across the genome which is associated with leaf size and shape. Most notably, chromosome c15 contained six QTLs, c17 contained four, c6 contained three and c1 and c9 contained two QTLs each, while c2, c3, c4, c10, c12, c18, c22 and c25 all contained one QTL each (Said et al., 2013). In addition, 106 microsatellite markers were used to investigate 323 G. hirsutum germplasms, treated by drought and salt, and 15 markers were found related to drought tolerance. For the drought tolerance, 12 markers showed negative allele affects and the remaining markers showed positive allele effects (Jia et al., 2014). Likewise, a field study conducted for two consecutive years under water‐deficit and well‐water conditions, 11 physiological and morphological traits were recorded. As a result of QTL mapping, 67 and 35 QTLs were identified under water‐deficit and well‐water conditions, respectively. Most notably, chromosome c16, c9 and c2 contained 13, 12 and 7 QTLs, respectively (Zheng et al., 2016).
Transgenic approach
Plants respond to multiple abiotic stress conditions at the molecular level by altering gene expression (up‐ or down‐regulation), which further regulates a number of proteins, and as a result, various biological functions are altered (Deeba et al., 2012). The regulation of genes involved in the stress response is one of the key factors in plants that cope with abiotic stresses and enhance tolerance against these conditions (Hozain et al., 2012). There are thousands of genes in plants, and a number of them are involved in drought stress. Different techniques were used to identify specific genes such as, the amplified fragment length polymorphism (AFLP) was used by Park et al. (2012), who identified several genes expressed under drought stress in cotton (G. hirsutum L.). In their study, heat‐shock protein‐related and ROS‐related transcripts were induced by water deficit. In another case, various stress‐related genes were identified by constructing normalized cDNA libraries of G. barabadense regarding drought‐, salt‐, heat‐, cold‐ and phosphorus‐deficit stresses (Zhou et al., 2016). It is possible to transfer specific traits or gene of interest, that is drought‐tolerant genes, from an organism of interest into another organism to obtain the desired characteristic by genetic engineering or biotechnology (Herdt, 2006). Recently, scientists transformed various drought‐tolerant genes into cotton, resulting in drought‐resistant plants (Table 4). Overexpressing of TsVP, an H+‐PPase gene from Thellungiella halophile in cotton improved shoot and root growth as compared to wild type. In addition, transgenic lines had higher chlorophyll content, improved photosynthesis and higher relative water content of leaves, and cell membrane damage was observed less than wild type. These properties improved root development and the lower solute potential resulting from higher solute content such as soluble sugars and free amino acids in the transgenic plants. These beneficial features enhanced drought tolerance in transgenic cotton, and seed cotton yield was 51% higher than wild‐type cotton plants (Lv et al., 2009). In another study, a gene, ScALDH21, from Syntrichia carninervis was transformed into cotton (Yang et al., 2016). Transgenic plants were checked in the green house as well as in the field for drought tolerance. Under field conditions, overexpressed transgenic lines showed greater plant height, larger bolls and greater fibre yield than wild type during different treatments of drought stress. It is due to improved proline and soluble sugars, greater photosynthetic rate and reduced lipid peroxidation in transgenic cotton as compared to wild type. These discoveries and other studies have led scientists to engineer drought‐tolerant plants (cotton) using genetic engineering methods, which are an effective technology at the present time. Thus, various transgenic plants, including cotton, have already been produced by inserting various stress‐related genes and then examined the plants for the specific traits, that is drought tolerance. However, field applications still need to be assessed because most of these experiments were performed in the laboratory and greenhouse conditions and did not produce appreciable results in the field.
Table 4.
Successful stories of genetically modified cotton with enhanced yield under drought stress
| Gene(s) | Promoter | Plant from which gene taken | Environmental condition | Abiotic stress type | Beneficial traits of transgenic cotton against drought stress | Effect on yield | References |
|---|---|---|---|---|---|---|---|
| ScALDH21 | CaMV 35S | Syntrichia caninervis | Greenhouse and field | Drought | Soluble sugar and proline content increased, higher peroxidase activity, reduced loss of net photosynthetic rate, reduced lipid peroxidation, greater plant height, larger bolls | Yield increased | Yang et al. (2016) |
| AtEDT1/HDG11 | CaMV 35S | A. thaliana | Laboratory Greenhouse and Field | Drought and salt | Soluble sugar and proline content increased, well‐developed roots, low stomatal density, increased ROS scavenging enzymes | 43% higher seeds | Yu et al. (2015) |
| SNAC1 | CaMV 35S | Rice | Greenhouse | Drought and salt | Enhanced proline content and root development, while transpiration rate decreased | 131% more bolls | Liu et al. (2014) |
| AVP1 | CaMV 35S | A. thaliana | Greenhouse and field | Drought and salt | Enhanced sequestration of ions and sugars into vacuole, reduced water potential, and enhanced root biomass | Increased 20% | Pasapula et al. (2011) |
| Osmotin | CaMV 35S | Tobacco | Greenhouse | Drought | Higher relative water content and proline level, while H2O2, lipid peroxidation, and electrolyte leakage were reduced | 57.6% more bolls | Parkhi et al. (2009) |
| TsVP | CaMV 35S | Thellungiella halophila | Greenhouse | Drought | Improved root and shoot growth, higher rate of photosynthesis and relative water content, while less cell membrane damaged | 42%–61% higher (Lumianyan 19) 27%–53% higher (Lumianyan 21) | Lv et al. (2009) |
| betA | CaMV 35S | Eschercia coli | Greenhouse | Drought | Increased photosynthesis, higher relative water content, better osmotic adjustment, less ion leakage and lipid membrane peroxidation | 3%–12% higher | Lv et al. (2007) |
Exogenous application of substances
Exogenous application of osmoprotectants and various plant growth regulators have been found effectively to enhance drought tolerance in cotton. Foliar application of osmoprotectants and plant hormones, including ABA, gibberellic acid (GA3), salicylic acid (SA), proline, glycinebetaine and polyamines, has been reported to relieve the effects of stress. These treatments elevated osmotic adjustment to improve turgor pressure and promoted accumulation of antioxidants to detoxify ROS, thus maintaining the integrity of membrane structures, enzymes and other macromolecules during drought conditions (Anjum et al., 2011). For example, the exogenous application of proline and glycinebetaine as a foliar spray has also been found to be effective in reducing the adverse effects of drought stress on cotton (Noreen et al., 2013). In this way, GA exogenous application enhanced the net rate of photosynthesis, transpiration rate and stomata conductance in cotton (Lichtfouse et al., 2009). Similarly, Zhao et al. (2013) exogenously sprayed ABA, JA and MeJA on cotton plants. GbRLK was differentially induced by JA and MeJA, but it was gradually up‐regulated when exposed to ABA treatment.
Concluding remarks and future perspectives
Currently, drought stress is responsible for extensive crop loss and will likely become worse in the future; thus, there is international interest in increasing drought‐tolerant crops. The goal of our study was to explore the mechanism of cotton under water‐deficit conditions. Scientists are attempting to induce drought tolerance in cotton as well as other important crops. The aim was to identify and enhance drought‐tolerant traits via QTL analysis, transgenic approaches and exogenous application of substances. Several genes have been identified and characterized by proteomic, transcriptomic and other omics that are induced by drought stress and the associated signalling and regulatory pathways in cotton plants. In comparison with Arabidopsis, the amount of data on drought‐regulated genes and their functions in cotton are inadequate. However, a few of these genes have been studied in cotton for their response to water‐deficit conditions, which is still in early stages. Transgenic cotton plants were mostly studied under greenhouse conditions or tested in the field under natural water‐deficit environments with a small amount (Table 4). It should be study in more realistic environment that is in the field that what really happens there. Usually, transgenic lines are developed by single gene transformation, which may not be as productive as if it had been developed by transferring a number of drought‐related genes. It seems interesting to transfer a number of prominent genes response to drought and yield in the same variety of cotton. The amount of data on drought‐associated cotton protein kinases is also limited. Only a few cotton protein kinases have been engineered and studied in Arabidopsis and tobacco (Table 2); however, there are no reports by engineering in cotton for enhancing drought tolerance. More work on cotton plants is needed, however, to link physiology, system biology and field performance. Understanding traits in cotton plants are associated with root architecture, stomatal conductance, photosynthesis and osmotic adjustment in drought stress. It is important to enhance the drought tolerance capability of cotton, and there is still much work to be performed to secure future generations from the upcoming crisis. Drought is a complex trait; however, rapid advances in the omics technologies will make it possible to use a system biology approach to understand cotton plants responses to drought stress and introduce drought‐tolerant cotton.
Acknowledgements
Funding was provided by the National Natural Science Foundation of China (31371675) and the Fundamental Research Funds for the Central Universities (2662016PY001). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. There is no conflict of interest.
References
- Ahmad, P. , Rasool, S. , Gul, A. , Akram, N.A. , Ashraf, M. and Gucel, S. (2016) Jasmonates: multifunctional roles in stress tolerance. Front. Plant Sci. 7, 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum, S.A. , Wang, L.C. , Farooq, M. , Hussain, M. , Xue, L.L. and Zou, C.M. (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 197, 177–185. [Google Scholar]
- Ashraf, M. , Athar, H.R. , Harris, P.J.C. and Kwon, T.R. (2008) Some prospective strategies for improving crop salt tolerance. Adv. Agron. 97, 45–110. [Google Scholar]
- Ashraf, M. (2010) Inducing drought tolerance in plants: recent advances. Biotechnol. Adv. 28, 169–183. [DOI] [PubMed] [Google Scholar]
- de Azvedo Neto, A.D. , Prisco, J.T. , Eneas‐Filho, J. , de Abreao, C.E.B. and Gomes‐Filho, E. (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation on leaves and roots of salt‐tolerant and salt‐sensitive maize genotypes. Environ. Exp. Bot. 56, 87–94. [Google Scholar]
- Bandurska, H. , Stroinski, A. and Kubis, J. (2003) The effect of jasmonic acid on the accumulation of ABA, proline and spermidine and its influence on membrane injury under water deficit in two barley genotypes. Acta Physiol. Plant 25, 279–285. [Google Scholar]
- Boudsocq, M. and Lauriere, C. (2005) Osmotic signaling in plants: multiple pathways mediated by emerging kinase families. Plant Physiol. 138, 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, M.D. (2008) Drought stress and reactive oxygen species. Plant Signal. Behav. 3, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, K.X. , Wirtz, M. , Phua, S.Y. , Estavillo, G.M. and Pogson, B.J. (2013) Balancing metabolites in drought: the sulfur assimilation conundrum. Trends Plant Sci. 18, 18–29. [DOI] [PubMed] [Google Scholar]
- Chastain, D.R. , Snider, J.L. , Collins, G.D. , Perry, C.D. , Whitaker, J. and Byrd, S.A. (2014) Water deficit in field‐grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. J. Plant Physiol. 171, 1576–1585. [DOI] [PubMed] [Google Scholar]
- Chastain, D.R. , Snider, J.L. , Choinski, J.S. , Collins, G.D. , Perry, C.D. , Whitaker, J. , Grey, T.L.et al. (2016) Leaf ontogeny strongly influences photosynthetic tolerance to drought and high temperature in Gossypium hirsutum. J. Plant Physiol. 199, 18–28. [DOI] [PubMed] [Google Scholar]
- Chaves, M.M. , Flexas, J. and Pinheiro, C. (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T.H. and Murata, N. (2011) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant, Cell Environ. 34, 1–20. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Liu, Z.H. , Feng, L. , Zheng, Y. , Li, D.D. and Li, X.B. (2013) Genome‐wide functional analysis of cotton (Gossypium hirsutum) in response to drought. PLoS ONE, 8, e80879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Li, W. , Hu, X. , Guo, J. , Liu, A. and Zhang, B. (2015a) A cotton MYB transcription factor, GbMYB5, is positively involved in plant adaptive response to drought stress. Plant Cell Physiol. 56, 917–929. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Wang, J. , Zhu, M. , Jia, H. , Liu, D. , Hao, L. and Guo, X. (2015b) A cotton Raf‐like MAP3K gene, GhMAP3K40, mediates reduced tolerance to biotic and abiotic stress in Nicotiana benthamiana by negatively regulating growth and development. Plant Sci. 240, 10–24. [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Lu, L. , Yang, Z. , Wu, Z. , Qin, W. , Yu, D. , Ren, Z.et al. (2016) GhCaM7‐like, a calcium sensor gene, influences cotton fiber elongation and biomass production. Plant Physiol. Biochem. 109, 128–136. [DOI] [PubMed] [Google Scholar]
- Chini, A. , Fonseca, S. , Fernández, G. , Adie, B. , Chico, J.M. , Lorenzo, O. , García‐Casado, G.et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature, 448, 666–671. [DOI] [PubMed] [Google Scholar]
- Chu, X. , Wang, C. , Chen, X. , Lu, W. , Li, H. , Wang, X. , Hao, L.et al. (2015) The cotton WRKY gene GhWRKY41 positively regulates salt and drought stress tolerance in transgenic Nicotiana benthamiana. PLoS ONE, 10, e0143022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas, L.H. , Becker, S.R. , Cruz, V.M.V. , Byrne, P.F. and Dierig, D.A. (2013) Root traits contributing to plant productivity under drought. Front. Plant Sci. 4, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danquah, A. , Zelicourt, A.D. , Colcombet, J. and Hirt, H. (2014) The role of ABA and MAPK signaling pathways in plant abiotic. Biotechnol. Adv. 32, 40–52. [DOI] [PubMed] [Google Scholar]
- Das, K. and Roychoudhury, A. (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS‐scavengers during environmental stress in plants. Front. Environ. Sci. 2, 53. [Google Scholar]
- Dawn news . (2016) http://www.dawn.com/news/1240448.
- Deeba, F. , Pandey, A.K. , Ranjan, S. , Mishra, A. , Singh, R. , Sharma, Y.K. , Shirke, P.A.et al. (2012) Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 53, 6–18. [DOI] [PubMed] [Google Scholar]
- Divya, K. , Jami, S.K. and Kirti, P.B. (2010) Constitutive expression of mustard annexin, AnnBj1 enhances abiotic stress tolerance and fiber quality in cotton under stress. Plant Mol. Biol. 73, 293–308. [DOI] [PubMed] [Google Scholar]
- Dong, T. , Park, Y. and Hwang, I. (2015) Abscisic acid: biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 58, 29–48. [DOI] [PubMed] [Google Scholar]
- Fang, Y. and Xiong, L. (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 72, 673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, Y. , Liao, K. , Du, H. , Xu, Y. , Song, H. , Li, X. and Xiong, L. (2015) A stress‐responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 66, 6803–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, S.S. and Tuteja, N. (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. [DOI] [PubMed] [Google Scholar]
- Gunapati, S. , Naresh, R. , Ranjan, S. , Nigam, D. , Hans, A. , Verma, P.C. , Pathre, U.V.et al. (2016) Expression of GhNAC2 from G. herbaceum, improves root growth and imparts tolerance to drought in transgenic cotton and Arabidopsis. Sci. Rep. 6, 24978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, M. , Liu, J.H. , Ma, X. , Luo, D.X. , Gong, Z.H. and Lu, M.H. (2016) The plant heat stress transcription factors (HSFs): structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 7, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, L. , Yang, X. , Wang, L. , Zhu, L. , Zhou, T. , Deng, J. and Zhang, X. (2013) Molecular cloning and functional characterization of a novel cotton CBL‐interacting protein kinase gene (GhCIPK6) reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 435, 209–215. [DOI] [PubMed] [Google Scholar]
- Heber, U. (2002) Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants. Photosyn. Res. 73, 223–231. [DOI] [PubMed] [Google Scholar]
- Heiber, I. , Stroher, E. , Raatz, B. , Busse, I. , Kahmann, U. , Bevan, M.W. , Dietz, K.J.et al. (2007) The redox imbalanced mutants of Arabidopsis differentiate signaling pathways for redox regulation of chloroplast antioxidant enzymes. Plant Physiol. 143, 1774–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnák, V. , Tatar, Ö. , Atasoy, G.D. , Martinková, J. , Çelen, A.E. , Hnilička, F. and Skalický, M. (2015) Growth and photosynthesis of Upland and Pima cotton: response to drought and heat stress. Plant Soil Environ. 61, 507–514. [Google Scholar]
- Herdt, R.W. (2006) Biotechnology in agriculture. Annu. Rev. Environ. Resour. 31, 265–295. [Google Scholar]
- Hetherington, A.M. and Woodward, F.I. (2003) The role of stomata in sensing and driving environmental change. Nature, 424, 901–908. [DOI] [PubMed] [Google Scholar]
- Hozain, M. , Abdelmageed, H. , Lee, J. , Kang, M. , Fokar, M. , Allen, R.D. and Holaday, A.S. (2012) Expression of AtSAP5 in cotton up‐regulates putative stress‐responsive genes and Improves the tolerance to rapidly developing water deficit and moderate heat stress. J. Plant Physiol. 169, 1261–1270. [DOI] [PubMed] [Google Scholar]
- Huang, Q.S. , Wang, H.Y. , Gao, P. , Wang, G.Y. and Xia, G.X. (2008) Cloning and characterization of a calcium dependent protein kinase gene associated with cotton fiber development. Plant Cell Rep. 27, 1869–1875. [DOI] [PubMed] [Google Scholar]
- Ichimura, K. , Shinozaki, K. , Tena, G. , Sheen, J. , Henry, Y. , Champion, A. , Kreis, M.et al. (2002) Mitogen‐activated protein kinase cascades in plants: a new nomenclature. Tends Plant Sci. 7, 301–308. [DOI] [PubMed] [Google Scholar]
- Iqbal, M. , Khan, M.A. , Naeem, M. , Aziz, U. , Afzal, J. and Latif, M. (2013) Inducing drought tolerance in upland cotton (Gossypium hirsutum L.), accomplishments and future prospects. World Appl. Sci. J. 21, 1062–1069. [Google Scholar]
- Jia, Y. , Sun, J. , Wang, X. , Zhou, Z. , Pan, Z. , He, S. , Pang, B.et al. (2014) Molecular diversity and association analysis of drought and salt tolerance in Gossypium hirsutum L. Germplasm. J. Integr. Agri. 13, 1845–1853. [Google Scholar]
- Jin, L.G. and Liu, J.Y. (2008) Molecular cloning, expression profile and promoter analysis of a novel ethylene responsive transcription factor gene GhERF4 from cotton (Gossypium hirstum). Plant Physiol. Biochem. 46, 46–53. [DOI] [PubMed] [Google Scholar]
- Khan, M.S. , Ahmad, D. and Khan, M.A. (2015) Utilization of genes encoding osmoprotectants in transgenic plants for enhanced abiotic stress tolerance. Elect. J. Biotechnol. 18, 257–266. [Google Scholar]
- Kombrink, E. (2012) Chemical and genetic exploration of jasmonate biosynthesis and signaling paths. Planta, 236, 1351–1366. [DOI] [PubMed] [Google Scholar]
- Kumar, B. , Pandey, D.M. , Goswami, C.L. and Jain, S. (2001) Effect of growth regulators on photosynthesis, transpiration and related parameters in water stressed cotton. Biol. Plantarum, 44, 475–478. [Google Scholar]
- Kuromori, T. , Miyaji, T. , Yabuuchi, H. , Shimizu, H. , Sugimoto, E. , Kamiya, A. , Moriyama, Y.et al. (2010) ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl Acad. Sci. USA, 107, 2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata, C. and Prasad, M. (2011) Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 62, 4731–4748. [DOI] [PubMed] [Google Scholar]
- Levi, A. , Ovnat, L. , Paterson, A.H. and Saranga, Y. (2009a) Photosynthesis of cotton near‐isogenic lines introgressed with QTLs for productivity and drought related traits. Plant Sci. 177, 88–96. [DOI] [PubMed] [Google Scholar]
- Levi, A. , Paterson, A.H. , Barak, V. , Yakir, D. , Wang, B. , Chee, P.W. and Saranga, Y. (2009b) Field evaluation of cotton near‐isogenic lines introgressed with QTLs for productivity and drought related traits. Mol. Breed. 23, 179–195. [Google Scholar]
- Levi, A. , Paterson, A.H. , Cakmak, I. and Saranga, Y. (2011) Metabolite and mineral analyses of cotton near isogenic lines introgressed with QTLs for productivity and drought‐related traits. Physiol. Plant. 141, 265–275. [DOI] [PubMed] [Google Scholar]
- Li, D. , Li, C. , Sun, H. , Liu, L. and Zhang, Y. (2012) Photosynthetic and chlorophyll fluorescence regulation of upland cotton (Gossiypium hirsutum L.) under drought conditions. Plant Omics J. 5, 432–437. [Google Scholar]
- Li, Y. , Zhang, L. , Wang, X. , Zhang, W. , Hao, L. , Chu, X. and Guo, X. (2013) Cotton GhMPK6a negatively regulates osmotic tolerance and bacterial infection in transgenic Nicotiana benthamiana, and plays a pivotal role in development. FEBS J. 280, 5128–5144. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zhang, L. , Lu, W. , Wang, X. , Wu, C.A. and Guo, X. (2014) Overexpression of cotton GhMKK4 enhances disease susceptibility and affects abscisic acid, gibberellin and hydrogen peroxide signalling in transgenic Nicotiana benthamiana. Mol. Plant Pathol. 15, 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L.B. , YU, D.W. , Zhao, F.L. , Pang, C.Y. , Song, M.Z. , Wei, H.L. , Fan, S.L.et al. (2015) Genome‐wide analysis of the calcium‐dependent protein kinase gene family in Gossypium raimondii. J. Integr. Agric. 14, 29–41. [Google Scholar]
- Liang, C. , Meng, Z. , Meng, Z. , Malik, W. , Yan, R. , Lwin, K.M. , Lin, F.et al. (2016) GhABF2, a bZIP transcription factor, confers drought and salinity tolerance in cotton (Gossypium hirsutum L.). Sci. Rep. 6, 35040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtfouse, E. , Navarrete, M. , Debaeke, P. , Souchère, V. , Alberola, C. and Menassieu, J. (2009) Agronomy for sustainable agriculture: a review. Agron. Sustain. Dev. 29, 1–6. [Google Scholar]
- Liu, G. , Li, X. , Jin, S. , Liu, X. , Zhu, L. , Nie, Y. and Zhang, X. (2014) Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS ONE, 9, e86895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loka, D.M. , Derrick, M. , Oosterhuis, D.M. and Ritchie, G.L. (2011) Water‐deficit stress in cotton. In Stress Physiology in Cotton ( Oosterhuis, D.M. , eds), pp. 37–72. Number Seven The Cotton Foundation Book Series. National Cotton Council of America. [Google Scholar]
- Long, L. , Gao, W. , Xu, L. , Liu, M. , Luo, X. , He, X. , Yang, X.et al. (2013) GbMPK3, a mitogen‐activated protein kinase from cotton, enhances drought and oxidative stress tolerance in tobacco. Plant Cell, Tissue Organ Cult. 116, 153–162. [Google Scholar]
- Lu, W. , Chu, X. , Li, Y. , Wang, C. and Guo, X. (2013) Cotton GhMKK1 induces the tolerance of salt and drought stress, and mediates defence responses to pathogen infection in transgenic Nicotiana benthamiana. PLoS ONE, 8, e68503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, L.J. (2010) Breeding for water‐saving and drought‐resistance rice (WDR) in China. J. Exp. Bot. 61, 3509–3517. [DOI] [PubMed] [Google Scholar]
- Luo, J. , Zhao, L.L. , Gong, S.Y. , Sun, X. , Li, P. , Qin, L.X. , Zhou, Y.et al. (2011) A cotton mitogen‐activated protein kinase (GhMPK6) is involved in ABA‐induced CAT1 expression and H2O2 production. J. Genet. Genomics, 38, 557–565. [DOI] [PubMed] [Google Scholar]
- Luo, H.H. , Zhang, Y.L. and Zhang, W.F. (2016) Effects of water stress and rewatering on photosynthesis, root activity, and yield of cotton with drip irrigation under mulch. Photosynthetica, 54, 65–73. [Google Scholar]
- Lv, S. , Yang, A. , Zhang, K. , Wang, L. and Zhang, J. (2007) Increase of glycinebetaine synthesis improves drought tolerance in cotton. Mol. Breed. 20, 233–248. [Google Scholar]
- Lv, S.L. , Lian, L.J. , Tao, P.L. , Li, Z.X. , Zhang, K.W. and Zhang, J.R. (2009) Overexpression of Thellungiella halophila H + ‐PPase (TsVP) in cotton enhances drought stress resistance of plants. Planta, 229, 899–910. [DOI] [PubMed] [Google Scholar]
- Ma, L. , Li, Y. , Chen, Y. and Li, X. (2016) Improved drought and salt tolerance of Arabidopsis thaliana by ectopic expression of a cotton (Gossypium hirsutum) CBF gene. Plant Cell Tiss. Organ Cult. 124, 583–598. [Google Scholar]
- Manavalan, L.P. , Guttikonda, S.K. , Tran, L.S.P. and Nguyen, H.T. (2009) Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 50, 1260–1276. [DOI] [PubMed] [Google Scholar]
- Mehrotra, R. , Bhalothia, P. , Bansal, P. , Basantani, M.K. , Bharti, V. and Mehrotra, S. (2014) Abscisic acid and abiotic stress tolerance – Different tiers of regulation. J. Plant Physiol. 171, 486–496. [DOI] [PubMed] [Google Scholar]
- Nakagami, H. , Pitzschke, A. and Hirt, H. (2005) Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 10, 339–346. [DOI] [PubMed] [Google Scholar]
- Ning, J. , Li, X. , Hicks, L.M. and Xiong, L. (2010) A Raf‐like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 152, 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor, G. , Veljovic‐Jovanovic, S. , Driscoll, S. , Novitskaya, L. and Foyer, C.H. (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann. Bot. 89, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreen, S. , Athar, H.U.R. and Ashraf, M. (2013) Interactive effects of watering regimes and exogenously applied osmoprotectants on earliness indices and leaf area index in cotton (Gossypium hirsutum L.) Crop. Pak. J. Bot. 45, 1873–1881. [Google Scholar]
- Parida, A.K. , Dagaonkar, V.S. , Phalak, M.S. , Umalkar, G. and Aurangabadkar, L.P. (2007) Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short‐term drought stress followed by recovery. Plant Biotechnol. Rep. 1, 37–48. [Google Scholar]
- Park, W. , Scheffler, B.E. , Bauer, P.J. and Campbell, B.T. (2012) Genome‐wide identification of differentially expressed genes under water deficit stress in upland cotton (Gossypium hirsutum L.). BMC Plant Biol. 12, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhi, V. , Kumar, V. , Sunilkumar, G. , Campbell, L.M. , Singh, N.K. and Rathore, K.S. (2009) Expression of apoplastically secreted tobacco osmotin in cotton confers drought tolerance. Mol. Breed. 23, 625–639. [Google Scholar]
- Pasapula, V. , Shen, G. , Kuppu, S. , Paez‐Valencia, J. , Mendoza, M. , Hou, P. and Payton, P. (2011) Expression of an Arabidopsis vacuolar H + ‐pyrophosphatase gene (AVP1) in cotton improves drought‐and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnol. J. 9, 88–99. [DOI] [PubMed] [Google Scholar]
- Popescu, S.C. , Popescu, G.V. , Bachan, S. , Zhang, Z. , Gerstein, M. , Snyder, M. and Dinesh‐Kumar, S.P. (2009) MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 23, 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Z.X. , Huang, B. and Liu, J.Y. (2008) Molecular cloning and functional analysis of an ERF gene from cotton (Gossypium hirsutum). Biochim. Biophys. Acta, 1779, 122–127. [DOI] [PubMed] [Google Scholar]
- Ratnayaka, H.H. , Molin, W.T. and Sterling, T.M. (2003) Physiological and antioxidant responses of cotton and spurred anoda under interference and mild drought. J. Exp. Bot. 54, 2293–2305. [DOI] [PubMed] [Google Scholar]
- Riaz, M. , Farooq, J. , Sakhawat, G. , Mahmood, A. , Sadiq, M. and Yaseen, M. (2013) Genotypic variability for root/shoot parameters under water stress in some advanced lines of cotton (Gossypium hirsutum L.). Gen. Mol. Res. 12, 552–561. [DOI] [PubMed] [Google Scholar]
- Riemann, M. , Dhakarey, R. , Hazman, M. , Miro, B. , Kohli, A. and Nick, P. (2015) Exploring Jasmonates in the Hormonal Network of Drought and Salinity Responses. Front. Plant Sci. 6, 1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said, J.I. , Lin, Z. , Zhang, X. , Song, M. and Zhang, J. (2013) A comprehensive meta QTL analysis for fiber quality, yield, yield related and morphological traits, drought tolerance, and disease resistance in tetraploid cotton. BMC Genom. 14, 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranga, Y. , Menz, M. , Jiang, C.X. , Wright, R.J. , Yakir, D. and Paterson, A.H. (2001) Genomic dissection of genotype x environment interactions conferring adaptation of cotton to arid conditions. Genome Res. 11, 1988–1995. [DOI] [PubMed] [Google Scholar]
- Sekmen, A.H. , Ozgur, R. , Uzilday, B. and Turkan, I. (2014) Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 99, 141–149. [Google Scholar]
- Seo, M. and Koshiba, T. (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci. 7, 41–48. [DOI] [PubMed] [Google Scholar]
- Shah, S.T. , Pang, C. , Fan, S. , Song, M. , Arain, S. and Yu, S. (2013) Isolation and expression profiling of GhNAC transcription factor genes in cotton (Gossypium hirsutum L.) during leaf senescence and in response to stresses. Gene, 531, 220–234. [DOI] [PubMed] [Google Scholar]
- Shen, H. , Liu, C. , Zhang, Y. , Meng, X. , Zhou, X. , Chu, C. and Wang, X. (2012) OsWRKY30 is activated by MAP kinases to confer drought tolerance in rice. Plant Mol. Biol. 80, 241–253. [DOI] [PubMed] [Google Scholar]
- Shi, J. , Zhang, L. , An, H. , Wu, C. and Guo, X. (2011) GhMPK16, a novel stress‐responsive group D MAPK gene from cotton, is involved in disease resistance and drought sensitivity. BMC Mol. Biol. 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, M. , Kumar, J. , Singh, S. , Singh, V.P. and Prasad, S.M. (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev. Environ. Sci. Biotechnol. 14, 407–426. [Google Scholar]
- Statista (2015) http://www.statista.com/statistics/263055/cotton-production-worldwide-by-top-countries/.
- Szalai, G. , Kellos, T. , Galiba, G. and Kocsy, G. (2009) Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 28, 66–80. [Google Scholar]
- Tan, J. , Tu, L. , Deng, F. , Wu, R. and Zhang, X. (2012) Exogenous jasmonic acid inhibits cotton fiber elongation. J. Plant Growth Regul. 31, 599–605. [Google Scholar]
- Tang, W. , Tu, L. , Yang, X. , Tan, J. , Deng, F. , Hao, J. , Guo, K.et al. (2014) The calcium sensor GhCaM7 promotes cotton fiber elongation by modulating reactive oxygen species (ROS) production. New Phytol. 202, 509–520. [DOI] [PubMed] [Google Scholar]
- Teige, M. , Scheikl, E. , Eulgem, T. , Doczi, R. , Ichimura, K. , Shinozaki, K. , Dangl, J.L.et al. (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell, 15, 141–152. [DOI] [PubMed] [Google Scholar]
- USDA (2015) http://www.ers.usda.gov/dataproducts/chartgallery/detail.aspx?chartId=52780.
- Uzilday, B. , Turkan, I. , Sekmen, A.H. , Ozgur, R. and Karakaya, H.C. (2012) Comparison of ROS formation and antioxidant enzymes in Cleome gynandra(C4) and Cleome spinosa(C3) under drought stress. Plant Sci. 182, 59–70. [DOI] [PubMed] [Google Scholar]
- Vinh, N.T. and Paterson, A.H. (2005) Genome mapping and its implication for stress resistance in plants. In Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches ( Ashraf, M. and Harris, P.J.C. , eds), pp. 725. Binghamton, NH, USA: The Haworth Press. [Google Scholar]
- Von Caemmerer, S. , Lawson, T. , Oxborough, K. , Baker, N.R. , Andrews, T.J. and Raines, C.A. (2004) Stomata conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J. Exp. Bot. 55, 1157–1166. [DOI] [PubMed] [Google Scholar]
- Wager, A. and Browse, J. (2012) Social network: JAZ protein interactions expand our knowledge of jasmonate signaling. Front. Plant Sci. 3, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Griffiths, R. , Ying, J. , McCourt, P. and Huang, Y. (2009) Development of drought‐tolerant (Brassica napus L.) through genetic modulation of ABA‐mediated stomata responses. Crop Sci. 49, 1539–1554. [Google Scholar]
- Wang, K. , Wang, Z. , Li, F. , Ye, W. , Wang, J. , Song, G. , Yue, Z.et al. (2012) The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet. 44, 1098–1103. [DOI] [PubMed] [Google Scholar]
- Wang, N.N. , Zhao, L.L. , Lu, R. , Li, Y. and Li, X.B. (2015) Cotton mitogen‐activated protein kinase4 (GhMPK4) confers the transgenic Arabidopsis hypersensitivity to salt and osmotic stresses. Plant Cell, Tissue Organ Cult. 123, 619–632. [Google Scholar]
- Wang, C. , Lu, W. , He, X. , Wang, F. , Zhou, Y. , Guo, X. and Guo, X. (2016) The cotton mitogen‐activated protein kinase kinase 3 functions in drought tolerance by regulating stomatal responses and root growth. Plant Cell Physiol. 57, 1629–1642. [DOI] [PubMed] [Google Scholar]
- Wasternack, C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 100, 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack, C. and Hause, B. (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 111, 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Hu, C. , Tan, Q. , Li, L. , Shi, K. , Zheng, Y. and Sun, X. (2015) Drought stress tolerance mediated by zinc‐induced antioxidative defense and osmotic adjustment in cotton (Gossypium Hirsutum). Acta Physiol. Plant 37, 167. [Google Scholar]
- Xing, Y. , Jia, W. and Zhang, J. (2008) AtMKK1 mediates ABA‐induced CAT1 expression and H2O2 production via _AtMPK6_‐coupled signaling in Arabidopsis. Plant J. 54, 440–451. [DOI] [PubMed] [Google Scholar]
- Xing, Y. , Chen, W.H. , Jia, W. and Zhang, J. (2015) Mitogen‐activated protein kinase kinase 5 (MKK5)‐mediated signalling cascade regulates expression of iron superoxide dismutase gene in Arabidopsis under salinity stress. J. Exp. Bot., 66, 5971–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Zhou, G. and Shimizu, H. (2010) Plant responses to drought and rewatering. Plant Signal. Behav. 5, 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi‐Shinozaki, K. and Shinozaki, K. (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57, 781–803. [DOI] [PubMed] [Google Scholar]
- Yan, H. , Jia, H. , Chen, X. , Hao, L. , An, H. and Guo, X. (2014) The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 55, 2060–2076. [DOI] [PubMed] [Google Scholar]
- Yang, H. , Zhang, D. , Li, X. , Li, H. , Zhang, D. , Lan, H. , Wood, A.J.et al. (2016) Overexpression of ScALDH21 gene in cotton improves drought tolerance and growth in greenhouse and field conditions. Mol. Breed. 36, 1–13. [Google Scholar]
- Yoshida, T. , Mogami, J. and Yamaguchi‐Shinozaki, K. (2014) ABA‐dependent and ABA‐independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 21, 133–139. [DOI] [PubMed] [Google Scholar]
- Yu, L.H. , Wu, S.J. , Peng, Y.S. , Liu, R.N. , Chen, X. , Zhao, P. , Xu, P.et al. (2015) Arabidopsis EDT1/HDG11 improves drought and salt tolerance in cotton and poplar and increases cotton yield in the field. Plant Biotechnol. J. 14, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Shen, G. , Kuppu, S. , Gaxiola, R. and Payton, P. (2011) Creating drought‐ and salt‐tolerant cotton by overexpressing a vacuolar pyrophosphatase gene. Plant Signal. Behav. 6, 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Li, Y. , Lu, W. , Meng, F. , Wu, C.A. and Guo, X. (2012) Cotton GhMKK5 affects disease resistance, induces HR‐like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotiana benthamiana. J. Exp. Bot. 63, 3935–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Zou, D. , Li, Y. , Sun, X. , Wang, N.N. , Gong, S.Y. , Zheng, Y.et al. (2014a) GhMPK17, a cotton mitogen‐activated protein kinase, is involved in plant response to high salinity and osmotic stresses and ABA signaling. PLoS ONE, 9, e95642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Wang, L. , Xu, X. , Cai, C. and Guo, W. (2014b) Genome‐wide identification of mitogen‐activated protein kinase gene family in Gossypium raimondii and the function of their corresponding orthologs in tetraploid cultivated cotton. BMC Plant Biol. 14, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Peng, J. , Chen, T.T. , Zhao, X.H. , Zhang, S.P. , Liu, S.D. , Dong, H.L.et al. (2014c) Effect of drought stress on lipid peroxidation and proline content in cotton roots. J. Anim. Plant Sci. 24, 1729–1736. [Google Scholar]
- Zhang, F. , Li, S. , Yang, S. , Wang, L. and Guo, W. (2015) Overexpression of a cotton annexin gene, GhAnn1, enhances drought and salt stress tolerance in transgenic cotton. Plant Mol. Biol. 87, 47–67. [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Gao, Y. , Zhang, Z. , Chen, T. , Guo, W. and Zhang, T. (2013) A receptor‐like kinase gene (GbRLK) from Gossypium barbadense enhances salinity and drought‐stress tolerance in Arabidopsis. BMC Plant Biol. 13, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J.Y. , Oluoch, G. , Riaz, K.M. , Wang, X.X. , Cai, X.Y. , Zhou, Z.L. , Wang, C.Y.et al. (2016) Mapping QTLs for drought tolerance in an F2: 3 population from an inter‐specific cross between Gossypium tomentosum and Gossypium hirsutum. Genet. Mol. Res. 15, 15038477. [DOI] [PubMed] [Google Scholar]
- Zhou, B. , Zhang, L. , Ullah, A. , Jin, X. , Yang, X.Y. and Zhang, X.L. (2016) Identification of Multiple Stress Responsive Genes by Sequencing a Normalized cDNA Library from Sea‐Land Cotton (Gossypium barbadense L.). PLoS ONE, 11, e0152927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y.N. , Shi, D.Q. , Ruan, M.B. , Zhang, L.L. , Meng, Z.H. , Liu, J. and Yang, W.C. (2013) Transcriptome analysis reveals crosstalk of responsive genes to multiple abiotic stresses in cotton (Gossypium hirsutum L.). PLoS ONE, 8, e80218. [DOI] [PMC free article] [PubMed] [Google Scholar]