How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait (original) (raw)
Abstract
Cuticular hydrocarbons (CHCs) cover the cuticles of virtually all insects, serving as a waterproofing agent and as a communication signal. The causes for the high CHC variation between species, and the factors influencing CHC profiles, are scarcely understood. Here, we compare CHC profiles of ant species from seven biogeographic regions, searching for physiological constraints and for climatic and biotic selection pressures. Molecule length constrained CHC composition: long-chain profiles contained fewer linear alkanes, but more hydrocarbons with disruptive features in the molecule. This is probably owing to selection on the physiology to build a semi-fluid cuticular layer, which is necessary for waterproofing and communication. CHC composition also depended on the precipitation in the ants' habitats. Species from wet climates had more alkenes and fewer dimethyl alkanes than those from drier habitats, which can be explained by different waterproofing capacities of these compounds. By contrast, temperature did not affect CHC composition. Mutualistically associated (parabiotic) species possessed profiles highly distinct from non-associated species. Our study is, to our knowledge, the first to show systematic impacts of physiological, climatic and biotic factors on quantitative CHC composition across a global, multi-species dataset. We demonstrate how they jointly shape CHC profiles, and advance our understanding of the evolution of this complex functional trait in insects.
Keywords: adaptation, climatic niche, cuticular hydrocarbons, selection pressure, viscosity, water loss rate
1. Introduction
The evolution of complex traits that fulfil multiple functions is one of the most challenging, but little understood topics in evolutionary biology. Cuticular hydrocarbon (CHC) profiles represent such a trait, covering the cuticle of nearly all insects investigated so far. CHCs serve multiple functions. Firstly, they prevent the insect from desiccation: insect cuticles bereft of their CHC layer lose water with a rate 10–100 fold higher than intact cuticles [1,2]. The waterproofing effect is caused by tight aggregation of the hydrophobic hydrocarbon molecules. Nevertheless, the CHC layer is not solid, but can rather be pictured as a viscous wax layer with a semi-liquid texture [3]. Secondly, CHCs mediate intra- and interspecific communication [4,5]. Serving as sex pheromones in many solitary insects [6,7], they represent the main communication channel in social insects, making them indispensable for the functioning of an insect colony. CHCs encode information about its carrier's colony membership, task within the colony and reproductive status [8–10]. Members of an insect colony can use information from their nest-mates' CHCs to decide on their tasks [11]. To homogenize recognition cues within the colony, nest-mates frequently exchange CHCs via trophallaxis and allogrooming [4]. According to the Gestalt model, this leads to a uniform colony odour, which is shared by all nest-mates as long as they are in contact with each other [12,13].
CHCs form complex profiles, with more than 100 different compounds on the same individual in many species [14]. A hydrocarbon molecule is characterized by the number and position of methyl groups and/or unsaturations, and its chain length. The simplest hydrocarbons are _n_-alkanes, i.e. linear chains of carbon atoms linked to hydrogen. Further CHC classes include mono-, di- and trimethyl alkanes (with one to three CH3 groups), alkenes and alkadienes (with one to two double bonds), and, more rarely, methyl-branched alkenes (with a methyl group and a double bond) [14].
CHC composition is largely genetically determined [15]. Chemical profiles within Drosophila also change across latitudinal gradients [16], suggesting that environmental factors can affect CHC evolution. Moreover, ant colonies from populations with social parasites show more diverse CHC profiles, suggesting that parasites select for higher cue diversity [17,18]. Besides, there are non-heritable CHC differences owing to environmental factors like food [19,20], host species [21], nest site material [22], short-term acclimation to climatic conditions [23,24] or changes in social status [25]. Except for sexual dimorphism [7], however, these intraspecific differences are largely restricted to quantitative variation of the same set of hydrocarbons.
By contrast, different species can possess highly different hydrocarbons [26]. Even sister species exhibit profiles with hardly any hydrocarbons in common [27–30]. This astonishing diversity raises the questions: (i) whether all theoretically possible combinations of CHC profiles occur in nature, or whether there are principal constraints, e.g. from the cuticular physiology, and (ii) which factors determine the evolution of these profiles. Although our knowledge on biosynthesis and function of CHC profiles has increased drastically in the last two decades, the ultimate causes behind CHC diversification are poorly known.
(a). Which factors affect cuticular hydrocarbon profiles?
It seems likely that interspecific variation in CHC profiles is caused by selection arising from their function as a communication signal and as a waterproofing agent, but might also be limited by physiological constraints. The hydrocarbon classes of insect CHC differ in physico-chemical properties, which determine their suitability for waterproofing and communication functions, and influence the viscosity of the CHC layer. Compared to methyl-branched or unsaturated hydrocarbons, _n-_alkane molecules aggregate most tightly because of van der Waals bonds. This effect increases at high chain lengths. At low temperatures, hydrocarbons with strong van der Waals bonds (e.g. _n-_alkanes and monomethyl alkanes) can crystallize [31,32]. This enhances waterproofing, but also increases the viscosity of the CHC layer, which is undesirable for substance diffusion and inter-individual CHC exchange and might therefore limit their function as a communication signal.
Compared to _n-_alkanes, methyl groups and especially unsaturations introduce disorder into the epicuticular layer, which reduce molecular aggregation. The degree of aggregation translates into the melting point _T_m. For the same chain lengths, _T_m decreases in the order _n-_alkanes, monomethyl alkanes, dimethyl alkanes, alkenes and alkadienes [33,34]. The melting point influences the waterproofing ability of a CHC layer: water loss through a CHC layer increases drastically if the ambient temperature is above _T_m [35], which has been shown, for example, in grasshoppers [2,36]. It is important to note that hydrocarbon mixtures possess a melting range rather than a melting point, with _T_m reflecting the midpoint of the melting range in these cases. Thus, melting occurs continuously over a broader temperature range rather than only at a specific temperature [37].
As waterproofing requirements change with climate, CHC profiles are supposed to be shaped by selection from the climatic conditions that insects are exposed to [38–40]. Indeed, tropical Drosophila melanogaster strains bear alkenes of longer chain length than temperate ones, possibly to endure higher desiccation stress [33,39]. One would therefore expect that, with increasing need for waterproofing, the percentage of _n-_alkanes and/or the average chain length of CHC profiles should increase. Consequently, hydrocarbon classes with lower waterproofing abilities (_n-_alkenes, alkadienes and methyl-branched alkenes) should be more abundant in species from wet climates, while those with better waterproofing abilities (_n-_alkanes, mono- and dimethyl alkanes) should be more abundant in species from drier sites.
At the same time, however, their function as communication cues requires that CHCs can be easily perceived by others, which is vital for their function as contact sex pheromone. In social insects, CHCs also need to be exchanged between nest-mates to create a common colony odour [13]. To this end, CHCs must be able to diffuse across an insect's body surface [3] to ensure uniform cuticle coating (i.e. an effective waterproofing) and a homogeneous composition of recognition cues. Thus, the CHC layer needs to maintain a certain degree of fluidity.
It is probable that variation of insect CHC profiles is constrained by these potentially conflicting requirements. CHC composition should trade-off high viscosity for waterproofing against low viscosity for substance diffusion and, hence, homogeneous CHC composition. Thus, one should expect that the percentage of compounds with disruptive features should increase with the average chain length of a CHC profile. Moreover, co-occurrence of substance classes may be non-random if they derive from the same or different biosynthetic pathways [41], but there may also be constraints on the simultaneous production of different substance classes. Finally, there are selection pressures arising from the communication function of CHCs. A recent study showed that Camponotus s.l. species living in a mutualistic association with other ants (parabiosis) bear systematically different CHC profiles with higher chain lengths and high quantities of alkadienes and methyl-branched alkenes, which is associated with high interspecific tolerance [27,42]. The selection pressure from being in mutualistic associations may additionally shape CHC profiles.
(b). Aim of the study
In this study, we asked how physiological constraints as well as climatic and biotic selection pressures shape CHC profiles. As different taxa may have found different solutions for similar problems, we focused on two ant genera with exceptionally high species richness and global distribution. We asked firstly whether there are constraints that lead to non-random CHC composition, concerning: (i) relationships between chain length and substance class composition, and (ii) co-occurrence of substance classes. Secondly, we tested how precipitation and temperature of an ant's habitat affect CHC class composition and chain length. Thirdly, we analysed whether parabiotic associations between ant species lead to convergent evolution of similar CHC traits in both genera. While several studies investigated the presence or the absence of hydrocarbons [40,43], effects on quantitative composition, i.e. the abundance of specific hydrocarbons or hydrocarbon classes, have to our knowledge not been investigated yet.
We analysed CHC profiles of 85 ant taxa of the genera Crematogaster (Formicidae: Myrmicinae) and Camponotus s.l. (sensu lato, including members of the newly resurrected genera Colobopsis and Dinomyrmex (Formicidae: Formicinae) [44]). The two groups are species-rich and globally distributed, which makes them suitable for global comparisons. Moreover, both are chemically highly diverse [29,42] and include parabiotic and non-parabiotic species. Our dataset covered seven biogeographic regions (Nearctic, Neotropical, Palaearctic, Australasian, southeast Asian, African and Malagasy) and a wide range of subgenera (electronic supplementary material, tables A1 and A2). By restricting our analyses to Crematogaster and Camponotus s.l., we obtained two sets of species with relatively low phylogenetic distance, such that phylogenetic effects should be low within each genus, while at the same time being able to analyse effects in species from different subfamilies.
2. Material and methods
(a). Study design
We analysed chemical profiles from workers of 38 described Camponotus s.l. and 42 Crematogaster species (electronic supplementary material, tables A1 and A2). The species were collected on five different continents and ranged from temperate to tropical species. All specimens were collected from the ground or the forest understorey. Thus, our samples only included foragers, which should experience higher desiccation stress than nurses or other workers within the nest [11]; furthermore, subterranean species or others with unusual habitats were avoided. The habitats ranged from tropical rainforest to deciduous forest and open shrubland (electronic supplementary material, table A2).
Usually, samples of the same species possessed similar quantitative and identical qualitative composition of CHC profiles [45]. In these cases, we considered one sample per species. In six species, we found two chemical types within the species which showed strong qualitative CHC variation. This concerned five Crematogaster species (Cr. macracantha, emeryana, difformis, onusta, levior) and Camponotus femoratus. Different chemotypes with genetic differentiation are already known for Cr. levior and Ca. femoratus ([29]; F. Menzel, B.B. Blaimer, T. Schmit 2013, unpublished data). For these six species, the presence of strongly different profiles suggests the existence of cryptic species [45], which is why we retained both chemotypes per species in the dataset.
Our dataset thus comprised 85 taxa: 39 Camponotus s.l. (including six Colobopsis and one Dinomyrmex species) and 46 Crematogaster taxa (electronic supplementary material, tables A1 and A2). They represent either described species, or one of two chemotypes within a species, i.e. putative cryptic species. For the sake of simplicity, we will use the term ‘species' for the 85 taxa henceforth. A part of these samples have been used for different analyses in [42].
(b). Cuticular hydrocarbon quantification
CHCs were obtained by immersing freeze-killed ants in hexane for 10 min. We extracted single workers or (in small species) pooled multiple workers to obtain sufficient CHC quantities for analysis in a single extract (average: 15.8 ± 2.5 s.e.). The extracts were analysed using a gas chromatograph coupled to a mass selective detector (Agilent GC 7890A and MSD 5975C as well as Agilent GC 6890A and MSD 5973) (GC-MS). The gas chromatograph was equipped with a HP5-MS capillary column (30 m× 0.25 mm ID; d.f. = 0.25 µm). Temperature was kept at 60°C for 2 min, then increased by 60°C min−1 up to 200°C and subsequently by 4°C min−1 to 320°C, where it remained constant for 10 min. Helium was used as carrier gas with a constant flow of 1.2 ml min−1. Analyses were run in splitless mode with an inlet temperature of 250°C. Electron impact mass spectra were recorded with an ionization voltage of 70 eV, a source temperature of 230°C and a quadrupole temperature of 150°C. Using this set-up, substances up to C41 can be detected.
To detect larger molecules (up to C50), the samples were additionally injected into the GC-MS equipped with a high temperature column (Agilent DB-1 HT and Phenomenex ZB-5HT; column dimensions as above). Temperature was raised from 60°C by 5°C min−1 up to 350°C and then kept constant for 10 min. The interface had a temperature of 350°C. All other settings were as above.
We identified all hydrocarbons based on diagnostic ions, M+ and retention indices, and quantified them using the total ion count. We included all hydrocarbons longer than C20 because CHCs shorter than C20 have not been reported from insect cuticles so far [46]. Owing to their strongly varying abundance among some Camponotus s.l. samples of the same species, _n-_C21 and _n-_C23 were excluded from further analysis (range: _n_-C21: 0–54%, _n_-C23: 0–16% within the same species), since we suspected these substances to be glandular contaminations. Extracts with large quantities of polar substances were fractionated over an SiOH column (Macherey-Nagel) with hexane as eluent and re-injected.
(c). Statistical analysis
(i). Choosing appropriate metrics to analyse cuticular hydrocarbon diversity
While tiny quantities of a substance may have a crucial role as a communication signal, physiological or ecological properties (like viscosity and waterproofing ability of the CHC layer) should depend more heavily on the abundant substances than on trace substances. Therefore, we chose relative abundances, instead of the presence or the absence of substances or homologous series [40].
From each CHC profile, we calculated the percentages of all hydrocarbon classes with a mean abundance of at least 2%: _n_-alkanes, monomethyl alkanes, dimethyl alkanes, alkenes, alkadienes and methyl-branched alkenes. Secondly, we calculated the median chain length of the CHC profile as the chain length with 50% of all hydrocarbons being shorter or equal and 50% being longer. This metric was based on CHC abundance of each hydrocarbon, not on the number of different substances [42].
(ii). Relation of cuticular hydrocarbon composition and chain length
To analyse the relationship of CHC composition and chain length, we used linear mixed-effects (LME) models with median chain length as an explanatory variable. In separate models, we analysed logit-transformed percentages of the six CHC classes as dependent variables. We allowed for unimodal effects by including second-order effects of the chain length.
(iii). Analysis of substance co-occurrence
To analyse how often hydrocarbon classes co-occurred in the same species, we defined a hydrocarbon class as present if its relative abundance exceeded 1%. Co-occurrence was analysed using Fisher tests on 2 × 2 contingency tables with the numbers of species that did or did not possess the two different substance classes (n = 85 species of Crematogaster and Camponotus s.l.). These analyses were performed for each pairwise combination of the six most common substance classes, and also included tri- plus tetramethyl alkanes, which were present in only a few species.
(iv). Analysis of climatic effects
We tested how hydrocarbon class composition and chain length is affected by the climate of the ants' origin. For this analysis, we excluded parabiotic species since they have aberrant CHC profiles and are restricted to the tropics, leaving n = 68 species. For each species, we obtained annual mean temperature and annual precipitation of the collection site from www.worldclim.org. Using linear mixed-effects models, we then tested the influence of temperature and precipitation (with interactions allowed) on the logit-transformed percentage of _n-_alkanes, monomethyl alkanes, dimethyl alkanes and _n-_alkenes, and the median chain length of the CHC profile. Alkadienes and methyl-branched alkenes were disregarded since they were too rare in non-parabiotic species.
(v). Effects of parabiotic lifestyle
Finally, we tested for effects of parabiotic lifestyle to confirm whether the effects found for Camponotus s.l. in a previous study [42] are similar in Crematogaster species. The abundance of each substance class and the median chain length were each tested in linear mixed-effects models as described above. To test for differences between Camponotus s.l. and Crematogaster, we included ‘subfamily’ as fixed factor in the analysis, and incorporated genus and subgenus as random factors (see below). Since parabioses only occur in the tropics, we restricted this analysis to tropical species (latitude <10°, n = 63 species) to avoid any bias from climatic effects.
(vi). Phylogenetic correction
Phylogenetic effects were incorporated into all models by including ‘subfamily’, ‘genus’ and ‘subgenus’ or ‘clade’ as random factors in a nested design. The genus Camponotus s.l. has recently been split up into the genera Camponotus s.str., Colobopsis and the monotypic Dinomyrmex (with the species D. gigas) by Ward et al. [44]. We accounted for this by using the three genera as random factors nested in the subfamily Formicinae. In the genus Camponotus s.str., we distinguished 11 subgenera: Camponotus, Myrmamblys, Myrmaphaenus, Myrmentoma, Myrmepomis, Myrmobrachys, Myrmopalpella, Myrmosericus, Myrmotarsus, Myrmothrix and Tanaemyrmex sensu Bolton [47]. For Crematogaster (subfamily Myrmicinae), we considered the three monophyletic clades Orthocrema, ‘global _Crematogaster_’ and ‘Australasian _Crematogaster_’ sensu Blaimer [48]. All models were analysed with ANOVA, and we consecutively removed non-significant factors or interactions until we obtained the model with the lowest Akaike information criterion.
3. Results
(a). Physiological constraints: cuticular hydrocarbon class and chain length
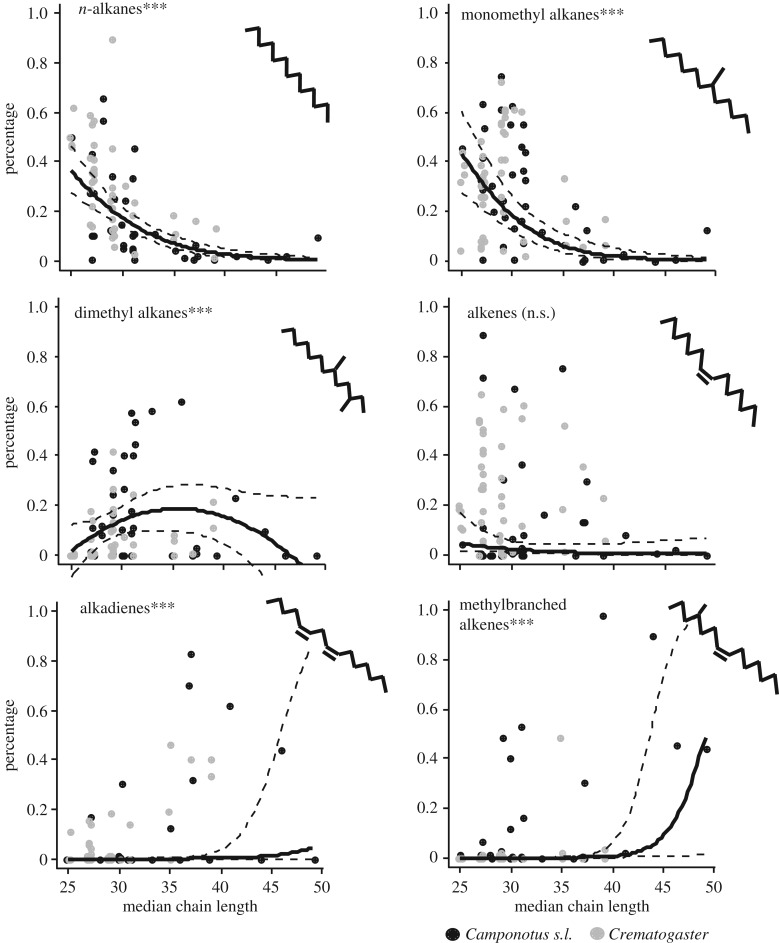
Our analysis revealed strong, and hitherto unknown, relationships between chain length and substance class composition of the CHC layer. The proportions of _n-_alkanes (LME: 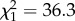 , p < 0.0001) and monomethyl alkanes (
, p < 0.0001) and monomethyl alkanes (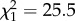 , p < 0.0001) strongly decreased with chain length (figure 1) across all species. Both substance classes aggregate more tightly than alkenes or dimethyl alkanes, thus increasing the viscosity of the CHC layer. By contrast, the percentage of hydrocarbons with disrupting features increased at high chain lengths, such as the percentage of alkadienes (
, p < 0.0001) strongly decreased with chain length (figure 1) across all species. Both substance classes aggregate more tightly than alkenes or dimethyl alkanes, thus increasing the viscosity of the CHC layer. By contrast, the percentage of hydrocarbons with disrupting features increased at high chain lengths, such as the percentage of alkadienes (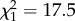 , p < 0.0001) and methyl-branched alkenes (
, p < 0.0001) and methyl-branched alkenes (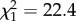 , p < 0.0001). Dimethyl alkanes were most abundant at intermediate chain lengths, but rare in profiles with very low or very high chain lengths. Only for this compound class, second-order effects were significant and retained in the best-fit model (first-order effect:
, p < 0.0001). Dimethyl alkanes were most abundant at intermediate chain lengths, but rare in profiles with very low or very high chain lengths. Only for this compound class, second-order effects were significant and retained in the best-fit model (first-order effect: 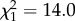 , p = 0.00019; second-order effect:
, p = 0.00019; second-order effect: 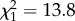 , p = 0.00021). The percentage of alkenes did not depend on chain length (
, p = 0.00021). The percentage of alkenes did not depend on chain length (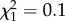 , p = 0.70).
, p = 0.70).
Figure 1.
Effects of median chain length of the total CHC profile on the percentage of different substance classes. Black circles, Camponotus s.l.; grey circles, Crematogaster. The lines represent model regressions with confidence intervals. For better visibility, jitter was added to the chain length values (_x_-axis). ***p < 0.001; **p<0.01; *p < 0.05; n.s., non-significant.
(b). Physiological constraints: co-occurrence of cuticular hydrocarbon classes
Several hydrocarbon classes showed non-random co-occurrence with other classes. Alkadienes were present only in species that also possessed alkenes (Fisher test: p < 0.0001), while 34 species had alkenes but lacked alkadienes. In analogy, dimethyl alkanes mostly occurred in species with monomethyl alkanes (p = 0.021) and tri- and tetramethyl alkanes only in species with dimethyl alkanes (p < 0.0001). Moreover, _n_-alkanes and monomethyl alkanes usually co-occurred (p = 0.016). However, the presence of methyl-branched alkenes was unrelated to the presence of either _n_-alkenes (p = 1) or monomethyl alkanes (p = 0.38).
Surprisingly, dimethyl alkanes were invariably absent from species with _n-_alkenes (Fisher test: p < 0.0001) or alkadienes (_p_ = 0.011). No such negative correlation was found for any other two substance classes (all _p_ > 0.3), except for a marginally negative relationship between _n_-alkanes and methyl-branched alkenes (p = 0.050).
Finally, we tested whether substance classes occurred more often among Camponotus s.l. or Crematogaster species. Indeed, more Camponotus s.l. than Crematogaster species possessed methyl-branched alkenes (19 out of 49 versus 7 out of 46 species; Fisher test: p = 0.00102). This difference was also detectable if only non-parabiotic tropical species were considered (Fisher test: p = 0.0097, n = 46). Similarly, trimethyl alkanes occurred in more Camponotus s.l. than Crematogaster species (p = 0.0014, n = 85), while alkenes were more common among Crematogaster species (p = 0.0048, n = 85). The occurrence of _n_-, monomethyl- and dimethyl alkanes, or alkadienes did not differ between genera (all p > 0.2).
(c). Environmental selection pressures: effects of climate
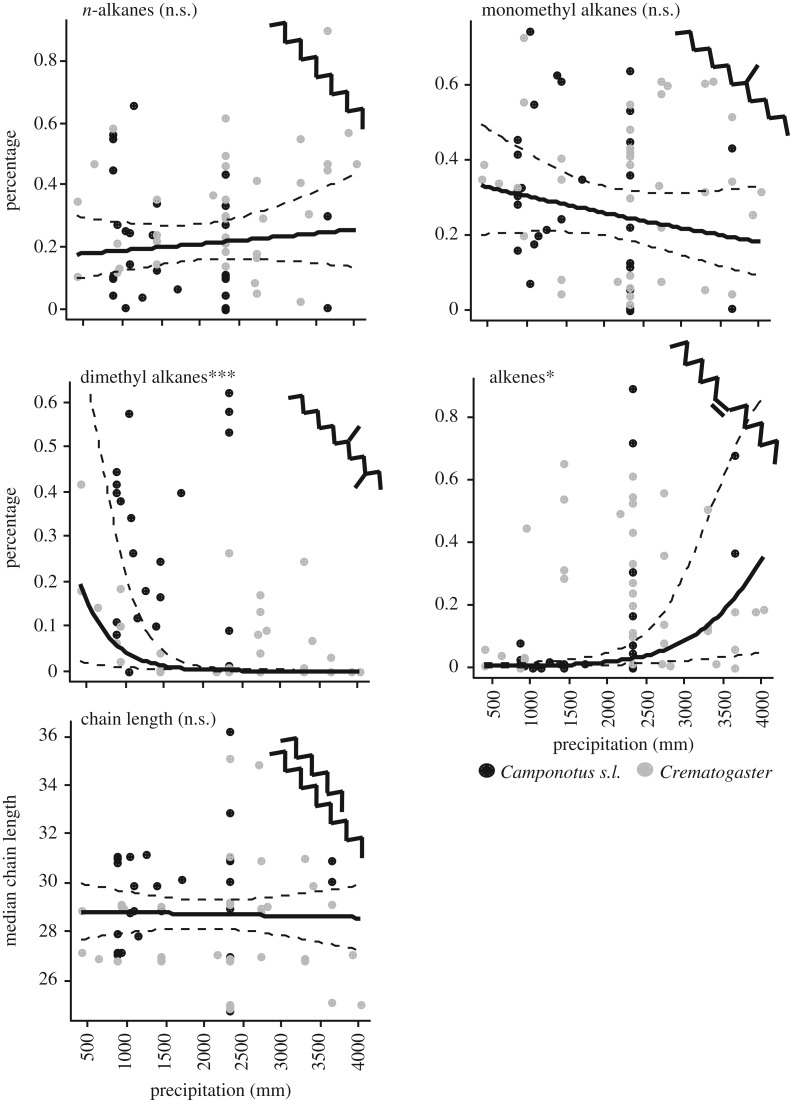
Hydrocarbon composition strongly depended on the annual precipitation, but not on the annual mean temperature of the collection site. In both genera, the percentage of dimethyl alkanes decreased with precipitation (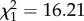 , p < 0.0001; figure 2). By contrast, the percentage of alkenes increased with precipitation (
, p < 0.0001; figure 2). By contrast, the percentage of alkenes increased with precipitation (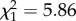 , _p_ = 0.015). Mean annual temperature did not affect dimethyl alkanes or alkenes, neither as single factor nor in interaction with humidity. The percentage of _n-_alkanes was not affected by precipitation, but marginally increased with temperature (temperature:
, _p_ = 0.015). Mean annual temperature did not affect dimethyl alkanes or alkenes, neither as single factor nor in interaction with humidity. The percentage of _n-_alkanes was not affected by precipitation, but marginally increased with temperature (temperature: 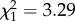 , _p_ = 0.070; figure 2). Monomethyl alkanes were not affected by temperature or precipitation (both _p_ > 0.2). The same was true for methyl-branched alkenes (both p > 0.1), which were very rare among the non-parabiotic species. Similar to alkenes, the abundance of alkadienes increased with increasing precipitation, but in alkadienes this effect became weaker at high annual temperatures (temperature : precipitation interaction:
, _p_ = 0.070; figure 2). Monomethyl alkanes were not affected by temperature or precipitation (both _p_ > 0.2). The same was true for methyl-branched alkenes (both p > 0.1), which were very rare among the non-parabiotic species. Similar to alkenes, the abundance of alkadienes increased with increasing precipitation, but in alkadienes this effect became weaker at high annual temperatures (temperature : precipitation interaction: 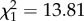 , p = 0.00030; data not shown). The median chain length was not affected by temperature or precipitation, with the final model only containing temperature (
, p = 0.00030; data not shown). The median chain length was not affected by temperature or precipitation, with the final model only containing temperature (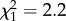 , p = 0.14).
, p = 0.14).
Figure 2.
Effects of annual precipitation at the collection site on the percentage of different substance classes and on median chain length (non-parabiotic species only). Black circles, Camponotus s.l.; grey circles, Crematogaster. The lines represent model regressions with confidence intervals. For better visibility, jitter was added to the precipitation values (_x_-axis) and, in the lowermost plot, to the chain length data (_y_-axis). ***p < 0.001; **p < 0.01; *p < 0.05; n.s., non-significant.
(d). Selection by parabiotic interactions
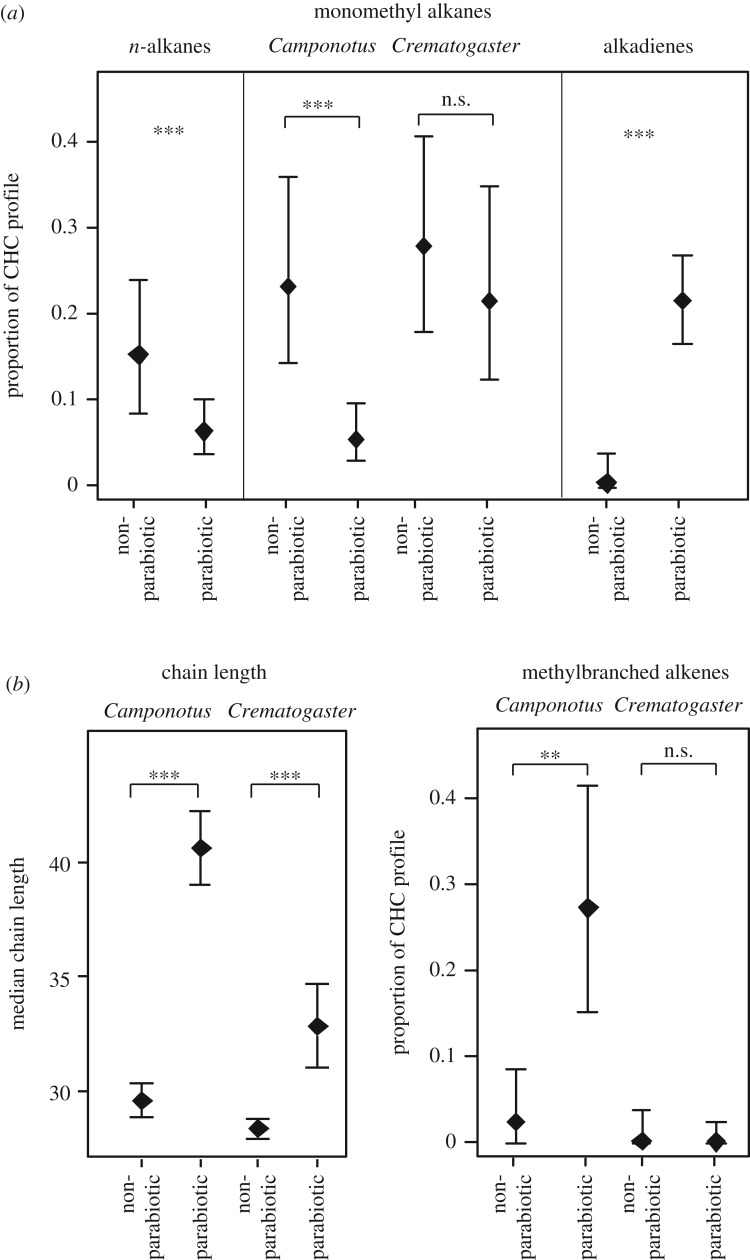
As expected, parabiotic species had more alkadienes (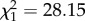 , p < 0.0001) and methyl-branched alkenes (
, p < 0.0001) and methyl-branched alkenes (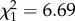 , p = 0.0097), all of which were close to zero among non-parabiotic species (only including tropical species; figure 3). However, while alkadienes occurred in parabiotic species of both taxonomic groups, methyl-branched alkenes were abundant only in parabiotic species of Camponotus s.l. (interaction subfamily : parabiotic lifestyle:
, p = 0.0097), all of which were close to zero among non-parabiotic species (only including tropical species; figure 3). However, while alkadienes occurred in parabiotic species of both taxonomic groups, methyl-branched alkenes were abundant only in parabiotic species of Camponotus s.l. (interaction subfamily : parabiotic lifestyle: 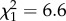 , p = 0.00999). By contrast, parabiotic species had fewer _n_-alkanes (
, p = 0.00999). By contrast, parabiotic species had fewer _n_-alkanes (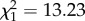 , p = 0.00028) than non-parabiotic ones, and parabiotic Camponotus s.l. species had fewer monomethyl alkanes than their non-parabiotic congeners (parabiotic lifestyle:
, p = 0.00028) than non-parabiotic ones, and parabiotic Camponotus s.l. species had fewer monomethyl alkanes than their non-parabiotic congeners (parabiotic lifestyle: 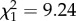 , p = 0.0024; interaction genus : parabiotic lifestyle:
, p = 0.0024; interaction genus : parabiotic lifestyle: 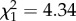 , p = 0.037). No differences were found for any other substance class. Median chain length was higher in parabiotic species (
, p = 0.037). No differences were found for any other substance class. Median chain length was higher in parabiotic species (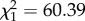 , p < 0.0001). This effect was stronger in Camponotus s.l. than in Crematogaster (interaction genus : parabiotic lifestyle:
, p < 0.0001). This effect was stronger in Camponotus s.l. than in Crematogaster (interaction genus : parabiotic lifestyle: 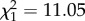 , p = 0.00089; figure 3).
, p = 0.00089; figure 3).
Figure 3.
Effects of the parabiotic lifestyle (a) on the abundance of different CHC classes and (b) on median chain length (tropical species only). The plots show model estimates (mean ± s.e.). The effects are shown separately for Camponotus s.l. and Crematogaster if they differed between the two taxa, but pooled if the interaction was non-significant. ***p < 0.001; **p < 0.01; *p< 0.05; n.s., non-significant.
4. Discussion
CHCs are shaped by physiological constraints and abiotic and biotic selection pressures owing to their dual function. Contrary to intraspecific variation, only few studies have investigated the exceptional CHC diversity across insect species so far [40,43]. Here, we identify evolutionary pressures that affect CHC profiles, and may have contributed to their diversification. In contrast to the above-mentioned studies, we focus on quantitative composition rather than the presence or absence of compounds. Even if the same substances are present in two profiles, differences in their quantitative composition may lead to very different ecological and physiological properties. Hence, quantitative composition should be biologically highly relevant.
(a). Are there selection pressures and constraints on cuticular hydrocarbon composition?
We showed that substance class composition and CHC chain length are linked and cannot vary independently. The percentage of tightly aggregating compounds (_n_-alkanes and monomethyl alkanes) decreased with median chain length, and there were no profiles with high chain lengths and high percentages of these hydrocarbons.
On a physiological level, the CHC layer experiences two basic selection pressures: firstly, its melting point must be high enough to waterproof the insect cuticle at ambient temperatures. Secondly, it must be fluid enough so that substances relevant for nest-mate recognition and/or waterproofing can spread over the cuticle [3,35] and be exchanged among nest-mates.
At equal chain lengths, pure _n_-alkanes have the highest melting points, thus enhancing the rigidity of the CHC layer [35]. This effect increases at higher chain lengths: lengthening of an _n-_alkane by a CH2 unit increases _T_m by approximately 2°C [2]. By contrast, methyl groups or double bonds disrupt the crystalline structure, thus reducing _T_m. The magnitude of _T_m reduction depends on the position of the methyl group(s) or unsaturation(s), but it is usually much larger than the effect of chain length [2].
In the species studied here, profiles with high chain length were always rich in methyl-branched alkenes and dienes, i.e. molecules with two disrupting structures each. Similarly, long-chain hydrocarbons (more than C35) in other ant genera were always accompanied by shorter-chain ones, and/or belonged to substance classes with disrupting features, such as di- or trimethyl alkanes (Atta colombica and Formica truncorum) [49,50] or methyl-branched alkenes (Nothomyrmecia macrops) [51].
Our analyses revealed that some substance classes frequently co-occurred with others. Alkadienes were present only in species with _n-_alkenes. Similarly, tri- and tetramethyl alkanes only occurred in species with dimethyl alkanes. This confirms previous results that more complex hydrocarbons are produced in the same biosynthetic pathways as simpler forms, which accounts for the observed co-occurrences [43]. Notably, however, di-, tri- or tetramethyl alkanes never co-occurred with unsaturated hydrocarbons. Although other Hymenoptera like bees or parasitoid wasps produce only alkenes or only methyl-branched CHCs, ants have been reported to frequently produce both [43]. Many ant species indeed produce both alkenes and monomethyl alkanes, but not alkenes and dimethyl alkanes. This fits data on European Formica species, which either possessed alkenes or dimethyl alkanes, but not both [52]. We have not been able to explain this up to now, but tentatively suggest that physiological constraints prevent the simultaneous activation of the two biosynthetic pathways. Methyl-branched alkanes derive from different amino acid precursors than unbranched hydrocarbons [41], and elongation with branched amino acids may conflict with the activity of desaturases. Interestingly, the presence of methyl-branched alkenes was independent from the presence of alkenes or methyl alkanes, suggesting that they are produced in yet a different pathway.
(b). Precipitation shapes cuticular hydrocarbon profiles
Ant hydrocarbon profiles showed a strong signal of the precipitation at their collection site: species with high alkene or alkadiene percentages stemmed from sites with high precipitation, while those with more dimethyl alkanes were from drier locations. By contrast, we found no effects of mean annual temperature. Alkenes offer worse waterproofing than _n-_alkanes, mono- or dimethyl alkanes [33]. Compared to an _n-_alkane, the insertion of a double bond reduces _T_m by 50°C or more, whereas _T_m of mono- and dimethyl alkanes is only 10–40°C lower [33]. Our data thus fit our prediction that high precipitation increases the percentage of compounds with low waterproofing ability.
Notably, CHC chain length was not affected by temperature or precipitation. This seems plausible given that chain length has a much weaker effect on _T_m than methyl groups or unsaturations [33,37]. Crematogaster and Camponotus s.l. species hence adapt to different climates via differences in CHC composition rather than differences in chain length. Nevertheless, they might adjust the average chain length of each homologous hydrocarbon series to acclimatize to the temperature of their habitats. Such effects, however, may easily be concealed by the high number of homologous series in a CHC profile. It is important to note that other taxa may apply different mechanisms to adapt to local climatic conditions. For example, despite a relatively low annual precipitation, many temperate Formica species produce alkenes, concomitantly with high percentages of _n-_alkanes [52]. The selection pressures we show here still allow a wide variety of CHC trait combinations, and different taxa may use different strategies within these constraints. This explains why coexisting species still have highly different CHC profiles, but it stresses the need to study quantitative, interspecific CHC variation across different taxa. Arthropods can also acclimatize to drier habitats by producing higher total CHC quantities [53,54]. This effect is much weaker than that of relative CHC composition since it does not change _T_m, but deserves further study in interspecific comparisons.
It is likely that the waterproofing requirements of an insect are determined by far more factors than the mere precipitation in its habitat. For example, seasonal fluctuations in temperature and rainfall may additionally create desiccation stress, e.g. during the dry season of an otherwise humid site. Especially temperate species face high seasonal and daily variation in temperature and humidity. It seems likely that CHC profiles differ in their waterproofing ability at fluctuating versus constant climates, such that seasonality exerts an additional selection pressure on CHC profiles.
A further open question concerns how different habitats or microhabitats in the same region affect CHC profiles. Species from humid habitats (like montane forests, swamplands, riparian or subterranean habitats) may well possess alkenes rather than dimethyl alkanes, even if the overall climate of the area is drier. The ant Formica candida is highly hygrophilous, and nests in swamps and bogs. Compared to sympatric Formica species, F. candida is the only one which produces alkadienes, while most congeners produce dimethyl alkanes [52]. This fits our results that alkadienes are generally common in species from humid habitats. By contrast, the profiles of desert species are characterized by saturated hydrocarbons, e.g. in scorpions [54], beetles [55,56] and ants [57].
(c). Effects of parabiotic lifestyle
Parabiotic species were characterized by higher chain lengths than non-parabiotic species. This was known for Camponotus s.l. [42], but here we showed convergent effects in Crematogaster. Interestingly, the few differences we found between the two taxa mostly concern methyl-branched alkenes and the effects of parabiotic lifestyle. Compared to Camponotus s.l., only a few Crematogaster species produced methyl-branched alkenes. This may explain why parabiotic Camponotus s.l. species possessed longer CHC chains than parabiotic Crematogaster: alkadienes might not offer a ‘disruption’ strong enough for extremely long CHC chains. That parabiotic species have fewer _n-_alkanes and monomethyl alkanes fits the physiological relation of chain length and _n-_alkane or monomethyl alkane abundance, especially the stronger effect in Camponotus s.l., which coincides with their higher chain lengths.
(d). Interactions of different selection pressures
Understanding how traits generate fitness trade-offs between different selection pressures is crucial to unravel the evolutionary causes of CHC diversification [58]. Here, such a trade-off might exist between climatic conditions, physiological constraints and the adaptation to the parabiotic lifestyle. The selection for high chain lengths in parabiotic species and the need to maintain CHC fluidity result in high percentages of alkadienes and methyl-branched alkenes. Such a profile, however, might not be viable in a dry climate. Hence, parabiotic species need to trade-off the ability to produce a long-chain CHC profile against waterproofing. Taken together, these constraints might explain why parabiotic species, at least those using long-chain hydrocarbons, are restricted to regions with high precipitation.
5. Conclusion
Our study revealed that CHC profiles in ants are shaped by constraints and different selection pressures in a predictable way. To our knowledge, this is the first study that shows how specific, quantitative CHC traits are inter-dependent and shaped by abiotic and biotic selection pressures. Our results open up a multitude of intriguing eco-evolutionary questions. Similar to morphological or physiological traits [59,60], a CHC profile may shed light on the climatic conditions, but also the biotic interactions an insect is involved in. Serving as intra- and interspecific recognition cues, it is likely that, similar to parabioses, insects adapt their CHC profiles to other biotic interactions, including predator–prey, parasitic or mutualistic relationships [17,61]. At the same time, ants need CHC profiles that can encode a multitude of information [4]. This may limit the evolution of very simple profiles or those dominated by _n-_alkanes, which are believed to convey little information [8].
Furthermore, owing to their role as a waterproofing agent, CHC profiles may be influenced by trait-based (micro-) habitat filtering, thus influencing community assembly [62,63]. Here, more research is needed to elucidate how abiotic and biotic factors influence CHC composition, and how they interact. Knowledge on these selection pressures might allow us to predict an insect's ability to adapt or acclimatize to changing climatic conditions.
Finally, it remains to be studied how CHC profiles are shaped by the need to withstand seasonal climatic fluctuations. Here, an important research area concerns the ability of insect individuals to adjust their CHC profiles to climatic conditions during their lifetime. It seems plausible that the waterproofing ability of an insect's CHC layer across a temperature gradient, coupled with the ability to change it during its lifetime, determines the climatic, and thus, the geographical range of the insect species.
Supplementary Material
Supplemental Tables S1 and S2: Additional information on the species investigated
Acknowledgements
We are grateful to Seiki Yamane (Kagoshima University, Japan), Shingo Hosoishi (Kyushu University, Japan) and Manfred Verhaagh (Museum for Natural History, Karlsruhe, Germany) for identification of samples of ours. We thank the Danum Valley Management Committee (DVMC), the Sarawak Forestry Department and the Malaysian Economic Planning Unit (EPU) for research permissions, and the Royal Society's South East Asia Rainforest Research Programme (SEARRP) and the staff at Danum Valley Field Center for logistic support and help in the field. Dr Arthur Chung (Forest Research Center, Sepilok) kindly supported our work as local collaborator in Malaysia. Furthermore, we thank Jérôme Orivel for support during our work in French Guiana and Jérôme Chave for research permission and logistic help for the Les Nouragues Research Station. We further thank Madagascar National Parks (MNP) and the Ministère de l'Environnement et des Forêts (MEFT) for delivering research authorizations in Madagascar, and Brian Fisher and his Madagascar ant team for logistical support during fieldwork. Lena Boettge and Heike Stypa are acknowledged for their assistance in the laboratories. We also thank Raphaël Boulay and an anonymous reviewer for helpful comments on the manuscript. All collections and research were conducted in accordance with all applicable laws and with the necessary permits of the respective countries.
Authors' contributions
T.S. and F.M. conceived of the study. F.M. and B.B.B. collected samples. F.M. performed chemical analysis and designed and conducted statistical analysis. All authors wrote the manuscript.
Competing interests
The authors declare no conflict of interest.
Funding
No funding has been received for this article.
References
- 1.Hadley NF. 1994. Water relations in terrestrial arthropods. Chapter 3: Cuticular transpiration. San Diego, CA: Academic Press. [Google Scholar]
- 2.Gibbs AG, Rajpurohit S. 2010. Cuticular lipids and water balance. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 100–120. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Geiselhardt S, Lamm S, Gack C, Peschke K. 2010. Interaction of liquid epicuticular hydrocarbons and tarsal adhesive secretion in Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). J. Comp. Physiol. 196, 369–378. ( 10.1007/s00359-010-0522-8) [DOI] [PubMed] [Google Scholar]
- 4.Leonhardt SD, Menzel F, Nehring V, Schmitt T. 2016. Ecology and evolution of communication in social insects. Cell 164, 1277–1287. ( 10.1016/j.cell.2016.01.035) [DOI] [PubMed] [Google Scholar]
- 5.Howard RW, Blomquist GJ. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50, 371–393. ( 10.1146/annurev.ento.50.071803.130359) [DOI] [PubMed] [Google Scholar]
- 6.Jallon JM. 1984. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 14, 441–478. ( 10.1007/BF01065444) [DOI] [PubMed] [Google Scholar]
- 7.Thomas ML, Simmons LW. 2008. Sexual dimorphism in cuticular hydrocarbons of the Australian field cricket Teleogryllus oceanicus (Orthoptera: Gryllidae). J. Insect Physiol. 54, 1081–1089. ( 10.1016/j.jinsphys.2008.04.012) [DOI] [PubMed] [Google Scholar]
- 8.D'Ettorre P, Lenoir A. 2010. Nestmate recognition. In Ant ecology (eds Lach L, Parr CL, Abbott KL), pp. 109–194. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Pamminger T, Foitzik S, Kaufmann KC, Schützler N, Menzel F. 2014. Worker personality and its association with spatially structured division of labor. PLoS ONE 9, e79616 ( 10.1371/journal.pone.0079616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebig J. 2010. Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 254–281. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Greene MJ, Gordon D. 2003. Cuticular hydrocarbons inform task decisions. Nature 423, 32 ( 10.1038/423032a) [DOI] [PubMed] [Google Scholar]
- 12.Boulay R, Hefetz A, Soroker V, Lenoir A. 2000. Camponotus fellah colony integration: worker individuality necessitates frequent hydrocarbon exchanges. Anim. Behav. 59, 1127–1133. ( 10.1006/anbe.2000.1408) [DOI] [PubMed] [Google Scholar]
- 13.Soroker V, Vienne C, Hefetz A, Nowbahari E. 1994. The postpharyngeal gland as a ‘Gestalt’ organ for nestmate recognition in the ant Cataglyphis niger. Naturwissenschaften 81, 510–513. ( 10.1007/s001140050120) [DOI] [Google Scholar]
- 14.Blomquist GJ, Bagnères A-G. 2010. Introduction: history and overview of insect hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 3–18. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.van Zweden JS, Dreier S, d'Ettorre P. 2009. Disentangling environmental and heritable nestmate recognition cues in a carpenter ant. J. Insect Physiol. 55, 159–164. ( 10.1016/j.jinsphys.2008.11.001) [DOI] [PubMed] [Google Scholar]
- 16.Frentiu FD, Chenoweth SF. 2010. Clines in cuticular hydrocarbons in two Drosophila species with independent population histories. Evolution 64, 1784–1794. ( 10.1111/j.1558-5646.2009.00936.x) [DOI] [PubMed] [Google Scholar]
- 17.Jongepier E, Foitzik S. 2016. Ant recognition cue diversity is higher in the presence of slavemaker ants. Behav. Ecol. 27, 304–311. ( 10.1093/beheco/arv153) [DOI] [Google Scholar]
- 18.Martin SJ, Helanterä H, Drijfhout FP. 2011. Is parasite pressure a driver of chemical cue diversity in ants? Proc. R. Soc. B 278, 496–503. ( 10.1098/rspb.2010.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang D, Silverman J. 2000. ‘You are what you eat’: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87, 412–416. ( 10.1007/s001140050752) [DOI] [PubMed] [Google Scholar]
- 20.Otte T, Hilker M, Geiselhardt S. 2014. The effect of dietary fatty acids on the cuticular hydrocarbon phenotype of an herbivorous insect and consequences for mate recognition. J. Chem. Ecol. 41, 32–43. ( 10.1007/s10886-014-0535-9) [DOI] [PubMed] [Google Scholar]
- 21.Kühbandner S, Hacker K, Niedermayer S, Steidle JLM, Ruther J. 2012. Composition of cuticular lipids in the pteromalid wasp Lariophagus distinguendus is host dependent. Bull. Entomol. Res. 102, 610–617. ( 10.1017/S000748531200017X) [DOI] [PubMed] [Google Scholar]
- 22.Heinze J, Foitzik S, Hippert A, Hölldobler B. 1996. Apparent dear-enemy phenomenon and environment-based recognition cues in the ant Leptothorax nylanderi. Ethology 102, 510–522. ( 10.1111/j.1439-0310.1996.tb01143.x) [DOI] [Google Scholar]
- 23.Wagner D, Tissot M, Gordon D. 2001. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 27, 1805–1819. ( 10.1023/A:1010408725464) [DOI] [PubMed] [Google Scholar]
- 24.Woodrow RJ, Grace JK, Nelson LJ, Haverty MI. 2000. Modification of cuticular hydrocarbons of Cryptotermes brevis (Isoptera: Kalotermitidae) in response to temperature and relative humidity. Environ. Entomol. 29, 1100–1107. ( 10.1603/0046-225X-29.6.1100) [DOI] [Google Scholar]
- 25.Thomas ML, Simmons LW. 2011. Short-term phenotypic plasticity in long-chain cuticular hydrocarbons. Proc. R. Soc. B 278, 3123–3128. ( 10.1098/rspb.2011.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin SJ, Drijfhout FP. 2009. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 35, 1151–1161. ( 10.1007/s10886-009-9695-4) [DOI] [PubMed] [Google Scholar]
- 27.Menzel F, Blüthgen N, Schmitt T. 2008. Tropical parabiotic ants: highly unusual cuticular substances and low interspecific discrimination. Front. Zool. 5, 16 ( 10.1186/1742-9994-5-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison WR, Witte V. 2011. Strong differences in chemical recognition cues between two closely related species of ants from the genus Lasius (Hymenoptera: Formicidae). J. Evol. Biol. 24, 2389–2397. ( 10.1111/j.1420-9101.2011.02364.x) [DOI] [PubMed] [Google Scholar]
- 29.Menzel F, Orivel J, Kaltenpoth M, Schmitt T. 2014. What makes you a potential partner? Insights from convergently evolved ant-ant symbioses. Chemoecology 24, 105–119. ( 10.1007/s00049-014-0149-2) [DOI] [Google Scholar]
- 30.Seppä P, Helanterä H, Trontti K, Punttila P, Chernenko A, Martin SJ, Sundström L. 2011. The many ways to delimit species: hairs, genes and surface chemistry. Myrmecol. News 15, 31–41. [Google Scholar]
- 31.Brooks L, Brunelli M, Pattison P, Jones GR, Fitch A. 2015. Crystal structures of eight mono-methyl alkanes (C26-C32) via single-crystal and powder diffraction and DFT-D optimization. IUCrJ 2, 490–497. ( 10.1107/S2052252515010271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maroncelli M, Qi SP, Strauss HL, Snyder RG. 1982. Nonplanar conformers and the phase behavior of solid n-alkanes. J. Am. Chem. Soc. 104, 6237–6247. ( 10.1021/ja00387a013) [DOI] [Google Scholar]
- 33.Gibbs A, Pomonis JG. 1995. Physical properties of insect cuticular hydrocarbons: the effects of chain lengths, methyl branching and unsaturation. Comp. Biochem. Physiol. 112B, 243–249. ( 10.1016/0305-0491(95)00081-X) [DOI] [Google Scholar]
- 34.Gibbs AG. 1998. Water-proofing properties of cuticular lipids. Am. Zool. 38, 471–482. ( 10.1093/icb/38.3.471) [DOI] [Google Scholar]
- 35.Gibbs AG. 2002. Lipid melting and cuticular permeability: new insights into an old problem. J. Insect Physiol. 48, 391–400. ( 10.1016/S0022-1910(02)00059-8) [DOI] [PubMed] [Google Scholar]
- 36.Rourke B, Gibbs A. 1999. Effects of lipid phase transitions on cuticular permeability: model membrane and in situ studies. J. Exp. Biol. 202, 3255–3262. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs A. 1995. Physical properties of insect cuticular hydrocarbons: model mixtures and lipid interactions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 112, 667–672. ( 10.1016/0305-0491(95)00119-0) [DOI] [Google Scholar]
- 38.Gibbs AG, Fukuzato F, Matzkin LM. 2003. Evolution of water conservation mechanisms in Drosophila. J. Exp. Biol. 206, 1183–1192. ( 10.1242/jeb.00233) [DOI] [PubMed] [Google Scholar]
- 39.Rouault J-D, Marican C, Wicker-Thomas C, Jallon J-M. 2004. Relations between cuticular hydrocarbon (HC) polymorphism, resistance against desiccation and breeding temperature; a model for HC evolution in D. melanogaster and D. simulans. Genetica 120, 195–212. ( 10.1023/B:GENE.0000017641.75820.49) [DOI] [PubMed] [Google Scholar]
- 40.van Wilgenburg E, Symonds MRE, Elgar MA. 2011. Evolution of cuticular hydrocarbon diversity in ants. J. Evol. Biol. 24, 1188–1198. ( 10.1111/j.1420-9101.2011.02248.x) [DOI] [PubMed] [Google Scholar]
- 41.Chung H, Carroll SB. 2015. Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 37, 822–830. ( 10.1002/bies.201500014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menzel F, Schmitt T. 2012. Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues. Evolution 66, 896–904. ( 10.1111/j.1558-5646.2011.01489.x) [DOI] [PubMed] [Google Scholar]
- 43.Kather R, Martin SJ. 2015. Evolution of cuticular hydrocarbons in the Hymenoptera: a meta-analysis. J. Chem. Ecol. 41, 871–883. ( 10.1007/s10886-015-0631-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward PS, Blaimer BB, Fisher BL. 2016. A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa 4072, 343–357. ( 10.11646/zootaxa.4072.3.4) [DOI] [PubMed] [Google Scholar]
- 45.Kather R, Martin SJ. 2012. Cuticular hydrocarbon profiles as a taxonomic tool: advantages, limitations and technical aspects. Physiol. Entomol. 37, 25–32. ( 10.1111/j.1365-3032.2011.00826.x) [DOI] [Google Scholar]
- 46.Blomquist GJ. 2010. Biosynthesis of cuticular hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnères A-G), pp. 35–53. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 47.Bolton B. 1995. A new general catalogue of the ants of the world. Cambridge, MA: Harvard University Press. [Google Scholar]
- 48.Blaimer B. 2012. Acrobat ants go global: origin, evolution and systematics of the genus Crematogaster (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 65, 421–436. ( 10.1016/j.ympev.2012.06.028) [DOI] [PubMed] [Google Scholar]
- 49.Akino T. 2006. Cuticular hydrocarbons of Formica truncorum (Hymenoptera: Formicidae): description of new very long chained hydrocarbon components. Appl. Entomol. Zool. 41, 667–677. ( 10.1303/aez.2006.667) [DOI] [Google Scholar]
- 50.Cvačka J, Jiroš P, Šobotník J, Hanus R, Svatoš A. 2006. Analysis of insect cuticular hydrocarbons using matrix-assisted laser desorption/ionization mass spectrometry. J. Chem. Ecol. 32, 409–434. ( 10.1007/s10886-005-9008-5) [DOI] [PubMed] [Google Scholar]
- 51.Brown WV, Jaisson P, Taylor RW, Lacey MJ. 1990. Novel internally branched, internal alkenes as major components of the cuticular hydrocarbons of the primitive Australian ant Nothomyrmecia macrops Clark (Hymenoptera: Formicidae). J. Chem. Ecol. 16, 2623–2635. ( 10.1007/BF00988074) [DOI] [PubMed] [Google Scholar]
- 52.Martin SJ, Helanterä H, Drijfhout FP. 2008. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linn. Soc. 95, 131–140. ( 10.1111/j.1095-8312.2008.01038.x) [DOI] [Google Scholar]
- 53.Hadley NF. 1977. Epicuticular lipids of the desert tenebrionid beetle, Eleodes armata: seasonal and acclimatory effects on composition. Insect Biochem. 7, 277–283. ( 10.1016/0020-1790(77)90025-7) [DOI] [Google Scholar]
- 54.Gefen E, Talal S, Brendzel O, Dror A, Fishman A. 2015. Variation in quantity and composition of cuticular hydrocarbons in the scorpion Buthus occitanus (Buthidae) in response to acute exposure to desiccation stress. Comp. Biochem. Physiol. Part A 182, 58–63. ( 10.1016/j.cbpa.2014.12.004) [DOI] [PubMed] [Google Scholar]
- 55.Hadley NF. 1978. Cuticular permeability of desert tenebrionid beetles: correlations with epicuticular hydrocarbon composition. Insect Biochem. 8, 17–22. ( 10.1016/0020-1790(78)90005-7) [DOI] [Google Scholar]
- 56.Geiselhardt SF, Geiselhardt S, Peschke K. 2006. Chemical mimicry of cuticular hydrocarbons: how does Eremostibes opacus gain access to breeding burrows of its host Parastizopus armaticeps (Coleoptera, Tenebrionidae)? Chemoecology 16, 59–68. ( 10.1007/s00049-005-0330-8) [DOI] [Google Scholar]
- 57.Dahbi A, Lenoir A, Tinaut A, Taghizadeh T, Franeke W, Hefetz A. 1996. Chemistry of the postpharyngeal gland secretion and its implication for the phylogeny of Iberian Cataglyphis species (Hymenoptera: Formicidae). Chemoecology 7, 163–171. ( 10.1007/BF01266308) [DOI] [Google Scholar]
- 58.Kunstler G, et al. 2016. Plant functional traits have globally consistent effects on competition. Nature 529, 204–207. ( 10.1038/nature16476) [DOI] [PubMed] [Google Scholar]
- 59.Gibb H, Stoklosa J, Warton DI, Brown AM, Andrew NR, Cunningham SA. 2015. Does morphology predict trophic position and habitat use of ant species and assemblages? Oecologia 177, 519–531. ( 10.1007/s00442-014-3101-9) [DOI] [PubMed] [Google Scholar]
- 60.Kraft NB, Valencia R, Ackerly DD. 2008. Functional traits and niche-based tree community assembly in an Amazonian forest. Science 322, 580–582. ( 10.1126/science.1160662) [DOI] [PubMed] [Google Scholar]
- 61.Lang C, Menzel F. 2011. Lasius niger ants discriminate aphids based on their cuticular hydrocarbons. Anim. Behav. 82, 1245–1254. ( 10.1016/j.anbehav.2011.08.020) [DOI] [Google Scholar]
- 62.McGill BJ, Enquist BJ, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. ( 10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 63.Kraft NJB, Ackerly DD. 2010. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 80, 401–422. ( 10.1890/09-1672.1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S2: Additional information on the species investigated