Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus (original) (raw)
Abstract
Nitric oxide (NO) is an important signaling molecule between cells which has been shown to have an inhibitory effect on some virus infections. The purpose of this study was to examine whether NO inhibits the replication cycle of the severe acute respiratory syndrome coronavirus (SARS CoV) in vitro. We found that an organic NO donor, _S_-nitroso-_N_-acetylpenicillamine, significantly inhibited the replication cycle of SARS CoV in a concentration-dependent manner. We also show here that NO inhibits viral protein and RNA synthesis. Furthermore, we demonstrate that NO generated by inducible nitric oxide synthase, an enzyme that produces NO, inhibits the SARS CoV replication cycle.
Severe acute respiratory syndrome (SARS), which is associated with a novel coronavirus (CoV), was first identified during fall 2002 in Guangdong Province, China (24, 32, 34). The mortality rate of SARS appears to range from 6 to 55% (12, 20, 21). Coronaviruses are enveloped single-stranded positive-sense RNA viruses with genomes of about 27 to 30 kb (21). Coronaviruses belong to the family Coronaviridae, in which SARS CoV forms a distinct group within the genus Coronavirus (9, 29).
Nitric oxide (NO) is an important signaling molecule between cells and is involved in a wide range of processes (9, 27). An antimicrobial activity of NO has been described for several bacteria and protozoa and for some viruses (1, 18, 27). NO is produced by three enzymes that catalyze the oxidation of l-arginine to NO and l-citrulline (9). Two of the enzymes, neuronal nitric oxide synthase (nNOS) and endothelial NOS (eNOS), are constitutively expressed and are calcium dependent (27). Inducible NOS (iNOS) is expressed only in activated cells and is calcium independent (11). The up-regulation of iNOS is common during an infection, and it is known that some viruses and bacteria are either inhibited or stimulated by increased levels of NO (1, 2, 11, 26, 30). It has also been demonstrated that iNOS is expressed after interferon stimulation in murine macrophages, mouse T cells, human hepatocytes, mononuclear cells, human airway epithelial cells, and alveolar macrophages (6, 13, 15, 25, 31, 33).
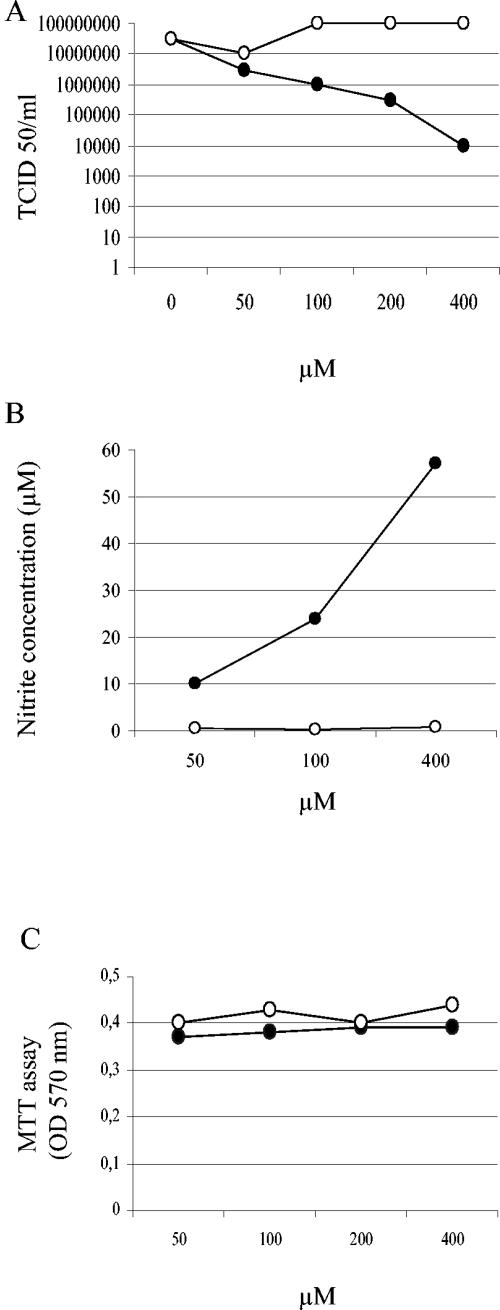
To investigate the role of NO in SARS CoV infection, we infected Vero E6 cells with SARS CoV at a multiplicity of infection (MOI) of 1. At 1 h postinfection (hpi), the cells were washed twice and then mock treated or treated with different concentrations of the organic NO donor _S_-nitroso-_N_-acetylpenicillamine (SNAP; Sigma, St. Louis, Mo.) or, as a negative control, _N_-acetylpenicillamine (NAP; Sigma), which lacks the NO-donating _S_-nitroso group (3, 7, 18, 19, 27). At 24 hpi, the amount of virus was deduced by the 50% tissue culture infective dose (TCID50), which was calculated from the cytopathic effect induced in cell culture by different dilutions of the harvested virus. As shown in Fig. 1A, SNAP inhibited the replication cycle of SARS CoV in a dose-dependent manner. Treatment with 100 μM SNAP resulted in a 2-log reduction in the yield of progeny virus, and the inhibitory effect was even more pronounced with 400 μM SNAP. As shown in Fig. 1B, the observed inhibitory effect correlated with the release of NO2 into the culture medium, as determined by the use of Griess reagent (14, 16, 28). To exclude the possibility that the detected antiviral effect of SNAP might have resulted from toxicity to the cells, we performed an MTT cell proliferation test (American Type Culture Collection). These results clearly excluded the possibility that the antiviral effect of SNAP was due to general cytotoxicity (Fig. 1C).
FIG. 1.
NO has an antiviral effect on SARS CoV infection. Vero E6 cells were infected with SARS CoV at an MOI of 1.0. At 1 hpi, the cells were treated with different concentrations of SNAP (•) and NAP (○). (A) Viruses was harvested at 24 hpi and titers were determined. (B) Nitrite concentrations produced at 24 h posttreatment with different concentrations of SNAP and NAP. (C) Cell viability, as determined by MTT assays. The mean values from two experiments are indicated.
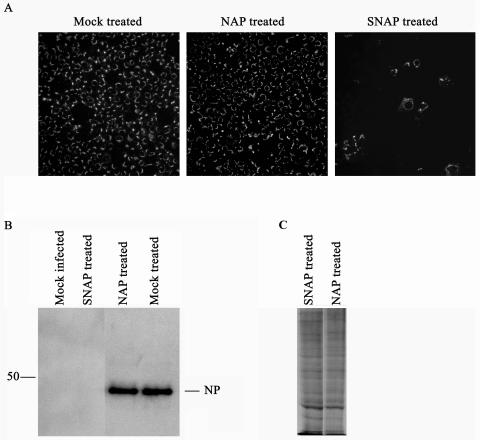
The inhibitory effect of NO on SARS CoV infection in Vero E6 cells was further demonstrated by an immunofluorescence assay and Western blotting as described previously (5), using rabbit polyclonal antibodies directed against SARS CoV NP (kindly provided by Luis Martínez-Sobrido and Adolfo García-Sastre). The antibodies were raised against purified recombinant NP of SARS CoV expressed in bacteria (L. Martínez-Sobrido, personal communication). Vero E6 cells were mock infected or infected with SARS CoV at an MOI of 1. At 1 hpi, the cells were mock treated or treated with SNAP or NAP (400 μM). At 12 or 24 hpi, the cells were fixed or lysed and then analyzed. The results clearly showed that treatment with 400 μM SNAP reduced the number of infected cells (Fig. 2A) and also demonstrated a significant reduction in NP expression (Fig. 2B).
FIG. 2.
Immunofluorescence assays and Western blot analyses revealed that SNAP inhibits the SARS CoV replication cycle. Vero E6 cells were infected with SARS CoV at an MOI of 1.0 and then treated with 400 μM SNAP or NAP at 1 hpi. Vero E6 cells were fixed with 80% acetone at 24 hpi and then analyzed by an immunofluorescence assay (A), lysed at 12 hpi and then analyzed by Western blotting (B), or pulsed with 250 μCi of radiation/ml for 1 h, lysed, and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (C).
To exclude the possibility that the reduction in NP expression was due to a common inhibition of protein translation, we mock treated Vero E6 cells or treated them with SNAP or NAP (400 μM). At 24 h posttreatment, the cells were pulsed with 250 μCi of radiation/ml for 1 h. The cells were lysed and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as described previously (22). The results clearly showed that the total amount of protein translation was not decreased in SNAP-treated cells compared to that in control cells (Fig. 2C).
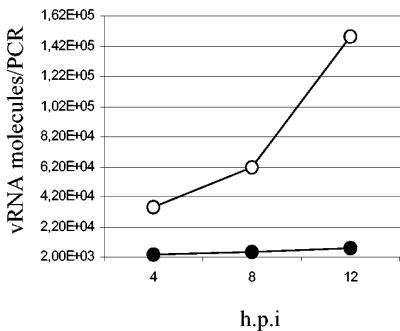
To investigate whether NO inhibits the viral RNA replication process of SARS CoV, we infected Vero E6 cells with SARS CoV at an MOI of 0.1. At 1 hpi, the cells were treated with 400 μM SNAP or NAP and then lysed by the use of Trizol (Gibco/Life Technologies/Invitrogen, Groningen, The Netherlands) at different times, and total RNAs were isolated as previously described (4). Viral RNAs were subsequently quantified by real-time PCR as described below. A ReSSQ SARS assay (LightUp Technologies AB, Stockholm, Sweden) was used for real-time PCR quantification with a LightCycler 1.0 instrument (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's instructions. One of the gene fragments targeted by the ReSSQ assay was designed to be almost identical to the fragment used in the SARS standard distributed by the Bernhard-Nocht Institute for Tropical Medicine (BNI) to enable the use of this standard for quantification; hence, all reported quantifications are based on the BNI standard. As shown in Fig. 3, we demonstrated that viral RNA production was significantly inhibited by 400 μM SNAP.
FIG. 3.
SNAP blocks viral RNA replication of SARS CoV. Vero E6 cells were infected at an MOI of 0.01 and then treated with 400 μM SNAP (•) or NAP (○) at 1 hpi. Treated cells were lysed by the use of Trizol at different times, and viral RNAs were quantified as described in the text.
To investigate the effect of iNOS on the replication cycle of SARS CoV in cell culture, we mock treated Vero E6 cells or treated them with 10 ng of recombinant human interleukin-1β (IL-1β) (1 U/ml; Peprotech, London, England)/ml, together with 400 U of recombinant human gamma interferon (IFN-γ) (Peprotech)/ml, to induce iNOS (26).
After 48 h of stimulation, the cells were mock infected or infected with SARS CoV at an MOI of 0.1. At 24 hpi, the virus was harvested and the titer was determined as described previously.
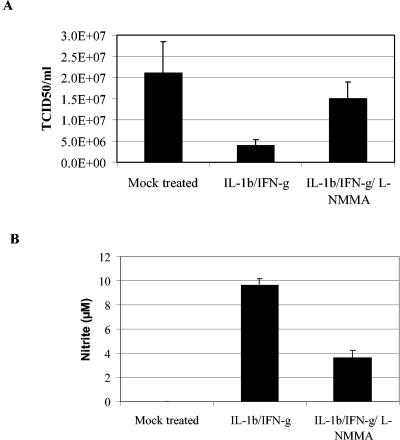
We found that the induction of iNOS reduced the yield of progeny virus by about 82% (from 2.1 × 107 to 3.9 × 106 TCID50) (Fig. 4A). This result confirmed a recent report which showed that IFN-γ has an antiviral effect on the replication cycle of SARS CoV in Vero cells (10).
FIG. 4.
iNOS has an inhibitory effect on the replication cycle of SARS CoV. Vero E6 cells were mock treated or treated with IL-1β and IFN-γ, with or without the iNOS inhibitor l-NMMA. At 48 h posttreatment, the cells were infected with SARS CoV at an MOI of 0.1. Progeny virus was harvested at 24 hpi, and the titer was deduced by calculating the TCID50 (A). Nitrite concentrations were determined by the use of Griess reagent (B). The mean values from three separate experiments are given, and error bars indicate standard deviations.
The measurement of NO levels (Fig. 4B) demonstrated that the concentration of nitrite produced by the cytokine treatment reached approximately the same level as that seen with 50 μM SNAP.
Most interestingly, we observed the same level of inhibition of the virus replication cycle with 50 μM SNAP as that with the cytokine treatment. In order to confirm that the NO production was dependent on iNOS induction, we treated cells with a 1 mM concentration of the iNOS inhibitor _N_G-monomethyl-l-arginine (l-NMMA) (8, 26, 27, 30), together with IL-1β and IFN-γ. l-NMMA significantly inhibited the production of NO and thereby restored the replication cycle of SARS CoV. However, it should be mentioned that the virus titer was still 30% lower than that of the control. One possible explanation for this observation may be that l-NMMA could not completely inhibit iNOS and that some NO was therefore still produced, which may have inhibited the virus (Fig. 4).
Our results demonstrated that NO specifically inhibits the replication cycle of SARS CoV, most probably during the early steps of infection, suggesting that the production of NO by iNOS results in an antiviral effect. However, the production of NO should be adjusted to exert antiviral rather than damaging effects. At present, there is no information concerning the levels of NO in SARS patients. Previous studies have shown that NO plays a role in the pathogenesis of influenza virus pneumonia in mice (2, 17). This pathological effect, however, has been suggested to be associated with the mouse model of pneumonia, since the peak of NO in infected humans was not associated with clinical symptoms (23). Thus, the role of NO during SARS infection in animal models and the level of NO in SARS patients constitute important areas for future studies.
Acknowledgments
We thank Luis Martínez-Sobrido and Adolfo García-Sastre (Department of Microbiology, Mount Sinai School of Medicine, New York, N.Y.) for kindly providing antibodies against SARS CoV.
REFERENCES
- 1.Adler, H., J. L. Beland, N. C. Del-Pan, L. Kobzik, J. P. Brewer, T. R. Martin, and I. J. Rimm. 1997. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS2). J. Exp. Med. 185**:**1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaike, T., Y. Noguchi, S. Ijiri, K. Setoguchi, M. Suga, Y. M. Zheng, B. Dietzschold, and H. Maeda. 1996. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc. Natl. Acad. Sci. USA 93**:**2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akarid, K., M. Sinet, B. Desforges, and M. A. Gougerot-Pocidalo. 1995. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. J. Virol. 69**:**7001-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, I., L. Bladh, M. Mousavi-Jazi, K. E. Magnusson, A. Lundkvist, O. Haller, and A. Mirazimi. 2004. Human MxA protein inhibits the replication of Crimean-Congo hemorrhagic fever virus. J. Virol. 78**:**4323-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson, I., M. Simon, Å. Lundkvist, M. Nilsson, A. Holmström, F. Elgh, and A. Mirazimi. 2004. Role of actin filaments in targeting of Crimean-Congo hemorrhagic fever virus nucleocapsid protein to perinuclear regions of mammalian cells. J. Med. Virol. 72**:**83-93. [DOI] [PubMed] [Google Scholar]
- 6.Asano, K., C. B. Chee, B. Gaston, C. M. Lilly, C. Gerard, J. M. Drazen, and J. S. Stamler. 1994. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc. Natl. Acad. Sci. USA 91**:**10089-10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benencia, F., and M. C. Courreges. 1999. Nitric oxide and macrophage antiviral extrinsic activity. Immunology 98**:**363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blond, D., H. Raoul, R. Le Grand, and D. Dormont. 2000. Nitric oxide synthesis enhances human immunodeficiency virus replication in primary human macrophages. J. Virol. 74**:**8904-8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher, J. L., C. Moali, and J. P. Tenu. 1999. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for l-arginine utilization. Cell Mol. Life Sci. 55**:**1015-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinatl, J., B. Morgenstern, G. Bauer, P. Chandra, H. Rabenau, and H. W. Doerr. 2003. Treatment of SARS with human interferons. Lancet 362**:**293-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman, J. W. 2001. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 1**:**1397-1406. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly, C. A., A. C. Ghani, G. M. Leung, A. J. Hedley, C. Fraser, S. Riley, L. J. Abu-Raddad, L. M. Ho, T. Q. Thach, P. Chau, K. P. Chan, T. H. Lam, L. Y. Tse, T. Tsang, S. H. Liu, J. H. Kong, E. M. Lau, N. M. Ferguson, and R. M. Anderson. 2003. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 361**:**1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geller, D. A., C. J. Lowenstein, R. A. Shapiro, A. K. Nussler, M. Di Silvio, S. C. Wang, D. K. Nakayama, R. L. Simmons, S. H. Snyder, and T. R. Billiar. 1993. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc. Natl. Acad. Sci. USA 90**:**3491-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126**:**131-138. [DOI] [PubMed] [Google Scholar]
- 15.Guo, F. H., H. R. De Raeve, T. W. Rice, D. J. Stuehr, F. B. Thunnissen, and S. C. Erzurum. 1995. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc. Natl. Acad. Sci. USA 92**:**7809-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao, Y. J., P. A. Piedra, G. L. Larsen, and G. N. Colasurdo. 2001. Induction and regulation of nitric oxide synthase in airway epithelial cells by respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 163**:**532-539. [DOI] [PubMed] [Google Scholar]
- 17.Karupiah, G., J. H. Chen, S. Mahalingam, C. F. Nathan, and J. D. MacMicking. 1998. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 188**:**1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane, T. E., A. D. Paoletti, and M. J. Buchmeier. 1997. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J. Virol. 71**:**2202-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin, Y. L., Y. L. Huang, S. H. Ma, C. T. Yeh, S. Y. Chiou, L. K. Chen, and C. L. Liao. 1997. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J. Virol. 71**:**5227-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manocha, S., K. R. Walley, and J. A. Russell. 2003. Severe acute respiratory distress syndrome (SARS): a critical care perspective. Crit. Care Med. 31**:**2684-2692. [DOI] [PubMed] [Google Scholar]
- 21.Marra, M. A., S. J. Jones, C. R. Astell, R. A. Holt, A. Brooks-Wilson, Y. S. Butterfield, J. Khattra, J. K. Asano, S. A. Barber, S. Y. Chan, A. Cloutier, S. M. Coughlin, D. Freeman, N. Girn, O. L. Griffith, S. R. Leach, M. Mayo, H. McDonald, S. B. Montgomery, P. K. Pandoh, A. S. Petrescu, A. G. Robertson, J. E. Schein, A. Siddiqui, D. E. Smailus, J. M. Stott, G. S. Yang, F. Plummer, A. Andonov, H. Artsob, N. Bastien, K. Bernard, T. F. Booth, D. Bowness, M. Czub, M. Drebot, L. Fernando, R. Flick, M. Garbutt, M. Gray, A. Grolla, S. Jones, H. Feldmann, A. Meyers, A. Kabani, Y. Li, S. Normand, U. Stroher, G. A. Tipples, S. Tyler, R. Vogrig, D. Ward, B. Watson, R. C. Brunham, M. Krajden, M. Petric, D. M. Skowronski, C. Upton, and R. L. Roper. 2003. The genome sequence of the SARS-associated coronavirus. Science 300**:**1399-1404. [DOI] [PubMed] [Google Scholar]
- 22.Mirazimi, A., and L. Svensson. 2000. ATP is required for correct folding and disulfide bond formation of rotavirus VP7. J. Virol. 74**:**8048-8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy, A. W., T. A. Platts-Mills, M. Lobo, and F. Hayden. 1998. Respiratory nitric oxide levels in experimental human influenza. Chest 114**:**452-456. [DOI] [PubMed] [Google Scholar]
- 24.Ng, L. F., M. Wong, S. Koh, E. E. Ooi, K. F. Tang, H. N. Leong, A. E. Ling, L. V. Agathe, J. Tan, E. T. Liu, E. C. Ren, L. C. Ng, and M. L. Hibberd. 2004. Detection of severe acute respiratory syndrome coronavirus in blood of infected patients. J. Clin. Microbiol. 42**:**347-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson, S., G. Bonecini-Almeida Mda, J. R. Lapa e Silva, C. Nathan, Q. W. Xie, R. Mumford, J. R. Weidner, J. Calaycay, J. Geng, N. Boechat, et al. 1996. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J. Exp. Med. 183**:**2293-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poljakovic, M., D. Karpman, C. Svanborg, and K. Persson. 2002. Human renal epithelial cells express iNOS in response to cytokines but not bacteria. Kidney Int. 61**:**444-455. [DOI] [PubMed] [Google Scholar]
- 27.Pope, M., P. A. Marsden, E. Cole, S. Sloan, L. S. Fung, Q. Ning, J. W. Ding, J. L. Leibowitz, M. J. Phillips, and G. A. Levy. 1998. Resistance to murine hepatitis virus strain 3 is dependent on production of nitric oxide. J. Virol. 72**:**7084-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimmelzwaan, G. F., M. M. Baars, P. de Lijster, R. A. Fouchier, and A. D. Osterhaus. 1999. Inhibition of influenza virus replication by nitric oxide. J. Virol. 73**:**8880-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rota, P. A., M. S. Oberste, S. S. Monroe, W. A. Nix, R. Campagnoli, J. P. Icenogle, S. Penaranda, B. Bankamp, K. Maher, M. H. Chen, S. Tong, A. Tamin, L. Lowe, M. Frace, J. L. DeRisi, Q. Chen, D. Wang, D. D. Erdman, T. C. Peret, C. Burns, T. G. Ksiazek, P. E. Rollin, A. Sanchez, S. Liffick, B. Holloway, J. Limor, K. McCaustland, M. Olsen-Rasmussen, R. Fouchier, S. Gunther, A. D. Osterhaus, C. Drosten, M. A. Pallansch, L. J. Anderson, and W. J. Bellini. 2003. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300**:**1394-1399. [DOI] [PubMed] [Google Scholar]
- 30.Saxena, S. K., A. Mathur, and R. C. Srivastava. 2001. Induction of nitric oxide synthase during Japanese encephalitis virus infection: evidence of protective role. Arch. Biochem. Biophys. 391**:**1-7. [DOI] [PubMed] [Google Scholar]
- 31.Taylor-Robinson, A. W., F. Y. Liew, A. Severn, D. Xu, S. J. McSorley, P. Garside, J. Padron, and R. S. Phillips. 1994. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur. J. Immunol. 24**:**980-984. [DOI] [PubMed] [Google Scholar]
- 32.Wong, C. K., C. W. Lam, A. K. Wu, W. K. Ip, N. L. Lee, I. H. Chan, L. C. Lit, D. S. Hui, M. H. Chan, S. S. Chung, and J. J. Sung. 2004. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136**:**95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie, Q. W., H. J. Cho, J. Calaycay, R. A. Mumford, K. M. Swiderek, T. D. Lee, A. Ding, T. Troso, and C. Nathan. 1992. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256**:**225-228. [DOI] [PubMed] [Google Scholar]
- 34.Yam, W. C., K. H. Chan, L. L. Poon, Y. Guan, K. Y. Yuen, W. H. Seto, and J. S. Peiris. 2003. Evaluation of reverse transcription-PCR assays for rapid diagnosis of severe acute respiratory syndrome associated with a novel coronavirus. J. Clin. Microbiol. 41**:**4521-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]