Association between hormone replacement therapy and subsequent stroke: a meta-analysis (original) (raw)
Abstract
Objectives To review completed trials assessing effect of hormone replacement therapy on subsequent risk of stroke, assessing stroke by pathological type, severity, and outcome.
Design Systematic review of randomised controlled trials identified from the Cochrane Library, Embase, and Medline; reviews; and reference lists of relevant papers.
Studies reviewed 28 trials, with 39 769 subjects, were identified.
Review measures Rates for cerebrovascular events analysed with a random effects model. Sensitivity analyses for heterogeneity included phase of prevention (primary or secondary), type of hormone replacement therapy (oestrogen alone or combined with progesterone), type of oestrogen (estradiol or conjugated equine oestrogen), size of trial (< 5000 or > 5000 patients), length of follow up (£ 3 years or > 3 years), sex (women only or men only), and trial quality (high or low).
Results Hormone replacement therapy was associated with significant increases in total stroke (odds ratio 1.29 (95% confidence interval 1.13 to 1.47), n = 28), non-fatal stroke (1.23 (1.06 to 1.44), n = 21), stroke leading to death or disability (1.56 (1.11 to 2.20), n = 14), ischaemic stroke (1.29 (1.06 to 1.56), n = 16), and a trend to more fatal stroke (1.28 (0.87 to 1.88), n = 22). It was not associated with haemorrhagic stroke (1.07 (0.65 to 1.75), n = 17) or transient ischaemic attack (1.02 (0.78 to 1.34), n = 22). Statistical heterogeneity was not present in any analysis.
Conclusions Hormone replacement therapy was associated with an increased risk of stroke, particularly of ischaemic type. Among subjects who had a stroke, those taking hormone replacement therapy seemed to have a worse outcome. Hormone replacement therapy cannot be recommended for the primary or secondary prevention of stroke.
Introduction
Sex steroid hormones are believed to provide women with endogenous protection against cerebrovascular events—premenopausal women have a lower risk of stroke than men of the same age,1,2 and the incidence of stroke in women increases rapidly after the menopause,3 coincident with diminished circulating levels of oestrogen and progesterone. As a result, hormone replacement therapy has been used widely for vascular prophylaxis in parallel with its known effects in reducing menopausal symptoms and bone loss. However, two meta-analyses of observational studies have suggested that hormone replacement therapy may increase risk of stroke, especially ischaemic stroke.4,5 Furthermore, the results of randomised controlled trials have given conflicting results, with studies either finding no benefit or apparent hazard. A recent non-systematic review of randomised controlled trials found that hormone replacement therapy was associated with an increased risk of stroke.6
The aim of this study was to review systematically the evidence from completed randomised controlled trials of hormone replacement therapy and subsequent stroke risk, in particular assessing stroke by pathological type, severity, and outcome.
Methods
_Literature search_—We identified publications from searches of the Cochrane Library, Embase, Medline (to May 2004), previous reviews,7-10 and reference lists from identified articles.
_Study selection_—We included completed, published, and non-confounded randomised controlled trials that compared hormone replacement therapy with a control group and that reported stroke events, or where such events could be calculated. Trials could include participants of either sex since early studies assessed the role of hormone replacement therapy in preventing vascular events in men. We excluded publications not in English or where event numbers were given for stroke and transient ischaemic attack combined and not separately.
_Quality assessment_—We assessed studies for quality of randomisation, blinding, reporting of withdrawals, generation of random numbers, and concealment of allocation. Trials scored one point for each area addressed, therefore receiving a score between 0 and 5 (highest level of quality).11
_Data abstraction_—All data were independently extracted by LJG and PMWB. Disparities were resolved by discussion.
_Study characteristics_—We recorded information on trial size, treatment regimen (oestrogen alone or plus progesterone), length of follow up, and outcome. Outcomes included stroke events (fatal and non-fatal), type of stroke (ischaemic, haemorrhagic, not known), and functional outcome (combined death and disability or dependency). Where data were available, we also recorded the number of transient ischaemic attacks (not included in the overall stroke outcome) and data related to intention to treat analyses.
Quantitative data synthesis
We analysed data using Stata (version 7) and Cochrane Review Manager (version 4.2). We assessed the effect of hormone replacement therapy on dichotomous outcomes from the odds ratio calculated with a random effects model since we expected the trials to be heterogeneous.
We used pre-specified sensitivity analyses to explain any heterogeneity, including phase of stroke prevention (primary or secondary), type of hormone replacement therapy (oestrogen only or oestrogen plus progesterone), type of oestrogen (estradiol or conjugated equine oestrogen), size of trial (£ 5000 or > 5000 patients), length of follow up (£ 3 years or > 3 years), sex, and quality of trial (high (5) or low (< 5)). We assessed interactions between subgroups and treatment. We examined publication bias using Eggers test.12
Results
Study characteristics
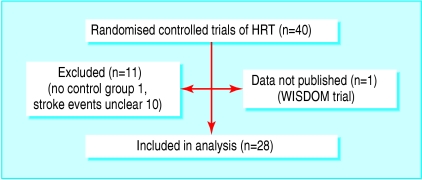
We identified 28 trials with 39 769 subjects for inclusion in our study (fig 1, table A on bmj.com).w1-w28 The trials varied in size between 59 subjectsw17 and 16 608.w26 Fifteen trials investigated primary prevention of stroke,w5-w7 w9-w12 w14 w17-w19 w22 w24 w26 w28 and 12 studied patients with prior vascular events (stroke,w1-w3 w21 ischaemic heart disease,w4 w8 w15 w16 w20 w23 w25 and venous thromboembolismw13). The average age of patients varied from 55 to 71. Three trials included men, with one trial of men exclusively.w1-w3 Three trials required that women should not have had a hysterectomy.w9 w20 w26 Follow up varied from 0.7 to 6.8 years. Twelve trials studied hormone replacement therapy with oestrogen alone, and 16 studied oestrogen plus progesterone. All trials, apart from five,w6 w14-w16 w27 were placebo controlled. Eleven trials, all small, did not record any stroke events.
Fig 1.
Results of literature search for randomised controlled trials of hormone replacement therapy (HRT) that reported stroke events
We excluded 12 trials (fig 1, table B on bmj.com), eight because they did not report vascular events,w30-w35 w37 w38 two because they did not distinguish between stroke and transient ischaemic attacks (total n = 685),w36 w39 and one because it did not have a control group.w40 The women's international study of long duration oestrogen after the menopause (WISDOM, n = 5664) was closed early after the release of data from the dual therapy arms of the women's health initiative trialw29; its data are yet to be published.
Data quality
Trials varied in their quality score11 from 2 to 5, median 5 (maximum score). All trials included were randomised, and 96% of trials gave adequate details of withdrawals.
Quantitative data synthesis
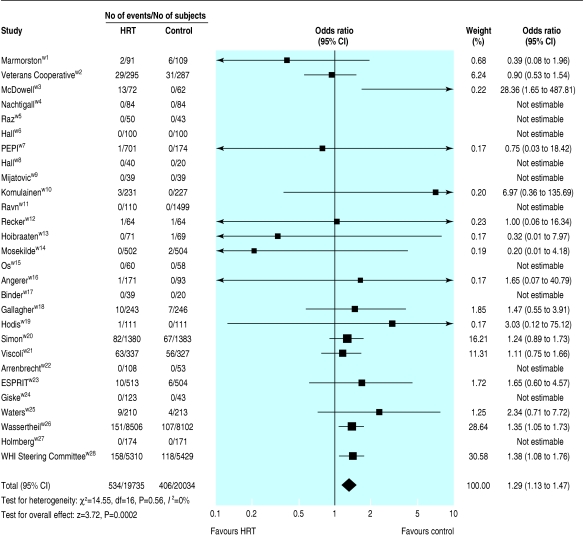
Stroke occurred in 2% of the participants randomised to no hormone replacement therapy, and this rate was significantly increased by a third (number needed to harm 147) in those randomised to hormone replacement therapy (table 1, fig 2). This increase in stroke resulted from an excess of ischaemic strokes but not primary intracerebral haemorrhage, as was seen in the women's health initiative dual trial.w26 An early increase in stroke occurred during the first six months of treatment in Viscoli et al's trial of secondary stroke prevention,w21 analogous to the early increase in coronary events seen in a trial of secondary prevention of coronary heart disease.w20 Both therapy arms of the women's health initiative trial (oestrogen alone and in combination with progesterone) were stopped prematurely because of therapy being associated with hazard.w26 w28
Table 1.
Effect of hormone replacement therapy on stroke and its type and outcome, and transient ischaemic attack
| No of trials | No of subjects | No of events | Control event rate (%) | Odds ratio*(95% CI), P value | Heterogeneity (P value) | |
|---|---|---|---|---|---|---|
| Stroke: | 28 | 39 769 | 940 | 2.03 | 1.29 (1.13 to 1.47), 0.0002 | 0.56 |
| Ischaemic | 16 | 23 426 | 443 | 1.59 | 1.29 (1.06 to 1.56), 0.01 | 0.59 |
| Haemorrhagic | 17 | 23 690 | 63 | 0.25 | 1.07 (0.65 to 1.75), 0.79 | 0.75 |
| Transient ischaemic attack | 22 | 10 050 | 233 | 2.13 | 1.02 (0.78 to 1.34), 0.86 | 0.86 |
| Outcome: | ||||||
| Fatal | 22 | 36 430 | 129 | 0.29 | 1.28 (0.87 to 1.88), 0.21 | 0.39 |
| Non-fatal | 21 | 36 230 | 710 | 1.72 | 1.23 (1.06 to 1.44), 0.007 | 0.45 |
| Death or dependency | 14 | 20 445 | 145 | 0.53 | 1.56 (1.11 to 2.20), 0.01 | 0.93 |
Fig 2.
Effects of hormone replacement therapy (HRT) on stroke events
A poor outcome after stroke, judged as combined death and dependency, was increased by half with hormone replacement therapy; we also found a non-significant increase in fatal stroke. This relation between hormone replacement therapy and severe stroke was present individually in three trials.w20 w21 w26 Hormone replacement therapy did not alter the rate of transient ischaemic attack (table 1). We found no statistical heterogeneity for any of the stroke outcomes.
Table 2 shows the results of our sensitivity analyses on several prognostic factors for the total stroke outcome. These results seem to be driven by the large women's health initiative trial, with significant effects being seen for the subgroups that included this study. However, we found no significant heterogeneity between trials examining primary versus secondary stroke prevention, oestrogen alone versus combination hormone replacement therapy, conjugated equine oestrogen versus estradiol, shorter versus longer follow up, smaller versus larger trials, those including exclusively men versus women alone, and high versus lower quality. We found no significant publication bias for the “all stroke” outcome (Eggers test P = 0.19).
Table 2.
Sensitivity analyses of the effect of hormone replacement therapy on total stroke
| No of trials | No of subjects | Control event rate (%) | Odds ratio (95% CI) | χ2 interaction | |
|---|---|---|---|---|---|
| Stroke, all | 28 | 39 769 | 2.03 | 1.29 (1.13 to 1.47) | |
| Stroke prevention: | |||||
| Primary | 16 | 33 236 | 1.40 | 1.37 (1.15 to 1.62) | 0.29 |
| Secondary | 12 | 6 533 | 5.33 | 1.18 (0.88 to 1.59) | |
| Hormone replacement therapy: | |||||
| Oestrogen alone | 12 | 14 256 | 3.05 | 1.21 (0.87 to 1.67) | 0.74 |
| Oestrogen plus progesterone | 16 | 25 513 | 1.46 | 1.32 (1.90 to 1.60) | |
| Type of oestrogen*: | |||||
| Estradiol | 13 | 4 569 | 3.02 | 1.17 (0.82 to 1.68) | 0.59 |
| Conjugated equine oestrogens | 13 | 33 194 | 2.11 | 1.30 (1.10 to 1.54) | |
| Trial size: | |||||
| Small (<5000) | 26 | 12 422 | 2.78 | 1.18 (0.96 to 1.46) | 0.33 |
| Large (>5000) | 2 | 27 347 | 1.88 | 1.37 (1.15 to 1.62) | |
| Length of follow up (years): | |||||
| Shorter (<3) | 22 | 8 027 | 2.83 | 1.15 (0.87 to 1.50) | 0.39 |
| Longer (>3) | 6 | 31 472 | 1.87 | 1.34 (1.15 to 1.56) | |
| Sex: | |||||
| Women only† | 26 | 38 926 | 1.90 | 1.31 (1.14 to 1.51) | — |
| Men only | 1 | 582 | 10.8 | 0.90 (0.53 to 1.54) | |
| Quality: | |||||
| High (5/5) | 15 | 34 987 | 2.33 | 1.29 (1.13 to 1.48) | 0.35 |
| Low (<5/5) | 13 | 4 782 | 0.27 | 1.71 (0.26 to 11.46) |
Discussion
This systematic review supports the results of individual trials and previous reviews finding that hormone replacement therapy does not reduce the risk of stroke in postmenopausal women. Indeed, it was associated with an overall 29% increase in the risk of stroke. This effect was driven by an increase in ischaemic but not haemorrhagic stroke. Importantly, the severity of stroke was increased with hormone replacement therapy, since the frequency of a poor functional outcome, judged as combined death and disability or dependency, was 56% higher in those randomised to therapy. Similarly, fatal stroke was non-significantly increased.
Possible explanations for results
Why hormone replacement therapy should increase ischaemic stroke and its severity when biological plausibility and some previous observational studies suggested it might protect against cerebrovascular events remains unclear. Firstly, it is possible that the results of the studies we reviewed are wrong, but this is unlikely since in none of the studies did hormone replacement therapy reduce stroke—25 trials had neutral results, and three found that therapy increased stroke risk.
Secondly, the trials used either oestrogen alone or combination hormone replacement therapy. Although long term therapy with oestrogen alone can promote uterine cancer, this would not explain an increase in stroke. In contrast, adding a progestogen could have had detrimental effects since progestogens can promote atherogenesis and vasoconstriction.13 This is particularly relevant for medroxyprogesterone acetate, which was used in most of the trials of combination therapy. However, we found no heterogeneity between trials of oestrogen alone and combination therapy, suggesting that oestrogen itself, given as estradiol or conjugated equine oestrogen, might be the culprit.
Thirdly, within-class differences in hormone replacement therapy may mean that the most appropriate type of oestrogen has yet to be tested adequately; the trials assessed either conjugated equine oestrogens or estradiol but not other types such as phytoestrogen.14 However, there was no evidence for statistical heterogeneity between the trials with respect to type of oestrogen.
Fourthly, the dose of oestrogen (and progestogen if present) may have been too high. The usual starting doses of conjugated equine oestrogen and estradiol in Britain in postmenopausal women are 0.625 mg and 1 mg respectively, although the dose may be titrated up if menopausal vasomotor symptoms persist. These doses are below those used in several of the trials.
Fifthly, the delivery route may be important since substantial pharmacological differences exist between oral and transdermal administration of oestrogen, especially regarding hepatic first pass metabolism.
Lastly, several of the trials may have been too short, with a median length of less than three years, contrasting with the earlier observational studies. Of note, the trials by Simon et al and Viscoli et al showed an early vascular hazard that disappeared later.w20 w21 The hazard during the first year of treatment seems to reflect the development of a thrombophilic state that may not persist. This raises the possibility that an extended follow up would have revealed long term benefit. An analogous situation exists with statin treatment, whereby benefit was found in trials with longer rather than shorter follow up.15 Nevertheless, the women's health initiative trial, the largest of the trials we reviewed, had follow up for more than five years and yet found no beneficial effect on stroke risk in either of its therapy arms.
Conclusions
We have found that the use of hormone replacement therapy is associated with an increased risk of stroke, typically ischaemic in type and severe in nature. Hormone replacement therapy cannot be recommended for the primary or secondary prevention of stroke.
What is already known on this topic
Postmenopausal women have a greater risk of stroke than premenopausal women
Hormone replacement therapy has been used widely for vascular prophylaxis and to reduce menopausal symptoms and bone loss
Some randomised controlled trials have shown that hormone replacement therapy may increase the risk of stroke
What this study adds
Hormone replacement therapy is associated with an increased risk of stroke, especially of ischaemic type
Hormone replacement therapy cannot be recommended for the primary or secondary prevention of stroke
Supplementary Material
[extra: Search strategy, references w1- w40]
 Details of the search strategy used, of the trials identified in the search, and of references w1-w40 are on bmj.com
Details of the search strategy used, of the trials identified in the search, and of references w1-w40 are on bmj.com
Contributors: PMWB conceived of the study, collated data, and drafted the paper and is guarantor. LJG collated data, performed statistical analyses, and revised the paper.
Funding: PMWB is Stroke Association professor of stroke medicine. LJG is funded, in part, by the Stroke Association (TSA 01/03) and BUPA Foundation. The Division of Stroke Medicine receives core funding from the Stroke Association.
Completing interests: None declared.
Ethical approval: None required.
Amendment
This is Version 2 of the paper. In this version table 1 has been corrected to show the control event rate for the outcome “Death or dependency” as 0.53% [not 0.1%]. Also, in the discussion, in the section “Possible explanations for results,” the second sentence now reads: “Firstly, it is possible that the results of the studies we reviewed are wrong, but this is unlikely since in none of the studies did hormone replacement therapy reduce stroke—25 [not 11] trials had neutral results, and three found that therapy increased stroke risk.”
References
- 1.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA 1991;265: 1861-7. [PubMed] [Google Scholar]
- 2.Kannel WB, Thom TJ. Incidence, prevalence and mortality of cardiovascular disease. In: Schlant RC, Alexander RW, eds. The heart. New York: McGraw-Hill, 1994: 185-97.
- 3.Wenger N, Speroff L, Packard B. Cardiovascular health and disease in women. N Engl J Med 1993;329: 247-56. [DOI] [PubMed] [Google Scholar]
- 4.Paganini-Hill A. Hormone replacement therapy and stroke: risk, protection or no effect? Maturitas 2001;38: 243-61. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HD, Humphrey LL, Hygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy. Scientific review. JAMA 2002;288: 872-81. [DOI] [PubMed] [Google Scholar]
- 6.Beral V, Banks E, Reeves G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet 2002;360: 942-4. [DOI] [PubMed] [Google Scholar]
- 7.Wren BG. Megatrials of hormonal replacement therapy. Drugs Aging 1998;12: 343-8. [DOI] [PubMed] [Google Scholar]
- 8.Zec RF, Trivedi MA. Effects of hormone replacement therapy on cognitive aging and dementia risk in postmenopausal women: a review of ongoing large-scale, long-term clinical trials. Climacteric 2002;5: 122-34. [PubMed] [Google Scholar]
- 9.Collins P. Clinical cardiovascular studies of hormone replacement therapy. Am J Cardiol 2002;90(suppl): 30-4F. [DOI] [PubMed] [Google Scholar]
- 10.Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women. J Gen Intern Med 2004;19: 791-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352: 609-13. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315: 629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badimon L, Bayes-Genis A. Effects of progestogens on thrombosis and atherosclerosis. Hum Reprod Update 1999;5: 191-9. [DOI] [PubMed] [Google Scholar]
- 14.Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med 2001;161: 1161-72. [DOI] [PubMed] [Google Scholar]
- 15.Collins R, Armitage J. High-risk elderly patients prosper from cholesterol-lowering therapy [commentary]. Lancet 2002;360: 1618-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[extra: Search strategy, references w1- w40]