A Virus that Infects a Hyperthermophile Encapsidates A-Form DNA (original) (raw)
. Author manuscript; available in PMC: 2017 Jul 17.
Published in final edited form as: Science. 2015 May 22;348(6237):914–917. doi: 10.1126/science.aaa4181
Abstract
Extremophiles, microorganisms thriving in extreme environmental conditions must have proteins and nucleic acids that are stable at extremes of temperature and pH. The non-enveloped, rod-shaped virus SIRV2 infects the hyperthermophilic acidophile Sulfolobus islandicus which lives at 80° C and pH 3. We have used cryo-EM to generate a three-dimensional reconstruction of the SIRV2 virion at ~ 4 Å resolution, which revealed a novel form of virion organization. While almost half of the capsid protein is unstructured in solution, this unstructured region folds in the virion into a single extended α-helix which wraps around the DNA. The DNA is entirely in A-form, suggesting a common mechanism with bacterial spores for protecting DNA in the most adverse environments.
Extreme geothermal environments, with temperatures above 80° C, are the habitat of hyperthermophilic archaeal DNA viruses which parasitize Archaea (1). These viruses have more than 92% of genes without homologs in databases (2, 3), unique protein folds (4) and unique mechanisms of viral egress (5). The high diversity of virion morphotypes may underpin virion morphogenesis and DNA packaging which could determine the high stability of the virions. Viruses from the family Rudiviridae (6) consist of a non-enveloped, helically arranged nucleoprotein composed of dsDNA and thousands of copies of a 134-residue protein. To understand the mechanisms stabilizing rudiviral DNA in natural habitats of host cells, which involve high temperatures (~80°C) and low pH values (~pH 3), we have analysed by cryo-EM the rudivirus SIRV2 (6) which infects the hyperthermophilic acidophilic archaeon Sulfolobus islandicus (7). Members of the archaeal genus Sulfolobus maintain their cytoplasmic pH neutral at pH 5.6–6.5 (8, 9). SIRV2 is therefore exposed to a wide range of pH values: from about pH 6 in the cellular cytoplasm, where it assembles and maturates (10), to pH 2–3 in the extracellular environment. We performed our studies at pH 6. SIRV2 is stable a in a wide range of temperatures: from −80°C, the temperature at which the virus can be stored for years without loss of infectivity, to 80°C, the temperature of its natural environment. The overall morphology of the virion is maintained whether imaging using negative stain at 75° C (11) or cryo-EM with a sample at 4° C prior to vitrification (Fig. 1a).
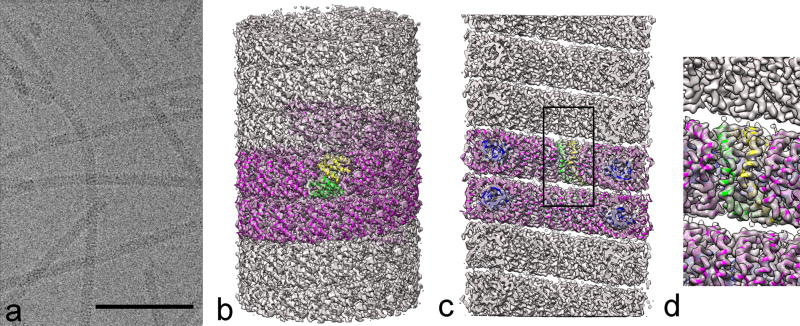
Figure 1. Cryo-EM and three-dimensional reconstruction of SIRV2.
a, Micrograph showing frozen/hydrated SIRV2 virions in vitreous ice. The space bar is 1,000 Å. b, A side view of the reconstructed virion with a ribbon model for the protein (magenta). The asymmetric unit in the virion contains a protein dimer, and one is shown with one chain in yellow and the other in green. c, A cutaway view showing the hollow lumen with the all α-helical protein segments that line the lumen. These α-helices wrap around the dsDNA (blue) and encapsidate it. d, A closeup view of the region shown within the rectangle in c.
Electron cryo-micrographs of SIRV2 (Fig. 1a) showed strong helical striations in most of the virions with a periodicity of 42 Å. Three-dimensional reconstruction was performed using the Iterative Helical Real Space Reconstruction (IHRSR) method (12), after first determining the helical symmetry. Only one solution (with 14.67 subunits per turn of the 42 Å pitch helix) yielded a reconstruction with recognizable secondary structure, almost all α-helical, and a resolution of ~ 3.8 Å in the more ordered interior which surrounds the DNA (Fig. S2). The asymmetric unit was a symmetrical dimer, the α-helices of which were wrapping around a continuous double stranded DNA. The DNA was in an A-form, in contrast to the B-form DNA observed in icosahedral bacteriophages (13–15).
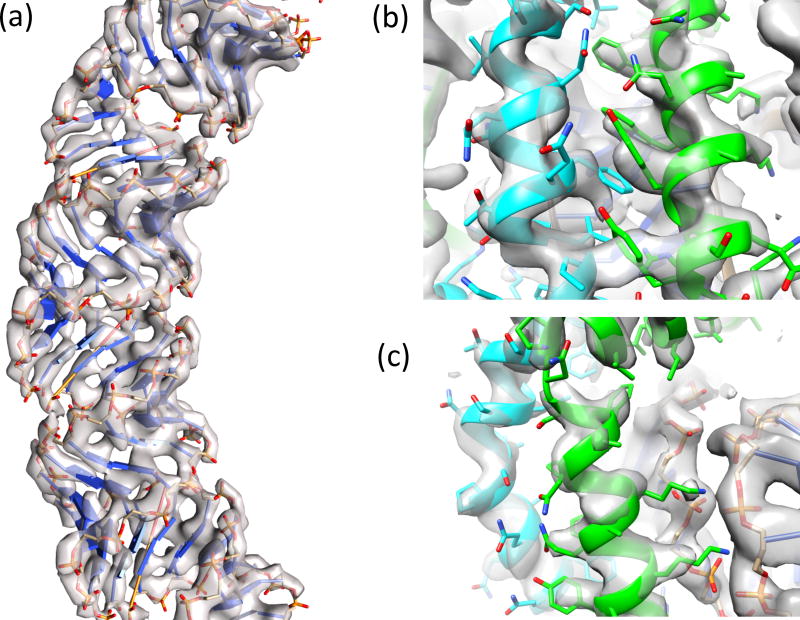
The atomic structure of SIRV2 was determined and refined using Rosetta guided by restraints from the EM data (16). We began by docking a model of A-form DNA into the map. The resulting model showed good agreement with the experimental data, where the rigid phosphate groups were well defined (Fig. 2a,c). A dimeric crystal structure from a close homolog with 88% identity (PDB id: 3f2e) was docked into the map. However, this model lacked 51 N-terminal residues, the first 46 of which were shown by NMR to be unstructured in the monomer in solution and not part of the fragment crystallized (17). In the cryo-EM reconstruction, these residues formed helices wrapping around the DNA. Since the NMR studies were done at pH 6, the same pH used for our cryo-EM studies, the gain in structure of these residues is associated with assembly, and not a change in pH. These N-terminal residues were built into the density map using RosettaCM (18). A representative model was chosen from a well-converged, low-energy ensemble (Fig. S4); this model shows good agreement with the sidechain density in the map at both protein-protein interfaces (Fig. 2b) and protein-DNA interfaces (Fig. 2c). Seven N-terminal residues could not be placed in the density.
Figure 2. The refined atomic model in the three-dimensional reconstruction.
a, The density allows accurate tracing of the nucleotide chain. b, The interface between helices within the asymmetric unit features large numbers of aromatic residues, which aid in correct registration of the sequence. c, Sidechains at the protein-DNA interface are well-defined in density.
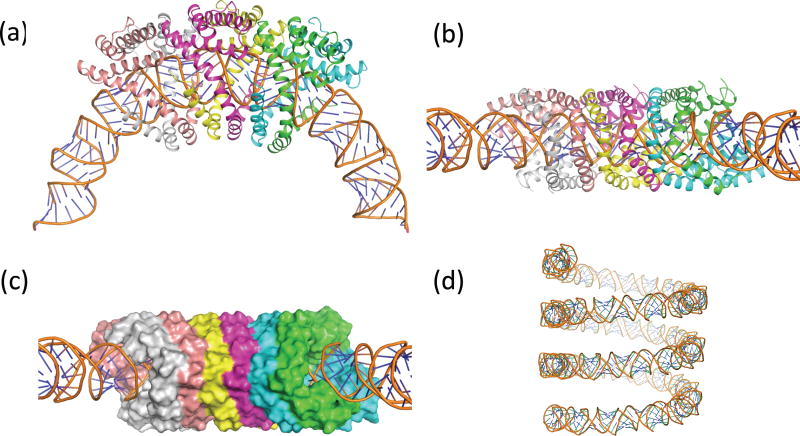
The final structure reveals that the N-terminal residues form a helix-turn-helix motif encapsulating the A-form DNA, with helices from each subunit in the asymmetric unit packing in an antiparallel configuration (Fig. 3a,b). Proline residues in this region (Pro27, Pro39, and Pro50) allow some helical deformation to tightly wrap the DNA. These interdigitated helices form a solvent-inaccessible surface surrounding the DNA (Fig. 3c). The DNA was confirmed as A-form (19), where the parameters, including an average base pair inclination of 25°, negative slide (average = −1.6) and a negative x-displacement (average = −4.8) match A-form DNA. The average phosphate-phosphate distance along the DNA backbone is 5.9 Å, as opposed to ~ 7.0 Å for B-DNA. The diameter of the DNA is ~ 24 Å. At this resolution, the sugar pucker is not discernable. A slight bulge in the DNA occurs near the dimer interface, where a buried arginine sidechain (Arg73) interacts with one of the DNA phosphate groups, leading to some local deviations from A-form DNA. Model bias was tested by starting with B-form, which converged to the same final model (Fig. S5). The DNA (Fig. 3d), whose axis is at a helical radius of ~ 60 Å, has three right-handed superhelical turns every 44 (=3*14.67) repeats (turns) of the DNA. So there are 528 bp (=44*12) per 47 right handed turns, which yields an overall twist of 11.2 bp/turn (Fig. 3d).
Figure 3. The SIRV2 protein dimer helices fully encapsulate the DNA.
a, Three asymmetric units of the virion are shown, illustrating how the N-terminal helices wrap around the DNA, forming antiparallel helix-helix packing. b, Side view. c, A surface view of the protein (using a 1.4 Å probe radius). d, The right-handed solenoidal supercoiling of the DNA, with three turns shown.
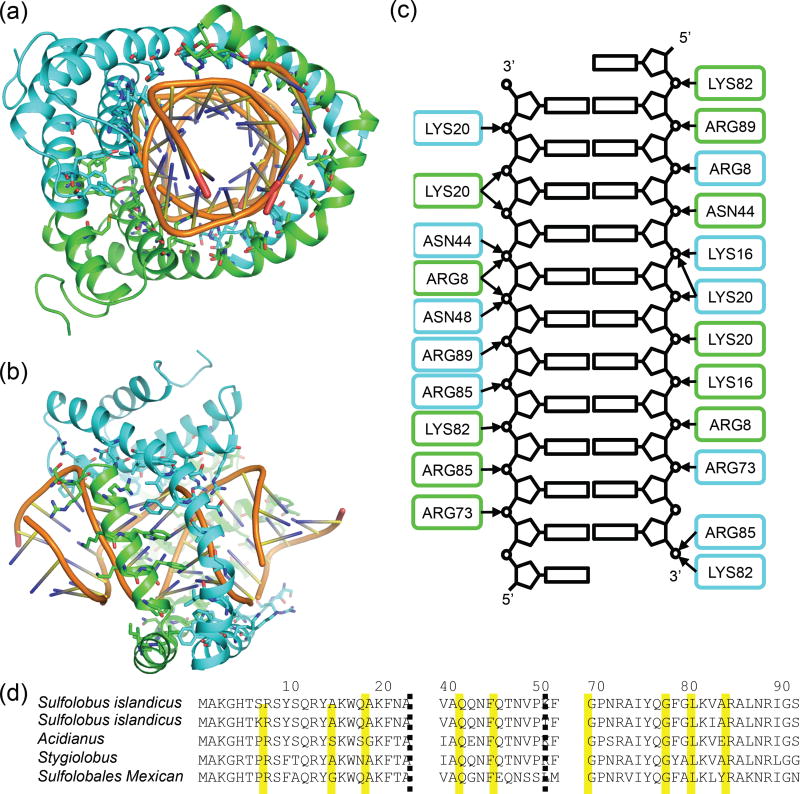
DNA-protein contacts (Fig. 4a–c) are largely polar, with nine conserved sidechains directly interacting with the DNA phosphate groups: Arg8, Lys16, Lys20, Asn44, Asn48, Arg73, Lys82, Arg85, and Arg89; the backbone of Ser9 also makes contact with the phosphate backbone of the DNA. There are also several hydrophobic contacts with the DNA, most notably the aromatic residues Trp17, Phe21, Phe24, and Phe52, as well as Val37. All of these residues are conserved in related rudiviruses (Fig. 4d), suggesting a similar method of DNA stabilization and protection. Extensive protein-DNA interactions in SIRV2 virions alleviate the necessity to package charge-neutralizing counterions (13, 14). Within the dimer, protein-protein interfaces are largely hydrophobic. The interface is extensive, comprising about 17% of each monomer’s surface area, with a total interface area of 1,491 Å2. Five aromatic residues (Tyr10, Tyr14, Trp17, Phe21 and Phe45) form a well-packed barrier separating DNA from solvent. The protein-protein interface between adjacent dimers also forms a largely hydrophobic interface, with 1,706 Å2 of contact area between dimers on both sides. Interactions between dimers across the groove of the helix are weak and largely polar.
Figure 4. An overview of protein-DNA contacts in the virion.
a, The protein-DNA interface is mainly polar, with largely Arg and Lys sidechains contacting phosphate groups in the DNA. b, Side view. c, A schematic indicating all the polar protein-DNA contacts; the coloring of each subunit is the same as in (a) and (b). d, A multiple sequence alignment with related archaeal rod-shaped viruses indicates that all these contacts are well conserved. SIRV2, Sulfolobus islandicus rod-shaped virus 2; ARV1, Acidianus rod-shaped virus 1; SRV, Stygiolobus rod-shaped virus; SMRV1, Sulfolobales Mexican rudivirus 1. The yellow boxes indicate the residues that are interacting with the DNA backbones.
Small acid-soluble proteins (SASPs) are responsible for protecting DNA in gram positive bacterial spores (20) and are largely unstructured in solution (21–24), but become α-helical upon binding dsDNA (25, 26). Almost half of the SIRV2 capsid protein is unstructured in solution (17), and this portion becomes α-helical when bound to DNA in the virion. The binding of SASPs to DNA is saturable, with saturation occuring at an SASP/DNA weight ratio of ~ 3:1 (27). In the SIRV2 virion, we have now shown that the weight ratio of capsid protein to DNA is 3.5:1. The binding of the SASPs to DNA induces a dimerization of the SASPs (25), and the asymmetric unit in the virion contains a symmetrical dimer of the coat protein. The binding of SASPs to DNA induces a transition from B-DNA to A-DNA (27) and an en masse transition of DNA from B-form to A-form can be induced in bacterial cells, suggesting that A-form plays an unrecognized role in stabilizing DNA under adverse conditions such as dessication (28). Sequence analysis and structural comparison (with 2z3x.pdb) do not show obvious homology, suggesting that these similarities could have arisen as a result of convergent evolution.
Supplementary Material
Supplement
Acknowledgments
This work was supported by NIH GM035269 (to E.H.E.). The map and model have been deposited to the EMDB and PDB, with accession numbers EMD-6310 and 3J9X.PDB, respectively.
References
- 1.Prangishvili D. The wonderful world of archaeal viruses. Annu Rev Microbiol. 2013;67:565–585. doi: 10.1146/annurev-micro-092412-155633. [DOI] [PubMed] [Google Scholar]
- 2.Prangishvili D, Garrett RA, Koonin EV. Evolutionary genomics of archaeal viruses: unique viral genomes in the third domain of life. Virus research. 2006;117:52–67. doi: 10.1016/j.virusres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Krupovic M, Prangishvili D, Hendrix RW, Bamford DH. Genomics of bacterial and archaeal viruses: dynamics within the prokaryotic virosphere. Microbiology and molecular biology reviews : MMBR. 2011;75:610–635. doi: 10.1128/MMBR.00011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krupovic M, White MF, Forterre P, Prangishvili D. Postcards from the edge: structural genomics of archaeal viruses. Advances in virus research. 2012;82:33–62. doi: 10.1016/B978-0-12-394621-8.00012-1. [DOI] [PubMed] [Google Scholar]
- 5.Prangishvili D, Quax TE. Exceptional virion release mechanism: one more surprise from archaeal viruses. Current opinion in microbiology. 2011;14:315–320. doi: 10.1016/j.mib.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Prangishvili D, Arnold HP, Gotz D, Ziese U, Holz I, Kristjansson JK, Zillig W. A novel virus family, the Rudiviridae: Structure, virus-host interactions and genome variability of the sulfolobus viruses SIRV1 and SIRV2. Genetics. 1999;152:1387–1396. doi: 10.1093/genetics/152.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaubert C, Danioux C, Oberto J, Cortez D, Bize A, Krupovic M, She Q, Forterre P, Prangishvili D, Sezonov G. Genomics and genetics of Sulfolobus islandicus LAL14/1, a model hyperthermophilic archaeon. Open biology. 2013;3:130010. doi: 10.1098/rsob.130010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubben M, Schafer G. Chemiosmotic energy conversion of the archaebacterial thermoacidophile Sulfolobus acidocaldarius: oxidative phosphorylation and the presence of an F0-related N,N'-dicyclohexylcarbodiimide-binding proteolipid. J Bacteriol. 1989;171:6106–6116. doi: 10.1128/jb.171.11.6106-6116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker-Austin C, Dopson M. Life in acid: pH homeostasis in acidophiles. Trends in microbiology. 2007;15:165–171. doi: 10.1016/j.tim.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Bize A, Karlsson EA, Ekefjard K, Quax TE, Pina M, Prevost MC, Forterre P, Tenaillon O, Bernander R, Prangishvili D. A unique virus release mechanism in the Archaea. Proc Natl Acad Sci U S A. 2009;106:11306–11311. doi: 10.1073/pnas.0901238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quemin ER, Lucas S, Daum B, Quax TE, Kuhlbrandt W, Forterre P, Albers SV, Prangishvili D, Krupovic M. First insights into the entry process of hyperthermophilic archaeal viruses. J Virol. 2013;87:13379–13385. doi: 10.1128/JVI.02742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egelman EH. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy. 2000;85:225–234. doi: 10.1016/s0304-3991(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 13.Yu TY, Schaefer J. REDOR NMR characterization of DNA packaging in bacteriophage T4. J Mol Biol. 2008;382:1031–1042. doi: 10.1016/j.jmb.2008.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overman SA, Aubrey KL, Reilly KE, Osman O, Hayes SJ, Serwer P, Thomas GJ., Jr Conformation and interactions of the packaged double-stranded DNA genome of bacteriophage T7. Biospectroscopy. 1998;4:S47–56. doi: 10.1002/(SICI)1520-6343(1998)4:5+S47::AID-BSPY63.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Earnshaw W, Casjens S, Harrison SC. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976;104:387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- 16.DiMaio FS, Li Y, Brunner X, Xu M, Conticello C, Egelman V, Marlovits EH, Cheng T, Baker Y. D., Atomic accuracy models from 4.5 Å cryo-electron microscopy data with density-guided iterative local rebuilding and refinement. Nature methods. 2015 doi: 10.1038/nmeth.3286. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szymczyna BR, Taurog RE, Young MJ, Snyder JC, Johnson JE, Williamson JR. Synergy of NMR, computation, and X-ray crystallography for structural biology. Structure. 2009;17:499–507. doi: 10.1016/j.str.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y, DiMaio F, Wang RY, Kim D, Miles C, Brunette T, Thompson J, Baker D. High-resolution comparative modeling with RosettaCM. Structure. 2013;21:1735–1742. doi: 10.1016/j.str.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu XJ, Olson WK. 3DNA: a software package for the analysis, rebuilding and visualization of three-dimensional nucleic acid structures. Nucleic Acids Res. 2003;31:5108–5121. doi: 10.1093/nar/gkg680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setlow P. I will survive: DNA protection in bacterial spores. Trends in microbiology. 2007;15:172–180. doi: 10.1016/j.tim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Setlow B, Setlow P. Binding to DNA protects alpha/beta-type, small, acid-soluble spore proteins of Bacillus and Clostridium species against digestion by their specific protease as well as by other proteases. J Bacteriol. 1995;177:4149–4151. doi: 10.1128/jb.177.14.4149-4151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes CS, Setlow P. Analysis of deamidation of small, acid-soluble spore proteins from Bacillus subtilis in vitro and in vivo. J Bacteriol. 1997;179:6020–6027. doi: 10.1128/jb.179.19.6020-6027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes CS, Illades-Aguiar B, Casillas-Martinez L, Setlow P. In vitro and in vivo oxidation of methionine residues in small, acid-soluble spore proteins from Bacillus species. J Bacteriol. 1998;180:2694–2700. doi: 10.1128/jb.180.10.2694-2700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes CS, Alarcon-Hernandez E, Setlow P. N-terminal amino acid residues mediate protein-protein interactions between DNA-bound alpha/beta -type small, acid-soluble spore proteins from Bacillus species. J Biol Chem. 2001;276:2267–2275. doi: 10.1074/jbc.M007858200. [DOI] [PubMed] [Google Scholar]
- 25.Hayes CS, Peng ZY, Setlow P. Equilibrium and kinetic binding interactions between DNA and a group of novel, nonspecific DNA-binding proteins from spores of Bacillus and Clostridium species. J Biol Chem. 2000;275:35040–35050. doi: 10.1074/jbc.M005669200. [DOI] [PubMed] [Google Scholar]
- 26.Lee KS, Bumbaca D, Kosman J, Setlow P, Jedrzejas MJ. Structure of a protein-DNA complex essential for DNA protection in spores of Bacillus species. Proc Natl Acad Sci U S A. 2008;105:2806–2811. doi: 10.1073/pnas.0708244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohr SC, Sokolov NV, He CM, Setlow P. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc Natl Acad Sci U S A. 1991;88:77–81. doi: 10.1073/pnas.88.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelan DR, Hiscox TJ, Rood JI, Bambery KR, McNaughton D, Wood BR. Detection of an en masse and reversible B- to A-DNA conformational transition in prokaryotes in response to desiccation. Journal of the Royal Society, Interface / the Royal Society. 2014;11:20140454. doi: 10.1098/rsif.2014.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettstetter M, Peng X, Garrett RA, Prangishvili D. AFV1, a novel virus infecting hyperthermophilic archaea of the genus acidianus. Virology. 2003;315:68–79. doi: 10.1016/s0042-6822(03)00481-1. [DOI] [PubMed] [Google Scholar]
- 30.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. Journal of Structural Biology. 2003;142:334–347. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 31.Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: Processing and visualization of images in 3D electron microscopy and related fields. Journal of Structural Biology. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 32.Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ. EMAN2: an extensible image processing suite for electron microscopy. Journal of Structural Biology. 2007;157:38–46. doi: 10.1016/j.jsb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nature methods. 2014;11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal PB, Henderson R. Optimal Determination of Particle Orientation, Absolute Hand, and Contrast Loss in Single-particle Electron Cryomicroscopy. Journal of Molecular Biology. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Settembre E, Xu C, Dormitzer PR, Bellamy R, Harrison SC, Grigorieff N. Near-atomic resolution using electron cryomicroscopy and single-particle reconstruction. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1867–1872. doi: 10.1073/pnas.0711623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement