Is Pelvic Floor Muscle Training Effective for Men With Poststroke Lower Urinary Tract Symptoms? A Single-Blinded Randomized, Controlled Trial (original) (raw)
Abstract
The aim of the current study was to evaluate the effect of pelvic floor muscle training in men with poststroke lower urinary tract symptoms. Thirty-one poststroke men, median age 68 years, were included in this single-blinded randomized controlled trial. Thirty participants, 15 in each group, completed the study. The intervention consisted of 3 months (12 weekly sessions) of pelvic floor muscle training in groups and home exercises. The effect was evaluated by the DAN-PSS-1 (Danish Prostate Symptom Score) questionnaire, a voiding diary, and digital anal palpation of the pelvic floor muscle. The DAN-PSS-1, symptom score indicated a statistical significant improvement (p < .01) in the treatment group from pretest to posttest, but not in the control group. The DAN-PSS-1, total score improved statistically significantly in both groups from pretest to posttest (treatment group: p < .01; control group: p = .03). The median voiding frequency per 24 hours decreased from 11 at pretest to 7 (36%; p = .04) at posttest and to 8 (27%; p = .02) at follow-up in treatment group, although not statistical significantly more than the control group. The treatment group but not the control group improved statistically significantly in pelvic floor muscle function (p < .01) and strength (p < .01) from pretest to posttest and from pretest to follow-up (p = .03; p < .01). Compared with the control group the pretest to posttest was significantly better in the treatment group (p = .03). The results indicate that pelvic floor muscle training has an effect for lower urinary tract symptoms, although statistical significance was only seen for pelvic floor muscle.
Keywords: male incontinence, stroke, overactive bladder, quality of life
Introduction
Lower urinary tract symptoms (LUTS); (Abrams et al., 2002) are highly prevalent in both male and female patients after stroke, ranging up to 94% (Tibaek et al., 2008; Williams, Srikanth, Bird, & Thrift, 2012). The poststroke symptoms are nocturia (Tibaek et al., 2008; Williams et al., 2012), urgency, frequent daytime voiding, urge incontinence, incomplete bladder emptying, and urinary tract infection (Ruffion et al., 2013).
Stroke has a profound effect on quality of life. For patients with LUTS, the impact is also substantial, leading to increasing limitations in social and physical activities with psychological (anxiety, depression, and isolation) aspects (Tibaek et al., 2008) as well as increased risk of falls (Divani, Vazquez, Barrett, Asadollahi, & Luft, 2009).
Pelvic floor muscle training (PFMT) is regarded as the first-line conservative treatment option for LUTSin neurologically healthy men (Gormley et al., 2012; Hay-Smith, Berghmans, & Burgio, 2009; Hunter, Glazener, & Moore, 2007; Newman, Guzzo, Lee, & Jayadevappa, 2014). It has also shown positive effects on LUTS in women with neurological diseases (De Ridder, Vermeulen, Ketelaer, Van Poppel, & Baert, 1999; Lucio, D’Ancona, Lopes, Perissinotto, & Damasceno, 2014; Lucio et al., 2010; McClurg, Ashe, Marshall, & Lowe-Strong, 2006; Tibaek, Gard, & Jensen, 2005). In women with urinary incontinence (UI) after a first stroke, 12 weeks of PFMT lead to significant reduction of UI leakage and frequency of daytime voiding while pelvic floor muscle (PFM) function increased significantly (Tibaek et al., 2005).
The goal of PFMT in stroke patients is to improve the contribution to the sphincter closure mechanism by motor learning and PFM awareness, function, strength, duration, and functional training. Furthermore, it is debated if an improved PFM can inhibit involuntary detrusor contraction (Fowler & Griffiths, 2010; Panicker, Fowler, & Kessler, 2015; Seseke et al., 2006; Zhang et al., 2005).
Although PFMT is a noninvasive treatment with no known side effects or complications, no randomized controlled trial (RCT) has evaluated its effect on poststroke LUTS in men. The aim of the current study was to evaluate the effect of PFMT on poststroke LUTS in men.
Material and Method
This study was part of a large, multifaceted study. The study focused on PFMT in poststroke men with LUTS (Tibaek et al., 2015).
Participants
Between February 1, 2010 and December 31, 2012, medical records from the Departments of Neurology at Glostrup and Herlev Hospitals, University of Copenhagen of poststroke men were successively screened according to inclusion and exclusion criteria. Inclusion criterion included (a) men, diagnosed with a stroke clinically defined according to the World Health Organization (1989) and confirmed by computed tomography or magnetic resonance imaging scan, (b) at least 1 month since their last stroke, (c) normal cognitive function (minimal mental examination score >25), (d) LUTS according to the International Continence Society definition (Abrams et al., 2002) with start or aggravation closely related to the stroke and measured by the Danish Prostatic Symptom Score (DAN-PSS-1) questionnaire (Meyhoff et al., 1993), (e) ability to walk independently indoors for at least 100m with or without walking aids, (f) outpatient and self-transported, (g) ability to visit the toilet independently, and (h) age >18 years. Exclusion criterion included (a) prior history of LUTS surgery, pelvic surgery, or trauma; (b) more than two strokes (diagnosed at a hospital); (c) other severe neurological disease including dementia; (d) severe dysphasia; (e) severe psychiatric disease; (f) prostate cancer; and (g) not speaking Danish or English.
Design
The design was an experimental prospective RCT using randomized, controlled, and single-blinded parallel groups. The trial started with a 4-week run-in period including an initial investigation sequence (pretest). This was followed by randomization to either a treatment group (TG) or a control group (CG).
After 12 weeks, the second investigation sequence was performed (posttest), with the final investigation sequence (follow-up) after a further 6 months. Randomization was based on a mathematical table delivered in blocks of 10 in sealed envelopes and managed by a person who did not participate further in the study.
Registration of variables from the pretest, posttest, and follow-up investigation sequences were managed by individuals (physiotherapists) who were blinded to the randomization code of the participants, while the physiotherapist treating the participants with PFMT was unblinded. The participants randomized for CG were all offered a 12-week PFMT program after the trial. The participants received verbal and written information and signed an informed consent before entry into the study. The study was approved by the Ethical Committee of Copenhagen County (H-B-2009-069) and registered in the Danish Register for Data protection and in the Clinical Trials.gov Protocol Registration System (Clinical Trials NTC01042249).
Intervention
The participants in the TG were treated in a systematic, controlled, intensive PFMT program over 3 months (12 weekly sessions and 60 minutes per session) by the same specialized physiotherapist. The treatment program consisted of the following topics: introduction (theory), home exercise, group treatment, and digital anal palpation of PFM. The PFMT program has been published previously (Tibaek et al., 2015). The participants in the CG followed the standard program of general rehabilitation without any specific LUTS treatment.
Outcome Measures
- The DAN-PSS-1 questionnaire (primary outcome). The DAN-PSS-1 questionnaire (Meyhoff et al., 1993) consists of 12 questions related to LUTS in the 2 weeks prior to response. Each question has two parts, Part A and Part B. In Part A, the participants are asked to state the frequency and severity of the symptoms (symptom score) and in Part B, the impact on their daily life of the symptoms if present (bother score). The respondents classify all answers in a four-ranked scale from 0 to 3 with zero being absence of symptoms or bother and three the maximum severity of symptoms or bother. The total DAN-PSS-1 index was calculated as symptom score × bother score. The DAN-PSS-1 questionnaire has been reported as reliable and valid in a sample of poststroke men and women (Tibaek & Dehlendorff, 2009; Tibaek, Jensen, Klarskov, Iversen, & Gard, 2006).
- The voiding diary (primary outcome). A 3-day voiding diary in a modified version (Larsson, Abrams, & Victor, 1991) measured: (a) time and frequency of voiding, (b) number of incontinence episodes, and (c) number of pads used.
- 24-Hour pad test (primary outcome). A 24-hour (h) pad test.
- Digital anal palpation of PFM (secondary outcome).
- PFM function. Best of 3 attempts graduated on a 4-point ordinal scale 0 = no PFM contraction, 1 = partial PFM contraction, 2 = PFM contraction + cocontraction with other related muscles, 3 = isolated PFM contraction (Tibaek et al., 2005).
- PFM strength. Best of three attempts graded 0 to 6 (nil, flicker, weak, moderate, good, strong, very strong) on a Danish version of the modified Oxford scale (Laycock, 1994). The participants were instructed to perform a maximal voluntary contraction of the pelvic floor muscle without cocontraction of other related muscles.
- PFM static endurance. The participants were instructed to maintain the contraction for as long as possible. Static endurance was the point of isometric fatigue at which the muscle contraction could no longer be maintained and was measured in seconds on a stopwatch.
- PFM dynamic endurance. Maximum number of 6-second PFM contractions with 6 seconds of relaxation in between. Dynamic endurance is the number of contractions after which contraction can no longer be sustained. The cutoff value was 40 contractions.
The test procedure was standardized and involved digital examination of the anal sphincter. It was performed for all participants by the same PT for whom the randomization code was blinded.
Statistical Analysis
Statistical analysis was carried out using IBM SPSS (Statistical Package of Social Science) version 20. Median and interquartile range (IQR) are presented for small samples. The null hypothesis between groups was tested by the Mann–Whitney U test and within groups using the Wilcoxon signed-rank test. In addition, the effect of PFMT was analyzed by comparing the change for each participant from test to test between the groups.
For all tests, the level for statistical significance was set to p < .05. Power calculations in which an assumption of a decrease of two points at DAN-PSS-1, total score indicated that, with 90% power and a significance level at p = .05, 120 participants would be needed for the study.
Results
Participants
Medical records of 1,812 poststroke men were screened for inclusion (Figure 1) and 516 were invited to participate. Thirty-one participants were eligible and randomized.
Figure 1.
Study profile.
Note. TG = treatment group; CG = control group.
Thirty participants, with a median age of 68 years (IQR: 60-74) completed the study, 15 in each group. At the 6-month follow-up, one participant from the CG dropped out.
Median PFMT sessions attendance rate in the TG was 11 sessions (IQR: 10-12) corresponding to 92%.
Baseline Characteristics
Demographic baseline characteristics of the study group are presented in Table 1. Neurological, urological, and physical baseline characteristics are presented in Table 2. There were no statistically significant differences between the TG and CG in any of the baseline characteristics. The DAN-PSS-1 questionnaires were complete in all participants, while data were missing from 9 to 11 (30% to 36%) participants for the 3-day voiding diary and 2 to 6 (6% to 20%) participants regarding the digital anal palpation of PFM.
Table 1.
Demographic Baseline Characteristics of Poststroke Men.
| Characteristic | Treatment group (n = 15), N (%) | Control group (n = 15), N (%) | p Value |
|---|---|---|---|
| Age, yearsa | 68 (57-73) | 70 (64-75) | .22 |
| Previous medical history, diseases, and risk factors | |||
| Stroke (one attack) | 0 (0) | 5 (33) | .13 |
| Hypertension | 4 (27) | 5 (33) | .78 |
| Transient ischemic attack | 0 (0) | 1 (7) | .78 |
| Diabetes mellitus | 2 (13) | 1 (7) | .78 |
| Coronary heart disease | 3 (20) | 0 (0) | .37 |
| Other diseases (arthritis, fractures, muscular disorders, etc.) | 5 (33) | 5 (33) | 1.00 |
| None | 7 (47) | 2 (13) | .13 |
| Smoking | |||
| Smoker | 2 (13) | 2 (13) | .49 |
| Former smoker | 3 (20) | 1 (7) | |
| Never smoker | 10 (67) | 12 (80) | |
| Alcohol, no. of consumed units per week | |||
| 0 | 5 (33) | 7 (47) | .33 |
| 0-6 | 5 (33) | 6 (40) | |
| 7-13 | 2 (13) | 1 (7) | |
| 14-21 | 2 (13) | 0 (0) | |
| >21 | 1 (7) | 1 (7) | |
| Social status | |||
| Employed | 5 (33) | 2 (13) | .57 |
| Unemployed | 0 (0) | 0 (0) | — |
| Early retirement/sick leave >3 months | 1 (7) | 3 (20) | |
| Retired | 9 (60) | 10 (67) | |
| Civil status | |||
| Single/divorced | 4 (27) | 4 (27) | .84 |
| Married/partnered | 10 (67) | 11 (73) | |
| Widower | 1 (7) | 0 (0) | |
| Current use of medication | |||
| Antiarrhythmics/antihypertensives | 10 (67%) | 6 (40%) | .22 |
| Statins | 12 (80%) | 10 (67%) | .54 |
| Anticoagulants/antithrombotics | 12 (80%) | 12 (80%) | 1.00 |
| Hypoglycemics agents | 1 (7%) | 0 (0%) | .77 |
| Analgesics | 1 (7%) | 1 (7%) | 1.00 |
| Antidepressants | 1 (7%) | 0 (0%) | .77 |
| Other | 5 (33%) | 7 (47%) | .54 |
Table 2.
Neurological, Urological, and Physical Baseline Characteristics of Poststroke Men.
| Characteristic | Treatment group (n = 15), N (%) | Control group (n = 15), N (%) | p Value |
|---|---|---|---|
| Neurological | |||
| Minimental state examination score (maximum score = 30)a | 29 (29-30) | 29 (28-30) | .22 |
| Barthel Index (maximum score = 100)a | 100 (100-100) | 100 (100-100) | .78 |
| Time since last stroke, daysa | 65 (50-87) | 55 (50-63) | .60 |
| Result of stroke verification by CT or MRI scan | |||
| Visible stroke at imaging | 12 (80) | 11 (73) | .78 |
| No visible stroke at imaging, but clinical stroke | 3 (20) | 4 (27) | |
| Subtypes of stroke | |||
| Infarct | 13 (87) | 10 (67) | .74 |
| Hemorrhage | 0 (0) | 2 (13) | |
| Infarct + hemorrhage | 0 (0) | 0 (0) | |
| No infarct | 1 (7) | 0 (0) | |
| No information | 1 (7) | 3 (20) | |
| Localization | |||
| Left hemisphere | 4 (27) | 6 (40) | .68 |
| Right hemisphere | 6 (40) | 7 (47) | |
| Bilateral | 2 (13) | 0 (0) | |
| Unclassified | 2 (13) | 2 (13) | |
| No information | 1 (7) | 0 (0) | |
| Urological | |||
| Past history of LUTS | |||
| None | 12 (80) | 11 (73) | .51 |
| Pharmacological treatment | 2 (13) | 4 (27) | |
| No information | 1 (7) | 0 (0) | |
| Current use of LUTSb medicine | |||
| Overactive bladder | 2 (13) | 4 (27) | .74 |
| Prostate | 1 (7) | 1 (7) | — |
| No medicine | 12 (80) | 10 (67) | .78 |
| Use of LUTS aids | |||
| Pads | 2 (13) | 2 (13) | 1.00 |
| Catheter | 0 (0) | 0 (0) | — |
| None | 13 (87) | 13 (87) | 1.00 |
| Physical activity a | |||
| Walking distance, meter | 2,000 (1,000-5,000) | 2,000 (1,000-4,000) | .68 |
| Sports/exercise, minutes per week | 0 (0-0) | 45 (0-120) | .81 |
| Use of walking aids | |||
| Crutch | 0 (0) | 0 (0) | .78 |
| Rollator | 0 (0) | 1 (7) | |
| Other walking aids | 0 (0) | 0 (0) | |
| None | 15 (100) | 14 (93) | |
| One leg stand, eyes open, secondsa | |||
| Dominant leg | 10 (5-29) | 3 (11-15) | .56 |
| Nondominant leg | 29 (4-30) | 10 (1-30) | .12 |
| Previous PFMT | |||
| Yes | 0 (0) | 2 (13) | .54 |
| No | 15 (100) | 13 (87) |
The DAN-PSS-1 Questionnaire
The results measured on the DAN-PSS-1 questionnaire are presented in Table 3.
Table 3.
Results of Pelvic Floor Muscle Training Within and Between Groups measured on the Danish Prostate Symptom Score (DAN-PSS-1) Questionnaire and the Voiding Diary in Poststroke Men.
| Treatment group (n = 15) | Control group (n = 15) | p Valuea | p Valueb |
|---|---|---|---|
| _DAN-PSS_-1 questionnaire | |||
| Symptom score, median (interquartile range) | |||
| Pretest (30/30) | 9 (7-11) | 10 (6-13) | .51 |
| Posttest (30/30) | 6 (4-10) | 5 (5-11) | .74 |
| Follow-up (30/30) | 7 (4-13) | 7 (2-12) | .59 |
| p Valuec | <.01* | .05 | .93i |
| p Valued | .04* | .46 | .06ii |
| p Valuee | .15 | .07 | .59iii |
| Bother score, median (interquartile range) | |||
| Pretest (30/30) | 7 (4-9) | 11 (6-13) | .13 |
| Posttest (30/30) | 3 (3-5) | 6 (3-12) | .13 |
| Follow-up (30/30) | 3 (1-12) | 6 (6-11) | .91 |
| p Valuec | <.01* | .01* | .93i |
| p Valued | .06 | .60 | .13ii |
| p Valuee | .16 | .02* | .45iii |
| Total score, median (interquartile range) | |||
| Pretest (30/30) | 11 (7-14) | 17 (8-24) | .25 |
| Posttest (30/30) | 3 (1-7) | 6 (4-22) | .15 |
| Follow-up (30/30) | 5 (1-19) | 8 (1-21) | .88 |
| p Valuec | <.01* | .03* | .59i |
| p Valued | .03* | .78 | .10ii |
| p Valuee | .12 | .09 | .85iii |
| Voiding diary | |||
| Voiding frequency, total per 24 hours, median (interquartile range) | |||
| Pretest (20/30) | 11 (6-10) | 11 (8-12) | .97 |
| Posttest (21/30) | 7 (5-10) | 8 (7-12) | .25 |
| Follow-up (19/30) | 8 (5-11) | 10 (7-10) | .60 |
| p Valuec | .04* | .89 | .09i |
| p Valued | .26 | .67 | .67ii |
| p Valuee | .02* | .46 | .84iii |
| Voiding frequency, daytime per 24 hours, median (interquartile range) | |||
| Pretest (20/30) | 8 (4-11) | 8 (5-9) | .65 |
| Posttest (21/30) | 6 (4-8) | 7 (6-9) | .35 |
| Follow-up (19/30) | 7 (3-10) | 8 (6-9) | .67 |
| p Valuec | .09 | 1.00 | .20i |
| p Valued | .35 | .93 | .84ii |
| p Valuee | .08 | .46 | .53iii |
| Voiding frequency, nighttime per 24 hours, median (interquartile range) | |||
| Pretest (20/30) | 2 (1-3) | 3 (2-4) | .11 |
| Posttest (21/30) | 1 (1-2) | 2 (1-3) | .11 |
| Follow-up (19/30) | 2 (1-3) | 2 (1-3) | .66 |
| p Valuec | .05 | .94 | .20i |
| p Valued | .04* | .23 | .04ii,* |
| p Valuee | .50 | .40 | .95iii |
Symptom Score
At 3 months, the results demonstrated a statistically significant decrease (p < .01) in symptom score for the TG from pretest to posttest, but not in the CG, although a trend (p = .05) was noted. The difference between the groups was, however, not significant. At follow-up, the results demonstrated a significant decrease (p = .04) in symptom score from posttest to follow-up in the TG which, however, was not a statistically significant difference from the CG, indicating no long-lasting effect of the intervention.
Bother Score
The results for bother score (Table 3) demonstrated a statistically significant decrease in both groups with no significant difference between the improvement in the TG compared with the CG. At follow-up, the bother score results demonstrated a trend to statistical significance (p = .06) in the TG from posttest to follow-up, but not in the CG. Furthermore, there was a significant difference (p = .02) in the CG from pretest to follow-up. However, none of these differences between groups were statistically significant.
Total Score
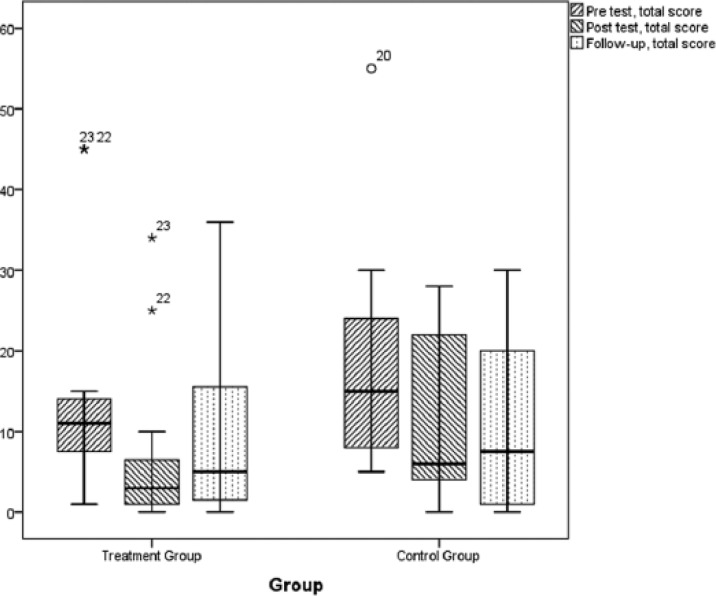
At 3 months, the total score results (Figure 2) demonstrated a significant decrease in both the TG (p < .01) and CG (p = .03), but the decreases were not significantly different from the decrease in the CG. At follow-up, the total score increased significantly (p = .03) in the TG from posttest to follow-up, but not in the CG.
Figure 2.
Results of pelvic floor muscle training measured on the Danish Prostate Symptom Score questionnaire, total score as presented as box plots.
Note. The symbols small circle and asterisk (*) in the box plot represents outliers, that is, results with a distance from the box ends (lower and upper quartile) that exceeds the height of the box (interquartile range) with a factor of more than 1.5 and 3, respectively. The numbers beside the symbols refers to the record number in the data.
The Voiding Diary
The results measured on the 3-day voiding diary are presented in Table 3.
Frequency of Voidings per 24 Hours
At 3 months, the TG voidings per 24 hours demonstrated a reduction in median value from 11 at pretest to 7 at posttest. This decrease of four (36%) voidings per 24 hours was statistically significant (p = .04) within the group. The median value of number of voidings in the CG was reduced insignificantly by three (27%) per 24 hours.
At follow-up, the TG median value indicated a statistically significant (p = .02) reduction by 3 (27%) from pretest to follow-up. However, for the CG, the median value was decreased by 1 (9%) and not significant.
No significant difference between TG and CG was demonstrated at pretest, posttest, and follow-up. Likewise, there was no significant difference between the groups.
Frequency of Voiding in the Nighttime
At 3 months, the median value of voiding frequency at night was reduced by 1 (50%) in the TG from pretest to posttest (p = .05). In the CG, the reduction was 1 (33%), not significant.
The frequency of nighttime voiding was reduced significantly for the TG (p = .04) from posttest to follow-up, but not for the CG.
There were statistically significant differences (p = .04) between groups for each participant between posttest and follow-up, but not between groups at pretest, posttest, or follow-up.
Number of UI Episodes and Used Pads
The recorded number of UI episodes per 24 hours was very small (TG: pretest = 3, posttest = 0, follow-up = 1; CG: pretest = 1, posttest = 0, follow-up = 0). Likewise, the number of pads used per 24 hours was (TG: pretest = 1, posttest = 0, follow-up = 0; CG: pretest = 0, posttest = 0, follow-up = 0). These numbers were too small for statistical analysis.
Digital Anal Palpation of PFM
The results are presented in Table 4.
Table 4.
Results of Pelvic Floor Muscle Training Measured on the Pelvic Floor Muscle in Poststroke Men.
| Treatment group | Control group | p Valuea | p Valueb |
|---|---|---|---|
| Pelvic floor muscle function, median (interquartile range) | |||
| Pretest (30/30) | 2 (2-2) | 2 (2-2) | 1.00 |
| Posttest (28/30) | 3 (2-3) | 2 (2-2) | .01* |
| Follow-up (24/30) | 2 (2-3) | 2 (2-2) | .25 |
| p Valuec | <.01* | 1.00 | .03i,* |
| p Valued | .48 | 1.00 | .53ii |
| p Valuee | .03* | 1.00 | .15iii |
| Pelvic floor muscle strength, median (interquartile range) | |||
| Pretest (30/30) | 3 (2-4) | 3 (2-4) | 1.00 |
| Posttest (28/30) | 5 (4-6) | 4 (4-5) | .07 |
| Follow-up (24/30) | 5 (4-6) | 4 (3-5) | .15 |
| p Valuec | <.01* | .10 | .03i,* |
| p Valued | .45 | .15 | .61ii |
| p Valuee | <.01* | .94 | .17iii |
| Pelvic floor muscle static endurance, seconds, median (interquartile range) | |||
| Pretest (30/30) | 14 (8-26) | 24 (13-30) | .33 |
| Posttest (28/30) | 29 (14-60) | 19 (7-29) | .10 |
| Follow-up (24/30) | 30 (14-60) | 25 (12-32) | .33 |
| p Valuec | .06 | .21 | .03i,* |
| p Valued | .86 | .74 | .82ii |
| p Valuee | .12 | .34 | .04iii,* |
| Pelvic floor muscle dynamic endurance, seconds, median (interquartile range) | |||
| Pretest (30/30) | 40 (21-40) | 32 (19-40) | .71 |
| Posttest (28/30) | 40 (40-40) | 40 (35-40) | .23 |
| Follow-up (24/30) | 40 (37-40) | 40 (30-40) | .65 |
| p Valuec | .05 | .21 | .29i |
| p Valued | .94 | .74 | .57ii |
| p Valuee | .18 | .34 | .91iii |
PFM Function
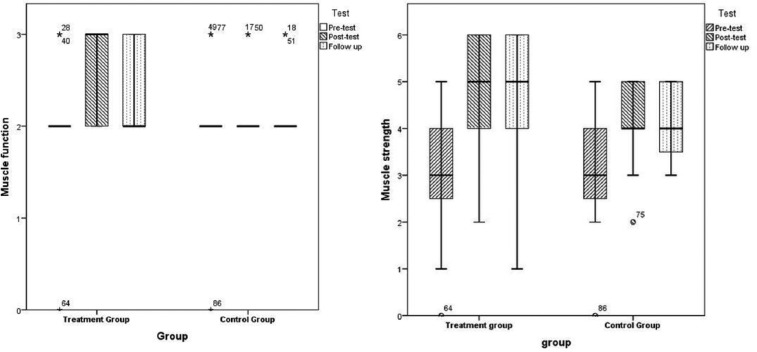
At 3 months, the results measured on PFM function for the TG demonstrated significant improvement (p < .01) from pretest to posttest compared with none in the CG (Figure 3), which was significantly better than the CG (p = .03).
Figure 3.
Results of pelvic floor muscle training measured on the pelvic floor muscle function and strength in poststroke men.
Note. The symbols small circle and asterisk (*) in the box plot represents outliers, that is, results with a distance from the box ends (lower and upper quartile) that exceeds the height of the box (interquartile range) with a factor of more than 1.5 and 3, respectively. The numbers beside the symbols refers to the record number in the data.
At follow-up, only the TG improved statistically significantly (p = .03) from pretest to follow-up.
PFM Strength
At 3 months, the results for the TG indicated significant improvement (p < .01) from pretest to posttest, which was significantly better compared with the CG (p = .03). The TG also improved significantly from pretest to follow-up (p < .01), but not significantly more than the CG.
PFM Static Endurance
At 3 months, the results on PFM static endurance demonstrated a trend to statistically significant improvement (p = .06) for the TG.
There was statistical significance (p = .03) between groups for each participant from pretest to posttest and from pretest to follow-up (p = .04).
PFM Dynamic Endurance
At 3 months, the results on PFM dynamic endurance demonstrated in the TG a trend to significant improvement (p = .05) from pretest to posttest but not in the CG. There were no significant differences between groups.
Adverse Events
No participants in either group reported any form of adverse event during the trial.
Discussion
This is, to the authors’ knowledge, the first RCT with 6-month follow-up aiming to evaluate the effect of PFMT in men with LUTS after a stroke indicating beneficial effect. However, the effect was not statistically significant between groups for patient-reported outcomes, in contrast to single-blinded observer-reported outcomes.
Despite the high prevalence of LUTS in poststroke men, the current study failed to include a large number of participants. Nevertheless, low dropout rate, high attendance at PFMT sessions, and no adverse effects were demonstrated.
The current results measured on the DAN-PSS-1 questionnaire indicated short-term effects within both groups, with most in the TG. The improvement in the CG, especially at posttest, can be explained not only by the placebo effect (Hrobjartsson & Gotzsche, 2010) but also by components such as self-monitoring voiding habits (Burgio, 2013) by rating the DAN-PSS-1 questionnaire and the voiding diary as well as learning effects resulting in self-PFMT by the digital anal palpation test.
The current results for the voiding frequency per 24 hours are consistent with previous results evaluating the effect of PFMT in women with poststroke UI (Tibaek et al., 2005). A panel of experts has in consensus suggested that, for healthy bladders, voiding frequency is approximately eight times per day and one or none per night (Lukacz, Whitcomb, Lawrence, Nager, & Luber, 2009). This rate was for women; men usually have lower voiding daytime frequency (Mueller et al., 2005).
Several participants in the current study did not complete the 3-day voiding diary, especially at the follow-up investigation, which weakens the reliability of the voiding diary as an outcome measure. At present, there is no optimal duration of days for rating voiding diaries in stroke patients.
The current short-term results for PFM function and PFM strength were consistent with the results for women (Tibaek et al., 2005). Moreover, the current study indicated statistical significance between groups for each participant in PFM function, PFM strength, and PFM static endurance. It also indicated statistical significance for the long-term effect within groups for PFM function and PFM strength.
It is surprising that, although none of the participants in the TG had previous knowledge of PFMT (Table 2), the participants in TG demonstrated a higher attendance rate at the PFMT sessions than did men with UI to PFMT sessions over 3 months following prostatectomy or transurethral resection of prostate (72% to 85% compared with the current 92%; Glazener et al., 2011).
Study Limitation
Several methodological issues in the current study need to be considered. The first issue is the small sample size of participants, leading to severe lack of statistical power. Approximately 1 out of 10 of those invited did not respond to the current invitation, half reported that they did not have LUTS, 1 out of 5 had previous LUTS but with no change in the condition after the stroke, and 1 out of 10 had LUTS but lacked resources to participate or did not want to do so.
The current data raise the question, “Why was it so difficult to involve these potential participants?” Perhaps the men regard their symptoms as normal (Lai, Wun, Luo, & Pang, 2011)? Perhaps they were too embarrassed or ashamed to report LUTS? Perhaps they had developed coping mechanisms which controlled their symptoms? Perhaps they did not like PFMT?
Identification of factors and barriers may improve the understanding of the attitude of poststroke men to LUTS and PFMT as the problem is significant in a substantial proportion of stroke patients. Likewise, an analysis of baseline characteristics for possible differences between included and nonincluded men may provide valuable information on this selection mechanism.
A second issue is the relatively large decrease measured at the follow-up compared with posttest. Almost all self-reported outcome data were worse in the TG at 6-month follow-up; for the PFMT the increased effect during intervention was stopped and the outcome data were at the same level as at posttest. All participants in the TG were strongly recommended to continue daily PFMT using their home-training program as in the intervention. However, education of participants is important to ensure consistency of the training and its monitoring. Perhaps using a training diary, PT telephone contact, or for some electronic knowledge participants advanced Internet methods might be important training supports (Piron, Tonin, Trivello, Battistin, & Dam, 2004).
The third issue is the validity and reliability of the digital anal palpation measurement of PFM. This methodological study remains in neurologic as well as nonneurologic healthy men and women with LUTS (Kruger, Dietz, Budgett, & Dumoulin, 2014).
The participants reported no side effects. However, side effects can occur and be negative (dry mouth, constipation, or irrigative symptoms) or positive (increased energy, absence of urinary infection and of obstipation, improved sexual function and satisfaction). Nonetheless, while many positive effects are known of physical activity, little is known about its effects related to PFMT.
By evaluating the effect of PFMT as a single intervention while an intervention with multiple components, such as urge suppression strategies (Burgio, 2013) and fluid restriction, may increase the effect. However, to understand the underlying mechanism of PFMT, it is needed to investigate the relative contributions of the individual components. Likewise, evaluating the effect of PFMT on LUTS in stroke patients with mild to moderate cognitive impairment needs future research.
Conclusion
The results of this study indicated that it is difficult to include men with stroke in a PFMT study; however, when they were included they were highly motivated and completed the study. The results of PFMT in poststroke men indicated its effectiveness by reducing LUTS and frequency of voidings, although no statistical significance was reported between groups except for PFM function and strength. It is recommended that further studies with larger sample sizes need to be carried out to understand the barriers to participation by men in such research and for exploring how PFMT works in men with poststroke LUTS.
Acknowledgments
The authors wish to thank all the patients for participation. Major thanks are also due to Anette Lia, Department of Neurology, Glostrup Hospital and Sille Malene G. Christensen, Department of Neurology, Herlev Hospital for their secretarial assistance. Thanks to all the staff at the Department of Physiotherapy and Occupational Therapy, Glostrup Hospital, University of Copenhagen.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Association of Danish Physiotherapists’ Research Foundation and Practice Foundation, the Foundation of 17.12.1981, Lykkefeldts Grant, the Foundation of Lundbeck (UCSF) and the Department of Physiotherapy and Occupational Therapy Glostrup Hospital, University of Copenhagen.
References
- Abrams P., Cardozo L., Fall M., Griffiths D., Rosier P., Ulmsten U., . . . Wein A. (2002). The standardisation of terminology of lower urinary tract function: Report from the Standardisation Sub-committee of the International Continence Society. Neurourology and Urodynamics, 21, 167-178. [DOI] [PubMed] [Google Scholar]
- Burgio K. L. (2013). Update on behavioral and physical therapies for incontinence and overactive bladder: The role of pelvic floor muscle training. Current Urology Reports, 14, 457-464. doi: 10.1007/s11934-013-0358-1 [DOI] [PubMed] [Google Scholar]
- De Ridder D., Vermeulen C., Ketelaer P., Van Poppel H., Baert L. (1999). Pelvic floor rehabilitation in multiple sclerosis. Acta Neurologica Belgica, 99(1), 61-64. [PubMed] [Google Scholar]
- Divani A. A., Vazquez G., Barrett A. M., Asadollahi M., Luft A. R. (2009). Risk factors associated with injury attributable to falling among elderly population with history of stroke. Stroke, 40, 3286-3292. doi: 10.1161/STROKEAHA.109.559195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. J., Griffiths D. J. (2010). A decade of functional brain imaging applied to bladder control. Neurourology and Urodynamics, 29, 49-55. doi: 10.1002/nau.20740 [DOI] [PubMed] [Google Scholar]
- Glazener C., Boachie C., Buckley B., Cochran C., Dorey G., Grant A., . . . N’Dow J. (2011). Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): Two parallel randomised controlled trials. Lancet, 378(9788), 328-337. doi: 10.1016/S0140-6736(11)60751-4 [DOI] [PubMed] [Google Scholar]
- Gormley E. A., Lightner D. J., Burgio K. L., Chai T. C., Clemens J. Q., Culkin D. J., . . . Vasavada S. P. (2012). Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. Journal of Urology, 188(6 Suppl.), 2455-2463. doi: 10.1016/j.juro.2012.09.079 [DOI] [PubMed] [Google Scholar]
- Hay-Smith J., Berghmans B., Burgio K. L. (2009). Adult conservative management: Committee 12. In Abrams P., Cardozo L., Khoury S., Wein A. (Eds.), Incontinence (4th ed., pp. 1025-1108). Plymouth, MN: Health Publication. [Google Scholar]
- Hrobjartsson A., Gotzsche P. C. (2010). Placebo interventions for all clinical conditions. Cochrane Database of Systematic Reviews, 1, CD003974. doi: 10.1002/14651858.CD003974.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. F., Glazener C. M., Moore K. N. (2007). Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews, 2, CD001843. doi: 10.1002/14651858.CD001843.pub3 [DOI] [PubMed] [Google Scholar]
- Kruger J. A., Dietz H. P., Budgett S. C., Dumoulin C. L. (2014). Comparison between transperineal ultrasound and digital detection of levator ani trauma: Can we improve the odds? Neurourology and Urodynamics, 33, 307-311. doi: 10.1002/nau.22386 [DOI] [PubMed] [Google Scholar]
- Lai U. C., Wun Y. T., Luo T. C., Pang S. M. (2011). In a free healthcare system, why do men not consult for lower urinary tract symptoms (LUTS)? Asia Pacific Family Medicine, 10(1), 7. doi: 10.1186/1447-056X-10-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson G., Abrams P., Victor A. (1991). The frequency volume chart in detrusor instability. Neurourology and Urodynamics, 10, 553-543. [Google Scholar]
- Laycock J. (1994). Clinical evaluation of the pelvic floor. In Schüssler B., Laycock J., Norton P., Stanton S. L. (Eds.), Pelvic floor re-education (1st ed., pp. 42-48). London, England: Springer-Verlag. [Google Scholar]
- Lucio A., D’Ancona C., Lopes M., Perissinotto M., Damasceno B. (2014). The effect of pelvic floor muscle training alone or in combination with electrostimulation in the treatment of sexual dysfunction in women with multiple sclerosis. Multiple Sclerosis, 20, 1761-1768. doi: 10.1177/1352458514531520 [DOI] [PubMed] [Google Scholar]
- Lucio A. C., Campos R. M., Perissinotto M. C., Miyaoka R., Damasceno B. P., D’Ancona C. A. (2010). Pelvic floor muscle training in the treatment of lower urinary tract dysfunction in women with multiple sclerosis. Neurourology and Urodynamics, 29, 1410-1413. doi: 10.1002/nau.20941 [DOI] [PubMed] [Google Scholar]
- Lukacz E. S., Whitcomb E. L., Lawrence J. M., Nager C. W., Luber K. M. (2009). Urinary frequency in community-dwelling women: What is normal? American Journal of Obstetrics and Gynecology, 200(5), 552.e1-e7. doi: 10.1016/j.ajog.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurg D., Ashe R. G., Marshall K., Lowe-Strong A. S. (2006). Comparison of pelvic floor muscle training, electromyography biofeedback, and neuromuscular electrical stimulation for bladder dysfunction in people with multiple sclerosis: A randomized pilot study. Neurourology and Urodynamics, 25, 337-348. doi: 10.1002/nau.20209 [DOI] [PubMed] [Google Scholar]
- Meyhoff H. H., Hald T., Nordling J., Andersen J. A., Bilde T., Walter S. (1993). A new patient weighted symptom score (DAN-PSS-1): Clinical assessment of indications and outcomes of transurethral prostatectomy for uncomplicated benign prostatic hyperplasia. Scandinavian Journal of Urology and Nephrology, 27, 493-499. [DOI] [PubMed] [Google Scholar]
- Mueller E., Latini J., Lux M., Stablein U., Brubaker L., Kreder K., Fitzgerald M. P. (2005). Gender differences in 24-hour urinary diaries of asymptomatic North American adults. Journal of Urology, 173, 490-492. doi: 10.1097/01.ju.0000149947.28100.cd [DOI] [PubMed] [Google Scholar]
- Newman D. K., Guzzo T., Lee D., Jayadevappa R. (2014). An evidence-based strategy for the conservative management of the male patient with incontinence. Current Opinion in Urology, 24, 553-559. doi: 10.1097/MOU.0000000000000115 [DOI] [PubMed] [Google Scholar]
- Panicker J. N., Fowler C. J., Kessler T. M. (2015). Lower urinary tract dysfunction in the neurological patient: Clinical assessment and management. Lancet. Neurology, 14, 720-732. doi: 10.1016/S1474-4422(15)00070-8 [DOI] [PubMed] [Google Scholar]
- Piron L., Tonin P., Trivello E., Battistin L., Dam M. (2004). Motor tele-rehabilitation in post-stroke patients. Medical Informatics and the Internet in Medicine, 29, 119-125. doi: 10.1080/14639230410001723428 [DOI] [PubMed] [Google Scholar]
- Ruffion A., Castro-Diaz D., Patel H., Khalaf K., Onyenwenyi A., Globe D., . . . Edwards M. (2013). Systematic review of the epidemiology of urinary incontinence and detrusor overactivity among patients with neurogenic overactive bladder. Neuroepidemiology, 41, 146-155. doi: 10.1159/000353274 [DOI] [PubMed] [Google Scholar]
- Seseke S., Baudewig J., Kallenberg K., Ringert R. H., Seseke F., Dechent P. (2006). Voluntary pelvic floor muscle control: An fMRI study. NeuroImage, 31, 1399-1407. doi: 10.1016/j.neuroimage.2006.02.012 [DOI] [PubMed] [Google Scholar]
- Tibaek S., Dehlendorff C. (2009). Validity of the Danish Prostate Symptom Score questionnaire in stroke. Acta Neurologica Scandinavica, 120, 411-417. [DOI] [PubMed] [Google Scholar]
- Tibaek S., Gard G., Dehlendorff C., Iversen H. K., Erdal J., Biering-Sorensen F., . . . Jensen R. (2015). The effect of pelvic floor muscle training on sexual function in men with lower urinary tract symptoms after stroke. Topics in Stroke Rehabilitation, 22, 185-193. doi: 10.1179/1074935714Z.0000000019 [DOI] [PubMed] [Google Scholar]
- Tibaek S., Gard G., Jensen R. (2005). Pelvic floor muscle training is effective in women with urinary incontinence after stroke. Neurourology and Urodynamics, 24, 348-357. [DOI] [PubMed] [Google Scholar]
- Tibaek S., Gard G., Klarskov P., Iversen H. K., Dehlendorff C., Jensen R. (2008). Prevalence of lower urinary tract symptoms (LUTS) in stroke patients: A cross-sectional, clinical survey. Neurourology and Urodynamics, 27, 763-771. [DOI] [PubMed] [Google Scholar]
- Tibaek S., Jensen R., Klarskov P., Iversen H. K., Gard G. (2006). The Danish Prostatic Symptom Score (DAN-PSS-1) Questionnaire is reliable in stroke patients. Neurourology and Urodynamics, 25, 319-323. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1989). WHO Task Force on Stroke and other cerebrovascular disorders: Recommendation on stroke prevention, diagnosis and therapy. Stroke, 20, 1407-1431. [DOI] [PubMed] [Google Scholar]
- Williams M. P., Srikanth V., Bird M., Thrift A. G. (2012). Urinary symptoms and natural history of urinary continence after first-ever stroke: A longitudinal population-based study. Age and Ageing, 41, 371-376. doi: 10.1093/ageing/afs009 [DOI] [PubMed] [Google Scholar]
- Zhang H., Reitz A., Kollias S., Summers P., Curt A., Schurch B. (2005). An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. NeuroImage, 24, 174-180. doi: 10.1016/j.neuroimage.2004.08.027 [DOI] [PubMed] [Google Scholar]