Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer (original) (raw)
. Author manuscript; available in PMC: 2017 Nov 28.
Published in final edited form as: N Engl J Med. 2011 Dec 7;366(6):520–529. doi: 10.1056/NEJMoa1109653
Abstract
BACKGROUND
Resistance to endocrine therapy in breast cancer is associated with activation of the mammalian target of rapamycin (mTOR) intracellular signaling pathway. In early studies, the mTOR inhibitor everolimus added to endocrine therapy showed antitumor activity.
METHODS
In this phase 3, randomized trial, we compared everolimus and exemestane versus exemestane and placebo (randomly assigned in a 2:1 ratio) in 724 patients with hormone-receptor–positive advanced breast cancer who had recurrence or progression while receiving previous therapy with a nonsteroidal aromatase inhibitor in the adjuvant setting or to treat advanced disease (or both). The primary end point was progression-free survival. Secondary end points included survival, response rate, and safety. A preplanned interim analysis was performed by an independent data and safety monitoring committee after 359 progression-free survival events were observed.
RESULTS
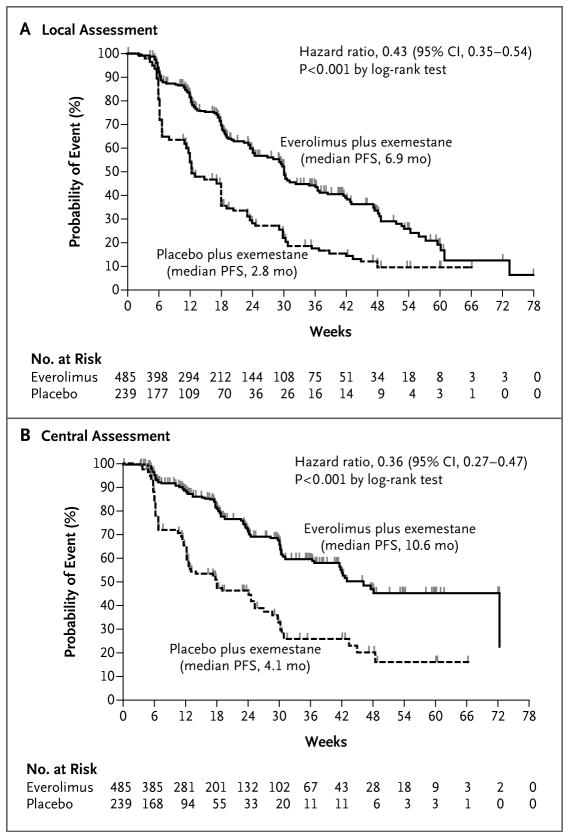
Baseline characteristics were well balanced between the two study groups. The median age was 62 years, 56% had visceral involvement, and 84% had hormone-sensitive disease. Previous therapy included letrozole or anastrozole (100%), tamoxifen (48%), fulvestrant (16%), and chemotherapy (68%). The most common grade 3 or 4 adverse events were stomatitis (8% in the everolimus-plus-exemestane group vs. 1% in the placebo-plus-exemestane group), anemia (6% vs. <1%), dyspnea (4% vs. 1%), hyperglycemia (4% vs. <1%), fatigue (4% vs. 1%), and pneumonitis (3% vs. 0%). At the interim analysis, median progression-free survival was 6.9 months with everolimus plus exemestane and 2.8 months with placebo plus exemestane, according to assessments by local investigators (hazard ratio for progression or death, 0.43; 95% confidence interval [CI], 0.35 to 0.54; P<0.001). Median progression-free survival was 10.6 months and 4.1 months, respectively, according to central assessment (hazard ratio, 0.36; 95% CI, 0.27 to 0.47; P<0.001).
CONCLUSIONS
Everolimus combined with an aromatase inhibitor improved progression-free survival in patients with hormone-receptor–positive advanced breast cancer previously treated with nonsteroidal aromatase inhibitors. (Funded by Novartis; BOLERO-2 ClinicalTrials .gov number, NCT00863655.)
Endocrine therapy is the cornerstone of treatment for patients with hormone-receptor (HR)–positive advanced breast cancer. In postmenopausal patients, aromatase inhibitors (e.g., letrozole and anastrozole) have become the treatment of choice in first-line therapy.1–5 Unfortunately, not all patients have a response to first-line endocrine therapy (primary or de novo resistance), and even patients who have a response will eventually relapse (acquired resistance). On disease progression, second-line treatment options include other classes of aromatase inhibitors (steroidal or nonsteroidal) and the estrogen-receptor (ER) antagonists fulvestrant and tamoxifen.6,7
The study of resistance to endocrine therapies in HR-positive breast cancer has aimed at identifying new therapeutic strategies that would enhance the efficacy of endocrine therapies.8 An emerging mechanism of endocrine resistance is aberrant signaling through the phosphatidylinositol 3-kinase (PI3K)–Akt–mammalian target of rapamycin (mTOR) signaling pathway.9–11 Growing evidence supports a close interaction between the mTOR pathway and ER signaling. A substrate of mTOR complex 1 (mTORC1), called S6 kinase 1, phosphorylates the activation function domain 1 of the ER, which is responsible for ligand-independent receptor activation.12,13
Everolimus (Afinitor, Novartis) is a sirolimus (formerly called rapamycin) derivative that inhibits mTOR through allosteric binding to mTORC1.14 In preclinical models, the use of everolimus in combination with aromatase inhibitors results in synergistic inhibition of the proliferation and induction of apoptosis.15 In a randomized, phase 2 study comparing neoadjuvant everolimus plus letrozole with letrozole alone in patients with newly diagnosed ER-positive breast cancer, the response rate for the combination was higher than that for letrozole alone.16 The Breast Cancer Trials of Oral Everolimus-2 (BOLERO-2) study reported here evaluated the efficacy and safety of the combination of everolimus and exemestane in patients with HR-positive breast cancer refractory to non-steroidal aromatase inhibitors.
METHODS
ROLES OF THE SPONSOR AND AUTHORS
The study was designed by the academic investigators and by representatives of the sponsor, Novartis. The data were collected with the use of the sponsor’s data-management systems and were analyzed by the sponsor’s statistical team. All authors vouch for the accuracy and completeness of the reported data and attest that the study conformed to the protocol and statistical analysis plan, available with the full text of this article at NEJM .org. Contributions to the interpretation of data and the subsequent writing, reviewing, and amending of the manuscript were made by all authors. The first draft of the manuscript was prepared by the first and last authors and by the trial’s lead physician at Novartis. No one who is not an author contributed to writing the manuscript.
PATIENTS
Eligible patients were postmenopausal women with ER-positive, human epidermal growth factor receptor type 2 (HER2)–nonamplified advanced breast cancer whose disease was refractory to previous letrozole or anastrozole, defined as recurrence during or within 12 months after the end of adjuvant treatment or progression during or within 1 month after the end of treatment for advanced disease. Letrozole or anastrozole did not have to be the most recent treatment before randomization, but recurrence or progression during receipt of the most recent systemic therapy had to be documented before randomization. Other previous anticancer endocrine treatments and a single prior chemotherapy regimen for advanced disease were also allowed.
Patients had to have at least one measurable lesion or mainly lytic bone lesions in the absence of measurable disease. Patients also had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less (on a scale from 0 to 5, with 0 indicating that the patient is fully active, 1 indicating that the patient is restricted in physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature, and 2 indicating that the patient is ambulatory and capable of all self-care but unable to work) and adequate organ and hematologic functions.17 Exclusion criteria included a history of brain metastases and previous treatment with exemestane or mTOR inhibitors.
Written informed consent was obtained from all patients before enrollment. The institutional review board at each participating center approved the study, which was conducted in accordance with the principles of Good Clinical Practice, the provisions of the Declaration of Helsinki, and other applicable local regulations. A steering committee supervised the conduct of the study, and an independent data and safety monitoring committee performed semiannual safety reviews and reviewed the interim efficacy results.
STUDY DESIGN AND TREATMENT
In this international, double-blind, phase 3 study, patients were randomly assigned to treatment with oral everolimus or matching placebo (at a dose of 10 mg daily), in conjunction with exemestane (25 mg daily). Randomization, at a 2:1 ratio in favor of the everolimus–exemestane group, was stratified according to the presence of visceral metastasis and previous sensitivity to endocrine therapy. The latter was defined as at least 24 months of endocrine therapy before recurrence in the adjuvant setting or a response or stabilization for at least 24 weeks of endocrine therapy for advanced disease.
The primary end point was progression-free survival, on the basis of radiographic studies assessed by the local investigators, with central assessment by an independent radiology committee used in a supportive analysis. Secondary end points included overall survival, overall response rate, clinical benefit rate, time to deterioration of ECOG performance status, safety, and quality of life, with the use of the European Organization for Research and Treatment of Cancer quality-of-life core questionnaire (QLQ-C30) and the breast cancer module (QLQ-BR23). Blood levels of everolimus and plasma levels of exemestane were assessed 4 weeks after randomization (both before and 2 hours after the medications were taken) in a subgroup of 80 patients. Plasma levels of estradiol were assessed at screening or day 1 before starting trial therapy and at week 4 for the same patients.
Treatment continued until disease progression, the development of unacceptable toxicity, or withdrawal of consent. The protocol provided detailed guidelines for dose interruptions or reductions for everolimus and matched placebo for adverse events. In such cases, two reductions in the everolimus or placebo dose were permitted: an initial reduction to 5 mg daily and a subsequent reduction to 5 mg every other day.
EFFICACY AND SAFETY ASSESSMENTS
Tumor assessment included computed tomographic (CT) scanning or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis at baseline and every 6 weeks until disease progression. Patients who discontinued one or both study treatments for any reason other than progression were required to follow the same schedule of assessments until progression. All imaging studies were required to be sent for central radiologic review. A bone scan or skeletal survey was required within 6 weeks before randomization. Abnormalities shown on bone scans were assessed by radiography, CT scanning with bone windows, or MRI before randomization and were assessed using the same method every 6 weeks. Hematologic function, biochemical measures, and vital signs were assessed at baseline and at each visit, and the lipid profile was assessed every 6 weeks. Adverse events were monitored continuously throughout the study and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.18
STATISTICAL ANALYSIS
The primary efficacy analysis (progression-free survival), based on local assessment, was a log-rank test stratified according to visceral metastases and previous hormone sensitivity. A total of 528 progression-free survival events were required for the final analysis, in order to detect a hazard ratio of 0.74 with 90% power with the use of a log-rank test and a two-look Lan–DeMets group-sequential design with an O’Brien–Fleming-type boundary19 at a one-sided cumulative 2.5% level of significance. Further assuming a median progression-free survival of 3.7 months in the control group,6 18 months of recruitment, a 10% rate of loss to follow-up, and a 2:1 randomization ratio in favor of the everolimus–exemestane group, 705 patients were to be randomly assigned. The study had a prespecified interim analysis after the observation of approximately 60% of the progression-free survival events (the event count was 359). At the time of the interim analysis, the data and safety monitoring committee was to disclose that the trial met its primary end point only if both analyses of progression-free survival (local and central assessments) crossed the thresholds of significance, as prospectively defined in the charter of the committee.
RESULTS
PATIENTS
A total of 724 women at 189 centers in 24 countries were randomly assigned to the combination either of everolimus and exemestane (485 patients, hereafter called the combination-therapy group) or exemestane and placebo (239 patients, hereafter called the exemestane-alone group), from June 2009 through January 2011 (Fig. 1 in the Supplementary Appendix, available at NEJM.org). Baseline characteristics were well balanced. The median age was 62 years, 56% of the patients had visceral involvement, and 76% had bone metastasis. Sixty-nine percent of the patients had measurable disease, and all other patients had at least one mainly lytic bone lesion. Thirty-six percent had metastases in at least three organs. According to local assessment, all patients had ER-positive tumors, and 72% had progesterone-receptor–positive disease. All patients had HER2-negative tumors (by protein or gene analysis), except 2 for whom the result was missing. Earlier therapies included letrozole or anastrozole (100%), tamoxifen (48%), fulvestrant (16%), and chemotherapy (68%), with a median of three previous therapies. The most recent therapy before randomization was letrozole or anastrozole in 74% of the patients (Table 1). By the protocol definition, 84% of the patients had previous sensitivity to endocrine therapy.
Table 1.
Patient and Tumor Characteristics at Baseline.*
| Characteristic | Everolimus and Exemestane (N = 485) | Placebo and Exemestane (N = 239) |
|---|---|---|
| Age (yr) | ||
| Median | 62 | 61 |
| Range | 34–93 | 28–90 |
| Race (%)† | ||
| White | 74 | 78 |
| Black | 3 | 1 |
| Asian | 20 | 19 |
| Other | 3 | 2 |
| Disease-free interval‡ | ||
| Median (mo) | 58 | 57 |
| Range (mo) | 1–340 | 5–316 |
| <12 mo (%) | 2 | 4 |
| 12–24 mo (%) | 5 | 6 |
| >24 mo (%) | 56 | 54 |
| No adjuvant therapy (%) | 31 | 31 |
| Previous sensitivity to endocrine therapy (%) | 84 | 84 |
| Visceral disease (%) | 56 | 56 |
| Measurable disease (%)§ | 70 | 68 |
| Metastatic site (%) | ||
| Lung | 29 | 33 |
| Liver | 33 | 30 |
| Bone | 76 | 77 |
| No. of metastatic sites (%) | ||
| 1 | 32 | 29 |
| 2 | 31 | 34 |
| ≥3 | 36 | 37 |
| ECOG performance status (%)¶ | ||
| 0 | 60 | 59 |
| 1 | 36 | 35 |
| 2 | 2 | 3 |
| Purpose of most recent treatment (%) | ||
| Adjuvant therapy | 21 | 16 |
| Treatment of advanced or metastatic disease | 79 | 84 |
| Previous treatment with letrozole or anastrozole (%) | 100 | 100 |
| Letrozole or anastrozole as most recent treatment (%) | 74 | 75 |
| Previous treatment with antiestrogen (%) | ||
| Any antiestrogen | 57 | 59 |
| Tamoxifen | 47 | 49 |
| Fulvestrant | 17 | 16 |
| Previous chemotherapy (%) | ||
| Neoadjuvant or adjuvant therapy only | 44 | 40 |
| Treatment of metastatic disease (with or without neoadjuvant or adjuvant therapy) | 26 | 26 |
| No. of previous therapies (%)[| | ](#TFN6) | |
| 1 | 16 | 18 |
| 2 | 30 | 30 |
| ≥3 | 54 | 53 |
TREATMENT
At the cutoff date (February 11, 2011), 296 patients were still receiving study treatment: 227 (47%) in the combination-therapy group and 69 (29%) in the exemestane-alone group. The median duration of exposure to everolimus was 14.6 weeks, as compared with 12.0 weeks of exposure to placebo; as for exposure to exemestane, the median duration was 17.4 weeks in the combination-therapy group versus 12.0 weeks in the exemestane-alone group. The most frequent primary reason for discontinuation was disease progression (37% in the combination-therapy group and 66% in the exemestane-alone group).
Data from the patients in the clinical pharmacology component of the trial showed that everolimus does not affect plasma concentrations of endogenous estradiol, and estradiol levels were not different between the two treatment groups (data not shown).
SAFETY
Serious adverse events, as defined in the protocol, were reported among 23% of patients in the combination-therapy group (11% attributed to study treatment) and 12% in the exemestane-alone group (1% attributed to study treatment). A higher percentage of patients discontinued everolimus in the combination-therapy group than discontinued placebo in the control group because of adverse events (19% vs. 4%) and withdrawal of consent (5% vs. 2%). For exemestane discontinuation, the corresponding numbers were 7% versus 3% and 7% versus 2%. In the combination-therapy group, seven deaths attributed to adverse events (1%) were reported during treatment or within 28 days after stopping treatment: two deaths from sepsis and one each from pneumonia, tumor hemorrhage, cerebrovascular incident, renal failure, and suicide. In the exemestane-alone group, one death from pneumonia (<1%) was reported during treatment.
The most common grade 3 or 4 adverse events were stomatitis (8% in the combination-therapy group vs. 1% in the exemestane-alone group), anemia (6% vs. <1%), dyspnea (4% vs. 1%), hyperglycemia (4% vs. <1%), fatigue (4% vs. 1%), and pneumonitis (3% vs. 0%) (Table 2). The time to deterioration of ECOG performance status and time to deterioration of quality of life (≥5%) were not statistically different between the two treatment groups (data not shown).
Table 2.
Adverse Events Irrespective of Relationship to Study Treatment (with at Least 10% Incidence in the Everolimus–Exemestane Group).
| Adverse Event | Everolimus and Exemestane (N = 482) | Placebo and Exemestane (N = 238) | ||||
|---|---|---|---|---|---|---|
| Any Event | Grade 3 Event | Grade 4 Event | Any Event | Grade 3 Event | Grade 4 Event | |
| percent | ||||||
| Stomatitis | 56 | 8 | 0 | 11 | 1 | 0 |
| Rash | 36 | 1 | 0 | 6 | 0 | 0 |
| Fatigue | 33 | 3 | <1 | 26 | 1 | 0 |
| Diarrhea | 30 | 2 | <1 | 16 | 1 | 0 |
| Decreased appetite | 29 | 1 | 0 | 10 | 0 | 0 |
| Nausea | 27 | <1 | <1 | 27 | 1 | 0 |
| Cough | 22 | 1 | 0 | 11 | 0 | 0 |
| Dysgeusia | 21 | <1 | 0 | 5 | 0 | 0 |
| Headache | 19 | <1 | 0 | 13 | 0 | 0 |
| Decreased weight | 19 | 1 | 0 | 5 | 0 | 0 |
| Dyspnea | 18 | 4 | 0 | 9 | 1 | <1 |
| Arthralgia | 16 | 1 | 0 | 16 | 0 | 0 |
| Anemia | 16 | 5 | 1 | 4 | <1 | <1 |
| Epistaxis | 15 | 0 | 0 | 1 | 0 | 0 |
| Vomiting | 14 | <1 | <1 | 11 | <1 | 0 |
| Peripheral edema | 14 | 1 | 0 | 6 | <1 | 0 |
| Pyrexia | 14 | <1 | 0 | 6 | <1 | 0 |
| Aspartate aminotransferase level increased | 13 | 3 | <1 | 6 | 1 | 0 |
| Constipation | 13 | <1 | 0 | 11 | <1 | 0 |
| Hyperglycemia | 13 | 4 | <1 | 2 | <1 | 0 |
| Pneumonitis | 12 | 3 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 12 | 2 | 1 | <1 | 0 | <1 |
| Asthenia | 12 | 2 | 0 | 3 | 0 | 0 |
| Alanine aminotransferase level increased | 11 | 3 | <1 | 3 | 2 | 0 |
| Pruritus | 11 | <1 | 0 | 3 | 0 | 0 |
| Insomnia | 11 | <1 | 0 | 8 | 0 | 0 |
| Back pain | 11 | 0 | 0 | 8 | 1 | 0 |
EFFICACY
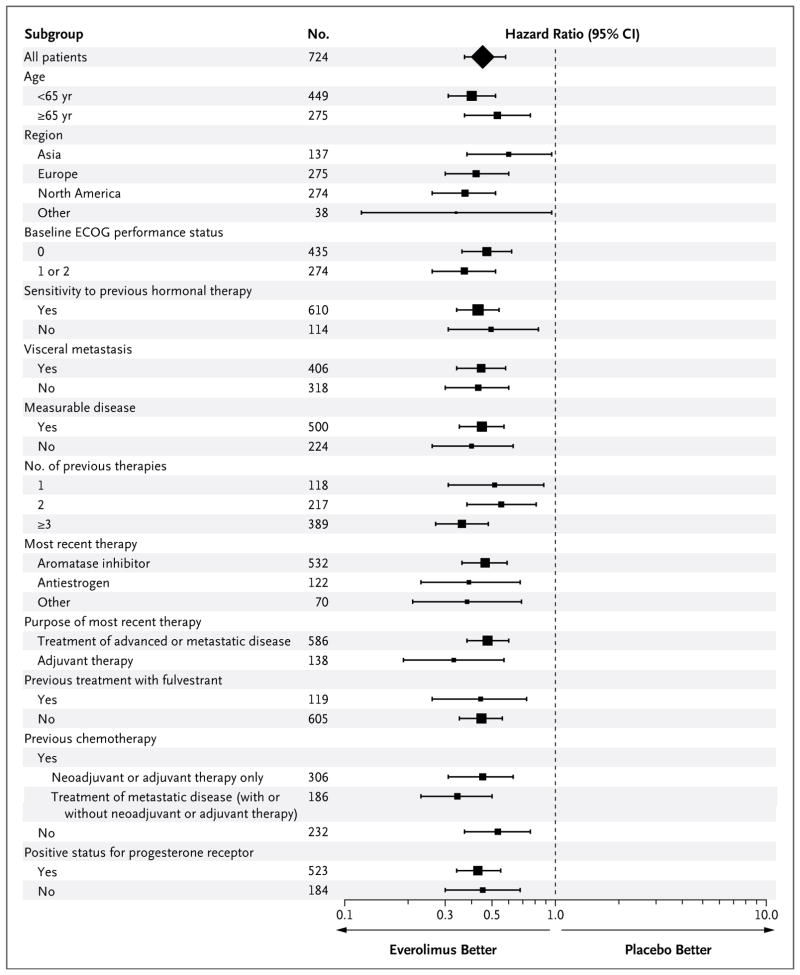
The trial met its primary end point, progression-free survival; the median progression-free survival, on the basis of radiographic studies assessed by the local investigators, was 6.9 months for everolimus plus exemestane versus 2.8 months for placebo plus exemestane (hazard ratio for progression or death, 0.43; 95% confidence interval [CI], 0.35 to 0.54; P<0.001) (Fig. 1 and Table 3). The median progression-free survivals on the basis of central assessment were 10.6 months and 4.1 months, respectively (hazard ratio, 0.36; 95% CI, 0.27 to 0.47; P<0.001) (Fig. 1 and Table 3). Both analyses crossed the prespecified thresholds for significance. The Kaplan–Meier estimates beyond week 36 should be interpreted with caution because of the small number of patients at risk and lack of adequate follow-up. The results for progression-free survival were also consistent across all subgroups (Fig. 2).
Figure 1. Kaplan–Meier Plot of Progression-free Survival.
Panel A shows progression-free survival on the basis of local assessment of radiographic studies, and Panel B shows central assessment. PFS denotes progression-free survival.
Table 3.
Efficacy Analysis on the Basis of Local and Central Assessment.*
| Variable | Everolimus and Exemestane (N = 485) | Placebo and Exemestane (N = 239) | P Value | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| Local assessment | ||||
| Progression-free survival | ||||
| Events — no. (%) | 202 (42) | 157 (66) | <0.001 | 0.43 (0.35–0.54) |
| Duration — mo | ||||
| Median | 6.9 | 2.8 | ||
| 95% CI | 6.4–8.1 | 2.8–4.1 | ||
| Best overall response — % | ||||
| Complete response | 0.4 | 0.0 | ||
| Partial response | 9.1 | 0.4 | ||
| Stable disease | 70.1 | 58.6 | ||
| Progressive disease | 9.9 | 31.4 | ||
| Unknown or too early | 10.5 | 9.6 | ||
| Objective response — % (95% CI) | 9.5 (7.0–12.4) | 0.4 (0.0–2.3) | <0.001 | |
| Central assessment | ||||
| Progression-free survival | ||||
| Events — no. (%) | 114 (24) | 104 (44) | <0.001 | 0.36 (0.27–0.47) |
| Duration — mo | ||||
| Median | 10.6 | 4.1 | ||
| 95% CI | 9.5–NR | 2.8–5.8 | ||
| Best overall response — % | ||||
| Complete response | 0.0 | 0.0 | ||
| Partial response | 7.0 | 0.4 | ||
| Stable disease | 74.6 | 64.4 | ||
| Progressive disease | 5.6 | 21.8 | ||
| Unknown or too early | 12.8 | 13.4 | ||
| Objective response — % (95% CI) | 7.0 (4.9–9.7) | 0.4 (0.0–2.3) | <0.001 |
Figure 2. Consistency of Results for Progression-free Survival across the Various Subgroups.
Scores for Eastern Cooperative Oncology Group (ECOG) performance status range from 0 to 5, with 0 indicating that the patient is fully active, 1 indicating that the patient is restricted in physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature, and 2 indicating that the patient is ambulatory and capable of all self-care but unable to work. The number of patients may not add up to 724 owing to missing baseline data. The size of each square is proportional to the number of patients in the subgroup. The data are shown on a semi-logarithmic scale.
Response rates, on the basis of local assessment, were 9.5% and 0.4% in the combination-therapy and exemestane-alone groups, respectively (P<0.001), and central assessment showed consistent results (Table 3). Overall survival results were immature at the time of the interim analysis, with a total of 83 deaths: 10.7% of patients in the combination-therapy group and 13.0% of those in the exemestane-alone group died. Patients and investigators continue to be unaware of study assignments and will remain so until survival results are mature for analysis.
DISCUSSION
The BOLERO-2 study showed that the addition of everolimus to exemestane significantly improves progression-free survival, with observed medians of 6.9 and 2.8 months, corresponding to a 57% reduction in the hazard ratio. These results were confirmed with the use of an independent, blinded radiologic assessment and were consistent across all subgroups. Our positive results are consistent with the outcomes of two other studies of everolimus and antiestrogen therapy in patients with HR-positive breast cancer.16,20 In one study involving patients with newly diagnosed breast cancer, neoadjuvant everolimus combined with letrozole improved the clinical response rate and decreased tumor-cell proliferation as compared with letrozole alone.16 More recently, in a randomized, phase 2 study involving 111 postmenopausal women with ER-positive advanced breast cancer previously treated with an aromatase inhibitor, the combination of everolimus and tamoxifen was associated with significantly improved progression-free survival relative to tamoxifen alone (8.6 months vs. 4.5 months, P = 0.002) and with significantly improved overall survival (median not reached vs. 24.4 months, P = 0.01).20 Taken together, these studies suggest that everolimus adds to the anticancer activity of antiestrogen therapy in a variety of clinical settings and with different classes of endocrine agents.
The magnitude of the observed benefit compares favorably with that of the limited options available to this group of patients with HR-positive advanced breast cancer. The ER down-regulator fulvestrant (at a standard dose of 250 mg monthly) was associated with activity similar to that of exemestane, with a median progression-free survival of 3.7 months.6 High-dose fulvestrant (500 mg monthly), as compared with standard-dose fulvestrant, provided only a modest improvement in median progression-free survival, from 5.5 to 6.5 months (hazard ratio, 0.80; P = 0.006). This improvement was less clear in patients whose most recent therapy was an aromatase inhibitor (hazard ratio, 0.85; P = 0.20) and in those who were considered to have had a response to the most recent endocrine therapy (hazard ratio, 0.85; P = 0.12).7 Our results also compare favorably to those shown with capecitabine and taxanes or anthracyclines, with a median progression-free survival duration of 6.2 months and 8.2 months, respectively, in patients with HR-positive disease.21
Combination therapy was associated with a higher incidence of adverse events than exemestane alone. The adverse events observed with everolimus plus exemestane are consistent with those reported with everolimus and other rapamycin analogues and include stomatitis, fatigue and asthenia, diarrhea, cough, pyrexia, and hyperglycemia.22,23 In the current study, a high percentage of patients discontinued everolimus because of a lack of tolerability. The longer treatment duration in the combination-therapy group might have contributed to the high discontinuation rate. Careful monitoring of patients and increased physician awareness of the safety profile of everolimus are warranted.
In summary, we report a phase 3 trial in patients with HR-positive advanced breast cancer showing that the addition of everolimus to endocrine therapy results in an improved clinical outcome. This benefit should be weighed against the side effects observed with everolimus. The potential of everolimus to benefit patient survival is not yet known.
Supplementary Material
Supplementary Appendix
Supplementary Protocol
Acknowledgments
Supported by Novartis, including funding for medical editorial assistance with the manuscript.
We thank the patients who participated in the BOLERO-2 trial; the investigators, study nurses, and clinical research associates from the individual trial centers who provided ongoing support; and Matthew Grzywacz of ApotheCom for medical editorial assistance with the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–42. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 2.Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–606. doi: 10.1200/JCO.2001.19.10.2596. [Erratum, J Clin Oncol 2001;19:3302.] [DOI] [PubMed] [Google Scholar]
- 3.Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol. 2000;18:3758–67. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 4.Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18:3748–57. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 5.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–91. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 6.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 7.Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600. doi: 10.1200/JCO.2010.28.8415. [Erratum, J Clin Oncol 2011;29:2293.] [DOI] [PubMed] [Google Scholar]
- 8.Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:1979–87. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 9.Burstein HJ. Novel agents and future directions for refractory breast cancer. Semin Oncol. 2011;38(Suppl 2):S17–S24. doi: 10.1053/j.seminoncol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Johnston SR. Clinical efforts to combine endocrine agents with targeted therapies against epidermal growth factor receptor/human epidermal growth factor receptor 2 and mammalian target of rapamycin in breast cancer. Clin Cancer Res. 2006;12:1061S–1068S. doi: 10.1158/1078-0432.CCR-05-2125. [DOI] [PubMed] [Google Scholar]
- 11.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Crosstalk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 12.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–8. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem. 2009;284:6361–9. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 14.Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–76. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–28. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 17.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events v3.0 (CTCAE), version 3.0. Bethesda, MD: Cancer Therapy Evaluation Program; Aug 9, 2006. ( http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) [Google Scholar]
- 19.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 20.Bachelot T, Bourgier C, Cropet C, et al. TAMRAD: a GINECO randomized phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients (pts) with hormone-receptor positive, HER2 negative metastatic breast cancer (MBC) with prior exposure to aromatase inhibitors (AI). Presented at the 33rd Annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 8–12, 2010. [Google Scholar]
- 21.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–60. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 22.Chow LWC, Sun Y, Jassem J, et al. Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer. Presented at the 29th Annual San Antonio Breast Cancer Symposium; San Antonio, TX. December 14–17, 2006. [Google Scholar]
- 23.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix
Supplementary Protocol