Cholangiocarcinoma — evolving concepts and therapeutic strategies (original) (raw)
. Author manuscript; available in PMC: 2018 Mar 1.
Published in final edited form as: Nat Rev Clin Oncol. 2017 Oct 10;15(2):95–111. doi: 10.1038/nrclinonc.2017.157
Abstract
Cholangiocarcinoma is a disease entity comprising diverse epithelial tumours with features of cholangiocyte differentiation: cholangiocarcinomas are categorized according to anatomical location as intrahepatic (iCCA), perihilar (pCCA), or distal (dCCA). Each subtype has a distinct epidemiology, biology, prognosis, and strategy for clinical management. The incidence of cholangiocarcinoma, particularly iCCA, has increased globally over the past few decades. Surgical resection remains the mainstay of potentially curative treatment for all three disease subtypes, whereas liver transplantation after neoadjuvant chemoradiation is restricted to a subset of patients with early stage pCCA. For patients with advanced-stage or unresectable disease, locoregional and systemic chemotherapeutics are the primary treatment options. Improvements in external-beam radiation therapy have facilitated the treatment of cholangiocarcinoma. Moreover, advances in comprehensive whole-exome and transcriptome sequencing have defined the genetic landscape of each cholangiocarcinoma subtype. Accordingly, promising molecular targets for precision medicine have been identified, and are being evaluated in clinical trials, including those exploring immunotherapy. Biomarker-driven trials, in which patients are stratified according to anatomical cholangiocarcinoma subtype and genetic aberrations, will be essential in the development of targeted therapies. Targeting the rich tumour stroma of cholangiocarcinoma in conjunction with targeted therapies might also be useful. Herein, we review the evolving developments in the epidemiology, pathogenesis, and management of cholangiocarcinoma.
Cholangiocarcinomas are diverse biliary epithelial tumours involving the intrahepatic, perihilar, and distal biliary tree1. Cholangiocarcinoma is the second most common hepatic malignancy after hepatocellular carcinoma (HCC), and the overall incidence of cholangiocarcinoma has increased progressively worldwide over the past four decades2–4. Intrahepatic cholangiocarcinomas (iCCAs) arise above the second-order bile ducts, whereas the cystic duct is the anatomical point of distinction between perihilar cholangiocarcinomas (pCCAs) and distal cholangiocarcinomas (dCCAs)1. Two histopathological subtypes of the disease are predominant: cancers with cylindrical, mucin-producing glands; and those with cuboidal, non-mucin-producing glands5. However, cholangiocarcinomas commonly have a mixture of these histopathological characteristics. Importantly, substantial differences exist in the molecular characteristics, biology, and management of the anatomical cholangiocarcinoma subtypes1.
Cholangiocarcinomas are aggressive tumours, and most patients have advanced-stage disease at presentation6. Diagnosing cholangiocarcinoma at an early stage remains a challenge owing to its ‘silent’ clinical character (most patients with early stage disease are asymptomatic), difficult to access anatomical location, and highly desmoplastic, paucicellular nature, which limits the sensitivity of cytological and pathological diagnostic approaches. Nonetheless, advanced cytological techniques, such as fluorescence in situ hybridization (FISH) and mutational analysis, have emerged as essential diagnostic modalities7,8.
Surgery is the preferred treatment option for all three disease subtypes, but a minority of patients (approximately 35%) have early stage disease that is amenable to surgical resection with curative intent6. Similarly, only a small subset of carefully selected patients with pCCA are candidates for liver transplantation following neoadjuvant chemoradiation9. Typically, iCCA is considered a formal contraindication for liver transplantation; however, results published in 2016 support liver transplantation as a treatment option for patients with ‘very early’ iCCA10. For patients with advanced-stage or unresectable cholangiocarcinoma, the available systemic therapies are of limited effectiveness: the median overall survival with the current standard-of-care chemotherapy regimen (gemcitabine and cisplatin) is <1 year11. The desmoplastic stroma and genetic heterogeneity both contribute to the resistance of cholangiocarcinoma to therapy; the rich tumour microenvironment fosters potent survival signals and might pose a barrier to the delivery of chemotherapy to the tumour. Advances in genetic profiling and classifications coupled with targeted therapies, radiation therapy, and immunotherapy might help improve survival outcomes of patients with this otherwise devastating malignancy. Herein, we review these advances, focusing on the current state-of-the-art and emerging concepts.
Evolving epidemiology
The anatomical subtypes of cholangiocarcinoma differ geographically in their incidence, presumably reflecting differences in the global distribution of risk factors, in addition to genetic variation. Risk factors for cholangiocarcinoma have previously been reviewed elsewhere1,12. Herein, we focus on the secular trends in the incidence of cholangiocarcinoma.
The incidence of iCCA and pCCA/dCCA
The international classification of cholangiocarcinoma does not, unfortunately, distinguish between pCCA and dCCA, and in this section we have aggregated these cancers together as ‘pCCA/dCCA’. Together, pCCA (50–60%) and dCCA (20–30%) account for approximately 80% of all cholangiocarcinomas diagnosed in the USA; the remaining 20% are iCCA13,14. The global incidence of cholangiocarcinoma is highest in northeast Thailand, with age-standardized incidence rates (ASIRs) of approximately 100 per 100,000 individuals among men and 50 per 100,000 individuals among women15; in the West, ASIRs range between 0.5–2.0 per 100,000 individuals15–17. The high incidence of cholangiocarcinoma in Thailand and neighbouring areas has been attributed to endemic liver fluke infection, in particular, with Opisthorchis viverrini15. Multiple studies reported that the incidence of iCCA increased by up to 10-fold, while the incidence of pCCA/dCCA decreased at a similar or slightly slower rate, over a 2–3-decade period around the turn of the 20th century in Australia, Japan, the USA, the UK, and across Europe3,4,18–21.
Given the poor prognosis of cholangiocarcinoma, patient mortality should parallel incidence rates. A study using data from the WHO revealed an overall decrease in age-standardized mortality rates (ASMR) among patients with pCCA/dCCA in the first decade of the 21st century across 13 European Union (EU) countries (−6% in males, −17% in females), the USA (−20%, −17%), Japan (−5%, −10%), and Australia (−69%, −28%)22. By contrast, overall ASMRs for iCCA increased by 36.5% in males and 36.2% in females across the 13 EU countries, with the largest increases in Austria, Spain, France, Germany, Italy, and Denmark22. ASMRs for iCCA also rose in the USA (by 11.2% in men and 13.8% in women) and Australia (30.2%, 19.5%), but remained stable in Japan (0.4%, 0.3%)22. Two other studies, however, demonstrated that the incidence of both iCCA and pCCA/dCCA remained stable in Burgundy, France23, and decreased in Denmark24. Furthermore, data from the North American Association of Central Cancer Registries indicate that the incidence of iCCA fell between 1998 and 2003 (annual percentage change (APC) −8% per year), then rose between 2003 and 2009 (APC 6% per year); the incidence of pCCA/dCCA increased between 1998 and 2003 (APC 9% per year), before plateauing from 2003 to 2009 (REF. 25).
Contributing factors
Several factors might explain the inconsistent trends in cholangiocarcinoma epidemiology, including some that are potentially artefactual. Cholangiocarcinoma classification in large epidemiological datasets is problematic, owing to the lack of differentiation between pCCA and dCCA. Furthermore, International Classification of Disease for Oncology (ICD-O;http://codes.iarc.fr/) editions change every few years, but are adopted by countries at different times. For example, the second edition of the ICD-O (ICD-O-2) assigned ‘Klatskin’ tumours (pCCA) a unique histology code, but this was cross-referenced to the topography code for intrahepatic rather than extrahepatic cholangiocarcinoma. Using the ICD-O-3, however, Klatskin tumours can be cross- referenced to either intrahepatic or extrahepatic cholangiocarcinoma. In the USA, the switch from ICD-O-2 to ICD-O-3 occurred in 2001, whereas in the UK, this switch did not occur until 2008 (REF.26). In a study of cholangiocarcinoma ASIRs between 1990 and 2008 in England and Wales26, a marked increase in iCCA and a decrease in pCCA/dCCA incidences were found, and remained evident after transferring all Klatskin tumours from intrahepatic to extrahepatic codes; however, only 1% of all cholangiocarcinomas were reportedly Klatskin, which cannot be a true reflection of all pCCA cases26. Of note, UK cancer registries reported that if a tumour site is unspecified, most would classify cholangiocarcinoma as intrahepatic26. In the same study26, an analysis of US Surveillance, Epidemiology, and End Results (SEER) data revealed that the ASIR of iCCA rose from 0.6 per 100,000 individuals in 1990 to 0.9 per 100,000 individuals in 2001; that year, concomitant with the uptake of ICD-O-3, the ASIRs for iCCA began to decrease, before plateauing at 0.6 per 100,000 individuals by 2007 (REF. 26). Conversely, ASIRs for pCCA/dCCA remained stable at around 0.8 per 100,000 individuals until 2001, and then began increasing, reaching 1.0 per 100,000 individuals by 2007 (REF. 26). These trends suggest that pCCA, the most-common subtype of cholangiocarcinoma, might have been misclassified as iCCA, the least common subtype, thereby falsely skewing the reported rates of iCCA.
Other studies have highlighted the misclassification of cholangiocarcinoma. Systematic under-reporting of the incidences of pancreatic cancer and cholangiocarcinoma was found by examining the concordance between Swedish cancer registries and patient registries: between 1990 and 2009, 44% of cholangiocarcinomas were reported only in the patient registries27. In Sweden, most deaths from liver cancer are classified by the Cancer Register as ‘unspecified’, and evidence indicates that the incidence of HCC is also under-reported28,29. The same classification and reporting issues probably apply to cholangiocarcinomas.
Whereas the incidence of iCCA has increased over the past 2–3 decades, a concomitant decline in the incidence of cancer of unknown primary (CUP) has been observed2. In a prospective, phase II trial involving patients with previously untreated CUP (n = 289)30, molecular tumour profiling enabled determination of the tissue of origin in 98% of patients. Of these, 18% of patients were predicted to have biliary tract cancer30. Hence, the enhanced clinical distinction between CUP and iCCA might be another factor contributing to the apparent increase in iCCA incidence31.
Aside from technical classification issues, and improvements in the accuracy and availability of diagnostic tools, several demographic trends could also be affecting the true incidence of cholangiocarcinoma subtypes, including rising obesity rates and the changing burden of chronic viral hepatitis (which are recognized risk factors for iCCA, as well as for HCC32); with improved antiviral therapy, the contribution of chronic viral hepatitis to the incidence of iCCA will probably decline in the future. Other demographic factors potentially influencing the incidence of cholangiocarcinoma include population migration between different risk areas.
In conclusion, the trends in cholangiocarcinoma incidence are complex and need to be interpreted with caution. Going forward, epidemiological data need to be recorded uniformly and accurately; this responsibility resides with both clinicians and cancer registries.
Standard of care: diagnosis and therapy
iCCA
Diagnosis
iCCA is typically detected as a hepatic mass lesion, often during routine imaging surveillance for HCC in patients with cirrhosis; in a cirrhotic liver, the differential diagnosis of HCC and iCCA can be difficult. Whereas arterial phase enhancement with subsequent delayed phase washout is diagnostic of HCCs33, dynamic gadolinium-enhanced MRI and CT scanning of iCCA yields an initial rim or peripheral arterial phase-enhancement pattern followed by centripetal enhancement in the delayed phases34,35. CT and MRI have comparable performance in the detection of primary and satellite iCCA lesions, but CT imaging is superior for the detection of vascular enhancement and, thus, assessment of resectability36(FIG. 1). Cancer antigen 19–9 (CA 19–9) is the primary serum biomarker used in the diagnosis of cholangiocarcinoma37,38, and CA 19–9 levels >1,000 U/ml have been associated with the presence of metastatic disease39. Of note, however, patients who are Lewis-antigen-negative (7% of the general population) have undetectable CA 19–9 levels40. A histopathological assessment of a biopsy specimen is essential for the diagnosis of iCCA.
Figure 1. Illustrative examples of the radiographic modalities used in the visualization of the different anatomical subtypes of cholangiocarcinoma.
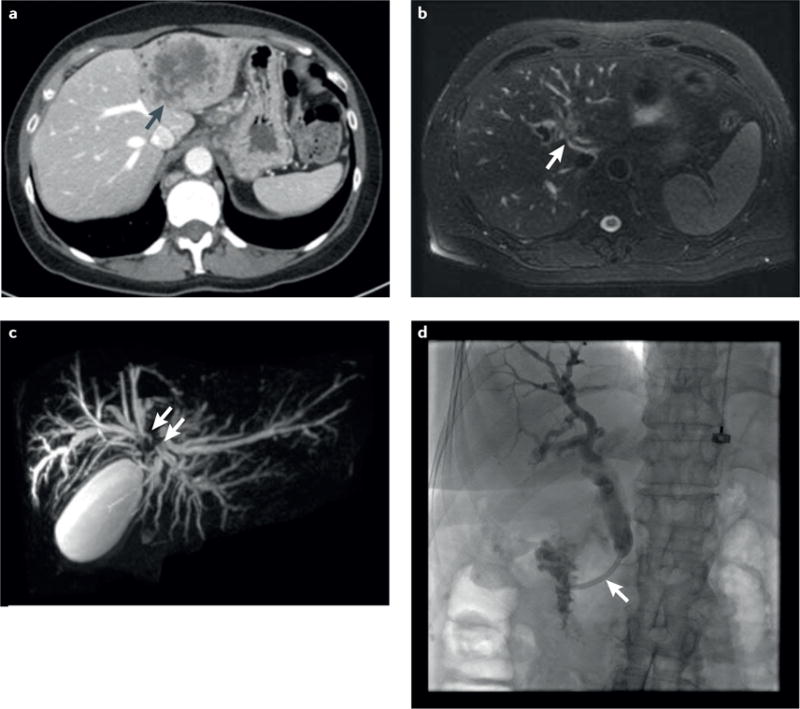
a | Axial CT image of a large, left lobe heterogeneous mass with peripheral bile-duct dilatation (black arrow) consistent with an intrahepatic cholangiocarcinoma (iCCA). The pattern of vascular enhancement on CT imaging, with initial rim enhancement followed by centripetal enhancement, helps distinguish iCCA from hepatocellular carcinoma, but does not enable assessment of resectability. b | Axial T2-weighted MRI scan of a circumferential, soft-tissue, perihilar mass (white arrow) consistent with a perihilar cholangiocarcinoma (pCCA). c | Coronal magnetic resonance cholangiopancreatography image of pCCA separating the right and left hepatic ducts (white arrows). d | Endoscopic retrograde cholangiopancreatography image of a malignant-appearing (‘dominant’) distal stricture (white arrow) consistent with a distal cholangiocarcinoma.
Surgical resection or liver transplantation
Surgical resection remains the mainstay of potentially curative therapy for iCCA (FIG. 2a), with median disease-free survival (DFS) durations of 12–36 months reported in various patient series41,42. Notably, the median overall survival of patients with R0-resected iCCA was 80 months in one cohort13. Predictors of short DFS durations include large tumour size, the presence of multiple liver lesions, and regional lymph-node involvement42. Cirrhosis is also an independent factor associated with unfavourable survival outcomes in patients with iCCA undergoing surgical resection43. iCCA has conventionally been considered a contraindication for liver transplantation owing to poor survival outcomes and a high risk of recurrence44,45. In 2014, however, a retrospective multicentre study demonstrated an excellent 5-year actuarial survival after liver transplantation of 73% in eight patients with cirrhosis and ‘very early’ iCCA, defined as single tumours ≤2 cm in diameter46. A follow-up study with a larger, international, multicentre cohort of patients found a 5-year survival of 65% in 15 patients with very early iCCA versus 45% in 33 patients with ‘advanced’ iCCA (single tumour >2 cm or multifocal disease)10. These studies indicate that liver transplantation might be an effective treatment option for a subset of cirrhotic patients with early iCCA.
Figure 2. Current clinical management algorithms for adult patients with cholangiocarcinoma.
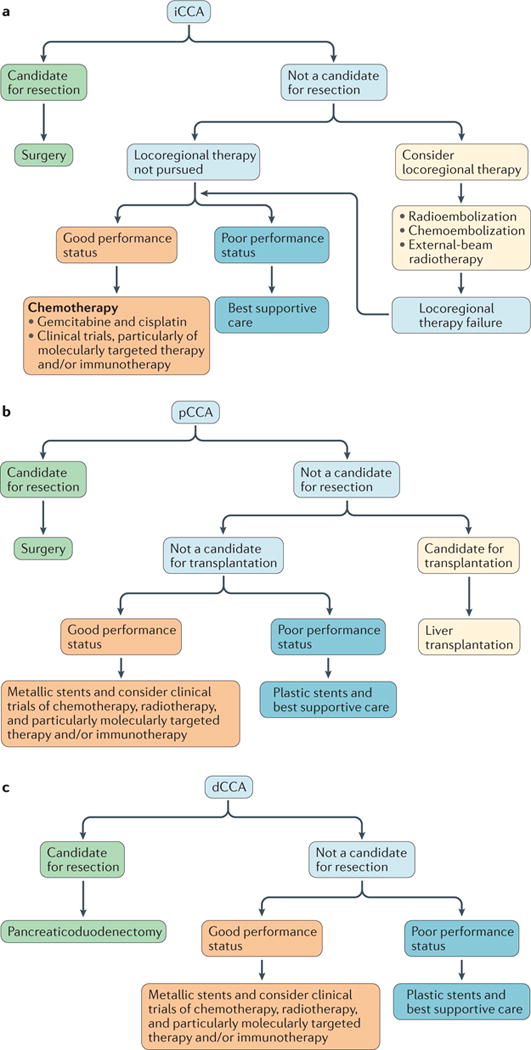
a | For patients with intrahepatic cholangiocarcinoma (iCCA).b | For those with perihilar cholangiocarcinoma (pCCA).c | For patients with distal cholangiocarcinoma (dCCA). Patients with unresectable pCCA/dCCA who are not candidates for liver transplantation and have a poor performance status generally have short survival durations; thus, the use of plastic stents is usually sufficient and probably more cost-effective than the use of metallic stents.
Locoregional therapies
Locoregional therapies are a reasonable treatment approach in patients with advanced-stage iCCA (FIG. 2a). In patients with localized, unresectable iCCA, transarterial chemoembolization (TACE) is considered a safe treatment option and is associated with median overall survival durations of 12–15 months47–49. In one such cohort, TACE with drug-eluting beads resulted in a median overall survival of 11.7 months, compared with 5.7 months with conventional TACE50. Radioembolization using yttrium-90 microspheres is an alternate treatment option for unresectable iCCA, with reasonable effectiveness (median overall survival durations of 11–22 months) and safety51,52. High-dose, conformal external-beam radiation therapy (EBRT) has emerged as an acceptable treatment for select patients with localized, unresectable iCCA (see ‘The evolving role of radiation therapy’ section). To date, no randomized controlled trials have compared different forms of locoregional therapy for iCCA. Patients who are not candidates for surgical resection or locoregional therapies should be considered for enrolment in a clinical trial of a targeted therapy (FIG. 2a).
pCCA
Diagnosis
A combination of CT and MRI with magnetic resonance cholangiopancreatography (MRCP) imaging is used for the detection of pCCA: MRI–MRCP has a higher level of diagnostic accuracy for the detection of biliary neoplastic invasion (FIG. 1), whereas CT enables a better assessment of vascular involvement53,54. The use of endoscopic ultrasonography (EUS) alone is associated with a high tumour detection rate compared with the use of CT or MRI, with better performance in the detection of dCCA versus pCCA (100% versus 83%, respectively)55. Fine-needle aspiration (FNA) during EUS carries a high risk of tumour seeding: among 191 patients with pCCA, 5 of 6 patients (83%) who underwent a transperitoneal primary tumour biopsy developed peritoneal metastases, compared with 14 of 175 (8%) of those who did not undergo a transperitoneal biopsy56. Endoscopic retrograde cholangiopancreatography (ERCP) has an integral role in pCCA management by enabling not only the detection of malignant biliary strictures, but also the acquisition of biliary brushing samples for cytological and genetic assessment.
A number of emerging cytological techniques have potential clinical utility in pCCA diagnosis (BOX 1). Conventional biliary cytology has a high specificity (97%) in the detection of pCCA, but limited sensitivity (43%)57, predominantly because cholangiocarcinomas are desmoplastic, paucicellular tumours potentially located in inaccessible regions of the biliary tree, causing difficulties in adequate specimen retrieval. FISH analyses have improved the diagnostic performance of conventional cytology. Chromosomal instability is a hallmark of cancer, and the diagnostic FISH assay involves the use of fluorescently labelled DNA probes to detect chromosomal aneusomy (gains or losses of chromosomal regions), with FISH polysomy indicating the presence of five or more cells with gains detected for two or more probes. An optimized FISH probe set targeting the 1q21, 7p12, 8q24, and 9p21 loci has been developed, and can detect pancreatobiliary malignancies, including cholangiocarcinoma, with a sensitivity and specificity of 93% and 100%, respectively7. Next-generation sequencing (NGS) for known or candidate oncogenic targets can enhance the diagnostic utility of conventional biliary cytology. In 33 patients with malignant-appearing pancreatobiliary strictures, NGS combined with cytology had a sensitivity of 85% in the detection of high-risk neoplasia or malignancy, compared with 67% for cytology alone58. Moreover, NGS revealed driver mutations in 24 patients, including KRAS, TP53, and_CDKN2A_ aberrations58.
Box 1. Diagnosis of perihilar cholangiocarcinoma (pCCA).
Various emerging cytological and genetic techniques that can be performed on biliary brush specimens, bile, and serum for the detection of pCCA based on the presence and/or abundance of characteristic molecular markers are listed below.
Cell-based assays on biliary brush specimens
- Conventional cytology, potentially with next-generation sequencing (NGS) of cellular material
- Fluorescence in situ hybridization, particularly with optimized probe sets
Molecular diagnostics on bile
- Analysis of microRNAs (miRNAs) from extracellular vesicles (EVs)
- NGS of cellular material (RNA and DNA)
- Mutational profiling of cell-free DNA (cfDNA)
Biomarkers in the peripheral circulation
- Serum levels of cancer antigen 19–9 (CA 19–9)
- Differentially methylated regions in circulating cfDNA
- Components of serum EVs, such as proteins and miRNAs
The cytological diagnosis of pCCA is not always possible, often necessitating a diagnosis based on clinical criteria (for example, a mass lesion and malignant-appearing stricture with elevated serum CA 19–9 levels); the major differential diagnosis for a perihilar stricture is pCCA versus IgG4 cholangiopathy59. Molecular profiling techniques, however, have the potential to improve cholangiocarcinoma diagnosis. For example, microRNAs (miRNAs) have emerged as promising diagnostic markers (BOX 1). Extracellular vesicles (EVs) are present in many biological fluids, including bile, and participate in intercellular communication; human biliary EVs contain abundant miRNA species60. A panel of miRNAs isolated from EVs in bile had a reported sensitivity of 67% and a specificity of 96% for the diagnosis of cholangiocarcinoma60. Furthermore, a separate proteomic analysis indicated that greater levels of oncogenic proteins are present in EVs obtained from cultures of human cholangiocarcinoma cells versus those derived from nonmalignant human cholangiocytes61. In addition, Severino et al.62demonstrated that patients with malignant biliary strictures have a significantly higher concentration of EVs in bile than those with nonmalignant strictures (2.4 × 1015 versus 1.6 × 1014 nanoparticles/l in the discovery cohort,P <0.0001; 4.0 × 1015 versus 1.3 × 1014 nanoparticles/l in the verification cohort,P <0.0001). Moreover, these authors identified an EV proteomic signature that can help discriminate malignant from common nonmalignant bile-duct strictures62.
Genomic and molecular advances have increased the clinical utility of circulating tumour DNA (ctDNA) or cell-free DNA63. The plasma concentration of ctDNA correlates with tumour size and stage; hence, ‘liquid biopsy’ approaches have the potential to be used for prognostication and disease monitoring in the management of cancer63 (BOX 1). In 69 patients with cholangiocarcinoma (94% with pCCA) and 95 individuals without cancer64, analyses of serum cell-free DNA revealed a panel of four genes that had differentially methylated regions (DMRs) in patients with cholangiocarcinoma (HOXA1, PRKCB,CYP26C1, and PTGDR). This DMR ctDNA panel had a sensitivity and a specificity of 83% and 93%, respectively, in the detection of cholangiocarcinoma64.
Surgical resection or liver transplantation
Surgical resection of pCCA is a potentially curative option for patients without the following exclusion criteria: bilateral involvement of the second-order bile ducts, bilateral or contralateral vascular involvement, presence of metastatic disease, and underlying primary sclerosing cholangitis (PSC). PSC is associated with underlying chronic parenchymal disease and a field defect that can be eliminated by liver transplantation, but not resection. The presence of regional lymphadenopathy, although not an absolute contraindication for resection, is associated with inferior patient outcomes65. Resection with curative intent often involves lobectomy with bile-duct resection, regional lymphadenectomy, and Roux-en-Y hepaticojejunostomy65. Surgical advances, such as extended lobectomy, vascular reconstruction, and techniques to increase remnant liver volume (including portal vein embolization and the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure), have facilitated the resection of tumours traditionally considered unresectable66–69.
Liver transplantation following neoadjuvant chemoradiation offers the best outcomes for patients with unresectable pCCA; however, only a minority of patients with early stage disease are candidates for this treatment option. Selection criteria — in an otherwise suitable candidate for liver transplantation — includes the presence of an unresectable tumour with a radial diameter of <3 cm, and the absence of intrahepatic or extrahepatic metastatic disease70. As alluded to previously, pCCA arising in the setting of PSC is best treated with liver transplantation regardless of resectability, owing to the field defect associated with this underlying chronic liver disease, which promotes carcinogenesis. Eligible patients typically undergo EBRT with radiosensitizing chemotherapy, brachytherapy, and maintenance oral chemotherapy before liver transplantation9. The 5-year DFS of patients with pCCA who underwent liver transplantation following neoadjuvant therapy was 65% across 12 US transplantation centres9. For patients with pCCA who are not candidates for surgical resection or liver transplantation, consideration should be given to enrolment in a clinical trial, particularly those evaluating targeted therapy (FIG. 2b; Supplementary information S1 (table)).
dCCA
Diagnosis
The same modalities that are used for the diagnosis of pCCA — CT, MRI–MRCP, ERCP, and EUS — are used to diagnose dCCA (FIG. 1). EUS with FNA of the lesion is usually diagnostic in patients with these tumours. The aforementioned molecular approaches to the diagnosis of pCCA might also be useful for the detection of dCCA.
Surgical resection
Surgical resection of dCCA typically entails a pancreaticoduodenectomy (Whipple procedure). In a large series of patients with cholangiocarcinoma undergoing surgical resection13, R0 resection was achieved in 78% of those with dCCA. In this cohort, dCCAs were mainly resected using a Whipple procedure; for smaller tumours, excision of the extrahepatic biliary tree with lymph-node dissection was used13. The 5-year overall survival of patients with dCCA was 23%, and was slightly higher (27%) if R0 resection was achieved (the median survival after R0 resection was 25 months)13. For patients with advanced-stage dCCA not amenable to resection, consideration should be given to enrolment in a clinical trial, potentially involving targeted therapy (FIG. 2c; Supplementary information S1 (table)).
Cytotoxic chemotherapies
The combination of gemcitabine and cisplatin is the current first-line chemotherapy for patients with advanced-stage cholangiocarcinoma not amenable to locoregional and surgical options, irrespective of anatomical disease subtype. Valle et al.11 reported a median survival of 11.7 months with this combination versus 8.1 months with gemcitabine alone; however, almost 40% of this cohort of patients in the UK had gallbladder cancer. Moreover, the 95% CI of the hazard ratio (HR) for death crossed one for the pCCA and dCCA subgroups11. A subsequent meta-analysis71, which incorporated data from the UK study11 and a Japanese study72, among others, reported similar results for the gemcitabine and cisplatin regimen, with a median overall survival of 11.7 months — and 11.1 months in the UK and Japanese study cohorts specifically. These data indicate that, at least for patients with advanced-stage pCCA/dCCA, enrolment in clinical trials of novel therapies could be considered in lieu of treatment with the current standard-of-care chemotherapy regimen (FIG. 2).
In the adjuvant setting, capecitabine has demonstrated efficacy in patients who had undergone surgical resection for cholangiocarcinoma or gallbladder cancer: the median overall survival was 51 months in the treatment arm compared with 36 months in the observation arm73. Results of a phase III trial conducted in France, however, demonstrated that adjuvant chemotherapy with gemcitabine and oxaliplatin (GEMOX), initiated 3 months after R0 or R1 resection of biliary tract cancer, did not significantly improve recurrence-free survival compared with placebo (HR 0.83, 95% CI 0.58–1.19; _P_= 0.31)74. More evidence is needed to clarify the role of adjuvant chemotherapy in the treatment of cholangiocarcinoma.
The evolving role of radiation therapy
Technological advances have improved the safety and effectiveness of radiation therapy for cholangiocarcinoma75. High-resolution, multiphase helical CT and multiparametric MRI of the liver and biliary tree have enabled more-precise determination of cancer location and the extent of radiotherapy targeting. Moreover, CT-based treatment planning and dose calculation enables accurate estimation of radiation doses delivered to the tumour and nonmalignant tissues76,77. In addition, advanced EBRT techniques, such as 3D conformal radiotherapy (3D–CRT) and intensity-modulated radiotherapy (IMRT), are used to deliver conformal radiation to the target while sparing nonmalignant tissues. Alternatively, charged-particle (proton or carbon) beams have a more-favourable physical dose-deposition profile than that of conventional X-ray beams, which might yield advantages in sparing nonmalignant tissues78 (FIG. 3). Consequently, accelerated and hypofractionated regimens, including stereotactic body radiation therapy (SBRT), have been used to deliver high-dose, ablative EBRT to patients with cholangiocarcinoma78–80. Image-guided, high-dose-rate brachytherapy can also be used as primary treatment or to provide a radiation boost for selected patients with localized disease81,82. Together, these technological advances might enable escalation of the radiotherapy dose to biliary tumours and/or improved protection of nonmalignant tissues, thus improving the therapeutic ratio for radiotherapy in the treatment of cholangiocarcinoma.
Figure 3. Proton radiotherapy of intrahepatic cholangiocarcinoma (iCCA).
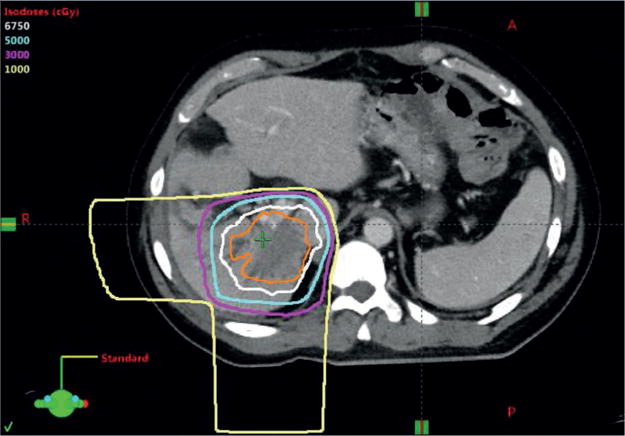
Proton-beam radiotherapy plan for a patient with localized, unresectable iCCA, with a total radiation dose of 6,750 cGy delivered in 15 fractions over 3 weeks. The orange line depicts the tumour. The white, cyan, magenta, and yellow lines represent the 6,750, 5,000, 3,000, and 1,000 cGy isodose lines, respectively. Radiation is delivered in two beams from the right lateral (R) and posterior (P) directions (as indicated by the 1,000 cGy isodose lines). Proton beams have no ‘exit dose’ deposition, which for this patient, enabled complete sparing of the left lobe of the liver, stomach, and bowel from radiation exposure.
For patients with resected cholangiocarcinoma, data from retrospective studies indicate a benefit from postoperative EBRT with concurrent chemotherapy, especially in patients with lymph-node-positive or resection-margin-positive disease83–85. Results of a multi-institutional, single-arm phase II study86demonstrated the safety and promising efficacy of adjuvant therapy consisting of gemcitabine plus capecitabine followed by conformal EBRT with concurrent capecitabine for patients with resected pCCA/dCCA and gallbladder cancer. The majority of patients (81%) received IMRT86. In the 54 patients with resected pCCA/dCCA, the 2-year overall survival and local control rates were 68% and 87%, respectively; no differences in overall survival or DFS were observed between patients with R0 versus R1 resection86. These results support the need for high-quality studies of adjuvant chemoradiotherapy for patients with resected cholangiocarcinoma.
Studies have demonstrated the safety and efficacy of high-dose, conformal EBRT for patients with localized, unresectable iCCA78,80. In a single-institution retrospective analysis80 involving 79 patients with localized, unresectable iCCA treated with high-dose, conformal EBRT (35–100 Gy, median 58.05 Gy, in 3–30 fractions), the median overall survival was 30 months. In a multi-institutional single-arm phase II study78, 37 patients with localized, unresectable iCCA received hypofractionated proton-beam therapy with a median dose of 58.05 Gy in 15 fractions delivered daily over 3 weeks. The median and 2-year overall survival was 22.5 months and 46.5%, respectively; the 2-year local control rate was 94%, and most recurrences occurred at extrahepatic sites78. These outcomes formed the basis for an ongoing multi-institutional phase III trial to assess the role of high-dose, conformal EBRT after initial gemcitabine and cisplatin chemotherapy (NCT02200042).
For patients with localized, unresectable pCCA/ dCCA, the role of radiotherapy remains unclear. Retrospective analyses of large observational cohorts suggest a modest benefit from radiotherapy, although these analyses are hampered by considerable inherent biases87,88. By contrast, in single-institution retrospective series89–91, long-term DFS has been reported for a small subset of patients treated with definitive chemoradiotherapy. Randomized trials are needed to better define the relative roles of contemporary treatments for localized, unresectable pCCA/dCCA, including systemic therapies and modern locoregional radiotherapy (FIG. 2).
Emerging molecularly-directed therapies
Molecular pathogenesis
The marked intertumoural and intratumoural heterogeneity of cholangiocarcinoma has contributed to the lack of effective targeted therapies for this deadly disease. Moreover, in most clinical trials, investigators have grouped together patients with different subtypes of the disease, under the broad definition of ‘biliary tract cancer’, rather than stratifying patients according to the presence of relevant oncogenic drivers. Molecular profiling studies have better delineated the genomic and transcriptomic landscape of each cholangiocarcinoma subtype (FIG. 4). Comprehensive whole-exome and transcriptome sequencing in a large cohort of 260 patients with biliary tract cancers, including 145 with iCCA, 86 with pCCA/dCCA, and 29 with gallbladder cancer, revealed potentially targetable genetic driver alterations in ~40% of patients92. In this study by Nakamura et al.92, the repertoire of genetic alterations varied across the different cholangiocarcinoma subtypes. For example, recurrent mutations in IDH1, IDH2,FGFR1, FGFR2, FGFR3,EPHA2, and BAP1 were noted predominantly in iCCAs, whereas ARID1B, ELF3,PBRM1, PRKACA, and PRKACB_mutations occurred preferentially in pCCA/dCCA92. The characteristic genomic signatures associated with the different genetic aberrations in each disease subtype contribute to their distinct biological behaviour. Notably, fibroblast growth factor receptor 2 (FGFR2) fusions that result in ligand-independent activation of this receptor-tyrosine kinase were identified exclusively in patients with iCCA92, consistent with prior observations93–97. Novel gene fusions involving PRKACA or PRKACB, which encode catalytic subunits of protein kinase A, were detected only in pCCA/dCCA92. The discovery of these aberrations is important because gene fusions are often targetable driver events. ELF3 was another novel candidate driver gene identified in this study92, primarily in pCCA/dCCA. Inactivating mutations in_ELF3 have since been identified in dCCA samples in two other genomic analyses98,99; thus, the ETS-related transcription factor ELF3 probably acts as a tumour suppressor in cholangiocarcinoma. In keeping with data reported by Nakamura et al.92, targeted sequencing of selected cancer-related genes in a study of 28 iCCA samples revealed potentially actionable alterations in IDH1,IDH2, FGFR2, KRAS, PTEN, and CDKN2A, among others95. The most common alterations involved_ARID1A_, IDH1, IDH2, and_TP53_ (each identified in 36% of the tumours), as well as MCL1 (amplified in 21% of tumours)95.
Figure 4. Evolving molecular stratification of cholangiocarcinoma (CCA) and therapeutic implications.
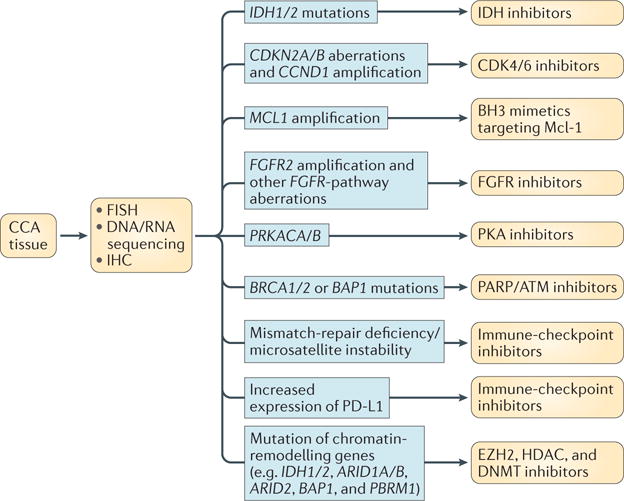
Emerging and conventional analytical techniques, such as RNA and/or DNA sequencing, fluorescence in situ hybridization (FISH), and immunohistochemistry (IHC), can be used for the detection of molecular aberrations in CCA tissue obtained via biopsy or surgery. The listed molecular alterations represent potential therapeutic targets in CCA. ATM, ataxia-telangiectasia mutated; BH3, BCL-2 homology domain 3; CDK4/6, cyclin-dependent kinases 4 and 6; DNMT, DNA methyltransferase; EZH2, enhancer of zeste homolog 2; FGFR, fibroblast growth factor receptor; HDAC, histone deacetylase; IDH, isocitrate dehydrogenase; Mcl-1, induced myeloid leukaemia cell differentiation protein Mcl-1; PARP, poly [ADP-ribose] polymerase; PD-L1, programmed cell death 1 ligand 1; PKA, protein kinase A.
Discrete carcinogenic exposures might induce distinct somatic alterations in patients with cholangiocarcinomas, as highlighted by whole-exome sequencing data from 108 liver-fluke-related and 101 non-liver-fluke-related tumours100: non-liver-fluke-related iCCAs had a higher prevalence of mutations in_IDH1_ or IDH2 (encoding isocitrate dehydrogenase [NADP] cytoplasmic (IDH1) and mitochondrial (IDH2)), and loss-of-function mutations in the tumour-suppressor gene_BAP1_ (encoding the epigenetic regulator BRCA1-associated protein 1 (BAP1))100. By contrast, mutations in the tumour-suppressor gene TP53 were a more frequent occurrence in liver-fluke-related cholangiocarcinomas100. These findings suggest that distinct causative aetiologies determine the mutational landscape of cholangiocarcinoma.
An integrated genomic analysis of predominantly liver-fluke-negative, hepatitis-negative iCCAs by The Cancer Genome Atlas (TCGA) investigators101identified inactivating mutations in tumour-suppressor genes, including_ARID1A_, ARID1B, BAP1,TP53, and PTEN, and gain-of-function mutations in the oncogenes IDH1, IDH2,BRAF, and KRAS — recapitulating the aforementioned findings. Recurrent focal losses of CDKN2A, encoding p16INK4A, which inhibits the cyclin-dependent kinases CDK4 and CDK6 (as well as p14ARF, which also indirectly inhibits CDK4 and CDK6), were observed in 47% of the tumours101 — a substantially higher proportion than reported previously (7–15%)95,102. Consistent with prior reports92,95,103,104, mutations in IDH1 or_IDH2_ were detected exclusively in iCCA, and were highly enriched in a novel, distinct molecular iCCA subtype identified through cluster-of-cluster analysis of gene-expression, DNA-methylation, and copy-number profiles101. Interestingly, this subtype was associated with high and low levels of mitochondrial and chromatin-modifier gene expression, respectively, including probable epigenetic silencing of ARID1A101, which encodes a subunit of the SWI/SNF chromatin-remodelling complex. Two other molecular subtypes of iCCA were defined, one comprising tumours enriched for BAP1 mutations and/or FGFR2 fusions, and the other enriched for_CCND1_ amplification101.
Molecularly targeted therapies
Receptor-tyrosine-kinase inhibitors
Several selective and nonselective small-molecule inhibitors of FGFRs are currently being investigated in early phase clinical trials involving patients with advanced-stage solid-organ malignancies, including cholangiocarcinoma (Supplementary information S1 (table)). The pan-FGFR inhibitor NVP-BGJ398, having demonstrated potential in preclinical models of cholangiocarcinoma105, is currently being investigated in a phase II study in patients with advanced-stage cholangiocarcinoma harbouring_FGFR_ alterations (NCT02150967). An interim analysis of data from this study indicated that NVP-BGJ398 has impressive antitumour activity, with a disease-control rate of 82%, and a manageable safety profile106. Erdafitinib is another orally active, pan-FGFR inhibitor107, and is being investigated in clinical trials. In a phase I dose-escalation study (NCT01703481), erdafitinib had a manageable safety profile at doses associated with clinical responses; among 23 response-evaluable patients with solid tumours harbouring FGFR-pathway alterations, four patients had a confirmed response to treatment with erdafitinib, one had an unconfirmed partial response, and 16 had stable disease108. A phase II trial of erdafitinib is currently ongoing (NCT02699606). Other FGFR-selective inhibitors currently being evaluated in patients with advanced-stage solid-organ malignancies include derazantinib (NCT01752920), TAS-120 (NCT02052778), Debio 1347 (NCT01948297), and INCB054828 (NCT02924376, NCT02393248). Ponatinib, a nonselective tyrosine-kinase inhibitor, has shown promising efficacy in patients with advanced-stage iCCA with _FGFR2_fusions93, and is currently being evaluated in a phase II trial in this population (NCT02265341; Supplementary information S1 (table)).
Inhibition of heat-shock protein 90 (HSP90) is an alternative to direct FGFR-kinase inhibition in _FGFR2_-fusion-driven cancers. HSP90 is a molecular chaperone required for essential cellular housekeeping functions, such as protein folding and mediating post-translational protein homeostasis, as well as for maintenance of oncoprotein stability109. As proof of this concept, the selective HSP90 inhibitor ganetespib induced loss of fusion protein expression, inhibition of oncogenic signalling, and consequent cancer-cell cytotoxicity in FGFR-fusion-driven bladder cancer110. Moreover, ganetespib had a synergistic combinatorial benefit with NVP-BGJ398 in preclinical models, with a change in average tumour volume relative to the vehicle-treated animals of −23% for ganetespib alone, −20% for NVP-BGJ398 alone, and −66% for the combination110.
ROS1 kinase fusion proteins have an oncogenic role in several malignancies, including cholangiocarcinoma; an immunoaffinity profiling study revealed FIG–ROS1 gene fusions in 2 of 23 patients with cholangiocarcinoma (8.7%)111. In a mouse orthotopic allograft model, expression of the FIG–ROS1 fusion accelerated iCCA tumour development and inactivation of this fusion had the converse effect, indicating that ROS1 fusions are potent oncoproteins and a potential therapeutic target in cholangiocarcinoma112. Of note, a gene fusion involving the ROS1-related kinase ALK (EML4–ALK) has also been detected in a patient with iCCA92. The ALK and ROS1 inhibitor ceritinib is currently being evaluated in two phase II trials in patients with ROS1-positive or ALK-positive advanced-stage pCCA or iCCA (NCT02374489; Supplementary information S1 (table)), or advanced-stage gastrointestinal malignancies, including cholangiocarcinoma (NCT02638909). Entrectinib, a selective tyrosine-kinase inhibitor with activity against ROS1 and ALK (as well as TRKA, TRKB, and TRKC), is also being evaluated in a phase II study involving patients with advanced-stage solid tumours harbouring _ROS1_or ALK fusions (NCT02568267).
Activating mutations of the proto-oncogene KRAS are a frequent occurrence (11–25%, depending on disease subtype) in cholangiocarcinomas92,95,101, and are associated with unfavourable progression-free survival (PFS) and overall survival95,102,113.KRAS activation upregulates signalling via downstream pathways, including the RAF–MEK–ERK (MAPK) pathway. Accordingly, KRAS_-mutant cholangiocarcinomas might be amenable to MEK inhibition. Results of a phase II study of selumetinib in patients with metastatic biliary cancer demonstrated a median PFS of 3.7 months and a median overall survival of 9.8 months114. In a subsequent phase Ib study in patients with advanced-stage biliary tract cancer, the combination of selumetinib, gemcitabine, and cisplatin conferred a median PFS of 6.4 months115. Neither of these studies involved patient selection based on_KRAS mutation status. BRAF mutations can also occur in cholangiocarcinoma (predominantly in iCCAs), albeit at a low frequency (3–5%)102,113,116. In eight patients with_BRAF_ V600-mutated cholangiocarcinoma, treatment with the oral BRAF inhibitor vemurafenib led to a partial response in one patient117.
Tyrosine-kinase signalling via the hepatocyte growth factor receptor MET is essential to a myriad of cellular processes required for cell survival. An integrated molecular analysis identified a proliferation class of iCCAs (62% of all iCCAs) characterized by activation of MET, EGFR, and MAPK signalling118; however, the results of early phase clinical trials of MET or EGFR inhibitors in patients with cholangiocarcinoma have been disappointing. A phase I study119 of the MET inhibitor tivantinib in combination with gemcitabine in patients with solid tumours, including cholangiocarcinoma, demonstrated partial responses and stable disease in 20% and 46% of patients, respectively; one patient with cholangiocarcinoma had a partial response. Cabozantinib, a multikinase inhibitor with activity against MET and VEGFR2, had limited activity (median PFS 1.8 months) and substantial toxicity in unselected patients with cholangiocarcinoma120. Moreover, MET expression did not correlate with patient outcomes in this study120. The combination of sorafenib, a multikinase inhibitor with activity against VEGFR and RAF family kinases, and the EGFR inhibitor erlotinib had disappointing clinical activity against advanced-stage biliary tract cancer121. In fact, this phase II study121 was terminated early owing to suboptimal PFS and overall survival. A phase II trial of the anti-HER2 antibody–drug conjugate trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced-stage malignancies, including cholangiocarcinoma, is currently ongoing (NCT02999672; Supplementary information S1 (table)). Umbrella and basket trial designs could facilitate the testing of these agents in what are essentially very rare molecular subtypes of cholangiocarcinoma.
Therapeutics targeting epigenetic alterations
The aforementioned genetic profiling studies have revealed that mutations affecting epigenetic regulators, such as IDH1, IDH2, BAP1, and ARID1A, are common in cholangiocarcinomas92,95,100,101; thus, epigenetic therapies are a promising endeavour122. Small-molecule inhibitors of mutant IDH1 or IDH2 have shown favourable efficacy in preclinical studies123,124; consequently, orally bioavailable inhibitors have entered clinical trials. Preliminary results from a phase I trial of AG-120 (NCT02073994; Supplementary information S1 (table)), an inhibitor of mutant IDH1, in a dose-escalation and dose-expansion cohort of patients with cholangiocarcinoma harbouring IDH1 mutations indicated a favourable safety profile125. Moreover, among 20 response-evaluable patients with cholangiocarcinoma treated with AG-120 in this study125, one had a partial response and 11 had stable disease. ClarIDHy, a global, multicentre, double-blind, placebo-controlled phase III trial involving 186 patients with_IDH1_-mutant cholangiocarcinoma, is currently underway (NCT02989857). Enasidenib, a first-in-class, oral, selective inhibitor of mutant IDH2, has demonstrated activity in preclinical models of acute myeloid leukaemia (AML)126–128. Consequently, enasidenib has been granted priority review by the FDA for patients with AML harbouring an IDH2 mutation. Enasidenib is currently being investigated in a multicentre phase I/II trial in patients with _IDH2_-mutant advanced-stage solid tumours, including iCCA (NCT02273739; Supplementary information S1 (table)).
Of note, _IDH_-mutant iCCA cells are dependent on SRC activity for survival; the SRC kinase inhibitor dasatinib induced tumour regression of mouse _IDH_-mutant tumour xenografts129. This preclinical work provided the basis for a phase II trial of dasatinib in patients with advanced-stage _IDH_-mutant iCCA (NCT02428855; Supplementary information S1 (table)). In addition, the TCGA analysis suggests that_IDH_-mutant cholangiocarcinomas probably have epigenetic silencing of the SWI/SNF chromatin-remodelling complex protein ARID1A101. In fact, mutation or silencing of SWI/SNF chromatin remodelling subunits, including ARID1A, ARID1B, ARID2, BAP1, PBRM1, SMARCA2, SMARCA4, and SMARCAD1, is a frequent occurrence in cholangiocarcinomas (and other cancers)92,101,130. Notably, tumours with mutations in genes encoding members of the SWI/SNF complex are dependent on the histone methyltransferase activity of EZH2 and, hence, are potentially susceptible to EZH2 inhibitors130. Indeed, EZH2 is typically overexpressed in cholangiocarcinomas, and EZH2 upregulation is correlated with a poor prognosis131,132. Furthermore, preclinical data indicate that EZH2 inhibition, in combination with gemcitabine, synergistically inhibits cholangiocarcinoma- cell proliferation133. Several active clinical trials are investigating EZH2 inhibitors, such as tazemetostat, but primarily in patients with haematopoietic or rhabdoid tumours. Trials of such agents in patients with cholangiocarcinoma are warranted.
The recurrent, inactivating mutations in chromatin regulators, including BAP1, ARID1A,ARID1B, ARID2, PBRM1, SMARCA2, SMARCA4, and_SMARCAD1_, support the notion that cholangiocarcinoma has an epigenetically-inclined mutational spectrum92,122,134,135. Loss of expression of ARID1A and PBRM1 seems to be a late event in cholangiocarcinoma carcinogenesis136,137. Several small-molecule inhibitors targeting chromatin-remodelling proteins are under investigation in preclinical and clinical studies of cholangiocarcinoma. These agents include histone deacetylase (HDAC) inhibitors, such as vorinostat, romidepsin, and valproic acid, and DNA methyltransferase (DNMT) inhibitors, including azacitidine and decitabine138–142. Results of a phase I/II study of valproic acid in 12 patients with advanced-stage pancreaticobiliary tract cancers indicate promising antitumour activity, with one patient achieving a partial response, 10 having stable disease, and one having progressive disease143.
Novel potential targeted therapies
Mesothelin, a cell-surface protein expressed in nonmalignant mesothelial cells, is often aberrantly expressed in cholangiocarcinomas, and is associated with advanced-stage and metastatic disease, and unfavourable overall survival144,145. Thus, this protein is an attractive target for therapy. Anetumab ravtansine, an anti-mesothelin antibody–drug conjugate, is being tested in a phase I trial open for enrolment of patients with advanced-stage cholangiocarcinoma with aberrant mesothelin expression (NCT03102320; Supplementary information S1 (table)).
The recurrent focal losses of CDKN2A, a gene encoding the proteins p16INK4A and p14ARF that are essential negative regulators of cell-cycle progression92,95,101, highlight the potential of CDK4/6 inhibitors, such as ribociclib and palbociclib, in the treatment of cholangiocarcinoma. These agents are approved treatments for breast cancer, and are in clinical trials for a range of other solid-organ malignancies (NCT03065062, NCT02022982), although the efficacy of these agents remains to be evaluated in patients with cholangiocarcinoma.
Somatic mutations of the tumour-suppressor genes_BRCA1_ and BRCA2 have been reported in cholangiocarcinomas92,102._BRCA_-mutated tumours are often sensitive to poly [ADP-ribose] polymerase (PARP) inhibition. Accordingly, in a retrospective clinical analysis in patients with_BRCA_-mutated cholangiocarcinoma (_n_= 18), one of the four patients who received PARP inhibitors had a sustained disease response with a PFS duration of 42.6 months146. Although PARP inhibitors and inhibitors of ataxia-telangiectasia mutated (ATM), another DNA-repair protein, are currently being evaluated in multiple clinical trials for _BRCA_-mutated breast cancer, they have yet to be prospectively evaluated in patients with cholangiocarcinoma. A phase II trial of the PARP inhibitor niraparib is, however, planned in patients with advanced-stage malignancies, including cholangiocarcinoma, and with known mutations in BAP1 and other DNA double-strand break repair pathway genes — excluding, for an unspecified reason,BRCA1/2 mutations (NCT03207347; Supplementary information S1 (table)).
Immunotherapy for cholangiocarcinoma
Immunotherapy in oncology
The immune system holds the remarkable potential to recognize and destroy aberrant cancer cells, but is regulated by a complex network of immune checkpoints that prevent uncontrolled immune activation. Cancers harness several mechanisms of immune escape to restrain or evade antitumour immune responses, including modulation of the local tumour microenvironment to create an immunosuppressive milieu; expression of immune-checkpoint proteins, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1); and loss of MHC expression. The exact mechanisms underlying the immune escape of cholangiocarcinomas remain to be elucidated. Immune-checkpoint inhibitors, antibodies that block the inhibitory interactions between CTLA-4 or PD-1 and their cognate ligands (FIG. 5), have demonstrated robust and durable antitumour activity in subsets of patients across a variety of tumour types, coupled with low rates of immune-mediated toxicity147. Indeed, various immune-checkpoint inhibitors have now been approved for use in the treatment of several malignancies. Ongoing studies of these agents, combination therapies, and novel adoptive-cell therapies148 show great promise to identify novel indications, improve upon the current response rates, refine treatment selection and sequencing, and address therapy resistance.
Figure 5. Biological rationale for the ongoing clinical trials of immunotherapies for cholangiocarcinoma.
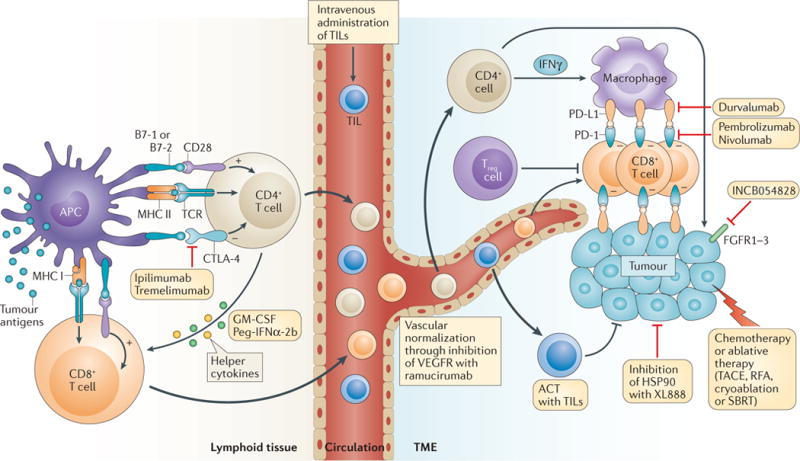
The mechanisms of action or targets of the immunotherapy combinations currently being tested in the ongoing trials listed in TABLE 1 are represented schematically. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) transmits inhibitory signals that limit T-cell priming by antigen-presenting cells (APCs), such as dendritic cells, in lymphoid organs, which can restrict responses to tumour antigens; thus, blockade of this inhibitory immune-checkpoint protein using the monoclonal antibodies ipilimumab or tremelimumab can enhance the activation of T cells with the capacity to recognize tumour cells. Similarly, programmed cell death 1 ligand 1 (PD-L1) is an inhibitory immune-checkpoint protein commonly expressed by tumour cells and immune cells in the tumour microenvironment (TME). Antibodies targeting PD-L1, such as durvalumab, or its receptor programmed cell death protein 1 (PD-1), such as pembrolizumab or nivolumab, can inhibit immunosuppressive signalling in T cells capable of recognizing tumour cells, potentiating anticancer immune responses. In combination with immune-checkpoint inhibition, intravenous adoptive transfer of tumour-infiltrating lymphocytes (TILs) isolated from the TME and expanded ex vivo might enhance anticancer immunity. Alternatively, targeting the vascular endothelial growth factor receptor 2 (VEGFR2) with the monoclonal antibody ramucirumab might enhance T-cell recruitment into the TME, as a result of normalization of the dysfunctional tumour vasculature, and can also have direct, beneficial immunological effects, for example, on tumour-associated macrophages. Immune-checkpoint inhibitors are also being combined with helper cytokines that might potentiate anticancer immunity, such as granulocyte- macrophage colony-stimulating factor (GM-CSF) and pegylated IFNα-2b (Peg-IFNα-2b), as well as small-molecular inhibitors of targets relevant to cholangiocarcinoma, such as fibroblast growth factor receptors (FGFR1–3) and heat-shock protein 90 (HSP90). ACT, adoptive cell therapy; MHC I, major histocompatibility complex class I; RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy; TACE, transarterial chemoembolization; TCR, T-cell receptor; Treg cell, regulatory T cell.
Rationale for and risks of immunotherapy in cholangiocarcinoma
In cholangiocarcinoma, a number of clinical and epidemiological factors might determine both the efficacy, and the potential risks associated with immunotherapy. A number of chronic infections, such as liver-fluke disease, viral hepatitis B and C, and bacterial pyogenic cholangitis, are established risk factors for cholangiocarcinoma1,149. Notably, immune- checkpoint inhibitors and other immunotherapies have shown promising efficacy in other tumours commonly associated with viral infections, such as head and neck cancer, Hodgkin lymphoma, Merkel-cell carcinoma, and HCC150, and this relationship is thought to be mediated, in part, by the presentation of non-self or neoantigens associated with viral infections150–152. Notably, transcriptome sequencing and clustering of gene-expression profiles revealed a subgroup of patients with cholangiocarcinomas with a high mutational load, resulting in abundant tumour-specific neoantigens, and enrichment for expression of immune-related genes, including genes encoding inhibitory immune-checkpoint proteins92. Interestingly, this patient subgroup had the poorest prognosis92. These findings support the hypothesis that some patients with cholangiocarcinoma might benefit from immune-checkpoint inhibition to ‘release the brake’ on an existing anticancer immune response.
Indeed, a substantial proportion of cholangiocarcinomas are surrounded by a reactive tumour stroma, populated by host cells including cancer-associated fibroblasts, endothelial cells, and immune cells, including tumour-associated macrophages (TAMs)153,154. These stromal elements produce soluble factors including various interleukins, growth factors, and cytokines, which in turn can promote tumour-cell proliferation, survival, and invasiveness, and modulate anticancer immune responses. In a small retrospective study involving 39 patients with cholangiocarcinoma, high numbers of alternatively activated, ‘M2-like’ TAMs, which are generally considered to be immunosuppressive, were associated with unfavourable disease-free survival155. Thus, targeting stromal cells, such as immunosuppressive TAMs or cancer-associated fibroblasts156–158, might prove to be a beneficial therapeutic strategy, particularly in combination with immunotherapy (FIG. 5; TABLE 1).
Table 1.
Selected immunotherapy clinical trials for cholangiocarcinoma
| Immunotherapy approach | Trial description | Key eligibility criteria | ClinicalTrials.gov reference |
|---|---|---|---|
| Checkpoint inhibitor monotherapy | |||
| Pembrolizumab (anti-PD-1 antibody) | Single-arm, open-label phase II trial; single-centre, single-arm, open-label, phase II trial | Advanced-stage CCA, with disease progression after first-line therapy, amenable to tumour-tissue sampling; advanced-stage solid tumours, including CCA, amenable to tumour-tissue sampling | NCT03110328; NCT02628067 |
| Nivolumab (anti-PD-1 antibody) | Single-arm, open-label, phase II trial | Advanced-stage CCA, with disease progression after systemic therapy (no more than two prior lines of systemic therapy) | NCT02829918 |
| Durvalumab (anti-PD-L1 antibody) | Multicentre, open-label, phase I trial | Advanced-stage solid tumours, including CCA, refractory to standard therapy, with at least one radiographically measurable lesion | NCT01938612 |
| Dual checkpoint inhibition | |||
| Nivolumab + ipilimumab (anti-CTLA-4 antibody) | Multicentre, randomized, phase II trial; single-arm, open-label, phase II trial | Advanced-stage CCA and radiographically measurable disease; advanced-stage rare tumours, including CCA, with tumour progression after standard systemic therapy | NCT03101566; NCT02834013 |
| Durvalumab + tremelimumab (anti-CTLA-4 antibody) | Multicentre, open-label, phase I trial | Advanced-stage solid tumours, including CCA | NCT01938612 |
| Checkpoint inhibition plus microenvironmental targeting | |||
| Pembrolizumab + GM-CSF | Randomized, open-label, phase II trial | Advanced-stage CCA | NCT02703714 |
| Pembrolizumab + Peg-IFNα-2b | Multicentre, single-arm, open-label, phase II trial | Advanced-stage CCA, with tumour progression after prior systemic therapy | NCT02982720 |
| Pembrolizumab + ramucirumab (anti-VEGFR2 antibody) | Multicentre, open-label, phase I trial | Advanced-stage solid tumours, including CCA, with tumour progression after one or two prior systemic therapies, and with availability of tumour tissue for biomarker analysis | NCT02443324 |
| Checkpoint inhibition plus ablative local therapy | |||
| Tremelimumab+TACE, RFA, cryoablation, or SBRT | Open-label, phase I trial | Advanced-stage liver cancer, including CCA, after at least one line of systemic therapy | NCT01853618 |
| Durvalumab + tremelimumab + TACE, RFA, or cryoablation | Open-label, phase I/II trial | Advanced-stage liver cancer, including CCA, with at least two tumour lesions | NCT02821754 |
| Checkpoint inhibition plus chemotherapy | |||
| Pembrolizumab + mFOLFOX6 regimen | Open-label, phase I trial | Advanced-stage gastrointestinal cancers, including CCA, amenable tumour-tissue sampling | NCT02268825 |
| Pembrolizumab + capecitabine- oxaliplatin | Open-label, phase II trial | Advanced-stage CCA, with at least one focus of metastatic disease amenable to pretreatment and on-treatment biopsies | NCT03111732 |
| Nivolumab + gemcitabine–cisplatin | Multicentre, randomized, open-label, phase II trial | Advanced-stage CCA, with least one radiographically measurable focus of disease | NCT03101566 |
| Durvalumab + tremelimumab + gemcitabine–cisplatin | Open-label, phase II trial | Advanced-stage CCA, with at least one measurable lesion | NCT03046862 |
| Checkpoint inhibition plus molecularly targeted therapy | |||
| Pembrolizumab + INCB054828 (FGFR1–3 inhibitor) | Open-label, phase I/II trial | Advanced-stage solid tumours, including CCA, with genetic alterations in FGF or_FGFR_ genes | NCT02393248 |
| Pembrolizumab + XL888 (HSP90 inhibitor) | Open-label, phase Ib trial | Advanced-stage gastrointestinal malignancies, including CCA, after failure of at least one prior therapy | NCT03095781 |
| Checkpoint inhibition plus adoptive cell therapy | |||
| Pembrolizumab + tumour-infiltrating lymphocytes | Open-label, phase II trial | Advanced-stage solid tumours, including CCA, refractory to standard therapy | NCT01174121 |
Prevalent hepatic dysfunction and the propensity for biliary obstruction in patients with cholangiocarcinoma is associated with high rates of adverse events in studies of cytotoxic therapies11, and raises concerns regarding an increased risk of immune-mediated hepatobiliary toxicity, such as cholestasis or hepatitis, with immune-checkpoint inhibition. Reassuringly, in the phase I/II CheckMate 040 trial159, the incidence of grade 3 or 4 immune-mediated transaminase elevation among 214 patients with HCC who received the PD-1 inhibitor nivolumab was approximately 4% (similar to the rates reported for patients with other tumour types), without any reported treatment-related hepatic decompensation. Autoimmune diseases, such as PSC and inflammatory bowel disease, are also known risk factors in a subset of patients with cholangiocarcinoma, raising additional concerns regarding the risk of flares in pre-existing colitis or biliary tract disease with the use of immune-activating therapies in this population. Of note, patients with underlying autoimmune disease have typically been excluded from clinical trials of immunotherapies; thus, the safety of such treatments in this subset of patients with cholangiocarcinoma remains uncertain.
Candidate biomarkers of response to immunotherapy
Many candidate biomarkers of a response to immune-checkpoint inhibition have emerged from studies relating to a range of tumour types. The most-studied biomarker to date is the PD-1 ligand, PD-L1; any expression of PD-L1 on tumour cells, and/or higher levels of tumour PD-L1 expression have both been associated with sensitivity to immune-checkpoint- inhibitor monotherapy in some tumour types, including melanoma and non-small-cell lung cancer (NSCLC), but with conflicting results in other diseases160–162. In studies of small numbers of cholangiocarcinoma tumour samples (n = 54–99), PD-L1 expression has been reported in 9–72% of specimens163–165, and on 46–63% of immune cells within the tumour microenvironment164,165. These data indicate that a substantial proportion of cholangiocarcinomas might be amenable to therapy with PD-1 or PD-L1 inhibitors. Further investigation of PD-L1 as a biomarker for anti-PD-1 and anti-PD-L1 therapies is required in order to understand the effects of important covariates, including tumour-cell versus immune-cell expression, primary versus metastatic lesion sampling, prior treatment exposure, and concurrent therapies, as well as the specific assay and cut-off points used.
Certain tumour genetic aberrations have also been associated with a likelihood of response to immune-checkpoint inhibitors, which might relate to the expression of neoantigens capable of eliciting an antitumour T-cell response. One example is the presence of tumour DNA mismatch repair (MMR) deficiency and/or microsatellite instability (MSI), which is associated with high rates and durability of responses to immune-checkpoint blockade across multiple tumour types166,167. Indeed, the anti-PD-1 antibody pembrolizumab has been approved by the FDA for the treatment of patients with unresectable or metastatic MMR-deficient and/or MSI-high solid tumours that progressed after prior therapy (when no satisfactory alternative treatment is available), independent of histology — which would include those with cholangiocarcinoma (https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm). Notably, MMR deficiency has been reported to occur in 5–10% of cholangiocarcinomas168. In addition to MMR deficiency, the cumulative tumour mutational burden has been correlated with responsiveness to immune-checkpoint inhibitors in some cancers, including melanoma, NSCLC, and urothelial carcinoma169–171. In a whole-exome-sequencing study of 231 cholangiocarcinoma tumour samples92, a median of 39 and 35 somatic nonsynonymous mutations were identified in intrahepatic and extrahepatic cholangiocarcinomas, respectively; overall, ~6% of the cholangiocarcinomas had evidence of hypermutation (mutation rates of >11.13 per megabase; median number of 641 nonsilent mutations), with concurrent MMR deficiency and/or MSI detected in about 36% of these hypermutated tumours92. For comparison, in patients with NSCLC who derived durable clinical benefit from pembrolizumab (partial or stable response lasting >6 months), the median number of nonsynonymous mutations was 302 (REF. 169). These data suggest that immune-checkpoint blockade and immune-modulating therapies could be promising options for the subgroup of patients with cholangiocarcinomas harbouring high mutational loads.
Emerging clinical data from immune-targeted therapies in cholangiocarcinoma
At present, the clinical data on immunotherapy in cholangiocarcinoma and other biliary tract cancers are limited. Interim safety and efficacy data from the KEYNOTE-028 basket trial (NCT02054806) of the anti-PD-1 antibody pembrolizumab have been reported for a small cohort of patients with PD-L1-positive biliary tract cancer163; 37 of 89 patients screened (41.6%) had PD-L1 expression on ≥1% of tumour cells by immunohistochemistry, 24 of whom enrolled in the study (20 with cholangiocarcinoma, four with gallbladder carcinoma)163. Of these 24 patients, four (17%, three with cholangiocarcinoma and one with gallbladder carcinoma) had a partial response, and four (17%) had stable disease163. The duration of partial response was protracted, with the median PFS not reached at the time of reporting. The rate of grade 3 toxicities was 16.7%, with no patients experiencing grade ≥4 toxicities, nor any marked hepatotoxicity163. The promising safety and efficacy of pembrolizumab in the KEYNOTE-028 biliary cancer cohort prompted a successor biliary cancer cohort of 100 patients in the ongoing KEYNOTE-158 basket trial (NCT02628067; TABLE 1).
Patients with MMR-deficient cholangiocarcinoma have also demonstrated responsiveness to treatment with immune-checkpoint inhibitors166,167,172. Among 86 patients with MMR-deficient tumours, encompassing 12 different tumour types including cholangiocarcinoma (n = 4), PD-1 blockade with pembrolizumab resulted in objective radiographic responses in 53% of patients, and in 25% of patients with cholangiocarcinoma (one of the patients with cholangiocarcinoma had a complete response, and the other three had stable disease, for a disease-control rate of 100%)172; median PFS and overall survival were not reached at the time of publication172. These provocative preliminary clinical data hold promise for immunotherapy approaches in cholangiocarcinoma, while underscoring the importance of biomarker development to identify patients who are most likely to respond, and to guide the rational selection of combination therapies. A number of clinical trials evaluating novel immunotherapy approaches in patients with cholangiocarcinoma are currently ongoing (TABLE 1).
Conclusions
Cholangiocarcinomas are anatomically distinct and genetically heterogeneous tumours. Current modalities for establishing a cholangiocarcinoma diagnosis are insufficient, as detection of the disease at a sufficiently early stage to enable potentially curative surgical therapies remains an arduous task. Novel biomarkers that merit further investigation include DNA-methylation markers, non-coding RNAs, and peptide panels60,173–175. Thus, one can envision the application of advanced technologies such as proteomic analysis by mass spectrometry or 2D gel electrophoresis, and microRNA analysis for the detection of cholangiocarcinoma biomarkers in biological specimens, including bile, serum, or stool samples. In addition, FISH could potentially be used to detect novel gene fusions in patients with cholangiocarcinoma.
An enhanced understanding of the driver genetic aberrations in each disease subtype is integral to establishing a precision medicine approach to cholangiocarcinoma therapy. Moreover, recently described gene fusions and mutations in cholangiocarcinoma need further investigation in functional studies and clinical trials. Emerging therapies that hold considerable promise include FGFR inhibitors and IDH1 and/or IDH2 inhibitors, as well as immunotherapies. Identification of biomarkers for the selection of patients harbouring pertinent genetic aberrations is an essential factor in targeted therapy. In future trials, patients should be stratified according to disease subtype and genetic drivers. Such biomarker-driven trials will be imperative in the development of effective medical therapies for cholangiocarcinoma. The extensive interactions and crosstalk between the various signalling pathways involved in cholangiocarcinoma carcinogenesis highlights the importance of combination therapeutic approaches. In particular, the combination of molecularly targeted agents and immunotherapy with immune-checkpoint inhibitors merits further investigation.
Supplementary Material
Sup Table
Key points.
- Each anatomical subtype of cholangiocarcinoma, intrahepatic (iCCA), perihilar (pCCA) and distal (dCCA), has a distinct epidemiology, biology, and prognosis, thus necessitating different management approaches
- Fluorescence in situ hybridization (FISH) has improved the diagnostic performance of conventional cytology for the detection of pCCA and dCCA; several emerging diagnostic modalities, including liquid biopsy techniques, might further improve cholangiocarcinoma diagnosis
- Neoadjuvant chemoradiotherapy followed by liver transplantation offers the best outcomes for a subset of patients with pCCA; liver transplantation might also be an option for patients with very early stage iCCA
- Emerging evidence indicates that high-dose, conformal external-beam radiation therapy is a potential treatment option for patients with localized, unresectable iCCA
- An enhanced understanding of the potential driver genetic aberrations in cholangiocarcinomas has heralded several novel drugs for advanced-stage disease, including FGFR inhibitors and IDH inhibitors; targeted therapy and immunotherapy combinations also hold promise
Acknowledgments
The authors thank Ms Courtney Hoover for her excellent secretarial support. The work of the authors is supported by the US NIH (grants DK59427 to G.J.G., 1R03CA212877-01 to R.K.K., and DK84567 to the Mayo Center for Cell Signalling in Gastroenterology), and by the Mayo Foundation. S.I.I. has also received support from the Cholangiocarcinoma Foundation and from the Mayo Center for Cell Signalling in Gastroenterology (Pilot & Feasibility Award P30DK084567).
Footnotes
Author contributions
All authors made substantial contributions to all aspects of the preparation of this manuscript.
Competing interests statement
R.K.K has received research support from Agios, Eli Lilly, Merck, and Novartis, via her institution, for conduct of clinical trials in cholangiocarcinoma. S.I.I., S.A.K., C.L.H., and G.J.G. declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
References
- 1.Ilyas SI, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. Oncologist. 2016;21:594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SA, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 4.Taylor-Robinson SD, et al. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut. 2001;48:816–820. doi: 10.1136/gut.48.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardinale V, et al. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2:272–280. doi: 10.3978/j.issn.2304-3881.2013.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarnagin WR, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr Fritcher EG, et al. An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology. 2015;149:1813–1824. doi: 10.1053/j.gastro.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 8.Gonda TA, et al. Mutation profile and fluorescence in situ hybridization analyses increase detection of malignancies in biliary strictures. Clin Gastroenterol Hepatol. 2017;15:913–919. doi: 10.1016/j.cgh.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Darwish Murad S, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapisochin G, et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64:1178–1188. doi: 10.1002/hep.28744. [DOI] [PubMed] [Google Scholar]
- 11.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 12.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeOliveira ML, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakeeb A, et al. Cholangiocarcinoma A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 17.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer. 2006;94:1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Alvaro D, et al. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490–495. doi: 10.1016/j.dld.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Bertuccio P, et al. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24:1667–1674. doi: 10.1093/annonc/mds652. [DOI] [PubMed] [Google Scholar]
- 23.Lepage C, et al. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54:306–310. doi: 10.1016/j.jhep.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 24.Jepsen P, Vilstrup H, Tarone RE, Friis S, Sorensen HT. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99:895–897. doi: 10.1093/jnci/djk201. [DOI] [PubMed] [Google Scholar]
- 25.Altekruse SF, et al. Geographic variation of intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and hepatocellular carcinoma in the United States. PLoS ONE. 2015;10:e0120574. doi: 10.1371/journal.pone.0120574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SA, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Kilander C, Mattsson F, Ljung R, Lagergren J, Sadr-Azodi O. Systematic underreporting of the population-based incidence of pancreatic and biliary tract cancers. Acta Oncol. 2014;53:822–829. doi: 10.3109/0284186X.2013.857429. [DOI] [PubMed] [Google Scholar]
- 28.Duberg AS, Hultcrantz R. Misleading figures on trends in mortality from hepatocellular carcinoma in Europe. Hepatology. 2009;49:336. doi: 10.1002/hep.22671. [DOI] [PubMed] [Google Scholar]
- 29.Torner A, et al. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology. 2017;65:885–892. doi: 10.1002/hep.28775. [DOI] [PubMed] [Google Scholar]
- 30.Hainsworth JD, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol. 2013;31:217–223. doi: 10.1200/JCO.2012.43.3755. [DOI] [PubMed] [Google Scholar]
- 31.Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371:757–765. doi: 10.1056/NEJMra1303917. [DOI] [PubMed] [Google Scholar]
- 32.Bridgewater J, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Rimola J, et al. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791–798. doi: 10.1002/hep.23071. [DOI] [PubMed] [Google Scholar]
- 34.Iavarone M, et al. Contrast enhanced CT-scan to diagnose intrahepatic cholangiocarcinoma in patients with cirrhosis. J Hepatol. 2013;58:1188–1193. doi: 10.1016/j.jhep.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Kim SH, et al. Typical and atypical imaging findings of intrahepatic cholangiocarcinoma using gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. J Comput Assist Tomogr. 2012;36:704–709. doi: 10.1097/RCT.0b013e3182706562. [DOI] [PubMed] [Google Scholar]
- 36.Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB. 2008;10:106–109. doi: 10.1080/13651820801992617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 38.Levy C, et al. The value of serum CA 19–19 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 39.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19–9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 40.Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24:139–154. doi: 10.1055/s-2004-828891. [DOI] [PubMed] [Google Scholar]
- 41.Choi SB, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 42.Endo I, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 43.Li YY, et al. Prognostic value of cirrhosis for intrahepatic cholangiocarcinoma after surgical treatment. J Gastrointest Surg. 2011;15:608–613. doi: 10.1007/s11605-011-1419-8. [DOI] [PubMed] [Google Scholar]
- 44.Pascher A, Jonas S, Neuhaus P. Intrahepatic cholangiocarcinoma: indication for transplantation. J Hepatobiliary Pancreat Surg. 2003;10:282–287. doi: 10.1007/s00534-002-0731-9. [DOI] [PubMed] [Google Scholar]
- 45.Robles R, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265–271. doi: 10.1097/01.sla.0000108702.45715.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapisochin G, et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant. 2014;14:660–667. doi: 10.1111/ajt.12591. [DOI] [PubMed] [Google Scholar]
- 47.Kiefer MV, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117:1498–1505. doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- 48.Park SY, et al. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66:322–328. doi: 10.1016/j.crad.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Vogl TJ, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer. 2012;131:733–740. doi: 10.1002/ijc.26407. [DOI] [PubMed] [Google Scholar]
- 50.Kuhlmann JB, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24:437–443. doi: 10.1097/MEG.0b013e3283502241. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann RT, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105–116. doi: 10.1007/s00270-011-0142-x. [DOI] [PubMed] [Google Scholar]
- 52.Rafi S, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36:440–448. doi: 10.1007/s00270-012-0463-4. [DOI] [PubMed] [Google Scholar]
- 53.Masselli G, Manfredi R, Vecchioli A, Gualdi G. MR imaging and MR cholangiopancreatography in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings. Eur Radiol. 2008;18:2213–2221. doi: 10.1007/s00330-008-1004-z. [DOI] [PubMed] [Google Scholar]
- 54.Ruys AT, et al. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85:1255–1262. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohamadnejad M, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc. 2011;73:71–78. doi: 10.1016/j.gie.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 56.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB. 2011;13:356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:783–789. doi: 10.1016/j.gie.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Dudley JC, et al. Next-generation sequencing and fluorescence in situ hybridization have comparable performance characteristics in the analysis of pancreaticobiliary brushings for malignancy. J Mol Diagn. 2016;18:124–130. doi: 10.1016/j.jmoldx.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka A, et al. Clinical features, response to treatment, and outcomes of IgG4-related sclerosing cholangitis. Clin Gastroenterol Hepatol. 2017;15:920–926. doi: 10.1016/j.cgh.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 60.Li L, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60:896–907. doi: 10.1002/hep.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arbelaiz A, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2017. http://dx.doiorg/10.1002/hep.29291. [DOI] [PubMed]
- 62.Severino V, et al. Extracellular vesicles in bile as markers of malignant biliary stenoses. Gastroenterology. 2017;153:495–504. doi: 10.1053/j.gastro.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 63.Wan JC, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 64.Yang J, et al. Detection of cholangiocarcinoma by assay of methylated DNA markers in plasma. Gastroenterology. 2017;152:S1041–S1042. [Google Scholar]
- 65.Nagorney DM, Kendrick ML. Hepatic resection in the treatment of hilar cholangiocarcinoma. Adv Surg. 2006;40:159–171. doi: 10.1016/j.yasu.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Hemming AW, Mekeel K, Khanna A, Baquerizo A, Kim RD. Portal vein resection in management of hilar cholangiocarcinoma. J Am Coll Surg. 2011;212:604–613. doi: 10.1016/j.jamcollsurg.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 67.Hong YK, et al. The efficacy of portal vein embolization prior to right extended hemihepatectomy for hilar cholangiocellular carcinoma: a retrospective cohort study. Eur J Surg Oncol. 2011;37:237–244. doi: 10.1016/j.ejso.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 68.Schnitzbauer AA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405–414. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 69.Tschuor C, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion — an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39:1230–1235. doi: 10.1016/j.ejso.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 70.Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23:692–697. doi: 10.1111/j.1432-2277.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 71.Valle JW, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 72.Okusaka T, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Primrose JN, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study [abstract] J Clin Oncol. 2017;35(Suppl. 15):4006. [Google Scholar]
- 74.Edeline J, et al. Gemox versus surveillance following surgery of localized biliary tract cancer: results of the PRODIGE 12-ACCORD 18 (UNICANCER GI) phase III trial. J Clin Oncol. 2017;35:225–225. [Google Scholar]
- 75.Crane CH, Koay EJ. Solutions that enable ablative radiotherapy for large liver tumors: fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer. 2016;122:1974–1986. doi: 10.1002/cncr.29878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan CC, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kavanagh BD, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–S107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 78.Hong TS, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2016;34:460–468. doi: 10.1200/JCO.2015.64.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tse RV, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 80.Tao R, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016;34:219–226. doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patel S, Ragab O, Kamrava M. Another solution that enables ablative radiotherapy for large liver tumors: percutaneous interstitial high-dose rate brachytherapy. Cancer. 2016;122:2766. doi: 10.1002/cncr.30128. [DOI] [PubMed] [Google Scholar]
- 82.Mukewar S, et al. Endoscopically inserted nasobiliary catheters for high dose-rate brachytherapy as part of neoadjuvant therapy for perihilar cholangiocarcinoma. Endoscopy. 2015;47:878–883. doi: 10.1055/s-0034-1392044. [DOI] [PubMed] [Google Scholar]
- 83.Hammad AY, et al. Is Radiotherapy warranted following intrahepatic cholangiocarcinoma resection? The impact of surgical margins and lymph node status on survival. Ann Surg Oncol. 2016;23:912–920. doi: 10.1245/s10434-016-5560-1. [DOI] [PubMed] [Google Scholar]
- 84.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 85.Jia AY, et al. Intensity-modulated radiotherapy following null-margin resection is associated with improved survival in the treatment of intrahepatic cholangiocarcinoma. J Gastrointest Oncol. 2015;6:126–133. doi: 10.3978/j.issn.2078-6891.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ben-Josef E, et al. SWOG S0809: a phase II Intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015;33:2617–2622. doi: 10.1200/JCO.2014.60.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shinohara ET, Mitra N, Guo M, Metz JM. Radiotherapy is associated with improved survival in adjuvant and palliative treatment of extrahepatic cholangiocarcinomas. Int J Radiat Oncol Biol Phys. 2009;74:1191–1198. doi: 10.1016/j.ijrobp.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 88.Pollom EL, et al. Does radiotherapy still have a role in unresected biliary tract cancer? Cancer Med. 2017;6:129–141. doi: 10.1002/cam4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Foo ML, Gunderson LL, Bender CE, Buskirk SJ. External radiation therapy and transcatheter iridium in the treatment of extrahepatic bile duct carcinoma. Int J Radiat Oncol Biol Phys. 1997;39:929–935. doi: 10.1016/s0360-3016(97)00299-x. [DOI] [PubMed] [Google Scholar]
- 90.Ghafoori AP, et al. Radiotherapy in the treatment of patients with unresectable extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2011;81:654–659. doi: 10.1016/j.ijrobp.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mansour JC, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB. 2015;17:691–699. doi: 10.1111/hpb.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakamura H, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–1010. doi: 10.1038/ng.3375. [DOI] [PubMed] [Google Scholar]
- 93.Borad MJ, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10:e1004135. doi: 10.1371/journal.pgen.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graham RP, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1630–1638. doi: 10.1016/j.humpath.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 95.Ross JS, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu YM, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sia D, et al. Massive parallel sequencing uncovers actionable FGFR2–PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087. doi: 10.1038/ncomms7087. [DOI] [PubMed] [Google Scholar]
- 98.Gingras MC, et al. Ampullary cancers harbor ELF3 tumor suppressor gene mutations and exhibit frequent WNT dysregulation. Cell Rep. 2016;14:907–919. doi: 10.1016/j.celrep.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yachida S, et al. Genomic sequencing identifies ELF3 as a driver of ampullary carcinoma. Cancer Cell. 2016;29:229–240. doi: 10.1016/j.ccell.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan-On W, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet. 2013;45:1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 101.Farshidfar F, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18:2780–2794. doi: 10.1016/j.celrep.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Churi CR, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS ONE. 2014;9:e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borger DR, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kipp BR, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552–1558. doi: 10.1016/j.humpath.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 105.Ilyas SI, et al. A hippo and fibroblast growth factor receptor autocrine pathway in cholangiocarcinoma. J Biol Chem. 2016;291:8031–8047. doi: 10.1074/jbc.M115.698472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Javle M. A phase 2 study of BGJ398 in patients (pts) with advanced or metastatic FGFR-altered cholangiocarcinoma (CCA) who failed or are intolerant to platinum-based chemotherapy [abstract] J Clin Oncol. 2016;34(Suppl. 4):335. [Google Scholar]
- 107.Perera TPS, et al. Discovery and pharmacological characterization of JNJ-42756493 (erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol Cancer Ther. 2017;16:1010–1020. doi: 10.1158/1535-7163.MCT-16-0589. [DOI] [PubMed] [Google Scholar]
- 108.Tabernero J, et al. Phase I dose-escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2015;33:3401–3408. doi: 10.1200/JCO.2014.60.7341. [DOI] [PubMed] [Google Scholar]
- 109.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 110.Acquaviva J, et al. FGFR3 translocations in bladder cancer: differential sensitivity to HSP90 inhibition based on drug metabolism. Mol Cancer Res. 2014;12:1042–1054. doi: 10.1158/1541-7786.MCR-14-0004. [DOI] [PubMed] [Google Scholar]
- 111.Gu TL, et al. Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma. PLoS ONE. 2011;6:e15640. doi: 10.1371/journal.pone.0015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saborowski A, et al. Mouse model of intrahepatic cholangiocarcinoma validates FIG–ROS as a potent fusion oncogene and therapeutic target. Proc Natl Acad Sci USA. 2013;110:19513–19518. doi: 10.1073/pnas.1311707110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu AX, et al. Genomic profiling of intrahepatic cholangiocarcinoma: refining prognosis and identifying therapeutic targets. Ann Surg Oncol. 2014;21:3827–3834. doi: 10.1245/s10434-014-3828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bekaii-Saab T, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29:2357–2363. doi: 10.1200/JCO.2010.33.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bridgewater J, et al. A phase 1b study of selumetinib in combination with cisplatin and gemcitabine in advanced or metastatic biliary tract cancer: the ABC-04 study. BMC Cancer. 2016;16:153. doi: 10.1186/s12885-016-2174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goeppert B, et al. BRAF V600E-specific immunohistochemistry reveals low mutation rates in biliary tract cancer and restriction to intrahepatic cholangiocarcinoma. Mod Pathol. 2014;27:1028–1034. doi: 10.1038/modpathol.2013.206. [DOI] [PubMed] [Google Scholar]
- 117.Hyman DM, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sia D, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–840. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pant S, et al. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann Oncol. 2014;25:1416–1421. doi: 10.1093/annonc/mdu157. [DOI] [PubMed] [Google Scholar]
- 120.Goyal L, et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer. 2017;123:1979–1988. doi: 10.1002/cncr.30571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El-Khoueiry AB, et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer. 2014;110:882–887. doi: 10.1038/bjc.2013.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Rourke CJ, Munoz-Garrido P, Aguayo EL, Andersen JB. Epigenome dysregulation in cholangiocarcinoma. Biochim Biophys Acta. 2017. [DOI] [PubMed]
- 123.Rohle D, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang F, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 125.Burris H, et al. The first reported results of AG-120, a first-in-class, potent inhibitor of the IDH1 mutant protein, in a phase I study of patients with advanced IDH1-mutant solid tumors, including gliomas. Mol Cancer Ther. 2015;14(12)(Suppl. 2):L04–05. [Google Scholar]
- 126.Amatangelo MD, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130:732–741. doi: 10.1182/blood-2017-04-779447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kats LM, et al. A pharmacogenomic approach validates AG-221 as an effective and on-target therapy in IDH2 mutant AML. Leukemia. 2017;31:1466–1470. doi: 10.1038/leu.2017.84. [DOI] [PubMed] [Google Scholar]
- 128.Thomas D, Majeti R. Optimizing next-generation AML therapy: activity of mutant IDH2 inhibitor AG-221 in preclinical models. Cancer Discov. 2017;7:459–461. doi: 10.1158/2159-8290.CD-17-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saha SK, et al. Isocitrate dehydrogenase mutations confer dasatinib hypersensitivity and SRC dependence in intrahepatic cholangiocarcinoma. Cancer Discov. 2016;6:727–739. doi: 10.1158/2159-8290.CD-15-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim KH, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med. 2015;21:1491–1496. doi: 10.1038/nm.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakagawa S, et al. Enhancer of zeste homolog 2 (EZH2) promotes progression of cholangiocarcinoma cells by regulating cell cycle and apoptosis. Ann Surg Oncol. 2013;20(Suppl. 3):S667–S675. doi: 10.1245/s10434-013-3135-y. [DOI] [PubMed] [Google Scholar]
- 132.Tang B, et al. EZH2 elevates the proliferation of human cholangiocarcinoma cells through the downregulation of RUNX3. Med Oncol. 2014;31:271. doi: 10.1007/s12032-014-0271-6. [DOI] [PubMed] [Google Scholar]
- 133.Nakagawa S, et al. Epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A inhibits the growth of cholangiocarcinoma cells. Oncol Rep. 2014;31:983–988. doi: 10.3892/or.2013.2922. [DOI] [PubMed] [Google Scholar]
- 134.Fujimoto A, et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun. 2015;6:6120. doi: 10.1038/ncomms7120. [DOI] [PubMed] [Google Scholar]
- 135.Jiao Y, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luchini C, et al. PBRM1 loss is a late event during the development of cholangiocarcinoma. Histopathology. 2017;71:375–382. doi: 10.1111/his.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sasaki M, Nitta T, Sato Y, Nakanuma Y. Loss of ARID1A expression presents a novel pathway of carcinogenesis in biliary carcinomas. Am J Clin Pathol. 2016;145:815–825. doi: 10.1093/ajcp/aqw071. [DOI] [PubMed] [Google Scholar]
- 138.Baradari V, Hopfner M, Huether A, Schuppan D, Scherubl H. Histone deacetylase inhibitor MS-275 alone or combined with bortezomib or sorafenib exhibits strong antiproliferative action in human cholangiocarcinoma cells. World J Gastroenterol. 2007;13:4458–4466. doi: 10.3748/wjg.v13.i33.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 140.Kwak TW, Kim DH, Jeong YI, Kang DH. Antitumor activity of vorinostat-incorporated nanoparticles against human cholangiocarcinoma cells. J Nanobiotechnol. 2015;13:60. doi: 10.1186/s12951-015-0122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sriraksa R, Limpaiboon T. Histone deacetylases and their inhibitors as potential therapeutic drugs for cholangiocarcinoma — cell line findings. Asian Pac J Cancer Prev. 2013;14:2503–2508. doi: 10.7314/apjcp.2013.14.4.2503. [DOI] [PubMed] [Google Scholar]
- 142.Wang B, et al. Sodium valproate inhibits the growth of human cholangiocarcinoma in vitro and in vivo Gastroenterol Res Pract. 2013;2013:374593. doi: 10.1155/2013/374593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iwahashi S, et al. Effects of valproic acid in combination with S-1 on advanced pancreatobiliary tract cancers: clinical study phases I/II. Anticancer Res. 2014;34:5187–5191. [PubMed] [Google Scholar]
- 144.Kawamata F, et al. Intracellular localization of mesothelin predicts patient prognosis of extrahepatic bile duct cancer. Int J Oncol. 2012;41:2109–2118. doi: 10.3892/ijo.2012.1662. [DOI] [PubMed] [Google Scholar]
- 145.Nomura R, et al. Mesothelin expression is a prognostic factor in cholangiocellular carcinoma. Int Surg. 2013;98:164–169. doi: 10.9738/INTSURG-D-13-00001.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Golan T, et al. Overall survival and clinical characteristics of BRCA-associated cholangiocarcinoma: a multicenter retrospective study. Oncologist. 2017;22:804–810. doi: 10.1634/theoncologist.2016-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martin-Liberal J, et al. The expanding role of immunotherapy. Cancer Treat Rev. 2017;54:74–86. doi: 10.1016/j.ctrv.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 148.Feldman SA, Assadipour Y, Kriley I, Goff SL, Rosenberg SA. Adoptive cell therapy — tumor-infiltrating lymphocytes, T-cell receptors, and chimeric antigen receptors. Semin Oncol. 2015;42:626–639. doi: 10.1053/j.seminoncol.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santana-Davila R, Bhatia S, Chow LQ. Harnessing the immune system as a therapeutic tool in virus-associated cancers. JAMA Oncol. 2017;3:106–112. doi: 10.1001/jamaoncol.2016.4574. [DOI] [PubMed] [Google Scholar]
- 151.Ott PA, Hodi FS. The B7-H1/PD-1 pathway in cancers associated with infections and inflammation: opportunities for therapeutic intervention. Chin Clin Oncol. 2013;2:7. doi: 10.3978/j.issn.2304-3865.2012.11.05. [DOI] [PubMed] [Google Scholar]
- 152.Tashiro H, Brenner MK. Immunotherapy against cancer-related viruses. Cell Res. 2017;27:59–73. doi: 10.1038/cr.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brivio S, Cadamuro M, Strazzabosco M, Fabris L. Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World J Hepatol. 2017;9:455–468. doi: 10.4254/wjh.v9.i9.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Raggi C, Invernizzi P, Andersen JB. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: molecular networks and biological concepts. J Hepatol. 2015;62:198–207. doi: 10.1016/j.jhep.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 155.Hasita H, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101:1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mertens JC, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ilyas SI, et al. Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis. J Biol Chem. 2014;289:22835–22849. doi: 10.1074/jbc.M114.563064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aguiar PN, et al. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9:499–506. doi: 10.2217/imt-2016-0150. [DOI] [PubMed] [Google Scholar]
- 161.Carbognin L, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS ONE. 2015;10:e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 163.Bang YJ, et al. Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: interim results of KEYNOTE-028 [abstract] Eur J Cancer. 2015;51(Suppl. 3):S112. [Google Scholar]
- 164.Gani F, et al. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23:2610–2617. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]
- 165.Fontugne J, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8:24644–24651. doi: 10.18632/oncotarget.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Le DT, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Naboush A, Roman CA, Shapira I. Immune checkpoint inhibitors in malignancies with mismatch repair deficiency: a review of the state of the current knowledge. J Investig Med. 2017;65:754–758. doi: 10.1136/jim-2016-000342. [DOI] [PubMed] [Google Scholar]
- 168.Silva VW, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016;5:62. doi: 10.21037/cco.2016.10.04. [DOI] [PubMed] [Google Scholar]
- 169.Rizvi NA, et al. Cancer immunology Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rosenberg JE, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Le DT, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andresen K, et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61:1651–1659. doi: 10.1002/hep.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lankisch TO, et al. Bile proteomic profiles differentiate cholangiocarcinoma from primary sclerosing cholangitis and choledocholithiasis. Hepatology. 2011;53:875–884. doi: 10.1002/hep.24103. [DOI] [PubMed] [Google Scholar]
- 175.Metzger J, et al. Urine proteomic analysis differentiates cholangiocarcinoma from primary sclerosing cholangitis and other benign biliary disorders. Gut. 2013;62:122–130. doi: 10.1136/gutjnl-2012-302047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sup Table