Acute Effect of Empagliflozin on Fractional Excretion of Sodium and eGFR in Youth With Type 2 Diabetes (original) (raw)
Early markers of diabetic kidney disease (DKD), including hyperfiltration and elevated albumin excretion, are common in youth with type 2 diabetes (T2D) (1). Despite the morbidity associated with the development of future DKD in youth-onset T2D, pharmacotherapeutic options to lower hyperglycemia are limited to metformin and insulin, the only drugs approved for use in pediatric patients.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors reduce blood pressure, weight, and hyperglycemia in adults with T2D. Furthermore, a 38% reduction in cardiovascular death and a 39% reduction in incident or worsening nephropathy have been demonstrated with the selective SGLT2 inhibitor empagliflozin in patients with established cardiovascular disease (2,3). In young adults with type 1 diabetes, treatment with the SGLT2 inhibitor empagliflozin for 8 weeks was associated with natriuresis, increased renal vascular resistance, and reductions in glomerular filtration rate (GFR) (using inulin clearances) and renal blood flow (assessed by _p_-aminohippuric acid clearance) in those with baseline hyperfiltration (4). This renal hemodynamic profile suggested that the 20% reduction in hyperfiltration observed in this trial was due to afferent vasoconstriction and a decline in intraglomerular hypertension (4). In adults with T2D, the reduction in intraglomerular hypertension is typically reflected by a mean reduction in estimated GFR (eGFR) of 2–4 mL/min/1.73 m2 after treatment initiation, followed by a stabilization of eGFR decline compared with placebo during long-term treatment (3). It is, however, not known whether these renal hemodynamic effects occur acutely, if at all, in youth-onset T2D. Accordingly, our aim was to examine whether eGFR effects occur within 24 h of a single exposure to empagliflozin in youth-onset T2D.
In this open-label, randomized, parallel-group study (reg. no. NCT02121483, clinicaltrials.gov), 27 youth with T2D (mean age 14.1 years, 67% girls, mean BMI 35.5 kg/m2) received a single dose of empagliflozin, either 5, 10, or 25 mg. The effects of empagliflozin on fractional sodium excretion (FeNA+) and eGFR (calculated by the Zappitelli combined creatinine and cystatin C equation) were investigated by pooling the three doses of empagliflozin for analyses (to increase the number of observations for meaningful analyses). Two ANCOVA models were fitted to examine change in FeNA+ and eGFR from baseline. Both models included empagliflozin dose and sex as fixed effects and baseline HbA1c and BMI standard deviation score, in addition to baseline FeNA+ or baseline eGFR depending on the dependent outcome, as continuous covariates. Hyperfiltration was defined as eGFR >119.1 mL/min/1.73 m2, namely, 2 SD above mean eGFR by the Zappitelli equation in obese youth in the National Health and Nutrition Examination Survey (NHANES) (5).
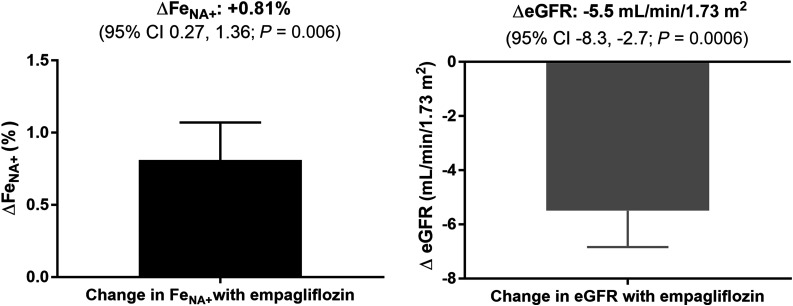
At baseline, mean FeNA+ was 0.55 ± 0.46%, and eGFR was 113.4 ± 15.6 mL/min/1.73 m2. After a single dose of empagliflozin, the adjusted mean FeNA+ increased and eGFR decreased (P = 0.006 and P = 0.0006 vs. baseline, respectively) (Fig. 1). Participants with hyperfiltration (n = 8) at baseline experienced a change in adjusted eGFR in response to empagliflozin of −6.7 ± 9.7 mL/min/1.73 m2, compared with −5.0 ± 6.0 mL/min/1.73 m2 in those with normofiltration (n = 19), although we cannot rule out that this difference reflects regression toward the mean.
Figure 1.
Changes from baseline in FeNA+ and eGFR after single-dose administration of empagliflozin (n = 27), adjusted for baseline value, age, sex, HbA1c, and BMI standard deviation score. Directions of effect on FeNA+ and eGFR were consistent across the three doses of empagliflozin tested.
There are limitations in this study, including small sample size and calculated rather than measured GFR. Nevertheless, we have shown that SGLT2 inhibition following a single dose of empagliflozin is associated with increased natriuresis and attenuation of elevated GFR in youth with T2D, suggesting a reduction in intraglomerular pressure. These findings are consistent with adult data and may yield potential to reduce the risk of future DKD in youth with T2D. Further mechanistic studies are required to better understand changes in renal function in response to SGLT2 inhibition, and longer-term trials examining renal protective effects in youth with diabetes are warranted.
Article Information
Funding. P.B. receives salary support from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (T32-DK063687, K23-DK116720-01), in addition to research support from the Thrasher Research Fund, JDRF, International Society for Pediatric and Adolescent Diabetes, and Center for Women’s Health Research at the University of Colorado. D.Z.I.C. receives support from the Canadian Institutes of Health Research, as well as Diabetes Action Canada, a Strategy for Patient-Oriented Research initiative supported by the Canadian Institutes for Health Research. D.Z.I.C. also receives operating funding from the Heart & Stroke Richard Lewar Centre of Excellence in Cardiovascular Research and Banting & Best Diabetes Centre, University of Toronto. D.Z.I.C. is the recipient of a University of Toronto Department of Medicine Merit Award.
Duality of Interest. L.L. has served as a consultant for Eli Lilly and Company, Sanofi, Novo Nordisk, Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, Menarini, Johnson & Johnson, LifeScan/Animas, Insulet, Roche Diagnostics, and Dexcom. W.V.T. has served as a consultant for AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk, Sanofi, and Takeda. G.S., S.H., M.v.E., J.G., and J.M. are employees of Boehringer Ingelheim. D.Z.I.C. receives operating funding from Boehringer Ingelheim, Janssen, AstraZeneca, and Merck. D.Z.I.C. has received consulting fees and/or speaking honoraria from Boehringer Ingelheim, Janssen, AstraZeneca, Merck, Mitsubishi Tanabe, Sanofi, and AbbVie. Funding for this trial was provided by the Boehringer Ingelheim and Eli Lilly and Company Diabetes Alliance. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.B., J.M., and D.Z.I.C. wrote the manuscript and researched the data. L.L., W.V.T., S.H., M.v.E., and J.G. reviewed/edited the manuscript and researched the data. G.S. performed the statistical analyses, reviewed/edited the manuscript, and researched the data. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and approved the final version. P.B., J.M., and D.Z.I.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
J.M. and D.Z.I.C. are co–senior authors.
References
- 1.Bjornstad P, Nehus E, El Ghormli L, et al.; TODAY Study Group . Insulin sensitivity and diabetic kidney disease in children and adolescents with type 2 diabetes: an observational analysis of data from the TODAY clinical trial. Am J Kidney Dis 2018;71:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 3.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 4.Cherney DZ, Perkins BA, Soleymanlou N, et al. . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 5.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 2011;6:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]