Cholesterol Modulates Cellular TGF-β Responsiveness by Altering TGF-β Binding to TGF-β Receptors (original) (raw)
. Author manuscript; available in PMC: 2018 Aug 24.
Published in final edited form as: J Cell Physiol. 2008 Apr;215(1):223–233. doi: 10.1002/jcp.21303
Abstract
Transforming growth factor-β (TGF-β) responsiveness in cultured cells can be modulated by TGF-β partitioning between lipid raft/caveolae- and clathrin-mediated endocytosis pathways. The TbR-II/TbR-I binding ratio of TGF-β on the cell surface has recently been found to be a signal that controls TGF-β partitioning between these pathways. Since cholesterol is a structural component in lipid rafts/caveolae, we have studied the effects of cholesterol on TGF-β binding to TGF-β receptors and TGF-β responsiveness in cultured cells and in animals. Here we demonstrate that treatment with cholesterol, alone or complexed in lipoproteins, decreases the TbR-II/TbR-I binding ratio of TGF-β while treatment with cholesterol-lowering or cholesterol-depleting agents increases the TbR-II/TbR-I binding ratio of TGF-β in all cell types studied. Among cholesterol derivatives and analogs examined, cholesterol is the most potent agent for decreasing the TbR-II/TbR-I binding ratio of TGF-β. Cholesterol treatment increases accumulation of the TGF-β receptors in lipid rafts/caveolae as determined by sucrose density gradient ultracentrifugation analysis of cell lysates. Cholesterol/LDL suppresses TGF-β responsiveness and statins/β-CD enhances it, as measured by the levels of P-Smad2 and PAI-1 expression in cells stimulated with TGF-β. Furthermore, the cholesterol effects observed in cultured cells are also found in the aortic endothelium of atherosclerotic ApoE-null mice fed a high cholesterol diet. These results indicate that high plasma cholesterol levels may contribute to the pathogenesis of certain diseases (e.g., atherosclerosis) by suppressing TGF-β responsiveness.
Transforming growth factor-β (TGF-β) is a family of 25-kDa structurally homologous dimeric growth factors or cytokines with three members in mammalian species (TGF-β1, TGF-β2, and TGF-β3) (Massague, 1990; Roberts, 1998). TGF-β regulates various physiological processes including cell proliferation, cell differentiation, extracellular matrix synthesis and immune response. It has been implicated in carcinogenesis, tissue fibrosis, autoimmune disease, and cardiovascular disease (Massague, 1990; Roberts, 1998).
TGF-β effectively exerts its activity at subpicomolar concentrations. It is also capable of sustaining or potentiating its activity by autoinduction in certain cell types (Van Obberghen-Schilling et al., 1988; McCartney-Francis et al., 1990; Flanders et al., 1995). The potency of TGF-β activity appears to be regulated at several levels including transcription and post-translation of TGF-β, both of which have been studied extensively (Wakefield et al., 1990; Taipale et al., 1994; Munger et al., 1997; Schmidt et al., 2003). Recently, a novel regulation occurring on cell-surface type-I and type-II TGF-β receptors (TβR-I and TβR-II) has been identified in cultured cells (Di Guglielmo et al., 2003; Ito et al., 2004; Mitchell et al., 2004; Le Roy and Wrana, 2005; Chen et al., 2006). TGF-β responsiveness (Smad-dependent) in cultured cells is regulated, depending on TGF-β or TGF-β receptor partitioning between lipid raft/caveolae-mediated and clathrin-mediated endocytosis pathways. Lipid raft/caveolae-mediated endocytosis results in rapid degradation of TGF-β and suppressed TGF-β responsiveness whereas clathrin-mediated endocytosis leads to endosomal signaling and promoted TGF-β responsiveness. A signal that controls TGF-β or TGF-β receptor partitioning between the two distinct endocytic pathways and resultant TGF-β responsiveness appears to be the TβR-II/TβR-I binding ratio of TGF-β (TGF-β bound to TβR-II/TGF-β bound to TβR-I) as determined by 125I-TGF-β1 affinity labeling (Huang and Huang, 2005; Chen et al., 2006). TβR-I and TβR-II have high affinity for each other and form oligomeric complexes in the absence of the ligand TGF-β (Chen et al., 1997). The TGF-β receptor oligomeric complexes localized in lipid rafts contain more TβR-I than TβR-II and thus exhibit a low TβR-II/TβR-I binding ratio of TGF-β (Huang and Huang, 2005; Chen et al., 2006). The TGF-β receptor oligomeric complexes localized in non-lipid raft microdomains of the plasma membrane contain more TβR-II than TβR-I, exhibit a high TβR-II/TβR-I binding ratio of TGF-β, and mediate endosomal signaling following TGF-β binding and clathrin-mediated endocytosis. TβR-I and TβR-II have preferred lipid-raft/caveolae and non-lipid raft microdomain localization, respectively because TβR-I and TβR-II have high affinity for caveolin-1 (a lipid-raft/caveolae resident protein) and adaptor protein-2 (a protein involved in clathrin-mediated endocytosis), respectively (Razani et al., 2001; Yao et al., 2002). TGF-β responsiveness can be potentiated or suppressed by altering the expression of TβR-I and TβR-II or changing the cell-surface environment and plasma membrane components (Huang and Huang, 2005; Chen et al., 2006). For example, microvascular endothelial cells grown on 2D gel exhibit a higher TβR-II/TβR-I protein level ratio and more potent TGF-β growth inhibitory response as compared to those found in cells grown on 3D gel (Sankar et al., 1996). Cell-surface heparan sulfate negatively modulates TGF-β responsiveness in epithelial cells by decreasing the TβR-II/TβR-I binding ratio of TGF-β without altering the protein levels of these receptors (Chen et al., 2006).
Cholesterol is an important structural component of lipid rafts/caveolae in plasma membranes (Harder and Simons, 1997; Galbiati et al., 2001). Cholesterol depletion by cholesterol binding agents such as β-methylcyclodextrin (β-CD) and nystatin has commonly been used as an experimental approach to examine the functional role of lipid raft/caveolae localization of resident membrane proteins (Park et al., 1998). Cholesterol depletion redirects resident membrane proteins to non-lipid raft microdomains by decreasing formation of or destabilizing lipid rafts/caveolae and influences the activities of these membranes proteins. Since lipid rafts/caveolae mediate TGF-β degradation, resulting in suppressed TGF-β responsiveness (Di Guglielmo et al., 2003; Chen et al., 2006), we hypothesize that cholesterol treatment of cells results in increased lipid raft/caveolae localization of TGF-β receptors (via increasing formation of or stabilizing lipid rafts/caveolae) and suppressed TGF-β responsiveness. To test this hypothesis, we have studied the effects of cholesterol on TGF-β binding to TGF-β receptors and TGF-β responsiveness in several cell types. In this study, we demonstrate that treatments of cells with cholesterol, LDL or VLDL decreases the TβR-II/TβR-I binding ratio of TGF-β and suppresses TGF-β responsiveness (as determined by measurement of TGF-β-stimulated Smad2 phosphorylation and plasminogen activator inhibitor-1 expression) while treatment with cholesterol-lowering agents or other cholesterol-depleting agents increases the TβR-II/TβR-I binding ratio of TGF-β and potentiates TGF-β responsiveness. We also show that cholesterol increases the accumulation of TβR-I and TβR-II in lipid rafts/caveolae. Furthermore, we show that the cholesterol effects in cultured cells (exhibiting a low TβR-II/TβR-I binding ratio of TGF-β and a low level of P-Smad2) are also detected in the aortic endothelium of ApoE-null mice fed a high cholesterol diet.
Materials and Methods
Materials
Na125I (17 Ci/mg) and [methyl-3H] thymidine (67 Ci/mmol) were purchased from ICN Radiochemicals (Irvine, CA). High molecular mass protein standards (myosin, 205 kDa; β-galactosidase, 116 kDa; phosphorylase, 97 kDa; bovine serum albumin, 66 kDa), chloramine-T, bovine serum albumin (BSA), LDL, VLDL, HDL, fluvastatin, lovastatin, cholesterol (>99% pure), cholest-4-ene-3-one, disuccinimidyl suberate (DSS), nystatin, β-cyclodextrin (β-CD), and lipoprotein (a) were obtained from Sigma (St. Louis, MO). 25-Hydroxycholesterol, 7-dehydrocholesterol (7-DHC), 7-ketocholestanol, 7-ketocholesterol 5-cholesten-7-one and 4-cholesten-3-one were obtained from Steraloids (Newport, RI). Oxidized 7-DHC (oxy-7-DHC) was prepared by air drying a thin film of 7-DHC (initially dissolved in chloroform) at ambient room temperature, protected from exposure to light with aluminum foil, for 1 week. Reverse-phase HPLC revealed a mixture of polar products, but no remaining 7-DHC. Phospho-Smad2 (P-Smad2) antibody was obtained from Cell Signaling Technology, Inc. (Danvers, MA). TGF-β1 was purchased from Austral Biologicals (San Ramon, CA). Mouse polyclonal antibodies to the transferrin receptor 1 (TfR1) were obtained from Zymed Laboratories (San Francisco, CA). Rabbit polyclonal antibodies to caveolin-1, TβR-I (ALK5), TβR-II, and α-actin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The luciferase assay system was obtained from Promega (Madison, WI).
Cell culture
Mink lung epithelial cells (Mv1Lu cells), Chinese hamster ovary cells (CHO-K1 cells), bovine aorta endothelial cells (BAEC) and normal rat kidney fibroblasts (NRK cells) were maintained in DMEM or DMEM-F12 containing 10% fetal calf serum with or without bFGF.
125I-TGF-β1 affinity labeling
Cells grown on 6-well dishes in DMEM or DMEM-F12 containing 10% fetal calf serum were treated with 50 μg/ml cholesterol or cholesterol analogs/derivatives, 5 or 50 μg protein/ml LDL, HDL or VLDL in serum-free medium at 37°C for 1 h or 1 μM fluvastatin or lovastatin in serum-free medium (volume: 1 ml) at 37°C for 18 h. 125I-TGF-β1 affinity labeling was then performed at 0°C using the cross-linking agent DSS according to the published procedures (Cheifetz et al., 1988; Huang et al., 2003). 125I-TGF-β1 affinity-labeled cell lysates were analyzed by 7.5% SDS–PAGE followed by autoradiography or quantification using a PhosphoImager. For all experiments, cholesterol or cholesterol analogs/derivatives were prepared as the stock solution with a concentration of 25 or 50 mg/ml in ethanol. The final concentration of ethanol in the medium was 0.2%. In all of the cell types used in the experiments, the TβR-I, which was affinity-labeled by 125I-TGF-β1 using the bifunctional reagent DSS, was identified as ALK5 as evidenced by immunoprecipitation of 125I-TGF-β1-TβR-I complexes using specific antibodies to alk5.
Northern blot analysis
Cells grown to confluence on 12-well dishes in DMEM containing 10% fetal calf serum were treated with several concentrations of cholesterol or vehicle only in serum-free DMEM (volume: 0.5 ml/well) at 37°C for 1 h. The final concentration of ethanol in the medium was 0.2%. The cholesterol-treated cells were then incubated with 50 or 100 pM TGF-β1 at 37°C for 2 h. The transcripts of plasminogen activator inhibitor-1 (PAI-1) and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) in the cell lysates were analyzed by Northern blot analysis and quantified with a PhosphoImager which yields a linearity from 9,000 to 100,000 arbitrary units of the transcript intensity.
Western blot analysis
Cells grown to near confluence on 12-well dishes were treated with cholesterol or vehicle only in serum-free DMEM (volume: 0.5 ml/well) at 37°C for 1 h. The treated cells were further incubated with 50 or 100 pM TGF-β1 at 37°C for 30 min (for determining Smad2 phosphorylation) or for 2 h (for determining the protein levels of TβR-I and TβR-II). Cell lysates were analyzed by 7.5% SDS–PAGE and Western blot analysis using anti-Smad2, anti-P-Smad2, anti-caveolin-1, anti-TβR-I (ALK5) or anti-TβR-II antibodies as described (Huang et al., 2003). The antigens on the blots were visualized by using horseradish peroxidase-conjugated anti-rabbit IgG antibody and the enhanced chemiluminescence (ECL) system as described (Huang et al., 2003). The relative amounts of antigen bands on X-ray films were quantified by densitometry.
125I-TGF-β affinity labeling and Western blot analysis of aortic endothelium from ApoE-null and wild-type mice
Female (2–3 month old) ApoE-null and wild-type mice (C57BL/6) were fed with high-cholesterol (2%) and normal diets for 4–6 weeks and then sacrificed. The plasma levels of cholesterol in ApoE-null mice fed high-cholesterol and normal diets for 4–6 weeks were estimated to be 1,000–1,400 mg/dl and 500–700 mg/dl, respectively based on the standard assay procedures. The high-cholesterol diet did not significantly affect the plasma levels of cholesterol in wild-type mice (100–120 mg/dl). ApoE-null mice fed a high-cholesterol diet exhibited typical atherosclerotic lesions (e.g., fatty streaks and plaques) in the aorta as described previously (Palinski et al., 1994). ApoE-null mice fed normal diet had no significant atherosclerotic lesions in the aorta. Aorta (~3 cm length) was removed and cut lengthwise to expose the intima and then incubated with 100 pM 125I-TGF-β1 in binding buffer containing 2 mg/ml BSA (Huang et al., 2003). After 2.5 h at 0°C, 125I-TGF-β1-affinity labeling was performed using DSS (Huang et al., 2003). The aortas were then extensively washed and the intimal endothelium was scrapped off from the luminal surface of aorta using a razor blade. The aortic endothelium was extracted with 1% Triton X-100. The Triton X-100 extracts were then analyzed by 7.5% SDS–PAGE and autoradiography or quantification using a PhosphoImager. Under the experimental conditions, 125I-TGF-β1-affinity labeling occurred mainly in the intimal endothelium as evidenced by the observation that the majority of 125I-TGF-β1 radioactivity (>90%) and factor VIII antigen in aortic endothelium could be removed by scrapping off intimal endothelium from the luminal surface of the aorta using a razor blade. For Western blot analysis of aortic endothelium, aorta (~3 cm length) was cut lengthwise. Intimal endothelium was obtained by scrapping off the endothelium from the luminal surface of aorta using a razor blade and extracted with 1% Triton X-100. An equal protein amount (~200 μg protein) of Triton X-100 extracts was subjected to 7.5% SDS–PAGE followed by Western blot analysis using antibodies to P-Smad2, Smad2, TβR-I (ALK5), TβR-II and α-actin and quantification by ECL and densitometry.
Sucrose density gradient ultracentrifugation
Mv1Lu and NRK cells were grown to near confluence in 100-mm dishes (5–10 × 106 cells per dish) and incubated with cholesterol (50 μg/ml) at 37°C for 1 h and then incubated with TGF-β1 (100 pM) for 1 h. After being washed with ice-cold phosphate-buffered saline, cells were scraped into 0.85 ml of 500 mM sodium carbonate, pH 11.0. Homogenization was carried out with 10 strokes of a tight-fitting Dounce homogenizer followed by three 20-sec bursts of ultrasonic disintegrator, (Soniprep 150; Fisher Scientific, Pittsburgh, PA) to disrupt cellular membranes as described previously (Ito et al., 2004; Chen et al., 2006). The homogenates were analyzed by sucrose density gradient ultracentrifugation as described previously (Ito et al., 2004; Chen et al., 2006). Ten 0.5-ml fractions were collected from the top of the tube, and a portion of each fraction was analyzed by SDS–PAGE followed by Western blot analysis using antibodies to TβR-I, TβR-II, caveolin-1, and TfR1. The relative amounts of TβR-I and TβR-II on the blot were quantified by densitometry.
Statistical analysis
One-way ANOVA was used to compare groups. Comparisons with the control group and experimental group were performed using the unpaired t-test and the Mann–Whitney test. P < 0.05 was considered significant.
Results
Treatment of cells with cholesterol decreases the TβR-II/TβR-binding ratio of TGF-β on the cell surface
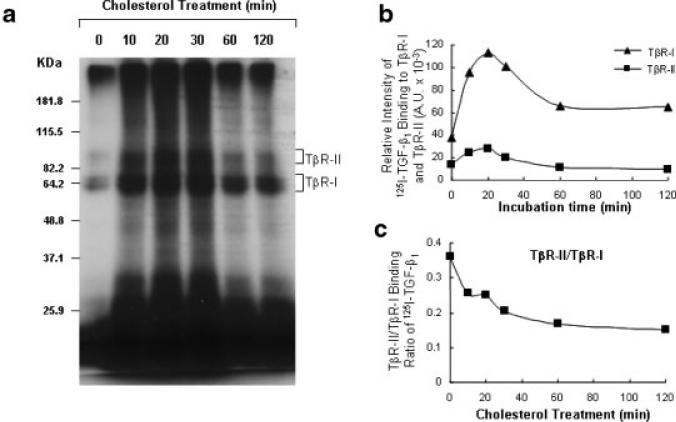
The TβR-II/TβR-I binding ratio of TGF-β has been shown to be positively correlated with TGF-β responsiveness in several cell systems (McCaffrey et al., 1995; Sankar et al., 1996; Coppa et al., 1997; McCaffrey et al., 1997; Letamendia et al., 1998; Eickelberg et al., 2002; Quan et al., 2004; Chen et al., 2006). We recently reported that the TβR-II/TβR-I binding ratio of TGF-β in TGF-β receptor oligomeric complexes on the cell surface is a signal determining TGF-β partitioning between lipid raft/caveolae- and clathrin-mediated endocytosis pathways and resultant TGF-β responsiveness (Huang and Huang, 2005; Chen et al., 2006). When the TβR-II/TβR-I binding ratio of TGF-β increases, more receptor-bound TGF-β (as Complex I, which contains more TβR-II than TβR-I and exists in non-lipid raft microdomains) undergoes clathrin-mediated endocytosis and generates signaling in endosomes, leading to promotion of TGF-β responsiveness; when the TβR-II/TβR-I binding ratio of TGF-β decreases, more receptor-bound TGF-β (as Complex II, which contains more TβR-I than TβR-II and exists in lipid rafts/caveolae) undergoes lipid raft/caveolae-mediated endocytosis and rapid degradation, resulting in suppression of TGF-β responsiveness (Chen et al., 2006). To determine if cholesterol treatment alters the TβR-II/TβR-I binding ratio of TGF-β, we first determined the time course of the effect of cholesterol treatment on the TβR-II/TβR-I binding ratio of 125I-TGF-β1 in Mv1Lu cells. These cells were treated with 50 μg/ml cholesterol at 37°C for several time periods. 125I-TGF-β1 binding to TβR-I and TβR-II was then determined by affinity labeling at 0°C using the bifunctional cross-linking agent DSS followed by 7.5% SDS–PAGE and autoradiography or quantification using a PhosphoImager. As shown in Figure 1, treatment of Mv1Lu cells with 50 μg/ml cholesterol increased binding of 125I-TGF-β1 to TβR-I in a time-dependent manner (Fig. 1A). The cholesterol-induced increase of 125I-TGF-β1 binding to TβR-I was observed within 10 min. Cholesterol increased 125I-TGF-β1 binding to TβR-I by –2-fold after a 60- or 120-min incubation, whereas it moderately affected 125I-TGF-β1 binding to TβR-II after the same time incubation (Fig. 1B). Cholesterol treatment for 60 or 120 min appeared to decrease the TβR-II/TβR-I binding ratio of 125I-TGF-β1 from 0.37 (in control cells treated without cholesterol) to 0.18 (Fig. 1C). These results indicate that cholesterol exerts its effect promptly.
Fig. 1.
Time dependence of the cholesterol effect on 125I-TGF-β1 binding to TβR-I and TβR-II in Mv1Lu cells. Cells were treated with 50 μg/ml cholesterol or vehicle only at 37°C for the time periods as indicated and 125I-TGF-β1-affinity labeling was performed. 125I-TGF-β1 affinity-labeled TGF-β receptors were analyzed by 7.5% SDS–PAGE and autoradiography (a) and quantified with a PhosphoImager(b). The bracket indicates the locations of 125I-TGF-β1 affinity-labeled TβR-I and TβR-II. The TβR-II/TβR-I ratio of 125I-TGF-β1 affinity-labeled TβR-II and TβR-I is plotted against the time of treatment with cholesterol (c).
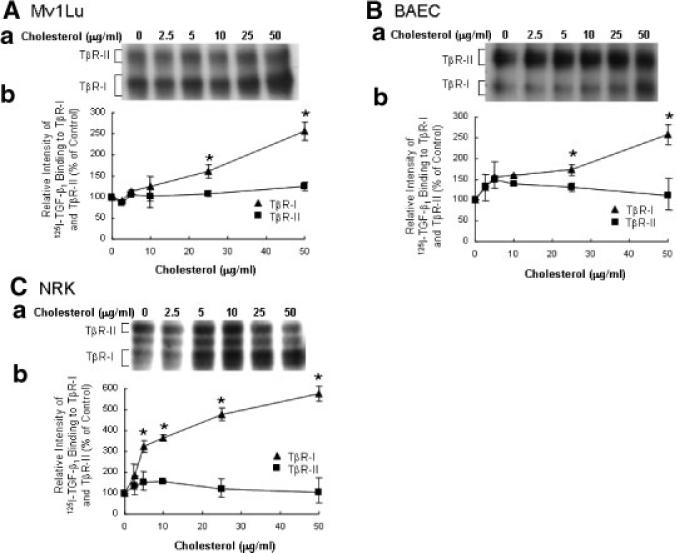
We then performed analysis of the effect of cholesterol treatment on 125I-TGF-β1 binding to TβR-I and TβR-II in several cell types. Mv1Lu, BAEC, and NRK cells were treated for 1 h with increasing concentrations of cholesterol. The binding of 125I-TGF-β1 to TβR-I and TβR-II in these cells was then determined by 125I-TGF-β1 affinity labeling at 0°C. As shown in Figure 2A–C, cholesterol treatment effectively increased the binding of 125I-TGF-β1 to TβR-I but slightly affect the binding of 125I-TGF-β1 to TβR-II in these three cell types. At 50 μg/ml, cholesterol increased 125I-TGF-β1 binding to TβR-I by ~2.5-fold, ~2.5-fold, and ~5.7-fold in Mv1Lu, BAEC, and NRK cells, respectively (Fig. 2A–C). Thus, cholesterol (50 mg/ml) decreased the TβR-II/TβR-I binding ratio of 125I-TGF-β1 from 0.4 to 0.2, 3.5 to 1.4, and 2.0 to 0.4 in Mv1Lu, BAEC, and NRK cells, respectively. These results suggest that pretreatment of cells with cholesterol decreases the TβR-II/TβR-I binding ratio of 125I-TGF-β1 on the cell surface of all three cell types studied.
Fig. 2.
Effect of increasing concentrations of cholesterol on 125I-TGF-β1 binding to TβR-I and TβR-II in Mv1Lu (A), BAEC (B), and NRK (C) cells. Cells were treated with increasing concentrations of cholesterol as indicated. 125I-TGF-β1 affinity labeling of TβR-I and TβR-II in treated cells was performed and analyzed. A representative of a total of three analyses is shown (a). The relative amounts of 125I-TGF-β1 affinity-labeled TβR-I and TβR-II in cells treated without cholesterol were taken as 100%. The quantitative data from three analyses is shown (b). The data bar represents the mean ± SD. *Significantly higher than that in cells treated with vehicle alone: P < 0.001.
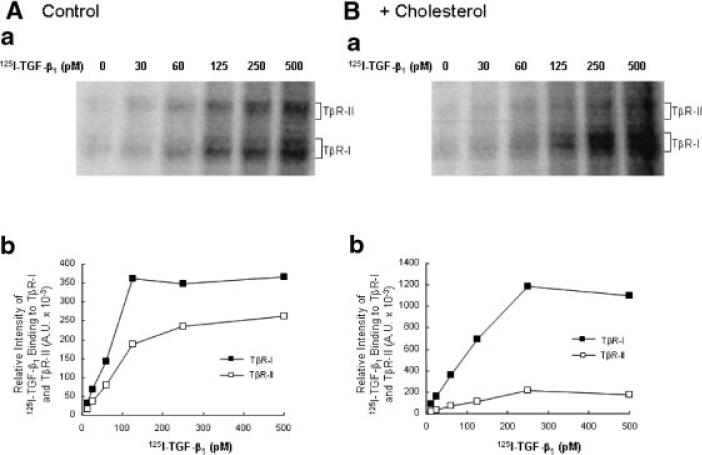
Cholesterol does not affect the affinity of TGF-β binding to TGF-β receptors
To determine if cholesterol treatment affects the affinity of TGF-β1 binding to TβR-I and TβR-II, Mv1Lu cells were treated with 50 μg/ml cholesterol at 37°C for 1 h and then incubated with increasing concentrations of 125I-TGF-β1 at 0°C for 2.5 h. 125I-TGF-β1 affinity labeling was performed. 125I-TGF-β1 affinity-labeled TGF-β receptors were then analyzed by 7.5% SDS–PAGE and autoradiography (Fig. 3Aa,Ba) or quantification using a PhosphoImager (Fig. 3Ab,Bb). Cholesterol (50 μg/ml) appeared to increase 125I-TGF-β1 (125 pM) binding to TβR-I by ~2-fold (from ~350 to ~700 × 103 A.U.) but did not increase 125I-TGF-β1 binding to TβR-II (from ~180 to ~120 × 10–3 A.U.; Fig. 3Bb vs. Fig. 3Ab). The half-maximum concentrations of 125I-TGF-β1 binding to TβR-I and TβR-II were ~80–100 pM in Mv1Lu cells treated with and without cholesterol. The half-maximum concentrations, ~80–100 pM, were close to the apparent Kds of TGF-β1 binding to TβR-I and TβR-II (Huang et al., 2003). These results suggest that cholesterol treatment significantly increases 125I-TGF-β1 binding to TβR-I, thus decreasing the TβR-II/TβR-I binding ratio of 125I-TGF-β1 but does not alter the affinities of 125I-TGF-β1 binding to either TβR-I or TβR-II.
Fig. 3.
Effect of cholesterol on binding of increasing concentrations of 125I-TGF-β1 to TβR-I and TβR-II in Mv1Lu cells. Cells were treated with 50 μg/ml cholesterol (B) or vehicle only (A) at 37°C for 1 h. 125I-TGF-β1 affinity labeling was performed and analyzed (a). The relative amounts of 125I-TGF-β1 affinity-labeled TβR-I and TβR-II, which were quantified with a PhosphoImager, are plotted against TGF-β1 concentrations (b).
Lipoproteins and statins alter the TβR-II/TβR-I binding ratio of TGF-β on the cell surface
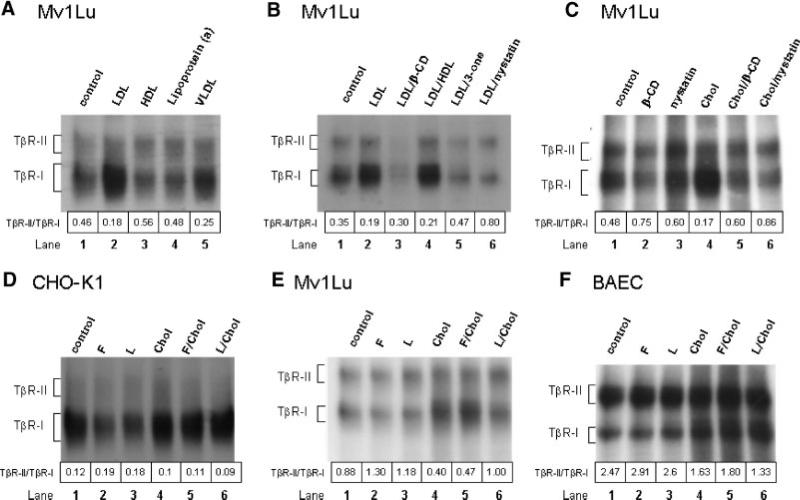
To further define the physiological significance of the cholesterol effect on TGF-β1 binding to TGF-β receptors, we determined the effect of pretreatment at 37°C for 1 h with cholesterol-containing lipoproteins (LDL, VLDL, and HDL), nystatin (a cholesterol-binding agent; Wang et al., 1998) and β-CD (Subtil et al., 1999) 4-cholesten-3-one (a cholesterol derivative) and cholesterol, alone or in combination, on 125I-TGF-β1 binding to TβR-I and TβR-II (as determined by 125I-TGF-β1 affinity labeling) in Mv1Lu cells. As shown in Figure 4A, LDL (50 μg protein/ml) and VLDL (5 μg protein/ml) increased 125I-TGF-β1 binding to TβR-I but not TβR-II, and thus decreased the TβR-II/TβR-I binding ratio of 125I-TGF-β1 from 0.46 to 0.18 and 0.25, respectively (lanes 2 and 5 vs. lane 1). HDL (50 μg protein/ml) slightly decreased 125I-TGF-β1 binding to TβR-I and thus increased the TβR-II/TβR-I binding ratio of 125I-TGF-β1 from 0.46 to 0.56 (Fig. 4 A, lane 3 vs. lane 1). Lipoprotein (a) (50 μg/ml), an atherogenic lipoprotein which exerts its atherogenic effect via a cholesterol-independent mechanism (Kojima et al., 1991), did not significantly affect 125I-TGF-β1 binding to TβR-I or TβR-II (Fig. 4 A, lane 4 vs. lane 1). The LDL- or cholesterol-induced increase of 125I-TGF-β1 binding to TβR-I was abolished in the presence of β-CD, nystatin, or 4-cholesten-3-one, (Fig. 4B, lanes 3, 5, and 6 vs. lane 2 and Fig. 4C, lanes 5 and 6 vs. lane 4). For example, the TβR-II/TβR-I binding ratio of 125I-TGF-β1 in cells treated with both LDL or cholesterol and β-CD or nystatin was similar to that of cells treated with β-CD or nystatin alone (Fig. 4C, lanes 5 and 6 vs. lanes 2 and 3). Treatment of cells with β-CD or nystatin alone decreased 125I-TGF-β1 binding to TβR-I and thus increased the TβR-II/TβR-I binding ratio of 125I-TGF-β1 as compared to untreated control cells (Fig. 4C, lanes 2 and 3 vs. lane 1). These results indicate that LDL and VLDL significantly increase 125I-TGF-β1 binding to TβR-I, resulting in a decreased TβR-II/TβR-I binding ratio of 125I-TGF-β1. Since treatment with cholesterol-binding agents β-CD or nystatin abolishes LDL- or cholesterol-induced increase of 125I-TGF-β1 binding to TβR-I, these results also suggest that LDL decreases the TβR-II/TβR-I binding ratio of 125I-TGF-β1 via the cholesterol component rather than other lipid or protein components.
Fig. 4.
Effects of LDL/VLDL/HDL, fluvastatin/lovastatin and nystatin/β-CD in the presence or absence of cholesterol on 125I-TGF-β1 binding to TβR-I and TβR-II in Mv1Lu (A–C,E), CHO-K1 (D) and BAEC (F) cells. Cells were treated with LDL (50 μg protein/ml), VLDL (5 μg protein/ml), HDL (50 μg protein/ml), lipoprotein (a) (10 μg/ml), fluvastatin (F; 1 μM), lovastatin (L; 1 μM), nystatin (Nys; 25 μg/ml), β-CD (8 mM), cholest-4-en-3-one (3-one; 50 μg/ml) or cholesterol (Chol; 50 μg/ml) alone or combination of two. After 1 h at 37°C or 16 h at 37°C (only for fluvastatin and lovastatin), 125I-TGF-β1 affinity labeling was performed. For cells treated with fluvastatin or lovastatin, 125I-TGF-β1 affinity labeling was performed following incubation with cholesterol (Chol; 50 μg/ml) or vehicle (ethanol) only at 37°C for 1 h. The relative amounts of 125I-TGF-β1 affinity-labeled TβR-I and TβR-II were quantified with a PhosphoImager. The TβR-II/TβR-I binding ratio of 125I-TGF-β1 was estimated. The numbers above the lane sequence denote the TβR-II/TβR-I binding ratios of 125I-TGF-β1.
Since statins are cholesterol-lowering agents (Alberts, 1988; Yuan et al., 1991), we reasoned that pretreatment of cells with statins (1 μM, at 37°C for 16 h) should reduce the cholesterol content in the plasma membrane (due to cholesterol metabolism or turnover), thereby decreasing TGF-β1 binding to TβR-I and increasing the TβR-II/TβR-I binding ratio of TGF-β1 (i.e., the converse of the cholesterol-induced increase of TGF-β1 binding to TβR-I). As shown in Figure 4D–F, treatment with fluvastatin (F) or lovastatin (L) decreased 125I-TGF-β1 binding to TβR-I in CHO-K1, Mv1Lu, and BAEC cells, respectively (Fig. 4D–F, lanes 2 and 3 vs. lane 1) and thus increased the TβR-II/TβR-I binding ratio of 125I-TGF-β1 from 0.12 to 0.19 or 0.18, 0.88 to 1.3 or 1.18 and 2.47 to 2.91 or 2.6 in CHO-K1, Mv1Lu, and BAEC cells, respectively. The statin effect was abolished by incubation of cells with cholesterol prior to 125I-TGF-β1 affinity labeling in CHO-K1, Mv1Lu, and BAEC cells (Fig. 4 D–F, lanes 5 and 6 vs. lanes 2 and 3), suggesting that the statin effect is due to its cholesterol-lowering properties.
Among cholesterol derivatives and analogs examined, cholesterol is the most potent for decreasing the TβR-II/TβR-I binding ratio of TGF-β on the cell surface
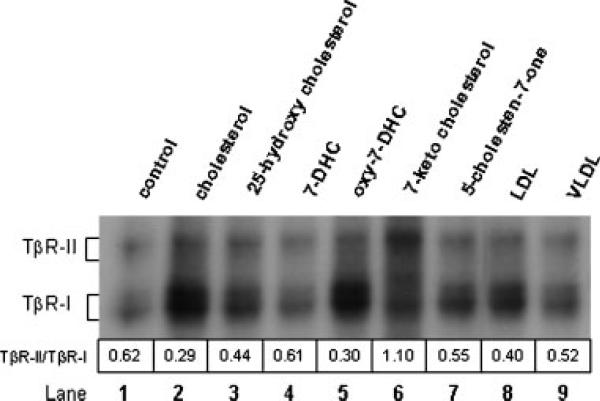
To define the specificity of the cholesterol effect, we determined the effects of cholesterol analogs and derivatives on 125I-TGF-β1 binding to TβR-I and TβR-II in Mv1Lu cells. These cells were treated with 50 μg/ml of cholesterol or related oxysterol derivatives (25-hydroxycholesterol, 7-DHC, oxy-7-DHC, cholest-5-en-7-one and 7-ketocholesterol) at 37°C for 1 h. 125I-TGF-β1 binding to TGF-β receptors was then determined by affinity labeling. Under the experimental conditions, treatment of Mv1Lu cells with cholesterol analogs and derivatives did not affect cell viability. As shown in Figure 5, cholesterol was the most potent of these compounds in increasing 125I-TGF-β1 binding to TβR-I (lane 2 vs. lanes 3, 4, 6, and 7) and decreasing the TβR-II/TβR-I binding ratio of 125I-TGF-β1 from 0.62 to 0.29 (lane 2 vs. lane 1). Curiously, a mixture of oxysterols produced by exhaustive air oxidation of 7-DHC in darkness (“oxy-7-DHC”) (lane 5) appeared to be nearly as potent as cholesterol in this regard, notably much more so than an equivalent amount of pure 7-DHC (lane 4).
Fig. 5.
Effects of cholesterol analogs and derivatives on 125I-TGF-β1 binding to TβR-I and TβR-II in Mv1Lu cells. Mv1Lu cells were treated with 50 μg/ml of cholesterol and cholesterol analogs/derivatives including 25-hydroxycholesterol, 7-DHC, 7-ketocholesterol, 7β,8β-epoxycholesterol, 7β-hydroxycholesterol, oxy-7-DHC, 50 μg/ml LDL and 5 μg/ml VLDL. After 1 h at 37°C, 125I-TGF-β1 affinity labeling was performed, analyzed by autoradiography and quantified with a PhosphoImager. The TβR-II/TβR-I binding ratios of 125I-TGF-β1 were estimated. The numbers above the lane sequence denote the TβR-II/ TβR-I binding ratios of 125I-TGF-β1.
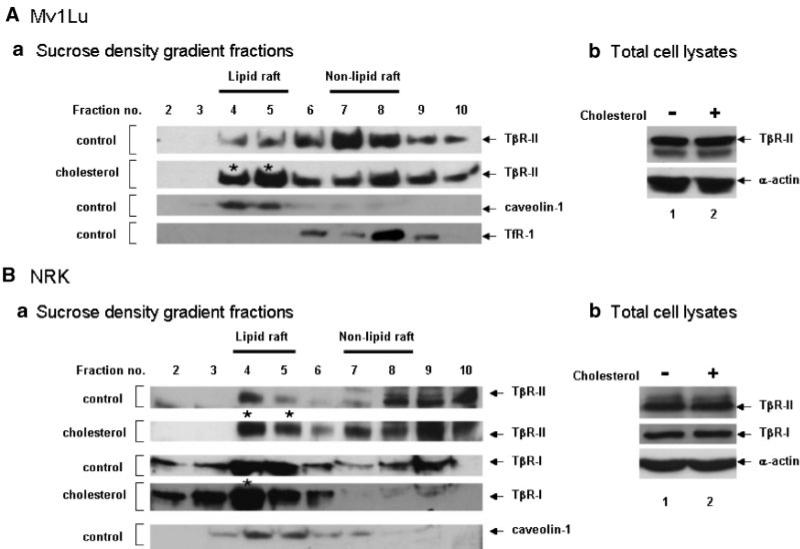
Cholesterol increases accumulation of TGF-β receptors in lipid rafts/caveolae
As demonstrated above, cells treated with cholesterol exhibited a low TβR-II/TβR-I binding ratio of 125I-TGF-β on the cell surface, which is the characteristic property of TGF-β receptor complexes localized in lipid rafts/caveolae (Chen et al., 2006). We then examined the localization of the TGF-β receptors in lipid rafts/caveolae of the plasma membrane in cells treated with 50 μg/ml cholesterol or vehicle (ethanol) only using sucrose density gradient ultracentrifugation analysis (Ito et al., 2004; Chen et al., 2006). Sucrose density gradient ultracentrifugation has been a standard procedure used to separate lipid rafts/caveolae from other microdomains in plasma membranes. As shown in Figure 6A,B, TβR-II was mainly localized in non-lipid raft fractions (fractions 7 and 8, which contain TfR1) of Mv1Lu and NRK cells. TβR-I was not analyzed in Mv1Lu cells due to the poor reactivity of the anti-TβR-I antibody (used in the experiment) to mink TβR-I. After treatment with cholesterol, TβR-I and TβR-II were enriched in the lipid raft/caveolae fractions (fractions 4 and 5) of the plasma membrane in Mv1Lu and NRK cells. Treatment with cholesterol did not affect the total amounts of TβR-I and TβR-II proteins (Fig. 6 Ab,Bb) and localization of caveolin-1 and TfR1 (data not shown) in these cells. These results suggest that cholesterol increases accumulation of TGF-β receptors in lipid rafts/caveolae. They are also consistent with the observation of a low TβR-II/TβR-I binding ratio of TGF-β in cells treated with cholesterol. A low TβR-I/TβR-II binding ratio of TGF-β is the characteristic property of cell-surface TGF-β receptor oligomeric complexes localized in lipid rafts/caveolae (Huang and Huang, 2005; Chen et al., 2006).
Fig. 6.
Sucrose density gradient ultracentrifugation analysis of TβR-I and TβR-II in Mv1Lu (A) and NRK (B) cells treated with and without cholesterol. Cells were treated with 50 μg/ml cholesterol or vehicle only at 37°C for 1 h. The cell lysates from these treated cells were directly analyzed by Western blot analysis using antibodies to TβR-I and TβR-II (b) and subjected to sucrose density gradient ultracentrifugation. The sucrose density gradient fractions were then analyzed by Western blot analysis (a). The arrow indicates the locations of TβR-I, TβR-II, caveolin-1, and TfR1. Fractions 4 and 5 contained lipid rafts/caveolae (which contained caveolin-1), whereas fractions 7 and 8 represented non-lipid raft fractions, which contained TfR1. * Indicates the increased amount of TβR-I or TβR-II in the fraction as compared to that of the untreated control.
Cholesterol suppresses TGF-β responsiveness
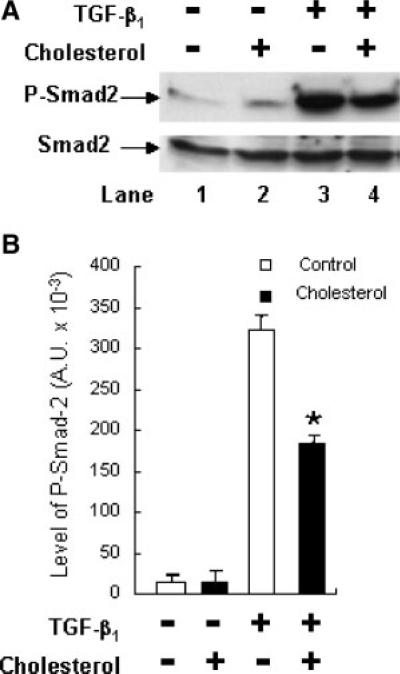
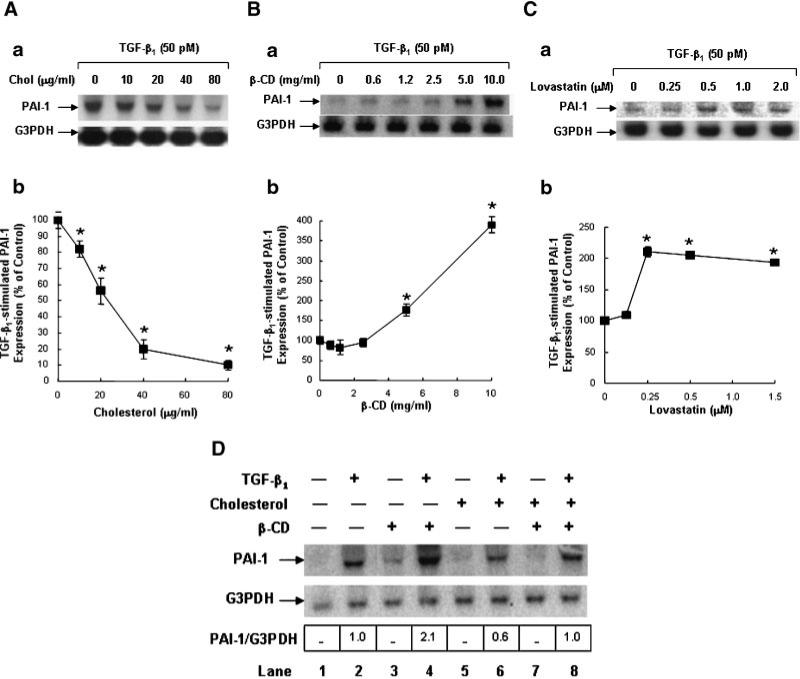
Since lipid rafts/caveolae have been shown to mediate down-regulation of TGF-β-stimulated signaling and thus TGF-β responsiveness (Di Guglielmo et al., 2003; Le Roy and Wrana, 2005; Huang and Huang, 2005), the finding of cholesterol-induced localization of TβR-I and TβR-II in lipid rafts/caveolae suggests that treatment of cells with cholesterol should suppress TGF-β-stimulated signaling and TGF-β responsiveness. To test this, we determined the effect of cholesterol on TGF-β-stimulated Smad2 phosphorylation, which is a key signaling event leading to TGF-β responsiveness (Heldin et al., 1997; Massague, 1998; Miyazono et al., 2000; Moustakas et al., 2002; Feng and Derynck, 2005). Mv1Lu cells were treated with 50 μg/ml cholesterol at 37°C for 1 h and then incubated with 50 pM TGF-β1 at 37°C for 30 min. P-Smad2 and Smad2 in the cell lysates were analyzed by 7.5% SDS–PAGE followed by Western blot analysis using anti-P-Smad2 and anti-Smad2 antibodies and ECL and quantified by densitometry. As shown in Figure 7, cholesterol treatment attenuated TGF-β1-stimulated Smad2 phosphorylation by ~50% in these cells (Fig. 7A, lane 4 vs. lane 3, and Fig. 7B). To determine the effect of cholesterol on TGF-β responsiveness, we analyzed the PAI-1 expression in cells treated with and without cholesterol and then stimulated with and without TGF-β1. TGF-β-stimulated PAI-1 expression is mediated by the Smad signaling pathway (Lund et al., 1987; Heldin et al., 1997; Massague, 1998). We used Northern blot analysis and quantification using a PhosphoImager which provided a linearly quantitative measurement. Mv1Lu cells were treated with increasing concentrations of cholesterol at 37°C for 1 h and then further incubated with 50 pM TGF-β1 at 37°C for 2 h. The transcripts of PAI-1 and G3PDH in the cell lysates were analyzed by Northern blot analysis and quantification using a PhosphoImager. G3PDH expression was used as an internal control. As shown in Figure 8A, cholesterol attenuated TGF-β1-stimulated PAI-1 expression in a dose-dependent manner (Fig. 8Aa). At 10 and 40 μg/ml, cholesterol attenuated PAI-1 expression by ~20 and ~80%, respectively in these cells (Fig. 8Ab). No significant PAI-1 expression was observed in control cells treated without TGF-β1 (data not shown). To define the role of endogenous cholesterol in TGF-β responsiveness, we determined the effect of the cholesterol-depleting agent β-CD and cholesterol-lowering agent lovastatin on PAI-1 expression stimulated by TGF-β1 in Mv1Lu cells. As shown in Figure 8B,C, treatment of cells with increasing concentrations of β-CD or lovastatin correspondingly enhanced TGF-β1-stimulated PAI-1 expression in these cells (Fig. 8Ba,Ca). β-CD (5 μg/ml) and lovastatin (0.25 mM) enhanced TGF-β1-stimulated PAI-1 expression by ~2- to 4-fold (Fig. 8Bb,Cb). The effects of β-CD and lovastatin were abolished when β-CD-treated cells were later treated with cholesterol (50 μg/ml; Fig. 8D, lane 8 vs. lanes 4 and 2) and when lovastatin-treated cells were later treated with cholesterol (data not shown). As described above, no significant PAI-1 expression was found in control cells treated without TGF-β1 (Fig. 8D, lane 1). It is important to note that the exposure time of the autoradiogram shown in Figure 8Aa was longer than those in Figure 8Ba,Ca. For this reason, the PAI-1 band at 0 μg/ml of cholesterol in Figure 8Aa is more apparent than those at 0 μg/ml of cholesterol in Figure 8Ba,Ca. This was intended to clearly show the inhibitory effect of cholesterol in Figure 8Aa and the stimulatory effect of β-CD and lovastatin in Figure 8Ba,Ca, respectively. These results suggest that cholesterol suppresses TGF-β responsiveness and that β-CD potentiates it. These results also suggest that β-CD or lovastatin enhances responsiveness by depleting cholesterol from the plasma membrane.
Fig. 7.
Effect of cholesterol on Smad2 phosphorylation in Mv1Lu cells stimulated with TGF-β1. Cells were treated with 50 μg/ml cholesterol or vehicle only at 37°C for 1 h and then further incubated with 50 pM TGF-β1 at 37°C for 30 min. P-Smad2 and total Smad2 in the cell lysates were analyzed by immunoblotting (A). The quantitative analysis of the immunoblots expressed as arbitrary units (A.U.) is shown (B). The data bar represents the mean ± SD (n = 3). *Significantly lower than that in cells treated with TGF-β1 only: P < 0.001.
Fig. 8.
Effects of cholesterol (A,D), β-CD (B,D) and lovastatin (C) on PAI-1 expression in Mv1Lu cells stimulated with TGF-β1. Cells were treated with increasing concentrations of cholesterol (A) and of β-CD (B), 50 μg/ml cholesterol (D) or 5 μg/ml β-CD (D) at 37°C for 1 h or with and without lovastatin (0.5 μM) at 37°C for 16 h (C) and incubated with 50 μg/ml cholesterol or vehicle only (B–D) and further incubated with 50 pM TGF-β1 (A–D) at 37°C for 2 h. Northern blot analysis of PAI-1 and G3PDH was performed. The relative amounts of the transcripts were quantified with a PhosphoImager. The ratio of the relative amounts of PAI-1 and G3PDH transcripts in cells treated without TGF-β1 and cholesterol was taken as 100% or onefold expression of PAI-1. A representative of a total of three analyses is shown (a). The quantitative analysis of the immunoblots is shown (b). The data bar represents the mean ± SD. *Significantly lower than that in cells treated with TGF-β1 only: P < 0.001.
The aortic endothelium of ApoE-null mice fed a high-cholesterol diet exhibits a low TβR-II/TβR-binding ratio of TGF-β
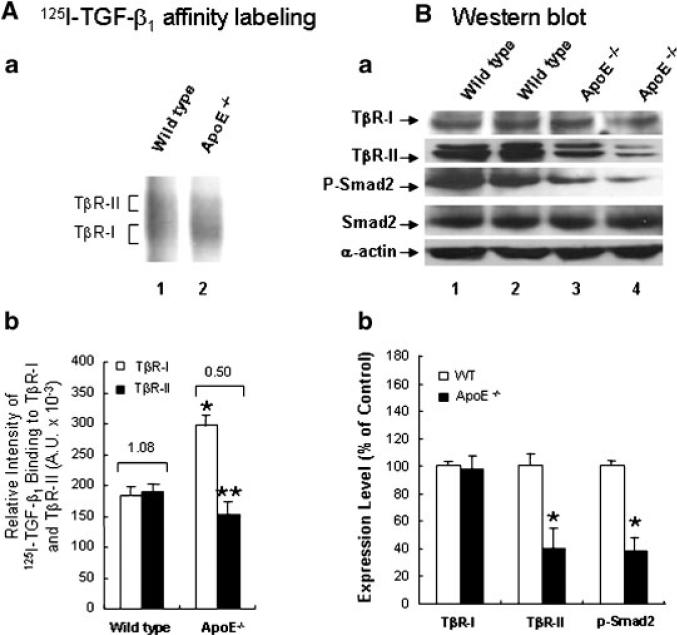
To define the in vivo relevance of the effect of cholesterol, we performed 125I-TGF-β1 affinity labeling and determined the level of P-Smad2 in the aortic endothelium of wild-type mice and ApoE-null mice fed a high-cholesterol (2%) or normal diet. ApoE-null mice fed a high cholesterol diet have commonly been used as an animal system to study the mechanisms by which hypercholesterolemia initiates and/or facilitates atherogenesis. ApoE-null mice fed a high-cholesterol diet show typical atherosclerotic lesions such as fatty streaks and plaques in the aorta as described (Palinski et al., 1994). To determine the TβR-II/TβR-I binding ratio of 125I-TGF-β1 in aortic endothelium, the aortas were cut lengthwise to expose the endothelium and incubated with 125I-TGF-β1 in binding buffer. After 2.5 h at 0°C, 125I-TGF-β1 affinity labeling with DSS was performed; the 125I-TGF-β1 affinity-labeled aortic endothelium was scrapped off from the luminal surface of the aorta and extracted with 1% Triton X-100. The Triton X-100 extracts, which contained factor VIII (an endothelial cell marker), were then analyzed by 7.5% SDS–PAGE and autoradiography. As shown in Figure 9A, the aortic endothelium from ApoE-null mice exhibited increased 125I-TGF-β1 binding to TβR-I and concomitantly decreased 125I-TGF-β1 binding to TβR-II (Fig. 9Aa, lane 2 vs. lane 1). The TβR-II/TβR-I binding ratios of 125I-TGF-β1 in ApoE-null mice appeared to be lower than those found in wild-type mice (Fig. 9Ab, 0.5 vs. 1.08). A low TβR-II/TβR-I binding ratio of 125I-TGF-β1 was also found in cultured BAEC cells treated with cholesterol (50 μg/ml) as compared with that found in control cells (Fig. 4F, lane 4 vs. lane 1).
Fig. 9.
A low TβR-II/TβR-I binding ratio of 125I-TGF-β1 (A) and a low level of P-Smad2 (B) in the aortic endothelium of ApoE-null mice fed a high-cholesterol diet. A: 125I-TGF-β affinity labeling—the aortic endothelium from wild-type and ApoE-null (ApoE–/–) mice fed a high-cholesterol diet (a, lanes 1 and 2, respectively) were affinity-labeled with 125I-TGF-β using DSS. 125I-TGF-β affinity-labeled TGF-β receptors were analyzed by SDS–PAGE followed by autoradiography (a) and quantitation using a PhosphoImager (b). Are presentative of a total of five animals each analyzed is shown (a). The data bar represents the mean ± SD. *Significantly higher than wild-type mice: P < 0.001; **significantly lower than wild-type mice: P < 0.05 (b). The number on the top of the bar charts is the estimated TβR-II/TβR-II binding ratio of 125I-TGF-β1 (b). B: Western blot analysis—the aortic endothelium from wild-type (a, lanes 1 and 2) and ApoE-null mice (ApoE–/–) (a, lanes 3 and 4) mice fed a high-cholesterol diet were extracted with 1% Triton X-100. Equal protein amounts (~200 μg) of the Triton X-100 extracts were then subjected to Western blot analysis using antibodies to Smad2, P-Smad2, TβR-I, TβR-II, and α-actin. Two representatives (lanes 1 and 2 and lanes 3 and 4) of a total of five animals each analyzed are shown (a). Quantitative analysis of the immunoblots is shown (b). The data bar represents the mean ± SD. *Significantly lower than wild-type mice: P < 0.001 (b).
To examine the TGF-β responsiveness, the level of P-Smad2 in the aortic endothelium of wild-type and ApoE-null mice fed a high cholesterol diet was analyzed by quantitative Western blot analysis following 7.5% SDS–PAGE. P-Smad2 has been used as an indicator for TGF-β responsiveness in aortas and other tissues (Kalinina et al., 2004; Phipps et al., 2004). As shown in Figure 9B, the relative levels of P-Smad2 were lower in ApoE-null mice fed a high cholesterol diet than those in wild-type mice fed a high-cholesterol diet (Fig. 9Ba, lanes 3 and 4 vs. lanes 1 and 2). The levels of P-Smad2 in ApoE-null mice fed a high cholesterol diet were estimated to be ~40% of those found in wild-type mice (Fig. 9Bb). The TβR-I protein levels in ApoE-null mice were similar to those found in wild-type mice (Fig. 9Ba, lanes 3 and 4 vs. lanes 1 and 2, and Fig. 9Bb). However, the TβR-II protein levels in ApoE-null mice were ~40% of those seen in wild-type mice (Fig. 9Ba, lanes 3 and 4 vs. lanes 1 and 2, and Fig. 9Bb). These results suggest that the aortic endothelium of atherosclerotic mice exhibit a low TβR-II/TβR-I binding ratio of TGF-β1 and a low level of P-Smad2, both of which are characteristic of suppressed TGF-β responsiveness as observed in cultured cells treated with cholesterol.
Discussion
In this communication, we demonstrate that cells treated with cholesterol, alone or complexed as lipoproteins, decrease the TβR-II/TβR-I binding ratio of TGF-β1 on the cell surface. Conversely, cholesterol-lowering agents and cholesterol-depleting agents increase the TβR-II/TβR-I binding ratio of TGF-β1. Cholesterol may exert this effect by integrating into the plasma membrane as evidenced by the following observations: (1) cholesterol acts rapidly. It effectively increases TGF-β1 binding to TβR-I within 10-min; (2) the cholesterol effect is reversible. It can be reversed by incubation of cholesterol-treated cells with 1% β-CD prior to 125I-TGF-β1 affinity labeling; and (3) the cholesterol effect on 125I-TGF-β1 binding to TGF-β receptors can be reproduced using plasma membranes purified from mouse liver (unpublished results), suggesting that cholesterol treatment is capable of increasing formation of or stabilizing lipid rafts/caveolae in the plasma membrane in a cell-free system.
Among the various sterols and cholesterol analogs examined, cholesterol exhibited the most potent activity in increasing 125I-TGF-β1 binding to TβR-I and promoting the formation of Complex II (Huang and Huang, 2005; Chen et al., 2006), which mainly utilizes the lipid raft/caveolae endocytosis pathway. This is consistent with prior reports indicating that cholesterol is a preferred sterol to form lipid rafts/caveolae with sphingolipids and phospholipids. Wang et al. (2004) identified several cholesterol derivatives and other sterols which behave like cholesterol in their ability to be inserted into lipid rafts, using an in vitro phospholipid binding assay. Subsequent studies by Keller et al. (2004), using both an in vitro model system and in vivo studies with rats, demonstrated that 7-DHC is as competent as cholesterol with regard to promoting lipid raft formation in an in vitro system. However, 7-DHC and most of the other sterols analyzed in the present study were unable to function like cholesterol in forming or stabilizing lipid rafts/caveolae in our 125I-TGF-β1 affinity labeling assay. This suggests that the TGF-β1 binding assay may be a useful system to study the structural and functional role of cholesterol in lipid rafts/caveolae in cells. Interestingly, while pure 7-DHC was a poor replacement for cholesterol in the 125I-TGF-β1 binding affinity labeling assay, exhaustively oxidized 7-DHC (a complex mixture of as yet undefined oxysterols) was nearly as potent as cholesterol in increasing 125I-TGF-β1 binding to TβR-I. This finding is currently not understood.
Cholesterol treatment appears to promote localization of TβR-I and TβR-II in lipid rafts/caveolae, presumably by promoting the formation of or stabilizing lipid rafts/caveolae. In the presence of ligand, TβR-I and TβR-II (as Complex II, which is characterized by a low TβR-II/TβR-I binding ratio of TGF-β1) localized in lipid rafts/caveolae undergo rapid degradation, resulting in suppressed TGF-β responsiveness (Huang and Huang, 2005; Chen et al., 2006). Depletion of cholesterol from the plasma membrane may result in decreased formation of, or destabilization of, lipid rafts/caveolae, thereby increasing localization of TβR-I and TβR-II (as Complex I, which is characterized by a high TβR-II/TβR-I binding ratio of TGF-β1) in non-lipid raft microdomains of the plasma membrane (Chen et al., 2006). This results in subsequent clathrin-mediated endocytosis, endosomal signaling and promoted cellular responsiveness (Huang and Huang, 2005; Chen et al., 2006). For example, depletion of cholesterol by β-CD from the plasma membrane results in potentiated TGF-β responsiveness as demonstrated by enhanced PAI-1 expression.
Cholesterol is a major causative agent for atherosclerotic cardiovascular disease. The mechanisms by which cholesterol initiates and/or facilitates atherogenesis have been studied extensively but remain poorly understood. In this communication, we demonstrate that cultured cells treated with cholesterol and the aortic endothelium in ApoE-null mice fed a high cholesterol diet share similar characteristics of suppressed TGF-β responsiveness. Aortic endothelial cells of ApoE-null mice fed a high cholesterol diet exhibit a low TβR-II/TβR-I binding ratio of TGF-β and a low level of P-Smad2 as compared with wild-type mice fed a high cholesterol diet. Notably, treatment of cultured cells with cholesterol does not affect the expression of either TβR-I or TβR-II whereas the aortic endothelium of ApoE-null mice fed a high cholesterol diet exhibit attenuated expression of TβR-II and little change in expression of TβR-I. The decreased expression of TβR-II protein in the aortic endothelium of these mice may be due to the chronic effect of increased cholesterol on vascular cells in the aortic endothelium of these animals. This possibility is supported by the observation that treatment of BAEC cells with 5 or 15 μg/ml cholesterol in medium containing 10% fetal calf serum for 3 days specifically down-regulates the TβR-II protein level without significantly affecting the TβR-I protein level (unpublished results). A strong causal link between atherosclerosis and low TGF-β responsiveness in vascular cells and/or low TGF-β levels in plasma has been demonstrated in several relevant in vivo models (McCaffrey et al., 1997; Grainger et al., 2000; Mallat et al., 2001; Reckless et al., 2001; Robertson et al., 2003; Li et al., 2006). The chronically suppressed TGF-β responsiveness in vascular cells caused by hypercholesterolemia should contribute to the pathogenesis of atherosclerosis.
Acknowledgments
We thank Dr. Steven J. Fliesler and Dr. Xianlin Han for providing oxy-7-DHC and ApoE-null mice, I-Hua Liu for performing Northern blot analysis, Drs. Rebecca Berman, Franklin W. Huang, Frank E. Johnson, and Michael A. Moxley for critical review of the manuscript and Chris Mables for typing the manuscript. This work was supported by U.S.P.H.S. (National Institutes of Health) grants CA38808 (JSH) and AR052578 (SSH).
Contract grant sponsor: U.S.P.H.S. (National Institutes of Health);
Contract grant numbers: CA38808, AR052578.
Literature Cited
- Alberts AW. Discovery, biochemistry and biology of lovastatin. Am J Cardiol. 1988;62:11. doi: 10.1016/0002-9149(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Cheifetz S, Bassols A, Stanley K, Ohta M, Greenberger J, Massague J. Heterodimeric transforming growth factor β. Biological properties and interaction with three types of cell surface receptors. J Biol Chem. 1988;263:10783–10789. [PubMed] [Google Scholar]
- Chen YG, Liu F, Massague J. Mechanism of TGFβ receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-L, Huang SS, Huang JS. Cellular heparan sulfate negatively modulates transforming growth factor-β responsiveness in epithelial cells. J Biol Chem. 2006;281:11506–11514. doi: 10.1074/jbc.M512821200. [DOI] [PubMed] [Google Scholar]
- Coppa A, Mincione G, Lazzereschi D, Ranieri A, Turco A, Lucignano B, Scarpa S, Ragano-Caracciolo M, Colletta G. Restored expression of transforming growth factor β type II receptor in k-ras-transformed thyroid cells, TGF β-resistant, reverts their malignant phenotype. J Cell Physiol. 1997;172:200–208. doi: 10.1002/(SICI)1097-4652(199708)172:2<200::AID-JCP7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Centrella M, Reiss M, Kashgarian M, Wells RG. Betaglycan inhibits TGF-β signaling by preventing type I-type II receptor complex formation. Glycosaminoglycan modifications alter βglycan function. J Biol Chem. 2002;277:823–829. doi: 10.1074/jbc.M105110200. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Holder MG, Winokur TS. Autoinduction of mRNA and protein expression for transforming growth factor-β S in cultured cardiac cells. J Mol Cell Cardiol. 1995;27:805–812. doi: 10.1016/0022-2828(95)90087-x. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Mosedale DE, Metcalfe JC, Bottinger EP. Dietary fat and reduced levels of TGFβ1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J Cell Sci. 2000;113:2355–2361. doi: 10.1242/jcs.113.13.2355. [DOI] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Huang SS, Huang JS. TGF-β control of cell proliferation. J Cell Biochem. 2005;96:447–462. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- Huang SS, Ling TY, Tseng WF, Huang YH, Tang FM, Leal SM, Huang JS. Cellular growth inhibition by IGFBP-3 and TGF-β1 requires LRP-1. FASEB J. 2003;17:2068–2081. doi: 10.1096/fj.03-0256com. [DOI] [PubMed] [Google Scholar]
- Ito T, Williams JD, Fraser DJ, Phillips AO. Hyaluronan regulates transforming growth factor-β1 receptor compartmentalization. J Biol Chem. 2004;279:25326–25332. doi: 10.1074/jbc.M403135200. [DOI] [PubMed] [Google Scholar]
- Kalinina N, Agrotis A, Antropova Y, Ilyinskaya O, Smirnov V, Tararak E, Bobik A. Smad expression in human atherosclerotic lesions: Evidence for impaired TGF-β/Smad signaling in smooth muscle cells of fibrofatty lesions. Arterioscler Thromb Vasc Biol. 2004;24:1391–1396. doi: 10.1161/01.ATV.0000133605.89421.79. [DOI] [PubMed] [Google Scholar]
- Keller RK, Arnold TP, Fliesler SJ. Formation of 7-dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith-Lemli-Opitz syndrome. J Lipid Res. 2004;45:347–355. doi: 10.1194/jlr.M300232-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Harpel PC, Rifkin DB. Lipoprotein (a) inhibits the generation of transforming growth factor β: An endogenous inhibitor of smooth muscle cell migration. J Cell Biol. 1991;113:1439–1445. doi: 10.1083/jcb.113.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letamendia A, Lastres P, Botella LM, Raab U, Langa C, Velasco B, Attisano L, Bernabeu C. Role of endoglin in cellular responses to transforming growth factor-β. A comparative study with betaglycan. J Biol Chem. 1998;272:18891–18895. doi: 10.1074/jbc.273.49.33011. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Li D, Liu Y, Chen J, Velchala N, Amani F, Nemarkommula A, Chen K, Rayaz H, Zhang D, Liu H, Sinha AK, Romeo F, Hermonat PL, Mehta JL. Suppression of atherogenesis by delivery of TGFβ1ACT using adeno-associated virus type 2 in LDLR knockout mice. Biochem Biophys Res Commun. 2006;344:701–707. doi: 10.1016/j.bbrc.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Lund LR, Riccio A, Andreasen PA, Nielsen LS, Kristensen P, Laiho M, Saksela O, Blasi F, Dano K. Transforming growth factor-β is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 1987;6:1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-β signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. [See comment]. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-β family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Consigli S, Du B, Falcone DJ, Sanborn TA, Spokojny AM, Bush HL., Jr. Decreased type II/type I TGF-β receptor ratio in cells derived from human atherosclerotic lesions. Conversion from an antiproliferative to profibrotic response to TGF-β1. J Clin Invest. 1995;96:2667–2675. doi: 10.1172/JCI118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey TA, Du B, Consigli S, Szabo P, Bray PJ, Hartner L, Weksler BB, Sanborn TA, Bergman G, Bush HL., Jr. Genomic instability in the type II TGF-β1 receptor gene in atherosclerotic and restenotic vascular cells. J Clin Invest. 1997;100:2182–2188. doi: 10.1172/JCI119754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney-Francis N, Mizel D, Wong H, Wahl L, Wahl S. TGF-β regulates production of growth factors and TGF-β by human peripheral blood monocytes. Growth Factors. 1990;4:27–35. doi: 10.3109/08977199009011007. [DOI] [PubMed] [Google Scholar]
- Mitchell H, Choudhury A, Pagano RE, Leof EB. Ligand-dependent and -independent transforming growth factor-β receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell. 2004;15:4166–4178. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, ten Dijke P, Heldin CH. TGF-β signaling by Smad proteins. Adv Immunol. 2000;75:115–157. doi: 10.1016/s0065-2776(00)75003-6. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-β signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-β: Structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- Palinski W, Ord VA, Plump AS, Breslow JL, Steinberg D, Witztum JL. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- Park H, Go YM, St. John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem. 1998;273:32304–32311. doi: 10.1074/jbc.273.48.32304. [DOI] [PubMed] [Google Scholar]
- Phipps S, Benyahia F, Ou TT, Barkans J, Robinson DS, Kay AB. Acute allergen-induced airway remodeling in atopic asthma. Am J Respir Cell Mol Biol. 2004;31:626–632. doi: 10.1165/rcmb.2004-0193OC. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Zhang XL, Bitzer M, von Gersdorff G, Bottinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem. 2001;276:6727–6738. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- Reckless J, Rubin EM, Verstuyft JB, Metcalfe JC, Grainger DJ. A common phenotype associated with atherogenesis in diverse mouse models of vascular lipid lesions. J Vasc Res. 2001;38:256–265. doi: 10.1159/000051054. [DOI] [PubMed] [Google Scholar]
- Roberts AB. Molecular and cell biology of TGF-β. Miner Electrolyte Metab. 1998;24:111–119. doi: 10.1159/000057358. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar S, Mahooti-Brooks N, Bensen L, McCarthy TL, Centrella M, Madri JA. Modulation of transforming growth factor β receptor levels on microvascular endothelial cells during in vitro angiogenesis. J Clin Invest. 1996;97:1436–1446. doi: 10.1172/JCI118565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Geigenmueller S, Voelker W, Seiler P, Buddecke E. Exogenous nitric oxide causes overexpression of TGF-β1 and overproduction of extracellular matrix in human coronary smooth muscle cells. Cardiovasc Res. 2003;58:671–678. doi: 10.1016/s0008-6363(03)00322-5. [DOI] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-β 1 associates to fibroblast extracellular matrix via latent TGF-β binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor β 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- Wakefield L, Kim SJ, Glick A, Winokur T, Colletta A, Sporn M. Regulation of transforming growth factor-β subtypes by members of the steroid hormone superfamily. J Cell Sci Suppl. 1990;13:139–148. doi: 10.1242/jcs.1990.supplement_13.13. [DOI] [PubMed] [Google Scholar]
- Wang MM, Sugar IP, Chong PL. Role of the sterol superlattice in the partitioning of the antifungal drug nystatin into lipid membranes. Biochemistry. 1998;37:11797–11805. doi: 10.1021/bi980290k. [DOI] [PubMed] [Google Scholar]
- Wang J, Megha, London E. Relationship between sterol/steroid structure and participation in ordered lipid domains (lipid rafts): Implications for lipid raft structure and function. Biochemistry. 2004;43:1010–1018. doi: 10.1021/bi035696y. [DOI] [PubMed] [Google Scholar]
- Yao D, Ehrlich M, Henis YI, Leof EB. Transforming growth factor-β receptors interact with AP2 by direct binding to β2 subunit. Mol Biol Cell. 2002;13:4001–4012. doi: 10.1091/mbc.02-07-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JN, Tsai MY, Hegland J, Hunninghake DB. Effects of fluvastatin (XU 62-320), an HMG-CoA reductase inhibitor, on the distribution and composition of low density lipoprotein subspecies in humans. Atherosclerosis. 1991;87:147–157. doi: 10.1016/0021-9150(91)90017-w. [DOI] [PubMed] [Google Scholar]