Hypoplastic Left Heart Syndrome: Current Considerations and Expectations (original) (raw)
. Author manuscript; available in PMC: 2018 Aug 27.
Published in final edited form as: J Am Coll Cardiol. 2012 Jan 3;59(1 Suppl):S1–42. doi: 10.1016/j.jacc.2011.09.022
Abstract
In the recent era, no congenital heart defect has undergone a more dramatic change in diagnostic approach, management, and outcomes than hypoplastic left heart syndrome (HLHS). During this time, survival to the age of 5 years (including Fontan) has ranged from 50% to 69%, but current expectations are that 70% of newborns born today with HLHS may reach adulthood. Although the 3-stage treatment approach to HLHS is now well founded, there is significant variation among centers. In this white paper, we present the current state of the art in our understanding and treatment of HLHS during the stages of care: 1) pre-Stage I: fetal and neonatal assessment and management; 2) Stage I: perioperative care, interstage monitoring, and management strategies; 3) Stage II: surgeries; 4) Stage III: Fontan surgery; and 5) long-term follow-up. Issues surrounding the genetics of HLHS, developmental outcomes, and quality of life are addressed in addition to the many other considerations for caring for this group of complex patients.
Keywords: congenital heart defects, Fontan procedure, Glenn procedure, hypoplastic left heart syndrome, Norwood procedure
In the recent era, no congenital heart defect has undergone a more dramatic change in diagnostic approach, management, and outcomes than hypoplastic left heart syndrome (HLHS). Although just over 30 years ago, comfort care was the only option, there are now a number of therapeutic options available for families, though there continues to be a debate as to the optimal treatment approach. Although the 3-stage treatment approach to HLHS is now well founded, there is significant variation among centers (1). The goals of Stage I palliation are to relieve systemic outflow tract obstruction, provide nonrestrictive coronary blood flow and adequate pulmonary blood flow, and create a nonrestrictive atrial septal defect. The second stage eliminates the existing, high-pressure, arterial or ventricular source of pulmonary blood flow and connects the superior vena cava (SVC) with the pulmonary artery. Conversion to a bidirectional superior cavopulmo- nary shunt results in reduced pressure and volume work for the single ventricle, improved circulatory efficiency because the source of pulmonary blood flow is now more desaturated venous blood rather than an arteriovenous admixture, generally higher arterial saturation, and growth potential. The third stage directs the remaining desaturated blood returning from the lower body to the pulmonary arteries.
Despite the effort devoted to this condition, there remains a lack of definitive evidence of cause and agreement on many management issues. In this white paper, we present the current state of the art in our understanding and treatment of HLHS during the stages of care: 1) pre-Stage I: fetal and neonatal assessment and management; 2) Stage I: perioperative care, interstage monitoring, and management strategies; 3) Stage II: surgeries; 4) Stage III: Fontan surgery; and 5) long-term follow-up. Issues surrounding the genetics of HLHS, developmental outcomes and quality of life will be addressed.
Pre-Stage I Considerations
Prenatal Diagnosis and Outcome
Possible mechanisms of development of HLHS.
The ability to identify and follow the fetus with HLHS with fetal echocardiography has shown the progressive nature of HLHS and highlighted the importance of abnormal flow patterns in the mechanisms of development of HLHS. The structures are all generally present albeit severely hypoplastic, or may be atretic, and at least some forms of HLHS occur relatively late in development after embryogenesis. Although fetal demise has been reported, most pregnancies reach term gestation with relatively normal growth and development of other organ systems although with an increased prevalence of central nervous system abnormalities (2, 3).
There are likely several inciting mechanisms resulting in the underdevelopment of the left ventricle (LV). In fetal life, the LV is predominantly filled by flow through the foramen ovale and any perturbation of flow into or out of the LV may result in growth impairment. It has been observed that the fetus with HLHS has a smaller foramen ovale than the fetus with a normal heart (4). In addition, there is a known association between HLHS and an anatomic abnormality of the atrial septum, namely posterior deviation of the septum primum (5). In this anomaly, the superior edge of the septum primum is deviated posterior and leftward, attaching anomalously to the left atrial wall, restricting atrial level shunting. An intact atrial septum in association with HLHS has also been observed in utero (6); often, there is a small communication early in gestation that closes over time. This diagnosis carries a very poor prognosis.
In addition to atrial septal anomalies, HLHS may result primarily from abnormal development of the cardiac valves or the left ventricle itself, caused by an intrinsic genetic abnormality or cause. The ventricle often appears dilated and echo bright with poor systolic function. Endocardial fibroelastosis, a poorly understood phenomenon whereby the endocardium of the LV becomes fibrotic, is often observed (7). Fetal restrictive cardiomyopathy is present with endocardial fibroelastosis, resulting in elevation of LV end-diastolic and left atrial pressures, and subsequent diminution of flow through the foramen ovale into the left heart. Typically, the LV initially appears dilated, poorly contractile, and larger than the right ventricle (RV), and later in gestation, hypoplastic in comparison to the normally growing RV (Fig. 1) (8, 9). In some forms of the disease, there is an inherent abnormality of the mitral (parachute, arcade) and/or the aortic valve (bicuspid or unicuspid), and multiple animal models have produced left ventricular hypoplasia as a result of the introduction of left-sided obstruction (inflow or outflow) (10, 11).
Figure 1. Fetal Echocardiograms.
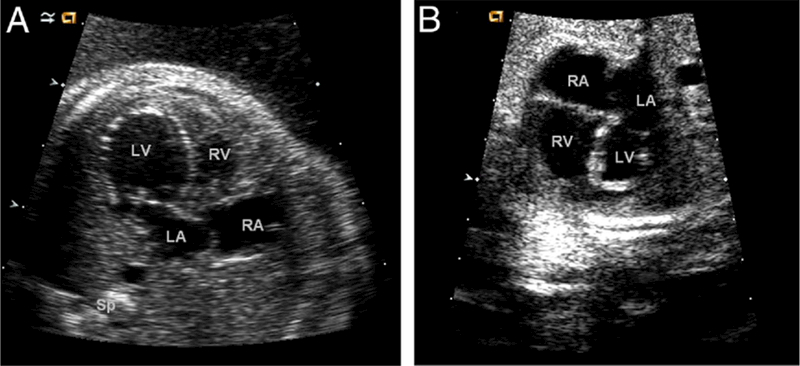
(A) Four-chamber view of a fetal echocardiogram at 20 weeks’ gestation demonstrates a dilated left ventricle with echo bright endocardium suggestive of endocardial fibroelastosis. The position of the atrial septum suggests abnormal left atrial to right atrial shunting in utero. (B) Four-chamber view of a fetal echocardiogram in the same fetus imaged at 33 weeks’ gestation demonstrates that the left ventricle has become hypoplastic. The echo bright endocardium is even more evident. LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle; Sp = spine.
Fetal Flow Patterns in HLHS
The normal fetal circulation allows both ventricles to contribute to the work of supplying blood for the developing fetus and permits immediate postnatal adaptation to terrestrial existence. As a consequence, there are important communications between the pulmonary and systemic circulations, including the foramen ovale and the ductus arteriosus. As a consequence of these communications, if one ventricle should be hypoplastic, the contralateral ventricle can compensate, permitting essential normal growth and development of the remaining organ systems.
In HLHS, in utero shunting across the atrial septum is reversed from the normal pattern. The minimal blood flow that enters the left atrium from the pulmonary veins must predominantly cross the atrial septum into the right atrium. The mixture of pulmonary and systemic venous blood then passes from the RV into the pulmonary artery. A small amount of blood enters the branch pulmonary arteries, whereas the majority goes through the ductus arteriosus. In the most extreme form of HLHS with aortic atresia, the myocardial and cerebral circulations are supplied solely by the ductus in a retrograde fashion. The lower body blood flow is also provided by the ductus arteriosus. This “adaptation” allows for hemodynamic stability during fetal life. However, flow inefficiencies are poorly tolerated in the fetus with HLHS. For example, severe tricuspid regurgitation results in volume overload and systemic venous hypertension, and may eventually cause hydrops fetalis (12). In rare forms of HLHS, there is severe mitral regurgitation with a markedly dilated left atrium. In these cases, the LV may actually be enlarged, though noncontractile, and may impact RV performance in utero. Finally, ductal constriction may occur in cases of maternal exposure to arachadonic acid inhibitors such as indomethacin or aspirin; this negatively affects right ventricular performance and systemic perfusion in these patients.
Impact of Fetal Diagnosis on Outcome
Infants with HLHS present in different ways. Many infants are now diagnosed prenatally and are physiologically stable at presentation; some infants are diagnosed due to a murmur or cyanosis that is discovered in the newborn nursery prior to discharge; and still other infants are diagnosed only after becoming acutely and critically ill following ductal closure.
There is conflicting data regarding the impact of fetal diagnosis on surgical outcome in neonates with HLHS. The majority of reports have concluded that mortality is not reduced if a prenatal diagnosis is made (13–15), though some have reported improved survival (16). In most cases, the inherent risks associated with the Norwood procedure likely outweigh the benefit of prenatal recognition of the disease. Though mortality may not be significantly altered, there is an improvement in morbidity when HLHS is diagnosed before birth. Infants with a prenatal diagnosis of HLHS have overall better pre-operative condition, including lower lactate levels (17), and better renal function (13). Neurological events that carry a poor prognosis, such as post-operative seizures, occur in fewer patients with a prenatal diagnosis of HLHS (13), and this is likely due to the rapid initiation of prostaglandin therapy and the prevention of cardiovascular collapse that occurs with ductal constriction or closure. As expected, a prenatal diagnosis of HLHS does not protect against neurodevelopmental abnormalities. Microcephaly and impaired somatic growth may be more prevalent in this population, though conflicting studies exist (18, 19).
Prenatal recognition of disease allows families to prepare for a child with a life-threatening defect by meeting with the multidisciplinary team that will care for their newborn and learning about the short- and long-term prognosis of the disease. Counseling also provides an opportunity to discuss the option of pregnancy termination or comfort care after birth. Genetic testing and evaluation for extracardiac anomalies has become imperative for prognosis. Genetic syndromes in which HLHS has been seen include Turner syndrome, trisomy 13, trisomy 18, Holt-Oram, Smith- Lemli-Opitz, partial trisomy 9, Jacobsen syndrome, and others (20). Extracardiac anomalies associated with HLHS include agenesis of the corpus callosum, diaphragmatic hernia, and omphalocele, among others (21). It is well recognized that genetic disorders and extracardiac anomalies in association with a diagnosis of HLHS carry a worse prognosis (22). Finally, prenatal diagnosis allows for potential fetal intervention. In select cases, prenatal balloon dilation of the aortic valve has been associated with decreased progression of left ventricular hypoplasia (23). Fetal atrial septostomy to provide an adequate atrial communication in fetuses with HLHS and intact atrial septum may also improve prognosis for this particularly high-risk subset of patients (23).
In the high-risk fetus with unfavorable anatomy, consideration for fetal listing for heart transplantation may be offered, and increases the potential window of opportunity for a donor organ to become available (24). In the current era of improved Stage I palliation, this option is rarely pursued.
Pre-Operative Assessment and Management
HLHS and related functional single RV conditions remain the highest risk and costliest group of lesions among the commonly occurring congenital heart defects (25). Regardless of presentation, infants with HLHS require careful management during the interim period between diagnosis and surgery. The goals of pre-operative management include clinical stabilization, complete definition of cardiac anatomy, recognition of noncardiac diagnoses, and family education.
Pre-operative medical management varies tremendously between institutions and providers (1,26–29). Neonates with HLHS require continuous intravenous infusion of prostaglandin E1 (PGE) to maintain ductal patency for adequate systemic blood flow. Infants who present in cardiac shock need immediate, effective resuscitation and often require intubation, volume expansion, and inotropic support. For all neonates, pulmonary vascular resistance (PVR) falls following birth, and for neonates with HLHS, ensuring adequate systemic perfusion (i.e., balancing the systemic and pulmonary circulations) becomes crucial. Some institutions use medical management including intubation and hypoventilation or inhaled nitrogen or carbon dioxide to increase PVR and redirect cardiac output to the body (30). Other management strategies seek to increase overall cardiac output via inotropic support, whereas some institutions pursue early surgical intervention prior to significant decrease in PVR.
Transthoracic echocardiography is used to determine patency of the ductus arteriosus, presence of an adequate atrial level communication, myocardial function, and degree of tricuspid regurgitation. Treatment and stabilization of secondary organ system involvement and impairment requires prompt diagnosis and treatment to optimize the pre-operative status.
The patient with HLHS and an intact or nearly intact atrial septum presents a particularly challenging clinical scenario. The decision to intervene using transcatheter versus surgical techniques varies by institution. In either procedure, the primary goal is to reduce the obstruction at the atrial level and then allow for recovery before performing the complete first-stage palliation, as banding is usually not well tolerated in the critical newborn.
These important pre-operative days allow for family education, which is particularly important if the cardiac lesion was not diagnosed prenatally. Although most centers counsel and encourage a staged palliation approach (31), some centers focus on primary transplantation (32). In recent years, and with significant improvements in outcomes, controversy has developed as to whether comfort care should still be offered as a treatment option (33, 34).
In centers located at altitude, pre-operative issues (e.g., pulmonary overcirculation) and management are similar. In the long term, unpublished and anecdotal evidence suggests similar outcomes and no significant correlation between altitude and PVR, pulmonary artery pressure, or trans- pulmonary gradient at pre-bidirectional cavopulmonary anastomosis and pre-Fontan.
Neonatal Treatment Strategies
Three basic strategies for the neonatal management of HLHS have evolved over the last 4 decades: surgical palliation with a Norwood procedure, hybrid palliation with surgical bilateral pulmonary artery banding and transcatheter ductal stenting, and orthotopic transplantation. Each strategy has a common set of objectives: provide unobstructed systemic cardiac output, a controlled source of pulmonary blood flow, a reliable source of coronary blood flow, and unobstructed egress of pulmonary venous drainage.
In the average-risk newborn, the optimal timing for surgical or hybrid palliation is not known and may be center specific. Although after 30 days, risks increase, the physiological parameters of pulmonary vascular resistance, ventricular performance, and atrioventricular valve competency are the determinants of palliative feasibility and success.
Norwood Procedure
Theoretical surgical strategies have long been put forth for palliation of HLHS, and several unsuccessful attempts occurred during the 1970s, but it was Norwood and colleagues who achieved the first real success in the 1980s (35–46). In addition to the formidable surgical obstacles to be overcome, this early success was codependent on the simultaneous developments in neonatal management. Among these developments was the use of PGE for maintenance of ductal patency that permitted resuscitation of profoundly ill neonates prior to complex surgery (47–57).
The Norwood procedure with modified Blalock-Taussig shunt.
In the classic Norwood procedure, pulmonary blood flow is provided by a Blalock-Taussig (BT) shunt, which directs blood from the innominate or subclavian artery to the pulmonary arteries via a polytetrafluoroethylene tube. Due to the lower PVR relative to systemic vascular resistance, there is continuous forward flow into the BT shunt, not only throughout systole, but also during diastole. This results in lower systemic diastolic blood pressure and “coronary steal” that may result in decreased myocardial perfusion (58–60). Utilizing nuclear imaging at rest and after administration of adenosine, coronary arterial flow and oxygen delivery have been shown to be significantly decreased in patients after the Norwood procedure compared with patients after anatomic repair of a congenital heart defect. It has been suggested that this relative coronary arterial insufficiency secondary to the coronary steal that occurs with a BT shunt may play an important role in the significant mortality of the palliated patient (61–63).
The Norwood procedure with RV-to-pulmonary artery conduit.
Early in the development of the operation that bears his name, Dr. William Norwood attempted to use an RV-to-pulmonary-artery conduit/shunt (RV-PA) to supply pulmonary artery blood flow (44). Although this source of pulmonary blood flow was abandoned in favor of the BT shunt, decades later, several authors have resurrected this technique in an attempt to address the issue of coronary artery steal. The RV-PA has the advantage of eliminating the diastolic runoff and coronary artery steal (46,59,64,65), but the ventriculotomy adds the risks of direct myocardial injury and arrhythmias (66).
A number of historically controlled case series have reported a decrease in hospital mortality with the RV-PA compared with the BT shunt (46,67–69). Sano et al. (46) reported an 89% hospital survival with the RV-PA compared with 53% with the BT shunt, and similar results were reported by Pizarro et al. (69) (BT shunt 70%, RV-PA 92%) and Mair et al. (68) (BT shunt 72%, PV-PA conduit 93%). Other recent, nonrandomized studies have shown no improvement in hospital survival comparing the 2 shunts (63,67,70,71).
SVR (Single Ventricle Reconstruction) Trial.
In an attempt to resolve the question of which Norwood modification is superior, the SVR trial was undertaken (19). The trial, a Pediatric Heart Network, 15-center, National Institutes of Health-sponsored, randomized trial compared the BT shunt and RV-PA. Patients with single RV malformations undergoing a Norwood procedure were randomized to receive either a BT shunt or RV-PA. The primary endpoint was death or transplantation at 1 year. Secondary endpoints included hospital course, RV function by echo, pulmonary artery size by angiography, unintended cardiovascular interventions, and serious adverse events and complications between shunts. Between May 2005 and July 2008, 555 newborns were enrolled.
The RV-PA was found to be superior to the BT shunt (26% vs. 36%, p = 0.01) for the primary endpoint of death or transplant at 12 months. All patients enrolled were followed annually until study close out, with a final mean follow-up of 32 ± 11 months. Although there was a significantly higher risk of mortality in the BT shunt at 12-month endpoint, this was no longer significant with the longer follow-up.
The need for cardiopulmonary resuscitation during the Norwood hospitalization was greater in the BT shunt group (20% vs. 13%, p = 0.04), whereas unintended cardiovascular interventions on the shunt or neoaorta were more common in the RV-PA group (92 vs. 70 per 100 infants, p = 0.003). Echo measures of RV end-diastolic volume and ejection fraction were both superior for the RV-PA group up to Stage II, but had equalized by 14 months. Pulmonary artery size was larger in the BT shunt group (169 vs. 145, p = 0.009). The complication rate was higher in the RV-PA group (5.3 vs. 4.7 complications per infant, p = 0.002), although the percentage of infants with at least 1 complication was the same in both groups at 91%.
Post-Operative Management Strategies
The newborn with univentricular anatomy has a high risk of shock before and after an initial palliative surgical procedure. The syndrome of inadequate cardiac output, characterized by reduced systemic oxygen delivery, high systemic oxygen extraction, and anaerobic end-organ dysfunction, is a stereotypical finding following neonatal cardiac surgery. Myocardial edema and post-ischemic systolic and diastolic dysfunction result in reduced stroke volume, and the metabolic response to trauma and inflammatory stimulus from cardiopulmonary bypass result in increased oxygen demand. The superimposition of these processes results in a high risk of shock (inadequate oxygen supply/demand economy) in the first 6 to 12 h after surgery following both complete repair of 2 ventricle defects and palliation of single-ventricle lesions (72–75). The newborn with HLHS has additional vulnerabilities: total ventricular mass—the source for mechanical circulatory energy—is reduced; the parallel anatomy of pulmonary and systemic circulations results in obligate desaturation of arterial blood, and the need exists for double the normal total cardiac output from a single ventricle. These superimposed vulnerabilities are implicated in the high mortality risk and are the focus of the strategies to mitigate that risk.
Optimizing oxygen delivery.
Achieving normal systemic oxygen delivery at the lowest total cardiac output requires an arteriovenous oxygen saturation difference of 20% to 25% and a pulmonary to systemic blood flow ratio (Qp/Qs) close to 1.0 (76–80). These conditions are not reliably met using standard monitoring; because univentricular output is apportioned by the balance of system and pulmonary resistances, arterial blood pressure and saturation will be relatively unchanged as systemic vascular resistance rises or falls (78, 81). Standard perioperative hemodynamic monitoring provides inadequate warning of circulatory failure, resulting in a high rate of cardiac arrest, cardiopulmonary resuscitation, extracorporeal circulatory support, and organ dysfunction in this population. In addition to improved operative techniques and perfusion strategies, application of venous oxygen (SvO2) monitoring with invasive devices or near- infrared spectroscopy, and pharmacological control of vascular resistance in the postoperative period have been associated with reduced operative mortality to <10% (79,80,82,83).
Balancing the circulation: managing pulmonary vascular resistance.
Early efforts to address circulatory failure recognized that pulmonary overcirculation would result in an increase inarterial oxygen saturation (SaO2) if systemic blood flow were maintained. Because venous oxygen measures were difficult to obtain, the variability in systemic blood flow was a theoretic concept (84) not visible at the bedside, and clinical management focused on preventing a rise in SaO2. Manipulation of inspired gas mixtures to control PVR, particularly inspired carbon dioxide (CO2), was reported to increase stability after Norwood palliation for HLHS (85–87). Both reduced fraction of inspired O2 and inspired CO2 will acutely lower SaO2, but only hyper- carbia will improve systemic oxygen delivery and cerebral oxygenation (30,88,89).
Balancing the circulation: targeting systemic venous oxygenation.
After reports of the usefulness of intermittent measures of SvO2 (76), the use of continuous venous oximetry via an oximetric catheter placed in the SVC at the time of surgery has become more common. Routine use of venous oximetry during the first 48 h after surgery (Fig. 2) has been associated with improved early and intermediate survival, fewer complications, and improved neurodevelopment at 4 to 6 years of age, particularly when SvO2 is >50% (78,80,90). More recently, application of near-infrared spectroscopy as a measure of regional venous-weighted oxygen saturation can provide a continuous and non- invasive estimate of SvO2 (91–93). Strategies measuring regional venous-weighted oxygen saturation in both cerebral and noncerebral regions provide better estimates of SvO2 and stronger relationship to outcome (93–95).
Figure 2. Complication Risk Associated With Superior Venous Oximetry.
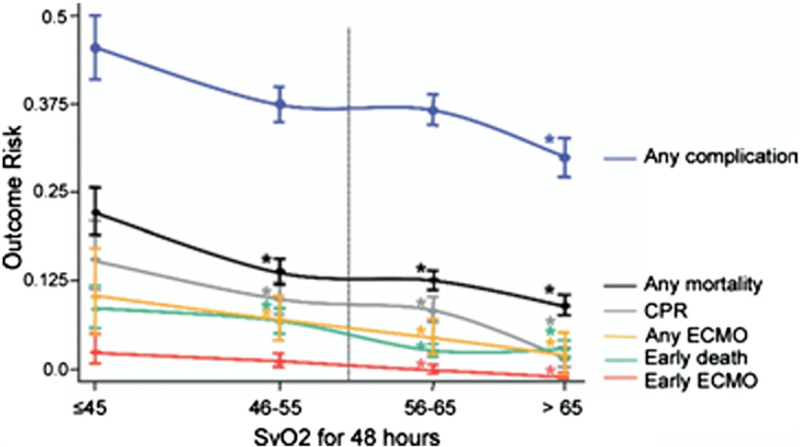
Risk of complication according to post-operative superior venous oximetry saturation (SvO2) assessed hourly during first 48 h. *Significant difference from risk at lower SvO2 in time-series regression. CPR = cardiopulmonary resuscitation; ECMO = extracorporeal membrane oxygenator. Reprinted with permission from Tweddell et al. (80).
Early experience with venous oximetry demonstrated the occurrence of life-threatening falls in SvO2 without significant perturbations in SaO2, blood pressure, or heart rate (Fig. 3). Episodes of falling SvO2, and thus reduced systemic oxygen delivery, were ineffectively managed by ventilator and medical gas manipulation, but rather reversed with additional anesthetic and inotropic support. These observations led to strategies that targeted measures of oxygen delivery and control of systemic vascular resistance to avoid circulatory collapse.
Figure 3. Hemodynamic Monitoring in the Immediate Post-Operative Period.
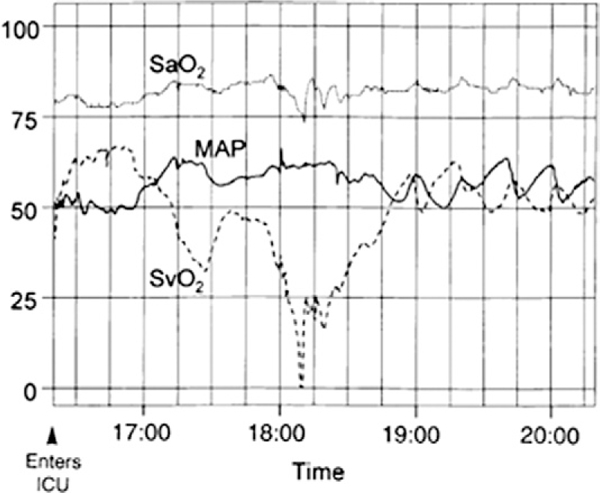
Multichannel recording of the arterial saturation (SaO2), mean arterial blood pressure (MAP) and superior vena cava saturation (SvO2) during the first 4 h after the Norwood procedure. Two episodes of decreased SvO2 were identified. Fall in SvO2 was mirrored by changes in MAP. Fall in SvO2 was initially mirrored by changes in SaO2, but with a marked decline in SvO2, the SaO2 decreased as well. These changes indicate that acute changes in SvO2 can occur and are not reliably identified by changes in SaO2 or MAP. Reprinted with permission from Tweddell et al. (73).
Balancing the circulation: managing systemic vascular resistance.
Alpha-adrenergic blockade has been the afterload-reducing agent most extensively studied in this population. Unlike nitrovasodilators, phenoxybenzamine and phentolamine directly block the systemic vasoconstriction that results from increased endogenous or exogenous catecholamines. Phenoxybenzamine has been shown to be successful in attenuating the expected low cardiac output syndrome (Fig. 4) (73–74,96) and to increase SvO2 and reduce Qp/Qs over a wide range of SaO2 and blood pressure, reducing the vulnerability to runaway vasoconstriction that precedes cardiovascular collapse (81). Effective control of systemic vascular resistance has been associated with reduced incidence of early circulatory collapse (73,97,98).
Figure 4. Superior Venous Saturation During the First 48 h After Norwood Procedure.
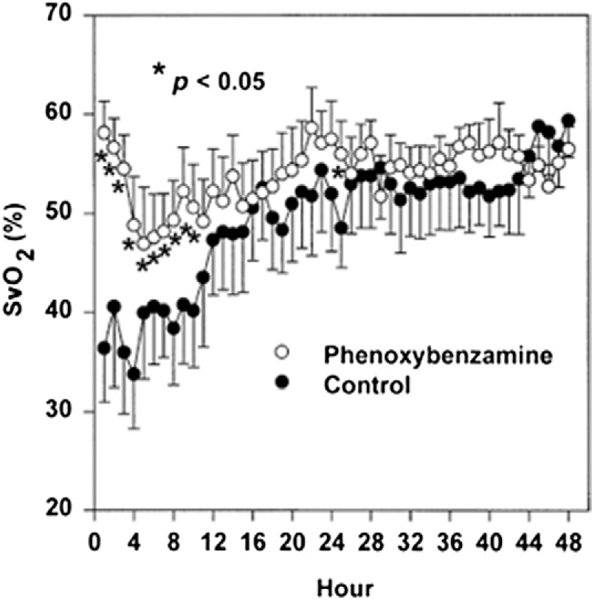
The SvO2 was significantly higher during hours 1 to 10 in infants treated with phenoxybenzamine (0.25 mg · kg at commencement of cardiopulmonary bypass + selective use of continuous infusion 0.25 · mg · kg · day) than in those treated with milrinone (load 50 _μ_g · kg · min prior to separation from bypass + continuous infusion 0.5 _μ_g · kg · min after surgery). Reprinted with permission from Tweddell et al. (73).
Adjunctive therapies.
Maintenance of systemic oxygen delivery is dependent on optimizing cardiac output and arterial oxygen content. Optimal cardiac output requires attention to volume status (preload), vascular resistance (afterload), heart rate, rhythm, and myocardial contractility, whereas arterial oxygen content is predominately dependent on hemoglobin and arterial saturation. In addition to venous oximetry or near-infrared spectroscopy and pulse oximetry, central venous pressure (CVP) and invasive arterial blood pressure monitoring, electrocardiography, capnography, urine output, and biochemical assessment of perfusion should be part of the routine perioperative monitoring. Adjunctive medical therapy may include inotropic agents and/or vasoactive medications. Sedative-analgesic medications can be used for reduction in systemic vascular resistance, but also have the advantage of reducing metabolic demands, allowing for better matching of oxygen consumption to oxygen delivery. In the presence of strategies that prioritize afterload reduction to balance the circulation, ventilator management can be targeted at preventing atelectasis while avoiding hypocarbia, inspired oxygen rather than promoting disproportionate hypoxia, and avoiding excessive work of breathing. Delayed sternal closure is commonly employed to reduce the risk of tamponade physiology, and has been associated with less circulatory collapse and a reduced need for mechanical circulatory support (99). Finally, extracorporeal membrane oxygenator (ECMO) support may be needed for infants with inadequate systemic oxygen delivery or to rescue infants with acute cardiovascular collapse that most commonly occurs from cardiogenic shock or acute shunt obstruction. In a multicenter randomized control trial of infants who had Stage I palliation, approximately 75% had delayed sternal closure, 10% were placed on ECMO during the postoperative period, and 15% required cardiopulmonary resuscitation (19).
Outcomes and Complications
Outcomes have improved over the last 3 decades, likely due to broad improvements in perioperative care (79). Singlecenter retrospective analyses have identified factors in perioperative care and technical modifications associated with improved outcomes, and several recent large series report survival rates between 74% and 93% (13,22,79,100). The Society of Thoracic Surgeons Congenital Heart Surgery Database has shown an improvement in hospital survival from 68.6% of 303 reported cases in 2002 to 81.4% of 2,320 cases in 2009 (Table 1). The post-operative period is significant for morbidity from both cardiac and noncardiac etiologies, often related to decreased cardiac output. Multiple studies report the need for chest compressions in 10% to 17% of patients and the emergent use of ECMO support in 7% to 10% (19,22,80,101). Arrhythmias, most commonly supraventricular tachycardia, occur in 14% to 15% of patients, and junctional ectopic tachycardia, ventricular tachycardia, and complete heart block have been reported (80, 101).
Table 1.
Hospital Discharge Mortality for Patients Undergoing Stage I Palliation, 2002–2009
| Year | Patients Undergoing Stage 1 Palliation, n | Deaths Prior to Discharge, n | Discharge Survival, % |
|---|---|---|---|
| 2002 | 303 | 95 | 68.60 |
| 2003 | 391 | 119 | 69.60 |
| 2004 | 297 | 75 | 74.70 |
| 2005 | 658 | 140 | 78.70 |
| 2006 | 1,155 | 234 | 79.70 |
| 2007 | 1,535 | 276 | 82.00 |
| 2008 | 1,879 | 334 | 82.20 |
| 2009 | 2,320 | 432 | 81.40 |
Bleeding with coagulopathy and product replacement are essentially universal problems following the Norwood procedure, yet little data exist to characterize the bleeding, transfusion use or specific coagulation abnormalities in these patients. A recent study of patients following the Norwood procedure found chest tube output for the first 24 h was 20.9 ± 21.9 ml/kg, and the patients received 14.5 ± 20.2 ml/kg of red cell transfusions (102). Data for the use of component therapy, recombinant factor VIIa, or topical hemostatic agents for correction of coagulopathy and control of bleeding in this group of patients is lacking.
The use of mechanical ventilation is required, on average, for 3 to 7 days (80, 101). Prolonged chylothorax and the use of supplemental oxygen for treatment of excessive cyanosis not due to inadequate pulmonary blood flow are also not uncommon post-operative issues and affect mechanical ventilation duration.
Infection due to impaired cardiac output, cyanosis, prolonged intensive care unit stay, central venous access, and invasive monitoring complicates approximately 10% of patients and was the sixth leading cause of death following Stage I palliation (76,101,103).
The most common neurological abnormality identified in the post-operative period is seizures and is associated with neurodevelopmental delay (101, 104). The incidence of seizures varies depending on whether clinical or electroencephalogram-identified seizures are reported. Clinical seizures occur in up to 4% to 17%, and electroencephalogram seizures were identified in 22% of postoperative Norwood patients (80,105–107). Similarly, identification of other central nervous system injury depends on the method of detection. Stroke and intracranial hemorrhage occur at a rate of approximately 5%, and the risk extends outside the perioperative period due to the ongoing, obligatory intracardiac shunt (80, 101). With more sophisticated imaging techniques, the identification of ischemic lesions increases. Pre- and post-operative magnetic resonance imaging (MRI) scanning has detected ischemic events in >20% of patients with HLHS undergoing the Norwood procedure (108, 109). Phrenic nerve injury has an incidence of <5% (101), whereas recurrent laryngeal nerve injury as documented by vocal cord paralysis on laryngoscopy has been reported in 8% to 9% of patients (101,110,111).
Renal dysfunction (defined as an elevation of creatinine) has been reported in up to 13% of patients during the post-operative period, and oliguria with hyperkalemia in 2.5% (80, 101). Although peritoneal dialysis is used by some groups in as many as one-quarter of their patients to remove excess water in the absence of the usual indications for dialysis, one large reported experience used dialysis in <2% of patients (80,101,112).
Biochemical evidence of hepatic dysfunction, such as elevation of transaminases and even hepatic cellular necrosis, has been reported in patients with HLHS, but these appear to be rare and resolve with improvement in cardiac output (101, 113). The incidence of necrotizing enterocolitis varies from 1% to 18% (101, 103), and the spectrum is broad. Feeding difficulties are common (gastroesophageal reflux has been reported in up to 9% of patients) and add to the length of stay (103). The use of nasogastric and gastrostomy tubes for feeding is used in up to one-quarter of patients in some series (103, 114).
Late complications can be defined as those that occur after hospital discharge and prior to Stage II palliation and are commonly anatomic lesions initially addressed at the time of Stage I palliation. In a series of 122 postmortem evaluations, the mechanism of death was associated with residual lesions in approximately three-quarters of the patients. Most commonly, impairment of coronary artery perfusion, excessive pulmonary blood flow, obstruction of pulmonary arterial blood flow, and neoaortic arch obstruction were found (76). Aortic arch obstruction is not well tolerated following the Norwood procedure, and most centers have a low threshold for transcatheter interventions to relieve residual obstruction.
Progressive decline in function with or without the development of tricuspid valve insufficiency occurs in a subset of patients. It is conceivable that this is due in some cases to coronary insufficiency, whether due to congenital coronary anomalies or as a consequence of an obstructive connection to the native ascending aorta. Recurrent or residual arch obstruction has been reported in up to 33% of Fontan survivors in some series and may be technique and materials dependent (115–117). As a cause of interstage death, arch obstruction is associated with poor weight gain and decreased ventricular function (76,114,118). Successful interventional catheterization may ameliorate the impact of recurrent arch obstruction (119, 120). Excessive cyanosis is also common and may be due to shunt obstruction, branch pulmonary artery stenosis, or rarely, a restrictive atrial communication (114, 121). If these late complications are detected in a timely manner during the interstage period, morbidity and mortality can be reduced significantly (114).
Hybrid (Combination Surgery/Interventional Catheterization)
In 1992, Gibbs and colleagues proposed palliating a newborn with HLHS using percutaneous patent ductus arteriosus (PDA) stent implantation and surgical bilateral pulmonary artery banding without cardiopulmonary bypass (122). These and subsequent efforts by Ruiz and others (123, 124) had poor outcomes. Over the last decade, the approach has seen renewed interest, improved results, and as a result, greater though nonuniform adoption. In those who have not adopted the concept as standard, some still employ the technique as a “rescue” procedure for high-risk HLHS and single-ventricle patients or as a bridge to heart transplant in infants with HLHS (125, 126).
Galantowicz and Cheatham with one of the largest U.S. experiences to date have settled on the following approach: 1) placement of surgical bilateral pulmonary artery bands via a small median sternotomy off cardiopulmonary bypass, and PDA stent delivery through a surgically placed sheath in the main pulmonary artery above the pulmonary valve; and 2) subsequent balloon atrial septostomy in a separate procedure 1 to 2 weeks later. The delay allows the left atrium to enlarge and permits the use of a larger balloon, which has decreased the need for repeat septostomy. Only retrograde aortic arch obstruction with the PDA fully open is considered a contraindication to the hybrid Stage I palliation. In attempting to predict which patients are at risk for retrograde aortic arch obstruction, one echo study using showed a tendency for the aortic root to be smaller, the angle between the aortic isthmus and PDA to be larger, and retrograde aortic arch Doppler velocities to be higher (127). In this scenario, obstruction of the retrograde orifice by stent struts can lead to coronary insufficiency. To avoid hemodynamic issues related to retrograde obstruction, one center has advocated the use of the “reverse BT shunt” at the time of hybrid palliation to protect coronary blood flow, but this is not universally applied in hybrid palliation (128, 129). A completely percutaneous Stage I procedure remains elusive as the technology for safe and effective internal pulmonary artery bands is not yet available.
In Europe, Akintuerk et al. reported an overall actuarial survival after hybrid palliation of 83% with a 21% combined mortality for patients through Stage II repair (130–133). In the current era, Galantowicz et al. have results similar to those reported by Akintuerk et al. (134–139). A recent multicenter study of 7 institutions examined all forms of hybrid procedures performed. Of the 128 procedures, single-ventricle circulation was present in 60% of the procedures. The most common hybrid intervention was PDA stent placement, accounting for 55 of 128 (43%) procedures, the majority of which (87%) were performed at the same time as surgical banding of the branch pulmonary arteries. Sixteen adverse events occurred in 15 of 128 (12%) procedures. The only major or catastrophic adverse event in a patient with HLHS was in a 7-day-old infant who required multiple cardioversions for atrial flutter and supraventricular tachycardia during the procedure. Although the study size was relatively small, the study suggests HLHS patients may have a lower incidence of adverse events if the procedure is performed using a direct approach with surgical exposure rather than a percutaneous approach. The impact that the hybrid approach will have on neurodevelopmental outcomes remains unanswered.
Transplantation
The use of orthotopic transplantation for initial palliation in neonates with HLHS was introduced by Leonard Bailey in the mid 1980s after development of the model in laboratory animals (140). Infants and children waiting for hearts in the United States have the highest waitlist mortality of all solid organ recipients (17%) and has increased progressively over the past 2 decades (141). Though a preferred therapeutic modality in a few centers (32, 142), given that waiting times for neonates in most regions approach several months and Stage 1 reconstructive palliation at most centers carry acceptable results, primary heart transplant for HLHS is rarely offered in this era, and is, in general, limited to neonates with severe RV dysfunction and/or moderate-to- severe tricuspid regurgitation. Because the limited supply of donor organs contributes to significant mortality while patients are waiting for a suitable donor, attempts have been made to increase the donor pool through the use of ABO-incompatible donors (143). In those who received transplants, perioperative mortality is higher than for older children, but longer-term outcomes are overall better, with current 30-day, 1-, 5-, and 10-year survival rates of 80% to 85%, 75%, 65%, and 60%, respectively (144).
Interstage Morbidity and Mortality
Following successful Stage I palliation, even the most stable of survivors remain at risk for acute hemodynamic decompensation during the interstage period (the time from hospital discharge following Stage I palliation until Stage II palliation). Interstage death remains an unfortunate, but not uncommon, occurrence, with published rates of 2% to 16% (61,82,145–147). The presence of residual, recurrent, or progressive anatomic lesions such as a restrictive atrial septum, stenosis/obstruction of the shunt or conduit, aortic arch, and/or pulmonary arteries, or tricuspid valve insufficiency has been associated with interstage death (76, 148). In addition, the occurrence of a simple childhood illness such as a respiratory tract infection or gastroenteritis as well as fever, may cause hypovolemia, hypoxemia, and/or increased systemic vascular resistance and may place an interstage infant with minimal cardiovascular reserve at great risk for interstage morbidity and mortality (149). Infants discharged home following Stage I palliation, warrant heightened surveillance during the interstage period.
Home Monitoring and Other Outcomes Programs
Conventional management of interstage infants consists of routine outpatient evaluation by a pediatric cardiologist and primary care provider along with parents observing their infant at home for signs of respiratory distress or poor perfusion and alerting the medical team as warranted. This level of monitoring has proven inadequate in limiting interstage death. In fact, infants who die during the interstage period had been evaluated by a physician within days of death and not uncommonly, interstage death occurred with 24 h of the first symptom (150).
The current best practice for interstage care is heightened surveillance of this at-risk population through participation in a home monitoring program (114, 151). The goal of home monitoring is to provide a simple, reliable, in-home method of detecting worsening systemic oxygenation, acute dehydration, or growth failure based on the hypothesis that early, at-home, detection of these parameters may indicate the development of serious anatomic lesions or an intercurrent illness and allow for life-saving intervention.
Home monitoring usually consists of supplying the family with an infant scale and pulse oximeter at home. Parents are asked to obtain and record a daily weight and oxygen saturation, as well as track enteral intake volumes in a log book. The parents are counseled to notify the cardiology team if a breach of pre-determined criteria occurs. The goal of the program is to provide a simple, reliable, in-home method of detecting worsening systemic oxygenation, acute dehydration, or growth failure based on the hypothesis that early, at-home, detection of these parameters may indicate the development of serious anatomic lesions or an intercurrent illness and allow for life-saving intervention. The home monitoring program established in 2000 (114) enrolls all patients after Norwood palliation. When these infants are discharged from the hospital, they are sent home with a digital infant scale and pulse oximeter for daily assessments of weight and oxygen saturation, and a standardized form for recording these parameters as well as daily enteral intake volumes. The parents are counseled to notify the cardiac care team if a breach of pre-determined criteria occurs.
Concerning physiological criteria include arterial saturation <75% or >90%, acute weight loss of 30 g or more, inability to gain 20 g over 2 to 3 days, or enteral intake <100 ml/kg/day (149). If a breach of criteria has occurred, infants need evaluation by a healthcare provider within 24 h or less based on the severity of the breach and the presence of compounding factors. An additional strategy utilized to expand the surveillance that home monitoring provides is the implementation of a weekly follow-up phone call to parents by a member of the cardiac team to assess nutritional parameters and other trends in an effort to prevent call criteria breaches.
Over the past decade, implementation of a home monitoring program has been associated with improved interstage survival at several centers (82,114,146). In one series, 128 infants were home monitored over a period of 8 years. Ninety-eight percent (125 of 128) of the infants survived to Stage II palliation. A breach of home monitoring criteria occurred in 62% of home-monitored infants and resulted in 106 hospital admissions, 88% of which required additional medical or surgical intervention (152).
Another interstage strategy targeted at improving outcomes for infants discharged home following Stage I palliation is the implementation of a specialized cardiology clinic dedicated to outpatient care of interstage infants. The availability of a high-risk or interstage specialty clinic provides an opportunity for frequent in-depth evaluation of these fragile infants as frequently as every 1 to 2 weeks without overburdening a general cardiology clinic setting (153). Clinic evaluation is provided by a dedicated cardiac team consisting of pediatric cardiologists, nurse practitioners, and clinic nurses familiar with the multifaceted needs of interstage infants and their families. In order to provide the greatest benefit, the clinic should be multidisciplinary, with routine patient evaluation by a dietician, speech and feeding specialist, social worker, and case manager. Advantages of a high-risk or interstage clinic include a venue for constant reassessment of the infant’s cardiorespiratory status and review of home monitoring data as well as the opportunity for frequent, thorough evaluation of nutritional status including oral-motor feeding skills. Growth failure is a well-described finding in this population, and inadequate nutrition contributes significantly to interstage morbidity and mortality (154).
Interventional Procedures
There are numerous studies demonstrating the usefulness of interventional catheterization after the Norwood procedure. Although some procedures occur in the acute post-operative setting, most often due to unexplained cyanosis, the majority are performed during the interstage period either as a result of detected or suspected anatomic abnormalities, or as planned pre-Stage II catheterizations. Interventions address obstructions/stenoses in the BT shunt or RV-PA conduit, pulmonary arteries, aortic arch, and/or the acquisition/ persistence of aortopulmonary (APCs) and venovenous collaterals (VVCs).
Stenotic BT shunt and RV-PA conduits with resulting increased cyanosis often lead to immediate evaluation and intervention. Although surgery may be used to alleviate shunt or conduit obstruction, many centers rely on the effectiveness of catheter intervention (155–166).
In the immediate post-operative time period, various transcatheter methods have been used to treat thrombosed BT shunts. Mechanical disruption using catheter manipulation and/or balloon angioplasty, pharmacological dissolution with urokinase or recombinant tissue plasminogen activator, and rheolytic catheter thrombectomy have been used individually or in combination (158–160,165). In cases of extreme cyanosis, the use of ECMO prior to catheter intervention has been described (164).
Shunt stenosis is more common than occlusion and is often due to a physical narrowing of the BT shunt or RV-PA conduit. Multiple studies have shown the success of balloon angioplasty for shunt stenosis, with recalcitrant lesions responding to stent placement (155–157,161,163,165,166). More recently, the institution of the Sano modification resulted in documented cases of RV-PA conduit stenoses. Several published reports demonstrated endovascular stent placement in obstructed RV-PA conduits to be effective and safe (163,167–170). Complications include hypotension and blood loss, less commonly complete heart block and bradycardia, and rarely, cardiac arrest and death.
In addition to pulmonary blood source obstruction, pulmonary artery obstruction has also been diagnosed and intervened upon in the catheterization laboratory. Outside of deferring until the time of Stage II surgery to plasty pulmonary arteries, several studies reported successful balloon dilatation of pulmonary arteries at the pre-operative catheterization for pulmonary artery stenosis (171–173). For lesions resistant to balloon dilation, stent placement has been utilized between stages, although many institutions would favor surgical repair to stent placement in patients of this size.
Obstruction to systemic blood flow is also a serious and well-documented finding in HLHS patients, with studies finding as many as one-third of patients developing arch obstruction (118,120,174–176). Although studies have demonstrated technical success in treating the arch obstruction (118,120,172–177), high morbidity and mortality was noted in this group of patients (118,176,177). Recent studies have reported similar survival rates between this group and control groups without recoarctation (120). Blood loss requiring transfusion, decreased lower extremity perfusion, and transient arrhythmia ranked among the most common complications. Rarely, obstructive intimal flap development or aortic damage requiring surgical repair occurred.
The final 2 interventions that occur between Stage I and II involve vessel embolization. Venovenous collaterals and left SCV to coronary sinus, or pulmonary venous connections can cause cyanosis in patients after Stage II. One study found almost one-third of their patients developed venous collaterals after superior cavopulmonary anastomosis (178). Other studies reported successful coil closure of these venous collaterals with resulting increased saturation (166, 179). The effect of venous collateral embolization on long-term morbidity remains unknown.
Finally, embolization of APCs can occur during interstage catheterization. Some centers do occlude large APCs to avoid volume loading the single ventricle. However, the clinical significance and actual indications for closure are not currently known or documented. Although one study showed prolonged post-operative pleural effusions in Fon- tan patients with significant APCs (180), 2 studies demonstrated the lack of impact of APC flow on post-operative hemodynamics, pleural effusions, or outcome (181, 182). The most common risk associated with vessel occlusion would be coil dislodgment.
Stage II (Glenn; Hemi-Fontan)
Stage II palliation is the conversion from a high pressure (RV or aorta) “arterial” source of pulmonary blood flow to a venous source through anastomosis of the SVC to the pulmonary arteries (183–187). In the case of bilateral superior caval veins both SVCs are connected to the pulmonary arteries. The shunt or RV-PA conduit is generally ligated and/or divided at the same time. Stage II is most commonly performed at 4 to 6 months of age, but may be safely performed at <3 months of age (188).
After a bidirectional Glenn shunt, an extracardiac conduit is most commonly used to complete the Fontan procedure. However, it is possible to allow the SVC to remain connected to the heart with patch closure of the superior vena caval/right atrial junction. This patch is removed at the time of Fontan completion allowing creation of an intra- atrial lateral tunnel. This hemi-Fontan procedure was described by Norwood and Jacobs and involves a side-to-side anastomosis of the SVC to the right pulmonary artery with homograft patch augmentation of central pulmonary arteries, creating a baffle between the pulmonary artery and the right atrium (185, 189). Following a hemi-Fontan procedure, the Fontan completion is usually performed by creation of an intra-atrial lateral tunnel. There is no conclusive evidence that the type of superior cavopulmonary connection (i.e., Glenn vs. hemi-Fontan) has a significant impact on late outcomes for patients with HLHS (185,190,191).
The Glenn is most commonly performed using cardiopulmonary bypass with widely varying strategies ranging from normothermia without cross-clamping to deep hypothermic circulatory arrest (192–194). Techniques have been described that allow creation of a bidirectional Glenn shunt without the use of cardiopulmonary bypass; however, these are not widely utilized (195–197).
The bidirectional Glenn shunt and hemi-Fontan are physiologically identical and achieve a number of goals: reduced work of providing pulmonary blood flow and volume loading of the heart, and improved circulatory system efficiency, given the source of pulmonary blood flow is more desaturated venous blood rather than an arteriovenous admixture (185,198–200). The arterial saturations are generally improved (though still cyanotic) and the completion of the Glenn heralds in a period of decreased risk compared with the preceding interstage period. Intermediate staging with a Glenn/hemi-Fontan reduces the risk of mortality and morbidity at the subsequent Fontan procedure (183,185,189).
Management of additional sources of pulmonary blood flow at the time of superior cavopulmonary anastomosis remains controversial. Leaving an additional source of pulmonary blood flow (BT shunt or RV-PA conduit) has the potential advantage that the increased flow may enhance pulmonary artery growth; however, the increased flow is not tolerated in all patients and occasionally leads to unacceptable elevation of SVC pressure. There is no definite evidence that leaving additional sources of pulmonary blood flow improves outcomes (201–202).
Stage II requires a low PVR. A PVR of <2 Wood units has been associated with improved survival (199). This maturation includes growth of the pulmonary vascular bed such that the ratio of arterioles to alveoli increases, combined with a reduction in the thickness of the arteriolar smooth muscle (203). The age at which pulmonary maturation is sufficient to permit conversion from an arterial source of pulmonary blood flow to a SVC-pulmonary artery connection is not precisely established and may be dependent on a number of factors such as age at presentation, the size of the arterial source of pulmonary blood flow, presence of a restrictive atrial septal defect and presence or absence of parenchymal lung disease. Whereas in the past, Stage II was arbitrarily performed at 6 months of age, it is now routinely performed at 3 to 4 months of age and has been performed in patients as young as 1 month of age (193) to limit the duration of the vulnerable interstage period.
Diagnostic studies prior to Stage II.
Prior to the use of cardiac MRI, the debate existed about whether patients required catheterization prior to Stage II. However, due to limitations of echocardiography to reliably define pulmonary artery and arch anatomy, studies reported only a subset of low-risk patients with adequate echo evaluation who qualified for noninvasive testing only (171,204,205). With the increased use and improvement of cardiac MRI, the question surrounding the need for pre-Stage II catheterization resurfaced.
Two studies showed the ability of MRI to define aortic and pulmonary artery anatomy in infants (206, 207). One retrospective study of HLHS patients reported MR sensitivity of 86% and specificity 97% for neoaortic obstruction (1 false-negative result that detected a lesser degree of aortic narrowing and 1 false positive that did not require repair upon surgical inspection), and sensitivity of 100% and specificity 94% for LPA stenosis (2 false-positive results) with routine right pulmonary artery reconstruction for all patients at this institution (208). They concluded that MR could replace catheterization given their institution’s preference for surgical repair of pulmonary artery stenosis and coarctation.
The only prospective study randomized patients without pulmonary vein stenosis, pulmonary hypertension, severe ventricular dysfunction, severe atrioventricular valvar regurgitation, known large APCs or VVCs, or coarctation of the aorta into MRI versus catheterization. This study showed that the patients undergoing catheterization had higher rates of minor adverse events and longer post-study hospital stays, and found no detectable differences in immediate or short-term post-operative outcomes (209). However, they specifically did not randomize patients with suspected vascular lesions or hemodynamic compromise as these patients all had catheterizations.
The need for pre-Stage II catheterization remains a controversy in the literature. For those with clearly no hemodynamic or anatomic vulnerabilities, the studies support the ability of good-quality echocardiograms and MRIs to supply pre-operative data (171,204,205,208–210). In the setting of coarctation, pulmonary artery or vein stenosis, APCs, VVCs, or elevated PVR, the data remain mixed (190,209,211). Center preference for angioplasty versus surgical repair for pulmonary artery stenosis and coarctation clearly affects the decision for noninvasive imaging versus catheterization. Finally, the inability to coil collateral vessels with MRI remains a reason for some institutions to support catheterization prior to Stage II (211), despite the lack of data with regard to its impact on long-term morbidity or outcomes (180–182,212).
Outcomes.
The mortality for Stage II remains low. Among several large recent series, hospital mortality was virtually zero with a 1-year survival of >95% (193, 213). Patients undergoing Stage II at an earlier age do have increased utilization of hospital resources and are initially more cyanotic (Fig. 5) (193). Patients undergoing earlier Stage II appear to progress to Fontan completion in the usual fashion (214). As a consequence, indications for Stage II have changed from an arbitrary age criteria to patient- specific factors such as worsening cyanosis, congestive heart failure, decreased function, and/or poor weight gain (215). Stage II is frequently combined with additional procedures to address arch obstruction, a restrictive atrial septal defect, or tricuspid valve insufficiency.
Figure 5. Oxygen Saturation by Age at Surgery for Stage II Palliation.
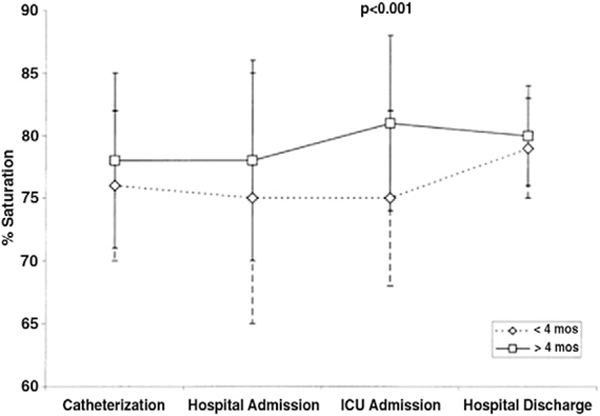
Patients undergoing early (<4 months) Stage II palliation initially had lower arterial saturation although they were not different than older patients at the time of hospital discharge. Reprinted with permission from Jaquiss et al. (193).
General complications.
Complications following Stage II are substantially less than following Stage I. The complications center on the procedure itself and the unique physiology of the cavopulmonary connection. Venous return from the superior circulation, head, and arms, including the substantial cerebral blood flow, is directed across the pulmonary vascular bed. Some degree of CVP elevation is a sine qua non of this procedure. The elevation of CVP is due to the in-series addition of the pulmonary vascular bed to the SVC drainage. SVC pressure early after Stage II is commonly in the high teens, but more significant elevation will result in impaired cerebral blood flow and a decrease in pulmonary blood flow resulting in cyanosis (213). This constellation is recognizable as SVC syndrome and requires prompt investigation into identification of reversible causes, including SVC anastomotic narrowing, pulmonary artery hypoplasia., or a restrictive atrial septal defect (216–218). Through the same mechanism of backward transmission of elevated pressure to the pulmonary veins and pulmonary artery, severe tricuspid valve insufficiency or an elevated RV end-diastolic pressure can also result in elevation of CVP. Mechanical ventilation will raise intrathoracic pressure, adding to the pressure needed to drive blood across the pulmonary vascular bed. Early extubation will result in a decrease in CVP and should be part of the post-operative management strategy of patients following Stage II surgery (217). The use of pulmonary vasodilators such as inhaled nitric oxide or sildenafil may be effective in the patient with a borderline or reactive pulmonary vascular bed (219, 220). If no anatomic issue is identified, and elevation of the CVP and cyanosis persist despite pulmonary vasodilator therapy, takedown to an arterial source of pulmonary blood flow should be considered. Additional causes of cyanosis (SaO2 <70%) early after Stage II include pulmonary parenchymal disease resulting in pulmonary venous desaturation. Venous collaterals that diverting flow from the SVC can also result in excessive cyanosis by decreasing pulmonary blood flow (221). The CVP helps to differentiate the causes of cyanosis following Stage II. If the CVP is elevated, then anatomic obstruction, tricuspid valve insufficiency, elevated RV end- diastolic pressure, and pulmonary parenchymal disease should be considered. If the CVP is low, venovenous collaterals are more likely.
Additional complications include arrhythmias, phrenic nerve injury, and embolic complications. The most common arrhythmia following Stage II is sinus node dysfunction. This is more common following the hemi-Fontan than the bidirectional Glenn shunt, presumably, but is an early phenomenon, and by hospital discharge, the incidence is equal (6% to 8%) between the 2 (191). Phrenic nerve injury can occur during Stage II because the nerve comes into jeopardy during dissection of the SVC (222). Inability to wean from positive-pressure ventilation may be an indication for diaphragm plication. With the obligatory right-to- left shunt, patients following Stage II are at risk of embolic complications, especially from femoral or lower extremity intravenous access (223). Anticoagulation is commonly used to prevent thrombotic complications in small infants with central venous lines in place, and care should be taken to prevent air bubbles from entering intravenous lines.
Pleural effusions.
All patients with single-ventricle physiology, particularly those with HLHS, are at increased risk for developing effusions in the pleural, pericardial, and peritoneal spaces (224–227). The rate of pleural effusions in patients with functional single ventricles ranges from 12% to 45% (228–231). Persistent pleural effusions may require long-term chest tube drainage, dietary modifications, repletion of serum proteins (clotting factors, immunoglobulin, and albumin), fluid restriction, introduction of medications, and further procedures (228,230,232). Effusions are the principal cause of prolonged hospital stays following singleventricle palliative surgeries (230). Therefore, care of the child with HLHS necessitates careful attention to factors that contribute to effusions and vigilance to prevent effusive complications.
On the most fundamental level, effusions result from either physical disruption of capillaries and lymphatic vessels, or physiological disruption of their function. Conditions that support the development of effusions when the vessels are intact include high hydrostatic pressure and/or low oncotic pressure in the vascular space, and low hydrostatic pressure and/or high oncotic pressure in the tissue compartment or potential spaces. Among patients with HLHS, the specific hemodynamic characteristics that favor these conditions include elevated RV end-diastolic pressure (231) (such as from poor ventricular compliance or significant neoaortic valve regurgitation), tricuspid valve stenosis or regurgitation, obstruction at the atrial septal level or pulmonary veins, elevated PVR (233), obstruction at any level between the systemic and pulmonary microvasculature, and significant aortopulmonary collaterals (230). The inadvertent surgical disruption of lymphatic channels during repair may increase oncotic pressure in tissue spaces and potential spaces due to extravasation of chylous fluid and compromise of local lymphatic function.
Factors that enhance the degree to which vascular and lymphatic structures behave as porous membranes also promote the development of effusions. In patients with HLHS, activation of the inflammatory and complement cascades during the course of surgical interventions may induce capillary leakage (234). Hormonal influences that contribute to fluid retention and elevated systemic venous pressures, such as elevated aldosterone (235) and atrial natriuretic hormone (236) may also promote effusions.
Strategies aimed to prevent or reduce effusive complications address these hydrostatic, oncotic, inflammatory. and hormonal factors. Most data about such interventions around Stage II come from retrospective reviews. In 42 patients with HLHS undergoing bidirectional Glenn operations, a reduced incidence of persistent pleural effusions was associated with use of the Sano modification versus use of the BT shunt in the Stage I palliation. Hypothetically,improved development of the pulmonary arteries (greater pulmonary artery diameter and less discrepant branch pulmonary artery sizes), also associated with use of the Sano approach, may have led to improved pulmonary artery hemodynamics (237). In a series of patients with functional single ventricles, the presence of accessory pulmonary blood flow was associated with a > 8-fold increase in risk of prolonged pleural effusions following bidirectional Glenn (238). These authors postulated that accessory pulmonary blood flow may increase pressures in systemic venous pathways, “steal” from systemic circulation, contribute to low systemic cardiac output, and promote activation of the renin-angiotensin system. Further review of 142 such cases suggested that elimination of accessory pulmonary blood flow was associated with significantly improved survival and reduced incidence of persistent effusions (233).
Several perioperative strategies are generally considered to improve hemodynamics and reduce the likelihood of effusions after Stage II palliation (Table 2). Despite judicious care to prevent or minimize pleural effusions following Stage II palliation for HLHS, some patients suffer with prolonged pleural effusions. If the effusions persist, a stepwise increase in therapy is commonly employed: low-fat diet, medical therapy with an angiotensin-converting enzyme (ACE) inhibitor or octreotide, total parenteral nutrition, exploration for identification of a lymphatic leak, thoracic duct ligation, and pleurodesis (227,239,240).
Table 2.
Strategies for Reducing the Likelihood of Effusion Development Following Stage II Palliation
| • Minimize anatomical obstructions in cavopulmonary circuit |
|---|
| • Optimize atrioventricular and semilunar valve function |
| • Maintain lowest central venous pressure possible (using ultrafiltration and diuretics) |
| • Minimize pulmonary vascular resistance (using selective and nonselective pulmonary vasodilators) |
| • Enhance systemic output (using inotropes and systemic afterload reduction) |
| • Enhance diastolic function (using lusitropic agents) |
| • Minimize systemic inflammatory response (using ultrafiltration and antiinflammatory therapies) |
| • Reduce lymphatic flow (avoiding enteral exposure to long-chain fatty acids, considering the use of the somatostatin analog, octreotide) |
Stage III (Fontan Operation; Total Cavopulmonary Connection)
In 1971, Dr. Francois Fontan became the first to place the pulmonary and systemic circulations in series with one ventricle, creating the named procedure (241). This original operation, as well as several modifications, was soon realized to be of benefit, not only to patients with tricuspid atresia, but to many patients with single-ventricle physiology. In 1988, de Leval et al. introduced the concept of the total cavopulmonary connection (TCPC) as an alternative to the atriopulmonary Fontan (Fig. 6), and by the early 1990s, staging towards the TCPC (i.e., the 3-stage approach) was being advocated (109, 242).
Figure 6. Fontan Surgical Techniques.
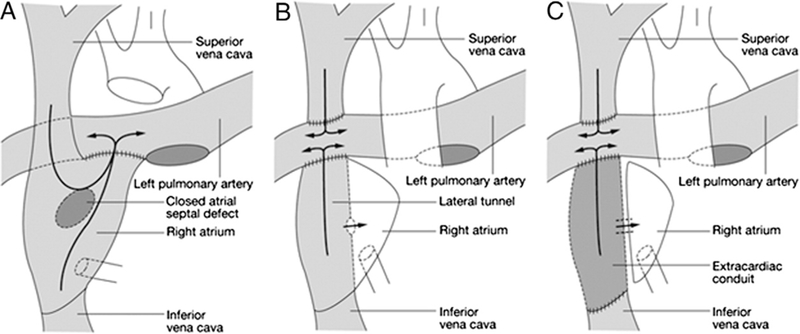
The original atriopulmonary Fontan (A) has been replaced with the lateral tunnel (B) and extracardiac conduit (C) Fontan. Reprinted with permission from de Leval (488).
Although the Fontan circulation has become the accepted final arrangement for the single-ventricle pathway, controversy in timing of the TCPC still remains. Many complete the Fontan circulation when children are 2 to 3 years of age to minimize the end-organ exposure to cyanosis (243, 244). However, other programs delay Fontan until it is physiologically indicated (i.e., decreasing saturations or symptoms of cyanosis with activity) and therefore complete the Fontan in the 3- to 5-year-old timeframe (245). This strategy may be supported by the literature demonstrating that younger age at Fontan is associated with prolonged recovery and Fontan failure (244,246,247). Regardless of timing, the Fontan circulation results in several physiological and anatomic consequences stemming from the increased pressure seen in the Fontan circulation and include right atrial dilation, inefficient flow dynamics, baffle thrombus, repeated subclinical pulmonary emboli, and atrial arrhythmias. These can coalesce to cause the Fontan circulation to fail (248–250). This is magnified in HLHS patients in whom there is a systemic RV and accompanying tricuspid valve, which are at risk for failure over time. RV dysfunction will lead to dilation and tricuspid insufficiency. Likewise, primary tricuspid insufficiency will lead to RV volume overload and dysfunction (251). This potential for negative synergy merits an aggressive approach towards tricuspid valve repair at the time of TCPC that is essential for longevity.
Extracardiac Conduit Versus Lateral Tunnel Fontan
The lateral tunnel (LT) Fontan connection has been the most extensively studied and most used configuration for completion of the TCPC. The use of the extracardiac conduit (ECC) technique to complete the Fontan, connecting the inferior vena cava to the pulmonary arteries via a conduit (most often Gore-Tex [W. L. Gore & Associates, Elkton, Maryland] and ranging in size from 18 to 24 mm, most commonly 18 to 20 mm), was introduced in 1990 (252), although it was not until this past decade that it gained widespread popularity. The ECC has several theoretical advantages, including flexibility in anatomically difficult situations (i.e., heterotaxy), the avoidance of sinus node manipulation, decreased suture lines and pressure in the right atrium (decreasing arrhythmogenic potential), low potential for dilation, and avoidance of cardioplegic arrest (253). The avoidance of cardiac arrest, fibrillatory arrest, and even circulatory arrest may preserve cardiac function, though this has not been clearly demonstrated when studying all single ventricles undergoing Fontan (254, 255). Advantages for the LT include growth potential and all of the advantages associated with the avoidance of a conduit, particularly in the low-pressure, right-sided circulation, which include the risk of thromboembolism. As with the ECC, these theoretical LT advantages have not been clearly demonstrated (253, 255). Fluid dynamic, geometric, and mathematical models have confirmed the hemodynamic advantages of both the LT and ECC techniques (256, 257).
Even though the advantages of avoiding cardiac arrest, systemic cooling, and extensive atrial manipulation appear obvious, studies comparing the LT and ECC Fontan procedures have not consistently shown an advantage of either technique (253–255). Also, there are many series reporting excellent outcomes in which either the ECC or LT strategy is almost exclusively used (245,258–261). HLHS patients are unique patient cohorts that often highlight subtle differences between surgical and medical therapies because of their tenuous hemodynamic state and reliance on a systemic RV and tricuspid valve. Unfortunately, there has not been a study in this cohort to see whether the theoretical advantages of either the LT or ECC Fontan are magnified to a point of consistently changing outcomes.
Fenestration.
Fenestration has been employed to minimize post-operative complications, including low post-operative cardiac output, pleural and pericardial effusions, and ascites, as well as long-term complications such as diminished exercise performance, and protein-losing enteropathy (PLE) (246,262–266). Despite the potential benefits of fenestration, drawbacks include the risks of systemic embolization, systemic desaturation, and need for late catheter interventions for fenestration closure (264,267,268). There have been a number of studies including one from the Pediatric Heart Network Fontan cross-sectional study group (269), as well as a prospective randomized study by Lemler et al. (266) that have demonstrated that fenestration decreases length of post-operative pleural effusions requiring chest tubes and hospital length of stay.
The emergence of the ECC Fontan in many programs has led to questioning the need for fenestration, and several centers have adopted a highly selective strategy for Fontan fenestration with encouraging and possibly even better results than those after fenestrated Fontan (245,270–272). Fenestration is reserved for the highest-risk patients, which may include those undergoing significant concomitant procedures or single-lung Fontan palliation, patients with elevated PVR or transpulmonary gradient, those having significant atrioventricular valve regurgitation or poor ventricular function, and patients with intracardiac anatomy not amenable to extracardiac conduit (263, 264). HLHS patients are at particular risk for poor systemic ventricular function and atrioventricular valve insufficiency and, therefore, may require fenestration more often than patients with other types of single ventricles. However, this has not been consistently seen in centers that are using fenestration selectively (245). These centers believe that the HLHS candidates for TCPC currently are better candidates than in the past because of the advances in all aspects of patient management, from improved outcomes after first- and second-stage single-ventricle palliation, as well as better medical management and interstage surveillance. Centers using the nonfenestrated Fontan strategy also tend to palliate their patients at an older age (3 to 5 years) (243, 245). Salazar et al. (245) reported similar outcomes over a 6-year period whether patients were fenestrated or not. The nonfenestrated patients had a shorter hospital and intensive care unit length of stay and less need for reintervention. These outcomes are confounded by the higher rate of extubation in the operating room for nonfenestrated patients, which this center, in the past, has shown to result in a shorter duration of intensive care unit and hospital stay, chest tube requirement, and lower resource utilization. The immediate reduction in Fontan baffle pressure and ability to mobilize these patients early may explain these findings (273).
As the pre-operative state (i.e., hemodynamics and/or age), operative techniques (i.e., ECC), and post-operative care (i.e., immediate extubation and mobilization) of Fon- tan patients continue to change, the benefit of fenestration must be readdressed. It is also very important to realize that practices and patient populations (i.e., ventricular morphology, age at Fontan, operating room extubation) at different centers may vary greatly and thus so may the usefulness of fenestration in any particular practice. With this in mind, the cohort of HLHS should be separately evaluated in the current era with regard to fenestration, controlling for important factors such as age.
Cardiopulmonary bypass.
In the late 1990s, with the spreading popularity of the ECC Fontan, surgeons began to investigate how completion of the TCPC could occur without cardiopulmonary bypass. Starting in 1998, a series of reports from different centers established the feasibility and safety of avoiding bypass when creating an ECC Fontan (274–277). The proinflammatory and vasoactive substances caused by cardiopulmonary bypass and their ill effect on the myocardium, the coagulation system, and the pulmonary vasculature have been well established (234). However, this relationship is dependent on time, the conduct of bypass, and many other factors. Therefore, whether avoidance of bypass during Fontan completion has clinical significance is unclear. Off-bypass Fontan completion has been demonstrated to attenuate proinflammatory markers; however, multiple studies have been unable to establish consistent clinical improvements (278, 279). The largest and most recent series to evaluate Fontan completion without cardiopulmonary bypass found no difference in immediate postoperative pressures or in early outcomes including chest tube drainage, arrhythmias, or mechanical ventilation (280). Although avoidance of cardiopulmonary bypass at completion Fontan in HLHS is safe and can be done with consistent results, it appears at this juncture to have no clinical benefit to patient care and will require more study if it is to become widely adopted.
Outcomes.
Hospital mortality after Fontan completion for HLHS is excellent, with short-term survival averaging >95% (258,259,281,282). Intermediate and long-term survival rates are 77% to 95% at 5 years and 72% to 91% at 10 years (245,258,281). Right ventricular morphology continues to be a risk factor highly associated with long-term mortality and heart failure after Fontan.
Fifty percent to 70% of newborns with HLHS will survive the 3 surgeries and live to the age of 5 (31,79,283). Increasingly, there is recognition that the major determinants of outcome following surgical intervention for HLHS have less to do with intraoperative support strategies than with patient-related factors such as gestational age, associated genetic syndromes, and genetic susceptibility, such as apolipoprotein polymorphisms (284, 285).
International Experience
Although a complete review of the international perspective on HLHS is beyond the scope of this paper, differences exist in both logistical and management approaches to the patient with HLHS. Perhaps one of the greatest differences between the United States and abroad is the concept of regionalization.
Larger center volumes and regionalization of patients with complex congenital heart disease has been shown to improve outcomes (286). Numerous countries, including Sweden and the United Kingdom, and the continent of Australia use this approach in the treatment of children with congenital heart disease, especially HLHS.
As in the United States, pre-operative management strategies are not standardized (26). In some centers in Germany, for example, to minimize pulmonary hyperperfusion and an increase in vascular resistance, avoidance of pre-operative ventilation and inotropic support are combined with systemic afterload reduction (29).
Most European centers perform the Norwood operation with a modified BT shunt rather than an RV-PA conduit, although some centers have adopted this modification with improved outcomes (283). A small number advocate the hybrid approach (131), and transplantation as an initial option is rarely used due to poor donor availability. In the Stage I post-operative period, afterload reduction includes phosphodiesterase inhibitors, sodium nitroprusside, or the alpha blocker phentolamine (83). Phenoxybenzamine is rarely in use in Europe due to the long half life.
The bidirectional Glenn is preferred over the hemi- Fontan for the second stage, whereas for the final stage, great differences exist in the type of procedure performed and the timing. One explanation of the timing differences (Germany early, United Kingdom later) could be insurance system related. Both the use of fenestrations and/or anticoagulation varies throughout Europe.
Pleural Effusions
The same principles underlying the development of effusions in the patient with HLHS undergoing Glenn or hemi-Fontan repairs apply to the patient proceeding to Fontan. In particular, specific hemodynamic factors that seem to increase the incidence of pleural effusions include higher mean pre-operative pulmonary artery pressure (230, 287) and higher mean post-operative CVP (230). Retrospective data comparing bypass versus off-bypass procedures, LT versus ECC, and modified ultrafiltration versus none have mixed results (255,279,287,288). However, a prospective randomized trial of the Fontan with and without fenestration suggests use of fenestration decreases the duration of post-operative effusions and reduces the length of hospital stay (266). Fenestration may result in creation of a “pop-off valve” to reduce CVPs and thereby reduce the hemodynamic conditions that lead to pleural effusion, particularly for patients with high pulmonary vascular resistance.
One retrospective review of nearly 100 patients undergoing Fontan suggested that operating during the winter respiratory viral season was associated with a higher risk of pleural effusions (230). As a result, some have suggested that if the Fontan cannot be postponed past the respiratory viral season, screening for subclinical respiratory syncytial virus may detect patients at high risk for effusive complications and other morbidities.
Chronic Medications
Afterload reduction.
Increased afterload is a fundamental feature of baseline hemodynamics in patients with HLHS. In patients with good functional outcome following the Fontan repair, preload conditions and ventricular contractility may be normal, but measures of increased afterload (including arterial elastance, end-systolic elastance and pressure, and CVP) account for reduced cardiac index and exercise performance (289). In single-ventricle patients, reducing both systemic afterload and PVR might theoretically improve cardiac performance and clinical outcome
Most large centers discharge HLHS patients on ACE inhibitors (27), and ACE inhibitors have documented success in treating children with heart failure (290). However, case-control studies of captopril and enalapril, and randomized, controlled trials of enalapril and lisinopril suggest that ACE inhibitors are not associated with decreased pleural drainage or effusions in the weeks following Fontan surgery (235, 291), improved exercise capacity years following surgery (288), general somatic growth, favorable ventricular remodeling and function, or Ross classification of congestive heart failure (292, 293).
ACE inhibitors may be associated with improved neuro- developmental outcome (292), improved endothelial function (294), and reduced renal injury (295) following the Fontan. Therefore, on a case-by-case basis, each patient on ACE inhibitors should be monitored to ensure that measurable benefit justifies use. Angiotensin receptor blockers, such as valsartan, are newer afterload-reducing agents employed in pediatric cardiovascular diseases, but no evidence exists for use in patients with HLHS following any stage of palliation.
Success in treating children with pulmonary hypertension and recent understanding of endothelial dysfunction in patients with single ventricles (295) and patients with Fontan circulation (296) has led to use of selective pulmonary vasodilators and endothelin receptor blockers. Use of sildenafil, a phosodiesterase-5 inhibitor, improved plastic bronchitis in a single case of a child with HLHS following Fontan surgery (297). A randomized, controlled study of sildenafil showed that a single dose improved cardiac index and exercise capacity in teens and young adults with Fontan circulation (298), and a recent abstract reporting on a double-blind, placebo-controlled crossover trial in 28 children and teens with Fontan circulation suggests sildenafil improves myocardial performance and may increase cardiac output (299). The dual-endothelin receptor antagonist, bosentan, has also been associated with improved symptoms and reduced pulmonary artery pressure, pulmonary vascular resistance, and pulmonary blood flow in patients with Fontan circulation (300–302). Further trials of these afterload reducers in patients with HLHS would clearly be warranted and helpful.
Anticoagulation and hematologic issues.
Thromboembolic events (TE), which occur more frequently after Fontan operation than for any other form of cardiac surgery except prosthetic valve replacement, may be due to inherited, acquired, and/or associated factors (e.g., cyanosis) (303). One-quarter of patients with Fontan physiology are at risk for the development of thrombus in the venous system because of the low-flow state of the cavopulmonary baffle (267). Levels of protein C, factors II, V, VII, IX, and X, plasminogen, fibrinogen, and antithrombin III (ATIII) are significantly lower in Stage I infants (304). Stage II patients have significantly lower levels of protein C, protein S, ATIII, and factors II, V, VII, and X (305). Despite these coagulation factor abnormalities, these patients do not commonly have an increased risk for clinical TE complications, with the isolated documented exception being Stage II patients with elevated levels of factor II and low levels of ATIII (306). Fontan patients have been shown to exhibit low levels of protein C, factors II, V, VII, and X, plasminogen, and ATIII, and elevated levels of factor VIII. Monitoring factor VIII level may help identify a subset of patients at risk for thrombosis, especially those with low levels of protein C (307).
Thromboembolic events after Fontan occur in a bimodal distribution peaking during the first post-operative year, and again 10 years later (249,308–312). Several crosssectional series report the incidence of TE to be between 0.8% (313) and 33% (314), and mortality following TE is as high as 25% in pediatric series (315). Risk factors for thrombosis include dehydration, low-flow state, stasis in the Fontan or pulmonary circuit, increased venous pressure, right-to-left shunt, hepatic dysfunction, PLE, prolonged post-operative immobilization, azygous continuation of the inferior vena cava (316), blind cul-de-sacs, prosthetic material, hypercoagulable states, ventricular dysfunction, atrial arrhythmias, and pulmonary embolism. The incidence of cerebrovascular accidents is reported as 1.4% to 19% (311, 315).
There is no consensus in the literature as to the optimal type of anticoagulation therapy or whether therapy is warranted at all (309,317,318). Aspirin is often used at a dose of 1 to 5 mg/kg/day. Clopidogrel, a glycoprotein IIb/IIIa inhibitor and antiplatelet aggregation agent, may provide an additive antiplatelet effect if combined with aspirin. Mutations in the CYP2C19 gene render certain patients unable to respond to clopidogrel. Warfarin is a vitamin K antagonist and is usually recommended in Fontan patients with documented thrombosis, poor hemodynamics (with or without chronic venous hypertension), those undergoing Fontan revision, and those with atrial tachyarrhythmias (310,318,319).
A recent multicenter, prospective, randomized trial comparing heparin/warfarin with aspirin as primary thromboprophylaxis found no significant difference in safety or efficacy in the first 2 years after Fontan. In the 111 patients (57 received aspirin, 54 received heparin/warfarin), the overall thrombosis rate was 23% (18% aspirin, 9% heparin/warfarin) despite medical prophylaxis. Major bleeding occurred in 1 patient in each group (320).
Patients with thrombosis while receiving aspirin should be switched to warfarin or low molecular weight heparin (LMWH). Although LMWH has become more commonly used in pediatric patients due to its ease of monitoring (anti-factor Xa level 4 h after an injection) and its relative lack of interference by other drugs or diet, there is no consensus on the benefits of LMWH over warfarin, and reports of failure of LMWH as bridging therapy exist.
Thrombolytic therapy is reserved for those patients with thrombosis of immediate clinical importance. Tissue plasminogen activator, which converts endogenous plasminogen to plasmin, is most commonly employed. There are a significant number of bleeding complications associated with thrombolytic therapy in children, occurring in 30% to 68% of patients (321).
Currently, long-term anticoagulation therapy is influenced by the provider’s preference, anticipated risk for thrombosis, presence of arrhythmias, and patient’s functional status. As a result of the multiple factors contributing to thrombus formation, it is unlikely that a single therapy or drug will provide complete prophylaxis.
Long-Term Outcomes
The original selection criteria for patients undergoing atrio- pulmonary connection for tricuspid atresia were laid out by Choussat and Fontan (Table 3) and remain relevant physiologic risk factors for Fontan outcomes (322). Because Fontan physiology requires systemic venous baffle pressure to be higher than left atrial pressure in order for the systemic and pulmonary circulations to work in series, many of the long-term outcomes post-Fontan may be a reflection of this relatively elevated pressure in the systemic venous system.
Table 3.
Original Criteria for Fontan’s Operation: The “10 Anatomic and Physiologic Commandments”
| • Age 4–15 yrs |
|---|
| • Sinus rhythm |
| • Normal drainage of the caval veins |
| • Normal right atrial volume |
| • Low pulmonary artery pressure (≤15 mm Hg) |
| • Pulmonary resistance <4 WU/m2 |
| • Adequate pulmonary artery size (PA/AO ratio >0.75) |
| • Normal ventricular function |
| • No mitral insufficiency |
| • No pulmonary artery distortion (from the BT shunt) |
Over the past 2 decades, the outcome after Fontan operations has improved, with actuarial survival of 87% at 20 years in one study for all underlying diagnoses (265). The current literature on long-term outcome after the Fontan operation reflects primarily single LV physiology; however, studies increasingly include single RVs and mixed physiology. Regardless of underlying diagnosis, late complications present ongoing management challenges in the adult Fon- tan patient. These long-term outcomes (262) include impaired systemic ventricular systolic and diastolic function, progressive hypoxemia, elevated pulmonary vascular resistance, and complications including arrhythmias, thromboembolism, and hepatic dysfunction. Maximal exercise tolerance is impaired in individuals with a Fontan and worsens with age as well. The majority of these changes, demonstrated late after Fontan operation, are not specific to HLHS. However, “failing Fontan” physiology, including the development of plastic bronchitis and PLE, may be increasingly prevalent in the HLHS Fontan population.
RV morphology in a Fontan patient may not increase intermediate-term mortality; however, it is associated with poorer ventricular and valvular function, as well as heart failure-related death in the long term (323). Gradual attrition in Fontan patients is most frequently secondary to thromboembolic or heart failure-related deaths and sudden cardiac death (thought to be associated with arrhythmia). Risk factors associated with late thromboembolic mortality include lack of antiplatelet or anticoagulant therapy and intracardiac thrombus, whereas heart failure-related mortality is predicted by the development of PLE, high right atrial pressures, and single RV morphology (281), and therefore may become more prevalent as the HLHS Fontan population ages.
Long-term outcomes in the Fontan circulation are reflected in exercise capacity. These individuals demonstrate a unique physiological response to exercise, and although there is a range of exercise responses, most individuals have reduced exercise tolerance. The etiology of this limitation is multifactorial and involves cardiac, pulmonary, systemic venous, and muscular contributions. Most well defined is an inadequate preload reserve limiting inotropic cardiac augmentation (324). During exercise, the increase in pulmonary blood flow leads to an increase in pressure, because the pulmonary vasculature is unable to vasodilate as in normal biventricular physiology. This increased demand is reflected into the Fontan circulation, and without a subpulmonary ventricle to increase pressure, LV filling is diminished and cardiac output does not increase normally. There is likely also diastolic dysfunction of the single ventricle with aging that further impairs passive LV filling (325).
These physiological changes can be measure with cardiopulmonary exercise testing (326). In patients with Fontan circulation, peak oxygen uptake is diminished for age, peak heart rate is decreased, with likely chronotropic incompetence, and arterial oxygen saturation is also decreased at peak exercise. Cardiac output is subnormal, secondary to reduced stroke volume, heart rate response, and diminished pulmonary venous return. Ventilatory anaerobic threshold is below normal values, and the ventilatory equivalent for carbon dioxide is higher than normal ranges for age. With training and cardiac rehabilitation, many of these parameters may demonstrate improvement (327). Further study of the effect of structured cardiopulmonary rehabilitation in the adult Fontan patient are underway.
“Routine” Follow-Up and Testing
As improved early outcomes lead to greater longevity in post-Fontan patients, caregivers are increasingly faced with recognizing and managing long-term complications of Fon- tan physiology including the “failing Fontan.” The failing Fontan population is broad and may include individuals limited by impaired ventricular function but also includes patients with preserved ventricular function with additional devastating and challenging complications such as PLE and plastic bronchitis. Essential to treatment in these patients is vigilance and a high index of suspicion to make an early diagnosis, with hopes that persistent optimization of the entirety of the Fontan circulation and physiology will aid in decreasing the rates of “failing Fontan” as the new generation of HLHS patients age.
There are no standard algorithms for long-term follow-up of children and adults who have undergone staged palliation for HLHS, but suggested guidelines have been published (328). The Fontan circulation is an unnatural cardiovascular state with risk for pathophysiological aberration that does not diminish over time. In fact, risks likely increase as these patients age. Patients with Fontan physiology have elevated systemic venous pressure, low cardiac output, and endothelial dysfunction (329–331). Over their lifetime, these significant morbidities require aggressive long-term follow-up and surveillance with cardiac testing to identify potentially serious complications. Moreover, transition to adult congenital heart disease specialists should occur as patients enter adulthood. Of note, the oldest patients with HLHS who have experienced “current” Fontan strategies are only now entering their third decade of life, and as such, the long-term consequences of the Fontan physiology in the setting of a single RV remain unknown.
If patients are clinically well without significant complications, visits to the cardiologists can be every 6 to 12 months. In addition to routine vital signs, physical examination should include pulse oximetry and upper and lower extremity blood pressures (to assess for recurrent distal arch obstruction). As part of a thorough history, patients should be asked about development of diarrhea and/or abdominal swelling (signs of PLE), palpitations, and syncope and exercise intolerance. Testing at regular intervals should include echocardiography, electrocardiograms, Holter monitoring, and exercise stress testing. Some suggest the addition of other tests to this long-term regimen including cardiac MRI, cardiac catheterization, and laboratory screening for PLE, coagulation, and/or hepatic abnormalities.
Echocardiography as a screening tool is useful to assess for ventricular dysfunction, tricuspid regurgitation, and function of the neoaortic root and valve. Neoaortic root dilation and valve regurgitation are common after the Fontan palliation and have as yet unknown long-term consequences (332). Echocardiography can be useful in identifying thrombus formation though thrombus can be missed on transthoracic imaging. If there is a high index of suspicion, transesophageal echocardiography may be required (314, 318). In patients with known thrombus formation, a coagulation profile with consultation from hematology is warranted.
Assessment for symptomatic and asymptomatic arrhythmias is useful as many can be treated with medical therapy. Electrocardiograms and Holter monitors may identify some of these rhythm disturbances. When symptoms are infrequent, transtelephonic event monitoring may be useful. Exercise stress testing is also useful to determine whether there is sinus node dysfunction or exercise-induced arrhythmias.
More controversial is whether other monitoring should be routinely performed in patients with HLHS after the Fontan procedure. Cardiac MRI, though noninvasive, often requires conscious sedation. The benefits of cardiac MRI include accurate assessment of ventricular ejection fraction and cardiac output, good visualization of branch pulmonary arteries, and estimation of the impact of aortopulmonary collaterals on the Fontan circulation (333, 334). Echocardiography cannot accurately and consistently provide the same information and some suggest routine cardiac MRI every 3 to 5 years after the Fontan procedure. Cardiac catheterization is no longer routinely performed after Fontan completion because of the inherent risks of an invasive procedure. However, catheterization provides important hemodynamic information and allows for catheter-directed interventions such as fenestration enlargement or closure, coil embolization of aortopulmonary collateral vessels, and dilation of residual or recurrent distal arch obstruction. In some centers, cardiac catheterization is recommended in the asymptomatic HLHS patient 3 to 5 years after Fontan and/or before they transition to adult cardiology caretakers (328).
Liver pathology runs the spectrum of congestion to cirrhosis and hepatocellular carcinoma after the Fontan operation (335). Some patients with failing Fontan circulation are found to have hepatic cirrhosis and thus are no longer candidates for cardiac transplantation, whereas others have undergone heart-liver transplant. Unfortunately, there is no good screening tool for cirrhosis. In patients with Fontan circulation, abnormal liver histology may be patchy and missed on single liver biopsies. No laboratory values definitively make the diagnosis or are markers for early stages of the disease (336). Despite these obstacles, some suggest that screening for liver abnormalities should be a part of routine follow-up in patients with Fontan physiology and that a liver biopsy before adulthood is warranted. Both PLE and hepatic cirrhosis require consultation with gastrointestinal specialists.
Associated Conditions
Protein-losing enteropathy.
PLE is an excessive loss of proteins across the intestinal mucosa, and its occurrence in Fontan patients was first reported in 1980 (337). Loss of protein in the bowel may be secondary to lymphatic distension as a result of elevated systemic venous pressure; however, PLE has been seen in patients with normal Fontan pressures, and the exact etiology of PLE remains elusive. Presenting symptoms include peripheral edema, ascites, and pleural or pericardial effusions, unlike the gastrointestinal symptoms of diarrhea and abdominal pain seen in patients with a primary gastrointestinal, rather than cardiac, etiology (338).
The prevalence of PLE as cited in the literature ranges from 3.7% to 24% (339), with a cumulative risk at 10 years of 13.4% (338). Risk factors associated with PLE include non-LV anatomy as in HLHS, long hospital stay at the time of Fontan surgery, prolonged cardiopulmonary bypass time, and renal failure in the immediate post-operative period (338, 340). High systemic venous pressure, elevated right atrial diastolic or ventricular end-diastolic pressure, and high mesenteric vascular resistance (341, 342) have all been associated with PLE in Fontan patients (343). Neither medical nor surgical interventions guarantee complete resolution in all patients with PLE. PLE after Fontan operation is associated with a very high mortality and morbidity rate with a 5-year actuarial survival rate of 46% (338, 344); transplantation does improve overall survival in this subset of patients (345). The diagnosis of PLE is made clinically with supporting lab results including hypoalbuminemia, hypoproteinemia, hypocalcemia, lymphocytopenia, elevated stool alpha-1 antitrypsin, and increased alpha-1 antitrypsin clearance.
Medical management of PLE should include treatment optimization of medical therapy (diuretics, afterload reduction), use of parenteral albumin, and dietary changes including sodium restriction, and a low-fat, high-protein, medium-chain triglyceride diet. Pulmonary vasodilators (346) have been used to lower elevated Fontan pressures, and systemic steroids (347, 348) and heparin may stabilize intestinal capillary endothelial cell membranes. Intervention to relieve obstruction in the Fontan pathway, closure of residual systemic to pulmonary arterial shunts and aortopulmonary collaterals (349), and creation of fenestrations in the Fontan circuit (350) have also been suggested. Pacing to create atrioventricular synchrony and improve cardiac output (351), conversion of the atriopulmonary Fontan (352) to an extracardiac conduit (353), and heart transplantation (354, 355) have been proposed, though mortality remains high.
Plastic bronchitis.
Plastic bronchitis is a rare but potentially fatal complication after Fontan operation characterized by recurrent expectoration of long, branching bronchial casts which may manifest as airway obstruction. Although the pathogenesis is not well defined, similar factors to the development of PLE may contribute, including increased systemic venous pressure, elevated pulmonary venous pressure, and endobronchial lymphatic leakage. Diagnosis is generally made at the time of clinical pulmonary decompensation, although computed tomography can identify early stages as well as used to guide bronchoscopy or monitor treatment response (356). Treatment modalities are primarily reported in the literature as case reports, and include interventions such as optimization of heart failure therapy and similar medical therapy to PLE (357), Fontan fenestration (358), short-term, high-frequency jet ventilation in intubated patients (359), aerosolized urokinase or tissue plasminogen activator (360), pulmonary arterial vasodilators (297), thoracic duct ligation, and cardiac transplantation have been proposed.
Arrhythmias.
One of the major causes of long-term morbidity and mortality in patients following the Fontan procedure is arrhythmia. In a study of long-term survival of 260 Fontan patients with a median follow-up of 12.2 years, the majority of deaths outside of the perioperative period were classified as sudden and were presumed to be arrhythmic in origin (281). The incidence of arrhythmia in Fontan patients range from 7% to 50% depending on Fontan type, era, and years of follow-up (361–364).
Chronic arrhythmia in the Fontan population has been associated with multiple hemodynamic issues, and a lower functional status has independently been associated with the development of intra-atrial re-entrant tachycardia (IART) following Fontan (362). Several atrial arrhythmias have been reported following the Fontan procedure, including atrial tachycardia, atrial fibrillation, and sinus node dysfunction (365, 366). Table 4 lists potential or well recognized risk factors for the development of supraventricular tachycardia (362, 367).
Table 4.
Risk Factors for the Development of SVT
| • Elevated pre-operative pulmonary artery pressures |
|---|
| • Pre-operative arrhythmia |
| • Older age at Fontan operation |
| • Type of Fontan repair |
| • Longer follow-up interval |
| • Increased right atrial size and pressure |
| • Sinus node dysfunction |
| • Heterotaxy |
| • Atrioventricular valve regurgitation |
| • Post-operative SVT |
There have been several studies assessing the relationship of arrhythmia formation and type of Fontan. Historically, these studies have suggested the extracardiac Fontan operation has a lower incidence of post-operative arrhythmias when compared with the LT (8% to 10% vs. 30% to 50%) (368, 369). More recently, a multicenter cohort study of Fontan patients did not find a difference in the prevalence of IART between these subgroups. This difference may relate to patient age at the time of study.
Ventricular rates typically range from 110 to 250 beats/min during atrial tachycardia as opposed to the usually 50 to 70 beats/min seen when in normal sinus rhythm. Thus, persistent mild tachycardia >100 beats/min at rest should raise the suspicion of atrial tachycardia. Symptoms may be nonspecific, such as general malaise, abdominal pain, or nausea, or include palpitations (62%), fatigue (44%), dyspnea (23%), edema (21%), presyncope (21%), or syncope (3%) due to rapid 1:1 atrioventricular conduction (361). Therapeutic modalities for acute cardioversion of IART include transesophageal or intra- atrial pacing, intravenous diltiazem or ibutilide, or synchronized cardioversion; evaluation for atrial thrombus prior to electrical or medical cardioversion is advised as chronic anticoagulation may be needed. Propafenone, beta-blockers, sotalol, and dofetilide have all been used in Fontan patients. Unfortunately, medical therapy tends to be effective in only one-third of patients (361, 370).
Transcatheter ablation may decrease the frequency of tachycardia episodes and the need for repeated cardioversion and anti-arrhythmic drugs. Although an acute success of 72% has been reported in Fontan patients, recurrence rates are high, ranging from 30% to 60% (371, 372). Atrial re-entry circuits tend to be multiple, and may occur in the pulmonary venous atrium (372, 373). Improved anatomic mapping techniques and higher energy sources may improve the short-term outcome of catheter ablation, but the marked atrial hypertrophy in atriopulmonary Fontan patients limits catheter ablation success.
Pacemaker implantation in Fontan patients is reported to be 23% by 20 years of follow-up (265). Pacing strategies have progressed from basic ventricular pacing for bradycardia, to sophisticated antitachycardia pacing algorithms for atrial arrhythmias (374). Epicardial leads are generally utilized due to limitations of venous accessibility and risk for thrombus formation. Chronic atrial pacing may decrease atrial ectopy and the ability to trigger tachycardia. Implantable cardioverter- defibrillators have been used in patients following the Fontan procedure, but no organized retrospective or prospective study of utility or risk factors has been undertaken. Novel implantable cardioverter-defibrillator configurations are often required in congenital heart disease patients, specifically those with single ventricles and usually consist of an epicardial pace/sense lead on the ventricle and defibrillator coil placed in the pericardial, thoracic or subcutaneous space (375). These devices pose significant technical challenges and have a higher failure rate than conventional devices (376).
Conversion of the initial Fontan anastomosis to an extracardiac total cavopulmonary connection with arrhythmia surgery and pacemaker implantation, has demonstrated the ability to eliminate IART and atrial fibrillation, while improving exercise capacity in selected patients (377, 378). Indications for conversion include Fontan pathway obstruction, cyanosis, exercise intolerance, and/or refractory arrhythmias. During midterm follow-up, the modified right atrial maze procedure, intended to address the multiplicity of atrial re-entrant circuits, has essentially eliminated recurrence of IART. The left atrial maze essentially eliminates the recurrence of atrial fibrillation, but has been associated with recurrent organized tachycardia in approximately 15% of patients (379). The maze procedures are not suitable for focal atrial tachycardia, or tachycardia utilizing accessory connections; these mechanisms are ideally treated with catheter ablation techniques. One report on 127 Fontan conversions demonstrated a surgical mortality of 1.6% and a subsequent need for transplantation in 6% (380). Optimal therapeutic approaches are selected based on the type of prior Fontan procedure, mechanism of tachycardia, and associated comorbidities. Efforts to limit the development of bradycardia, perhaps with earlier atrial pacing, and modification of the Fontan surgery are needed to minimize tachycardia development from this palliative surgical intervention.
Adult Congenital Considerations
Survival.
Until recently, studies have shown those born with HLHS in the early 1980s had <30% chance of surviving to adulthood (149, 381). Most of the mortality occurred early within the first year of life, and stabilized over the next 10 years. Given the overall improvement in survival for HLHS, larger percentages are reaching adulthood than in the past. In a study of over 300 patients (53% HLHS) at a single institution, from 1992 to 1999, overall 1-year mortality following the Fontan procedure was 6.6%, and decreased to 1% after 1994. HLHS was not associated with increased morality compared with other univentricular anatomies (323). Therefore, the expectation that 70% of HLHS patients will survive to adulthood may be achievable.
However, reaching adulthood does not imply continued success. Outcome data for the post-surgical Fontan population suggest the morbidity and mortality risks are substantial. Since there are no long-term data regarding adults with repaired HLHS, conclusions can be drawn from the current Fontan population with additional caveats related to aortic arch repair, and a systemic RV. In a multicenter study from European adult congenital heart disease (ACHD) programs, morbidity and mortality were assessed for a variety of CHD lesions over a 5-year period. With 4,110 patients followed (including 198 Fontan patients), the highest mortality was found in those with cyanotic heart disease and Fontan circulation. The Fontan patients had an 8.2% 5-year mortality. In comparison, repaired tetralogy of Fallot, coarctation of the aorta, and D-transposition of the great arteries all were <3% over the same time period (382). Although the cause of death was not specifically stated, the Fontan patients had significant morbidity, with the overall highest percentage of cerebral vascular accidents and supraventricular arrhythmias over the 5-year follow-up. Also, the Fontan group had significant heart failure that worsened over time, with 10% classified as heart failure functional class III/IV. In another study from Toronto evaluating the mean age of death in ACHD patients, those with univen- tricular anatomy status post-Fontan had an astonishing mean age of death <30 yrs (383). The most common cause of death was perioperative. Therefore, assuming the repaired HLHS patients would, at a minimum, follow a similar survival pattern, we would expect a continual decline in survival once reaching adulthood.
Morbidity.
Complications leading to significant morbidity in adults after Fontan operation are well established, and the adult with repaired HLHS carries additional “potential” risks yet to be fully clarified. Known risks, however, include ongoing risk of arrhythmias, thromboembolism, heart failure, exercise intolerance, PLE, Fontan circulation obstruction, pulmonary vein obstruction (right pulmonary veins with atrial-to-pulmonary connection), hepatic dysfunction, and cyanosis from residual or developing right-to-left shunts.
Two large European ACHD studies found at least a 50% incidence of supraventricular arrhythmias, and 3% for ventricular arrhythmias (382, 384). Intra-atrial re-entry tachycardia is the most common form of arrhythmia in this population, and re-entrant circuits tend to be multiple. Any arrhythmia presentation should initiate a work-up for hemodynamic etiologies. Sudden death, presumably from arrhythmias, is found to be the cause of death in approximately 30% of adults with Fontan circulation (383).
Over time, functional class seems to worsen. In a study following 39 adults with Fontan circulation, functional class worsened from predominately class II 10 years out from repair, to functional class III 20 years from Fontan operation (385). A morphologic LV was associated with a higher VO2 max. In general, patients have a lower exercise capacity than expected with a VO2 max % predicted ranging from 55% to 63% (386). With improved surgical techniques, that is, from atrial pulmonary connections to LT and extracardiac Fontan, some of the morbidity related to atrial arrhythmias and heart failure will hopefully improve and will be sustained into adulthood.
There are additional potential complications to consider in the adult with repaired HLHS. Close attention to ventricular function and clinical signs and symptoms of heart failure are warranted in the adult patient, and studies evaluating the benefits of medical therapies are indicated. Additionally, previous studies have demonstrated aortic root and valve abnormalities in those with repaired HLHS. In a study of 53 patients, median follow-up of 9.2 years, 98% developed progressive neoaortic root dilation with a z-score >2. Neoaortic valve regurgitation progressed over time in 49% (332). Therefore, with some follow-up reaching into adolescence, early data suggest neoaortic root dilation and neoaortic valve regurgitation are potential problems and warrant close surveillance. Lastly, unique to the repaired HLHS Fontan patient is an obligatory aortic arch repair. Whether the arch will have a tendency toward restenosis, dilation/aneurysm formation, or both is yet to be determined and also requires close surveillance. It is unclear whether the adult with repaired HLHS will have a risk, similar to the adult with repaired coarctation of the aorta, for systemic hypertension, cerebrovascular disease, and premature coronary artery disease.
Follow-Up.
As repaired HLHS patients enter adulthood, the recommended follow-up should reflect our current knowledge of potential complications as stated above. Recently published ACHD care guidelines do not specifically address the repaired HLHS patient (387). However, recommendations for the care of those with Fontan circulation and aortic arch repair are applicable, with follow-up performed by staff with expertise in ACHD:
- ■
Lifelong, at a minimum yearly, follow-up by cardiologists; - ■
Periodic echocardiography; - ■
Periodic monitoring of liver health, including serum albumin and liver function as well as imaging studies; - ■
Periodic cardiac MRI to evaluate the thoracic aorta; - ■
Warfarin for patients with right-to-left shunts, atrial thrombus, atrial arrhythmias, and/or a TE; - ■
Close monitoring for arrhythmias and consultation with electrophysiologists should be considered.
Pregnancy.
Women with Fontan circulation are able to achieve successful pregnancy and delivery. However, the hemodynamic changes associated with pregnancy, delivery, and post-partum period have the potential to lead to significant complications. The expected increase in plasma volume and heart rate during pregnancy would increase the already substantial risk for atrial arrhythmias. Cardiac output increases and systemic vascular resistance is reduced during pregnancy and usually is well tolerated with Fontan circulation. However, at the time of delivery, cardiac output must increase further and immediately after delivery systemic afterload increases along with a drop in preload from blood loss. This set of hemodynamic changes, especially if ventricular systolic function is diminished, has the potential to be detrimental to maternal Fontan circulation.
Thromboembolic events are increased as a consequence of the expected hypercoagulable state during pregnancy. With Fontan circulation and an existing right-to-left shunt, the risk for a cerebral vascular event would potentially increase during pregnancy.
There are only limited studies retrospectively evaluating women following Fontan operation that have either attempted pregnancy or become pregnant. In one study, a higher than expected incidence of infertility and miscarriages were found when Fontan patients attempted pregnancy (388). In another study of 14 women with Fontan circulation who became pregnant, only 2 cardiovascular events occurred. One developed supraventricular tachycardia, and 1 developed heart failure symptoms during pregnancy. Both had noncomplicated deliveries and survived (389). It is recommended that all women undergo prepregnancy evaluation with a team consisting of high-risk obstetricians, anesthesiologists, and ACHD experts.
Transplant.
Heart transplantation may be utilized as rescue therapy at all stages of palliation for HLHS (390) and most often after completion Fontan for single-ventricle anatomies (391–393). One large multicenter study reviewed outcomes of 97 Fontan patients listed at 17 pediatric centers from 1993 to 2001. In this series, 25% were <4 years of age at time of listing, only 70 of 97 patients survived to transplant, and 1-year survival was 76%, which is in contrast to the current overall survival of >90% for other pediatric recipients (394). Infection was the most common cause of death (30%). Importantly, PLE resolved in all 34 patients who survived >30 days after transplant (393).
There is no clear consensus on the optimal timing of transplantation, and suggested indications for transplantation are summarized in Table 5. In one small series of patients with single-ventricle physiology, there was a significant difference in outcomes between patients transplanted following bidirectional Glenn (100% late survival, n = 9) versus after Fontan (33% survival, n = 6). These authors concluded that for selected high-risk single-ventricle patients, transplant should be considered as an alternative to Fontan completion (393). However, a recent large multi-center study from the Pediatric Heart Transplant Study group found similar outcomes for patients listed after bidirectional Glenn (n = 189) versus those transplanted after Fontan (n = 194); in this series, patients transplanted with Fontan who became ventilator dependent had poor survival (395).
Table 5.
Indications for Heart Transplantation With HLHS
| • Primary transplantation: infants with HLHS and poor RV function |
|---|
| • HLHS s/p Stage I palliation: poor candidates for proceeding to Stage 2 |
| • HLHS s/p Stage II palliation: poor RV function ± moderate/severe TR |
| • HLHS s/p Stage III palliation with Fontan procedure and: |
| ○ Ventricular systolic dysfunction |
| ○ Ventricular diastolic dysfunction |
| ○ Protein-losing enteropathy |
| ○ Plastic bronchitis |
| ○ Refractory arrhythmias (and not suitable for Fontan conversion surgery) |
| ○ Cyanosis: saturation <80% (Fontan), <70% (Glenn); pulmonary AVMs |
A further factor that affects decisions about timing of transplantation is that children listed for transplant have the highest waiting list mortality in solid organ transplantation. In one multicenter series, 20.6% of listed Fontan patients died waiting (393). A review of U.S. registry data of 3,098 children listed for transplant from 1999 to 2006 found 17% of patients died waiting, with risk factors for death including HLHS, ECMO, ventilator support, congenital heart disease, dialysis, and nonwhite ethnicity (141).
Transplant centers in the United States, as well as Canada and Europe have improved waitlist times by listing infants for ABO-incompatible transplantation, which has improved donor scarcity, with acceptable outcomes (396). However, in the United States at the present time, only infants <18 months of age are eligible for ABO-incompatible transplants. Patients with single-ventricle physiology are particularly difficult to support while listed with measures other than ventilation and high-dose ino- tropes, as no ideal ventricular assist device exists. Limited success has been reported with ECMO as well as with the Berlin Heart Ventricular Assist Device, which is awaiting U.S. Food and Drug Administration approval (397, 398).
Children who survive to transplant after Stage I, II, or III palliation commonly have multiple complex technical surgical challenges and medical comorbidities, such as renal, pulmonary, and hepatic insufficiency, which complicate their post-operative course. In addition to these challenges, many of these patients have the added concern of “presensitization.” Single-ventricle patients may develop preformed antibodies to donor human leukocyte antigens, either due to multiple prior blood transfusions, or the presence of prior homograft material (396). Of rescue transplants in children who had HLHS, half had a panel reactive antibody test >10% (390), which may cause morbidity from antibody-mediated rejection (399), and lead to a higher risk of allograft vasculopathy (400). Most transplant centers are incorporating protocols to address sensitization, attempting to desensitize patients at the time of transplant, for example with plasmapheresis, intravenous immunoglobulin (401), and utilizing virtual cross-match protocols, so as not to limit the donor selection pool (402).
Other Topics
Fetal Interventions
Fetal cardiac interventions can be performed for 2 types of HLHS. Most enticingly, it can be performed for severe aortic stenosis (AS) in an attempt to prevent progression to HLHS. At the other end of the spectrum, it can be performed for the otherwise lethal HLHS with intact atrial septum in an attempt to improve perinatal survival and surgical outcome.
Fetal aortic stenosis with evolving HLHS.
The first fetal cardiac interventions (FCI) for AS with LV dilation and dysfunction were performed by Maxwell et al. (403) in London, United Kingdom, in the late 1980s, and this defect remains the major focus of current FCI (404). Although there are only a few studies describing the natural history of fetal AS and the evolution to HLHS, there are no papers proposing otherwise (405–407). Conventional thinking has proposed that valvar AS is the dominant lesion resulting in LV myocardial and mitral valve damage and dysfunction with resultant growth arrest and HLHS at birth. This is likely oversimplifying the pathogenesis, because a primary genetic disorder that simultaneously affects myocardial and valvar development may be a significant contributor to the development and evolution of HLHS. Despite a hiatus in FCI during the 1990s, the hypotheses behind HLHS in this unique subgroup have remained an area of focus in the field of fetal therapy (408–410). To this end, since 2000, the cardiology and perinatal groups in Boston, and other centers around the world have continued to pursue AS dilation in mid-gestation to prevent progression to HLHS, with increasing technical and clinical success (411–416). Despite increasing experience and technical success, fetal loss from the procedure remains around 10% to 15%. If fetal therapy is considered, early referral to a FCI center for evaluation is important because AS, LV dysfunction, and irreversible damage can progress rapidly. Patient selection for FCI for AS continues to evolve. Currently, FCI is not recommended, but instead, close follow-up is undertaken in fetuses with AS with preserved LV function and antegrade arch flow because they might achieve a biventricular repair without FCI. Additionally, FCI is not recommended in fetuses where the LV has gone through the dilation phase and is shrinking, generating low pressure, and has significant endocardial fibroelastosis. Current selection of “appropriate” candidates for FCI are those who are predicted to evolve to HLHS (moderate or more LV dysfunction and retrograde arch flow) but whose LV is still dilated and generating at least fetal systemic pressure as measured by mitral regurgitation jet velocity or aortic stenosis gradient (>20 mm Hg or similar to gestational age) (414, 417).
HLHS with highly restrictive or intact atrial septum.
There are several convincing papers illustrating the highly lethal nature of this subgroup of HLHS (6,418,419). Thus, prenatal creation of an adequate sized atrial septal defect should, in theory, allow for decompression of pulmonary venous return prenatally, and potentially benefit lung and pulmonary vascular development. Fetal creation of an atrial defect can be achieved via percutaneous, transpulmonary parenchymal, right or left atrial puncture, with balloon or stent dilation of the commonly thick atrial septum (420, 421). Although creating an adequate atrial defect (>2.5 mm) prenatally in this group will likely lead to improved clinical stability in the neonatal period, it is likely that the pulmonary parenchymal and pulmonary arterial and venous damage previously incurred during gestation may make these patients poor candidates for early surgical and medium-term survival. Preferably, an atrial defect should be created as early as possible (20 to 25 weeks) to potentially prevent or reverse this damage. However, cannula puncture through the thin atrial wall can lead to fatal hemopericar- dium in mid-gestation. Thus, most procedures for this defect have been performed in the third trimester. Although the short-term survival in published series may be better than the natural history, the medium-term outcome is unknown (421). Not surprisingly, some patients, both those who underwent a successful fetal intervention and those born with an intact, highly restrictive atrial septum, have developed late pulmonary vein stenosis and pulmonary hypertension. Therefore, it behooves us in this field to work towards the creation of novel and miniaturized tools that would enable procedures to be performed earlier in gestation to allow creation of a larger atrial septal defect on a thinner atrial septum, more time for pulmonary parenchymal, lymphatic, and vascular remodeling, and a more stable perinatal transition.
Genetics/Genomics
In addition to hypoplasia of the LV and ascending aorta, the pathological anatomy of the aortic and mitral valves defines HLHS (422–424). Pathology series have shown aortic valve malformation is universal, and mitral valve abnormalities are common, with tricuspid and/or pulmonary valve dysplasia less frequently observed (425–428). The presence of bicuspid aortic valve (BAV) in family members of HLHS probands, the observation that HLHS is part of the in utero natural history of aortic stenosis (414), and the frequent occurrence of left- and right-sided valve dysplasia in HLHS probands suggests that HLHS is a severe form of valve malformation (428).
Genetic origins of HLHS
HLHS is heritable and has been linked with several cytogenetic abnormalities, including Turner and Jacobsen syndromes (20,429,430), and heterozygous mutations in NKX2.5 and GJA1 have been reported in a small number of cases (431, 432). However, the genetic basis of HLHS remains largely unknown. Family clustering of HLHS and other cardiovascular malformations has long been known. Pedigree analyses have been interpreted as indicating simple Mendelian inheritance of HLHS (433), but this interpretation has been challenged (434). To determine the size of the genetic effect (heritability) in families ascertained by a child with HLHS proband, echocardiograms were performed on participating family members using a sequential sampling strategy. All HLHS patients had aortic valve hypoplasia and dysplasia; in addition, dysplasia of the mitral (94%), tricuspid (56%), and pulmonary (11%) valves was also noted. Over half the families had >1 affected individual, and 36% of participants had cardiovascular malformations, including 11% with BAV. Maximum likelihood- based variance decomposition showed that HLHS is a complex trait with a heritability of h2 = 0.99 (428).
HLHS links to chromosomes 10q and 6q and is genetically related to BAV. To demonstrate this, family-based nonparametric genome-wide linkage analysis to identify disease loci for HLHS and evaluate the genetic relationship between HLHS and BAV was performed. Families ascertained by either HLHS (n = 33) or BAV (n = 102) proband were evaluated. The recurrence risk ratio of BAV in HLHS families (8.05) was nearly identical to that in BAV families (8.77). In addition, 5 suggestive loci suggesting linkage were identified (435). These studies indicate that HLHS is determined largely by genetic effects, and specifically, the linkage to multiple loci identifies HLHS as genetically heterogeneous, and suggest complex inheritance of HLHS.
The grouping of left-sided malformations (BAV, aortic coarctation, and HLHS) as causally related was based on epidemiology studies that identified these defects in the same kindreds (436, 437). Further support is provided by the occurrence of these defects in patients with Turner syndrome (429), and identical twins with discordant phenotypes of BAV and HLHS (428). In addition, when families exhibiting these phenotypes are grouped, heritability is identified (438). However, the first evidence of a direct genetic relationship between HLHS and BAV came from the identification of shared loci in a combined cohort of HLHS and BAV families (435); still, the majority of linkage peaks, including the strongest HLHS and BAV loci, could not be confirmed in a combined cohort. This finding suggests that the genetic relationship between HLHS and BAV may not be as strong as has been presumed (439) and suggests that future genetic studies should focus on specific phenotypes to reduce background noise. In addition, several studies have questioned simple Mendelian inheritance of HLHS and BAV (428,434,438,440,441) and suggested they are complex traits.
Neurodevelopment Outcomes
There are now numerous studies of neurodevelopmental outcome after palliative surgery or transplantation for HLHS. There is a distinctive pattern of neurodevelopmen- tal and behavioral dysfunction characterized by mild cognitive impairment, impaired social interaction, and deficits in core communication skills including pragmatic language, as well as inattention, impulsive behavior, and impaired executive function (442, 443). School-age survivors more commonly require remedial services including tutoring, special education, and physical, occupational, and speech therapy.
There are several factors that may pose additional risks for neurodevelopmental outcome in HLHS. It is now widely recognized that prior to birth, children with CHD may have impaired brain growth as well as delayed brain maturity. A recent study demonstrated that fetal brain growth is inversely proportional to the proportion of combined ventricular output through the aortic valve (444). It is also recognized that somatic growth in the first year of life—a critical time for brain development—may play a major role in later developmental outcome.
For those children undergoing the Norwood procedure, aortic arch reconstruction historically has necessitated the use of deep hypothermic circulatory arrest (DHCA). Prolonged periods of DHCA—generally greater than 40 min—have been associated with later neurological impairment in some studies. As such, certain investigators have suggested that regional low-flow perfusion be used to augment cerebral blood flow during deep hypothermia. To date, the neuroprotective benefits of this strategy in HLHS surgery are unproven. A single-institution randomized trial comparing DHCA with regional low-flow perfusion did not demonstrate any difference in the primary outcome measure, the Bayley Scales of Infant Development-II, assesses at 1 year of age (445).
Infant heart transplantation has been undertaken in many centers as an alternative management approach to reconstructive surgery. In general, these studies have reported intermediate-term neurodevelopmental outcomes quite comparable to the assessment following staged surgical palliation (446). Interestingly, one study found that patients who waited longer for transplantation had significantly lower scores on cognitive testing, estimating a 4.5-point fall in IQ for each month on the waiting list. This finding is in keeping with numerous other studies that have reported hospital length of stay to be an important predictor of later developmental status. To date, there are very limited data regarding neurodevelopmental outcome following hybrid- type management of HLHS. Hopefully, recently instituted studies can shed light on neurological implications of this novel approach.
In reviewing published reports, group data must be interpreted with caution: “HLHS” represents a heterogeneous spectrum of disease, both from an anatomic and a physiological perspective, with multiple risk factors for adverse neurodevelopmental outcomes. Patients with genetic syndromes, low birth weight, shock at presentation, or with intraoperative or post-operative complications may have a guarded neurological prognosis, whereas those with a smooth transition to the operating room, an anatomically and physiologically sound repair, and uneventful postoperative course are likely to do well over the long run. Future research should be directed at optimal pre- and post-operative oxygen delivery, further improvements in intraoperative support techniques, and a better understanding and recognition of “neurological resuscitation” after complex open heart surgery in neonates to not only optimize outcomes but to define realistic, risk-adjusted outcome data so that realistic expectations may be obtained.
Effects of Altitude: Attempts at Minimizing Pulmonary Vascular Resistance
Energy efficiency in the Fontan circuit is dependent on low vascular resistance. Altitude may impact outcomes of cavo- pulmonary palliation by elevating baseline pulmonary artery pressures and PVR attributable to pulmonary vasoconstriction and alveolar hypoxia. Thus, in theory, cavopulmonary palliation of single-ventricle physiology should be less effective at elevated altitude. However, the elevation at which clinically significant deterioration in the Fontan palliation occurs is uncertain. Some patients may seemingly do well at elevations of 1,600 m or less, whereas others do poorly. One study noted a trend toward increased survival at altitude in fenestrated patients with mean pulmonary artery pressure >15 mm Hg (447). In a more recent retrospective study at a mean altitude of 1,600 m, the average pre-bidirectional Glenn pulmonary arterial pressure was 15.4 mm Hg and transpulmonary gradient of 8.1 mm Hg (448). Despite these elevated values for this patient cohort, a substantial proportion of patients had favorable outcomes after cavopulmonary palliation, with a survival after bidirectional Glenn of 92.4% at 5 years, and freedom from palliation failure, defined as death, transplant, bidirectional Glenn/Fontan takedown, or revision of 81% at 5 years. Factors for palliation failure at elevated altitude include pulmonary artery pressure >15 mm Hg, and transpulmonary gradient >8 mm Hg, and higher mean altitude than those patients who were asymptomatic at follow-up (1,706 ± 270 m versus 1,579 ± 313 m, p < 0.05). It is interesting that a portion of patients undergoing heart transplantation for failed Fontan circulation may unmask pulmonary vascular disease once cardiac output through the pulmonary circulation is normalized (449). Most centers performing a Fontan procedure at moderate altitude include a fenestration because lack of fenestration at the time of the initial procedure is associated with marginal status in the midterm.
Quality of Life/Air Travel
The complex nature of HLHS and limited information on long-term physical and psychosocial morbidity demand attention to quality-of-life (QOL) issues for survivors. Strategies to minimize limitations and empower parents to pursue therapies, such as early intervention for motor or cognitive delay, should be used to achieve optimal functioning (450). It is important for providers and parents to understand that high treatment burden does not necessarily translate to poor QOL (33), and the majority of patients and families will adapt well to the challenges faced (451, 452).
Findings from QOL research in CHD have been inconsistent (453). This is due in part to methodological issues such as small sample size, use of proxy-reporters, and variable approaches to the measurement of QOL (454, 455). The inconsistency in findings also reflects that the psychological response to the stress of chronic illness is highly individual and is influenced by many factors including personal perceptions, expectations, and values that likely exert far more influence than a specific diagnosis (451,452,456).
Severity of disease is not a reliable predictor of QOL. Other outcomes, such as New York Heart Association functional status, level of education, employment, or exercise tolerance, although they may contribute to QOL, are not adequate substitutes for assessment of QOL directly. The presence of functional limitations, cognitive delays, comorbid conditions, and a higher perceived daily burden of disease have been found to be related to reports of poorer QOL (453,457–460). Patients, parents, and clinicians may have different perspectives on which aspects of a disease and treatment impact QOL (461).
Most studies exploring QOL in CHD have involved heterogeneous groups of patients. Only 2 studies were identified that examined QOL in HLHS exclusively, and both have limitations. In a sample of 38 infants and children with HLHS operated on in the 1990s, parents reported the lowest QOL in infants following Stage II surgical palliation. In the portion of the sample greater than 5 years of age and after Fontan completion, psychosocial function was noted to be lower than a healthy reference sample, but physical function was not different (462). In 18 children with HLHS born from 1993 to 2005 and assessed at 2.7 to 10.6 years of age, parents reported that their children had lower selfesteem, more psychosomatic symptoms, and lower peer acceptance than age- and gender-matched healthy children. The presence of neurological sequelae was an added risk factor for poorer outcomes (459).
Several studies have explored QOL and functional health status in survivors of the Fontan procedure for multiple forms of single ventricle CHD (455). In one study, 67 adults living with single-ventricle CHD reported QOL scores that were similar to a healthy population. Older age and residual cyanosis were related to poorer outcomes (463). Using a cardiac-specific QOL measure, QOL scores were reported to be significantly lower for those with single-ventricle CHD compared with biventricular CHD for children (age 8 to 12 years), adolescents (age 12 to 18 years), and by parent-proxy report (464). A multicenter, cross-sectional study of 537 children with Fontan physiology at a mean age of 11.9 years explored QOL, heart rhythm, exercise tolerance, and the presence of other physical morbidities (458, 465). In this study, parent-reported scores for quality of physical health were approximately 1 standard deviation below normal, and scores for psychosocial health were one-half standard deviation lower than normative values.
Prospective, longitudinal assessment of QOL and its determinants is needed to understand the impact of this disease. Emphasis should be placed on seeking self-report from adolescents and adults as they adjust to increasing demands for social and role functioning.
Air Travel
There are no specific guidelines regarding air travel for patients with CHD, and providers should address specific concerns with their individual patients. Concern has been raised that high altitudes may induce hypoxemia in patients with heart or lung disease. One study examined the effects of altitude in adults with cyanotic CHD using both simulated and actual flight conditions. In both patients and control subjects, transcutaneous SaO2 decreased at a maximum cabin altitude of 8,000 feet. Reductions in SaO2 as compared with sea level values averaged 8.8% in patients and 7.0% in control subjects. These changes were well tolerated, and all patients returned to baseline values in ground conditions (466). A subsequent study documented safe air travel for patients with Eisenmenger syndrome and acyanotic CHD (467). Both studies concluded that there is no justification for limiting air travel in these patient populations.
Patients who require supplemental oxygen need to make special arrangements with their airline prior to air travel. A prescription for oxygen should be provided to the patient. Policies for onboard oxygen vary by carrier, and oxygen service may not be provided. Federal regulations prohibit passengers from bringing or checking their own oxygen canisters on a flight. Some brands of oxygen concentrators are allowed, or canisters can be purchased for use during the flight (468, 469). Patients with pacemakers traveling via air should carry a pacemaker identification card and request a private security screening that does not involve a metal detector or hand-wanding (468).
Cardiac Resynchronization
Very little data are available regarding the use of resynchronization therapy in children, and even less in multisite pacing in patients with single ventricles. Friedberg et al. showed that patients with HLHS, a group that is known to be at high risk of RV failure, do indeed have RV mechanical dyssynchrony unrelated to electrical dyssynchrony (470). In 17 children with HLHS, they found that although electrical dyssynchrony was not seen in this group (median QRS duration of 93 ms [71 to 140 ms]), patients did have significant abnormalities in strain and strain rate when compared with controls (time to peak strain 37 ± 35 ms in patients with HLHS vs. 8 ± 8 ms in controls). This suggests part of the ventricular dysfunction often seen in HLHS may be related to mechanical dyssynchrony and may be amenable to resynchronization therapy.
Bacha et al. (471) Zimmerman et al. (472) evaluated the use of multisite pacing in patients with univentricular hearts in an acute post-operative setting. They compared acute hemodynamic parameters (systolic blood pressure, cardiac index) as well as 3-dimensional echo measurements before and after a brief (20 min) pacing intervention of atrial- multisite ventricular pacing. Ventricular leads were placed on the outflow tract near the semilunar valve, on the right side of the anterior wall, near the atrioventricular groove, and on the ventricular apex. Simultaneous ventricular stimulation at 2 sites was used for this study. The authors found significant improvement in mechanical synchrony, as measured by 3-dimensional echo, which resulted in hemodynamic improvement.
There have been 3 large studies of chronic resynchronization in CHD patients (473–475). Each of these studies had a subgroup of patients with single-ventricle physiology, but the underlying diagnosis of HLHS was found in only a small number of patients. The evaluation of chronic resynchronization therapy by Dubin et al. (475) included 7 patients with single ventricles. Patients had no change in ejection fraction (as measured by radionuclide scan) but did have a decrease in QRS duration. Clinical improvement was seen in 2 of the 7. Janousek et al. (474) had 4 patients with a functional single ventricle (1 HLHS), with clinical improvement (New York Heart Association functional class) seen in 3 patients. Cechin et al. (473) 13 patients with single-ventricle physiology (473) and found clinical improvement in 7 of 11 patients, and ejection fraction improvement in 10 of 11 patients. These data, although somewhat encouraging, need to be interpreted with caution; there are extremely limited data regarding the use of multisite pacing in patients with univentricular hearts, and there are no data regarding appropriate lead placement in these patients. It appears from Friedberg et al. (470) that the substrate of mechanical dyssynchrony is present, and that there may be some potential benefit of this therapy in this complex group, but much more research needs to be done as to where and how resynchronization occurs.
Computational Simulation
The role of computational simulations in the treatment of HLHS patients is increasing, although currently still limited in its direct clinical application. Although there have been many advances in patient-specific modeling and simulation techniques in recent years, these tools are not yet used as standard of care in day-to-day clinical practice. In a recent review article, DeGroff (476) issued a “call to arms” to increase the sophistication and clinical impact of Fontan simulations. In particular, this paper cited a need for, among other things, more realistic methods for modeling respiration, wall compliance, exercise, and increased anatomic sophistication. Above all else, the biggest need in increasing clinical applicability is validation with in vivo data, and direct comparison of simulation parameters to clinical outcomes and patient data.
With that said, there have been impressive advances in the areas of patient-specific modeling, fluid structure interaction, closed-loop circulation modeling, and surgery optimization in recent years. Simulations are now being used to evaluate new surgical designs for the Fontan and Glenn, as well as the first-stage Norwood and BT shunt procedures. These tools are now poised to make significant contributions towards clinical decision making and surgical planning, particularly in the customization of surgeries for individual patients.
The clinical impact of simulation methods on the Fontan procedure began with pioneering computational simulations by de Leval et al. that compared energy loss associated with the standard T-junction Fontan procedure with a newly proposed “offset” model (477, 478). This work subsequently led to the adoption of the offset model as the currently preferred surgical method.
During the ensuing 10 years, there has been ongoing and increasing interest in modeling the Fontan circulation, with contributions in several clinically relevant areas. Simulations have been used to study exercise hemodynamics following the Fontan surgery (479, 480). This helps to fill a much- needed gap, as current diagnostic methods have limited capabilities for determining in vivo flow and pressure during exercise.
Most of the recent Fontan simulation work has focused on a single energy-loss parameter for evaluating Fontan performance. Simulations are now being used to evaluate a broader range of parameters that are believed to be clinically relevant, including wall shear stress (Fig. 7), particle residence times, hepatic flow distribution, and changes in pressure levels and pressure drops (481, 482).
Figure 7. Computational Simulation as an Emerging Tool.
Representative Fontan model showing angiography and computational simulation-derived measures of wall shear stress. The red areas indicate higher levels of shear (when compared with blue or green).
Patient-specific simulations also provide a means to evaluate new surgical methods and interventions at no risk to the patient. As an example of this, simulations have recently been used to evaluate a new bifurcated Y-graft design for the ECC Fontan surgery, and the design has proven to decrease energy losses and improve flow distribution in both idealized and patient specific models (483, 484). Surgery optimization methods are now being expanded to take advantage of sophisticated and efficient tools developed for optimal shape design in the aeronautics industry (485).
Multiscale modeling and lumped parameter networks have been increasing in sophistication in the past several years, and it is now possible to model the entire circulatory system as a closed loop. Whereas uncoupled simulations provide insight only into local hemodynamics, full circulatory models are also able to account for global changes in heart rate, cardiac output, and other parameters. These tools have recently been applied to both the Fontan and Norwood surgeries (486).
Ongoing efforts are also underway to design and simulate mechanical circulatory support devices for Fontan patients. Recent work has pioneered an expandable rotary impeller pump, which is being tested in simulations and mock circuits. Simulations are also being used to design next- generation pediatric ventricular assist devices, which may hold promise for bridge to transplant use in single-ventricle patients (487).
Summary
The dramatic improvements in the treatment and outcomes for HLHS over the past 3 decades have been accomplished through the efforts of many dedicated providers, families, and patients. Current successes and expectations that 70% of newborns born today with HLHS may reach adulthood are exciting, yet one must always remember that the current surgical strategies remain palliative. Caution must be exercised because a great deal remains to be understood as it relates to this group of patients, their QOL, their long-term morbidities, and the sequelae of recent surgical modifications. The work of the Joint Commission on Congenital Heart Disease, the recent publication from the first multicenter randomized surgical trial in congenital heart disease (SVR), and the increased awareness surrounding the CHD population (both pediatric and adult). all herald a new “infancy” in the field of CHD and should give both providers and family members a great deal of hope for the future. Emerging knowledge around fetal interventions, genetics of the disease, computational simulations, and neurodevelopmental outcomes will all likely shape our understanding and may affect future management decisions.
Acknowledgments
Dr. Feinstein has received research grant support from Pfizer and GlaxoSmithKline. Dr. Dubin has received fellowship funding from Medtronic. Dr. Ivy receives salary support for being a consultant for Actelion, Gilead, Pfizer, and United Therapeutics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Schidlow DN, Anderson JB, Klitzner TS, et al. Variation in interstage outpatient care after the Norwood procedure: a report from the Joint Council on Congenital Heart Disease National Quality Improvement Collaborative. Congenit Heart Dis 2011;6:98–107. [DOI] [PubMed] [Google Scholar]
- 2.Galindo A, Nieto O, Villagra´ S, Grañeras A, Herraiz I, Mendoza A. Hypoplastic left heart syndrome diagnosed in fetal life: associated findings, pregnancy outcome and results of palliative surgery. Ultrasound Obstet Gynecol 2009;33:560–6. [DOI] [PubMed] [Google Scholar]
- 3.Hinton RB, Andelfinger G, Sekar P, et al. Prenatal head growth and white matter injury in hypoplastic left heart syndrome. Pediatr Res 2008;64:364–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feit LR, Copel JA, Kleinman CS. Foramen ovale size in the normal and abnormal human fetal heart: an indicator of transatrial flow physiology. Ultrasound Obstet Gynecol 1991;1:313–9. [DOI] [PubMed] [Google Scholar]
- 5.Chin AJ, Weinberg PM, Barber G. Subcostal two-dimensional echocardiographic identification of anomalous attachment of septum primum in patients with left atrioventricular valve underdevelopment. J Am Coll Cardiol 1990;15:678–81. [DOI] [PubMed] [Google Scholar]
- 6.Rychik J, Rome JJ, Collins MH, DeCampli WM, Spray TL. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol 1999;34:554–60. [DOI] [PubMed] [Google Scholar]
- 7.Lurie PR. Changing concepts of endocardial fibroelastosis. Cardiol Young 2010;20:115–23. [DOI] [PubMed] [Google Scholar]
- 8.Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol 1989;25:341–3. [DOI] [PubMed] [Google Scholar]
- 9.Danford DA, Cronican P. Hypoplastic left heart syndrome: progression of left ventricular dilation and dysfunction to left ventricular hypoplasia in utero. Am Heart J 1992;123:1712–3. [DOI] [PubMed] [Google Scholar]
- 10.Fishman N, Hof R, Rudolph A, Heymann M. Models of congenital heart disease in fetal lambs. Circulation 1978;58:354–64. [DOI] [PubMed] [Google Scholar]
- 11.Harh JY, Paul MH, Gallen WJ, Friedberg DZ, Kaplan S. Experimental production of hypoplastic left heart syndrome in the chick embryo. Am J Cardiol 1973;31:51–6. [DOI] [PubMed] [Google Scholar]
- 12.Silverman N, Kleinman C, Rudolph A, et al. Fetal atrioventricular valve insufficiency associated with nonimmune hydrops: a twodimensional echocardiographic and pulsed Doppler ultrasound study. Circulation 1985;72:825–32. [DOI] [PubMed] [Google Scholar]
- 13.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics 2001; 107:1277–82.. [DOI] [PubMed] [Google Scholar]
- 14.Satomi G, Yasukochi S, Shimizu T, Takigiku K, Ishii T. Has fetal echocardiography improved the prognosis of congenital heart disease? Comparison of patients with hypoplastic left heart syndrome with and without prenatal diagnosis. Pediatr Int 1999;41:728–32.. [DOI] [PubMed] [Google Scholar]
- 15.Sivarajan V, Penny DJ, Filan P, Brizard C, Shekerdemian LS. Impact of antenatal diagnosis of hypoplastic left heart syndrome on the clinical presentation and surgical outcomes: the Australian experience. J Paediatr Child Health 2009;45:112–7.. [DOI] [PubMed] [Google Scholar]
- 16.Tworetzky W, McElhinney DB, Reddy VM, Brook MM, Hanley FL, Silverman NH. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation 2001;103:1269–1273.. [DOI] [PubMed] [Google Scholar]
- 17.Verheijen PM, Lisowski LA, Stoutenbeek P, et al. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg 2001;121:798–803.. [DOI] [PubMed] [Google Scholar]
- 18.Shillingford AJ, Ittenbach RF, Marino BS, et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young 2007;17:189–195.. [DOI] [PubMed] [Google Scholar]
- 19.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the norwood procedure for single-ventricle lesions. N Engl J Med 2010;362:1980–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye M, Coldren C, Lai X Liang, et al. Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Hum Mol Genet 2010;19:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natowicz M, Chatten J, Clancy R, et al. Genetic disorders and major extracardiac anomalies associated with the hypoplastic left heart syndrome. Pediatrics 1988;82:698–706.. [PubMed] [Google Scholar]
- 22.Stasik CN, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg 2006;131:412–7.. [DOI] [PubMed] [Google Scholar]
- 23.McElhinney DB, Tworetzky W, Lock JE. Current status of fetal cardiac intervention. Circulation 2010;121:1256–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock-Barziv SM, McCrindle BW, West LJ, Dipchand AI. Waiting before birth: outcomes after fetal listing for heart transplantation. Am J Transplant 2008;8:412–8.. [DOI] [PubMed] [Google Scholar]
- 25.Hospital Stays, Hospital Charges, and In-Hospital Deaths Among Infants with Selected Birth Defects—United States, 2003. MMWR Morb Mortal Wkly Rep 2007;56:25–9.. [PubMed] [Google Scholar]
- 26.Johnson B, Mussatto K, Uhing M, Zimmerman H, Tweddell J, Ghanayem N. Variability in the preoperative management of infants with hypoplastic left heart syndrome. Pediatr Cardiol 2008;29: 515–20. [DOI] [PubMed] [Google Scholar]
- 27.Wernovsky G, Ghanayem N, Ohye RG, et al. Hypoplastic left heart syndrome: consensus and controversies in 2007. Cardiol Young 2007;17:75–86. [DOI] [PubMed] [Google Scholar]
- 28.Graham E, Bradley S, Atz A. Preoperative management of hypoplastic left heart syndrome. Expert Opin Pharmacother 2005;6:687–93. [DOI] [PubMed] [Google Scholar]
- 29.Stieh J, Fischer G, Scheewe J, et al. Impact of preoperative treatment strategies on the early perioperative outcome in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2006;131: 1122–9.e2. [DOI] [PubMed] [Google Scholar]
- 30.Tabbutt S, Ramamoorthy C, Montenegro LM, et al. Impact of inspired gas mixtures on preoperative infants with hypoplastic left heart syndrome during controlled ventilation. Circulation 2001;104: I-159–64. [DOI] [PubMed] [Google Scholar]
- 31.Bove EL, Ohye RG, Devaney EJ. Hypoplastic left heart syndrome: conventional surgical management. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004;7:3–10. [DOI] [PubMed] [Google Scholar]
- 32.Chrisant MRK, Naftel DC, Drummond-Webb J, et al. Fate of infants with hypoplastic left heart syndrome listed for cardiac transplantation: a multicenter study. J Heart Lung Transplant 2005;24: 576–82. [DOI] [PubMed] [Google Scholar]
- 33.Wernovsky G The paradigm shift toward surgical intervention for neonates with hypoplastic left heart syndrome. Arch Pediatr Adolesc Med 2008;162:849–54. [DOI] [PubMed] [Google Scholar]
- 34.Kon AA. Healthcare providers must offer palliative treatment to parents of neonates with hypoplastic left heart syndrome. Arch Pediatr Adolesc Med 2008;162:844–8. [DOI] [PubMed] [Google Scholar]
- 35.Sinha SN, Rusnak SL, Sommers HM, Cole RB, Muster AJ, Paul MH. Hypoplastic left ventricle syndrome: analysis of thirty autopsy cases in infants with surgical considerations. Am J Cardiol 1968;21: 166–73. [DOI] [PubMed] [Google Scholar]
- 36.Deely W, Ehlers K, Levin A, Engle M. Hypoplastic left heart syndrome. Anatomic, physiologic, and therapeutic consideraons. Am J Dis Child 1971;121:168–75. [DOI] [PubMed] [Google Scholar]
- 37.Knott H, Doty D. Experimental bypass of the left ventricle. J Thorac Cardiovasc Surg 1977;74:436–9. [PubMed] [Google Scholar]
- 38.Doty D, Knott H. Hypoplastic left heart syndrome. Experience with an operation to establish functionally normal circulation. J Thorac Cardiovasc Surg 1977;74:624–30. [PubMed] [Google Scholar]
- 39.Mohri H, Horiuchi T, Haneda K, et al. Surgical treatment for hypoplastic left heart syndrome: case reports. J Thorac Cardiovasc Surg 1979;78:223–8. [PubMed] [Google Scholar]
- 40.Milo S, Ho S, Anderson R. Hypoplastic left heart syndrome: can this malformation be treated surgically? Thorax 1980;35:351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doty D, Marvin W Jr., Schieken R, Lauer R. Hypoplastic left heart syndrome: successful palliation with a new operation. J Thorac Cardiovasc Surg 1980;80:148–52. [PubMed] [Google Scholar]
- 42.Behrendt D, Rocchini A. An operation for the hypoplastic left heart syndrome: preliminary report. Ann Thorac Surg 1981;32:284–8. [DOI] [PubMed] [Google Scholar]
- 43.Norwood W, Kirklin J. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol 1980;45:87–91. [DOI] [PubMed] [Google Scholar]
- 44.Norwood W, Lang P, Casteneda A, Campbell D. Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 1981;82:511–9. [PubMed] [Google Scholar]
- 45.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med 1983;308: 23–6. [DOI] [PubMed] [Google Scholar]
- 46.Sano S, Ishino K, Kawada M, et al. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2003;126:504–9. [DOI] [PubMed] [Google Scholar]
- 47.Freed MD, Heymann MA, Lewis AB, Roehl SL, Kensey RC. Prostaglandin E1 infants with ductus arteriosus-dependent congenital heart disease. Circulation 1981;64:899–905. [DOI] [PubMed] [Google Scholar]
- 48.Linday LA, Engle MA. Prostaglandin treatment of newborns with ductal-dependent congenital heart disease. Pediatr Ann 1981;10: 29–38. [PubMed] [Google Scholar]
- 49.Donahoo JS, Roland JM, Kan J, Gardner TJ, Kidd BS. Prostaglandin E1 as an adjunct to emergency cardiac operation in neonates. J Thorac Cardiovasc Surg 1981;81:227–31. [PubMed] [Google Scholar]
- 50.Schleman MM, Casta A, Mongkolsmai C, Black IF. Prostaglandin E1 in the neonate with heart disease. Adv Prostaglandin Thromboxane Res 1980;7:879–81. [PubMed] [Google Scholar]
- 51.Olley PM, Coceani F, Rowe RD, Swyer PR. Clinical use of prostaglandins and prostaglandin synthetase inhibitors in cardiac problems of the newborn. Adv Prostaglandin Thromboxane Res 1980;7:913–6. [PubMed] [Google Scholar]
- 52.Yabek SM, Mann JS. Prostaglandin E1 infusion in the hypoplastic left heart syndrome. Chest 1979;76:330–1. [DOI] [PubMed] [Google Scholar]
- 53.Browdie DA, Norberg W, Agnew R, Altenburg B, Ignacio R, Hamilton C. The use of prostaglandin E1 and Blalock-Taussig shunts in neonates with cyanotic congenital heart disease. Ann Thorac Surg 1979;27:508–13. [DOI] [PubMed] [Google Scholar]
- 54.Graham TP Jr., Atwood GF, Boucek RJ Jr. Pharmacologic dilatation of the ductus arteriosus with prostaglandin E1 in infants with congenital heart disease. South Med J 1978;71:1238–41,46. [DOI] [PubMed] [Google Scholar]
- 55.Lewis AB, Takahashi M, Lurie PR. Administration of prostaglandin E1 in neonates with critical congenital cardiac defects. J Pediatr 1978;93:481–5. [DOI] [PubMed] [Google Scholar]
- 56.Neutze JM, Starling MB, Elliott RB, Barratt-Boyes BG. Palliation of cyanotic congenital heart disease in infancy with E-type prostaglandins. Circulation 1977;55:238–41. [DOI] [PubMed] [Google Scholar]
- 57.Olley PM, Coceani F. Proceedings: emergency treatment of certain cyanotic heart defects with prostaglandin E2. Br Heart J 1976;38:877. [PubMed] [Google Scholar]
- 58.Khouri EM, Gregg DE, Rayford CR. Effect of exercise on cardiac output, left coronary flow and myocardial metabolism in the unanesthetized dog. Circ Res 1965;17:427–37. [DOI] [PubMed] [Google Scholar]
- 59.Ohye RG, Ludomirsky A, Devaney EJ, Bove EL. Comparison of right ventricle to pulmonary artery conduit and modified Blalock- Taussig shunt hemodynamics after the Norwood operation. Ann Thorac Surg 2004;78:1090–3. [DOI] [PubMed] [Google Scholar]
- 60.Donnelly JP, Raffel DM, Shulkin BL, et al. Resting coronary flow and coronary flow reserve in human infants after repair or palliation of congenital heart defects as measured by positron emission tomography. J Thorac Cardiovasc Surg 1998;115:103–10. [DOI] [PubMed] [Google Scholar]
- 61.Azakie A, Merklinger SL, McCrindle BW, et al. Evolving strategies and improving outcomes of the modified Norwood procedure: a 10-year single-institution experience. Ann Thorac Surg 2001;72: 1349–53. [DOI] [PubMed] [Google Scholar]
- 62.Charpie JR, Dekeon MK, Goldberg CS, Mosca RS, Bove EL, Kulik TJ. Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex congenital heart disease. J Thorac Cardiovasc Surg 2000;120:73–80. [DOI] [PubMed] [Google Scholar]
- 63.Mahle WT, Spray TL, Gaynor JW, Clark BJ. Unexpected death after reconstructive surgery for hypoplastic left heart syndrome. Ann Thorac Surg 2001;71:61–5. [DOI] [PubMed] [Google Scholar]
- 64.Kishimoto H, Kawahira Y, Kawata H, Miura T, Iwai S, Mori T. The modified norwood palliation on a beating heart. J Thorac Cardiovasc Surg 1999;118:1130–2. [DOI] [PubMed] [Google Scholar]
- 65.Sano S, Ishino K, Kawada M, Honjo O. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2004;7:22–31. [DOI] [PubMed] [Google Scholar]
- 66.Takabayashi S, Shimpo H, Yokoyama K, Onoda K, Mitani Y. Reduced regional right ventricular wall motion after transventricular repair of tetralogy of Fallot. J Thorac Cardiovasc Surg 2007;133: 1656–8. [DOI] [PubMed] [Google Scholar]
- 67.Mahle WT, Cuadrado AR, Tam VKH. Early experience with a modified norwood procedure using right ventricle to pulmonary artery conduit. Ann Thorac Surg 2003;76:1084–8. [DOI] [PubMed] [Google Scholar]
- 68.Mair R, Tulzer G, Sames E, et al. Right ventricular to pulmonary artery conduit instead of modified Blalock-Taussig shunt improves postoperative hemodynamics in newborns after the norwood operation. J Thorac Cardiovasc Surg 2003;126:1378–84. [DOI] [PubMed] [Google Scholar]
- 69.Pizarro C, Malec E, Maher KO, et al. Right ventricle to pulmonary artery conduit improves outcome after stage I Norwood for hypoplastic left heart syndrome. Circulation 2003;108:II155–60. [DOI] [PubMed] [Google Scholar]
- 70.Azakie A, Martinez D, Sapru A, Fineman J, Teitel D, Karl TR. Impact of right ventricle to pulmonary artery conduit on outcome of the modified norwood procedure. Ann Thorac Surg 2004;77:1727–33. [DOI] [PubMed] [Google Scholar]
- 71.Tabbutt S, Dominguez TE, Ravishankar C, et al. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock Taussig shunt. Ann Thorac Surg 2005;80:1582–91. [DOI] [PubMed] [Google Scholar]
- 72.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 1995;92:2226–35. [DOI] [PubMed] [Google Scholar]
- 73.Tweddell JS, HoiFman GM, Fedderly RT, et al. Phenoxybenzamine improves systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg 1999;67:161–7. [DOI] [PubMed] [Google Scholar]
- 74.Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003;107:996–1002. [DOI] [PubMed] [Google Scholar]
- 75.Tweddell J, Hoffman G. Postoperative management in patients with complex congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5:187–205. [DOI] [PubMed] [Google Scholar]
- 76.Bartram U, Grunenfelder J, Van Praagh R. Causes of death after the modified Norwood procedure: a study of 122 postmortem cases. Ann Thorac Surg 1997;64:1795–802. [DOI] [PubMed] [Google Scholar]
- 77.Rossi AF, Sommer RJ, Lotvin A, et al. Usefulness of intermittent monitoring of mixed venous oxygen saturation after stage I palliation for hypoplastic left heart syndrome. Am J Cardiol 1994;73:1118–23. [DOI] [PubMed] [Google Scholar]
- 78.Hoffman GM, Ghanayem NS, Kampine JM, et al. Venous saturation and the anaerobic threshold in neonates after the Norwood procedure for hypoplastic left heart syndrome. Ann Thorac Surg 2000;70: 1515–20. [DOI] [PubMed] [Google Scholar]
- 79.Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation 2002;106: I-82–9. [PubMed] [Google Scholar]
- 80.Tweddell JS, Ghanayem NS, Mussatto KA, et al. Mixed venous oxygen saturation monitoring after stage 1 palliation for hypoplastic left heart syndrome. Ann Thorac Surg 2007;84:1301–11. [DOI] [PubMed] [Google Scholar]
- 81.Hoffman GM, Tweddell JS, Ghanayem NS, et al. Alteration of the critical arteriovenous oxygen saturation relationship by sustained afterload reduction after the norwood procedure. J Thorac Cardiovasc Surg 2004;127:738–745. [DOI] [PubMed] [Google Scholar]
- 82.Srinivasan C, Sachdeva R, Morrow WR, et al. Standardized management improves outcomes after the Norwood procedure. Congenit Heart Dis 2009;4:329–337. [DOI] [PubMed] [Google Scholar]
- 83.Furck AK, Hansen JH, Uebing A, Scheewe J, Jung O, Kramer H-H. The impact of afterload reduction on the early postoperative course after the Norwood operation — a 12-year single-centre experience. Eur J Cardiothorac Surg 2010;37:289–95. [DOI] [PubMed] [Google Scholar]
- 84.Barnea O, Santamore WP, Rossi A, Salloum E, Chien S, Austin EH. Estimation of oxygen delivery in newborns with a univentricular circulation. Circulation 1998;98:1407–13. [DOI] [PubMed] [Google Scholar]
- 85.Jobes DR, Nicolson SC, Steven JM, Miller M, Jacobs ML, Norwood WI. Carbon dioxide prevents pulmonary overcirculation in hypoplastic left heart syndrome. Ann Thorac Surg 1992;54:150–1. [DOI] [PubMed] [Google Scholar]
- 86.Mora G, Pizarro C, Jacobs M, Norwood W. Experimental model of single ventricle. Influence of carbon dioxide on pulmonary vascular dynamics. Circulation 1994;90:II43–6. [PubMed] [Google Scholar]
- 87.Riordan CJ, Randsbaek F, Storey JH, Montgomery WD, Santamore WP, Austin EH 3rd. Effects of oxygen, positive end-expiratory pressure, and carbon dioxide on oxygen delivery in an animal model of the univentricular heart. J Thorac Cardiovasc Surg 1996;112: 644–54. [DOI] [PubMed] [Google Scholar]
- 88.Ramamoorthy C, Tabbutt S, Kurth CD, et al. Effects of inspired hypoxic and hypercapnic gas mixtures on cerebral oxygen saturation in neonates with univentricular heart defects. Anesthesiology 2002; 96:283–8. [DOI] [PubMed] [Google Scholar]
- 89.Taeed R, Schwartz SM, Pearl JM, et al. Unrecognized pulmonary venous desaturation early after Norwood palliation confounds Gp:Gs assessment and compromises oxygen delivery. Circulation 2001;103: 2699–704. [DOI] [PubMed] [Google Scholar]
- 90.Hoffman GM, Mussatto KA, Brosig CL, et al. Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg 2005;130: 1094–100. [DOI] [PubMed] [Google Scholar]
- 91.Tortoriello TA, Stayer SA, Mott AR, et al. A noninvasive estimation of mixed venous oxygen saturation using near-infrared spectroscopy by cerebral oximetry in pediatric cardiac surgery patients. Paediatr Anaesth 2005;15:495–503. [DOI] [PubMed] [Google Scholar]
- 92.Ranucci M, Isgro G, De La Torre T, Romitti F, Conti D, Carlucci C. Near-infrared spectroscopy correlates with continuous superior vena cava oxygen saturation in pediatric cardiac surgery patients. Paediatr Anaesth 2008;18:1163–9. [DOI] [PubMed] [Google Scholar]
- 93.Kaufman J, Almodovar M, Zuk J, Friesen R. Correlation of abdominal site near-infrared spectroscopy with gastric tonometry in infants following surgery for congenital heart disease. Pediatr Crit Care Med 2008;9:62–8. [DOI] [PubMed] [Google Scholar]
- 94.Chakravarti SB, Mittnacht AJC, Katz JC, Nguyen K, Joashi U, Srivastava S. Multisite near-infrared spectroscopy predicts elevated blood lactate level in children after cardiac surgery. J Cardiothorac Vasc Anesth 2009;23:663–7. [DOI] [PubMed] [Google Scholar]
- 95.Phelps HM, Mahle WT, Kim D, et al. Postoperative cerebral oxygenation in hypoplastic left heart syndrome after the Norwood procedure. Ann Thorac Surg 2009;87:1490–4. [DOI] [PubMed] [Google Scholar]
- 96.Chang AC, Atz AM, Wernovsky G, Burke RP, Wessel DL. Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med 1995;23:1907–14. [DOI] [PubMed] [Google Scholar]
- 97.De Oliveira NC, Ashburn DA, Khalid F, et al. Prevention of early sudden circulatory collapse after the Norwood operation. Circulation 2004;110:II-133–8. [DOI] [PubMed] [Google Scholar]
- 98.Wright GE, Crowley DC, Charpie JR, Ohye RG, Bove EL, Kulik TJ. High systemic vascular resistance and sudden cardiovascular collapse in recovering norwood patients. Ann Thorac Surg 2004;77: 48–52. [DOI] [PubMed] [Google Scholar]
- 99.Tabbutt S, Duncan BW, McLaughlin D, Wessel DL, Jonas RA, Laussen PC. Delayed sternal closure after cardiac operations in a pediatric population. J Thorac Cardiovasc Surg 1997;113:886–93. [DOI] [PubMed] [Google Scholar]
- 100.Gaynor JW, Mahle WT, Cohen MI, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg 2002;22: 82–9. [DOI] [PubMed] [Google Scholar]
- 101.Wernovsky G, Kuijpers M, Van Rossem MC, et al. Postoperative course in the cardiac intensive care unit following the first stage of Norwood reconstruction. Cardiol Young 2007;17:652–65. [DOI] [PubMed] [Google Scholar]
- 102.Blackwood J, Joffe AR, Robertson CMT, et al. Association of hemoglobin and transfusion with outcome after operations for hypoplastic left heart. Ann Thorac Surg 2010;89:1378–84.e2. [DOI] [PubMed] [Google Scholar]
- 103.Jeffries HE, Wells WJ, Starnes VA, Wetzel RC, Moromisato DY. Gastrointestinal morbidity after norwood palliation for hypoplastic left heart syndrome. Ann Thorac Surg 2006;81:982–987. [DOI] [PubMed] [Google Scholar]
- 104.Gaynor JW, Jarvik GP, Bernbaum J, et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg 2006;131:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clancy RR, Sharif U, Ichord R, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia (Series 4) 2005;46: 84–90. [DOI] [PubMed] [Google Scholar]
- 106.Andropoulos DB, Mizrahi EM, Hrachovy RA, et al. Electroenceph- alographic seizures after neonatal cardiac surgery with high-flow cardiopulmonary bypass. Anesth Analg 2010;110:1680–5. [DOI] [PubMed] [Google Scholar]
- 107.Gaynor JW, Nicolson SC, Jarvik GP, et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg 2005;130:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leu- komalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2004;127:692–704. [DOI] [PubMed] [Google Scholar]
- 109.Dent CL, Spaeth JP, Jones BV, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 2005;130:1523–30. [DOI] [PubMed] [Google Scholar]
- 110.Sachdeva R, Hussain E, Moss MM, et al. Vocal cord dysfunction and feeding difficulties after pediatric cardiovascular surgery. J Pediatr 2007;151:312–5.e2. [DOI] [PubMed] [Google Scholar]
- 111.Skinner ML, Halstead LA, Rubinstein CS, Atz AM, Andrews D, Bradley SM. Laryngopharyngeal dysfunction after the Norwood procedure. J Thorac Cardiovasc Surg 2005;130:1293–301. [DOI] [PubMed] [Google Scholar]
- 112.Edwards L, Morris KP, Siddiqui A, Harrington D, Barron D, Brawn W. Norwood procedure for hypoplastic left heart syndrome: BT shunt or RV-PA conduit? Arch Dis Child Fetal Neonatal Ed 2007;92:F210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ichihashi K, Matsui A, Yanagisawa M, Yamada S. Hepatic cell necrosis with congenital heart disease in the newborn. Acta Paediatr Jpn 1991;33:87–92. [DOI] [PubMed] [Google Scholar]
- 114.Ghanayem NS, Hoffman GM, Mussatto KA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg 2003;126:1367–1375. [DOI] [PubMed] [Google Scholar]
- 115.Bautista-Hernandez V, Marx GR, Gauvreau K, et al. Coarctectomy reduces neoaortic arch obstruction in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2007;133:1540–6. [DOI] [PubMed] [Google Scholar]
- 116.Lamers L, Frommelt P, Musatto K, Mitchell M, Tweddell J. Abstract 2351: decreased incidence of recurrent coarctation of the aorta following the Norwood procedure (abstr). Circulation 2007; 116:II515. [Google Scholar]
- 117.Burkhart HM, Ashburn DA, Konstantinov IE, et al. Interdigitating arch reconstruction eliminates recurrent coarctation after the Norwood procedure. J Thorac Cardiovasc Surg 2005;130:61–5. [DOI] [PubMed] [Google Scholar]
- 118.Soongswang J, McCrindle B, Jones T, Vincent R, Hsu D, Kuhn M. Outcomes of transcatheter balloon angioplasty of obstruction in the neo-aortic arch after the Norwood operation. Cardiol Young 2001; 11:54–61. [DOI] [PubMed] [Google Scholar]
- 119.Ballweg JD, Dominguez TE, Ravishankar C, et al. Re-coarctation following stage 1 reconstruction does not adversely affect survival or outcome at Fontan completion (abstr). 35th Annual Meeting of the Western Thoracic Surgical Association. Banff, Alberta, Canada: Western Thoracic Surgical Association, 2009:109–10. [Google Scholar]
- 120.Zeltser I, Menteer J, Gaynor JW, et al. Impact of re-coarctation following the Norwood operation on survival in the balloon angioplasty era. J Am Coll Cardiol 2005;45:1844–1848. [DOI] [PubMed] [Google Scholar]
- 121.Jonas R, Lang P, Hansen D, Hickey P, Castaneda A. First-stage palliation of hypoplastic left heart syndrome. The importance of coarctation and shunt size. J Thorac Cardiovasc Surg 1986;92:6–13. [PubMed] [Google Scholar]
- 122.Gibbs JL, Rothman MT, Rees MR, Parsons JM, Blackburn ME, Ruiz CE. Stenting of the arterial duct: a new approach to palliation for pulmonary atresia. Brit Heart J 1992;67:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruiz CE, Gamra H, Zhang HP, Garcia EJ, Boucek MM. Stenting of the ductus arteriosus as a bridge to cardiac transplantation in infants with the hypoplastic left-heart syndrome. N Engl J Med 1993;328:1605–8. [DOI] [PubMed] [Google Scholar]
- 124.Gibbs JL, Wren C, Watterson KG, Hunter S, Hamilton JR. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: a new approach to palliation for the hypoplastic left heart syndrome. Br Heart J 1993;69:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Artrip JH, Campbell DN, Ivy DD, et al. Birth weight and complexity are significant factors for the management of hypoplastic left heart syndrome. Ann Thorac Surg 2006;82:1252–9. [DOI] [PubMed] [Google Scholar]
- 126.Bacha EA, Daves S, Hardin J, et al. Single-ventricle palliation for high-risk neonates: The emergence of an alternative hybrid stage I strategy. J Thorac Cardiovasc Surg 2006;131:163–171.e2. [DOI] [PubMed] [Google Scholar]
- 127.Egan MJ, Hill SL, Boettner BL, et al. Predictors of retrograde aortic arch obstruction after hybrid palliation of hypoplastic left heart syndrome. Pediatr Cardiol 2011;32:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caldarone C, Benson L, Holtby H, Van Arsdell G. Main pulmonary artery to innominate artery shunt during hybrid palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2005;130:e1–2. [DOI] [PubMed] [Google Scholar]
- 129.Caldarone CA, Benson L, Holtby H, Li J, Redington AN, Van Arsdell GS. Initial experience with hybrid palliation for neonates with single-ventricle physiology. Ann Thorac Surg 2007;84:1294–300. [DOI] [PubMed] [Google Scholar]
- 130.Akintuerk H, Michel-Behnke I, Valeske K, et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation 2002;105:1099–103. [DOI] [PubMed] [Google Scholar]
- 131.Akinturk H, Michel-Behnke I, Valeske K, et al. Hybrid transcatheter-surgical palliation. Pediatr Cardiol 2007;28:79–87. [DOI] [PubMed] [Google Scholar]
- 132.Michel-Behnke I, Akintuerk H, Marquardt I, et al. Stenting of the ductus arteriosus and banding of the pulmonary arteries: basis for various surgical strategies in newborns with multiple left heart obstructive lesions. Heart 2003;89:645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muller M, Akinturk H, Schindler E, et al. A combined stage 1 and 2 repair for hypoplastic left heart syndrome: anaesthetic considerations. Paediatr Anaesth 2003;13:360–5. [DOI] [PubMed] [Google Scholar]
- 134.Hill SL, Galantowicz M, Cheatham JP. Emerging strategies in the treatment of hlhs: combined transcatheter & surgical techniques. Pediatr Cardiol Today 2003;1:1–4. [Google Scholar]
- 135.Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol 2005;26:190–9. Erratum in: Pediatr Cardiol 2005;26:307. [DOI] [PubMed] [Google Scholar]
- 136.Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg 2008;85:2063–71. [DOI] [PubMed] [Google Scholar]
- 137.Holzer R, Wood A, Chisolm J, et al. Atrial septal interventions in patients with hypoplastic left heart syndrome. Catheter Cardiovasc Interv 2008;72:696–704. [DOI] [PubMed] [Google Scholar]
- 138.Fenstermaker B, Berger G, Rowland D, et al. Interstage echocardio- graphic changes in patients undergoing hybrid stage I palliation for hypoplastic left heart syndrome. J Am Soc Echocardiogr 2008;21: 1222–8. [DOI] [PubMed] [Google Scholar]
- 139.Holzer R, Green J, Bergdall V, et al. An animal model for hybrid stage I palliation of hypoplastic left heart syndrome. Pediatr Cardiol 2009;30:922–7. [DOI] [PubMed] [Google Scholar]
- 140.Bailey L Role of cardiac replacement in the neonate. J Heart Transplant 1985;4:506 −9. [PubMed] [Google Scholar]
- 141.Almond CSD, Thiagarajan RR, Piercey GE, et al. Waiting list mortality among children listed for heart transplantation in the United States. Circulation 2009;119:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boucek RJ, Chrisant M. Cardiac transplantation for hypoplastic left heart syndrome. Cardiol Young 2004;14:83–7. [DOI] [PubMed] [Google Scholar]
- 143.West LJ, Pollock-Barziv SM, Dipchand AI, et al. ABO- incompatible heart transplantation in infants. N Engl J Med 2001; 344:793–800. [DOI] [PubMed] [Google Scholar]
- 144.Kirk R, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official pediatric heart transplantation report—2010. J Heart Lung Transplant 2010;29:1119–28. [DOI] [PubMed] [Google Scholar]
- 145.Hehir DA, Dominguez TE, Ballweg JA, et al. Riskfactors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg 2008;136:94–99.e3. [DOI] [PubMed] [Google Scholar]
- 146.Furck AK, Uebing A, Hansen JH, et al. Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: a 12-year single-center survey. J Thorac Cardiovasc Surg 2010;139:359–65. [DOI] [PubMed] [Google Scholar]
- 147.Tweddell JS, Hoffman GM, Fedderly RT, et al. Patients at risk for low systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg 2000;69:1893–9. [DOI] [PubMed] [Google Scholar]
- 148.Jonas R, Hansen D, Cook N, Wessel D. Anatomic subtype and survival after reconstructive operation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 1994;107:1121–7; discussion 1127–8. [PubMed] [Google Scholar]
- 149.Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ 3rd. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation 2000;102:III136–41. [DOI] [PubMed] [Google Scholar]
- 150.Tsao S, Pigula F, Siewers R, et al. Viral illness may be detrimental to patients with HLHS post stage1 Norwood procedure. Paper presented at: Midwest Pediatric Cardiology Meeting; 2000; WI: September 15, 2000. [Google Scholar]
- 151.Kugler JD III RHB, Rosenthal GL, et al. Development of a pediatric cardiology quality improvement collaborative: from inception to implementation. From the Joint Council on Congenital Heart Disease Quality Improvement Task Force. Congenit Heart Dis 2009;4:318–28. [DOI] [PubMed] [Google Scholar]
- 152.Rudd N, Steltzer M, Buckles C, et al. Home surveillance program detects at-risk infants following Norwood palliation. Cardiology 2008: 11th Annual Update on Pediatric Cardiovascular Disease- Abstracts. Cardiol Young 2008;18:191–235. [Google Scholar]
- 153.Nieves J Safely transitioning the fragile neonate to a home environment by utilizing a focused neonatal outpatient clinic. Presented at: the 12th Annual Update on Pediatric and Congenital Cardiovascular Disease, 2009; February 4–8, 2009; Paradise Island, Nassau, Bahamas. [Google Scholar]
- 154.Kelleher D, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition 2006;22:237–44. [DOI] [PubMed] [Google Scholar]
- 155.Parsons JM, Ladusans EJ, Qureshi SA. Balloon dilatation of a stenosed modified (polytetrafluoroethylene) Blalock-Taussig shunt. Brit Heart J 1989;62:228–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marasini M, Dalmonte P, Pongiglione G, et al. Balloon dilatation of critically obstructed modified (polytetrafluoroethylene) Blalock- Taussig shunts. Am J Cardiol 1994;73:405–7. [DOI] [PubMed] [Google Scholar]
- 157.Wang J-K, Wu M-H, Chang C-I, Chiu I-S, Lue H-C. Balloon angioplasty for obstructed modified systemic-pulmonary artery shunts and pulmonary artery stenoses. J Am Coll Cardiol 2001;37: 940–7. [DOI] [PubMed] [Google Scholar]
- 158.Robinson A, Fellows KE, Bridges ND, Rome JJ. Effectiveness of pharmacomechanical thrombolysis in infants and children. Am J Cardiol 2001;87:496–9, A8. [DOI] [PubMed] [Google Scholar]
- 159.Whisenant BK, Baim DS, Kuntz RE, Garcia LA, Ramee SR, Carrozza JP. Rheolytic thrombectomy with the Possis AngioJet: technical considerations and initial clinical experience. J Invasive Cardiol 1999;11:421–6. [PubMed] [Google Scholar]
- 160.Singh M, Tiede DJ, Mathew V, et al. Rheolytic thrombectomy with Angiojet in thrombus-containing lesions. Catheter Cardiovasc Interv 2002;56:1–7. [DOI] [PubMed] [Google Scholar]
- 161.Zahn EM, Chang AC, Aldousany A, Burke RP. Pediatric interventions: emergent stent placement for acute Blalock-Taussig shunt obstruction after stage 1 Norwood surgery. Catheter Cardiovasc Diagn 1997;42:191–4. [DOI] [PubMed] [Google Scholar]
- 162.Lee KJ, Humpl T, Hashmi A, Nykanen DG, Williams WG, Benson LN. Restoration of aortopulmonary shunt patency. Am J Cardiol 2001;88:325–8. [DOI] [PubMed] [Google Scholar]
- 163.Petit CJ, Gillespie MJ, Kreutzer J, Rome JJ. Endovascular stents for relief of cyanosis in single-ventricle patients with shunt or conduit- dependent pulmonary blood flow. Catheter Cardiovasc Interv 2006; 68:280–6. [DOI] [PubMed] [Google Scholar]
- 164.Kogon B, Villari C, Shah N, et al. Occlusion of the modified Blalock-Taussig shunt: unique methods of treatment and review of catheter-based intervention. Congenit Heart Dis 2007;2:185–90. [DOI] [PubMed] [Google Scholar]
- 165.Gillespie MJ, Rome JJ. Transcatheter treatment for systemic-to- pulmonary artery shunt obstruction in infants and children. Catheter Cardiovasc Interv 2008;71:928–35. [DOI] [PubMed] [Google Scholar]
- 166.Moszura T, Zubrzycka M, Michalak K, et al. Acute and late obstruction of a modified Blalock-Taussig shunt: a two-center experience in different catheter-based methods oftreatment. Interact Cardiovasc Thorac Surg 2010;10:727–31. [DOI] [PubMed] [Google Scholar]
- 167.Eicken A, Genz T, Sebening W. Stenting of stenosed shunts in patients after a Norwood-Sano operation. Catheter Cardiovasc Interv 2006;68:301–3. [DOI] [PubMed] [Google Scholar]
- 168.Dahnert I, Riede FT, Razek V, et al. Catheter interventional treatment of Sano shunt obstruction in patients following modified Norwood palliation for hypoplastic left heart syndrome. Clin Res Cardiol 2007;96:719–22. [DOI] [PubMed] [Google Scholar]
- 169.Muyskens S, Nicolas R, Foerster S, Balzer D. Endovascular stent placement for right ventricle to pulmonary artery conduit stenosis in the Norwood with Sano modification. Congenit Heart Dis 2008;3: 185–90. [DOI] [PubMed] [Google Scholar]
- 170.Desai T, Stumper O, Miller P, et al. Acute interventions for stenosed right ventricle-pulmonary artery conduit following the right-sided modification of Norwood-Sano procedure. Congenit Heart Dis 2009;4:433–9. [DOI] [PubMed] [Google Scholar]
- 171.Brown DW, Gauvreau K, Moran AM, et al. Clinical outcomes and utility of cardiac catheterization prior to superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg 2003;126:272–81. [DOI] [PubMed] [Google Scholar]
- 172.del Cerro M, Fernndez A, Espinosa S, et al. Interventional catheterization after the Norwood procedure. Rev Esp Cardiol 2008;61: 146–53. [PubMed] [Google Scholar]
- 173.Moszura T, Mazurek-Kula A, Dryzek P, et al. Interventions complementing surgery as part ofmultistage treatment for hypoplastic left heart syndrome: one center’s experience. Pediatr Cardiol 2009;30: 106–13. [DOI] [PubMed] [Google Scholar]
- 174.Zellers TM. Balloon angioplasty for recurrent coarctation of the aorta in patients following staged palliation for hypoplastic left heart syndrome. Am J Cardiol 1999;84:231–3. [DOI] [PubMed] [Google Scholar]
- 175.Chessa M, Dindar A, Vettukattil JJ, et al. Balloon angioplasty in infants with aortic obstruction after the modified stage I Norwood procedure. Am Heart J 2000;140:227–31. [DOI] [PubMed] [Google Scholar]
- 176.Tworetzky W, McElhinney DB, Burch GH, Teitel DF, Moore P. Balloon arterioplasty of recurrent coarctation after the modified Norwood procedure in infants. Catheter Cardiovasc Interv 2000;50: 54–8. [DOI] [PubMed] [Google Scholar]
- 177.Moore JW, Spicer RL, Mathewson JW, Kirby WC. High-risk angioplasty. Coarctation of the aorta after Norwood Stage 1. Tex Heart Inst J 1993;20:48–50. [PMC free article] [PubMed] [Google Scholar]
- 178.Magee AG, McCrindle BW, Mawson J, Benson LN, Williams WG, Freedom RM. Systemic venous collateral development after the bidirectional cavopulmonary anastomosis: prevalence and predictors. J Am Coll Cardiol 1998;32:502–8. [DOI] [PubMed] [Google Scholar]
- 179.Andrews RE, Tulloh RMR, Anderson DR. Coil occlusion of systemic venous collaterals in hypoplastic left heart syndrome. Heart 2002;88:167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Spicer RL, Uzark KC, Moore JW, Mainwaring RD, Lamberti JJ. Aortopulmonary collateral vessels and prolonged pleural effusions after modified Fontan procedures. Am Heart J 1996;131:1164–8. [DOI] [PubMed] [Google Scholar]
- 181.McElhinney DB, Reddy VM, Tworetzky W, Petrossian E, Hanley FL, Moore P. Incidence and implications of systemic to pulmonary collaterals after bidirectional cavopulmonary anastomosis. Ann Tho- rac Surg 2000;69:1222–8. [DOI] [PubMed] [Google Scholar]
- 182.Bradley SM, McCall MM, Sistino JJ, Radtke WAK. Aortopulmonary collateral flow in the Fontan patient: does it matter? Ann Thorac Surg 2001;72:408–15. [DOI] [PubMed] [Google Scholar]
- 183.Kanter K Management of single ventricle and cavopulmonary connection In: Sellke F, del Nido P, Swanson S, eds. Sabiston and Spencer’s Surgery of the Chest, 8th Edition Philadelphia, PA: Saunders Elsevier, 2010:2041–54. [Google Scholar]
- 184.Ring W Cavopulmonary shunts and the Hemi-Fontan operation In: Kaiser L, Kron I, Spray T, eds. Mastery of Cardiothoracic Surgery. Philadelphia, PA: Lippincott-Raven, 1998:839–47. [Google Scholar]
- 185.Jacobs M Hypoplastic left heart syndrome In: Kaiser L, Kron I, Spray T, eds. Mastery of Cardiothoracic Surgery. Philadelphia, PA: Lipponcott-Ravens, 1998:858–66. [Google Scholar]
- 186.Kogon BE, Plattner C, Leong T, Simsic J, Kirshbom PM, Kanter KR. The bidirectional Glenn operation: a risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg 2008;136: 1237–42. [DOI] [PubMed] [Google Scholar]
- 187.Scheurer M, Hill E, Vasuki N, et al. Survival after bidirectional cavopulmonary anastomosis: analysis of preoperative risk factors. J Thorac Cardiovasc Surg 2007;134:82–9, 9.e1. [DOI] [PubMed] [Google Scholar]
- 188.Manning PB, Mayer JE Jr., Wernovsky G, Fishberger SB, Walsh EP. Staged operation to fontan increases the incidence of sinoatrial node dysfunction. J Thorac Cardiovasc Surg 1996;111:833–40. [DOI] [PubMed] [Google Scholar]
- 189.Norwood WI, Jacobs ML. Fontan’s procedure in two stages. Am J Surg 1993;166:548–51. [DOI] [PubMed] [Google Scholar]
- 190.Kanter KR, Vincent RN, Raviele AA. Importance of acquired systemic-to-pulmonary collaterals in the Fontan operation. Ann Thorac Surg 1999;68:969–74. [DOI] [PubMed] [Google Scholar]
- 191.Cohen MI, Bridges ND, Gaynor JW, et al. Modifications to the cavopulmonary anastomosis do not eliminate early sinus node dysfunction. J Thorac Cardiovasc Surg 2000;120:891–901. [DOI] [PubMed] [Google Scholar]
- 192.Douglas WI, Goldberg CS, Mosca RS, Law IH, Bove EL. Hemi- Fontan procedure for hypoplastic left heart syndrome: outcome and suitability for Fontan. Ann Thorac Surg 1999;68:1361–7. [DOI] [PubMed] [Google Scholar]
- 193.Jaquiss RDB, Ghanayem NS, Hoffman GM, et al. Early cavopul- monary anastomosis in very young infants after the Norwood procedure: impact on oxygenation, resource utilization, and mortality. J Thorac Cardiovasc Surg 2004;127:982–9. [DOI] [PubMed] [Google Scholar]
- 194.Sano S, Huang S-C, Kasahara S, Yoshizumi K, Kotani Y, Ishino K. Risk factors for mortality after the Norwood procedure using right ventricle to pulmonary artery shunt. Ann Thorac Surg 2009;87: 178–86. [DOI] [PubMed] [Google Scholar]
- 195.Liu J, Lu Y, Chen H, Shi Z, Su Z, Ding W. Bidirectional Glenn procedure without cardiopulmonary bypass. Ann Thorac Surg 2004; 77:1349–52. [DOI] [PubMed] [Google Scholar]
- 196.Jonas R Commentary on should the bidirectional Glenn procedure be performed through a thoracotomy without cardiopulmonary bypass? J Thorac Cardiovasc Surg 1999;118:367–8. [DOI] [PubMed] [Google Scholar]
- 197.Jahangiri M, Keogh B, Shinebourne EA, Lincoln C. Should the bidirectional Glenn procedure be performed through a thoracotomy without cardiopulmonary bypass? J Thorac Cardiovasc Surg 1999; 118:367–8. [DOI] [PubMed] [Google Scholar]
- 198.Hopkins RA, Armstrong BE, Serwer GA, Peterson RJ, Oldham HN. Physiological rationale for a bidirectional cavopulmonary shunt. A versatile complement to the Fontan principle. J Thorac Cardiovasc Surg 1985;90:391–8. [PubMed] [Google Scholar]
- 199.Bridges N, Jonas R, Mayer J, Flanagan M, Keane J, Castaneda A. Bidirectional cavopulmonary anastomosis as interim palliation for high-risk Fontan candidates. Early results. Circulation 1990;82:IV 170–6. [PubMed] [Google Scholar]
- 200.Jacobs ML, Rychik J, Rome JJ, et al. Early reduction of the volume work of the single ventricle: the hemi-fontan operation. Ann Thorac Surg 1996;62:575–6. [PubMed] [Google Scholar]
- 201.Gray R, Altmann K, Mosca R, et al. Persistent antegrade pulmonary blood flow post-glenn does not alter early post-Fontan outcomes in single-ventricle patients. Ann Thorac Surg 2007;84:888–93. [DOI] [PubMed] [Google Scholar]
- 202.Yoshida M, Yamaguchi M, Yoshimura N, Murakami H, Matsuhisa H, Okita Y. Appropriate additional pulmonary blood flow at the bidirectional Glenn procedure is useful for completion of total cavopulmonary connection. Ann Thorac Surg 2005;80:976–81. [DOI] [PubMed] [Google Scholar]
- 203.Rabinovitch M, Haworth S, Castaneda A, Nadas A, Reid L. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation 1978;58:1107–22. [DOI] [PubMed] [Google Scholar]
- 204.Fraisse A, Colan SD, Jonas RA, Gauvreau K, Geva T. Accuracy of echocardiography for detection of aortic arch obstruction after stage I Norwood procedure. Am Heart J 1998;135:230–6. [DOI] [PubMed] [Google Scholar]
- 205.McMahon C, Eidem B, Bezold L, et al. Is cardiac catheterization a prerequisite in all patients undergoing bidirectional cavopulmonary anastomosis? J Am Soc Echocardiogr 2003;16:1068–72. [DOI] [PubMed] [Google Scholar]
- 206.Geva T, Greil G, Marshall A, Landzberg M, Powell A. Gadolinium- enhanced 3-dimensional magnetic resonance angiography of pulmonary blood supply in patients with complex pulmonary stenosis or atresia: comparison with x-ray angiography. Circulation 2002;106: 473–8. [DOI] [PubMed] [Google Scholar]
- 207.Tsai-Goodman B, Geva T, Odegard K, Sena L, Powell A. Clinical role, accuracy, and technical aspects of cardiovascular magnetic resonance imaging in infants. Am J Cardiol 2004;94:69–74. [DOI] [PubMed] [Google Scholar]
- 208.Muthurangu V, Taylor AM, Hegde SR, et al. Cardiac magnetic resonance imaging after stage I Norwood operation for hypoplastic left heart syndrome. Circulation 2005;112:3256–3263. [DOI] [PubMed] [Google Scholar]
- 209.Brown DW, Gauvreau K, Powell AJ, et al. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis in infants with functional single ventricle: a prospective randomized trial. Circulation 2007;116:2718–25. [DOI] [PubMed] [Google Scholar]
- 210.Fogel MA. Is routine cardiac catheterization necessary in the management of patients with single ventricles across staged Fontan reconstruction? No! Pediatr Cardiol 2005;26:154–8. [DOI] [PubMed] [Google Scholar]
- 211.Nakanishi T Cardiac catheterization is necessary before bidirectional Glenn and Fontan procedures in single ventricle physiology. Pediatr Cardiol 2005;26:159–61. [DOI] [PubMed] [Google Scholar]
- 212.Triedman JK, Bridges ND, Mayer JE, Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol 1993;22:207–15. [DOI] [PubMed] [Google Scholar]
- 213.Lai L, Laussen P, Cua C, et al. Outcomes after bidirectional Glenn operation: Blalock-Taussig shunt versus right ventricle-to-pulmonary artery conduit. Ann Thorac Surg 2007;83:1768–73. [DOI] [PubMed] [Google Scholar]
- 214.Jaquiss RDB, Siehr S, Ghanayem N, et al. Early cavopulmonary anastomosis after Norwood procedure results in excellent Fontan outcome. Ann Thorac Surg 2006;82:1260–5. [DOI] [PubMed] [Google Scholar]
- 215.Ghanayem N, Tweddell J, Hoffman G, Mussatto K, Jaquiss RDB. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol Young 2006;16 Suppl 1:61–6. [DOI] [PubMed] [Google Scholar]
- 216.Mainwaring RD, Lamberti JJ, Uzark K. The bidirectional Glenn procedure: palliation of the univentricular heart. Advances in cardiac surgery 1994;5:115–40. [PubMed] [Google Scholar]
- 217.Ishiwata T, Kondo C, Nakanishi T, Nakazawa M, Imai Y, Momma K. Nonobstructive ASD creation to qualify patients for the Fontan operation: effects on pulmonary hypertension due to restrictive left atrioventricular valve and interatrial communication. Catheter Car- diovasc Interv 2002;56:528–32. [DOI] [PubMed] [Google Scholar]
- 218.Jonas RA. Intermediate procedures after first-stage Norwood operation facilitate subsequent repair. Ann Thorac Surg 1991;52:696–700. [DOI] [PubMed] [Google Scholar]
- 219.Yahagi N, Kumon K, Tanigami H, et al. Cardiac surgery and inhaled nitric oxide: indication and follow-up (2–4 years). Artif Organs 1998;22:886–91. [DOI] [PubMed] [Google Scholar]
- 220.Nemoto S, Umehara E, Ikeda T, Itonaga T, Komeda M. Oral sildenafil ameliorates impaired pulmonary circulation early after bidirectional cavopulmonary shunt. Ann Thorac Surg 2007;83:e11–3. [DOI] [PubMed] [Google Scholar]
- 221.McElhinney DB,Reddy VM, Hanley FL,Moore P. Systemic venous collateral channels causing desaturation after bidirectional cavopul- monary anastomosis: evaluation and management. J Am Coll Cardiol 1997;30:817–24. [DOI] [PubMed] [Google Scholar]
- 222.Joho-Arreola A, Bauersfeld U, Stauffer U, Baenziger O, Bernet V. Incidence and treatment of diaphragmatic paralysis after cardiac surgery in children. Eur J Cardiothorac Surg 2005;27:53–7. [DOI] [PubMed] [Google Scholar]
- 223.Jonas R, Jonas RA. Cardiac surgery and neurological injury in children. Heart Lung Circ 2000;9:16–22. [DOI] [PubMed] [Google Scholar]
- 224.Wegria R, Zekert H, Entrup RW, et al. Effect of an increase in systemic venous pressure on the formation and evacuation of lymph. Acta Cardiol 1964;19:193–216. [PubMed] [Google Scholar]
- 225.Wegria R, Zekert H, Walter KE, et al. Effect of systemic venous pressure on drainage of lymph from thoracic duct. Am J Physiol 1963;204:284–8. [DOI] [PubMed] [Google Scholar]
- 226.Berdat P, Belli E, Lacour-Gayet F, Planch C, Serraf A. Additional pulmonary blood flow has no adverse effect on outcome after bidirectional cavopulmonary anastomosis. Ann Thorac Surg 2005;79: 29–36. [DOI] [PubMed] [Google Scholar]
- 227.Thompson LD, McElhinney DB, Culbertson CB, et al. Perioperative administration of angiotensin converting enzyme inhibitors decreases the severity and duration of pleural effusions following bidirectional cavopulmonary anastomosis. Cardiol Young 2001;11: 195–200. [DOI] [PubMed] [Google Scholar]
- 228.Mott AR, Spray TL, Gaynor JW, et al. Improved early results with cavopulmonary connections. Cardiol Young 2001;11:3–11. [DOI] [PubMed] [Google Scholar]
- 229.Jacobs ML, Norwood WI. Fontan operation: influence of modifications on morbidity and mortality. Ann Thorac Surg 1994;58:945–51. [DOI] [PubMed] [Google Scholar]
- 230.Fedderly RT, Whitstone BN, Frisbee SJ, Tweddell JS, Litwin SB. Factors related to pleural effusions after Fontan procedure in the era of fenestration. Circulation 2001;104:I-148–51. [DOI] [PubMed] [Google Scholar]
- 231.Pizarro C, Mroczek T, Gidding SS, Murphy JD, Norwood WI. Fontan completion in infants. Ann Thorac Surg 2006;81:2243–9. [DOI] [PubMed] [Google Scholar]
- 232.Cava J, Bevandic S, Steltzer M, Tweddell J. A medical strategy to reduce persistent chest tube drainage after the fontan operation. Am J Cardiol 2005;96:130–3. [DOI] [PubMed] [Google Scholar]
- 233.Mainwaring RD, Lamberti JJ, Uzark K, Spicer RL, Cocalis MW, Moore JW. Effect of accessory pulmonary blood flow on survival after the bidirectional Glenn procedure. Circulation 1999;100:II-151–6. [DOI] [PubMed] [Google Scholar]
- 234.Mainwaring RD, Lamberti JJ, Hugli TE. Complement activation and cytokine generation after modified Fontan procedure. Ann Thorac Surg 1998;65:1715–20. [DOI] [PubMed] [Google Scholar]
- 235.Franqois K, Bove T, De Groote K, et al. Pleural effusions, water balance mediators and the influence of lisinopril after completion Fontan procedures. Eur J Cardiothorac Surg 2009;36:57–62. [DOI] [PubMed] [Google Scholar]
- 236.Alkan T, Sarioglu A, Samanli UB, et al. Atrial natriuretic peptide: could it be a marker for postoperative recurrent effusions after Fontan circulation in complex congenital heart defects? ASAIO J 2006;52: 543–8. [DOI] [PubMed] [Google Scholar]
- 237.Caspi J, Pettitt TW, Mulder T, Stopa A. Development of the pulmonary arteries after the norwood procedure: comparison between Blalock-Taussig shunt and right ventricular-pulmonary artery conduit. Ann Thorac Surg 2008;86:1299–304. [DOI] [PubMed] [Google Scholar]
- 238.Mainwaring RD, Lamberti JJ, Uzark K, Spicer RL. Bidirectional Glenn: is accessory pulmonary blood flow good or bad? Circulation 1995;92:294–7. [DOI] [PubMed] [Google Scholar]
- 239.Wright S, Mainwaring R. Octreotide for management of chylothorax following bidirectional Glenn in a three-month-old infant. J Card Surg 2009;24:216–8. [DOI] [PubMed] [Google Scholar]
- 240.Mainwaring RD, Lamberti JJ, Carter TL, Moore JW, Nelson JC. Renin, angiotensin II, and the development of effusions following bidirectional Glenn and Fontan procedures. J Card Surg 1995;10: 111–8. [DOI] [PubMed] [Google Scholar]
- 241.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pridjian AK, Mendelsohn AM, Lupinetti FM, et al. Usefulness of the bidirectional Glenn procedure as staged reconstruction for the functional single ventricle. Am J Cardiol 1993;71:959–62. [DOI] [PubMed] [Google Scholar]
- 243.Hirsch JC, Goldberg C, Bove EL, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg 2008; 248:402–10. [DOI] [PubMed] [Google Scholar]
- 244.Salvin JW, Scheurer MA, Laussen PC, et al. Factors associated with prolonged recovery after the Fontan operation. Circulation 2008;118: S171–6. [DOI] [PubMed] [Google Scholar]
- 245.Salazar J, Zafar F, Siddiqui K. Fenestration during Fontan palliation: now the exception instead of the rule. J Thorac Cardiovasc Surg 2010;140:129–36. [DOI] [PubMed] [Google Scholar]
- 246.Gentles TL, Mayer JJE, Gauvreau K, et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg 1997;114:376–91. [DOI] [PubMed] [Google Scholar]
- 247.Van Arsdell GS, McCrindle BW, Einarson KD, et al. Interventions associated with minimal Fontan mortality. Ann Thorac Surg 2000; 70:568–74. [DOI] [PubMed] [Google Scholar]
- 248.Morales DLS, Dibardino DJ, Braud BE, et al. Salvaging the failing Fontan: lateral tunnel versus extracardiac conduit. Ann Thorac Surg 2005;80:1445–52. [DOI] [PubMed] [Google Scholar]
- 249.Jahangiri M, Ross DB, Redington AN, Lincoln C, Shinebourne EA. Thromboembolism after the Fontan procedure and its modifications. Ann Thorac Surg 1994;58:1409–13. [DOI] [PubMed] [Google Scholar]
- 250.Morales D, Fraser C. Surgical strategies for the failing systemic ventricle In: Chang A, Towbin J, eds. Heart Failure in Children and Young Adults: From Molecular Mechanisms to Medical and Surgical Strategies. Philadelphia, PA: Saunders Elsevier, 2006:591–606. [Google Scholar]
- 251.Ohye RG, Gomez CA, Goldberg CS, Graves HL, Devaney EJ, Bove EL. Tricuspid valve repair in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2004;127:465–72. [DOI] [PubMed] [Google Scholar]
- 252.Marcelletti C, Corno A, Giannico S, Marino B. Inferior vena cava-pulmonary artery extracardiac conduit. A new form of right heart bypass. J Thorac Cardiovasc Surg 1990;100:228–32. [PubMed] [Google Scholar]
- 253.Robbers-Visser D, Miedema M, Nijveld A, et al. Results of staged total cavopulmonary connection for functionally univentricular hearts; comparison of intra-atrial lateral tunnel and extracardiac conduit. Eur J Cardiothorac Surg 2010;37:934–41. [DOI] [PubMed] [Google Scholar]
- 254.Kumar SP, Rubinstein CS, Simsic JM, Taylor AB, Saul JP, Bradley SM. Lateral tunnel versus extracardiac conduit fontan procedure: a concurrent comparison. Ann Thorac Surg 2003;76:1389–97. [DOI] [PubMed] [Google Scholar]
- 255.Fiore AC, Turrentine M, Rodefeld M, et al. Fontan operation: a comparison of lateral tunnel with extracardiac conduit. Ann Thorac Surg 2007;83:622–30. [DOI] [PubMed] [Google Scholar]
- 256.KrishnankuttyRema R, Dasi LP, Pekkan K, et al. Quantitative analysis of extracardiac versus intraatrial fontan anatomic geometries. Ann Thorac Surg 2008;85:810–7. [DOI] [PubMed] [Google Scholar]
- 257.Amodeo A, Grigioni M, Oppido G, et al. The beneficial vortex and best spatial arrangement in total extracardiac cavopulmonary connection. J Thorac Cardiovasc Surg 2002;124:471–8. [DOI] [PubMed] [Google Scholar]
- 258.Hirsch JC, Ohye RG, Devaney EJ, Goldberg CS, Bove EL. The lateral tunnel Fontan procedure for hypoplastic left heart syndrome: results of 100 consecutive patients. Pediatr Cardiol 2007;28:426–32. [DOI] [PubMed] [Google Scholar]
- 259.Mitchell ME, Ittenbach RF, Gaynor JW, Wernovsky G, Nicolson S, Spray TL. Intermediate outcomes after the Fontan procedure in the current era. J Thorac Cardiovasc Surg 2006;131:172–80. [DOI] [PubMed] [Google Scholar]
- 260.Meyer D, Zamora G, Wernovsky G, et al. Outcomes of the Fontan procedure using cardiopulmonary bypass with aortic cross-clamping. Ann Thorac Surg 2006;82:1611–8. [DOI] [PubMed] [Google Scholar]
- 261.Kim S-J, Kim W-H, Lim H-G, Lee J-Y. Outcome of 200 patients after an extracardiac Fontan procedure. J Thorac Cardiovasc Surg 2008;136:108–16. [DOI] [PubMed] [Google Scholar]
- 262.Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol 2008;52:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hosein RBM, Clarke AJB, McGuirk S, et al. Factors influencing early and late outcome following the Fontan procedure in the current era. The ‘Two Commandments’? Eur J Cardiothorac Surg 2007;31: 344–52. [DOI] [PubMed] [Google Scholar]
- 264.Bridges N, Lock J, Castaneda A. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 1990;82:1681–9. [DOI] [PubMed] [Google Scholar]
- 265.Ono M, Boethig D, Goerler H, Lange M, Westhoff-Bleck M, Breymann T. Clinical outcome of patients 20 years after Fontan operation—effect of fenestration on late morbidity. Eur J Cardiothorac Surg 2006;30:923–9. [DOI] [PubMed] [Google Scholar]
- 266.Lemler MS, Scott WA, Leonard SR, Stromberg D, Ramaciotti C. Fenestration improves clinical outcome of the fontan procedure: a prospective, randomized study. Circulation 2002;105:207–12. [DOI] [PubMed] [Google Scholar]
- 267.Monagle P, Karl T. Thromboembolic problems after the Fontan operation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2002;5:36–47. [DOI] [PubMed] [Google Scholar]
- 268.Kaulitz R, Ziemer G, Paul T, Peuster M, Bertram H, Hausdorf G. Fontan-type procedures: residual lesions and late interventions. Ann Thorac Surg 2002;74:778–85. [DOI] [PubMed] [Google Scholar]
- 269.Atz A, Travison T, McCrindle B. Impact of surgical fenestration in a cohort ofFontan patients (abstr). J Am Coll Cardiol 2009;53:361A.19161887 [Google Scholar]
- 270.Thompson LD, Petrossian E, McElhinney DB, et al. Is it necessary to routinely fenestrate an extracardiac Fontan? J Am Coll Cardiol 1999;34:539–44. [DOI] [PubMed] [Google Scholar]
- 271.Hsu D, Quaegebeur J, Ing F, Selber E, Lamour J, Gersony W. Outcome after the single-stage, nonfenestrated Fontan procedure. Circulation 1997;96:II-335–40. [PubMed] [Google Scholar]
- 272.Harada Y, Uchita S, Sakamoto T, et al. Do we need fenestration when performing two-staged total cavopulmonary connection using an extracardiac conduit? Interact Cardiovasc Thorac Surg 2009;9: 50–4. [DOI] [PubMed] [Google Scholar]
- 273.Morales DLS, Carberry KE, Heinle JS, McKenzie ED, Fraser CD Jr, Diaz LK. Extubation in the operating room after Fontan’s procedure: effect on practice and outcomes. Ann Thorac Surg 2008;86:576–82. [DOI] [PubMed] [Google Scholar]
- 274.Uemura H, Yagihara T, Yamashita K, Ishizaka T, Yoshizumi K, Kawahira Y. Establishment of total cavopulmonary connection without use of cardiopulmonary bypass. Eur J Cardiothorac Surg 1998; 13:504–7. [DOI] [PubMed] [Google Scholar]
- 275.McElhinney DB, Petrossian E, Reddy VM, Hanley FL. Extracardiac conduit fontan procedure without cardiopulmonary bypass. Ann Thorac Surg 1998;66:1826–8. [DOI] [PubMed] [Google Scholar]
- 276.Tam VKH, Miller BE, Murphy K. Modified Fontan without use of cardiopulmonary bypass. Ann Thorac Surg 1999;68:1698–703. [DOI] [PubMed] [Google Scholar]
- 277.Yetman A, Drummond-Webb J, Fiser W, et al. The extracardiac Fontan procedure without cardiopulmonary bypass: technique and intermediate-term results. Ann Thorac Surg 2002;74:S1416–21. [DOI] [PubMed] [Google Scholar]
- 278.Kawahira Y, Uemura H, Yagihara T. Impact of the off-pump fontan procedure on complement activation and cytokine generation. Ann Thorac Surg 2006;81:685–9. [DOI] [PubMed] [Google Scholar]
- 279.Shikata F, Yagihara T, Kagisaki K, et al. Does the off-pump Fontan procedure ameliorate the volume and duration of pleural and peritoneal effusions? Eur J Cardiothorac Surg 2008;34:570–5. [DOI] [PubMed] [Google Scholar]
- 280.Navabi MA, Rastegar SM, Kiani A, et al. Avoiding cardiopulmonary bypass in extracardiac cavopulmonary connection: does it really matter? J Thorac Cardiovasc Surg 2010;139:1183–8. [DOI] [PubMed] [Google Scholar]
- 281.Khairy P, Fernandes SM, Mayer JE Jr. et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85–92. [DOI] [PubMed] [Google Scholar]
- 282.d’Udekem Y, Iyengar A, Cochrane A, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation 2007;116:I157–64. [DOI] [PubMed] [Google Scholar]
- 283.McGuirk SP, Griselli M, Stumper OF, et al. Staged surgical management of hypoplastic left heart syndrome: a single institution 12 year experience. Heart 2006;92:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg 2003;126:1736–45. [DOI] [PubMed] [Google Scholar]
- 285.Gaynor JW, Wernovsky G, Jarvik GP, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg 2007;133:1344–53.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Hirsch J, Gurney J, Donohue J, Gebremariam A, Bove E, Ohye R. Hospital mortality for Norwood and arterial switch operations as a function of institutional volume. Pediatr Cardiol 2008;29:713–7. [DOI] [PubMed] [Google Scholar]
- 287.Mascio CE, Wayment M, Colaizy TT, Mahoney LT, Burkhart HM. The modified Fontan procedure and prolonged pleural effusions. Am Surg 2009;75:175–7. [DOI] [PubMed] [Google Scholar]
- 288.Koutlas TC, Gaynor JW, Nicolson SC, Steven JM, Wernovsky G, Spray TL. Modified ultrafiltration reduces postoperative morbidity after cavopulmonary connection. Ann Thorac Surg 1997;64:37–43. [DOI] [PubMed] [Google Scholar]
- 289.Senzaki H, Masutani S, Ishido H, et al. Cardiac rest and reserve function in patients with Fontan circulation. J Am Coll Cardiol 2006;47:2528–2535. [DOI] [PubMed] [Google Scholar]
- 290.Momma K ACE inhibitors in pediatric patients with heart failure. Paediatr Drugs 2006;8:55–69. [DOI] [PubMed] [Google Scholar]
- 291.Heragu N, Mahony L. Is captopril useful in decreasing pleural drainage in children after modified Fontan operation? Am J Cardiol 1999;84:1109–12. [DOI] [PubMed] [Google Scholar]
- 292.Hsu DT, Zak V, Mahony L, et al. Enalapril does not improve growth or ventricular function in infants with single ventricle: a multicenter clinical trial (abstr). Circulation 2009;120:S560–1. [Google Scholar]
- 293.Hsu D, Mital S, Ravishankar C, et al. Rationale and design of a trial of angiotensin-converting enzyme inhibition in infants with single ventricle. Am Heart J 2009;157:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Jin SM, Noh CI, Bae EJ, Choi JY, Yun YS. Impaired vascular function in patients with Fontan circulation. Int J Cardiol 2007;120: 221–6. [DOI] [PubMed] [Google Scholar]
- 295.Anne P, Du W, Mattoo TK, Zilberman MV. Nephropathy in patients after Fontan palliation. Int J Cardiol 2009;132:244–7. [DOI] [PubMed] [Google Scholar]
- 296.Natarajan S, Heiss C, Yeghiazarians Y, Fineman J, Teitel D, Tacy T. Peripheral arterial function in infants and young children with one-ventricle physiology and hypoxemia. Am J Cardiol 2009;103: 862–6. [DOI] [PubMed] [Google Scholar]
- 297.Haseyama K, Satomi G, Yasukochi S, Matsui H, Harada Y, Uchita S. Pulmonary vasodilation therapy with sildenafil citrate in a patient with plastic bronchitis after the Fontan procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2006;132:1232–3. [DOI] [PubMed] [Google Scholar]
- 298.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio F. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. Eur Heart J 2008;29: 1681–7. [DOI] [PubMed] [Google Scholar]
- 299.Goldberg DJ, Szwast AL, Marino BS, et al. Abstract 2161: sildenafil improves ventricular performance in children and young adults after the Fontan operation (abstr). Circulation 2009;120:S603–b. [Google Scholar]
- 300.Apostolopoulou SC, Papagiannis J, Rammos S. Bosentan induces clinical, exercise and hemodynamic improvement in a pre-transplant patient with plastic bronchitis after Fontan operation. J Heart Lung Transplant 2005;24:1174–6. [DOI] [PubMed] [Google Scholar]
- 301.Votava-Smith JK, Perens GS, Alejos JC. Bosentan for increased pulmonary vascular resistance in a patient with single ventricle physiology and a bidirectional Glenn shunt. Pediatr Cardiol 2007; 28:314–6. [DOI] [PubMed] [Google Scholar]
- 302.Ovaert C, Thijs D, DewolfD, et al. The effect of bosentan in patients with a failing Fontan circulation. Cardiol Young 2009:1–9. [DOI] [PubMed] [Google Scholar]
- 303.Horigome H, Murakami T, Isobe T, Nagasawa T, Matsui A. Soluble P-selectin and thrombomodulin-protein C-Protein S pathway in cyanotic congenital heart disease with secondary erythrocytosis. Thromb Res 2003;112:223–7. [DOI] [PubMed] [Google Scholar]
- 304.Odegard KC, McGowan FX, DiNardo JA, et al. Coagulation abnormalities in patients with single-ventricle physiology precede the Fontan procedure. J Thorac Cardiovasc Surg 2002;123:459–65. [DOI] [PubMed] [Google Scholar]
- 305.Cheung EWY, Chay G, Ma ESK, Cheung Y-F. Systemic oxygen saturation and coagulation factor abnormalities before and after the fontan procedure. Am J Cardiol 2005;96:1571–5. [DOI] [PubMed] [Google Scholar]
- 306.Butenas S, van’t Veer C, Mann KG. ‘‘Normal” thrombin generation. Blood 1999;94:2169–78. [PubMed] [Google Scholar]
- 307.Odegard KC, Zurakowski D, DiNardo JA, et al. Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage I through Fontan completion. J Thorac Cardiovasc Surg 2009;137:934–41. [DOI] [PubMed] [Google Scholar]
- 308.Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg 2001;71:1990–4. [DOI] [PubMed] [Google Scholar]
- 309.Seipelt RG, Franke A, Vazquez-Jimenez JF, et al. Thromboembolic complications after Fontan procedures: comparison of different therapeutic approaches. Ann Thorac Surg 2002;74:556–62. [DOI] [PubMed] [Google Scholar]
- 310.Jacobs ML, Pourmoghadam KK. Thromboembolism and the role of anticoagulation in the Fontan patient. Pediatr Cardiol 2007;28: 457–64. [DOI] [PubMed] [Google Scholar]
- 311.Kaulitz R, Ziemer G, Rauch R, et al. Prophylaxis of thromboembolic complications after the Fontan operation (total cavopulmonary anas-tomosis). J Thorac Cardiovasc Surg 2005;129:569–75. [DOI] [PubMed] [Google Scholar]
- 312.Hanseus K, Bjorkheim G, Sorenson G. Formation of thrombus and thromboembolism after the bidirectional Glenn anastomosis, total cavopulmonary connection and the Fontan operation. Cardiol Young 1998;8:211–6. [Google Scholar]
- 313.Mahnke CB, Boyle GJ, Janosky JE, Siewers RD, Pigula FA. Anticoagulation and incidence of late cerebrovascular accidents following the Fontan procedure. Pediatr Cardiol 2005;26:56–61. [DOI] [PubMed] [Google Scholar]
- 314.Balling G, Vogt M, Kaemmerer H, Eicken A, Meisner H, Hess J. Intracardiac thrombus formation after the Fontan operation. J Thorac Cardiovasc Surg 2000;119:745–51. [DOI] [PubMed] [Google Scholar]
- 315.Monagle P, Cochrane A, McCrindle B, Benson L, Williams W, Andrew M. Editorial: thromboembolic complications after fontan procedures—the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg 1998;115:493–8. [DOI] [PubMed] [Google Scholar]
- 316.Konstantinov IE, Puga FJ, Alexi-Meskishvili VV. Thrombosis of intracardiac or extracardiac conduits after modified Fontan operation in patients with azygous continuation of the inferior vena cava. Ann Thorac Surg 2001;72:1641–4. [DOI] [PubMed] [Google Scholar]
- 317.Jacobs ML, Pourmoghadam KK, Geary EM, et al. Fontan’s operation: is aspirin enough? Is Coumadin too much? Ann Thorac Surg 2002;73:64–8. [DOI] [PubMed] [Google Scholar]
- 318.Walker HA, Gatzoulis MA. Prophylactic anticoagulation following the Fontan operation. Heart 2005;91:854–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Shirai LK, Rosenthal DN, Reitz BA, Robbins RC, Dubin AM. Arrhythmias and thromboembolic complications after the extracardiac Fontan operation. J Thorac Cardiovasc Surg 1998;115:499–505. [DOI] [PubMed] [Google Scholar]
- 320.Monagle P, Cochrane A, Roberts R, et al. Abstract 1157: a multicenter randomized trial comparing Heparin/warfarin versus aspirin as primary thromboprophylaxis for two years after Fontan procedure in children (abstr). Circulation 2008;118:S651. [Google Scholar]
- 321.Monagle P, Chan A, Massicotte P, Chalmers E, Michelson A. Antithrombotic therapy in children: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126: 645S–87. [DOI] [PubMed] [Google Scholar]
- 322.Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five- to fifteen-year follow-up after Fontan operation. Circulation 1992;85:469–96. [DOI] [PubMed] [Google Scholar]
- 323.Gaynor JW, Bridges ND, Cohen MI, et al. Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? J Thorac Cardiovasc Surg 2002;123:237–45. [DOI] [PubMed] [Google Scholar]
- 324.La Gerche A, Gewillig M. What limits cardiac performance during exercise in normal subjects and in healthy Fontan patients? Int J Pediatr 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Nakamura Y, Yagihara T, Kagisaki K, Hagino I, Kobayashi J. Ventricular performance in long-term survivors after Fontan operation. Ann Thorac Surg 2011;91:172–80. [DOI] [PubMed] [Google Scholar]
- 326.Takken T, Tacken MH, Blank AC, Hulzebos EH, Strengers JL, Helders PJ. Exercise limitation in patients with Fontan circulation: a review. J Cardiovasc Med (Hagerstown) 2007;8:775–81. [DOI] [PubMed] [Google Scholar]
- 327.Singh TP, Curran TJ, Rhodes J. Cardiac rehabilitation improves heart rate recovery following peak exercise in children with repaired congenital heart disease. Pediatr Cardiol 2007;28:276–9. [DOI] [PubMed] [Google Scholar]
- 328.Wernovsky G, Rome J, Tabbutt S, et al. Guidelines for the outpatient management of complex congenital heart disease. Congenit Heart Dis 2006;1:10–26. [DOI] [PubMed] [Google Scholar]
- 329.Shachar G, Fuhrman B, Wang Y, Lucas R Jr, Lock J. Rest and exercise hemodynamics after the Fontan procedure. Circulation 1982;65:1043–8. [DOI] [PubMed] [Google Scholar]
- 330.Hsia T-Y, Khambadkone S, Deanfield JE, Taylor JFN, Migliavacca F, de Leval MR. Subdiaphragmatic venous hemodynamics in the Fontan circulation. J Thorac Cardiovasc Surg 2001;121:436–47. [DOI] [PubMed] [Google Scholar]
- 331.Mahle W, Todd K, Fyfe D. Endothelial function following the Fontan operation. Am J Cardiol 2003;91:1286–8. [DOI] [PubMed] [Google Scholar]
- 332.Cohen MS, Marino BS, McElhinney DB, et al. Neo-aortic root dilation and valve regurgitation up to 21 years after staged reconstruction for hypoplastic left heart syndrome. J Am Coll Cardiol 2003;42:533–40. [DOI] [PubMed] [Google Scholar]
- 333.Margossian R, Schwartz M, Prakash A, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol 2009;104:419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Whitehead KK, Sundareswaran KS, Parks WJ, Harris MA, Yoga- nathan AP, Fogel MA. Blood flow distribution in a large series of patients having the Fontan operation: a cardiac magnetic resonance velocity mapping study. J Thorac Cardiovasc Surg 2009;138:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg 2005;129:1348–52. [DOI] [PubMed] [Google Scholar]
- 336.Kiesewetter CH, Sheron N, Vettukattill JJ, et al. Hepatic changes in the failing Fontan circulation. Heart 2007;93:579–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Crupi G, Locatelli G, Tiraboschi R, Villani M, De Tommasi M, Parenzan L. Protein-losing enteropathy after Fontan operation for tricuspid atresia (imperforate tricuspid valve). Thorac Cardiovasc Surg 1980;28:359–63. [DOI] [PubMed] [Google Scholar]
- 338.Feldt RH, Driscoll DJ, Offord KP, et al. Protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg 1996;112: 672–80. [DOI] [PubMed] [Google Scholar]
- 339.Malcic I, Sauer U, Stern H, et al. The influence of pulmonary artery banding on outcome after the Fontan operation. J Thorac Cardiovasc Surg 1992;104:743–7. [PubMed] [Google Scholar]
- 340.Powell AJ, Gauvreau K, Jenkins KJ, Blume ED, Mayer JE, LockJE. Perioperative risk factors for development of protein-losing enteropathy following a Fontan procedure. Am J Cardiol 2001;88:1206–9. [DOI] [PubMed] [Google Scholar]
- 341.Ostrow AM, Freeze H, Rychik J. Protein-losing enteropathy after fontan operation: investigations into possible pathophysiologic mechanisms. Ann Thorac Surg 2006;82:695–700. [DOI] [PubMed] [Google Scholar]
- 342.Rychik J Protein-losing enteropathy after Fontan operation. Con- genit Heart Dis 2007;2:288–300. [DOI] [PubMed] [Google Scholar]
- 343.Hess J, Kruizinga K, Bijleveld CM, Hardjowijono R, Eygelaar A. Protein-losing enteropathy after Fontan operation. J Thorac Cardiovasc Surg 1984;88:606–9. [PubMed] [Google Scholar]
- 344.Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Proteinlosing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg 1998;115:1063–73. [DOI] [PubMed] [Google Scholar]
- 345.Griffiths ER, Kaza AK, Wyler von Ballmoos MC, et al. Evaluating failing Fontans for heart transplantation: predictors of death. Ann Thorac Surg 2009;88:558–63, discussion 563–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Uzun O, Wong JK, Bhole V, Stumper O. Resolution of proteinlosing enteropathy and normalization of mesenteric Doppler flow with sildenafil after Fontan. Ann Thorac Surg 2006;82:e39–40. [DOI] [PubMed] [Google Scholar]
- 347.Rothman A, Snyder J. Protein-losing enteropathy following the Fontan operation: resolution with prednisone therapy. Am Heart J 1991;121:618–9. [DOI] [PubMed] [Google Scholar]
- 348.Rychik J, Piccoli DA, Barber G. Usefulness of corticosteroid therapy for protein-losing enteropathy after the Fontan procedure. Am J Cardiol 1991;68:819–21. [DOI] [PubMed] [Google Scholar]
- 349.Masetti P, Marianeschi SM, Cipriani A, Iorio FS, Marcelletti CF. Reversal of protein-losing enteropathy after ligation of systemic pulmonary shunt. Ann Thorac Surg 1999;67:235–6. [DOI] [PubMed] [Google Scholar]
- 350.Jacobs ML, Rychik J, Byrum CJ, Norwood WI Jr. Protein-losing enteropathy after Fontan operation: resolution after baffle fenestration. Ann Thorac Surg 1996;61:206–8. [DOI] [PubMed] [Google Scholar]
- 351.Cohen MI, Rhodes LA, Wernovsky G, Gaynor JW, Spray TL, Rychik J. Atrial pacing: an alternative treatment for protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg 2001;121:582–3. [DOI] [PubMed] [Google Scholar]
- 352.Sheikh AM, Tang AT, Roman K, et al. The failing Fontan circulation: successful conversion of atriopulmonary connections. J Thorac Cardiovasc Surg 2004;128:60–6. [DOI] [PubMed] [Google Scholar]
- 353.Weinstein S, Chan D. Extracardiac Fontan conversion, cryoablation, and pacemaker placement for patients with a failed Fontan. Semin Thorac Cardiovasc Surg 2005;17:170–8. [DOI] [PubMed] [Google Scholar]
- 354.Brancaccio G, Carotti A, D’Argenio P, Michielon G, Parisi F. Protein-losing enteropathy after Fontan surgery: resolution after cardiac transplantation. J Heart Lung Transplant 2003;22:484–6. [DOI] [PubMed] [Google Scholar]
- 355.Sierra C, Calleja F, Picazo B, Martinez-Valverde A. Protein-losing enteropathy secondary to Fontan procedure resolved after cardiac transplantation. J Pediatr Gastroenterol Nutr 1997;24:229–30. [DOI] [PubMed] [Google Scholar]
- 356.Goo HW, Jhang WK, Kim YH, et al. CT findings of plastic bronchitis in children after a Fontan operation. Pediatr Radiol 2008;38:989–93. [DOI] [PubMed] [Google Scholar]
- 357.Barber BJ, Burch GH, Tripple D, Balaji S. Resolution of plastic bronchitis with atrial pacing in a patient with fontan physiology. Pediatr Cardiol 2004;25:73–6. [DOI] [PubMed] [Google Scholar]
- 358.Wilson J, Russell J, Williams W, Benson L. Fenestration of the Fontan circuit as treatment for plastic bronchitis. Pediatr Cardiol 2005;26:717–9. [DOI] [PubMed] [Google Scholar]
- 359.Zahorec M, Kovacikova L, Martanovic P, Skrak P, Kunovsky P. The use of high-frequency jet ventilation for removal of obstructing casts in patients with plastic bronchitis. Pediatr Crit Care Med 2009;10: e34–6. [DOI] [PubMed] [Google Scholar]
- 360.Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med 2005;6:76–8. [DOI] [PubMed] [Google Scholar]
- 361.Ghai A, Harris L, Harrison DA, Webb GD, Siu SC. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol 2001;37:585–92. [DOI] [PubMed] [Google Scholar]
- 362.Stephenson EA, Lu M, Berul CI, et al. Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross-sectional study. J Am Coll Cardiol 2010;56:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Giannico S, Hammad F, Amodeo A, et al. Clinical outcome of 193 extracardiac Fontan patients: the first 15 years. J Am Coll Cardiol 2006;47:2065–73. [DOI] [PubMed] [Google Scholar]
- 364.Gewillig M, Wyse RK, de Leval MR, Deanfield JE. Early and late arrhythmias after the Fontan operation: predisposing factors and clinical consequences. Br Heart J 1992;67:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kurer C, Tanner C, Norwood W, Vetter V. Perioperative arrhythmias after Fontan repair. Circulation 1990;82:IV190–4. [PubMed] [Google Scholar]
- 366.Cohen M, Wernovsky G, Vetter V, et al. Sinus node function after a systematically staged Fontan procedure. Circulation 1998;98: II352–8, discussion II358−9. [PubMed] [Google Scholar]
- 367.Fishberger SB, Wernovsky G, Gentles TL, et al. Factors that influence the development of atrial flutter after the fontan operation. J Thorac Cardiovasc Surg 1997;113:80–6. [DOI] [PubMed] [Google Scholar]
- 368.Ovroutski S, Dahnert I, Alexi-Meskishvili V, Nurnberg JH, Hetzer R, Lange PE. Preliminary analysis of arrhythmias after the Fontan operation with extracardiac conduit compared with intra-atrial lateral tunnel. Thorac Cardiovasc Surg 2001;49:334–7. [DOI] [PubMed] [Google Scholar]
- 369.Azakie A, McCrindle BW, Van Arsdell G, et al. Extracardiac conduit versus lateral tunnel cavopulmonary connections at a single institution: impact on outcomes. J Thorac Cardiovasc Surg 2001;122: 1219–28. [DOI] [PubMed] [Google Scholar]
- 370.Deal BJ, Mavroudis C, Backer CL. Arrhythmia management in the Fontan patient. Pediatr Cardiol 2007;28:448–56. [DOI] [PubMed] [Google Scholar]
- 371.Collins KK, Love BA, Walsh EP, Saul JP, Epstein MR, Triedman JK. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol 2000;86:969–74. [DOI] [PubMed] [Google Scholar]
- 372.Triedman JK, Alexander ME, Berul CI, Bevilacqua LM, Walsh EP. Electroanatomic mapping of entrained and exit zones in patients with repaired congenital heart disease and intra-atrial reentrant tachycardia. Circulation 2001;103:2060–5. [DOI] [PubMed] [Google Scholar]
- 373.De Groot N, Blom N, Wall EEV, Schalij M. Different mechanisms underlying consecutive, postoperative atrial tachyArrhythmias in a Fontan patient. Pacing Clin Electrophysiol 2009;32:e18–20. [DOI] [PubMed] [Google Scholar]
- 374.Tsao S, Deal BJ, Backer CL, Ward K, Franklin WH, Mavroudis C. Device management of arrhythmias after Fontan conversion. J Thorac Cardiovasc Surg 2009;138:937–40. [DOI] [PubMed] [Google Scholar]
- 375.Stephenson EA, Batra AS, Knilans TK, et al. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol 2006;17:41–6. [DOI] [PubMed] [Google Scholar]
- 376.Radbill AE, Triedman JK, Berul CI, et al. System survival of nontransvenous implantable cardioverter-defibrillators compared to transvenous implantable cardioverter-defibrillators in pediatric and congenital heart disease patients. Heart Rhythm 2010;7:193–8. [DOI] [PubMed] [Google Scholar]
- 377.Mavroudis C, Backer CL, Deal BJ, Johnsrude CL. Fontan conversion to cavopulmonary connection and arrhythmia circuit cryoablation. J Thorac Cardiovasc Surg 1998;115:547–56. [DOI] [PubMed] [Google Scholar]
- 378.Marcelletti CF, Hanley FL, Mavroudis C, et al. Revision of previous Fontan connections to total extracardiac cavopulmonary anastomosis: A multicenter experience. J Thorac Cardiovasc Surg 2000;119: 340–6. [DOI] [PubMed] [Google Scholar]
- 379.Mavroudis C, Deal BJ, Backer CL, et al. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg 2007;84:1457–66, discussion 1465–6. [DOI] [PubMed] [Google Scholar]
- 380.Dearani J, Mavroudis C, Quintessenza J, et al. Surgical advances in the treatment of adults with congenital heart disease. Curr Opin Pediatr 2009;21:565–72. [DOI] [PubMed] [Google Scholar]
- 381.Tibballs J, Kawahira Y, Carter BG, Donath S, Brizard C, Wilkinson J. Outcomes of surgical treatment of infants with hypoplastic left heart syndrome: an institutional experience 1983–2004. J Paediatr Child Health. 2007;43:746–51. [DOI] [PubMed] [Google Scholar]
- 382.Engelfriet P, Boersma E, Oechslin E, et al. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. Eur Heart J 2005;26:2325–33. [DOI] [PubMed] [Google Scholar]
- 383.Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol 2000;86:1111–6. [DOI] [PubMed] [Google Scholar]
- 384.van der Velde ET, Vriend JWJ, Mannens MM, Uiterwaal CS, Brand R, Mulder BJ. CONCOR, an initiative towards a national registry and DNA-bank of patients with congenital heart disease in the Netherlands: rationale, design, and first results. Eur J Epidemiol 2005;20:549–57. [DOI] [PubMed] [Google Scholar]
- 385.van den Bosch A, Roos-Hesselink J, Van Domburg R, Bogers AJJC, Simoons M, Meijboom F. Long-term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol 2004;93: 1141–5. [DOI] [PubMed] [Google Scholar]
- 386.Ohuchi H, Yasuda K, Hasegawa S, et al. Influence of ventricular morphology on aerobic exercise capacity in patients after the Fontan operation. J Am Coll Cardiol 2001;37:1967–74. [DOI] [PubMed] [Google Scholar]
- 387.Warnes C, Williams R, Bashore T, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). J Am Coll Cardiol 2008;52:e1–121. [DOI] [PubMed] [Google Scholar]
- 388.Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Pregnancy and delivery in women after Fontan palliation. Heart 2006;92:1290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Canobbio MM, Mair DD, van der Velde M, Koos BJ. Pregnancy outcomes after the Fontan repair. J Am Coll Cardiol 1996;28:763–7. [DOI] [PubMed] [Google Scholar]
- 390.Jacobs JP, Quintessenza JA, Chai PJ, et al. Rescue cardiac transplantation for failing staged palliation in patients with hypoplastic left heart syndrome. Cardiol Young 2006;16:556–62. [DOI] [PubMed] [Google Scholar]
- 391.Gamba A, Merlo M, Fiocchi R, et al. Heart transplantation in patients with previous Fontan operations. J Thorac Cardiovasc Surg 2004;127:555–62. [DOI] [PubMed] [Google Scholar]
- 392.Bernstein D, Naftel D, Chin C, et al. Outcome of listing for cardiac transplantation for failed Fontan: a multi-institutional study. Circulation 2006;114:273–80. [DOI] [PubMed] [Google Scholar]
- 393.Michielon G, Parisi F, Di Carlo D, et al. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg 2003;24:502–10. [DOI] [PubMed] [Google Scholar]
- 394.Kirk R, Edwards L, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: Twelfth Official Pediatric Heart Transplantation Report-2009. J Heart Lung Transplant 2009;28:993–1006. [DOI] [PubMed] [Google Scholar]
- 395.Kovach J, Blume E, Naftel D. Comparison of risk factors and outcomes for pediatric patients listed for heart transplantation after bidirectional Glenn and after Fontan: a multi-institutional study. J Heart Lung Transplant 2010;29:2:S71. [DOI] [PubMed] [Google Scholar]
- 396.Dipchand AI, BarZiv SMP, Manlhiot C, West LJ, VanderVliet M, McCrindle BW. Equivalent outcomes for pediatric heart transplantation recipients: ABO-blood group incompatible versus ABO- compatible. Am J Transplant 2010;10:389–97. [DOI] [PubMed] [Google Scholar]
- 397.Nathan M, Baird C, Fynn-Thompson F, et al. Successful implantation of a Berlin heart biventricular assist device in a failing single ventricle. J Thorac Cardiovasc Surg 2006;131:1407–8. [DOI] [PubMed] [Google Scholar]
- 398.Calvaruso D, Ocello S, Salviato N, et al. Implantation of a Berlin Heart as single ventricle by-pass on Fontan circulation in univen- tricular heart failure. ASAIO J 2007;53:e1–2. [DOI] [PubMed] [Google Scholar]
- 399.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody- mediated rejection of cardiac transplants. J Heart Lung Transplant 2006;25:153–9. [DOI] [PubMed] [Google Scholar]
- 400.Wu GW, Kobashigawa JA, Fishbein MC, et al. Asymptomatic antibody-mediated rejection after heart transplantation predicts poor outcomes. J Heart Lung Transplant 2009;28:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Holt DB, Lublin DM, Phelan DL, et al. Mortality and morbidity in pre-sensitized pediatric heart transplant recipients with a positive donor crossmatch utilizing peri-operative plasmapheresis and cytolytic therapy. J Heart Lung Transplant 2007;26:876–82. [DOI] [PubMed] [Google Scholar]
- 402.Zangwill S, Ellis T, Stendahl G, Zahn A, Berger S, Tweddell J. Practical application of the virtual crossmatch. Pediatr Transplant 2007;11:650–4. [DOI] [PubMed] [Google Scholar]
- 403.Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Brit Heart J 1991;65:256–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Kohl T, Sharland G, Allan LD, et al. World experience of percutaneous ultrasound-guided balloon valvuloplasty in human fetuses with severe aortic valve obstruction. Am J Cardiol 2000;85:1230–3. [DOI] [PubMed] [Google Scholar]
- 405.Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart 1997;77:205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.McCaffrey FM, Sherman FS. Prenatal diagnosis of severe aortic stenosis. Pediatr Cardiol 1997;18:276–81. [DOI] [PubMed] [Google Scholar]
- 407.Makikallio K, McElhinney DB, Levine JC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation 2006;113:1401–5. [DOI] [PubMed] [Google Scholar]
- 408.Kohl T, Szabo Z, Suda K, et al. Fetoscopic and open transumbilical fetal cardiac catheterization in sheep: potential approaches for human fetal cardiac intervention. Circulation 1997;95:1048–53. [DOI] [PubMed] [Google Scholar]
- 409.Kohl T, Witteler R, Strmper D, et al. Operative techniques and strategies for minimally invasive fetoscopic fetal cardiac interventions in sheep. Surg Endosc 2000;14:424–30. [DOI] [PubMed] [Google Scholar]
- 410.Kohl T, Strumper D, Witteler R, et al. Fetoscopic direct fetal cardiac access in sheep : an important experimental milestone along the route to human fetal cardiac intervention. Circulation 2000;102:1602–4. [DOI] [PubMed] [Google Scholar]
- 411.Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation 2004;110:2125–31. [DOI] [PubMed] [Google Scholar]
- 412.Marshall A, Tworetzky W, Bergersen L, et al. Aortic valvuloplasty in the fetus: technical characteristics of successful balloon dilation. J Pediatr 2005;147:535–9. [DOI] [PubMed] [Google Scholar]
- 413.Selamet Tierney ES, Wald RM, McElhinney DB, et al. Changes in left heart hemodynamics after technically successful in-utero aortic valvuloplasty. Ultrasound Obstet Gynecol 2007;30:715–20. [DOI] [PubMed] [Google Scholar]
- 414.McElhinney DB, Marshall AC, Wilkins-Haug LE, et al. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation 2009;120:1482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Mizrahi-Arnaud A, Tworetzky W, Bulich L, et al. Pathophysiology, management, and outcomes of fetal hemodynamic instability during prenatal cardiac intervention. Pediatr Res 2007;62:325–30. [DOI] [PubMed] [Google Scholar]
- 416.Vogel M, Wilkins-Haug L, McElhinney D, et al. Reversible ductus arteriosus constriction due to maternal indomethacin after fetal intervention for hypoplastic left heart syndrome with intact/restrictive atrial septum. Fetal Diagn Ther 2010;27:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Johnson P, Maxwell DJ, Tynan MJ, Allan LD. Intracardiac pressures in the human fetus. Heart 2000;84:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Vlahos AP, Lock JE, McElhinney DB, van der Velde ME. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: outcome after neonatal transcatheter atrial septostomy. Circulation 2004;109:2326–30. [DOI] [PubMed] [Google Scholar]
- 419.Glatz J, Tabbutt S, Gaynor JW, et al. Hypoplastic left heart syndrome with atrial level restriction in the era of prenatal diagnosis. Ann Thorac Surg 2007;84:1633–8. [DOI] [PubMed] [Google Scholar]
- 420.Marshall A, Levine J, Morash D, et al. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn 2008;28:1023–8. [DOI] [PubMed] [Google Scholar]
- 421.Marshall AC, van der Velde ME, Tworetzky W, et al. Creation of an atrial septal defect in utero for fetuses with hypoplastic left heart syndrome and intact or highly restrictive atrial septum. Circulation 2004;110:253–8. [DOI] [PubMed] [Google Scholar]
- 422.Lev M Pathologic anatomy and interrelationship of hypoplasia of the aortic tract complexes. Lab Invest 1952;1:61–70. [PubMed] [Google Scholar]
- 423.Noonan JA, Nadas AS. The hypoplastic left heart syndrome; an analysis of 101 cases. Pediatr Clin North Am 1958;5:1029–56. [DOI] [PubMed] [Google Scholar]
- 424.Tchervenkov CI, Jacobs JP, Weinberg PM, et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol Young 2006;16:339–68. [DOI] [PubMed] [Google Scholar]
- 425.Bharati S, Lev M. The surgical anatomy of hypoplasia of aortic tract complex. J Thorac Cardiovasc Surg 1984;88:97–101. [PubMed] [Google Scholar]
- 426.Stamm C, Anderson RH, Ho SY. The morphologically tricuspid valve in hypoplastic left heart syndrome. Eur J Cardiothorac Surg 1997;12:587–92. [DOI] [PubMed] [Google Scholar]
- 427.Bharati S, Nordenberg A, Brock RR, Lev M. Hypoplastic left heart syndrome with dysplastic pulmonary valve with stenosis. Pediatr Cardiol 1984;5:127–30. [DOI] [PubMed] [Google Scholar]
- 428.Hinton RB Jr., Martin LJ, Tabangin ME, Mazwi ML, Cripe LH, Benson DW. Hypoplastic left heart syndrome is heritable. J Am Coll Cardiol 2007;50:1590–5. [DOI] [PubMed] [Google Scholar]
- 429.Surerus E, Huggon IC, Allan LD. Turner’s syndrome in fetal life. Ultrasound Obstet Gynecol 2003;22:264–7. [DOI] [PubMed] [Google Scholar]
- 430.Grossfeld PD, Mattina T, Lai Z, et al. The 11q terminal deletion disorder: a prospective study of 110 cases. Am J Med Genet A 2004;129A:51–61. [DOI] [PubMed] [Google Scholar]
- 431.Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE). Mutat Res 2001; 479:173–86. [DOI] [PubMed] [Google Scholar]
- 432.Elliott DA, Kirk EP, Yeoh T, et al. Cardiac homeobox gene NKX2–5 mutations and congenital heart disease: associations with atrial septal defect and hypoplastic left heart syndrome. J Am Coll Cardiol 2003;41:2072–6. [DOI] [PubMed] [Google Scholar]
- 433.Shokeir MH. Hypoplastic left heart. Evidence for possible autosomal recessive inheritance. Birth Defects Orig Artic Ser 1974;10:223–7. [PubMed] [Google Scholar]
- 434.Holmes LB, Rose V, Child AH, Kratzer W. Hypoplastic left heart. Evidence for possible autosomal recessive inheritance. Comment. Birth Defects Orig Artic Ser 1974;10:228–30. [PubMed] [Google Scholar]
- 435.Hinton RB, Martin LJ, Rame-Gowda S, Tabangin ME, Cripe LH, Benson DW. Hypoplastic left heart syndrome links to chromosomes 10q and 6q and is genetically related to bicuspid aortic valve. J Am Coll Cardiol 2009;53:1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Ferencz C, Rubin JD, McCarter RJ, et al. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol 1985;121:31–6. [DOI] [PubMed] [Google Scholar]
- 437.Brenner JI, Berg KA, Schneider DS, Clark EB, Boughman JA. Cardiac malformations in relatives of infants with hypoplastic left- heart syndrome. Am J Dis Child 1989;143:1492–4. [DOI] [PubMed] [Google Scholar]
- 438.McBride KL, Pignatelli R, Lewin M, et al. Inheritance analysis of congenital left ventricular outflow tract obstruction malformations: segregation, multiplex relative risk, and heritability. Am J Med Genet A 2005;134A:180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.McBride KL, Zender GA, Fitzgerald-Butt SM, et al. Linkage analysis of left ventricular outflow tract malformations (aortic valve stenosis, coarctation of the aorta, and hypoplastic left heart syndrome). Eur J Hum Genet 2009;17:811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Wessels MW, van de Laar IM, Roos-Hesselink J, et al. Autosomal dominant inheritance of cardiac valves anomalies in two families: extended spectrum ofleft-ventricular outflow tract obstruction. Am J Med Genet A 2009;149A:216–25. [DOI] [PubMed] [Google Scholar]
- 441.Martin LJ, Ramachandran V, Cripe LH, et al. Evidence in favor of linkage to human chromosomal regions 18q, 5q and 13q for bicuspid aortic valve and associated cardiovascular malformations. Hum Genet 2007;121:275–84. [DOI] [PubMed] [Google Scholar]
- 442.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wer- novsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics 2000;105:1082–9. [DOI] [PubMed] [Google Scholar]
- 443.Goldberg CS, Schwartz EM, Brunberg JA, et al. Neurodevelopmen- tal outcome of patients after the fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr 2000;137:646–52. [DOI] [PubMed] [Google Scholar]
- 444.Limperopoulos C Disorders of the fetal circulation and the fetal brain. Clin Perinatol 2009;36:561–77. [DOI] [PubMed] [Google Scholar]
- 445.Goldberg CS, Bove EL, Devaney EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: Outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg 2007;133:880–7.e1. [DOI] [PubMed] [Google Scholar]
- 446.Mahle W, Visconti K, Freier MC, et al. Relationship of surgical approach to neurodevelopmental outcomes in hypoplastic left heart syndrome. Pediatrics 2006;117:e90–7. [DOI] [PubMed] [Google Scholar]
- 447.Day RW, Orsmond GS, Sturtevant JE, Hawkins JA, Doty DB, McGough EC. Early and intermediate results of the Fontan procedure at moderately high altitude. Ann Thorac Surg 1994;57:170–6. [DOI] [PubMed] [Google Scholar]
- 448.Malhotra S, Ivy DD, Mitchell M, et al. Performance of cavopulmo- nary palliation at elevated altitude: midterm outcomes and risk factors for failure. Circulation 2008;118:S177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Mitchell MB, Campbell DN, Ivy D, et al. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg 2004;128:693–702. [DOI] [PubMed] [Google Scholar]
- 450.Rempel G, Harrison M, Williamson D. Is “treat your child normally” helpful advice for parents of survivors of treatment of hypoplastic left heart syndrome? Cardiol Young 2009;19:135–44. [DOI] [PubMed] [Google Scholar]
- 451.Thompson R, Gustafson K. Adaptation to Chronic Childhood Illness. 1st ed. Washington, DC: American Psychological Association, 1999. [Google Scholar]
- 452.Wallander J, Thompson R, Alriksson-Schmidt A. Psychosocial adjustment of children with chronic physical conditions In: Roberts M, ed. Handbook of Pediatric Psychology. New York, NY: Guilford Press, 2003:772. [Google Scholar]
- 453.Latal B, Helfricht S, Fischer J, Bauersfeld U, Landolt M. Psychological adjustment and quality of life in children and adolescents following open-heart surgery for congenital heart disease: a systematic review. BMC Pediatr 2009;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Moons P, Van Deyk K, Budts W, De Geest S. Caliber of quality- of-life assessments in congenital heart disease: a plea for more conceptual and methodological rigor. Arch Pediatr Adolesc Med 2004;158:1062–9. [DOI] [PubMed] [Google Scholar]
- 455.Pike NA, Evangelista LS, Doering LV, Koniak-Griffin D, Lewis AB, Child JS. Health-related quality of life: a closer look at related research in patients who have undergone the Fontan operation over the last decade. Heart Lung 2007;36:3–15. [DOI] [PubMed] [Google Scholar]
- 456.Drotar D Health status and quality of life In: Naar-King S, Ellis D, Frey M, eds. Assessing Children’s Well-Being : A Handbook of Measures. Mahwah, NJ: Lawrence Erlbaum Associates, 2004. [Google Scholar]
- 457.Moons P, Van Deyk K, De Geest S, Gewillig M, Budts W. Is the severity of congenital heart disease associated with the quality of life and perceived health of adult patients? Heart 2005;91:1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.McCrindle BW, Williams RV, Mitchell PD, et al. Relationship of patient and medical characteristics to health status in children and adolescents after the Fontan procedure. Circulation 2006;113: 1123–9. [DOI] [PubMed] [Google Scholar]
- 459.Mellander M, Berntsson L, Nilsson B. Quality of life in children with hypoplastic left heart syndrome. Acta Paediatr 2007;96:53–7. [DOI] [PubMed] [Google Scholar]
- 460.Rose M, Kohler K, Kohler F, Sawitzky B, Fliege H, Klapp BF. Determinants of the quality of life of patients with congenital heart disease. Qual Life Res 2005;14:35–43. [DOI] [PubMed] [Google Scholar]
- 461.Marino BS, Tomlinson RS, Drotar D, et al. Quality-of-life concerns differ among patients, parents, and medical providers in children and adolescents with congenital and acquired heart disease. Pediatrics 2009;123:e708–15. [DOI] [PubMed] [Google Scholar]
- 462.Williams DL, Gelijns AC, Moskowitz AJ, et al. Hypoplastic left heart syndrome: valuing the survival. J Thorac Cardiovasc Surg 2000;119:720–31. [DOI] [PubMed] [Google Scholar]
- 463.Saliba Z, Butera G, Bonnet D, et al. Quality of life and perceived health status in surviving adults with univentricular heart. Heart 2001;86:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Marino BS, Shera D, Wernovsky G, et al. The development of the pediatric cardiac quality of life inventory: a quality of life measure for children and adolescents with heart disease. Qual Life Res 2008;17: 613–26. [DOI] [PubMed] [Google Scholar]
- 465.Clark B, Sleeper L, Anderson P. The Fontan cross-sectional study: testing for correlations among health-related quality of life, maximal exercise performance, and ventricular mass-to-volume ratio (abstr). Circulation 2004;110:III441. [Google Scholar]
- 466.Harinck E, Hutter PA, Hoorntje TM, et al. Air travel and adults with cyanotic congenital heart disease. Circulation 1996;93:272–6. [DOI] [PubMed] [Google Scholar]
- 467.Broberg CS, Uebing A, Cuomo L, Thein SL, Papadopoulos MG, Gatzoulis MA. Adult patients with Eisenmenger syndrome report flying safely on commercial airlines. Heart 2007;93:1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.TSA. Hidden Disabilities. Available at: http://www.tsa.gov/travelers/airtravel/specialneeds/editorial_1374.shtm. Accessed November 8, 2011.
- 469.NHOPA. Airline Travel with Oxygen. Available at: http://www.homeoxygen.org/airline-travel-with-oxygen. Accessed November 8, 2011.
- 470.Friedberg MK, Silverman NH, Dubin AM, Rosenthal DN. Mechanical dyssynchrony in children with systolic dysfunction secondary to cardiomyopathy: a Doppler tissue and vector velocity imaging study. J Am Soc Echocardiogr 2007;20:756–63. [DOI] [PubMed] [Google Scholar]
- 471.Bacha EA, Zimmerman FJ, Mor-Avi V, et al. Ventricular resynchronization by multisite pacing improves myocardial performance in the postoperative single-ventricle patient. Ann Thorac Surg 2004;78: 1678–83. [DOI] [PubMed] [Google Scholar]
- 472.Zimmerman FJ, Starr JP, Koenig PR, Smith P, Hijazi ZM, Bacha EA. Acute hemodynamic benefit of multisite ventricular pacing after congenital heart surgery. Ann Thorac Surg 2003;75:1775–80. [DOI] [PubMed] [Google Scholar]
- 473.Cecchin F, Frangini PA, Brown DW, et al. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: five years experience in a single institution. J Cardiovasc Electrophysiol 2009;20:58 −65. [DOI] [PubMed] [Google Scholar]
- 474.Janousek J, Gebauer RA, Abdul-Khaliq H, et al. Cardiac resynchronisation therapy in paediatric and congenital heart disease: differential effects in various anatomical and functional substrates. Heart 2009; 95:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Dubin AM, Janousek J, Rhee E, et al. Resynchronization therapy in pediatric and congenital heart disease patients: an international multicenter study. J Am Coll Cardiol 2005;46:2277–83. [DOI] [PubMed] [Google Scholar]
- 476.DeGroff CG. Modeling the fontan circulation: where we are and where we need to go. Pediatr Cardiol 2008;29:3–12. [DOI] [PubMed] [Google Scholar]
- 477.de Leval MR, Dubini G, Migliavacca F, et al. Use of computational fluid dynamics in the design of surgical procedures: application to the study of competitive flows in cavopulmonary connections. J Thorac Cardiovasc Surg 1996;111:502–13. [DOI] [PubMed] [Google Scholar]
- 478.Dubini G, de Leval MR, Pietrabissa R, Montevecchi FM, Fumero R. A numerical fluid mechanical study of repaired congenital heart defects. Application to the total cavopulmonary connection. J Bio- mech 1996;29:111–21. [DOI] [PubMed] [Google Scholar]
- 479.Marsden AL, Vignon-Clementel IE, Chan FP, Feinstein JA, Taylor CA. Effects of exercise and respiration on hemodynamic efficiency in CFD simulations of the total cavopulmonary connection. Ann Biomed Eng 2007;35:250–63. [DOI] [PubMed] [Google Scholar]
- 480.Whitehead KK, Pekkan K, Kitajima HD, Paridon SM, Yoganathan AP, Fogel MA. Nonlinear power loss during exercise in singleventricle patients after the Fontan: insights from computational fluid dynamics. Circulation 2007;116:I165–71. [DOI] [PubMed] [Google Scholar]
- 481.Marsden A, Reddy V, Shadden S, Chan F, Taylor C, Feinstein J. A new multiparameter approach to computational simulation for Fon- tan assessment and redesign. Congenit Heart Dis 2010;5:104–17. [DOI] [PubMed] [Google Scholar]
- 482.Sundareswaran K, de Zelicourt D, Sharma S, et al. Correction of pulmonary arteriovenous malformation using image-based surgical planning. J Am Coll Cardiol Img 2009;2:1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Soerensen DD, Pekkan K, de Zelicourt D, et al. Introduction of new optimized total cavopulmonary connection. Ann Thorac Sur 2007;83:2182–90. [DOI] [PubMed] [Google Scholar]
- 484.Marsden AL, Bernstein AJ, Reddy VM, et al. Evaluation of a nove Y-shaped extracardiac Fontan baffle using computational fluid dy namics. J Thorac Cardiovasc Surg 2009;137:394–403.e2. [DOI] [PubMed] [Google Scholar]
- 485.Marsden AL, Feinstein JA, Taylor CA. A computational framewor for derivative-free optimization of cardiovascular geometries. Com put Methods Appl Mech Eng 2008;197:1890–905. [Google Scholar]
- 486.Bove E, Migliavacca F, de Leval M, et al. Use of mathemati modeling to compare and predict hemodynamic effects of th modified Blalock-Taussig and right ventricle-pulmonary arter shunts for hypoplastic left heart syndrome. J Thorac Cardiovasc Sur 2008:312–20. [DOI] [PubMed] [Google Scholar]
- 487.Hochareon P, Manning KB, Fontaine AA, Tarbell JM, Deutsch S Fluid dynamic analysis of the 50 cc Penn State artificial heart unde physiological operating conditions using particle image velocimetry J Biomech Eng 2004;126:585–93. [DOI] [PubMed] [Google Scholar]
- 488.de Leval MR. The Fontan circulation: a challenge to William Harvey? Nat Clin Pract Cardiovasc Med 2005;2:202–8. [DOI] [PubMed] [Google Scholar]