Interventions to reduce inappropriate prescribing of antibiotics for acute respiratory tract infections: summary and update of a systematic review (original) (raw)
Short abstract
Objective
Antibiotic overuse contributes to antibiotic resistance and adverse consequences. Acute respiratory tract infections (RTIs) are the most common reason for antibiotic prescribing in primary care, but such infections often do not require antibiotics. We summarized and updated a previously performed systematic review of interventions to reduce inappropriate use of antibiotics for acute RTIs.
Methods
To update the review, we searched MEDLINE®, the Cochrane Library (until January 2018), and reference lists. Two reviewers selected the studies, extracted the study data, and assessed the quality and strength of evidence.
Results
Twenty-six interventions were evaluated in 95 mostly fair-quality studies. The following four interventions had moderate-strength evidence of improved/reduced antibiotic prescribing and low-strength evidence of no adverse consequences: parent education (21% reduction, no increase return visits), combined patient/clinician education (7% reduction, no change in complications/satisfaction), procalcitonin testing for adults with RTIs of the lower respiratory tract (12%–72% reduction, no increased adverse consequences), and electronic decision support systems (24%–47% improvement in appropriate prescribing, 5%–9% reduction, no increased complications).
Conclusions
The best evidence supports use of specific educational interventions, procalcitonin testing in adults, and electronic decision support to reduce inappropriate antibiotic prescribing for acute RTIs without causing adverse consequences.
Keywords: Antibiotics, resistance, overuse, review, acute respiratory tract infections, adverse consequences
Introduction
Antibiotic resistance is a serious public health problem. In the United States, approximately 23,000 people die of antibiotic-resistant infections every year.1 Although the reasons for increasing antibiotic resistance are multifactorial, including the use of antibiotics in livestock and underdevelopment of new antibiotics, a key factor is outpatient antibiotic overuse.1 Research has shown that a multitude of diverse factors may influence overuse of antibiotics for acute respiratory tract infections (RTIs), including location, environment (i.e., clinic type, time, and resources), patient demographics, patient and/or clinician preferences, clinician specialty and experience, and clinician–patient communication and shared decision-making.2–4 Hence, studies on reducing inappropriate antibiotic use for acute RTIs have employed a variety of approaches and have targeted various factors. In this review, we categorized studies according to their approach and intended target. Interventions to improve antibiotic use are intended to achieve a variety of outcomes, including slower development of antibiotic resistance, decreased use of any antibiotic in situations for which antibiotics are not effective, increased use of a recommended antibiotic when one is indicated, fewer adverse drug events, and decreased healthcare costs. However, these positive effects should not come at the expense of under-treatment of patients who truly need antibiotics, potentially increasing the risk of undesirable outcomes (“adverse consequences”) such as hospitalization, medical complications, additional clinic visits, time off of work and/or school, patient dissatisfaction, or a longer symptom duration. Adverse consequences can also occur for patients whose condition is unlikely to require antibiotics for resolution; for example, patients expecting a prescription may be disappointed and even seek care elsewhere. Clinicians may also experience adverse consequences from an intervention (e.g., electronic medical record alert fatigue or increased time required to participate in trainings). Although the weight or value of specific adverse consequences varies according to the perspective, such consequences must be taken into account when assessing the impact of an intervention aimed at reducing antibiotic use.
The best settings for such interventions may be those in which there is a high prevalence of the disease, antibiotics are commonly prescribed, and there is a reasonably high risk of prescribing an antibiotic when one is not warranted. Acute RTIs, which include a broad group of diagnoses such as bronchitis and acute otitis media, are highly prevalent, frequently do not require an antibiotic (i.e., are self-limiting infections or are caused by viral infections),5 and are the most common reason for antibiotic prescriptions in the primary care setting. Acute RTIs account for approximately 70% of primary diagnoses in adults presenting for ambulatory care office visits with a chief symptom of cough.6 A 2013 report regarding healthy adults visiting outpatient offices and emergency departments for acute bronchitis revealed that prescriptions for antibiotics were given at 73% of visits from 1996 to 20107 despite the fact that most cases of acute bronchitis are caused by viral pathogens for which antibiotics are not helpful. Similarly, a 2014 analysis of data from the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey indicated that 60% of children diagnosed with pharyngitis in the United States from 1997 to 2010 were prescribed antibiotics8 despite the fact that only about 37% of pharyngitis episodes are caused by bacteria. It must be assumed that some antibiotics prescribed in these studies were unnecessary (i.e., inappropriate).
In this report, we summarize and update a large, complex comparative effectiveness review (CER) of the evidence of effectiveness of all potential interventions designed to reduce inappropriate antibiotic use for acute RTIs while not causing adverse consequences. Prior reviews have not covered all possible interventions (including the rapidly developing area of point-of-care diagnostic tests), nor have they considered both benefits and potential adverse consequences of interventions.
Methods
This report is based in part on a systematic review conducted for the Agency for Healthcare Research and Quality (AHRQ);9 this manuscript updates the evidence and focuses on prescribing and adverse consequences, while the full report also includes other outcomes (e.g., knowledge, attitudes). We followed the current standard methods for AHRQ systematic reviews,9 including obtaining input from experts and the public, and our protocol is registered with PROSPERO.10 Detailed methods (search strategies, inclusion criteria, and data abstraction) are available in the AHRQ report.9
Search strategy
For the original CER, we searched MEDLINE® and the Cochrane Library from 1990 through June 2016 using a peer-reviewed strategy that included terms for interventions aimed at improving antibiotic prescribing for acute RTIs in the outpatient setting. The electronic search strategy is available in the full report.11 We updated the search through January 2018 for the present manuscript. We defined acute RTIs as acute bronchitis, acute otitis media (AOM), pharyngitis/tonsillitis, rhinitis, sinusitis, and other viral syndromes and excluded community-acquired pneumonia, acute exacerbations of chronic obstructive pulmonary disease, bronchiectasis, or other chronic underlying lung diseases.5 The search had no language limits and no study design limits. For the CER, we also searched reference lists of included studies, reviewed information from point-of-care diagnostic test manufacturers, and consulted a panel of experts that convened for the AHRQ review.9,10
Study selection and data extraction
We included randomized controlled trials (RCTs) and comparative observational studies that studied a single or multifaceted intervention compared with usual care and that reported antibiotic prescribing outcomes. We screened systematic reviews to identify studies. Citations were screened by one reviewer, and any studies deemed ineligible were screened by a second reviewer. Selected studies were then dually reviewed.12 The outcomes were overall antibiotic prescribing (or use if reported), appropriate versus inappropriate prescribing as defined per study, and measures of adverse consequences (return visits, hospitalization, duration of symptoms, patient satisfaction, etc.). The study characteristics and results were abstracted by one reviewer and checked by a second. All differences in judgment were resolved through consensus.
Critical appraisal and data synthesis
Given that the percentage of acute RTIs for which antibiotics are prescribed commonly exceeds the known prevalence of RTIs for which antibiotics would be effective, we considered a reduction in overall antibiotic prescribing (or use) to be a meaningful measure of an intervention’s effectiveness, in addition to measures that more explicitly specified a reduction of “inappropriate” antibiotic prescribing (or use). The quality of trials was assessed based on predefined criteria related to randomization and allocation concealment, outcome assessment and blinding, and amount and handling of missing data, resulting in a rating of good, fair, or poor using dual review and consensus.13 The observational study criteria included questions on selection bias, attrition bias, specification and ascertainment of outcomes, and statistical analysis, and these studies were required to have controlled for potential confounding or temporal trends to be deemed good or fair quality.13
Data from clinically and methodologically similar studies were pooled using a random-effects model.14 We evaluated statistical heterogeneity using the I2 statistic. According to AHRQ methodology, we graded the strength of evidence as high, moderate, low, or insufficient for key outcomes based on methodological limitations of the body of evidence, consistency of study findings, directness of outcome measurement, and precision of estimates.15
Results
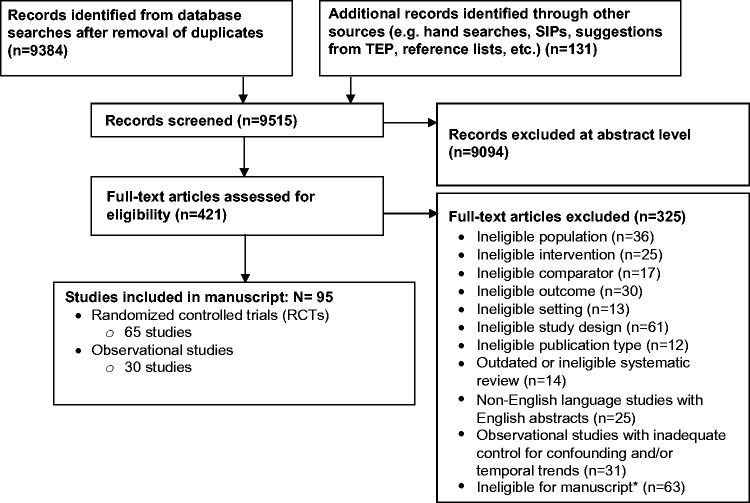
In our original CER, we included 82 (88%) mostly fair-quality studies (88 publications): 57 RCTs and 25 observational studies. For this update, we screened 2486 citations published since the original search (June 2016) and included 13 additional studies (8 RCTs, 5 observational studies) in 14 publications.16–29 The study characteristics and quality assessment for studies included in the CER can be found in the AHRQ report,11 and studies added in this update can be found in Table 1. Cumulatively, there were 95 (86%) mostly fair-quality studies: 65 RCTs and 30 observational studies (Figure 1). Most studies were multisite RCTs targeting broad populations of children and adults with any acute RTI (Table 2) and included 101,443 clinics or clinicians and 7,452,357 patients or parents. Educational and clinical strategies were most widely studied. Sore throat, pharyngitis, and tonsillitis were the most common types of RTI; cough was most common in studies of communication interventions. While all studies reported the change in overall prescribing, appropriate or inappropriate prescribing was reported in only 10 studies (10.4%). The proportion of studies conducted in the United States was 35% overall and ranged widely across intervention categories, from 16% for clinical and point-of-care testing strategies to 80% for system-level strategies.
Table 1.
Studies of interventions to reduce inappropriate antibiotic prescribing in acute RTIs since 2016
| Authors, yearCountry | Patient population | Study designSample size | Study interventions |
|---|---|---|---|
| Patient or caregiver interventions | |||
| Alexandrino et al., 201716Portugal | Acute uncomplicated RTIChildren <3 years old | RCT177 caregivers | Education caregivers of children <3 years old attending daycare versus no intervention |
| Alexandrino et al., 201717Portugal | Acute uncomplicated RTIChildren <3 years old | RCT138 caregivers | Education caregivers of children <3 years old attending daycare versus nasal clearing protocol, both, or no intervention |
| Lee et al., 201722Singapore | Acute uncomplicated RTI | RCT914 Patients | Patient education versus no intervention |
| Clinician interventions | |||
| Breakell et al., 201818England | Bronchiolitis | Pre–post101 patients | Education on National Institute for Clinical Excellence (NICE) guidance |
| Cioffi et al., 201619Italy | Acute uncomplicated RTIChildren | RCT23 clinicians, 792 patients | Rapid WBC testing plus delayed antibiotic prescribing versus delayed prescribing only |
| Do et al., 201620Vietnam | Acute uncomplicated RTIPrimary care | RCT2037 patients | CRP point-of-care testing versus no intervention |
| Hoa et al., 201721Vietnam | Acute uncomplicated RTIChildren | RCT206 clinicians | Education plus posters and quizzes versus no intervention |
| Link et al., 201623 | Acute bronchitis | Pre–post | Education and communication training |
| Little et al., 201724England | Uncomplicated LRTI | Prospective cohort28,883 patients | Delayed prescribing versus immediate prescribing versus no antibiotics |
| Magin et al., 201825Magin et al., 201626Australia | Upper RTI and acute bronchitis | Longitudinal856 clinicians | Education of trainee GPs |
| Ouldali et al., 201727France | Acute RTI (pediatric) | Interrupted time-series7 pediatric EDs | Guideline implementation, education, and feedback |
| Persell et al., 201628USA | Acute uncomplicated RTIPrimary care clinics | RCT28 clinicians | Education and 1 of 3 behavioral interventions via computer: accountable justifications, alternatives, and peer comparison versus no intervention |
| Sharp et al., 201729USA | Acute sinusitisPrimary care | RCT126 clinics | Electronic decision support (plus single education al intervention) versus no intervention |
Figure 1.
Results of literature search
Table 2.
Summary of characteristics of studies included in review
| Study characteristic | Category | All studies | Educational | Communication | Clinical and POC | System level | Multidimensionala |
|---|---|---|---|---|---|---|---|
| Design | RCTs (% Total, % Cluster RCT) | 65 (68%, 47%) | 23 (66%, 50%) | 5 (100%, 80%) | 29 (78%, 25%) | 5 (50%, 60%) | 15 (54%, 64%) |
| Observational studies | 31 (33%) | 12 (33%) | 0 | 8 (22%) | 5 (50%) | 13 (46%) | |
| Total (% of all studies) | 96 (100%) | 35 (36%) | 5 (5%) | 37 (39%) | 10 (10%) | 28 (29%) | |
| Study quality | Good | 10 (10%) | 7 (20%) | 0 | 4 (11%) | 1 (10%) | 0 |
| Fair | 86 (90%) | 28 (80%) | 5 (100%) | 33 (89%) | 9 (90%) | 28 (100%) | |
| Total (% of all studies) | 96 (100%) | 35 (36%) | 5 (6%) | 37 (39%) | 10 (10%) | 28 (29%) | |
| Sample size | Clinic/Clinicianb | 101,443 | 14,821 | 450 | 2,465 | 2,833 | 82,236 |
| Patient/Caregiverg | 7,452,357 | 6,708548 | 12,364 | 144,145 | 355,868 | 595,955 | |
| Population | Adult | 28 (30%) | 9 (26%) | 2 (40%) | 14 (38%) | 3 (30%) | 6 (21%) |
| Child or both | 68 (71%) | 26 (74%) | 3 (60%) | 23 (62%) | 7 (70%) | 22 (79%) | |
| Total (% of all studies) | 96 (100%) | 35 (36%) | 5 (5%) | 37 (39%) | 10 (10%) | 28 (29%) | |
| Duration of intervention | Range | 3 weeks – 4 years | 1 month – 4 years | 4 months – 10 months | 1 month – 4 years | 11 months – 4 years | 3 weeks – 4 years |
| Duration of follow-up | Range | 1 day – 4 years | 1 day – 4 years | 28 days – 3 months | 1 day – 2 years | 2 weeks – 3 years | 1 week – 1 year |
| Location | United States | 34 (35%) | 15 (43%) | 1 (20%) | 6 (16%) | 8 (80%) | 9 (32%) |
| Other | 62 (65%) | 20 (57%) | 4 (80%) | 31 (84%) | 2 (20%) | 19 (68%) | |
| Total (% of all studies) | 96 (100%) | 35 (36%) | 5 (6%) | 37 (39%) | 10 (10%) | 28 (29%) | |
| Multisite or single sitec | Multisite | 81 (84%) | 32 (91%) | 5 (100%) | 27 (73%) | 9 (90%) | 26 (93%) |
| Single site | 15 (16%) | 3 (9%) | 0 | 10 (27%) | 1 (10%) | 2 (7%) | |
| Total (% of all studies) | 96 (100%) | 35 (36%) | 5 (5%) | 37 (39%) | 10 (10%) | 28 (29%) | |
| Type of infection targetede | Acute bronchitis | 23 (24%) | 11 (31%) | 1 (20%) | 4 (12%) | 4 (40%) | 8 (29%) |
| Acute otitis media | 21 (22%) | 13 (37%) | 1 (20%) | 3 (9%) | 3 (30%) | 5 (18%) | |
| Sore throat/pharyngitis/tonsillitis | 32 (33%) | 12 (34%) | 2 (40%) | 9 (26%) | 5 (50%) | 11 (39%) | |
| Rhinitis | 7 (7%) | 3 (9%) | 1 (20%) | 2 (6%) | 2 (20%) | 2 (7%) | |
| Sinusitis | 22 (23%) | 6 (17%) | 1 (20%) | 5 (15%) | 4 (40%) | 9 (32%) | |
| Cough and common cold | 16 (17%) | 3 (9%) | 3 (60%) | 9 (26%) | 2 (20%) | 6 (21%) | |
| Any acute RTI | 65 (68%) | 23 (66%) | 4 (80%) | 21 (62%) | 6 (60%) | 15 (54%) | |
| Total (% of all studies)e | 96 e | 35 (36%) | 5 (5%) | 37 (39%) | 10 (10%) | 28 (29%) |
Studies differed substantially in the intervention target (e.g., patient, clinician, both; specific age group; or diagnosis), mode (population-level or individual-level), duration, frequency, and intensity; in outcome selection and assessment; and in the level of detail with respect to the patient characteristics, interventions, and outcomes reported. In addition, while there were several studies involving combinations of multifaceted interventions, they were mostly “one-off” combinations, limiting the strength of the evidence. This level of heterogeneity is often characteristic of complex multicomponent interventions and can be a challenge to constructing a framework for organizing the evidence synthesis. This is because the evidence can be conceptually amalgamated or split by various types of characteristics, and there is no agreed-upon single best approach for doing so.24
As a consequence of this variability, the results of the evidence synthesis could not be presented as a simple framework of “winners” and “losers.” We grouped the evidence for specific types of interventions into four hierarchical categories based on the direction and strength of evidence of benefits (prescribing outcomes) and adverse consequences (e.g., return clinic visits). In Table 3, we provide an overview of which interventions had low-, moderate-, or high-strength evidence according to these categories, as well as interventions for which evidence was insufficient to draw conclusions. Table 4presents the findings for interventions with evidence of both a benefit and lack of adverse consequences. Note that the studies varied in how the data were reported; e.g., some reported only the relative change in prescribing (not absolute) or reported on a specific infection (e.g., AOM). For the ease of decision-makers, this approach emphasizes the subset of interventions with the highest combined level of favorable evidence of both benefits and harms and contrasts it with interventions with either mixed evidence or no evidence of harms and/or evidence of either no effect or a negative effect on prescribing. As shown in Table 3, five interventions had evidence that was insufficient to draw conclusions for any included outcome because of methodological limitations, imprecision due to small sample sizes, and inconsistency of findings across studies.
Table 3.
Summary of evidence findings by category of intervention*
| Intervention category | Specific intervention | Studies(n, type) | Benefit and no increase in ACs | Benefit but mixed data on ACs | Benefit but no data on ACs | No benefit | Increased prescribing | Insufficient evidence |
|---|---|---|---|---|---|---|---|---|
| Education | Clinic or private setting education of parents of children at risk for aRTI | 5 RCTs | X | |||||
| Public education campaigns for parents | 2 non-RCTs | X | ||||||
| Combined patient/parent education campaign and clinician education | 7 RCTs | X | ||||||
| Clinician education | 5 RCTs 7 non-RCTs | X | ||||||
| Clinic-based education for parents of children ≤24 months old with acute otitis media | 1 RCT | X | ||||||
| Clinical | Delayed versus immediate prescribing | 8 RCTs 1 non-RCT | X | |||||
| Electronic decision support (with ≥50% use) | 4 RCTs | X | ||||||
| Electronic behavioral interventions | 1 RCT | X | ||||||
| Decision rules (paper) | 1 RCT | X | ||||||
| Comm | Communication training for clinicians | 4 RCTs | X | |||||
| Point-of-care testing | Procalcitonin (adults) | 4 RCTs | X | |||||
| Procalcitonin (children) | 1 RCT | X | ||||||
| Rapid viral testing (adults) | 1 RCT | X | ||||||
| Streptococcal antigen (rapid strep) | 3 RCTs | X | ||||||
| C-reactive protein | 7 RCTs | X | ||||||
| Influenza (children) | 4 RCTs | X | ||||||
| Tympanometry (children) | 1 RCT | X | ||||||
| Multidimensional | Clinician education + audit/feedback | 2 RCTs 1 non-RCT | X | |||||
| Clinician education +clinical algorithm | 1 non-RCT | X | ||||||
| Patient/clinician education plus audit & feedback | 3 non-RCTs | X | ||||||
| Patient/clinician education plus communication training plus audit & feedback | 1 RCT 1 non-RCT | X | ||||||
| Audit & feedback, patient education, or both | 1 RCT | X | ||||||
| Delayed prescribing +patient education | 2 RCTs | X | ||||||
| Patient education + electronic decision support + delayed prescribing + audit & feedback | 1 non-RCT | X | ||||||
| Peer academic detailing (education, encouraging delayed prescribing) +audit & feedback | 1 non-RCT | X | ||||||
| Nurse telephone care and audit/feedback | 1 RCT | X | ||||||
| Communication training + electronic decision support (prescribing agreements)+ audit/feedback | 1 RCT | X | ||||||
| CRP + communication training | 2 RCTs | X | ||||||
| Clinician and patient education plus CRP | 7 non-RCTs | X | ||||||
| Rapid WBC plus delayed prescribing | 1 RCT | X | ||||||
| Guideline implementation, clinician education, audit/feedback | 1 non-RCT | X |
Table 4.
Interventions with evidence of benefits in antibiotic prescribing for acute RTI and not causing adverse consequences
| Intervention | Antibiotic prescribing | Appropriateness of prescribing | Adverse consequences | ||
|---|---|---|---|---|---|
| Baseline or control group rate | Absolute changeRelative effect | Strength of evidence | Baseline/controlAbsolute changeRelative effectStrength of evidence | Impact on outcomesAll low strength of evidence | |
| Combined patient/parent education and clinician education | 37% to 59%(5 RCTs) | −7.3% (95% CI, 4.0–10.6)OR, 0.56 (95% CI, 0.36 − 0.87) to OR, 0.62 (95% CI, 0.54 − 0.75)(5 RCTs) | Moderate | Children with pharyngitis: 37.1%–10.4%(1 RCT)OR 0.62 (95% CI 0.54 to 0.75)Low strength of evidence | No difference in patient or parent satisfaction (2 RCTs |
| Adults with acute RTIs: 43%–9.7% (1 RCT)NRLow strength of evidence | No difference in AOM complications (1 observational study). | ||||
| Clinic-based education of parents of children up to age 14 years | 40.8% (1 RCT) | −21.3% (1 RCT)Pooled OR, 0.39(95% CI, 0.26–0.58)(2 RCTs) | Moderate | NR | No difference in return visits (2 RCTs). |
| Public education campaigns for parents (prescribing for child) | 37% to 44% | NRURTI: OR, 0.75 (95% CI, 0.69–0.81)AOM: OR, 0.65 (95% CI, 0.59–0.72) Pharyngitis: OR, 0.93 (95% CI, 0.89–0.97) (2 observational studies) | Low | NR | No difference in diagnosis of complications and decrease in subsequent visits (1 observational study). |
| Procalcitonin (adults) | 37% to 97% | −12% to −72%OR, 0.14 (95% CI, 0.09–0.22)Acute bronchitis: OR, 0.15 (95% CI, 0.10–0.23)(1 SR of 4 RCTs) | Moderate | NR | No difference in number of days of limited activity, missing work, or continuing symptoms at 28 days for URTI or LRTI in primary care (1 RCT) |
| No difference in AE/lack of efficacy (1 RCT) or hospitalizations (1 RCT) | |||||
| No difference in mortality or treatment failure at 30 days in acute bronchitis/URTI in primary care or ED;URTI or LRTI in primary care (4 RCTs) | |||||
| Electronic decision support (systems with ≥50% use per patient case) | 38% to 47% | −9.2%RR, 0.73 (95% CI, 0.58–0.92)(3 RCTs) | Moderate | 38% to 47%−13% to 24%(2 RCTs)Moderate strength of evidence | No difference in healthcare utilization or complications (1 RCT) |
Interventions that improved appropriate prescribing or reduced overall prescribing of antibiotics without increasing adverse consequences
Three education interventions, procalcitonin testing, and electronic decision support were the only interventions with evidence of improved prescribing without adverse consequences (Table 4).
Education interventions
Three education-based interventions were found to have a benefit with evidence of not increasing adverse consequences. A clinic-based educational intervention for parents of pediatric patients had the largest reduction in overall antibiotic prescribing among the education interventions (−21.3%) without increasing the number of return office visits. Public education campaigns aimed at parents of young children reduced prescribing (e.g., for AOM: combined odds ratio [OR] = 0.65, 95% confidence interval [CI] = 0.26–0.58, two observational studies, I2 not estimable), decreased return office visits, and did not increase potential complications. Combining clinician and patient or parent education interventions resulted in smaller reductions in overall prescribing (−7.3%) compared with other education strategies, but this combination also improved appropriate prescribing with no negative impact on medical complications or patient satisfaction.
Procalcitonin point-of-care testing
Procalcitonin was the only point-of-care test with evidence of any benefit and was restricted to adults. Use of procalcitonin testing in the emergency department or outpatient setting reduced overall prescribing. The wide range in absolute reductions was related to a wide variation in baseline prescribing, and larger reductions were associated with greater baseline prescribing. There was no negative impact on the days of missed work, days with limited activity, symptom duration, hospitalizations, or a combined outcome of adverse events and efficacy.
Electronic decision support systems
Electronic decision support systems led to modest reductions in overall antibiotic prescribing (−9.2%) and improvements in appropriate prescribing for acute RTI (13%–24% improvement), but only with more frequent use of the system (i.e., used in ≥50% of patient cases). This was accomplished without affecting health care utilization or complications. Evidence of less frequent use of the system was insufficient due to inconsistency.
Interventions that reduced overall prescribing of antibiotics but had a mixed impact on adverse consequences
Some interventions had evidence of reducing antibiotic prescribing but mixed evidence of reducing adverse consequences (i.e., they showed evidence of not affecting some outcomes but worsening others).
Communication training
Interventions to improve clinicians’ communication with patients (including shared decision-making interventions) regarding antibiotic prescribing decisions reduced overall prescribing, with the effect ranging from 9% to 26%; however, evidence of symptom improvement was conflicting. There was a slightly longer duration of symptoms but better health ratings at 2 weeks and insufficient evidence for other outcomes.
Delayed prescribing
Compared with immediate prescribing, various delayed prescribing methods reduced antibiotic use by 34% to 76% without affecting return visits or the duration of symptoms. However, delayed prescribing decreased patient satisfaction.
C-reactive protein measurement
Measurement of the serum C-reactive protein (CRP) concentration reduced overall prescribing for acute RTIs from 13% to 33% in the trials; the prescribing reductions ranged widely depending in part on the baseline prescribing level. CRP measurement increased return visits within 4 weeks (risk ratio = 1.64, 95% CI = 1.35–2.00, four RCTs, I2 = 0%).
Multifaceted interventions
Clinician communication training combined with CRP measurement resulted in a large reduction in overall prescribing (combined OR = 0.30, 95% CI = 0.26–0.36, two RCTs, I2 not estimable). There was no impact on return visits, diagnostic testing use, or days off work; however, there was an increase in hospitalizations at 1 month (combined OR = 4.65, 95% CI = 1.21–17.87, two RCTs, I2 not estimable) and duration of symptoms. Although statistically significant, the absolute differences were small (1.1% vs. 0.2% hospitalization at 30 days, 5 vs. 6 days symptom duration). The reasons for even a small increase in the risk of hospitalization were unclear in these two trials involving >4,000 patients.
Interventions that reduced overall prescribing of antibiotics but had no evidence or insufficient evidence of adverse consequences
Rapid strep testing for sore throat, rapid viral testing (multi-viral polymerase chain reaction) in adults, clinician education combined with audit and feedback, nurse telephone care combined with audit and feedback, rapid white blood cell count testing combined with delayed prescribing, and clinician communication training combined with electronic decision support and audit and feedback had low- to moderate-strength evidence of improved prescribing outcomes but no evidence on potential harms. Clinician education alone and combined clinician and patient education, audit and feedback, CRP measurement, and academic detailing had low-strength evidence of reducing overall prescribing, but evidence regarding other outcomes was insufficient to draw conclusions. The evidence on adverse consequences was insufficient because of combinations of methodological limitations, imprecision due to few studies reporting a given outcome, and inconsistency in findings across studies.
Interventions with no effect or increased prescribing of antibiotics
Clinic-based education for parents of children aged ≤24 months with AOM, public education campaigns aimed at adults, clinician education combined with audit and feedback, point-of-care testing for influenza in children, and tympanometry in children with suspected AOM had no impact on overall prescribing.25–31
Audit and feedback, patient education (a pamphlet), or the combination resulted in increased prescribing, although patient education alone and audit and feedback combined with patient education increased prescribing at a lower rate than in the control group.32 Using the adult algorithm for procalcitonin test results in children_increased_ prescribing of antibiotics with a related increase in adverse events.33
Other considerations
In our CER, we examined several factors identified a priori that could potentially have an effect on the results of studies of interventions to improve antibiotic prescribing for acute RTIs.
Methods for assessing appropriate prescribing
Significant improvement in appropriate prescribing of antibiotics was found in 7 of the 10 studies that measured appropriateness.28,30–38 Improvement was seen for each of the three methods used to assess appropriate prescribing: ICD-9 codes or diagnostic category (reduction of 13%–24%), guideline adherence (reduction of <1%–22%), and symptom duration in patients with pharyngitis or sinusitis (reduction of 10%–24%).
Intended target of intervention
Absolute reductions in prescribing were greater when the target was the patient or parent in educational interventions, and combining patient and clinician education did not result in clearly greater reductions. The intended target population did not affect other outcomes. Communication training for clinicians had evidence of a benefit while similar training for patients did not, although this evidence was sparse.
Baseline prescribing rates
Baseline prescribing rates varied extremely widely across studies (from <10% to >90%), and several studies noted temporal trends of declining prescribing during the study period. In general, the magnitude of the reduction in overall antibiotic prescribing correlated with the prescribing rate at baseline, such that locations with higher prescribing at baseline showed greater reductions.
Discussion
This summary and update of a CER of interventions to improve antibiotic prescribing for acute RTIs included a heterogeneous group of interventions that varied in their number (i.e., single or multiple), targets, mode, duration, frequency, and intensity of interventions as well as in the outcomes studied and variation in reporting of important factors such as the characteristics of patients, interventions, and outcomes. The outcomes were grouped into categories regarding the prescribing of antibiotics and other related outcomes, such as adverse consequences of the interventions (e.g., increased return visits). With this complex network of interventions and possible outcomes, we organized the findings into groups according to evidence of a benefit plus or minus evidence of adverse consequences. Notably, the adverse consequences reported may have differing value or weights to individual patients or clinicians; however, evaluating this issue was beyond the scope of our work.
While all 96 mostly fair-quality studies reported the change in overall prescribing, only 10% reported the changes in appropriate prescribing. The studies used a variety of definitions and methods of ascertainment for appropriate prescribing. Three types of education interventions, procalcitonin testing, and electronic decision support were the only interventions with evidence of improved prescribing and no adverse consequences (details on these interventions can be found in the AHRQ report and inTable 1 for newer studies). Several other interventions improved prescribing, but lacked adequate evidence of adverse consequences. Tympanometry or parent education (alone) for suspected AOM, clinician education plus audit/feedback, and influenza testing in children had at least low-strength evidence that they were each ineffective, and adult procalcitonin test algorithms used for children _increased_antibiotic prescribing.
Because the evidence base represents heterogeneous study methods and settings, there may be variability in the real-world results. Even with moderate-strength evidence, further study is needed to present a more complete picture of the relevant outcomes.
The multiple layers of findings in this study exemplify a gray area that has inhibited implementation of specific interventions more broadly across the United States, as outlined by Gonzales et al.35 Challenges to employing interventions to reduce inappropriate antibiotic use include the potential for unintentionally causing adverse consequences and the logistics of implementing interventions. The concern regarding adverse consequences can be addressed by selecting interventions from the short list of interventions with evidence of some benefit and at least some evidence of not increasing adverse consequences. While implementation of several of the interventions is likely to be most achievable by organized or integrated health systems or public health organizations, determining which interventions might best be implemented in a given setting or by a particular clinician requires close evaluation of the evidence and characteristics of the intervention, population, and setting that can be found in the AHRQ evidence report.9 The combination of procalcitonin measurement and clinical evaluation has shown promise for use as a decision aid for excluding clinically relevant lower respiratory bacterial infections (e.g., pneumonia) and determining when to safely withhold antibiotics in adults with low serum procalcitonin concentrations (<0.1–0.25 µg/L) and likely viral lower respiratory infections; however, its limited availability in the United States is a primary barrier to its use.
It is possible that interventions with evidence of improved antibiotic prescribing but without evidence related to adverse consequences (e.g., communication strategies, including shared decision-making) may not cause adverse clinical consequences. Given the importance of balancing considerations of the benefit and potential harm in the use of these potentially valuable interventions, further research into possible adverse consequences is clearly needed. Similarly, further research is needed to elucidate potential adverse consequences for interventions with evidence of a benefit but with mixed evidence of adverse consequences (e.g., delayed prescribing, CRP measurement, communication training, and communication training with CRP measurement). Such research should include evaluations of patients’ and clinicians’ values related to specific adverse consequences, particularly because some of these interventions have already been recommended.38 Arguably, some interventions are unlikely to cause serious adverse consequences (e.g., patient education) and may not require conclusive evidence to establish that fact.
This work adds to a fairly robust body of reviews on this general topic.39–42 The reviews are generally more narrowly focused on specific types of interventions, but they have broadly concluded that multifaceted educational interventions, clinician education, delayed prescribing, CRP measurement, and procalcitonin measurement may be effective in certain settings without assessing adverse outcomes. Our review adds significant depth by providing an updated search, evaluating adverse consequences, and including strength-of-evidence assessments. While our findings overlap with some others, they are not identical because of differences in intervention types (e.g., inclusion of point-of-care tests), intervention goals (e.g., quality improvement), indication/disease, and outcomes (e.g., inclusion of adverse consequences).
Even with a large body of evidence, there are important limitations and gaps in the body of evidence that should be considered when designing future studies. Most of the studies described herein only reported on overall prescribing, neglecting the important outcomes of appropriate prescribing, antibiotic resistance, or the potential consequences of reduced prescribing. Only electronic decision support and the combined parent–clinician educational intervention had evidence of improving appropriate prescribing. However, the definition of appropriateness in these studies was simplistic and the methods of measurement were less than robust. The inability to accurately measure appropriate prescribing is a major gap in the evidence. For overall prescribing, our ability to judge the meaningfulness of the magnitude of reductions was limited by the general lack of established parameters regarding minimally important differences. While many studies used a difference of 15% (versus usual care) in sample size calculations, there is no agreement on what percent reduction is meaningful in terms of improving resistance to antibiotics. Similarly, the change in prescribing is closely tied to the baseline prescribing rates, such that the measurement of change should take this level into account. Another drawback of the body of evidence is variation in geographic study locations, with 35% and 64% inside and outside the United States, respectively (52% in European countries). This is an issue for two reasons: the baseline or background prescribing rate varies by country, sometimes widely, and the healthcare systems, cultural attitudes, and behaviors of clinicians and patients may vary enough in other countries to reduce the generalizability of the findings. Reporting issues and small numbers of studies assessing similar interventions limited analysis of the evidence according to these factors.
Although there were numerous studies, many were flawed, and this area of research seems to be more immature than the volume of publications suggests. Better agreement on several issues is needed before this field of study can fully mature. For example, among the many ill-defined outcomes, the highest priority is the need for agreement on defining and measuring the appropriateness of antibiotic use in acute RTI. Similarly, we need evidence on the possible correlation between improvements in improved overall or appropriate prescribing and reduced antibiotic resistance, including what degree of reduction in overall prescribing is clinically important. Future studies must also regularly measure adverse outcomes. We suggest that the use of complex intervention concepts in both the design and reporting of studies will improve the consistency of key elements across studies such that cumulative results can lead to stronger conclusions, particularly in evaluating which combinations of interventions result in greater improvements than single interventions without increasing adverse consequences.24
Potential limitations in our review methods and procedures include the lack of standard search terms that uniformly cover all interventions and the limitation of the studies to non-English language papers that had an abstract in English. However, we do not believe that we excluded important information using these methods. We had limited ability to assess potential publication and reporting bias because of few opportunities to pool studies and the lack of availability of study protocols.
Conclusions
There is evidence that several interventions can effectively reduce inappropriate use of antibiotics in acute RTI without adverse consequences; the best evidence supports clinic-based education for parents, public campaigns for parents combined with clinician education, procalcitonin testing in adults, and electronic decision support. The magnitude of the benefit varied, and evidence on modifying factors was inadequate. Evidence for numerous other interventions was inadequate to draw conclusions in favor of their implementation. Future research must better define and measure key outcomes (e.g., appropriate prescribing); assess adverse consequences; compare interventions, sustainability, and resource use; and evaluate effect-modifiers.
Acknowledgments
The authors gratefully acknowledge the following individuals for their contributions to this project: Andrew Hamilton, MLS, for performing the literature search; Leah Williams, BS, for editing the manuscript; Elaine Graham, MLS, for assisting with the project management; Jessica Griffin, MS and Sujata Thakurta, MPA:HA, for contributing to topic refinement; Rebecca Holmes, MD, MS, for assisting with the quality assessment of observational studies; and Ryan Stoner, MA and Laura LaLonde for performing the data extraction and citation management (all were employees of Oregon Health & Science University at the time of the project and were paid to conduct the work using AHRQ contract funds). The authors would also like to thank the AHRQ Task Order Officer, Elisabeth Kato, MD, MRP, for providing guidance in developing the scope of the review.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review was initially funded by the Agency for Healthcare Research and Quality, Contract #HHSA290201200014I. Updates to the original work were performed without additional funding.
References
- 1.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed November 19, 2014.
- 2.Barlam TF, Morgan JR, Wetzler LM, et al. Antibiotics for respiratory tract infections: a comparison of prescribing in an outpatient setting. Infect Control Hosp Epidemiol 2015; 36: 153–159. [DOI] [PubMed] [Google Scholar]
- 3.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60: 1308–1316. [DOI] [PubMed] [Google Scholar]
- 4.May L, Gudger G, Armstrong P, et al. Multisite exploration of clinical decision making for antibiotic use by emergency medicine providers using quantitative and qualitative methods. Infect Control Hosp Epidemiol 2014; 35: 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence. Respiratory tract infections – antibiotic prescribing. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. NICE clinical guideline 69 [pdf]. 2008; http://www.nice.org.uk/guidance/cg69/resources/guidance-respiratory-tract-infections-antibiotic-prescribing-pdf. Accessed October 16, 2013. [PubMed]
- 6.Metlay JP, Stafford RS, Singer DE. National trends in the use of antibiotics by primary care physicians for adult patients with cough. Arch Intern Med 1998; 158: 1813–1818. [DOI] [PubMed] [Google Scholar]
- 7.Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. Jama 2014; 311: 2020–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dooling KL, Shapiro DJ, Van Beneden C, et al. Overprescribing and inappropriate antibiotic selection for children with pharyngitis in the United States, 1997–2010. JAMA Pediatrics 2014; 168: 1073–1074. [DOI] [PubMed] [Google Scholar]
- 9.McDonagh M, Peterson K, Winthrop K, et al. Improving Antibiotic Prescribing for Uncomplicated Acute Respiratory Tract Infections. Comparative Effectiveness Review No. 163. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2012-00014-I.) AHRQ Publication No. 15(16)-EHC033-EF. Rockville, MD: Agency for Healthcare Research and Quality; January 2016. http://www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed]
- 10.McDonagh M, Peterson K, Buckley D, et al. Interventions to improve appropriate antibiotic use for acute respiratory tract infections. PROSPERO 2014:CRD42014010094 Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014010094Accessed October 22 2014.
- 11.McDonagh M, Peterson K, Winthrop K, et al. Improving Antibiotic Prescribing for Uncomplicated Acute Respiratory Tract Infections. Comparative Effectiveness Review No. 163. (Prepared by the Pacific Northwest Evidence-based Practice Center under Contract No. 290-2012-00014-I.) AHRQ Publication No. 15(16)-EHC033-EF. Rockville, MD: Agency for Healthcare Research and Quality; January 2016. (Electronic Search Strategies can be found at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/antibiotics-respiratory-infection_research.pdf-page=184; Individual Study Quality Assessments can be found at:https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/antibiotics-respiratory-infection_research.pdf-page=443; Individual Study Characteristics and Results can be found at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/antibiotics-respiratory-infection_research.pdf-page=184; Strength of Evidence Ratings for each intervention/outcome pair can be found at:https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/antibiotics-respiratory-infection_research.pdf-page=668).
- 12.McDonagh M, Peterson K, Raina P. Avoiding bias in selecting studies. Methods guide for comparative effectiveness reviews. Agency for Healthcare Research and Quality 2013. [PubMed] [Google Scholar]
- 13.McDonagh MS, Jonas DE, Gartlehner G, et al. Methods for the drug effectiveness review project. BMC Med Res Methodol 2012; 12: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 15.Berkman N, Lohr K, Ansari M. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the agency for healthcare research and quality: an update. methods guide for comparative effectiveness reviews. _Agency for Healthcare Research and Quality_2013. [PubMed]
- 16.Alexandrino AM, Santos RI, Melo MC, et al. Designing and evaluating a health education session on respiratory infections addressed to caregivers of children under three years of age attending day-care centres in Porto, Portugal: A community-based intervention. Eur J Gen Pract 2017; 23: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrino AS, Santos R, Melo C, et al. Caregivers' education vs rhinopharyngeal clearance in children with upper respiratory infections: impact on children's health outcomes. Eur J Pediatr 2017; 176: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 18.Breakell R, Thorndyke B, Clennett J, et al. Reducing unnecessary chest X-rays, antibiotics and bronchodilators through implementation of the NICE bronchiolitis guideline. Eur J Pediatr 2018; 177: 47–51. [DOI] [PubMed] [Google Scholar]
- 19.Cioffi L, Limauro R, Sassi R, et al. Decreased antibiotic prescription in an Italian pediatric population with nonspecific and persistent upper respiratory tract infections by use of a point-of-care white blood cell count, in addition to antibiotic delayed prescription strategy. Glob Pediatr Health 2016; 3: 2333794X15615771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do NT, Ta NT, Tran NT, et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Glob Health 2016; 4:e633–e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoa NQ, Thi Lan P, Phuc HD, et al. Antibiotic prescribing and dispensing for acute respiratory infections in children: effectiveness of a multi-faceted intervention for health-care providers in Vietnam. Glob Health Action 2017; 10: 1327638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MHM, Pan DST, Huang JH, et al. Results from a patient-based health education intervention in reducing antibiotic use for acute upper respiratory tract infections in the private sector primary care setting in Singapore. Antimicrob Agents Chemother 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Link TL, Townsend ML, Leung E, et al. Reducing inappropriate antibiotic prescribing for adults with acute bronchitis in an urgent care setting: a quality improvement initiative. Adv Emerg Nurs J 2016; 38: 327–335. [DOI] [PubMed] [Google Scholar]
- 24.Little P, Stuart B, Smith S, et al. Antibiotic prescription strategies and adverse outcome for uncomplicated lower respiratory tract infections: prospective cough complication cohort (3C) study. BMJ 2017; 357: j2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magin P, Tapley A, Morgan S, et al. Reducing early career general practitioners' antibiotic prescribing for respiratory tract infections: a pragmatic prospective non-randomised controlled trial. Fam Pract 2018; 35: 53–60. [DOI] [PubMed] [Google Scholar]
- 26.Magin PJ, Morgan S, Tapley A, et al. Changes in early-career family physicians' antibiotic prescribing for upper respiratory tract infection and acute bronchitis: a multicentre longitudinal study. Fam Pract 2016; 33: 360–367. [DOI] [PubMed] [Google Scholar]
- 27.Ouldali N, Bellettre X, Milcent K, et al. Impact of implementing national guidelines on antibiotic prescriptions for acute respiratory tract infections in pediatric emergency departments: an interrupted time series analysis. Clin Infect Dis 2017; 65: 1469–1476. [DOI] [PubMed] [Google Scholar]
- 28.Persell SD, Doctor JN, Friedberg MW, et al. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016; 16: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharp AL, Hu YR, Shen E, et al. Improving antibiotic stewardship: a stepped-wedge cluster randomized trial. Am J Manag Care 2017; 23: e360–e365. [PubMed] [Google Scholar]
- 30.Briel M, Langewitz W, Tschudi P, et al. Communication training and antibiotic use in acute respiratory tract infections. A cluster randomised controlled trial in general practice. Swiss Med Wkly 2006; 136: 241–247. [DOI] [PubMed] [Google Scholar]
- 31.Samore MH, Bateman K, Alder SC, et al. Clinical decision support and appropriateness of antimicrobial prescribing: a randomized trial. JAMA 2005; 294: 2305–2314. [DOI] [PubMed] [Google Scholar]
- 32.Litvin CB, Ornstein SM, Wessell AM, et al. Use of an electronic health record clinical decision support tool to improve antibiotic prescribing for acute respiratory infections: the ABX-TRIP study. J Gen Intern Med 2013; 28: 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes-Morales H, Flores-Hernandez S, Tome-Sandoval P, et al. A multifaceted education intervention for improving family physicians' case management. Fam Med 2009; 41: 277–284. [PubMed] [Google Scholar]
- 34.Davis RL, Wright J, Chalmers F, et al. A cluster randomized clinical trial to improve prescribing patterns in ambulatory pediatrics. PLoS Clin Trials 2007; 2: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med 2013; 173: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RH, MacKenzie TD, Leeman-Castillo B, et al. Optimizing antibiotic prescribing for acute respiratory tract infections in an urban urgent care clinic. J Gen Intern Med 2003; 18: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen R, Allaert FA, Callens A, et al. Medico-economic evaluation of an educational intervention to optimize children uncomplicated nasopharyngitis treatment in ambulatory care . Med Mal Infect 2000; 30: 691–698. [Google Scholar]
- 38.Meeker D, Knight TK, Friedberg MW, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med 2014; 174: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Sullivan JW, Harvey RT, Glasziou PP, et al. Written information for patients (or parents of child patients) to reduce the use of antibiotics for acute upper respiratory tract infections in primary care. Cochrane Database Syst Rev 2016; 11: CD011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odermatt J, Friedli N, Kutz A, et al. Effects of procalcitonin testing on antibiotic use and clinical outcomes in patients with upper respiratory tract infections. An individual patient data meta-analysis. Clin Chem Lab Med 2017; 56: 170–177. [DOI] [PubMed] [Google Scholar]
- 41.Spurling GK, Del Mar CB, Dooley L, et al. Delayed antibiotic prescriptions for respiratory infections. Cochrane Database Syst Rev 2017; 9: CD004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tonkin-Crine SK, Tan PS, van Hecke O, et al. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: an overview of systematic reviews. Cochrane Database Syst Rev 2017; 9: CD012252. [DOI] [PMC free article] [PubMed] [Google Scholar]