Can the plasma PD-1 levels predict the presence and efficiency of tumor-infiltrating lymphocytes in patients with metastatic melanoma? (original) (raw)
Abstract
Background:
The immune response in melanoma patients is locally affected by presence of tumor-infiltrating lymphocytes (TILs), generally divided into brisk, nonbrisk, and absent. Several studies have shown that a greater presence of TILs, especially brisk, in primary melanoma is associated with a better prognosis and higher survival rate.
Patients and Methods:
We investigated by enzyme-linked immunosorbent assay (ELISA) the correlation between PD-1 levels in plasma and the presence/absence of TILs in 28 patients with metastatic melanoma.
Results:
Low plasma PD-1 levels were correlated with brisk TILs in primary melanoma, whereas intermediate values correlated with the nonbrisk TILs, and high PD-1 levels with absent TILs. Although the low number of samples did not allow us to obtain a statistically significant correlation between the plasma PD-1 levels and the patients’ overall survival depending on the absence/presence of TILs, the median survival of patients having brisk type TILs was 5 months higher than that of patients with absent and nonbrisk TILs.
Conclusions:
This work highlights the ability of measuring the plasma PD-1 levels in order to predict the prognosis of patients with untreated metastatic melanoma without a BRAF mutation at the time of diagnosis.
Keywords: brisk TILs, immune response, melanoma, plasma PD-1, plasma PD-L1, tumor-infiltrating lymphocytes
Introduction
Melanoma is the most aggressive form of skin cancer, causing more than 70% of skin cancer-related deaths and accounting for 3% of new cancer cases estimated in Italy (http://www.registri-tumori.it). According to the Italian Cancer Society, an annual average incidence of 12.5 new melanoma diagnoses per 100,000 males and 13.1 per 100,000 females were registered.1 It is considered to be the second most frequent neoplasm in youth, with global incidence rates continuously increasing over the last few decades, resulting in a poor prognosis with an extremely low 5-year survival rate in the case of metastatic tumors.2,3 Cutaneous melanoma can be divided into four major subtypes: nodular melanoma, superficial spreading, lentigo maligna melanoma (LMM), and acral lentiginous.4 The melanoma onset is caused by complex interactions between genetic, phenotypic, and environmental factors.5,6 Melanomas have been shown to be genetically and phenotypically heterogeneous tumors harboring different genetic alterations in three main oncogenes: BRAF, NRAS, and c-KIT.7 Large-scale analysis of melanoma exome data discovered six novel melanoma genes (PPP6C, RAC1, SNX31, TACC1, STK19, and ARID2), three of which, RAC1, PPP6C, and STK19, harbored potentially targetable mutations. Moreover, RB and p53 pathways are deregulated in this type of malignancy.8 Mutations in genes encoding the SWI/SNF chromatin-remodeling enzymes such as ARID1A (BAF250A/SMARCF1), ARID1B (BAF250B), ARID2 (BAF200), and SMARCA4 (BRG1) are involved in melanoma, in addition to other chromatin organization/histone modification proteins (EZH1,EZH2,SETD2, and TRRAP).8
Currently, prevention and early diagnosis are the most effective strategies for decreasing the occurrence of this tumor type and improving its prognosis.9 The management of advanced metastatic melanoma represents an interesting challenge, because chemotherapy and previous immunological therapies showed only a modest efficacy in curing patients.10,11 Currently, melanoma is considered to be one of the most immunogenic tumors, with a tremendous ability to stimulate the immune reactions in the host organism, by harboring a tumor microenvironment (TME) composed of a variety of immune cells, including T lymphocytes and macrophages. These immune cells produce a wide spectrum of cytokines, chemokines, growth factors, and interleukins (ILs).12–14 Experimental evidence has shown the capacity of the immune system to be exploited in the fight against melanoma.15 Therefore, the therapeutic options aimed at enhancing the host immune response were thoroughly investigated. Several targeted agents and new immune checkpoint inhibitors (e.g. ipilimumab, nivolumab, pembrolizumab) determined clinically important progress in treating metastatic melanoma, and improving the prognosis of patients.16–19 Indeed, new agents approved by the US Food and Drug Administration (FDA) since 2011 have played a key role in improving the survival rates of patients with metastatic melanoma.20–23 Monoclonal antibodies targeting mainly programmed death 1 (PD-1) receptor and its ligand (PD-L1), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) showed clinically significant advances in clinical practice.24–26 PD-1, mostly expressed on the surface of activated T cells, B cells, and myeloid cells, is a type I transmembrane receptor that belongs to the CD28/CTLA-4 protein superfamily and is involved in the modulation of immune response. The binding of PD-1 to its specific ligands PD-L1 and PD-L2 causes the inhibition of the T-cell activity, induction of their apoptosis, hindering the T-cell-mediated targeting of tumor cells and the secretion of IL-1, IL-4, IL-10, and interferon (IFN)-gamma.27 Increased PD-1/PD-L1 expression levels in tumors are associated with a poor prognosis. The use of monoclonal antibodies blocking PD-1 and PD-L1 may restore the activity of cancer-specific T cells, leading to improvements in the overall survival (OS) and progression-free survival (PFS) of cancer patients.28,29
The prognosis of patients with cutaneous melanoma is mainly associated with the location and depth of the primary tumor, and the presence or absence of loco-regional and metastatic disease30 Moreover, other factors, assessed in primary tumor, may also affect this prognosis, including the presence of tumor-infiltrating lymphocytes (TILs), ulceration, and tumor regression.31–33 Unlike the noninfiltrating lymphocytes, TILs represent a selected population of lymphocytes with a higher specific immunological reactivity against tumor cells.31 Indeed, the presence of TILs, which are generally divided into brisk type (infiltrating the entire base of the invasive tumor), nonbrisk type (infiltrating melanoma only focally), and absent (not present, or not infiltrating tumor), has been shown to locally influence the immune response to melanoma, contributing to give this tumor a high immunogenic potential.34,35 Experimental evidence suggested that a higher abundance of TILs, especially of brisk type, in primary melanoma is associated with a more favorable prognosis and a higher survival rate, regardless of the type of detected mutation, tumor degree and stage, and histological subtype.36–38 Furthermore, the analysis of the immunoregulatory gene expression profiles in patients with primary melanomas showed that the several classes of TILs are characterized by a immunophenotypic and functional heterogeneity.39 Unlike some studies that have reported a certain discordance in the PD-L1 expression levels between primary melanomas and their metastases in 50% of patients, owing to an expression heterogeneity in melanoma metastases,40 instead a few other histopathology studies35,41 seem to support the idea of a concordance of prognostic and predictive significance of TILs between primary melanomas and their lymph node metastases, probably owing to a matched clonality of TILs in the primary lesion and metastatic sites with regard to T-cell receptor.
However, several factors render the TILs to be unable to eradicate the tumor completely, such as a low number of specific-tumor lymphocytes, senescent or anergic TILs, high number of immunosuppressive cells in the TME (regulatory T cells, myeloid-derived suppressor cells, etc.), and release of immunosuppressive factors (e.g. indoleamine 2,3-dioxygenase).42
Recent studies revealed an association between high PD-1/PD-L1 expression levels in tumor and antitumor immune response.40,43 Higher PD-L1 expression values positively correlate with a better response to PD-1/PD-L1 agents in patients with metastatic melanoma.44 The aim of our study is to investigate the correlation between plasma PD-1/PD-L1 expression levels and presence/absence/class of TILs in patients with metastatic melanoma, thereby determining the potential ability of the circulating PD-1 levels in predicting the prognosis of patients with metastatic advanced melanoma.
Patients and methods
Study population
In the period from January 2016 to March 2018, 41 patients diagnosed with metastatic melanoma were consecutively enrolled and treated at the Section of Medical Oncology of the Department of Surgical, Oncological, and Oral Sciences of the University of Palermo (Italy). These patients were selected and considered eligible for our study based on the following inclusion criteria: (i) histologically confirmed and well documented diagnosis of unresectable metastatic melanoma; (ii) patients older than 18 years with Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) ⩽ 2; (iii) therapeutic treatment-naïve patients at the moment of blood sampling harboring no BRAF mutations; (iv) availability of peripheral blood from affected patients for the plasma isolation.
All patients were recruited in the study before starting treatment with PD-1 immune checkpoint inhibitors (nivolumab and pembrolizumab). We have excluded the BRAF mutated patients in order to ensure as much as possible the homogeneity of our sample population, because only patients without BRAF mutation at the diagnosis can be subjected to a first-line treatment with PD-1 immune checkpoint inhibitors. In addition, the exclusion of BRAF mutated patients can be justified also by the awareness that BRAF mutation alone is not sufficient to induce a detectable antitumor immune response.40
Patients with a familial melanoma history or active or suspected autoimmune disease were excluded from the study. Written informed consent was obtained from each recruited patient and the study (G-Land 2017, approval number: 01-03-2017) was approved by ethical committee (Comitato Etico Palermo 1) of the university-affiliated hospital AOUP ‘P. Giaccone’ of Palermo. All clinical information for each enrolled patient was anonymously recorded and coded. All enrolled individuals were from a Southern Italian region (Sicily). Only 28 out of 41 recruited consecutive patients were included in our investigation, because it was not possible to obtain the data concerning the presence/absence of TILs from the histological reports of the other 13 patients, owing to the difficulty of identifying the primary tumor directly at the time of diagnosis.
The clinicopathological characteristics of the enrolled patients with metastatic melanoma were summarized in Table 1.
Table 1.
Clinical and pathological features of patients with metastatic melanoma.
| Number of Patients | % | |
|---|---|---|
| All patients | 41 | |
| Sex | ||
| Male | 23 | 56 |
| Female | 18 | 44 |
| Age at diagnosis (years) | ||
| 21–30 | 2 | 4.88 |
| 31–40 | 8 | 19.51 |
| 41–50 | 13 | 31.71 |
| 51–60 | 6 | 14.63 |
| 61–70 | 5 | 12.20 |
| > 70 | 7 | 17.07 |
| Mean age 50,6 | ||
| Relapse | ||
| Yes | 32 | 78 |
| No | 9 | 22 |
| ‘Primary tumor site | ||
| Limbs (acral) | 11 | 26.82 |
| Head and neck | 7 | 17.07 |
| Back | 7 | 17.07 |
| Uvea | 5 | 12.20 |
| Chest | 2 | 4.88 |
| Abdomen | 2 | 4.88 |
| Mucosal | 2 | 4.88 |
| Unknown | 5 | 12.20 |
| Histological subtype | ||
| Superficial spreading (SSM) | 11 | 26.83 |
| Nodular (NM) | 6 | 14.63 |
| Acral lentiginous (ALM) | 1 | 2.44 |
| NA | 23 | 56.1 |
| Growth phase | ||
| Radial (RGP) | 2 | 4.88 |
| Vertical (VGP) | 14 | 34.15 |
| Radial-vertical | 1 | 2.44 |
| NA | 24 | 58.53 |
| Breslow thickness | ||
| < 1 mm | 3 | 7.32 |
| 1-2 mm | 8 | 19.51 |
| 2-4 mm | 6 | 14.63 |
| > 4 mm | 11 | 26.83 |
| NA | 13 | 31.71 |
| Clark level | ||
| I | 2 | 4.88 |
| II | 4 | 9.75 |
| III | 7 | 17.07 |
| IV | 5 | 12.20 |
| V | 3 | 7.32 |
| NA | 20 | 48.78 |
| Ulceration | ||
| Present | 15 | 36.58 |
| Absent | 13 | 31.71 |
| NA | 13 | 31.71 |
| TILs | ||
| Brisk | 10 | 24.39 |
| Nonbrisk | 6 | 14.63 |
| Absent | 12 | 29.27 |
| NA | 13 | 31.71 |
| TILs density | ||
| Mild | 6/16 | 37.5 |
| Moderate | 1/16 | 6.25 |
| Marked | 1/16 | 6.25 |
| NA | 8/16 | 50.0 |
| Tumor regression | ||
| Present | 4 | 9.75 |
| Absent | 16 | 39.03 |
| NA | 21 | 51.22 |
| Microsatellitosis | ||
| Present | 1 | 2.44 |
| Absent | 6 | 14.63 |
| NA | 34 | 82.93 |
| Metastatic site | ||
| Lymph nodes | 31 | 75.61 |
| Lung | 19 | 46.34 |
| Liver | 16 | 39.02 |
| Skin | 14 | 34.15 |
| Bones | 9 | 22.0 |
| CNS | 8 | 19.51 |
| Soft Tissues | 8 | 19.51 |
| Adrenal Gland | 6 | 14.63 |
| Others | 18 | 43.90 |
| Metastasis surgery | ||
| Yes | 15 | 36.58 |
| No | 26 | 63.42 |
Sample collection and plasma isolation
The peripheral blood samples from patients untreated with immunotherapy were processed within 2 h of collection, by centrifugation at 2,200_g_ for 15 min at 4°C in presence of EDTA. The isolated supernatants (plasma fractions) were aliquoted in cryotubes and stored at −80°C until their use for subsequent analysis.
Determination of soluble PD-1 and PD-L1 concentrations in plasma
The plasma PD-1 and PD-L1 levels were measured by specific homemade enzyme-linked immunosorbent assay (ELISA) assays not yet commercially available. As some discrepancies were observed when using kits obtained from different sources, specific ELISA assays produced by DYNABIO S.A. (Parc de Luminy, Marseille France) according to our specifications were used. These specifications included: (i) verification by tandem mass spectrometry of the antigen sequence; (ii) optimization of the assay by testing all combinations of available monoclonal antibodies in capture and detection, targeting maximal signal/background ratio and sensitivity (combinations of two or more antibodies in coating or detection were also tested to improve performances); (iii) checking sample compatibility (serum versus plasma, interference of the matrix); and (iv) ensure that assay can be run at room temperature for easy handling and robustness.
Both ELISAs followed the same protocol: all steps were run at room temperature. Plates were coated overnight with the antibody selected for antigen, then washed. Remaining binding sites were blocked to minimize background. The next steps all ended with plate washing. For the PD-L1 assay, all steps were performed under shaking. Samples to be tested were incubated for 3 h. Then, the biotinylated antibody selected for detection was incubated for 30 min, followed by incubation for 15 min with the avidine–peroxidase conjugate. Finally, the substrate 3,3’,5,5’-tetramethylbenzidine (TMB) was incubated for 15 min, the reaction stopped with H2SO4 and the Optical Density (OD) read at 450 nm. Concentrations were established by comparison with a range obtained with known concentrations of the recombinant antigen.
Studies comparing concentrations of the two markers measured in serum and plasma from the same blood collection showed that apparent concentrations in serum were at least 10 times lower than in plasma. This observation showed that clotting determined the apparent loss of a large part of the assayed proteins. Because the mechanism of such loss is unknown, determination of protein concentrations in serum might be affected by factors other than the patient clinical status. Consequently, the use of serum samples could be misleading and should be avoided. For this reason, all samples assayed in this study were plasmas. We also observed in both ELISAs an interference of the plasma matrix, which becomes negligible when plasma samples are diluted at least 1/5. In the present study, all plasma samples were at least diluted 1/5 before assay.
Details of the two ELISA assays are reported in Table 2.
Table 2.
Characteristics of ELISA assays for PD-1 and PD-L1.
| PD-L1 | PD-1 | |
|---|---|---|
| Coating antibody | α-PD-L1 1.8 +α PD-L1 2.1 | α-PD-1 6.4 |
| Detection antibody (biotinylated) | A-PD-L1 1.3.1 | α-PD-1 3.1 |
| Detection limit (pg/ml) | 20 | 50 |
Statistical analysis
Student’s t and analysis of variance (ANOVA) tests were used to perform analyses of correlation between TILs and plasma PD-1 and PD-L1 levels, respectively. Survival analysis was performed using the Kaplan–Meier method and log-rank test. Data were generated using the MedCalc software for Windows, version 18.2.1 (MedCalc Software, Ostend, Belgium). Fisher’s exact test was used to evaluate the immunotherapy response based on the plasma PD-1 and PD-L1 levels.
p values <0.05 were considered statistically significant.
Results
Clinico-pathological features of patients with metastatic melanoma
A total of 41 patients with metastatic melanoma who met the previously established inclusion criteria (see the Patients and Methods section) were recruited and studied at the Section of Medical Oncology of the University of Palermo (Italy), by defining the clinical and pathological features of their melanomas.
A total of 23 patients were men and 18 were women, with an average age of 50.6 years (range 23–74) at the time of diagnosis. A total of 32 out of 41 patients (78%) witnessed disease relapse. Primary tumors were mainly located in the limbs in 26.82% of patients (11), head–neck and back in 34.14% (7 patients for each site), uvea in 12.2% (5), and chest, abdomen, and mucosal in 14.64% (2 patients for each site). The most frequent histological tumor subtypes of patients enrolled in this study were superficial spreading melanoma (26.83%), followed by nodular melanoma (14.63%), and acral lentiginous melanoma (2.44%), where no cases of LMM were observed.
Regarding the analysis of prognostic factors, a vertical growth phase melanoma was observed in 14 patients (34.15%), a IV-level Breslow thickness in 11 (26.83%), a Clark level of III and V in 7 and 3 patients, respectively (17.07% and 7.32%), ulceration in 15 cases (36.58%), tumor regression in 4 (9.75%), and microsatellitosis in 1 individual only (2.44%). Data on TILs was available only for 28 out of 41 patients. The presence of TILs was detected in 18 patients by immunohistochemical analysis, whereas a lack of TILs was observed in 12 individuals. About a third of patients (14) was already harboring metastasis at the time of diagnosis, and 3 patients died during our investigation. The main sites of metastatic localization were the lymph nodes in 31 patients (75.61%), followed by lung in 19 (46.34%), liver in 16 (39.02%), and skin in 14 cases (34.15%). No patients possessed BRAF mutations. The clinical and pathological features of 41 studied cases of metastatic melanoma were summarized in Table 1.
Correlation between plasma PD-1 expression levels and presence/typology of TILs in patients with metastatic melanoma
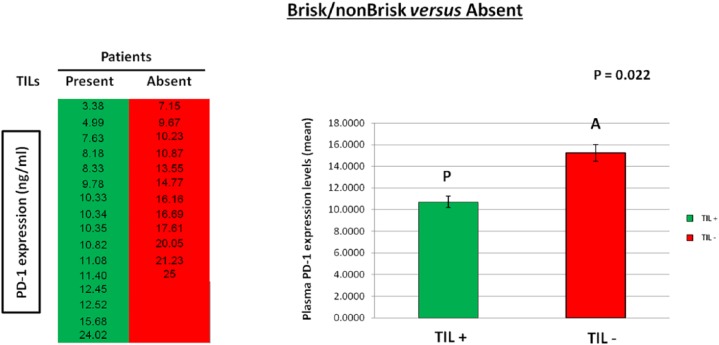
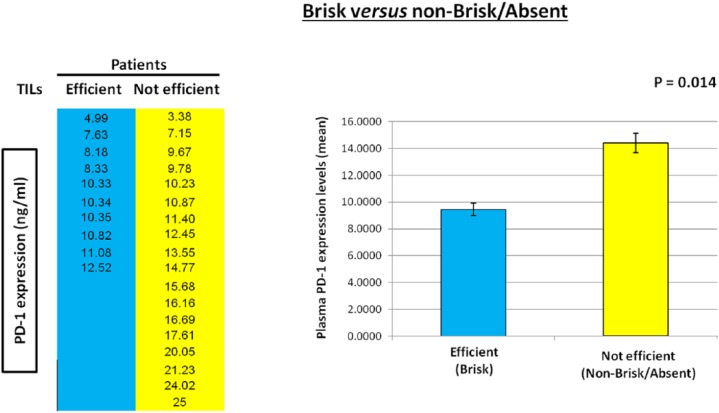
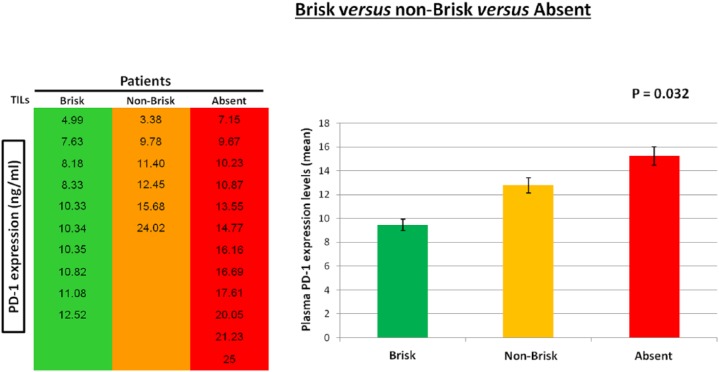
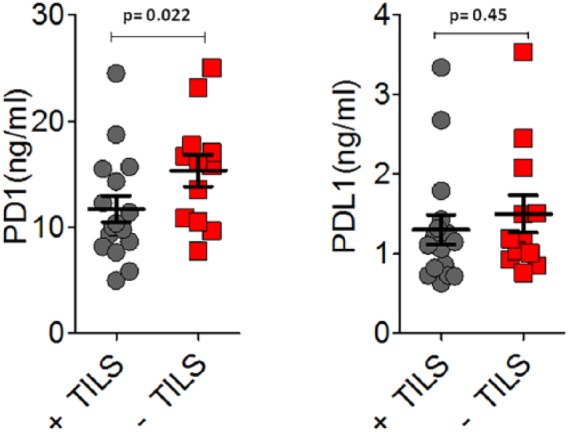
As experimental evidence showed that TILs locally affect the host immune response to melanoma31,35,37 and PD-1 expression is also associated with an antitumor immune response,40,45,46 41 patients with metastatic melanoma were enrolled and studied in order to investigate the presence of a potential correlation between the plasma PD-1 expression levels with the presence/absence and typology of TILs in melanoma primary tumors. The analysis of expression levels of the PD-1 immune checkpoint in plasma of 41 patients was performed using a specific ad hoc developed ELISA assay. The histological reports, in addition to clinical and pathological data of the patients, have been carefully examined to extrapolate information on TILs. We obtained the data concerning TILs from the histological reports only for 28 out of the 41 examined patients, because this information is not always reported by pathologists, owing to the difficulty of identifying the primary tumor directly at the time of diagnosis in some patients with metastatic melanoma. Consequently, 13 out of 41 patients were excluded from our investigation owing to the lack of their TIL data. All information concerning presence/absence/typology/density of TILs in the primary tumors of 28 patients, in addition to other patient clinical data, have been collected in a specific database. TILs were observed in the primary tumors of 16 out of 28 patients, 10 were of brisk type and 6 were of nonbrisk type, whereas TILs were completely absent in 12 patients (Figure 1). The moderate presence of peritumoral lymphocytic infiltrate was considered as absence of TILs, whereas a slight amount of intratumoral infiltrate was considered as presence of TILs. Plasma PD-1 levels were analyzed based on: (i) the presence/absence of TILs, (ii) their efficiency (brisk TILs versus nonbrisk/absent TILs); and (iii) their typology (brisk versus nonbrisk versus absent TILs) (Figure 2). Importantly, our analysis revealed that the presence of TILs, both of brisk and nonbrisk type, in the primary melanomas correlates with low/intermediate plasma PD-1 levels, whereas the absence of TILs correlates with higher plasma PD-1 levels (p = 0.022) (Figure 3). In addition, efficient TILs (brisk type) in the primary melanoma are associated in a statistically significant manner with lower plasma PD-1 levels, whereas the nonefficient TILs (nonbrisk type/absent) show higher PD-1 expression values (p = 0.014) (Figure 4). Finally, low plasma PD-1 levels have been shown to be statistically associated with brisk TILs in primary melanoma, intermediate values with nonbrisk TILs, and higher expression levels with absent TILs (p = 0.032) (Figure 5).
Figure 1.
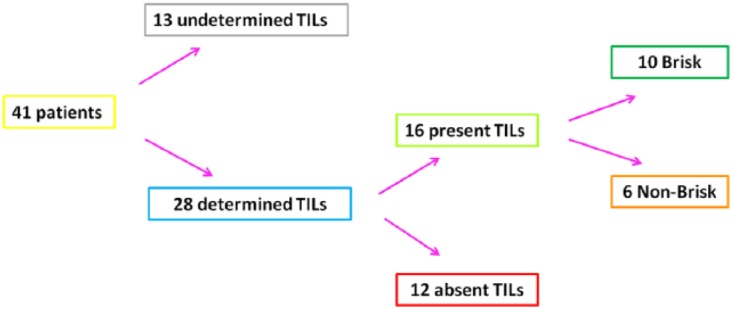
Characterization and classification of tumor-infiltrating lymphocytes (TILs) in patients with metastatic melanoma.
Data about the presence/absence and typology of TILs, extracted by the histological reports and regarding the examined patients, were summarized in this scheme.
Figure 2.
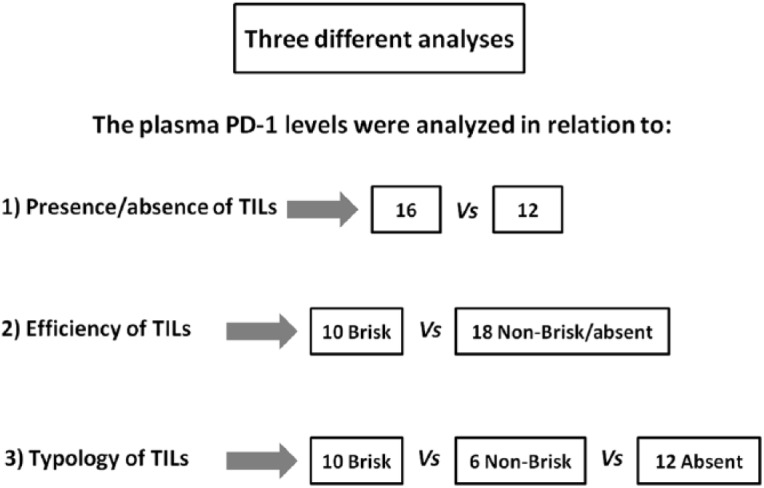
Analysis of the association between plasma PD-1 levels and tumor-infiltrating lymphocytes (TILs).
This scheme describes three different analysis approaches used to evaluate the plasma PD-1 expression levels in relation to the (1) presence/absence of TILs (brisk/nonbrisk versus absent), (2) efficiency of TILs (brisk versus nonbrisk/absent), and (3) class of TILs (brisk versus nonbrisk versus absent).
Figure 3.
Association between plasma PD-1 levels and present/absent tumor-infiltrating lymphocytes (TILs).
The presence of TILs (brisk and nonbrisk) in primary melanoma is associated in a statistically significant manner with low/intermediate plasma PD-1 levels. Data are represented as mean ± SD (standard deviation). Statistical analyses were performed by Student’s t test. Values of p < 0.05 were estimated to be statistically significant.
Figure 4.
Association between plasma PD-1 levels and efficient/not efficient tumor-infiltrating lymphocytes (TILs).
Efficient TILs (brisk) in primary melanoma are associated in a statistically significant manner with low plasma PD-1 levels, whereas not efficient TILs (nonbrisk/absent) show higher plasma PD-1 expression values. Data are represented as mean ± SD (standard deviation). Statistical analyses were performed by Student’s t test. Values of p < 0.05 were estimated to be statistically significant.
Figure 5.
Association between plasma PD-1 levels and typology of tumor-infiltrating lymphocytes (TILs).
Brisk TILs are associated in a statistically significant manner with low plasma PD-1 levels, nonbrisk TILs with intermediate values, and absent TILs with higher expression levels. Data are represented as mean ± SD (standard deviation). Statistical analyses were performed by one-way analysis of variance (ANOVA) test. Values of p < 0.05 were estimated to be statistically significant.
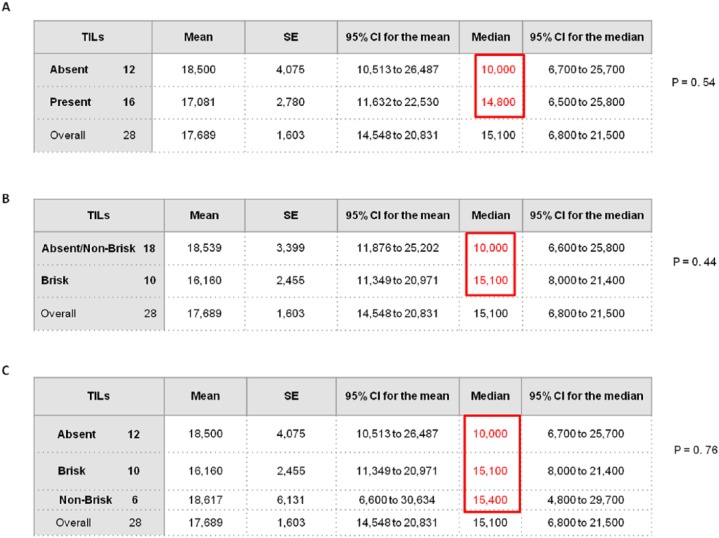
Although the small number of analyzed samples did not allow us to demonstrate a statistically significant association between the plasma PD-1 expression levels and PFS depending on the absence or presence of TILs (brisk/nonbrisk), the median PFS of patients with brisk/nonbrisk TILs and low/intermediate plasma PD-1 levels was approximately 5 months longer than that of patients with absent TILs (Figure 6A and C). Furthermore, when patients with nonbrisk type TILs were included in the same group along with those with absent TILs, the difference between survival medians of the two groups was about 5 months longer in patients with brisk TILs (Figure 6B).
Figure 6.
Association between plasma PD-1 expression levels and median PFS in patient groups with different typology of TILs.
Based on plasma PD-1 levels, survival analysis was carried out on different patient subpopulations in relation to the (A) presence/absence of TILs (brisk/nonbrisk versus absent), (B) efficiency of TILs (brisk versus nonbrisk/absent), and (C) class of TILs (brisk versus nonbrisk versus absent).
CI, Confidence Interval; PFS, progression-free survival; SE, standard error; TIL, tumor-infiltrating lymphocyte.
A further analysis aimed to assess the potential correlation between plasma PD-L1 expression levels and TILs showed no statistically significant association between presence of TILs (brisk and nonbrisk) in primary tumor and plasma PD-L1 levels in 28 patients with metastatic melanoma (Figure 7).
Figure 7.
Analysis of association between plasma PD-L1 levels and tumor-infiltrating lymphocytes (TILs).
As shown by the scatter plot, no statistically significant correlation between presence of TILs in primary melanoma and plasma PD-L1 levels was observed.
Association between plasma PD-1/PD-L1 levels and immunotherapy response
The baseline PD-1/PD-L1 expression levels in the plasma of 28 _BRAF_-wild type patients subsequently subjected to first-line immunotherapeutic treatment (pembrolizumab or nivolumab) were correlated with different types of therapy response: progressive disease (PD), stable disease (SD), partial response (PR), and complete response (CR). The best tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.47 This analysis allowed us to divide patients into two groups with different prognosis based on the levels of PD-1/PD-L1 compared with the median value (12.28 ng/ml for PD-1 and 1.23 ng/ml for PD-L1). Although the small number of analyzed samples did not allow us to demonstrate a statistically significant association (p = 0.07 for PD-1 and p = 0.55 for PD-L1), two groups with good and poor prognosis were identified for both PD-1 and PD-L1. Notably, the analysis regarding PD-1 provided correlation data close to be statistically significant (p = 0.07). A total of 13 out of 28 patients with baseline plasma PD-1 levels lower than the median showed more frequently SD (53%) and PR (8%), whereas 15 patients with values above the median exhibited more frequently PD (73%) (Table 3). Likewise, 16 patients with baseline plasma PD-L1 levels below the median exhibited more frequently SD (43%) and PR (6%), whereas 12 patients with values above the median showed more frequently PD (67%) (Table 4). Therefore, despite the lack of statistical significance, patients under immunotherapy with baseline PD-1 and PD-L1 expression below their respective median values appeared to show a better clinical outcome compared with those with plasma PD-1 and PD-L1 levels above the medians.
Table 3.
Contingency table between median PD-1 expression and immunotherapy response.
| Immunotherapy treatment response | ||||
|---|---|---|---|---|
| PD-1 (ng/ml) | Total | PD | SD | PR |
| <12.28 | 13 | 5 (38%) | 7 (53%) | 1 (8%) |
| ⩾12.28 | 15 | 11 (73%) | 4 (27%) | 0 (0%) |
Table 4.
Contingency table between median PD-L1 expression and immunotherapy response.
| Immunotherapy treatment response | ||||
|---|---|---|---|---|
| PD-L1 (ng/ml) | Total | PD | SD | PR |
| <1.23 | 16 | 8(50%) | 7(43%) | 1(6%) |
| ⩾1.23 | 12 | 8(67%) | 4(33%) | 0(0%) |
Discussion
Melanoma is a poorly differentiated high-grade malignancy arising from cells producing melanin pigments called melanocytes. In recent years, its incidence has shown a gradual increase, leading to a unfavorable prognosis in the presence of advanced metastatic disease.3
New therapies targeting immune checkpoints, especially the CTLA-4 and PD-1/PD-L1 pathways, have been recently introduced into clinical practice. Along with the introduction of new targeted agents, such as BRAF and MEK inhibitors and immune checkpoint inhibitors, significant improvements in survival of patients with metastatic melanoma were observed;48 however, the identification of new biomarkers able to predict prognosis and therapy response remains one of major objectives of clinical cancer research, in order to select patient subsets able to benefit from a specific therapeutic treatment. Therefore, the development of personalized clinical approaches is required in order to hamper the occurrence of resistance to therapies and, consequently, preventing tumor recurrence.49
Melanoma shows a high immunogenic potential suggested by the presence of TILs in the TME. TILs have been shown to be involved in the process of cancer immunoediting and, according to Clark’s classification adopted by the College of American Pathologist, are divided into brisk, nonbrisk, and absent. In addition to this classification, the current grading system considers other parameters including the density of TILs (mild, moderate, and marked) and their distribution pattern (focal, multifocal, and diffused).35,50 Numerous studies over the years have shown that TILs, especially the brisk type, detected in primary melanoma, are correlated with a good prognosis, increased survival rate, and tumor regression,31,38,51,52 whereas absent- or nonbrisk-type TILs are considered as predictive factors of sentinel lymph node positivity.53,54 The presence of an intratumor lymphocytic infiltrate showed no survival improvement for early stage melanomas or in the radial growth phase.55 Some recent studies have also highlighted the role of TILs as potential predictive biomarkers of response to immunotherapy, by associating a higher abundance of CD8+ TILs with a better response to anti-PD-1 therapy.56 Assessing TILs in multifocal tumors and lymph node metastasis or other metastatic sites, as well as elucidating their role in the tumor heterogeneity, will be the main objective of research in the near future.
The binding of PD-L1 to its PD-1 receptor, expressed on TILs, causes TIL inactivation and thereby preserves melanoma from the immune-mediated cell lysis. In many types of human tumors, including melanoma, non-small cell lung cancer, and renal carcinoma, the PD-L1 expression was associated with poor prognosis, as it induces host immunosuppression.57 In addition, PDL-1/PD-1 expression levels are predictive biomarkers of the clinical outcome to PDL-1/PD-1 axis inhibitors in a variety of solid tumors, including bladder cancer, gastroesophageal cancer, Merkel cell cancer, head and neck cancer, and small cell lung cancer.58
Currently, there are no studies in the literature that have focused on the potential prognostic relevance resulting from the correlation between soluble forms of PD-1 and PD-L1 in plasma and the presence/efficiency of TILs in metastatic melanoma. As several studies have shown that the TME affects melanoma prognosis and therapy response, TILs locally affect the immune response to melanoma and there is a correlation between PD-1/PD-L1 expression and antitumor immune response, therefore, we have decided to investigate the potential association between PD-1/PD-L1 expression levels in the plasma of 28 patients with metastatic melanoma and presence/absence/typology of TILs in paired primary melanomas. Indeed, one of the major issues often detected in some patients with metastatic melanoma at diagnosis is the loss of prognostic information deriving from TILs (which we know are favorable prognostic indicators), because it is not always possible to identify the primary tumor lesion in which the characterization of TILs is usually performed. In our study, we have chosen to detect these soluble molecules in plasma fraction instead of serum fraction, because the levels detected in serum are 10-fold lower than the plasma levels in the same patient, as described in the ‘Material and methods’ section. In addition, because the tissue biopsy showed several limitations mainly due to the invasiveness of the technique, low amount of tissue sample available for analysis, and poor evaluation dynamicity (static data), our study was focused on the analysis of the plasma PD-1/PD-L1 dosage, using the liquid biopsy as a tool to obtain a more dynamic profile reflecting the real image of the TME in melanoma. The innovation and originality of our work was to bypass the restrictions related to the tissue biopsy, by carrying out a low-invasive repeated sequential study on an easily available material such as plasma.
Our study showed that as PD-1 plasma levels increase, the likelihood of finding nonbrisk and absent TILs in primary tumor increases, resulting in worsening of the prognosis of patients with metastatic melanoma. In contrast, lower plasma PD-1 levels are statistically correlated with a greater presence of brisk-type TILs in primary lesions, suggesting a more favorable prognosis. Therefore, these results show how the plasma PD-1 expression levels can be correlated, in a statistically significant way, not only with the presence or absence of TILs, but also with their efficiency (brisk/nonbrisk). However, no statistically significant correlation was observed between the PD-L1 expression levels in plasma of the 28 examined melanoma patients and presence of TILs in the corresponding primary tumors. In addition, despite the lack of statistical significance owing to the small size of the analyzed sample, our investigation revealed that patients harboring brisk-type TILs in their primary tumors showed a median PFS that was approximately 5 months longer than that of patients with nonbrisk or absent TILs.
Another subsequent analysis was performed in order to correlate the baseline plasma PD-1 and PD-L1 levels with different responses to immunotherapy, allowing us to identify two potential subsets of patients with better or worse clinical outcome. In general, higher levels are associated with a bad clinical outcome, because the patients showed more frequently a disease progression (PD), whereas lower plasma PD-1 and PD-L1 levels are correlated with a better response to therapy (PR and SD).
Therefore, this work suggests, for the first time, the potential role of the plasma PD-1 levels to predict prognosis and obtain more information about the presence/typology of TILs also in patients with metastatic melanoma at the time of diagnosis. In conclusion, our study reveals that the plasma dosage of the soluble PD-1 form could be used to predict prognosis and clinical outcome of advanced melanoma patients under immunotherapy. As PD-1 has been shown to be mainly expressed on the surface of activated TILs, we can reasonably hypothesize that low PD-1 levels in plasma may reflect an enrichment of TILs in the TME, whereas high plasmatic levels of this immune checkpoint may represent an impoverishment by the TME of lymphocytic infiltrate. However, a greater number of studies is needed to further investigate the releasing mechanism of the soluble form of this immune checkpoint from tumors or stromal cells.
Although this study provides significant and useful information to current knowledge in the field, it shows some potential limitations, including the restricted number of analyzed patients, the heterogeneity of the clinical–pathological features (histological subtype, primary tumor localization, and metastatic site), the low statistical power regarding a few analyses, the difficulty of identifying the primary tumor directly at the time of diagnosis resulting in lacking data about TILs on the histological report, and the poor information concerning the distribution pattern of TILs (focal, multifocal, diffused). In addition to the diagnosis of the advanced stage disease, the failure to identify the primary lesion may be due also to events of tumor regression that may determine a tumor understaging. Furthermore, regarding the methodology, an interference of the plasma matrix may be detected during the execution of the ELISA essays. However, this issue has been solved through a 1/5 dilution of the sample.
Lastly, our study confirmed the promising role of TILs as potential biomarkers reflecting the host immune response against tumors. In the future, typing the different TILs subpopulations could have a strong clinical relevance not only in the identification of clusters of patients with different prognosis, but also in the selection of the most suitable patients for a specific treatment with immune checkpoint inhibitors. Indeed, a more in-depth understanding of the specificity and immunobiology of the different lymphocyte subgroups is needed in order to better interpret the prognostic and predictive significance of TILs. As Teng et al.59 have defined four different TMEs in melanoma, based on the presence of TILs and PD-L1 expression, in order to predict the most effective immunotherapy treatment, in the same way, our results could provide an important tool to identify new patient stratification patterns based on the presence/typology of TILs in primary tumor and plasma PD-1 expression. In addition, TIL activity could be enhanced also by the combined use of multiple immunotherapeutic strategies. Finally, investigating the molecular mechanisms underlying the cross-talk between melanoma and immune cells within the TME and identifying factors that drive tumor infiltration will be the key to predicting the heterogeneous clinical outcomes in patients with metastatic melanoma.
Acknowledgments
The authors thank Dr. Chiara Drago for English language revision.
Footnotes
Funding: This work was supported by the Consorzio Interuniversitario Nazionale per la Bio-Oncologia (CINBO).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Lorena Incorvaia, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Giuseppe Badalamenti, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Gaetana Rinaldi, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Juan Lucio Iovanna, Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR 7258, Aix-Marseille Université and Institut Paoli-Calmettes, Parc Scientifique et Technologique de Luminy, Marseille, France.
Daniel Olive, Team Immunity and Cancer, Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR 7258, Aix-Marseille Université and Institut Paoli-Calmettes, Marseille, France;.
Mirna Swayden, Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR 7258, Aix-Marseille Université and Institut Paoli-Calmettes, Parc Scientifique et Technologique de Luminy, Marseille, France.
Lidia Terruso, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Bruno Vincenzi, Medical Oncology Department, University Campus Bio-Medico, Rome, Italy.
Fabio Fulfaro, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Viviana Bazan, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
Antonio Russo, Section of Medical Oncology, Department of Surgical, Oncological and Oral Sciences, University of Palermo, Via del Vespro 129, 90127 Palermo, Italy.
Daniele Fanale, Department of Surgical, Oncological and Oral Sciences, Section of Medical Oncology, University of Palermo, Palermo, Italy.
References
- 1.Ferretti S, Crocetti E, Buzzoni C, et al. Italian cancer figures: North and South are getting closer. Epidemiol Prev 2011; 35: 96–99. [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Fanale D, Bronte G, Russo A. Targeted therapies for solid tumors – A handbook for moving toward new frontiers in cancer treatment. In: Russo A, Rosell R, Rolfo C. (eds) Current clinical pathology. New York: Human Press; 2015: 211–227. [Google Scholar]
- 4.Tas F, Keskin S, Karadeniz A, et al. Noncutaneous melanoma have distinct features from each other and cutaneous melanoma. Oncology 2011; 81: 353–358. [DOI] [PubMed] [Google Scholar]
- 5.Oba-Shinjo SM, Correa M, Ricca TI, et al. Melanocyte transformation associated with substrate adhesion impediment. Neoplasia 2006; 8: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Lorenzo S, Fanale D, Corradino B, et al. Absence of germlineCDKN2A mutation in Sicilian patients with familial malignant melanoma: could it be a population-specific genetic signature? Cancer Biol Ther 2015; 17: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sini MC, Doneddu V, Paliogiannis P, et al. Genetic alterations in main candidate genes during melanoma progression. Oncotarget 2018; 9: 8531–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodis E, Watson Ian R, Kryukov Gregory V, et al. A landscape of driver mutations in melanoma. Cell 2012; 150: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curiel-Lewandrowski C, Chen SC, Swetter SM. Screening and prevention measures for melanoma: is there a survival advantage? Curr Oncol Rep 2012; 14: 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eggermont AMM. Advances in systemic treatment of melanoma. Ann Oncol 2010; 21: vii339–vii344. [DOI] [PubMed] [Google Scholar]
- 11.CiRen B, Wang X, Long Z. The evaluation of immunotherapy and chemotherapy treatment on melanoma: a network meta-analysis. Oncotarget 2016; 7: 81493–81511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camisaschi C, Vallacchi V, Castelli C, et al. Immune cells in the melanoma microenvironment hold information for prediction of the risk of recurrence and response to treatment. Expert Rev Mol Diagn 2014; 14: 643–646. [DOI] [PubMed] [Google Scholar]
- 13.Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol 2012; 23: viii10–viii14. [DOI] [PubMed] [Google Scholar]
- 14.Gajewski TF. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin Cancer Res 2006; 12: 2326s–2330s. [DOI] [PubMed] [Google Scholar]
- 15.Passarelli A, Mannavola F, Stucci LS, et al. Immune system and melanoma biology: a balance between immunosurveillance and immune escape. Oncotarget 2017; 8: 106132–106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long GV, Atkinson V, Cebon JS, et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial. Lancet Oncol 2017; 18: 1202–1210. [DOI] [PubMed] [Google Scholar]
- 17.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371: 1867–1876. [DOI] [PubMed] [Google Scholar]
- 18.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372: 30–39. [DOI] [PubMed] [Google Scholar]
- 19.Ngiow SF, Knight DA, Ribas A, et al. BRAF-targeted therapy and immune responses to melanoma. OncoImmunology 2014; 2: e24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna TP, Nguyen P, Baetz T, et al. A population-based study of survival impact of new targeted and immune-based therapies for metastatic or unresectable melanoma. Clin Oncol 2018; 30: 609–617. [DOI] [PubMed] [Google Scholar]
- 21.Davey RJ, van der Westhuizen A, Bowden NA. Metastatic melanoma treatment: combining old and new therapies. Crit Rev Oncol Hematol 2016; 98: 242–253. [DOI] [PubMed] [Google Scholar]
- 22.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372: 320–330. [DOI] [PubMed] [Google Scholar]
- 23.Cooper ZA, Frederick DT, Ahmed Z, et al. Combining checkpoint inhibitors and BRAF-targeted agents against metastatic melanoma. OncoImmunology 2014; 2: e24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Song Y, Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J Hematol Oncol 2017; 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 2014; 588: 368–376. [DOI] [PubMed] [Google Scholar]
- 26.Topalian SL. Targeting immune checkpoints in cancer therapy. JAMA 2017; 318: 1647. [DOI] [PubMed] [Google Scholar]
- 27.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2016; 8: 2171–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 2015; 125: 3384–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merelli B, Massi D, Cattaneo L, et al. Targeting the PD1/PD-L1 axis in melanoma: biological rationale, clinical challenges and opportunities. Crit Rev Oncol Hematol 2014; 89: 140–165. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet 2005; 365: 687–701. [DOI] [PubMed] [Google Scholar]
- 31.Badalamenti G, Fanale D, Incorvaia L, et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol. Epub ahead of print 30 January 2018. DOI: 10.1016/j.cellimm.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Bønnelykke-Behrndtz ML, Schmidt H, Christensen IJ, et al. Prognostic stratification of ulcerated melanoma. Am J Clin Pathol 2014; 142: 845–856. [DOI] [PubMed] [Google Scholar]
- 33.Cooper PH. Regression in thin malignant melanoma. Arch Dermatol 1985; 121: 1127. [PubMed] [Google Scholar]
- 34.Gooden MJM, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011; 105: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mihm MC, Mule JJ. Reflections on the histopathology of tumor-infiltrating lymphocytes in melanoma and the host immune response. Cancer Immunol Res 2015; 3: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clemente CG, Mihm MC, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996; 77: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 37.Fortes C, Mastroeni S, Mannooranparampil TJ, et al. Tumor-infiltrating lymphocytes predict cutaneous melanoma survival. Melanoma Res 2015; 25: 306–311. [DOI] [PubMed] [Google Scholar]
- 38.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 2012; 30: 2678–2683. [DOI] [PubMed] [Google Scholar]
- 39.Weiss SA, Han SW, Lui K, et al. Immunologic heterogeneity of tumor-infiltrating lymphocyte composition in primary melanoma. Hum Pathol 2016; 57: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obeid JM, Erdag G, Smolkin ME, et al. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: correlation with tumor-infiltrating immune cells and clinical outcome. OncoImmunology 2016; 5: e1235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diem S, Hasan Ali O, Ackermann CJ, et al. Tumor infiltrating lymphocytes in lymph node metastases of stage III melanoma correspond to response and survival in nine patients treated with ipilimumab at the time of stage IV disease. Cancer Immunol Immunother 2017; 67: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014; 35: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu-Monette ZY, Zhang M, Li J, et al. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol 2017; 8: 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdel-Rahman O, Fouad M. A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy 2016; 8: 665–674. [DOI] [PubMed] [Google Scholar]
- 45.Chapon M, Randriamampita C, Maubec E, et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol 2011; 131: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 46.Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: friend or foe for immunotherapy? OncoImmunology 2017; 7: e1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 48.Ascierto PA, Flaherty K, Goff S. Emerging strategies in systemic therapy for the treatment of melanoma. In: American Society of Clinical Oncology Educational Book. Chicago, IL: American Society of Clinical Oncology Annual Meeting Faculty, 2018, pp. 751–758. [DOI] [PubMed] [Google Scholar]
- 49.Dobbin KK, Cesano A, Alvarez J, et al. Validation of biomarkers to predict response to immunotherapy in cancer: volume II — clinical validation and regulatory considerations. J Immunother Cancer 2016; 4: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee N, Zakka LR, Mihm MC, et al. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology 2016; 48: 177–187. [DOI] [PubMed] [Google Scholar]
- 51.Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer 2017; 117: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol 2013; 31: 4252–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burton AL, Roach BA, Mays MP, et al. Prognostic significance of tumor infiltrating lymphocytes in melanoma. Am Surg 2011; 77: 188–192. [PubMed] [Google Scholar]
- 54.Taylor RC, Patel A, Panageas KS, et al. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol 2007; 25: 869–875. [DOI] [PubMed] [Google Scholar]
- 55.Ladányi A. Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma. Pigment Cell Melanoma Res 2015; 28: 490–500. [DOI] [PubMed] [Google Scholar]
- 56.Uryvaev A, Passhak M, Hershkovits D, et al. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Med Oncol 2018; 35: 25. [DOI] [PubMed] [Google Scholar]
- 57.Efremova M, Rieder D, Klepsch V, et al. Targeting immune checkpoints potentiates immunoediting and changes the dynamics of tumor evolution. Nat Commun 2018; 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khunger M, Hernandez AV, Pasupuleti V, et al. Programmed cell death 1 (PD-1) ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: a systematic review and meta-analysis. JCO Prec Oncol 2017; 1: 1–15. [DOI] [PubMed] [Google Scholar]
- 59.Teng MWL, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75: 2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]