Synapsins regulate α-synuclein functions (original) (raw)
Abstract
The normal function of α-synuclein (α-syn) remains elusive. Although recent studies suggest α-syn as a physiologic attenuator of synaptic vesicle (SV) recycling, mechanisms are unclear. Here, we show that synapsin—a cytosolic protein with known roles in SV mobilization and clustering—is required for presynaptic functions of α-syn. Our data offer a critical missing link and advocate a model where α-syn and synapsin cooperate to cluster SVs and attenuate recycling.
Keywords: alpha synuclein, synapsin, neurotransmission
Significant effort has been spent in deciphering the normal function of α-synuclein (α-syn), a presynaptic protein involved in neurodegeneration. An emerging consensus is that α-syn acts as a physiologic attenuator of neurotransmitter release. Modest α-syn overexpression suppresses exocytosis in various cells—including neurons (1, 2)—and impaired transmission is the first phenotype in an in vivo model of α-syn overexpression (3). Although phenotypes of single-gene α-syn–knockout mice are mild, reduced striatal dopamine stores suggest enhanced release (4). Knockouts of multiple synuclein genes reveal increases in dopamine release in vivo (5) as well as enhanced transmission in hippocampal slices (6) (but see ref. 7).
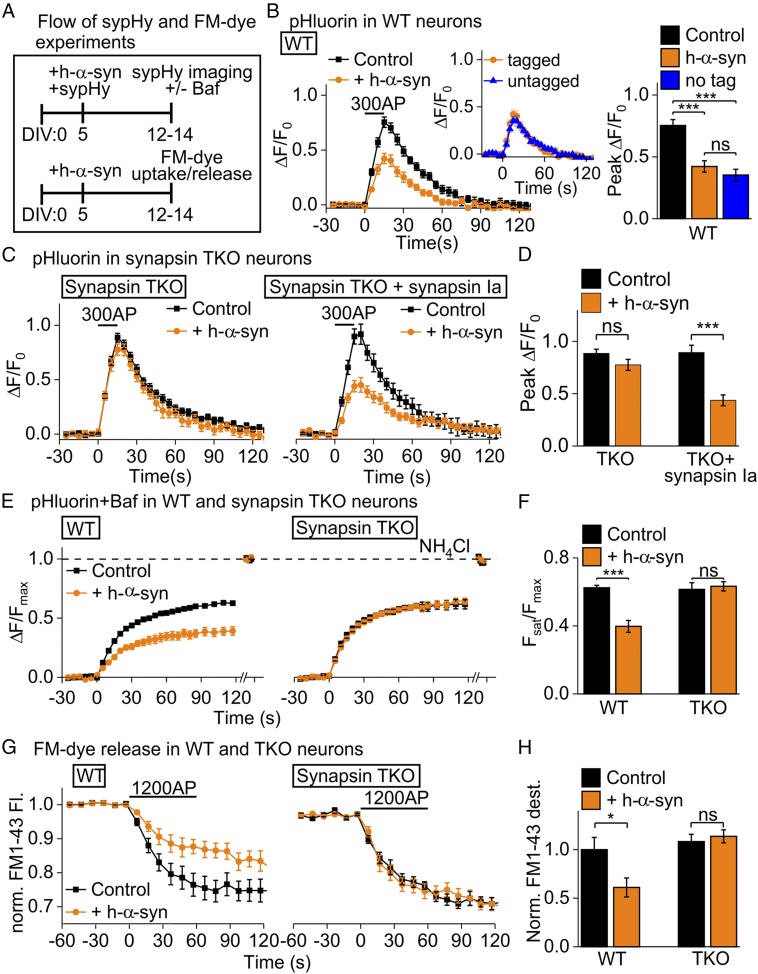
The mechanism by which α-syn attenuates release is unclear and controversial. Although most studies show that α-syn affects exocytosis (8–10), some suggest slowing of endocytosis (11, 12). We proposed that α-syn facilitates synaptic vesicle (SV) clustering, restricting mobility and egress of SVs to the active zone, attenuating exocytosis (10). Collaboration of protein networks is a common theme at synapses, yet molecules regulating α-syn–mediated SV attenuation are unknown. Like synucleins, synapsins are a family of cytosolic regulators of SV mobilization and clustering (13). Here, we explore putative links between α-syn and synapsins using cultured hippocampal neurons from synapsin triple-knockout (TKO) mice (14, 15). We used synaptophysin I-pHluorin (sypHy) and the styryl-dye FM1-43 (FM) to report exo/endocytosis (ref. 15 and Fig. 1_A_). While modest human α-syn (hα-syn) overexpression in wild-type (WT) neurons reduced SV recycling (Fig. 1_B_), surprisingly, there was no effect in TKO neurons (Fig. 1 C, Left and D). Reintroduction of synapsin Ia restored hα-syn–induced attenuation (Fig. 1 C, Right and D), confirming synapsins’ critical role. Selective blocking of SV reacidification (15) confirmed the role for synapsins in α-syn–induced attenuation of exocytosis (Fig. 1 E and F), as did FM-dye experiments (Fig. 1 G and H).
Fig. 1.
Synapsins are required for α-syn–mediated synaptic attenuation. (A) Experimental design: cultured hippocampal neurons were transduced (AAV1/2) at 5 d in vitro (DIV); sypHy/FM1-43 were imaged at 12 to 14 DIV. (B) SypHy in WT neurons. Modest overexpression of hα-syn-mCherry (184% ± 10%) attenuated sypHy responses in WT neurons (Control: mCherry); (Inset) similar effect of untagged hα-syn (stimulation: 10 V/cm, 20 Hz; n = 9 to 13, >30 synapses per coverslip, ≥3 cultures; one-way ANOVA, Tukey’s post hoc analysis). (C) SypHy in synapsin TKO neurons. Hα-syn failed to attenuate synaptic responses in TKO neurons (Left); reintroduction of tag blue fluorescent protein (mTagBFP)-synapsin Ia (Right) reinstated hα-syn–induced synaptic attenuation. (D) Quantification of data in C (n = 6 to 15; t test). (E) Evaluation of exocytosis. Bafilomycin A (Baf) blocked SV reacidification after endocytosis. Hα-syn reduced exocytosis in WT neurons (Left), but not in TKO neurons (Right). Dashed line indicates total SV pool revealed by NH4Cl. (F) Quantification of data in E (n = 6 to 17; t test). (G) FM-dye release. Neurons loaded with FM1-43 were stimulated to evaluate exocytosis. Hα-syn reduced FM-dye release in WT neurons, but not in TKO neurons. (H) Quantification of data in G (n = 5 to 9; t test). ns, not significant, *P < 0.05, ***P < 0.001.
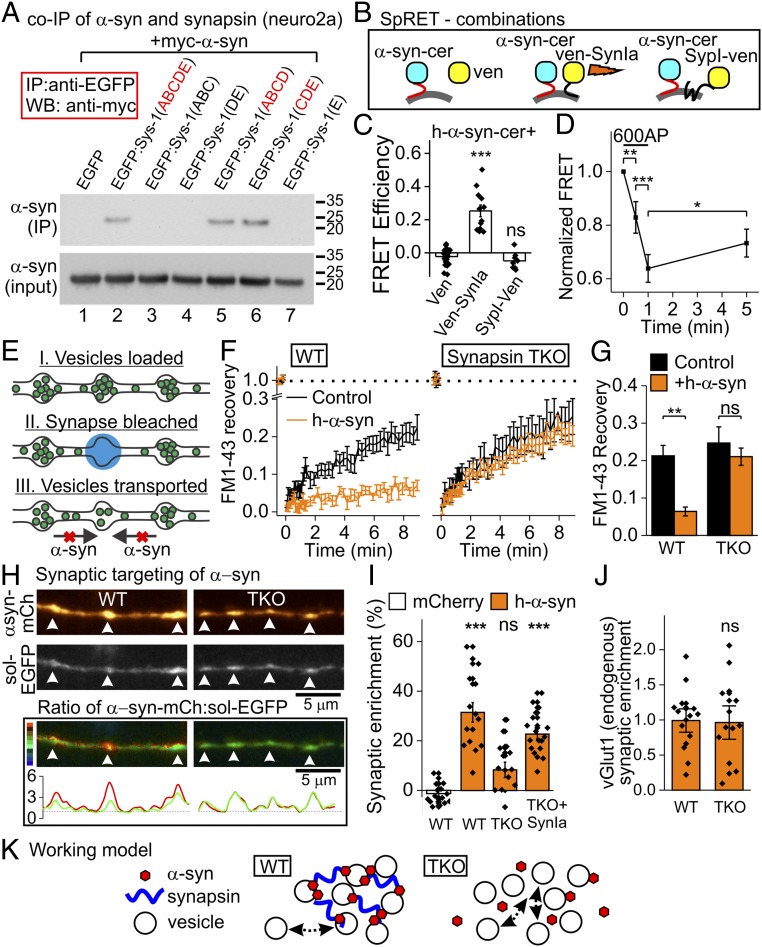
Do α-syn and synapsin interact? Although previous screens imply so (16, 17), this has not been validated at synapses. Indeed, α-syn and synapsin Ia coimmunoprecipitated (co-IPd) in neuro2a cells, likely via synapsin C/D domains (Fig. 2_A_). Using SpRET-a sensitive spectral fluorescence resonance energy transfer (FRET) assay (18)—at synapses, we found association of hα-syn with synapsin, but not with soluble Venus or with the membrane-spanning SV protein synaptophysin I, arguing against nonspecific FRET (Fig. 2 B and C). Also, hα-syn/synapsin FRET diminished after stimulation (Fig. 2_D_), in line with activity-induced dispersal of α-syn and synapsin (19). How do synapsins affect α-syn function? Previously, using fluorescence recovery after photobleaching (FRAP) of FM1-43, we found that α-syn inhibited SV exchange between adjacent synaptic boutons, likely due to α-syn–induced SV clustering (ref. 9 and Fig. 2_E_). Interestingly, this effect was also lost in TKO neurons (Fig. 2 F and G), suggesting mechanistic links among α-syn, synapsin, and SV clustering. Finally, synaptic enrichment of hα-syn—as evaluated by a rigorous ratiometric method (14)—was reduced in TKO neurons but restored by synapsin Ia expression (Fig. 2 H and I). This effect is likely unrelated to SV distribution, which was similar in WT and TKO neurons expressing hα-syn (Fig. 2_J_; vGlut1 as SV marker). Our data suggest that synapsins facilitate α-syn–SV interactions, either locally or by influencing axonal transport of α-syn. Additionally, α-syn may insert into a synapsin/SV liquid phase that assists SV clustering and alter it (20). Together with the companion paper (21), our data reveal functional partners of α-syn at synapses, offer a framework for interpreting α-syn biology, and open avenues for research into the synucleinopathies.
Fig. 2.
Interaction of α-syn and synapsin in neuronal cell lines and synapses. (A) Coimmunoprecipitation (co-IP) of synapsin and α-syn. Neuro2a cells were cotransfected with enhanced green fluorescent protein (EGPF)-synapsin Ia (or its deletions) and myc-hα-syn, and then immunoprecipitated with an anti-EGFP antibody (Millipore). Full-length EGFP-synapsin I (but not EGFP alone) interacted with α-syn (lanes 1 and 2). Synapsin C/D domains (lanes 5 and 6) are critical for this interaction [Bottom: inputs; anti-myc (Abcam); repeated twice]. (B) FRET combinations. Donor: hα-syn-cerulean (α-syn-cer); acceptor: soluble Venus (ven), Venus-synapsin Ia (ven-SynIa), or the SV protein synaptophysin I-Venus (SypI-ven). (C) FRET data. Note FRET in synapses between hα-syn-cerulean and Venus-synapsin Ia, but not soluble Venus (control). No FRET was seen with synaptophysin I-Venus (n = 8 to 26; one-way ANOVA, Tukey’s post hoc analysis). (D) FRET between hα-syn and synapsin Ia was reduced by stimulation, recovering during rest (n = 101 synapses in 3 experiments; Friedman’s ANOVA, post hoc analysis: Wilcoxon’s test/Bonferroni’s correction). (E) FM-FRAP schematic. SVs are loaded with FM1-43 and a single bouton is bleached. Hα-syn inhibits intersynaptic SV traffic and FM recovery. (F) FM-FRAP experiments. Hα-syn dampens recovery in WT neurons (Left), but not in TKO neurons (Right). (G) Quantification of data in F (n = 10 to 17 experiments, 3 synapses per experiment; t test). (H, Top) Synaptic enrichment. Hα-syn-mCherry and soluble EGFP (volume filler) in WT and TKO neurons. (H, Bottom) Ratio images (scale to left) and intensity curves; red indicates α-syn, green indicates EGFP. (I) Quantification of data in H. Hα-syn-mCherry (vs. soluble mCherry) is enriched in WT, but not in TKO boutons. Reintroduction of synapsin Ia restored α-syn synaptic enrichment (n = 15 to 18; one-way ANOVA, Tukey’s post hoc analysis). (J) Synaptic enrichment of endogenous vGlut1 (normalized by WT) is similar in WT and TKO neurons overexpressing hα-syn (n = 14 to 15; t test). (K) Synapsins help α-syn associate with SVs, thereby facilitating SV clustering [α-syn also binds VAMP2 to help clustering (21), not shown]. Loss of synapsins decreases α-syn targeting and disrupts clustering of SVs. ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Acknowledgments
This work was funded by Israel Science Foundation Grant 1427/12 (to D.G.) and NIH/National Institute on Aging Grants P50AG005131 and R01AG048218 (to S.R.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Garcia-Reitböck P., et al. , SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 133, 2032–2044 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen K. E., et al. , Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J. Neurosci. 26, 11915–11922 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundblad M., Decressac M., Mattsson B., Björklund A., Impaired neurotransmission caused by overexpression of α-synuclein in nigral dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 109, 3213–3219 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abeliovich A., et al. , Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Anwar S., et al. , Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 31, 7264–7274 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greten-Harrison B., et al. , αβγ-synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc. Natl. Acad. Sci. U.S.A. 107, 19573–19578 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burré J., et al. , Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemani V. M., et al. , Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65, 66–79 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott D., Roy S., α-synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 32, 10129–10135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., et al. , α-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr. Biol. 24, 2319–2326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busch D. J., et al. , Acute increase of α-synuclein inhibits synaptic vesicle recycling evoked during intense stimulation. Mol. Biol. Cell 25, 3926–3941 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J., et al. , α-synuclein mutation inhibits endocytosis at mammalian central nerve terminals. J. Neurosci. 36, 4408–4414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song S. H., Augustine G. J., Synapsin isoforms and synaptic vesicle trafficking. Mol. Cells 38, 936–940 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gitler D., et al. , Molecular determinants of synapsin targeting to presynaptic terminals. J. Neurosci. 24, 3711–3720 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orenbuch A., et al. , Synapsin selectively controls the mobility of resting pool vesicles at hippocampal terminals. J. Neurosci. 32, 3969–3980 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betzer C., et al. , Identification of synaptosomal proteins binding to monomeric and oligomeric α-synuclein. PLoS One 10, e0116473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaltieri M., et al. , α-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J. Cell Sci. 128, 2231–2243 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Levy S., et al. , SpRET: Highly sensitive and reliable spectral measurement of absolute FRET efficiency. Microsc. Microanal. 17, 176–190 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Fortin D. L., et al. , Neural activity controls the synaptic accumulation of alpha-synuclein. J. Neurosci. 25, 10913–10921 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milovanovic D., Wu Y., Bian X., De Camilli P., A liquid phase of synapsin and lipid vesicles. Science 361, 604–607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J., et al. , Functional cooperation of α-synuclein and VAMP2 in synaptic vesicle recycling. Proc. Natl. Acad. Sci. U.S.A. 116, 11113–11115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]