Regression From Prediabetes to Normal Glucose Regulation and Prevalence of Microvascular Disease in the Diabetes Prevention Program Outcomes Study (DPPOS) (original) (raw)
Abstract
OBJECTIVE
Regression from prediabetes to normal glucose regulation (NGR) was associated with reduced incidence of diabetes by 56% over 10 years in participants in the Diabetes Prevention Program Outcomes Study (DPPOS). In an observational analysis, we examined whether regression to NGR also reduced risk for microvascular disease (MVD).
RESEARCH DESIGN AND METHODS
Generalized estimating equations were used to examine the prevalence of aggregate MVD at DPPOS year 11 in people who regressed to NGR at least once (vs. never) during the Diabetes Prevention Program (DPP). Logistic regression assessed the relationship of NGR with retinopathy, nephropathy, and neuropathy, individually. Generalized additive models fit smoothing splines to describe the relationship between average A1C during follow-up and MVD (and its subtypes) at the end of follow-up.
RESULTS
Regression to NGR was associated with lower prevalence of aggregate MVD in models adjusted for age, sex, race/ethnicity, baseline A1C, and treatment arm (odds ratio [OR] 0.78, 95% CI 0.65–0.78, P = 0.011). However, this association was lost in models that included average A1C during follow-up (OR 0.95, 95% CI 0.78–1.16, P = 0.63) or diabetes status at the end of follow-up (OR 0.92, 95% CI 0.75–1.12, P = 0.40). Similar results were observed in examination of the association between regression to NGR and prevalence of nephropathy and retinopathy, individually. Risk for aggregate MVD, nephropathy, and retinopathy increased across the A1C range.
CONCLUSIONS
Regression to NGR is associated with a lower prevalence of aggregate MVD, nephropathy, and retinopathy, primarily due to lower glycemic exposure over time. Differential risk for the MVD subtypes begins in the prediabetes A1C range.
Introduction
The Diabetes Prevention Program Outcomes Study (DPPOS) is the follow-up of the Diabetes Prevention Program (DPP), which was a randomized controlled clinical trial examining whether it is possible to prevent or delay the onset of diabetes. The study continues to follow participants—now for nearly 20 years—to determine the enduring impact of the formerly randomized treatments (intensive lifestyle intervention, metformin, or placebo) on microvascular disease, cardiovascular disease, cancer, and aspects of aging (1). To date, the DPPOS has provided evidence that vascular disease can be reduced when diabetes is prevented or delayed, with particular effectiveness of lifestyle therapy in women (21% lower composite microvascular disease vs. placebo) (1) and metformin in men (11% lower presence of coronary artery calcium vs. placebo) (2). The many anticipated outcomes of the DPPOS may ultimately determine whether prediabetes is recognized as simply an earlier form of diabetes.
Current management of prediabetes is largely limited to behavior modification to facilitate weight loss—not necessarily normalization of plasma glucose concentration. This is relevant, as a post hoc analysis from the DPPOS revealed a 31% increased risk for diabetes in people who did not regress from prediabetes to normoglycemia despite being randomized to the intensive lifestyle modification arm (vs. those who did regress who had been randomized to placebo) (3). It is likely that this group represents people at a more advanced stage in their disease course and who are therefore less responsive to the lifestyle intervention, thus highlighting the need to follow plasma glucose concentration during preventive interventions. The increase in diabetes risk was in direct contrast to a 56% reduction in diabetes incidence for collective participants who had restored normoglycemia at least once during the DPP compared with those who never did. Further, regression was associated with a protective cardiovascular phenotype despite the use of fewer medications for blood pressure and lipids (4).
Hence, the current analysis sought to examine whether regression from prediabetes to normoglycemia is also associated with a lower prevalence of aggregate microvascular disease and whether this would be due to, or independent of, lower cumulative glycemic exposure. We also sought to compare the impact of regression to normoglycemia and the relative contribution of glycemia to retinopathy, nephropathy, and neuropathy, individually.
Research Design and Methods
Participants
The DPP was a randomized clinical trial performed at 27 research centers in the U.S. that enrolled overweight or obese adults with prediabetes determined on one occasion. BMI ≥24 kg/m2, elevated fasting glucose 95–125 mg/dL (5.3–6.9 mmol/L)—for all but those at the American Indian centers, for whom it was <125 mg/dL (<6.9 mmol/L)—and 2-h plasma glucose levels of 140–199 mg/dL (7.8–11.0 mmol/L) were requisite entry criteria into DPP; by American Diabetes Association criteria (5), 100% had impaired glucose tolerance with 85% also having impaired fasting glucose. The DPPOS is the follow-up of DPP and includes 2,775 persons (85% of the original cohort as of data lock) on 2 January 2014 (1). Detailed methods have previously been published (6,7), and the protocol is available at http://www.bsc.gwu.edu/dpp. Institutional review boards at each center approved the protocol, and all participants gave written informed consent prior to participation.
Interventions
During the DPP, participants were randomized to 1) an intensive lifestyle intervention (low-fat diet and exercise >150 min/week for a goal of 7% body weight reduction), 2) metformin 850 mg twice daily, or 3) matching double-blinded placebo. Median follow-up during DPP was 3.2 years followed by a 10-month “bridge” period (8) during which all participants, including those who had been randomized to the intensive lifestyle intervention arm during DPP, were offered group-based lifestyle sessions prior to the start of DPPOS (7). Open-label metformin was also continued in participants initially randomized to metformin during DPP but was discontinued when progression to diabetes required management outside of the protocol or for reasons of safety and/or tolerability. Median follow-up in DPPOS was 15 years (range 14–17) from randomization to the closing date of this analysis (2 January 2014).
Outcomes
The primary outcome of the DPP and DPPOS is the development of diabetes, defined as fasting plasma glucose ≥126 mg/dL (≥7.0 mmol/L [checked semiannually]) and/or 2-h glucose ≥200 mg/dL (≥11.1 mmol/L [checked annually]) during a 75-g oral glucose tolerance test (OGTT) (5). Once diabetes is confirmed on a second OGTT, the participant is classified as having diabetes irrespective of subsequent plasma glucose values. Normal glucose regulation (NGR) (aka normoglycemia) is defined as fasting plasma glucose levels <100 mg/dL (5.6 mmol/L) and 2-h plasma glucose levels <140 mg/dL (7.8 mmol/L) at least once on an annual OGTT during the active intervention phase of DPP.
Prevalence of aggregate microvascular disease is a coprimary outcome of DPPOS. During the follow-up in DPPOS, microvascular disease was defined as follows:
- retinopathy: diagnosed on seven-field stereoscopic fundus photography by Early Treatment of Diabetic Retinopathy Study (ETDRS) score ≥20 in either eye, or treatment of retinopathy with laser photocoagulation or intravitreal injections, assessed once during DPPOS in 2012 or 2013, and/or
- neuropathy: measured as absence of light touch sensation (<8 of 10 applications detected on the dorsum of the great toe) measured with a 10-g Semmes-Weinstein monofilament (9), assessed annually throughout DPPOS, and/or
- nephropathy: assessed by albuminuria ≥30 mg/g creatinine in a spot urine collection on two consecutive tests, or an estimated glomerular filtration rate <45 mL/min/1.73 m2 on two consecutive tests, based on annual serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (10), or renal failure (end-stage renal disease, dialysis, or transplantation, assessed annually throughout DPPOS). Participants who had met the nephropathy criteria previously and were taking blood pressure–lowering medication(s) at the final assessment were considered to have reached the nephropathy outcome even if they did not meet albuminuria or estimated glomerular filtration rate criteria at that time.
For improvement of our detection of the outcomes, the current analysis defines the aggregate microvascular disease outcome as the occurrence of one or more of the microvascular disease subtypes as opposed to the average prevalence of the three subtypes as has been used to define the microvascular disease outcomes previously (1). We also examined each component of the aggregate microvascular disease outcome individually.
Potential Effect Modifiers, Mediators, and Confounders
We examined the influence of several factors that could potentially modify or confound the association between NGR status and microvascular disease. There was no interaction between regression to NGR and treatment group on aggregate microvascular disease (possibly due to small numbers); hence, subsequent analyses were not stratified. Sequential models thus adjusted for baseline age, sex, race/ethnicity, and treatment group, conceptualized as potential confounders, as well as average A1C or diabetes development during follow-up, conceptualized as potential mediators. The follow-up period was defined starting at the time of randomization until the time microvascular outcomes were assessed in DPPOS year 11 (14–17 years postrandomization). A time-to-event analysis could not be done due to differences in ascertainment of the microvascular outcomes and the fact that preexisting microvascular disease was not an exclusion to enroll into DPP. A1C values collected after an adjudicated microvascular outcome had been reached are censored in this analysis to avoid subsequent treatment bias in the data.
Statistical Analyses
Descriptive statistics were used to compare baseline demographic characteristics between those who had ever versus never achieved NGR during DPP. Generalized estimating equations were used to examine the prevalence of aggregate microvascular disease during DPPOS in participants who ever versus never attained NGR at any time during DPP. Logistic models assessed the association of regression to NGR with individual microvascular disease subtypes (retinopathy, nephropathy, and neuropathy). The impact of A1C (as a continuous variable) is per 1 percentage point difference. Initial models were univariate (model 1), while subsequent models were sequentially adjusted for baseline age, sex, race/ethnicity, baseline A1C (model 2), model 2 adjustments plus treatment group (model 3), model 3 adjustments plus average A1C over time (model 4), and model 3 adjustments plus diabetes status during DPPOS follow-up (model 5). A sensitivity analysis added dietary factors, physical activity level, smoking, and known genes related to diabetes to the models, and they did not materially influence the association of interest (NGR and outcomes) or on the models exploring the mediation by A1C or diabetes status (data not shown). Generalized additive models were used, and cubic, smoothing splines (i.e., degrees of freedom = 4) were fitted, to estimate the prevalence of aggregate and individual microvascular outcomes at DPPOS year 11, as a smooth function of average A1C levels during DPPOS follow-up.
Results
Baseline Characteristics
Baseline characteristics of participants who were ever versus never able to restore NGR during DPP are shown in Table 1. Overall, approximately one-third of participants returned to NGR at some point during DPP, and this did not differ by sex, race/ethnicity, or the use of aspirin or hormone-replacement therapy at baseline, except that more American Indians achieved NGR. The latter finding likely relates to the lower fasting glucose criteria for enrollment in this group. Consistent with our previous results (3,4,11), participants who did not regress to NGR were older, were more likely to have hypertension and/or dyslipidemia at baseline, and were more likely to develop diabetes (data not shown) and had higher average A1C over time (Table 1) compared with those who attained NGR status during DPP.
Table 1.
Baseline characteristics of people who never versus ever achieved NGR during DPP
| Never NGR (sample size 2,181) | Ever NGR (sample size 1,053) | P | |
|---|---|---|---|
| Race | |||
| White | 1,206 (55) | 562 (53) | 0.0017 |
| Black | 437 (20) | 208 (20) | |
| Hispanic | 346 (16) | 162 (15) | |
| Asian | 101 (5) | 41 (4) | |
| American Indian | 91 (4) | 80 (8) | |
| Sex | |||
| Male | 714 (33) | 329 (31) | 0.3946 |
| Female | 1,467 (67) | 724 (69) | |
| Medical history at baseline DPP | |||
| Hypertension* | 496 (23) | 183 (17) | 0.0006 |
| Dyslipidemia | 793 (36) | 345 (27) | 0.0448 |
| Hormone-replacement use | 521 (24) | 239 (27) | 0.4541 |
| Age | 51.1 ± 10.8 | 49.7 ± 10.5 | 0.0004 |
| Baseline A1C (%) | 5.96 ± 0.51 | 5.81 ± 0.47 | <0.0001 |
| Average A1C during follow-up | 6.10 ± 0.70 | 5.76 ± 0.49 | <0.0001 |
| Aspirin (frequency/week) | 1.92 ± 1.55 | 1.90 ± 1.49 | 0.7371 |
Association of Regression to NGR (Ever Versus Never) During DPP With Prevalent Microvascular Disease in DPPOS
Regression to NGR was associated with lower odds of aggregate microvascular disease in the first three models (model 1, odds ratio [OR] 0.70, 95% CI 0.59–0.84, P < 0.001; model 2, OR 0.77, 95% CI 0.64–0.92, P = 0.005; and model 3, OR 0.78, 95% CI 0.65–0.95, P = 0.011) (Table 2). However, the significance of this association was lost in models 4 and 5, which included average A1C over time (OR 0.95, 95% CI 0.78–1.16, P = 0.63) or diabetes status at follow-up (OR 0.92, 95% CI 0.75–1.11, P = 0.40) (Table 2), respectively. Similar results were observed for the association between regression to NGR and prevalence of nephropathy and retinopathy, individually (Table 2). There was no interaction between diabetes status and microvascular disease in the univariate model employing generalized estimating equations; hence, no subsequent analyses were conducted stratifying by diabetes status.
Table 2.
Impact of ever versus never regressing to NGR in DPP on microvascular outcomes in DPPOS
| | OR | 95% CI | P | | | | ------------------------------------------------------------------ | ----------- | ----- | ----- | ------ | | Lower limit | Upper limit | | | | | Aggregate MVD | | | | | | Adjustment covariates | | | | | | Model 1: univariate (unadjusted) | 0.704 | 0.588 | 0.843 | <0.001 | | Model 2: adjusted for age, sex, race/ethnicity, baseline A1C | 0.765 | 0.635 | 0.922 | 0.005 | | Model 3: model 2 adjustments + treatment group | 0.784 | 0.649 | 0.947 | 0.011 | | Model 4: model 3 adjustments + average A1C during follow-up | 0.953 | 0.783 | 1.160 | 0.629 | | Model 5: model 3 adjustments + diabetes status at end of follow-up | 0.916 | 0.750 | 1.121 | 0.395 | | Nephropathy | | | | | | Adjustment covariates | | | | | | Model 1: univariate (unadjusted) | 0.638 | 0.500 | 0.813 | <0.001 | | Model 2: adjusted for age, sex, race/ethnicity, baseline A1C | 0.695 | 0.542 | 0.892 | 0.004 | | Model 3: model 2 adjustments + treatment group | 0.689 | 0.535 | 0.888 | 0.004 | | Model 4: model 3 adjustments + average A1C during follow-up | 0.808 | 0.623 | 1.049 | 0.109 | | Model 5: model 3 adjustments + diabetes status at end of follow-up | 0.887 | 0.678 | 1.161 | 0.384 | | Retinopathy | | | | | | Adjustment covariates | | | | | | Model 1: univariate (unadjusted) | 0.660 | 0.500 | 0.880 | 0.004 | | Model 2: adjusted for age, sex, race/ethnicity, baseline A1C | 0.675 | 0.508 | 0.898 | 0.007 | | Model 3: model 2 adjustments + treatment group | 0.672 | 0.504 | 0.897 | 0.007 | | Model 4: model 3 adjustments + average A1C during follow-up | 0.850 | 0.630 | 1.147 | 0.288 | | Model 5: model 3 adjustments + diabetes status at end of follow-up | 0.738 | 0.544 | 1.001 | 0.051 | | Neuropathy | | | | | | Adjustment covariates | | | | | | Model 1: univariate (unadjusted) | 0.810 | 0.610 | 1.070 | 0.131 | | Model 2: adjusted for age, sex, race/ethnicity, baseline A1C | 0.950 | 0.700 | 1.280 | 0.738 | | Model 3: model 2 adjustments + treatment group | 1.024 | 0.754 | 1.392 | 0.878 | | Model 4: model 3 adjustments + average A1C during follow-up | 1.144 | 0.834 | 1.571 | 0.404 | | Model 5: model 3 adjustments + diabetes status at end of follow-up | 1.055 | 0.761 | 1.464 | 0.747 |
Association Between Average A1C Over Time and Prevalent Aggregate Microvascular Disease
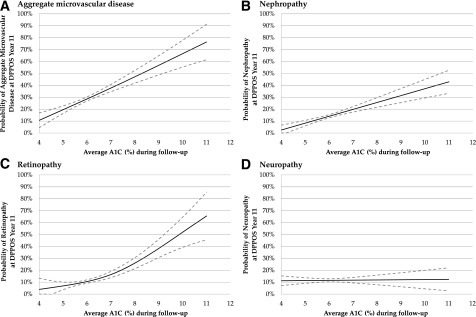
Because lack of diabetes and lower A1C during follow-up explained, in large part, the association between regression to NGR and lower prevalence of microvascular disease, additional investigation was undertaken to model the associations between average A1C during the follow-up and aggregate microvascular disease, as well as for A1C and the subtypes (below). The smoothed relationship (and pointwise 95% CIs) between average A1C levels during follow-up and aggregate microvascular disease prevalence at DPPOS year 11 is plotted in Fig. 1_A_. As shown, the risk of aggregate microvascular disease increases from 10% to nearly 80% across the A1C range 4–11%. The slope of the A1C × aggregate microvascular disease curve was not different comparing A1C <6.5% (normoglycemia and prediabetes) vs. ≥6.5% (diabetes) ranges, indicating no inflection point (P = 0.763).
Figure 1.
Predicted, unadjusted prevalence of aggregate MVD (retinopathy, nephropathy, and/or neuropathy) (A), nephropathy (B), retinopathy (C), and neuropathy (D) as a function of A1C levels during DPPOS follow-up. Solid lines represent a smoothed, fitted relationship, whereas the dotted lines represent the pointwise 95% CI.
Association Between Average A1C Over Time and Prevalent Microvascular Disease Subtypes
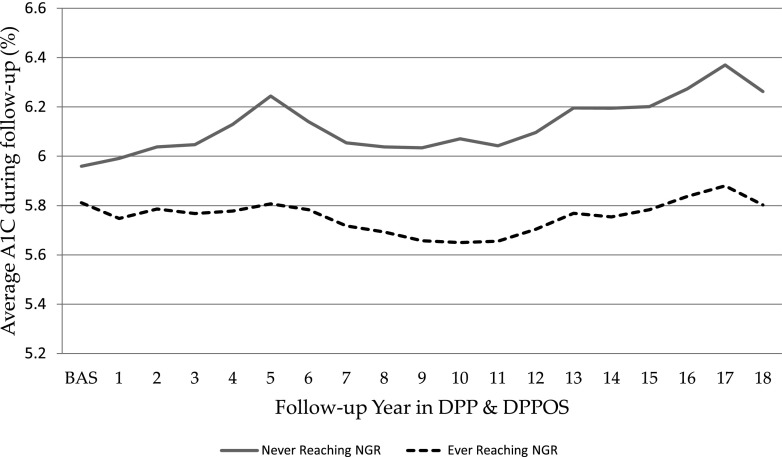
Prevalence of nephropathy increased steadily across the A1C range studied, reaching an approximate prevalence of 40% at an A1C of 11% (Fig. 1_B_). Prevalence of retinopathy was <10% for A1C <6% but rose steeply after an A1C of 6%, reaching an estimated prevalence of 65% at an A1C of 11% (Fig. 1_C_). Prevalence of peripheral neuropathy was ∼12% and not different across A1C 4–11% (Fig. 1_D_). The average A1C over the DPP and DPPOS follow-up (included in this analysis) is shown in Fig. 2.
Figure 2.
A1C over DPP and DPPOS for participants who ever versus never regressed from prediabetes to normoglycemia (includes those who have and have not developed diabetes). BAS, baseline.
Conclusions
A number of landmark trials have convincingly demonstrated that diabetes can be prevented or delayed in people with prediabetes (reviewed in 12). An emerging body of evidence suggests that complications can also be prevented in people with prediabetes receiving early intervention aimed at reducing body weight, lipids, blood pressure, and/or plasma glucose (13–15). Results from the current analysis add to this growing body of literature by demonstrating that regression from prediabetes to normoglycemia (assessed by both fasting and 2-h plasma glucose concentrations) is associated with a lower prevalence of aggregate microvascular disease, as well as nephropathy and retinopathy, individually, due to lower cumulative glycemic exposure over time.
Limiting cumulative glycemic exposure remains central in diabetes care. The current results demonstrate that this is also true for people with prediabetes who have not or will not develop overt diabetes. Despite the mild dysglycemic state that defines prediabetes, we observed a 22–30% lower prevalence of aggregate microvascular disease in participants with prediabetes who regressed to normoglycemia (models 1–3). This finding was explained by a lower average A1C over time (model 4) and lower risk for diabetes (model 5). Microvascular complications can and do occur in people with prediabetes (16–18), and the collective evidence has been deemed sufficient to result in treatment guidelines for people with prediabetes (19–21)—largely resembling those for diabetes itself. Guidelines put forth by the American Association of Clinical Endocrinologists specifically advocate for the restoration of normoglycemia and multiple risk factor intervention for the prevention of atherosclerotic cardiovascular disease in people with prediabetes (22). Altogether, the paradigm of treating prediabetes to prevent complications is directly akin to our goals for people with diabetes and argues against the notion of a “pre” disease.
Benchmarks of care for diabetes are based on the A1C level where the classic microvascular complications emerge (23). Importantly, however, controversy exists as to whether the relationship between A1C and microvascular disease is continuous (24) or curvilinear (16), casting a degree of ambiguity on the timing for intervention. For example, the DETECT-2 Collaboration did observe an inflection point between glycemic measures and retinopathy in people without diabetes (17), whereas long-term follow-up of people with early diabetes in the UK Prospective Diabetes Study (UKPDS) shows the presence of micro- and macrovascular disease across the A1C range, including what is now considered the at-risk “prediabetes” A1C range (i.e., 5.7–6.4%) (25), according to the American Diabetes Association (26). The current analysis is consistent with the latter observation, as we found an ∼10% increase in the probability of having aggregate microvascular disease at DPPOS year 11 (roughly from 25 to 35%) (Fig. 2_A_) when A1C changes from A1C 5.7–6.4%, with no clear inflection point. Likewise, the relationship between average A1C over time and nephropathy also appeared relatively linear. Together, it may be time to revisit whether prediabetes is actually an earlier form of diabetes (27).
It is alluring to imagine an A1C threshold below which patients are fully protected from diabetes complications (28). This quest has proven less straightforward than is widely acknowledged. Accordingly, a number of previous reviews have revisited the A1C threshold where diabetes-related microvascular complications occur (25,29–31). Even for retinopathy (which experts believe to be the most diabetes-specific complication), when A1C thresholds are calculated, they range widely from 5.2 to 7.8% (30). Kowall and Rathmann (30) cite a number of reasons for the discrepant findings including differing criteria for and method of defining retinopathy, statistical methodology, population ethnicity influence on A1C, and the use of nonstandardized A1C assays. Results from the DPPOS provide valuable longitudinal data in a well-described cohort. The current analysis highlights different relationships between the microvascular disease subtypes and A1C over time. For example, prevalence of nephropathy showed a linear increase over the A1C range, whereas the relationship between retinopathy and A1C was curvilinear, and no relationship was seen for neuropathy and A1C from 4 to 11%. This observation suggests that neuropathy—as assessed by sensation to light touch—is less related to hyperglycemia (or the accompanying metabolic milieu) compared with retinopathy and possibly nephropathy. It is also consistent with the relatively poor sensitivity and specificity for A1C to predict neuropathy highlighted in a recent review (30). In the era of precision medicine, these findings may have implications for the timing of glucose-lowering intervention based on someone’s risk for a particular microvascular disease subtype.
Despite the risk for diabetes-related complications for people with prediabetes, goals for this patient population are largely limited to diabetes prevention via lifestyle modification (32). For example, the DPP inspired the Centers for Disease Control and Prevention (CDC)-funded National Diabetes Prevention Program (NDPP). The NDPP aims to make the lifestyle curriculum developed for DPP available to the public to prevent diabetes in the U.S. population. One major shortfall, however, is the fact that few NDPP programs follow plasma glucose level or A1C (33) leaving the only true metric for success the national diabetes incidence rate. In contrast, pursuit of regression from prediabetes to normoglycemia—by way of lifestyle modification with or without medical therapy—is measurable and actionable in a clinical setting and can reduce the risk for both diabetes and diabetes-related complications, even if only transiently.
Collection and analysis of data from the DPPOS continue to provide valuable insights into the natural history and impact of preventive efforts on the development of diabetes and its complications. Nevertheless, several limitations of our current analysis warrant mention. This analysis is post hoc conducted postintervention during observational follow-up, and results should be viewed as hypothesis generating. Associations do not confirm causation. For example, people with prediabetes may spontaneously regress to normoglycemia due to inherently lower risk for diabetes rather than, or in addition to, a treatment response (34). We did not analyze the glycemic status of people confirmed as having diabetes who also could have later regressed to prediabetes or NGR. Thus far, prevalence of complications, especially for the individual components of microvascular disease, has been very low, and the complications data were ascertained with different schedules. This is in part because the average A1C over follow-up has been relatively low (i.e., only seven participants have average A1C >10% during follow-up). In addition, the lower sensitivity of our methods of detection for some of the microvascular disease end points complicate direct comparisons of individual microvascular disease outcomes. Presence of microvascular disease was not an exclusion to enroll into DPP.
In conclusion, regression from prediabetes to normoglycemia is associated with a lower prevalence of aggregate microvascular disease, nephropathy, and retinopathy due to lower glycemic exposure over time. The current analysis also highlights different relationships between the microvascular disease subtypes and glycemia over time. Timing for glucose-lowering intervention(s) may well need to change as tools are developed to determine individual risk for microvascular disease and its subtypes.
Supplementary Material
Supplementary Data
Article Information
Acknowledgments. The DPP Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS.
Funding and Duality of Interest. During the DPP and DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study and collection, management, analysis, and interpretation of the data (U01-DK-048489). The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development; the National Institute on Aging; the National Eye Institute; the National Heart, Lung, and Blood Institute; the National Cancer Institute; the Office of Research on Women’s Health; the National Institute on Minority Health and Health Disparities; the Centers for Disease Control and Prevention; and the American Diabetes Association. LifeScan, Inc.; Health o meter; Hoechst Marion Roussel, Inc.; Merck-Medco Managed Care, Inc.; Merck and Co.; Nike Sports Marketing; Slim Fast Foods Co.; and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. Lipha (Merck-Santé) provided medication and LifeScan, Inc., donated materials during the DPP and DPPOS. This research was also supported, in part, by the Intramural Research Program of the NIDDK. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP. McKesson BioServices Corp.; Matthews Media Group, Inc.; and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., provided support services under subcontract with the Coordinating Center. L.P. has received personal fees for speaking and/or consulting from Novo Nordisk, Sanofi, Boehringer Ingelheim, AstraZeneca, Merck, Janssen, WebMD, Medscape, and UpToDate. All authors in the writing group had access to all data. No other potential conflicts of interest relevant to this article were reported.
The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. The funding agencies were not represented on the writing group, although all members of the Steering Committee had input into the report’s contents. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions. L.P. proposed the analysis, interpreted data, and wrote and edited the manuscript. L.P., Q.P., E.B.S., R.R.K., G.A.B., S.D.-J., N.H.W., R.B.G., S.E.K., W.C.K., N.M., and D.D. edited the manuscript, and the entire DPP Research Group planned and conducted the clinical trial and obtained the study data. L.P., Q.P., E.B.S., R.R.K., G.A.B., S.D.-J., N.H.W., R.B.G., S.E.K., W.C.K., N.M., and D.D. approved the final version of the manuscript. Q.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
*
A complete list of centers, investigators, and staff for the Diabetes Prevention Program Research Group can be found in the Supplementary Data online.
Contributor Information
Collaborators: Diabetes Prevention Program Research Group, George A. Bray, Kishore Gadde, Annie Chatellier, Jennifer Arceneaux, Amber Dragg, Crystal Duncan, Frank L. Greenway, Daniel Hsia, Erma Levy, Monica Lockett, Donna H. Ryan, David Ehrmann, Margaret J. Matulik, Kirsten Czech, Catherine DeSandre, Barry J. Goldstein, Kevin Furlong, Kellie A. Smith, Wendi Wildman, Constance Pepe, Ronald B. Goldberg, Jeanette Calles, Juliet Ojito, Sumaya Castillo-Florez, Hermes J. Florez, Anna Giannella, Olga Lara, Beth Veciana, Steven M. Haffner, Helen P. Hazuda, Maria G. Montez, Kathy Hattaway, Carlos Lorenzo, Arlene Martinez, Tatiana Walker, Richard F. Hamman, Dana Dabelea, Lisa Testaverde, Denise Anderson, Alexis Bouffard, Tonya Jenkins, Dione Lenz, Leigh Perreault, David W. Price, Sheila C. Steinke, Edward S. Horton, Catherine S. Poirier, Kati Swift, Enrique Caballero, Barbara Fargnoli, Ashley Guidi, Mathew Guido, Sharon D. Jackson, Lori Lambert, Kathleen E. Lawton, Sarah Ledbury, Jessica Sansoucy, Jeanne Spellman, Steven E. Kahn, Brenda K. Montgomery, Wilfred Fujimoto, Robert H. Knopp, Edward W. Lipkin, Ivy Morgan-Taggart, Anne Murillo, Lonnese Taylor, April Thomas, Elaine C. Tsai, Dace Trence, Abbas E. Kitabchi, Samuel Dagogo-Jack, Mary E. Murphy, Laura Taylor, Jennifer Dolgoff, Debra Clark, Uzoma Ibebuogu, Helen Lambeth, Harriet Ricks, Lily M.K. Rutledge, Judith E. Soberman, Mark E. Molitch, Boyd E. Metzger, Mariana K. Johnson, Mimi M. Giles, Diane Larsen, Samsam C. Pen, David M. Nathan, Mary Larkin, Charles McKitrick, Heather Turgeon, Ellen Anderson, Laurie Bissett, Kristy Bondi, Enrico Cagliero, Kali D’Anna, Linda Delahanty, Jose C. Florez, Valerie Goldman, Peter Lou, Alexandra Poulos, Elyse Raymond, Christine Stevens, Beverly Tseng, Elizabeth Barrett-Connor, Mary Lou Carrion-Petersen, Lauren N. Claravall, Jonalle M. Dowden, Javiva Horne, Diana Leos, Sundar Mudaliar, Jean Smith, Simona Szerdi Janisch, Karen Vejvoda, F. Xavier Pi-Sunyer, Jane E. Lee, Sandra T. Foo, Susan Hagamen, David G. Marrero, Kieren J Mather, Susie M Kelly, Paula Putenney, Marcia A. Jackson, Gina McAtee, Ronald T. Ackermann, Carolyn M. Cantrell, Edwin S. Fineberg, Angela Hadden, Mario S. Kirkman, Erin O’Kelly, Paris J. Phillips, Robert E. Roach, Vanita Ratner, Sue Aroda, Catherine Shapiro, Peggy Bavido-Arrage, Gabriel Gibbs, Renee Uwaifo, Mohammed F. Wiggins, Karol Saad, Medhat Watson, Sujata Botrous, Maria Jinagouda, Claudia Budget, Perpetua Conzues, Kathy Magpuri, Kathy Ngo, Neil H. Xapthalamous, Angela L. White, Samia Brown, Prajakta Das, Tamara Khare-Ranade, Ana Stich, Cormarie Santiago, Christopher D. Wernimont, Sherita Saudek, Tracy Hill Golden, Frederick L. Whittington, Jeanne M. Brancati, Alicia Clark, Dawn Greene, Henry Jiggetts, John Mosley, Richard R. Reusing, Shawne Rubin, Evonne Stephens, David S. Utsey, Karwyn S. Schade, Claire Adams, Penny Hemphill, Janene L. Hyde, Kathleen Canady, Ysela Colleran, Doris A. Gonzales, Carolyn Hernandez-McGinnis, Jill King, Janet O. Crandall, Gilda Brown, Elsie Trandafirescu, Helena Adorno, Angela Duffy, Jennifer Goldstein, Helen Lukin, Dorothy Martinez, Harry Pompi, Jonathan Shamoon, Elizabeth A. Scheindlin, Judith Walker, Trevor Wylie-Rosett, Andrea Orchard, Susan Kriska, M. Kaye Jeffries, Marie Kramer, Catherine Smith, Stephanie Benchoff, Jessica Guimond, Debra Pettigrew, Linda Rubinstein, Elizabeth Semler, Valarie Venditti, Richard F. Weinzierl, Narleen K. Arakaki, Mae K. Baker-Ladao, Nina E. Isonaga, Marjorie K. Bermudez, John S. Mau, Robin E. Melish, William C. Yamamoto, Norman Knowler, Alvera Cooeyate, Mary A. Enote, Camille Hoskin, Carol A. Natewa, Kelly J. Percy, Vickie L. Acton, Roz Andre, Shandiin Barber, Brian C. Begay, Sherron Bucca, Jeff Cook, Charlotte Curtis, Matthew S. Dodge, Jason Doughty, Justin Kurland, Martia Glass, Robert L. Glass, Louise E. Hanson, Kathleen M. Ingraham, Jonathan Kobus, Catherine Krakoff, Cherie Manus, Sara McCabe, Tina Michaels, Julie A. Morgan, Christopher Nelson, Robert J. Piromalli, Sandra Roy, Miranda Sangster, Darryl P. Smart, Rachel Tonemah, Charlton Williams, Sarah Wilson, Marinella Fowler, Michael Temprosa, Tina Larsen, Hanna Brenneman, Sharon L. Sherif, Solome Edelstein, Julie Abebe, Melanie Bamdad, Joel Barkalow, Tsedenia Bethepu, Nicole Bezabeh, Jackie Butler, Caitlin E. Callaghan, Costas Carter, Gregory M. Christophi, Mary Dwyer, Yuping Foulkes, Robert Gao, Adrienne Gooding, Nisha Gottlieb, Heather Grover, Ashley Hoffman, Kathleen Hogan Tjaden, Richard Jablonski, Preethy Katz, John M. Kolinjivadi, Yong Lachin, Qing Ma, Susan Pan, Alla Reamer, Elizabeth M. Sapozhnikova, Andrea M. Venditti, Linda Kriska, Valerie Semler, Santica Weinzierl, Greg Marcovina, John Strylewicz, Judith Albers, Sanford Fradkin, Christine Garfield, Edward Lee, Gregg, and Ping Zhang
References
- 1.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg RB, Aroda VR, Bluemke DA, et al.; Diabetes Prevention Program Research Group . Effect of long-term metformin and lifestyle in the Diabetes Prevention Program and its outcome study on coronary artery calcium. Circulation 2017;136:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE; Diabetes Prevention Program Research Group . Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet 2012;379:2243–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perreault L, Temprosa M, Mather KJ, et al.; Diabetes Prevention Program Research Group . Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program Outcomes Study. Diabetes Care 2014;37:2622–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S5–S10 [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program Research Group The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venditti EM, Bray GA, Carrion-Petersen ML, et al.; Diabetes Prevention Program Research Group . First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes 2008;32:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olaleye D, Perkins BA, Bril V. Evaluation of three screening tests and a risk assessment model for diagnosing peripheral neuropathy in the diabetes clinic. Diabetes Res Clin Pract 2001;54:115–128 [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF; Diabetes Prevention Program Research Group . Regression from pre-diabetes to normal glucose regulation in the Diabetes Prevention Program. Diabetes Care 2009;32:1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cefalu WT, Buse JB, Tuomilehto J, et al. . Update and next steps for real-world translation of interventions for type 2 diabetes prevention: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2016;39:1186–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsson LMS, Sjöholm K, Karlsson C, et al. . Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol 2017;5:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trial Research Group . Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003;290:486–494 [DOI] [PubMed] [Google Scholar]
- 15.Li G, Zhang P, Wang J, et al. . Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Clinical practice recommendations 1997. Diabetes Care 1997;20(Suppl. 1):S1–S70 [PubMed] [Google Scholar]
- 17.Colagiuri S, Lee CM, Wong TY, Balkau B, Shaw JE, Borch-Johnsen K; DETECT-2 Collaboration Writing Group . Glycemic thresholds for diabetes-specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011;34:145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCance DR, Hanson RL, Charles MA, et al. . Comparison of tests for glycated haemoglobin and fasting and two hour plasma glucose concentrations as diagnostic methods for diabetes. BMJ 1994;308:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association Summary of revisions: Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S4–S5 [DOI] [PubMed] [Google Scholar]
- 20.Garber AJ, Abrahamson MJ, Barzilay JI, et al. . Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2017 executive summary. Endocr Pract 2017;23:207–238 [DOI] [PubMed] [Google Scholar]
- 21.Rosenzweig JL, Ferrannini E, Grundy SM, et al.; Endocrine Society . Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008;93:3671–3689 [DOI] [PubMed] [Google Scholar]
- 22.Garber AJ, Abrahamson MJ, Barzilay JI, et al. . Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2018 executive summary. Endocr Pract 2018;24:91–120 [DOI] [PubMed] [Google Scholar]
- 23.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 24.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN; DCCT/EDIC Research Group . Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial—revisited. Diabetes 2008;57:995–1001 [DOI] [PubMed] [Google Scholar]
- 25.Stratton IM, Adler AI, Neil HA, et al. . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- 27.Perreault L, Færch K, Gregg EW. Can cardiovascular epidemiology and clinical trials close the risk management gap between diabetes and prediabetes? Curr Diab Rep 2017;17:77. [DOI] [PubMed] [Google Scholar]
- 28.Sattar N, Preiss D. HbA1c in type 2 diabetes diagnostic criteria: addressing the right questions to move the field forwards. Diabetologia 2012;55:1564–1567 [DOI] [PubMed] [Google Scholar]
- 29.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care 2011;34(Suppl. 2):S184–S190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowall B, Rathmann W. HbA1c for diagnosis of type 2 diabetes. Is there an optimal cut point to assess high risk of diabetes complications, and how well does the 6.5% cutoff perform? Diabetes Metab Syndr Obes 2013;6:477–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren B, Pankow JS, Matsushita K, et al. . Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2017;5:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association 5. Prevention or delay of type 2 diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S51–S54 [DOI] [PubMed] [Google Scholar]
- 33.Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med 2011;1:480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Bennett PH. Transient impaired glucose tolerance in Pima Indians: is it important? BMJ 1988;297:1438–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data