Cell Swelling and a Nonselective Cation Channel Regulated by Internal Ca2+ and ATP in Native Reactive Astrocytes from Adult Rat Brain (original) (raw)
Abstract
Hypoxia–ischemia and ATP depletion are associated with glial swelling and blebbing, but mechanisms involved in these effects remain incompletely characterized. We examined morphological and electrophysiological responses of freshly isolated native reactive astrocytes (NRAs) after exposure to NaN3, which depletes cellular ATP. Here we report that NaN3 caused profound and sustained depolarization attributable to activation of a novel 35 pS Ca2+-activated, [ATP]i-sensitive nonselective cation (NCCa-ATP) channel, found in >90% of excised membrane patches. The channel was impermeable to Cl−, was nearly equally permeable to monovalent cations, with permeabilities relative to K+ being PCs+/PK+(1.06) ≈ PNa+/PK+(1.04) ≈ PRb+/PK+(1.02) ≈ PLi+/PK+(0.96), and was essentially impermeable to Ca2+ and Mg2+(PCa2+/PK+≈ PMg2+/PK+< 0.001), with intracellular Mg2+ (100 μm to 1 mm) causing inward rectification. Pore radius, estimated by fitting relative permeabilities of organic cations to the Renkin equation, was 0.41 nm. This channel exhibited significantly different properties compared with previously reported NCCa-ATP channels, including different sensitivity to block by various adenine nucleotides (EC50of 0.79 μm for [ATP]i, with no block by AMP or ADP), and activation by submicromolar [Ca]i. The apparent dissociation constant for Ca2+ was voltage dependent (0.12, 0.31, and 1.5 μm at −40, −80, and −120 mV, respectively), with a Hill coefficient of 1.5. Channel opening by [ATP]i depletion was accompanied by and appeared to precede blebbing of the cell membrane, suggesting participation of this channel in cation flux involved in cell swelling. We conclude that NRAs from adult rat brain express a 35 pS NCCa-ATP channel that may play an important role in the pathogenesis of brain swelling.
Keywords: cation channel, Ca2+, ATP, cell swelling, astrocyte, brain injury, patch clamp
Swelling of glial cells is part of the cytotoxic or cellular edema response that characterizes brain damage in cerebral ischemia and traumatic brain injury and is a major cause of morbidity and mortality (Staub et al., 1993; Kimelberg et al., 1995). A number of mediators have been identified that initiate swelling of glial elements, including elevation of extracellular K+, acidosis, release of neurotransmitters, and free fatty acids (Kempski et al., 1991; Rutledge and Kimelberg, 1996; Mongin et al., 1999).
Inhibition of ATP synthesis also causes glial cell swelling and blebbing, and, if sufficiently severe, plasma membrane disruption and cell death (Jurkowitz-Alexander et al., 1993). Mechanisms of swelling involved in ATP depletion remain incompletely characterized (Lomneth and Gruenstein, 1989; Juurlink et al., 1992; Rose et al., 1998). In energized cells, however, an equivalent degree of osmotic swelling induced by ouabain-mediated inhibition of the Na+/K+-ATPase pump does not produce large depolarization, blebbing, or cell death (Jurkowitz-Alexander et al., 1992; Brismar and Collins, 1993), implicating mechanisms other than pump failure as critical to swelling of glial cells.
Nonselective cation channels that are activated by intracellular Ca2+ and inhibited by intracellular ATP (NCCa-ATP channels) have been identified in a number of cell types, both native and cultured, but not in astrocytes (Sturgess et al., 1987; Gray and Argent, 1990; Rae et al., 1990;Champigny et al., 1991; Popp and Gogelein, 1992; Ono et al., 1994). Overall, these channels comprise a heterogeneous group with incompletely defined characteristics. They exhibit single-channel conductances in the range of 25–35 pS, discriminate poorly between Na+ and K+, are impermeable to anions and, for the most part, to divalent cations, and they are blocked by similar concentrations of the adenine nucleotides ATP, ADP, and AMP on the cytoplasmic side. The function of these channels remains enigmatic, in part because unphysiological concentrations of Ca2+ are generally required for channel activation.
We examined the morphological and electrophysiological responses of glial cells after exposure to NaN3, a metabolic toxin used to induce “chemical hypoxia” (Swanson, 1992). We studied freshly isolated native reactive astrocytes (NRAs) from adult rat brain, a model system similar to that characterized previously by us (Perillan et al., 1999, 2000), except that cells were not cultured but were stored at 4°C until studied, within 24 hr of isolation from the brain. Here we report that NRAs from adult brain express a 35 pS nonselective cation channel that is activated by depletion of [ATP]i at physiological concentrations of [Ca2+]i. This NCCa-ATP channel, newly identified in NRAs and present in >90% of membrane patches, exhibited significantly different properties, including activation by submicromolar [Ca]i and different sensitivity to block by various adenine nucleotides when compared with previously reported NCCa-ATP channels. Opening of this channel by ATP depletion, which caused profound depolarization, preceded blebbing of the cell membrane, suggesting participation of this channel in cation flux involved in cell swelling. Demonstration of a putative role for this channel in ischemia–hypoxia-induced cell swelling may be of clinical significance.
MATERIALS AND METHODS
Cell preparation. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland. NRAs from adult brain were obtained from gelatin sponges (Gelfoam; Upjohn Co., Kalamazoo, MI) implanted into a stab wound in the parietal lobe of 8-week-old Wistar rats as described previously (Perillan et al., 1999). Sponge pieces were harvested at 8 d and washed three times in PBS, pH 7.4. Washed pieces were placed in an Eppendorf tube containing artificial CSF (aCSF) composed of: 124 mm NaCl, 5 mm KCl, 1.3 mm MgCl2, 2 mm CaCl2, 26 mm NaHCO3, and 10 mm d-glucose, pH 7.4, ≈290 mOsm, with 20 U/ml papain, 10 mg/ml trypsin inhibitor, and 0.01% DNase (Worthington, Lakewood, NJ). The digestion system was transferred to an incubator (90% humidified air–10% CO2, 37°C) for 20 min and was gently triturated every 5 min. The cell suspension was centrifuged at 3,000 rpm for 1 min, and pelleted cells were resuspended in aCSF and stored at 4°C until studied.
The cell isolation protocol yielded cells of various sizes, ranging from 11 to 45 μm in diameter, some phase bright and others phase dark. A subgroup of phase-bright cells had multiple short but distinct cell processes that were shorter than the cell soma. Except for occasional red blood cells, >95% of cells were GFAP-positive when examined by immunofluorescence (Perillan et al., 1999, 2000). For the experiments reported here, we studied only larger (≈30 μm diameter), phase-bright cells with short processes (less than one cell length).
Electrophysiology. Experiments were performed at room temperature, 22–25°C, within 24 hr of cell isolation. An aliquot of cells was placed in the recording chamber filled with extracellular bath solution (see below for composition). After viable cells adhered to the surface, flushing with excess solution washed away residual debris not removed previously by centrifugation. Membrane currents were amplified (Axopatch 200A; Axon Instruments, Foster City, CA) and sampled on-line at 5 kHz using a microcomputer equipped with a digitizing board (Digidata 1200A; Axon Instruments) and running Clampex software (version 8.0; Axon Instruments). Membrane currents were recorded in intact cells using both the cell-attached and the nystatin perforated whole-cell configurations (Horn and Marty, 1988) and in cell-free isolated membrane patches using both the inside-out and outside-out configurations (Hamill et al., 1981). Patch-clamp pipettes, pulled from borosilicate glass (Kimax; Fisher Scientific, Pittsburgh, PA), had resistances of 6–8 MΩ for single-channel recordings and 2–4 MΩ for experiments using the nystatin-perforated whole-cell technique. The bath electrode was a Ag/AgCl pellet (Clark Electromedical Instruments, Reading, UK) that was placed directly in the bath, except when the bath [Cl−] was altered, in which case an agar bridge made with 3m KCl was used to connect to the bath.
Cells with seal resistance of <3 GΩ and access resistance of >50 MΩ were discarded. Macroscopic membrane currents were measured during step pulses (600 msec) or during ramp pulses (−140 to +50 mV at 0.32 mV/msec) from a holding potential of −67 mV. For some experiments (see Fig. 2D-F), we used cell-attached patches to measure single-channel currents. For these experiments, calculation of the reversal potential (_E_rev) of a channel requires knowledge of the actual cell membrane potential (_E_m). Experiments were performed assuming _E_m of 0 mV after addition of NaN3 (see Fig. 2A). After single-channel data collection, the recording was converted from a cell-attached to a conventional whole-cell configuration to measure_E_m. Measurements of_E_m, made within 30 sec of gaining access to the cytoplasm, were successful for 6 of 10 cells, in which values (mean ± SD) of _E_m of −5.2 ± 2.7 mV were obtained. This mean value was used to compute the proper value of _E_rev for the single-channel recordings.
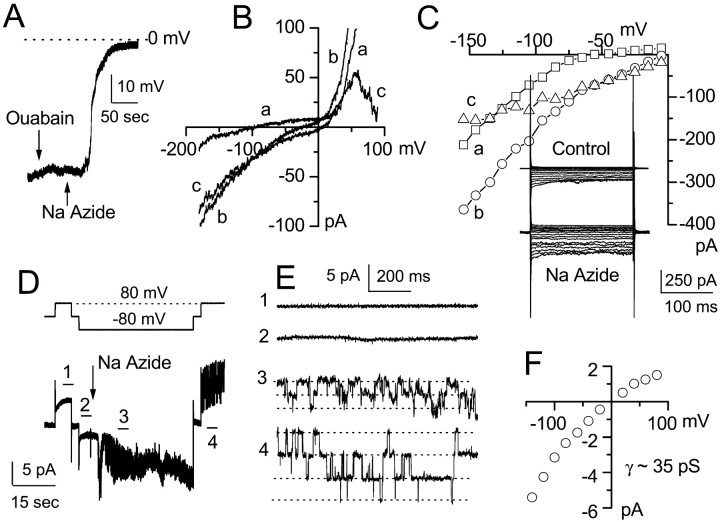
Fig. 2.
NaN3-induced ATP depletion elicits depolarizing inward current attributable to opening of 35 pS channel.A, Current-clamp recording showing resting potential near E_K (≈60 mV, 10 mm KCl). One minute exposure to 1 mm ouabain (down arrow) depolarized the cell <5 mV, with recovery after washout; 3 min exposure to 1 mm NaN3 (up arrow) caused rapid depolarization to near 0 mV.B, Voltage-clamp recordings during ramp pulses before (a) and after (b) NaN3 show a net increase in inward current with drug; the difference current (c) indicates a reversal potential near 0 mV. C, Original records (inset) and current–voltage curves during step pulses before (a) and after (b) NaN3, with the difference current (c) also illustrated. D, Cell-attached patch recording of current (bottom panel) recorded at −80, 0, and 80 mV (top panel), before and after 1 mmNaN3 (drug added at arrow).E, Current records at higher temporal resolution obtained from the segments marked with the corresponding numbers in_D. F, Single-channel current–voltage relationship for four cell-attached patches showing a 35 pS conductance with inward rectification that reverses near 0 mV.
Recording solutions. For whole-cell macroscopic recordings (see Fig. 2A-C), we used a nystatin perforated-patch technique with a bath solution containing (in mm): 130 NaCl, 10 KCl, 1 CaCl2, 1 MgCl2, 32.5 HEPES, and 12.5 glucose, pH 7.4. The pipette solution contained (in mm): 55 KCl, 75 K2SO4, 8 MgCl2, and 10 HEPES, pH 7.2. Nystatin (50 mg; Calbiochem, La Jolla, CA) was dissolved in 1 ml of dimethylsulfoxide (DMSO). Working solutions were made before the experiment by adding 16.5 μl of nystatin stock solution to 5 ml of the base pipette solution to yield a final concentration of nystatin of 165 μg/ml and DMSO of 3.3 μl/ml. The composition of the pipette solution proposed by Korn et al. (1991) and used by others to study astrocytes (Walz et al., 1994) replaced with K2SO4 some of the KCl that would otherwise be included. The SO42− anion, unlike Cl−, is not permeable through the nystatin pore. Reducing the pipette [Cl−] reduces the driving force for Cl− into the cell, thereby minimizing osmotic swelling of the cell that might otherwise occur during electrophysiological recording (Horn and Marty, 1988).
For cell-attached patch recording (see Fig.2D,E), we used a bath solution containing (in mm): 130 NaCl, 10 KCl, 1 CaCl2, 1 MgCl2, 32.5 HEPES, and 12.5 glucose, pH 7.4. The pipette contained (in mm): 145 KCl, 1 MgCl2, 0.2 CaCl2, 5 EGTA, and 10 HEPES, pH 7.3. The measured osmolarity of the extracellular solution was ≈300 mOsm (Precision Systems, Natick, MA).
For most inside-out patch recording (see Figs. 6-8), we used a bath solution containing (in mm): 145 CsCl, 1.5 CaCl2, 1 MgCl2, 5 EGTA, 32.5 HEPES, and 12.5 glucose, pH 7.4. The pipette contained (in mm): 145 CsCl, 1 MgCl2, 0.2 CaCl2, 5 EGTA, and 10 HEPES, pH 7.3. For other inside-out patch recordings (see Figs. 3, 9), Cs+ in both of the above solutions was replaced with equimolar K+. For the inorganic cation substitution experiments (see Fig. 4), Cs+ in the bath was replaced with K+, and Cs+in the pipette was replaced by equimolar concentrations of individual test ions, except when using Ca2+ or Mg2+, in which case we used a concentration of 75 mm to facilitate seal formation (Cook et al., 1990).
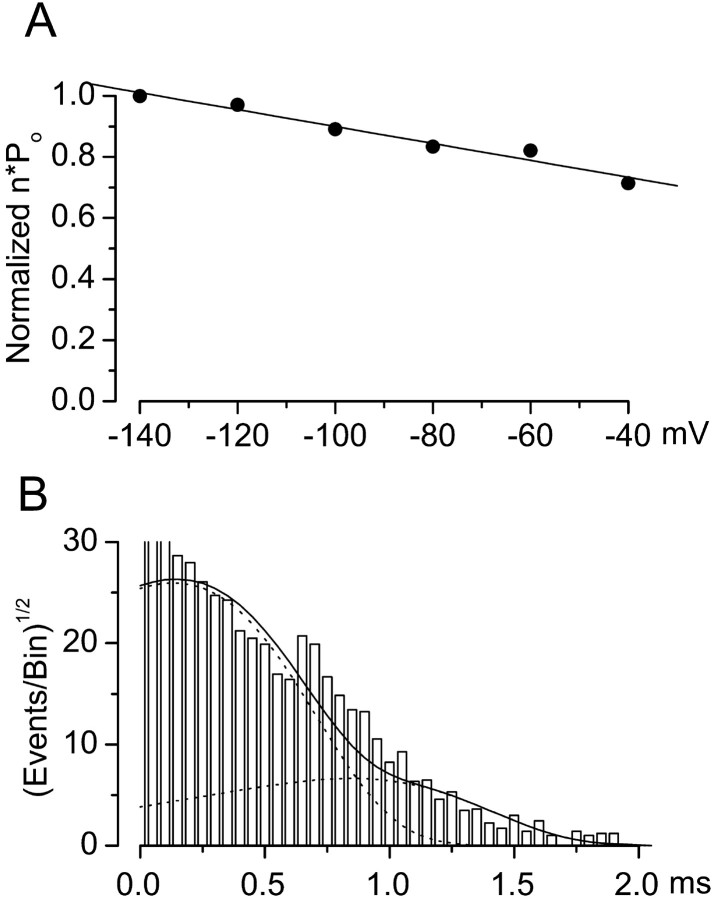
Fig. 6.
Voltage dependence of open-channel probability and open dwell times. A, Channel open probabilities (n · Po) at −140 mV ≤_V_m ≤ −40 mV were normalized to values at −140 mV and plotted (filled circles); linear regression gave a slope of −2.87 × 10−3mV−1, which was not significantly different from zero (p = 0.09). Data are mean values from five patches. B, Open channel events from 1-min-long continuous records obtained at −80 mV were compiled into a probability density histogram with a square root axis for the ordinate and a logarithmic axis for the abscissa; the _solid line_represents the probability density function (Eq. 4 in Materials and Methods) with values of τ1 = 2.7 msec, τ2 = 8.3 msec, _a_1 = 1493, and _a_2 = 580, with broken lines denoting each of the two components individually.
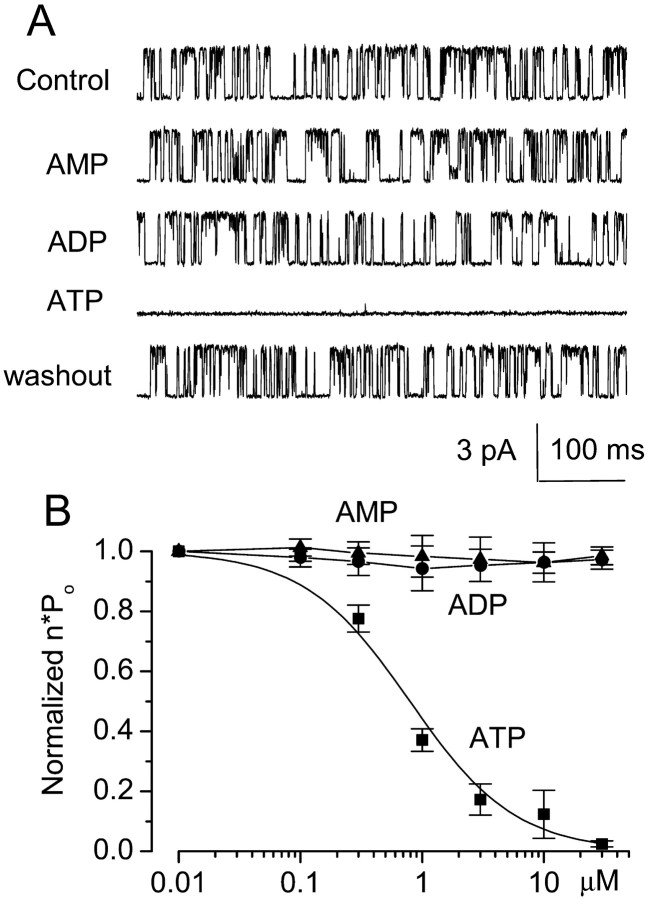
Fig. 7.
Cytoplasmic ATP but not AMP or ADP inhibits 35 pS channel opening. A, Openings of the 35 pS channel in an inside-out patch under control conditions and after successive addition and subsequent washout of 1 mm AMP, 1 mm ADP, and 1 mm ATP; inward cationic current is plotted_upward_. B, Normalized open channel probability (n · Po) with different concentrations of adenine nucleotides; data with ATP were fit to a standard logistic equation, with a Hill coefficient of 1 and half-maximum inhibition of 0.79 μm. Values plotted are means ± SE from five, five, and six patches for AMP, ADP, and ATP, respectively.
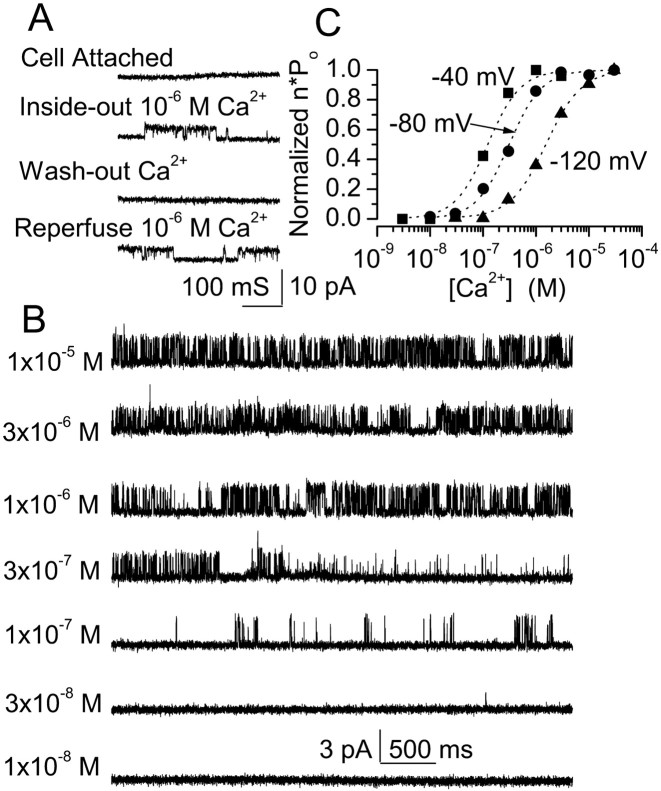
Fig. 8.
Ca2+ dependence of the 35 pS channel. A, Cell-attached patch shows no activity when recorded for >10 min; conversion from a cell-attached to an inside-out configuration in a bath solution containing 1 μmCa2+ resulted in activation of a 35 pS channel. Channel activity was lost in Ca2+-free solution and was restored with 1 μm Ca2+; inward cationic current is plotted upward. B, Original current records obtained from one patch in an inside-out configuration at _E_m of −80 mV. [Ca2+] in the bath was changed as indicated; inward cationic current is plotted upward.C, Values of n · Pomeasured in 1 min continuous recordings from four to nine patches at the potentials and [Ca2+] indicated; for each patch, data were normalized to the values obtained at 3 μm [Ca2+]. Average data were fit to a standard logistic equation with a Hill coefficient of 1.5 and half-maximum values of 0.12, 0.31, and 1.5 μm at −40, −80, and −120 mV, respectively.
Fig. 3.
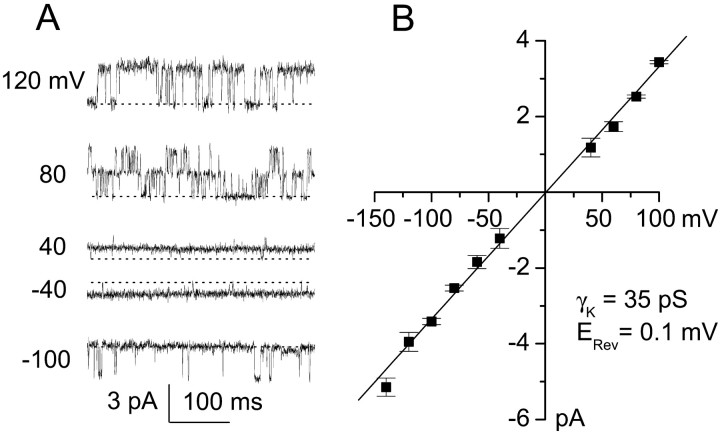
Single-channel currents recorded in an inside-out patch. A, Original records were obtained during test pulses to the potentials indicated, with equimolar K+ on both sides of the membrane. Broken line indicates channel closing; outward cationic current is plotted upward. B, Data (mean ± SD) on single-channel amplitudes at different potentials from four patches are plotted; fit of the data indicated a slope conductance of 35.2 pS and an extrapolated reversal potential (_E_rev) of +0.1 mV, with no apparent rectification.
Fig. 9.
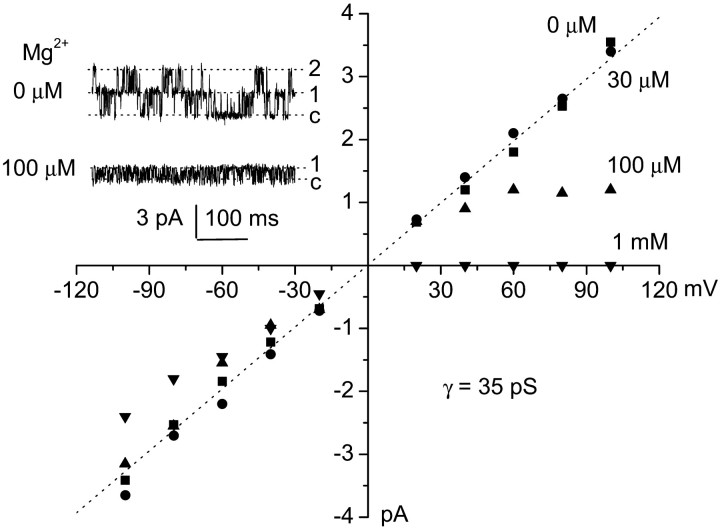
Intracellular Mg2+ causes inward rectification. Single-channel records obtained in an inside-out configuration with 0 μm (inset,top) and 100 μm (inset,bottom) Mg2+ on the cytoplasmic side;E_m of +80 mV. c denotes channel closing; outward cationic current is plotted_upward. Plot of mean single channel amplitude at different potentials studied with equimolar K+ on both sides of the membrane and 0 μm, 30 μm, 100 μm, and 1 mm Mg2+ on the cytoplasmic side; broken line indicates 35 pS conductance. All data are from the same patch as Figure3A.
Fig. 4.
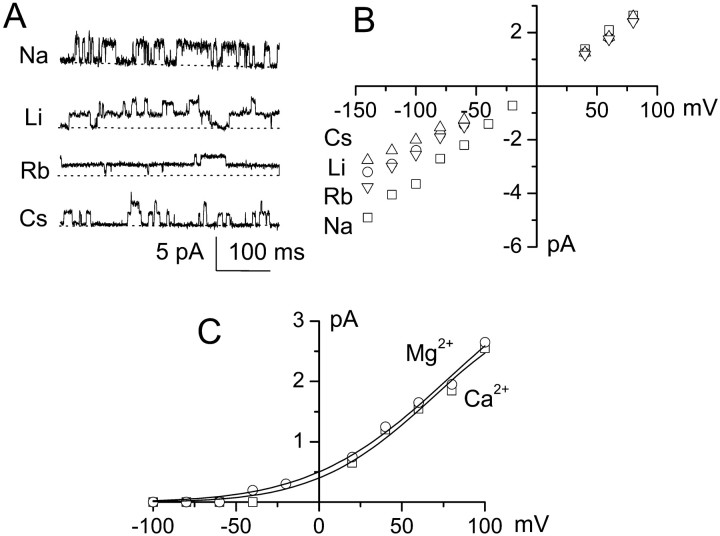
Relative permeabilities of 35 pS channel.A, Single-channel records obtained at _E_of −100 mV, showing the 35 pS channel conducting the various alkaline ions indicated. Broken lines indicate channel closings; inward cationic current is plotted upward.B, Plot of single-channel amplitude versus voltage for various alkaline ions. Values of _E_rev were estimated by linear extrapolation. Permeabilities relative to K+, calculated using Equation 1 in Materials and Methods, were PCs+/PK+(1.06) ≈ PNa+/PK+(1.04) ≈ PRb+/PK+(1.02) ≈ PLi+/PK+(0.96); data are mean values for five to seven patches for each cation.C, Plot of single-channel amplitude versus voltage for Ca2+ and Mg2+. Values of_E_rev, estimated from fits to an exponential function (lines), were more negative than −150 mV for both Ca2+ and Mg2+; permeabilities relative to K+, calculated using Equation 2 in Materials and Methods, were < 0.001. Data are mean values for four and six patches for Ca2+ and Mg2+, respectively.
For outside-out patch recording, we used the pipette solution containing (in mm): 145 CsCl, 1 MgCl2, 0.2 CaCl2, 5 EGTA, and 10 HEPES, pH 7.3. The standard bath solution contained (in mm): 145 CsCl, 1.5 CaCl2, 1 MgCl2, 5 EGTA, 32.5 HEPES, and 12.5 glucose, pH 7.4. For the organic cation substitution experiments (see Fig. 5), Cs+ in the bath was replaced with equimolar concentrations of test cation.
Fig. 5.
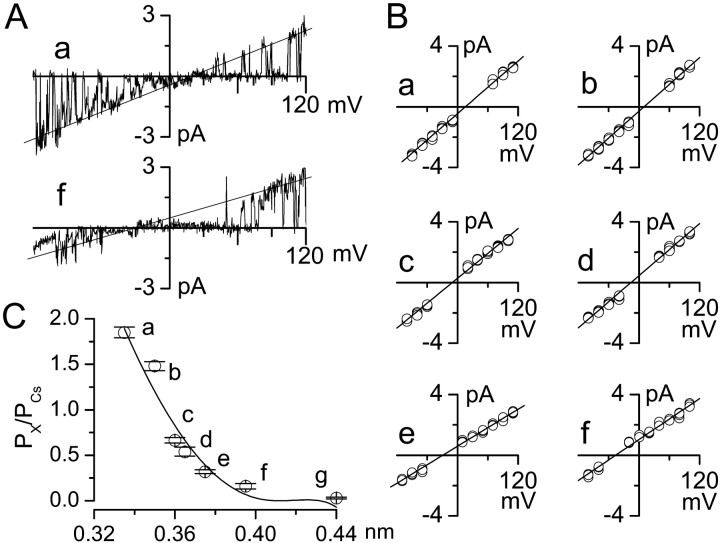
Pore size of 35 pS channel. A, Single-channel currents obtained in outside-out patches with Cs+ in the pipette and methanolamine (a) and Tris (f) in the bath. B, Current–voltage relationships obtained with methanolamine (a), guanidium (b), ethanolamine (c), diethylamine (d), piperazine (e), and Tris (f) in the bath. C, Channel pore size was estimated from the relationship between the permeability (relative to Cs+) and the molecular radius of a series of monovalent organic cations. Values marked a–f are from the same data as in B; the value marked _g_was obtained with _N-_methylglucamine. The solid line is a least-squares fit to the Renkin equation (see Materials and Methods), with extrapolation to Px/PCs = 0 indicating an equivalent pore radius of 0.41 nm; data are mean ± SE values from four to five patches.
For experiments requiring low concentration of free Ca2+ in the bath solution (see Fig. 8), Ca2+–EGTA-buffered solution was used, and free [Ca2+] was calculated using the program WEBMAXC, version 2.10 (www.stanford.edu/∼cpatton/maxc.html). For [Ca2+] of 1 μm, we used 5 mm EGTA and 4.5 mmCa2+ salt. [Ca2+] of 1 μm was also used in solutions to test intracellular ATP and Mg2+ activities (see Figs. 7, 9).
Data analysis. Single-channel amplitudes used to calculate slope conductance (see Figs. 2F, 3B,4B,C, 9) were obtained by fitting a Gaussian function to an all-points amplitude histogram of records obtained at various potentials. To calculate open-channel probability (n · Po) at various potentials and with different test agents (see Figs. 6-8), the all-points histogram was fit to a Gaussian function, and the area under the fitted curve for the open channel was divided by the area under the fitted curve for the closed plus open channel. Values of n · Po at different concentrations of test agents (see Figs. 7B, 8C) were fit to a standard logistic equation using a least-squares method.
We assessed the relative permeability of monovalent cations using inside-out patches with K+ in the bath (see Fig. 4B). The reversal potential for current flow (_E_rev) was estimated from a fit of the data to a linear equation, and relative permeabilities (Px+/PK+) were calculated using the Goldman–Hodgkin–Katz (GHK) equation (Goldman 1943; Hodgkin and Katz, 1949) (Eq. 1): PX+/PK+= [K+]i/[X+]o· exp(E_rev ·_F/RT), where F, R, and T have their usual meaning.
We assessed the relative permeability of divalent cations using inside-out patches with K+ in the bath (see Fig. 4C). _E_rev was estimated from a fit of the data to an exponential function, and values of PCa2+/PK+and PMg2+/PK+were calculated using the GHK equation (Eq. 2): PX2+/ PK+= [K+]i/4[X2+]o· (ν2 + ν), where ν = exp(E_rev ·_F/RT) (Lewis, 1979; Rae et al., 1990).
Relative permeabilities of monovalent organic cations, obtained as above, were used to estimate the pore size of the channel (see Fig.5B) (Cook et al., 1990). The Stokes–Einstein radius (_r_SE) was calculated from the limiting conductivities (λ) of the ions with the following formula:_r_SE · λ = constant , with the constant being determined from the behavior of tetraethylammonium (TEA) at 25°C, for which λ = 44.9 cm2Ω−1equiv−1and _r_SE = 0.204 nm. The Stokes–Einstein radius was then converted to the molecular radius using correction factors read off from Robinson and Stokes (1970), their Figure 6.1. The equivalent limiting conductance for ethanolamine was obtained from the same reference, and those of other ions were calculated from their molecular weights by the formula MW0.5 · λ = constant, with the constant being determined by the value for ethanolamine at 25°C: MW = 62.1, and λ = 4.42 cm2Ω−1equiv−1. Relative permeabilities (Px+/PCs+) were then plotted against the calculated ionic radii. The effect of solute size on the rate of penetration (permeability) through pores is expressed by the Renkin equation (Renkin, 1955) (Eq. 3): Px+/PCs+= c · [1 − (r/R)]2 · [1 − 2.104(r/R) + 2.09(r/R)3 − 0.95(r/R)5], in which c, a constant factor, is 510 and is related to drag of a sphere moving through a viscous liquid in a cylinder (Dwyer et al., 1980; Preisig and Berry, 1985), and r and R are the radius of the solute and radius of the pore, respectively.
For open-channel dwell times (see Fig. 6B), we used records with single-channel openings obtained during test pulse to_E_m of −80 mV, filtered at 1 kHz (−3 dB; rise time, 330 μsec). As suggested previously (Sigworth and Sine, 1987), the distribution of open times was compiled after conversion to the logarithm of the time interval, using 10 bins per decade for the abscissa of the histogram and a square root axis for the ordinate. Using this transformation, the probability density function (pdf) for a double-exponential distribution is as follows (Eq. 4): pdf = {_a_1 · exp[_z_1 − exp(_z_1)] + _a_2 · exp[_z_2 − exp(z_2)]}0.5, where_z_1 = [ln(t) −_ln(τ1)],z_2 = [ln(t) −_ln(τ2)], t is time, and_a_1 and_a_2 relate to the number of open events having time constants of τ1 and τ2, respectively.
Junction potentials, which generally did not exceed 5 mV, were determined with an electrometer by measuring the diffusion potential established across a dialysis membrane and were subtracted when appropriate. Holding currents were not subtracted from any of the recordings. Difference currents (see Fig. 2B) were obtained by simply subtracting current records before and after perfusing NaN3, with no other processing being used. Data were fit to a Gaussian function and the Renkin equation using the Levenberg–Marquardt algorithm and to Equation 4 using the maximum likelihood method (MLM) as implemented in pClamp 8.0 (Axon Instruments).
Scanning electron microscopy. To study cell blebbing and swelling, freshly isolated cells were exposed at room temperature to NaN3, and then, after various time intervals, cells were fixed using iced 4% formaldehyde plus 1% glutaraldehyde for 24 hr and then dehydrated using serial concentrations (35, 50, 75, 95, and 100%) of ethanol (Jewell et al., 1982). Specimens were critical point dried (Tousimis, Rockville, MD), gold coated (Technics), and viewed using an AMR 1000 scanning electron microscope.
RESULTS
Morphological changes with NaN3
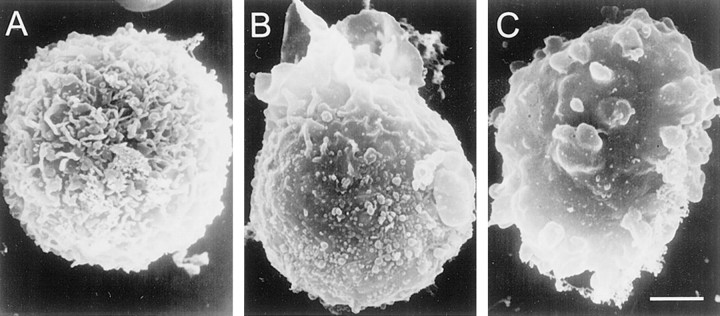
We first sought to establish that ATP depletion would result in swelling of freshly isolated NRAs, as reported previously for cultured glial cells (Jurkowitz-Alexander et al., 1992, 1993). When examined using a scanning electron microscope, the surfaces of NRAs were highly complex, exhibiting small membrane evaginations and fine processes that decorated the entire cell surface (Fig.1A). Exposure of NRAs to NaN3 (1 mm) caused changes in the surface appearance, characterized early on by loss of complex structure and development of surface blebs (Fig.1B), followed later by a grossly swollen appearance with complete loss of fine structure and formation of multiple large blebs (Fig. 1C). Phase-contrast microscopy was also useful for assessing this process. Although fine structure could not be resolved, blebbing was readily appreciated 10–15 min after exposure to NaN3 (10 experiments). It is generally considered that morphological changes of the sort observed here are attributable to loss of cytoskeletal integrity, combined with action of an osmotic force that causes swelling of the cell. To assess the contribution of the osmotic gradient to cell swelling, we repeated the experiment in the presence of mannitol, an impermeant oncotic agent. Mannitol (50 mm), at a concentration sufficient to increase osmolarity of the extracellular solution from 300 to 350 mOsm, delayed bleb formation >30 min after exposure to NaN3(three experiments) (Jurkowitz-Alexander et al., 1993). Similar results, including cell membrane blebbing and delay of blebbing by mannitol, were obtained when cellular ATP was depleted using exposure to NaCN (2.5 mm) plus 2-deoxyglucose (10 mm) (Johnson et al., 1994) (three experiments), suggesting that the effect of NaN3 was attributable in fact to ATP depletion and not to any other nonspecific effect of drug (Harvey et al., 1999).
Fig. 1.
Cell blebbing and swelling after NaN3-induced ATP depletion. Scanning electron micrographs of freshly isolated native reactive astrocytes. Formaldehyde–glutaraldehyde fixation was initiated under control conditions (A), 5 min after exposure to 1 mm NaN3 (B), and 25 min after exposure to 1 mm NaN3(C). Scale bar, 12 μm.
General electrophysiological properties of NRAs
We next measured the resting potential and whole-cell macroscopic currents, because electrophysiological properties of freshly isolated NRAs had not been described previously. In the large phase-bright cells with short processes that are the subject of this report, over 95% of cells (46 of 48 cells) had resting potentials (_E_m of −68.7 ± 2.4 and −97.1 ± 3.1 mV) near _E_K of −67 and −95 mV for [K+]o of 10 and 3 mm, respectively (Fig.2A), suggesting that our enzymatic dissociation method had not appreciably harmed the cells.
Whole-cell macroscopic currents were characterized by small inward currents at negative potentials, large outward currents at positive potentials, and a flat “plateau” region at intermediate potentials (Fig. 2B, a), consistent with previous observations in primary cultured cells of the same origin (Perillan et al., 1999, 2000). Inward currents negative to the K+ equilibrium potential (_E_K) were usually <100 pA, much smaller than values reported in cultured neonatal astrocytes (Ransom and Sontheimer, 1995) but consistent with findings in astrocytes freshly isolated from injured brain (Bordey and Sontheimer, 1998;Schroder et al., 1999). The large outward currents in these cells were partially blocked by charybdotoxin (100 nm; six cells), iberiotoxin (100 nm; seven cells), and tetraethylammonium chloride (5 mm; nine cells), suggesting the presence of a large conductance Ca2+-activated K+ channel (Perillan et al., 1999). The outward current that remained in the presence of charybdotoxin could be further blocked by 4-aminopyridine (5 mm; seven cells) and exhibited kinetic properties typical of a delayed rectifier K+ channel. Outward currents were not further characterized in these cells. Consistent with our previous report (Perillan et al., 1999), fast inward voltage-dependent currents attributable to Na+ channels were observed in <1% of NRAs (2 of >200 cells).
NaN3 elicits depolarizing inward current attributable to 35 pS channel
We used current-clamp recordings to investigate the effect of ATP depletion by NaN3 in NRAs. For these experiments, a nystatin perforated-patch method was used to ensure that the metabolic disruption would come from drug application and not from cell dialysis. Extracellular application of NaN3 (1 mm; room temperature) resulted in a large and swift depolarization of the cells (Fig. 2A). In 6 of 10 cells, NaN3 rapidly depolarized the cells to_E_m of ≈0 mV (−5.2 ± 2.7 mV). Depolarization usually started ≈1 min after addition of NaN3, was complete in <3 min, and was irreversible on washout of drug. The magnitude of the depolarization observed with NaN3 far exceeded the small reversible depolarization induced by ouabain (1 mm) (Brismar and Collins, 1993), a known Na+/K+-ATPase blocker (Fig. 2A), indicating that pump failure was not the cause of the large depolarization observed after exposure to NaN3.
The time course of depolarization with NaN3 was appreciably more rapid than the time course for development of cell membrane blebbing observed with the same treatment. Also, neither the time course nor the magnitude of the depolarization was affected by raising the extracellular osmolarity with 50 mm mannitol (three experiments), a treatment that substantially delayed bleb formation. Together, these observations suggested that depolarization was a primary event, not secondary to cell swelling or stretch (Ubl et al., 1988; Christensen and Hoffmann, 1992; Kim and Fu, 1993; Korbmacher et al., 1995).
Voltage-clamp recordings showed that exposure to NaN3 resulted in a net increase of inward current in NRAs. Recordings obtained using both ramp (Fig.2B) and step pulses (Fig. 2C) showed significantly larger currents after NaN3 (Fig.2B, b, C, b). A plot of the “difference currents,” obtained by subtracting the current–voltage curve before drug from that after drug (Fig.2B, c, C, c), indicated that the new current turned on by NaN3reversed near 0 mV. A reversal potential near 0 mV suggested that the NaN3-induced current might be attributable to a nonselective cation conductance.
Cell-attached patch recordings were used to further characterize the NaN3-induced current. Exposure to NaN3 elicited single-channel currents in 4 of 12 patches that had been completely silent before addition of drug (Fig.2D,E). After addition of NaN3, recordings at low temporal resolution revealed a large increase in current variance (Fig.2D, 3, 4) that, after increasing temporal resolution, was revealed to be attributable to single-channel events (Fig. 2E, 3,4). The amplitudes of single-channel events recorded at different membrane potentials are plotted in Figure2F, showing that NaN3 activated a single-channel conductance of ≈35 pS that exhibited weak inward rectification when measured in the cell-attached configuration. Additional experiments performed in the cell-attached configuration with the pipette solution supplemented with various drugs showed that the 35 pS NaN3-induced single-channel currents were not blocked by 10 mm TEA, 5 mm 4-AP, 100 nmiberiotoxin, 100 nm charybdotoxin, or 1 μm tetrodotoxin (four to six patches for each compound; data not shown), indicating that a typical K+ or Na+channel was not involved. Also, because 0.2 mmCa2+ was included in the pipette solution, these single-channel openings were unlikely to be attributable to monovalent cation influx via an L-type Ca2+ channel.
Similar depolarization and activation of a 35 pS channel were obtained when cellular ATP was depleted using exposure to NaCN (2.5 mm) plus 2-deoxyglucose (10 mm) (Johnson et al., 1994) (three experiments), suggesting that the effect of NaN3 was attributable in fact to ATP depletion and not to any other nonspecific effect of drug. In addition, direct application of NaN3 to outside-out patches studied with 1 mm ATP and 1 μmCa2+ in the pipette did not activate the 35 pS channel (n = 5), again indicating that ATP depletion, rather than the drug itself, was responsible for channel activation (Harvey et al., 1999).
Apart from ATP depletion, patch excision was also found to be a highly reliable method for channel activation. Of >120 cells studied in the cell-attached configuration, we recorded spontaneous channel activity attributable to a 35 pS conductance in only two cells, suggesting that this channel was typically silent in metabolically healthy cells. In contrast, a 35 pS channel was present in >90% (134 of 146 patches) of inside-out patches formed from NRAs not exposed to NaN3 or other metabolic toxins, suggesting that an intracellular element lost on patch excision might normally prevent channel activation.
We examined another potential mechanism of channel activation other than patch excision. Cell swelling is widely recognized as a stimulus that initiates regulatory volume decrease (RVD), a phenomenon accompanied by activation of various currents, including a nonselective cation channel in some systems (Ono et al., 1994). When membrane patches were studied in a cell-attached configuration, hyposmotic stimulation (210 mOsm) activated single-channel events but none exhibiting a 35 pS conductance (three experiments). This finding suggested that the depolarization and channel activation observed with NaN3 were not part of an RVD response secondary to NaN3-induced cell swelling and accorded with the previously noted observation that NaN3-induced depolarization preceded cell swelling.
Relative permeabilities and pore size
We further characterized the channel using membrane patches in the inside-out configuration. Original records obtained during test pulses to various potentials with equal [K+] on both sides of the membrane are shown in Figure3A. Amplitude histograms were constructed of events observed at potentials from −140 to +100 mV, and values (mean ± SE) for four patches are plotted (Fig.3B). Fit of the data to a linear equation indicated a slope conductance of 35.2 pS, with an extrapolated reversal potential (_E_rev) of +0.1 mV, close to the expected K+ reversal potentials (_E_K) of 0 mV. An apparent noise level of 0.4 pA (peak to peak; 1 kHz filter) precluded accurate resolution of channel openings at −30 mV < _E_m< +30 mV.
In addition to conducting K+, the 35 pS channel was also shown to transport a variety of alkaline ions (Fig.4A), indicating that it was a nonselective cation channel. Using inside-out patches, we measured the conductance of the channel with various alkaline ions in the pipette solution, including Cs+, Na+, Rb+, K+, and Li+, always with equimolar K+ in the bath solution. Na+ was shown to have a nearly equal slope conductance (32.6 pS) compared with K+ (35.2 pS), but the slope conductance was reduced with other cations (Fig. 4B). Values of_E_rev, estimated by linear extrapolation, were used to calculate (Eq. 1) relative permeabilities for the series of alkaline ions. Values for relative permeabilities were PCs+/PK+= 1.06, PNa+/PK+= 1.04, PRb+/PK+= 1.02, and PLi+/PK+= 0.96, indicating that this channel was nearly equally permeable to all monovalent cations.
We also assessed whether the 35 pS channel was permeable to anions such as Cl−. After measuring single-channel current amplitudes at different potentials with 145 mm KCl, we changed the bath solution to equimolar K+ gluconate. When an agar bridge was used, the solution change resulted in a change in_E_rev < 0.5 mV (six experiments), indicating that the 35 pS channel was essentially impermeable to anions.
We also investigated the permeability of the channel to the divalent cations Ca2+ and Mg2+ (Fig. 4C). When K+ in pipette solution was replaced with 75 mm Ca2+ or Mg2+, inward currents were not visible, even at very negative potentials. Fit of the current–voltage data to an exponential function gave estimates of_E_rev more negative than −150 mV for both Ca2+ and Mg2+. Using this value with the appropriate form of the GHK equation (Eq. 2) indicated relative permeabilities with respect to K+ of <0.001, signifying that this channel was essentially impermeable to divalent cations.
Because the 35 pS channel discriminated very poorly among monovalent inorganic cations (Fig. 4A,B), we performed experiments to measure channel permeability relative to Cs+ for a wide range of organic cations, with the aim of determining the equivalent pore size of the channel. Using an outside-out patch configuration, single-channel current–voltage relationships were measured (Fig.5A) and used to obtain_E_rev for a number of organic cations (Fig. 5B). Permeability ratios were then calculated using the GHK equation (Eq. 1), and, for each organic cation, mean values obtained from four to five patches were plotted against the hydrated molecular radius (Fig. 5C, open circles). The permeability ratios defined a smoothly declining series of values that were well fit by the Renkin equation (Eq. 3), which describes the permeation of a rigid sphere through a cylindrical pore (Renkin, 1955). Least-squares fit to the equation indicated an equivalent pore radius of 0.41 nm for the 35 pS channel (Fig. 5C), a value that compared favorably with a pore radius of 0.37 nm for the nicotinic ACh receptor channel (Adams et al., 1980) and 0.49 nm for the nonselective cation channel in epithelial cells (Cook et al., 1990).
Properties of 35 pS single channel
We measured two additional biophysical properties of the channel, the voltage dependence of the open channel probability, _n_· Po(_E_m), and the open dwell time characteristics. We measured n · Po at −140 mV ≤ _E_m ≤ −40 mV, and normalized all values to the value obtained at_E_m of −140 mV. Data obtained during continuous hyperpolarizing pulses of 1 min were pooled from 6–10 recordings from 10 patches at each potential (Fig.6A, filled circles). Linear regression of the data gave a slope of −2.9 × 10−3mV−1 (Fig. 6A,line), a value that was not significantly different from zero (p = 0.09), indicating that the open channel probability for the 35 pS channel was not appreciably voltage dependent over the range of potentials studied.
To quantify the open dwell time characteristics, we constructed a probability density histogram of events pooled from 1 min segments of data obtained during hyperpolarizing pulses to −80 mV (Fig.6B). Using the MLM methods (see Materials and Methods), logarithmically binned data were fit to the pdf (Eq. 4) with values of τ1 = 2.7 msec, τ2 = 8.3 msec,_a_1 = 1493, and_a_2 = 580 (Fig. 6B,solid line). This analysis confirmed that the 35 pS channel exhibited two distinct open states, as suggested by visual inspection of single-channel recording (Fig. 3A), with open channel dwell times comparable with values of 1 and 11 msec reported in cultured secretory epithelial cells (Cook et al., 1990). The short open state was dominant, as indicated by the finding that 72% of openings were from the closed to the short open state, versus 28% from the closed to the long open state.
Inhibition by [ATP]i
We hypothesized that the 35 pS nonselective cation channel might be inhibited by intracellular ATP, based on the finding that this channel was turned on after exposure to NaN3(Fig. 2) or to NaCN plus 2-deoxyglucose (data not shown), which are known to deplete intracellular ATP (Harvey et al., 1999). This hypothesis also accorded with the observation that the 35 pS channel was seldom observed in cell-attached patches from healthy cells but became evident in >90% of patches after conversion to an inside-out configuration.
We used inside-out patches to test the hypothesis that the channel was sensitive to block by ATP on the cytoplasmic side of the membrane. Patches were studied using Cs+ as the charge carrier to ensure that no K+channel, such as Kir2.3 or KATP, was contributing to patch activity. With no ATP and 1 μmCa2+ in the bath, the 35 pS channel exhibited vigorous openings (Fig.7A,Control). Channel availability was unaffected by 1 mm AMP or ADP, but 1 mm ATP caused profound diminution in channel activity, an effect that was readily reversed on washout (Fig. 7A). We measured the open channel probability (n · Po) at different [ATP]i, normalized these values to that obtained at [ATP]i of 0 mm, and fitted these values to a standard logistic equation. As shown in Figure 7B, the 35 pS channel was blocked by [ATP]i in a dose-dependent manner. Half maximum inhibition (IC50) was observed at [ATP]i of 0.79 μm with a Hill coefficient of 1, and channel activity was completely abolished at [ATP]i of >30 μm. In contrast, ADP (six patches), AMP (four patches), and adenosine (four patches) had no effect on the 35 pS nonselective cation channel in inside-out patches (Fig.7A).
Activation by [Ca2+]i
Apart from ATP, the Ca2+concentration on the cytoplasmic side of the membrane was also found to regulate activity of the 35 pS channel. As shown in Figure8A, transforming a cell-attached patch with no apparent channel activity to an inside-out patch in a bath solution containing 1 μmCa2+ resulted in activation of the channel. Channel activity was totally lost by perfusing the cytoplasmic side with a Ca2+-free solution containing 10 mm EGTA, and activity was restored by replacement of 1 μmCa2+ (Fig. 8A).
We further examined the relationship between channel activity and [Ca2+]i using inside-out patches studied at _E_m of −80 mV. For these experiments, care was taken to study only patches containing a single channel. Changing [Ca2+]i clearly affected activity of the nonselective cation channel (Fig.8B). When free [Ca2+]i was <30 nm, no channel activity was apparent. With [Ca2+]i of >30 nm, the open probability (n · Po) increased in accordance with the [Ca2+]i, up to ≈1 μm of [Ca2+]i at which activity was near maximum.
The effect of Ca2+ on channel availability was found to depend on membrane voltage. Values of n · Po from four to nine patches obtained at three different potentials,_E_m of −40, −80, and −120 mV, were normalized to values observed with 3 μm[Ca2+]i. These data were fit to a standard logistic equation using a Hill coefficient of 1.5 and half-maximum values of 0.12, 0.31, and 1.5 μm at −40, −80, and −120 mV, respectively (Fig. 8C). These data indicated that channel activity was strongly dependent on [Ca2+]i at physiologically relevant concentrations and that the effect of Ca2+ was voltage dependent, consistent with a Ca2+ binding site inside the electric field of the membrane.
Internal Mg2+ causes rectification
Recognizing that certain channels are sensitive to intracellular Mg2+ (Chuang et al., 1997; Perillan et al., 2000), we sought to determine whether the channel rectification observed in cell-attached patch recordings (Fig. 2F) might be attributable to intracellular Mg2+. Using inside-out patches studied with equimolar K+ on both sides of the membrane, we varied [Mg2+] on the cytoplasmic side. Single-channel records and channel amplitudes observed with different [Mg2+]i are shown (Fig. 9; same patch as Fig.3A). As observed in Figure 3, no rectification was evident with [Mg2+]i of ≤30 μm, but at [Mg2+]i of ≥100 μm, increasingly strong rectification was present. At 100 μm, Mg2+ appeared to produce a flickery block, but this was not studied in detail (Fig. 9, inset). Similar results were obtained in five other patches. If rectification by internal Mg2+ accounts for the slight inward rectification observed in cell-attached patches (Fig.2F), the degree of rectification observed suggests that, in NRAs, 30 μm < [Mg2+]i < 100 μm.
Other properties
No rundown of channel activity was observed when recording inside-out patches over prolonged periods of time (>1 hr), a finding that contrasts sharply with observations on NCCa-ATP channels in other systems in which channel activity may be lost within seconds or minutes of patch excision (Sturgess et al., 1987; Cook et al., 1990).
We also studied inside-out patches formed from NRAs isolated by the same method but cultured with 5% serum for 1 week (20 patches), as well as astrocytes isolated by classical methods (Perillan et al., 2000) from neonatal rat brain and cultured for 1–2 weeks (18 patches). In these preparations, inside-out patch recordings revealed no single channel events of 35 pS, suggesting that the 35 pS channel that we observed in freshly isolated NRAs was not constitutively expressed.
DISCUSSION
The principal finding of this study was the identification in freshly isolated NRAs of a Ca2+-activated nonselective cation channel that is activated by metabolic compromise associated with reduced cytosolic ATP. The basis for the identification rests on detailed electrophysiological characterization of the channel at both the whole-cell macroscopic and single-channel levels. This channel was essentially silent under normal conditions and was rapidly activated after disturbance of mitochondrial respiration, resulting in a strong depolarization of the cell that was followed by osmotic gradient-driven cell blebbing and swelling.
The features of the NCCa-ATP channel in NRAs related to its pore properties were similar to NCCa-ATP channels identified in other cells. These characteristics include poor selectivity for monovalent cations, immeasurable permeability for anions and for divalent cations, and an appreciable pore size allowing measurable conductance of small organic molecules. The channel was almost equally permeable to a variety of monovalent cations, with a relative permeability sequence of PCs+/PK+(1.06) ≈ PNa+/PK+(1.04) ≈ PRb+/PK+(1.02) ≈ PLi+/PK+(0.96), which is comparable with previous observations in other preparations (Cook et al., 1990; Ono et al., 1994). With regard to permeability of the divalent cations Ca2+ and Mg2+, we were unable to demonstrate any inward current, even at very negative potentials, when either of these ions was the lone charge carrier in the pipette, and, in both cases, permeabilities relative to K+ were estimated to be <0.001. This finding of relative impermeability to divalent cations is consistent with observations in most other preparations (Rae et al., 1990; Popp and Gogelein, 1992; Ono et al., 1994), although in some (Cook et al., 1990), measurable permeability to Ca2+ was reported. Although the mechanism was not studied in detail, we also found that physiological concentrations of Mg2+ appeared to block the channel from inside (Popp and Gogelein, 1992), resulting in inward rectification similar to that observed in other channels such as Kir2.3 (Chuang et al., 1997; Perillan et al., 2000). Finally, in a separate series of experiments, we measured relative permeabilities of small organic cations and found that fitting of these data to the Renkin equation indicated a relative pore radius of 0.41 nm, similar to the pore size reported for the NCCa-ATP channel in a secretory epithelial cell line (Cook et al., 1990). Overall, these pore characteristics do not distinguish the NCCa-ATPchannel in NRAs from that in other preparations.
Conversely, the two principal features of the NCCa-ATP channel related to its regulation, sensitivity to Ca2+ and sensitivity to adenine nucleotides, differed in important ways from the characteristics of NCCa-ATP channels in other cells. In previous reports, NCCa-ATP channels have generally been shown to require fairly high concentrations of Ca2+ for activation, with no activity observed until Ca2+ was raised either above 1 μm (Gray and Argent, 1990; Popp and Gogelein, 1992; Thorn and Petersen, 1992; Ono et al., 1994) or even above 100 μm (Sturgess et al., 1987; Cook et al., 1990; Rae et al., 1990; Champigny et al., 1991). One early report suggested sensitivity to lower Ca2+ concentrations, but this was apparently a transient phenomenon (Maruyama and Petersen, 1984). For the most part, the requirement for high Ca2+ concentrations has remained unexplained and has raised doubt about a physiological role for the channel (Cook et al., 1990). Moreover, previous attempts at activating the channel in cell-attached patches by metabolic poisoning (2,4-dinitrophenol and Na iodoacetate) have reportedly failed (Rae et al., 1990). In contrast, in the present study, we found in inside-out patches at −80 mV that the threshold for activation was [Ca2+]i of ≈30 nm, and the EC50 was [Ca2+]i of 0.31 μm, indicating that the NCCa-ATPchannel in NRAs could easily be activated by ATP depletion at physiological concentrations of Ca2+. Indeed, we readily demonstrated robust channel activation by NaN3-induced and by NaCN plus 2-deoxyglucose-induced ATP depletion at physiological [Ca2+]i in cell-attached patches, as well as in whole-cell recordings that used nystatin perforated patches to prevent disturbance of cytosolic [Ca2+]. In addition, we found that Ca2+ sensitivity depended on membrane voltage, increasing with depolarization. This not only signifies that Ca2+ binds within the electric field of the membrane, but it also provides an intrinsic mechanism for positive feedback, reinforcing channel opening with increasing depolarization of the cell.
The second principal regulatory feature regarding which NCCa-ATP channel in NRAs differs from that in other cells concerns sensitivity to adenine nucleotides. In previous reports, ATP, ADP, and AMP were all shown to effectively block the NCCa-ATP channel, with AMP showing somewhat greater efficacy than ATP, and with half-maximum block being observed with [ATP]i of 8–20 μm (Sturgess et al., 1987; Paulais and Teulon, 1989). Nonhydrolyzable analogues of ATP also serve as effective blockers (Sturgess et al., 1986). In contrast, in NRAs, ADP and AMP were completely without effect, and half-maximum block was observed with [ATP]i of 0.8 μm. Overall, the nucleotide sensitivity observed here resembles more closely that reported for KATP channels, which show much less sensitivity to ADP than to ATP and which are virtually insensitive to AMP (Cook and Hales, 1984; Misler et al., 1986). Together, the different Ca2+- and adenine nucleotide-sensitivities exhibited by the NCCa-ATP channel in NRAs point to this being a new channel distinct from previously described NCCa-ATP channels in other preparations, although additional molecular characterization will be required to confirm this.
The normal physiological role of the NCCa-ATP in NRAs remains to be determined. Its abundance alone in freshly isolated cells would suggest that it serves an important function, and its rapid loss during culture suggests that some constituent of the unique_in vivo_ environment is required for its expression. When opened in the presence of physiological ion gradients, with an inwardly directed electrochemical gradient for Na+larger than the outward K+ gradient, a nonselective cation channel such as this will act essentially as an Na+ channel, except that its reversal potential will not be positive but will be near 0 mV. Therefore, with partial activation, this channel will deliver an influx of Na+ that will depolarize the cell and decrease the Na+ gradient across the cell membrane. Although difficult to predict precisely, in astrocytes in general, depolarization would favor opening of voltage-dependent channels, and a diminished Na+ gradient would compromise activity of certain transport systems, including those for Ca2+ and glutamate (Rose et al., 1998). With full activation, the cell will depolarize completely to 0 mV, with Na+ influx sufficient to generate an osmotic gradient and cause cell blebbing and swelling, as seen in our experiments. Compromise of the Na+/Ca2+exchanger by the reduced Na+ gradient would favor an increase in [Ca2+]i, providing a second mechanism for positive feedback besides the intrinsic voltage dependence of Ca2+ affinity that would further contribute to channel opening. Whether the cell can recover from such an event is not known, but recovery might depend in part on the magnitude of the osmotic gradient and on whether Ca2+ influx pathways are activated by these events. Although our experiments provide no clear data regarding normal function of this channel, our data strongly suggest that, under pathological conditions associated with decreased cellular ATP, this channel appears to become activated, initiating a sequence of monovalent cation influx that results in depolarization and cell swelling.
In summary, we provide electrophysiological evidence that a heretofore undescribed nonselective cation channel activated by internal Ca2+ and blocked by internal ATP is expressed in native reactive astrocytes from injured adult brain and that this channel is involved in cell swelling secondary to ATP depletion.
Footnotes
This work was supported by a Merit Review Award from the Veterans Administration (Baltimore Veterans Administration, Baltimore, MD). We thank Drs. Vladimir Gerzanich and Xing Li for valuable discussions during the course of this study, and Dr. Jia Bi Yang for expert technical assistance.
Correspondence should be addressed to Dr. J. Marc Simard, Department of Neurosurgery, University of Maryland School of Medicine, 22 South Greene Street, Suite 12SD, Baltimore, MD 21201-1595. E-mail:msimard@surgery1.umaryland.edu.
REFERENCES
- 1.Adams DJ, Dwyer TM, Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980;75:493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 3.Brismar T, Collins VP. Effect of external cation concentration and metabolic inhibitors on membrane potential of human glial cells. J Physiol (Lond) 1993;460:365–383. doi: 10.1113/jphysiol.1993.sp019476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champigny G, Verrier B, Lazdunski M. A voltage, calcium, and ATP sensitive non selective cation channel in human colonic tumor cells. Biochem Biophys Res Commun. 1991;176:1196–1203. doi: 10.1016/0006-291x(91)90412-z. [DOI] [PubMed] [Google Scholar]
- 5.Christensen O, Hoffmann EK. Cell swelling activates K+ and Cl- channels as well as nonselective, stretch-activated cation channels in Ehrlich ascites tumor cells. J Membr Biol. 1992;129:13–36. doi: 10.1007/BF00232052. [DOI] [PubMed] [Google Scholar]
- 6.Chuang H, Jan YN, Jan LY. Regulation of IRK3 inward rectifier K+ channel by m1 acetylcholine receptor and intracellular magnesium. Cell. 1997;89:1121–1132. doi: 10.1016/s0092-8674(00)80299-8. [DOI] [PubMed] [Google Scholar]
- 7.Cook DI, Poronnik P, Young JA. Characterization of a 25-pS nonselective cation channel in a cultured secretory epithelial cell line. J Membr Biol. 1990;114:37–52. doi: 10.1007/BF01869383. [DOI] [PubMed] [Google Scholar]
- 8.Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 9.Dwyer TM, Adams DJ, Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980;75:469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman DE. Potential impedance and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray MA, Argent BE. Non-selective cation channel on pancreatic duct cells. Biochim Biophys Acta. 1990;1029:33–42. doi: 10.1016/0005-2736(90)90433-o. [DOI] [PubMed] [Google Scholar]
- 12.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 13.Harvey J, Hardy SC, Ashford ML. Dual actions of the metabolic inhibitor, sodium azide on K(ATP) channel currents in the rat CRI-G1 insulinoma cell line. Br J Pharmacol. 1999;126:51–60. doi: 10.1038/sj.bjp.0702267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol (Lond) 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewell SA, Bellomo G, Thor H, Orrenius S, Smith M. Bleb formation in hepatocytes during drug metabolism is caused by disturbances in thiol and calcium ion homeostasis. Science. 1982;217:1257–1259. doi: 10.1126/science.7112127. [DOI] [PubMed] [Google Scholar]
- 17.Johnson ME, Gores GJ, Uhl CB, Sill JC. Cytosolic free calcium and cell death during metabolic inhibition in a neuronal cell line. J Neurosci. 1994;14:4040–4049. doi: 10.1523/JNEUROSCI.14-07-04040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurkowitz-Alexander MS, Altschuld RA, Hohl CM, Johnson JD, McDonald JS, Simmons TD, Horrocks LA. Cell swelling, blebbing, and death are dependent on ATP depletion and independent of calcium during chemical hypoxia in a glial cell line (ROC-1). J Neurochem. 1992;59:344–352. doi: 10.1111/j.1471-4159.1992.tb08910.x. [DOI] [PubMed] [Google Scholar]
- 19.Jurkowitz-Alexander MS, Altschuld RA, Haun SE, Stephens RE, Horrocks LA. Protection of ROC-1 hybrid glial cells by polyethylene glycol following ATP depletion. J Neurochem. 1993;61:1581–1584. doi: 10.1111/j.1471-4159.1993.tb13662.x. [DOI] [PubMed] [Google Scholar]
- 20.Juurlink BH, Chen Y, Hertz L. Use of cell cultures to differentiate among effects of various ischemia factors on astrocytic cell volume. Can J Physiol Pharmacol [Suppl] 1992;70:S344–S349. doi: 10.1139/y92-281. [DOI] [PubMed] [Google Scholar]
- 21.Kempski O, von Rosen S, Weigt H, Staub F, Peters J, Baethmann A. Glial ion transport and volume control. Ann NY Acad Sci. 1991;633:306–317. doi: 10.1111/j.1749-6632.1991.tb15622.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim D, Fu C. Activation of a nonselective cation channel by swelling in atrial cells. J Membr Biol. 1993;135:27–37. doi: 10.1007/BF00234649. [DOI] [PubMed] [Google Scholar]
- 23.Kimelberg HK, Rutledge E, Goderie S, Charniga C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab. 1995;15:409–416. doi: 10.1038/jcbfm.1995.51. [DOI] [PubMed] [Google Scholar]
- 24.Korbmacher C, Volk T, Segal AS, Boulpaep EL, Fromter E. A calcium-activated and nucleotide-sensitive nonselective cation channel in M-1 mouse cortical collecting duct cells. J Membr Biol. 1995;146:29–45. doi: 10.1007/BF00232678. [DOI] [PubMed] [Google Scholar]
- 25.Korn SJ, Marty A, Conner JA, Horn R. Perforated patch recording. In: Conn PM, editor. Methods in neuroscience. Electrophysiology and microinjection. Academic; San Diego: 1991. pp. 364–373. [Google Scholar]
- 26.Lewis CA. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol (Lond) 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomneth R, Gruenstein EI. Energy-dependent cell volume maintenance in UC-11MG human astrocytomas. Am J Physiol. 1989;257:C817–C824. doi: 10.1152/ajpcell.1989.257.4.C817. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama Y, Petersen OH. Single calcium-dependent cation channels in mouse pancreatic acinar cells. J Membr Biol. 1984;81:83–87. doi: 10.1007/BF01868812. [DOI] [PubMed] [Google Scholar]
- 29.Misler S, Falke LC, Gillis K, McDaniel ML. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci USA. 1986;83:7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mongin AA, Cai Z, Kimelberg HK. Volume-dependent taurine release from cultured astrocytes requires permissive [Ca2+]i and calmodulin. Am J Physiol. 1999;277:C823–C832. doi: 10.1152/ajpcell.1999.277.4.C823. [DOI] [PubMed] [Google Scholar]
- 31.Ono S, Mougouris T, DuBose TD, Jr, Sansom SC. ATP and calcium modulation of nonselective cation channels in IMCD cells. Am J Physiol. 1994;267:F558–F565. doi: 10.1152/ajprenal.1994.267.4.F558. [DOI] [PubMed] [Google Scholar]
- 32.Paulais M, Teulon J. A cation channel in the thick ascending limb of Henle's loop of the mouse kidney: inhibition by adenine nucleotides. J Physiol (Lond) 1989;413:315–327. doi: 10.1113/jphysiol.1989.sp017656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perillan PR, Li X, Simard JM. K+ inward rectifier currents in reactive astrocytes from adult rat brain. Glia. 1999;27:213–225. [PubMed] [Google Scholar]
- 34.Perillan PR, Li X, Potts EA, Chen M, Bredt DS, Simard JM. Inward rectifier K+ channel Kir2.3 (IRK3) in reactive astrocytes from adult rat brain. Glia. 2000;31:181–192. doi: 10.1002/1098-1136(200008)31:2<181::aid-glia90>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Popp R, Gogelein H. A calcium and ATP sensitive nonselective cation channel in the antiluminal membrane of rat cerebral capillary endothelial cells. Biochim Biophys Acta. 1992;1108:59–66. doi: 10.1016/0005-2736(92)90114-2. [DOI] [PubMed] [Google Scholar]
- 36.Preisig PA, Berry CA. Evidence for transcellular osmotic water flow in rat proximal tubules. Am J Physiol. 1985;249:F124–F131. doi: 10.1152/ajprenal.1985.249.1.F124. [DOI] [PubMed] [Google Scholar]
- 37.Rae JL, Dewey J, Cooper K, Gates P. A non-selective cation channel in rabbit corneal endothelium activated by internal calcium and inhibited by internal ATP. Exp Eye Res. 1990;50:373–384. doi: 10.1016/0014-4835(90)90138-k. [DOI] [PubMed] [Google Scholar]
- 38.Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. J Neurophysiol. 1995;73:333–346. doi: 10.1152/jn.1995.73.1.333. [DOI] [PubMed] [Google Scholar]
- 39.Renkin EM. Filtration, diffusion, and molecular seiving through porous cellulose membranes. J Gen Physiol. 1955;38:225–243. [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson RA, Stokes RH. Electrolyte solutions, Chap 6, pp 118–132. Butterworths; London: 1970. [Google Scholar]
- 41.Rose CR, Waxman SG, Ransom BR. Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci. 1998;18:3554–3562. doi: 10.1523/JNEUROSCI.18-10-03554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutledge EM, Kimelberg HK. Release of [3H]-d-aspartate from primary astrocyte cultures in response to raised external potassium. J Neurosci. 1996;16:7803–7811. doi: 10.1523/JNEUROSCI.16-24-07803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder W, Hager G, Kouprijanova E, Weber M, Schmitt AB, Seifert G, Steinhauser C. Lesion-induced changes of electrophysiological properties in astrocytes of the rat dentate gyrus. Glia. 1999;28:166–174. [PubMed] [Google Scholar]
- 44.Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staub F, Peters J, Kempski O, Schneider GH, Schurer L, Baethmann A. Swelling of glial cells in lactacidosis and by glutamate: significance of Cl−-transport. Brain Res. 1993;610:69–74. doi: 10.1016/0006-8993(93)91218-h. [DOI] [PubMed] [Google Scholar]
- 46.Sturgess NC, Hales CN, Ashford ML. Inhibition of a calcium-activated, non-selective cation channel, in a rat insulinoma cell line, by adenine derivatives. FEBS Lett. 1986;208:397–400. doi: 10.1016/0014-5793(86)81056-0. [DOI] [PubMed] [Google Scholar]
- 47.Sturgess NC, Hales CN, Ashford ML. Calcium and ATP regulate the activity of a non-selective cation channel in a rat insulinoma cell line. Pflügers Arch. 1987;409:607–615. doi: 10.1007/BF00584661. [DOI] [PubMed] [Google Scholar]
- 48.Swanson RA. Astrocyte glutamate uptake during chemical hypoxia in vitro. Neurosci Lett. 1992;147:143–146. doi: 10.1016/0304-3940(92)90580-z. [DOI] [PubMed] [Google Scholar]
- 49.Thorn P, Petersen OH. Activation of nonselective cation channels by physiological cholecystokinin concentrations in mouse pancreatic acinar cells. J Gen Physiol. 1992;100:11–25. doi: 10.1085/jgp.100.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ubl J, Murer H, Kolb HA. Ion channels activated by osmotic and mechanical stress in membranes of opossum kidney cells. J Membr Biol. 1988;104:223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- 51.Walz W, Gimpl G, Ohlemeyer C, Kettenmann H. Extracellular ATP-induced currents in astrocytes: involvement of a cation channel. J Neurosci Res. 1994;38:12–18. doi: 10.1002/jnr.490380104. [DOI] [PubMed] [Google Scholar]