Structural brain magnetic resonance imaging of pediatric twins (original) (raw)
Abstract
To explore the relative impact of genetic and nongenetics factors on human brain anatomy during childhood and adolescence development, a collaborative team from the Child Psychiatry Branch of the National Institute of Mental Health and Virginia Commonwealth University is applying structural equation modeling to brain morphometric data acquired via magnetic resonance imaging from a large sample of monozygotic and dizygotic pediatric subjects. In this report, we discuss methodologic issues related to pediatric neuroimaging twin studies and synthesize results to date from the project. Current sample size from the ongoing longitudinal study is approximately 150 twin pairs. Consistent themes are: (1) heritability is high and shared environmental effects low for most brain morphometric measures; (2) the cerebellum has a distinct heritability profile; (3) genetic and environmental factors contribute to the development of the cortex in a regional and age specific manner; and (4) shared genetic effects account for more of the variance than structure specific effects. Understanding of influences on trajectories of brain development may shed light on the emergence of psychopathology during childhood and adolescence and ultimately may guide therapeutic interventions. Hum Brain Mapp 2007. © 2007 Wiley‐Liss, Inc.
Keywords: child development, adolescent development, heredity, neuroanatomy, cerebellum
INTRODUCTION
The Child Psychiatry Branch of the National Institute of Mental Health is conducting a longitudinal pediatric brain imaging study of typically and atypically developing children and adolescents. The long‐term strategy is to: (1) map the trajectories of brain morphometric measures; (2) discern the influences on those trajectories; and (3) apply understanding of influences to guide interventions. The first component, mapping developmental trajectories, is well underway and indicates a general pattern of increasing white matter volumes and inverted U shaped trajectories of gray matter volumes with different peak volumes for different regions [Giedd et al., 1999]. One of the fundamental challenges for component two, understanding what factors affect the trajectories, is to discern genetic from nongenetic influences. Twin studies are best suited to address these types of questions and therefore, in 2001, we began collaborating with a team of experts in twin research at the Virginia Institute for Psychiatric and Behavioral Genetics and Department of Psychiatry, Virginia Commonwealth University.
Pediatric neuroimaging twin studies, although presenting more methodological obstacles, may offer advantages over adult studies in some domains. Most neuropsychiatric disorders manifest during childhood or adolescence and even many disorders with adult onset, such as schizophrenia, are increasingly conceptualized as developmental in origin. Studying the developing brain in premorbid conditions or early in the course of illness, before the effects of long term impairment or treatment interventions, may sharpen the interpretation of brain differences to the core features of the illnesses. Particularly important is to consider that differences in the trajectories of development may in some cases be more informative than the final adult differences. For instance, in our longitudinal study, looking at the relationship between cortical thickness and IQ differences in age by cortical thickness developmental curves were more predictive of IQ than differences in cortical thickness at age 20 years [Shaw et al., 2006].
In this report, we will discuss some of the methodologic considerations and challenges in conducting pediatric neuroimaging twin studies and summarize current results from the Child Psychiatry Branch/VCU collaborative project.
METHODS
Study Design
Initial power analysis projected sample size requirements of 100 MZ pairs and 100 same sex DZ pairs (50 of each sex) scanned at ∼2‐year intervals for at least three useable longitudinal data points.
Subjects
All subjects were screened via an initial telephone interview, parent and teacher rating versions of the Child Behavior checklist [Achenbach and Ruffle, 2000], and physical and neurological assessment. Exclusion criteria included psychiatric diagnosis in the subject or a first degree relative, and head injury or other conditions that might have affected gross brain development.
For twin recruitment, advertisements specified that the MRI study sought twins between the ages of 5 and 18, with no learning disabilities, neurological problems or behavioral disorders. The screening process involved phone interviews, behavioral questionnaires mailed to parents and teachers, an in‐person clinical interview, family history assessment, as well as a physical and neurological exam. Exclusion criteria included having a lifetime history of physical, neurological, or psychiatric abnormalities, learning disabilities, or psychiatric illness oneself, or in either one first‐degree relative or more than 20% of second‐degree relatives. Approximately one in four families responding to the advertisements met inclusion criteria. Twins were included in the analysis only if quantifiable MRI scans free from motion or other artifact were obtained on both twins at the same age. Written assent from the child and written consent from a parent were obtained for each participant. The study protocol was approved by the institutional review board of the National Institute of Mental Health. Zygosity was determined by DNA analysis of buccal cheek swabs using 9‐21 unlinked short tandem repeat loci for a minimum certainty of 99%, by BRT Laboratories (Baltimore, MD).
Image Acquisition
All subjects were scanned on the same GE 1.5 Tesla Signa scanner using the same three‐dimensional spoiled gradient recalled echo in the steady state (3D SPGR) imaging protocol (axial slice thickness = 1.5 mm, time to echo = 5 ms, repetition time = 24 ms, flip angle = 45°, acquisition matrix = 192 × 256, number of excitations = 1, and field of view = 24 cm). A clinical neuroradiologist evaluated all scans and no gross abnormalities were reported.
Image Analysis
Image analysis was performed via collaboration with the Montreal Neurological Institute. The native MRI scans are registered into standardized stereotaxic space using a 9 degrees of freedom linear transformation [Collins et al., 1994] and corrected for nonuniformity artifacts [Sled et al., 1998]. The registered and corrected volumes are segmented into white matter, gray matter, and cerebrospinal fluid (CSF) using a neural net classifier [Zijdenbos et al., 2002]. Region of interest analysis is performed by combining tissue classification information with a probabilistic atlas derived from an independent sample of adult scans. The regions that have been validated by comparison with other methods are the following: midsagittal area of the corpus callosum, volumes of the cerebellum, caudate nucleus, and lateral ventricles, and gray and white matter volumes of the total cerebrum, frontal lobes, parietal lobes, and temporal lobes [Collins et al., 1995].
Statistical Analysis
Methodologic tools
A general objective of twin studies is to divide the variance in a phenotype into genetic and nongenetic sources by analyzing differing correlations among MZ and DZ twins [Neale, 1992]. Structural equation modeling (SEM) and its mathematically equivalent visual analog, path analysis, provide a powerful and flexible framework way to model causal and correlational influences on both observed and unobserved (i.e. latent) traits. SEM analyses typically use numeric optimization of a likelihood function to produce parameter values that provide the best fit to the data [Neale et al., 2002]. The optimization strategy begins with more or less arbitrary start values for model parameters and calculation of an initial fit based on those start values. The output informs modification of the parameters and the process is repeated until the likelihood of the observed data is maximized, that is, the model best approximates the data.
For continuous measures such as volumetric data, the calculation of likelihood is based on the probability distribution function. If there are m observed variables on each twin, the multivariate normal probability density function of a column vector of twins observed scores xi is given by:
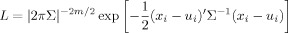 |
(1) |
|---|
where Σ is the predicted covariance matrix, and ui is the (column) vector of predicted means of the variables [Neale, 2003]. This represents the likelihood of observing record i, given the population mean and variance and assuming normality. The joint likelihood of the N independent pairs in the sample is computed as the product of the likelihoods of all pairs.
Mx, a software program developed by one of the authors (M.N.) is used to parcellate the variance of neuroanatomic structures into genetic and nongenetic factors; depending on the hypothesis being tested, other details will vary from model to model. Mx is the most commonly used software package for the analysis of genetically informative samples, and can also be applied to many types of multivariate problems that are likely to manifest during the course of the project.
Path diagrams can be constructed to model the variance attributed by different latent variables (see Fig. 1). In models for classical twin studies, three latent variables are usually defined: a, c, and e, representing additive genetic, shared environmental, and unique environmental dimensions, respectively [Evans et al., 2002; Neale, 1992]. The E term also includes measurement error. Parameters A or C or both can be removed from the model to generate submodels (i.e. AE, CE, and E) that can be tested via likelihood ratio (χ 2) test. The models can also include parameters to account for effects of age and sex on morphometric measures. Further, these models can be expanded to include siblings of twin pairs and singleton families, which can greatly increase power to detect influences of the shared environment (see Fig. 2). This “extended twin design” assumes that the shared environment operates similarly in both twins and singleton births, with respect to the phenotype of interest. In our sample, families contained a twin pair and up to three additional siblings, or singleton families with up to five members in total. The use of a large singleton sample can increase precision in the estimates of neuroanatomic variance due to a greater number of observed covariance statistics [Posthuma and Boomsma, 2000; Posthuma et al., 2000], as well as within‐subject covariance in multivariate models.
Figure 1.
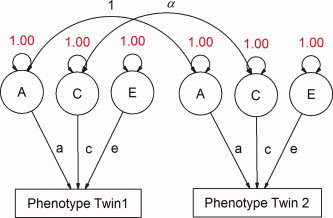
Path diagram depicting the classical ACE model for univariate variance component analysis. With MZ and DZ twins, three latent variables can be identified representing an additive genetic factor (A), a shared environmental factor (C) and a unique environmental factor (E). Paths depict the strength of the relationship between latent factors and the observed phenotype and are analogous to beta weights in linear regression. α represents the genetic correlation between twins (1 for MZ pairs, Â1/2 for DZ pairs). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
Figure 2.
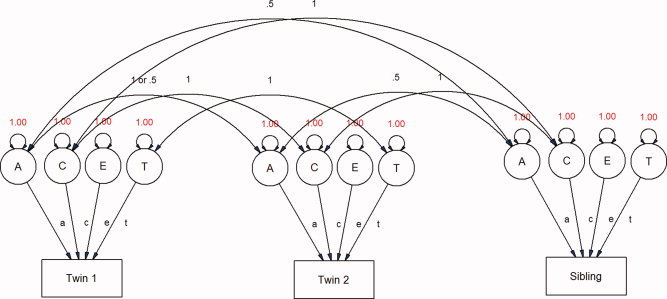
The extended twin design. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
Multivariate analysis
Multivariate analyses allow assessment of the degree to which the same genetic or environmental factors contribute to multiple neuroanatomic structures. Like the univariate variables, these interstructure correlations can be parceled into relationships of either genetic or environmental origin. This knowledge is vitally important for interpretation of most of the twin data including understanding the impact of genes which may affect distributed neural networks and interventions that may have global brain impacts.
Multivariate models can be simple extensions of the ACE model, but may also use information about cross‐twin, cross‐trait correlations that allows for the determination of which underlying factors cause phenotypes to covary (see Fig. 3). Multivariate modeling also increases the precision of parameter estimates—including the univariate a, c, and e estimates—since far more observed statistics are used. These properties are useful for assessing the underlying causes of covariance or for modeling changes in phenotypes over time with longitudinal designs.
Figure 3.
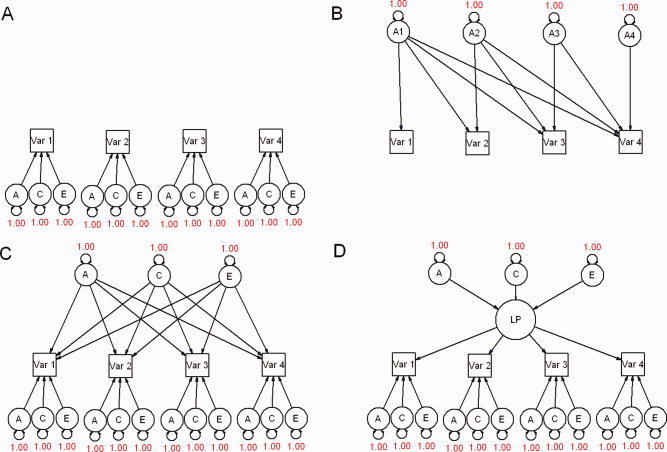
Examples of extensions into multivariate analyses. For simplicity, only one member of a twin pair is shown in each model. A: Univariate analyses run in parallel with four observed variables. There is no covariance between phenotypes. B: Cholesky decomposition, with only genetic factors shown. The triple Cholesky would include a series of C and E factors with the same pattern as A. C: Independent pathways model. D: Common pathways model. The influences of A, C, and E are mediated through common, latent phenotypes (LP). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.\]
There are three commonly used multivariate factor models in behavioral genetics analysis, the Cholesky decomposition, the independent pathways (i.e. biometric) model, and the common pathways (i.e. psychometric) model; path diagrams for each are given in Figure 3. The most fully parameterized is the Cholesky decomposition, which deconstructs any n × n positive definite variance–covariance matrix into an n x n triangular matrix, postmultiplied by its transpose, and places few a priori constraints on the fitting of the data [Evans et al., 2002; Neale, 1992; SAS Institutes, 2000]. Cholesky models are typically used as a saturated model against which the fit of more restrictive models may be compared, or to calculate genetic correlations.
Independent pathways models (IPM) allow genetic, shared environmental, and unique environmental common factors to affect observed variables directly, while in common pathways models (CPM) these factors exert their influence through a shared, latent phenotype (Fig. 3C,D). In both models, each observed variable is permitted a residual variance term, which can also be parsed into A, C, and E components. While IPMs are conceptually simpler, CPMs, require fewer parameters (when the number of factors is constant across models, e.g., 1A, 1C, and 1E factor vs. Phenotypic factor) and thus are favored by the rules of parsimony. Both CPMs and IPMs are nested submodels ofthe Cholesky decomposition, and for a given number of common factors the CPM is nested within IPM. Depending on the number of observed variables, both of these classes of models can be expanded to allow for multiple genetic, shared environmental, or unique environmental common factors.
Age‐by‐heritability interactions
An important aspect of developmental twin studies is assessment of age by heritability interactions (Age*A). Although longitudinal data is obviously better suited to address Age*A questions, a novel extension of the ACE model allows for modeling age effects on phenotypic variance using cross‐sectional data [Purcell, 2002]. The principle is similar to regression, but rather than affecting the mean phenotypic value, the moderator variable adjusts the magnitude of the influence of the latent variables A, C, and E on phenotype (see Fig. 3). Thus, in the interaction model, the influence of the latent variable A on phenotype for individual i and moderator variable M (in this case, age), is (a 1 + a 2)*Mi rather than simply a 1 Since the effects of these latent variables are inferred through phenotypic variance and cross‐twin covariance, the result of the expanded g × e model is to allow for changes in variance (i.e. heteroscedasticity) and covariance of phenotype along the dimension of the moderating environmental trait. The twin design allows for the determination of which variance component(s) are responsible for the interaction, and thus to test whether these effects are statistically significant.
SUMMARY OF RESULTS TO DATE
A univariate study addressing heritability of brain morphometric measures [Wallace et al., 2006] and a multivariate study addressing shared genetic or environmental effects across regions [Schmitt et al., 2007] have been the initial reports from this ongoing project.
Univariate Study
The sample size for this report was 90 monozygotic twin pairs, 38 same‐sex dyzygotic twin pairs, and 158 unrelated typically developing singletons. Brain morphometry measures were midsagittal area of the corpus callosum, volumes of the cerebellum, caudate nucleus, and lateral ventricles, and gray and white matter volumes of the total cerebrum, frontal lobes, parietal lobes, and temporal lobes. Shared environmental effects (_c_2) were negligible and additive genetic effects (_a_2) highly significant for all structures examined. Additive genetic effects for total cerebrum and lobar volumes (including gray and white matter subcompartments) ranged from 0.77 to 0.88, for the caudate nucleus was 0.80, for corpus callosum 0.85, for cerebellum 0.49, and for the lateral ventricles 0.31. Significant age‐by‐heritability interactions were observed with gray matter volume heritabilities decreasing over time and white matter volume heritabilities increasing over time.
Multivariate Analysis
A multivariate analysis was performed on 127 pairs of monozygotic twins (mean age = 11.6, SD = 3.3; age range = 5.6–18.7; 74 [58%] male, 53 female) and 36 pairs of same‐sex dizygotic twins (mean age = 11.0, SD = 3.7; age range = 5.5‐18.2; 18 [60%] male, 12 female) [Schmitt et al., 2007]. The brain measures examined were the cerebrum, thalamus, lateral ventricles, telencephalic subcortical nuclei, corpus callosum, and cerebellum.
In general, within‐structure, cross‐twin correlations were substantially higher in the MZ than in the DZ twins, suggesting a strong genetic impact on brain volumes' variation. Factor analysis indicated that much of the genetic effect was accounted for by two common factors. One strongly influenced variance of cerebrum, thalamus, and basal ganglia, with factor loadings (analogous to standardized partial regression coefficients) of about 0.85. This factor also accounted for a substantial proportion of the genetic variance of the cerebellum, and had a low but statistically significant effect on corpus callosum, but no impact on lateral ventricular volumes. The second genetic factor predominantly comprised the modest genetic effects on ventricular volume, with a statistically significant negative factor loading on the basal ganglia compartment.
Although the overall contribution from environmental sources was much less than from genetic, analysis indicated that two factors also accounted for a substantial portion of the environmental effects. One environmental factor primarily contributed to variance in all deep structures (thalamus, basal ganglia, lateral ventricles, and corpus callosum), with the ventricles negatively correlated with the other variables. The second environmental factor represented shared effects on the cerebrum, lateral ventricles, and cerebellum.
Structure‐specific factors contributed far less variance than the common factors with the exception of the corpus callosum where genetic factors specific to that structure accounted for 69% of the variance. Less than 10% of the variance in corpus callosum size could be explained by genetic sources that also affected other structures in the analysis.
DISCUSSION/SYNTHESIS
Consistent themes from analysis of data from the early stages of this ongoing project are: (1) heritability is high and shared environmental effects low for most brain morphometric measures; (2) the cerebellum has a distinct heritability profile; (3) genetic and environmental factors contribute to the development of the cortex in a regional and age specific manner; and (4) shared genetic effects account for more of the variance than structure specific effects.
High Heritability of Brain Morphometry
Highly heritable brain morphometric measures provide biological markers for inherited traits, and may serve as targets for genetic linkage and association studies. These intermediate phenotypes may increase the power to detect quantitative trait loci (QTLs) influencing critical behavioral functions and liability to psychopathology [Gottesman and Gould, 2003]. A greater understanding of the forces that guide brain development will help provide a heuristic for developing and implementing more effective interventions in the treatment of brain‐based disorders.
Smaller structures, perhaps because of a greater proportion of measurement error, tend to have lower heritability values and white matter volumes tend to more heritable than gray matter volumes. Lower heritability for gray matter is consistent with the notion of plastic synapses changing in response to environment and activity.
Cerebellum
The cerebellum is the least heritable of the structures we have examined (although wide confidence intervals merit cautious interpretation). Pediatric cerebellar development is also noteworthy as being the most sexually dimorphic and amongst the latest to reach peak volume [Lenroot et al., 2005]. These features make the cerebellum a prime target for pediatric neuroimaging studies. Prominent environmental influences on cerebellar development are consistent with its preferential susceptibility to insults such as alcohol, lead, or anoxia. Postnatal neurogenesis of cerebellar Purkinje cells may also be related to environmental susceptibility [Welsh et al., 2002], although the relationship between this process and volumetric changes remains to be elucidated. Also unclear is whether the unique development and heritability characteristics of the cerebellum are related to its divergence from the other regions soon after neural tube formation; while cerebrum, corpus callosum, and subcortical structures all are derived from the embryonic prosencephalon, cerebellar tissue is primarily derived from the rombencephalon [Kandel et al., 2000].
Age‐by‐Heritability Interactions
Vulnerabilities of highly heritable neuropsychiatric illnesses such anxiety, bipolar disorder, depression, eating disorder, psychosis, and substance abuse are presumably present at birth. However, the peak age for emergence of symptoms in all of these disorders is during adolescence. Age related changes in heritability may be linked to the timing of gene expression and related to the age of onset of disorders. Knowledge of when certain brain structures are particularly sensitive to genetic or environmental influences during development could also have important educational and/or therapeutic implications.
For lobar volumes total variance attributable to both additive genetic and unique environmental factors increased with time, though the proportion of that increased variance attributable to genetic or nongenetic factors differed between gray and white matter components. Specifically, for white matter additive genetic effects account for a greater proportion of the variance with increasing age whereas for gray matter unique environment effects account for a greater proportion of the variance with increasing age. As heritability is derived from the proportion of variance accounted for by genetic factors gray matter heritability thus decreases with age while white matter heritability increases with age.
Shared Genetic Effects
The multivariate analysis demonstrates that not only do genes play the predominant role in generating the observed volumetric diversity, but that most of the genetic variance is determined by genes that are shared between the major gross neural subdivisions. This finding is concordant with evolutionary genetic models of brain development which hypothesize global, genetically‐mediated differences in cell division as the driving force behind interspecies differences in total brain volume [Finlay and Darlington, 1995] as well as with the radial unit hypothesis of neocortical expansion proposed by Rakic [1995].
Comparative neuroanatomic analyses of multiple mammalian species have shown that total brain volume is highly correlated with regional volumes, irrespective of region (including neocortex, striatum, thalamus, and cerebellum), and accounts for the vast majority (>96%) of the observed volumetric variance in all regions measured except for the olfactory bulb [Darlington et al., 1999; Fishell, 1997]. Such strong correlations are thought to reflect a generalized adaptation to specific selective pressures; although it is more expensive, in terms of energy, to expand the computational resources of the entire brain when only specific functions are needed, the molecular adjustments required are far fewer than those required to completely repattern gross neural architecture.
Theoretically, genetic associations between neuroanatomic structures could arise via numerous putative mechanisms; several general models can be considered while interpreting the present data. First, brain volumes may be related genetically via ubiquitous gene products involved in basic cellular metabolism, cell growth, differentiation, or other global processes expressed throughout neuroectodermal derivatives [Rakic, 1995]. For example, functional variation in housekeeping genes or cell cycle regulators might be expected to produce genetic correlations between all brain regions that express them (and perhaps other tissues), if they produce downstream effects on volumetric measures via changes in cell proliferation or survival.
Second, correlations in brain volumes may represent vestigial relationships generated prenatally between structures with shared ontological origins. Region‐specific expression of transcription factors during neuroembryonic patterning would be one example of gene products producing regional correlations during embryogenesis. In this case, one would expect stronger genetic relationships among structures whose development diverged more recently (e.g., thalamic and hypothalamic volumes to be correlated via their shared diencephalic origin).
Third, functional interrelationships between structures may generate volumetric correlations via morphological changes associated with increased connectivity. This hypothesis is essentially a generalization of the Protocortex model, which states that neocortical development is determined by extrinsic influences, such as effects of thalamic innervation [O'Leary, 1989; Schlaggar and O'Leary, 1991]. Thus, one might expect structures in the visual processing network, such as V1 and the lateral geniculate nucleus, to be structurally correlated despite being potentially unrelated spatially or ontologically.
Finally, genetic correlations may result from shared supra‐regional gene expression triggered postnatally, in the present data from birth to the age of scan acquisition, approximately mid‐childhood.
Though the unique environment had a relatively minor effect on the volumes of the structures measured, our relatively large sample allowed us to describe its role on the correlations between structures with high precision. We found that structures in spatial proximity were significantly positively correlated via individual‐specific environmental factors shared between multiple anatomic regions. In other words, the environment tends to influence nearby structures similarly.
CONCLUSION
In the past decade few disciplines in neuroscience have undergone as dramatic growth as genetics and neuroimaging. Brain imaging studies of twins lie at the interface of these burgeoning disciplines. Although still in its nascent stages and underpowered to detect important nuances of gene–brain relationships, these preliminary findings suggest a few emerging principles that may help guide ongoing investigations. Future directions will include acquiring additional time points from the ongoing longitudinal study to allow more sophisticated modeling of age by heritability interactions, analysis of novel morphometric and physiologic measures, and linking the neuroanatomy and heritability findings to cognitive and behavioral measures. The rapid progress in the application of neuroimaging techniques to the study of twins promises to provide increasingly powerful tools to elucidate the biological basis of cognition, emotion, and behavior in health and illness.
REFERENCES
- Achenbach TM,Ruffle TM( 2000): The Child Behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 21: 265–271. [DOI] [PubMed] [Google Scholar]
- Collins DL,Neelin P,Peters TM,Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Collins DL,Holmes CJ,Peters TM,Evans AC ( 1995): Automatic 3‐D model‐based neuroanatomical segmentation. Hum Brain Mapp 3: 190–208. [Google Scholar]
- Darlington RB,Dunlop SA,Finlay BL ( 1999): Neural development in metatherian and eutherian mammals: Variation and constraint. J Comp Neurol 411: 359–368. [PubMed] [Google Scholar]
- Evans DM,Gillespie NA,Martin NG ( 2002): Biometrical genetics. Biol Psychol 61: 33–51. [DOI] [PubMed] [Google Scholar]
- Finlay BL,Darlington RB ( 1995): Linked regularities in the development and evolution of mammalian brains. Science 268: 1578–1584. [DOI] [PubMed] [Google Scholar]
- Fishell G ( 1997): Regionalization in the mammalian telencephalon. Curr Opin Neurobiol 7: 62–69. [DOI] [PubMed] [Google Scholar]
- Giedd JN,Blumenthal J,Jeffries NO,Castellanos FX,Liu H,Zijdenbos A,Paus T,Evans AC,Rapoport JL ( 1999): Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2: 861–863. [DOI] [PubMed] [Google Scholar]
- Gottesman II,Gould TD ( 2003): The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636–645. [DOI] [PubMed] [Google Scholar]
- Kandel ER,Schwartz JH,Jessell TM ( 2000): Principles of Neural Science. New York: Health Professions Division, McGraw‐Hill. [Google Scholar]
- Lenroot RK,Shaw P,Taylor K,Greenstein D,Clasen L,Evans A,Giedd J ( 2005): Gender‐specific longitudinal changes in cortical thickness. Kona, HI: American College of Neuropsychopharmacology. [Google Scholar]
- Neale MC ( 2003): Twins studies: Software and algorithms In: Cooper DN,Hoboken NJ, editors. Encyclopedia of the Human Genome. London: Nature Publishing; p 88–96. [Google Scholar]
- Neale Michael C.,Cardon Lon R. ( 1992): Methodology for Genetic Studies of Twins and Families. Dordrecht: Kluwer Academic. [Google Scholar]
- Neale MCB, SM,Xie G,Maes HH ( 2002): Mx: Statistical Modeling. Richmond,VA: Virginia Commonwealth University. [Google Scholar]
- O'Leary DD ( 1989): Do cortical areas emerge from a protocortex? Trends Neurosci 12: 400–406. [DOI] [PubMed] [Google Scholar]
- Posthuma D,Boomsma DI ( 2000): A note on the statistical power in extended twin designs. Behav Genet 30: 147–158. [DOI] [PubMed] [Google Scholar]
- Posthuma D,De Geus EJ,Neale MC,Hulshoff Pol HE,Baare WEC,Kahn RS,Boomsma D ( 2000): Multivariate genetic analysis of brain structure in an extended twin design. Behav Genet 30: 311–319. [DOI] [PubMed] [Google Scholar]
- Purcell S ( 2002): Variance components models for gene‐environment interaction in twin analysis. Twin Res 5: 554–571. [DOI] [PubMed] [Google Scholar]
- Rakic P ( 1995): A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends Neurosci 18: 383–388. [DOI] [PubMed] [Google Scholar]
- SAS Institute ( 2000): SAS, version 8. Cary, NC: SAS Institutes, Inc. [Google Scholar]
- Schlaggar BL,O'Leary DD ( 1991): Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science 252 (5012): 1556–1560. [DOI] [PubMed] [Google Scholar]
- Schmitt JE,Wallace GL,Rosenthal MA,Molloy EA,Ordaz S,Lenroot R,Clasen LS,Blumenthal JD,Kendler KS,Neale MC,Giedd JN ( 2007): A multivariate analysis of neuroanatomic relationships in a genetically informative pediatric sample. Neuroimage 35: 70–82. [DOI] [PubMed] [Google Scholar]
- Shaw P,Greenstein D,Lerch J,Clasen L,Lenroot R,Gogtay N,Evans A,Rapoport J,Giedd J ( 2006): Intellectual ability and cortical development in children and adolescents. Nature 440: 676–679. [DOI] [PubMed] [Google Scholar]
- Sled JG,Zijdenbos AP,Evans AC ( 1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Wallace GL,Schmitt JE,Lenroot RK,Viding E,Ordaz S,Rosenthal MA,Molloy E,Clasen L,Kendler KS,Neale MC,Giedd JN ( 2006): A pediatric twin study of brain morphometry. J Child Psychol Psychiatry 47: 987–993. [DOI] [PubMed] [Google Scholar]
- Welsh JP,Yuen G,Placantonakis DG,Vu TQ,Haiss F,O'Hearn E,Molliver ME,Aicher SA ( 2002): Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol 89: 331–59. [PubMed] [Google Scholar]
- Zijdenbos AP,Forghani R,Evans AC ( 2002): Automatic “pipeline” analysis of 3‐D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging 21: 1280–1291. [DOI] [PubMed] [Google Scholar]