Cognitive behaviour therapy for chronic fatigue syndrome in adults (original) (raw)
Notes
Editorial note
This 2008 review predates the mandatory use of GRADE methodology to assess the strength of evidence, and the review is no longer current. It should not be used for clinical decision‐making. The author team is no longer available to maintain the review.
Abstract
Background
Chronic fatigue syndrome (CFS) is a common, debilitating and serious health problem. Cognitive behaviour therapy (CBT) may help to alleviate the symptoms of CFS.
Objectives
To examine the effectiveness and acceptability of CBT for CFS, alone and in combination with other interventions, compared with usual care and other interventions.
Search methods
CCDANCTR‐Studies and CCDANCTR‐References were searched on 28/3/2008. We conducted supplementary searches of other bibliographic databases. We searched reference lists of retrieved articles and contacted trial authors and experts in the field for information on ongoing/completed trials.
Selection criteria
Randomised controlled trials involving adults with a primary diagnosis of CFS, assigned to a CBT condition compared with usual care or another intervention, alone or in combination.
Data collection and analysis
Data on patients, interventions and outcomes were extracted by two review authors independently, and risk of bias was assessed for each study. The primary outcome was reduction in fatigue severity, based on a continuous measure of symptom reduction, using the standardised mean difference (SMD), or a dichotomous measure of clinical response, using odds ratios (OR), with 95% confidence intervals (CI).
Main results
Fifteen studies (1043 CFS participants) were included in the review. When comparing CBT with usual care (six studies, 373 participants), the difference in fatigue mean scores at post‐treatment was highly significant in favour of CBT (SMD ‐0.39, 95% CI ‐0.60 to ‐0.19), with 40% of CBT participants (four studies, 371 participants) showing clinical response in contrast with 26% in usual care (OR 0.47, 95% CI 0.29 to 0.76). Findings at follow‐up were inconsistent. For CBT versus other psychological therapies, comprising relaxation, counselling and education/support (four studies, 313 participants), the difference in fatigue mean scores at post‐treatment favoured CBT (SMD ‐0.43, 95% CI ‐0.65 to ‐0.20). Findings at follow‐up were heterogeneous and inconsistent. Only two studies compared CBT against other interventions and one study compared CBT in combination with other interventions against usual care.
Authors' conclusions
CBT is effective in reducing the symptoms of fatigue at post‐treatment compared with usual care, and may be more effective in reducing fatigue symptoms compared with other psychological therapies. The evidence base at follow‐up is limited to a small group of studies with inconsistent findings. There is a lack of evidence on the comparative effectiveness of CBT alone or in combination with other treatments, and further studies are required to inform the development of effective treatment programmes for people with CFS.
Plain language summary
Cognitive behaviour therapy for chronic fatigue syndrome
Chronic fatigue syndrome (CFS) is a very common and disabling condition, in which people suffer from persistent symptoms of fatigue that are unexplained. Cognitive behaviour therapy is a psychological therapy model that is commonly used to treat a range of psychological and chronic pain conditions. This review aimed to find out whether CBT is effective for CBT, both as a standalone treatment and in combination with other treatments, and whether it is more effective than other treatments used for CFS. The review included 15 studies, with a total of 1043 CFS participants. The review showed that people attending for CBT were more likely to have reduced fatigue symptoms at the end of treatment than people who received usual care or were on a waiting list for therapy, with 40% of people in the CBT group showing clinical improvement, in contrast with 26% in usual care. At follow‐up, 1‐7 months after treatment ended, people who had completed their course of CBT continued to have lower fatigue levels, but when including people who had dropped out of treatment, there was no difference between CBT and usual care. The review also compared CBT against other types of psychological therapy, including relaxation techniques, counselling and support/education, and found that people attending for CBT was more likely to have reduced fatigue symptoms at the end of treatment than those attending for other psychological therapies. Physical functioning, depression, anxiety and psychological distress symptoms were also more reduced when compared with other psychological therapies. However at follow‐up, the results were inconsistent and the studies did not fit well together, making it difficult to draw any conclusions. Very few studies reported on the acceptability of CBT and no studies examined side effects. Only two studies compared the effectiveness of CBT against other treatments, both exercise therapy, and just one study compared a combination of CBT and other treatments with usual care. More studies should be carried out to establish whether CBT is more helpful than other treatments for CFS, and whether CBT in combination with other treatments is more helpful than single treatment approaches.
Background
Description of the condition
Chronic fatigue syndrome (CFS) is a condition characterised by persistent fatigue and significant associated disability, but without evident physical or psychological disorder to explain the problem. Sufferers endure fatigue which may cause considerable and persisting debility. They may also experience a lack of understanding from others, including health professionals. This may be exacerbated by normal or equivocal findings on physical examination or investigation despite the sufferer's experience of illness. It is an important health care issue, presenting major problems for both its sufferers and for health services. Whilst fatigue is so common as to be almost a normal consequence of life (Ridsdale 1989), many of the population experience fatigue which is much more severe or long‐lasting. About one in three of the UK population report substantial fatigue, and one in five report fatigue having been present for over six months (Cox 1987). Some of these sufferers have physical or psychological disorders which cause fatigue. Nevertheless, a significant proportion of people with disabling and chronic fatigue appear to have a disorder which is medically unexplained. The term CFS describes this more defined, and serious, problem, which is thought to affect up to 1% of the general population (Wessely 1995), with a reported range of 0.006% up 3% depending on the setting and criteria used (Afari 2003).
CFS has had many names in recent decades. These include Royal Free disease, Iceland disease, neurasthenia, myalgic encephalomyelitis ('ME'), and post‐viral fatigue syndrome. All these appear to describe groups of patients with a similar problem, that is, persistent medically unexplained fatigue which causes disability and distress. The term CFS has been adopted and clearly defined in order to foster research on this common problem. Opinions regarding the cause have tended to focus on either mainly physical or mainly psychological explanations. There is increasing awareness in a range of illnesses of the potential interaction of physical and psychological factors in the development and maintenance of the disorder. Such illnesses include irritable bowel syndrome, low back pain and CFS.
Many people with CFS in the community attend their general practitioners for assessment and treatment. Some attend alternative practitioners, disappointed with the results of orthodox medical practice. Others are referred to out‐patient clinics for assessment. Referral to a variety of specialist clinics, including general medicine, endocrinology, infectious diseases, neurology, and psychiatry, reflects uncertainty and, often, disagreement among doctors and sufferers about the causes of CFS. Sufferers' and professionals' struggle to understand the illness may lead to unsatisfactory patient‐professional relationships, and dissatisfied patients.
Description of the intervention
Until recently, research studies have been unable to offer neither a convincing explanation of the causes of CFS nor evidence for effective treatments. Often, sufferers have been advised to limit the demands placed upon their bodies, in order to promote recovery and prevent deterioration, which may lead to further impairment of quality of life. Treatment strategies for CFS include psychological, physical and pharmacological interventions. Randomised controlled trials (RCTs) have been carried out to assess the effectiveness of treatments as diverse as exercise therapy (Fulcher 1997, Wearden 1998), self‐help treatment (Chalder 1997), antidepressants (Behan 1995, Vercoulen 1996, Wearden 1998) and dietary supplementation with fatty acids (Warren 1999) and folic acid (Kaslow 1989). Over the last 15 years, an increasing number of trials for CFS have focused attention on psychological therapies, including supportive 'non‐directive' interventions and treatments that draw from cognitive and behavioural approaches.
How the intervention might work
First developed in the 1960s, and later manualised as cognitive therapy (CT) (Beck 1979), cognitive behavioural therapy (CBT) incorporates elements of both behavioural therapy (BT) and cognitive therapy approaches. CBT facilitates the identification of unhelpful, anxiety‐provoking thoughts, and challenges these negative automatic thoughts and dysfunctional underlying assumptions through collaborative 'hypothesis‐testing', using behavioural tasks of diary‐keeping and validity‐testing of beliefs between sessions, together with skills training within sessions.
For the treatment of CFS, CBT combines a rehabilitative approach of a graded increase in activity with a psychological approach addressing thoughts and beliefs about CFS that may impair recovery. A gradual increase in activity, negotiated and agreed with the patient, can be used as a 'behavioural experiment', in which core beliefs, such as about the link between increased activity and worsened physical symptoms, can be tested. Catastrophic interpretations of transient increases in physical symptoms can be challenged by orthodox cognitive therapy techniques, such as the 'double column technique', in which the original, anxious, unhelpful thought is written alongside new, more realistic, more helpful alternatives.
Recent developments in CBT include 'third wave' approaches, including mindfulness‐based therapy (Kabat‐Zinn 1991; Segal 2002), compassion‐focused therapy (Gilbert 2005) and acceptance and commitment therapy (Hayes 2003). Originally focusing on the treatment of depression (Segal 2002), and now being used with a number of other disorders, including CFS, mindfulness training incorporates meditation techniques and teaches participants to observe thoughts, emotions and sensations without trying to escape, avoid or change them (Kabat‐Zinn 1991).
Why it is important to do this review
The current body of evidence for CBT remains limited to narrative synthesis within generic CFS reviews (NICE 2007; Chambers 2006) or to meta‐analysis of mean effect sizes (Malouff 2008). Furthermore, potential heterogeneity has been largely based on qualitative assessment and the impact of symptom severity and healthcare setting are uncertain moderators of effect (NICE 2007). An in‐depth, up‐to‐date, systematic review of CBT alone and in combination with other treatments for CFS is of key importance to inform treatment decision by patients, clinicians and policy‐makers. This review is central in a programme of Cochrane reviews for CFS, which also cover exercise therapy (Edmonds 2004), pharmacological treatments (Rawson 2007) and complementary approaches, including acupuncture (Zhang 2006) and traditional Chinese herbal medicine (Adams 2007).
Objectives
1. To examine the effectiveness and acceptability of CBT for CFS compared with usual management or waiting list control 2. To examine the comparative effectiveness and acceptability of CBT for CFS against other interventions (immunological, pharmacological, other psychological therapies, exercise alone, complementary/alternative and supplements) For the purposes of the updated version of this review, a third objective has been added: 3. To examine the effectiveness and acceptability of CBT in combination with other interventions against CBT alone or against other interventions alone
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs), both published and unpublished, in any language, were included in the review.
Cluster RCTs, in which centres or therapists were randomised to intervention or control groups were included, provided that the specific aim of the study was to examine the effect of the intervention.
Types of participants
Patient characteristics and setting Trials of male and female participants over the age of 16 were included, irrespective of culture and setting
Diagnosis Trials of patients who fulfilled the following diagnostic criteria for CFS were included: 1) fatigue was the principal symptom 2) the fatigue was medically unexplained, i.e. did not appear to be adequately explained by abnormalities found on examination or investigation, by diagnosed physical disorder, or by psychiatric disorder including eating disorders, alcohol or drug misuse, psychosis, bipolar affective disorder or severe depressive illness 3) the fatigue was of sufficient severity to significantly disable or distress the patient 4) the fatigue was over six months in duration*
Trials involving several disorders were included, provided that over 90% of the patients had a primary diagnosis of CFS according to the above criteria. Trials in which less than 90% of participants had a primary diagnosis of CFS were included in the review if data limited to CFS participants were provided
* Although there are a number of differences between diagnostic criteria used for CFS (Sharpe 1991; Lloyd 1988; Fukuda 1994; Carruthers 2003), they are all consistent in specifying a duration of at least six months since onset.
Comorbidity Studies involving participants with comorbid physical or common mental disorders were eligible for inclusion, as long as the comorbidity was secondary to the diagnosis of CFS. However, studies involving patients with a comorbid psychiatric diagnosis of substance‐related disorder, schizophrenia or psychotic disorder were excluded.
Types of interventions
Intervention Trials of experimental interventions which met the following criteria were included: 1) The psychological treatment(s) of interest incorporated attempted modification of thoughts and beliefs about symptoms and illness and attempted modification of behavioural responses to symptoms and illness, such as rest, sleep and activity. 2) The intervention was clearly described and was supported by appropriate references 3) No restrictions were imposed on the length of treatment, in terms of duration or number of sessions, or time between sessions 4) Both individual and group treatment modalities were eligible for inclusion
In the original version of the review, the way in which modification of thoughts, beliefs, rest, and activity was attempted was used to delineate two 'types' of CBT. 'Type A' attempted to increase activity and reduce rest time in a systematic manner, independent of symptoms, towards 'normal' levels. 'Type B' attempted to tailor the patient's rest and activity towards levels which were compatible with the limitations imposed by the disorder. Therefore, Type B CBT did not explicitly attempt to increase the patient's physical or psychological capacity beyond improving their ability to 'cope' with their disabilities.
For the purposes of updating the review, following discussion between the review authors, it was agreed that distinguishing between the two types of CBT described above was not feasible. This approach to categorising studies was therefore not adopted. Instead, interventions were categorised according to their theoretical underpinning, as follows: 1) Traditional CBT models, including cognitive behavioural approaches drawing from Beckian principles (Beck 1979). 2) Recently developed CBT models, including mindfulness training (Kabat‐Zinn 1991), compassion‐focused therapy (Gilbert 2005) and acceptance and commitment therapy (Hayes 2003).
Control conditions The following control interventions were included: 1) Minimal or usual medical management (also sometimes described in trials as standard care, treatment as usual or waiting list), to be defined here as being elements of clinic attendance, investigation, reassurance, and simple advice, without explicit psychological therapy, and also including naturalistic prescribing of medication and/or other interventions 2) Immunological therapy, to include immunoglobulin, staphylococcus toxoid and interferon 3) Pharmacotherapy, to include hydrocortisone preparations and antidepressants (all classes) 4) Non‐CBT psychological therapies, to include non‐directive (supportive) therapies, behavioural relaxation training and psychodynamic therapy 5) Exercise as a stand‐alone intervention 6) Complementary/alternative therapies, to include homeopathy, acupuncture, massage 7) Supplements, to include general supplements, essential fatty acids and other mineral and vitamin based preparations
Combination therapy For the purposes of the updated review, CBT in combination with other interventions was investigated, with therapies combined with CBT categorised as immunological, pharmacological, complementary/alternative and supplements. Exercise in the form of graded exercise or paced activity, was assumed to be an integral component of CBT.
Main comparisons For the purposes of the updated review, the following main comparisons were planned: 1. CBT versus usual care (to include treatment as usual and waiting list) 2. CBT versus immunological therapies 3. CBT versus pharmacological therapies 4. CBT versus other psychological therapies 5. CBT versus exercise 6. CBT versus complementary/alternative therapies 7. CBT versus nutritional supplements 8. CBT + other interventions (see control condition categories) versus usual care 9. CBT + other interventions versus CBT alone 10. CBT + other interventions versus other interventions alone
Types of outcome measures
Primary outcomes
The principal outcome used in this review was fatigue, measured as follows: 1) Reduction in fatigue severity (continuous), measured using the Chalder Fatigue Scale (Chalder 1993), a 14 item self‐rated scale measuring physical and mental fatigue, sometimes shortened to 11 items, or any other validated fatigue scale. 2) Clinical recovery or improvement (dichotomous), based on diagnostic interview or defined cut‐off on validated scales, as specified by trial authors.
Secondary outcomes
- Improvement in overall physical functioning, measured using rating scales, either patient‐rated such as the Physical Function dimension of the Short Form‐36 (Ware 1993), or clinician‐rated, such as the Karnofsky index (Karnofsky 1948). Other possible measures included timed walking tests and tests of strength or aerobic capacity 2) Depression symptoms, using measures such as Hamilton Rating Scale for Depression (HRSD) (Hamilton 1960), Beck Depression Inventory (BDI) (Beck 1987) or Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983) 3) Anxiety symptoms, using measures such as State Trait Anxiety Inventory (STAI) (Spielberger 1983) or Beck Anxiety Inventory (BAI) (Beck 1988) 4) General psychological distress, using measures such as Symptom Checklist‐90 (SCL‐90) or General Health Questionnaire 5) Quality of life, using instruments such as Short Form 36 (SF‐36) (Ware 1993) 6) Acceptability of treatment, measured through: a) self‐rated acceptability/satisfaction scales b) dropout rates from trials due to adverse effects or ineffectiveness 7) Adverse effects: proportion of participants experiencing one or more adverse effects of treatment ( adverse effects being defined as those that are behavioural in basis e.g. self‐harm, substance misuse, suicidal attempts/suicide, violence to others) 8) Overall dropout rates from trials 9) Occupational outcomes, including employment status and work absence 10)Health service resource use e.g. primary care consultation rate, secondary care referral rate, use of alternative practitioners
Outcomes were classified as post treatment, short term follow‐up (I ‐6 months post‐treatment), medium term follow‐up (7‐12 months post‐treatment) and long term (longer than 12 months).
Search methods for identification of studies
Electronic searches
- The Depression, Anxiety and Neurosis Cochrane Review Group Trials Registers were searched during December 2006, with an updating search carried out in March 2008.
CCDANCTR‐Studies was searched on 28/3/2008 using the terms
Diagnosis = chronic fatigue syndrome
and
Intervention = *therap*'.
CCDANCTR‐References was searched on 28/3/2008 using the terms
Full‐text = fatigue or CFS
- Cochrane Central Register of Controlled Trials (CENTRAL; Issue 4, 2006) was searched using the search terms (chronic and fatigue and syndrome or CFS or myalgic encephalomyelitis) within title, abstract or keywords. 3) Index to Theses of Great Britain and Ireland (from 1716 to 14 December 2006) was searched at www.theses.com using advanced search (stemming on, fuzziness level 4) as follows: (chronic AND fatigue AND syndrome) OR (myalgic AND encephalomyelitis). 4) We searched during January 2007 for ongoing studies at http://www.clinicaltrials.gov (free text search for 'chronic fatigue' or 'myalgic encephalomyelitis'), and http://www.controlled‐trials.com (all registers in the meta‐register of controlled trials, (chronic and fatigue and syndrome) or cfs, or myalgic and encephalomyelitis.
Searching other resources
1) Citation checking Citation lists of potentially relevant papers obtained by these methods were searched for further relevant trials, as were the reference lists of those further relevant trials. In addition, the citation lists of relevant and recent systematic reviews were perused (NICE 2007; Chambers 2006; Cho 2005; Clark 2005; Rimes 2005; Carruthers 2003; CFS/ME WG 2002; RACPWG 2002; Mulrow 2001).
2) Personal contact Known specialists in the field and principal authors of those studies, identified by the above methods, were contacted and asked to provide details of additional trials, published or unpublished, of which they were aware.
Data collection and analysis
Selection of studies
Two review authors (JP and EM or VH) independently decided whether each potential trial fulfilled inclusion criteria. Review authors were not blinded to the names of the authors, institutions, journal of publication, and results, when they applied the inclusion criteria. Any disagreement on the eligibility of a study was discussed with the third review author, the decisions documented and, where necessary, the authors of the studies were contacted for further information.
Data extraction and management
Full data extraction, using a standardised data extraction sheet, was performed by two review authors independently (EM and ET) on studies selected for inclusion in the review. Information was collected regarding the characteristics of participants, characteristics of interventions (including 'type' of CBT), characteristics of outcome measures, and results. Data on the number with each outcome event was sought by allocated treatment group, irrespective of compliance and whether or not the patient was deemed ineligible or otherwise excluded from treatment or follow‐up, in order to allow an intention‐to‐treat analysis. If any of this information was not available in the published trial, it was sought by correspondence with the trial authors.
Assessment of risk of bias in included studies
Methodological quality in the context of a systematic review of treatment trials means the extent to which design is likely to have reduced the impact of bias on the results. For the purposes of the updated version of the review, methodological quality was assessed using the Cochrane Collaboration tool for assessing risk of bias. The following domains of risk of bias were used: 1) Sequence generation: Was the allocation sequence adequately generated? 2) Allocation concealment: Was allocation adequately concealed? 3) Blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: Was knowledge of the allocated intervention adequately prevented during the study? 4) Incomplete outcome data for each main outcome or class of outcomes: Were incomplete outcome data adequately addressed? 5) Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting? 6) Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
Trials were allocated to three categories within and across studies as follows: 1. Low risk of bias 2. Unclear risk of bias 3. High risk of bias.
During this procedure, review authors (EM and VH) worked independently of each other. The review authors were not blinded to the names of the authors, institutions, journal of publication and results of the trials. The review authors compared their results, and any disagreements were resolved by discussion. In the event of disagreement, a third review author (JP) was consulted, the decisions documented, and where necessary, the authors of the studies were contacted for further information.
Measures of treatment effect
Continuous outcomes: where studies used the same outcome measure for a comparison, data were pooled by calculating the weighted mean difference (WMD). Where different measures were used to assess the same outcome for a comparison, data were pooled by calculating the standardised mean difference (SMD), using 95% confidence intervals.
Dichotomous outcomes: dichotomous outcomes were analysed by calculating an odds ratio (OR) for each comparison, with the uncertainty in each result expressed using 95% confidence intervals (CIs). For studies where the number of participants showing clinical response were not presented in the original articles, but means and standard deviations were reported for continuous symptomatology scales, the number of responders was calculated and imputed from continuous data using a validated statistical method (Furukawa 2005). When overall results were significant, the number needed to treat (NNT) to produce one outcome was calculated by combining the overall relative risk with an estimate of the prevalence of the event in the control group of the trials.
Unit of analysis issues
Where studies had two or more active treatment arms to be compared against TAU, data were managed as follows: Continuous data ‐ means, SDs and number of participants for each active treatment group were pooled across treatment arms as a function of the number of participants in each arm (Law 2003) to be compared against the control group. As an alternative strategy, the active comparison considered to be of greatest relevance was selected (e.g. CBT was selected in preference to CT or BT arms). Dichotomous data ‐ active treatment groups were collapsed into a single arm for comparison against the control group, or the control group was split equally into two.
Dealing with missing data
Missing dichotomous data were managed through intention to treat (ITT) analysis, in which it was assumed that patients who dropped out after randomisation had a negative outcome. Best/worse case scenarios were also calculated for the clinical response outcome, in which it was assumed that dropouts in the active treatment group had positive outcomes and those in the control group had negative outcomes (best case scenario), and that dropouts in the active treatment group had negative outcomes and those in the control group had positive outcomes (worst case scenario), thus providing boundaries for the observed treatment effect.
Missing continuous data were either analysed on an endpoint basis, including only participants with a final assessment, or analysed using last observation carried forward to the final assessment (LOCF) if LOCF data were reported by the trial authors. Where SDs were missing, attempts were made to obtain these data through contacting trial authors. Where SDs were not available from trial authors, they were calculated from t‐values, confidence intervals or standard errors, where reported in articles (Deeks 1997). If these additional figures were not available or obtainable, the study data were not included in the comparison of interest.
Assessment of heterogeneity
Statistical heterogeneity was formally tested using the natural approximate chi‐square test, which provides evidence of variation in effect estimates beyond that of chance. Since the chi‐squared test has low power to assess heterogeneity where a small number of participants or trials are included, the p‐value was conservatively set at 0.1. Heterogeneity was also tested using the I2 statistic, which calculates the percentage of variability due to heterogeneity rather than chance, with I2 values over 50% indicating strong heterogeneity (Higgins 2003).
Assessment of reporting biases
Where sufficient numbers of trials allowed a meaningful presentation, funnel plots were constructed to establish the potential influence of publication bias.
Data synthesis
A fixed effect model was used in the first instance to combine data. Where there was evidence of statistical heterogeneity, results were recalculated using a random effects model, in order to obtain a more conservative estimate.
Subgroup analysis and investigation of heterogeneity
Clinical characteristics were examined in subgroup analyses to compare their influence on the size of the treatment effect. Where sufficient studies were available for inclusion, subgroup analyses were performed for: 1) Type of control condition (minimal management/standard care vs waiting list) 2) Modality of treatment (individual therapy vs group therapy) 3) Therapy process (face‐to‐face contact versus self‐help) 4) Therapist experience/qualifications (delivered by trained therapists vs trainee therapists) 5) Increased activity introduced as component of intervention versus tailoring activity/rest to fit current limitations or not specified 6) Number of CBT sessions (up to and including 8 sessions vs more than 8 sessions) 7) Severity/chronicity of CFS symptomatology at baseline 8) Setting (clinic‐based versus home‐based) These subgroup analyses were also used to examine potential sources of clinical heterogeneity.
Sensitivity analysis
Where sufficient studies were available for inclusion, sensitivity analyses were planned to test the robustness of the findings obtained by removing studies based on the following internal validity criteria: 1) Inadequate allocation concealment 2) Use of less stringent diagnostic inclusion criteria, such as those set out by Lloyd 1988. 3) Dropout rate higher than 20% 4) Lack of formal testing of fidelity to psychological therapy manual These sensitivity analyses were also used to examine potential sources of methodological heterogeneity.
Results
Description of studies
Results of the search
An updating search of CCDANCTR‐Studies and CCDANCTR‐References was performed in March 2008. Of 14 studies identified through the search of CCDANCTR‐Studies, seven were selected for inclusion in the review. Three of the studies had been included in the original review (Deale 1996; Lloyd 1993; Sharpe 1993) and a further four studies were included in the updated review (King 1999; O'Dowd 2000; Prins 2001; Jason 2007). The search of CCDANCTR‐References identified an additional two studies for inclusion (Huibers 2004; Strang 2002).
A further 20 full articles on potentially eligible studies, including two unpublished papers and a PhD thesis, were obtained through supplementary searches and personal communication with experts in the field. Of those studies, six were selected for inclusion in the review (Barrett 1992; Ridsdale 2004; Russell 2001; Strang 2002; Whitehead 2002; Stevens 1999). Two studies (Chalder 1997; Powell 2001) are currently awaiting assessment. Futher details are provided below.
Included studies
In total, the combined searches identified 15 completed studies that were eligible for inclusion in the review. Descriptive information for each study, including an appraisal of risk of bias for methodological domains, is presented in the Characteristics of Included Studies table.
Design All the studies were of a randomised trial design, with randomisation at the patient level for 14 trials. One trial (Whitehead 2002) randomised general practices to intervention or control groups.
The overall duration of trials including follow‐up ranged from 6 weeks (Strang 2002) to 14 months (Prins 2001), with a mean duration of 7.5 months. Four studies (Barrett 1992, Russell 2001, Strang 2002, Surawy 2005) measured final outcomes immediately after the termination of intervention (no follow‐up). The longest period of follow‐up after intervention was 8 months (Huibers 2004, O'Dowd 2000). The overall mean duration of follow‐up after intervention of 3.8 months for the 11 studies for which it could be calculated.
Seven studies reported obtaining ethical approval, and nine studies stated that patient consent had been obtained. Two studies made no mention of ethical approval or patient consent (King 1999; Russell 2001).
Sample sizes The mean sample size of studies was 91 subjects, ranging from 18 subjects in a randomised trial component of a larger series of exploratory studies (Surawy 2005), to 278 subjects in a trial conducted at three centres (Prins 2001). Nine studies reported using power calculation to estimate the required sample size prior to subject recruitment.
Comparison groups Studies used between two and four arms to conduct comparisons between groups. Not all arms of all studies were used in this review. Lloyd 1993 used a four arm trial combining psychological therapy with active or placebo immunologic therapy; only the two placebo arms were used in this review. Ridsdale 2004 used three arms comprising CBT, graded exercise therapy (GET), and a 'booklet and usual care' (BUC) group. This last group was described as a prospective cohort (not being randomised at the same time as the other groups), and was not used for comparison in this review.
Setting Eight studies were conducted in the UK, three in the USA, two in The Netherlands, and two in Australia.
Four studies were conducted in a primary care setting (general practice surgeries) (Huibers 2004; Ridsdale 2004; King 1999; Whitehead 2002), and eight in secondary care (hospital outpatients or psychology department clinics). The setting of the remaining three studies was unclear. Two studies by doctoral researchers (Stevens 1999, Strang 2002) recruited subjects from sources such as physicians, psychiatrists, advertisements and local CFS support groups, but did not specify the setting in which intervention occurred. The remaining study (Surawy 2005) did not specify sources of subject recruitment or the setting of intervention.
Participants The combined sample size across the 15 included studies was 1374 participants, of which 1043 participants met the inclusion criteria for this review. In three studies, participants with a diagnosis of CFS rather than chronic fatigue symptoms were selected for inclusion (Huibers 2004; King 1999; Ridsdale 2004). One study included additional treatment arms that were not eligible for the purposes of this review (Lloyd 1993). Ten studies gave reasons why not all patients eligible for inclusion were recruited to the study, of which seven studies reported the total number of patients eligible for inclusion. Only five studies reported the total number of patients assessed for eligibility.
Eleven studies provided extensive demographic information on their samples such as marital and occupational status, as well as age and gender. The remaining four studies (Lloyd 1993, Russell 2001, Whitehead 2002; Surawy 2005) provided only minimal demographic information comprising gender, mean age, or age range.
Age of participants Of the ten studies which stated age criteria for subject recruitment (or the age range of their eventual sample), four studies included participants under the age of 18 years, as well as adult participants. The lower age range of these studies was 17 years for one study (Lloyd 1993), 16 years for two studies (King 1999, Ridsdale 2004), and 15 years for one study (Whitehead 2002). The oldest upper age range was 75 years (King 1999, Ridsdale 2004). Six studies provided the mean age of their sample, and a further eight studies provided the mean ages of comparison groups from which the weighted overall mean age could be calculated. The overall mean age of subjects was 40.9 years in these 14 studies. One study (Surawy 2005) did not provide a mean age of the sample or comparison groups.
Gender composition of participants All studies reported the gender composition of their samples. 70.1% of subjects were female.
Duration of illness Three studies provided the mean duration of illness of their sample, and a further nine studies provided the mean illness duration of comparison groups from which the weighted overall mean illness duration could be calculated. The mean duration of illness in these subjects was 56.5 months (4.7 years).
Inclusion criteria All studies specified inclusion criteria requiring that participants had fatigue as their main or major complaint, the minimum duration of fatigue being six months in 12 studies, at least four months in one study (Huibers 2004), and at least three months in the two remaining studies (King 1999, Ridsdale 2004). There was heterogeneity of other recruitment criteria between studies. Seven studies used the 'CDC' ('Fukuda') criteria (Fukuda 1994) for inclusion. One of these studies (Prins 2001), waived the requirement of four of eight additional symptoms included in the CDC criteria to be present. Three studies (Sharpe 1996, Deale 1996, Surawy 2005) used the 'Oxford' criteria (Sharpe 1991) for participant inclusion. The sample in Deale 1996 fulfilled CDC as well as Oxford criteria. Two studies (Barrett 1992, Lloyd 1993) used the 'Australian' criteria (Lloyd 1988).
Three studies did not use standard CFS criteria. Two of these studies (King 1999, Ridsdale 2004) used a score of at least 4 on the Chalder fatigue scale (Chalder 1993) as the basis for inclusion. The remaining study (Huibers 2004) included subjects who scored 35 or more on the Dutch Checklist Individual Strength; Vercoulen 1999) and who had been completely absent from work for 6‐26 weeks on health grounds. These studies thus had samples suffering from chronic fatigue, of which a sub‐sample met CDC chronic fatigue syndrome criteria (28% in King 1999, 21% in Ridsdale 2004, and 44% in Huibers 2004). Only these subgroups were used in the current review.
Exclusion criteria Twelve studies specified exclusionary criteria, and two (Strang 2002, Whitehead 2002) did not. The remaining study (Russell 2001) did not stipulate any specific exclusion criteria, but did describe an assessment process for "readiness to engage in the rehabilitation process, group suitability, and the presence of other complicating psychological difficulties". It is not, however, clear whether this process resulted in exclusion of subjects. Eight studies excluded subjects with current mental disorder such as depression or bipolar disorder (n=3), psychosis (n=6), somatisation disorder (n=1), substance use (n=2), or who "were at significant risk of suicide or required urgent psychiatric treatment" (n=1). Two studies excluded those on psychiatric medication e.g. antidepressants, unless the dose was stable. Six studies excluded subjects whose symptoms were attributable to a comorbid condition or a diagnosis other than CFS. Other conditions excluding participation included pregnancy (n=2), learning difficulties precluding completion of questionnaires (n=1), organic brain syndromes (n=1), severe obesity (n=1), and asthma or ischaemic heart disease contraindicating a step‐test (n=1).
Other exclusionary criteria were: likely inability to attend all sessions e.g. non ambulatory subjects (n=9), insufficient English language to allow participation (n=4), ongoing physical investigations (n=2), fertility treatment (n=1), previous immunologic therapy (n=1), involvement in other previous or current CFS research (n=1).
Assessment of participants for recruitment In eleven studies, inclusion or exclusion criteria were applied by interviewing the subject to obtain a problem history. Some of these studies also made use of further measures such as structured or semi‐structured psychiatric interviews e.g. SCID (n=5), questionnaires or rating scales (n=7), physical examination of the patient (n=4), and laboratory investigations such as full blood count, biochemistry, autoantibody screening, thyroid function tests, and screening for infectious disease (n=6).
Two studies (Stevens 1999, Strang 2002) used a validated questionnaire as the sole method of determining recruitment eligibility, and two studies (Whitehead 2002, Ridsdale 2004) recruited subjects by referral from general practitioners who had themselves made a diagnosis of CFS using respective study criteria.
Interventions Comparison groups Ten studies compared a CBT model of therapy against a wait list (WL) (n=5) or 'treatment as usual' (TUC) control condition (n=6). Three of these studies (Barrett 1992, Prins 2001, O'Dowd 2000) had additional comparison arms consisting of guided support/education to control for non‐specific effects of therapy. A further study (Lloyd 1993) similarly compared CBT versus a TUC group, but both groups also attended clinic biweekly to receive intramuscular injections of saline as placebo for dialyzable lymphocyte extract (DLE). One study (Jason 2007) compared CBT with three comparison groups comprising cognitive therapy, anaerobic activity therapy, and relaxation therapy. The remaining studies compared CBT with comparison groups comprising relaxation therapy (Deale 1996), psychodynamic counselling (King 1999) and graded exercise therapy (Ridsdale 2004).
Manualisation Seven studies reported using manuals or treatment protocols for the CBT intervention (Deale 1996; Huibers 2004; Jason 2007; King 1999; Ridsdale 2004; Strang 2002; Prins 2001). Three studies reported testing of fidelity to manuals through audio or videotaping a random selection of CBT sessions (Jason 2007; King 1999; Prins 2001) and two studies measured fidelity to manuals through research team meetings (Deale 1996) or through supervision/checking written records (Huibers 2004).
Model of CBT Twelve studies used a 'traditional' model of CBT. Three studies deviated from traditional models of CBT (in terms of underlying theory as opposed to delivery). One of these studies (Stevens 1999) used a chronobiologically‐oriented treatment protocol (Nixon 1995), focusing on "sleep‐hygiene education, biofeedback‐assisted relaxation training, breathing re‐training, and cognitive therapy" (Stevens 1999). Another study (Strang 2002) used an element of personal mastery training (Reich 1981) alongside traditional CBT, and one study (Surawy 2005) used mindfulness‐based stress reduction (MBSR; Kabat‐Zinn 1991) and mindfulness‐based cognitive therapy (Segal 2002) as the basis of its treatment model.
Ten studies (Barrett 1992, Lloyd 1993, Sharpe 1993, Deale 1996, Stevens 1999, Prins 2001, Strang 2002, Whitehead 2002, O'Dowd 2000, Jason 2007) attempted to increase activity and reduce rest time as part of the CBT intervention. Other studies either attempted to tailor the patients' rest and activity towards levels compatible with limitations imposed by fatigue, or made no mention of activity/exercise in their intervention.
Delivery of intervention Ten studies delivered therapy on a one‐to‐one therapist to participant basis, including one study (Lloyd 1993) that invited subjects to bring a close family member with them, and a further study (Whitehead 2002) that used a booklet and diary keeping in addition to one‐to‐one GP contact. Five studies used a group therapy format, with group sizes of 8 or fewer subjects (Russell 2001), 8‐10 subjects (Strang 2002), 8‐12 subjects (O'Dowd 2000) or of unspecified size (Barrett 1992, Surawy 2005).
Therapist characteristics In six studies, therapists conducting CBT were clinical psychologists, psychiatrists, and/or trained cognitive (‐behavioural) therapists. One study (Barrett 1992) used a doctoral level psychologist as therapist, after an initial session with a psychiatrist. Two other studies (Stevens 1999, Strang 2002) also used doctoral level psychologists or their trained assistants to delivery therapy. Two studies (Whitehead 2002, Huibers 2004) used GPs to deliver therapy. One study (Jason 2007) used trained nurses with experience of delivering psychotherapy. Three studies did not specify the nature and training of therapists.
Supervision and protocol fidelity checks Six studies (Deale 1996, Prins 2001, King 1999, Huibers 2004, Ridsdale 2004, Jason 2007) specified that the therapists were supervised, and a further study (Whitehead 2002) offered telephone support to GP therapists, but this did not amount to formal supervision. Four studies (Prins 2001, King 1999, Huibers 2004, Jason 2007) assessed treatment fidelity (e.g. through blind assessment of video‐taped sessions by experienced therapists), and a further study (Deale 1996) stated that regular meetings were held to ensure sessions adhered to the treatment protocol.
Total, frequency and length of intervention sessions The frequency of sessions ranged from weekly in five studies (Barrett 1992, Sharpe 1993, Deale 1996, Stevens 1999, Surawy 2005), once every two weeks (Lloyd 1993, Prins 2001, Ridsdale 2004, O'Dowd 2000, Jason 2007), or with a variable gap between sessions not exceeding three weeks (Deale 1996, Strang 2002, Whitehead 2002, Huibers 2004). One study (King 1999) did not specify the frequency of sessions.
The minimum number of sessions specified was five to seven (Huibers 2004), and the maximum was sixteen (Sharpe 1993, Prins 2001), though in one study (Whitehead 2002) participants were asked to visit their GPs for therapy weekly or once every two weeks over 12 months.
Session length ranged from a minimum of 30 minutes (Huibers 2004) to a maximum of 150 minutes in a group format including a 30 minute break (Russell 2001). The modal length of session was 60 minutes. One study did not specify the length of treatment sessions (Surawy 2005), and in a further study (Whitehead 2002) session length was set by the treating GPs.
For the eleven studies in which total protocol treatment time could be determined, the minimum time was 4½ hours (Ridsdale 2004), and the maximum time 30 hours (including breaks) (Russell 2001). The mean total treatment specified in these studies' protocols was approximately 13 hours. In the five studies (Prins 2001, King 1999, Huibers 2004, Ridsdale 2004, Jason 2007) reporting actual treatment adherence across conditions, the mean time spent in CBT or control treatment was approximately eight hours.
Non‐intervention elements of care programmes Of the five studies (Deale 1996, Stevens 1999, Prins 2001, Huibers 2004, O'Dowd 2000) reporting whether non‐intervention aspects of treatment protocols were similar, only one (Prins 2001) noted differences (forbidding participants in the CBT condition to have physical investigations related to chronic fatigue syndrome during the intervention, but allowing participants in the 'guided support' and 'natural course' conditions to do so). Five studies explicitly stated that psychiatric medication e.g. antidepressants and anxiolytic drugs, was not forbidden by protocol. A further study (Prins 2001) noted that other treatments for CFS were forbidden by trial protocol (but made no mention of psychiatric medication). One study (Sharpe 1993) noted that two participants (7%) in its TUC condition received supportive psychotherapy, and that one participant (3%) in the CBT condition received counselling as part of vocational training. A further study (Huibers 2004) noted that 18% of CBT and 31% of TUC participants received "psychosocial co‐interventions" during the intervention period.
Outcomes A wide range of outcomes were used in the included studies. These include measures of this review's primary outcome (fatigue), as well as secondary outcomes such as physical functioning, depression, anxiety, acceptability of treatment, quality of life, occupational outcome, treatment compliance, dropout rate, health service resource use, illness attribution, and adverse effects. A range of rating methods were used including self‐report questionnaires (e.g. Chalder fatigue scale; Chalder 1993), observer‐rated outcomes (e.g. Karnofsky performance status scale; Karnofsky 1948) and physiological measures (e.g. step test, white cell count).
The most common measure of fatigue was the Chalder fatigue scale, followed by the Profile of Mood States (POMS) fatigue scale (McNair 1992). The most common measures of physical functioning were the Karnofsky scale and physical functioning subscale of the Medical Outcomes Survey (MOS) SF‐36 scale (Ware 1993). Commonly used measures of depression included the Beck Depression Inventory (Beck 1961), and Hospital Anxiety and Depression Scale (HADS; Zigmond 1983), the latter also being the most common measure of anxiety along with the Beck Anxiety Inventory (Beck 1990). Quality of life was most often measured by the SF‐36 in its entirety (including physical and mental functioning subscales). Other outcomes had more heterogeneous measures.
Six studies (Sharpe 1993, Deale 1996, Prins 2001, Huibers 2004, Ridsdale 2004, O'Dowd 2000) used pre‐determined criteria for successful outcomes on major measures which were deemed to represent clinical improvement. These outcomes were either of physical functioning (n=4) and/or fatigue (n=2).
Eight studies detailed dropout rates during treatment. Five studies reported dropout rates across the duration of the trial including follow‐up. One study (Whitehead 2002) presented data as to how many patients were using activity diaries at different points of follow‐up. One study reported only the mean dropout rate across all conditions (Jason 2007).
Excluded studies
CCDANCTR‐Studies and CCDANCTR‐Refs searches identified two studies (Friedberg 1994; Burgess 2001) that could not be immediately excluded by perusal of title or abstract, but were deemed not to meet the inclusion criteria for the review after scrutinising the full articles. Another ten studies identified through supplementary searches or personal communication with experts in the field were excluded after obtaining the full articles (Allen 2006, Bleichhardt 2004, Butler 1991, Candy 2004, Donta 2003, Gielissen 2006, Goudsmit 1996, Lyles 2003, Soderberg 2001, Taylor 2004). These are detailed in the table entitled Characteristics of Excluded Studies, with reasons for their exclusion from the updated version of this review.
Ongoing studies Searches of CCDANCTR‐Studies and CCDANCTR‐Refs identified a total of six ongoing studies (Wearden 2006, White 2005; Bleijenberg 2008; Carney 2008; Gibson‐Saxty 2002; Vissers 2008) that appear to meet the inclusion criteria for this review. No findings have yet been reported or obtained from these studies.
Studies awaiting assessment Two studies were identified which may fulfil review criteria but could not be included in the present review. The first study (Chalder 1997) randomised participants to either a self‐help booklet with specific advice (incorporating cognitive behavioural elements), or a no treatment control group. Participants were primary care patients complaining of fatigue who had previously presented with a possible viral illness. Inclusion criteria were deliberately broad, but included a subgroup of participants who met 'Oxford' criteria for CFS (12% of sample). However, the review authors have as yet been unable to obtain this subgroup data for inclusion in the review.
The second study (Powell 2001), compared "evidence based‐explanation of symptoms that encouraged graded activity" against standardised medical care. Delivery of intervention was in three groups (minimum, telephone, and maximum intervention groups) with varying amounts of face‐to‐face contact. Whilst each intervention included some cognitive‐behavioural principles, the review authors have been unable to establish with the study authors which, if any, of these interventions might satisfy inclusion criteria for this review.
Risk of bias in included studies
The methodological quality of the 15 studies included in the review was classified according to the Cochrane Collaboration's tool for assessing the risk of bias. The risk of bias in each of the six domains of validity (sequence generation, allocation concealment, blinding of participants/personnel/outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias) was rated independently by two review authors as 'high', 'low' or 'unclear', and differences between authors were reconciled. The final ratings are reported in the Characteristics of Included Studies table. A risk of bias summary graph (Figure 1) and summary figure (Figure 2) are also presented.
1.
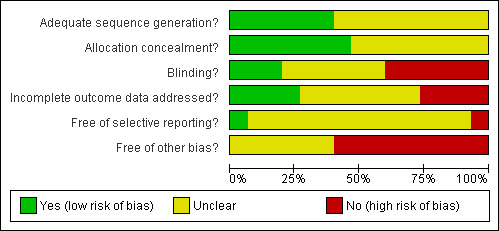
Risk of bias graph: review authors' judgement about each risk of bias item presented as percentages across all included studies
2.
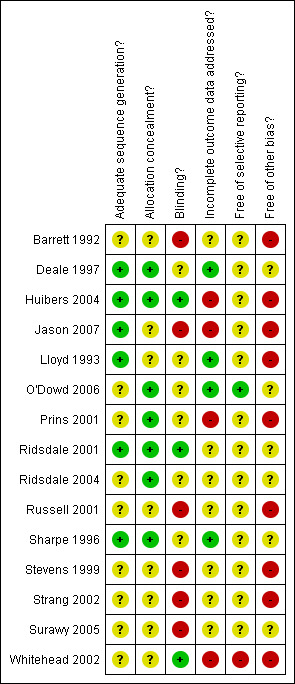
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation Whilst all studies specified that participants were randomly allocated to conditions (or GP practices randomised to treatment or control conditions in the case of Whitehead 2002), only seven studies specified the method of generating the sequence leading to random allocation, whether by computer (n=3) or random number table (n=4). No study specified a sequence generation method that was quasi‐ or non‐random, therefore these seven studies received a 'low' risk of bias rating for sequence generation, with the remaining eight studies receiving a rating of 'uncertain'.
Eight studies reported using blocked randomisation, and four studies stratified their samples, by presence of major depressive disorder (Sharpe 1993), by source of referral (Deale 1996), by length of work absence (Huibers 2004), or by duration and severity of fatigue (Ridsdale 2004). Allocation concealment Method of concealing assignment to condition from investigators and participants was described by seven studies through the use of numbered/opaque envelopes (variously described as being opened in front of the participant, immediately before his/her first session, or being handled by third parties).
Nine studies conducted preliminary univariate analyses of demographic and historical characteristics to check that randomisation had resulted in appropriate comparability of groups for demographic characteristics, with six reporting differences between groups. Sharpe 1993 reported that significantly fewer CBT participants were actively employed, and spent significantly more days in bed than TUC subjects. Deale 1996 reported that CBT participants were on average seven years older than the relaxation group. A greater number of the CBT group in Strang 2002 received disability compensation than the wait list group, while the CBT patients in Whitehead 2002 were younger and had a shorter duration of illness than their TUC counterparts. CBT subjects in Ridsdale 2004 had a longer median duration of fatigue than GET participants. In O'Dowd 2000, the CBT group had twice as many males as the other two arms.
Ten studies reported baseline differences between groups on outcome variables, either explicitly or via inference from confidence intervals in data tables. Of these ten studies, five reported that pre‐treatment differences did indeed exist. Stevens 1999 reported their CBT group had more morning insomnia, awoke more at night, had worse SF‐36 scores, and outperformed the WL group on step‐testing. The CBT participants in Whitehead 2002 were significantly more fatigued as measured by the Chalder fatigue scale. TUC subjects in Huibers 2004 were significantly more distressed as measured by the SCL‐90 scores but had better physical functioning as measured by MOS SF‐36 scores, whilst the CBT subjects in Ridsdale 2004 were more fatigued on the Chalder fatigue scale. CBT and anaerobic activity therapy groups in Jason 2007 had higher scores on a self‐efficacy measure than subjects in the relaxation therapy condition.
Blinding
One trial (O'Dowd 2000) stated that "both the participants and those administering the assessments were unaware of which cohort the subject was in", and indeed described its protocol as a "double‐blind randomised controlled trial". The only formal test of participant blinding to treatment group was in the case of active or placebo DLE injection in Lloyd 1993 (which described itself as a 'double blind, randomised, placebo controlled study'), which showed participants estimations of whether they received active or placebo DLE bore no significant correlation with actual treatment received. Only one study (Huibers 2004) specifically mentioned that researchers and treatment providers were not blinded to treatment allocation.
Of the four studies comparing CBT with other psychological/exercise therapy interventions, only two (King 1999 and Jason 2007) assessed treatment fidelity. Of these, one study (King 1999) used four independent assessors of recorded sessions and found differentiation in the management approaches of the two groups according to specified protocol. The other (Jason 2007) used a licensed and masters level psychologist (who had acceptable inter‐rater reliability) to rate a random sample of sessions according to therapists' use of techniques unique to the four treatment conditions, and indeed found the conditions were discriminant according to corresponding treatment manuals. A third study (Deale 1996) did not formally assess treatment fidelity, but did hold regular meetings to ensure protocol adherence. Two other studies (Prins 2001, Huibers 2004) also assessed treatment fidelity, not to differentiate CBT from other psychological or graded exercise treatment, but to check quality of CBT intervention.
The outcome variables in ten studies were assessed by self‐report scales or were based on physiological data (e.g. step‐test, white cell count). The remaining five studies included outcome measures that were based on observer assessment. Three of these studies used the Karnofsky scale. Of these, one study (Prins 2001) used "an independent clinical psychologist" to rate Karnofsky score who by this description is presumed to have been blind. Another study (Sharpe 1993) reported using a blinded researcher to rate participants Karnofsky status, on the basis of an interview made with participants by another researcher; however, the blinding status of the researcher conducting the interview was not specified. The remaining study to use the Karnofsky scale (Lloyd 1993) made no mention of blinding of assessor.
Two further studies used assessors to rate the change of participants on clinical dimensions. One study (Jason 2007) included a clinician global impression of change in the patient's overall functioning. No mention of blinding to treatment group was made. One study (Deale 1996) included an assessor rating of participants' degree of fatigue and disability through a structured interview. The assessors for this outcome were blinded through no test of blind was made.
Incomplete outcome data
Dropout rates Whilst not all studies differentiated between dropouts during therapy and those lost to follow‐up assessment, 14 studies reported dropout rates between baseline and end of treatment (or first post‐treatment assessment) by comparison group. The remaining study (Jason 2007) reported a mean drop‐out rate across all conditions.
Studies used varying criteria to define a dropout. For example, Ridsdale 2004 used a conservative estimate of failing to complete all six of the sessions of therapy specified in the study protocol, whereas Jason 2007 reports a participant was deemed to have dropped out if they completed four or fewer sessions out of 13 specified. Some studies (e.g. O'Dowd 2000) defined dropouts not on the basis of intervention sessions attended, but instead on whether participants attended follow‐up assessment or not. Another study (Prins 2001) used different criteria for dropout between groups; in the TUC group, only participants not attending assessments were classified as dropouts. In the CBT group, stopping coming to therapy was defined as a dropout, whereas in the guided support group, frequent non‐attendance was not classified as a dropout (a dropout was only counted if the participant formally declared an intention to withdraw). Whitehead 2002 reported dropouts as those unavailable for assessment at six months (31% of subjects). However, over 70% of subjects had terminated use of their treatment diaries or GP contact by that time.
The mean aggregate reported dropout rate of all 15 studies was 16.4%. Dropout rates ranged from 0% (Sharpe 1993) to 40% (Whitehead 2002), with six studies (Prins 2001, King 1999, Strang 2002, Whitehead 2002, Ridsdale 2004, Jason 2007) reporting dropout rates of over 20%. For the 14 studies reporting dropout rates by comparison group, the mean dropout rate for the CBT intervention was 16.8%. Of the four studies comparing CBT with another active psychological/graded exercise therapy, only one (Jason 2007) detailed that there were no differences between dropout rates of comparison groups (mean 25%). Dropout rates were broadly similar in the other three studies: 10% CBT vs 13.3% relaxation (Deale 1996); 36.3% CBT vs. 31.3% counselling (King 1999); and 28.6% versus 40% graded exercise therapy (Ridsdale 2004). Dropout rates between comparison groups also appeared broadly similar in the three studies comparing CBT with a guided support/education arm: 6.7% CBT versus 0% education and support (Barrett 1992); 36.6% CBT versus 30.9% guided support (Prins 2001); and 5.8% versus 10% education and support (O'Dowd 2000).
Reasons for dropouts Five studies (Barrett 1992, Lloyd 1993, Deale 1996, Stevens 1999, Whitehead 2002) reported reasons for dropouts during treatment by comparison group. In none of these studies did the reasons for dropout between groups appear dissimilar. A further study (Huibers 2004) reported reasons for dropout by CBT group but not for any other comparison group, and two other studies (King 1999, Ridsdale 2004) gave aggregate reasons for dropout not detailed by comparison groups. Four studies (Deale 1996, King 1999, Huibers 2004, Ridsdale 2004) investigated differences between dropouts and completers on demographic and baseline variables.
Method of dealing with missing data Five studies (Lloyd 1993, Prins 2001, Russell 2001, Whitehead 2002, O'Dowd 2000) did not provide means and/or standard deviation (SD) data for outcome variables. Baseline data for completers was also not inferable from the data presented by King 1999. Where data was required for this review, authors were contacted. Data were received from the authors of Prins 2001 (with the exception of two variables which were unobtainable) and King 1999.
Eight studies reported how they dealt with missing data (from dropouts or due to another reason such as inability/unwillingness to complete a walking test). Seven of these studies (Sharpe 1993, Deale 1996, Prins 2001, King 1999, Huibers 2004, Ridsdale 2004, O'Dowd 2000) presented sensitivity analyses (examining any differential effect of carrying forward data, using multiple value imputation, or abolishing dropouts from final analysis) or employed a full 'intention to treat' analysis carrying forward previous data values or assuming a negative outcome for dropouts.
Selective reporting
Only one study (O'Dowd 2000) made reference to deviation from its trial protocol, and only one study (Barrett 1992) specified an outcome variable which it did not report (delayed type hypersensitivity skin‐test). Two other studies indicated outcomes for which they did not then provide data (SF‐36 in King 1999; HADS in Whitehead 2002).
Whilst Lloyd 1993 collected data concerning the adverse effects of DLE injection, data referring to adverse effects of psychological treatment was not systematically presented by any study. One study (Sharpe 1993) noted reasons given by subjects for any deterioration during treatment, with two of four CBT participants who were subjectively 'worse' or 'much worse' after treatment blaming the treatment for deterioration. Huibers 2004 noted that 'no adverse event attributable to CBT was reported'. Jason 2007 reported numbers of participants who endorsed 'worse' or 'unchanged' to a 'Treatment made me feel' statement, but did not differentiate between the two categories or further investigate reasons, making inference of adverse effects impossible.
Other potential sources of bias
Five studies (Barrett 1992, Stevens 1999, Russell 2001, Strang 2002, Surawy 2005) made use of a wait‐list control condition, a design feature that may affect the natural course of fatigue or other symptoms, either in a directive direction through the implicit suggestion that recovery should be placed 'on hold' until treatment, or in a positive direction, through anticipated 'rescue' by treatment.
Less than half of the included studies reported the use of manuals or treatment protocols to standardise the CBT provided, and only three studies reported testing treatment fidelity through assessment of a random selection of video or audio tapes of sessions (King 1999; Jason 2007; Prins 2001), thus introducing uncertainty that therapists provided and adhered to CBT interventions in sessions.
Effects of interventions
Of 15 studies included in the review, 12 studies contributed data to primary and secondary outcomes, and three studies contributed to the secondary outcome of dropout only. Statistical heterogeneity was examined for each outcome, and where indicated to be statistically significant, I2 figures were reported in the text. The fixed effects model was used for all outcomes unless otherwise stated in the text. Subgroup analyses of the primary outcome were conducted where data from at least two studies were available for each sub‐group.
COMPARISON 01: COGNITIVE BEHAVIOUR THERAPY vs USUAL CARE Nine studies contributed to this comparison at post‐treatment (Prins 2001, Huibers 2004, O'Dowd 2000, Barrett 1992, Strang 2002, Lloyd 1993, Russell 2001, Whitehead 2002) with an additional study providing data at follow‐up (Sharpe 1993). The studies by Lloyd 1993, Russell 2001 and Whitehead 2002 provided post‐treatment data for the secondary outcome of attrition only. For the purposes of this comparison, short and medium‐term follow‐up outcomes were combined.
Primary outcome Seven studies contributed data to the primary outcome meta‐analysis. Measures used to assess fatigue symptoms and clinical response comprised the fatigue subscale of the CIS (Huibers 2004, Prins 2001), Chalder Fatigue Scale (O'Dowd 2000, Surawy 2005), POMS fatigue scale (Barrett 1992), Fatigue Severity Scale (Strang 2002) and a single‐item fatigue Likert scale (Sharpe 1993).
1) Reduction in fatigue severity Post treatment (Graph 01 01) Five traditional CBT studies and one mindfulness/compassion‐focused CBT study (373 participants in total) contributed to this outcome. The difference in fatigue mean scores between the CBT group and usual care group was highly significant in favour of the CBT group (SMD ‐0.39, 95% CI ‐.0.60 to ‐0.19). No statistical heterogeneity was indicated.
Short/medium term follow up (Graph 01 09) Four traditional CBT studies (330 participants in total) contributed to this outcome. The difference in fatigue severity mean scores between the CBT and usual care group was highly significant in favour of CBT (SMD ‐0.47, 95%CI ‐0.69 to ‐0.25). No statistical heterogeneity was indicated.
2) Clinical response Post treatment (Graph 01 02) Four studies (371 participants) contributed to this outcome. A total of 40% of participants in the CBT group showed clinical response to treatment, in contrast with 26% in the treatment as usual care group. The difference between the two groups was highly significant (OR 0.47, 95% CI 0.29 to 0.76). No statistical heterogeneity was indicated.
Short term follow up (Graph 01 10) Three studies (353 participants) contributed to this outcome. No significant difference in clinical response rates was indicated between the CBT and usual care groups (OR 1.03, 95% CI 0.53 to 2.00). Some heterogeneity was indicated (I2=40%), and a random effects model was used.
Secondary outcomes
1) Improvement in physical functioning Post treatment (Graph 01 03) Three traditional CBT studies and one mindfulness/compassion‐focused CBT study (318 participants in total) contributed to this outcome. Measures used to assess physical functioning comprised Karnofsky performance status scale (one study) and SF‐36 physical functioning subscale (three studies). The difference in physical functioning mean scores between the CBT group and the usual care group was not significant (SMD 0.11, 95%CI ‐0.32 to 0.54). Significant heterogeneity was indicated (I2 = 68%) and a random effects model was used.
Short/medium term follow‐up (Graph 01 11) Three traditional CBT studies (275 participants) contributed to this outcome. The difference in physical functioning mean scores between the CBT group and the usual care group was not significant (SMD 0.15, 95%CI ‐0.21 to 0.51). Significant heterogeneity was indicated (I2 = 53%) and a random effects model was used.
2) Reduction in depression symptoms Post treatment (Graph 01 04) Three traditional CBT studies and one mindfulness/compassion focused CBT study (183 participants in total) contributed to this outcome. Measures used to assess depression symptoms comprised the depression subscale of the HADS (two studies) and BDI (two studies). The difference in depression mean scores between the CBT group and the usual care was not significant (SMD ‐0.24, 95%CI ‐0.53 to 0.05). No statistical heterogeneity was indicated.
Short/medium term follow‐up (Graph 01 12) Two traditional CBT studies (149 participants) contributed to this outcome. Both studies used the HADS depression subscale to measure depression symptoms. The difference in depression mean scores between the CBT group and the usual care group was significant (MD ‐1.39, 95%CI ‐2.60 to ‐0.18). No significant heterogeneity was indicated.
3) Reduction in anxiety symptoms Post treatment (Graph 01 05) Three traditional CBT studies and one mindfulness/compassion focused CBT study (183 participants in total) contributed to this outcome. Measures used to assess anxiety symptoms comprised the anxiety subscale of the HADS (two studies), BAI (one study) and the STAI (one study). The difference in anxiety mean scores between the CBT group and usual care was significant (SMD ‐0.30, 95%CI ‐0.59 to ‐0.01). No statistical heterogeneity was indicated.
Short/medium term follow‐up (Graph 01 13) Two traditional CBT studies (149 participants in total) contributed to this outcome. Both studies used the HADS anxiety subscale of HAD to measure anxiety symptoms. The difference in anxiety mean scores between the CBT group and the usual care group was not significant (MD ‐1.38, 95% CI ‐2.91 to 0.16). No statistical heterogeneity was indicated.
4) Reduction in psychological distress Post treatment (Graph 01 06) Three traditional CBT studies (191 participants in total) contributed to this outcome. Measures used to assess psychological distress symptoms comprised the SCL‐90 (one study), the GHQ (one study) and a single item Likert scale of distress (one study). The difference in mean scores between the CBT group and usual care did not reach significance (SMD ‐0.27, 95%CI ‐0.56 to 0.01). No significant heterogeneity was indicated.
Short term follow‐up (Graph 01 14) One traditional CBT study (61 participants) contributed to this outcome. The difference in psychological distress mean scores between the CBT group and the usual care group was not significant (MD ‐0.24, 95% CI ‐28.95 to 28.37).
5) Improvement in quality of life Post treatment (Graph 01 07) One traditional CBT study (184 participants) contributed to this outcome. The measure used to assess improvement in quality of life comprised the Euro‐Qol. The difference in QoL improvement rates between the CBT group and the usual care group was not significant (OR 1.19, 95%CI 0.58 to 2.46).
Short term follow‐up (Graph 01 15) One traditional CBT study (125 participants) contributed to this outcome. The difference in QoL mean scores between the CBT group and the usual care group was significant (MD 8.00, 95% CI 0.68 to 15.32).
6) Acceptability of treatment No studies contributed to this outcome at post treatment or follow‐up.
7) Adverse effects No studies contributed to this outcome at post treatment or follow‐up.
8) Overall dropout rates Post treatment (Graph 01 08) Eight traditional CBT studies and one mindfulness/compassion‐focused study (586 participants in total) contributed data to this outcome. The attrition rate was 20.6% in the CBT group and 13.9% in the usual care group. The difference in attrition rate between the CBT group and the usual care group was significant in favour of usual care (OR 1.70, 95% CI 1.10 to 2.63). No statistical heterogeneity was indicated.
Short/medium term follow‐up (Graph 01 16) Four traditional CBT studies (413 participants in total) contributed data to this outcome. One study had no dropouts. The attrition rate was 22.7% in the CBT group and 14.8% in the usual care group. The difference was significant in favour of the usual care group (OR 1.46, 95% CI 0.52 to 4.10). Statistical heterogeneity was indicated (I2 = 56%) and a random effects model was used.
9) Occupational outcomes Short/medium term follow‐up (Graph 01 17 and Graph 01 18) Two traditional CBT studies contributed data to this outcome. They recorded occupational outcome in quite different ways, and therefore their results were not combined. One study (61 participants) reported the number of days of absenteeism during the 12‐month follow‐up period. The difference between the CBT group and usual care group was not significant (MD 4.35, 95% CI ‐50.06 to 58.76) (Graph 01 17). The other study (60 participants) reported improvement in work status at 12 months. The difference between the CBT group and the usual care group was significant (RR 3.17, 95% CI 1.47 to 6.81).
10) Health service resource use No studies contributed data on health service resource use.
Sub‐group analyses Subgroup analyses were conducted for the outcome of reduction in fatigue symptoms at post treatment.
a) Individual vs group therapy (Graph 05 01) Two studies used an individual CBT modality and four studies used group CBT. A highly significant difference in effect was shown for individual therapy and a significant difference of lesser magnitude was shown for group therapy when compared with usual care.
b) Treatment as usual vs waiting list (Graph 05 02) Three studies used a treatment as usual condition and three studies used a waiting list as the control condition. A highly significant difference in effect was shown for the CBT group when compared with treatment as usual. In contrast a non‐significant difference in effect was shown for the CBT group when compared with the waiting list condition.
c) Increased activity as component of CBT (Graph 05 03) Four studies incorporated an increased activity component into the CBT intervention and two studies tailored activity and rest to the needs of the individual or did not specify an activity schedule. A highly significant difference in effect was shown for the CBT group in which increased activity was incorporated when compared with usual care. In contrast, a non‐significant difference in effect was shown for the studies in which increased activity was not part of the CBT intervention when compared with usual care.
d) Number of sessions (Graph 05 04) Four studies offered eight or fewer sessions of CBT, and two studies offered more than eight sessions of CBT. A highly significant difference in effect of similar magnitude was shown for both CBT groups when compared with usual care.
COMPARISON O2: COGNITIVE BEHAVIOURAL THERAPY vs OTHER PSYCHOLOGICAL THERAPIES Four studies contributed to this comparison at post‐treatment (Barrett 1992, Deale 1996, Prins 2001, O'Dowd 2000), with two additional studies contributing data at short‐term (King 1999) and medium term (Jason 2007) follow‐up. The studies compared a traditional CBT approach with relaxation (Deale 1996, Jason 2007), counselling (King 1999), guided support (Prins 2001) and education and support (O'Dowd 2000, Barrett 1992).
Primary outcome All six studies contributed data to the primary outcome meta‐analysis. Measures used to assess fatigue symptoms and clinical response comprised the fatigue subscale of the CIS (Prins 2001), Chalder Fatigue Scale (O'Dowd 2000, Deale 1996, King 1999), POMS fatigue scale (Barrett 1992) and Fatigue Severity Scale (Jason 2007).
1) Reduction in fatigue severity Post treatment (Graph 02 01) Four studies (313 participants in total) contributed to this outcome. The difference in fatigue mean scores between the CBT group and other psychological therapies group was highly significant in favour of the CBT group (SMD ‐0.43, 95% CI ‐0.65 to ‐0.20). No statistical heterogeneity was indicated.
Short term follow up (Graph 02 09) Four studies (294 participants in total) contributed to this outcome. The difference in fatigue severity mean scores between the CBT group and other psychological therapies group was significant in favour of CBT (SMD ‐0.47, 95%CI ‐0.83 to ‐0.11). Significant heterogeneity was indicated (I2 = 54%) and a random effects model was used.
2) Clinical response Post treatment (Graph 02 02) Three studies (349 participants in total) contributed to this outcome. A total of 48% of participants in the CBT group showed clinical response to treatment, in contrast with 27% in the other psychological therapies group. The difference between the two groups was highly significant in favour of CBT (OR 0.35, 95% CI 0.19 to 0.62). No statistical heterogeneity was indicated.
Short/medium term follow up (Graph 02 10) Three studies (219 participants in total) contributed to this outcome. The difference in clinical response rates between the CBT and other psychological therapies groups was not significant (OR 0.39, 95% CI 0.14 to 1.12). Statistical heterogeneity was indicated (I2=59%) and a random effects model was used.
Long‐term follow‐up One study (60 participants in original study, 53 participants in follow‐up study) contributed to this outcome. A total of 13/25 (52%) of participants in the CBT group no longer met the UK diagnostic criteria for CFS, in comparison with 11/28 (39%) participants in the other psychological therapies group, who had received relaxation (Fisher's exact test p=0.42). A total of 6/25 (24%) participants in the CBT group had made a complete recovery in comparison with one participant (4%) in the relaxation group (Fisher's exact test p=0.02).
Secondary outcomes
1) Improvement in physical functioning Post treatment (Graph 02 03) Three studies (286 participants in total) contributed to this outcome. Measures used to assess physical functioning comprised the Karnofsky performance status scale (one study) and SF‐36 physical functioning subscale (two studies). The difference in physical functioning mean scores between the CBT group and the other psychological therapies group was significant (SMD 0.53, 95%CI 0.10 to 0.97). Significant heterogeneity was indicated (I2 = 68%) and a random effects model was used.
Short term follow‐up (Graph 02 11) Three studies (257 participants in total) contributed to this outcome. The difference in physical functioning mean scores between the CBT group and the other psychological therapies group was highly significant (SMD 0.71, 95%CI 0.27 to 1.14). Statistical heterogeneity was indicated (I2 = 64%) and a random effects model was used.
2) Reduction in depression symptoms Post treatment (Graph 02 04) Three studies (175 participants in total) contributed to this outcome. Measures used to assess depression symptoms comprised the depression subscale of the HADS (one study) and BDI (two studies). The difference in depression mean scores between the CBT group and other psychological therapies group was significant (SMD ‐0.46, 95%CI ‐0.76 to ‐0.16). No statistical heterogeneity was indicated.
Short term follow‐up (Graph 02 12) Three studies (257 participants in total) contributed to this outcome. The difference in depression mean scores between the CBT group and the other psychological therapies group was non‐significant (SMD ‐0.03, 95%CI ‐0.50 to 0.44). Statistical heterogeneity was indicated (I2 = 57%) and a random effects model was used.
3) Reduction in anxiety symptoms Post treatment (Graph 02 05) Two studies (122 participants in total) contributed to this outcome. Measures used to assess anxiety symptoms comprised the anxiety subscale of the HADS (one study) and the STAI (one study). The difference in anxiety mean scores between the CBT group and other psychological therapies group was significant (SMD ‐0.41, 95%CI ‐0.71 to ‐0.11). No statistical heterogeneity was indicated.
Short term follow‐up (Graph 02 13) Two studies (124 participants in total) contributed to this outcome. The difference in anxiety mean scores between the CBT group and the other psychological therapies group was not significant (SMD ‐0.03, 95% CI ‐2.96 to 3.02). Statistical heterogeneity was indicated (I2 = 66%) and a random effects model was used.
4) Reduction in psychological distress Post treatment (Graph 02 06) Three studies (175 participants in total) contributed to this outcome. Measures used to assess psychological distress symptoms comprised the GHQ (two studies) and a single item Likert scale of distress (one study). The difference in mean scores between the CBT group and other psychological therapies was significant (SMD ‐0.41, 95%CI ‐0.71 to ‐0.11). No statistical heterogeneity was indicated.
Short term follow‐up (Graph 02 14) One study (88 participants) contributed to this outcome. The difference in psychological distress mean scores between the CBT group and the other psychological therapies group was significant (MD ‐3.60, 95% CI ‐7.07 to ‐0.13).
5) Improvement in quality of life Post treatment (Graph 02 07) One study (124 participants) contributed to this outcome. The measure used to assess improvement in quality of life comprised the Euro‐Qol. The difference in QoL mean scores between the CBT group and the other psychological therapies group was significant (MD 10.00, 95%CI 3.43 to 16.57).
Short term follow‐up (Graph 02 15) One study (116 participants) contributed to this outcome. The difference in QoL mean scores between the CBT group and the other psychological therapies group was highly significant (WMD 13.00, 95% CI 5.48 to 20.52).
6) Acceptability of treatment a) Satisfaction with care/therapy (short‐term follow‐up) One study contributed to this outcome (Deale 1996). A total of 21/27 participants (78%) reported being satisfied or very satisfied with the treatment outcome, in comparison with 13/26 (50%) in the other psychological therapies group (relaxation) (Chi‐square 2.1, p<0.05).
b) Dropout due to adverse effects No studies contributed to this outcome at post treatment or follow‐up.
7) Adverse effects No studies contributed to this outcome at post treatment or follow‐up.
8) Overall dropout rates Post treatment (Graph 02 08) Four studies (377 participants in total) contributed data to this outcome. The attrition rate was 24.7% in the CBT group and 20.3% in the other psychological therapies group. The difference in attrition rate between the two groups was not significant (OR 1.34, 95% CI 0.81 to 2.20). No statistical heterogeneity was indicated.
Short term follow‐up (Graph 02 16) Four studies (394 participants in total) contributed data to this outcome. The attrition rate was 27.2% in the CBT group and 23.6% in the other psychological therapies group. The difference in attrition rate between the two groups was not significant (OR 1.23, 95% CI 0.76 to 1.97). No statistical heterogeneity was indicated.
9) Occupational outcomes No studies contributed to this outcome at post treatment or follow‐up.
10) Health service resource use No studies contributed to this outcome at post treatment or follow‐up.
COMPARISON 03: COGNITIVE BEHAVIOURAL THERAPY vs EXERCISE One study contributed data to this comparison at post‐treatment (Ridsdale 2004) and one study contributed data to this comparison at follow‐up (Jason 2007).
Primary outcome Both studies contributed data to the primary outcome meta‐analysis. Measures used to assess fatigue symptoms comprised the Chalder Fatigue Scale (Ridsdale 2004) and the Fatigue Severity Scale (Jason 2007).
1) Reduction in fatigue severity Post treatment (Graph 03 01) One study (36 participants) contributed to this outcome. The difference in fatigue mean scores between the CBT group and exercise group was not significant (MD ‐2.52, 95% CI ‐7.34 to 2.30).
Follow‐up No studies contributed data to this outcome
2) Clinical response Post treatment (Graph 03 02) One study (36 participants) contributed data to this outcome. The difference in clinical response rates between the CBT and exercise groups was not significant (OR 0.47, 95% CI 0.10 to 2.17).
Medium term follow up (Graph 03 03) One study (57 participants) contributed data to this outcome. The difference in clinical response rates between the CBT and exercise groups was not significant (OR 0.10, 95% CI 0.01 to 1.94).
Secondary outcomes No studies contributed data to secondary outcomes at post treatment or at follow‐up.
COMPARISON O4: COGNITIVE BEHAVIOURALTHERAPY COMBINED WITH OTHER INTERVENTIONS VS USUAL CARE One study contributed to this comparison at post‐treatment (Stevens 1999), and compared CBT combined with biofeedback, sleep hygiene, relaxation and aerobic exercise against usual care.
Primary outcome One study contributed towards the primary outcome meta‐analysis. The measure used to assess fatigue symptoms comprised the POMS fatigue scale.
1) Reduction in fatigue severity Post treatment (Graph 04 01) One study (27 participants) contributed to this outcome. The difference in fatigue mean scores between the combination CBT group and the usual care group was significant in favour of the combination CBT group (WMD ‐7.12, 95%CI ‐12.83 to ‐1.41).
Follow‐up No studies contributed data to this outcome.
2) Clinical response No studies contributed data to this outcome at post treatment or follow‐up.
Secondary outcomes
1) Improvement in physical functioning Post treatment (Graph 04 02) One study (27 participants) contributed to this outcome. The measure used to assess physical functioning comprised the SF‐36 physical functioning subscale. The difference in physical functioning mean scores between the combination CBT group and usual care group was significant (MD 8.38, 95%CI 0.99 to 15.78).
Follow‐up No studies contributed data to this outcome
2) Overall dropout rates Post treatment (Graph 04 03) One study (29 participants) contributed data to this outcome. The difference in attrition rate between the two groups was not significant (OR 0.16, 95% CI 0.01 to 3.68).
Follow‐up No studies contributed data to this outcome
Other secondary outcomes No studies contributed data to other secondary outcomes at post treatment or follow‐up.
Consideration of publication bias Funnel plots were produced for the Comparison 01 primary outcome of reduction of anxiety symptoms (6 studies). Visual inspection of the funnel plot indicated possible asymmetry, which might suggest that small trials with negative outcomes were not included in the review. However, the small number of studies included in the funnel plot limits further meaningful interpretation.
Discussion
Summary of main results
In the original review, no meta‐analyses were performed, since only three studies were eligible for inclusion at that time, and each study used a different control intervention. This updated version of the review includes an additional 12 studies conducted since 1999. All 15 included studies provided some data for meta‐analyses, and four comparisons were performed. A summary of the main findings for each comparison follows.
Cognitive behavioural therapy versus usual care Based on a homogeneous group of six studies (373 participants), the review provides evidence that at post‐treatment, CBT was more effective than usual care in reducing the severity of fatigue symptoms in CFS patients who had persisted with treatment. The robustness of this finding is supported by the outcome of clinical response (4 studies, 371 participants), with a 40% response rate for CBT compared to 26% for usual care. However, at short to medium term follow‐up (rather than immediately post‐treatment), the evidence for the effectiveness of CBT was inconclusive. Anxiety symptoms were lower at post treatment in patients who completed CBT sessions than those assigned to usual care, but not at follow‐up. Patients assigned to usual care were less likely to drop out of treatment than those assigned to CBT.
Four sub‐group analyses were conducted. The first sub‐group analysis found that individual CBT interventions were highly effective in reducing fatigue severity compared with usual care at post treatment, whereas the difference between a group modality of CBT and usual care only just reached significance. The second found that CBT was highly effective in reducing fatigue severity when compared with treatment as usual control, and, in contrast, there was no significant difference between CBT and waiting list control. The third found that CBT in which increased activity and reduced rest was a component of treatment was highly effective in reducing fatigue severity compared with usual care, and, in contrast, CBT without focus on increased activity, or in which this was not specified, was no more effective than usual care. Finally, both 8 or fewer sessions of CBT and more than 8 sessions were highly effective in reducing fatigue severity compared with usual care. Given the non‐randomisation of studies into subgroups coupled with the small number of studies, the significance of these findings should be interpreted with caution.
Cognitive behavioural therapy versus other psychological therapies Based on a homogeneous group of four studies (309 participants) the review provides evidence that CBT was more effective than other psychological therapies in reducing the severity of fatigue symptoms in CFS patients persisting with treatment at post treatment. This finding is supported by the clinical response outcome (3 studies, 259 participants), with a 48% response rate for CBT in contrast with 28% for other psychological therapies. Patients who completed CBT sessions achieved a greater improvement in physical functioning, together with lower depression, anxiety and psychological distress symptoms at post‐treatment than those who completed other psychological therapy sessions. At short term follow‐up, the difference between CBT and other psychological therapies was inconsistent, and statistical heterogeneity was indicated.
Cognitive behavioural therapy versus exercise The evidence is limited to two studies, both of which independently showed a lack of difference between CBT and exercise in reducing fatigue levels or increasing clinical response, at post treatment and at medium term follow‐up.
Cognitive behavioural therapy combined with other interventions versus usual care The evidence is limited to a single study of 27 participants, which showed that at post‐treatment, CBT combined with biofeedback, relaxation and aerobic exercise was more effective in reducing fatigue severity than usual care.
Overall completeness and applicability of evidence
Participants The majority of studies included in this review were conducted in outpatient departments or specialist clinics, and two thirds of studies excluded patients who were 'incapable' of attending sessions or confined to wheelchairs/bed. In limiting the population to those patients who were more mobile, the findings from this review may not be representative of more severely affected individuals. Nevertheless, chronicity was a key clinical feature for participants in included studies, with mean duration of illness estimated at 4.7 years.
The updated version of the review includes four trials in which recruitment was wholly or partially conducted in primary care settings (Huibers 2004; King 1999; Ridsdale 2004; Whitehead 2002). This potentially increases the generalisability of the findings to individuals with less disabling and severe symptoms. However, with each of these four studies, this review restricted the analysed dataset to the subgroup of participants diagnosed with CFS. The rationale for this was the minimisation of diagnostic heterogeneity, although it is acknowledged that this may have reduced the applicability of the findings to primary care settings.
Interventions The updated review shows that the largest body of evidence comprises traditional CBT interventions adapted for CFS populations, in which increasing activity and reducing rest time are core features of treatment. In line with the emergence of newer 'third wave' CBT interventions, however, a recently conducted study of mindfulness CBT has also been included in the review (Surawy 2005). In this intervention, participants are encouraged to observe symptoms, thoughts and emotions without attempting to avoid or change them. It seems likely that further trials informed by compassion focused and mindfulness principles will be conducted and included in future updates.
Given the complex and often chronic clinical presentation of CFS, it seems conceivable that a combination of interventions might be required for the optimal treatment of this disorder. In updating this review, it had been hoped to include evidence from trials of combination therapies. Surprisingly, however, despite an exhaustive search of the literature, the evidence base for combination therapy appears to contain just two studies (Stevens 1999; Lloyd 1993). This precludes the ability to draw conclusions on which interventions might be more effective in combination rather than as stand‐alone treatments.
During this review it was also planned to conduct formal comparisons between CBT alone and other active interventions alone. However, to date, the evidence base is limited to just two studies, both of which compared CBT to a manualised exercise intervention. One additional group of studies (Barrett 1992; Deale 1996; O'Dowd 2000; Prins 2001; King 1999) compared CBT with other psychological therapy interventions, including relaxation, education and support and counselling. However, these interventions were largely intended as attention placebo conditions, rather than as active treatments.
Outcomes An encouraging feature of recent trials examining the effectiveness of CBT for CFS is the use of more extended follow‐up periods. The majority of studies conducted over the last eight years incorporated follow‐up time points 6‐12 months post‐treatment, with one study (Deale 1996) conducting a five year follow‐up with 88% of the original sample, thus providing a more clinically relevant measure of effectiveness and cost‐effectiveness.
Effective treatments are of little value to health services unless they are acceptable to the recipients ‐ poor acceptability will reduce treatment uptake and adherence. Only three trials included in this review incorporated the outcome of satisfaction with treatment (Deale 1996; Jason 2007; Ridsdale 2004), with usable data available from one study only (Deale 1996). Given the understandable ambivalence of many CFS patients towards a psychological treatment for symptoms that are experienced as physical, the under‐investigation of acceptability of treatment is an important omission. Other outcomes which are of high relevance to the individual with CFS, including adverse effects, health‐related quality of life, and occupational outcomes, are also under‐evaluated in the included studies.
Quality of the evidence
Historically, trials of psychological therapy have provided poor quality information on the specific randomisation procedure used to assign participants to groups. Therefore, it is encouraging that nearly half of the studies included in the updated review described and used appropriate methods of sequence generation and allocation concealment. Nevertheless, many studies failed to describe their randomisation procedure, and, therefore, it is not possible to assess the extent to which selection bias may have occurred in this group of studies.
Blinding of therapists and participants is not feasible in trials of psychological therapy, although it is notable that two studies in the review did make strenuous attempt to reduce or avoid contamination between groups (Prins 2001; O'Dowd 2000). Whilst the use of blinded assessors is feasible in psychological therapy trials, the majority of studies used subjective self‐report measures to collect psychological outcomes. In general, little information was provided on the data collection process, and the extent to which response bias may have occurred is uncertain. Of the five studies that used clinician‐rated measures, only three studies reported the use of independent or blinded assessors (Deale 1996; Prins 2001; Sharpe 1993) to minimise detection bias.
Waiting list is frequently employed as an ethical 'no treatment' condition, to ensure that all participants eventually receive the 'active' treatment. This approach was used in almost half of all studies comparing CBT against usual care. However, sub‐group analyses showed that, at post‐treatment, waiting list patients had reduced fatigue symptoms equivalent to those of patients attending for treatment. A possible explanation is that being on the waiting list for treatment had a 'holding' positive influence, decreasing the apparent effect of the CBT intervention.
Because psychological therapy involves the application of complex techniques over a period of time, it is important from a methodological perspective that each intervention is operationally defined by detailed protocols (Borkovec 2001). One of the methodological weaknesses of trials included in this review was the apparent failure of eight of the fifteen studies to use manuals or treatment protocols to more effectively standardise the CBT provided. Most studies did, however, employ therapists who were experienced in the psychological model under examination. Testing of therapists' fidelity to treatment manuals through the systematic or random checking of audiotapes by independent clinicians is an additional key methodological aspect of psychological therapy studies. This ensures that any observed treatment effect can be attributed to specific components and characteristics of the model. However, only three of the fifteen included studies reported testing therapists' treatment fidelity through assessing recordings of sessions (Prins 2001; King 1999; Jason 2007). Therefore, there is no certainty in most of the studies that therapists were adhering to the required psychological model.
Four studies controlled for therapist attention by using low intensity interventions such as education and support or relaxation. When compared against these placebo interventions, CBT appeared to achieve a more consistently positive effect across all outcomes than when compared against usual care, a finding that appears counter‐intuitive. It is possible that the findings could be explained by the greater use of naturalistic treatments in usual care conditions. However, another plausible explanation is the influence of researcher allegiance to CBT, as in three of these studies the therapist was also the primary investigator (Barrett 1992; Deale 1996; O'Dowd 2000). In addition, in the study by Prins 2001, the control condition was group therapy with less emphasis given to regular attendance, in contrast with the CBT intervention, which was individual in modality. Whilst it is possible, therefore, that the effect of CBT may have been over‐estimated when compared with the low intensity conditions, it should nevertheless be acknowledged that CBT and placebo conditions appeared to be managed equally across studies in terms of manualisation, fidelity checking, and therapist qualifications/experience.
Whilst the overall attrition rate across studies was reasonably low at 16.4%, six of the fifteen studies had dropout rates of higher than 20%, and only five studies reported reasons for attrition in full for each group. Therefore, it is not certain to what extent there might have been systematic differences between dropouts and completers between groups that could have influenced treatment outcomes, leading to an underestimate or overestimate of effect. In contrast, however, missing data was well managed in most studies, with nine studies conducting a sensitivity analysis, analyses using intention to treat principles, or last observation carried forward.
Potential biases in the review process
Strengths A core strength of this review is the extensive, rigorous and highly sensitive search strategy used to locate high quality evidence. As a result, randomised evidence from unpublished as well as published studies were included in the review, none of which have been included in previous high quality systematic reviews. Furthermore, vigorous efforts made to obtain missing data resulted in additional data from three investigators (King 1999; Huibers 2004; Prins 2001), and responses from two further investigators who may be able to provide access to their datasets in future updates (Chalder 1997; Russell 2001). It seems likely that all or almost all evidence that should have been included was included and that the occurrence of publication bias may have been minimised. The increased number of studies, and their relative homogeneity, permitted a quantitative synthesis of some of the data, thus improving the precision of the estimates of the effectiveness of CBT
A further strength is the diversity of the authors' areas of expertise, coupled with collaborative and transparent review methods, ensuring that the potential for bias was minimised. Two independent review authors were involved in every stage of the review process and a third author mediated when necessary. During study selection, the authors' different perspectives helped to inform inclusion‐exclusion decisions when, for example, it was difficult to assess the extent to which interventions were sufficiently CBT‐based to be included in the review. Of note, in the case of one study where it was not possible to reach agreement on its inclusion, the authors of the study concerned (Powell 2001) were invited to cast the deciding vote.
Limitations Although every effort was made to obtain missing data from trial authors, it was not possible in every case to obtain these data, and therefore the included studies are not represented fully in the meta‐analyses.
Given that formal blinding of participants and clinicians to treatment arm is not inherently possible in trials of psychological therapy, the decision was taken to assess the risk of bias for the blinding domain on the basis of outcome assessor blinding only, whilst acknowledging that our decision to do this could have under‐estimated the extent to which clinicians' and participants' knowledge of group assignation influenced the true effect.
Whilst the identification and inclusion of four unpublished studies (Barrett 1992; Russell 2001; Strang 2002; Stevens 1999) is regarded as a strength of the updated review in terms of reducing publication bias, it should nevertheless be acknowledged that these trials have not been through full peer review and may have been especially vulnerable to bias.
Agreements and disagreements with other studies or reviews
The key high quality review of evidence in this area is that by Chambers and colleagues (Chambers 2006), which helped to inform the UK's NICE Clinical Guideline on the care of people with CFS‐ME (NICE 2007). The Chambers 2006 review addressed a very wide range of therapeutic modalities, including not only CBT and graded exercise, but also immunological, complementary, pharmacological, and nutritional therapies. Like this review, it noted a marked increase in the size and the quality of the evidence‐base for interventions for CFS. It concluded that two interventions, CBT and graded exercise, appeared to be effective, but that there was either evidence of lack of effectiveness or a lack of evidence for other interventions. However, the conclusions drawn by Chambers 2006 for CBT interventions were based on a smaller dataset than that provided in this review, included non‐randomised controlled trials, and no meta‐analyses were undertaken. A recently published systematic review and meta‐analysis by Malouff 2008, using broader inclusion criteria, and based on the calculation of a pooled effect size rather than head to head comparisons, has drawn similiar conclusions to those of Chambers 2006.The updated findings from the current review provide robust high quality evidence to support those reported by Chambers 2006 and Malouff 2008 for the effectiveness of CBT interventions for CFS, and in addition, is able to demonstrate the homogeneity of the evidence.
Authors' conclusions
Implications for practice.
CBT is more effective than usual care for reducing fatigue symptoms in adults with CFS, with 40% of participants assigned to CBT showing clinical response at post‐treatment, in comparison with 26% assigned to usual care control. The benefits of CBT in sustaining clinical response and reduction of fatigue symptoms at short and medium term follow‐up are inconclusive. The benefits of CBT in improving physical functioning and reducing depression, anxiety and psychological distress at post treatment and at follow‐up are also uncertain.
CBT may be more effective in reducing fatigue symptoms at post treatment than psychological therapies using relaxation techniques or a support and education approach, and may also be more effective in improving physical functioning and reducing depression, anxiety and psychological distress at post‐treatment. However these findings are based on a small body of evidence in which other therapies were designed as attention placebo controls, which limits confidence in the findings obtained. The body of evidence for CBT compared with relaxation or support and education at short and medium term follow‐up is very small, heterogeneous and inconclusive.
CBT may be an acceptable form of treatment for CFS, although this evidence is currently based on a small number of individual studies. Whilst the overall attrition rate from CBT interventions is reasonably low, at 16%, reasons for dropout were under‐reported in studies, and may not only have been due to low acceptability or ineffectiveness of CBT.
Currently there is a lack of available evidence on the effectiveness of CBT as a stand‐alone intervention or in combination with other interventions compared with usual care or other types of treatment (including immunological therapies, pharmacological therapies, exercise, complementary/alternative therapies and nutritional supplements) for CFS.
Implications for research.
The body of evidence for the effectiveness of CBT for CFS has grown considerably in quantity and methodological quality over the past eight years. The studies included in this review demonstrated some methodological strengths, including the increasing use of appropriate randomisation procedures, attempts to control for therapist attention and time, and follow‐up assessments conducted 6‐12 months after the completion of treatment. Further specific recommendations to enhance the internal validity of future studies include the recruitment of larger appropriately powered samples, the use of treatment as usual control conditions rather than waiting list, systematic assessment of psychological therapy fidelity to manuals/protocols, and further use of blinded outcome assessors. To increase the applicability of the findings to CFS individuals, clinicians and policy makers, future studies should include adverse effects, acceptablity and quality of life measures, together with cost‐effectiveness, health service resource use and occupational outcomes.
Whilst the evidence base for CBT as a stand‐alone treatment for CFS continues to expand, there is a surprising lack of high quality evidence on the effectiveness of CBT alone or in combination with other treatments to inform the development of clinical management programmes for people with CFS. Given the complex and chronic clinical presentation of CFS, coupled with the evidence that CBT alone is effective for no more than 40‐48% of patients at the end of treatment, trials examining CBT in combination with other interventions to increase treatment effectiveness should be prioritised.
To date trials have been largely conducted in secondary or primary care settings, recruiting patients who are sufficiently mobile to attend out‐patient clinics for CBT sessions. Studies based in domicilary or in‐patient settings should be considered in order to establish the extent to which CBT is effective for those who are severely disabled with CFS.
Whilst the review provides some very preliminary findings for the effectiveness of CBT using an individual modality and using increased activity, further study of these aspects of the CBT interventions are required in order to be able to draw valid conclusions on their superior benefit.
There are a number of trials currently in progress (Bleijenberg 2008; Carney 2008; Gibson‐Saxty 2002; Vissers 2008; Wearden 2006; White 2005), which will help to strengthen the evidence base on CBT interventions for CFS. The findings of these studies are awaited with interest.
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2021 | Amended | To further clarify the status of this review, the following text has been added as an editorial note: "This 2008 review predates the mandatory use of GRADE methodology to assess the strength of evidence, and the review is no longer current. It should not be used for clinical decision‐making. The author team is no longer available to maintain the review." |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 15 February 2021 | Amended | To clarify the status of this review, the following text has been added as an editorial note: "This 2008 review predates the mandatory use of GRADE methodology to assess the strength of evidence, and the review is no longer current. The author team is no longer available to maintain the review." |
| 6 July 2020 | Amended | To clarify the status of this review on cognitive behaviour therapy, the following text has been added to the Abstract: “This review was last updated in 2008 and is no longer current. The author team is no longer available to maintain the review.” |
| 13 February 2009 | Amended | Data on Employment status (Improvement over 12 months) added for Sharpe 1993 (see Graph 1.18), following confirmation of the correct time point by the trialists |
| 1 November 2008 | Amended | Authors updated |
| 6 May 2008 | Amended | Converted to new review format. |
| 2 April 2008 | New citation required and conclusions have changed | Updated through Department of Health Incentive Scheme |
Notes
Acknowledgements
We would like to aknowledge Dr J Couper, St Vincent's Hospital, University of Melbourne, Australia, for his contribution on the original version of the review.
We are most grateful to the following authors for kindly providing us with subgroup data from their trials: Dr Marcus Huibers, Department of Epidemiology, Maastricht University, The Netherlands Dr Judith Prins, University Medical Centre Nijmegen, The Netherlands Dr Leone Ridsdale, Academic Neuroscience Centre, Institute of Psychiatry, London, UK
We also acknowledge the following authors for responding to our request for further information: Professor Trudie Chalder, Academic Department of Psychological Medicine, Institute of Psychiatry, London, UK Dr Vanessa Russell, Adult Psychological Services (East Riding), Willerby, UK Professor Andrew Lloyd, University of New South Wales, Sydney, Australia Mr Paul Seed, Divison of Reproduction and Endocrinology, King's College London Professor Peter Campion, Postgraduate Medical Institute, University of Hull
Data and analyses
Comparison 1. Cognitive behaviour therapy versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Reduction in fatigue severity at post‐treatment | 6 | 373 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.60, ‐0.19] |
| 1.1.1 Traditional CBT | 5 | 356 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.61, ‐0.19] |
| 1.1.2 Mindfulness/compassion focused CBT | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.17, 0.74] |
| 1.2 Clinical response at post‐treatment | 4 | 371 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.29, 0.76] |
| 1.2.1 Traditional CBT | 3 | 353 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.29, 0.78] |
| 1.2.2 Mindfulness/compassion focused CBT | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.06, 2.70] |
| 1.3 Improvement in overall physical functioning at post‐treatment | 4 | 318 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.32, 0.54] |
| 1.3.1 Traditional CBT | 3 | 301 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.40, 0.61] |
| 1.3.2 Mindfulness/compassion focused CBT | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.81, 1.10] |
| 1.4 Reduction in depression symptoms at post ‐treatment | 4 | 183 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.53, 0.05] |
| 1.4.1 Traditional CBT | 3 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.53, 0.08] |
| 1.4.2 Mindfulness/compassion focused CBT | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.34, 0.59] |
| 1.5 Reduction in anxiety symptoms at post ‐treatment | 4 | 183 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.59, ‐0.01] |
| 1.5.1 Traditional CBT | 3 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.63, ‐0.01] |
| 1.5.2 Mindfulness/compassion focused CBT | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.06, 0.85] |
| 1.6 Reduction in psychological distress at post ‐treatment | 3 | 191 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.56, 0.01] |
| 1.6.1 Traditional CBT | 3 | 191 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.56, 0.01] |
| 1.6.2 Mindfulness/compassion‐focused CBT | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 1.7 Improvement in overall quality of life at post‐treatment | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.58, 2.46] |
| 1.7.1 Traditional CBT | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.58, 2.46] |
| 1.7.2 Mindfulness/compassion‐focused CBT | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.8 Dropout at post treatment | 9 | 586 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.10, 2.63] |
| 1.8.1 Traditional CBT | 8 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.13, 2.75] |
| 1.8.2 Mindfulness/compassion focused therapy | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 8.35] |
| 1.9 Reduction in fatigue severity at follow‐up (short/medium‐term) | 4 | 330 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.69, ‐0.25] |
| 1.9.1 Traditional CBT | 4 | 330 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.69, ‐0.25] |
| 1.10 Clinical response at follow‐up (short/medium‐term) | 3 | 353 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.53, 2.00] |
| 1.10.1 Traditional CBT | 3 | 353 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.53, 2.00] |
| 1.11 Improvement in overall physical functioning at follow‐up (short/medium‐term) | 3 | 275 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.21, 0.51] |
| 1.11.1 Traditional CBT | 3 | 275 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.21, 0.51] |
| 1.12 Reduction in depression symptoms at follow‐up (short/medium‐term) | 2 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐1.39 [‐2.60, ‐0.18] |
| 1.12.1 Traditional CBT | 2 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐1.39 [‐2.60, ‐0.18] |
| 1.13 Reduction in anxiety symptoms at follow‐up (short/medium‐term) | 2 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐2.91, 0.16] |
| 1.13.1 Traditional CBT | 2 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐2.91, 0.16] |
| 1.14 Reduction in psychological distress at follow‐up (short/medium‐term) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐28.85, 28.37] |
| 1.14.1 Traditional CBT | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐28.85, 28.37] |
| 1.15 Improvement in overall quality of life at follow‐up (short/medium term) | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 8.00 [0.68, 15.32] |
| 1.15.1 Traditional CBT | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 8.00 [0.68, 15.32] |
| 1.16 Dropout at follow‐up (short/medium‐term) | 4 | 413 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.52, 4.10] |
| 1.16.1 Traditional CBT | 4 | 413 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.52, 4.10] |
| 1.17 Employment status: days of registered absenteeism over 12 months | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 4.35 [‐50.06, 58.76] |
| 1.17.1 Traditional CBT | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 4.35 [‐50.06, 58.76] |
| 1.18 Employment status: Improvement over 12 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.47, 6.81] |
| 1.18.1 Traditional CBT | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.47, 6.81] |
1.1. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 1: Reduction in fatigue severity at post‐treatment
1.2. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 2: Clinical response at post‐treatment
1.3. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 3: Improvement in overall physical functioning at post‐treatment
1.4. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 4: Reduction in depression symptoms at post ‐treatment
1.5. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 5: Reduction in anxiety symptoms at post ‐treatment
1.6. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 6: Reduction in psychological distress at post ‐treatment
1.7. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 7: Improvement in overall quality of life at post‐treatment
1.8. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 8: Dropout at post treatment
1.9. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 9: Reduction in fatigue severity at follow‐up (short/medium‐term)
1.10. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 10: Clinical response at follow‐up (short/medium‐term)
1.11. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 11: Improvement in overall physical functioning at follow‐up (short/medium‐term)
1.12. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 12: Reduction in depression symptoms at follow‐up (short/medium‐term)
1.13. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 13: Reduction in anxiety symptoms at follow‐up (short/medium‐term)
1.14. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 14: Reduction in psychological distress at follow‐up (short/medium‐term)
1.15. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 15: Improvement in overall quality of life at follow‐up (short/medium term)
1.16. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 16: Dropout at follow‐up (short/medium‐term)
1.17. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 17: Employment status: days of registered absenteeism over 12 months
1.18. Analysis.

Comparison 1: Cognitive behaviour therapy versus usual care, Outcome 18: Employment status: Improvement over 12 months
Comparison 2. Cognitive behaviour therapy versus other psychological therapies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Reduction in fatigue severity at post‐treatment | 4 | 313 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.65, ‐0.20] |
| 2.2 Clinical response at post‐treatment | 3 | 349 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.19, 0.62] |
| 2.3 Improvement in overall physical functioning at post‐treatment | 3 | 286 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.10, 0.97] |
| 2.4 Reduction in depression symptoms at post‐treatment | 3 | 175 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.76, ‐0.16] |
| 2.5 Reduction in anxiety symptoms at post‐treatment | 2 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.80, ‐0.08] |
| 2.6 Reduction in psychological distress at post‐treatment | 3 | 175 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.71, ‐0.11] |
| 2.7 Improvement in overall quality of life at post‐treatment | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 10.00 [3.43, 16.57] |
| 2.8 Dropout at post treatment | 4 | 377 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.81, 2.20] |
| 2.9 Reduction in fatigue severity at follow‐up (short‐term) | 4 | 294 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.83, ‐0.11] |
| 2.10 Clinical response at follow‐up (short/medium‐term) | 3 | 219 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.12] |
| 2.11 Improvement in overall physical functioning at follow‐up (short‐term) | 3 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [0.27, 1.14] |
| 2.12 Reduction in depression symptoms at follow‐up (short‐term) | 3 | 177 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.50, 0.44] |
| 2.13 Reduction in anxiety symptoms at follow‐up (short‐term) | 2 | 124 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐2.96, 3.02] |
| 2.14 Reduction in psychological distress at follow‐up (short‐term) | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐7.07, ‐0.13] |
| 2.15 Improvement in overall quality of life at follow‐up (short‐term) | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | 13.00 [5.48, 20.52] |
| 2.16 Dropout during follow‐up (short‐term) | 4 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.76, 1.97] |
2.1. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 1: Reduction in fatigue severity at post‐treatment
2.2. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 2: Clinical response at post‐treatment
2.3. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 3: Improvement in overall physical functioning at post‐treatment
2.4. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 4: Reduction in depression symptoms at post‐treatment
2.5. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 5: Reduction in anxiety symptoms at post‐treatment
2.6. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 6: Reduction in psychological distress at post‐treatment
2.7. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 7: Improvement in overall quality of life at post‐treatment
2.8. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 8: Dropout at post treatment
2.9. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 9: Reduction in fatigue severity at follow‐up (short‐term)
2.10. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 10: Clinical response at follow‐up (short/medium‐term)
2.11. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 11: Improvement in overall physical functioning at follow‐up (short‐term)
2.12. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 12: Reduction in depression symptoms at follow‐up (short‐term)
2.13. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 13: Reduction in anxiety symptoms at follow‐up (short‐term)
2.14. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 14: Reduction in psychological distress at follow‐up (short‐term)
2.15. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 15: Improvement in overall quality of life at follow‐up (short‐term)
2.16. Analysis.

Comparison 2: Cognitive behaviour therapy versus other psychological therapies, Outcome 16: Dropout during follow‐up (short‐term)
Comparison 3. Cognitive behaviour therapy versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Reduction in fatigue severity at post treatment | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐2.52 [‐7.34, 2.30] |
| 3.2 Clinical response at post treatment | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.17] |
| 3.3 Clinical response at follow‐up (medium‐term) | 1 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
3.1. Analysis.

Comparison 3: Cognitive behaviour therapy versus exercise, Outcome 1: Reduction in fatigue severity at post treatment
3.2. Analysis.

Comparison 3: Cognitive behaviour therapy versus exercise, Outcome 2: Clinical response at post treatment
3.3. Analysis.

Comparison 3: Cognitive behaviour therapy versus exercise, Outcome 3: Clinical response at follow‐up (medium‐term)
Comparison 4. Cognitive behaviour therapy combined with other interventions versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Reduction in fatigue severity at post treatment | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐7.12 [‐12.83, ‐1.41] |
| 4.1.1 CBT + biofeedback + relaxation + aerobic exercise | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐7.12 [‐12.83, ‐1.41] |
| 4.2 Improvement in overall physical functioning at post treatment | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 8.38 [0.98, 15.78] |
| 4.2.1 CBT + biofeedback + relaxation + aerobic exercise | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 8.38 [0.98, 15.78] |
| 4.3 Dropout at post treatment | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.68] |
| 4.3.1 CBT + biofeedback + relaxation + aerobic exercise | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.68] |
4.1. Analysis.

Comparison 4: Cognitive behaviour therapy combined with other interventions versus usual care, Outcome 1: Reduction in fatigue severity at post treatment
4.2. Analysis.

Comparison 4: Cognitive behaviour therapy combined with other interventions versus usual care, Outcome 2: Improvement in overall physical functioning at post treatment
4.3. Analysis.

Comparison 4: Cognitive behaviour therapy combined with other interventions versus usual care, Outcome 3: Dropout at post treatment
Comparison 5. Subgroup analyses (Comparison 1).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Individual versus group therapy: reduction in fatigue at post treatment | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Individual | 2 | 201 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.76, ‐0.20] |
| 5.1.2 Group | 4 | 172 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, 0.00] |
| 5.2 Usual care versus waiting list: reduction in fatigue at post treatment | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Usual care control | 3 | 290 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.72, ‐0.25] |
| 5.2.2 Waiting list control | 3 | 83 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.51, 0.35] |
| 5.3 Increased activity versus activity and rest/not specified: reduction in fatigue at post treatment | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 Increased activity | 4 | 293 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.65, ‐0.18] |
| 5.3.2 Activity and rest/not specified | 2 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.76, 0.13] |
| 5.4 8 or fewer sessions versus more than 8 sessions: reduction in fatigue at post treatment | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.4.1 8 or fewer sessions | 4 | 207 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.62, ‐0.06] |
| 5.4.2 More than 8 sessions | 2 | 166 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.77, ‐0.15] |
5.1. Analysis.

Comparison 5: Subgroup analyses (Comparison 1), Outcome 1: Individual versus group therapy: reduction in fatigue at post treatment
5.2. Analysis.

Comparison 5: Subgroup analyses (Comparison 1), Outcome 2: Usual care versus waiting list: reduction in fatigue at post treatment
5.3. Analysis.

Comparison 5: Subgroup analyses (Comparison 1), Outcome 3: Increased activity versus activity and rest/not specified: reduction in fatigue at post treatment
5.4. Analysis.

Comparison 5: Subgroup analyses (Comparison 1), Outcome 4: 8 or fewer sessions versus more than 8 sessions: reduction in fatigue at post treatment
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrett 1992.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ not specified 2. Allocation concealment: uncertain ‐ not specified 3. Blinding: high ‐ self‐report or physiological measures (except SIP‐O rated by significant other); delayed expectations of waiting list control group vulnerable to response bias; no information on how and when measures were completed 4. Incomplete outcome data: uncertain ‐ not all results available on all measures, for which reasons not specified; low dropout rate but no ITT analysis, SIP‐O completed for 58% of sample only; no information on how and when measures completed 5. Selective outcome reporting: uncertain ‐ delayed‐type hypersensitivity skin test not reported but otherwise not specified 6. Other sources of bias: high ‐ interventions clearly described but any manualisation or fidelity testing not reported; medication use measured pre and post treatment, but prevalence not reported; esearcher was also therapist across both groups (potential contamination) | |
| Participants | Setting: secondary care ‐ exact setting unclear Sample population: previously assessed by CFS research unit or from specialist outpatient clinic Sample size: 43 Inclusion criteria: 'Australian' CFS criteria (Lloyd et al 1990) Exclusion criteria: non‐English speaking, symptoms attributable to comorbidity, concurrent depression, history of psychosis, substance abuse Baseline characteristics: comparison groups similar pre‐treatment on demographic and outcome variables | |
| Interventions | 1: 'cognitive behavioural group intervention'; group setting (size unspecified); 9 sessions of 90 minutes over 9 weeks; manualisation not specified; treatment fidelity not specified; one session with psychiatrist, 8 sessions with unspecified therapist (supervision not specified); goal of therapy to address anxiety, depression, illness attribution, and control; examined role of thoughts, stress, emotion, overbreathing; techniques of progressive muscle relaxation, stress reduction, and goal setting, with one session focussing on increasing physical activity. 2: 'education and support control'; non‐directive educational group programme designed to control for non‐specific elements of CBT such as clinic attendance, social support etc. 3: 'waiting list control' | |
| Outcomes | 1. MEASURES: BDI, STAI, SIP‐S, SIP‐O, IBQ (general hypochondriasis), IBQ (disease conviction), IBQ (affective disturbance), self‐monitored activity (derived from West‐Haven‐Yale Multidimensional Pain inventory), distress rating, CD4 white cell count, CD8 white cell count, POMS fatigue scale, expectancy rating, credibility rating, evaluation rating, delayed type hypersensitivity skin test (not reported) 2. FOLLOW‐UP TIMES: 9 weeks (immediately post‐treatment) | |
| Notes | Mean duration of fatigue 98 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
Deale 1996.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: low ‐ random number table, prepared in random perumted blocks stratified for source of referral 2. Allocation concealment: low ‐ sealed envelopes opened immediately before first session 3. Blinding: uncertain ‐ self‐report or interview with blind assessor at 3 months; no information on how assessments were completed post‐treatment, 1 month and 6 month follow‐ups 4. Incomplete outcome data: low ‐ low drop‐out rate, intention‐to‐treat analysis, and last observation carried forward analysis, showing pattern of results similar to main analysis 5. Selective outcome reporting: uncertain ‐ study protocol not available 6. Other sources of bias: uncertain ‐ research team met fortnightly to ensure protocol adherence but no formal testing of treatment fidelity reported; interventions conducted by single therapist who was primary investigator; some baseline inequivalence between groups (see below) | |
| Participants | Setting: secondary; hospital outpatient clinic Sample population: consecutive referrals from primary/secondary care to CFS outpatient clinic Sample size: 60 Inclusion criteria: 'Oxford' and CDC criteria Exclusion criteria: somatisation disorder, severe depression, ongoing physical investigations, concurrent new therapy, recent (less than 3 months) change in antidepressant/anxiolytic therapy, inability to attend treatment sessions Baseline characteristics: CBT group older (38 yrs as opposed to 31 in control group) and had more social class I & II participants, but otherwise comparison groups similar on demographic, psychiatric, and outcome variables | |
| Interventions | 1: 'cognitive behaviour therapy'; individual; 13 sessions of approximately 60 mins over 24 weeks (total therapist contact time approximately 15 hours); manualised; therapists unspecified (supervised); treatment fidelity assured by regular research team meetings; aim to show patients that activity could be increased steadily and safely without exacerbating symptoms; graded exercise plan; homework tasks and daily diaries 2: 'relaxation'; aim to teach skills in progressive muscular relaxation, visualization, and rapid relaxation. | |
| Outcomes | 1. MEASURES: SF‐36 physical functioning subscale, good outcome on SF‐36 (increase of 50 or more or end score of 83+), work & social adjustment scale, progression towards long term goals rating, fatigue problem rating, Chalder fatigue scale, GHQ‐12, BDI, global improvement self‐rating, satisfaction with treatment outcome self‐rating, usefulness of treatment self‐rating, assessor rating of physical functioning at 3 month follow‐up, assessor rating of fatigue 3 month follow‐up 2. FOLLOW‐UP TIMES: 1 month post‐treatment, 3 months, 6 months | |
| Notes | Mean duration of fatigue 48 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | sealed envelopes opened immediately before first session |
Huibers 2004.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: low ‐ computer generated random number table in blocks of four stratified according to duration of absenteeism 2. Allocation concealment: low ‐ opaque sealed envelopes administered by third party 3. Blinding: low ‐ patients masked to allocation system and existance/procedures of other groups; self‐report measures completed on computer or by post 4. Incomplete outcome data: high ‐ apparently imbalanced dropout rate between groups (with reasons not given for control group); intention‐to‐treat analysis and exploratory sub‐group analyses presented 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol 6. Other sources of bias: high ‐ 18% of CBT group received other psychosocial interventions during study in contrast with 31% of control group; treatment fidelity assessed by consisted only of supervisors checking registration forms rather than taping of sessions | |
| Participants | Setting: secondary; GP practices Sample population: patients on sick leave recruited via local occupational health service Sample size: 151 Inclusion criteria: severe fatigue as defined as 35+ on Dutch CIS for 4 months or more as one of main health problems; complete absenteeism from work for 6‐26 weeks Exclusion criteria: medical condition which explained fatigue; receiving another intervention for fatigue; previously classified psychiatric disorder; receiving current psychological therapy; absenteeism from work caused primarily by non‐health related problem Baseline characteristics: any differences on pre‐treatment demographic or outcome variables not specified | |
| Interventions | 1: 'cognitive behavioural therapy'; individual; 5‐7 sessions of 30 minutes over 4 months; manualised; treatment fidelity assessed by supervisors on basis of GPs records and supervision sessions (but no results of fidelity assessment reported); GPs (trained in two 5 hr workshops) were therapists (supervised); goals of therapy to diminish fatigue, establish work resumption and other personal goals by assessment of perpetuating factors, addressing sense of loss of control, disturbed sleep pattern, unbalanced physical activites, providing helpful cognitions, and planning systematic and gradual work resumption 2: 'no treatment' (usual GP care) | |
| Outcomes | 1. MEASURES: CIS score, number of work resumers, registered absenteeism, clinical recovery (defined by CIS <35 with self‐report work resumption), perceived recovery, SF‐36 physical functioning subscale, SCL‐90, self‐efficacy, psychological attributions, somatic attributions, # visits to GP in intervention period 2. FOLLOW‐UP TIMES: 4 months (immediately after treatment), 8 months, 12 months | |
| Notes | Mean duration of fatigue approximately 27 months; 44% of sample met CDC criteria for CFS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | opaque sealed envelopes administered by third party |
Jason 2007.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: low ‐ computer generated using SPSS software 2. Allocation concealment: uncertain ‐ not specified 3. Blinding: high ‐ measures self‐report (method of completion not reported) except clinician rating of patient's overall functioning which is not specified as blinded 4. Incomplete outcome data: high ‐ considerable dropout rate which is not broken down by treatment or reasons for dropout specified; used linear unbiased predictor model to deal with missing data where at least 2 points were available 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol 6. Other sources of bias: high ‐ intervention was individualised and dose varied by subject, but these differences not reported or recorded; fidelity ratings of audiotaped sessions presented and although expert found 4 arms to be distinct, interventions share a number of components and study underpowered to detect true differences; risk of selection bias due to recruitment process (word of mouth/media/physician referral) | |
| Participants | Setting: Secondary care ‐ exact setting unclear Sample population: referred by physicians, recruited from CFS support groups, or self‐referral by way of media/word of mouth Sample size: 114 Inclusion criteria: Fukuda (CDC) criteria Exclusion criteria: under 18 years, pregnant, non‐English speaking, deemed unable to attend treatment sessions, symptoms attributable to ‘physical’ cause on interview w/ Komaroff 1996 instrument, examination or investigations; BMI greater than 45; symptoms attributable to Axis 1 disorder on SCID Baseline characteristics: comparison groups similar pre‐treatment on demographic and psychiatric variables; no analysis of pre‐treatment similarity on outcome variables | |
| Interventions | 1: 'cognitive behaviour therapy'; individual; 13 sessions of 45 minutes over 26 weeks; manualised; treatment fidelity assessed by assessed by masters level/licensed psychologists via random sample of audiotaped sessions, therapists were two registered nurses with previous experience and training in psychotherapy (supervised by a clinical psychologist); therapy involved evaluation of the effect of gradual, consistent increases in activity, utilising strategies other than avoidance with behavioural homework and diary‐keeping 2: 'cognitive therapy'; a broad‐based cognitive approach to better tolerate and reduce stress and symptoms, and to lessen self‐criticism, without structured schedules of increasing activity or exercise (instead pacing activity and avoiding overexertion) 3: 'anaerobic activity therapy'; encouraged individualised, constructive and pleasurable activities accompanied by reinforcement of progress, using self‐monitoring diaries, behavioural homework, 4: 'relaxation treatment'; progressive muscle relaxation, stretching, autogenic training, breathing focus techniques with homework and diary keeping | |
| Outcomes | 1. MEASURES: SF‐36, fatigue severity scale, BDI, BAI, self‐efficacy, perceived stress scale, pain severity (brief pain inventory), pain interference with general activities (brief pain inventory), quality of life scale, 6 minute walking test, symptoms (sore throat, tender nodes, muscle pain, joint pain, impaired memory, unrefreshing sleep, post‐exertional malaise, headaches, participant satisfaction with treatment, employment status, participant rating of usefulness of treatment, participant rating of improvement, clinician rating of patient's overall functioning 2. FOLLOW‐UP TIMES: baseline, 12 month follow‐up | |
| Notes | Mean duration of fatigue not specified but the authors state that it was "about 5 years longer than [other studies of CBT for CFS]"; in addition, subjects were aged "about 10 years older in age than [other studies of CBT for CFS]"; participants received US$150 for successful completion of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
King 1999.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: low ‐ computer generated combinations in blocks of 10 2. Allocation concealment: low ‐ opaque sealed envelopes, taken in sequence to each baseline assessment and opened in front of the patient 3. Blinding: low ‐ self‐report measures, completed by post 4. Incomplete outcome data: uncertain ‐ high dropout rate and reasons for dropout not reported; sensitivity and intention‐to‐treat analyses reported 5. Selective outcome reporting: uncertain ‐ study protocol not available but no results presented for SF‐36 scores 6. Other sources of bias: uncertain ‐ although counselling was selected as anactive comparison, the hypothesis was that CBT would be more effective ‐ the manner of report of eventual equivalence suggests author allegiance to the cognitive behavioural paradigm; subgroup analysis of CFS participants appears post‐hoc | |
| Participants | Setting: primary care; general practices Sample population: all patients meeting study criteria referred by GPs complaining of fatigue as main or important problem Sample size: 160 Inclusion criteria: greater than 3 months fatigue as indexed by score of 4 or more on Chalder fatigue scale Exclusion criteria: outside study age range (16 to 75 years), three months or more duration of fatigue, abnormal FBC/ESR/TFTs within previous 6 months, concurrent physical problems judged to be cause of fatigue, recent change in drug regimen, psychotic illness, unable to read English, learning difficulty precluding completion of questionnaire, concurrent psychological/psychiatric treatment, patient unable to attend Baseline characteristics: no testing for differences on pre‐treatment demographic, psychiatric, or outcome variables | |
| Interventions | 1: 'counselling'; qualified counsellors offering psychodynamic approach 2: 'cognitive behaviour therapy'; individual; 6 treatments of 60 minutes over period unspecified period; manualised; treatment fidelity assessed by independent rating of sampling of recorded sessions; therapists were qualified CBT therapists (supervised during trial); used activity planning, consistent and realistic activity given patient's responsibilities, addressed negative self‐beliefs, expectations, self‐esteem, establishment of sleep routine, and relapse prevention | |
| Outcomes | 1. MEASURES: Chalder fatigue score, number of cases of fatigue on Chalder fatigue score, HADS Anxiety, HADS depression, on antidepressants, social adjustment score, satisfaction with therapy 2. FOLLOW‐UP TIMES: 3 months, 6 months (duration of treatment not specified) | |
| Notes | Mean duration of fatigue approximately 27 months; 44% of sample met CDC criteria for CFS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | opaque sealed envelopes, taken in sequence to each baseline assessment and opened in front of the patient |
Lloyd 1993.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: low ‐ computer generated 2. Allocation concealment: uncertain ‐ not specified (use of clouded vials for immunologic treatment) 3. Blinding: uncertain ‐ double blinding for immunologic treatments (with test of blind); self‐report or physiological measures apart from Karnsofsky scale with blinding unspecified 4. Incomplete outcome data: low ‐ single dropout though reasons not specified; available case analysis 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol 6. Other sources of bias: high ‐ participants also received placebo injections for dialyzable lymphocyte extract; symptomatic improvement correlated with belief that subjects had received active DLE; CBT sessions also sometimes involved other family member and ranged in length from 30‐60 minutes therefore of possibly inconsistent intensity; no manualisation or fidelity testing reported | |
| Participants | Setting: secondary care ‐ exact setting unclear Sample population: not specified Sample size: 90 in entire study (including active DLE arms); 44 in placebo arms included in this review Inclusion criteria: 'Australian' CFS criteria (Lloyd et al 1990) Exclusion criteria: previous immunologic therapy, non‐ambulatory, incapable of attending clinic every 2 weeks, alternative medical explanation for symptoms assessed by history, physical examination and investigations Baseline characteristics: comparison groups similar pre‐treatment on demographic and outcome variables | |
| Interventions | 1: 'active DLE injections plus cognitive behavioural therapy' (not included in this review) 2: 'active DLE injections only' (not included in this review) 3: 'placebo injections plus cognitive behavioural therapy'; individual (participant could choose to bring close family member); one 60 minute session and five 30‐60 minute sessions over 12 weeks; manualisation not specified; treatment fidelity not specified; therapists were psychiatrists (supervision not specified); subject received booklet with treatment rationale; goal of therapy to shift perceptions of being helpless victim, to re‐establishing social activities; schedule of home based graded exercise; visited clinic every two weeks for 16 weeks for DLE placebo injection. 4: 'placebo injections only'; visited clinic every two weeks for 16 weeks for DLE placebo injection. | |
| Outcomes | 1. MEASURES: quality of life visual analogue scales, nonsedentary hours activity/day, Karnofsky score, POMS fatigue scale, POMS confusion scale, POMS depression score, CD4 white cell count, CD8 white cell count, delayed type hypersensitivity skin test 2. FOLLOW‐UP TIMES: 7 months (3 months post‐treatment) | |
| Notes | Mean dutaion of fatigue 66 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | not specified (use of clouded vials for immunologic treatment) |
O'Dowd 2000.
| Study characteristics | ||
|---|---|---|
| Methods | 1. Sequence generation: uncertain ‐ prepared by third party statistician using blocked randomisation but method of sequence generation not specified 2. Allocation concealment: low ‐ details provided in sequentially numbered opaque sealed envelopes 3. Blinding: uncertain ‐ self‐report measures; subjects not informed of existence of other groups; some outcome measures relying on assessment by blind assessor who was instructed not to enquire about treatment undertaken, and to stop participants divulging such information; 7 patients did not receive the treatment to which they were randomly assigned (for clinical and ethical reasons) demonstrating contamination and leakage 4. Incomplete outcome data: low ‐ low dropout rate, apparently balanced between groups, with reasons for dropouts specified; analysis method ensured patients with partial data were not excluded, based on assumption that data were missing at random 5. Selective outcome reporting: low ‐ authors detail deviations from trial protocol, which included non‐usage of subanaerobic exercise test and conduction of 18 month follow‐up assessments by post 6. Other sources of bias: uncertain ‐ referral methods changed half way through trial; changes in therapists during trial; interventions guided by programme rather than manual, and assessment of treatment fidelity not specified | |
| Participants | Setting: secondary care; pain management clinic Sample population: patients managed in local GP practices with presentation consistent with CFS Sample size: 153 Inclusion criteria: CDC criteria Exclusion criteria: concurrent severe mental illness, planned or concurrent rehabilitation, inability to attend all sessions, ongoing physical investigations Baseline characteristics: detailed comparison of pre‐treatment differences on demographic, psychiatric and outcome variables; twice as many males in CBT group than EAS/SMC groups | |
| Interventions | 1: 'cognitive behavioural therapy'; group (8‐12 individuals); 8 sessions of 120 minutes over 16 weeks; manualisation not specified; treatment fidelity not specified; therapists were clinical psychologists, specialist physiotherapists, and senior occupational therapists (supervision not specified); therapy attempted to modify thoughts and beliefs about symptoms and illness, and behavioural responses such as rest, sleep and activity, with monitoring of activity and teaching of exercises to be paced up in small increments (with reduction if significant exacerbation of symptoms occurred) 2: 'education and support' ; sharing of experiences and learning of basic relaxation skills serving as a control for the non‐specific elements of group therapy 3: 'standard medical care'; patients managed as usual in primary care | |
| Outcomes | 1. MEASURES: SF‐36 physical functioning subscale, SF‐36 mental health subscale, perceived fatigue, incremental shuttle walk test, normal walking speed, HADS anxiety, HADS depression, GHQ, Chalder fatigue scale, HU13, mood ‐ alertness, mood – hedonic tone, mood ‐ anxiety, recall – total words recalled/correct words/incorrect words, simple reaction time – reaction time/trials completed, repeated digits detection – reaction time/hit rate/false alarms, number of subjects with SF‐36 scores within range of normal population (physical functioning subscale/mental health subscale), number of patients with SF‐36 scores at least 15% greater than baseline (physical functioning subscale/mental health subscale) 2. FOLLOW‐UP TIMES: 6 months (2 months post‐treatment), 12 months | |
| Notes | Mean duration of fatigue not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | details provided in sequentially numbered opaque sealed envelopes |
Prins 2001.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ allocated sequentially by blockwise randomisation separately for each centre (but method of sequence generation not specified) 2. Allocation concealment: low ‐ sealed envelopes opened in presence of patient before first session 3. Blinding: uncertain ‐ measures self‐report except for Karnofsky scale which was administered by 'independent clinical psychologist', but exact nature of blinding not specified 4. Incomplete outcome data: high ‐ considerable dropout rate in intervention groups and no reasons provided for dropouts provided; intention‐to‐treat analysis performed 5. Selective outcome reporting: uncertain ‐ no reference to study protocol 6. Other sources of bias: high ‐ no testing for pre‐treatment differences between comparison groups is reported; CBT group conducted singly but guided support conducted in groups; subjects in CBT group forbidden to have further physical investigations but control group free to do so; frequent non‐attendance in guided support group had no consequences for further treatment or dropout status and resulted in significant difference in mean hours of attendance between groups | |
| Participants | Setting: secondary care; specialist outpatient clinic at teaching hospital Sample population: patients with primary complaint of fatigue referred to specialist outpatient clinic Sample size: 278 Inclusion criteria: CDC criteria with exception of 4 of 8 additional symptoms being present Exclusion criteria: greater than 90 minutes travelling time from study centre, previous/current participation in CFS research, pregnancy, fertility treatment, less than 18 years or over 60 years. Baseline characteristics: demographic characteristics of subjects reported but no statistics analysing pre‐treatment differences between comparison groups on demographic, psychiatric, or outcome variables | |
| Interventions | 1: 'cognitive behaviour therapy'; individual; 16 sessions of 60 minutes over 8 months; treatment protocol used; sessions audiotaped and treatment fidelity assessed by independent judge; therapists were psychologists, psychiatrists, health scientists (supervised throughout trial); therapy emphasised control rather than physician/prescription dependenc, challenging fatigue related cognitions; subjects encouraged to attain and maintain base level of physical activity needed to prevent bursts of activity and resultant extreme fatigue; final sessions dealt with relapse prevention and further improvement of self‐control 2: ' guided support group'; approximately 8 patients in each group given non‐directive and client‐centred counselling by social worker; no treatment protocol specified 3: 'natural course'; no other assessments or treatments (subjects free to have other examinations or treatments) | |
| Outcomes | 1. MEASURES: number improving significantly on fatigue subscale of CIS, number improving significantly on Karnofsky scale, number improving significantly on self‐rated improvement, CIS, SIP, Karnofsky scale, EuroQol, mean hours working in job per day 2. FOLLOW‐UP TIMES: 8 months (immediately after treatment), 14 months | |
| Notes | Mean duration of fatigue approximately 66 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | sealed envelopes opened in presence of patient before first session |
Ridsdale 2004.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ states random combinations but method of sequence generation not specified 2. Allocation concealment: low ‐ opaque sealed envelopes administered by third party in presence of patient following baseline assessment 3. Blinding: uncertain ‐ self‐report measures or physiological measures (step‐test) ‐ blinding not specified 4. Incomplete outcome data: uncertain ‐ possible patient treatment preference bias ‐ more patients reported lack of faith in allocated treatment as a reason for not starting or for stopping GET compared to CBT; only 60% of patients in GET attended 6 sessions in contrast with 71% in CBT; intention‐to‐treat and sensitivity analyses presented 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol ‐ third (originally unfunded) arm introduced during trial but not used in this review 6. Other sources of bias: uncertain ‐ baseline differences between comparison groups (see below) | |
| Participants | Setting: primary care; GP surgeries but therapists were trained cognitive behavioural therapists Sample population: patients presenting to GP with main complaint of unexplained fatigue of greater than 3 months Sample size: 40 Inclusion criteria: greater than 3 months fatigue as indexed by score of 4 or more on Chalder fatigue scale Exclusion criteria: outside study age range (16‐75), recent change in drug regimen, abnormal FBC/ESR/TFTs within previous 6 months, unable to read English, concurrent physical problems judged to be cause of fatigue, asthma/ischaemic heart disease contraindicating step test, psychotic illness, organic brain syndrome, substance dependency, current psychological treatment Baseline characteristics: CBT group more fatigued than GET group; otherwise similar on pre‐treatment demographic, psychiatric, and outcome variables | |
| Interventions | 1: 'cognitive behavioural therapy'; individual; 6 sessions of 45 minutes over 3 months; manualised; treatment fidelity not assessed; therapists were cognitive behavioural therapists (supervised); treatment based on model focusing on perpetuating factors, ensuring levels of activity and rest are consistent and realistic given patients responsibilities, involving activity planning, homework, establishing a sleep routine, and other cognitive interventions to challenge negative beliefs regarding the symptom of fatigue, self‐expectations or self‐esteem, with relapse prevention addressed 2: 'graded exercise therapy'; developed from GET protocol designed for patients with chronic fatigue syndrome; aims for gradual but progressive increase in aerobic activities, usually walking 3: 'prospective cohort' (this third 'control' arm is not included in the present review as it was randomised after the other two groups) | |
| Outcomes | 1. MEASURES: Chalder fatigue score, mean fatigue (combined 3 and 8 month data), number recovered on Chalder 1993 criteria, proportion recovered using Cleare 1999 criteria, HAD depression, HAD anxiety, WASA functional impairment, illness attributions 2. FOLLOW‐UP TIMES: 3 months (immediately after treatment), 8 months | |
| Notes | Mean duration of fatigue 24 months; 29% of sample met CDC criteria for CFS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | opaque sealed envelopes administered by third party in presence of patient following baseline assessment |
Russell 2001.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ not specified 2. Allocation concealment: uncertain ‐ not specified 3. Blinding: high ‐ self‐report measures; process of questionnaire completion not reported; delayed expectations of waiting list control group vulnerable to reponse bias 4. Incomplete outcome data: uncertain ‐ low dropout rate but reasons for dropouts unspecified 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol 6. Other sources of bias: high ‐ manualisation if intervention or testing of treatment fidelity not reported; no information on qualifications of therapists provided; no information on other treatments e.g. antidepressants received by each group; no reporting of treatment adherence | |
| Participants | Setting: secondary care; exact setting unspecified Sample population: patients referred by GP to specialist immunology clinic who received a diagnosis of CFS Sample size: 25 Inclusion criteria: CDC criteria Exclusion criteria: none specified apart from assessment by psychologist and physiotherapist for readiness to engage in therapy, but not clear if this resulted in any exclusions Baseline characteristics: minimal demographic characteristics supplied; no testing for demographic or psychiatric differences at baseline; comparison groups similar pre‐treatment outcome measures | |
| Interventions | 1: 'group rehabilitation'; group (maximum size 8 participants); 12 sessions of 150 minutes over 12 weeks; manualisation not specified; treatment fidelity not specified; therapists not specified (supervision not specified); approach offered a cognitive explanation for the maintenance of symptoms, challenged unhelpful attitudes and expectations, emphasised overactivity/rest cycle and relapse prevention 2: 'wait list control' | |
| Outcomes | 1. MEASURES: LHS, Chalder fatigue scale, HAD total, HAD anxiety, HAD depression, HAD anxiety number of cases ‘definitely disturbed’, HAD depression number of cases ‘definitely disturbed’, IPQ identity subscale/time line/consequences/control/cure, ATQ negative/positive thoughts 2. FOLLOW‐UP TIMES: 3 months (immediately after treatment) | |
| Notes | Mean duration of fatigue approximately 60 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | not specified |
Sharpe 1993.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: low ‐ random number table 2. Allocation concealment: low ‐ labelled cards in sealed numbered envelopes 3. Blinding: uncertain ‐ self‐report measures, semistructured interview (unclear if assessor blind), Karnofsky scale (secondary assessor blinded but based on data from primary, possibly unblinded, assessor), walking test (unclear if assessor blind) 4. Incomplete outcome data: low ‐ contacted single dropout (no change since last assessment); conducted intention‐to‐treat analysis 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol 6. Other sources of bias: uncertain ‐ groups not equivalent at baseline; assessment of treatment fidelity not reported or attendance at sessions by participants | |
| Participants | Setting: secondary care (urban general and psychiatric hospitals) Sample population: consecutive referrals to hospital infectious diseases outpatient clinic Sample size: 60 Inclusion criteria: 'Oxford' CFS criteria Exclusion criteria: concurrent depression, history of bipolar disorder, schizophrenia, substance abuse, concurrent psychotherapy, urgent need for psychiatric treatment, risk of suicide, recent (less than 3 months) change in drug treatment or change in condition, unavailable for follow‐up Baseline characteristics: comparison groups similar pre‐treatment on demographic, psychiatric, and outcome variables | |
| Interventions | 1: 'medical care plus cognitive behavioural therapy'; individual; 16 sessions of 60 minutes over 16 weeks; manualisation not specified; treatment fidelity not specified; therapists were research clinical psychologist, consultant psychologist, psychiatrist (supervised by experienced cognitive therapist); encouraged biopsychosocial understanding of illness, active problem solving with gradual, consistent increases in activity, addressed perfectionism & self‐criticism 2: 'medical care alone'; subject reassured they had no serious organic disease; told they had chronic fatigue syndrome, advised to increase physical activity by as much as they felt able, and discharged to GP care | |
| Outcomes | 1. MEASURES: Karnofsky scale – achievement of satisfactory outcome (greater than 80 points), Karnofsky scale – improvement of at least 10 points, interference with daily activities, improvement in employment status, number of days in bed each week, 6 minute walking distance, fatigue (Likert), HADS anxiety, HADS depression, self‐rated overall change, illness beliefs and coping behaviour statement endorsement e.g. "cause is a virus", frequency of avoidance of exercise 2. FOLLOW‐UP TIMES: 5 months (one month post‐treatment), 8 months, 12 months | |
| Notes | Mean duration of fatigue 32 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | labelled cards in sealed numbered envelopes |
Stevens 1999.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ not specified 2. Allocation concealment: uncertain ‐ not specified 3. Blinding: high ‐ self‐report or physiological measures except stepping test with blinding unspecified; no information on procedure for collecting post‐treatment questionnaires; delayed expectations of wait list control group vulnerrable to reponse bias 4. Incomplete outcome data: uncertain ‐ low dropout rate and reasons specified, but no sensitivity analysis 5. Selective outcome reporting: uncertain ‐ not specified 6. Other sources of bias: high ‐ recruitment based on referrals from physicians/local support groups with possible selection bias; inequivalence of groups at baseline (see below); no testing of treatment fidelity reported | |
| Participants | Setting: secondary care ‐ exact setting unclear Sample population: recruited from physicians or local CFS groups Sample size: 29 Inclusion criteria: CDC criteria Exclusion criteria: organic aetiology for condition identified by GP in previous 12 months Baseline characteristics: no differences on psychiatric or demographic variables reported except control group significantly more likely to have received a diagnosis of fibromyalgia; comparison groups similar pre‐treatment on outcome variables except intervention group had more morning insomnia, awoke more at night, had worse SF‐36 scores and outperformed control group on stepping test | |
| Interventions | 1:'chronobiological treatment programme'; individual; 8 sessions of 60 minutes over 9 weeks; manualisation not specified; treatment fidelity not specified; therapists were doctoral level psychologist or 4 'trained assistants' (supervision not specified); used sleep hygiene education, biofeedback assisted relaxation, breathing retraining, graded aerobic exercise, and cognitive therapy; diary keeping and home based‐exercise programme 2:'wait‐list control'; no intervention sessions | |
| Outcomes | 1. MEASURES: moderate‐hard exercise (hrs/wk), relaxation hrs/wk, stress hrs/day), nighttime insomnia, morning insomnia, nights per week awakening at night, CESD (center for epidemiological studies depression score), POMS Profile of Moods Scale total score/fatigue score/vigour score, SF‐36 physical functioning subscale/mental health subscale, CFS symptoms check‐list score, forehead EMG, temperature, heart rate, breaths per minute, electrodermal response, amplitude, end tidal carbon dioxide, exercise test step duration exercise test step level, exercise test rate of perceived exertion 2. FOLLOW‐UP TIMES: 9 weeks (1 week post‐treatment) | |
| Notes | Mean duration of fatigue not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | not specified |
Strang 2002.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ not specified 2. Allocation concealment: uncertain ‐ not specified 3. Blinding: high ‐ self‐report measures but no information on process of completion; delayed expectations of wait list control group vulnerable to response bias 4. Incomplete outcome data: uncertain ‐ substantial dropout rate with no reasons for dropouts specified; no intention‐to‐treat or last observation carried forward analyses 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol 6. Other sources of bias: high ‐ primary investigator was also therapist; no testing of treatment fidelity reported; no treatment adherence reported; no information on other treatments received during trial e.g. antidepressants | |
| Participants | Setting: secondary; exact setting unclear Sample population: referred to study by practitioners (internists, rheumatologists, psychiatrists and psychologists), newspaper advertisements, fliers, and local support organisations Sample size: 51 Inclusion criteria: CDC criteria Exclusion criteria: none specified Baseline characteristics: comparison groups similar pre‐treatment on demographic and outcome variables, except a greater number in treatment group received disability compensation | |
| Interventions | 1: 'cognitive behavioural intervention'; group (8‐10 subjects per group); 6 sessions of 90 minutes over 6‐9 weeks; treatment manualised; therapist was author (doctoral level psychologist) (supervision not specified); treatment fidelity not specified; therapy had elements of personal mastery training, concentrating on realistic goal setting, gradual increases in activity level, cognitive restructuring of maladaptive cognitions, increasing perceived control over symptoms and life events 2: 'wait list control' | |
| Outcomes | 1. MEASURES: fatigue severity scale, SF‐36, average fatigue, fatigue related cognitions scale, structure of coping questionnaire (mastery subscale); internality, powerful others, and chance (internality subscale), perceived health competence, positive and negative affect schedule, BDI, BAI 2. FOLLOW‐UP TIMES: 6‐9 weeks (immediately after treatment) | |
| Notes | Mean duration of fatigue 144 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | not specified |
Surawy 2005.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ not specified 2. Allocation concealment: uncertain ‐ not specified 3. Blinding: high ‐ self‐report measures; for intervention group, self‐report measures taken at first interview and after completion of group ‐ acceptability of programme especially vulnerable to response bias; delayed expectations of wait list control group vulnerable to reponse bias; control group sent questionnaires by post therefore no 'minimum contact' with research team 4. Incomplete outcome data: uncertain ‐ one dropout, but reasons not given 5. Selective outcome reporting: uncertain ‐ no reference to trial protocol 6. Other sources of bias: uncertain ‐ unclear whether principal investigator was therapist, manualisation or test of treatment fidelity not reported; no information on other treatment received (e.g. antidepressants) during the trial | |
| Participants | Setting: secondary care; exact setting unclear Sample population: patients with CFS due to be offered CBT and who would have had to wait more than 3 months Sample size: 18 Inclusion criteria: 'Oxford' criteria Exclusion criteria: no primary diagnosis of CFS, unable to travel to group therapy, diagnosis of major depression/psychosis Baseline characteristics: no information given on differences between comparison groups | |
| Interventions | 1: 'group mindfulness training'; group (number of participants not specified); 8 sessions of unspecified length over 8 weeks; therapists unspecified (supervision unspecified); manualisation not specified; treatment fidelity not specified; programme based on mindfulness based stress reduction and mindfulness based cognitive therapy 2: Wait list control | |
| Outcomes | 1. MEASURES: HADS anxiety, HADS depression, Chalder fatigue scale, SF‐36 physical functioning subscale 2. FOLLOW‐UP TIMES: 2 months (immediately post‐treatment) | |
| Notes | Mean duration of fatigue not specified; all patients attended 4 or more sessions but mean number of sessions attended is not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | not specified |
Whitehead 2002.
| Study characteristics | ||
|---|---|---|
| Methods | Risk of bias: 1. Sequence generation: uncertain ‐ practices rather than patients randomised to treatment group (method of sequence generation not specified) 2. Allocation concealment: uncertain ‐ not specified 3. Blinding: low ‐ low risk of contamination due to randomisation by practice; self‐report measures completed by post 4. Incomplete outcome data: high ‐ relatively high dropout rate, reported as patient rather than practice dropout; no intention‐to‐treat analysis; standard deviations missing for all continuous outcomes 5. Selective outcome reporting: high ‐ no reference to trial protocol but no HADS data reported 6. Other sources of bias: high ‐ pre‐treatment differences in age and symptom duration by group (see below), suggesting GPs may have been more willing to include fatigued patients in the study; lack of information reported on the number of GP practices recruited into the study, therefore not possible to estimate the effect of clustering; no description of management package, and cognitive elements; unclear if sessions in which GPs apparently used the package with CFS patients were recorded; no information on the number of eligible patients and the number who declined; no information on whether patients recruited to study did fulfil CDC criteria (though GPs were trained to refer patients according to criteria); higher uptake from control surgeries, suggests reluctance by intervention GP practices to participate | |
| Participants | Setting: primary; GP surgeries Sample population: patients at GP surgeries identified as meeting CDC criteria by GPs Sample size: 65 Inclusion criteria: CDC criteria Exclusion criteria: none specified Baseline characteristics: intervention subjects were younger and had a shorter duration of illness than controls, as well as having a higher level of fatigue at baseline | |
| Interventions | 1: 'intervention group'; individual/bibliotherapy; subjects urged to visit GPs weekly or every two weeks to discuss diaries, goals and progress; manualised; therapists were GPs trained by investigator (informal supervision available by telephone); treatment fidelity not assessed; subjects received booklet containing explanatory models of CFS, kept focused diaries of activity aiming for a gradual increase in activity at appropriate level and rate, addressing beliefs and behaviours around symptoms and recovery from CFS, exploring validity of negative thoughts around symptom attribution and recovery 2: 'control group'; did not receive intervention | |
| Outcomes | 1. MEASURES: Chalder fatigue scale, LHS, HAD anxiety, HAD depression 2. FOLLOW‐UP TIMES: 6 months, 12 months | |
| Notes | Mean duration of fatigue approximately 30 months; five patients used diaries for one month or less, a further five ceased using them within 3 months; eight used them for 6 months or more, and four used them for 12 months or more | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | not specified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 2006 | Participants: not CFS population. |
| Bleichhardt 2004 | Participants: not CFS population. |
| Burgess 2001 | Intervention: Comparison conducted between modalities of CBT (telephone CBT versus outpatient care) |
| Butler 1991 | Study type: case series. |
| Candy 2004 | Participants: not CFS population. Intervention: brief psycho‐educational intervention without specific cognitive element. |
| Donta 2003 | Participants: not CFS population. |
| Friedberg 1994 | Study type: non‐randomised, controlled trial. |
| Gielissen 2006 | Participants: not CFS population |
| Goudsmit 1996 | Study type: not RCT Type of intervention: not CBT |
| Hatcher 1998 | Intervention: dothiepin and graded activity, with no CBT intervention included |
| Lyles 2003 | Study type: not RCT Type of intervention: not CBT |
| Soderberg 2001 | Intervention: psychological therapy under investigation not CBT‐oriented in theoretical approach |
| Taylor 2004 | Intervention: psychological therapy under investigation no sufficiently CBT‐oriented in theoretical approach |
| Zhang 1989 | Unable to obtain hard copy of article Thought unlikely to be either an RCT or CBT‐oriented |
Characteristics of studies awaiting classification [ordered by study ID]
Chalder 1997.
| Methods | Randomised controlled trial |
|---|---|
| Participants | Patients with chronic fatigue and chronic fatigue symdrome |
| Interventions | Patient leaflet, based on cognitive behavioural principles, together with 15‐minute advice‐based session with research nurse |
| Outcomes | |
| Notes |
Powell 2001.
| Methods | Randomised controlled trial |
|---|---|
| Participants | patients with chronic fatigue syndrome |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Bleijenberg 2008.
| Study name | |
|---|---|
| Methods | Randomised controlled trial |
| Participants | Patients who are severely fatigued and disabled |
| Interventions | i) Group cognitive behavioural therapy (16 2‐hour sessions) in large groups. 2) Group CBT in small groups, 3) Waiting list control |
| Outcomes | CIS subscale fatigue severity, disabilities (Sickness Impact profile), SF‐36 physical functioning |
| Starting date | 01/01/2006 |
| Contact information | |
| Notes |
Carney 2008.
| Study name | |
|---|---|
| Methods | Randomised controlled trial, single blind, active control, parrallel assignment |
| Participants | Patients with chronic fatigue syndrome |
| Interventions | 1) Behavioral therapy targeted to sleep problems (behavioral insomnia therapy), 2) Usual care |
| Outcomes | Total wake time, total sleep time, sleep efficiency, sleep habits |
| Starting date | September 2007 |
| Contact information | |
| Notes |
Gibson‐Saxty 2002.
| Study name | |
|---|---|
| Methods | Randomised controlled trial |
| Participants | Patients with chronic fatigue syndrome |
| Interventions | 1) Group cognitive behavioral therapy (10 sessions), 2) Waiting list |
| Outcomes | Not stated |
| Starting date | 2002 |
| Contact information | |
| Notes |
Vissers 2008.
| Study name | |
|---|---|
| Methods | Randomised controlled trial |
| Participants | Patients with chronic fatigue syndrome |
| Interventions | 1) Multidisciplinary disability resolution (MDR) program (12‐18 months), 2) Care as usual |
| Outcomes | Off‐claim, number of hours in paid job, fatigue severity, functional impairment, physical limitations, psychological well‐being, pain |
| Starting date | 01/04/2006 |
| Contact information | |
| Notes |
Wearden 2006.
| Study name | FINE Trial |
|---|---|
| Methods | |
| Participants | 360 subjects; Inclusion: 18+, fulfill 'Oxford' criteria and 4+ on Chalder fatigue scale; exclusion: fatigue explained by active medical condition, schizophrenia, bipolar, dementia, eating disorder, substace abuse, insufficient English, incapable of giving informed consent, current psychological therapy for CFS/ME, or who have had pragmatic rehabilitation therapy in last year |
| Interventions | 1) Pragmatic rehabilitation 2) supportive listening 3) treatment as usual by GP |
| Outcomes | Primary: SF‐36, EuroQol, Chalder fatigue scale; Secondary: step test, max heart rate on step test, HADS anxiety, HADS depression, sleep scale |
| Starting date | 2006 |
| Contact information | AJ Wearden, School of Psychological Sciences, University of Manchester, Manchester, M13 9PL |
| Notes |
White 2005.
| Study name | PACE Trial |
|---|---|
| Methods | |
| Participants | 600 subjects; Inclusion: 18+, 'Oxford' criteria AND 6+ on Chalder fatigue scale AND 65 or less on SF‐36; exclusion: relevant alternative medical diagnosis, significant risk of self‐harm or psychiatric condition excluded in 'Oxford' criteria, considered unable to complete trial therapy or measures, previously received treatment similar to those in the trial |
| Interventions | 1) standardised specialist medical care alone 2) standardised specialist medical care plus adaptive pacing therapy 3) standardised specialist medical care plus cognitive behaviour therapy 4) standardised specialist medical care plus graded exercise therapy |
| Outcomes | Primary: Chalder fatigue score, SF‐36; secondary: Chalder fatigue scale likert scoring; clinical global impression change, HADS anxiety, HADS depression, WASA, EuroQol, 6 minute walking test, step test, Borg scale, client service receipt inventory, CDC symptoms likert, PHQ15, participant satisfaction at 52 weeks |
| Starting date | Recruitment began March 2005 |
| Contact information | Peter D White, Department of Psychological Medicine, Queen Mary School of Medicine and Dentistry, St Bartholomew's Hospital, London, UK |
| Notes |
Contributions of authors
JP conceived and conducted original review, and was responsible for selection of studies, obtaining missing data, interpreting data and drawing conclusions in updated review
EWM was responsible for data extraction of included studies, selection of studies, obtaining missing data, assessment of risk of bias, writing of the description of studies and methodological quality of included studies sections, and characteristics of included studies and excluded studies tables in updated review
ET was responsible for data extraction of included studies in updated review
VH was responsible for updating the methods section, selection of studies, assessment of risk of bias, data entry, writing the results section, interpreting the data, writing the discussion and drawing conclusions in updated review
Sources of support
Internal sources
- University of Oxford Department of Psychiatry, UK
- Institute of Psychiatry, King's College London, UK
External sources
- The Wellcome Trust, UK
- The Medical Research Council, UK
- Oxford & Anglia NHS Research & Development Directorate, UK
- Department of Health, UK
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Barrett 1992 {unpublished data only}
- Barrett E. Cognitive-behaviour therapy in the management of chronic fatigue syndrome. M Psych University of Sydney 1992.
Deale 1996 {published data only}
- Deale A, Chalder T, Marks I, Wessely S. Cognitive behavior therapy for chronic fatigue syndrome: A randomized controlled trial. American Journal of Psychiatry 1997;154:408-14. [DOI] [PubMed] [Google Scholar]
- Deale A, Chalder T, Marks I, Wessely S. Cognitive behaviour therapy for chronic fatigue syndrom: A randomised controlled trial. In: South Thames Research and Development, Open Day Feb 28. 1996.
- Deale A, Chalder T, Wessely S. Illness beliefs and treatment outcome in chronic fatigue syndrome. Journal of Psychosomatic Research 1998;45:77-83. [DOI] [PubMed] [Google Scholar]
- Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: a 5-year follow-up study. American Journal of Psychiatry 2001;158(12):2038-42. [DOI] [PubMed] [Google Scholar]
- Peakman M, Deale A, Field R, Mahalingam M, Wessely S. Clinical improvement in chronic fatigue syndrome is not associated with lymphocyte subsets of function or activation. Clinical Immunology and Immunopathology 1997;82(1):83-91. [DOI] [PubMed] [Google Scholar]
- Quarmby L, Rimes KA, Deale A, Wessely S, Chalder T. Cognitive-behaviour therapy for chronic fatigue syndrome: Comparison of outcomes within and outside the confines of a randomised cotrolled trial. Behaviour Research and Therapy 2007;45(6):1085-94. [DOI] [PubMed] [Google Scholar]
- Wessely S. Cognitive behaviour therapy versus relaxation for Chronic Fatigue Syndrome: OUtcome at five year follow-up. National Research Register 1999.
Huibers 2004 {published data only}
- Huibers MJ, Beurskens AJ, Van Schayck CP, Baselmans E, Metsemakers JF, Knottnerus JA et al. Efficacy of cognitive-behavioural therapy by general practitioners for unexplained fatigue among employees: Randomised controlled trial. British Journal of Psychiatry 2004;184:240-6. [DOI] [PubMed] [Google Scholar]
Jason 2007 {published data only (unpublished sought but not used)}
- Jason LA, Torres-Harding S, Friedberg F, Corradi K, Njoku MG, Donalek J et al. Non-pharmacologic interventions for CFS: A randomized trial. Journal of Clinical Psychology in Medical Settings 2007;14:275-96. [Google Scholar]
King 1999 {published data only}
- Chalder T, Godfrey E, Ridsdale L, King M, Wessely S. Predictors of outcome in a fatigued population in primary care following a randomized controlled trial. Psychological Medicine 2003;33(2):283-7. [DOI] [PubMed] [Google Scholar]
- Chisholm D, Godfrey E, Ridsdale L, Chalder T, King M, Seed P, Wallace P, Wessely S. Chronic fatigue in general practice: Economic evaluation of counselling versus cognitive behaviour therapy. British Journal of General Practice 2001;51(462):15-8. [PMC free article] [PubMed] [Google Scholar]
- Godfrey E, Chalder T, Ridsdale L, Seed P, Ogden J. Does the therapeutic alliance affect outcome in the context of an RCT of CBT versus counselling for chronic fatigue in primary care? In: 34th Annual Conference of the British Association for Behavioural and Cognitive Psychotherapies. Vol. July 19-21. Warwick, 2006:81-2.
- Godfrey E, Chalder T, Ridsdale L, Seed P, Ogden J. Investigating the active ingredients of cognitive behaviour therapy and counselling for patients with chronic fatigue in primary care: developing a new process measure to assess treatment fidelity and predict outcome. British Journal of Clinical Psychology 2007;46(3):253-72. [DOI] [PubMed] [Google Scholar]
- Godfrey E. Aspects of the therapeutic alliance associated with a good outcome: An analysis of the process of therapy taken from a randomised controlled trial of CBT versus counselling for patients with chronic fatigue in primary care. In: 28th Annual Conference of the British Association for Behavioural and Cognitive Psychotherapies. Vol. July 19-22. London, 2000.
- King MB. Randomised controlled trial of counselling or CBT for patients with chronic fatigue in general practitioners. National Research Register 1999.
- Ridsdale L, Godfrey E, Chalder T, Seed P, King M, Wallace P, Wessely S. Chronic fatigue in general practice: Is counselling as good as cognitive behaviour therapy? British Journal of General Practice 2001;51(462):19-24. [PMC free article] [PubMed] [Google Scholar]
- Ridsdale L, Godfrey E, Chalder T, Seed P, King M, Wallace P, Wessely S and Fatigue Trialists' Group. Chronic fatigue in general practice: is counselling as good as cognitive behaviour therapy? A UK randomised trial. British Journal of General Practice 2001;51:19-24. [PMC free article] [PubMed] [Google Scholar]
Lloyd 1993 {published data only}
- Lloyd AR, Hickie I, Brockman A, Hickie C, Wilson A, Dwyer J et al. Immunologic and psychologic therapy for patients with chronic fatigue syndrome: a double-blind, placebo-controlled trial. American Journal of Medicine 1993;94:197-203. [DOI] [PubMed] [Google Scholar]
O'Dowd 2000 {published data only}
- O'Dowd H, Gladwell P, Rogers C, Hollinghurst S, Gregory A. Cognitive behavioural therapy in chronic fatigue syndrome: A randomised controlled trial of an outpatient group programme. In: 33rd Annual Conference of the British Association for Behavioural and Cognitive Psychotherapies. Vol. July 21-23. Canterbury, 2005:78-9.
- O'Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A. Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme. Health Technology Assessment 2006;10(37):iii-iv, ix-x, 1-121. [DOI] [PubMed] [Google Scholar]
- O'Dowd H. Cognitive behavioural therapy in chronic fatigue syndrome (CFS): A randomised controlled trial of an outpatient group programme. Current Controlled Trials 2000. [DOI] [PubMed]
Prins 2001 {published data only}
- Bazelmans E, Prins JB, Hoogveld S, Bleijenberg G. Manual-based cognitive behaviour therapy for chronic fatigue syndrome: therapists' adherence and perceptions. Cognitive Behaviour Therapy 2004;33(3):143-50. [DOI] [PubMed] [Google Scholar]
- Knoop JA, Prins JB, Bleijenberg G. Is CBT an effective treatment for pain symptoms in chronic fatigue syndrome? In: 32nd Congress of the British Association for Behavioural and Cognitive Psychotherapies (jointly with European Association of Behavioural and Cognitive Therapies). Manchester, 2004 September 7-11:212-3.
- Prins J, Bleijenberg G, Royweler EK, Meer J. Effect of psychiatric disorders on outcome of cognitive-behavioural therapy for chronic fatigue syndrome. British Journal of Psychiatry 2005;187:184-5. [DOI] [PubMed] [Google Scholar]
- Prins JB, Bleijenberg G, Bazelmans E, Elving LD, Boo TM, Severens JL et al. Cognitive behaviour therapy for chronic fatigue syndrome: a multicentre randomised controlled trial. Lancet 2001;357:841-7. [DOI] [PubMed] [Google Scholar]
- Prins JB, Bos E, Huibers MJ, Servaes P, Werff SP, van der Meer JW et al. Social support and the persistence of complaints in chronic fatigue syndrome. Psychotherapy and Psychosomatics 2004;73(3):174-82. [DOI] [PubMed] [Google Scholar]
- Severens JL, Prins JB, Wilt GJ, Meer JW, Bleijenberg G. Cost-effectiveness of cognitive behaviour therapy for patients with chronic fatigue syndrome. QJM 2004;97(3):153-6. [DOI] [PubMed] [Google Scholar]
Ridsdale 2004 {published and unpublished data}
- Ridsdale L, Darbishire L, Seed PT. Is graded exercise better than cognitive behaviour therapy for fatigue? A UK randomized trial in primary care. Psychological Medicine 2004;34:37-49. [DOI] [PubMed] [Google Scholar]
Russell 2001 {unpublished data only}
- Russell V, Gaston AM, Lewin RJP, Atkinson CM, Campion PD. Group rehabilitation for adult chronic fatigue syndrome. unpublished article.
Sharpe 1993 {published and unpublished data}
- Sharpe M, Hawton K, Peto T. Cognitive behaviour therapy for the chronic fatigue syndrome. BMJ 1998;312:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M, Hawton K, Simkin S, Surawy C, Hackmann A, Klimes I, Peto T, Warrell D, Seagroatt S. Cognitive behavior therapy for chronic fatigue syndrome: a randomized controlled study. Verhaltenstherapie 1998;8(2):118-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M, Hawton K, Simkin S, Surawy C, Hackmann A, Klimes I et al. Cognitive behaviour therapy for the chronic fatigue syndrome: a randomized controlled trial. British Medical Journal 1996;312:22-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M. Non-pharmacological approaches to treatment. In: Chronic Fatigue Syndrome: Ciba Foundation Symposium. Vol. 173, 298-308. Chichester: John Wiley & Sons, 1993. [DOI] [PubMed] [Google Scholar]
Stevens 1999 {unpublished data only}
- Stevens M, Gevirtz R, Verity L, Wiederhold M. Chronic fatigue syndrome: a chronobiologically oriented controlled treatment outcome study. unpublished article 1999.
Strang 2002 {unpublished data only}
- Strang JM. Treatment of chronic fatigue syndrom: a cognitive-behavioral approach to enhance personal mastery. Dissertation: Arizona State University August 2002.
Surawy 2005 {published data only}
- Surawy C, Roberts J, Silver A. The effect of mindfulnes training on mood and measures of fatigue, activity, and quality of life in patients with chronic fatigue syndrome on a hospital waiting list: a series of exploratory studies. Behavioural and Cognitive Psychotherapy 2005;33:103-9. [Google Scholar]
Whitehead 2002 {published data only}
- Whitehead L, Campion P. Can general practitioners manage chronic fatigue syndrome. Journal of Chronic Fatigue Syndrome 2002;10(1):55-64. [Google Scholar]
References to studies excluded from this review
Allen 2006 {published data only}
- Allen L, Woolfolk R, Escobar J, Gara M, Hamer R. Cognitive-behavioral therapy for somatization disorder. Archives of Internal Medicine 2006;166:1512-8. [DOI] [PubMed] [Google Scholar]
Bleichhardt 2004 {published data only}
- Bleichhardt G, Timmer B, Rief W. Cognitive-behavioural therapy for patients with multiple somatoform symptoms--a randomised controlled trial in tertiary care. Journal of Psychosomatic Research 2004;56:449-54. [DOI] [PubMed] [Google Scholar]
Burgess 2001 {published data only}
- Burgess M, Chalder T. Telephone cognitive behaviour therapy for chronic fatigue syndrome in secondary care. Behavioural and Cognitive Psychotherapy 2001;29(4):447-55. [DOI] [PubMed] [Google Scholar]
Butler 1991 {published data only}
- Bonner D, Ron M, Chalder T, Butler S, Wessely S. Chronic fatigue syndrome: a follow up study. Journal of Neurology, Neurosurgery and Psychiatry 1994;57:617-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler S, Chalder T, Ron M, Wessely S. Cognitive behaviour therapy in chronic fatigue syndrome. Journal of Neurology, Neurosurgery and Psychiatry 1991;54:153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Candy 2004 {published data only}
- Candy, B Chalder, T Cleare, A Wessely, S Hotopf, M. A randomised controlled trial of a psycho-educational intervention to aid recovery in infectious mononucleosis.. Journal of Psychosomatic Research 2004;57:89-94. [DOI] [PubMed] [Google Scholar]
Donta 2003 {published data only}
- Donta ST, Clauw DJ, Engel CC Jr, Guarino P Peduzzi P, Williams DA et al. Cognitive behavioral therapy and aerobic exercise for Gulf War veterans' illnesses: a randomized controlled trial. JAMA 2003;289(11):1396-404.. [DOI] [PubMed] [Google Scholar]
Friedberg 1994 {published data only}
- Friedberg F, Krupp LB. A comparison of cognitive behavioral treatment for chronic fatigue syndrome and primary depression. Clinical Infectious Diseases 1994;18(Suppl. Clinical Infectious Diseases 1994;18(Suppl 1):105-10. [DOI] [PubMed] [Google Scholar]
Gielissen 2006 {published data only}
- Gielissen MF, Verhagen S, Witjes F, Bleijenberg G. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: a randomized controlled trial. J Clin Oncol 2006;24(30):4882-7.. [DOI] [PubMed] [Google Scholar]
Goudsmit 1996 {unpublished data only}
- Goudsmit EM. The psychological aspects and management of chronic fatigue syndrome. Unpublished PhD Thesis, Brunel University 1996.
Hatcher 1998 {published data only}
- Hatcher S. A randomised double-blind placebo controlled trial of dothiepin and graded activity in the treatment of chronic fatigue syndrome. personal communication 1998.
Lyles 2003 {published data only}
- Lyles JS, Hodges A, Collins C, Lein C, Given CW, Given B et al. Using nurse practitioners to implement an intervention in primary care for high-utilizing patients with medically unexplained symptoms. General Hospital Psychiatry 2003;25(2):67-73. [DOI] [PubMed] [Google Scholar]
Soderberg 2001 {published data only}
- Soderberg S, Evengard, B. Short-term group therapy for patients with chronic fatigue syndrome. Psychotherapy and Psychosomatics 2001;70:108-11. [DOI] [PubMed] [Google Scholar]
Taylor 2004 {published data only}
- Taylor R. Quality of life and symptom severity for individuals with chronic fatigue syndrome: Findings from a randomized clinical trial. The American Journal of Occupational Therapy 2004;58:35-43. [DOI] [PubMed] [Google Scholar]
Zhang 1989 {published data only}
- Zhang X. Short-term psycotherapy of 107 neurasthenia cases. Acta Academiae Medicinae Hebie 1989;10(4):349-51. [Google Scholar]
References to studies awaiting assessment
Chalder 1997 {published and unpublished data}
- Chalder T, Wallace P, Wessely S. Self-help treatment of chronic fatigue in the community: A randomized controlled trial. British Journal of Health Psychology 1997;2:189-97. [Google Scholar]
Powell 2001 {published data only}
- Powell P, Bentall RP, Nye FJ, Edwards HT. Radomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. BMJ 2001;322:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Bleijenberg 2008 {unpublished data only}
- Bleijenberg G. The effectiveness of cognitive behavioural therapy in groups for patients with chronic fatigue syndrome (CFS): a randomised controlled study. controlled-trials.com 2008.
Carney 2008 {unpublished data only}
- Carney C and Jones-Alexander J. Behavioral insomnia therapy with chronic fatigue syndrome. clinicaltrials.gov 2008.
Gibson‐Saxty 2002 {unpublished data only}
- Gibson-Saxty J. Group therapy for chronic fatigue syndrome. National Research Register 2002.
Vissers 2008 {unpublished data only}
- Vissers H. The efficacy and predicting variables of a multidisciplinary disability resolution (MDR) program for CFS patients receiving long term disability benefits from income protection insurers. controlled-trials.com 2008.
Wearden 2006 {unpublished data only}74156610
- Wearden AJ, Riste L, Dowrick C, Chew-Graham C, Bentall RP, Morriss RK, Peters S, Dunn G, Richardson G, Lovell K, Powell P. Fatigue Intervention by Nurses Evaluation - The FINE Trial. A randomised controlled trial of nurse led self-help treatment for patients in primary care with chronic fatigue syndrome: study protocol. BMC Medicine 2006;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2005 {unpublished data only}
- White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R. Protocol for the PACE trial: A randomised controlled trial of adaptive pacing, cognitive behaviour therapy and graded exercise as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy. BMC Neurology 2007;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 2007
- Adams D, Wu T, Tai S, Wiebe N, Vohra S. Traditional Chinese medicinal herbs for idiopathic chronic fatigue and chronic fatigue syndrome. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD006348. [DOI: 10.1002/14651858.CD006348] [DOI] [PubMed] [Google Scholar]
Afari 2003
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. American Journal of Psychiatry 160;2:221-36. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [DOI] [PubMed] [Google Scholar]
Beck 1979
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guildford Press, 1979. [Google Scholar]
Beck 1987
- Beck AT, Steer R. Beck depression inventory: manual. San Antonio, TX: Psychological Corporation, 1987. [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology 1988;56:893-7. [DOI] [PubMed] [Google Scholar]
Behan 1995
- Behan PO, Hannifah H. 5-HT reuptake inhibitors in CFS. EOS Rivista di Immunologia ed Immunofarmacologia 1995;15 (1-2):66-9. [Google Scholar]
Borkovec 2001
- Borkovec TD, Ruscio AM. Psychotherapy for generalized anxiety disorder. Journal of Clinical Psychiatry 2001;62(Suppl 11):15-19. [PubMed] [Google Scholar]
Carruthers 2003
- Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. Journal of Chronic Fatigue Syndrome 2003;11(1):7-97. [Google Scholar]
CFS/ME WG 2002
- CFS/ME Working Group. Report of the CFS/ME Working Group to the Chief Medical Officer. London: Department of Health, 2002. [Google Scholar]
Chalder 1993
- Chalder T. Development of a fatigue scale. Journal of Psychosomatic Research 1993;37:147-53. [DOI] [PubMed] [Google Scholar]
Chambers 2006
- Chambers D, Bagnall AM, Hempel S, Forbes C. Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review. Journal of the Royal Society of Medicine 2006;99:506-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2005
- Cho, JH, Hotopf M, Wessely S. The placebo response in the treatment of chronic fatigue syndrom: a systematic review and meta-analysis. Psychosomatic Medicine 2005;87:301-313. [DOI] [PubMed] [Google Scholar]
Clark 2005
- Clark LV, White PD. The role of deconditioning and therapeutic exercise in chronic fatigue syndrome (CFS). Journal of Mental Health 2005;14(3):237-52. [Google Scholar]
Cox 1987
- Cox B, Blaxter M, Buckle A, et al. The health and lifestyle survey. London: Health Promotion Research Trust, 1987. [Google Scholar]
Deeks 1997
- Deeks J. Are you sure that's a standard deviation? (part 1). Cochrane News 1997;10:11-2. [Google Scholar]
Edmonds 2004
- Edmonds M, McGuire H, Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD003200. [DOI: 10.1002/14651858.CD003200.pub2] [DOI] [PubMed] [Google Scholar]
Fukuda 1994
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Annals of Internal Medicine 1994;121(12):953-9. [DOI] [PubMed] [Google Scholar]
Fulcher 1997
- Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ 1997;314(7095):1647-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furukawa 2005
- Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N. Imputing response rates from means and standard deviations in meta-analyses. International Clinical Psychopharmacology 2005;1:49-52. [DOI] [PubMed] [Google Scholar]
Gilbert 2005
- Gilbert P (Ed). Compassion: Conceptualisations, Research and Use in Psychotherapy. Routledge, 2005. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 2003
- Hayes, Steven C, Kirk D Strosahl, Kelly G. Acceptance and Commitment Therapy : An Experiential Approach to Behavior Change. The Guilford Press, 2003. [Google Scholar]
Higgins 2003
- Higgins JT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(6):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabat‐Zinn 1991
- Kabat-Zinn J. Full catastrophe living: The programme of the stress reduction clinic at the Unviersity of Massachusetts Medical Centre. New York: Delta, 1991. [Google Scholar]
Karnofsky 1948
- Karnofsky D, Abelmann W, Craver L, Burchenal J. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948;1:634-56. [Google Scholar]
Kaslow 1989
- Kaslow JE, Rucker L, Onishi R. Liver extract-folic acid-cyanocobalamin vs placebo for chronic fatigue syndrome. Archives of Internal Medicine 1989;149(11):2501-3. [PubMed] [Google Scholar]
Law 2003
- Law J, Garrett Z, Nye C. Speech and language therapy interventions for children with primary speech and language delay or disorder. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd 1988
- Lloyd A, Wakefield D, Boughton C, Dwyer J. What is myalgic encephalomyelitis? Lancet 1988;1:1286-7. [DOI] [PubMed] [Google Scholar]
Malouff 2008
- Malouff JM, Thorsteinsson EB, Rooke SE, Bhullar N, Schutte NS. Efficacy of cognitive behavioral therapy for chronic fatigue syndrome: A meta-analysis. Clinical Psychology Review 2008;28:736-745. [DOI] [PubMed] [Google Scholar]
McNair 1992
- McNair DM, Lorr M, Dropppleman LF. Profile of Mood States Manual. San Diego: Educational and Industrial Testing Service, 1992. [Google Scholar]
Mulrow 2001
- Mulrow CD, Ramirez G, Cornell JE, Allsup K. Defining and managing chronic fatigue syndrome. In: Evidence Report/Technology Assessment No 42. San Antonio Evidence-based Practice Center, University of Texas, 2001. [PMC free article] [PubMed] [Google Scholar]
NICE 2007
- Chronic fatigue syndrome/myalgic encephatomyelitis (or encephalopathy): diagnosis and management of CFS/ME in adults and children. National Institute of Health and Clinical Excellence (2007) Clinical Guideline 53.
Nixon 1995
- Nixon PGF. Hyperventilation and Chronic Fatigue Syndrome. Queen's Journal of Medicine 1995;88:73-74. [PubMed] [Google Scholar]
RACPWG 2002
- Royal Australasian College of Physicians Working Group. Chronic fatigue syndrome: Clinical practice guidelines. Medical Journal of Australia 2002;176:S17-S55. [PubMed] [Google Scholar]
Rawson 2007
- Rawson KM, Rickards H, Haque S, Ward C. Pharmacological treatments for chronic fatigue syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No: CD006813. [DOI: 10.1002/14651858.CD006813] [DOI] [Google Scholar]
Reich 1981
- Reich JW, Zautra AJ. Life events and persona causation: some relationships with satisfaction and distress. Journal of Personality and Social Psychology 1981;41(5):1002-12. [DOI] [PubMed] [Google Scholar]
Ridsdale 1989
- Ridsdale L. Chronic fatigue in family practice. Journal of Family Practice 1989;29:486-8. [PubMed] [Google Scholar]
Rimes 2005
- Rimes K, Chalder T. Treatments for chronic fatigue syndrome. Occupational Medicine 2005;55:32-9. [DOI] [PubMed] [Google Scholar]
Segal 2002
- Segal Z, Williams JMG, Teasdale JD. Mindfulness based cognitive therapy for depression: A new approach for preventing relapse. New York: Guildford Press, 2002. [Google Scholar]
Sharpe 1991
- Sharpe M, Archard L, Banatvala J, et al. Chronic fatigue syndrome: guidelines for research. Journal of the Royal Society of Medicine 1991;84:118-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
Vercoulen 1996
- Vercoulen JH, Swanink CM, Zitman FG, Vreden SG, Hoofs MP, Fennis JF. Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet 1996;347(9005):858-61. [DOI] [PubMed] [Google Scholar]
Vercoulen 1999
- Vercoulen JH, Alberts M, Bleijenberg G. The Checklist Individual Strength (CIS). Gedragstherapie 1999;32:31-6. [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: New England Medical Centre, 1993. [Google Scholar]
Warren 1999
- Warren G, McKendrick M, Peet M. The role of essential fatty acids in chronic fatigue syndrome. A case-controlled study of red-cell membrane essential fatty acids (efa) and a placebo-controlled treatment study with high dose of efa. Acta Neurologica Scandinavica 1999;99(2):112-6. [DOI] [PubMed] [Google Scholar]
Wearden 1998
- Wearden AJ, Morriss RK, Mullis R, Strickland PL, Pearson DJ, Appleby L. Randomised, double-blind, placebo-controlled trial of fluoxetine and graded exercise for chronic fatigue syndrome. British Journal of Psychiatry 1998;172:485-90. [DOI] [PubMed] [Google Scholar]
Wessely 1995
- Wessely S. The epidemiology of chronic fatigue syndrome. Epidemiologic Reviews 1995;17:139-51. [DOI] [PubMed] [Google Scholar]
Zhang 2006
- Zhang W, Liu ZS, Wu Taixiang, Peng WN. Acupuncture for chronic fatigue syndrome. Cochrane Database of Systematic Reviews 2006, Issue Issue 2. Art. No: CD006010. [DOI: 10.1002/14651858.CD006010] [DOI] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361-70. [DOI] [PubMed] [Google Scholar]