Antibiotics for the common cold and acute purulent rhinitis (original) (raw)
Abstract
Background
It has long been believed that antibiotics have no role in the treatment of common colds yet they are often prescribed in the belief that they may prevent secondary bacterial infections.
Objectives
To determine the efficacy of antibiotics compared with placebo for reducing general and specific nasopharyngeal symptoms of acute upper respiratory tract infections (URTIs) (common colds). To determine if antibiotics have any influence on the outcomes for acute purulent rhinitis and acute clear rhinitis lasting less than 10 days before the intervention. To determine whether there are significant adverse outcomes associated with antibiotic therapy for participants with a clinical diagnosis of acute URTI or acute purulent rhinitis.
Search methods
For this 2013 update we searched CENTRAL 2013, Issue 1, MEDLINE (March 2005 to February week 2, 2013), EMBASE (January 2010 to February 2013), CINAHL (2005 to February 2013), LILACS (2005 to February 2013) and Biosis Previews (2005 to February 2013).
Selection criteria
Randomised controlled trials (RCTs) comparing any antibiotic therapy against placebo in people with symptoms of acute upper respiratory tract infection for less than seven days, or acute purulent rhinitis less than 10 days in duration.
Data collection and analysis
Both review authors independently assessed trial quality and extracted data.
Main results
This updated review included 11 studies. Six studies contributed to one or more analyses related to the common cold, with up to 1047 participants. Five studies contributed to one or more analyses relating to purulent rhinitis, with up to 791 participants. One study contributed only to data on adverse events and one met the inclusion criteria but reported only summary statistics without providing any numerical data that could be included in the meta‐analyses. Interpretation of the combined data is limited because some studies included only children, or only adults, or only males; a wide range of antibiotics were used and outcomes were measured in different ways. There was a moderate risk of bias because of unreported methods details or because an unknown number of participants were likely to have chest or sinus infections.
Participants receiving antibiotics for the common cold did no better in terms of lack of cure or persistence of symptoms than those on placebo (risk ratio (RR) 0.95, 95% confidence interval (CI) 0.59 to 1.51, (random‐effects)), based on a pooled analysis of six trials with a total of 1047 participants. The RR of adverse effects in the antibiotic group was 1.8, 95% CI 1.01 to 3.21, (random‐effects). Adult participants had a significantly greater risk of adverse effects with antibiotics than with placebo (RR 2.62, 95% CI 1.32 to 5.18) (random‐effects) while there was no greater risk in children (RR 0.91, 95% CI 0.51 to 1.63).
The pooled RR for persisting acute purulent rhinitis with antibiotics compared to placebo was 0.73 (95% CI 0.47 to 1.13) (random‐effects), based on four studies with 723 participants. There was an increase in adverse effects in the studies of antibiotics for acute purulent rhinitis (RR 1.46, 95% CI 1.10 to 1.94).
Authors' conclusions
There is no evidence of benefit from antibiotics for the common cold or for persisting acute purulent rhinitis in children or adults. There is evidence that antibiotics cause significant adverse effects in adults when given for the common cold and in all ages when given for acute purulent rhinitis. Routine use of antibiotics for these conditions is not recommended.
Plain language summary
Antibiotics for the common cold, an infection of the upper respiratory tract
Most people around the world will have one or more common cold episodes every year. Except in low‐income countries, the common cold is one of the most cited reasons for people to use antibiotics, even more so if the mucus from their nose is coloured (acute purulent rhinitis). However, common colds are caused by viruses, which do not respond to antibiotics, and antibiotics can cause side effects, especially diarrhoea. Overuse of antibiotics leads to bacteria becoming resistant to antibiotics.
To find out whether antibiotics work for the common cold we identified studies that compared one group of people taking an antibiotic with another group of people taking a medication that looked similar but contained no antibiotic (a placebo). We found six studies of the common cold, with 1047 participants and five studies of acute purulent rhinitis, with 791 participants. Many of the studies had flaws which might have biased the results, especially because many of the participants probably had chest or sinus infections that the researchers did not know about.
Results suggest that antibiotics do not work for either the common cold or for acute purulent rhinitis and many people are affected by antibiotic side effects.
Background
The International Classification of Health Problems in Primary Care (ICHPPC) (Spector 1995) defines an acute upper respiratory tract infection (URTI), the common cold, as an illness with evidence of acute inflammation of the nasal or pharyngeal mucosa and the absence of other specifically defined respiratory conditions, for example streptococcal tonsillitis, laryngitis, bronchitis, pneumonia, asthma and hay fever (ICHPPC 1986). A common cold is commonly regarded as a self limiting viral illness that is experienced annually by the majority of the population. A practical definition is an acute illness with some of the following symptoms: rhinitis (not hay fever or allergic rhinitis), sore throat (not streptococcal pharyngitis), with or without fever, cough and/or productive sputum/purulent sputum.
There is evidence of high usage of antibiotics for the common cold (viral URTI) in spite of doubts about the efficacy of such therapy (McGregor 1995; Spector 1995). Although it is known that viruses are the causative agent, many patients presenting to their general practitioners receive antibiotics. In one study, URTI was the most common reason for new consultations in general practice and the second most common reason for the prescribing of an antibiotic (McAvoy 1994). In a New Zealand study, computerised records of 100,222 consultations from 17 General Practices were examined over one year (McGregor 1995). Seventy‐eight per cent of patients with an URTI received antibiotics. About one‐third of these medications were expensive broad‐spectrum antibiotics. In a more recent study, 49% of persons with URTIs received antibiotics (Ochoa 2000).
There are two published reviews of antibiotics for treating URTIs. One review found no benefit of antibiotics for preventing pneumonia in patients with URTI (Gadomski 1993). Another review found that antibiotics had no benefit in children and that there was no strong evidence of significant adverse effects (Fahey 1998).
The presence of purulent nasal discharge (or a runny nose with coloured discharge) has repeatedly been shown to be an important determinant of antibiotic prescribing for respiratory tract infections for both adults and children (Arroll 2000; Gonzales 1999; Mainous 1997). In one study, no General Practitioners said they would give antibiotics for clear rhinitis, yet 72% said they would for purulent rhinitis (Arroll 2000). A survey found that primary care physicians were far more likely to prescribe an antibiotic for a patient with a cold involving coloured nasal discharge than for a patient without coloured nasal discharge (Mainous 1997). Another study found purulent nasal discharge was a stronger predictor of prescribing antibiotics than any other patient characteristic (Gonzales 1999).
Guidelines usually advise against antibiotics for acute purulent rhinitis (Rosenstein 1998; Snow 2001) yet evidence supporting such recommendations comes from a limited number of small studies of varying methodological quality. Some studies have found no evidence that antibiotics reduce the duration of acute purulent rhinitis (Todd 1984) whereas a recent larger study reported that treatment with amoxicillin reduced the duration of purulent rhinorrhoea; although there was no significant difference between the groups in terms of general symptom improvement (De Sutter 2002).
It is important to obtain an estimate of the effectiveness of antibiotics for the common cold to support judicious use of these important medicines, particularly because URTIs are so common. If ineffective, as has long been thought, widespread use of antibiotics is not only a poor use of health funds but also a cause of unnecessary morbidity from adverse effects and of development of resistant strains (Arason 1996; Verkatesum 1995). If antibiotics were shown to be effective for the common cold then society may be willing to tolerate the increase of resistance with an appropriate reduction in the symptoms. Other reviews to date have not specifically considered the effect of antibiotics in acute purulent rhinitis. The current review expands on previous reviews by considering the effects of antibiotics on the speed of resolution of common cold symptoms in both adults and children, and by examining the evidence for antibiotics in both acute purulent rhinitis and acute clear rhinitis.
Description of the condition
The common cold, or acute upper respiratory tract infection (URTI), is an illness with evidence of acute inflammation of the nasal (rhinitis) or pharyngeal mucosa (sore throat) and the absence of other specifically defined respiratory conditions, for example streptococcal tonsillitis, laryngitis, bronchitis, pneumonia, asthma and hay fever. Acute purulent rhinitis is the presence of coloured discharge, from one or both nostrils, that has persisted for less than 10 days. The choice of less than 10 days is based on the inclusion criteria for the Cochrane Review on chronic purulent rhinitis which included patients with 10 or more days of purulent rhinitis (Morris 2007).
Description of the intervention
The intervention in this review is antibiotics of any kind or placebo given to participants within seven days of the onset of common cold symptoms.
How the intervention might work
Antibiotics are thought to work on bacterial complications of the common cold, as they have no direct effect on the viruses.
Why it is important to do this review
There is evidence that too many antibiotics are being used. Of the top 18 medications prescribed in New Zealand in 2008, three were antibiotics. Two of them are mainly used for respiratory infections and the number of prescriptions totaled 1,608,000 for a population of about four million people (Pharmac 2008).
Objectives
- To determine the efficacy of antibiotics compared with placebo for reducing general and specific nasopharyngeal symptoms of acute URTIs (common colds).
- To determine if antibiotics have any influence on the outcomes for acute purulent rhinitis and acute clear rhinitis lasting less than 10 days before the intervention.
- To determine whether there are significant adverse outcomes associated with antibiotic therapy for participants with a clinical diagnosis of acute URTI or acute purulent rhinitis.
Methods
Criteria for considering studies for this review
Types of studies
All trials in which participants with the diagnosis of acute upper respiratory tract infection (URTI) were randomly assigned to treatment with an antibiotic or a placebo; also all trials in which the majority of participants had acute purulent rhinitis of less than 10 days duration. We included trials allowing concurrent use of other medications if they allowed equal access for participants in both the antibiotic and placebo group.
We excluded studies for the following reasons.
- If they involved the use of an active substance (such as cough mixtures or anti‐pyretics/analgesics) instead of a placebo as these substances may help or suppress symptoms, thereby giving a false measure of the antibiotic's effectiveness. Trials comparing one antibiotic with another, or trials comparing the use of antibiotics versus other medications, were not included.
- If antibiotics were given prophylactically, that is given to asymptomatic individuals, for many weeks to prevent symptoms of acute URTI.
- If more than 5% of participants had throat swabs positive for beta haemolytic streptococcal infection. In the sore throat review by Del Mar 1992, the lowest percentage of participants with streptococci on throat swab was 8%, hence our choice of 5%. Two studies (Haight 1954; Hardy 1956) were excluded by this criterion but neither had data that could be analysed. We accept the contradiction that some studies may include participants with streptococcal infections or colonisation due to the fact that they have not performed throat cultures. There is also the issue of contaminants, which may be as high as 57% in children under 15 years with pharyngitis (Kaplan 1971), hence our desire to remove studies with high proportions of participants with positive throat cultures.
- If not randomised.
- If participants had past histories of serious illness, for example chronic obstructive respiratory disease where antibiotics have been shown to be effective in treating exacerbations.
- If the participants had been given the diagnosis of bronchitis. The International Classification of Health Problems in Primary Care (ICHPPC) definition of bronchitis (Spector 1995) is a definite cough with abnormal chest signs: scattered or generalised, coarse or moist sounds, or wheeze. This definition is not always used by trialists and probably not by General Practitioners (Arroll 2001).
- If the participants had more than seven days of symptoms at the time of study entry. This is an arbitrary duration to avoid post viral syndromes.
- If participants were treated with two or more medications, that is in situations where co‐interventions could be an issue.
- If participants were diagnosed with pharyngitis not conforming to the ICHPPC (Spector 1995).
- If they did not include a placebo arm.
Types of participants
The analysis of the effect of antibiotics on general symptoms of the common cold included participants of all ages who had been diagnosed with an acute URTI and had symptoms for seven days or less. We excluded trials in which the majority of participants had been diagnosed with pharyngitis or bronchitis conforming to the International Classification of Health Problems in Primary Care (ICHPPC 1986) definition. Lower respiratory tract signs were accepted in participants with the above symptoms, so long as the majority of participants in the study did not have these signs and pneumonia was ruled out.
Types of interventions
Antibiotic therapy versus placebo. We included trials which allowed concurrent use of other medications if they allowed equal access for participants in both the antibiotic and placebo group. We excluded studies if they did not compare antibiotic with placebo but instead used aspirin or antitussives in the control group.
Types of outcome measures
The outcome measures included persistence of symptoms of nasopharyngeal inflammation (rhinitis, sore throat and sneezing), global rating of health and any adverse effects.
Primary outcomes
- For the common cold: persisting symptoms.
- For acute purulent rhinitis: persistent purulent rhinitis.
Secondary outcomes
- For both the common cold and acute purulent rhinitis: adverse effects.
- For the common cold: sore throat, loss of time at work, loss of appetite, sneezing.
- For purulent rhinitis: persisting purulent rhinitis on currently available medication.
Search methods for identification of studies
Electronic searches
For this 2013 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 1, part of The Cochrane Library, www.thecochranelibrary.com (accessed 26 March 2013), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (March 2005 to February 2013), EMBASE (January 2010 to February 2013), CINAHL (2005 to February 2013), LILACS (2005 to February 2013) and Biosis Previews (2005 to February 2013).
We used the following search strategy to search MEDLINE and CENTRAL. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted this search strategy to search EMBASE (Appendix 1), CINAHL (Appendix 2), LILACS (Appendix 3) and Biosis Previews (Appendix 4). The search strategy has been amended since the last published version as the authors have now extended the review to include persistent nasal discharge (rhinosinusitis). Details of earlier searches are in Appendix 5.
MEDLINE (Ovid)
1 Respiratory Tract Infections/ 2 (upper adj3 respiratory tract infection*).tw. 3 (upper adj3 respiratory infection*).tw. 4 urti.tw. 5 Common Cold/ 6 common cold*.tw. 7 head cold*.tw. 8 coryza.tw. 9 Rhinitis/ 10 rhinit*.tw. 11 rhinosinusit*.tw. 12 nasosinusit*.tw. 13 (rhinorrhoea or rhinorrhoea).tw. 14 Nasal Obstruction/ 15 ((runny or running or discharg* or congest* or blocked or stuff* or dripping) adj2 (nose* or nasal)).tw. 16 pharyngitis/ or nasopharyngitis/ 17 (pharyngit* or nasopharyngit*).tw. 18 rhinopharyngit*.tw. 19 Paranasal Sinus Diseases/ 20 exp Sinusitis/ 21 sinusit*.tw. 22 exp Laryngitis/ 23 laryngit*.tw. 24 sore throat*.tw. 25 or/1‐24 26 exp Anti‐Bacterial Agents/ 27 antibiotic*.tw,nm. 28 (amoxicillin* or amoxycillin* or ampicillin* or penicillin* or tetracycline* or erythromycin* or oxytetracycline* or azithromycin* or ciprofloxacin* or pivampicillin* or cefuroxime* or augmentin* or co‐trimoxazole* or cefoxitin* or ceftriaxone* or cefixime* or norfloxacin* or ceftazidime* or cefaclor* or ofloxacin*).tw,nm. 29 exp Sulfamethoxazole/ 30 (sulphamethoxazole or sulfamethoxazole or sulfisoxazole).tw,nm. 31 or/26‐30 32 25 and 31
Searching other resources
We wrote to the trial authors of all the included studies and asked them if they knew of any unpublished material. We did not find any additional papers by this mechanism.
Data collection and analysis
Selection of studies
The two review authors (BA, TK) independently checked the abstracts from the searches and selected the papers. We resolved disagreements through discussion. The Herne 1980 trial was translated from French to English. One paper was excluded because a placebo was not used in the control group (Sutrisna 1991).
Data extraction and management
The two review authors (BA, TK) selected the relevant abstracts after the full papers were obtained. The two review authors (BA, TK) independently extracted data and we resolved disagreements through discussion.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool for assessing bias (Higgins 2011) with consideration of: adequate sequence generation, concealment of allocation, adequate blinding, incomplete outcome reporting and freedom from selective reporting. The two review authors (BA, TK) independently assessed trial quality and any differences were resolved through discussion.
Measures of treatment effect
We used the risk ratio (RR) exclusively for dichotomous data.
Unit of analysis issues
All trials were standard parallel design randomised controlled trials (RCTs). The individual patient was the unit of analysis.
Dealing with missing data
None of the trials in this review except De Sutter 2002 conducted an intention‐to‐treat (ITT) analysis. All our results are per protocol analyses.
Assessment of heterogeneity
We assessed heterogeneity using the I2 statistic. When the I2 statistic was over 50% we changed the analysis from fixed‐effect to random‐effects.
Assessment of reporting biases
The majority of our studies were negative and this, to some extent, guards against publication bias. The one positive study may have included participants with bacterial conditions such as streptococcal tonsillitis.
Data synthesis
We conducted a meta‐analysis using the Review Manager (RevMan 2012) software.
Subgroup analysis and investigation of heterogeneity
There was no subgroup analysis.
Sensitivity analysis
We conducted a sensitivity analysis on the main analysis of persisting symptoms and omitted the trial by Herne 1980. This changed the I2 statistic from 54% to 0%. The Herne trial was the outlier in that it was the only statistically significant trial in the common cold section of the review and may have contained participants with streptococcal tonsillitis. We conducted another sensitivity analysis with the adverse effects for all the trials. This showed a statistically significant result in adults but not in children. The other sensitivity analysis was in the persistent purulent rhinitis outcome where the Herne trial was added for both clear and purulent rhinitis. When the Herne trial was added as purulent, the reduction in symptoms was just statistically significant. This did not change the conclusions.
Results
Description of studies
Results of the search
See Appendix 2 for details of previous searches. In the 2009 search we identified 545 papers. None of them met the inclusion criteria for this review. The 2013 search of the electronic resources retrieved 1128 records.
Included studies
Types of participants
Ten studies contributed data to the meta‐analyses. A further trial (Gordon 1974) reported only summary statistics, without providing any numerical data that could be included in the meta‐analyses. Six studies contributed to one or more analyses of common cold outcomes (up to 1047 participants) and five contributed to one or more analyses of purulent rhinitis outcomes (up to 791 participants). Two contributed to both (Herne 1980; Taylor 1977). One study contributed to the adverse effects but did not contribute to the other outcome data (Howie 1970). Four of the trials in this review included children only (Gordon 1974; Lexomboon 1971; Taylor 1977; Todd 1984). Vogt 1966 included participants recruited from paediatricians' offices, so there is an implication that these were all children. The remaining studies included:
- adolescents and adults with no upper limit of age (Herne 1980; Hoaglund 1950; Kaiser 1996);
- males aged 20 to 49 years (Howie 1970); and
- adults up to 69 years (McKerrow 1961).
Three studies examined men only (Herne 1980; Hoaglund 1950; Howie 1970).
Types of outcome measures
Only four studies measured the effect of antibiotics on symptoms (Herne 1980; Howie 1970; Taylor 1977; Todd 1984). The denominator for one of these, Howie 1970, was episodes of illness rather than the number of participants, which prevented these data from being pooled with other studies. However, adverse effects in this study were based on individuals, hence adverse effects reported have been added to those from other studies (except for outcomes from five participants who had adverse effects with more than one course of antibiotics and were removed from the analysis).
Types of interventions
Antibiotics used included:
- a form of tetracycline, in five studies (Herne 1980; Hoaglund 1950; Howie 1970; Lexomboon 1971; McKerrow 1961);
- penicillin, ampicillin and amoxicillin, and amoxicillin/clavulanic acid, in five studies (De Sutter 2002; Gordon 1974; Kaiser 1996; Lexomboon 1971; Taylor 1977);
- erythromycin, in one study (Gordon 1974);
- co‐trimoxazole, in one study (Taylor 1977);
- cephalosporin, in one study (Todd 1984);
- an antibacterial called xibornol (no longer on the market in France or elsewhere) in one study (Herne 1980) and also mentioned by Reynolds 1996; and
- topical nitrofurazone, in one study (Vogt 1966).
Nasopharyngeal cultures
Kaiser 1996 performed an analysis by culture status of three nasopharyngeal pathogens: Haemophilus influenzae (H. influenzae),Moraxella catarrhalis (M. catarrhalis) and Streptococcus pneumoniae (S. pneumoniae). The only trial to do throat swabs on almost all (88 out of 89) participants was Gordon 1974. These participants were included in the analysis. Herne 1980 excluded some participants from the analysis because they developed a red, pustular tonsillitis thought to be due to beta haemolytic streptococcus.
Inclusion criteria
The inclusion criteria were either contained in the methods section or in the title of the study. They were:
- in Gordon 1974, participants with minor respiratory illness (in the title) and symptoms referable to the respiratory tract, not sick enough for the use of a placebo to be a risk;
- in Herne 1980, non‐severe upper respiratory tract infection (in the methods);
- in Hoaglund 1950, common cold (in the title) and local and constitutional symptoms (in the methods);
- in Howie 1970, minor respiratory illness (in the title) and presence of cough, spit, purulent spit and purulent nasal discharge;
- in Kaiser 1996, common cold (in the title) and symptoms of nasal congestion and rhinorrhoea (in the methods);
- in Lexomboon 1971, upper respiratory tract infection (URTI) (in the title) and symptoms of URTI within the last 48 hours (in the methods);
- in McKerrow 1961 common cold (in the title);
- in Taylor 1977 participants with presumed viral respiratory infection (in the title) and exclusion of participants with beta haemolytic streptococcus on throat swabs, otitis media and pneumonia;
- in Todd 1984, non‐transparent anterior nasal discharge (in the methods section).
Two included studies (De Sutter 2002; Vogt 1966) focused on acute purulent rhinitis.
Excluded studies
We excluded 31 studies. The major reason for exclusion was that they were not placebo‐controlled (usually compared to another antibiotic or an over‐the‐counter medication). There were also a number of trials conducted in the military which used prophylactic antibiotics.
Risk of bias in included studies
All of the included trials were double‐blind evaluations comparing antibiotic with a placebo. It was assumed that blinding was satisfactory because all studies included a statement that medication was given in a double‐blind manner and un‐blinding of participants was not reported. However, no formal description of blinding was included in any of the trials.
The method of randomisation was marginally satisfactory for some of the studies. In Hoaglund 1950 the pharmacist dispensed the medication "in rotation". The method of randomisation was "haphazard" in the study by Vogt 1966, hence there is some concern about the methods of this study. In Lexomboon 1971 the allocation was determined by the selection of coloured strips and only the pharmacist knew the allocation. The method of randomisation for the De Sutter 2002 study was by computer‐generated random number and the medications (capsules) were identical.
All but two of the studies had either inclusion criteria that selected cases of acute viral respiratory tract infection and/or had the term viral or minor respiratory infection or common cold in the title of the study. The exception was Todd 1984, where the children had non‐transparent nasal discharge. While there was some variation in the inclusion criteria (for example, Gordon 1974 found 13% of children under two years old and 16% of two‐ to four‐year olds had clinical signs in the chest) the study groups generally included participants with acute URTIs. There were no extractable data in this study so the results were not pooled.
Loss to follow‐up was an issue for a number of the studies; only one analysed results on an ITT basis (De Sutter 2002).
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
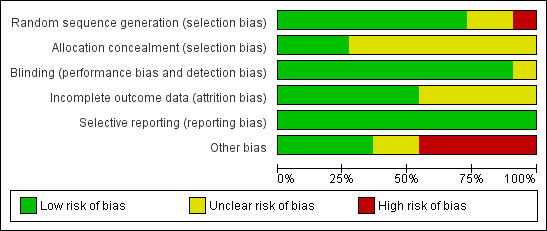
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
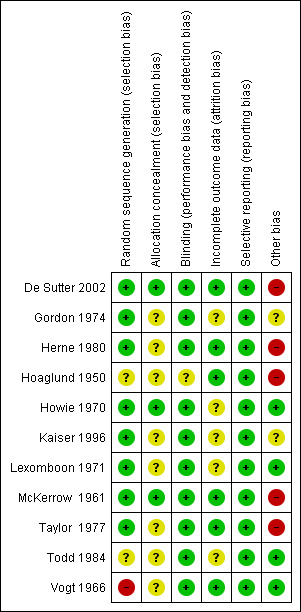
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
There was a range of different methods of randomisation. They ranged from computerised randomisation (De Sutter 2002), randomised and no details (Herne 1980), dispensed in rotation by the pharmacist (Hoaglund 1950), and random selection of coloured strips from a box (only the pharmacists knew the allocation) (Lexomboon 1971).
Blinding
All trials had identical active and placebo medications.
Incomplete outcome data
The drop‐out rate ranged from 0% (Gordon 1974; Hoaglund 1950) to 25% (Todd 1984). There do not seem to be any differential drop‐out rates so it is unlikely that this would affect the outcomes.
Selective reporting
There was no evidence of selective reporting.
Other potential sources of bias
The potential for bias in a trial of a predominantly viral condition is that of bacterial co‐morbidity. In the De Sutter 2002 trial more then 60% of the participants had facial pain and some of these may have had bacterial sinusitis. In the study by Herne 1980 there were a number of participants with streptococcal tonsillitis. Both these co‐morbidities could over estimate the effectiveness of treatment and possibly did so in the Herne 1980 trial. The fact that the overall pooling showed no effect means that these trials did not affect the outcome.
Effects of interventions
All analyses used the fixed‐effect model unless otherwise stated.
Lack of cure and persisting symptoms
Studies reported different outcome measures but all those with analysable data reported some general aspect of improvement. This was defined as:
- persistence of clinical symptoms and signs at day five (Herne 1980);
- no benefit in 24 hours (Hoaglund 1950);
- symptoms persistent or worse at five days (Kaiser 1996);
- not better at day seven (Lexomboon 1971);
- no cure or not improved in three days (McKerrow 1961);
- not returning to normal activity (Taylor 1977); and
- persistence of purulent nasal discharge at day 10 (De Sutter 2002).
The risk ratio (RR) of no cure or persistence was 0.95 (95% confidence interval (CI) 0.59 to 1.51, random‐effects) (Analysis 1.1). When we analysed results for children and adults separately, there was no significant finding for lack of cure or persistence of symptoms in either group (Analysis 1.3; Analysis 1.4). Herne 1980 was the only study to show a benefit with antibiotics. Seven participants were removed from the analysis by the investigators because of tonsillitis and the review authors were concerned that there may have been more in this study. When Herne 1980 was removed from the analysis, the RR of no cure or persistence increased to 1.11 (95% CI 0.70 to 1.76) (Analysis 1.2).
1.1. Analysis.
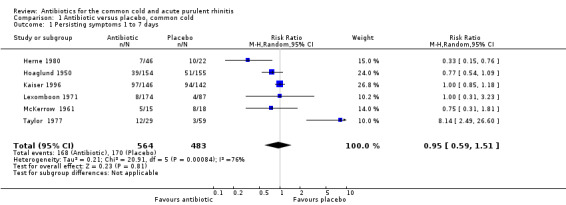
Comparison 1 Antibiotic versus placebo, common cold, Outcome 1 Persisting symptoms 1 to 7 days.
1.3. Analysis.
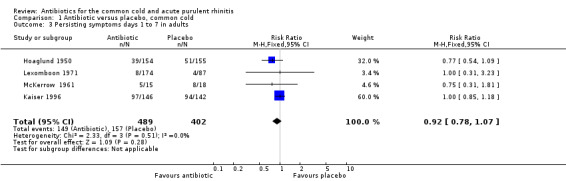
Comparison 1 Antibiotic versus placebo, common cold, Outcome 3 Persisting symptoms days 1 to 7 in adults.
1.4. Analysis.
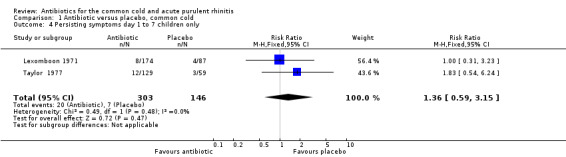
Comparison 1 Antibiotic versus placebo, common cold, Outcome 4 Persisting symptoms day 1 to 7 children only.
1.2. Analysis.
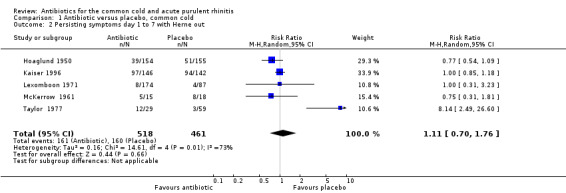
Comparison 1 Antibiotic versus placebo, common cold, Outcome 2 Persisting symptoms day 1 to 7 with Herne out.
Howie 1970 reported outcomes using episodes of illness as the denominator, which meant that the data could not be pooled with the other studies. This study analysed antibiotic versus placebo by signs and symptoms (cough, purulent nasal discharge, spit and purulent spit) and found no significant differences.
Analysis by type of antibiotic was not undertaken for any of the outcomes. The results for different types of antibiotics were added together in situations where more than one antibiotic was used in a study.
The only study not to contribute to this lack of cure and persistence of symptoms analysis (other than Howie) was Gordon 1974. Their results were expressed as P values rather than a count of participants with symptoms and they found that placebo was better at relief of symptoms than ampicillin (P = 0.05); there was no significant difference between placebo and erythromycin or placebo and penicillin. Only two participants grew beta haemolytic streptococcus in that study so it was decided to leave the study in this review.
Sensitivity analysis of lack of cure and persisting symptoms
Delay starting antibiotic and low dose antibiotic
Hoaglund 1950 assessed the outcome of treatment at 24 hours after medication was started, that is sooner than antibiotics are thought to work. McKerrow 1961 used 15 mg tablets three times daily for the three tetracycline groups in their study. Today this dose would be regarded as sub‐therapeutic. Analysis excluding these two studies did not significantly change the RR (Analysis 1.5).
1.5. Analysis.
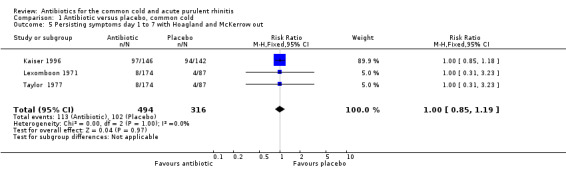
Comparison 1 Antibiotic versus placebo, common cold, Outcome 5 Persisting symptoms day 1 to 7 with Hoagland and McKerrow out.
Positive throat swabs
Kaiser 1996 found a significant benefit (cure) for antibiotics only in the subset of participants who had positive nasopharyngeal aspirates for any one of three respiratory pathogens (H. influenzae,M. catarrhalis and S. pneumoniae), which occurred in 20% of the study participants. Open treatment was prescribed for 11 (39%) participants on placebo and three (10%) participants assigned to amoxicillin/clavulanic acid. When Kaiser 1996 was excluded from the analysis there was no significant change in the effect (RR 1.00 95% CI 0.45 to 2.22) (Analysis 1.6). We acknowledge the contradiction of including a study with some participants who had a bacterial diagnosis on retropharyngeal swabs while excluding studies with participants who had positive throat swabs for streptococcus.
1.6. Analysis.
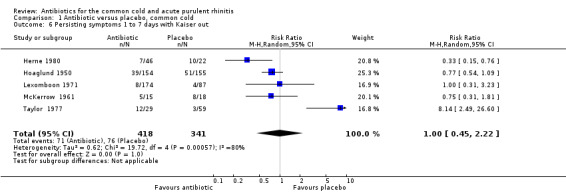
Comparison 1 Antibiotic versus placebo, common cold, Outcome 6 Persisting symptoms 1 to 7 days with Kaiser out.
Active medication in the control group
In Ackerman 1968, penicillin and tetracycline were compared with a control group taking dextromethorphan (Robitussin), which is a cough suppressant and for which there was some evidence that it was effective. It thus cannot be assumed to be an inert placebo. A sensitivity analysis adding Ackerman 1968 to the other studies made no significant difference to the RR. Also, our review was directed at antibiotics versus placebo. Consequently this paper has been left out of the final analysis.
Smoking
Six of the 10 reviewed studies were in adults but smoking was considered in only one of these studies (Howie 1970). In this study there was no significant difference between active and placebo treatments in terms of benefit to smokers or non smokers (P value > 0.05).
Other outcomes
Sore throat
Only two studies reported on sore throat persisting at day seven as an outcome (Herne 1980; Taylor 1977). The RR was 0.47 95% CI 0.12 to 1.82 for persisting sore throat, with no significant difference between the treatment groups (Analysis 4.1).
4.1. Analysis.
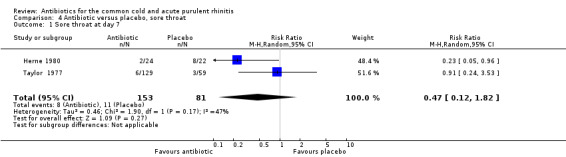
Comparison 4 Antibiotic versus placebo, sore throat, Outcome 1 Sore throat at day 7.
Loss of appetite
There was only one study which reported the lack of return of appetite at day eight (Taylor 1977). The RR of a lack of return of appetite was 0.97, 95% CI 0.44 to 2.12 (Analysis 6.1). Return of appetite was not specified a priori as an item warranting analysis.
6.1. Analysis.
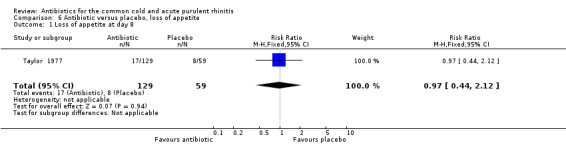
Comparison 6 Antibiotic versus placebo, loss of appetite, Outcome 1 Loss of appetite at day 8.
Loss of time at work
The only study to report loss of time at work was Howie 1970, where the RR for any work loss per episode of illness was 0.88, 95% CI 0.69 to 1.13 (Analysis 5.1).
5.1. Analysis.
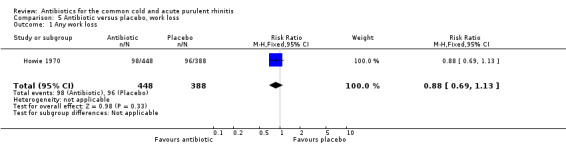
Comparison 5 Antibiotic versus placebo, work loss, Outcome 1 Any work loss.
Sneezing
None of the studies reported sneezing as an outcome.
Adverse effects
Six studies reported adverse effects experienced by individual participants. When all studies were combined there was a significant difference for adverse effects (RR 1.80, 95% CI 1.01 to 3.21; random‐effects model) but there was a high level of heterogeneity (Analysis 1.7). This appeared to be due to a difference between adults and children because when analysed separately there was a significant difference for adults (RR 2.62, 95% CI 1.32 to 5.18; random‐effects model) (Analysis 1.8) but not for children (RR 0.91, 95% CI 0.51 to 1.63) Analysis 1.9; heterogeneity for the child analysis was not significant. McKerrow 1961 did not distinguish between the pneumoconiosis group and the office (unit) group so the proportion of adverse effects in the office unit group were allocated in proportion to the number of participants in the two parts of the study; with this study removed the increase in adverse effects with antibiotics was not significant (RR1.68, 95% CI 0.67 to 4.20) (Analysis 1.10). Some studies did not report adverse effects (Gordon 1974; Herne 1980; Hoaglund 1950; Lexomboon 1971; Vogt 1966). In the purulent rhinitis studies there was a statistically significant increase in adverse effects (RR 1.46, 95% CI 1.1 to 1.94) (Analysis 3.4).
1.7. Analysis.
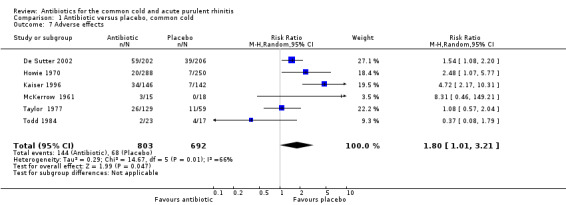
Comparison 1 Antibiotic versus placebo, common cold, Outcome 7 Adverse effects.
1.8. Analysis.
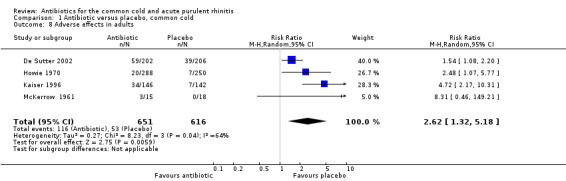
Comparison 1 Antibiotic versus placebo, common cold, Outcome 8 Adverse effects in adults.
1.9. Analysis.
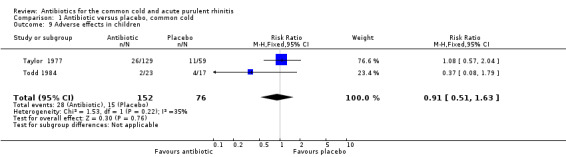
Comparison 1 Antibiotic versus placebo, common cold, Outcome 9 Adverse effects in children.
1.10. Analysis.
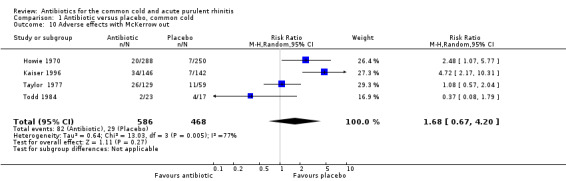
Comparison 1 Antibiotic versus placebo, common cold, Outcome 10 Adverse effects with McKerrow out.
3.4. Analysis.
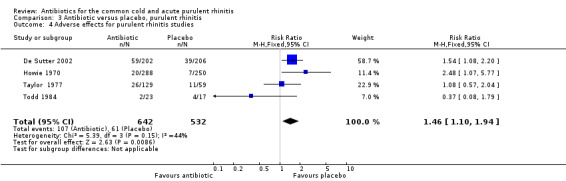
Comparison 3 Antibiotic versus placebo, purulent rhinitis, Outcome 4 Adverse effects for purulent rhinitis studies.
Rhinitis purulent and clear
The study by Herne 1980 did not specify if the rhinitis was purulent or not. When this study was excluded, pooling the other four antibiotic versus placebo studies for purulent rhinitis gave a RR of 0.73 (95% CI 0.47 to 1.13; random‐effects model) (Analysis 3.1). When Herne 1980 was added to the purulent group, the RR was 0.67 (95% CI 0.43 to 1.04; random‐effects model) for persisting purulent rhinitis (Analysis 3.2). Because xibornol and inhaled nitrofurazone are no longer available, pooling the three studies plus the tetracycline arm of the Herne study gave a RR of 0.74 (95% CI 0.46 to 1.18; random‐effects model) for persisting purulent rhinitis on currently available antibiotics (Analysis 3.3). It is worth noting that in Howie 1970 there was no significant benefit from antibiotics in participants with purulent nasal discharge but this study used illnesses, not individuals, as the denominator. Antibiotics also appeared to be not effective for clear rhinitis when Herne was added as clear rhinitis (RR 0.58, 95% CI 0.23 to 1.48; random‐effects model) (Analysis 2.1). Five studies reported purulent rhinitis with no additional explanation (Howie 1970; Kaiser 1996; Taylor 1977; Todd 1984; Vogt 1966). De Sutter 2002 defined purulent rhinitis on a purely clinical basis (that is, the recruiting doctors made the decision). Nineteen per cent of those in Kaiser 1996 had radiologically confirmed sinusitis. Fifty‐three per cent to 56% of participants in De Sutter 2002 reported unilateral facial pain. The incidence of unilateral facial pain was not reported in six trials (Herne 1980; Howie 1970; Kaiser 1996; Taylor 1977; Todd 1984; Vogt 1966).
3.1. Analysis.
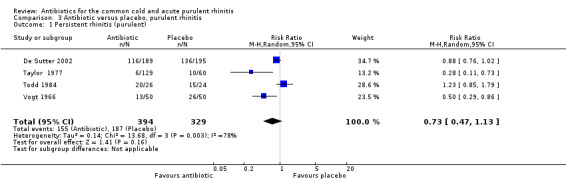
Comparison 3 Antibiotic versus placebo, purulent rhinitis, Outcome 1 Persistent rhinitis (purulent).
3.2. Analysis.
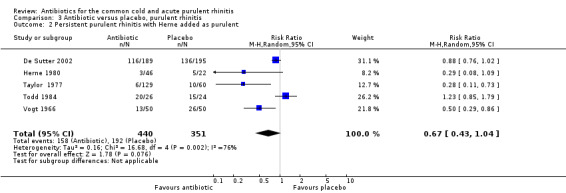
Comparison 3 Antibiotic versus placebo, purulent rhinitis, Outcome 2 Persistent purulent rhinitis with Herne added as purulent.
3.3. Analysis.
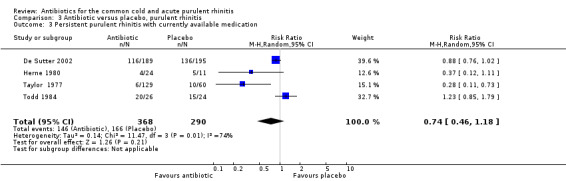
Comparison 3 Antibiotic versus placebo, purulent rhinitis, Outcome 3 Persistent purulent rhinitis with currently available medication.
2.1. Analysis.
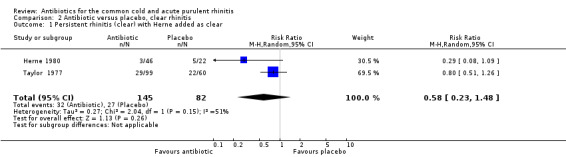
Comparison 2 Antibiotic versus placebo, clear rhinitis, Outcome 1 Persistent rhinitis (clear) with Herne added as clear.
Discussion
While the International Classification of Health Problems in Primary Care (ICHPPC) gives one definition of upper respiratory tract infection (URTI), it is not clear how rigidly this definition applies in practice. The review authors have accepted that the diagnosis of URTI is made on clinical grounds and treatment decisions are made on the basis of that clinical decision. Although the range of inclusion criteria appears wide, the review authors accept that the majority of participants in each study in this review were suffering from viral URTIs. Only three studies reported their findings in terms of the lower respiratory tract. Gordon 1974 reported 13% of children under two years old and 13% of those over six years to have lower respiratory tract signs. Forty‐three per cent (84 out of 197) of participants in Taylor 1977 had auscultatory evidence of a more extensive peripheral airways disease.
Herne 1980 included seven participants with bronchitis: four in the tetracycline group, two in the xibornol group and one in the placebo group. In the context we assumed this diagnosis was mainly due to bronchospasm. We excluded studies with significant numbers of participants with streptococci on throat swabs (see Characteristics of excluded studies table). We included Gordon 1974, which found 2% of participants (2 of 89) with cultures of beta haemolytic streptococci. Seven participants were excluded from Herne 1980 because the pharynx looked as though the participants had clinical streptococcal tonsillitis.
Other studies did not test for throat bacteria. Cultures that are positive for streptococci may include contaminants as well as cases of true infection. The issue of bacterial involvement is a concern for reviews of bronchitis, sore throats and upper respiratory tract infections. There is no overlap with Fahey's bronchitis review (Fahey 1998) but there are two studies (Howie 1970; Kaiser 1996) that are also in the Smith 2011 bronchitis review.
The review by Del Mar 1992 on managing sore throat included one study that is in our review (Taylor 1977). Clearly the larger the proportion of illnesses caused by bacteria that exist among the cases of viral illness the more likely the findings are to show a benefit for antibiotics. There is no other way of defining the disease of interest other than perhaps doing nasopharyngeal aspirations, as done in Kaiser 1996, and throat cultures on all participants. This is not current clinical practice and the purpose of our review is to be relevant to clinical practice.
The a priori outcomes for analysis included persistence of symptoms of nasopharyngeal inflammation, global rating of health and adverse effects. The summary risk ratio (RR) for general improvement or cure was 0.95 (95% CI 0.59 to 1.51) (Analysis 1.1). In contrast with the Cochrane Review by Smith 2011, there was no over‐reporting of significant findings. Indeed, there was a tendency to under‐report findings and, hence, less likelihood of a type one statistical error.
Most studies did not report adverse effects. However, the summary RR for adverse effects from the four studies in adults that had analysable data was significant. The majority of adverse effects were gastrointestinal, which is consistent with clinical anecdote. In the two studies in children there was no significant adverse effect from antibiotics, which is consistent with the findings of Fahey 1998.
The symptoms of nasopharyngeal inflammation chosen for analysis were sore throat and runny nose, either purulent or clear. No studies reported sneezing as an outcome. Six trials provided outcome data on nasopharyngeal inflammation (De Sutter 2002; Herne 1980; Howie 1970; Todd 1984; Taylor 1977; Vogt 1966). Data from Howie 1970 were not in a form that could be used in this review but indicated no benefit from antibiotics for purulent nasal discharge. Pooling the purulent rhinitis data suggested that antibiotics are not beneficial for this condition. For runny nose with a clear discharge there was no significant benefit when Herne 1980 and Taylor 1977 were pooled. As noted above, the definition of the type of rhinitis in Herne 1980 was not given and hence it was added to both clear and purulent rhinitis. There is collateral information about this in the literature. In an acute bronchitis study (Stott 1976) there was a lower incidence of runny nose at day seven in the doxycycline group than in the placebo group (P < 0.01). There is a Cochrane Review on persisting rhinosinusitis (more than 10 days duration) which shows a benefit from treating with antibiotics (Morris 2007).
Sore throats
The finding of no benefit from antibiotics for sore throat was expected. We would suggest the reader read the Cochrane Review on antibiotics for sore throat (Spinks 2011). The role of bacteria in upper respiratory tract infections, either as a causal factor or a complication, is highlighted in Kaiser 1996. The authors make a convincing argument for the role of three types of bacteria (H. influenzae, M. catarrhalis and S. pneumoniae). It would be helpful to see this work repeated in another centre. Treatment in this subgroup resulted in one day less of symptoms.
Sinusitis or purulent rhinitis
The difference between sinusitis and acute purulent rhinitis is not clear. There is presumably an overlap: 19% of the participants in Kaiser 1996 had radiological sinusitis and between 53% and 56% of the participants in De Sutter 2002 had unilateral facial pain. In one study, which was excluded because of a greater than 10‐day duration of purulent rhinitis, all participants had to have facial X‐rays free of abnormalities (Haye 1998). In this study azithromycin was significantly more effective than placebo on the outcome of purulent rhinitis. This suggests that participants may be able to have purulent rhinitis without sinus involvement. These issues need to be taken into account in future studies. The methodological quality of the studies before 1990 was low and hence it is difficult to draw firm conclusions from these.
Generalisability
Although only three of the studies were conducted in general practice (De Sutter 2002; Howie 1970; Taylor 1977), the others seemed to represent a cross‐section of participants seen in primary care settings. This indicates that our results are generalisable to the wider primary care setting if the assumption is made that participants can self refer to these secondary care settings and use them as primary care providers. Other study settings were:
- a military base (Hoaglund 1950); unit staff (McKerrow 1961);
- hospital outpatients (Kaiser 1996; Lexomboon 1971);
- paediatricians in private practice (Vogt 1966);
- casualty clinic (Gordon 1974); and
- military base (Herne 1980; Todd 1984).
The heterogeneity of the pooled data for adverse effects from all the studies appears to come from the difference between adverse effects in adults and children. There was no heterogeneity when they were analysed separately. In one arm of Taylor 1977 the group taking co‐trimoxazole had more adverse effects than the placebo group, while the amoxycillin group had fewer. None of the children were given amoxycillin/clavulanic acid, which contributed a lot to the adverse effects in Kaiser 1996. This finding in children is consistent with Fahey 1998. That review included the two studies in our review and three which we excluded. This negative finding in children relates mainly to the unexpected findings in Taylor 1977. There is no methodological reason for this and our assumption would be that this is a chance (and erroneous) finding. The expected finding would be for children to get more adverse effects in the antibiotic group, as was found in the Cochrane Review on antibiotics for acute otitis media (Venekamp 2013). The adverse effects in the purulent rhinitis group were statistically significant.
Summary of main results
The RR for lack of cure or persistence of symptoms is 0.95, 95% CI 0.59 to 1.51 (Analysis 1.1). The overall RR for adverse effects for antibiotics given to participants with the common cold is 1.8, 95% CI 1.01 to 3.21 (Analysis 1.7) and for purulent rhinitis is 1.46, 95% CI 1.1 to 1.94 (Analysis 3.4). The overall RR for the antibiotic treatment of persisting acute purulent rhinitis is 0.73, 95% CI 0.47 to 1.13 (Analysis 3.1). In summary, there is no benefit for antibiotics for the common cold or acute purulent rhinitis but there is an increase in adverse effects.
Overall completeness and applicability of evidence
There is unclear risk of bias across the studies. All of the trials were randomised controlled trials (RCTs). However, methods of randomisation were not always clear. All the medications were identical in each trial. Most of the participants had a viral upper respiratory tract infection but some may have had bacterial chest or sinus infections.
Quality of the evidence
See comments in previous section.
Potential biases in the review process
The major bias is likely to be the inclusion of participants with bacterial disease such as streptococcal tonsillitis or bacterial sinusitis. The fact that the pooled result was not significant may mean this is a small consideration.
Agreements and disagreements with other studies or reviews
The findings of this study are consistent with two reviews in children, Gadomski 1993 and Fahey 1998, which found no benefit for antibiotics.
Authors' conclusions
Implications for practice.
Antibiotics offer no benefit in the initial treatment of the common cold (acute upper respiratory tract infections (URTIs)). Antibiotics should not be given in the first instance as they will not improve the symptoms and adult participants will be affected by their adverse effects. Antibiotics offer no benefit for acute purulent rhinitis while there is an increase in adverse effects. However, if the symptoms persist for more than 10 days then antibiotic therapy may be beneficial (Morris 2007) and clinicians may wish to negotiate the use of them with patients, taking into account the resistance issues.
Implications for research.
Further research is needed on the role of pathogenic nasopharyngeal bacteria and their presence in URTIs. Studies need to be more diligent in reporting adverse effects and symptoms.
Feedback
Antibiotics for the common cold
Summary
METHODS and METHODOLOGICAL QUALITIES OF INCLUDED STUDIES The reviewers use a scoring system for methodological quality, whereby each trial is given a numerical score on a scale of 1 to 12 points, which they say is described in the Cochrane Handbook. The Handbook in fact advises against composite scores as they are not transparent. In the Methodological quality of included studies section, the reviewer's then say that they do not think that these scores are an accurate measure of study quality. Furthermore, trial quality is not incorporated into interpretation of the results. It is therefore difficult for the reader to assess how systematic bias in the included studies might have affected the findings reported in the review.
It is not clear how concealment of allocation in each trial was assessed. Have the reviewers erroneously used reporting of double blinding in the trials to assess concealment of the allocation sequence? The trials Howie 1970, Kaiser 1996 and Taylor 1977 are reported as having adequate concealment of allocation (A) yet there is no description of how this was achieved. In the trials Hoaglund 1950 and Lexomboom 1971, allocation concealment is reported as unclear (B) yet, according to the Table of characteristics of included studies, in the latter only the pharmacist knew of allocation. Also, why have the reviewers use the option (D) not to assign a score for allocation concealment to the trial Sutrisna 1991? The reader can not find any information in the review about adequacy of allocation concealment for the trial Gordon 1974.
The statement, in Methodological quality of included studies, that "Loss to follow‐up was an issue for a number of studies..." needs to be expanded and loss to follow‐up in each trial should be documented in the table of characteristics of included studies. Presumably the denominators used in the analyses are participants who were evaluable? How might loss to follow‐up have affected the findings in the review (worst case and best case scenario)?
RESULTS In the meta‐analysis of General improvement, it would be better to present the Hoaglund study separately if, as the reviewer's say, there is good biological reason why measuring this outcome at 24 hours does not make sense.
It is questionable to calculate numbers needed to treat and numbers needed to harm from pooled data without qualification of the conditions to which they apply, and they should not be reported without confidence intervals.
There are several inconsistencies in the numerical data reported in the text compared with the graphs.
CONCLUSIONS The statement, in Implications for practice, that "many participants will get adverse effects" from antibiotics is not supported by evidence presented in the review. The principle of using a random effect model of analysis where there is significant heterogeneity, without going on to explore possible reasons to explain it, is questionable.
REFERENCES The references should be listed in the appropriate reference sections rather than included as text at the end of the conclusions.
The above comment was made as part of a collaborative effort coordinated by Ole Olsen at the Nordic Cochrane Centre. All new reviews on Cochrane Library 1998.4 were selected and critically read by a set of methodologists, comments were coordinated and finally fed back. The general results of the survey will be presented at the Cochrane Colloquium in Rome, October 1999.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
Author's response:
I thought the comments made were very helpful to us. I have been struggling with the numbers needed to treat information and the recent BMJ article and some stuff from the EBM mailbase has been useful. I can see the advantages of an electronic database where changes can be continuously made.
METHODS and METHODOLOGICAL QUALITIES OF INCLUDED STUDIES 1. The two papers which did not contribute to the review for benefit were the two high scoring studies. Howie with a score of 9 and Gordon with a score of 8. The others either had a 6 or a 7 and we did not feel that there was any face validity in using such a small score difference to meta‐analyse the others (referring to The Cochrane Library 1999, Issue 2 ‐ the commentator may be referring to the original review which was in fact a draft and put on the Cochrane database by mistake).
2. We assumed that if the authors said they randomised the study then there was adequate concealment of allocation. The study by Sutrisna has been eliminated from the final review as it did not have a placebo control group.
3. The worst case scenario found that the adverse effects became statistically significant when added to the adverse effects of antibiotics but did not change the findings when added to placebo. There was no change for treatment or control when the missing participants were added for general improvement.
RESULTS 4. Removing the Hoaglund study did not alter the results nor did removing the McKerrow study where very low doses of tetracycline (15 mg) were used (these would be considered to be sub‐therapeutic today).
5. We agree with this comment and quote Smeeth et al BMJ 1999;318:1548‐51 that it is best not to use NNT derived from meta‐analyses as there are differences in baseline risk. In the next update there are no statistically significant findings apart from runny nose (both purulent and clear in one study). Hence there will be no NNT or NNH. The adverse effects findings have changed as there were 5 participants in the trial by Howie who had side effects on more than one occasion and hence contributed more to the adverse effects. With those 5 participants removed the adverse effects confidence interval now includes one.
6. There were two typographical errors in translating the tables to the data form. Correction of this did not alter the findings.
CONCLUSIONS 7. The commentator may be looking at the original version of the review which as explained above was a draft version. The current version has "there is a significant increase in adverse effects associated with antibiotic use. RR 1.37; 95% CI 1.34 to 1.39". This is consistent with findings of other meta‐analyses of antibiotics versus placebo, e.g. Sanders S, Glasziou PP, Del Mar C, Rovers M. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2004, Issue 1. Art. No.: CD000219. DOI: 10.1002/14651858.CD000219.pub2. In the next version of the review we have removed 5 participants from the adverse effect intervention group. This makes the adverse event findings non‐significant and hence our conclusions will need to note that they are not significant but are very similar to those in other meta‐analyses of antibiotic versus placebo which were significant.
8. This will be added to the next version.
Contributors
Heather McIntosh
What's new
| Date | Event | Description |
|---|---|---|
| 26 February 2013 | New search has been performed | Searches conducted. We found no new trials. Numbers from the De Sutter 2002 trial with the outcome of persistent purulent rhinitis were corrected. |
| 26 February 2013 | New citation required but conclusions have not changed | There is no substantive change in the statistical results. Our conclusions remain unchanged. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 11 August 2009 | Amended | Searches conducted. No new studies were found. We have made a few amendments, for example, changing Herne 1980 to one antibiotic group and Taylor 1977 to one antibiotic group. This has the impact of reducing the weight for these studies in the random‐effects analysis, hence this is a conservative bias but our conclusions remain unchanged. Antibiotics are not effective as an initial treatment for patients with the common cold. Our analysis now shows no benefit for antibiotics for acute purulent rhinitis and our recommendation is that they should not be used initially. It may be reasonable to negotiate the use of antibiotics with patients if the purulent rhinitis persists for more than 10 days. However, even in these cases, most patients get better without antibiotics. |
| 18 July 2008 | Amended | Converted to new review format. |
| 8 March 2005 | New search has been performed | In this updated review (2005) an additional four studies were identified and added to the review. The first, Herne 1980, was in French, which necessitated translation into English. The addition of this result has not altered the findings of the common cold systematic review. The other papers added to the review were the studies by De Sutter 2002, Todd 1984 and Vogt 1966, which dealt with the treatment of purulent rhinitis. It was pointed out to us by peer reviewers that two studies, Howie 1970 and Kaiser 1996, included patients with purulent rhinitis and hence we decided to expand the review to include patients with acute purulent rhinitis. While there is some variation in the findings, they are suggestive of there being a benefit from antibiotics for this condition. In the past, guidelines have suggested not using antibiotics as they are not effective. The review authors suggest not recommending antibiotics in the first instance as most people will get better without them. |
| 16 November 2004 | Feedback has been incorporated | Feedback comment and reply added. |
| 31 July 2001 | New search has been performed | Searches conducted. |
| 20 July 1997 | New search has been performed | Review first published Issue 4, 1998. |
Acknowledgements
The Charitable Trust of the Auckland Faculty of the Royal New Zealand College of General Practice (RNZCGP) for a grant to undertake the literature search. Thanks to Elspeth Kay for editing the penultimate version in Arroll 2005. The authors also wish to thank Leonard Leibovici, Mark Jones, Janet Yarrow and Dennis Conrad for commenting on the draft Arroll 2005 review.
Appendices
Appendix 1. Embase.com search strategy
#35. #31 AND #34 1,818 12 Sep 2011 #34. #32 OR #33 891,645 12 Sep 2011 #33. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR allocat*:ab,ti OR assign*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 851,315 12 Sep 2011 #32. 'randomized controlled trial'/de OR 'single blind procedure'/de OR 'double blind procedure'/de OR 'crossover procedure'/de AND [embase]/lim 248,094 12 Sep 2011 #31. #24 AND #30 16,060 12 Sep 2011 #30. #25 OR #26 OR #27 OR #28 OR #29 #29. sulfamethoxazole*:ab,ti OR sulphamethoxazole*:ab,ti OR sulfisoxazole*:ab,ti AND [embase]/lim 8,672 12 Sep 2011 #28. 'sulfamethoxazole'/de AND [embase]/lim 12,140 12 Sep 2011 #27. amoxicillin*:ab,ti OR amoxycillin*:ab,ti OR ampicillin*:ab,ti OR penicillin*:ab,ti OR tetracyclin*:ab,ti OR erythromycin*:ab,ti OR oxytetracyclin*:ab,ti OR azithromycin*:ab,ti OR ciprofloxacin*:ab,ti OR pivampicillin*:ab,ti OR cefuroxim*:ab,ti OR augmentin*:ab,ti OR cotrimoxazole*:ab,ti OR 'co‐trimoxazole':ab,ti OR cefoxitin*:ab,ti OR ceftriaxome*:ab,ti OR cefixime*:ab,ti OR norfloxacin*:ab,ti OR ceftazidime*:ab,ti OR cefaclor*:ab,ti OR ofloxacin*:ab,ti AND [embase]/lim 115,374 12 Sep 2011 #26. antibiotic*:ab,ti AND [embase]/lim 180,884 12 Sep 2011 #25. 'antibiotic agent'/exp AND [embase]/lim 720,198 12 Sep 2011 #24. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 74,116 12 Sep 2011 #23. 'sore throat'/de AND [embase]/lim 6,511 12 Sep 2011 #22. laryngit*:ab,ti AND [embase]/lim 1,058 12 Sep 2011 #21. 'laryngitis'/de AND [embase]/lim 1,909 12 Sep 2011 #20. 'sinusitis'/de OR 'rhinosinusitis'/de OR 'paranasal sinusitis'/de OR 'bacterial sinusitis'/de OR 'viral sinusitis'/de AND [embase]/lim 14,866 12 Sep 2011 #19. 'paranasal sinus disease'/de OR 'sinus congestion'/de AND [embase]/lim 1,468 12 Sep 2011 #18. nasopharyngit*:ab,ti OR rhinopharyngit*:ab,ti AND [embase]/lim 551 12 Sep 2011 #17. 'rhinopharyngitis'/de AND [embase]/lim 3,825 12 Sep 2011 #16. 'pharyngitis'/de AND [embase]/lim 8,874 12 Sep 2011 #15. ((runny OR running OR discharg* OR congest* OR blocked OR stuff* OR dripping) NEAR/2 (nose* OR nasal)):ab,ti AND [embase]/lim 3,257 12 Sep 2011 #14. 'nose infection'/de OR 'nose obstruction'/de AND [embase]/lim 5,392 12 Sep 2011 #13. rhinorrhea:ab,ti OR rhinorrhoea:ab,ti AND [embase]/lim 3,101 12 Sep 2011 #12. rhinosinusit*:ab,ti OR nasosinusit*:ab,ti AND [embase]/lim 3,154 12 Sep 2011 #11. 'rhinosinusitis'/de AND [embase]/lim 3,163 12 Sep 2011 #10. rhinit*:ab,ti AND [embase]/lim 17,599 12 Sep 2011 #9. 'rhinitis'/de AND [embase]/lim 10,191 12 Sep 2011 #8. 'common cold symptom'/de AND [embase]/lim 180 12 Sep 2011 #7. coryza:ab,ti AND [embase]/lim 203 12 Sep 2011 #6. 'head cold':ab,ti OR 'head colds':ab,ti AND [embase]/lim 12 12 Sep 2011 #5. 'common cold':ab,ti OR 'common colds':ab,ti AND [embase]/lim 2,147 12 Sep 2011 #4. 'common cold'/de AND [embase]/lim 3,895 12 Sep 2011 #3. urti:ab,ti AND [embase]/lim 443 12 Sep 2011 #2. 'upper respiratory tract infection':ab,ti OR 'upper respiratory tract infections':ab,ti OR 'upper respiratory infection':ab,ti OR 'upper respiratory infections':ab,ti AND [embase]/lim 5,163 12 Sep 2011 #1. 'upper respiratory tract infection'/de AND [embase]/lim 11,596 12 Sep 2011
Appendix 2. CINAHL (Ebsco) search strategy
S40 S30 and S39 368 S39 S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 159158 S38 (MH "Quantitative Studies") 6818 S37 (MH "Placebos") 6019 S36 TI placebo* OR AB placebo* 17833 S35 TI random* OR AB random* 86958 S34 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) OR AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) 13087 S33 TI clinical* N1 trial* OR AB clinical* N1 trial* 24104 S32 PT 49013 S31 (MH "Clinical Trials+") 97955 S30 S23 and 291751 S29 S24 or S25 or S26 or S27 or S28 29666 S28 TI (sulfamethoxazole or sulphamethoxazole or sulfisoxazole ) OR AB (sulfamethoxazole or sulphamethoxazole or sulfisoxazole) 372 S27 (MH "Sulfamethoxazole+") OR (MH "Sulfisoxazole") 473 S26 TI (amoxicillin* or amoxycillin* or ampicillin* or penicillin* or tetracyclin* or erythromycin* or oxytetracyclin* or azithromycin* or ciprofloxacin* or pivampicillin* or cefuroxim* or augmentin* or co‐trimoxazole* or cefoxitin* or ceftriaxone* or cefixime* or norfloxacin* or ceftazidime* or cefaclor* or ofloxacin*) OR AB (amoxicillin* or amoxycillin* or ampicillin* or penicillin* or tetracyclin* or erythromycin* or oxytetracyclin* or azithromycin* or ciprofloxacin* or pivampicillin* or cefuroxim* or augmentin* or co‐trimoxazole* or cefoxitin* or ceftriaxone* or cefixime* or norfloxacin* or ceftazidime* or cefaclor* or ofloxacin*) 4235 S25 TI antibiotic* OR AB antibiotic* 12608 Edit S25 S24 (MH "Antibiotics+") 22197 Edit S24 S23 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 11580 S22 TI sore throat* OR AB sore throat* 502 S21 TI laryngit* OR AB laryngit* 110 S20 (MH "Laryngitis+") 569 S19 TI sinusit* OR AB sinusit* 118 Edit S19 S18 (MH "Sinusitis") OR (MH "Rhinosinusitis") 2204 S17 (MH "Paranasal Sinus Diseases") 346 S16 TI (pharyngit* or nasopharyngit* or rhinopharyngit* ) OR AB (pharyngit* or nasopharyngit* or rhinopharyngit*) 477 S15 (MH "Pharyngitis") 912 Edit S15 S14 TI ( runny N2 nasal or running N2 nasal or discharg* N2 nasal or congest* N2 nasal or blocked N2 nasal or stuff* N2 nasal or dripping N2 nasal ) OR AB ( runny N2 nasal or running N2 nasal or discharg* N2 nasal or congest* N2 nasal or blocked N2 nasal or stuff* N2 nasal or dripping N2 nasal ) 302 Edit S14 S13 TI (runny N2 nose* or running N2 nose* or discharg* N2 nose* or congest* N2 nose* or blocked N2 nose* or stuff* N2 nose* or dripping N2 nose*) OR AB (runny N2 nose* or running N2 nose* or discharg* N2 nose* or congest* N2 nose* or blocked N2 nose* or stuff* N2 nose* or dripping N2 nose*) 116 S12 (MH "Nasal Obstruction") 501 S11 TI (rhinorrhea or rhinorrhoea) OR AB (rhinorrhea or rhinorrhoea) 242 S10 TI (rhinit* or rhinosinusit* or nasosinusit*) OR AB (rhinit* or rhinosinusit* or nasosinusit*) 2333 S9 (MH "Rhinitis") 1628 S8 TI coryza OR AB coryza 23 S7 TI "head cold*" OR AB "head cold*" 3 S6 TI "common cold*" OR AB "common cold*" 428 S5 (MH "Common Cold") 1343 S4 TI urti OR AB urti 102 S3 TI "upper respiratory infection*" OR AB "upper respiratory infection*" 289 S2 TI "upper respiratory tract infection*" OR AB "upper respiratory tract infection*" 515 S1 (MH "Respiratory Tract Infections") 2937
Appendix 3. LILACS (BIREME) search strategy
Mh respiratory tract infections OR Tw respiratory tract infection$ OR Tw Infecciones del Sistema Respiratorio OR Tw Infecções Respiratórias OR Tw urti OR Mh common cold OR Tw common cold$ OR Tw Resfriado Común OR Tw Resfriado Comum OR Tw corzya OR Mh rhinitis OR Tw rhinit$ OR Tw rhinosinusit$ OR Tw nasosinusit$ OR Tw rhinorrhea OR Tw rhinorrhoea OR Tw Rinorrea OR Tw Rinorréia OR Mh nasal obstruction OR Tw Obstrucción Nasal OR Tw Obstrução Nasal OR Tw runny nose$ OR Tw running nose$ OR Tw nose$ discharge$ OR Tw nose$ congest$ OR Tw congest$ nose$ OR Tw blocked nose$ OR Tw nose$ block$ OR Tw stuff$ nose$ OR Tw dripping nose$ OR Tw nasal congest$ OR Tw congest$ nasal OR Tw nasal discharg$ OR Tw blocked nasal OR Tw stuff* nasal OR Mh pharyngitis OR Tw pharyngit$ OR Tw faringit$ OR Tw nasopharyngit$ OR Tw rhinopharyngit$ OR Tw nasofaringit$ OR Mh paranasal sinus diseases OR Tw Enfermedades de los Senos Paranasales OR Tw Doenças dos Seios Paranasais OR Mh sinusitis OR Tw sinusit$ OR Mh laryngitis OR Tw laryngit$ OR Tw laringit$ OR Tw sore throat$ [Words] and Mh Anti‐Bacterial Agents OR Tw antibiotic$ OR Tw Agentes Antibacterianos OR Tw Antibióticos OR Mh penicillins OR Tw penicil$ OR Tw amoxicil$ OR Mh amoxicillin OR Mh ampicillin OR Tw ampicil$ OR Mh pivampicillin OR Tw pivampicil$ OR Mh tetracycline OR Tw tetracycline OR Tw tetraciclina OR Mh erythromycin OR Tw erythromycin OR Tw eritromicina OR Mh oxytetracycline OR Tw oxytetracycline OR Tw Oxitetraciclina OR Mh azithromycin OR Tw azithromycin OR Tw azitromicina OR Mh ciprofloxacin OR Tw ciprofloxacin$ OR Mh cefuroxime OR Tw cefuroxim$ OR Tw augmentin OR Tw co‐trimoxazole OR Mh cefoxitin OR Tw cefoxitin$ OR Mh ceftriaxone OR Tw ceftriaxon$ OR Mh cefixime OR Tw cefixim$ OR Mh norfloxacin OR Tw norfloxacin$ OR Mh ceftazidime OR Tw ceftazidim$ OR Mh cefaclor OR Tw cefaclor OR Mh ofloxacin OR Mh ofloxacin$ OR Mh sulfamethoxazole OR Tw sulfamethoxazol$ OR Tw sulfametoxazol$ OR Tw sulphamethoxazole OR Tw sulfisoxazole [Words] and Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method OR Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR w aleator$ OR Mh research design OR Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$ [Words]
Appendix 4. Biosis Previews (ISI Thomson) search strategy
#10 #9 AND #8 DocType=All document types; Language=All languages; #9 Topic=(random* or placebo*) OR Topic=("clinical trial*") OR Topic=((singl* or doubl*) NEAR/1 blind*) DocType=All document types; Language=All languages; #8 #7 AND #6 DocType=All document types; Language=All languages; #7 Topic=(antibiotic*) OR Topic=(amoxicillin* or amoxycillin* or ampicillin* or penicillin* or tetracyclin* or erythromycin* or oxytetracyclin* or azithromycin* or ciprofloxacin* or pivampicillin* or cefuroxime* or augmentin* or co‐trimoxazole* or cefoxitin* or ceftriaxone* or cefixime* or norfloxacin* or ceftazidime* or cefaclor* or ofloxacin*) OR Topic=(sulphamethoxazole* or sulfamethoxazole or sulfisoxazole) DocType=All document types; Language=All languages; #6 #5 OR #4 OR #3 OR #2 OR #1 DocType=All document types; Language=All languages; #5 Topic=(sinusit* or laryngit* or pharyngit* or "sore throat*") DocType=All document types; Language=All languages; #4 Topic=((nose* or nasal) NEAR/2 (runny or running or blocked or congest* or discharg* or stuff* or dripping)) DocType=All document types; Language=All languages; #3 Topic=(rhinitis or rhinosinusit* or nasosinusit* or rhinopharyngit* or nasopharyngit*) OR Topic=(rhinorrhea or rhinorrhoea) DocType=All document types; Language=All languages; #2 Topic=("common cold*") OR Topic=("head cold*") OR Topic=(coryza) DocType=All document types; Language=All languages; #1 Topic=(upper NEAR/3 "respiratory tract infection*") OR Topic=(upper NEAR/3 "respiratory infection*") OR Topic=(urti) DocType=All document types; Language=All languages;
Appendix 5. Details of previous search strategies
Details of A018 2009 search update
For this 2009 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 3) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (1966 to July 2009) and EMBASE (1980 August 2009).
The following search strategy was used to search MEDLINE and CENTRAL. The MEDLINE search strategy was combined with the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). This search strategy was adapted to search EMBASE (Appendix 1). (Details of earlier searches are in Appendix 2).
MEDLINE (Ovid)
1 exp Respiratory Tract Infections/ 2 upper.tw. 3 1 and 2 4 (upper adj3 respiratory tract infection*).tw. 5 (upper adj3 respiratory infection*).tw. 6 urti.tw. 7 Common Cold/ 8 common cold*.tw. 9 exp Rhinitis/ 10 rhinit*.tw. 11 ((runny or blocked) adj2 nose*).tw. 12 ((nasal or nose) adj2 (discharge or congest*)).tw. 13 rhinosinusit*.tw. 14 exp Pharyngitis/ 15 pharyngit*.tw. 16 Nasopharyngitis/ 17 nasopharyngit*.tw. 18 head cold*.tw. 19 exp Laryngitis/ 20 laryngit*.tw. 21 or/3‐20 22 exp Anti‐Bacterial Agents/ 23 antibiotic*.tw,nm. 24 (amoxicillin* or amoxicillin* or ampicillin* or penicillin* or tetracycline* or erythromycin* or oxytetracycline* or azithromycin* or ciprofloxacin* or pivampicillin* or cefuroxime* or augmentin* or co‐trimoxazole* or cefoxitin* or ceftriaxone* or cefixime* or norfloxacin* or ceftazidime* or cefaclor* or ofloxacin*).tw,nm. 25 or/22‐24 26 25 and 21
EMBASE search strategy
18. #14 AND #17 17. #15 OR #16 16. random*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR placebo*:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti 15. 'randomized controlled trial'/exp OR 'single blind 277,843 11 Aug 2009 procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 14. #9 AND #13 13. #10 OR #11 OR #12 12. amoxicillin*:ab,ti OR amoxycillin*:ab,ti OR ampicillin*:ab,ti OR penicillin*:ab,ti OR tetracycline*:ab,ti OR erythromycin*:ab,ti OR oxytetracycline*:ab,ti OR azithromycin*:ab,ti OR ciprofloxacin*:ab,ti OR pivampicillin*:ab,ti OR cefuroxime*:ab,ti OR augmentin*:ab,ti OR 'co trimoxazole':ab,ti OR cefoxitin*:ab,ti OR ceftriaxone*:ab,ti OR cefixime*:ab,ti OR norfloxacin*:ab,ti OR ceftazidime*:ab,ti OR cefaclor*:ab,ti 11. antibiotic*:ab,ti 10. 'antibiotic agent'/exp 9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 8. ((nasal OR nose) NEAR/2 (discharge OR congest*)):ab,ti 7. ((runny OR blocked) NEAR/2 nose*):ab,ti 6. rhinit*:ab,ti OR pharyngit*:ab,ti OR rhinopharyngit*:ab,ti OR nasopharyngit*:ab,ti OR laryngit*:ab,ti 5. 'rhinitis'/exp OR 'rhinosinusitis'/exp OR 'pharyngitis'/exp OR 'laryngitis'/exp 4. 'common cold':ab,ti OR 'common colds':ab,ti OR 'head cold':ab,ti OR 'head colds':ab,ti 3. 'common cold'/exp 2. 'upper respiratory tract infection':ab,ti OR 'upper respiratory tract infections':ab,ti OR 'upper respiratory infection':ab,ti OR 'upper respiratory infections':ab,ti OR urti:ab,ti 1. 'upper respiratory tract infection'/exp
Searching other resources
We wrote to the trial authors of all the included studies and asked them if they knew of any unpublished material. We did not find any additional papers by this mechanism.
Details of the 2005 A018 update
For the 2005 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2005, Issue 1); MEDLINE (January 1966 to March, Week 1, 2005); EMBASE (1980 to December 2004), the Family Medicine Database (1908, volume 1 to 1993, volume 13; this database was discontinued in 1993) and reference lists of articles, and we contacted principal investigators. All languages were included in the search strategy. Principal investigators were contacted to seek unpublished literature but none was found.
MEDLINE search strategy for 2005 update
1 exp Anti‐Bacterial Agents/ 2 (antibiotic$ or amoxicillin$ or amoxycillin$ or ampicillin$ or penicillin$ or tetracycline$ or erythromycin$ or oxytetracycline$ or azithromycin$ or amoxycillin$ or ciprofloxacin$ or pivampicillin$ or cefuroxime$ or augmentin$ or co‐trimoxazole$ or cefoxitin$ or ceftriaxone$ or cefixime$ or norfloxacin$ or ceftazidime$ or cefaclor$ or ofloxacin$).mp. 3 or/1‐2 4 exp respiratory tract infections/ 5 upper.mp. 6 4 and 5 7 (upper respiratory tract infection$ or URTI$).mp. [mp=title, original title, abstract, name of substance, MeSH subject heading] 8 exp common cold/ 9 common cold.mp. 10 exp rhinitis/ 11 rhinitis.mp. 12 exp pharyngitis/ 13 pharyngitis.mp. 14 exp nasopharyngitis/ 15 nasopharyngitis.mp. 16 exp laryngitis/ 17 laryngitis.mp. 18 or/6‐17 19 3 and 18 20 randomized controlled trial.pt. 21 controlled clinical trial.pt. 22 randomized controlled trials.sh. 23 random allocation.sh. 24 double blind method.sh. 25 single blind method.sh. 26 or/20‐25 27 Animals/ 28 Human/ 29 27 not 28 30 26 not 29 31 clinical trial.pt. 32 exp clinical trials/ 33 (clin$ adj25 trial$).ti,ab. 34 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 35 placebos.sh. 36 placebo$.ti,ab. 37 random$.ti,ab. 38 or/31‐37 39 38 not 29 40 30 or 39 41 19 and 40
The above search terms were also used to search CENTRAL and were adapted to search EMBASE but no additional studies were found.
Details of A014 2005 search update
In previous versions of the review, we searched CENTRAL (The Cochrane Library 2002, Issue 1), MEDLINE (1966 to 2002) and EMBASE (1990 to 2002).
In this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2005, Issue 1) which includes the ARI Group's specialised trials register; MEDLINE (1966 to April Week 3, 2005), EMBASE (1997 to December 2004) and the references of relevant articles. Authors and pharmaceutical companies were contacted.
MEDLINE (OVID) and CENTRAL were searched using the following terms which were combined with the highly sensitive search strategy devised by Dickersin (Dickersin 1994).
1 exp SINUSITIS/ 2 sinusitis 3 exp RHINITIS/ 4 rhinitis 5 exp Paranasal Sinus Diseases/ 6 paranasal sinus disease$ 7 exp NASOPHARYNGITIS/ 8 nasopharyngitis 9 exp Common Cold/ 10 common cold$ 11 (rhinorrhoea or rhinorrhea) 12 nasal discharge 13 or/1‐12 14 exp Anti‐Bacterial Agents/ 15 antibiotic$ 16 (amoxicillin or amoxycillin or ampicillin or azithromicin or azithromycin or cefaclor or penicillin or sulphamethoxazole or sulfisoxazole) 17 or/14‐16 18 13 and 17
The above terms were modified to search for trials in EMBASE (WebSPIRS).
OLD MEDLINE was accessed via the National Library of Medicine Gateway and combined with the following terms to identify randomised controlled trials: random: OR "double‐blind" OR "double blind" OR "single‐blind" OR "single blind" OR placebo: OR "clinical trial:" OR "drug therapy".
We searched the list of references in relevant publications for reports of randomised controlled trials. Written communication with the authors of trials included in the review and with major pharmaceutical companies (with offices in Australia) that manufacture antibiotics was undertaken. There were no language restrictions.
Data and analyses
Comparison 1. Antibiotic versus placebo, common cold.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persisting symptoms 1 to 7 days | 6 | 1047 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.59, 1.51] |
| 2 Persisting symptoms day 1 to 7 with Herne out | 5 | 979 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.70, 1.76] |
| 3 Persisting symptoms days 1 to 7 in adults | 4 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.07] |
| 4 Persisting symptoms day 1 to 7 children only | 2 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.59, 3.15] |
| 5 Persisting symptoms day 1 to 7 with Hoagland and McKerrow out | 3 | 810 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.19] |
| 6 Persisting symptoms 1 to 7 days with Kaiser out | 5 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.45, 2.22] |
| 7 Adverse effects | 6 | 1495 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.01, 3.21] |
| 8 Adverse effects in adults | 4 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 2.62 [1.32, 5.18] |
| 9 Adverse effects in children | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.63] |
| 10 Adverse effects with McKerrow out | 4 | 1054 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.67, 4.20] |
Comparison 2. Antibiotic versus placebo, clear rhinitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent rhinitis (clear) with Herne added as clear | 2 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.48] |
Comparison 3. Antibiotic versus placebo, purulent rhinitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent rhinitis (purulent) | 4 | 723 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.47, 1.13] |
| 2 Persistent purulent rhinitis with Herne added as purulent | 5 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.04] |
| 3 Persistent purulent rhinitis with currently available medication | 4 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.46, 1.18] |
| 4 Adverse effects for purulent rhinitis studies | 4 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.10, 1.94] |
Comparison 4. Antibiotic versus placebo, sore throat.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sore throat at day 7 | 2 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.12, 1.82] |
Comparison 5. Antibiotic versus placebo, work loss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any work loss | 1 | 836 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.13] |
Comparison 6. Antibiotic versus placebo, loss of appetite.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Loss of appetite at day 8 | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.44, 2.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De Sutter 2002.
| Methods | Randomised controlled trial, double‐blind | |
|---|---|---|
| Participants | 12 years old or older with respiratory tract infection and purulent rhinorrhoea | |
| Interventions | Amoxicillin 500 mg 3 times daily for 10 days | |
| Outcomes | Duration of general illness (NS); duration of pain (NS) and duration of purulent rhinorrhoea (P = 0.007). Persisting purulent rhinitis in amoxicillin group 116/189 and in the placebo group 136/195 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Adequate: sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding maintained due to identical capsules and confirmed by asking physicians and participants to guess what group they were in |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Almost identical follow‐up in both arms; 42/416 (10%) drop‐out |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Possibility that > 60% of participants may have had sinusitis (see Table 1 in De Sutter 2002 paper) |
Gordon 1974.
| Methods | Randomised controlled trial, double‐blind | |
|---|---|---|
| Participants | Children at a casualty department, symptoms referable to respiratory tract, rhinitis most common 78% to 95%, most had cough and wheeze, no antibiotics in previous week, no pneumonitis 63% of children had wheeze in the under 2‐year age group while 22% had wheeze in the 2 years to 4 years age group. In children under 2 years 13% had clinical signs in the chest and 13% in those over the age of 6 years. 88 had streptococcal throat swabs, only 2 of which were positive. Number of drop‐outs = 89 | |
| Interventions | Ampicillin, penicillin, erythromycin palmitate each 125 mg 4 x daily and in over 2‐year olds given double dose; duration not stated. Medication given in numbered bottles and the code was not broken until the end of the trial | |
| Outcomes | 89 children started Outcomes at 5 days: placebo better than ampicillin (P = 0.05) for relief of symptoms while antibiotics no better for symptoms or signs | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | Process not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical numbered bottles |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Any child who took less than half the medication was excluded; no number is given and it appears that the report concerns only those 89 remaining (0% drop‐out) |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Unclear risk | "Most of the children had cough or wheeze suggesting that the intrathoracic passages were involved" |
Herne 1980.
| Methods | Randomised controlled trial, double‐blind | |
|---|---|---|
| Participants | 75 military men aged 16 to 23 years with acute seasonal upper respiratory tract infections with no obvious severe signs. 7 were removed from the analysis because they clinically appeared to have streptococcal tonsillitis, therefore n1 = 75, n2 = 68 (i.e. 7 removed). No drop‐outs | |
| Interventions | Xibornol 250 mg 2 tablets 3 times daily or tetracycline 250 mg 2 tablets 3 times per day or matching placebo | |
| Outcomes | Persisting symptoms (Table 7 in Herne 1980) xibornol 3/22, tetracycline 4/24 and placebo 10/22 therefore all antibiotics 7/46. Antibiotics were significantly better than placebo for fever at day 5 and for residual clinical signs and symptoms. Rhinitis 3/46 for antibiotic and 5/22 for placebo (not stated if it was purulent or clear); sore throat 2/46 for antibiotic and 8/22 for placebo. No adverse effects were reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Refer to randomisation and double‐blinded but no details |
| Allocation concealment (selection bias) | Unclear risk | Process not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up apart from 7 drop‐outs with streptococcal tonsillitis in approximately equal numbers. 7/75 drop‐out (9%) |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | Possibility that more participants may have had bacterial rather than viral infections, for example pharyngitis/rhinopharyngitis 21 participants and tonsillitis 10 participants (in Table 1 of Herne 1980) |
Hoaglund 1950.
| Methods | Dispensed in rotation by pharmacist | |
|---|---|---|
| Participants | Healthy young males with local and constitutional symptoms and thick nasal discharge, military recruits. No drop‐outs, n = 309 | |
| Interventions | Placebo, aureomycin 1 g/day for first 69 participants, then aureomycin 2 g/day in the remaining 85 | |
| Outcomes | No benefit in 24 hours: 39/154 (antibiotic), 51/155 (placebo) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Medication dispensed by pharmacist "in rotation" |
| Allocation concealment (selection bias) | Unclear risk | No process is described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Given aureomycin or inert yellow capsules but it is not stated that the placebo was identical |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We assume 100% follow‐up in this military study |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | High risk | 24‐hour outcome assessment possibly too short for any antibacterial effect |
Howie 1970.
| Methods | Randomised controlled trial, double‐blind; medicine sent to participants in advance of illness | |
|---|---|---|
| Participants | Given at home to male participants, 20 to 49 years, from Glasgow General Practices; start medicine if cold or influenza‐like illness not getting better after 2 days. No physical exam hence no lower respiratory tract signs reported. Of 829 potential participants, 293 took antibiotics and 250 took placebo. 66 were excluded because of chronic respiratory signs or symptoms and 198 took no medication. 22 returned no cards | |
| Interventions | Demethylchlortetracycline 300 mg twice daily for 5 days and matching placebo; given 2 days after onset | |
| Outcomes | Purulent sputum/spit/purulent nasal discharge and cough ‐ all NS ‐ the denominator is the number of illnesses not individuals. Adverse effects 2.9% (placebo) and 9.5% (antibiotic) or 25/293 (antibiotic) and 7/250 (placebo) (NS). 5 participants had adverse effects more than once therefore change 25 to 20 and 293 to 288 (participants as denominator)Work loss: 98/448 on antibiotic treatment and 96/388 on placebo Work loss 1.1 average days versus 1.5 per illness on placebo. Work loss 22% (antibiotic) and 25% (placebo); more than 10 days 2.9% (antibiotic) versus 5.3% (placebo) | |
| Notes | Denominator is illnesses, except for adverse effects and work loss which are numbers of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated to be randomised |
| Allocation concealment (selection bias) | Low risk | Adequate code number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo stated to be matching and the difference was not detectable to the medically qualified participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Both groups got similar numbers of treatments; 28/857 people did not return their symptom cards; the report is therefore on the remaining 829; 66 participants who may have had chronic bronchitis were excluded from the main analysis as were 40 illnesses (not participants) where people attended their doctor rather than take the trial medication. 28/829 (3.3%) dropped out and 106 were excluded |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | Yes |
Kaiser 1996.
| Methods | Randomised controlled trial, double‐blind | |
|---|---|---|
| Participants | Adult participants aged over 16 with a history of acute upper respiratory infection with nasal congestion or rhinorrhoea or pharyngitis who presented with a common cold to the hospital outpatient department. Exclusion = facial pain, sinusitis, purulent pharyngitis, purulent or chronic bronchitis, bronchopneumonia, use of antibiotics in previous 10 days, allergy to aminopenicillins. Each patient had a nasopharyngeal aspiration for bacteriological culture (n = 288; excluding 26 drop‐outs) | |
| Interventions | 5 days of co‐amoxiclav 375 mg tid for 5 days or a placebo | |
| Outcomes | Table 2 (in Kaiser 1996) at day 5. Persistent or worse 97/146 (antibiotic) and 94/142 (placebo)Adverse effects 34/146 (antibiotic) and 7/142 (placebo); GI disturbances or diarrhoeaSignificant finding for cure with antibiotics in the group that were culture positive for S. pneumoniae, M. catarrhalis or H. influenzae (58 participants; 27% versus 4%; P value 0.001) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated randomly assigned but did not state how it was done |
| Allocation concealment (selection bias) | Unclear risk | Process not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Bottles were numbered otherwise same for intervention and control |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6.2% drop‐out rate |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Unclear risk | 19% of participants had sinusitis on X‐ray which may have influenced the group with positive nasopharyngeal swabs |
Lexomboon 1971.
| Methods | ? random selection of coloured strips from a box; only pharmacists knew of allocation | |
|---|---|---|
| Participants | Hospital outpatients children 6 months to 12 years with upper respiratory symptoms and fever 64% had bacterial pathogens in nasopharyngeal samples. Drop‐outs not mentioned, n = 261 | |
| Interventions | Penicillin V for 7 days 30 mg/kg, tetracycline 40 mg/kg for 7 days and placebo syrup | |
| Outcomes | Failure of treatment in penicillin 3/86, 5/88 tetracycline and 4/87 in placebo | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Therapeutic regimen determined by selection of coloured chips from a box |
| Allocation concealment (selection bias) | Unclear risk | Process depended on blinding of selecting physician remaining intact throughout the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only the pharmacy knew the contents of the syrups implying the clinicians and the participants did not know |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Whole report includes only those in whom there was no definitive clinical diagnosis (this is appropriate) and those who attended at least 3 of the 4 follow‐up visits ‐ the number failing to attend for follow‐up is not mentioned |
| Selective reporting (reporting bias) | Low risk | Yes, apart from those not returning for follow‐up |
| Other bias | Low risk | Yes |
McKerrow 1961.
| Methods | Randomised controlled trial | |
|---|---|---|
| Participants | Normal participants from research unit. The pneumoconiosis group in the factory is not included. No drop‐outs. Aged 15 to 69 years, n = 33. This study included 2 groups. An office group otherwise healthy and a factory group some of whom had pneumoconiosis. The factory subgroup was excluded | |
| Interventions | Tetracycline 15 mg 3 daily for 3 days or oxytetracycline 15 mg or chlortetracycline 15 mg 3 daily. The antibiotic was chosen according to the sensitivity reactions of the participants' saliva. In factory 22 took placebo and 28 took active medication; in the unit 15 took medication and 18 took placebo | |
| Outcomes | First cold. Table 3 (in McKerrow 1961) unit office data. No cure or improvement in 3 days. 5/15 (A ‐ all tetracycline), 8/18 (placebo). Adverse events for both groups 9/43 antibiotic 0/40 placebo. Adverse effects in unit group assigned to tetracycline = 3/15 and 0/18 placebo | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There was mention of allocation at random (2‐step: first random to active versus control; then random allocation for active group to an antibiotic to which their flora were sensitive) |
| Allocation concealment (selection bias) | Low risk | An elaborate process described to mask the fact that there was no identical dummy tablet for the chlortetracycline |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although there was no identical dummy tablet for the chlortetracycline, the authors were satisfied that their elaborate process kept the medication identity hidden; they went to particular lengths to ensure this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | None detected |
| Other bias | High risk | The trial was for 3 days and they used very low doses of tetracycline, e.g. 15 mg (today the doses would be at least 10 times those doses) |
Taylor 1977.
| Methods | Randomised controlled trial with 3 groups; double‐blind | |
|---|---|---|
| Participants | Nasopharyngitis, pharyngo‐tonsillitis and bronchitis Children aged 2 to 10 years in general practice. 43% had auscultatory evidence of more extensive airways disease (n = 188 excluding 9 drop‐outs) | |
| Interventions | Placebo, co‐trimoxazole 40 mg/5 ml, amoxicillin 125/5 ml for 5 days | |
| Outcomes | Numbers with activity not returned day 7 Co‐trimoxazole = 6/75, amoxicillin = 6/54, placebo = 3/59Appetite not returned day 7 Co‐trimoxazole = 11/75, amoxicillin = 11/54, placebo = 11/59Symptoms at day 8 Runny nose clear = amoxicillin 8/54, co‐trimoxazole = 21/75, placebo = 22/59Runny nose purulent at day 8 Amoxicillin = 3/54, co‐trimoxazole = 3/75, placebo = 9/59Sore throat at day 8 Amoxicillin = 3/54, co‐trimoxazole = 3/75, placebo = 3/59Adverse effects at day 8 (rash, vomiting and diarrhoea) Co‐trimoxazole = 18/75, amoxicillin = 8/54, placebo = 11/59 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The term "randomly assigned" was used |
| Allocation concealment (selection bias) | Unclear risk | Process not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not clear if medications were masked but word "double‐blind" used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% did not complete (as they were placed on open antibiotic in the meantime) but unlikely to affect the results |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted |
| Other bias | High risk | 43% of participants had lower respiratory signs so may not all have had common colds |
Todd 1984.
| Methods | Randomised controlled trial, placebo‐controlled, 4 groups | |
|---|---|---|
| Participants | Children older than 2 months of age in an army medical centre with non‐transparent anterior nasal discharge. Normal tympanic membranes, no evidence of foreign body, no history of allergic rhinitis and no adverse reactions to the study drugs. 142 started, 107 analysed, 35 drop‐outs | |
| Interventions | Cephalexin oral 25 to 50 mg/kg/day max 2 g and pseudoephedrine/triprolidine 1 to 4 teaspoons per day and matching placebos. Medication code broken if group A streptococcus found on nasopharyngeal cultures and excluded if developed a treatable illness, for example otitis media | |
| Outcomes | Day 5 to 6 nasal purulent discharge 22/29 cephalexin + pseudoephedrine/triprolidine, 20/26 cephalexin + placebo, 17/27 pseudoephedrine/triprolidine + placebo, 15/24 placebo + placeboDrug adverse effect (rash, hyperactivity, diarrhoea, vomiting) 2/23, placebo 4/17 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequentially into pre‐randomised groups (we do not understand what this means) |
| Allocation concealment (selection bias) | Unclear risk | Process not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Identical appearing placebos", neither parents not health professionals were aware of the contents of the medications |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 35 drop‐outs due to developing another treatable illness; failure to return for follow‐up; non‐confirmed compliance, leaving 107. A further 9 grew Group A streptococci and were put on antibiotics but the outcomes table (Table 6) appears to include 107 participants at the highest count and there is a lot of missing data. 35/142 dropped out (25%) |
| Selective reporting (reporting bias) | Low risk | No selection beyond the missing data |
| Other bias | Low risk | None noted |
Vogt 1966.
| Methods | Randomised controlled trial with 2 groups | |
|---|---|---|
| Participants | Paediatric population, median age in each group between 1 and 2 years | |
| Interventions | Nasal phenylephrine with nitrofurazone 0.02% (intervention) and phenylephrine (control) | |
| Outcomes | Clearance of purulent rhinitis at 4 days | |
| Notes | 43 of the 50 intervention group and 41 of the control group were given an oral antibiotic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | May not have been formally randomised; participants were "haphazardly assigned" to groups |
| Allocation concealment (selection bias) | Unclear risk | Process not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The medication was probably put into identical bottles that were marked only with a code number. Neither patient nor physicians knew the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up, information was collected from every patient |
| Selective reporting (reporting bias) | Low risk | Yes |
| Other bias | Low risk | No other biases noted |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackerman 1968 | Excluded as had active control, i.e. Robitussin, a cough suppressant. Used penicillin V 100,000 units 4 times daily for 10 days, tetracycline 50 mg 4 times daily for 10 days, compared with Robitussin. Results: no difference in shortening the median duration of illness or in preventing secondary complications |
| Banks 1965 | Excluded as used ascorbic acid as control group. Results: no difference between tetracycline, spiramycin and ascorbic acid |
| Banks 1969 | This paper is a reappraisal of 2 studies reported in Medical Officer 1968;119:7‐8. Excluded as control groups had ascorbic acid and kaolin This was a preventive trial of the common cold in 1962 to 1963 and another trial in 1965 to 1967 for preventive treatment of the common cold. Excluded as ascorbic acid plus spiramycin was compared with spiramycin |
| Bateson 1961 | Not randomised and controlled |
| Bessel‐Lorck 1959 | Excluded as antibiotics used for prophylaxis |
| Burke 1956 | Excluded as antibiotics given prophylactically. Results: in children awaiting tonsillectomy antibiotics were associated with 25 colds compared with 60 on calcium tablets (P < 0.01). The antibiotic group had 30 weeks school absence while the group on calcium tablets had 80 weeks school absence (P < 0.001) |
| Cronk 1954 | Excluded as control group had salicylamide in their medication. Results: better response to salicylamide than to penicillin |
| Darelid 1993 | Excluded as study subjects had cough for 10 days minimum; M. catarrhalis in 75% of children. An open study with control group untreated. Results: 88% better in erythromycin group versus 36% in untreated group (P < 0.0001) |
| Fraser 1962 | Excluded as used aspirin in control participants. Results: phenoxymethylpenicillin and oxytetracycline were no better than aspirin |
| Gottfarb 1994 | Excluded as study subjects had cough for 10 days minimum. Results: 71% improved on antibiotic while 22% improved on placebo |
| Gupta 1997 | Excluded as had no placebo control group |
| Haight 1954 | Excluded as 28.7% had group A beta haemolytic streptococcus. Results: in non‐bacterial group there was no difference between erythromycin and penicillin and placebo |
| Hardy 1956 | Excluded as not randomised into treatment groups, i.e. supposedly rotated medication but left with unequal numbers in each of 4 groups. The authors of the paper expressed their concern over the adequacy of randomisation. Also excluded as at least 14% had Group A beta haemolytic streptococci. Results: no benefit from antibiotics |
| Haye 1998 | All participants had symptoms for more than 10 days |
| Jones 1953 | Excluded as used acetylsalicylic acid in control participants. Results: no benefit from antibiotics |
| Knox 1962 | Excluded as used acetylsalicylic acid in control participants. Results: no difference between antibiotics and aspirin |
| Kuh 1949 | Excluded as antibiotics given prophylactically |
| Lapin 1984 | Excluded as antibiotics given prophylactically. Penicillin group experienced a lower rate of upper respiratory tract infections and a reduction in the number of febrile days compared with the no prophylaxis group |
| Lockhart 1961 | Excluded as not randomised or controlled. Results: not able to assess any benefit |
| Marlow 1989 | Excluded as did not meet the entry criteria for URTI, i.e. no rhinitis. Results: statistically significant improvement in sickness but not in soreness of throat |
| McLane 1952 | Excluded as control group received APC (acetylsalicylic acid, phenacetin and caffeine) and/or antihistamine. Results: APC and antihistamine and procaine penicillin were more effective at relieving nasopharyngeal symptoms and preventing secondary complications than APC alone or with antihistamine |
| Reinert 1991 | Excluded because antibiotic combined with topical cortisone. Results: the tixocortol neomycin combination was more effective at controlling symptoms at 7 days than the placebo |
| Ritchie 1958 | Corrupted randomisation. Results: the proportion of participants who had full colds was consistently lower in the antibiotic group than in the control group |
| Seal 1953 | Excluded as antibiotics given prophylactically and focus on streptococcal tonsillitis. Results: both penicillin and chlortetracycline were effective in the prevention of streptococcal infections during the period of administration |
| Sulman 1958 | Excluded as not randomised. Results: showed a benefit for chloramphenicol |
| Sutrisna 1991 | Excluded as there was no placebo control group. Results: antibiotic no better than control group |
| Townsend 1960 | Excluded as used prophylactic antibiotics. Results: no benefit from antibiotics |
| Townsend 1962 | Excluded as control group not obtained by randomisation. Results: no benefits from antibiotics |
| Traisman 1955 | Excluded as not randomised ‐ simply divided in to 4 groups. Results: no benefit from antibiotics |
| Wald 1991 | Patients were children less than 2 years age who had persistent nasal discharge for at least 10 days that had not begun to improve before trial entry. RCT with 6 children in antibiotic group, 7 children on placebo, evaluated at 10 days when 5/6 treatment and 2/7 placebo group had resolved |
| Walker 1955 | Excluded as was using antibiotic prophylactically. Results: no benefit from antibiotics |
| Wynn‐Williams 1961 | Excluded as participants had complicated past histories for inclusion. Results: 58% reduction in bronchitis in tetracycline group and 49% reduction in the placebo group. The average durations of time off school were 1.9 days and 2.9 days respectively. No statistical testing was reported |
Contributions of authors
Both review authors (BA, TK) read the abstracts and extracted the data. Discrepancies were resolved by discussion. BA pooled the data and both review authors were involved with the writing.
Sources of support
Internal sources
- No sources of support supplied
External sources
- The Charitable Trust of the Auckland Faculty of the Royal New Zealand College of General Practitioners, New Zealand.
- The National Prescribing Service of Australia (NPS), Australia.
Declarations of interest
There are no known conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
De Sutter 2002 {published data only}
- Sutter AI, Meyere MJ, Christiaens TC, Duriel ML, Peersman W, Maeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea?. Journal of Family Practice 2002;51:317‐23. [PubMed] [Google Scholar]
Gordon 1974 {published data only}
- Gordon M, Lovell S, Dugdale AE. The value of antibiotics in minor respiratory illness in children. Medical Journal of Australia 1974;1:304‐6. [PubMed] [Google Scholar]
Herne 1980 {published data only}
- Herne N. Double blinded randomized trial comparing xibornol, tetracycline and placebo in seasonal upper respiratory tract infections [Essai comparatif en doble aveugle randomise xibornol, tetracycline et placebo dans les infections saisonnieres des vioes aeriennes superiours]. Medicines et Maladies Infectieuses 1980;10:185‐90. [Google Scholar]
Hoaglund 1950 {published data only}
- Hoaglund RJ, Dietz EN, Myers PW, Cosand HC. Aureomycin in the treatment of the common cold. New England Journal of Medicine 1950;243:773‐5. [DOI] [PubMed] [Google Scholar]
Howie 1970 {published data only}
- Howie JG, Clark GA. Double‐blind trial of early demethylchlortetracycline in minor respiratory illness in general practice. Lancet 1970;2:1099‐102. [DOI] [PubMed] [Google Scholar]
Kaiser 1996 {published data only}
- Kaiser L, Lew D, Hirshel B, Auckenthaler R, Morabia A, Heald A, et al. Effects of antibiotic treatment in the subset of common‐cold patients who have bacteria in nasopharyngeal secretions. Lancet 1996;1996:1507‐10. [DOI] [PubMed] [Google Scholar]
Lexomboon 1971 {published data only}
- Lexomboon U, Duangmani C, Kusalasai V, Sunakorn P, Olson LC, Noyes HE. Evaluation of orally administered antibiotics for treatment of upper respiratory infections in Thai children. Journal of Pediatrics 1971;78:772‐8. [DOI] [PubMed] [Google Scholar]
McKerrow 1961 {published data only}
- McKerrow CB, Oldham PD, Thomson S. Antibiotics and the common cold. Lancet 1961;1:185‐7. [Google Scholar]
Taylor 1977 {published data only}
- Taylor B, Abbott GD, Kerr MM. Amoxycillin and co‐trimoxazole in presumed viral respiratory infections in childhood: a placebo controlled trial. BMJ 1977;2:552‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Todd 1984 {published data only}
- Todd JK, Todd N, Damanto J, Todd WA. Bacteriology and treatment of purulent nasopharyngitis: a double blind, placebo‐controlled evaluation. Pediatric Infectious Diseases 1984;3:226‐32. [DOI] [PubMed] [Google Scholar]
Vogt 1966 {published data only}
- Vogt FC. Medical management of purulent rhinitis. A double‐blind comparison of vasoconstrictor agent alone with a combination of vasoconstrictor and antimicrobial drugs. Clinical Pediatrics 1966;5(9):547‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackerman 1968 {published data only}
- Ackerman BD. Treatment of undifferentiated respiratory infections in infants. Clinical Pediatrics 1968;7:391‐5. [DOI] [PubMed] [Google Scholar]
Banks 1965 {published data only}
- Banks HS. Common cold: controlled clinical trials. British Medical Journal 1965;2:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banks 1969 {published data only}
- Banks HS, Ritchies JM. Re‐appraisal: a new look at the common cold. Journal of the Royal College of General Practitioners 1969;18:166‐72. [PMC free article] [PubMed] [Google Scholar]
Bateson 1961 {published data only}
- Bateson PR, Allison RM, Wall T, Sons. The treatment of the common cold with antibiotics in a factory population. British Journal of Clinical Practice 1961;15:543‐9. [PubMed] [Google Scholar]
Bessel‐Lorck 1959 {published data only}
- Bessel‐Lorck. Prophylaxis for common cold in adolescents in a skiing camp. Die Medizinische Welt 1959;10:2126‐7. [PubMed] [Google Scholar]
Burke 1956 {published data only}
- Burke JB. Prophylactic sulphadimidine in children subject to recurrent infections of upper respiratory tract. British Medical Journal 1956;March 10:538‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cronk 1954 {published data only}
- Cronk GO, Naumann DE, McDermott K. A controlled study of oral penicillin G in the treatment of non‐specific upper respiratory infections. American Journal of Medicine 1954;16:804‐9. [DOI] [PubMed] [Google Scholar]
Darelid 1993 {published data only}
- Darelid J, Lofgren S, Malmvall BE. Erythromycin treatment is beneficial for longstanding Moraxella catarrhalis associated cough in children. Scandinavian Journal of Infectious Diseases 1993;25:323‐9. [DOI] [PubMed] [Google Scholar]
Fraser 1962 {published data only}
- Fraser PK, Hatch LA, Hughes KEA. A comparison between aspirin and antibiotics in the treatment of minor respiratory infections. Lancet 1962;1:614‐7. [DOI] [PubMed] [Google Scholar]
Gottfarb 1994 {published data only}
- Gottfarb P, Bruaner A. Children with persistent cough‐outcome with treatment and role of Moraxella catarrhalis. Scandinavian Journal of Infectious Diseases 1994;26:545‐51. [DOI] [PubMed] [Google Scholar]
Gupta 1997 {published data only}
- Gupta S, Bhushan V, Srivastava G. The therapeutic trial of chloramphenicol, Eskaycillin (ampicillin) and co‐trimoxazole in respiratory tract infection of childhood. Indian Paediatrics 1977;14:391‐3. [PubMed] [Google Scholar]
Haight 1954 {published data only}
- Haight TH, Kahn FH, Ziegra SR. Efficacy of erythromycin in the treatment of acute respiratory infections. US Armed Forces Medical Journal 1954;5:1405‐22. [PubMed] [Google Scholar]
Hardy 1956 {published data only}
- Hardy LM, Traismam HS. Antibiotics and chemotherapeutic agents in the treatment of uncomplicated respiratory infections in children. Journal of Pediatrics 1956;48:146‐56. [DOI] [PubMed] [Google Scholar]
Haye 1998 {published data only}
- Haye R, Lingaas E, Hoivik HO, Odegard T. Azithromycin versus placebo in acute infectious rhinitis with clinical symptoms but without radiological signs of maxillary sinusitis. European Journal of Clinical Microbiology and Infectious Diseases 1998;17:309‐12. [DOI] [PubMed] [Google Scholar]
Jones 1953 {published data only}
- Jones PN, Bigham RS Jr, Manning PR. Use of antibiotics in nonbacterial respiratory infections. Journal of the American Medical Association 1953;153(4):262‐4. [DOI] [PubMed] [Google Scholar]
Knox 1962 {published data only}
- Knox JDE. Comparison between aspirin and anti‐biotics in minor respiratory infections. Lancet 1962;1:1358‐9. [DOI] [PubMed] [Google Scholar]
Kuh 1949 {published data only}
- Kuh C, Collen MF. Mass penicillin prophylaxis: an experiment with negative results. Journal of the American Medical Association 1949;140:1324‐8. [DOI] [PubMed] [Google Scholar]
Lapin 1984 {published data only}
- Lapin JH. Prophylaxis of upper respiratory infections in children treated with oral penicillin. Journal of Pediatrics 1948;32:119‐24. [DOI] [PubMed] [Google Scholar]
Lockhart 1961 {published data only}
- Lockhart R. Treatment of common cold by Terramycin. Health Bulletin 1961;19:12‐3. [Google Scholar]
Marlow 1989 {published data only}
- Marlow RA, Torrez AJ, Haxby D. The treatment of non‐streptococcal pharyngitis with erythromycin. A preliminary study. Family Medicine 1989;21:425‐7. [PubMed] [Google Scholar]
McLane 1952 {published data only}
- McLane RA. Clinical evaluation of combined drug therapy in acute upper respiratory infections. Journal of the Medical Society of New Jersey 1952;49:509‐10. [PubMed] [Google Scholar]
Reinert 1991 {published data only}
- Reinert P, Narcy P, Paliwoda A, Rouffiac E. Evaluation of tisocortal pivalate‐neomycin combination versus a placebo excipient in acute rhinopharyngitis in children. Annals de Pediatrie 1991;38:503‐8. [PubMed] [Google Scholar]
Ritchie 1958 {published data only}
- Ritchie JM. Antibiotics in small doses for the common cold. Lancet 1958;1:618‐21. [DOI] [PubMed] [Google Scholar]
Seal 1953 {published data only}
- Seal JR. The prophylaxis of acute respiratory infections with oral penicillin or chlortetracycline ‐ proceedings of the symposium on antibiotics. Antibiotics Annual. Medical Encyclopedia Inc, 1953‐4.
Sulman 1958 {published data only}
- Sulman FG. Chloramphenicol and control of the common cold. Lancet 1958;ii:1355‐6. [Google Scholar]
Sutrisna 1991 {published data only}
- Sutrisna B, Freruchs RR, Reingold AL. Randomised controlled trial of effectiveness of ampicillin in mild acute respiratory infections in Indonesian children. Lancet 1991;338:471‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsend 1960 {published data only}
- Townsend EH. Chemoprophylaxis during respiratory infections in private pediatric practice. American Journal of Diseases in Children 1960;99:566‐73. [DOI] [PubMed] [Google Scholar]
Townsend 1962 {published data only}
- Townsend EH, Radebaugh JF. Prevention of complications of respiratory illness in pediatric practice. New England Journal of Medicine 1962;266:683‐9. [DOI] [PubMed] [Google Scholar]
Traisman 1955 {published data only}
- Traisman HS. A controlled study in the uses of chemotherapeutic and antibiotic agents in uncomplicated upper respiratory infections in children. Illinois Medical Journal 1955;107:221‐4. [PubMed] [Google Scholar]
Wald 1991 {published data only}
- Wald ER. Purulent nasal discharge. Pediatric Infectious Diseases Journal 1991;10(4):329‐33. [DOI] [PubMed] [Google Scholar]
Walker 1955 {published data only}
- Walker SH. Tetracycline in the control of upper respiratory infections of infants. Antibiotics Annual. First Edition. New York: Medical Encyclopedia, Inc, 1955‐6:341‐4. [PubMed] [Google Scholar]
Wynn‐Williams 1961 {published data only}
- Wynn‐Williams SH. Tetracycline in the control of common upper respiratory infections of infants. Antibiotic Annual. First Edition. New York: Medical Encyclopedia, Inc, 1961:341‐4. [PubMed] [Google Scholar]
Additional references
Arason 1996
- Arason A, Kristinsson KG, Sigurdsson JA, Stefansdottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ 1996;313:387‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arroll 2000
- Arroll B, Goodyear‐Smith F. General practitioner management of upper respiratory tract infections: when are antibiotics prescribed?. New Zealand Medical Journal 2000;113:493‐6. [PubMed] [Google Scholar]
Arroll 2001
- Arroll B, Kenealy T. Antibiotics for acute bronchitis. Four reviews and still no answers: our clinical definitions are at fault. BMJ 2001;322:939‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Mar 1992
- Mar C. Managing sore throat: a literature review, II. Do antibiotics confer benefit?. Medical Journal of Australia 1992;156:644‐9. [PubMed] [Google Scholar]
Fahey 1998
- Fahey T, Stocks N, Thomas T. Quantitative systematic review of randomised controlled trials comparing antibiotic with placebo for acute cough in adults. BMJ 1998;316:906‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadomski 1993
- Gadomski AM. Potential interventions for preventing pneumonia among young children: lack of effect of antibiotic treatment for upper respiratory infections. Pediatric Infectious Disease Journal 1993;12:115‐20. [DOI] [PubMed] [Google Scholar]
Gonzales 1999
- Gonzales R, Barrett P, Steiner J. The relation between purulent manifestations and antibiotic treatment of upper respiratory tract infections. Journal of General Internal Medicine 1999;14:151‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
ICHPPC 1986
- ICHPPC‐2. International Classification of Health Problems in Primary Care. Third Edition. Oxford: Oxford University Press, 1986. [Google Scholar]
Kaplan 1971
- Kaplan EL, Top FH, Dudding BA, Wannamake L. Diagnosis of streptococcal pharyngitis: differentiation of active infection from the carrier state in the symptomatic child. Journal of Infectious Diseases 1971;123:490‐501. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester: Wiley‐Blackwell, 2011. [Google Scholar]
Mainous 1997
- Mainous AG, Hueston WJ, Eberlain C. Colour of respiratory discharge and antibiotic use. Lancet 1997;350:1077. [DOI] [PubMed] [Google Scholar]
McAvoy 1994
- McAvoy B, Davis P, Raymont A, Gribben B. The Waikato Medical Care Survey. New Zealand Medical Journal 1994;107:387‐433. [PubMed] [Google Scholar]
McGregor 1995
- McGregor A, Dovey S, Tilyard M. Antibiotic use in upper respiratory tract infections in New Zealand. Family Practice 1995;12:166‐70. [DOI] [PubMed] [Google Scholar]
Morris 2007
- Morris PS, Leach AJ. Antibiotics for persistent nasal discharge (rhinosinusitis) in children. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001094.pub2] [DOI] [PubMed] [Google Scholar]
Ochoa 2000
- Ochoa C, Eiros JM, Inglada L, Vallano A, Guerra L. Assessment of antibiotic prescription in acute respiratory infections in adults. The Spanish study group on antibiotic treatment. Journal of Infection 2000;41:73‐80. [DOI] [PubMed] [Google Scholar]
Pharmac 2008
- Pharmac. Annual review. www.pharmac.govt.nz (accessed 5 January 2010) 2008; Vol. section 2:16.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Reynolds 1996
- Reynolds JEF, Parfitt K (editors). Martindale: The Extra Pharmacopeia. 1st Edition. London: Royal Pharmaceutical Society, 1996. [Google Scholar]
Rosenstein 1998
- Rosenstein N, Phillips W, Gerber M, Marcy S, Schwartz B, Dowell S. The common cold ‐ principles of judicious use of antimicrobial agents. Pediatrics 1998;101:181‐4. [Google Scholar]
Smith 2011
- Smith S, Fahey T, Smucny J, Becker L. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD000245.pub2] [DOI] [PubMed] [Google Scholar]
Snow 2001
- Snow V, Mottur‐Pilson C, Gonzales R. Principles of appropriate antibiotic use for treatment of non specific upper respiratory tract infections in adults. Annals of Internal Medicine 2001;134:487‐9. [DOI] [PubMed] [Google Scholar]
Spector 1995
- Spector SL. The common cold: current therapy and natural history. Journal of Allergy and Clinical Immunology 1995;95:1133‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinks 2011
- Spinks A, Glasziou PP, Mar C. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD000023.pub3] [DOI] [PubMed] [Google Scholar]
Stott 1976
- Stott NC, West RR. Randomised controlled trial of antibiotics in patients with cough and purulent sputum. British Medical Journal 1976;2:556‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venekamp 2013
- Venekamp RP, Sanders S, Glasziou PP, Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000219.pub3] [DOI] [PubMed] [Google Scholar]
Verkatesum 1995
- Verkatesum P, Innes JA. Antibiotic resistance in common acute respiratory pathogens. Thorax 1995;50:481‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Arroll 1998
- Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000247.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arroll 2002
- Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD000247.pub2] [DOI] [PubMed] [Google Scholar]
Arroll 2005
- Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD000247.pub2] [DOI] [PubMed] [Google Scholar]
Arroll 2010
- Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD000247.pub2] [DOI] [PubMed] [Google Scholar]