Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases (original) (raw)
Summary
Background
The cutaneous manifestations of COVID‐19 disease are poorly characterized.
Objectives
To describe the cutaneous manifestations of COVID‐19 disease and to relate them to other clinical findings.
Methods
We carried out a nationwide case collection survey of images and clinical data. Using a consensus we described five clinical patterns. We later described the association of these patterns with patient demographics, the timing in relation to symptoms of the disease, the severity and the prognosis.
Results
The lesions may be classified as acral areas of erythema with vesicles or pustules (pseudo‐chilblain) (19%), other vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%) and livedo or necrosis (6%). Vesicular eruptions appear early in the course of the disease (15% before other symptoms). The pseudo‐chilblain pattern frequently appears late in the evolution of the COVID‐19 disease (59% after other symptoms), while the rest tend to appear with other symptoms of COVID‐19. The severity of COVID‐19 shows a gradient from less severe disease in acral lesions to more severe in the latter groups. The results are similar for confirmed and suspected cases, in terms of both clinical and epidemiological findings. Alternative diagnoses are discussed but seem unlikely for the most specific patterns (pseudo‐chilblain and vesicular).
Conclusions
We provide a description of the cutaneous manifestations associated with COVID‐19 infection. These may help clinicians approach patients with the disease and recognize cases presenting with few symptoms.
What is already known about this topic?
- Previous descriptions of cutaneous manifestations of COVID‐19 were case reports and mostly lacked illustrations.
What does this study add?
- We describe a large, representative sample of patients with unexplained skin manifestations and a diagnosis of COVID‐19, using a consensus method to define morphological patterns associated with COVID‐19.
- We describe five clinical patterns associated with different patient demographics, timing and prognosis, and provide illustrations of these patterns to allow for easy recognition.
Linked Editorial: Hay et al. Br J Dermatol 2020; 183:3–4.
In December 2019, the first cases of pneumonia with unknown cause were reported in Wuhan, China.1 The new pathogen, called SARS‐CoV‐2, was isolated from samples of the lower respiratory tract of infected patients,2 and the resulting disease was called COVID‐19 (Coronavirus Disease 2019). SARS‐CoV‐2 has rapidly spread, reaching the level of a pandemic disease.
COVID‐19 can affect different organ systems, probably including the skin. There are few descriptions of the cutaneous manifestations of COVID‐19. Twenty per cent of patients in an Italian medical ward had cutaneous lesions, described as rash or urticaria and including one case of ‘chickenpox‐like’ lesions.3 Other case reports describe a rash mistaken for dengue,4 acro‐ischaemia in children5 and critical patients,6 plaques on the heels,7 and urticaria.8,9 Most of these reports lack clinical images, due to safety concerns,10 and they describe few patients in hospital settings.
There is no previous detailed classification or description of the cutaneous manifestations of COVID‐19. This information may prove useful to manage patients and to recognize paucisymptomatic patients, and might provide prognostic information. The recognition of paucisymptomatic patients could also be helpful for epidemiological control, especially in areas where diagnostic tests are scarce.11
For all of these reasons we conducted a nationwide case collection survey among dermatologists, to allow a quick description of the cutaneous manifestations of COVID‐19 disease and to relate them to other clinical findings.
Materials and methods
From the start of the study until 8 April 2020 (the last available data), the World Health Organization considered Spain an area of SARS‐CoV‐2 local transmission.12 With the support of the Spanish Academy of Dermatology, we asked all Spanish dermatologists (many of them relocated to the acute care of patients during the COVID‐19 pandemic) to include patients in this study for 2 weeks. All patients were included who had an eruption of recent onset (previous 2 weeks) and no clear explanation, and suspected (patients presenting with compatible symptoms) or confirmed COVID‐19 (with laboratory confirmation of SARS‐CoV‐2, irrespectively of clinical signs and symptoms), using the definitions of the European Centre for Disease Prevention and Control.13 A standardized questionnaire was used, and pictures taken for most of the patients. Expecting four or five patterns of similar incidence, we had assumed that collecting 60 confirmed cases would be adequate for an initial description. In the middle of the recruitment period, we had identified 120 cases. Their photographs were independently reviewed by a group of four dermatologists without knowing about the rest of the clinical information, and a consensus was reached on the cutaneous patterns of disease. These patterns were applied to the whole dataset of pictures and were further refined without knowledge of the rest of the clinical information. These morphological diagnostic data were later merged with the rest of the clinical information for analysis.
In most areas, viral tests were especially scarce in this period and were rarely done for less severe cases or cases with a clear diagnosis. Due to the low sensitivity of some diagnostic tests and their scarcity, we accepted cases with clinical diagnosis of the disease (suspected cases) but performed a sensitivity analysis to check that the results did not change when including only confirmed patients. Analysis consisted of description of the data and distribution tests (χ2‐test for qualitative variables and anova for quantitative variables) and was done using Stata 16 (StataCorp, College Station, TX, USA).
The study was authorized by an ethics committee (HUGCDN: 2020‐172‐1‐COVID‐19) and the Spanish Drug Agency (ACG‐CLO‐2020‐01), and was included in EnCEPP (EUPAS34469). All patients, or their next of kin in the case of minors, gave their informed consent to participate and an explicit consent to use their pictures in publications.
Results
We collected data on 429 cases from 3 to 16 April 2020, during the peak of the epidemic in Spain. Five cases were excluded for being compatible with other diagnosis (three herpes zoster and two psoriasis). Also, 31 patients with cutaneous lesions were excluded for not meeting the definition of confirmed or suspected COVID‐19, and 18 were excluded because of missing information. The overall impression was that the majority of the excluded patients showed similar lesions, mostly described as acral. The final sample included 375 patients. The case fatality rate in the sample was 1·9%.
Clinical patterns
Consensus following review of the images led to the description of five major clinical patterns (Appendix S1; see Supporting Information). Nearly all of the patients could be classified into these groups, and a few unusual cases are highlighted in the description. The groups are as follows.
1. Acral areas of erythema–oedema with some vesicles or pustules (pseudo‐chilblain) (19% of cases). These lesions may resemble chilblains and have purpuric areas, affecting the hands and feet (Figure 1a, b). They were usually asymmetrical.
Figure 1.
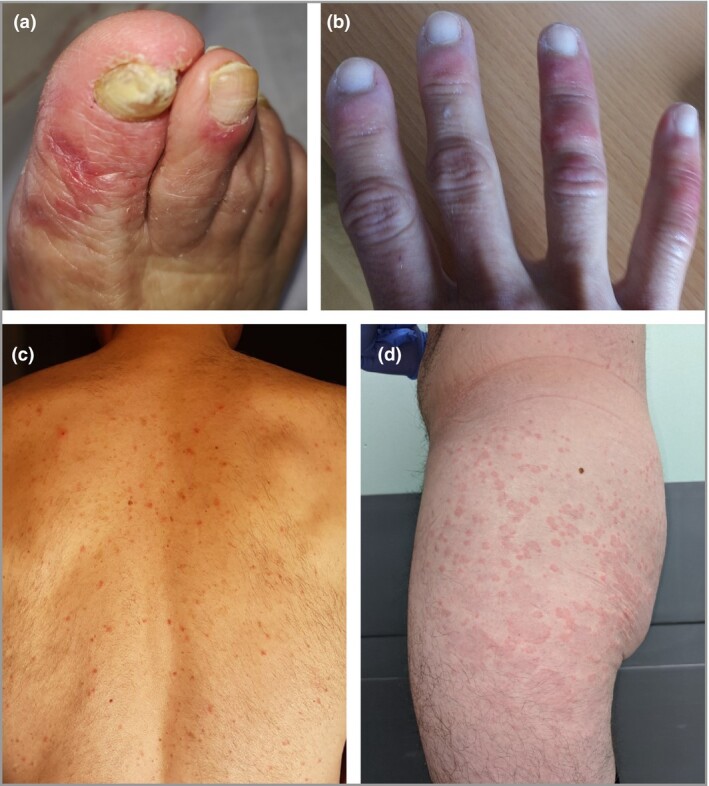
All of the patients shown had confirmed COVID‐19. (a, b) Acral areas of erythema–oedema with vesicles or pustules (pseudo‐chilblain). (c) Monomorphic (i.e. at same stages) disseminated vesicles. (d) Urticarial lesions.
2. Other vesicular eruptions (9%). Some presented on the trunk and consisted of small monomorphic vesicles (i.e. at same stages, unlike polymorphic vesicles in chickenpox) (Figure 1c). They may also affect the limbs, have haemorrhagic content, and become larger or diffuse.
3. Urticarial lesions (19%) (Figure 1d). These are mostly distributed on the trunk or dispersed. A few cases were palmar.
4. Other maculopapules (47%). Some of these cases showed perifollicular distribution and varying degrees of scaling (Figure 2a). Some were described as being similar to pityriasis rosea. Purpura was also sometimes present, either punctiform or on larger areas. A few cases showed infiltrated papules on the extremities, mostly the dorsum of the hands, that look pseudovesicular (Figure 2b) or resemble erythema elevatum diutinum or erythema multiforme (Figure 2c).
Figure 2.

All of the patients shown had confirmed COVID‐19. (a) Maculopapular eruption. Some of the lesions are perifollicular. (b) Acral infiltrated papules (pseudovesicular). (c) Acral papules (erythema multiforme like). (d) Livedoid areas.
5. Livedo or necrosis (6%). These patients showed different degrees of lesions suggesting occlusive vascular disease, including areas of truncal or acral ischaemia (Figure 2d).
A few patients showed other manifestations such as enanthem or purpuric flexural lesions.
Dermatologists also perceived an increased number of cases of herpes zoster in patients with COVID‐19.
Characteristics associated with each clinical pattern
The different clinical patterns were associated with differences in demographics and in other clinical manifestations (Tables 1 and 2). Pseudo‐chilblain lesions affected younger patients, lasted longer (mean 12·7 days), took place later in the course of COVID‐19 disease and were associated with less severe disease (in terms of hospital admission, pneumonia, intensive care unit admission or mortality). These lesions could cause pain (32%) or itch (30%). Vesicular lesions appeared in middle‐aged patients, lasted for a mean of 10·4 days, appeared more commonly than the other types (15%) before other symptoms and were associated with medium severity. Itching was common (68%).
Table 1.
Characteristics of the 375 patients with COVID‐19, and the therapy and prognosis of each group
| Characteristics | Pseudo‐chilblain | Vesicular | Urticarial | Maculopapules | Livedo/necrosis | _P_‐value |
|---|---|---|---|---|---|---|
| Number of patients (% of row) | 71 (19) | 34 (9) | 73 (19) | 176 (47) | 21 (6) | |
| Female | 48 (68) | 19 (56) | 47 (64) | 98 (56) | 10 (48) | 0·28 |
| Age (years), mean ± SD | 32·5 ± 21·8 | 45·6 ± 20 | 48·7 ± 19·9 | 55·3 ± 20·2 | 63·1 ± 17·3 | < 0·001 |
| Smoking | 7 (10) | 2 (6) | 12/60 (20%)a | 21/140 (15%)b | 2/13 (15%)c | 0·40 |
| Cough | 37 (52) | 25 (74) | 48 (66) | 135 (77) | 14 (67) | 0·004 |
| Dyspnoea | 18 (25) | 12 (35) | 30 (41) | 100 (57) | 11 (52) | < 0·001 |
| Fever | 44 (62) | 24 (71) | 55 (75) | 140 (80) | 17 (81) | 0·068 |
| Asthenia | 37 (52) | 21 (62) | 47 (64) | 110 (63) | 11 (52) | 0·49 |
| Headache | 27 (38) | 12 (35) | 24 (33) | 55 (31) | 9 (43) | 0·74 |
| Nausea, vomiting, diarrhoea | 17 (24) | 8 (24) | 18 (25) | 58 (33) | 6 (29) | 0·52 |
| Anosmia, ageusia | 13 (18) | 10 (29) | 21 (29) | 40 (23) | 6 (29) | 0·51 |
| Pneumonia | 10 (14) | 10 (29) | 38 (52) | 110 (63) | 15 (71) | < 0·001 |
| Hospital admission | 9 (13) | 11 (32) | 32 (44) | 107 (61) | 18 (86) | < 0·001 |
| ICU or noninvasive mechanical ventilation | 2 (3) | 2 (6) | 8 (11) | 21 (12) | 7 (33) | 0·004 |
| COVID‐19 status | < 0·001 | |||||
| Suspected case | 42 (59) | 17 (50) | 24 (33) | 54 (31) | 4 (19) | |
| Confirmed case | 29 (41) | 17 (50) | 49 (67) | 122 (69) | 17 (81) | |
| Duration of cutaneous eruption (days), mean ± SD | 12·7 ± 8 | 10·4 ± 9·3 | 6·8 ± 7·8 | 8·6 ± 6·8 | 9·4 ± 5·4 | < 0·001 |
| Presence of cutaneous symptoms | 52 (73) | 28 (82) | 69 (95) | 112 (64) | 6 (29) | < 0·001 |
| Pain | 23 (32) | 3 (9) | 1 (1) | 4 (2) | 1 (5) | |
| Burning | 8 (11) | 2 (6) | 1 (1) | 9 (5) | 2 (10) | |
| Itch | 21 (30) | 23 (68) | 67 (92) | 99 (56) | 3 (14) | |
| Treatment | 38 (54) | 26 (76) | 52 (71) | 138 (78) | 16 (76) | 0·004 |
| With paracetamol or without treatment | 65 (92) | 29 (85) | 54 (74) | 120 (68) | 13 (62) | < 0·001 |
| Paracetamol | 32 (45) | 21 (62) | 33 (45) | 82 (47) | 8 (38) | 0·43 |
| NSAIDs | 11 (15) | 2 (6) | 6 (8) | 16 (9) | 1 (5) | 0·48 |
| Chloroquine, hydroxychloroquine | 6 (8) | 7 (21) | 23 (32) | 79 (45) | 11 (52) | < 0·001 |
| Lopinavir, ritonavir | 3 (4) | 2 (6) | 13 (18) | 54 (31) | 6 (29) | < 0·001 |
| Tocilizumab | 2 (3) | 1 (3) | 4 (5) | 9 (5) | 3 (14) | 0·34 |
| Systemic corticosteroids | 1 (1) | 3 (9) | 7 (10) | 21 (12) | 6 (29) | 0·004 |
| Azithromycin | 3 (4) | 7 (21) | 13 (18) | 39 (22) | 2 (10) | 0·005 |
| Patient survival | 71 (100) | 34 (100) | 73 (100) | 172 (98) | 19 (90) | 0·055 |
Table 2.
Temporal relationship with other manifestations of COVID‐19
| Timing of cutaneous signs with respect to other symptoms | Pseudo‐chilblain | Vesicular | Urticarial | Maculopapules | Livedo/necrosis | Total |
|---|---|---|---|---|---|---|
| Before, n (%) | 5 (7) | 5 (15) | 3 (4) | 8 (5) | 1 (5) | 22 |
| Same time, n (%) | 24 (34) | 19 (56) | 43 (61) | 108 (61) | 18 (86) | 212 |
| After, n (%) | 42 (59) | 10 (29) | 25 (35) | 60 (34) | 2 (10) | 139 |
| Total | 71 | 34 | 71a | 176 | 21 | 373 |
Urticarial and maculopapular lesions showed very similar patterns of associated findings. They lasted for a shorter period (mean 6·8 days for urticarial and 8·6 for maculopapular), usually appeared at the same time as the other symptoms and were associated with more severe COVID‐19 disease (2% mortality in the maculopapular sample). Itching was very common for urticariform lesions (92%) and occurred in 56% of cases of maculopapular lesions. Livedoid or necrotic lesions were seen in older patients with more severe disease (10% mortality). However, the manifestations of COVID‐19 in this group were more variable, including transient livedo, with some having COVID‐19 that did not require hospitalization.
The severity of the associated disease followed a gradient, from less severe disease in pseudo‐chilblain to most severe in patients with livedoid presentations, as shown by the increasing percentages of pneumonia, hospital admission and intensive care requirements.
Of 71 patients with pseudo‐chilblain, only one had a previous history of perniosis. The percentage with confirmed presence of SARS‐CoV‐2 in this group was 41%; lower than in the other morphological groups (Table 1).
Patients in the group with urticarial eruptions were receiving drugs more commonly than those with pseudo‐chilblain or vesicular lesions, but less commonly than those with maculopapules or livedoid lesions, in relationship with increased severity.
We identified three familial clusters with lesions. One family had two siblings with pseudo‐chilblain and another showing a generalized vesicular eruption with suspected COVID‐19. Another two families showed clusters of lesions but did not have symptoms of respiratory COVID‐19 and did not enter the study; each of the two families included two children, who simultaneously developed pseudo‐chilblains.
We reproduced the same analysis using only confirmed cases of COVID‐19, and the results are similar (Tables S1 and S2; see Supporting Information).
Discussion
We have described five cutaneous clinical patterns and several subpatterns associated with COVID‐19. These patterns appear at different times in the disease, and are associated with different duration, severity and probably prognosis.
Previous publications have described some of these patterns but are based on very few cases. They also lack photography or use inadequate terms, like ‘chickenpox‐like’ for monomorphic lesions or ‘acro‐ischaemic’ for acral areas of erythema–oedema with some vesicles or pustules. No temporal relationship with symptoms or prognosis has previously been described.
One strength of our study is that the description of clinical patterns has been done by experts based only on morphology. The resulting patterns were shown to allow for easy classification of patients and to correlate with differences in demographics and severity.
Given the large number and distribution of participants, the sample is likely to be representative of the overall distribution of cutaneous lesions in COVID‐19. However, we cannot define the source population, and, lacking a denominator, we have no measures of the incidence of clinical manifestations, only relative ones. We have omitted patients in the spectrum of severe disease due to difficulties in obtaining consent. This explains the low case fatality rate. However, descriptions of the lesions in these patients are less useful for diagnosis, as their diagnosis is usually obvious. Patients in the general population without clinical or virological confirmation of COVID‐19 disease were also under‐represented. We thought that this restrictive admission of reports was needed to increase the specificity of the results.
During the study period, testing was not done in most cases of mild disease. As we aimed to describe the lesions in less severe cases, we accepted both confirmed and suspected cases in our study. The results show that both groups showed similar cutaneous lesions (Appendix S1; see Supporting Information) and epidemiological results (Tables S1 and S2; see Supporting Information). Patients excluded for lack of COVID‐19 diagnostic criteria (n = 31) also had similar patterns, confirming that the inclusion of suspected patients did not bias the results.
As the study describes a short period of follow‐up, it is better defined as a cross‐sectional design rather than a cohort. Data on the duration and severity of the disease and the outcome are limited to the time when the patient was observed. It is possible that some of the patients with less severe disease will worsen with time. Against this limitation, the data show that the less severe forms were described late in the evolution of the disease, and have a longer duration, so it is unlikely that they will worsen over time.
Our study included any unexplained cutaneous lesions in patients with COVID, so it is possible that some of them have alternative causes. Pseudo‐chilblain may look like perniosis, and as these lesions appear later in the evolution and are less commonly associated with virological confirmation, it is possible that they are not related to the COVID‐19. We think that the pseudo‐chilblain pattern is linked to COVID‐19 because pseudo‐chilblain appeared in a warm weather period, dermatologists perceived a greatly increased incidence, and patients frequently had COVID‐19 contacts. Only one of the 71 patients had a previous history of chilblain. Overall, 29 of 71 (41%) had SARS‐CoV‐2 confirmed and we found three simultaneous familial clusters. The late appearance of pseudo‐chilblains might explain the frequently negative polymerase chain reaction results.14 Monomorphic disseminated vesicular lesions and acral vesicular–pustulous lesions are probably quite specific and their appearance is coherent with lesions in other viral exanthemas.
Most of the urticarial and maculopapular lesions might not be very helpful for diagnosis, as these are common and may have many different causes. Drug reactions may be an important and difficult differential diagnosis. The patients with these presentations had more severe disease and received more drugs. Regarding their relationship with the other manifestations, urticarial and maculopapular lesions may be considered similar.
Livedoid and necrotic lesions were relatively uncommon, and appeared mostly in elderly patients and those with severe disease. As the number of patients is lower for this subset the information is less precise. In two case reports livedoid lesions were transient.15 These might be primary lesions of COVID‐19 or simply indicate complications leading to vascular occlusion, as COVID‐19 has been linked to alterations in coagulation and vascular damage.6,16,17
It is unusual, from our previous experience with cutaneous manifestations of viral diseases, that a single virus can lead to several different clinical patterns, especially as different patterns do not coexist in the same patient. Patients who may be classified as having more than one pattern are very uncommon. A hypothesis to explain this polymorphism may be that some of them have alternative causes, or there are differences in the virus or the host. The fact that some of the lesions, even in patients with confirmed COVID‐19, are similar to those in other viral infections (notably parvovirus),18 and the perceived increased number of cases of zoster, raises the possibility of some of these being the result of coinfection and uncertainty as to whether SARS‐CoV‐2 is responsible for this.
In terms of arousing suspicion of COVID‐19, we feel that pseudo‐chilblain and vesicular lesions may be useful as indicators of disease. They uncommonly (10 of 373 cases with data) presented preceding other symptoms in our sample. Pseudo‐chilblain lesions more commonly appear later during the disease and are not associated with severe disease, so they might be more useful as epidemiological markers than for diagnosis. It is possible that the sampling strategy might bias this result, and pseudo‐chilblain might appear without other COVID‐19 symptoms more commonly in the general population. Urticarial lesions may be due to many causes and mostly did not precede other symptoms in our study, so they are unlikely to lead to diagnosis. Regarding maculopapular lesions, they tend to co‐occur with other symptoms, and most of them are not specific. A few subtypes, such as the pseudovesicular type (Figure 2b) and those resembling erythema elevatum diutinum (Appendix S1; see Supporting Information) or erythema multiforme (Figure 2c), could lead to suspicion of a diagnosis. Livedoid or necrotic lesions occur late in the evolution and are probably unhelpful for diagnosis. However, they fit nicely with the idea of vascular damage due to COVID‐19.
In conclusion, we provide a description of the cutaneous manifestations associated with COVID‐19. These may help clinicians approach patients with the disease and recognize cases with few symptoms. The usefulness of these patterns for diagnosis should be confirmed in clinical use. We suggest that further research could be improved by having more tests to confirm COVID‐19 and to exclude other infections, and by describing clinicopathological correlation and some of the patterns that have been grouped in our study.
Acknowledgments
M.A. Descalzo‐Gallego provided support with data management and analysis. We thank the following Spanish dermatologists for their generous contributions: E. Cruz Gómez, L. Carnero, Z. Martínez de Lagrán, A. Do Campo, A. Estébanez Corrales, A. González Quesada, A. Mateos Mayo, A. Perea Polak, A. Pulido, A. Tuneu Valls, A.C. Villanueva Álvarez‐Santullano, A.G. Angulo, A.M. Rosell Díaz, C. Nadal, D. Caro Gutiérrez, D. Jiménez Gallo, E. Fernández Cogolludo, E. Roo Rodríguez, E. San Juan Lasser, G. Mele i Ninot, G. Sais, H. Perandones, I. Molina López, I. Poveda, I. Balaguer Franch, J. Boix, J. Galán Sánchez, J. Melgosa, J. Tercedor, J.A. Llamas Carmona, J.L. López Estebaranz, M. Ara, L. Galvany Rossell, L. Marqués Martín, L. Navarro, M. de Troya, J.L. Galán, M. Elosua, M. Escoda, M. García Bustunduy, M. Martín Dorado, M. Nieto Benito, M. Sidro, M.A. Arregui, M.C. Fuente Lázaro, M.V. Ara Martín, N. Eiris, P. Burgos‐Blasco, P. Pasquali, G. Petiti, E. Ledo, R. Linares Navarro, R. Taberner Ferrer, A. Juárez Martín, S. Aparicio Fernández, A. Juárez Martín, T. Toledo Pastrana, Y. Yelamos, A. González Quesada, A. Viñolas, A. Suárez, A. Estébanez, M. Ubals, B. Espadafor, P. Fernández, M.L. Fernández, T. Repiso, E. Agut, X. García Navarro, G. Ruiz Carrillo, D. Jiménez, L.M. Nieto Benito, J. Monte Serrano, M. Alegre, M. Ferrer, V. García‐Patos Briones, M. Velasco Guidonet, Á. Gómez Tomás, P. Castro García, V. Cabezas Calderón, D. Vega‐Díez, R. Pérez‐Mesonero and M. González‐Cañete. This work is part of the PhD degree of Alba Catalá, in the Department of Medicine, Dermatology, Universidad Autónoma de Barcelona.
Supplementary Material
bjd19163-sup-0001-SupinfoS1
Appendix S1 Photographic atlas.
bjd19163-sup-0002-SupinfoS2
Table S1 Characteristics of the confirmed cases.
Table S2 Temporal relationship with other manifestations in confirmed cases of COVID‐19.
bjd19163-sup-0003-VideoS1
Video S1 Author video.
bjd19163-sup-0004-PowerpointS1
Powerpoint S1 Journal Club Slide Set.
References
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; DOI: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- Li Q, Guan X, Wu P. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34:e212–13. [DOI] [PubMed] [Google Scholar]
- Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 2020; 82:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzota F, Troccoli T. Acute acro‐ischemia in the child at the time of COVID‐19. Available at: https://www.fip-ifp.org/wp-content/uploads/2020/04/acroischemia-ENG.pdf (last accessed 4 May 2020).
- Zhang Y, Cao W, Xiao M. et al. [Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia]. Zhonghua Xue Ye Xue Za Zhi 2020; 41:E006 (in Chinese). [DOI] [PubMed] [Google Scholar]
- Estebanez A, Perez‐Santiago L, Silva E. et al. Cutaneous manifestations in COVID‐19: a new contribution. J Eur Acad Dermatol Venereol 2020; DOI: 10.1111/jdv.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JJ, Dong X, Cao YY. et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; DOI: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Lu S, Lin J, Zhang Z. et al. Alert for non‐respiratory symptoms of Coronavirus Disease 2019 (COVID‐19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID‐19 patients. J Med Virol 2020; DOI: 10.1002/jmv.25776. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Nieto D, Ortega‐Quijano D, Segurado‐Miravalles G. et al. Comment on: Cutaneous manifestations in COVID‐19: a first perspective. Safety concerns of clinical images and skin biopsies. J Eur Acad Dermatol Venereol 2020; DOI: 10.1111/jdv.16470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet. COVID‐19: learning from experience. Lancet 2020; 395:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Novel coronavirus situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (last accessed 4 May 2020).
- European Centre for Disease Prevention and Control. Case definition and European surveillance for COVID‐19, as of 2 March 2020. Available at: https://www.ecdc.europa.eu/en/case-definition-and-european-surveillance-human-infection-novel-coronavirus-2019-ncov (last accessed 4 May 2020).
- Romani J, Baselga E, Mitja O. et al. Chilblain and acral purpuric lesions in Spain during COVID confinement: retrospective analysis of 12 cases. Actas Dermosifiliogr 2020; DOI: 10.1016/j.adengl.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient livedo reticularis. J Am Acad Dermatol 2020; DOI: 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiao M, Zhang S. et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020; 382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe A, Birckel E, Krieger S. et al. A distinctive skin rash associated with Coronavirus Disease 2019? J Eur Acad Dermatol Venereol 2020; DOI: 10.1111/jdv.16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjd19163-sup-0001-SupinfoS1
Appendix S1 Photographic atlas.
bjd19163-sup-0002-SupinfoS2
Table S1 Characteristics of the confirmed cases.
Table S2 Temporal relationship with other manifestations in confirmed cases of COVID‐19.
bjd19163-sup-0003-VideoS1
Video S1 Author video.
bjd19163-sup-0004-PowerpointS1
Powerpoint S1 Journal Club Slide Set.